Abstract
Carbon-ion radiotherapy (CIRT) holds promise in the treatment of glioblastoma, an aggressive X-ray–resistant brain tumor. However, since glioblastoma cells show a highly invasive nature, carbon-ion (C-ion) irradiation of normal tissues surrounding the tumor is inevitable. Recent studies have revealed the existence of neural stem cells in the adult brain. Therefore, the damaging effect of C-ion beams on the neural stem cells has to be carefully considered in the treatment planning of CIRT. Here, we investigated the growth and death mode of human neural stem cells (hNSCs) and glioblastoma A172 cells after X-ray or C-ion beam irradiation. The X-ray dose resulting in a 50% growth rate (D50) was 0.8 Gy in hNSCs and 3.0 Gy in A172 cells, while the D50 for C-ion beams was 0.4 Gy in hNSCs and 1.6 Gy in A172 cells; the relative biological effectiveness value of C-ion beams was 2.0 in hNSCs and 1.9 in A172 cells. Importantly, both X-rays and C-ion beams preferentially induced apoptosis, not necrosis, in hNSCs; however, radiation-induced apoptosis was less evident in A172 cells. The apoptosis-susceptible nature of the irradiated hNSCs was associated with prolonged upregulation of phosphorylated p53, whereas the apoptosis-resistant nature of A172 cells was associated with a high basal level of nuclear factor kappa B expression. Taken together, these data indicate that apoptosis is the major cell death pathway in hNSCs after irradiation. The high sensitivity of hNSCs to C-ion beams underscores the importance of careful target volume delineation in the treatment planning of CIRT for glioblastoma.
Keywords: carbon-ion (C-ion) beams, neural stem cells, glioblastoma, radiosensitivity, apoptosis
INTRODUCTION
Glioblastoma is the most aggressive type of primary brain tumor and accounts for ∼50% of all primary brain tumor cases [1]. Standard therapy for glioblastoma consists of maximum feasible surgical resection followed by concurrent chemoradiotherapy [2]. However, the clinical outcome of this therapy is unsatisfactory and many patients die within 2 years [1]. The X-ray–resistant behavior of glioblastoma observed in the clinic is also supported by the basic research [3, 4]. These data suggest that enhancement of radiotherapy intensity for glioblastoma is urgently needed.
Carbon-ion radiotherapy (CIRT) has generated a great deal of interest as a highly intensive radiotherapy compared with conventional X-ray radiotherapy, owing to the intensive energy deposition and the high cell-killing effect. Several clinical studies showing the excellent results of CIRT for X-ray–resistant tumors [5] highlights the potential of CIRT as a curative treatment modality for glioblastoma. On the other hand, glioblastoma has a highly invasive nature that allows infiltration of tumor cells to adjacent normal tissues. Therefore, in radiotherapy for glioblastoma, irradiation to normal cells surrounding the tumor is inevitable, even using carbon-ion (C-ion) beams [6].
Recent research has indicated that the subventricular zone (SVZ), a frequent predisposition site of glioblastoma, is vulnerable to X-ray irradiation; irradiation of the SVZ can lead to late toxicity such as neurocognitive disorders [7]. Importantly, it is known that the SVZ harbors neural stem cells (NSCs) playing pivotal roles in self-renewal during neurogenesis after brain damage [8]. Although several previous studies have investigated the sensitivity of NSCs to X-rays and γ-rays [9–14], the effect of C-ion beams on NSCs is not well understood. The present study aimed to determine the biological basis for optimal treatment planning strategy in CIRT for glioblastoma. To this end, we evaluated the sensitivities of human NSCs (hNSCs) and glioblastoma cells to C-ion beams and X-rays, and analyzed the mode of cell death.
MATERIALS AND METHODS
Cell culture
hNSCs derived from NIH-approved H9 (WA09) human embryonic stem cells (TP53-wild-type) [15] were cultured in KnockOut™ DMEM/F-12 supplemented with 2% StemPro® Neural Supplement, 2 mM GlutaMAX, 20 ng/ml basic fibroblast growth factor, and 20 ng/ml epidermal growth factor. The cell culture dishes were coated with CTS™ CELLstart™ substrate in Dulbecco's Phosphate-Buffered Saline CTS™ supplemented with calcium chloride and magnesium chloride. Human glioblastoma A172 cells (TP53-wild-type; ATCC, VA, USA) were cultured in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS; Biowest, Nuaille, France), 20 mM 2-[4-(2-hydroxyethyl)-1-piperazinyl] ethanesulfonic acid, 4 mM L-glutamine, and 1% penicillin–streptomycin. In the cell growth and death assays, the culture media for both cell lines was replaced with fresh media, or fresh media was added every 48 h after cell seeding. All reagents except for FBS were purchased from Gibco (Carlsbad, CA, USA).
Irradiation
Cells were irradiated with X-rays or C-ion beams. X-ray irradiation (150-kVp, ∼1 Gy/min) was performed using MBR-1520R-4 (Hitachi Power Solutions, Ibaraki, Japan). C-ion beam irradiation (290 MeV/nucleon; an average linear energy transfer at the center of a 6-cm spread-out Bragg peak, 50 keV/μm) was performed at Gunma University Heavy Ion Medical Center (GHMC; Gunma, Japan) [16].
Cell growth assay
hNSCs and A172 cells were collected using TrypLE™ Express (Gibco) and trypsin-EDTA solution (Immuno-Biological Laboratories, Gunma, Japan), respectively, and suspended in culture medium. The cells were counted using a hemocytometer just before irradiation (0 h) and every 24 h after irradiation. The relative growth rate of cells receiving a given dose was calculated by dividing the difference in the number of cells between 0 h and 72 h by that of non-irradiated cells (n.b. the non-irradiated cells showed exponential growth within 72 h). The dose–response curves were generated by fitting the data plots to the exponential approximation, from which D50 (a dose giving 50% growth rate) was calculated. Finally the relative biological effectiveness (RBE) values of C-ion beams were calculated by dividing the D50 for X-rays by that for C-ion beams [17, 18].
Cell death assay
Radiation-induced apoptosis and necrosis were determined by fluorescence staining with acridine orange (Dojindo laboratories, Kumamoto, Japan) and ethidium bromide (Wako, Osaka, Japan) (Supplementary Fig. 1) as previously described [19, 20]. The percentages of apoptotic or necrotic cells were calculated with normalization to the total number of cells. The dose–response curves were generated using the polynomial approximation by KaleidaGraph software (Hulinks, Tokyo, Japan) (Supplementary Fig. 2) as previously described [21]. The RBE values were calculated using the coefficients of the linear components of the curve-fitting–formulae (Supplementary Table 1). At least 400 cells were counted per sample.
Immunoblot analysis
Immunoblot analysis was performed as previously described [22]. The antibodies used were listed in Supplementary Table 2.
Statistical analysis
Data are presented as the mean ± standard deviation (SD) of three independent experiments. Statistical significance was assessed by a one-way ANOVA followed by Tukey's post-hoc test. A P-value of <0.05 was considered statistically significant.
RESULTS AND DISCUSSION
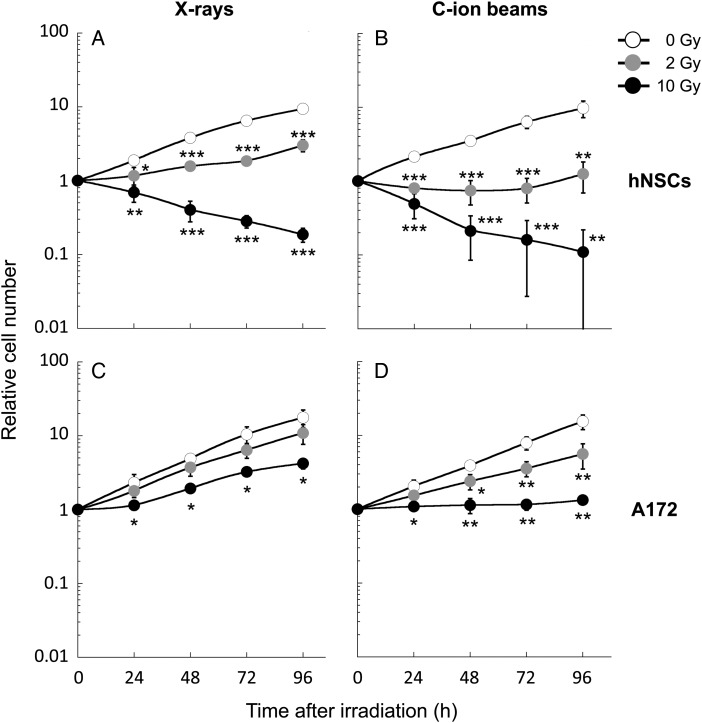
First we examined the sensitivity of hNSCs and A172 cells to X-rays or C-ion beams. The radiosensitivity was assessed by counting the total number of adherent cells on culture dishes because hNSCs lack colony-forming ability [23]. In the absence of irradiation, the cell growth speed was comparable between hNSCs and A172 cells (Fig. 1); the population doubling time was 28.2 ± 1.5 h in hNSCs and 23.9 ± 1.7 h in A172 cells. X-ray irradiation for 2 Gy and 10 Gy significantly suppressed the growth of hSNCs (Fig. 1A). The X-ray irradiation also suppressed the growth of A172 cells. However, the effect was much smaller than that observed in hNSCs; A172 cells kept proliferating after irradiation for 10 Gy (Fig. 1C). C-ion beams showed a greater growth-suppression effect in both cell lines than that induced by X-rays (Fig. 1B and D). Notably, 10 Gy C-ion beams completely suppressed the growth of A172 cells over 96 h post-irradiation (Fig. 1D).
Fig. 1.
Proliferation of hNSCs and A172 cells irradiated with X-rays or carbon-ion (C-ion) beams. The number of cells was counted every 24 h after irradiation and is shown after normalization to the number of non-irradiated controls at 0 h. (A) hNSCs irradiated with X-rays. (B) hNSCs irradiated with C-ion beams. (C) A172 cells irradiated with X-rays. (D) A172 cells irradiated with C-ion beams. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the corresponding control.
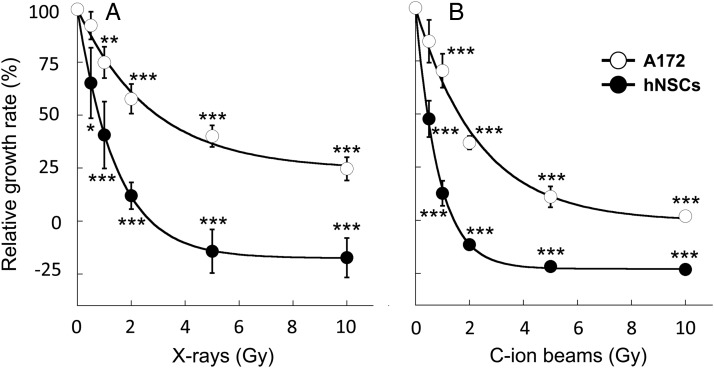
To further investigate the effect of X-ray and C-ion beam irradiation on the growth of hNSCs and A172 cells, we generated the dose–response curves (Fig. 2) and calculated the D50 and the RBE values (Supplementary Table 1). The D50 for X-rays was 0.8 Gy in hNSCs and 3.0 Gy in A172 cells. The D50 for C-ion beams was 0.4 Gy in hNSCs and 1.6 Gy in A172 cells. Consequently, the RBE value of C-ion beams at the D50 was 2.0 in hNSCs and 1.9 in A172 cells. The RBE value in A172 cells in the present study was comparable with the previously reported RBE value in glioblastoma cells assessed by clonogenic assay [24], indicating the validity of the cell-number counting method for radiosensitivity assessment. Together, these data indicate that hNSCs are ∼4-fold more sensitive to X-rays and C-ion beams than A172 cells; the RBE values of C-ion beams over X-rays were comparable between hNSCs and A172 cells; C-ion beams more effectively suppress the growth of A172 cells than do X-rays.
Fig. 2.
Dose–response curves of hNSCs and A172 cells irradiated with X-rays or carbon-ion (C-ion) beams. The relative growth rate was calculated by dividing the difference in the number of cells irradiated with a given dose between 0 and 72 h by the difference in the number of non-irradiated cells between 0 and 72 h. The data plots of the relative growth rate were fitted to the exponential approximation to generate dose–response curves. (A) X-rays. (B) C-ion beams. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with the corresponding control.
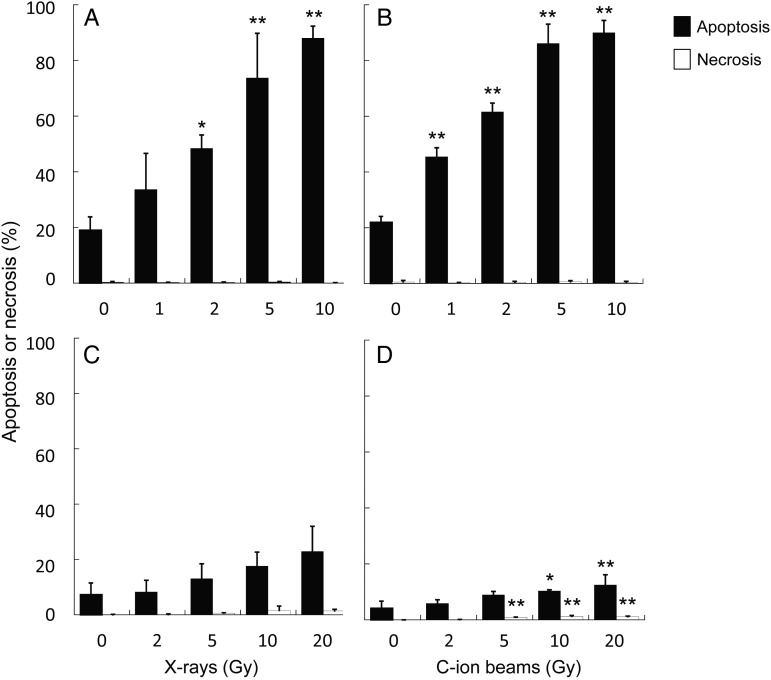
To further investigate the effect of X-rays and C-ion beams on hNSCs and A172 cells, we next assessed the induction of apoptosis and necrosis after irradiation. hNSCs showed remarkably high sensitivity to apoptosis after X-ray irradiation; ∼90% of X-ray–irradiated hNSCs underwent apoptosis (Fig. 3A). Efficient apoptosis induction in hNSCs was also observed after C-ion beam irradiation, and was slightly greater than that observed after X-ray irradiation (Fig. 3B). Necrosis was rarely observed in hNSCs after X-ray or C-ion beam irradiation (Fig. 3A and B). These results indicate that the high sensitivity to radiation-induced apoptosis underlies the high sensitivity to X-rays and C-ion beams in hNSCs. In contrast, A172 cells did not show evident induction of apoptosis or necrosis after X-ray irradiation (Fig. 3C). C-ion beams significantly induced apoptosis and necrosis in A172 cells, although the fractions were small (Fig. 3D). These results indicate the apoptosis-resistant nature of A172 cells.
Fig. 3.
Apoptosis and necrosis induction by X-ray or carbon-ion (C-ion) beam irradiation in hNSCs and A172 cells. Cells were simultaneously stained with acridine orange and ethidium bromide at 72 h after irradiation and analyzed for apoptosis and necrosis (see Materials and Methods and Supplementary Fig. 1 for definitions of apoptosis and necrosis). (A) hNSCs irradiated with X-rays. (B) hNSCs irradiated with C-ion beams. (C) A172 cells irradiated with X-rays. (D) A172 cells irradiated with C-ion beams. The percentages of apoptotic or necrotic cells were calculated following normalization to the total number of cells. At least 400 cells were counted per sample. *P < 0.05 and **P < 0.01 compared with the corresponding control.
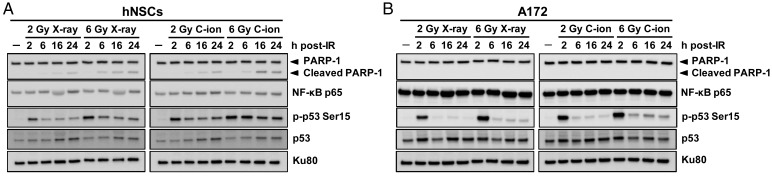
Finally we investigated the mechanisms underlying the difference in the level of the radiation-induced apoptosis between hNSCs and A172 cells by immunoblot analysis (Fig. 4). In hNSCs, the levels of cleavage of poly (ADP-ribose) polymerase-1 (PARP-1), a marker for apoptosis [25], were increased after irradiation, and were greater for C-ion beams than for X-rays. In contrast, cleaved PARP-1 was undetectable in the X-ray or C-ion beam–irradiated A172 cells. These results were consistent with the results of the cell death assay, demonstrating the greater induction of apoptosis by C-ion beam irradiation than by X-ray irradiation in hNSCs, and the apoptosis-resistant nature of A172 cells after both X-ray and C-ion beam irradiation (Fig. 3). Interestingly, in hNSCs but not in A172 cells, phosphorylation of p53 at Ser15 was maintained at high levels after X-ray and C-ion beam irradiation over 24 h. Since phosphorylation of p53 at Ser15 plays a key role in inducing apoptosis after ionizing irradiation [26, 27], these data suggest that the prolonged upregulation of p53 phosphorylation contributes to the apoptosis-susceptible nature of hNSCs. Previous study demonstrated the involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling in irradiation-induced apoptosis in hNSCs [14]. Therefore, we checked the TRAIL expression; however, it was undetectable in our experimental systems (data not shown). On the other hand, we found that A172 cells, but not hNSCs, express high levels of the p65 subunit of nuclear factor kappa B (NF-κB) regardless of the presence or absence of X-ray or C-ion beam irradiation. It is known that activated NF-κB suppresses apoptosis induction by inhibiting caspase 3 and caspase 9 [28]. Moreover, the aberrant constitutive activation of NF-κB is known to be one of the major mechanisms underlying the treatment-resistant nature of glioblastoma [29]. With these facts taken together, these data suggest that the high basal level of NF-κB expression contributes to the apoptosis-resistant nature of A172 cells after X-ray and C-ion beam irradiation.
Fig. 4.
Immunoblot analysis for the apoptosis-associated proteins in hNSCs and A172 cells after X-ray or carbon-ion (C-ion) beam irradiation. Cells were irradiated with 2 or 6 Gy of X-rays or C-ion beams. Levels of PARP-1 cleavage, p65 subunit of NF-κB (NF-κB p65), phosphorylated p53 at Ser15 (p-p53 Ser15) and p53 were detected by immunoblot analysis at 2, 6, 16 and 24 h post irradiation (IR). Ku80 was used as a loading control. (A) hNSCs. (B) A172 cells.
The high sensitivity of hNSCs to radiation-induced apoptosis may partly be attributable to the fact that stem cells are programmed to preferentially undergo apoptosis to prevent the inheritance of damaged DNA, such as mutations, by daughter cells [30]. In accordance with this, hNSC showed a moderately high (∼20%) background for apoptosis (Fig. 3A and B). In contrast, it is likely that modes for growth suppression other than apoptosis may occur in the irradiated A172 cells, because the RBE value calculated from the apoptosis data is much lower than that calculated from the cell growth data (0.7 vs 1.9; Supplementary Table 1 and Supplementary Fig. 2). Previous studies have demonstrated the induction of senescence and mitotic catastrophe in C-ion beam–irradiated glioblastoma cells [31, 32]. Such modes of cell arrest should be analyzed in the future.
The RBE value of 3.0 is used for CIRT in Japan. This value is based on the results of the clonogenic assay using human salivary gland tumor cells [16, 33]. However, recent studies suggest that the sensitivities of cells to X-rays and C-ion beams varies widely among cancer types and, more importantly, among normal tissues [34, 35]. In relation to this, the results of the present study will provide an insight into the RBE value of C-ion beams in NSCs.
In summary, the results of this study indicate that (i) hNSCs are highly sensitive to X-rays and C-ion beams, relative to glioblastoma cells; (ii) the RBE value of C-ion beams is comparable between hNSCs and glioblastoma cells; (iii) the high sensitivity of hNSCs to X-rays and C-ion beams may be partly due to their high susceptibility to apoptosis; and (iv) cellular outcomes other than apoptosis and necrosis may partly contribute to the efficient growth-suppression effect of C-ion beams in glioblastoma cells. To the best of our knowledge, this is the first report of the RBE value of C-ion beams in hNSCs. The high sensitivity of hNSCs to C-ion beams underscores the importance of careful target volume delineation in the treatment planning of CIRT for glioblastoma.
FUNDING
This work was supported by a Grant-in-Aid for Young Scientists (Start-up) (No. 25893030) from the Japan Society for the Promotion of Science ( JSPS) and by the Program for Strategic Young Researcher Overseas Visits Program for Accelerating Brain Circulation. Funding to pay the Open Access publication charges for this article was provided by operational expense at project of GHMC.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Ms Yakou for technical assistance and Drs Ando, Inoue, Nakayama, and Otsu for their valuable comments. This work was performed as part of the research project with heavy ions at the GHMC.
REFERENCES
- 1.Oike T, Suzuki Y, Sugawara K, et al. Radiotherapy plus concomitant adjuvant temozolomide for glioblastoma: Japanese mono-institutional results. PLoS One 2013;8:e78943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66. [DOI] [PubMed] [Google Scholar]
- 3.Li HF, Kim JS, Waldman T. Radiation-induced Akt activation modulates radioresistance in human glioblastoma cell. Radiat Oncol 2009;4:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y, Kim KH, Lee J, et al. Wnt activation is implicated in glioblastoma radioresistance. Lab Invest 2012;92:466–73. [DOI] [PubMed] [Google Scholar]
- 5.Nakano T, Suzuki Y, Ohno T, et al. Carbon beam therapy overcomes the radiation resistance of uterine cervical cancer originating from hypoxia. Clin Cancer Res 2006;12:2185–90. [DOI] [PubMed] [Google Scholar]
- 6.Giese A, Bjerkvig R, Berens ME, et al. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 2003;21:1624–36. [DOI] [PubMed] [Google Scholar]
- 7.Iuchi T, Hatano K, Kodama T, et al. Phase 2 trial of hypofractionated high-dose intensity modulated radiation therapy with concurrent and adjuvant temozolomide for newly diagnosed glioblastoma. Int J Radiat Oncol Biol Phys 2014;88:793–800. [DOI] [PubMed] [Google Scholar]
- 8.Anderson V, Yeates KO. Introduction: Pediatric traumatic brain injury: New frontiers in clinical and translational research. In: Anderson V, Yeates KO. (eds). Pediatric Traumatic Brain Injury: New Frontiers in Clinical and Translational Research. Cambridge: Cambridge University Press, 2010, 1–6. [Google Scholar]
- 9.Guida P, Vazquez ME, Otto S. Cytotoxic effects of low- and high-LET radiation on human neuronal progenitor cells: induction of apoptosis and TP53 gene expression. Radiat Res 2005;164:545–51. [DOI] [PubMed] [Google Scholar]
- 10.Acharya MM, Lan ML, Kan VH, et al. Consequences of ionizing radiation–induced damage in human neural stem cells. Free Radic Bio Med 2010;49:1846–55. [DOI] [PubMed] [Google Scholar]
- 11.Lan ML, Acharya MM, Tran KK, et al. Characterizing the radioresponse of pluripotent and multipotent human stem cells. PLoS One 2012;7:e50048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isono M, Otsu M, Konishi T, et al. Proliferation and differentiation of neural stem cells irradiated with X-rays in logarithmic growth phase. Neurosci Res 2012;73:263–8. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida Y, Suzuki Y, Al-Jahdari WS, et al. Evaluation of the relative biological effectiveness of carbon ion beams in the cerebellum using the rat organotypic slice culture system. J Radiat Res 2012;53:87–92. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov VN, Hei TK. A role for TRAIL/TRAIL-R2 in radiation-induced apoptosis and radiation-induced bystander response of human neural stem cells. Apoptosis 2014;19:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marei HES, Althani A, Afifi N, et al. Gene expression profiling of embryonic human neural stem cells and dopaminergic neurons from adult human substantia nigra. PLoS One 2011;6:e28420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohno T, Kanai T, Yamada S, et al. Carbon ion radiotherapy at the Gunma University Heavy Ion Medical Center: new facility set-up. Cancers 2011;3:4046–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys 1999;44:201–10. [DOI] [PubMed] [Google Scholar]
- 18.Kagawa K, Murakami M, Hishikawa Y, et al. Preclinical biological assessment of proton and carbon ion beams at Hyogo ion beam medical center. Int J Radiat Oncol Biol Phys 2002;54:928–38. [DOI] [PubMed] [Google Scholar]
- 19.Leite M, Quinta-Costa M, Leite PS, et al. Critical evaluation of techniques to detect and measure cell death—study in a model of UV radiation of the leukaemic cell line HL60. Anal Cell Pathol 1999;19:139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi A, Matsumoto H, Yuki K, et al. High-LET radiation enhanced apoptosis but not necrosis regardless of p53 status. Int J Radiat Oncol Biol Phys 2004;60:591–7. [DOI] [PubMed] [Google Scholar]
- 21.Meijer AE, Kronqvist US, Lewensohn R, et al. RBE for the induction of apoptosis in human peripheral lymphocytes exposed in vitro to high-LET radiation generated by accelerated nitrogen ions. Int J Radiat Biol 1998;73:169–77. [DOI] [PubMed] [Google Scholar]
- 22.Shibata A, Moiani D, Arvai AS, et al. DNA double-strand break repair pathway choice is directed by distinct MRE11 nuclease activities. Mol Cell 2014;53:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galli R, Gritti A, Bonfanti L, et al. Neural stem cells: an overview. Circ Res 2003;92:598–608. [DOI] [PubMed] [Google Scholar]
- 24.Tsuboi K, Tsuchida Y, Nose T, et al. Cytotoxic effect of accelerated carbon beams on glioblastoma cell lines with p53 mutation: clonogenic survival and cell-cycle analysis. Int J Radiat Biol 1998;74:71–9. [DOI] [PubMed] [Google Scholar]
- 25.D'Amours D, Sallmann FR, Dixit VM, et al. Gain-of-function of poly(ADP-ribose) polymerase-1 upon cleavage by apoptotic proteases: implications for apoptosis. J Cell Sci 2001;114:3771–8. [DOI] [PubMed] [Google Scholar]
- 26.Unger T, Sionov RV, Moallem E, et al. Mutations in serines 15 and 20 of human p53 impair its apoptotic activity. Oncogene 1999;18:3205–12. [DOI] [PubMed] [Google Scholar]
- 27.Saito S, Goodarzi AA, Higashimoto Y, et al. ATM mediates phosphorylation at multiple p53 sites, including Ser46, in response to ionizing radiation. J Biol Chem 2002;277:12491–4. [DOI] [PubMed] [Google Scholar]
- 28.Dai Y, Liu M, Tang W, et al. Smac-mimetic sensitizes prostate cancer cells to TRAIL-induced apoptosis via modulating both IAPs and NF-kappaB. BMC Cancer 2009;9:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bredel M, Scholtens DM, Yadav AK, et al. NFKBIA deletion in glioblastomas. N Engl J Med 2011;364:627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu JC, Lerou PH, Lahav G. Stem cells: balancing resistance and sensitivity to DNA damage. Trends Cell Biol 2014;5:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oishi T, Sasaki A, Hamada N, et al. Proliferation and cell death of human glioblastoma cells after carbon-ion beam exposure: morphologic and morphometric analyses. Neuropathology 2008;28:408–16. [DOI] [PubMed] [Google Scholar]
- 32.Alphonse G, Maalouf M, Battiston-Montagne P, et al. p53-independent early and late apoptosis is mediated by ceramide after exposure of tumor cells to photon or carbon ion irradiation. BMC Cancer 2013;13:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys 1999;44:201–10. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki M, Kase Y, Yamaguchi H, et al. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in Chiba (HIMAC) carbon-ion beams. Int J Radiat Oncol Biol Phys 2000;48:241–50. [DOI] [PubMed] [Google Scholar]
- 35.Ando K, Kase Y. Biological characteristics of carbon-ion therapy. Int J Radiat Biol 2009;85:715–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.