Abstract
Tetrahymena is a useful eukaryotic model for biochemistry and molecular cell biology studies. We previously demonstrated that targeted ectopic DNA elimination, also called co-Deletion (coDel), can be induced by the introduction of an internal eliminated sequence (IES)-target DNA chimeric construct. In this study, we demonstrate that coDel occurs at most of the loci tested and can be used for the production of somatic gene KO strains. We also showed that coDel at two loci can be simultaneously induced by a single transformation; thus, coDel can be used to disrupt multiple gene loci in a single cell. Therefore, coDel is a useful tool for functional genetics in Tetrahymena and further extends the usefulness of this model organism.
Keywords: Tetrahymena, DNA elimination, RNAi, gene knockout
THE ciliated protozoan Tetrahymena thermophila can grow at an exceptionally high rate (its doubling time is approximately 2 hr) and can reach a high density (a few million cells per milliliter) under simple and inexpensive culture conditions (reviewed in Orias et al. 2000). In combination with robust genetic manipulation methods (reviewed in Chalker 2012), Tetrahymena is a useful model eukaryote for biochemistry and molecular cell biology studies (reviewed in Collins and Gorovsky 2005).
Three strategies for loss-of-function genetic studies have been established for this organism. The first strategy is a germline knockout (KO), in which one of the two gene copies in the diploid micronucleus (MIC) is replaced with a drug-resistance gene by homologous recombination, and two heterozygous strains are then sexually crossed to obtain a homozygous germline KO strain (Cassidy-Hanley et al. 1997). The second strategy is somatic KO, in which one of the ∼45 copies of a gene in the polyploid macronucleus (MAC) is replaced with a drug-resistance gene by homologous recombination, and “phenotypic assortment” is used to obtain cells with the drug-resistance gene at all loci (Merriam and Bruns 1988). Phenotypic assortment is possible because MAC chromosomes are randomly segregated into daughter cells in vegetative cell division and a stepwise increase of the drug in culture selects for cells that have more drug-resistance genes. The final strategy is gene knockdown (KD) by RNA interference (RNAi), in which a construct expressing long hairpin RNA that is complementary to the gene of interest is introduced into a nonessential locus in the MAC and small RNAs produced from the hairpin RNA post-transcriptionally silence the expression of the gene (Howard-Till and Yao 2006).
Although the first two gene KO strategies have been reliably used to study individual gene functions, they are not suitable for high-throughput genetic screening because the integration of a KO construct into the MIC germline occurs at very low efficiency and the phenotypic assortment process used in somatic KOs require the careful control of drug concentrations in each strain and culture step. Moreover, it takes ∼1 month to obtain KO strains using these methods. For germline KO, two sexual crosses are necessary to produce a homozygous KO, and the Tetrahymena have a sexually immature stage that lasts ∼2 weeks after the first sexual cross. For somatic KO, phenotypic assortment requires at least 2–3 weeks for completion. In contrast to the gene KO strategies, RNAi KD can be done in a shorter period of time and is thus suitable for high-throughput genetic screening. However, RNAi KD often only incompletely shuts down expression of a target gene (Howard-Till and Yao 2006; Cheng et al. 2010; Chung and Yao 2012; Lukaszewicz et al. 2013) and may thus result in the misinterpretation of the gene function.
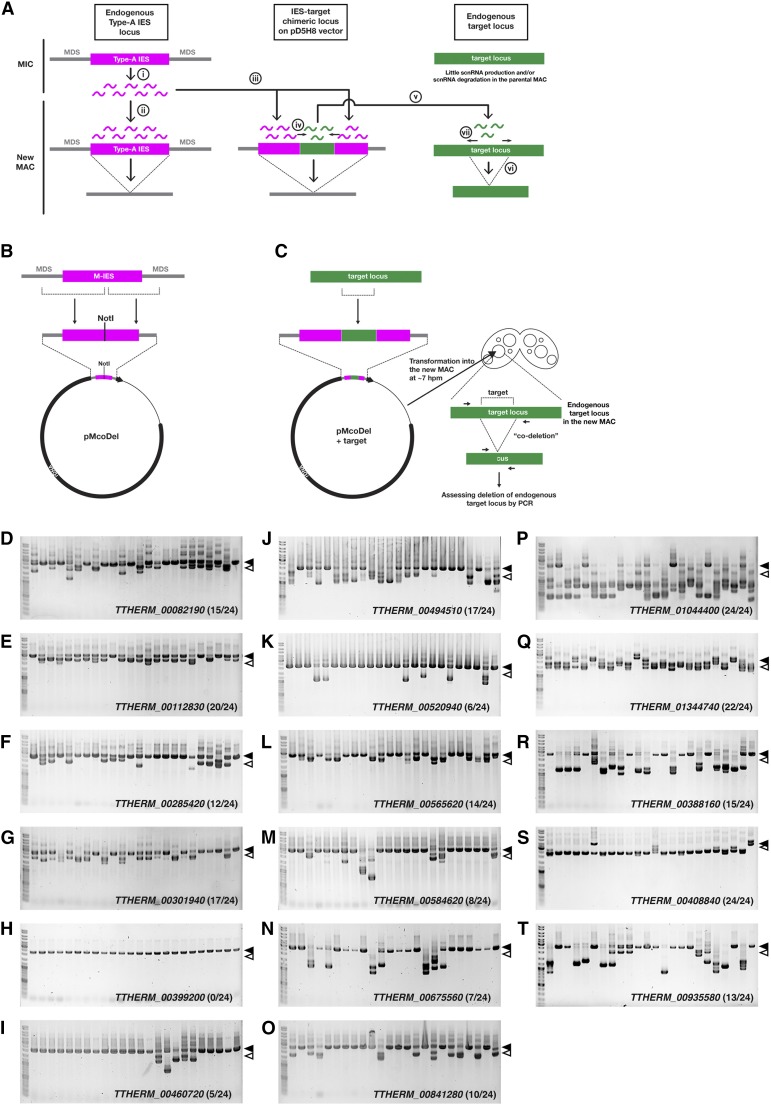
During the development of the new MAC of Tetrahymena, >8000 DNA segments called internal eliminated sequences (IESs) were reproducibly eliminated. IESs are recognized by 26- to 32-nt small RNAs called scnRNAs (Schoeberl et al. 2012). IESs can be classified into two types: type A IESs that are recognized by scnRNAs produced by themselves and type B IESs that are recognized by scnRNAs produced by type A IESs (Noto et al. 2015). In a recent study, we demonstrated that ectopic DNA elimination can be induced in a targeted manner through the introduction of a chimeric construct of a type A IES and a fragment of a target locus into the new MAC (Noto et al. 2015). We call this phenomenon co-Deletion (coDel). Several pieces of experimental evidence indicate that coDel is induced by the molecular mechanism shown schematically in Figure 1A. First, scnRNAs produced from the endogenous type A IES in the MIC (Figure 1A, i) recognize the endogenous copy of the IES in the new MAC (Figure 1A, ii) and the type A IES in the introduced chimeric construct (Figure 1A, iii); scnRNA production is then induced from the target sequence adjacent to the type A IES (Figure 1A, iv), which recognizes the endogenous target locus in trans (Figure 1A, v) and thus induces ectopic DNA elimination.
Figure 1.
Versatility of coDel. (A) The proposed actions of early and late scnRNAs in coDel. Early scnRNAs produced from an endogenous type A IES in the MIC (i) interact not only with the endogenous type A IES (ii) but also with the type A IES on the pMcoDel vector introduced into the new MAC (iii). The latter interaction triggers late scnRNA production from the adjacent target sequence in cis (iv), which then interacts with the endogenous target locus in trans (v) and induce ectopic DNA elimination (vi). Late scnRNAs likely further induces production of late scnRNAs in cis at the target locus (vii). (B) Construction of the pMcoDel vector. (C) A schematic of the coDel experiment. A PCR-amplified fragment (∼580–710 bp) of each target gene was inserted into the NotI site of pMcoDel vector and introduced into the conjugating cells at ∼7 hpm. Progeny cells with the pMcoDel-derived rDNA were then selected and ectopic elimination of the endogenous target gene loci in the MAC analyzed by genomic PCR. (D–T) Results of coDel experiments. Sequences complementary to the indicated target loci (D–Q) conjugation-specific genes; (R and S) growing cell-specific genes; (T) starved cell-specific genes) were cloned into pMcoDel and introduced into the new MACs of conjugating wild-type cells. Deletions at the endogenous target loci in progeny cells were analyzed by PCR (C). The number of progeny lines showing any deletions at the endogenous target loci was determined. The solid and open arrowheads, respectively, indicate the expected positions of PCR products from the target loci without deletions and with deletions corresponding to the exact target sequences.
Because coDel can be induced by the simple introduction of an IES-target DNA chimeric construct, we reasoned that coDel would be suitable as a gene KO technology in Tetrahymena. In this study, we tested the versatility and specificity of coDel as a tool to produce somatic gene KO strains.
Materials and Methods
Strains and culture conditions
Wild-type B2086 and CU428 strains of T. thermophila were obtained from the Tetrahymena Stock Center at Cornell University. Cells were grown in SPP medium (Gorovsky et al. 1975) containing 2% proteose peptone at 30°. For conjugation, growing cells (∼5–7 × 105/ml) of two different mating types were washed, prestarved (∼12–24 hr), and mixed in 10 mM Tris (pH 7.5) at 30°.
coDel
pMcoDel vector (Noto et al. 2015; see also Figure 1B) was digested with NotI, and the target sequences were inserted by Gibson Assembly (NEB). The genomic location of the targets and the primer sets used for their amplification are listed in Table S1. The primer sequences are listed in Supporting Information, Table S2. To insert the XRN2 and XRN4 target sequences into the pMcoDel vector, two target sequences were first connected by overlapping PCR before insertion via Gibson Assembly. The vectors were introduced into conjugating wild-type cells using a biolistic gun, and the cells were cultured in 10 mM Tris (pH. 7.5) overnight and for an additional ∼3 hr in 1× SPP. Next, the cells possessing pMcoDel-derived rDNAs were selected using 100 µg/ml paromomycin in 1× SPP. Total genomic DNA was then extracted from paromomycin-resistant cells using a NucleoSpin Tissue Kit (Macherey-Nagel), and DNA eliminations at the endogenous target loci were determined by PCR using the primer sets listed in Table S1.
Production of gene KO strains and DNA elimination assays
Progeny lines showing higher coDel efficiency were selected and cultured for 12 passages. In each passage, 5 µl of the previous culture was incubated in 1 ml 1× SPP at 30° for 1–2 days. Next, 6–8 clones from each cell line were isolated, and their target locus was analyzed by genomic PCR. Clones showing a complete lack of the target gene were established as KO strains. Combinations of KO strains that mate were determined by directly checking their mating ability. DNA elimination of the Tlr1 element was analyzed by DNA FISH, as described previously (Noto et al. 2010).
Complementation assay
pHA-pur4 was produced by replacing the neo gene of pHA-neo4 (Kataoka et al. 2010) with the puromycin-resistance gene (pac) of the pur4 cassette (Iwamoto et al. 2014). The pur4 cassette expresses a puromycin-resistance gene in the presence of cadmium ions. To make a DED1 rescue construct (Figure 6C), the 3′ flanking region of the DED1 gene was amplified by PCR using DED1_Res_3FW and DED1_Res_3RV and cloned into the XhoI site of the pHA-pur4 vector using Gibson Assembly (NEB). The resulting plasmid was then digested with BamHI, and the 5′ flanking region of DED1 gene, which was amplified by PCR using DED1_Res_5FW and DED1_ResHA_5RV, was inserted using Gibson Assembly (NEB). The sequences of the PCR primers are listed in Table S2. The resulting plasmid DNA was digested with BamHI and XhoI and introduced into the MACs of starved DED1 KO strains by biolistic gun. Then, cells were cultured in 1× SPP for ∼4 hr, and cells that had the rescue constructs were selected in 1× SPP containing 200 µg/ml puromycin (InvivoGen) and 1 µg/ml CdCl2. Phenotypic assortment was then performed until cells grew in 1–1.5 mg/ml puromycin.
Figure 6.
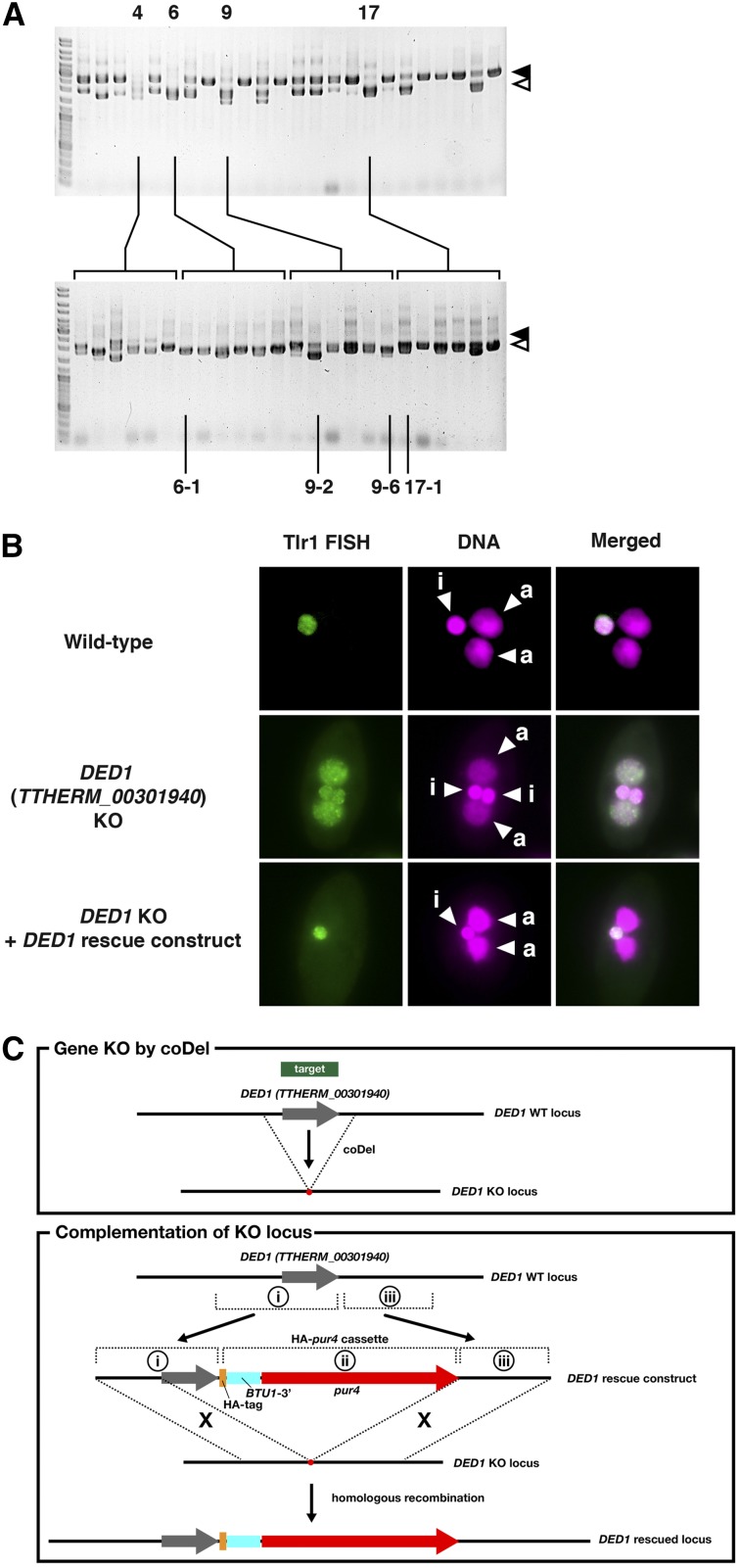
Establishment and analyses of DED1 KO strains produced by coDel. (A) Establishment of TTHERM_00301940 (DED1) KO strains by coDel. Progeny lines showing nearly complete deletion (nos. 4, 6, 9, and 17) in the coDel experiment shown in Figure 1G were cultured for 12 passages to allow random assortment of DED1 MAC locus and sexual maturation. Six clonal cell lines were then established from each progeny line and their DED1 locus analyzed by genomic PCR. Clones 6-1, 9-2, 9-6, and 17-1 were used as DED1 KO cells. (B) Analyses of DNA elimination. Exconjugants (progeny) of wild-type cells (top), DED1 KO cells (middle) or DED1 KO cells rescued with a DED1 rescue construct (bottom) at ∼36 hpm were used to detect Tlr1-IES elements by fluorescent in situ hybridization (Tlr-FISH, green). The DNA was stained with DAPI (DNA, magenta). The MICs (i) and the new MACs (a) are marked. (C) Complementation assay for DED1 KO locus. Top: A schematic drawing of DED1 KO by coDel. Bottom: A schematic drawing of the strategy for genetic rescue of DED1 KO. The DED1 rescue construct was produced by combining the 5′ flanking region and the coding region of the DED1 gene (i), HA-pur4 cassette (ii), and the 3′ flanking region of DED1 (iii) and the DED1 KO locus was replaced with the DED1 rescue construct by homologous recombination.
Data availability
Strains and plasmids used in this study are available upon request.
Results
coDel occurs at many target loci
In the previous study, we showed that coDel was induced at all three of the targeted loci (Noto et al. 2015). To ask if coDel occurs generally at different loci, we target 14 genes for coDel that are expressed predominantly at early conjugation stages (Figure S1). This was done because the complete deletion of an essential gene causes a loss of progeny and underestimation of coDel efficiency at that gene locus, whereas gene expression at early conjugation stages occurs in the maternal MAC and deletions from the new (zygotic) MAC most likely would not affect progeny viability.
We used the pMcoDel vector (Figure 1B) (Noto et al. 2015), in which the M-IES (a type A IES) (Austerberry et al. 1984) and its flanking MAC-destined sequences were inserted into pD5H8 (gift from Dr. Meng-Cao Yao, Academia Sinica, Taiwan). The pD5H8 vector contains Tetrahymena MIC rDNA with a paromomycin-resistant allele of the 17S rRNA (Spangler and Blackburn 1985). Upon introduction of pD5H8 into the new MAC, the rDNA is excised from the vector and forms an rDNA chromosome. At the 3′ nontranscribed region of the rDNA, we can insert a sequence of interest (in this study, we used M-IES and its flanking sequences) without disturbing the functions of the rDNA (Sweeney and Yao 1989). The unique NotI site was made in the middle of M-IES where we can insert the target DNA sequence (Figure 1B). Our experimental scheme is shown in Figure 1C. A PCR-amplified fragment (∼580–710 bp) of each target gene was inserted into the NotI site of the pMcoDel vector and introduced into the conjugating cells at ∼7 hpm. Progeny cells that have pD5H8-derived rDNA were then selected on the basis of their resistance to paromomycin and ectopic elimination of the endogenous target gene in the MAC analyzed by genomic PCR.
Of the 14 early conjugation-specific genes that were subjected to coDel using the strategy described above, ectopic DNA elimination occurred in the new MAC at 13 gene loci (Figure 1, D–T). These results indicate that coDel can be induced at many different genomic loci. DNA elimination induced by coDel removed regions wider than the targets at several of the tested loci (the open arrowheads in Figure 1, D–W, indicate the expected sizes of the PCR products lacking the exact target sequences). As previously suggested (Noto et al. 2015), this likely indicates that Late-scnRNAs further induce Late-scnRNA production at the target loci in cis, as shown in Figure 1A, vi.
To test whether coDel occurs independently of the expression pattern of the target gene, we targeted loci encoding two genes that were expressed predominantly in exponentially growing cells (Figure 1, R and S) and one gene that was expressed predominantly in starved cells (Figure 1T) for coDel. We found that coDel also occurs at these vegetative gene loci. Altogether, we conclude that coDel occurs at a variety of genomic loci, irrespective of their gene expression pattern.
coDel is efficiently induced by introduction of an IES-target chimeric construct into the early developing MAC
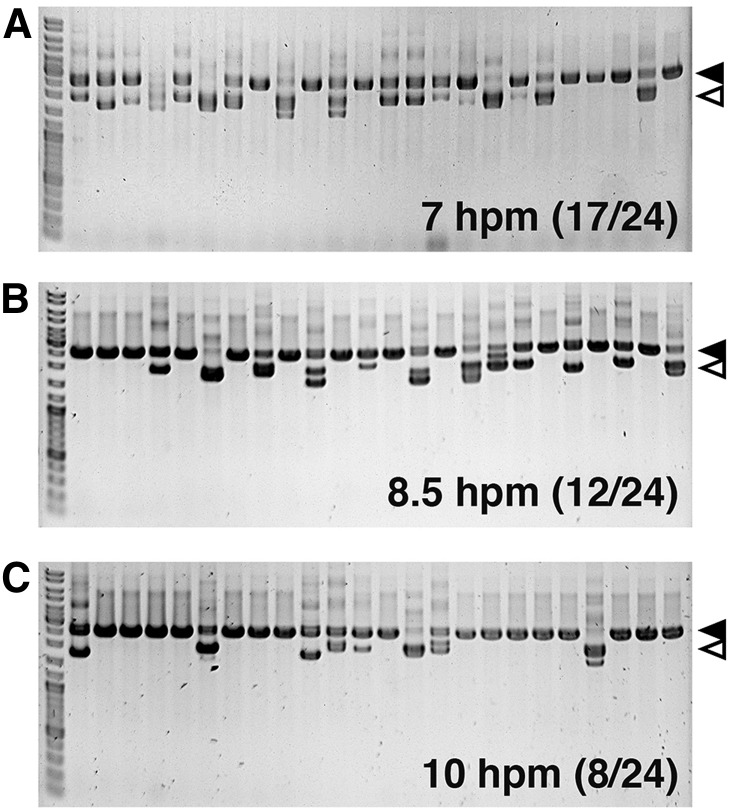
We then investigated whether the timing of the introduction of an IES-target chimeric construct affects the efficiency of coDel. For this purpose, we used the pMcoDel vector containing a 592-bp DNA fragment complementary to the TTHERM_00301940 locus, which was also used for the experiment shown in Figure 1G. This construct was introduced into conjugating cells at 7, 8.5, and 10 hpm, and DNA elimination at the TTHERM_00301940 locus in the new MAC of the progeny was analyzed by genomic PCR. It is important to note that the formation of the new MAC starts at ∼7 hpm but that most DNA eliminations occur at ∼12–16 hpm. We found that the earlier we introduced the construct, the higher the efficiency of coDel was (Figure 2). Therefore, introducing a coDel-inducing construct into the new MAC in its early development likely induces coDel more efficiently.
Figure 2.
Effect of the introduction of a coDel-inducing vector at different developmental stages. The pMcoDel–TTHERM_00301940 vector used for the experiment shown in Figure 1G was introduced into the conjugating cells at 7, 8.5, or 10 hpm and DNA eliminations at the endogenous TTHERM_00301940 loci in the MAC were analyzed by genomic PCR as in Figure 1. The number of progeny lines showing any deletions at the endogenous target loci was determined. The solid and open arrowheads, respectively, indicate the expected positions of PCR products from the target loci without deletions and with deletions corresponding to the exact target sequences.
Longer target sequences increase the efficiency for coDel
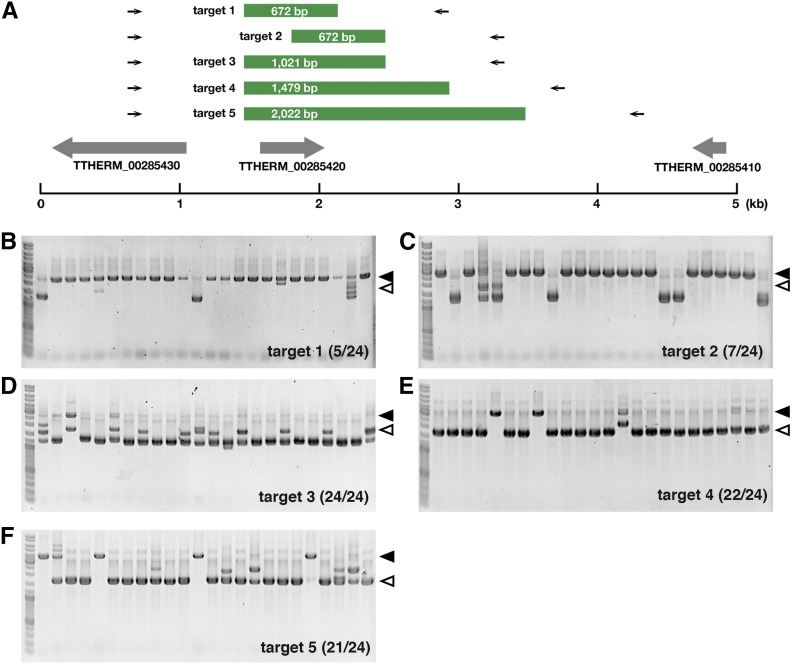
We then investigated whether an extension of the target sequence improves the efficiency of coDel. We prepared pMcoDel vectors with 672-, 672-, 1021-, 1479-, or 2022-bp DNA fragments complementary to the TTHERM_00285420 locus (Figure 3A) and introduced them into conjugating cells at 7 hpm to induce coDel. We found that coDel occurred at a higher frequency with the 1021-bp target sequence than with the two different 672-bp target sequences (Figure 3, B–D), whereas the 1021-, 1479-, and 2022-bp target sequences induced coDel at a similar efficiency (Figure 3, D–F). Therefore, we conclude that longer target sequences increase the efficiency for coDel and an ∼1-kb target sequence is necessary and sufficient to induce coDel at the highest efficiency at the TTHERM_00285420 locus in our experimental condition.
Figure 3.
Effect of the introduction of coDel-inducing vectors of different target length. (A) Schematic of the TTHERM_00285420 MAC locus. Green boxes indicates target sequences complementary to the TTHERM_00285420 gene. Primers used for genomic PCR for each target are shown as arrows adjacent to the target. (B–E) Results of the coDel experiments. The indicated targets were cloned into pMcoDel and introduced into the new MACs of the conjugating cells at 7 hpm. Deletions at the endogenous TTHERM_00285420 locus in the MAC were analyzed by genomic PCR with primers indicated in (A). The number of progeny lines showing any deletions at the endogenous target loci was determined. The solid arrowheads indicate PCR products from the target loci without deletions. The open arrowheads show the expected positions of PCR products from the target loci with deletions corresponding to the exact target sequences.
Off-target coDel occurs at extremely similar loci
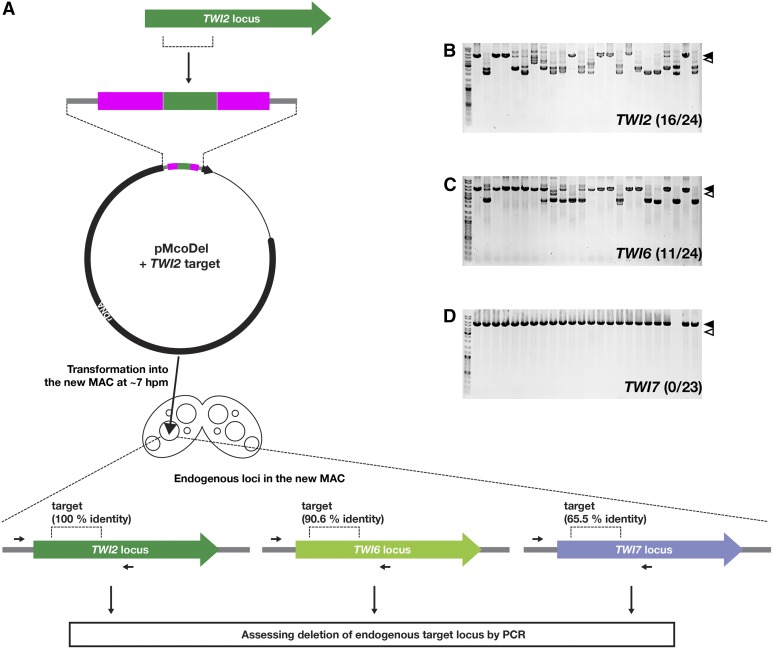
To test how much homology between a target sequence in the pMcoDel vector and a locus in the new MAC is necessary to induce coDel, we introduced a pMcoDel vector containing a 1022-bp DNA fragment that was complementary to part of the TWI2 coding sequence to determine whether other loci (Couvillion et al. 2009) were also targeted for coDel. TWI6 and TWI7 have 90.6 and 65.5% identity at the nucleotide level, respectively, to the target sequence for TWI2. We found that the introduction of the TWI2-targeting construct induced DNA elimination not only at the TWI2 locus (Figure 4A) but also at the TWI6 locus (Figure 4B). In contrast, we did not detect DNA elimination at the TWI7 locus (Figure 4C). These results suggest that off-target coDel can be induced at a locus that shares high similarity with a target sequence.
Figure 4.
Analysis of off-target coDel. (A) A schematic of the coDel experiment targeting the TWI2 locus. A part (1022 bp) of the TWI2 locus was cloned into pMcoDel and introduced into the new MACs of conjugating cells at 7 hpm. Deletions at the endogenous TWI2 locus in the MAC were analyzed by genomic PCR. Deletions at the TWI6 and TWI7 loci, which share 90.6 and 65.5% identities at the nucleotide level with the TWI2 locus, respectively, were also analyzed by genomic PCR. (B–D) Results of genomic PCR detecting deletions at the endogenous TWI2 (B), TWI6 (C), and TWI7 (D) loci in the MAC. PCR products from the same progeny lines were separated in the same lane positions. The number of progeny lines showing any deletions at the endogenous target loci was determined. The solid arrowheads indicate PCR products from the target loci without deletions. The open arrowheads show the expected positions of PCR products from the target loci with deletions corresponding to the exact target sequences.
Two-target loci can be simultaneously deleted by coDel
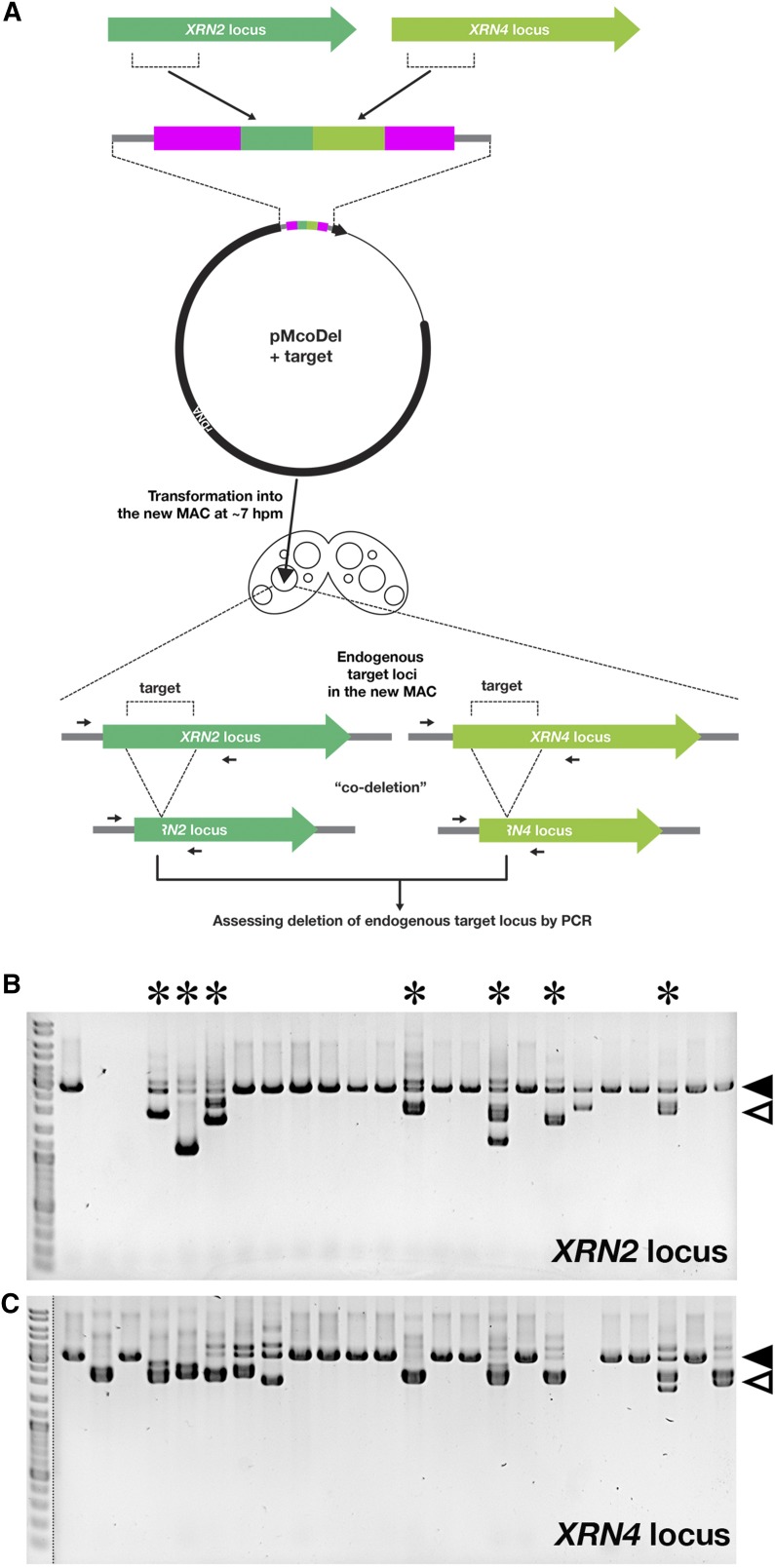
Because >2-kb target sequence can be inserted into the pMcoDel vector without reducing the coDel efficiency (Figure 3E) and an ∼1-kb target sequence is sufficient to efficiently induce coDel (Figure 3C), we reasoned that coDel may be simultaneously induced at two independent loci by introducing a pMcoDel vector with two target sequences. To test this idea, we introduced a pMcoDel vector containing 998 bp complementary to the XRN2 gene and 1083-bp DNA complementary to the XRN4 gene into conjugating cells at 7 hpm (Figure 5A). XRN2 and XRN4 share only 51.9% identity at the nucleotide level in their coding regions; thus, we expect no off-target coDel between these loci. We found that in 7 of the 24 cell lines, DNA elimination was induced at both XRN2 and XRN4 loci via the introduction of the coDel vector with two target sequences (Figure 5B, marked with asterisks). Therefore, coDel can be simultaneously induced at two independent loci using the pMcoDel vector. In most of the clones we tested, we observed either the simultaneous coDel of the both genes or no deletion of them. This tendency might be caused by introduction of the vector at variable stages of development, because coDel efficiency depends on timing of the introduction of a coDel vector (Figure 2).
Figure 5.
Double coDel. (A) A schematic of the coDel experiment simultaneously targeting XRN2 and XRN4 loci. A part of the XRN2 locus and a part of the XRN4 locus were cloned into pMcoDel and introduced into the new MACs of the conjugating cells at 7 hpm. Deletions at the endogenous XRN2 and XRN4 loci in the MAC were analyzed by genomic PCR. (B and C) Results of genomic PCR detecting deletions at the endogenous XRN2 (B) and XRN4 (C) loci in the MAC. PCR products from the same progeny lines were separated in the same lane positions. Asterisks indicate progeny lines showing deletions at both XRN2 and XRN4 loci. The solid arrowheads indicate PCR products from the target loci without deletions. The open arrowheads show the expected positions of PCR products from the target loci with deletions corresponding to the exact target sequences.
Gene KO strains can be produced by coDel
Next, we aimed to establish somatic KO strains for a gene by coDel and to analyze the function of the gene using the established KO strains. The establishment process of TTHERM_00301940 KO cell lines is shown in Figure 6A. In most of the cell lines subjected to coDel, some wild-type copies of the target loci remained (Figure 6A, top). This is likely because DNA elimination occurs when each new MAC has four to eight copies of the genome following one to two rounds of endoreplication (Allis et al. 1987) and because coDel does not always induce DNA elimination in all copies of the target locus. Therefore, to establish the TTHERM_00301940 KO strains, we first chose cell lines that had the highest ratios of deleted target loci to corresponding nondeleted (wild-type) loci (cell lines 4, 6, 9, and 17 in Figure 6A, top), cultured them through several passages to allow for the random assortment of MAC chromosomes, and then isolated single cells to find and establish clonal cell lines in which all of the target loci in the MACs were deleted (Figure 6A bottom). Several KO strains were then chosen and test crossed to each other to identify clones with different mating types. The clones 6-1, 9-2, 9-6, 17-1 of TTHERM_00301940 KO cells were selected for further study.
TTHERM_00301940 is exclusively expressed during conjugation and many conjugation-specific genes are required for DNA elimination (Mochizuki et al. 2002; Taverna et al. 2002; Liu et al. 2007; Cheng et al. 2010; Shieh and Chalker 2013; Woehrer et al. 2015); we sought to determine whether TTHERM_00301940 is also involved in DNA elimination. TTHERM_00301940 KO strains (6-1 × 9-6 [= cross 1 in Table 1] or 9-2 × 17-1 [= cross 2 in Table 1]) were mated, and the DNA elimination in their exconjugants (progeny) was analyzed by DNA FISH using probes complementary to the Tlr1 element. The Tlr1 element is a transposon-related IES that is moderately repeated in the MIC genome. In the exconjugants from the wild-type cells, DNA complementary to the Tlr1 element was detected in the MIC but not in the new MAC (Figure 6B, top, and Table 1). In contrast, DNA complementary to the Tlr1 element was detected in both the MIC and the new MAC in the exconjugants from the TTHERM_00301940 KO cells (Figure 6B, middle, and Table 1), indicating that TTHERM_00301940 plays an important role in DNA elimination. According to the phenotypes of TTHERM_00301940 KO cells, we decided to call this gene DNA Elimination Defective 1 (DED1).
Table 1. Results of DNA elimination assays.
| Genotypes | DNA elimination assay results | No. counted | Exconjugants | ||
|---|---|---|---|---|---|
| ++ | + | − | |||
| Wild type | 100 | 0 | 0 | 100 | |
| TTHERM_00079530 (COI5) KO | Cross 1 | 0 (no exconjugant) | — | — | — |
| Cross 2 | 0 (no exconjugant) | — | — | — | |
| TTHERM_00082190 KO | Cross 1 | 100 | 7 | 7 | 86 |
| Cross 2 | 100 | 9 | 5 | 86 | |
| TTHERM_00112830 KO | Cross 1 | 0 (no exconjugant) | — | — | — |
| Cross 2 | 0 (no exconjugant) | — | — | — | |
| TTHERM_00285420 (DED2) KO | Cross 1 | 100 | 73 | 25 | 2 |
| Cross 2 | 100 | 64 | 31 | 5 | |
| TTHERM_00301940 (DED1) KO | Cross 1 | 100 | 100 | 0 | 0 |
| Cross 2 | 100 | 100 | 0 | 0 | |
| TTHERM_00313180 KO | Cross 1 | 100 | 0 | 0 | 100 |
| Cross 2 | 100 | 0 | 0 | 100 | |
| TTHERM_00460720 KO | Cross 1 | 100 | 0 | 1 | 99 |
| Cross 2 | 100 | 0 | 2 | 98 | |
| TTHERM_00494510 KO | Cross 1 | 0 (no exconjugant) | — | — | — |
| Cross 2 | 0 (no exconjugant) | — | — | — | |
| TTHERM_00520940 KO | Cross 1 | 100 | 5 | 1 | 94 |
| Cross 2 | 100 | 9 | 0 | 91 | |
| TTHERM_00565620 KO | Cross 1 | 100 | 0 | 0 | 100 |
| Cross 2 | 100 | 0 | 0 | 100 | |
| TTHERM_00584620 KO | Cross 1 | 100 | 0 | 0 | 100 |
| Cross 2 | 100 | 0 | 0 | 100 | |
| TTHERM_00675560 KO | Cross 1 | 0 (no exconjugant) | — | — | — |
| Cross 2 | 0 (no exconjugant) | — | — | — | |
| TTHERM_00841280 KO | Cross 1 | 60 | 0 | 0 | 60 |
| Cross 2 | 100 | 0 | 0 | 100 | |
| TTHERM_01044400 KO | Cross 1 | 0 (no exconjugant) | — | — | — |
| Cross 2 | 0 (no exconjugant) | — | — | — | |
| TTHERM_01344740 KO | Cross 1 | 100 | 5 | 2 | 93 |
| Cross 2 | 100 | 2 | 3 | 95 | |
| TTHERM_00301940 (DED1) KO | Cross 1 | 100 | 0 | 0 | 100 |
| + DED1 rescue construct | Cross 2 | 100 | 0 | 0 | 100 |
Establishment of a complementation assay system
As shown above, coDel occurs not only at the target locus but also potentially at loci that have high homology to the target sequence (Figure 4). In addition, because the regulation of DNA elimination has not been fully elucidated, unintended DNA elimination might be induced during coDel. Therefore, it is important to confirm whether phenotypes of gene KO strains produced by coDel are caused only by the absence of the target genes and not by unintended disruption of nontarget genes.
We designed a genetic complementation system and tested it by complementing the DED1 KO strains. We produced the DED1 rescue construct (Figure 6C) by combining the 5′ flanking region and the coding region of DED1 (Figure 6C, i), the HA-pur4 cassette (Figure 6C, ii), and the 3′ flanking region of DED1 (Figure 6C, iii). The DED1 KO locus was replaced with the DED1 rescue construct by homologous recombination (Figure 6C). The HA-pur4 cassette expresses a puromycin-resistance gene (Iwamoto et al. 2014); thus, cells containing the rescue construct were selected on the basis of their resistance to puromycin. Phenotypic assortment was performed to increase copy number of DED1 rescue loci in the macronucleus to produce the DED1 rescue strains. The DED1 rescue strains were then mated with the DED1 KO cells, and the DNA elimination in their exconjugants was analyzed by DNA FISH using probes complementary to the Tlr1 element. We found that DNA complementary to Tlr1 was absent in the new MACs in these exconjugants (Figure 6B, bottom, and Table 1). Therefore, we conclude that the DNA elimination-defective phenotype of the DED1 KO cells was due to loss of the DED1 gene and that the rescue system above can be used for genetic complementation analyses of KO strains made by coDel.
CoDel can be used for functional genetic screening
Next, we aimed to perform functional genetic screening by coDel. For this purpose, we asked whether some of the early conjugation-specific genes subjected to coDel (Figure 1, E–U) are important for programmed DNA elimination, such as DED1. In addition, we studied two early conjugation-specific genes [TTHERM_00079530 (also called COI5; Woehrer et al. 2015) and TTHERM_00313180] that we subjected to coDel in our previous study (Noto et al. 2015). For TTHERM_00285420, TTHERM_00460720, and TTHERM_00675560, the original coDel attempts with ∼600- to 700-bp targets (Figure 1, F, I, and N) did not provide sufficient DNA elimination to produce KO strains. Therefore, we induced coDel for these genes using ∼900- to 1000-bp targets.
The establishment process of KO cells for these genes is shown in Figure S2. We established KO strains for the 14 genes (including DED1) in which we detected DNA elimination at the target locus by coDel. Then, two KO strains for each gene were mated, and the DNA elimination in their exconjugants was analyzed by DNA FISH for the Tlr1 element. A summary of the DNA elimination analyses is shown in Table 1. Because the KO cells for TTHERM_00079530, TTHERM_00112830, TTHERM_01044400, TTHERM_00494510, and TTHERM_00675560 did not produce exconjugants for unknown reasons, we could not check whether these genes played any role in DNA elimination. Two of 11 conjugation-specific genes were also determined as genes required for completing conjugation in our previous conventional homologous recombination-based gene KO studies (Woehrer et al. 2015). Because conjugation involves multiple complex processes, it is not surprising that many conjugation-specific genes are required for producing exconjugants.
The KO strains for the rest of the genes formed exconjugants and we found that in addition to DED1 KO cells, a strong DNA elimination defect was detected in TTHERM_00285420 KO cells. Therefore, we named TTHERM_00285420 DNA Elimination Defective 2 (DED2). The KO strains for TTHERM_00082190, TTHERM_01344740, TTHERM_00520940, and TTHERM_00460720 also showed weaker defects in DNA elimination, and these minor defects may be consequences of some disturbance in the conjugation progression. Altogether, we conclude that a medium-throughput functional genetic screen can be performed using coDel.
Discussion
CoDel as a tool for the production of gene KO strains
We detected coDel at 16 of the 17 target loci (Figure 1). These 16 loci include genes expressed in exponentially growing cells, starved cells, and conjugating cells. In addition, we previously showed that coDel can occur at noncoding genomic loci (Noto et al. 2015). Therefore, coDel can be induced at many different loci, irrespective of their transcriptional state and coding capacity. However, the current protocol for coDel is not suitable for production of gene KO strains at some loci, as we could not detect coDel at the TTHERM_00399200 locus. Currently it is not clear what makes the TTHERM_00399200 locus insensitive to coDel. We need a larger-scale study to determine whether there are necessary conditions for target loci to be eliminated by coDel and to make coDel a more versatile gene KO technology. Nonetheless, because gene KO strains can be produced in a week by coDel, instead of in a month by the conventional homologous recombination-based strategies (Cassidy-Hanley et al. 1997; Chalker 2012), coDel is a useful tool to facilitate Tetrahymena genetics.
High-throughput genetic screening
To make a somatic gene KO strain, all copies of a target gene in the polyploid MAC must be disrupted. This can be achieved either by coDel of all copies of a target gene or by coDel of some copies of a target gene followed by random assortment of MAC chromosomes and then choosing cells in which all copies of a target gene are eliminated. In Figure 6 and Table 1, all KO strains were produced by the latter strategy because some wild-type copies remained in many strains in which coDels were detected. Because the assortment and the following selection of KO cells is a cumbersome process, improving the efficiency of coDel is important for establishing this technology as a tool for high-throughput genetic screening. It may be useful to compare coDel efficiency using different IESs in a coDel-inducing vector to find a better IES for coDel experimentation. In addition, because we found that extension of the target sequence in the pMcoDel vector improves not only the number of progeny cells showing coDel but also the completeness of coDel within each progeny line (Figure 3), it would be important to use 1 kb or longer sequences as a target for coDel for a high-throughput genetic screening attempt. In addition, because we found that off-target coDel occurs when a locus shares ∼90% identity with a target sequence (Figure 4), using unnecessarily long target sequences should be avoided. In addition, target sequences need to be chosen carefully if a target locus shares high-sequence identity with other loci. Because off-target coDel could occur in any experimental design, it is always necessary to confirm a cause–effect relationship between coDel-induced disruption of a target gene and a detected phenotype by (i) observing multiple independent coDel cell lines and (ii) asking if reintroduction of the target gene into a coDel line can restore the phenotype.
Supplementary Material
Acknowledgments
This work was supported by an Austrian Science Fund (FWF) stand-alone grant (P26032-B22), the FWF Special Research Program (SFB) “RNA regulation of the transcriptome” (F4307-B09), and core funding from the Austrian Academy of Sciences.
Footnotes
Communicating editor: M. Johnston
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.178525/-/DC1.
Literature Cited
- Allis C. D., Colavito-Shepanski M., Gorovsky M. A., 1987. Scheduled and unscheduled DNA synthesis during development in conjugating Tetrahymena. Dev. Biol. 124: 469–480. [DOI] [PubMed] [Google Scholar]
- Austerberry C. F., Allis C. D., Yao M. C., 1984. Specific DNA rearrangements in synchronously developing nuclei of Tetrahymena. Proc. Natl. Acad. Sci. USA 81: 7383–7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy-Hanley D., Bowen J., Lee J. H., Cole E., VerPlank L. A., et al. , 1997. Germline and somatic transformation of mating Tetrahymena thermophila by particle bombardment. Genetics 146: 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalker D. L., 2012. Transformation and strain engineering of Tetrahymena. Methods Cell Biol. 109: 327–345. [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Vogt A., Mochizuki K., Yao M. C., 2010. A domesticated piggyBac transposase plays key roles in heterochromatin dynamics and DNA cleavage during programmed DNA deletion in Tetrahymena thermophila. Mol. Biol. Cell 21: 1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung P. H., Yao M. C., 2012. Tetrahymena thermophila JMJD3 homolog regulates H3K27 methylation and nuclear differentiation. Eukaryot. Cell 11: 601–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K., Gorovsky M. A., 2005. Tetrahymena thermophila. Curr. Biol. 15: R317–R318. [DOI] [PubMed] [Google Scholar]
- Couvillion M. T., Lee S. R., Hogstad B., Malone C. D., Tonkin L. A., et al. , 2009. Sequence, biogenesis, and function of diverse small RNA classes bound to the Piwi family proteins of Tetrahymena thermophila. Genes Dev. 23: 2016–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L., 1975. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 9: 311–327. [DOI] [PubMed] [Google Scholar]
- Howard-Till R. A., Yao M. C., 2006. Induction of gene silencing by hairpin RNA expression in Tetrahymena thermophila reveals a second small RNA pathway. Mol. Cell. Biol. 26: 8731–8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M., Mori C., Hiraoka Y., Haraguchi T., 2014. Puromycin resistance gene as an effective selection marker for ciliate Tetrahymena. Gene 534: 249–255. [DOI] [PubMed] [Google Scholar]
- Kataoka K., Schoeberl U. E., Mochizuki K., 2010. Modules for C-terminal epitope tagging of Tetrahymena genes. J. Microbiol. Methods 82: 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Taverna S. D., Muratore T. L., Shabanowitz J., Hunt D. F., et al. , 2007. RNAi-dependent H3K27 methylation is required for heterochromatin formation and DNA elimination in Tetrahymena. Genes Dev. 21: 1530–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A., Howard-Till R. A., Loidl J., 2013. Mus81 nuclease and Sgs1 helicase are essential for meiotic recombination in a protist lacking a synaptonemal complex. Nucleic Acids Res. 41: 9296–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam E. V., Bruns P. J., 1988. Phenotypic assortment in Tetrahymena thermophila: assortment kinetics of antibiotic-resistance markers, tsA, death, and the highly amplified rDNA locus. Genetics 120: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki K., Fine N. A., Fujisawa T., Gorovsky M. A., 2002. Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in Tetrahymena. Cell 110: 689–699. [DOI] [PubMed] [Google Scholar]
- Noto T., Kurth H. M., Kataoka K., Aronica L., DeSouza L. V., et al. , 2010. The Tetrahymena Argonaute-binding protein Giw1p directs a mature Argonaute-siRNA complex to the nucleus. Cell 140: 692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto T., Kataoka K., Suhren J. H., Hayashi A., Woolcock K. J., et al. , 2015. Small-RNA-mediated genome-wide trans-recognition network in Tetrahymena DNA elimination. Mol. Cell 59: 229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orias E., Hamilton E. P., Orias J. D., 2000. Tetrahymena as a laboratory organism: useful strains, cell culture, and cell line maintenance. Methods Cell Biol. 62: 189–211. [DOI] [PubMed] [Google Scholar]
- Schoeberl U. E., Kurth H. M., Noto T., Mochizuki K., 2012. Biased transcription and selective degradation of small RNAs shape the pattern of DNA elimination in Tetrahymena. Genes Dev. 26: 1729–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh A. W., Chalker D. L., 2013. LIA5 is required for nuclear reorganization and programmed DNA rearrangements occurring during Tetrahymena macronuclear differentiation. PLoS One 8: e75337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler E. A., Blackburn E. H., 1985. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J. Biol. Chem. 260: 6334–6340. [PubMed] [Google Scholar]
- Sweeney R., Yao M. C., 1989. Identifying functional regions of rRNA by insertion mutagenesis and complete gene replacement in Tetrahymena thermophila. EMBO J. 8: 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S. D., Coyne R. S., Allis C. D., 2002. Methylation of histone h3 at lysine 9 targets programmed DNA elimination in Tetrahymena. Cell 110: 701–711. [DOI] [PubMed] [Google Scholar]
- Woehrer S. L., Aronica L., Suhren J. H., Busch C. J., Noto T., et al. , 2015. A Tetrahymena Hsp90 co-chaperone promotes siRNA loading by ATP-dependent and ATP-independent mechanisms. EMBO J. 34: 559–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains and plasmids used in this study are available upon request.