Anterior ischemic optic neuropathy (AION), is classified as arteritic (giant cell arteritis; GCA) or non-arteritic (NAION). Varicella zoster virus (VZV) vasculopathy causes symptoms and signs of AION.1-4 Because VZV is present in temporal arteries (TAs) of most patients with GCA1,5 and ischemic optic nerve and retinal pathologies,2,3 we examined GCA-negative TAs from seven AION patients for VZV.
Case 1
79 year-old man developed acute vision loss right eye (OD) and worsened vision left eye (OS), preceded by jaw claudication. Visual acuity (VA) was no light perception (NLP) OD and count fingers (CF) OS (baseline 20/50 OD, 20/150 OS), with a right afferent pupillary defect (APD), bilateral disc edema, right disc hemorrhage and left peripapillary cotton wool spot (CWS). Erythrocyte sedimentation rate (ESR) was normal and C-reactive protein (CRP) was 78 (normal <4.9 mg/dL). Oral prednisone was started and TA biopsy (day-8) was GCA-negative. Valacyclovir was started at one month with prednisone taper. VA remained unchanged. Optic nerves developed pallor. TA biopsy was VZV-negative.
Case 2
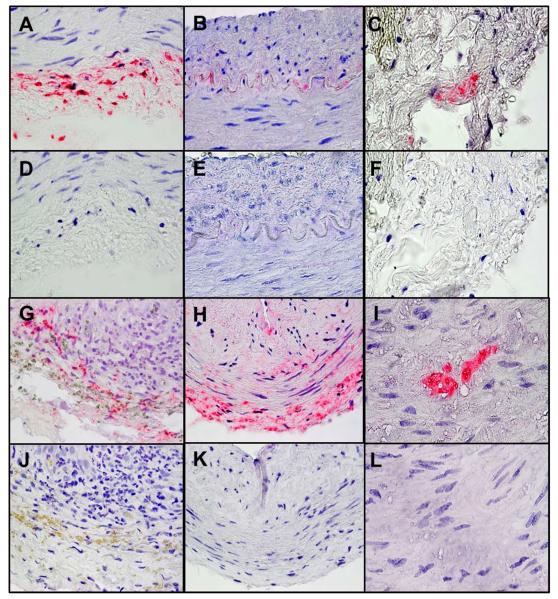
78 year-old woman suffered sudden painless vision loss OS preceded by jaw claudication. VA was 20/25-2 OD, 20/30 OS with left inferior visual field (VF) defect, left APD and left disc edema with CWS. ESR was normal; CRP was 25.9 (normal <10 mg/dL). Solumedrol was infused for 3 days with prednisone taper. Bilateral TA biopsies were GCA-negative; immunohistochemistry revealed VZV antigen (Fig 1B). CRP elevated during prednisone taper, and valacyclovir was added; CRP normalized 1 week later.
Figure 1.
Varicella zoster virus (VZV) in temporal arteries from patients with anterior ischemic optic neuropathy (AION). One-hundred 5-μm sections from each TA were cut and baked for 1 hour at 60°C. Every other slide (50 sections/TA) was deparaffinized and immunostained with either mouse anti-VZV glycoprotein E (a late viral protein indicative of productive infection) IgG1 antibody or control mouse IgG1 antibody (Dako) and examined by light microscopy; if VZV antigen was detected, adjacent sections were stained with hematoxylin and eosin and examined histologically, as described.5 VZV was detected in 5 temporal arteries from patients with AION. VZV antigen was seen in the adventitia of a positive control VZV-infected cadaveric cerebral artery (A, pink) 14 days post-infection in vitro. Note VZV antigen in cells adjacent to the internal elastic lamina of patient 2 (B), in the adventitia of patient 3 (C), in the adventitia and media of patient 4 (G), in the adventitia, media and intima of patient 5 (H), and in the media of patient 6 (I). No staining was seen in sections adjacent to those containing VZV antigen when mouse IgG1 antibody was substituted for mouse anti-VZV gE IgG1 antibody (D-F, J-L). Magnification 600X.
Case 3
53 year-old woman developed sudden painless vision loss OS. Five months later, VA was 20/25 OD, CF OS, with a left APD and diffuse left disc pallor with gliosis. ESR was 31; CRP was normal. TA biopsy was GCA-negative, but VZV-positive (Fig 1C).
Case 4
68 year-old woman noted painless vision loss OD with associated polymyalgia rheumatica, frontal headaches, jaw tightness, diplopia and VF change OD. ESR was 79; CRP was 14 (normal <1 mg/dL). TA biopsy was GCA-negative after 2 months of prednisone. Six months later during prednisone taper, she experienced further vision loss OD. Examination showed VA 20/150 OD, 20/40 OS, with right a APD and right disc edema with hemorrhages. ESR and CRP were normal. Re-evaluation of earlier TA biopsy revealed VZV antigen (Fig 1G) with adjacent GCA histopathology.
Case 5
81 year-old woman with history of sudden painless vision loss OS three years earlier, experienced new acute vision loss OD. Examination showed VA 20/100 OD, CF OS (baseline 20/40 OD and CF OS) with a left APD, right pallid disc edema with hemorrhage, and left disc pallor. ESR and CRP were normal. TA biopsy after one week of prednisone was GCA-negative but VZV-positive (Fig 1H). Oral acyclovir was added to prednisone taper, but vision did not improve.
Case 6
63 year-old man developed painless superior VF loss OD. Examination showed: VA 20/25 OD, 20/25-2 OS, bilateral superior arcuate scotomas, and bilateral inferior disc edema with hemorrhages. ESR and CRP were normal. Two months later, examination revealed progressive VF loss and additional right superior disc edema with hemorrhages. Six months later, examination showed progressive bilateral superior VF loss, bilateral disc pallor and left superior peripapillary subretinal hemorrhage. In four months, he lost inferior VF OS and developed left APD. TA biopsy was GCA-negative, but VZV-positive (Fig 1I).
Case 7
49 year-old man developed sudden loss of vision OD. Examination revealed VA: hand motion OD, 20/20 OS, with a right APD and right disc edema. ESR and CRP were normal. After intravenous corticosteroids, VA was 20/200 OD. Eleven months later, he developed sudden vision loss OS with VA 20/200 bilaterally and left disc edema with hemorrhage. Four months later, he developed bitemporal headache and vesicular lip lesions, and was treated with acyclovir. VA was now 20/200 OD, 20/60 OS. Three months later, TA biopsy was GCA-negative and VZV-negative.
Table summarizing cases available: www.aaojournal.org.
GCA-negative TA biopsies contained VZV antigen in 5/7 (71%) patients with AION. Importantly, most patients with VZV-positive biopsies had atypical AION features (vascular gliosis at optic disc, subretinal hemorrhage and progressive vision loss), suggesting that VZV vasculopathy in AION produces a wider range of ischemia than in classical GCA. Due to the multifocal nature of VZV vasculopathy and the rich innervation of the vascular supply to optic nerve and retina, VZV vasculopathy may produces a spectrum of ischemic injuries: anterior and posterior ION, retinal necrosis and central retinal artery occlusion.2-4 Thus, searching for VZV in atypical NAION cases with elevated cup/disc ratios, pain, retinal pathology or slow progression may identify individuals who could benefit from antiviral treatment. Additional prospective randomized controlled trials are needed to evaluate effects of antiviral therapy in these patients.
Two VZV-positive, biopsy-negative AION cases had clinical features consistent with GCA. In one patient, GCA pathology was subsequently found adjacent to VZV antigen. We recently reported a strong correlation between GCA pathology and VZV.5 Examining TA biopsies for VZV together with histopathology may increase diagnosis of GCA and prevent irreversible vision loss. Antiviral treatment may prevent future ischemic events in the fellow eye and brain by shortening exposure to long-term corticosteroids that potentiate VZV infection. In our study, it seems unlikely that corticosteroids confounded the results of VZV antigen testing. Of the 5 patients with VZV-positive TA biopsies, 2 received corticosteroids for ≤1 week, one for 2 months and the other 2 patients had no recent steroid exposure at the time of biopsy.
Overall, virological examination of TA biopsies in AION patients with or without clinical and laboratory findings for GCA may reveal VZV infection and should be included in the standard evaluation of AION patients. Extensive serial sections of TA biopsies should be examined for both GCA pathology and for VZV antigen, since patients whose TAs contain VZV may benefit from antiviral treatment.
Supplementary Material
Acknowledgments
Financial Support:
J.L.B. supported by the Guthy-Jackson Charitable Foundation and research grant EYO22936 from the National Institutes of Health; M.A.N. and D.G. supported by research grant AG032958 from the National Institutes of Health.
The funding organization had no role in the design or conduct of this research.
Abbreviations and Acronyms
- AION
anterior ischemic optic neuropathy
- AMD
age-related macular degeneration
- APD
afferent pupillary defect
- CF
count finger
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- GCA
giant cell arteritis
- NAION
non-arteritic anterior ischemic optic neuropathy
- NLP
no light perception
- OD
right eye
- OS
left eye
- TA
temporal artery
- VA
visual acuity
- VF
visual field
- VZV
varicella zoster virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
No conflicting relationship exists for any author.
References
- 1.Nagel MA, Khmeleva N, Boyer PJ, et al. Varicella zoster virus in the temporal artery of a patient with giant cell arteritis. J Neurol Sci. 2013;335:228–30. doi: 10.1016/j.jns.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel MA, Russman AN, Feit DO, et al. VZV ischemic optic neuropathy and subclinical temporal artery infection without rash. Neurology. 2013;80:220–2. doi: 10.1212/WNL.0b013e31827b92d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagel MA, Bennett JL, Khmeleva N, et al. Multifocal VZV vasculopathy with temporal artery infection mimics giant cell arteritis. Neurology. 2013;80:2017–21. doi: 10.1212/WNL.0b013e318294b477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathias M, Nagel MA, Khmeleva N, et al. VZV multifocal vasculopathy with ischemic optic neuropathy, acute retinal necrosis and temporal artery infection in the absence of zoster rash. J Neurol Sci. 2013;325:180–2. doi: 10.1016/j.jns.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilden D, White T, Khmeleva N, et al. Prevalence and distribution of varicella zoster virus in temporal arteries of patients with giant cell arteritis. Neurology. 2015 Feb 18;:pii. 10.1212/WNL.0000000000001409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.