Abstract
Testicular histological alterations following Sertoli cell cytoskeleton disruption are numerous. The Sertoli cell cytoskeleton is comprised of intermediate filaments, microtubules, microfilaments and their direct interacting proteins and performs essential functions including structural support of the seminiferous epithelium, apicobasal movement of elongate spermatids, and release of elongate spermatids from the seminiferous epithelium during spermiation. This review summarizes the histological changes occurring after disruption of the Sertoli cell cytoskeleton, including the signature lesion of seminiferous epithelium sloughing. By presenting examples of histological changes after exposure to toxins or toxicants directly affecting the Sertoli cell cytoskeleton or genetic manipulations of this cytoskeleton, the toxicologist observing similar histological changes associated with exposure to novel compounds can use this information to generate hypotheses about a potential mode of action.
Keywords: actin, cytoskeleton, histopathology microtubule, Sertoli, testis, microfilament tubulin, toxicology
Goal and Scope of the Review
The Sertoli cell controls many aspects of spermatogenesis, and disruption of Sertoli cell function is often invoked to explain the mode of action of agents that alter sperm production. When confronted with a histopathological change in the testis, the researcher is faced with the daunting task of identifying a plausible mode of action for this effect. One potential target is the Sertoli cell cytoskeleton. To aid the researcher in determining whether or not the Sertoli cell cytoskeleton is a plausible target, the goals of this review are twofold: to describe testicular histopathological alterations that may occur after perturbation of the Sertoli cell cytoskeleton and to provide potential mechanisms linking Sertoli cell cytoskeletal alterations to the histopathological outcome.
The scope of this review is limited to describing histopathological effects known, or likely, to be mediated via alterations to the Sertoli cell cytoskeleton. At the molecular level, this review will be limited to effects associated with perturbations to cytoskeletal filaments or proteins directly affecting cytoskeletal function. It will rely heavily on histopathological information derived from toxins or toxicants known to directly target cytoskeletal proteins. As in other cells, the Sertoli cell cytoskeleton is linked physically and functionally to numerous biological processes. For the Sertoli cell, one of these processes is adhesion to adjacent cells. Histopathology associated with disruption of Sertoli cell adhesion junctions is a topic for a companion review [see Cheng, this issue] and will not be described here. Non-Sertoli testicular cells use their cytoskeleton for important functions including movement of sperm from the seminiferous tubule to the epididymis, spermatid shape changes during spermiogenesis, and intercellular bridge connectivity between syncitial germ cells. However, histopathological changes associated with cytoskeletal perturbations within non-Sertoli testicular cells will not be described.
The Signature Histological Lesion: Seminiferous Epithelium Sloughing
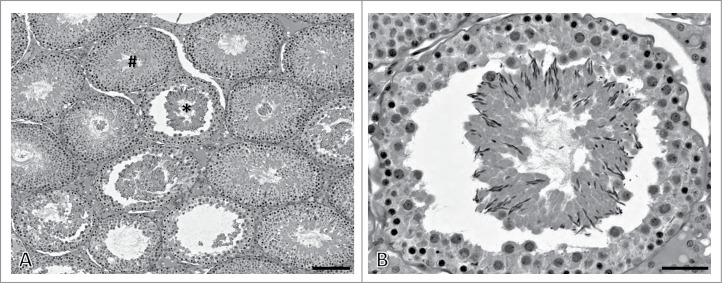
A basic function of the cytoskeleton in most cells is structural support, and this is especially true for the Sertoli cell because of its highly asymmetric shape.1,2 Many of the histological changes described in this review are not unique to agents acting via a cytoskeleton disruption mode of action. However, a histological observation of seminiferous epithelium sloughing into the seminiferous tubule lumen (Fig. 1) is a significant clue that the agent may be targeting the Sertoli cell cytoskeleton. Such an observation is a hallmark of testicular toxicants such as colchicine, vinblastine, and carbendazim that depolymerize Sertoli cell microtubules.3-5 Cellular sloughing may involve a portion of the seminiferous epithelium or encompass the circumference of the seminiferous epithelium. Additional information about this lesion is presented below.
Figure 1.
Seminiferous epithelium sloughing histopathology. (A) In this example, seminiferous epithelium sloughing is evident by detachment and separation of germ cell cohorts from the underlying epithelium (asterisk). Several seminiferous tubules in this image are in the process of sloughing or have sloughed material from other seminiferous tubule areas within the lumen. Other seminiferous tubules show no evidence of sloughing (hashtag). (B) This image is a higher magnification view of the seminiferous tubule in panel A with an asterisk. In this rat stage XI-XII seminiferous tubule, the entire elongate spermatid cohort has detached from the seminiferous epithelium. Some pachytene spermatocytes have also detached with the sloughed material. To capture these images, an adult Fisher 344 rat was exposed to 1% 2,5-hexanedione for 17 d followed by an additional exposure to 200 mg/kg carbendazim for 24 hours.44 The testis was immersion fixed in 10% neutral buffered formalin, embedded in glycol methacrylate, and 3 μm sections stained with periodic acid-Schiff's reagent and hematoxylin. Scale bar in panel A = 150 μm; scale bar in panel B = 50 μm.
Sertoli Cell Cytoskeleton Structure and Function
The cytoskeleton performs crucial roles in signal transduction processes, positioning and intracellular transport of organelles and macromolecular complexes, cell shape determination, and structurally linking cells through intercellular junctions into a cohesive tissue.6,7 These processes are performed by the filamentous cytoskeletal backbone and the plethora of associated proteins that interact with this backbone. In eukaryotic cells, 3 main types of filaments exist: intermediate filaments, actin-based microfilaments, and tubulin-based microtubules. These filaments are dynamic structures constantly being remodelled in response to different cellular processes. The assembly and disassembly of the cytoskeleton filaments are controlled by protein complexes that spatially and temporally determine cytoskeletal organization. By interacting with numerous associated proteins, cytoskeletal filaments serve as scaffolds for intercellular adhesion junctions, the movement of intracellular organelles, and changes in cell shape. If one envisions the cell as a factory building, the cytoskeleton serves as the structural posts and beams determining the overall shape of the building and the location of rooms within the building, the assembly line moving goods from one location to the other, and the foundation physically linking the factory to its environment. Unlike a factory, however, a cell can remodel its cytoskeleton in response to internal and external cues, and this capability is required for dynamic processes like spermatogenesis.
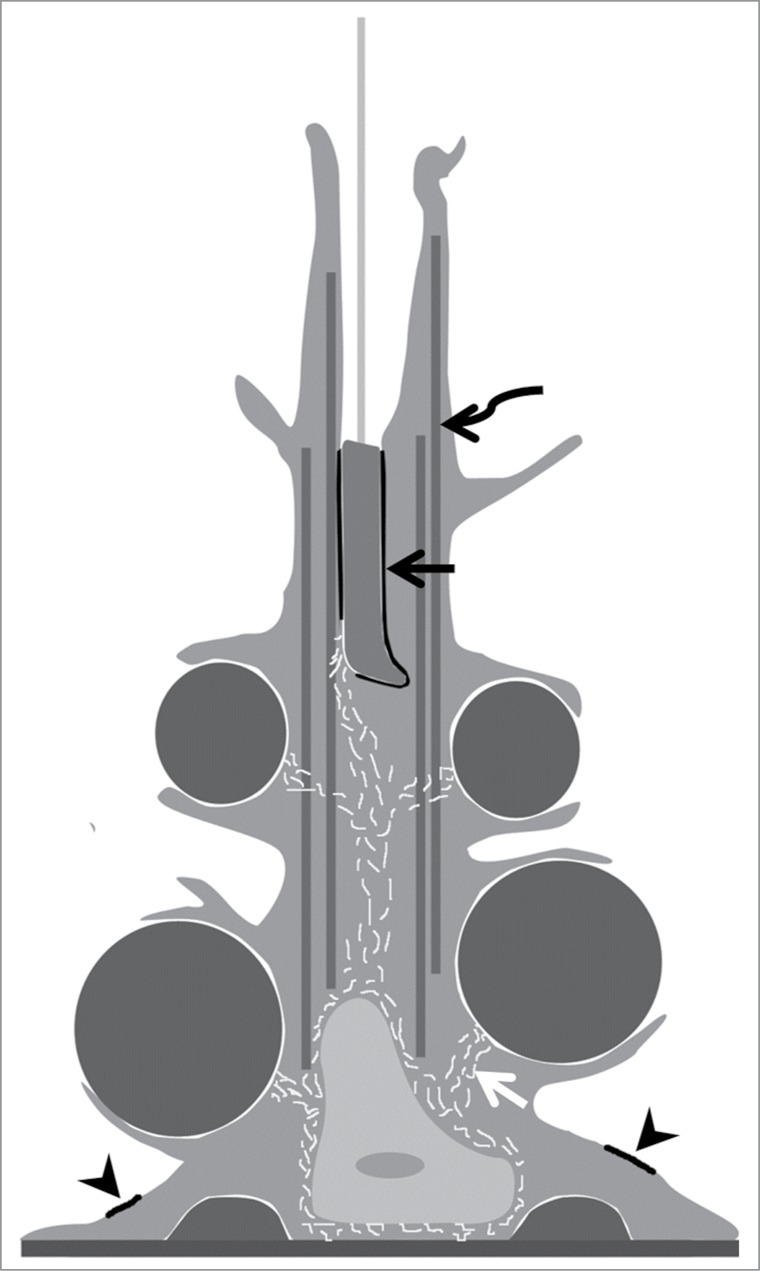
Within the Sertoli cell, microfilaments, intermediate filaments, and microtubules are located at spatially distinct subcellular sites and thus perform unique functions (Fig. 2). As in other cells, the Sertoli cell cytoskeleton functions in intercellular adhesion, intracellular movement, and structural support.8 Because of the unique interaction between the Sertoli cell and surrounding germ cells, these basic cytoskeletal processes are somewhat distinct in Sertoli cells. In most epithelia, tight junctions delineating apical and basal membranes are positioned near the apical pole, but Sertoli cell tight junctions are positioned near the basal cellular aspect, creating an elaborate apical cytoplasm. This apical cytoplasm takes on an appearance similar to a tree, having a relatively thick stalk with numerous long and tenuous processes extending from the stalk and enveloping differentiating germ cells.1,2 For structural support, the Sertoli cell cytoplasmic stalk and apical extensions contain numerous microtubules oriented parallel to the Sertoli cell long axis.9,10 This pattern also allows the Sertoli cell microtubules to function as a platform upon which microtubule motors attach to specialized Sertoli-spermatid adhesion junctions (ectoplasmic specializations) and move elongating spermatids within the seminiferous epithelium along the Sertoli cell apicobasal axis.11,12 Other functions ascribed to Sertoli cell microtubules include secretion of seminiferous tubule fluid,13 intracellular movement of germ cell residual bodies from the apical to basal Sertoli cell subcellular compartments,14,15 and release of mature spermatids from the seminiferous epithelium (spermiation).14,15
Figure 2.
The Sertoli cell cytoskeleton. Shown is a schematic diagram of one Sertoli cell, adjacent germ cells, and the Sertoli cell cytoskeleton. The Sertoli cell is columnar in form with cytoplasmic processes surrounding juxtaposed germ cells. Actin-based microfilaments are associated with basal ectoplasmic specializations (arrowheads) between adjacent Sertoli cell. Actin-based microfilaments are also located at apical ectoplasmic specializations and tubulobulbar complex junctions between the Sertoli cell and elongate spermatid nuclei (black arrow). Microtubules (curved arrow) extend from the Sertoli cell nucleus into apical cytoplasmic processes and are also juxtaposed to ectoplasmic specialization junctions between the Sertoli cell and elongate spermatid. Lastly, intermediate filaments (white arrow) surround the Sertoli cell nucleus and extend to desmosome junctions between the Sertoli cell and germ cells.
Sertoli cell actin-based microfilaments are abundant at 2 specialized testis-specific intercellular junctions. One of these junctions is the ectoplasmic specialization,12,16 and the other is the tubulobulbar complex17,18(also see Cheng, this issue). Ectoplasmic specialization junctions occur at 2 locations: a basal location associated with tight junctions between adjacent Sertoli cells (blood-testis barrier) and an apical location between step 8 and more mature spermatids and Sertoli cells. At the basal junction, a belt of hexagonally arranged actin filaments encircles the Sertoli cell periphery, while morphologically similar actin bundles appear to surround the elongating spermatid head at the apical junction. The primary function of ectoplasmic specializations is adhesion, but apical ectoplasmic specializations also perform the secondary role of elongate spermatid movement along microtubule tracks as previously mentioned. The adhesive function of both basal and apical ectoplasmic specializations must be modified to allow transit of spermatocytes (basal junction) or spermatid release (apical junction). The second Sertoli cell actin-based intercellular junction, the tubulobular complex, appears to be involved in turnover of both apical and basal ectoplasmic specializations. A hallmark of the tubulobulbar complex is a tube-like cytoplasmic process that extends into the Sertoli cell cytoplasm, and actin surrounds this process like a cuff. Given these considerations, Sertoli cell actin-based microfilaments appear crucial for blood-testis barrier function, positioning of germ cells within the seminiferous epithelium, and spermiation.
Intermediate filaments are the final Sertoli cell cytoskeletal component. While intermediate filaments of most epithelial cells are composed of cytokeratin, Sertoli cell intermediate filaments are polymers of vimentin.19 Intermediate filaments surround the Sertoli cell nucleus and extend from this location to adhesion junctions termed desmosome junctions (Fig. 2).20,21 Testicular desmosome junctions are found between adjacent Sertoli cells as well as between Sertoli cells and germ cells. Thus, Sertoli cell intermediate filaments seem poised for a role in intercellular adhesion in the seminiferous epithelium. However, little is known experimentally about Sertoli cell intermediate filament function. The lack of a specific small molecule inhibitor of intermediate filament function partially explains this information gap compared to Sertoli cell microfilaments and microtubules. In addition, mice lacking vimentin show no apparent testicular phenotype.22,23
Because of this information (or lack of understanding), any role of intermediate filaments in mediating histopathological changes in the testis after toxicant exposure remains speculative. An altered Sertoli cell intermediate filament distribution patt-ern has been observed after exposure to various testicular toxicants.24-26 However, it remains to be determined if this effect is likely to be a key event in the toxicant mode of action. Therefore, this review will focus on histopathology associated with Sertoli cell microfilament and microtubule disruption rather than Sertoli cell intermediate filament disruption.
Testicular Histopathology Associated with Sertoli cell Microtubule Disruption
Seminiferous epithelium sloughing
Substances that depolymerize Sertoli cell microtubules induce detachment and sloughing of the seminiferous epithelium into the seminiferous tubule lumen. The 2 most studied agents producing this effect are the plant-derived toxin colchicine and carbendazim, the toxic metabolite of the benzimidazole fungicide benomyl. Both carbendazim and colchicine bind to the β-tubulin building block subunit of microtubules and inhibit polymerization of tubulin subunits into microtubules.27 Seminiferous epithelium sloughing occurs within hours of high dose level colchicine or carbendazim administration4,5,28-30 (Table 1).
Table 1.
Seminiferous epithelium histopathology following Sertoli cell microtubule disruption
| Histopathology Observed | Treatment Inducing Histopathology | Proposed Mechanism | Notes* | References |
|---|---|---|---|---|
| Seminiferous epithelium sloughing | Colchicine Carbendazim |
Destabilization of Sertoli cell stalk by a lack of microtubule structural support | Sloughing may includes the entire circumference of the seminiferous epithelium Reported to be the most sensitive histological endpoint after Sertoli cell microtubule disruption by depolymerising agents Sloughing generally occurs between dissimilar germ cell cohorts with elongating spermatid cohorts being the most sensitive Sloughed material contains fragments of apical Sertoli cell cytoplasm attached by adhesion junctions to intact germ cells Stage dependent: stages III - V are the most resistant to sloughing Rete testis and efferent duct become occluded with sloughed seminiferous epithelium cellular material May result in persistent atrophy of the seminiferous epithelium |
30 57 34 4 35 28 29 5 33 |
| Abnormal seminiferous epithelium location of elongating spermatid nuclei | Colchicine Taxol Gamma tubulin overexpression |
Inhibition of elongate spermatid movement along Sertoli cell microtubules | Impaired basal movement of elongate spermatids resulting in an their apical location within stage IV - VI seminiferous tubules Impaired apical movement of elongate spermatids during stage VI resulting in step 19 spermatids located in the basal portion of seminiferous epithelium |
30 14 15 39 |
| Retained spermatids | Carbendazim 2,5-Hexanedione Gamma tubulin overexpression |
Impairment of Sertoli-elongate spermatid junction dynamics followed by Sertoli cell phagocytosis of the elongate spermatid | Retained step 19 spermatids may be present in basal, mid, or apical regions of the seminiferous epithelium Most often observed in stage IX – X seminiferous tubules |
33 14 56 |
| Seminiferous epithelium vacuolization |
Carbendazim 2,5-Hexanedione MAP7 knockout KATNAL1 knockout |
Dilation of Sertoli cell smooth endoplasmic reticulum | Vacuoles are large; one study defined vacuoles as being greater than 16 μm in diameter Vacuoles are observed in the basal compartment of the seminiferous epithelium Treatment-related vacuolization may be observed in only a low percentage of seminiferous tubules |
56 47 44 45 33 29 46 |
| Residual body retention | Gamma tubulin overexpression Taxol Colchicine |
Failure of residual body movement within the Sertoli cell from an apical to a basal location | Retained residual bodies are identified by their location in the apical seminiferous epithelium of stages IX - XIV seminiferous tubules |
14 15 39 |
| Seminiferous epithelium atrophy | Carbendazim 2,5-Hexanedione Colchicine MAP7 knockout |
Severe functional Sertoli cell deficit rendering its germ cell “nurse” function inoperable | Represents the “Sertoli cell-only” syndrome Generally a late histopathological finding and progressive in nature Seminiferous tubules are of small diameter, contain few basal spermatogonia, and Sertoli cell cytoplasm fills the tubule lumen May be induced by direct action of the toxic agent on the Sertoli cell such as seminiferous epithelium sloughing or may be secondary to efferent duct blockage |
14 47 34 58 28 |
| Enlarged seminiferous tubule lumen | Carbendazim | Rete testis and efferent duct occlusion along with sloughing of seminiferous epithelium | Sloughed seminiferous epithelium occludes the efferent duct leading to back pressure-induced expansion of the seminiferous tubule lumen because of continued seminiferous tubule fluid secretion Occlusion and seminiferous tubule back pressure induce atrophy of the seminiferous epithelium |
34 32 44 33 |
*Seminiferous tubule staging and spermiogenic steps refer to those developed for the rat
Typically, sloughing begins with detachment of apical elongate spermatid cohorts and may involve a portion of, or the entire circumference of, the seminiferous epithelium.31 Morphologically, sloughing has been defined as a separation of at least one germ cell layer from the seminiferous epithelium30,32 or the presence of detached cellular material in the tubule lumen with a diameter of at least 24 μm.33 Sensitivity to sloughing is stage dependent, with stages III-V (when elongate spermatids are buried deep within Sertoli cell crypts) being the least sensitive stages.4,30,31 With time or higher dose levels of microtubule depolymerising agents, more basal seminiferous epithelium layers may become involved, including the entire apical Sertoli cell cytoplasmic stalk with attached germ cells to the level of the blood-testis barrier.5,28
Extensive seminiferous epithelium sloughing produces the secondary effect of testicular atrophy. The lumen of all seminiferous tubules is connected to the epididymis by a collecting duct system comprised of the rete testis and efferent ducts. Given sufficient seminiferous epithelium sloughing, this cellular material is carried by seminiferous tubule fluid into the rete testis and efferent duct where it becomes entrapped and occludes the rete testis and efferent duct.28,34 The initial result of this occlusion is an increase in testis weight because of continued seminiferous tubule fluid secretion;32,34 however, the ultimate effects of occlusion are inhibition of seminiferous tubule fluid secretion, reduced testis weight, and atrophy of the seminiferous epithelium.5,28,32,34
As mentioned previously, a mechanism of seminiferous epithelium sloughing is the depolymerisation of Sertoli cell microtubules. At both the electron and light microscopy levels, sloughing is associated with reduced microtubule content within the Sertoli cell cytoplasmic stalk.5,30,31 Sloughed cellular material contains fragments of Sertoli cell cytoplasm along with germ cells,4,5,31 and germ cells remain attached to Sertoli cells via adhesion junctions.4,5 Based upon these data and the observation of sloughing of entire germ cell layers, the following hypothesis has been developed as the physical mechanism of sloughing.5,35 Sertoli cell microtubule depolymerisation induces displacement of the Sertoli cell cytoplasm toward the basal lamina, but the most apical portion of the Sertoli cell cytoplasm cannot be displaced toward the basal lamina because of the syncitial nature of attached germ cell cohorts. The tension thus created cleaves the Sertoli cell cytoplasmic stalk above the level of Sertoli-Sertoli adhesion junctions releasing the apical portion of the seminiferous epithelium.
Abnormal seminiferous epithelium location of elongating spermatid nuclei
During 2 phases of spermatogenesis, elongate spermatids appear to traverse nearly the entire length of the seminiferous epithelium.12,36 In the rat, elongate spermatid nuclei have an apical location in stage II-III seminiferous tubules but are located deep within Sertoli cell cytoplasmic crypts at stage V. Subsequently, elongate spermatid nuclei move from these crypts to the seminiferous epithelium apical surface during stage VI. As previously mentioned, spermatid translocation is hypothesized to involve movement of Sertoli/elongated spermatid ectoplasmic specialization junctions (with attached elongate spermatid nuclei) along Sertoli cell microtubule tracks12 (also see O’Donnell, this issue).
Agents that depolymerize or stabilize Sertoli cell microtubules may produce the histopathological observation of abnormal elongate spermatid nuclear position. While abnormal spermatid location has often been analyzed qualitatively, Fleming et al.14 quantified this endpoint by dividing the seminiferous epithelium of selected stages into apical and basal sectors and counting elongate spermatid nuclei within the sectors. Taxol is a plant-derived toxin that directly binds β-tubulin and stabilizes microtubules leading to abnormal microtubule dynamics and an excess of microtubules within cells.37,38 Exposure of the rat testis to taxol increases the number of microtubules in the Sertoli cell cytoplasm.15 Furthermore, step 19 elongate spermatid nuclei in taxol-exposed rats are positioned deep within Sertoli cell crypts in stage VII seminiferous tubules, rather than being in their normal apical position.15 Genetic overexpression of γ-tubulin (a protein that enhances microtubule polymerization) in rat Sertoli cells produces a histopathological effect on step 19 spermatid location similar to taxol exposure.14 Like microtubule stabilizing agents, colchicine is reported to inhibit the apical movement of elongate spermatids in the ground squirrel39 and to induce a more apical position of step 17 elongate spermatid nuclei in stage V rat seminiferous tubules.30 The hypothetical mechanism for these effects is an inhibition of elongate spermatid movement along Sertoli cell microtubules.
Residual body retention
During spermiation, excess elongate spermatid cytoplasm, RNA, and organelles are endocytosed into Sertoli cells as the residual body.40 Within Sertoli cells, the residual body is transported to the basal cytoplasm and fuses with lysosomes to catabolize residual body contents. In histological sections of control rat stage VIII seminiferous tubules, residual bodies are observed at the apical seminiferous epithelial surface and move toward the basal lamina and are degraded by stage X.41
In the rat, residual body retention is a histological observation of residual bodies within the apical seminiferous epithelium in stage X-XIV seminiferous tubules.14,15 Retained residual bodies have been observed after testicular exposure to agents that alter Sertoli cell microtubule polymerization dynamics, including colchicine,39 γ-tubulin overexpression,14 and taxol.15 The mechanism for this effect is unclear but is hypothesized to be disruption of microtubule-based Sertoli cell transport of residual bodies to the basal Sertoli cell cytoplasm.15
Retained spermatids
During spermiation that occurs at stage VIII of the rat seminiferous epithelium cycle, step 19 elongate spermatids are released from their adhesion to the apical Sertoli cell plasma membrane into the seminiferous tubule lumen. One of the more common testis histopathological observations is failure of step 19 spermatid release. Exposure of rodents to several toxicants, presumably acting via distinct modes of action, can induce spermatid retention;42 thus, retained spermatid histopathology is not unique to a Sertoli cell cytoskeleton disruption mode of action (also see O’Donnell, this issue).
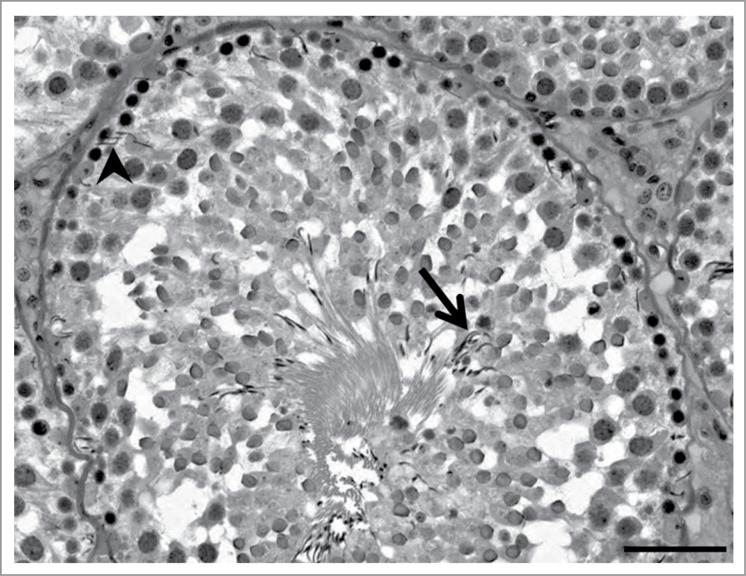
Retained spermatids are detected by the presence of condensed spermatid nuclei in stage IX-XIV seminiferous tubules (Fig. 3).14,33 Retained spermatid nuclei may appear at any position within the seminiferous epithelium and are likely phagocytosed and degraded by the Sertoli cell.43 It should be noted that the observations of retained spermatids and abnormal positioning of elongate spermatids within the seminiferous epithelium are distinguished by the seminiferous epithelial stage and spermiogenic step involved. Agents causing retained spermatids and targeting the Sertoli cell microtubule system include carbendazim,33 γ-tubulin overexpression,14 taxol,15 and 2,5-hexanedione.33 Because spermatid retention requires approximately one day to develop before it is observed histologically, it is not a sensitive endpoint for toxicant exposures of short duration.42 However, for toxicant exposures requiring several days or weeks before testicular histopathology is observed (such as 2,5-hexanedione), retained spermatid histopathology may be an early and sensitive marker of testicular injury.42 Like retention of spermatid residual bodies, the mechanism of spermatid retention after exposure to microtubule disupting agents is unclear but is speculated to involve abnormal microtubule-based transport of elongate spermatids that leads to an inhibition of spermiation.14 Another potential mechanism linked to Sertoli cell microtubules may be alteration of microtubule function at ectoplasmic specialization junctions between Sertoli cells and elongate spermatids.42
Figure 3.
Retained spermatids histopathology. In this rat stage X seminiferous tubule, step 19 spermatids are present in both the basal aspect (arrowhead) and apical aspect (arrow) of the seminiferous epithelium. In addition, this seminiferous tubule has a mottled appearance suggesting sloughing of the step 10 spermatid cohort may be occurring. Toxicant exposure in this example was a combination of 2,5-hexanedione and carbendazim, as described in the legend to Figure 1. Scale bar = 50 μm.
Seminiferous epithelium vacuolization
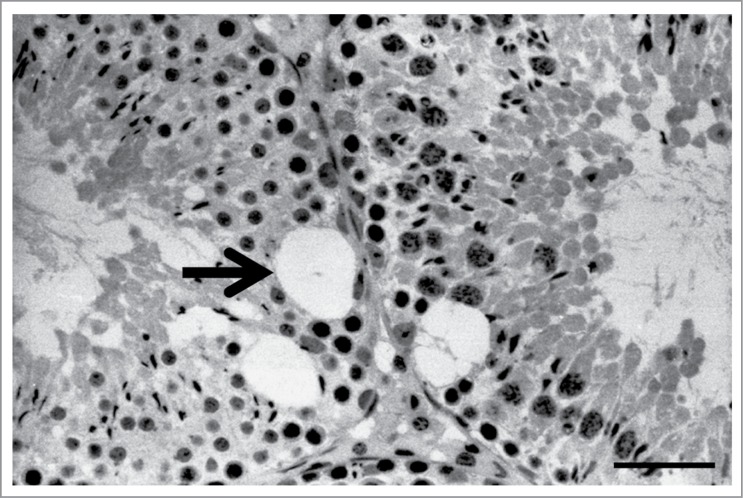
Like spermatid retention, seminiferous epithelium vacuolization is a relatively common histopathological observation associated with Sertoli cell injury that is observed after exposure to testicular toxicants with various modes of action. In this respect, Sertoli cell vacuolization is similar to retained spermatids histopathology. Seminiferous epithelium vacuolization is defined by the observation of large diameter (≥16 μm) vacuoles in the basal aspect of the seminiferous epithelium (Fig. 4).44 Seminiferous epithelium vacuoles are distended portions of the Sertoli cell smooth endoplasmic reticulum45 and may represent an early stage of Sertoli cell toxicity.33 Vacuoles may present in a stage-dependent manner, as is the case after rat exposure to the Sertoli cell microtubule stabilizing toxicant 2,5-hexanedione.45 Carbendazim also induces seminiferous epithelium vacuolization,29,44 as does genetic deletion of the microtubule associated proteins MAP7 and KATNAL1 in mouse Sertoli cells.46,47 How alterations to the Sertoli cell microtubule system cause vacuolization is unknown.
Figure 4.
Sertoli cell vacuolization histopathology. In these adjacent rat seminiferous tubules, large vacuoles (arrow) are seen in the basal half of the seminiferous epithelium. In this example, a young adult rat was exposed to 1% 2,5-hexanedione in the drinking water for 25 days.56 The testis was processes as described in the legend to Figure 1. Scale bar = 50 μm.
Seminiferous epithelium atrophy
As might be imagined, atrophy of the seminiferous epithelium is not an outcome limited to agents targeting the Sertoli cell cytoskeleton. Nevertheless, atrophy is observed following exposure to toxicants that both stabilize and depolymerize Sertoli cell microtubules. The typical presentation is a seminiferous tubule of small diameter having Sertoli cell and the occasional spermatogonial nuclei adjacent to the basement membrane. Sertoli cell cytoplasm fills the tubule, and there may be no obvious lumen present.
Atrophic seminiferous tubules typically develop after exposure to higher toxicant dose levels and/or longer time periods of exposure, and the mode of action for development of atrophic seminiferous tubules can vary. The carbendazim mode of action was described previously and is indirect occurring via occlusion of the efferent ducts with sloughed seminiferous epithelium. 2,5-Hexanedione-induced seminiferous tubule atrophy appears to be caused by a direct affect of the toxicant on the Sertoli cell, impairing the ability of Sertoli cells to physiologically support germ cell maturation.48
Testicular Histopathology Associated with Sertoli cell Microfilament Disruption
Within Sertoli cells, microfilaments are concentrated at intercellular junctions as described previously. Because testicular histopathology following disruption of these junctions is addressed in a companion review [see Cheng, this issue] and few agents causing testicular injury are known to directly target the microfilament system, this section is limited to describing the testicular phenotype following exposure to the microfilament depolymerising mycotoxin cytochalasin D. Unlike other agents reported to cause testicular effects by a putative microfilament-disrupting mode of action,49,50 cytochalasin D is known to directly bind to actin and depolymerize microfilaments.51 Testicular histopathology after siRNA-mediated knockdown of Sertoli cell cortactin expression is also described (Table 2).
Table 2.
Seminiferous epithelium histopathology following Sertoli cell microfilament disruption
| Histopathology Observed | Treatment Inducing Histopathology | Proposed Mechanism | Notes* | References |
|---|---|---|---|---|
| Malorientation of round spermatids | Cytochalasin D | Loss or lack of formation of the ectoplasmic specialization junction between round spermatids and Sertoli cells | Involves step 8 spermatids The acrosome of step 8 spermatids normally is pointed toward the seminiferous epithelium basal lamina but acrosome orientation becomes more random |
53 |
| Retained spermatids | siRNA-mediated cortactin knockdown | Alteration of tubulobulbar complex function and persistence of the ectoplasmic specialization junction between elongate spermatid and Sertoli cell | Spermatids retained past the normal stage of spermiation may be located at the seminiferous epithelium apical border or in a more basal location | 54 |
* Spermiogenic steps refer to those developed for the rat
Malorientation of round spermatids
Intratesticular injection of cytochalasin D depolymerizes Sertoli cell actin microfilaments, resulting in the abolishment of actin-based basal ectoplasmic specialization adhesion junctions between Sertoli cells and apical ectoplasmic specialization adhesion junctions between Sertoli cells and spermatids.52,53 In the rat, apical ectoplasmic specializations first form between Sertoli cells and step 8 round spermatids. At this spermiogenic step, the acrosome of round spermatids becomes oriented toward the basal aspect of the seminiferous tubule; however, cytochalasin D exposure randomizes the orientation of step 8 spermatids within the seminiferous epithelium.53 Presumably, the Sertoli-spermatid ectoplasmic specialization positions the spermatid nucleus such that the acrosome faces the basal lamina, and microfilament depolymerisation perturbs the ectoplasmic specialization allowing the spermatid nuclear position to become randomized.
Retained spermatids
Just prior to spermiation, actin-based tubulobulbar complexes form between Sertoli cells and step 19 rat spermatids and are hypothesized to function in disengagement of adhesive ectoplasmic specialization junctions between Sertoli cells and elongate spermatids.18 There are conflicting data on the effect of agents targeting the actin cytoskeleton on spermiation. Although rat cytochalasin D exposure is reported to inhibit tubulobulbar complex formation,15,53 spermiation failure was not reported.15 On the other hand, depletion of cortactin in mouse Sertoli cells by siRNA-mediated knockdown results in abnormal tubulobulbar complex morphology, persistence of apical ectoplasmic specializations, and spermiation failure.54 Cortactin is an actin-binding protein that modulates microfilament nucleation and branching pattern.55 In cortactin siRNA-exposed Sertoli cells, step 16 spermatid nuclei were observed in stage IX seminiferous epithelium, indicating abnormal spermatid retention.54 Thus, alterations to the Sertoli cell actin cytoskeleton may affect tubulobulbar complex function leading to an inhibition of spermiation.
Conclusion
The Sertoli cell cytoskeleton performs important roles in maintaining seminiferous epithelium structural integrity, movement of elongating spermatids during the seminiferous epithelial cycle, and adhesion and release of elongate spermatids. Although agents that target the Sertoli cell cytoskeleton can induce a plethora of histopathological alterations, many of these alterations are not specific to Sertoli cell cytoskeletal disruption. Despite this non-specificity, the investigator should consider a Sertoli cell cytoskeleton mode of action when confronted with any of the histological changes described in this review. If sloughing of the seminiferous epithelium is observed that includes germ cell cohorts and attached Sertoli cell cytoplasm, the researcher should consider this finding an excellent clue that the Sertoli cell cytoskeleton is a likely target.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The author would like to thank Dr. Kim Boekelheide and Susan Hall for providing histological images used in this manuscript and Drs. Bob Chapin, Mike Woolhiser, and Kim Boekelheide for critical reading of the manuscript prior to submission.
References
- 1. Wong V, Russell LD. Three-dimensional reconstruction of a rat stage V Sertoli cell: I. Methods, basic configuration, and dimensions. Am J Anat 1983; 167:143-61; PMID:6351582; http://dx.doi.org/ 10.1002/aja.1001670202 [DOI] [PubMed] [Google Scholar]
- 2. Wang F, Zhang Q, Cao J, Huang Q, Zhu X. The microtubule plus end-binding protein EB1 is involved in Sertoli cell plasticity in testicular seminiferous tubules. Exp Cell Res 2008; 314:213-26; PMID:17964570; http://dx.doi.org/ 10.1016/j.yexcr.2007.09.022 [DOI] [PubMed] [Google Scholar]
- 3. Correa LM, Nakai M, Strandgaard CS, Hess RA, Miller MG. Microtubules of the mouse testis exhibit differential sensitivity to the microtubule disruptors Carbendazim and colchicine. Toxicol Scie 2002; 69:175-82; PMID:12215672; http://dx.doi.org/ 10.1093/toxsci/69.1.175 [DOI] [PubMed] [Google Scholar]
- 4. Nakai M, Hess RA. Morphological changes in the rat Sertoli cell induced by the microtubule poison carbendazim. Tissue Cell 1994; 26:917-27; PMID:7886678; http://dx.doi.org/ 10.1016/0040-8166(94)90041-8 [DOI] [PubMed] [Google Scholar]
- 5. Russell LD, Malone JP, MacCurdy DS. Effect of the microtubule disrupting agents, colchicine and vinblastine, on seminiferous tubule structure in the rat. Tissue Cell 1981; 13:349-67; PMID:7314074; http://dx.doi.org/ 10.1016/0040-8166(81)90010-0 [DOI] [PubMed] [Google Scholar]
- 6. Brieher WM, Yap AS. Cadherin junctions and their cytoskeleton(s). Curr Opin Cell Biol 2013; 25:39-46; PMID:23127608; http://dx.doi.org/ 10.1016/j.ceb.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 7. Jaqaman K, Grinstein S. Regulation from within: the cytoskeleton in transmembrane signaling. Trends Cell Biol 2012; 22:515-26; PMID:22917551; http://dx.doi.org/ 10.1016/j.tcb.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol 2008; 636:186-211; PMID:19856169; http://dx.doi.org/ 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 9. Neely MD, Boekelheide K. Sertoli cell processes have axoplasmic features: an ordered microtubule distribution and an abundant high molecular weight microtubule-associated protein (cytoplasmic dynein). J Cell Biol 1988; 107:1767-76; PMID:2972729; http://dx.doi.org/ 10.1083/jcb.107.5.1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vogl AW. Changes in the distribution of microtubules in rat Sertoli cells during spermatogenesis. Anat Record 1988; 222:34-41; PMID:3056115; http://dx.doi.org/ 10.1002/ar.1092220107 [DOI] [PubMed] [Google Scholar]
- 11. Vaid KS, Guttman JA, Singaraja RR, Vogl AW. A kinesin is present at unique sertoli/spermatid adherens junctions in rat and mouse testes. Biol Reprod 2007; 77:1037-48; PMID:17855729; http://dx.doi.org/ 10.1095/biolreprod.107.063735 [DOI] [PubMed] [Google Scholar]
- 12. Vogl AW, Pfeiffer DC, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol 2000; 63:1-15; PMID:10770585; http://dx.doi.org/ 10.1679/aohc.63.1 [DOI] [PubMed] [Google Scholar]
- 13. Richburg JH, Redenbach DM, Boekelheide K. Seminiferous tubule fluid secretion is a Sertoli cell microtubule-dependent process inhibited by 2,5-hexanedione exposure. Toxicol Appl Pharmacol 1994; 128:302-9; PMID:7940545; http://dx.doi.org/ 10.1006/taap.1994.1210 [DOI] [PubMed] [Google Scholar]
- 14. Fleming SL, Shank PR, Boekelheide K. gamma-Tubulin overexpression in Sertoli cells in vivo. II: Retention of spermatids, residual bodies, and germ cell apoptosis. Biol Reprod 2003; 69:322-30; PMID:12672672; http://dx.doi.org/ 10.1095/biolreprod.102.011817 [DOI] [PubMed] [Google Scholar]
- 15. Russell LD, Saxena NK, Turner TT. Cytoskeletal involvement in spermiation and sperm transport. Tissue Cell 1989; 21:361-79; PMID:2479117; http://dx.doi.org/ 10.1016/0040-8166(89)90051-7 [DOI] [PubMed] [Google Scholar]
- 16. Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocrine Rev 2004; 25:747-806; PMID:15466940; http://dx.doi.org/ 10.1210/er.2003-0022 [DOI] [PubMed] [Google Scholar]
- 17. Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int Rev Cell Mol Biol 2013; 303:319-55; PMID:23445814; http://dx.doi.org/ 10.1016/B978-0-12-407697-6.00008-8 [DOI] [PubMed] [Google Scholar]
- 18. Vogl AW, Du M, Wang XY, Young JS. Novel clathrin/actin-based endocytic machinery associated with junction turnover in the seminiferous epithelium. Semin Cell Dev Biol 2013;55-64; PMID:24280271 [DOI] [PubMed] [Google Scholar]
- 19. Franke WW, Grund C, Schmid E. Intermediate-sized filaments present in Sertoli cells are of the vimentin type. Eur J Cell Biol 1979; 19:269-75; PMID:385322 [PubMed] [Google Scholar]
- 20. Mruk DD, Cheng CY. Desmosomes in the testis: Moving into an unchartered territory. Spermatogenesis 2011; 1:47-51; PMID:21866275; http://dx.doi.org/ 10.4161/spmg.1.1.15443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Russell L. Desmosome-like junctions between Sertoli and germ cells in the rat testis. Am J Anat 1977; 148:301-12; PMID:857631; http://dx.doi.org/ 10.1002/aja.1001480302 [DOI] [PubMed] [Google Scholar]
- 22. Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell 1994; 79:679-94; PMID:7954832; http://dx.doi.org/ 10.1016/0092-8674(94)90553-3 [DOI] [PubMed] [Google Scholar]
- 23. Vogl AW, Colucci-Guyon E, Babinet C. Vimentin intermediate filaments are not necessary for the development of a normal differentiated phenotype by mature Sertoli cells. Mol Biology Cell 1996; 7:555a. [Google Scholar]
- 24. Richburg JH, Boekelheide K. Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol Appl Pharmacol 1996; 137:42-50; PMID:8607140; http://dx.doi.org/ 10.1006/taap.1996.0055 [DOI] [PubMed] [Google Scholar]
- 25. Hall ES, Eveleth J, Boekelheide K. Two,5-Hexanedione exposure alters the rat Sertoli cell cytoskeleton. II. Intermediate filaments and actin. Toxicol Appl Pharmacol 1991; 111:443-53; PMID:1746025 [DOI] [PubMed] [Google Scholar]
- 26. Alam MS, Kurohmaru M. Disruption of Sertoli cell vimentin filaments in prepubertal rats: An acute effect of butylparaben in vivo and in vitro. Acta Histochem 2014; 682-7. [DOI] [PubMed] [Google Scholar]
- 27. Winder BS, Strandgaard CS, Miller MG. The role of GTP Binding and microtubule-associated proteins in the inhibition of microtubule assembly by carbendazim. Toxicol Sci 2001; 59:138-46; PMID:11134553; http://dx.doi.org/ 10.1093/toxsci/59.1.138 [DOI] [PubMed] [Google Scholar]
- 28. Nakai M, Hess RA, Moore BJ, Guttroff RF, Strader LF, Linder RE. Acute and long-term effects of a single dose of the fungicide carbendazim (methyl 2-benzimidazole carbamate) on the male reproductive system in the rat. J Androl 1992; 13:507-18; PMID:1293130 [PubMed] [Google Scholar]
- 29. Lim J, Miller MG. The role of the benomyl metabolite carbendazim in benomyl-induced testicular toxicity. Toxicol Appl Pharmacol 1997; 142:401-10; PMID:9070363; http://dx.doi.org/ 10.1006/taap.1996.8042 [DOI] [PubMed] [Google Scholar]
- 30. Allard EK, Johnson KJ, Boekelheide K. Colchicine disrupts the cytoskeleton of rat testis seminiferous epithelium in a stage-dependent manner. Biol Reprod 1993; 48:143-53; PMID:8418902; http://dx.doi.org/ 10.1095/biolreprod48.1.143 [DOI] [PubMed] [Google Scholar]
- 31. Nakai M, Miller MG, Carnes K, Hess RA. Stage-specific effects of the fungicide carbendazim on Sertoli cell microtubules in rat testis. Tissue Cell 2002; 34:73-80; PMID:12165241; http://dx.doi.org/ 10.1016/S0040-8166(02)00006-X [DOI] [PubMed] [Google Scholar]
- 32. Hess RA, Moore BJ, Forrer J, Linder RE, Abuel-Atta AA. The fungicide benomyl (methyl 1-(butylcarbamoyl)-2-benzimidazolecarbamate) causes testicular dysfunction by inducing the sloughing of germ cells and occlusion of efferent ductules. Fundam Appl Toxicol 1991; 17:733-45; PMID:1778360; http://dx.doi.org/ 10.1016/0272-0590(91)90181-3 [DOI] [PubMed] [Google Scholar]
- 33. Moffit JS, Bryant BH, Hall SJ, Boekelheide K. Dose-dependent effects of sertoli cell toxicants 2,5-hexanedione, carbendazim, and mono-(2-ethylhexyl) phthalate in adult rat testis. Toxicol Pathol 2007; 35:719-27; PMID:17763286; http://dx.doi.org/ 10.1080/01926230701481931 [DOI] [PubMed] [Google Scholar]
- 34. Hess RA, Nakai M. Histopathology of the male reproductive system induced by the fungicide benomyl. Histol Histopathol 2000; 15:207-24; PMID:10668211 [DOI] [PubMed] [Google Scholar]
- 35. Nakai M, Hess RA, Netsu J, Nasu T. Deformation of the rat Sertoli cell by oral administration of carbendazim (methyl 2-benzimidazole carbamate). J Androl 1995; 16:410-6; PMID:8575980 [PubMed] [Google Scholar]
- 36. Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann New York Acad Sci 1952; 55:548-73; PMID:13139144; http://dx.doi.org/ 10.1111/j.1749-6632.1952.tb26576.x [DOI] [PubMed] [Google Scholar]
- 37. Rao S, He L, Chakravarty S, Ojima I, Orr GA, Horwitz SB. Characterization of the Taxol binding site on the microtubule. Identification of Arg(282) in β-tubulin as the site of photoincorporation of a 7-benzophenone analogue of Taxol. J Biol Chem 1999; 274:37990-4; PMID:10608867; http://dx.doi.org/ 10.1074/jbc.274.53.37990 [DOI] [PubMed] [Google Scholar]
- 38. Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A 1980; 77:1561-5; PMID:6103535; http://dx.doi.org/ 10.1073/pnas.77.3.1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vogl AW, Linck RW, Dym M. Colchicine-induced changes in the cytoskeleton of the golden-mantled ground squirrel (Spermophilus lateralis) Sertoli cells. A J Anat 1983; 168:99-108; PMID:6637858; http://dx.doi.org/ 10.1002/aja.1001680110 [DOI] [PubMed] [Google Scholar]
- 40. Kerr JB, de Kretser DM. Proceedings: The role of the Sertoli cell in phagocytosis of the residual bodies of spermatids. J Reprod Fertil 1974; 36:439-40; PMID:4819326; http://dx.doi.org/ 10.1530/jrf.0.0360439 [DOI] [PubMed] [Google Scholar]
- 41. Russell LD, Ettlin RA, Sinha Hikim AP, Clegg ED. Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press, 1990. [Google Scholar]
- 42. Bryant BH, Yamasaki H, Sandrof MA, Boekelheide K. Spermatid head retention as a marker of 2,5-hexanedione-induced testicular toxicity in the rat. Toxicol Pathol 2008; 36:552-9; PMID:18467684; http://dx.doi.org/ 10.1177/0192623308317426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Russell LD. The perils of sperm release– ‘let my children go’. Int J Androl 1991; 14:307-11; PMID:1794915; http://dx.doi.org/ 10.1111/j.1365-2605.1991.tb01097.x [DOI] [PubMed] [Google Scholar]
- 44. Markelewicz RJ, Jr., Hall SJ, Boekelheide K. Two,5-hexanedione and carbendazim coexposure synergistically disrupts rat spermatogenesis despite opposing molecular effects on microtubules. Toxicol Sci 2004; 80:92-100; PMID:15141104; http://dx.doi.org/ 10.1093/toxsci/kfh140 [DOI] [PubMed] [Google Scholar]
- 45. Chapin RE, Morgan KT, Bus JS. The morphogenesis of testicular degeneration induced in rats by orally administered 2,5-hexanedione. Exp Mol Pathol 1983; 38:149-69; PMID:6832342; http://dx.doi.org/ 10.1016/0014-4800(83)90082-5 [DOI] [PubMed] [Google Scholar]
- 46. Smith LB, Milne L, Nelson N, Eddie S, Brown P, Atanassova N, O’Bryan MK, O’Donnell L, Rhodes D, Wells S, et al. . KATNAL1 regulation of sertoli cell microtubule dynamics is essential for spermiogenesis and male fertility. PLoS Genet 2012; 8:e1002697; PMID:22654668; http://dx.doi.org/ 10.1371/journal.pgen.1002697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Komada M, McLean DJ, Griswold MD, Russell LD, Soriano P. E-MAP-115, encoding a microtubule-associated protein, is a retinoic acid-inducible gene required for spermatogenesis. Genes Dev 2000; 14:1332-42; PMID:10837026 [PMC free article] [PubMed] [Google Scholar]
- 48. Boekelheide K, Fleming SL, Allio T, Embree-Ku ME, Hall SJ, Johnson KJ, Kwon EJ, Patel SR, Rasoulpour RJ, Schoenfeld HA, et al. . Two,5-hexanedione-induced testicular injury. Ann Rev Pharmacol Toxicol 2003; 43:125-47; PMID:12471174; http://dx.doi.org/ 10.1146/annurev.pharmtox.43.100901.135930 [DOI] [PubMed] [Google Scholar]
- 49. Hew KW, Heath GL, Jiwa AH, Welsh MJ. Cadmium in vivo causes disruption of tight junction-associated microfilaments in rat Sertoli cells. Biol Reprod 1993; 49:840-9; PMID:8218650; http://dx.doi.org/ 10.1095/biolreprod49.4.840 [DOI] [PubMed] [Google Scholar]
- 50. Wiebe JP, Kowalik A, Gallardi RL, Egeler O, Clubb BH. Glycerol disrupts tight junction-associated actin microfilaments, occludin, and microtubules in Sertoli cells. J Androl 2000; 21:625-35; PMID:10975408 [PubMed] [Google Scholar]
- 51. Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol 1987; 105:1473-8; PMID:3312229; http://dx.doi.org/ 10.1083/jcb.105.4.1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weber JE, Turner TT, Tung KS, Russell LD. Effects of cytochalasin D on the integrity of the Sertoli cell (blood-testis) barrier. Am J Anat 1988; 182:130-47; PMID:3400621; http://dx.doi.org/ 10.1002/aja.1001820204 [DOI] [PubMed] [Google Scholar]
- 53. Russell LD, Goh JC, Rashed RM, Vogl AW. The consequences of actin disruption at Sertoli ectoplasmic specialization sites facing spermatids after in vivo exposure of rat testis to cytochalasin D. Biol Reprod 1988; 39:105-18; PMID:3207792; http://dx.doi.org/ 10.1095/biolreprod39.1.105 [DOI] [PubMed] [Google Scholar]
- 54. Young JS, De Asis M, Guttman J, Vogl AW. Cortactin depletion results in short tubulobulbar complexes and spermiation failure in rat testes. Biol Open 2012; 1:1069-77; PMID:23213386; http://dx.doi.org/ 10.1242/bio.20122519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol : CB 2001; 11:370-4; PMID:11267876; http://dx.doi.org/ 10.1016/S0960-9822(01)00098-7 [DOI] [PubMed] [Google Scholar]
- 56. Johnson KJ, Hall ES, Boekelheide K. Two,5-Hexanedione exposure alters the rat Sertoli cell cytoskeleton. I. Microtubules and seminiferous tubule fluid secretion. Toxicol Appl Pharmacol 1991; 111:432-42; PMID:1746024; http://dx.doi.org/ 10.1016/0041-008X(91)90248-D [DOI] [PubMed] [Google Scholar]
- 57. Lim J, Miller MG. Role of testis exposure levels in the insensitivity of prepubertal rats to carbendazim-induced testicular toxicity. Fundam Appl Toxicol 1997; 37:158-67; PMID:9242589; http://dx.doi.org/ 10.1006/faat.1997.2315 [DOI] [PubMed] [Google Scholar]
- 58. Carter SD, Hess RA, Laskey JW. The fungicide methyl 2-benzimidazole carbamate causes infertility in male Sprague-Dawley rats. Biol Reprod 1987; 37:709-17; PMID:3676414; http://dx.doi.org/ 10.1095/biolreprod37.3.709 [DOI] [PubMed] [Google Scholar]