Abstract
Objective
To compare the benefits of fluocinolone acetonide implant therapy versus systemic corticosteroid therapy supplemented (when indicated) with immunosuppression for intermediate, posterior, and panuveitis.
Design
Additional follow-up of a randomized comparative effectiveness trial cohort
Participants
255 patients with intermediate, posterior or panuveitis who had been randomized to implant or systemic therapy.
Main Outcome Measures
Best-corrected visual acuity (BCVA), visual field mean deviation, activity of uveitis, and presence of macular edema (per Reading Center grading) were ascertained prospectively.
Methods
Trial participants were followed through 54 months from original randomization.
Results
The trajectory of visual function in uveitic eyes demonstrated a similar (p = 0.73) degree of modest (not statistically significant) improvement from baseline to 54 months in both groups (mean improvement in BCVA at 54 months: 2.4 and 3.1 letters in the implant and systemic group respectively). Many had excellent initial visual acuity, limiting the potential for improvement. The mean automated perimetry mean deviation score remained similar to baseline throughout 48 months’ follow-up in both groups. Overall control of inflammation was superior in the implant group at every time point assessed (p<0.016), although most eyes in the systemic therapy arm also had substantial inflammatory improvement, achieving complete control or low levels of inflammation. While macular edema improved significantly more often with implant treatment within the first six months, the systemic group gradually improved over time thereafter such that the proportions with macular edema converged in the two groups by 36 months and were overlapping thereafter (p=0.41 at 48 months).
Conclusions
Visual outcomes of fluocinolone acetonide implant and systemic treatment for intermediate, posterior, and panuveitis were similarly favorable through 54 months. The implant maintains a clear advantage in controlling inflammation through 54 months. Nevertheless, with systemic therapy a large majority of patients also experienced greatly improved inflammatory status. Macular edema improved equally with longer follow-up. Based on cost-effectiveness and side effect considerations, systemic therapy may be indicated as the initial treatment for many bilateral uveitis cases. However, implant therapy is a reasonable alternative approach, especially for unilateral cases, and in situations where systemic therapy is not feasible or is not successful.
Uveitis (intraocular inflammation) is an important cause of visual impairment in the United States1;2 and globally. 3–5 It affects a younger (working age) population6–8 than the most common, age-related eye diseases.9–11 Hence its impact in terms of years of vision lost and economic impact is particularly high, per case.12 Different parts of the eye can be affected, corresponding to which the classifications anterior uveitis, intermediate uveitis, anterior plus intermediate uveitis, posterior uveitis, and panuveitis have been used.13;14 While anterior uveitis is more commonly encountered,6–8 the other forms of uveitis have a higher risk of visual loss,15;16 and are more difficult to treat because of the limited impact of topical therapy on inflammation centered more deeply in the eye.
The Multicenter Uveitis Steroid Treatment (MUST) Trial,17 a comparative effectiveness trial, directly compared systemic corticosteroids (supplemented in 86% of cases by corticosteroid-sparing immunosuppressive drugs) versus a surgically placed fluocinolone acetonide intravitreous implant (approved by the United States Food and Drug Administration in 2005).18 The MUST Trial found that the alternative treatments had similar visual outcomes through two years; implant therapy was superior in terms of control of intraocular inflammation, had more ocular side effects without a substantial advantage with respect to systemic side effects, and was associated with a minimal advantage in quality of life outcomes.18 Taking into account three years’ follow-up—corresponding to the expected duration of implant therapy19—systemic therapy was more cost-effective as an initial treatment for bilateral disease, but for unilateral disease implant therapy was reasonably cost-effective relative to systemic therapy based on the available (limited, N = 31) data.20
To provide additional insight into the alternative treatments, here we report the visual function and control of inflammation results of additional follow-up of the MUST Trial cohort, providing 54-month results. A companion paper reports 54-month results regarding risks and quality of life.21
Methods
Study Design
The Multicenter Uveitis Steroid Treatment (MUST) Trial was a randomized (allocation ratio 1:1), partially masked, 23-center, parallel treatment comparative effectiveness trial (clinicaltrials.gov identifier NCT00132691). Patients were followed at least quarterly until a common closeout when the last participant completed two years’ follow-up, and then were enrolled into a long-term follow-up study (the MUST Trial Follow-up Study). Previous reports detail the MUST Trial protocol,17;18 which randomly assigned patients to fluocinolone acetonide implant therapy (in the eye(s) where it was indicated) or systemic therapy with corticosteroids supplemented by immunosuppressive therapy when indicated in a manner based on expert panel guidelines.22 During the MUST Trial Follow-up Study, the follow-up frequency was reduced to every six months, and the intensity of data collection and imaging was reduced, but the key outcomes of interest continued to be measured (see below). Completion of the trial phase and unmasking of trial results required that treatment protocols no longer could be mandated, but most patients’ uveitis was controlled satisfactorily at the time of entry into the MUST Trial Follow-up Study and they continued their originally assigned treatment. All patients provided written informed consent to participate in both the MUST Trial and the MUST Trial Follow-up Study; all governing institutional review boards provided approval of both protocols for the relevant period of follow-up. All research was conducted following the principles of the Declaration of Helsinki.
At the time of MUST Trial enrollment, participants had been 13 years of age or older with active or recently active (within ≤60 days) non-infectious intermediate, posterior or panuveitis in one or both eyes for which systemic corticosteroid therapy was indicated. Patients requiring systemic therapy for non-ocular indications at the time of enrollment and those for whom one of the treatments was contraindicated had been excluded. The 23 participating uveitis centers in the United States, the United Kingdom, and Australia continued to follow patients during the MUST Trial Follow-up Study. A small number of patients had completed 54 months’ follow-up prior to completion of the MUST Trial, and the majority completed ongoing follow-up under the MUST Trial Follow-up Study protocol with less frequent observations.
During the trial phase of the study, the treatment protocol for the implant arm called for initial implantation of a fluocinolone acetonide implant (0.59 mg, Bausch & Lomb, Rochester, NY) after quieting the anterior chamber, and for replacement of implants when recurrence of uveitis activity—to a level that would justify systemic corticosteroid therapy—was observed. Systemic therapy involved induction of control of active inflammation with high doses of systemic corticosteroids followed by tapering and maintenance with as low a dose of corticosteroids as possible, using corticosteroid-sparing immunosuppressive therapy if needed to achieve a suppressive dose that was tolerated and at a level bioequivalent to 7.5 mg/day of prednisone or less. Immunosuppressive drugs also were used for disease-specific indications enumerated by the expert panel, and in the event of failure of high dose corticosteroids to induce control of inflammation. The study-certified ophthalmologists managing the patients were permitted to select the immunosuppressive drug(s) to be used based on the suitability of the side effect profile for each individual patient, but use of each agent was protocol-driven. Topical corticosteroid use was permitted per best medical judgment, and use of periocular and intravitreous injections of corticosteroids were permitted for clearing of residual macular edema or to quiet the anterior chamber prior to implantation, but were not to be used as a primary method of control of inflammation. During the Follow-up Study, protocol-driven treatment was not mandated, but the treatment approaches were reviewed at site visits and found to be similar to those used during the trial phase.
In both phases of the study, best-corrected visual acuity was measured by study-certified visual acuity examiners as the number of letters read from standard logarithmic visual acuity charts.17;18 Visual field sensitivity was measured by automated perimetry and summarized by the mean deviation statistic.17;18 Uveitis activity was graded by unmasked clinicians,17;18 including anterior chamber cells and vitreous haze in a manner based on the Standardization of Uveitis Nomenclature Working Group-endorsed methods.14 Overall activity was determined by clinician judgment, and vitreous cells were graded ordinally based on the count of cells in 1 × 0.5 mm beam: grade 0 (none); trace (up to 5 cells); 1+ (6–10 cells); 2+ (11–20 cells); 3+ (21 up to 50 cells); 4+ (greater than 50 cells). Macular edema was counted as present when OCT measured macular thickness was more than two standard deviations thicker than the normal mean retinal thickness for the OCT machine used per masked reading center gradings.23
Statistical Analyses
Primary analyses were conducted “as randomized.” The primary analyses evaluated the ocular outcomes of all eyes having uveitis at enrollment in relation to treatment assignment, given that outcomes of mildly affected fellow eyes might have been affected by systemic therapy vs. lack of study therapy in the implant arm (there were a small number of eyes with uveitis for which clinicians thought implant therapy was not indicated (as previously reported).18
Longitudinal models used generalized estimating equations (GEE) to estimate parameters.24 For the visual acuity outcome, we used a saturated means model (including visit and visit-by-treatment interaction indicators) adjusting for the trial’s stratified randomization scheme (intermediate versus posterior/panuveitis). To account for both longitudinal and within subject (between eyes) correlation, we used a Toeplitz covariance structure. All available visit data from the study visits at six month intervals were incorporated into the model. To maintain the data structure, we used missing data indicators. The six month study visits were selected because the visit schedule changed in the transition to the follow-up study and the three month visits were not available between 24–54 months of follow-up for all participants. Because of the change in modeling (from inclusion of all three month visits in our previous report to including only six month visits), model estimates of treatment effects were slightly different than those reported previously,18 although the differences are small. Other analyses for measured outcomes used the same mean structure with a Toeplitz, unstructured, or exchangeable covariance matrix. Event-time analyses were used to estimate the cumulative proportion of uveitic eyes receiving 1, 2, or 3 implants during the first 54 months of follow-up while accounting for loss to follow-up. Analyses were conducted by the Statistical Analysis Committee (see Credit Roster, Supplementary Appendix 1).
Robust standard errors were computed for all models. The bootstrap was used to calculate standard error, confidence intervals, and p-values for outcomes where two eyes of the same individual were at risk (e.g., visual acuity in uveitic eyes). Bootstrap sampling was stratified by treatment group, unilateral vs. bilateral uveitis, and uveitis stratum (intermediate vs. posterior or panuveitis) with 5000 replicates. Following prior recommendations,25 all reported confidence intervals and p-values are nominal and not adjusted for multiple comparisons. Statistical analyses used SAS (SAS/STAT User’s Guide, Version 9.2, Cary, NC:SAS Institute), and R (The R Project for Statistical Computing, Version 2.13.1, http://www.r-project.org/, accessed on December 12, 2014).
Results
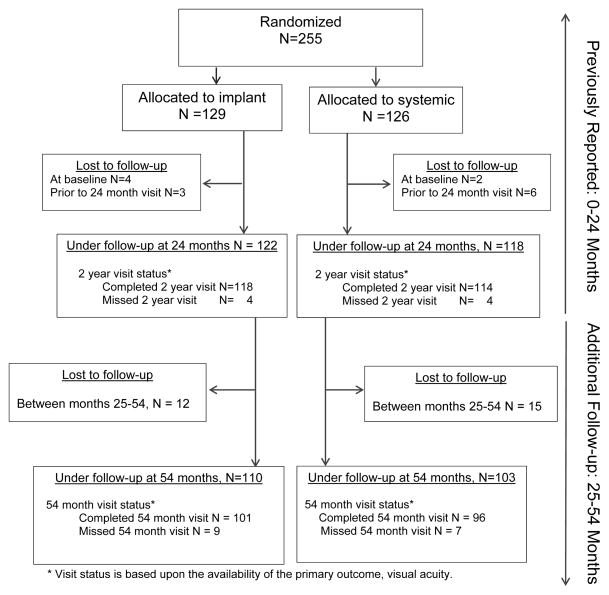
Between December 2005 and December 2008, 255 patients (479 eyes with uveitis) were enrolled in the MUST Trial and randomized to implant (n=129) or systemic (n=126) Therapy.18 At two years following randomization, 122 implant-assigned and 118 systemic-assigned patients remained under follow-up (see Figure 1). Among these, 110 (90%) implant-assigned patients (85% of those originally randomized to implant) and 103 (87%) systemic-assigned patients (82% of those originally randomized to systemic) remained under follow-up at or beyond 54 months. Based on the timing of enrollment, 16 completed 54 months’ follow-up prior to the completion of the MUST Trial; some follow-up occurred during the MUST Trial Follow-up Study for the others (N = 197). The primary outcome, best-corrected visual acuity, was collected at the 54 month visit for 101 and 96 individuals, respectively. As reported previously,18 demographic and clinical characteristics were distributed similarly at the study’s baseline between groups with the exception that eyes with uveitis in the implant group tended to have lesser visual field sensitivity than systemic group eyes with uveitis (median −5.7 dB vs −3.8 dB respectively).
Figure 1.
CONSORT flow diagram for the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study (FS) through 54 months’ follow-up).
* Visit status is based upon the availability of the primary outcome, visual acuity.
In the implant-assigned group, 122/129 (95%) patients received implant therapy. By 54 months, among uveitic eyes of implant-assigned patients, 87% (95% CI: 82, 91%) of eyes had received at least 1 implant; 8% (95% CI: 3%, 11%) of eyes with uveitis had received 2 implants; and 2% (95% CI: 0%, 7%) of eyes with uveitis had received 3 implants. (As previously noted, some second eyes with uveitis did not have sufficiently severe disease to require implant therapy). The proportion of implant-assigned patients taking systemic corticosteroids and/or immunosuppressive drugs declined to approximately 20% by 12 months following randomization, and remained at a similar level through 54 months, by which point 17% were receiving one or more systemic treatments (14% oral corticosteroids, 8% immunosuppressive drugs, and 4% biologics).
In the systemic group, 121/126 (96%) received systemic therapy at some point during follow-up. The proportion of individuals receiving one or more systemic therapies was high throughout the first 54 months, declining slightly by 54 months’ follow-up to 65% (39% oral steroids, 45% immunosuppression, and 9% biologics, some using more than one). The percentage of uveitic eyes that were treated with implant therapy grew over time, with 21% (95% CI: 13%, 28%) of patients (19% of uveitic eyes [95% CI: 12%, 26%]) having received implant therapy by 54 months. None of these cross-over cases received more than one implant during this follow-up period.
Visual Function
Visual Acuity
Among patients randomized, 101 (190 uveitic eyes; 78%) and 96 (179 uveitic eyes; 76%) individuals in the implant and systemic groups respectively completed best-corrected visual acuity (BCVA) measurement at the 54 month follow-up visit. Overall, 4,178 of 4,790 possible study visits for uveitic eyes (87%) provided BCVA for analysis through 54 months. Baseline values for BCVA and other clinical characteristics were similar among eyes lost to follow-up with respect to eyes continuing follow-up (see Table 1, available at www.aaojournal.org).
Table 1.
Comparison of baseline characteristics for uveitic eyes of patients completing or not completing the 54 month visit, in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study*
| Completed 54 month visit | Did not complete 54 month visit | P-value** | |
|---|---|---|---|
| Characteristics of Patients | N = 213 | N = 42 | |
| Age (years), Mean (Standard Deviation) | 46 (14) | 47 (19) | 0.83 |
| Female, N(%) | 158 (74%) | 33 (79%) | 0.55 |
| Race/Ethnicity, N(%) | |||
| White | 122 (57%) | 20 (48%) | 0.17 |
| Hispanic | 26 (12%) | 7 (17%) | |
| Black | 56 (26%) | 10 (24%) | |
| Other | 9 (4%) | 5 (12%) | |
| Duration of uveitis (years), median (25th–75th percentile (IQR)) | 3.9 (1.4, 8.1) | 3.4 (0.7, 6.8) | 0.17 |
| Bilateral uveitis, N(%) | 188 (88%) | 36 (86%) | 0.64 |
| Site of uveitis, N(%) | |||
| Intermediate | 86 (40%) | 11 (26%) | 0.08 |
| Posterior or Panuveitis | 127 (60%) | 31 (74%) | |
| Associated systemic inflammatory disease, N(%) | 55 (26%) | 14 (33%) | 0.32 |
| Bone density, N(%) | |||
| Normal | 109 (52%) | 23 (58%) | 0.71 |
| Osteopenia | 84 (40%) | 15 (38%) | |
| Osteoporosis | 17 (8%) | 2 (5%) | |
| Characteristics of Eyes‡ | E = 401 | E = 78 | |
| Eye-specific duration of uveitis (years), median (IQR) | 3.8 (1.3, 8.0) | 3.4 (0.7, 6.9) | 0.24 |
| Visual acuity, median (IQR), letters | 69 (52, 80) | 73 (41, 82) | 0.70 |
| Visual acuity 20/40 or better | 196 (49%) | 42 (54%) | 0.40 |
| Visual acuity 20/200 or worse | 56 (14%) | 18 (23%) | 0.16 |
| Visual field sensitivity (mean deviation), median (IQR), decibels | −5.0 (−9.3, −3.0) | −5.8 (−13.2, −2.7) | 0.19 |
| Active uveitis, N(%) | 311 (79%) | 62 (79%) | 0.99 |
| Anterior chamber cells, N(%) | |||
| 0 | 213 (53%) | 27 (35%) | 0.28 |
| 0.5+ | 112 (28%) | 25 (32%) | |
| 1+ or more | 76 (19%) | 26 (33%) | |
| Vitreous haze, N(%) | |||
| 0 | 124 (33%) | 17 (22%) | 0.46 |
| 1+ | 167 (45%) | 38 (50%) | |
| 2+ or more | 80 (22%) | 21 (28%) | |
| Lens opacities, N(%) | |||
| Absent | 90 (22%) | 16 (21%) | 0.87 |
| Present | 142 (35%) | 24 (31%) | |
| Aphakic or pseudophakic | 169 (42%) | 38 (49%) | |
| Intraocular pressure (mm Hg), median (IQR) | 14 (11.5, 17) | 14 (11.5, 17) | 0.82 |
| Macular edema (center point thickness > 240 m), N (%) | 125 (35%) | 30 (41%) | 0.48 |
For covariates where the counts do not add up to the total, the remaining observations are missing.
N=number of eyes.
P-values are calculated using the χ2 or Fisher’s exact text (categorical covariates) and Wilcoxon rank sum (continuous covariates) for the person-level covariates. P-values are calculated using GEE with exchangable covariance structure to account for two eyes from the same person for the eye-level covariates.
Eye specific characteristics are summarized for the 479 eyes with uveitis at enrollment.
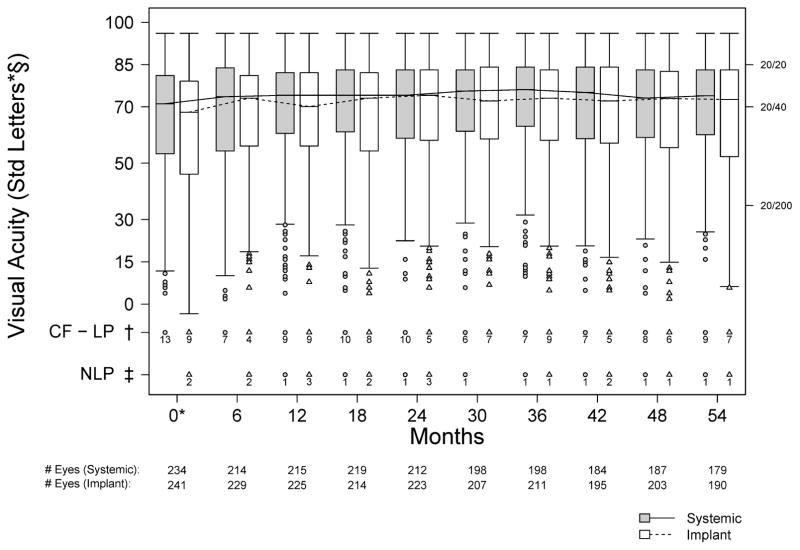
BCVA results through 54 months are summarized in Figure 2 and Table 2. Among uveitic eyes, 50% had a baseline BCVA of 20/40 or better. Mean BCVA showed a modest initial improvement in both groups; thereafter mean BCVA remained slightly better than at baseline through 54 months, with about 1/2 line improvement at 54 months with respect to baseline in both groups, a change which was not quite statistically significant in the individual groups (p = 0.086 and 0.052 for implant and systemic, respectively). No statistically significant or substantial differences were observed between treatment groups (range of differences observed 2.7 to −0.7 letters, with positive values favoring implant, p>0.16 at all time points). Neither were there statistically significant differences between groups in a sensitivity analysis “as treated” (those receiving implants vs. not; p>0.99). Results were similar for analyses of visual acuity in subjects’ better eyes (which may better reflect their overall experience), except less improvement from baseline was observed than for all eyes with uveitis, corresponding to a larger percentage (69%) of better eyes having BCVA of 20/40 or better at baseline. Results between groups also were similar when the analysis was restricted to eyes presenting with visual acuity worse than 20/40 (p=0.16).
Figure 2.
Distribution of VA for eyes with uveitis assigned to implant (grey) and systemic (white) therapy during the first 54 months of follow-up in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study. *Calculated from the subset of eyes with uveitis at enrollment; § 20/20 = 85 letters, 20/40 = 70 letters, 20/200 = 35 letters; † Count Fingers (CF), Hand Motion (HM), Light Perception (LP) denoted as −10 letters; ‡ No Light Perception (NLP) denoted as −25 letters.
Table 2.
Change in visual acuity over 54 months by implant vs. systemic treatment group in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study.
| Estimated Mean (SE)† | Estimated Mean Change from BL (SE)† | Estimated Treatment Effect (95% CI) †§ | |||||
|---|---|---|---|---|---|---|---|
| Sample size* | Implant | Systemic | Implant | Systemic | Implant - Systemic | P value | |
| Visual acuity (uveitic eyes) | |||||||
| Enrollment | 475 | 61.5 (2.4) | 64.8 (2.5) | n/a | n/a | n/a | |
| 12 months | 440 | 65.9 (2.4) | 68.1 (2.6) | 4.39 (1.37) | 3.35 (1.27) | 1.04 (−2.24, 4.61) | 0.569 |
| 24 months | 435 | 67.4 (2.4) | 68.0 (2.6) | 5.94 (1.38) | 3.21 (1.45) | 2.73 (−0.98, 6.70) | 0.165 |
| 36 months | 409 | 66.6 (2.4) | 69.1 (2.5) | 5.13 (1.46) | 4.31 (1.36) | 0.82 (−3.05, 5.02) | 0.675 |
| 48 months | 390 | 65.0 (2.5) | 67.7 (2.5) | 3.52 (1.51) | 2.91 (1.63) | 0.61 (−3.55, 5.17) | 0.781 |
| 54 months | 369 | 63.9 (2.5) | 67.9 (2.5) | 2.40 (1.40) | 3.12 (1.61) | −0.72 (−5.01, 3.54) | 0.737 |
| Visual acuity (better eye) | |||||||
| Enrollment | 254 | 71.6 (2.3) | 75.8 (2.0) | n/a | n/a | n/a | |
| 12 months | 234 | 74.8 (2.0) | 77.4 (2.3) | 3.13 (1.28) | 1.66 (1.07) | 1.47 (−1.80, 4.74) | 0.378 |
| 24 months | 232 | 75.5 (2.1) | 77.9 (2.2) | 3.83 (1.31) | 2.08 (1.13) | 1.75 (−1.64, 5.14) | 0.311 |
| 36 months | 217 | 74.9 (2.1) | 78.0 (2.2) | 3.25 (1.25) | 2.20 (1.19) | 1.05 (−2.34, 4.43) | 0.544 |
| 48 months | 208 | 74.1 (2.2) | 77.6 (2.2) | 2.44 (1.41) | 1.80 (1.35) | 0.64 (−3.19, 4.46) | 0.744 |
| 54 months | 197 | 73.8 (2.3) | 77.1 (2.3) | 2.20 (1.20) | 1.31 (1.50) | 0.89 (−2.88, 4.66) | 0.644 |
Number of eyes or individuals with data available at each visit.
Parameter estimates are calculated using generalized estimating equations assuming working independence between eyes and a Toeplitz covariance structure for the within-eye replicate measurements with robust standard errors. For the analysis of all uveitic eyes, bootstrap retaining the original treatment assignments with 1000 replicates is used to calculate the standard errors, confidence intervals, and p-values for the parameter estimates in order to adjust for between-eye correlation.
The treatment effect is defined as the between the two treatment groups (Implant – Systemic) at the enrollment visit and is defined as the difference in the change from baseline between the two treatment groups (Implant – Systemic) for all follow-up visits. Positive numbers favor implant treament; negative numbers favor systemic treatment.
CI = 95% confidence interval; SE = standard error.
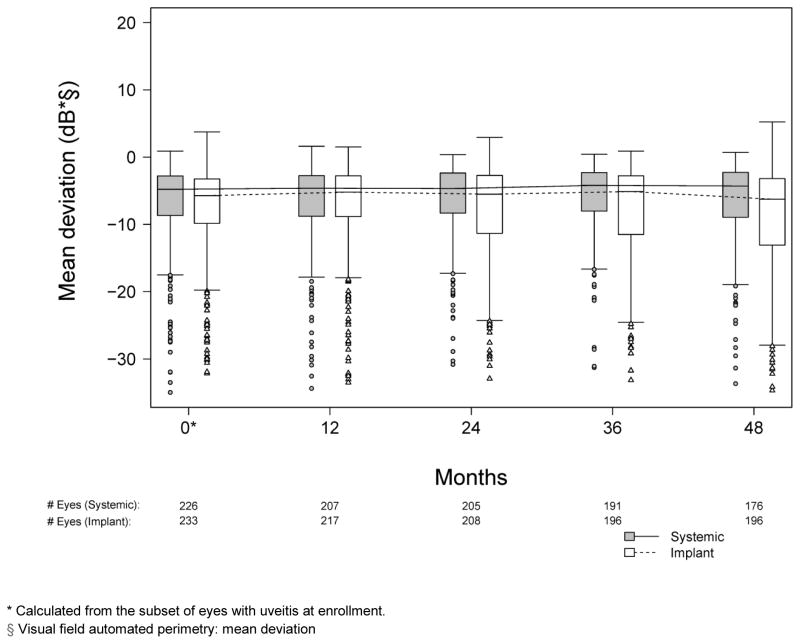
Visual field sensitivity, which had remained stable through the 24 months of follow-up during the trial portion of the study,18 continued to be stable through 54 months in both groups for the most part (see Figure 3, available at www.aaojournal.org). Median changes in mean deviation (MD) from baseline to each time point assessed were minimal and similar in the two groups (p > 0.30 for all four years, comparing change from baseline between treatment groups, data not shown). However, the implant group did tend to have more observations at the low MD end of the distribution, with the lower quartile being at a worse mean deviation than in the systemic group.
Figure 3.
Distribution of mean deviation at annual visits for eyes with uveitis assigned to implant (grey) and systemic (white) therapy during the first 48 months of follow-up in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study.
* Calculated from the subset of eyes with uveitis at enrollment.
§ Visual field automated perimetry: mean deviation
Uveitis Activity and Macular Edema
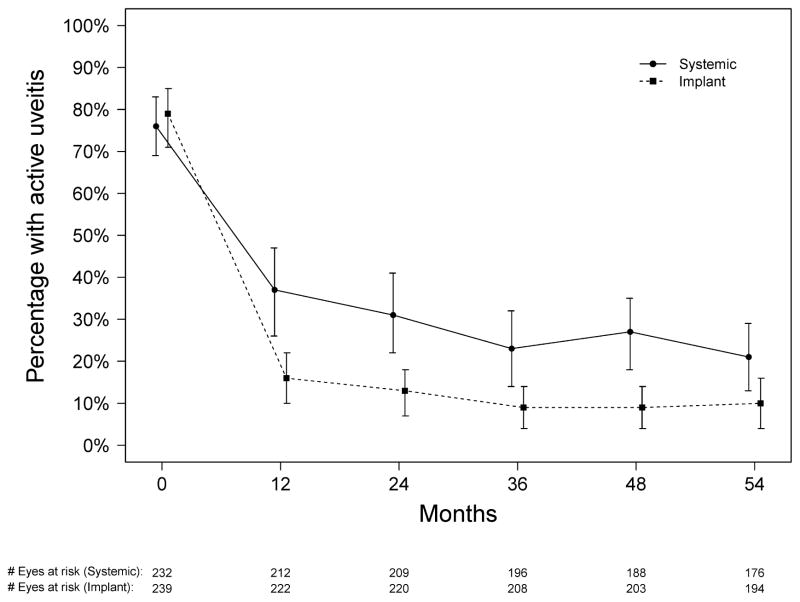
As previously reported,18 the majority of initially active eyes in both groups were controlled within 9 months of randomization, but significantly more were controlled in the implant group. Subsequently, in the implant group, the proportion remaining active remained similar (9–16%) through 54 months. In the systemic group, there tended to be a modest improvement over time in the proportion active, with 31% active at 24 months declining to 21% active at 54 months. Nevertheless, the implant group had a statistically significantly larger reduction in the odds of activity at every time point assessed (all p≤0.016; see Figure 4 and Table 3).
Figure 4.
Proportion of uveitic eyes with uveitis activity for those assigned to implant (dotted line) and systemic (solid line) therapy during the first 54 months of follow-up in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study.
Table 3.
Proportion of uveitic eyes with active disease and macular edema over 54 and 48 months, respectively, by implant vs. systemic therapy treatment group in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study.
| Estimated Proportion with Condition (95% CI) | Ratio of Odds Compared to Baseline Odds of Having Condition (95% CI) | Estimated Treatment Effect (95% CI) † | |||||
|---|---|---|---|---|---|---|---|
| Sample size* | Implant | Systemic | Implant | Systemic | Implant: Systemic | P value | |
| Uveitis Activity, % active | |||||||
| Enrollment | 471 | 79% (71%, 85%) | 76% (69%, 83%) | n/a | n/a | n/a | |
| 12 months | 434 | 16% (10%, 22%) | 37% (26%, 47%) | 0.05 (0.02, 0.09) | 0.19 (0.11, 0.30) | 0.27 (0.12, 0.55) | <0.001 |
| 24 months | 429 | 13% (7%, 18%) | 31% (22%, 41%) | 0.04 (0.02, 0.07) | 0.14 (0.07, 0.23) | 0.28 (0.13, 0.59) | <0.001 |
| 36 months | 404 | 9% (4%, 14%) | 23% (14%, 32%) | 0.03 (0.01, 0.05) | 0.09 (0.04, 0.16) | 0.30 (0.11, 0.69) | 0.006 |
| 48 months | 390 | 9% (4%, 14%) | 27% (18%, 35%) | 0.03 (0.01, 0.05) | 0.11 (0.06, 0.19) | 0.23 (0.09, 0.48) | <0.001 |
| 54 months | 371 | 10% (4%, 16%) | 21% (13%, 29%) | 0.03 (0.01, 0.06) | 0.08 (0.04, 0.14) | 0.35 (0.14, 0.82) | 0.016 |
| Macular edema, % | |||||||
| Enrollment | 436 | 41% (32%, 51%) | 40% (31%, 50%) | n/a | n/a | n/a | |
| 12 months | 385 | 21% (14%, 29%) | 30% (21%, 39%) | 0.39 (0.25, 0.57) | 0.64 (0.43, 0.93) | 0.60 (0.34, 1.05) | 0.064 |
| 24 months | 403 | 22% (14%, 30%) | 31% (22%, 40%) | 0.41 (0.25, 0.60) | 0.67 (0.46, 0.96) | 0.60 (0.33, 1.03) | 0.069 |
| 36 months | 359 | 22% (14%, 30%) | 23% (15%, 33%) | 0.39 (0.25, 0.58) | 0.46 (0.28, 0.71) | 0.86 (0.46, 1.49) | 0.615 |
| 48 months | 356 | 19% (12%, 27%) | 22% (15%, 31%) | 0.33 (0.19, 0.52) | 0.43 (0.28, 0.63) | 0.78 (0.39, 1.38) | 0.406 |
CI = 95% confidence interval; SE = standard error.
Sample size: the number of eyes with uveitis with data available at each visit.
At each of the follow-up time points, the “estimated treatment effect” is the ratio of odds ratios with 95% CIs. A ratio of odds ratios less than 1 favors implant.
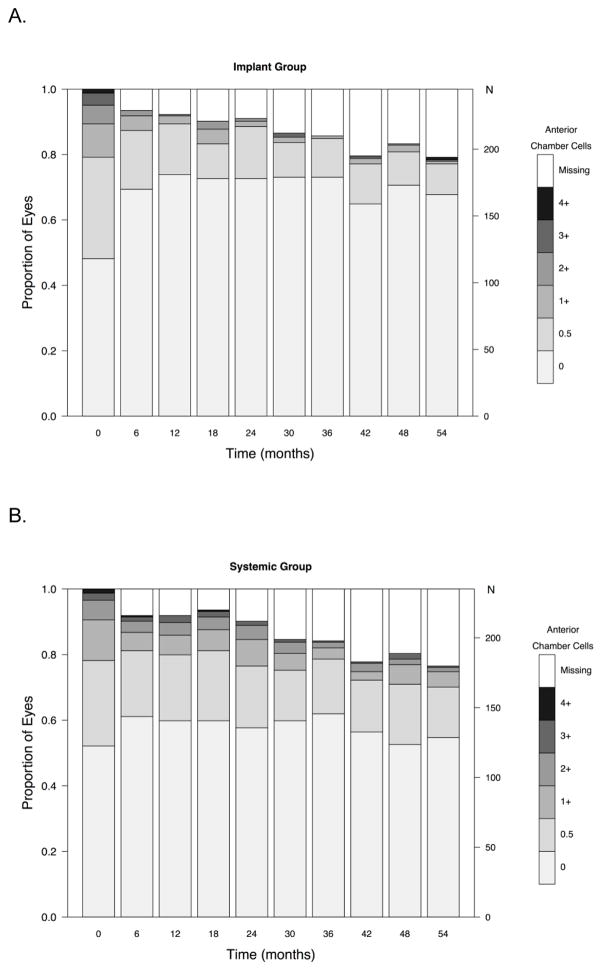
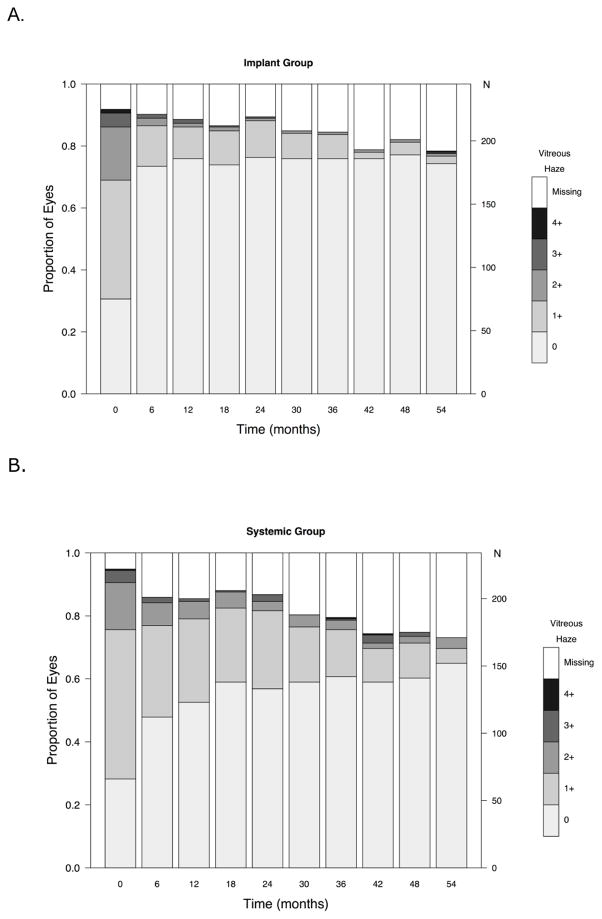
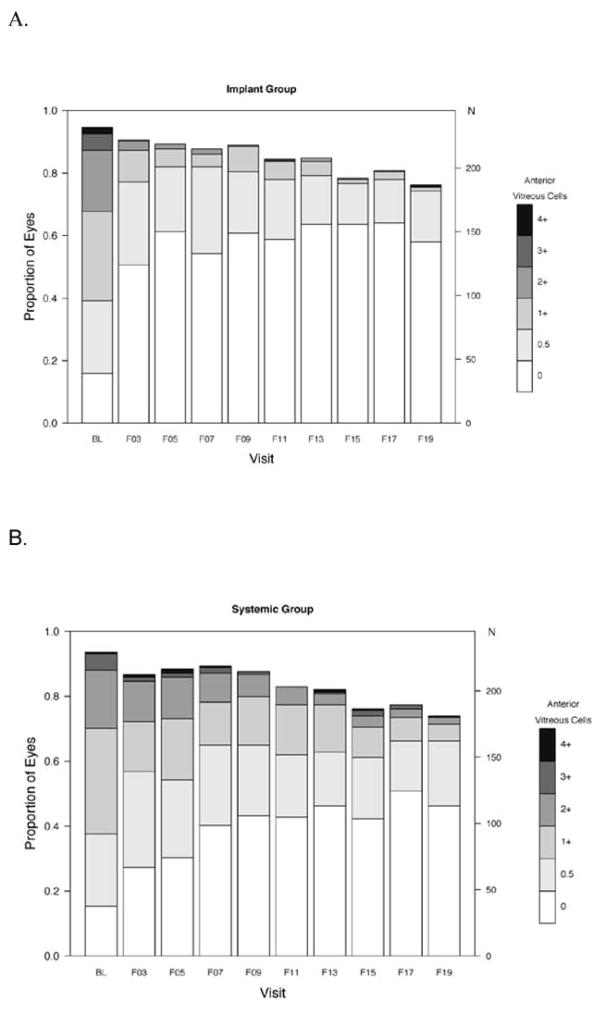
In evaluating the degree of activity, many of the eyes counted as active at any given point during follow-up showed improvement with respect to baseline. As shown in Figure 5 (available at www.aaojournal.org), the proportion with high grades of anterior chamber cells declined in both groups, although the increase in the odds of uveitic eyes achieving a grade of 0 was significantly more favorable in the implant group at all follow-up time-points (p < 0.01). There tended to be more eyes with anterior chamber cell grades of 1+ or higher in the systemic arm than the implant arm throughout follow-up, but the proportion was low in both groups. The proportion with the lower grades of vitreous haze also improved over time in both groups, but improved more quickly in the implant group (see Figure 6, available at www.aaojournal.org). The implant group had a larger increase in the odds of uveitic eyes having vitreous haze scores = 0 (clear) than the systemic group through the month 48 visit (range of ratio of odds ratios: 2.3 to 3.0; higher ratio of odds ratios favors implant; all p-values were<0.05 except at the 36 month visit p=0.054). At the 54 month visit, this difference narrowed (ratio of odds ratios = 1.9, 95% CI: 0.7, 6.2, p = 0.25). Although there is no consensus method for grading vitreous cells, based on the ordinal approach used in the MUST Trial and Follow-up Study the outcome of vitreous cells over time showed improvement in both groups with more improvement in the implant group (see Figure 7, available at www.aaojournal.org).
Figure 5.
Distribution of anterior chamber cell grades over time in (A) the fluocinolone acetonide (Implant Group) and (B) systemic therapy (Systemic Group) groups in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study. N=number of eyes.
Figure 6.
Distribution of vitreous haze grades over time in (A) the fluocinolone acetonide (implant) and (B) systemic therapy (systemic) groups in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study. N=number of eyes.
Figure 7.
Distribution of vitreous cell grades over time in (A) the fluocinolone acetonide (implant) and (B) systemic therapy (systemic) groups in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study. N=number of eyes.
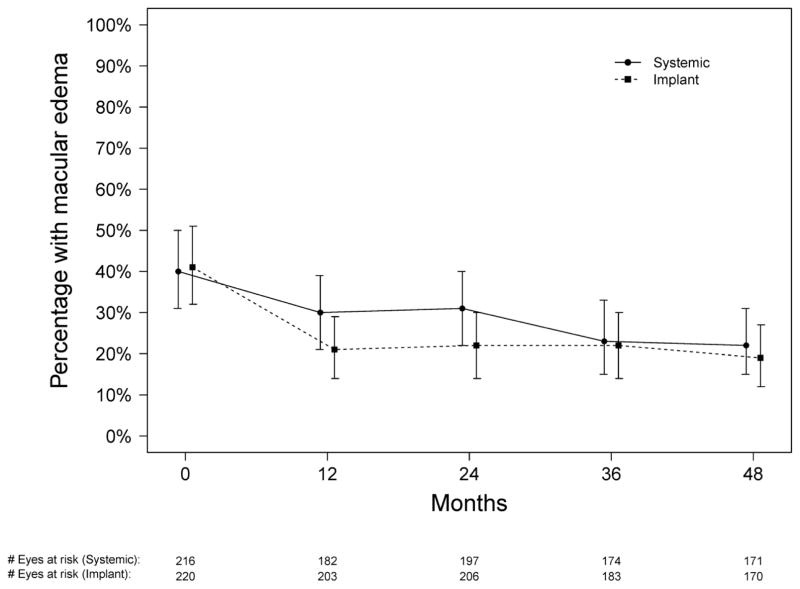
As reported previously,18 the proportion of eyes having a clinically meaningful degree of macular edema23 was similar for the two treatment groups at baseline, rapidly improved in the implant group and then remained steady, and gradually improved in the systemic therapy group to a degree that was not statistically significantly different from the implant group by 24 months (p=0.069). By 36 months, the proportion with macular edema had further improved in the systemic group while remaining steady in the implant group, and at 48 months both groups had achieved a similar reduction in the odds of having macular edema (p=0.41; see Table 2, and Figure 8 [available at www.aaojournal.org]), resulting in about half the proportion having macular edema in each group compared with baseline.
Figure 8.
Proportion of uveitic eyes with macular edema for those assigned to implant (dotted line) and systemic (solid line) therapy during the first 48 months of follow-up in the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study.
Discussion
In this cohort of patients randomized to systemic vs. fluocinolone implant therapy—with initially active or recently active intermediate, posterior and panuveitis—the mean BCVA did not differ significantly from baseline at the 54 months’ follow-up. Differences in the clinical course of BCVA in the two treatment arms were minimal throughout follow-up without statistically significant differences. Overall visual field sensitivity, as measured by mean deviation, also remained similar over time without differences between treatment groups, except for a modest amount more cases with lower visual field sensitivity in the implant group, likely corresponding to the higher risk of glaucoma in that group.21 These results suggest that the two treatment approaches yield similar visual outcomes through 54 months, with both approaches associated with stable to slightly improved vision (in a population where about half of eyes were 20/40 or better at baseline).
Implant therapy remained superior for control of intraocular inflammation throughout the initial 54 months’ follow-up. Specific evaluation of anterior chamber cell, vitreous haze and vitreous cell grades over time provided additional insight into the pattern of inflammatory response to the alternative treatments. Most eyes in both groups had substantially improved anterior chamber cells and vitreous haze, even if they did not entirely meet the higher bar of inflammation inactivity. The tendency for systemic therapy-assigned uveitic eyes to have a higher proportion of grade 1+ and higher anterior chamber cells and vitreous haze may reflect more frequent reactivations or an ongoing slightly greater degree of inflammation, or both, in the systemic arm. Occasional reactivations in the systemic therapy arm are to be expected, as medications may be tapered after extended control of inflammation, as part of best management practices, and this may result in relapse of inflammation in some instances. In contrast, implant therapy cannot be tapered when it might no longer be needed. Vitreous haze and vitreous cells improved more slowly than anterior chamber cells in the systemic therapy arm, the latter perhaps reflecting concomitant use of topical corticosteroids clearing anterior chamber cells faster, but reached a similarly low level after 54 months of management. This observation, that existing systemic therapies clear vitreous inflammation more slowly than implant therapy, suggests that long periods of follow-up may be needed to see vitreous haze effects of new immunosuppressive treatments for uveitis in drug licensing trials using vitreous haze as a primary outcome.
In our previous report through 24 months’ follow-up, macular edema improved faster with implant than systemic therapy, to a degree that was statistically significant at 6 months and marginal but non-significant at 12 and 24 months.18 Additional follow-up demonstrated that while the proportion still with macular edema in the implant arm remained steady through 54 months, the proportion with macular edema in the systemic therapy arm continued to improve such that the two groups had a similar proportion with macular edema from 36 months’ follow-up onward. To the extent that macular edema may cause irreversible damage to the macula if it persists long enough, earlier clearing of macular edema may represent an advantage of implant therapy. However, given the similar visual results of the two treatment arms any such advantage must be modest.
In the systemic therapy arm, the treatment protocol during the MUST Trial and the treatment guidelines during the Follow-up Study allowed for cessation of therapy in cases where therapy appeared no longer to be needed. While the use of systemic therapy in the systemic therapy arm did decline over time, the majority still were receiving treatment at 54 months, and control of inflammation was not universal, suggesting that remission does not rapidly occur in cases of uveitis of a spectrum similar to those eligible for this study. Replacement of implants occurred at a degree that was somewhat less than anticipated based on the expected 2.5–3 year duration of effect,26 with only 10% of uveitic eyes receiving 2 or more implants. Whether this observation is the result of longer duration of implant therapeutic effect than expected or differences in the remission rate between treatment arms will be a point of interest which longer-term follow-up of these subjects will address.
Limitations of the study primarily consist of losses to follow-up over the increasingly long follow-up time, and crossovers between treatment arms. Crossovers from systemic to implant therapy primarily reflected physician judgment that inflammatory control was inadequate, whereas use of systemic therapy in the implant arm often reflected an evolving need to treat systemic disease with systemic therapy. Crossovers between treatment arms, about 20% in each direction, may have diluted differences between treatment arms. However, given the overlapping visual function throughout follow-up time (including early time points with less crossover), crossovers are unlikely to have affected the primary visual acuity results. An approach of initial treatment with systemic therapy and “rescue” of systemic treatment failures with implant would be expected to result in similar outcomes to those reported here for the systemic therapy arm. It is possible that crossovers resulted in underestimation of the superiority of implant therapy in controlling inflammation, as cases uncontrollable by systemic therapy would have been more likely to cross over to implant therapy, suggesting that the advantage of implant therapy with respect to inflammatory control may have been underestimated.
Regarding macular edema, measurement using time-domain OCT during the beginning of the trial limited our definition of macular edema to a crude measure of macular thickness, which nevertheless correlated well with visual acuity.23 Because such measurements are valid (though less precise than spectral-domain OCT) and because differential measurement error would be very unlikely in a randomized study, use of time-domain OCT is unlikely to have affected assessment of the relative impact of the alternative treatments on macular edema. Regarding measurement of anterior chamber and vitreous inflammation, some forms of inflammation would be expected to lack these specific sites of inflammation, which may have led to underestimation of the early advantages of implant therapy for control of inflammation.
In summary, our results suggest that systemic and implant therapies yield similar visual outcomes through 54 months’ treatment. Given the greater cost of implant therapy for bilateral disease,20 and the higher risk of ocular complications in the implant arm without remarkable advantages with respect to systemic complications (see companion paper),21 our results suggest that systemic therapy may be the preferred initial therapy for most bilateral cases of active or recently active intermediate, posterior and panuveitis. Given the finding of consistent superiority of implant with respect to control of inflammation supplemented by anecdotal observations from patients in the trial (that a small number of cases are uncontrollable with practicable systemic therapy strategies) implant therapy appears to have a valuable role for severe cases failing systemic therapy. There also will be some cases where systemic therapy will not be feasible for one reason or another, and implant therapy seems a reasonable treatment approach in that situation. For unilateral cases, implant therapy appears to be reasonably cost-effective (better than the $50,000/QALY threshold).20 The question of whether substantial advantages or disadvantages regarding visual outcome become manifest over longer periods of time awaits ongoing follow-up of these patients. The use of ongoing therapy in the systemic therapy arm along with some continued activity suggests that the majority of patients will require ongoing treatment for these conditions for several years. Whether remission of uveitis differs between treatment arms also is a question that will be addressed by additional follow-up.
Supplementary Material
Acknowledgments
Financial Support: This study is supported by National Eye Institute Collaborative Agreements U10EY014655 (Dr. Jabs), U10EY014660 (Dr. Holbrook), and U10EY014656 (Dr. Altaweel). Bausch & Lomb provided support to the study in the form of donation of fluocinolone implants for patients randomized to implant therapy who were uninsured or otherwise unable to pay for implants, or were located at a site where implants could not be purchased (e.g., in the United Kingdom). Additional support was provided by Research to Prevent Blindness and the Paul and Evanina Mackall Foundation. A representative of the National Eye Institute participated in the conduct of the study, including the study design and the collection, management, analysis, and interpretation of the data, as well as in the review and approval of this manuscript.
Duke Eye Center, Durham, NC: Glenn J. Jaffe, MD (Director); Brenda Branchaud, COT; Paul Hahn, MD, PhD; Larry Koreen, MD, PhD, MPH; Eleonora (Nora) M. Lad; Phoebe Lin; Joseph Nissim Martel; Neha (Shah) Serrano, MD; Cindy Skalak, RN, COT; Lejla Vajzovic, MD. Former Members: Claxton Baer, MD; Joyce Bryant, COT; Sai Chavala, MD; Michael Cusick, MD; Shelley Day, MD; Pouya Dayani, MD; Justis Ehlers, MD; Muge Kesen, MD; Annie Lee, MD; Alex Melamud, MD; Jawad A. Qureshi, MD; Adrienne Williams Scott; Robert F. See, MD; Robert K. Shuler, MD; Megan Wood, MS, COA.
Emory University, Atlanta, GA: Steven Yeh, MD (Director); Alcides Fernandes, MD; Deborah Gibbs, COMT, CCRC; Donna Leef, COMT. Former Members: Daniel F. Martin, MD; Sunil Srivastava, MD.
Johns Hopkins University, Baltimore, MD: James P. Dunn, MD (Director); Hosne Begum, MBBS, MPH; Jeff Boring, COA; Kristen L. Brotherson, BS; Bryn Burkholder, MD; Nicholas J. Butler, MD; Dennis Cain; Mary A. Cook, BA, MS; David Emmert; Janis R. Graul; Mark Herring; Ashley Laing, MD; Theresa G. Leung, MD; Michael C. Mahon, BS; Ahmafreza Moradi, MD; Antonia Nwankwo, BS; Trucian L. Ostheimer, MD; Terry Reed, COA. Former Members: Ellen Arnold, BS; Patricia M. Barnabie, BS; Marie-Lynn Belair, MD; Stephen G. Bolton, CRNP; Joseph B. Brodine; Diane M. Brown, MSN, RN; Lisa M. Brune, BSN, RN; Anat Galor, MD; Theresa Gan, MD; Adam Jacobowitz, MD; Meera Kapoor; Sanjay Kedhar, MD; Stephen Kim, MD; Henry A. Leder, MD; Alison G. Livingston, BSN, RN; Yavette Morton; Kisten Nolan, BSN, MPH, RN; George B. Peters, MD, MBA; Priscilla Soto; Ricardo Stevenson, MD; Michelle Tarver-Carr, MD, PhD; Yue Wang, MD, PhD.
Massachusetts Eye Research and Surgery Institute, Cambridge, MA: C. Stephen Foster, MD (Director); Stephen D. Anesi, MD; Linda Bruner; Olga Ceron, MD; David M. Hinkle, MD; Nancy Persons; Bailey Wentworth. Former Members: Sarah Acevedo, MD; Fahd Anzaar, MD; Tom Cesca; Angelica Contero; Kayleigh Fitzpatrick; Faith Goronga; Jyothir Johnson; Karina Q. Lebron, MD; Danielle Marvell; Chandra Morgan; Nita Patel, MSW; Jennifer Pinto; Sana S. Siddique; Janet Sprague; Taygan Yilmaz.
National Eye Institute, Bethesda, MD: H. Nida Sen, MD, MHSc (Director); Michael Bono; Denise Cunningham, CRA; Darryl Hayes, COA; Dessie Koutsandreas, BS, COA; Robert B. Nussenblatt, MD; Patti R. Sherry, BSN; Gregory L. Short, COMT; Wendy Smith, MD; Alana Temple, BSN. Former Members: Allison Bamji, RN; Hanna Coleman, MD; Geetaniali Davuluri, MD; Lisa Faia, MD; Chloe Gottlieb, MD; Guy V. Jirawuthiworavong, MD; Julie C. Lew, MD; Richard Mercer, COT; Dominic Obiyor, BSN; Cheryl H. Perry, BSN; Natalia Potapova, MD; Eric Weichel, MD; Keith J. Wroblewski, MD; Steven Yeh, MD.
New York Eye & Ear Infirmary, New York, NY: Paul A. Latkany, MD (Director); Corinne Coonan; Andrea Honda, MD; Monica Lorenzo-Latkany, MD; Robert Masini, MD; Susan Morell; Angela Nguyen. Former Members: Jason Badamo; Kenneth M. Boyd; Matthew Enos; Jenny Gallardo; Jacek Jarczynski; Ji Yun Lee; Mirjana McGrosky; Ann Nour; Meredith Sanchez; Kate Steinberg.
Royal Victorian Eye & Ear Hospital, East Melbourne, Australia: Richard J. Stawell, MD (Director); Lisa Breayley; Carly D’Sylva; Elizabeth Glatz; Lauren Hodgson; Lyndell Lim, MD; Cecilia Ling, MD; Rachel McIntosh; Julie Morrison (Ewing); Andrew Newton; Sutha Sanmugasundram; Richard Smallwood; Ehud Zamir, MD. Former Members: Nicola Hunt; Lisa Jones; Ignatios Koukouras; Suzanne Williams.
Rush University Medical Center, Chicago, IL: Pauline T. Merrill, MD (Director); Danielle Carns; Len Richine; Denise L. Voskuil-Marre, BS; Kisung Woo. Former Members: Bruce Gaynes; Christina Giannoulis; Pam Hulvey; Elaine Kernbauer; Heena S. Khan, BA; Sarah J. Levine; Scott Toennessen; Eileen Tonner, CC, VA, DS.
Texas Retina Associates, Dallas, TX: Robert C. Wang, MD (Director); Hank Aguado; Sally Arceneaux; Karen Duignan; Gary E. Fish, MD; Nick Hesse; Diana Jaramillo; Michael Mackens. Former Members: Jean Arnwine; David Callanan, MD; Kimberly Cummings; Keith Gray; Susie Howden; Karin Mutz; Brenda Sanchez.
United Kingdom Institute of Ophthalmology, London, UK: Susan Lightman, MD, PhD (Director); Filis Ismetova, MD; Ashley Prytherch; Sophie Seguin-Greenstein; Oren Tomkins. Former Members: Asat Bar; Kate Edwards; Lavanish Joshi; Jiten Moraji; Ahmed Samy, MD; Timothy Stubbs; Simon Taylor, MD; Hamish Towler; Rebecca Tronnberg.
University of California at Los Angeles, Los Angeles, CA: Gary N. Holland, MD (Director); Robert D. Almanzor, COA; Jose Castellanos, COT; Jean Pierre Hubschman, MD; Ann K. Johiro, NP; Alla Kukuyev, MD; Ralph D. Levinson, MD; Colin A. McCannel, MD; Susan S. Ransome, MD. Former Members: Christine R. Gonzales, MD; Anurag Gupta, MD; Partho S. Kalyani, MD; Michael A. Kapamajian, MD; Peter J. Kappel, MD.
University of California at San Diego, San Diego, CA: Cheryl Arcinue, MD (Director); Janne Chuang; Giulio Barteselli, MD; Glenn Currie; Veronica Mendoza; Debbie Powell, BBA. Former Members: Tom Clark, BSc, CRA; Denine E. Cochran, COT, CCRC; William R. Freeman, MD; Joshua Hedaya, MD; Tiara Kemper; Igor Kozak, MD; Jacqueline M. LeMoine; Megan E. Loughran, BA; Luzandra Magana; Francesca Mojana, MD; Victoria Morrison, MD; Vivian Nguyen; Stephen F. Oster, MD.
University of California at San Francisco, San Francisco, CA: Nisha Acharya, MD (Director); David Clay; Salena Lee, OD; Mary Lew, BA; Todd P. Margolis, MD; Jay Stewart, MD; Ira G. Wong, MD. Former Members: Debra Brown; Claire M. Khouri, BA.
University of Illinois at Chicago, Chicago, IL (currently Northwestern University): Debra A. Goldstein, MD (Director); Andrea Birnbaum, MD, PhD; Andrea Degillio, CRA; Gemma De la Rosa, COT; Carmen Ramirez; Evica Simjanowski, CRA; Mariner Skelly, CRA. Former Members: Anna L. Castro-Malek, BA, CCRC; Catherine E. Crooke; Melody Huntley; Katrina Nash; Marcia Niec; Dimitry Pyatetsky, MD; Misel Ramirez; Zuzanna Rozenbajgier, MS; Howard H. Tessler, MD.
University of Miami, Miami, FL: Janet L. Davis, MD (Director); Thomas A. Albini, MD; Marie Chin. Former Members: Daniela Castaño; Ariana Elizondo, BS, CCRP; Macy Ho; Jaclyn L. Kovach, MD; Richard C-S Lin, MD; Efrem Mandelcorn; Jackie K-D Nguyen, MD; Aura Pacini; Susan Pineda; David A. Pinto; Jose Rebimbas; Kimberly E. Stepien, MD; Claudia Teran.
University of Michigan, Ann Arbor, MI: Susan G. Elner, MD (Director); Hillary Bernard; Linda Fournier, COT; Lindsay Godsey; Linda Goings; Richard Hackel; Moella Hesselgrave, COA; K. Thiran Jayasundera, MD; Robert Prusak; Pamela Titus, COT. Former Members: Melissa Bergeron, COA; Reneé Blosser, COMT; Rebecca Brown, COA; Carrie Chrisman-McClure; Julie R. Gothrup, COA; Stephen J. Saxe, MD; Deanna Sizemore, COA.
University of Pennsylvania, Philadelphia, PA: John H. Kempen, MD, PhD (Director); James Berger; Sheri Drossner; Joan C. DuPont; Albert M. Maguire, MD; Janice Petner; Stephanie Engelhard. Former Members: Tim Hopkins, MD; Dawn McCall; Monique McRay; Daniel Will, MD; Wei Xu, Jonathan Lo, Rebecca Salvo, Elizabeth Windsor; Laurel Weeney.
University of South Florida, Tampa, FL: Peter R. Pavan, MD (Director); Ken Albritton; JoAnn Leto; Brian Madow, MD; Lori Mayor; Scott E. Pautler, MD; Wyatt Saxon; Judy Soto. Former Members: Burton Goldstein, MD; Amy Klukoff; Lucy Lambright; Kim McDonald; Maria Ortiz; Susan Scymanky; Dee Dee Szalay.
University of Southern California, Los Angeles, CA: Narsing Rao, MD (Director); Tamara Davis; Jackie Douglass; Judith Linton; Margaret Padilla, CCRP; Sylvia Ramos; Narsing A. Rao, MD. Former Members: Alexia Aguirre; Lawrence Chong, MD; Lupe Cisneros, COA; Elizabeth Corona; Dean Eliott, MD; Amani Fawzi, MD; Jesse Garcia; Rahul Khurana, MD; Jennifer Lim, MD; Rachel Mead; Julie H. Tsai, MD.
University of Utah, Salt Lake City, UT: Albert Vitale, MD (Director); Paul S. Bernstein, MD, PhD; Bonnie Carlstrom, COA; James Gilman, CRA; Sandra Hanseen, COA; Paula Morris, CRA; Diana Ramirez; Kimberley Wegner, BS, CRC.
Virginia Eye Consultants, Norfolk, VA: John D. Sheppard, MD, MMSc (Director); Brianne Anthony; Amber Casper; Lisa Felix-Kent, COA; Jeanette Fernandez, COMT; Tari Johnson, BS; Stephen V. Scoper, MD. Former Members: R. Denise Cole; Nancy Crawford; Lisa Franklin; Krista Hamelin; Jen Martin; Rebecca Marx; Gregory Schultz, DD; Joseph Webb, BS; Pamela Yeager.
Vitreoretinal Consultants, Houston, TX (currently: Retina Consultants of Houston): Rosa Y. Kim, MD (Director); Matthew S. Benz, MD; David M. Brown, MD; Eric Chen, MD; Richard H. Fish, MD; Eric Kegley, BA, CRA, COA; Laura Shawver, CCRP, COT; Tien P. Wong, MD. Former Members: Rebecca De La Garza; Shayla Friday (Hay); Karin Mutz.
Washington University, St. Louis, MO: P. Kumar Rao, MD (Director); Eve Adcock; Rajendra S. Apte, MD, PhD; Amy Baladenski; Rhonda Curtis; Sarah Gould; Amanda Hebden; Jamie Kambarian; Charla Meyer; Sam Pistorius; Melanie Quinn; Greg Rathert. Former Members: Kevin J. Blinder, MD; Ashley Hartz; Pam Light; Gaurav K. Shah, MD; Russell VanGelder, MD, PhD.
Executive Committee: Douglas A. Jabs, MD, MBA (Chair); Michael M. Altaweel, MD; Janet T. Holbrook, PhD, MPH; John H. Kempen, MD, PhD; Natalie Kurinij, PhD.
Steering Committee: Douglas A. Jabs, MD, MBA (Chair); Robert D. Almanzor, COA; Michael M. Altaweel; Diane Brown; James P. Dunn, MD; Janet T. Holbrook, PhD, MPH; Gary N. Holland, MD; John H. Kempen, MD, PhD; Rosa Y. Kim, MD; Natalie Kurinij, PhD; Nancy Prusakowski, MS; Jennifer E. Thorne, MD, PhD. Former Members: Stephen G. Bolton, RN, BSN; Lisa M. Brune, RN, BSN; Tom Clark, CRA; James Gilman; Larry Hubbard, MAT; Daniel F. Martin, MD; Robert B. Nussenblatt, MD.
Data, Safety and Monitoring Committee: Voting members: Janet Wittes, PhD (Chair); William E. Barlow, PhD; Marc Hochberg, MD; Alice T. Lyon, MD; Alan G. Palestine, MD; Lee S. Simon, MD. Non-voting members: Michael M. Altaweel, MD; Janet T. Holbrook, PhD, MPH; Douglas A. Jabs, MD, MBA; Natalie Kurinij, PhD. Former Members: James T. Rosenbaum, MD; Harmon Smith, PhD.
Surgical Quality Assurance Committee: John H. Kempen, MD, PhD (Chair); Glenn J. Jaffe, MD. Former Members: Janet Davis, MD; James P. Dunn, MD; Daniel F. Martin, MD; Jennifer Thorne, MD; Albert Vitale, MD.
Medical Therapy Quality Assurance Committee: Jennifer E. Thorne, MD, PhD (Chair); Nisha R. Acharya, MD, MS; Douglas A. Jabs, MD, MBA; John H. Kempen, MD, PhD; Paul A. Latkany, MD; Albert T. Vitale, MD. Former Members: Robert B. Nussenblatt, MD, MPH; Russell VanGelder, MD, PhD.
Visual Function Quality Assurance Committee: Robert D. Almanzor, COA (Chair); Jeffrey A. Boring, COA; Deborah Gibbs, COMT, CCRC; Salena Lee, OD, FAAO; Nancy Prusakowski; Jennifer E. Thorne, MD, PhD (Advisor). Former Members: Judith Alexander, BA, CCRP; Wai Ping Ng, BS.
Glaucoma Committee: David S. Friedman, MD (Chair); Anna Adler, MS; Judith Alexander, BA, CCRP; Alyce Burke, MPH; Janet T. Holbrook, PhD, MPH; Joanne Katz, ScD; John H. Kempen, MD, PhD; Nancy Prusakowski, MS; Susan Reed, BS; Jennifer E. Thorne, MD, PhD; Husam Ansari, MD, PhD (consultant). Former Members: Nicholas Cohen, MS; Sanjukta Modak, MS; Wai Ping Ng, BS.
Statistical Analysis Committee: Elizabeth A. Sugar, PhD (Chair); Alyce E. Burke, MPH; Lea T. Drye, PhD; Janet T. Holbrook, PhD, MPH; Mark L. Van Natta, MHS. Former Members: Kevin Frick, PhD; JoAnn Katz, ScD; Thomas A. Louis, PhD; Sanjukta Modak, MS; David Shade, JD.
Chairman’s Office: Mount Sinai School of Medicine, New York, NY: Douglas A. Jabs, MD, MBA (Study Chairman); Karen Pascual, MBA; Jill S. Slutsky-Sanon, MPA. Former Members: Colby Glomp; Melissa A. Nieves, BA; Maria Stevens, CM, Amanda Allen; Yasmin Hilal, MHS.
Coordinating Center, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD: Janet T. Holbrook, PhD, MPH (Director); Francis Abreu, ScM; Alyce Burke, MPH; Anne Shanklin Casper, MA; Lea T. Drye, PhD; Cathleen Ewing; David S. Friedman, MD, PhD; Adante Hart, BS; Andrea Lears, BS; Shirley Li; Jill Meinert; Vinnette Morrison, BS; Deborah Nowakowski; Nancy Prusakowski, MS; Girlie Reyes, BS; Dave M. Shade, JD; Jacqueline Smith, AA; Karen Steuernagle; Elizabeth A. Sugar, PhD; Jennifer E. Thorne, MD, PhD; Mark Van Natta, MHS; Vidya Venugopal, MS; Tsung Yu, ScM. Former Members: Anna Adler, MS; Judith Alexander, BA; Jeff Boring, COA; Paul Chen; Nicholas Cohen, MS; Karen Collins; John Dodge; Kevin D. Frick, PhD; Rosetta Jackson; Christian Jimenez; Joanne Katz, ScD; Ariel Landers; Hope Livingston; Thomas A. Louis, PhD; Curtis L. Meinert, PhD; Sanjukta Modak, MS; Wai Ping Ng, BS; Sobharani Rayapudi, MD, ScM; Weijiang Shen, MS; Charles Shiflett, BS; Rochelle Smith, BS; Ada Tieman, MBA; James A. Tonascia, PhD; Richard Zheng, MS.
University of Wisconsin Fundus Photograph Reading Center, Madison, WI: Michael M. Altaweel, MD (Director); James Allan, BSc; Wendy K. Benz, PhD; Amitha Domalpally, MD; Kristine A. Johnson; Dawn J. Myers, BS; Jeong Won Pak, PhD; Susan Reed, BS; James L. Reimers, BA; Former Members: Debra J. Christianson, BS; Geoffrey Chambers, MS; Margaret A. Fleischli, AB, DVM; Jacquelyn Freund, MS; Kathleen E. Glander, BBA; Anne Goulding, BA; Vonnie Gama; Sapna Gangaputra, MD, MPH; Dennis Hafford; Susan E. Harris, MS; Larry D. Hubbard, MAT; Jeffrey M. Joyce, MS; Christina N. Kruse, BA; Lauren Nagle; Amy Remm, BS; Gwyn E. Padden-Lechten, BS; Alyson Pohlman, BA; Ruth A. Shaw, BS; Peggy Sivesind; Dennis Thayer; Erika Treichel, DVM; Kelly J. Warren, RN, BS, MSES; Sheila M. Watson, BS, DVM; James K. White, BME; Tara Wilhelmson, BS; Grace Zhang, BA. Mary K. Webster, BS.
Footnotes
The Credit Roster for the Multicenter Uveitis Steroid Treatment (MUST) Trial Research Group appears in Appendix 1, available at http://aaojournal.org.
Off label use of drugs: adalimumab, azathioprine, cyclosporine, cyclophosphamide, daclizumab, infliximab, methotrexate, mycophenolate mofetil, rituximab, tacrolimus.
ClinicalTrials.gov Identifier: NCT00132691
Conflicts of Interest: In the last three years, Dr. Kempen has served as a consultant for AbbVie, Alcon, Allergan, Can-Fite, Clearside, Lux Biosciences, Roche, and Xoma. Dr. Jabs serves on the Data and Safety Monitoring Committees for Applied Genetic Technologies Corporation and Novartis Pharmaceutical Corp and is a consultant to Santen. Dr. Thorne is a consultant for AbbVie, Gilead, and XOMA and receives research funding from Allergan.. Drs. Altaweel, Drye, Holbrook, and Sugar have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.National Advisory Eye Council Program Planning Subcommittee. Vision Research—A National Plan: 1983–1987. Volume 1: The 1983 Report of the National Advisory Eye Council. Bethesda, MD: National Institutes of Health, Public Health Service, U.S. Department of Health and Human Services; 1983. [Accessed November 13, 2014]. p. 13. Available at: http://ia700402.us.archive.org/34/items/visionresearchna01nati/visionresearchna01nati.pdf. [Google Scholar]
- 2.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–8. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- 3.Dandona L, Dandona R, John RK, et al. Population based assessment of uveitis in an urban population in southern India. Br J Ophthalmol. 2000;84:706–9. doi: 10.1136/bjo.84.7.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rathinam SR, Namperumalsamy P. Global variation and pattern changes in epidemiology of uveitis. Indian J Ophthalmol. 2007;55:173–83. doi: 10.4103/0301-4738.31936. [DOI] [PubMed] [Google Scholar]
- 5.ten Doesschate J. Causes of blindness in The Netherlands. Doc Ophthalmol. 1982;52:279–85. doi: 10.1007/BF01675857. [DOI] [PubMed] [Google Scholar]
- 6.Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. 2013;131:1405–12. doi: 10.1001/jamaophthalmol.2013.4237. [DOI] [PubMed] [Google Scholar]
- 7.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111:491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Suhler EB, Lloyd MJ, Choi D, et al. Incidence and prevalence of uveitis in Veterans Affairs Medical Centers of the Pacific Northwest. Am J Ophthalmol. 2008;146:890–6. doi: 10.1016/j.ajo.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Congdon N, Vingerling JR, Klein BE, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122:487–94. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DS, Wolfs RC, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–8. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman DS, O’Colmain BJ, Munoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 12.Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica. 2004;218:223–36. doi: 10.1159/000078612. [DOI] [PubMed] [Google Scholar]
- 13.Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluation of intraocular inflammatory disease. Am J Ophthalmol. 1987;103:234–5. doi: 10.1016/s0002-9394(14)74235-7. [DOI] [PubMed] [Google Scholar]
- 14.Standardization of Uveitis Nomenclature Working Group. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005;140:509–16. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durrani OM, Tehrani NN, Marr JE, et al. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. 2004;88:1159–62. doi: 10.1136/bjo.2003.037226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol. 1996;80:332–6. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Multicenter Uveitis Steroid Treatment Trial Research Group. Kempen JH, Altaweel MM, et al. The multicenter uveitis steroid treatment trial: rationale, design, and baseline characteristics. Am J Ophthalmol. 2010;149:550–61. doi: 10.1016/j.ajo.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Multicenter Uveitis Steroid Treatment Trial Research Group. Kempen JH, Altaweel MM, et al. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: the multicenter uveitis steroid treatment trial. Ophthalmology. 2011;118:1916–26. doi: 10.1016/j.ophtha.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callanan DG, Jaffe GJ, Martin DF, et al. Treatment of posterior uveitis with a fluocinolone acetonide implant: three-year clinical trial results. Arch Ophthalmol. 2008;126:1191–201. doi: 10.1001/archopht.126.9.1191. [DOI] [PubMed] [Google Scholar]
- 20.The Multicenter Uveitis Steroid Treatment Trial Follow-up Study Research Group. Cost-Effectiveness of Fluocinolone Acetonide Implant versus Systemic Therapy for Noninfectious Intermediate, Posterior, and Panuveitis. Ophthalmology. 2014;121:1855–62. doi: 10.1016/j.ophtha.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Multicenter Uveitis Steroid Treatment Trial Follow-up Study Research Group. Quality of Life and Risks Associated with Systemic Anti-inflammatory Therapy Versus Fluocinolone Acetonide Intraocular Implant for Intermediate, Posterior or Panuveitis: 54 month results of The Multicenter Uveitis Steroid Treatment Trial (MUST) and Follow-up Study. Ophthalmology. doi: 10.1016/j.ophtha.2015.06.043. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabs DA, Rosenbaum JT, Foster CS, et al. Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol. 2000;130:492–513. doi: 10.1016/s0002-9394(00)00659-0. [DOI] [PubMed] [Google Scholar]
- 23.Sugar EA, Jabs DA, Altaweel MM, et al. Identifying a clinically meaningful threshold for change in uveitic macular edema evaluated by optical coherence tomography. Am J Ophthalmol. 2011;152:1044–52. doi: 10.1016/j.ajo.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeger SL, Liang KY. An overview of methods for the analysis of longitudinal data. Stat Med. 1992;11:1825–39. doi: 10.1002/sim.4780111406. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine--reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–94. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 26.Jaffe GJ, Yang CH, Guo H, et al. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Invest Ophthalmol Vis Sci. 2000;41:3569–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.