Abstract
LIM homeobox 8 (Lhx8) is a highly conserved transcriptional factor with recently illustrated roles in cholinergic and GABAergic differentiation, and is expressed in neural crest derived craniofacial tissues during development. However, Lhx8 functions and signaling pathways are largely elusive. Here we showed that Lhx8 regulates dental mesenchyme differentiation and function via Wnt and TGFβ pathways. Lhx8 expression was restricted to dental mesenchyme from E11.5 to a peak at E14.5, and absent in dental epithelium. By reconstituting dental epithelium and mesenchyme in an E16.5 tooth organ, Lhx8 knockdown accelerated dental mesenchyme differentiation; conversely, Lhx8 overexpression attenuated dentin formation. Lhx8 overexpressed adult human dental pulp stem/progenitor cells in β-tricalcium phosphate cubes attenuated mineralized matrix production in vivo. Gene profiling revealed that postnatal dental pulp stem/progenitor cells upon Lhx8 overexpression modified several matrix related gene expression including Dspp, Cola1 and osteocalcin. Lhx8 transcriptionally activates Wnt and TGFβ pathways, and its attenuation upregulates multiple dentinogenesis genes. Together, Lhx8 regulates dentin development and regeneration by fine-turning Wnt and TGFβ signaling.
Keywords: Lhx8, dentinogenesis, transcriptional regulation, signal pathways, tooth regeneration
1. Introduction
Lhx8, formerly known as L3 and Lhx7, is a remarkably conserved transcriptional factor of the LIM-homeobox family among species [45]. In the developing central nervous system (CNS), Lhx8 is primarily expressed in the ventral telencephalon, specifically in the medial ganglionic eminence (MGE) of the basal forebrain, and in subsets of neurons in the striatum [1]. Lhx8 plays crucial roles in regulating the fate of cholinergic and GABAergic neurons [2, 45]. Lhx8 and Isl1 together regulate cholinergic differentiation [3, 4], whereas Lhx8 and Lhx6 together appear to promote GABAergic differentiation [5, 6]. Neural crest cells migrate from the midbrain and hindbrain regions at embryonic day 8.5 (E8.5) and form the branchial arches [7, 8]. The first branchial arch begins to acquire tooth-forming capability from E8.75 to E10.5 [9]. Lhx8 transcripts were detected in the neural crest-derived mesenchyme of the first branchial arch at E9.5, and are abundantly expressed in the dental mesenchyme at bud stage (E12.5) [1, 10–12]. However, the functional roles played by Lhx8 in multiple tissues are largely elusive.
Deletion of Lhx6 and/or Lhx8 has severe consequences. In Lhx8 deficient mice, forebrain cholinergic neuron progenitors survive but fail to generate cholinergic interneurons in the striatum and cholinergic projection neurons in the basal forebrain [13]. Lhx6/8 double knockout leads to arrested dental mesenchyme specification and tooth absence [11]. In contrast to a lack of craniofacial defects in Lhx6 null mutants, mice homozygous for Lhx8 mutation develop cleft palate[11, 14]. Lhx6/8 double homozygous knockout mice have multiple craniofacial defects including cleft palate and missing molar teeth[11]. Molar absence in Lhx6/8 double mutants results from failed specification of molar mesenchyme, rather than patterning defects in the first brachial arch[11]. A single functional copy of either Lhx6 or Lhx8 appears sufficient for molar development. Some of the roles played by Lhx6/8 may be interchangeable, although Lhx6 only partially compensates for Lhx8 in tooth development. Despite these observations of Lhx8 functions, independent of or in conjunction with Lhx6, little is known about Lhx8 signaling pathways that regulate mesenchyme development. Here, we discovered that Lhx8 silencing accelerated dental mesenchyme development, and conversely, Lhx8 overexpression reduced dentin differentiation and maturation. Profiling and pathway analysis of dental pulp stem/progenitor cells revealed that Lhx8 exerts its functions by fine-tuning Wnt/βcatenin and TGFβ pathways in addition to signal molecules that may complement Lhx8 functions. Thus, Lhx8 likely regulates mesenchyme development as a negative modulator for differentiation and maturation. Dental mesenchyme was adopted as a model to study Lhx regulation of mesenchyme development in the present work because mineralization of dental mesenchyme serves as a powerful and convenient tool.
2. Materials and methods
2.1 In Situ Hybridization
Mouse Lhx8 fragment plasmid (nt 1022–1185 of the mouse cDNA) was a gift from Drs. M. Grigoriou and V. Pachnis [15]. Following linearization with NotI and HindIII respectively, antisense and sense control RNA probes were synthesized by in vitro transcription with T3 or T7 RNA polymerase (MAXIscript® T3/T7 In Vitro Transcription Kit, Ambion), per manufacturer instructions using Digoxigenin (DIG)-11-UTP (Roche, Germany). Whole mount in situ hybridization of E11.5 embryos was performed per prior methods[16, 17]. Mandibular incisor and first molar tooth germs from CD-1 mice (Charles River Laboratories) at E12.5, E14.5, E16.5, E18.5, P1, P5, P7 and P21 were dissected in cold PBS (pH=7.4) and fixed with Z7 (0.5% w/v zinc chloride, 0.5% w/v zinc acetate, 0.05% w/v calcium acetate, 17.16 mM zinc trifluoroacetate, in 0.1M Tris-HCl, pH=7) for 30 min to 6 hrs at room temperature (RT) with gentle agitation. Tissues were transferred to 30% w/v sucrose and incubated at 4°C prior to OCT mounting. Frozen sections (10 μm) were cut for in situ hybridization[15]. NBT/BCIP from DIG Nucleic Acid Detection Kit (Roche) was used with constant color development for 24 hrs for all sections. Lhx8 transcripts were hybridized at 67°C with 1:5000 diluted anti-DIG-AP antibody (Roche). All animal experiments were approved by Columbia University IACUC.
2.2 Cell Culture
Proietics™ human Dental Pulp Stem Cells (DPSCs) were from the third molar of an anonymous adult female donor and cryopreserved at a primary passage (PT-5025, Lonza). These cells are positive for CD105, CD166, CD29, CD90, and CD73, negative for CD34, CD45, and CD133. DPSCs were maintained and expanded in DPSC BulletKit™ Medium (PT-3005, Lonza) at 37°C and 5% CO2. Cells were passaged at 80% confluence, with medium change every 2–3 days.
2.3 Lentivirus packaging and infection
The open reading frame of Lhx8 was cloned into pWPI vectors (Plasmid #12254, Addgene). Control pWPI or pWPI-Lhx8 (6.25μg), pMD2.G (Plasmid #12259, Addgene) (0.625 μg) and psPAX2 (Plasmid #12260, Addgene) (3.125μg) vectors were co-transfected into 80% confluent 293T cells using Calcium Phosphate Transfection Kit per manufacturer’s protocol (Invitrogen). Virus supernatant was collected 2 days after transfection was filtered with 0.45μm membrane and purified with ViraBind™ Lentivirus Purification Kit (Cell Biolabs) per manufacturer’s instructions. DPSCs were cultured to 30–50% confluence and infected with lentivirus in 8 μg/mL polybrene (Santa Cruz Biotechnology). Infected cells were sorted by FACS based on GFP expression (Suppl. Fig. 1A,B) and were further passaged 3–5 times.
2.4 In vitro recombination and organ culture
Pregnant CD-1 mice were purchased from Charles River Laboratories, with the date of vaginal plug appearance as Embryonic day 0.5 (E0.5). E16.5 tooth germs were harvested per conventional procedures[18, 19]. Briefly, the first mandibular molar tooth germs were dissected from E16.5 mouse embryo with fine forceps under dissection microscope. Dental epithelium and mesenchyme were dissociated in 50 U/mL dispase (Corning) at RT for 10.5 min, and followed by mechanical separation using 25G needles under dissection microscope. In knockdown experiments, dental mesenchyme was transfected with Cy3 labeled control siRNA or Lhx8 siRNA (Suppl. Table 1, Ambion) by incubating with Lipofectamine RNAiMAX (Life technologies)-siRNA duplex for 1hr at RT before recombination. In overexpression experiments, dental mesenchyme was incubated with Lhx8 over-expressing lentivirus, whereas dental epithelium was incubated in DPBS (HyClone, Thermo Scientific) followed by reconstitution[20], and culture for up to 7 days[21]. Transfection efficiency was tested by observing the Cy3 labeled or GFP postive cells with confocal microscope. Reconstituted tooth germs at day 3 were washed with cold PBS prior to nuclear staining with 6-diamidino-2-phenylindole (DAPI, Invitrogen) for 10 min RT. The effects of siLhx8 and lentivirus mediated Lhx8 overexpression were analyzed by histology and immunohistochemistry.
2.5 Cell proliferation and differentiation
Control and Lhx8 overexpressed dental pulp stem/progenitor cells (DPSCs) were seeded in 96-well plates at a density of 3×103 cells per well. Cell numbers were analyzed by Cell Counting Kit-8 (Sigma-Aldrich) at 450-nm absorbance. For odontogenic differentiation, control and Lhx8 overexpressed DPSCs were seeded at 70% confluence and cultured with DPSC BulletKit™ Medium for 24hrs before switched the odontogenic differentiation medium consisting of 100 μM ascorbic acid, 2 mM β-glycerophosphate, 10 nM dexamethasone (Sigma-Aldrich) and 100ng/mL rhBMP2 (R&D Systems)[22], with medium change every 2 days. In some groups, 200 ng/ml rhDKK1 (R&D Systems) or 10μM SB431542 (Abcam) were used to assay Wnt and TGFβ pathways. RNA samples were collected at day 0 and 14 for gene expression analysis and protein samples were collected at day 4 for western blotting. ALP staining (Alkaline Phosphatase Staining Kit II, Stemgent) was performed at day 7, and Alizarin red staining at day 21.
2.6 Cell migration assay
Cell migration assay was performed using 8-μm pore size Transwell@ cell culture insert (Corning) for 12-well plate. After 6-hr serum starvation, 2×105 DPSCs with Lhx8 knockdown, over-expression were placed onto the insert in 300-μl DPSC Basal Medium (PT-3927, Lonza) with 0.5% fetal bovine serum (FBS, Gibico), and subjected to migration induction medium (2% FBS in 700μl DPSC Basal Medium) in the lower chamber. Following 12-hr incubation at 37°C and 5% CO2, unmigrated cells in the upper chamber were removed whereas migrated cells in lower chamber were washed with PBS, fixed with 4% paraformaldehyde and counted by 0.1% crystal violet staining.
2.7 In vivo stem/progenitor cell transplantation
Porous β-tricalcium phosphate β-TCP scaffold (5×2 mm3; pore size; 200–500 μm; porosity: 50–70%) was seeded with 1×106 control or Lhx8 over-expressed DPSCs per scaffold, and were primed for odontogenic differentiation at 37°C and 5% CO2 for 24 hrs. The mice (6–8 wks, Harlan) were first anesthetized with isofluorane. Both control and Lhx8 over-expressing DPSCs were seeded on β-TCP scaffolds and were subcutaneously implanted into each side of the dorsum. Eight weeks later, animals were euthanatized, the transplants were collected and fixed with 4% paraformaldehyde. After fixation, samples were demineralized in 0.5M EDTA (pH 7.4) for 1 wk and then dehydrated. Paraffin sections (5 μm) were used for HE, Masson’s trichrome and immunohistochemical staining.
2.8 Histochemical analysis and immunohistochemistry
Tissue sections were stained with hematoxylin-eosin. Immunohistochemical staining was done using Anti-Mouse HRP-DAB Cell & Tissue Staining Kit (R&D Systems). Briefly, sections were deparaffinized, heat-retrieved, blocked and thereafter incubated with the primary antibodies (DSPP, sc-73632, 1:200, Santa Cruz Biotechnology) overnight at 4°C, followed by washing and incubation with HRP conjugated secondary antibodies. Images were developed with DAB finally. For staining of CTNNB1 (β-catenin) in cells, control and Lhx8 overexpressed cells were grown on the coverslip at the 70–80% confluence. Following blocking of nonspecific binding sites, cells were incubated with anti-CTNNB1 antibody (ab32572, 1:200, Abcam) overnight at 4°C. The experiments were repeated at least three times.
2.9 qPCR
Total RNA was isolated using Trizol (Invitrogen) per manufacturer instructions. cDNA synthesis was done with random hexamer primers using iScript™ cDNA Synthesis Kit (Bio-Rad). mRNA expression was measured by quantitative real-time PCR using SYBR method. The conditions were 10μL of SYBR® Green PCR Master Mix (Applied Biosystems), 0.5μM of each 5′ and 3′ primer, 4μL of sample and H2O to a final volume of 20μL. Samples were amplified for 45 cycles with a denaturation at 95°C for 5 sec, annealing and extension at 60°C for 34 sec. SYBR green fluorescence was measured to determine the amount of double-stranded DNA. Relative mRNA levels of target genes were normalized to 18S RNA or GAPDH levels and further compared with the control using the 2−ddCt method. Primers were listed in Suppl. Table 1.
2.10 Western Blot
Cells were washed with ice-cold PBS and protein was extracted in RIPA Lysis Buffer (Thermo Scientific) with Protease/Phosphatase Inhibitor Cocktail (Cell Signaling Technology). Proteins were separated on a NuPAGE® Novex® 4–12% Bis-Tris Protein Gel (1.0 mm), transferred to nitrocellulose membrane (Bio-Rad), and detected with anti Pro-COL1A1 (sc-8782, 1:200, Santa Cruz Biotechnology), anti-CTNNB1 (ab32572, 1:200, Abcam), anti-DSPP (sc-73632, 1:200, Santa Cruz Biotechnology), anti-LHX8 (ab137036, 1:500, Abcam) and anti-GAPDH (sc-25778, 1:200, Santa Cruz Biotechnology) antibodies. Image was developed with IR fluorescence & Odyssey using corresponding secondary antibodies (LI-COR).
2.11 Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as described[23]. Briefly, formaldehyde cross-linked proliferating DPSCs (5×107 cells/sample) were quenched with ice-cold 0.125 M glycine before subject to ChIP. Cells were first treated with hypotonic buffer and then the nucleic were pelleted and resuspended in 5 ml RIPA buffer containing Protease/Phosphatase Inhibitor Cocktail, and sonicated to shear the cross-linked DNA to an optimal fragment size ranging from 200 to 1000bp. 5% of the sonicated samples were collected as Input and the rest were subjected to next steps. The sonicated samples were precleared with Protein A beads (EMD Millipore) and then incubated with antibodies overnight at 4°C. Immunoprecipitation was performed with 1μg control IgG (ab6556, Abcam) and anti-Lhx8 (ab137036, Abcam) antibodies. The immunocomplexes were further incubated with protein A beads at room temperature for 1hr on an orbital shaker and then centrifuged. After 3 times of wash, the protein A-antibody-antigen-DNA complexes were eluted from the beads by reversal of crosslinking. Finally, immunoprecipitated DNA-protein complexes were treated with RNase A (Qiagen) and proteinase K (Boston BioProducts) before harvested for PCR analysis. ChIP-PCR primers were listed in Suppl. Table 1.
2.12 RNA-seq and data analysis
Polyadenylated fraction of the RNA isolated from control and Lhx8 overexpression DPSCs was subjected to RNA-sequencing at University of Rochester Genomics Research Center. Briefly, 150–300 ng total RNAs from each sample were polyA selected and single-end sequencing libraries were constructed using TruSeq RNA Sample Prep Kit as manually instructed (Illumina). The samples were then sequenced using the Illumina HiSeq sequencer. RNA-Seq reads were mapped to the human reference genome. Mapped reads were assembled into RNA transcripts using the Cufflinks. Fragments Per Kilobase of exon model per Million mapped fragments (FPKM) value (a normalized gene expression value that are comparable between different samples and genes) were estimated for each gene in each sample.
Functional enrichment analysis was restricted to significantly differentially expressed coding genes. Gene Ontology functional enrichment for biological processes was carried out using DAVID[24]. Lhx8 putative targets in the whole genome, which harbors the Lhx8 binding sites TAATTA[25] in the promoter region, were retrieved in the Msigdb (Gene Set: TAATTA_V$CHX10_01, Suppl. Table 6; MSigDB 3.0: www.broadinstitute.org/gsea/msigdb).)[26]. Two public microarray datasets (GSE32321 and GSE19488) were retrieved from the GEO database to find the tooth development related genes[27, 28]. Intersections between Lhx8 regulated genes, tooth development related genes, and Lhx8 putative targets were analyzed by VENN diagram. GSEA (Gene Set Enrichment Analysis)[26] was also used to identify enriched gene sets of Lhx8 regulated genes. Enrichment was compared between control and Lhx8 overexpression groups. The statistical significance of GSEA was analyzed using 1000 permutations. A positive enrichment score indicates that the specific molecular signature correlated with the Lhx8 phenotype.
2.13 Statistical analysis
All experiments were performed at least three times, and all quantitative data expressed as mean±SD, were analyzed by Student’s t-test or one-way ANOVA, with significance of p≤0.05.
3. Results
3.1 Lhx8 expression in neural tissues and neural crest derived dental mesenchyme during development
At E11.5, Lhx8 was expressed abundantly in maxillary and mandibular processes from which tooth develops, in addition to the frontal process in whole mount (Fig. 1A,B). At E12.5, Lhx8 was intensively and exclusively expressed in neural crest derived mesenchyme including dental mesenchyme, and was completely absent from dental and oral epithelium (Fig. 1D–F,T). At E14.5, Lhx8 expression was remarkably restricted to dental mesenchyme in both the molar (Fig. 1G–I) and incisor (Fig. 1U). At E16.5, Lhx8 expression was localized to dental papilla in the molar but with decreasing intensity (Fig. 1J,K). Notably, mesenchyme cells adjacent to dental epithelium had robust Lhx8 expression in comparison to mesenchyme cells farther from the epithelium (Fig. 1K, arrowheads). At E18.5 when dental papilla began to differentiate into odontoblasts for dentin matrix deposition, Lhx8 expression was attenuated and further restricted to the odontoblasts immediately adjacent to dental epithelium and sub-odontoblastic cells, particularly in the cuspal region (Fig. 1L,M,V, arrowheads). Postnatally, Lhx8 expression substantially diminished in dental mesenchyme (Fig. 1N–S, 1W–Y, arrowheads), including faint presence in the odontoblast layer at P21 (Fig. 1S, arrowheads). At P7, Lhx8 was sporadically observed in apical dental papilla (Fig. 1N,O, Q, Y, arrows). Besides dental tissues, Lhx8 expression was also detected in the medial mesenchyme of maxillary and mandibular arch where lips develop (Fig. 1U, asterisk), faintly in the alveolar bone surrounding tooth germs (Fig. 1R, asterisk) and in the medial ganglionic eminence (mge, Fig. 1D,G) of the basal forebrain, which is associated with cholinergic neuron differentiation. We pooled previously published data of Lhx8 expression in dental mesenchyme from E11.5 to E13.5 (Gene Datasets GSE32321[28]), E14.5, E17.5 (GSE19488[27]), and combined with our own transcriptomic data, yielding a quantitative plot of Lhx8 expression from E11.5 to P7 in Fig. 1C. Clearly, Lhx8 expression is robust during neural and dental development from E11.5 to a peak at E13.5 and E14.5, but significantly decreases from E17.5 to postnatal day 7, with committed odontoblasts expressing ~20% of Lhx8 in comparison to cells at E14.5 (Fig. 1C).
Fig. 1. Lhx8 expression in neural and craniofacial tissues.
(A) Whole-mount in situ hybridization reveals that Lhx8 is intensively expressed in the first branchial arch of E11.5 mouse embryo. (B) Magnification of frontal craniofacial portion of E11.5 mouse embryo in (A). (C) Lhx8 expression data of laser capture microdissected E11.5, E12.5, E13.5, E14.5, E17.5 molar mesenchyme (n=3 for each group) and adult odontoblasts (n=4) are normalized to Gapdh before comparison among groups. * P < 0.05. (D–S) Expression of Lhx8 in E12.5 neural crest derived mesenchyme of maxillary and mandibular process (D–F). Lhx8 is restricted in dental mesenchyme in E14.5 tooth germ (G–I) and dental papilla at E16.5 (J and K). Lhx8 expression gradually decreases and becomes further restricted in odontoblasts and sub-odontoblastic cells from E18.5 (L and M), P1 (N), P5 (O and P), P7 (Q), till P21 (R and S). (T–Y) Expression pattern of Lhx8 in mandibular mouse incisor at E12.5 (T), E14.5 (U), E18.5 (V), P1 (W), P5 (X) and P7 (Y) is similar to that in molars. fn, frontonasal process; mx, maxillary process; md, mandibular process; oe, oral epithelium; de, dental epithelium; dm, dental mesenchyme; mge, medial ganglionic eminence; dp, dental papilla; pu, dental pulp; od, odontoblast; am, ameloblast; e, enamel; d, dentin; m, molar; i, incisor. Scale: 500 μm. Data represent mean ± SD. * P<0.05.
3.2 Lhx8 negatively regulates mesenchyme differentiation and maturation
With a comprehensive portrait of Lhx8 expression in prenatal and postnatal tooth organs, we next explored its roles in morphogenesis and cell differentiation, with a primary focus on its function from E16.5 onwards, which has not been studied before. E16.5 dental mesenchyme was isolated from dental epithelium under dissection microscope and reconstituted (Fig. 2A–F). About 50% of the tooth germs were successfully reconstituted, which continued to develop and formed dentin (Suppl. Fig. 2). We then knocked down and over-expressed Lhx8 in two separate experiments. The liposome encapsulated Cy3 labeled RNAi duplex was efficiently transfected into E16.5 dental mesenchyme (Fig. 2O–Q). Endogenous Lhx8 was reduced to ~1/3 of the original level by siLhx8 qPCR (Fig. 2R). Both control siRNA and Lhx8 siRNA transfection did not affect the success rate of reconstituted tooth germ, which remained at ~50%. Surprisingly, Lhx8 knockdown led to accelerated dentinogenesis with increased dentin area and thickness in reconstituted tooth germs (Fig. 2G–I,K–M, S, T). Lhx8 knockdown further enhanced Laminin a1, a basement membrane components known to be produced by dental mesenchyme, in addition to a ~14 fold increase in mesenchyme derived Dspp, a key hallmark of odontoblastic differentiation (Fig. 2U), which is further confirmed by increased Dspp protein by immunohistochemistry in the siLhx8 sample (Fig. 2N with 2J as control).
Fig. 2. Lhx8 knockdown accelerates mesenchyme differentiation.
(A) Schematic representation of the Lhx8 intervention and dental epithelium and dental mesenchyme recombination procedures. Dental epithelium and mesenchyme of mandibular first molar at E16.5 are separated and then the dental mesenchyme is placed onto upper chamber of Transwell cell culture insert and further incubated with either RNAi or lentivirus for 1 hr at RT. Meanwhile the dental epithelium is kept in ice cold DPBS. Then the mesenchyme and epithelium are recombined and cultured for up to 7 days. (B and C) Representative data showing the location (B) and morphology (C) of the tooth germ of E16.5 mandibular first molar. (D–F) The top view of the recombined tooth germ right after recombination (D), 1 day culture (E), 3 days culture (F) and 7 days culture (G, control group; K, siLhx8 group). (H–N) The recombined tooth germs from control (G–J) and siLhx8 (K–N) were harvested at day 7 and chemically stained with HE (H, I, L and M) and immunostained with Dspp (J, N) (n=5). (O–Q) Immunofluorescence of the isolated E16.5 dental mesenchyme transfected with Lipofectamine RNAiMAX coated Cy3 labeled RNAi duplex before recombination. Strong positive Cy3 dots indicate high transfection efficiency. (R) Knockdown efficiency of Lhx8. (S, T) Quantification data of dentin area (S) and thickness (T). (U) Tooth development related gene expression were examined by qPCR in the recombined tooth germs 4 days after in vitro culture from both control and siLhx8 groups. Data presented are a representative of 3 experiments. de, dental epithelium; dm, dental mesenchyme; d, dentin; od, odontoblast; am, ameloblast; e, enamel. Scale bars: 1mm (B–F, Q and K, O–Q); 500 μm (I and M); 250 μm (J and N); Data represent mean ± SD. * P<0.05.
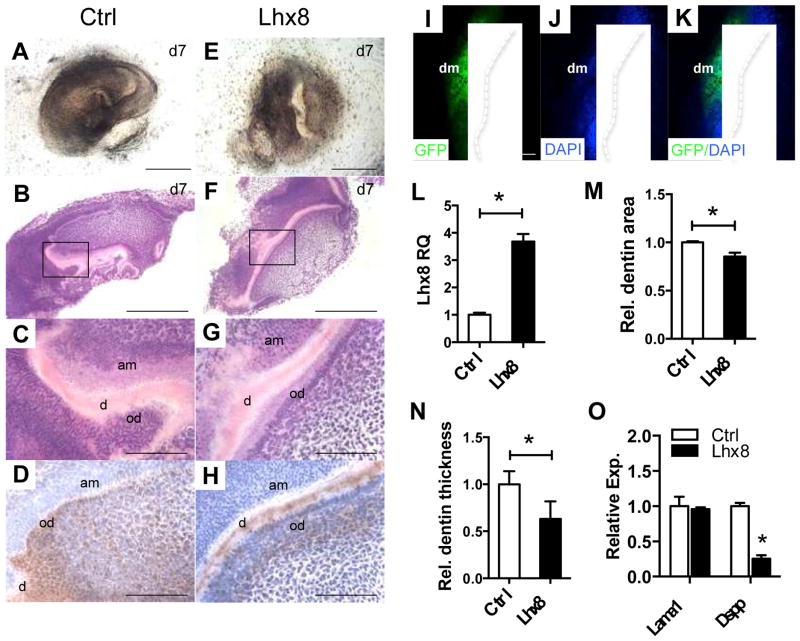
Conversely, lentivirus infection resulted in only ~10% success rate of reconstituted tooth germ, which should be attributable to the destruction of the mesenchyme and virus toxicity rather than gene intervention. In successfully reconstituted tooth germs, Lhx8 overexpression in dental mesenchyme delayed tooth development (Fig. 3A–D,E–H). Lhx8 lentivirus infection of E16.5 dental mesenchyme (Fig. 3I–K) induced a 2-fold Lhx8 increase (Fig. 3L), and significantly reduced dentin area and thickness (Fig. 3B,C, F, G, M, N) in addition to significantly reduced Dspp expression at both transcription (Fig. 3O) and protein (Fig. 3D,H) levels, further suggesting an inhibitory role of Lhx8 on dentinogenesis.
Fig. 3. Lhx8 overexpression attenuates mesenchyme differentiation and maturation.
(A–H) The recombined tooth germs from control (A–D) and Lhx8 overexpression (E–H) harvested at day 7 are chemically stained with HE (B, C, F and G) and immunostained with Dspp (D and H) (n=5). (I–K) The strong GFP expression in the isolated mesenchyme infected with lentivirus pWPI indicates high infection efficiency. (L) RT-PCR reveals the overexpression efficiency of Lhx8. (M and N) Quantification of dentin area (M) and thickness (N). (O) Tooth development related gene expression were examined by qPCR in the recombined tooth germs 4 days after in vitro culture from both control and Lhx8 overexpression groups. Data presented are a representative of 3 experiments. de, dental epithelium; dm, dental mesenchyme; d, dentin; od, odontoblast; am, ameloblast; e, enamel; Scale bars: 1 mm (A, B, E and F, I–K); 500 μm (C and G); 250 μm (D and H). Data represent mean ± SD. * P<0.05.
In an attempt to explain how Lhx8 regulates mesenchyme differentiation and maturation, we tested the effects of Lhx8 on cell proliferation, migration and mineralization. Postnatal mesenchyme stem/progenitor cells from dental pulp, which derives directly from dental papilla in which Lhx8 was robustly expressed (Fig. 1F, I, T, U), were adopted for their biological relevance and potential utility in tissue regeneration [7, 8]. Lhx8 overexpression had minimal effects on cell proliferation and migration (Suppl. Fig. 1C–G), suggesting perhaps its restricted roles in regulating mesenchyme differentiation and maturation without necessarily affecting cell motility and growth. Conversely, Lhx8 overexpression reduced mineralization, including reduced ALP staining after 7-day culture (Suppl. Fig. 1H–K, L–O) and decreased alizarin red staining after 21-day culture (Suppl. Fig. 1P–S). To confirm whether Lhx8 indeed had negative effects on mesenchyme differentiation and maturation, we performed an ectopic transplantation assay using control and Lhx8 overexpressed dental pulp cells in β-TCP cubes subcutaneously in mice. Along with abundant extracellular matrix and collagen (Fig. 4A, B, F, G), Dspp production was attenuated by Lhx8 overexpression (Fig. 4D,I), which was further quantified as decreased relative DSPP positive area (Fig. 4K), with similar cell numbers per field (Fig. 4L). Dentin-like structures in the scaffold were likely produced primarily by transplanted human cells per human mitochondria staining (Fig. 4E, J).
Fig. 4. Lhx8 overexpression delays the odontoblast differentiation.
(A–E) Control DPSCs were incubated in β-TCP scaffold in odontogenic medium for 24hr before transplanted into nude mice subcutaneously. Eight weeks later, samples were harvested for HE (A and B), Masson’s trichrome (C), DSPP (D) and human specific mitochondria staining (E). Data presented are representative of six different samples. (F–J) Lhx8 overexpressed DPSCs were treated same as above and harvested for HE (F–G), Masson’s trichrome (H), DSPP (I) and human specific mitochondria immunostaining (J). (K and L) DSPP expression was significantly lower than the control group (K) with similar total cell number (L). Data presented are representative of six different samples. b, blood vessels; m, extracellular matrix; c, implanted cells (DPSCs); s, scaffold residue; ms, mouse skin. Scale bars: 200μm.
3.3 Lhx8 overexpression reverts postnatal dental pulp stem/progenitor cells into E13.5 dental mesenchyme-like cells
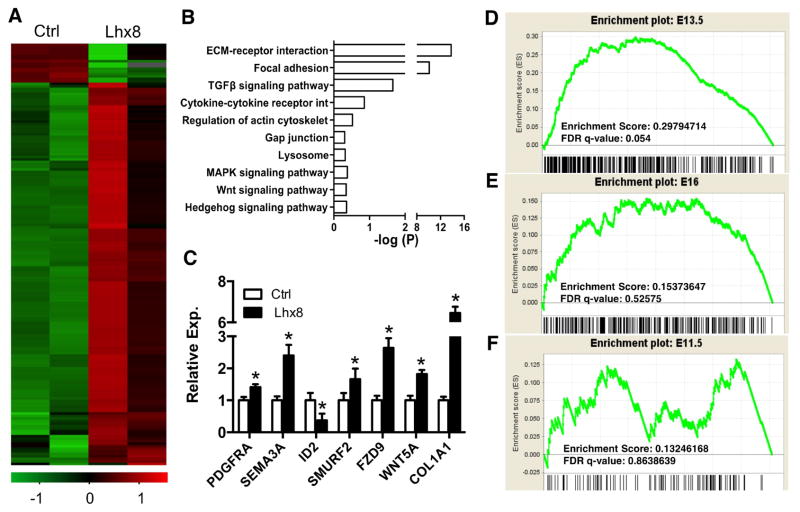
To further understand the molecular mechanisms of Lhx8 regulation of mesenchyme differentiation and maturation, we compared the transcriptome between native and Lhx8 over-expressed dental pulp stem/progenitor cells (DPSCs) by RNA-seq analysis. A total of 828 genes changed significantly upon Lhx8 over-expression: 141 downregulated and 687 upregulated (Fig. 5A, Suppl. Table 2). DAVID functional annotation analysis further revealed that many of the significantly altered genes were those involved in cell adhesion, extracellular matrix synthesis primarily via several signal pathways including TGFβ, Wnt and Hedgehog (Fig. 5B). The majority of the Wnt receptors were upregulated in Lhx8 overexpressed DPSCs (Suppl. Fig. 3). All of these enriched functional categories are important in both odontoblast differentiation and epithelium-mesenchyme crosstalk, both of which are critical for tooth development. qPCR analysis of selected genes corresponding to these enriched categories further confirmed the RNA-seq data (Fig. 5C).
Fig. 5. Postnatal dental pulp stem/progenitor cells upon Lhx8 overexpression gain E13.5 gene signatures.
(A) RNA-seq analysis of control and Lhx8 overexpressed human dental pulp stem cells. Heatmap of the per-row normalized 828 differentially expressed genes. (B) The differentially expressed 828 genes were submitted to DAVID for functional gene annotation. Lhx8 changed genes are involved in multiple development and disease pathways. (C) qPCR confirmation of the selected differentially expressed genes from different pathways of interest. (D–F) The ratio of gene expression between Lhx8 overexpression and control group are ranked before running GESA analysis. The E11.5, E13.5 and E16 gene sets (which denote the highest expression among the groups) were extracted from previously published data. GSEA analysis reveals that E13.5 geneset is enriched in Lhx8 overexpression group (D), whereas E11.5 (F) and E16 (E) groups are not enriched. Data represent mean ± SD. * P<0.05.
Next we extracted gene expression data from dental E11.5, E12.5, E13.5 (GSE32321)[28], E16, E18 and P3 (GSE19488)[27] from Gene Datasets, given not only Lhx8’s robust expression from E11.5 to E14.5, but also diminished expression from E16 to P7. Genes with higher expression in E11.5 than E12.5 and E13.5 were denoted as E11.5 signature genesets (Suppl. Table 3). Similarly, genes with higher expression in E13.5 than E12.5 and E11.5 were denoted as E13.5 genesets (Suppl. Table 4); genes with higher expression in E16 than E18 and P3 were denoted as E16 genesets (Suppl. Table 5). GSEA analysis revealed enrichment of E13.5 signature genes in Lhx8 overexpressed DPSCs (Fig. 5D), but a lack of enrichment of E16 (Fig. 5E) or E11.5 (Fig. 5F) signatures, further suggesting Lhx8’s roles perhaps by restricting dental mesenchyme cells in a stage similar to E13.5, with functional significance that E13.5 dental mesenchyme is capable of inducing tooth morphogenesis.
To further unlock key pathways that interact with Lhx8, we searched its potential direct targets from the 828 significantly altered genes (Fig. 5A, Suppl. Table 2). Lhx8 acts by binding the consensus sequence TAATTA in the promoter region of target genes (Fig. 6A). We crossed the 811 genes harboring Lhx8 binding sites TAATTA (Gene Set: TAATTA_V$CHX10_01, Suppl. Table 6)[26] with the 828 genes that were altered by Lhx8 overexpression. VENN diagram revealed a total of 58 overlapping genes (Fig. 6B,C). DAVID functional annotation analysis of these 58 genes again showed their direct roles in cell adhesion, extracellular matrix, and multiple signal pathways, especially TGFβ (Fig. 6D). We performed ChIP analysis on 7 randomly selected genes from 58 overlapping genes, and discovered that Lhx8 directly bounds to 5 promoters of the 7 genes (Fig. 6E), confirming the accuracy of our bioinformatics analysis.
Fig. 6. Lhx8 transcriptionally activates Wnt and TGFβ pathways.
(A) Sequence logo of the Lhx8 binding sites from the Uniprobe database. (B) Venn diagram based on the 811 genes harboring Lhx8 binding site and 828 differentially expressed genes shows that there are 58 genes overlapped in the two group, suggesting the possibility of being Lhx8 direct targets in the context of dental mesenchyme. (C) Heatmap of the 58 genes with each row normalized. (D) DAVID annotation of the 58 genes reveals that they are involved in multiple signal pathways similar as the 828 genes. (E) ChIP PCR analysis of the selected genes, indicating potential Lhx8 binding targets. Data represent mean ± SD. * P<0.05.
3.4 Lhx8 regulates mesenchyme development by fine-tuning Wnt and TGFβ pathways
Per our RNAseq data, Lhx8 impacts on multiple genes that are pivotal for Wnt and TGFβ pathways. Western blot revealed that Lhx8 overexpression increased CTNNB1 but decreased Dspp (Fig. 7A). Lhx8 overexpression further increased Topflash reporter luciferase activity (Fig. 7B). Consistent with increased CTNNB1 and Topflash activity, immunofluorescence staining revealed that Lhx8 upregulated CTNNB1 expression and its nuclear translocation (Fig. 7D,E), suggesting that Lhx8 enhances Wnt activity. Additionally, Lhx8 overexpression increased luciferase activity of Smad binding element (SBE) reporter (Fig. 7C), suggesting Lhx8 activation of TGFβ pathway. Furthermore, Lhx8 increased COL1A1 (Fig. 7F) and OCN (Fig. 7H) expression, but decreased DSPP (Fig. 7G). DKK1, a Wnt inhibitor, reversed Lhx8 effects on COL1A1, OCN, and DSPP (Fig. 7I–K). SB431542, an inhibitor of TGFβ signaling, reversed Lhx8 induction of COL1A1 and OCN, and nearly abolished Lhx8 mediated attenuation of DSPP (Fig. 7I–K). Thus, Wnt and TGFβ activation appears to reverse Lhx8’s inhibitory role on dental mesenchyme differentiation.
Fig. 7. Functional outcome of Lhx8-mediated Wnt and TGFβ pathways.
(A) Western blot analysis of COL1A1, CTNNB1, DSPP, LHX8 and GAPDH in control and Lhx8 overexpressed dental pulp stem/progenitor cells (DPSCs) after odontogenic differentiation for 4 days. GAPDH served as a loading control and data are representative of triplicate experiments. (B) Topflash or Fopflash reporter was co-transfected with control or Lhx8 overexpression vector into DPSCs. Lhx8 overexpression significantly increased the ratio of Topflash/Fopflash, indicating an activation of Wnt (n=3). *p<0.05. (C) 4×SBE reporter was co-transfected with control or Lhx8 overexpression vector into DPSC cells. Lhx8 overexpression significantly increased the 4×SBE reporter, indicating activation of TGFβsignal pathway (n=3). *p<0.05. (D and E) Immunostaining of β-catenin in control and Lhx8 overexpressed DPSC cells, and Lhx8 induces significant nuclear translocation of β-catenin. (F–M) Control and Lhx8 overexpressed DPSCs cells cultured in odontogenic differentiation for 14 days, with additional DKK1 or SB431542 treatment. COL1A1 (F and I), DSPP (G and J) and OCN (H and K) expression were detected by qPCR, with 18S RNA as an internal control (n=3). * P30.05. (L) During the initiation stage of tooth development, when Wnt and TGFβ are both needed, high level Lhx8 specifies dental mesenchyme by activating these two pathways. However, odontoblast differentiation of later stages is inhibited by Wnt and TGFβ. The waning of Lhx8 at this stage decreases Wnt and TGFβ activation, guaranteeing tooth development. (M) Alteration of Lhx8 leads to aberration of Wnt and TGFβ pathways and subsequent tooth development defect. Scale:100μm. Data represent mean ± SD. * P<0.05.
4. Discussion
These data suggest Lhx8 functions in mediating mesenchyme differentiation and maturation via Wnt and TGFβ pathways. As schematically summarized (Fig. 7L,M), it is conceivable that robust Lhx8 expression at E14.5 activates Wnt and TGFβ pathways, and is required for restricting dental mesenchyme from premature differentiation into odontoblasts (Fig. 7L). From E17.5 onwards, vanishing Lhx8 decreases Wnt and TGFβ activation in exchange of odontoblastic differentiation and maturation (Fig. 7M).
Wnt signaling plays promotes early tooth development[29–31], whereas excessive Wnt attenuates late tooth development [32, 33]. Inactivation of TGFβ type I receptor (ALK5) in neural crest cells leads to delayed tooth initiation and development [34]. Transgenic mice overexpressing active TGFβ in odontoblasts show a significant reduction in the tooth mineralization and defective dentin formation [35]. Besides Wnt and TGFβ signaling, our data provide clues that Lhx8 may participate in other pathways in tooth development such as hedgehog, which is significantly altered by Lhx8 overexpression and serves as one of the pivotal signaling pathways in tooth development[36].
Lhx8 overexpression in postnatal dental pulp stem/progenitor cells decreased 141 genes and increased 687 genes. Broadly regarded as transcriptional repressor [37–39], Lhx8 also transcriptionally activates target genes such as Shh [40]. Either transactivation or trans-repression is perhaps attributable to the co-factors interacting with Lhx8 on specific targets. Crossing co-factors for different Lhx8 targets, including known factors important for tooth development, may help reveal additional insight of Lhx8 mediated effects on dental mesenchyme.
Our study has several limitations. First, our ectopic transplantation model with Lhx8 over-expressed dental pulp stem/progenitor cells is perhaps not nearly as instructive for tooth regeneration as an orthotopic model with native dentin as a substrate. Second, adult dental pulp stem/progenitor cells are highly heterogeneous [44] and even with Lhx8 overexpression likely do not overwhelmingly transform into odontoblasts with the capacity as E14.5 dental mesenchyme cells for dentinogenesis. Third, Lhx8 overexpressed dental pulp stem/progenitor cells are in an artificial state and their RNASeq comparison with cells of the same population should be considered accordingly.
These data uncover a dynamic pattern of Lhx8 expression during embryonic and postnatal tooth development, and this dynamic expression is crucial for Lhx8 functions in mesenchyme development. Decoding the molecular network of Lhx8 appears to be crucial for our understanding of not only tooth development, but also tooth regeneration. E14.5 dental mesenchyme cells have the ability to induce tooth morphogenesis, when combined with non-dental epithelium[19, 41–44]. Lhx8 appears to maintain dental mesenchymal cells in an undifferentiated stage via Wnt and TGFβ pathways, and thus may be crucial in retaining the induction capacity of dental mesenchyme. Vanishing Lhx8 lifts the control on Wnt, TGFβ and other signaling pathways such as Shh, and promotes odontoblast differentiation and dentin formation. Our ongoing studies using orthotopic models and Lhx8 inducible expression systems may unravel perhaps stage-specific roles of Lhx8 in tooth development and regeneration.
Supplementary Material
Supplementary Figure 1 Effects of Lhx8 on cell proliferation, migration and mineralization
(A, B) DPSC cells were infected with control (A) and Lhx8 (B) overexpression lentivirus. Cells were then sorted based on the GFP expression intensity.
(C) Sorted control and Lhx8 overexpression cells were cultured in growth medium for indicated time and cell numbers were measured by CCK8. Lhx8 slightly increases cell proliferation (n=3). (D–G) Lhx8 overexpression (E) and knockdown cells (G), together with corresponding control cells (D and F), were subjected for transwell assay for measurement of migration ability. Overexpression of Lhx8 slightly increases migration, while knockdown decreases the migration ability. (H–K) Lhx8 overexpression (I) and knockdown cells (K), together with corresponding control cells (H and J), were cultured in odontogenic medium for 7 days and ALP activity was measured by ALP staining (n=3). Overexpression of Lhx8 significantly reduces ALP activity, while knockdown increases the activity. (L–O) Microscopic views of the above groups. Lhx8 overexpression (M) and knockdown cells (O), together with corresponding control cells (L and N). (P–S) Lhx8 overexpression (Q) and knockdown cells (S), together with corresponding control cells (P and R), were cultured in odontogenic medium for 14 days and mineralization was measured by Alizarin staining (n=3). Overexpression of Lhx8 significantly reduces Alizarin red staining, while knockdown increases Alizarin Red staining. Scale bars: 200μm. Data represent mean ± SD.
Supplementary Figure 2 Recombination and organ culture of E16.5 mouse tooth germs.
(A–C) E16.5 mandibular first molar tooth germs were isolated and digested to separate dental epithelium and dental mesenchyme, which were subsequently cultured to day 1(A), day 5(B) and day 7(C). (D) Polarization of dental epithelial and mesenchymal cells was observed as well as dentin formation by HE staitnig. od, odontoblast; am, ameloblast; d, dentin. Scale bars: 1mm(A–C), 500 μm (D).
Supplementary Figure 3 Lhx8 increases the expression of multiple Wnt receptors
Heatmap of selected Wnt receptors shows increase expression of most of the Wnt receptors, indicating an activation of Wnt by Lhx8.
Supplementary Table 1 Sequences used in this study
Supplementary Table 2 The 828 differentially expressed genes induced by Lhx8 overexpression
*FPKM means Fragments Per Kilobase of exon per Million fragments mapped, and defines the relative expression of each gene.
Supplementary Table 3 The E11.5 gene set
Supplementary Table 4 The E13.5 gene set
Supplementary Table 5 The E16 gene set
Supplementary Table 6 The Lhx8 binding site containing gene set
Acknowledgments
We thank R. Birdie, Q. Guo and J. Melendez for administrative and technical assistance. Mouse Lhx8 fragment plasmid (nt 1022–1185 of the mouse cDNA) was a gift from Drs. M. Grigoriou and V. Pachnis. Some of the experimental materials were supported by Guangdong Pioneer Grant (52000-3210002), and National Natural Science Foundation of China (81170932) to J.Q.L. M. Chen is supported by NIH/K12DE023583. The work was supported by NIH grants R01AR065023 and R01DE023112 to J.J. Mao.
Footnotes
Conflict of interest statement
J.J. Mao, C. Zhou, G. Yang and M. Chen hold a patent on the use of Lhx6/8 genes and gene products in tissue regeneration.
Author Contribution
C.Z. and G.Y. designed the experiments and were responsible for the technical design and primary technical undertaking, and conducted the experiments, collected and analyzed data, and drafted the manuscript. M.C. generated initial data of robust Lhx8 expression in odontoblast in a separate experiment, participated in the technical design and provided critical analysis of data. C.W., L.H., L.X. and D.C. assisted in various molecular assays and in vivo experiments. J.Q.L. co-mentored C.Z. and assisted in data analysis. J.J.M. conceived and designed the experiments, and oversaw the collection of results, data interpretation and wrote the manuscript. All authors read and approved the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grigoriou M, Tucker AS, Sharpe PT, Pachnis V. Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development. 1998;125:2063–74. doi: 10.1242/dev.125.11.2063. [DOI] [PubMed] [Google Scholar]
- 2.Manabe T, Tatsumi K, Inoue M, Matsuyoshi H, Makinodan M, Yokoyama S, et al. L3/Lhx8 is involved in the determination of cholinergic or GABAergic cell fate. Journal of neurochemistry. 2005;94:723–30. doi: 10.1111/j.1471-4159.2005.03261.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y, Marin O, Hermesz E, Powell A, Flames N, Palkovits M, et al. The LIM-homeobox gene Lhx8 is required for the development of many cholinergic neurons in the mouse forebrain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:9005–10. doi: 10.1073/pnas.1537759100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elshatory Y, Gan L. The LIM-homeobox gene Islet-1 is required for the development of restricted forebrain cholinergic neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:3291–7. doi: 10.1523/JNEUROSCI.5730-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachy I, Retaux S. GABAergic specification in the basal forebrain is controlled by the LIM-hd factor Lhx7. Developmental biology. 2006;291:218–26. doi: 10.1016/j.ydbio.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Fogarty M, Grist M, Gelman D, Marin O, Pachnis V, Kessaris N. Spatial genetic patterning of the embryonic neuroepithelium generates GABAergic interneuron diversity in the adult cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:10935–46. doi: 10.1523/JNEUROSCI.1629-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–9. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 8.Imai H, Osumi-Yamashita N, Ninomiya Y, Eto K. Contribution of early-emigrating midbrain crest cells to the dental mesenchyme of mandibular molar teeth in rat embryos. Developmental biology. 1996;176:151–65. doi: 10.1006/dbio.1996.9985. [DOI] [PubMed] [Google Scholar]
- 9.Lumsden AG. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development. 1988;103(Suppl):155–69. doi: 10.1242/dev.103.Supplement.155. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto K, Tanaka T, Furuyama T, Kashihara Y, Mori T, Ishii N, et al. L3, a novel murine LIM-homeodomain transcription factor expressed in the ventral telencephalon and the mesenchyme surrounding the oral cavity. Neuroscience letters. 1996;204:113–6. doi: 10.1016/0304-3940(96)12341-7. [DOI] [PubMed] [Google Scholar]
- 11.Denaxa M, Sharpe PT, Pachnis V. The LIM homeodomain transcription factors Lhx6 and Lhx7 are key regulators of mammalian dentition. Developmental biology. 2009;333:324–36. doi: 10.1016/j.ydbio.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shibaguchi T, Kato J, Abe M, Tamamura Y, Tabata MJ, Liu JG, et al. Expression and role of Lhx8 in murine tooth development. Archives of histology and cytology. 2003;66:95–108. doi: 10.1679/aohc.66.95. [DOI] [PubMed] [Google Scholar]
- 13.Fragkouli A, Hearn C, Errington M, Cooke S, Grigoriou M, Bliss T, et al. Loss of forebrain cholinergic neurons and impairment in spatial learning and memory in LHX7-deficient mice. The European journal of neuroscience. 2005;21:2923–38. doi: 10.1111/j.1460-9568.2005.04141.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Guo YJ, Tomac AC, Taylor NR, Grinberg A, Lee EJ, et al. Isolated cleft palate in mice with a targeted mutation of the LIM homeobox gene lhx8. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:15002–6. doi: 10.1073/pnas.96.26.15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stylianopoulou E, Lykidis D, Ypsilantis P, Simopoulos C, Skavdis G, Grigoriou M. A rapid and highly sensitive method of non radioactive colorimetric in situ hybridization for the detection of mRNA on tissue sections. PloS one. 2012;7:e33898. doi: 10.1371/journal.pone.0033898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Komatsu Y, Kishigami S, Mishina Y. In situ hybridization methods for mouse whole mounts and tissue sections with and without additional beta-galactosidase staining. Methods in molecular biology. 2014;1092:1–15. doi: 10.1007/978-1-60327-292-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piette D, Hendrickx M, Willems E, Kemp CR, Leyns L. An optimized procedure for whole-mount in situ hybridization on mouse embryos and embryoid bodies. Nature protocols. 2008;3:1194–201. doi: 10.1038/nprot.2008.103. [DOI] [PubMed] [Google Scholar]
- 18.Oshima M, Ogawa M, Yasukawa M, Tsuji T. Generation of a bioengineered tooth by using a three-dimensional cell manipulation method (organ germ method) Methods in molecular biology. 2012;887:149–65. doi: 10.1007/978-1-61779-860-3_14. [DOI] [PubMed] [Google Scholar]
- 19.Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, et al. The development of a bioengineered organ germ method. Nature methods. 2007;4:227–30. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 20.Song Y, Zhang Z, Yu X, Yan M, Zhang X, Gu S, et al. Application of lentivirus-mediated RNAi in studying gene function in mammalian tooth development. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:1334–44. doi: 10.1002/dvdy.20706. [DOI] [PubMed] [Google Scholar]
- 21.Narhi K, Thesleff I. Explant culture of embryonic craniofacial tissues: analyzing effects of signaling molecules on gene expression. Methods in molecular biology. 2010;666:253–67. doi: 10.1007/978-1-60761-820-1_16. [DOI] [PubMed] [Google Scholar]
- 22.Arakaki M, Ishikawa M, Nakamura T, Iwamoto T, Yamada A, Fukumoto E, et al. Role of epithelial-stem cell interactions during dental cell differentiation. The Journal of biological chemistry. 2012;287:10590–601. doi: 10.1074/jbc.M111.285874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raha D, Hong M, Snyder M. ChIP-Seq: a method for global identification of regulatory elements in the genome. In: Ausubel Frederick M, et al., editors. Current protocols in molecular biology. Unit 21. Chapter 21. 2010. pp. 19pp. 1–4. [DOI] [PubMed] [Google Scholar]
- 24.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.Berger MF, Badis G, Gehrke AR, Talukder S, Philippakis AA, Pena-Castillo L, et al. Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell. 2008;133:1266–76. doi: 10.1016/j.cell.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–50. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki H, Muramatsu T, Kwon HJ, Yamamoto H, Hashimoto S, Jung HS, et al. Down-regulated genes in mouse dental papillae and pulp. Journal of dental research. 2010;89:679–83. doi: 10.1177/0022034510366844. [DOI] [PubMed] [Google Scholar]
- 28.O’Connell DJ, Ho JW, Mammoto T, Turbe-Doan A, O’Connell JT, Haseley PS, et al. A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Science signaling. 2012;5:ra4. doi: 10.1126/scisignal.2002414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan G, Yang G, Zheng Y, Zhu X, Chen Z, Zhang Z, et al. The non-canonical BMP and Wnt/beta-catenin signaling pathways orchestrate early tooth development. Development. 2015;142:128–39. doi: 10.1242/dev.117887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar L, Sharpe PT. Inhibition of Wnt signaling by exogenous Mfrzb1 protein affects molar tooth size. Journal of dental research. 2000;79:920–5. doi: 10.1177/00220345000790040601. [DOI] [PubMed] [Google Scholar]
- 31.Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1(−/−) mice. Genes & development. 2002;16:3173–85. doi: 10.1101/gad.1035602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bae CH, Lee JY, Kim TH, Baek JA, Lee JC, Yang X, et al. Excessive Wnt/beta-catenin signaling disturbs tooth-root formation. Journal of periodontal research. 2013;48:405–10. doi: 10.1111/jre.12018. [DOI] [PubMed] [Google Scholar]
- 33.Scheller EL, Chang J, Wang CY. Wnt/beta-catenin inhibits dental pulp stem cell differentiation. Journal of dental research. 2008;87:126–30. doi: 10.1177/154405910808700206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao H, Oka K, Bringas P, Kaartinen V, Chai Y. TGF-beta type I receptor Alk5 regulates tooth initiation and mandible patterning in a type II receptor-independent manner. Developmental biology. 2008;320:19–29. doi: 10.1016/j.ydbio.2008.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thyagarajan T, Sreenath T, Cho A, Wright JT, Kulkarni AB. Reduced expression of dentin sialophosphoprotein is associated with dysplastic dentin in mice overexpressing transforming growth factor-beta 1 in teeth. The Journal of biological chemistry. 2001;276:11016–20. doi: 10.1074/jbc.M010502200. [DOI] [PubMed] [Google Scholar]
- 36.Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998;125:2803–11. doi: 10.1242/dev.125.15.2803. [DOI] [PubMed] [Google Scholar]
- 37.Hobert O, Westphal H. Functions of LIM-homeobox genes. Trends in genetics : TIG. 2000;16:75–83. doi: 10.1016/s0168-9525(99)01883-1. [DOI] [PubMed] [Google Scholar]
- 38.Bach I, Rodriguez-Esteban C, Carriere C, Bhushan A, Krones A, Rose DW, et al. RLIM inhibits functional activity of LIM homeodomain transcription factors via recruitment of the histone deacetylase complex. Nature genetics. 1999;22:394–9. doi: 10.1038/11970. [DOI] [PubMed] [Google Scholar]
- 39.Weimann M, Grossmann A, Woodsmith J, Ozkan Z, Birth P, Meierhofer D, et al. A Y2H-seq approach defines the human protein methyltransferase interactome. Nature methods. 2013;10:339–42. doi: 10.1038/nmeth.2397. [DOI] [PubMed] [Google Scholar]
- 40.Flandin P, Zhao Y, Vogt D, Jeong J, Long J, Potter G, et al. Lhx6 and Lhx8 coordinately induce neuronal expression of Shh that controls the generation of interneuron progenitors. Neuron. 2011;70:939–50. doi: 10.1016/j.neuron.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang N, Zhou J, Chen M, Schiff MD, Lee CH, Kong K, et al. Postnatal epithelium and mesenchyme stem/progenitor cells in bioengineered amelogenesis and dentinogenesis. Biomaterials. 2014;35:2172–80. doi: 10.1016/j.biomaterials.2013.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kollar EJ, Fisher C. Tooth induction in chick epithelium: expression of quiescent genes for enamel synthesis. Science. 1980;207:993–5. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- 43.Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. Journal of dental research. 2004;83:518–22. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- 44.Mao JJ, Prockop DJ. Stem cells in the face: tooth regeneration and beyond. Cell Stem Cell. 2012 Sep 7;11(3):291–301. doi: 10.1016/j.stem.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou C, Yang GD, Chen M, He L, Xiang L, Ricupero CL, Mao JJ, Ling J. Lhx6 and Lhx8: Cell Fate Regulators and Beyond. FASEB J. doi: 10.1096/fj.14-267500. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Effects of Lhx8 on cell proliferation, migration and mineralization
(A, B) DPSC cells were infected with control (A) and Lhx8 (B) overexpression lentivirus. Cells were then sorted based on the GFP expression intensity.
(C) Sorted control and Lhx8 overexpression cells were cultured in growth medium for indicated time and cell numbers were measured by CCK8. Lhx8 slightly increases cell proliferation (n=3). (D–G) Lhx8 overexpression (E) and knockdown cells (G), together with corresponding control cells (D and F), were subjected for transwell assay for measurement of migration ability. Overexpression of Lhx8 slightly increases migration, while knockdown decreases the migration ability. (H–K) Lhx8 overexpression (I) and knockdown cells (K), together with corresponding control cells (H and J), were cultured in odontogenic medium for 7 days and ALP activity was measured by ALP staining (n=3). Overexpression of Lhx8 significantly reduces ALP activity, while knockdown increases the activity. (L–O) Microscopic views of the above groups. Lhx8 overexpression (M) and knockdown cells (O), together with corresponding control cells (L and N). (P–S) Lhx8 overexpression (Q) and knockdown cells (S), together with corresponding control cells (P and R), were cultured in odontogenic medium for 14 days and mineralization was measured by Alizarin staining (n=3). Overexpression of Lhx8 significantly reduces Alizarin red staining, while knockdown increases Alizarin Red staining. Scale bars: 200μm. Data represent mean ± SD.
Supplementary Figure 2 Recombination and organ culture of E16.5 mouse tooth germs.
(A–C) E16.5 mandibular first molar tooth germs were isolated and digested to separate dental epithelium and dental mesenchyme, which were subsequently cultured to day 1(A), day 5(B) and day 7(C). (D) Polarization of dental epithelial and mesenchymal cells was observed as well as dentin formation by HE staitnig. od, odontoblast; am, ameloblast; d, dentin. Scale bars: 1mm(A–C), 500 μm (D).
Supplementary Figure 3 Lhx8 increases the expression of multiple Wnt receptors
Heatmap of selected Wnt receptors shows increase expression of most of the Wnt receptors, indicating an activation of Wnt by Lhx8.
Supplementary Table 1 Sequences used in this study
Supplementary Table 2 The 828 differentially expressed genes induced by Lhx8 overexpression
*FPKM means Fragments Per Kilobase of exon per Million fragments mapped, and defines the relative expression of each gene.
Supplementary Table 3 The E11.5 gene set
Supplementary Table 4 The E13.5 gene set
Supplementary Table 5 The E16 gene set
Supplementary Table 6 The Lhx8 binding site containing gene set