Drought inhibits cell division and expansion in the maize leaf growth zone by reducing antioxidant levels and increases photosynthetic capacity to allow for enhanced growth upon recovery.
Abstract
Drought is the most important crop yield-limiting factor, and detailed knowledge of its impact on plant growth regulation is crucial. The maize (Zea mays) leaf growth zone offers unique possibilities for studying the spatiotemporal regulation of developmental processes by transcriptional analyses and methods that require more material, such as metabolite and enzyme activity measurements. By means of a kinematic analysis, we show that drought inhibits maize leaf growth by inhibiting cell division in the meristem and cell expansion in the elongation zone. Through a microarray study, we observed the down-regulation of 32 of the 54 cell cycle genes, providing a basis for the inhibited cell division. We also found evidence for an up-regulation of the photosynthetic machinery and the antioxidant and redox systems. This was confirmed by increased chlorophyll content in mature cells and increased activity of antioxidant enzymes and metabolite levels across the growth zone, respectively. We demonstrate the functional significance of the identified transcriptional reprogramming by showing that increasing the antioxidant capacity in the proliferation zone, by overexpression of the Arabidopsis (Arabidopsis thaliana) iron-superoxide dismutase gene, increases leaf growth rate by stimulating cell division. We also show that the increased photosynthetic capacity leads to enhanced photosynthesis upon rewatering, facilitating the often-observed growth compensation.
Drought imposes a major limitation on crop productivity (Boyer, 1982). Currently, no less than 75% of the world’s freshwater supplies are utilized in agriculture, and it is more than likely that the expanding world population and unfavorable climate conditions will decrease its availability in the near future (Wallace, 2000). For example, climate change trends toward increasing drought are predicted to reduce U.S. maize (Zea mays) yields between 15% and 30% (Lobell et al., 2014). Therefore, increasing crop productivity under conditions of limiting water availability is of major importance. To achieve this, a systems-level understanding of how plant growth adapts to drought is a scientific requirement.
The inhibition of leaf growth is one of the earliest responses to limited water availability, leading to the reduction of transpiration and water conservation. This response can cost as much as 60% of the potential yield of a maize crop even in the absence of visual wilting symptoms (Ribaut et al., 1997).
At the cellular level, division and expansion in a plant’s growth zones determine organ- and plant-level growth responses to drought. The developing maize leaf provides an ideal model system in which to investigate such a growth zone at various organizational levels. Already in the 1980s and early 1990s, this system was used to study organ growth by kinematic analysis (Silk and Erickson, 1979). In recent years, it is increasingly being used for studies into the regulation of cell division and expansion (Rymen et al., 2007) and the environmental effects on these processes (Walter et al., 2009), redox regulation (Kravchik and Bernstein, 2013), hormone homeostasis (Nelissen et al., 2012), protein expression and phosphorylation (Riccardi et al., 1998; Bonhomme et al., 2012), and the development of the C4 photosynthetic system (Li et al., 2010; Majeran et al., 2010). Several of these studies are currently impossible in the model plant Arabidopsis (Arabidopsis thaliana) due to the small size of its meristematic and elongation zones. The steady-state growth after leaf emergence and the relatively large size of the leaf growth zone (approximately 1–2 cm for the meristem and approximately 4–6 cm for the elongation zone) are important advantages, allowing sampling for molecular and physiological analyses with subzonal resolution.
Next to a reduction of leaf area, plants adapt to drought by avoiding dehydration, due to the activation of mechanisms such as stomatal closure and the accumulation of osmolytes (Pro and soluble sugars) and by increasing drought tolerance through the induction of protective mechanisms against cell damage, such as the synthesis of dehydrins and late-embryogenesis-abundant proteins (Verslues et al., 2006). Drought stress also leads to the accumulation of reactive oxygen species (ROS), inducing cells to generate antioxidants and activate redox-regulating enzymes (Cruz de Carvalho, 2008). Although these responses have been studied extensively in mature tissues, little is known about their regulation in the growth zone and their interaction with growth processes (Considine and Foyer, 2014). Nevertheless, gene expression varies strongly between dividing, expanding, and mature cells (Beemster et al., 2005), and the impact of osmotic and salt stress on each of these processes is distinctly different (Skirycz and Inzé, 2010), urging more development-specific studies of the impact of drought.
Here, we use the growth zone of the maize leaf to investigate the effect of relatively mild drought that inhibits growth in the absence of other visual signs of stress and a more severe drought that leads to leaf rolling but still allows for continued growth.
RESULTS
Leaf Growth
We studied the effect of drought on the leaf growth of maize line B73. Control pots were watered daily to maintain a soil water content (SWC) of 54% throughout the experiments. For drought treatments, the soil was not watered until it reached 43% (mild stress, no wilting) and 34% SWC (severe stress, leaves wilting during the day; Supplemental Fig. S1), after which it was maintained at that level. We studied the fifth leaf, because it is the first to initiate and develop fully under stress conditions.
The treatments reduced final leaf length by 17% and 40%. This was associated with an even stronger inhibition of leaf elongation rate (LER) by 27% and 63% in mild and severe stress conditions, respectively (Table I), which was partly offset by an increased duration of the leaf growth period (data not shown). Kinematic analysis, based on measurements of LER, the cell length profile along the growth zone (Supplemental Fig. S2), and the length of the meristem determined by locating mitotic cells, showed that the decrease in LER is primarily due to a strongly reduced cell production in the meristem, whereas mature cell length showed only a small reduction that was not statistically significant (Table I). The decrease in cell production in the meristem, in turn, was caused by a reduction in division rate (and thus a prolonged cell cycle) and a smaller number of dividing cells (Table I), due to a smaller division zone (Supplemental Fig. S2). Severe drought also inhibited cell expansion rates by 39%, but a tripling of the time in the elongation zone compensated for this, so that mature cell length was not affected (Table I).
Table I. Kinematic analysis of the effect of drought on cell division and expansion in the growing maize leaf.
Results are averages of five independent experiments ± se. Statistical significance is based on Student’s t test, and P values > 0.05 are marked as NS (not significant). Parameters are as follows: leaf length (LL; measured on the fifth leaf at the time of harvesting; Supplemental Fig. S1), LER (calculated during the first 3 d after leaf appearance), mature cell length (Lmat), cell production rate (P), cell division rate (D), cell cycle duration (Tc), length of the meristem (Lmer), number of cells in the meristem (Nmer), time in the division zone (Tdiv), cell elongation rate (Rel), and time in the elongation zone (Tel).
| Parameter | Control | Mild Stress | Severe Stress | Percentage Change in Mild/Severe Stress |
|---|---|---|---|---|
| LL (mm) | 727 ± 15 | 603 ± 16 | 436 ± 23 | −17/−40 |
| LER (mm h−1) | 3.0 ± 0.1 | 2.2 ± 0.1 | 1.1 ± 0.2 | −27/−63 |
| Lmat (µm) | 134 ± 6 | 126 ± 7 | 117 ± 8 | NS/NS |
| P (cells h−1) | 22 ± 2 | 17 ± 1 | 9 ± 1 | −24/−58 |
| D (cell cell−1 h−1) | 0.029 ± 0.004 | 0.026 ± 0.003 | 0.016 ± 0.002 | NS/−44 |
| Tc (h) | 26 ± 2 | 28 ± 3 | 48 ± 7 | NS/+84 |
| Lmer (mm) | 13 ± 1 | 10 ± 1 | 10 ± 1 | NS/−26 |
| Nmer | 867 ± 58 | 685 ± 41 | 591 ± 17 | −21/−32 |
| Tdiv (h) | 253 ± 25 | 267 ± 26 | 437 ± 67 | NS/+73 |
| Rel (µm µm−1 h−1) | 0.040 ± 0.002 | 0.032 ± 0.003 | 0.024 ± 0.006 | NS/−39 |
| Tel (h) | 44 ± 3 | 60 ± 8 | 131 ± 30 | NS/+195 |
Microarray Analysis
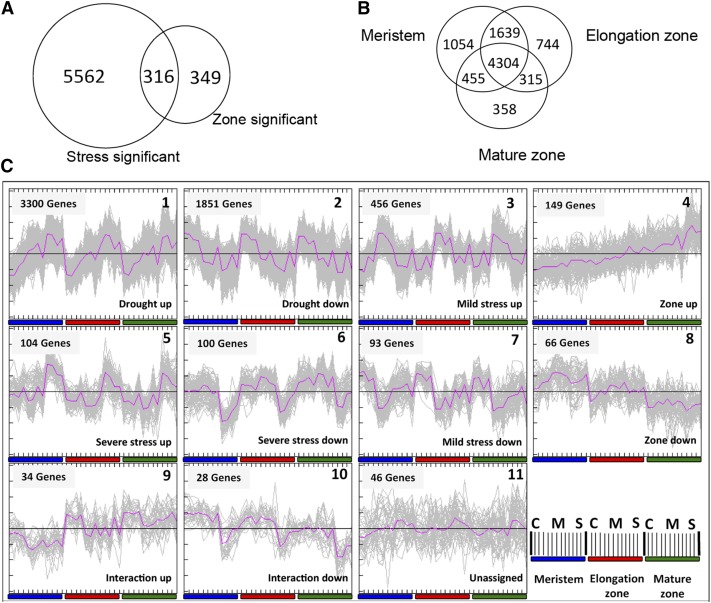
The cell length profile provides a map of the growth zone under each experimental condition, allowing sampling of equivalent meristem, elongation, and mature zones, although their spatial localization has shifted (Supplemental Fig. S2). We used two-color Agilent maize chips (Ma et al., 2008) in a hybridization design, involving three separate loops for the zones, in each of which the three treatments were contrasted (Supplemental Fig. S3). Out of 44,000 probes on the array, 16,850 transcripts were above background levels (defined as foreground [FG] > background [BG] + 2 sd) in at least one sample. The levels of 5,878 transcripts were significantly affected by drought (Bonferroni-corrected P < 0.05 and logarithm 2 Fold Change [log2FC] > 0.75), 665 transcripts levels varied between the zones (false discovery rate [FDR] < 0.05 and log2FC > 0.75), and 316 transcripts showed both responses (Fig. 1A). The relatively low number of transcripts showing significantly different levels between the three zones, compared with treatments (despite using a less stringent multiple testing correction), was probably due to the hybridization design: in contrast to the treatments within each segment, there were no direct comparisons between different zones in the design (Supplemental Fig. S3), limiting the statistical power in their comparison compared with that of the treatment effects. When comparing the effect of stress along the leaf axis, the majority of differentially affected transcripts (4,304) were common for the three zones (Fig. 1B). Only 358 genes were significantly affected in the mature tissues, compared with 744 in the elongation zone and 1,054 in the meristem, indicating that the strongest transcriptional responses occur in the growth zone and particularly in the meristem (Fig. 1B). Quality threshold clustering (Heyer et al., 1999) of the 6,227 differentially expressed transcripts (Fig. 1A) resulted in 10 clusters of transcript profile patterns (Fig. 1C; expression values of all genes and their associated cluster are provided in Supplemental Text S1). To identify the major processes represented by the transcription profiles in the different clusters, we performed a gene-enrichment analysis using PageMan (Usadel et al., 2006). The two largest clusters contained 3,300 and 1,851 genes with increasing and decreasing transcript levels in proportion to the severity of the stress conditions, respectively (Fig. 1C). It was striking that their opposite expression patterns translated into opposite enrichment and depletion of functional categories (Supplemental Fig. S4). The most prominent (z > 1.96) transcriptional shifts induced by the drought included the overrepresentation of transcripts related to photosynthesis/light reactions, cellulose synthesis, redox (ascorbate, gluthatione, dismutases, and catalases), oxidases, and the secondary metabolites isoprenoids and flavonoids among up-regulated transcripts. Inversely, there was an overrepresentation of lipid metabolism, fermentation, cell wall, amino acid metabolism, RNA regulation, DNA synthesis and repair, protein synthesis, signaling, and cell division and cell cycle among the down-regulated transcripts (Supplemental Fig. S4). The expression patterns indicated that these processes were affected proportionally to the level of stress, starting in mild stress, even before visible signs of wilting occur.
Figure 1.
Gene expression analysis in the growth zone in response to drought. A, Overview of the 6,227 significant (two-way ANOVA with Bonferroni correction for the stress and an FDR correction for the zone effect; P < 0.05 and log2FC > 0.75) gene transcripts on the microarray. B, Overview of the transcripts changed significantly in response to drought stress in each developmental zone (meristem, elongation, and mature) along the leaf axis (three independent one-way ANOVAs with FDR correction; P < 0.05 and log2FC > 0.75). C, Clustering of gene expression profiles by quality threshold clustering analysis (Heyer et al., 1999; Pearson correlation measure; cluster diameter = 0.5 and minimum cluster population = 20) of the expression profiles of 6,227 significantly modulated genes (P < 0.05 and log2FC > 0.75). The abscissa, which is enlarged for cluster 8, denotes three stress treatments (C = control, M = mild, and S = severe stress) for each zone (meristem, elongation, and mature) and four biological replicates (each one a pool of four plants) for each zone/treatment combination. The ordinate indicates normalized and median-centered expression levels. The colored bars show the corresponding growth phases based on the kinematic analysis (Supplemental Fig. S2).
In contrast, there were 545 (cluster 3) and 93 (cluster 7) transcripts whose expression was specifically up- or down-regulated under mild stress conditions (Fig. 1C). These represented a different set of functional classes. Minor carbohydrate metabolism, ATP synthesis, and ethylene-related transcripts were overrepresented among down-regulated transcripts (Supplemental Fig. S4).
Another 104 (cluster 5) and 100 (cluster 6) transcripts were specifically up- or down-regulated in response to severe stress (Fig. 1C). These were specifically enriched in RNA processing and binding and protein amino acid activation among the up-regulated transcripts and glycolysis, brassinosteroid metabolism, and abiotic stress/heat among the down-regulated transcripts (Supplemental Fig. S4). Taken together, these results show that the largest clusters represent pathways that respond proportionally to stress levels, while others are specifically affected by mild and severe stress.
Clusters 4 and 8 contained 149 and 66 transcripts that were gradually increasing and decreasing across the zones with the highest levels in the mature zone and in the meristem, respectively (Fig. 1C). We found a strong enrichment of major carbohydrate metabolism/Suc degradation, protein synthesis, and posttranscriptional modifications among the transcripts with the highest expression in proliferating cells, whereas amino acid metabolism, ethylene metabolism, drought/salt stress, nucleotide metabolism, RNA processing, protein targeting, and development were overrepresented among transcripts that were up-regulated in elongating and mature cells (Supplemental Fig. S4).
Finally, the smallest clusters, nine and 10 (34 and 28 transcripts), represented profiles where the effects of the developmental zone and stress were superimposed (Fig. 1C). These were enriched in lipid metabolism, protein degradation, and mitogen-activated protein kinase signaling (Supplemental Fig. S4).
In summary, this analysis shows that drought and developmental stage induce severe reprogramming of the maize leaf transcriptome. The largest number of transcripts in our data set are affected in all zones in proportion to drought levels, with smaller sets of transcripts responding specifically to mild or more severe drought and to developmental differences between the zones.
To understand the significance of these changes, we investigated in more detail the most prominent changes in the largest clusters: the down-regulation of DNA duplication and cell cycle gene expression, the up-regulation of the photosynthetic machinery for the light reactions, and changes in redox regulation.
Cell Cycle Regulation
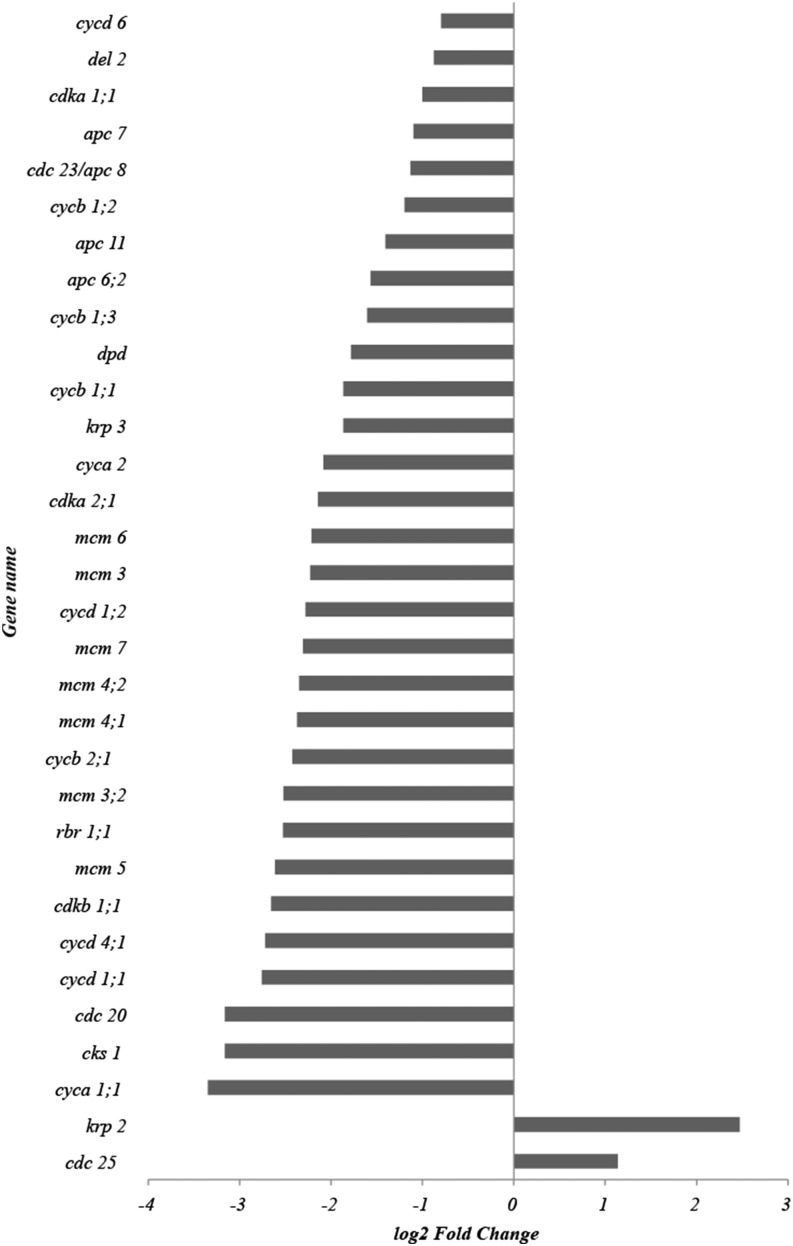
Our kinematic analysis shows that inhibited cell division is the main contributor to the reduced leaf growth in response to drought (Table I). The cell division cycle is transcriptionally regulated (Menges et al., 2005; De Veylder et al., 2007). Therefore, we investigated in detail the expression patterns of the maize cell cycle regulators, cyclin-dependent kinases (CDKs), cyclins, and their interacting proteins, identified earlier (Rymen et al., 2007; Supplemental Table S1). Of the 57 cell cycle-related genes on the array, the transcripts of 44 were detected above background levels (FG > BG + 2 sd), and 34 of these were significantly affected by drought (P < 0.05 and log2FC > 0.75), all but two of which were 2-fold or more down-regulated (Fig. 2). The only two up-regulated transcripts were a putative homolog of CELL DIVISION CYCLE25, the function of which in plants is disputed (Boudolf et al., 2006), and a homolog of the inhibitor KIP-RELATED PROTEIN (KRP2) in Arabidopsis (De Veylder et al., 2001). The latter change appears to be offset by an opposite effect on the expression of another member of the KRP family, krp3. Most striking, however, was the suppression of four cyclin-dependent kinases (cdka1;1, cdka2;1, cdkb1;1, and cdkb2;1) and 10 of their activating cyclins as well as retinoblastoma-related1;1, which acts as a master switch controlling E2 transcription factor (E2F) transcriptional activation downstream of CDKA for S-phase entry (Sabelli et al., 2013). Related to that, we found seven minichromosome maintenance transcripts that control DNA duplication (Chong et al., 1996; Aparicio et al., 1997), which are known targets of E2F transcription factors (Vandepoele et al., 2005), and four transcripts of genes encoding subunits of the anaphase-promoting complex (APC) that controls the cell cycle at M-phase exit (Eloy et al., 2006). These changes indicate that the reduced cell division activity is a consequence of transcriptional down-regulation of all stages of the cell cycle.
Figure 2.
Effect of drought stress on cell cycle gene expression in the meristem. Presented are fold change values of the 34 of 57 cell cycle genes present on the array (for the full list of cell cycle genes, see Supplemental Table S2) that have significantly affected expression levels (log2FC > 0.75 and P < 0.05, two-way ANOVA with Bonferroni correction) under drought conditions.
Photosynthesis
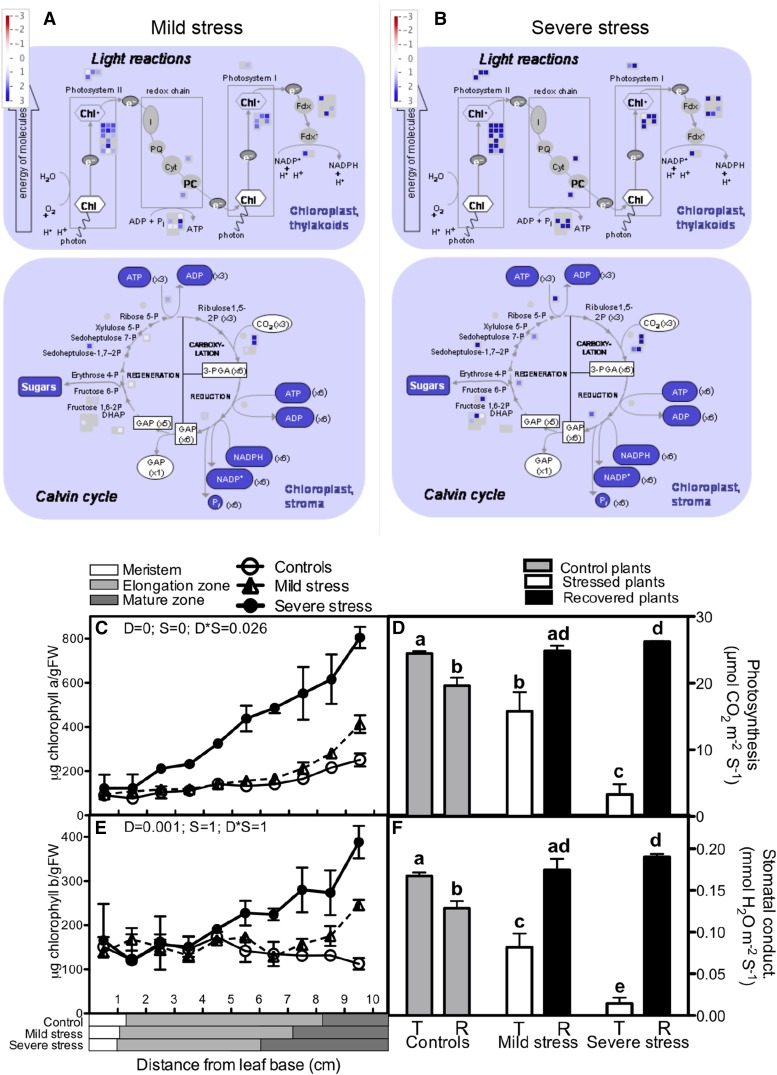
Transcripts encoding proteins for PSI and PSII and light reactions were most enriched among up-regulated transcripts (and underrepresented in down-regulated transcripts; Supplemental Fig. S4). This suggests a transcriptional up-regulation of the photosynthetic machinery along the whole-leaf growth zone under drought conditions. This is unexpected, given the growth inhibition and other studies showing down-regulation of the photosynthetic activity (Dwyer et al., 1992; Ashghizadeh and Ehsanzadeh, 2008) and inhibited expression of photosynthesis genes under drought stress (Hayano-Kanashiro et al., 2009).
To validate and understand the up-regulation of the photosynthetic apparatus under mild and severe drought stress (Fig. 3, A and B), we measured biochemical and physiological photosynthetic parameters. Consistent with the increased mRNA levels of photosynthesis-related transcripts (Fig. 3, A and B) and transcripts encoding enzymes involved in the synthesis of tetrapyrrole, which forms the active core of chlorophyll (Supplemental Fig. S4), chlorophyll content (types a and b) increased up to 5-fold by drought. The mild stress only affected the levels in the mature tissues, whereas in severe stress, chlorophyll already started to accumulate in the elongation zone (Fig. 3, C and E). However, in line with published results, our gas-exchange data indicated that photosynthesis in the mature part of the leaf is progressively inhibited by increasing drought stress levels (Fig. 3D). This inhibition was correlated with a strongly reduced stomatal conductance (Fig. 3F), indicating that, under drought conditions, photosynthesis in the mature parts of the leaf is limited by stomatal aperture.
Figure 3.
Changes in the photosynthetic machinery in the growth zone of the maize leaf under mild and severe drought stress. A and B, Transcript abundance of photosynthesis-related genes (log2FC) under mild (A) and severe (B) drought stress. Chl, Chlorophyll; I, pheophytin; PQ, plastoquinone; Cyt, cytochrome; PC, plastocyanin; FDX, ferredoxin; GAP, glyceraldehyde 3-phosphate; PGA, 3-phosphoglycerate; DHAP, dihydroxyacetone. C and E, Chlorophyll a (C) and chlorophyll b (E) content across the leaf axis in well-watered and stressed plants (a two-way ANOVA was used as a statistical test and P values for the two factors, drought [D] and segment [S], as well as the interaction between them [D*S]). Data are averages ± se (n = 3). The length of each developmental zone (meristem, elongation, and mature) is marked on the x axis for each treatment (control, mild, and severe stress) according to Supplemental Figure S2. FW, Fresh weight. D and F, Rates of photosynthesis (D) and stomatal conductance (F) before and after recovery of the stressed plants (T = treatment and R = recovery). Unstressed plants of the same age as the plants that were subjected to stress and allowed to recover were included as a control for ontogenetic differences. Student’s t test was used for statistical analysis, and significant differences (P < 0.05) are marked with different letters. Data are averages ± se (n = 5).
To reconcile the apparent contradiction of increased photosynthetic capacity with reduced carbon-assimilation rates in drought-stressed leaves, we hypothesized that the investment in the photosynthesis machinery facilitates enhanced carbon acquisition upon recovery. To functionally test this, we rewatered drought-stressed plants at 3 weeks after sowing (4–5 d after emergence of the fifth leaf). After 5 d of recovery, we measured the photosynthesis of the fifth leaf, which had developed under stress conditions. As a control, we measured leaves grown under control conditions but of the same age as the leaves from rewatered plants. In the control plants, photosynthesis decreased when the leaves matured (Fig. 3D). In contrast, the photosynthetic rates in the leaves that had recovered from the drought were 26% (mild stress; P = 0.044) and 33% (severe stress; P = 0.0005) higher than in controls of the same age and even 1% (mild stress; not significant) and 7% (severe stress; P = 0.003) higher than the control leaves that had just emerged. The increased photosynthesis upon recovery was accompanied by enhanced stomatal conductance (Fig. 3F). These results demonstrate that leaves developing under drought conditions increase their photosynthetic capacity to maximize photosynthesis upon recovery when the stomata are allowed to open.
Oxidative Stress
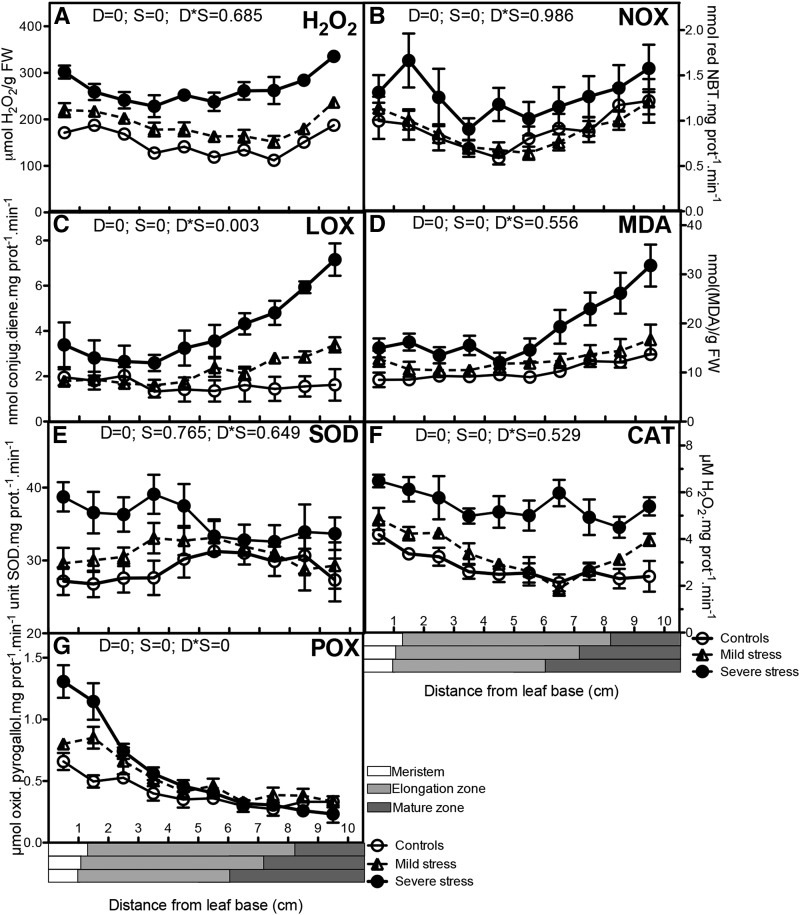
The observed transcriptional changes in redox control are in line with earlier observations that drought induces oxidative stress in mature leaves (Cruz de Carvalho, 2008; Kar, 2011). In such conditions, cellular redox homeostasis is often disturbed, as a consequence of extra ROS generation. A more oxidative environment may result in macromolecule damage (protein oxidation and lipid peroxidation) but also alters regulatory and signaling processes. Redox signaling is essential in a number of processes during plant growth and development, as it affects calcium fluxes (Foreman et al., 2003; Mazars et al., 2010) and regulates the activity of redox-sensitive enzymes, containing disulfide bonds (Klomsiri et al., 2011). In order to evaluate a potential link with the growth response, we characterized changes in redox status and regulation along the growth zone at the molecular level.
In contrast to most other ROS, hydrogen peroxide (H2O2) levels can be quantified. However, existing techniques are sensitive to the reactivity of the H2O2 molecule, which possibly creates artifacts (Cheeseman, 2006; Queval et al., 2008). Therefore, we compared different extraction and detection methods. Using an extraction in 5% (w/v) TCA and staining with Xylenol Orange, we observed a doubling of H2O2 content in severe stress conditions and a small increase across the growth zone under mild stress conditions (Fig. 4A). Extraction in a very distinct environment, phosphate buffer supplemented with catalase inhibitor, and quantification with both Xylenol Orange and Amplex Red H2O2/peroxidase assay on independent samples gave nearly identical results (Supplemental Fig. S5, A–C). This strongly suggests the independence of these data of extraction conditions and detection assays. Even under carefully controlled conditions, considerable variation is observed in H2O2 determinations, possibly related to extraction efficiency and the stability of the molecule (Cheeseman, 2006; Queval et al., 2008). Therefore, we also performed in-tissue H2O2 staining (3′,3-diaminobenzidine [DAB]; Thordal-Christensen et al., 1997). In this assay, H2O2 is captured inside the cells (Supplemental Fig. S5D). These results also confirmed that increasing drought levels progressively increased H2O2 throughout the growth zone. However, the effect is much less pronounced, particularly in the more mature tissues. Differences between DAB, Amplex Red, and Xylenol Orange measurements may be related to the limited penetration of DAB, which could be more prominent in mature cells with thicker cell walls. On the other hand, DAB staining is dependent on intracellular peroxidase activity, which also strongly decreases toward the mature cell zone (Fig. 4G). Despite the intrinsic advantages and disadvantages of each of these methods, they consistently indicate increased H2O2 levels in the growth zone in response to drought stress.
Figure 4.
Oxidative stress determinants and main antioxidant enzymes. Well-watered control plants were compared with mildly and severely stressed plants. Metabolite concentrations and enzyme activities were determined in each 1 cm of the leaf growth zone: H2O2 contents (A), NOX activity (B), LOX activity (C), malondialdehyde (MDA) contents (D), SOD activity (E), CAT activity (F), and POX activity (G). A two-way ANOVA was used as a statistical test, and P values for the two factors, drought (D) and segment (S), as well as the interaction between them (D*S), are presented. Data are averages ± se (n = 5). The length of each developmental zone (meristem, elongation, and mature) in each treatment (control, mild, and severe stress) is marked on the x axes of the graphs according to Supplemental Figure S2. FW, Fresh weight; NBT, nitroblue tetrazolium.
Increased ROS levels under stress originate primarily from increased photorespiration, altered electron transport in chloroplasts and mitochondria, and increases in RESPIRATORY BURST OXIDASE HOMOLOG (RBOH) activity. The latter activity (RBOH) represents membrane-bound NADPH-dependent oxidases (NOX) that catalyze the production of superoxide, which is converted to H2O2. The levels of zmrboh a and b transcripts were proportionally increased by mild and severe drought (Supplemental Table S2). NOX activity levels in mild stress were close to those in the control plants, whereas in the severely stressed plants, a dramatic increase occurred throughout the growth zone (Fig. 4B). NOX activity appeared to be suppressed in expanding cells in all conditions, which was not observed in the transcriptome data (Supplemental Table S2). Together, H2O2 and NOX activity data clearly indicated increased ROS levels throughout the growth zone in response to drought stress.
One effect of increased ROS levels is the potential increase of levels of oxidation of lipid molecules to lipid hydroperoxides. The activity of lipoxygenase enzymes (LOX) is also a major source of lipid peroxidation (Repetto et al., 2010). Out of six LOX enzymes annotated in our maize microarray data, the expression levels of four transcripts encoding LOX isoforms (lox2, lox6, lox10, and lox11) increased in response to drought stress, while the expression was highest in elongating and mature tissues (significant only for lox2 and lox10; Supplemental Table S2). During lipid peroxidation, small hydrocarbon fragments such as ketones and MDA are formed. Total LOX activity and MDA content closely followed the LOX transcript profiles in response to drought, increasing toward the mature part of the leaf (Fig. 4, C and D), demonstrating that drought induces oxidative damage in all regions of the leaf, with mature tissues showing the strongest response.
Enzymatic Oxidative Stress Defenses
To evaluate the response of the antioxidative defense system, we measured the expression and activity of the main antioxidant enzymes along the leaf growth zone. SUPEROXIDE DISMUTASE (SOD) is a metalloenzyme associated with copper, zinc, manganese, or iron (Fe) that catalyzes the dismutation of superoxide to H2O2 and oxygen. In the microarray data, six SOD-encoding transcripts were expressed above background levels. Four of these were 2- to 5-fold up-regulated by drought (Supplemental Table S2). The expression levels of five of these genes were highest in the meristem, compared with the mature parts of the leaf, but these changes were only significant for one homolog of the Arabidopsis FeSOD (Supplemental Table S2). The total SOD enzyme activity correlated with the transcriptional data, showing a progressive induction by drought and highest activity in the meristem (Fig. 4E).
Catalases (CATs) and peroxidases (POXs) are the primary scavengers of H2O2. Their activity protects plants in response to various stress factors (Castillo, 1992; Willekens et al., 1995). Of the three CAT-encoding transcripts identified in maize, two were up-regulated (cat1 and cat2) and one was down-regulated (cat3) by drought (Supplemental Table S2). The activity of the enzyme was highest in the meristem and was enhanced by drought, correlating with the transcript levels (Fig. 4F).
POXs are localized in the cell wall and vacuoles and use numerous substrates (Carpin et al., 1999). The transcript levels of two plasma membrane-bound POX isoforms (pmpox1 and pmpox3-1) were significantly up-regulated in response to severe drought only, whereas those of pmpox3-2 were significantly up-regulated in both stress levels (Supplemental Table S2). Again, the enzyme activity was consistent with the transcription data, with a higher activity in the meristem and up-regulation by drought (Fig. 4G).
Together, these results show a general correlation between the transcript-level changes of various antioxidant enzymes and their activity. Interestingly, the activity of POX and CAT, but also SOD under severe drought, was highest in the meristem at the base of the leaf, while the oxidative stress (determined by the activity of the enzymes NOX and LOX and the content of MDA) increased mainly in the mature zone. Our measurements indicate that antioxidant regulation in response to drought differs across the growth zone, the meristem being most actively protected.
P35S:AtFeSOD-Overexpressing Maize Has Increased ROS Tolerance and Improved Growth Rates under Control and Drought Stress Conditions
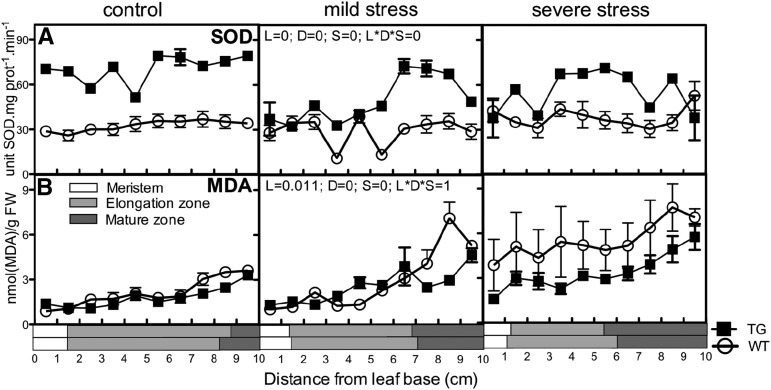
The increased activity of the antioxidant enzymes in the meristem, where inhibition of cell division is at the basis of the growth response, leads to the question of whether enhanced antioxidant production in this zone could reduce the growth inhibition imposed by drought. The expression of transgenic Arabidopsis FeSOD in chloroplasts enhanced oxidative stress tolerance in tobacco (Nicotiana tabacum) plants by protection of the plasma membranes and PSII (Van Camp et al., 1996; Van Breusegem et al., 1999). To test the effect of elevated FeSOD chloroplast-targeted expression in maize, Van Breusegem et al. (1999) produced transgenic maize lines overexpressing Arabidopsis FeSOD under the control of a 35S promotor, inducing enhanced tolerance toward methyl viologen (Paraquat) and improved growth under control and cold stress conditions. Because we found highest SOD levels in the meristem and increased levels in response to drought, we hypothesized that the improved growth of this line would also occur under drought conditions and opposite to the effect of drought (Table I), due to improved cell division. To prove this, we first validated that the overexpression of the Arabidopsis gene in this line resulted in increased SOD activity and improved redox state in the growth zone of maize leaves. Indeed, the activity of the enzyme was significantly higher throughout the growth zone in the FeSOD-overexpressing line than in the wild type in control and drought conditions (Fig. 5A). This increased activity led to lower levels of ROS, as evidenced by a significant reduction in the levels of MDA in the transgenic plants, showing reduced lipid peroxidation (Fig. 5B).
Figure 5.
SOD activity and MDA levels in the leaf growth zone of the P35S:ATFeSOD line and its wild type. Biochemical determination of SOD activity (A) and MDA levels (B) was done in the growing zone of maize leaves from control (well-watered) plants and plants exposed to mild and severe water stress, comparing wild-type (WT) and 35S-AtFESOD transgenic (TG) lines. A three-way ANOVA was used as a statistical test, and P values for the three factors, line (L), drought (D), and segment (S), as well as the interaction between them (L*D*S), are present on the middle graph for each band. Data are averages ± se (n = 3). The length of each developmental zone (meristem, elongation, and mature) is marked on the x axes of the graphs for each line (wild type and transgenic). FW, Fresh weight.
To test the effect of the transgene on growth, we subjected the AtFeSOD-overexpressing plants and the wild type (H99) to a kinematic analysis. We observed a 17% increase in LER of the transgenic plants under control conditions, confirming the previous observations (Van Breusegem et al., 1999), but also obtained 30% faster growth under mild drought and 9% under severe drought, respectively. Consistent with our hypothesis, these enhanced growth rates were due to increased cell production rates in the transgenic line, whereas no significant differences were observed between the cell lengths and cell elongation rates (Table II).
Table II. Kinematic analysis, describing the effect of mild and severe drought stress on cell division and cell expansion parameters during the steady-state growth of the fifth leaf of wild-type and transgenic plants overexpressing an Arabidopsis gene encoding an FeSOD enzyme.
A two-way ANOVA was used to determine statistically significant differences between the three treatments and the two maize lines, and P values > 0.05 are marked as NS (not significant). Data are averages ± se (n = 4). Parameters are as follows: leaf length (LL; measured on the fifth leaf at the time of harvesting; Supplemental Fig. S1), LER (calculated during the first 3 d after leaf appearance), mature cell length (Lmat), cell production rate (P), cell division rate (D), cell cycle duration (Tc), length of the meristem (Lmer), number of cells in the meristem (Nmer), time in the division zone (Tdiv), cell elongation rate (Rel), and time in the elongation zone (Tel).
| Parameter | Wild-Type Control | 35S-FeSOD Control | Wild-Type Mild Stress | 35S-FeSOD Mild Stress | Wild-Type Severe Stress | 35S-AtFeSOD Severe Stress | Treatment | Lines |
|---|---|---|---|---|---|---|---|---|
| LL (mm) | 802 ± 21 | 894 ± 8 | 662 ± 51 | 755 ± 43 | 402 ± 73 | 439 ± 54 | S | S |
| LER (mm h−1) | 2.5 ± 0.1 | 3.0 ± 0.1 | 1.7 ± 0.3 | 2.3 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.4 | S | S |
| Lmat (µm) | 135 ± 12 | 112 ± 11 | 126 ± 10 | 116 ± 4 | 103 ± 4 | 105 ± 4 | NS | NS |
| P (cells h−1) | 19 ± 2 | 28 ± 2 | 13 ± 2 | 20 ± 3 | 10 ± 2 | 11 ± 4 | S | S |
| D (cell cell−1 h−1) | 0.025 ± 0.003 | 0.033 ± 0.003 | 0.016 ± 0.002 | 0.023 ± 0.004 | 0.013395 ± 0.004 | 0.015 ± 0.005 | S | NS |
| Tc (h) | 29 ± 3 | 22 ± 2 | 46 ± 7 | 33 ± 5 | 62 ± 19 | 76 ± 32 | S | NS |
| Lmer (mm) | 20 ± 1 | 20 ± 0 | 15 ± 0 | 14 ± 1 | 11 ± 0 | 13 ± 1 | S | NS |
| Nmer | 810 ± 109 | 865 ± 122 | 799 ± 63 | 883 ± 46 | 782 ± 99 | 757 ± 100 | NS | NS |
| Rel (µm µm−1 h−1) | 0.031 ± 0.002 | 0.035 ± 0.002 | 0.021 ± 0.005 | 0.030 ± 0.003 | 0.014283 ± 0.001 | 0.017 ± 0.006 | S | NS |
| Tel (h) | 52 ± 6 | 42 ± 5 | 81 ± 19 | 64 ± 8 | 102 ± 4 | 133 ± 41 | S | NS |
DISCUSSION
Drought is one of the major factors limiting plant growth in ecological and agricultural contexts. Although its effects have been studied extensively, most molecular and physiological studies have focused on mature leaves of plants exposed to severe stress treatments that lead to clear signs of wilting. Our results show that well before such signs appear, growth is already inhibited up to 30%. These more mild stress levels are important determinants of crop yields in modern agricultural conditions (Skirycz et al., 2011).
Our studies confirm major differences in gene expression and physiology (Beemster et al., 2005; Li et al., 2010) between mature and growing tissues. This implies that, to understand the effect of drought on growth, growing tissues need to be analyzed separately. So far, such studies have been performed more extensively in maize roots and differences in the regulation of growth-related processes between the apical and basal regions of the primary root tip have been reported (Yamaguchi and Sharp, 2010). Similar to our results (Fig. 1C), specific changes in gene expression and protein composition in these zones were described between well-watered and drought-stressed plants (Zhu et al., 2007; Spollen et al., 2008).
Kinematic analysis shows that a significant decrease in cell division rates in the meristem is the main cause of the growth reduction (Table I). The microarray data obtained from the meristem show down-regulation of the entire cell cycle machinery (Fig. 2) and up-regulation of an inhibitor of cell cycle progression (krp2; De Veylder et al., 2001). It was demonstrated previously that CDKA kinase activity correlates with reduced cell division rates in maize leaves subjected to drought conditions (Granier et al., 2000), but the regulatory mechanism was not determined. Previously, we found that, in response to cold nights, cell cycle transcript levels in the maize leaf meristem correlated with inhibited cell division rates (Rymen et al., 2007). Generally, our results are consistent with other studies of cell cycle regulation and abiotic stress: a decrease of transcript levels of A- and B-type cyclins occurred in response to cold, drought, and salinity stress (West et al., 2004; Rymen et al., 2007; Kakumanu et al., 2012). Studies in Arabidopsis have shown that altered expression of A- and B-type cyclins indeed results in altered cell proliferation (Doerner et al., 1996; Vanneste et al., 2011). In addition to these core cell cycle regulators, several subunits of the APC/cyclosome were also down-regulated (Supplemental Table S1). This complex promotes the transition from anaphase to metaphase by the destruction of B-type cyclins, and increased levels of its subunit APC10 enhanced leaf growth (Eloy et al., 2011). Together, our results indicate a broad effect on multiple control points of the cell cycle rather than a single key regulator that is responsible for the reduced cell division activity.
The up-regulation of genes involved in the photosynthetic machinery (Fig. 3, A and B) in the microarray study was surprising, particularly given the reduced rates of photosynthesis in this study (Fig. 3D) and other published studies (Dwyer et al., 1992; Ashghizadeh and Ehsanzadeh, 2008; Hayano-Kanashiro et al., 2009). In other studies, either little effect (Chaves et al., 2009) or down-regulation (Kilian et al., 2007; Hayano-Kanashiro et al., 2009; Humbert et al., 2013) of photosynthesis-related transcripts was observed in response to drought and other abiotic stresses. However, most of these results have been obtained by sampling mature leaves and, therefore, may relate to the maintenance of the fully developed photosynthetic system during stress conditions. Our results indicate that leaves developing in drought conditions, in contrast, increase their photosynthetic capacity, possibly to compensate for the smaller size of their leaves. This potential can be used upon recovery from the drought, when the stomata open. To our knowledge, these changes have not been reported at the transcriptional level. They represent an important finding and may explain at least to some extent the often-observed phenomenon that stressed plants upon recovery grow faster than unstressed control plants, so that the effect of the stress on plant size is reduced (Hayano-Kanashiro et al., 2009; Xu et al., 2009).
The observed increase in chlorophyll levels in the stressed plants could be also linked to the redox status in the leaf. We showed a significant increase in the activity of redox enzymes in the growth zone of the maize leaf. It has been shown that there is a direct link between ROS levels and photosynthetic activity during leaf development, which could additionally influence plant growth and leaf aging (Chen and Gallie, 2006).
The growth reduction under drought stress could also be explained by changes in the redox status of the stressed plants. Our measurements show a significant increase in H2O2 levels, especially in severe stress conditions. Besides their oxidative effect, H2O2 and other ROS are demonstrated to play a role in growth-related processes as signaling molecules. In mammalian cells, it is well established that ROS can act as positive growth regulators depending on their concentration and pulse duration (Sauer et al., 2001; Menon and Goswami, 2007). In plants, ROS are involved in the regulation of several processes (for review, see Considine and Foyer, 2014), including both cell division and cell elongation. For example, low concentrations of ROS are needed to induce cell proliferation (Fehér et al., 2008) and cell differentiation (Tsukagoshi et al., 2010). H2O2 is shown to block cell cycle progression (Reichheld et al., 1999; Kovtun et al., 2000) and is needed for cell elongation (Rodríguez et al., 2002). ROS also play a role in cell wall stiffening and thus may inhibit cell expansion (Hohl et al., 1995; Schopfer, 1996). Our measurements show that H2O2 in maize leaves progressively increases with the severity of the drought treatments, suggesting a risk of oxidative damage in the meristem, elongation, and maturation zones. Therefore, the observed inhibition of both cell division and cell expansion in response to drought can possibly be linked to the negative impact of elevated H2O2 in the corresponding leaf zones directly on the regulation of these two processes.
Our analysis of redox regulation shows that different antioxidant systems dominate in specific parts of the growth zone during drought stress. POX is mainly active in the meristem and in the very beginning of the elongation zone, whereas SOD and CAT activity decrease slightly over the growth zone, with highest activity in the meristem. SOD activity is most strongly up-regulated in this zone. The meristem, therefore, appears to be the part of the leaf, with highest antioxidant enzymatic activity (Fig. 4, E–G). Similarly, salinity stress induced higher transcription of genes involved in antioxidant protection in young compared with old cells across the maize leaf (Kravchik and Bernstein, 2013). Possibly as a consequence, the mature zone shows higher levels of lipid peroxidation during drought stress (Fig. 4E). High MDA, in turn, can also affect cellular processes such as gene expression and activate defense responses (Weber et al., 2004).
The increases in lipid peroxidation also correlate with increased LOX activity in the more mature leaf segments (Fig. 4C). This could possibly point to increased stress-related jasmonate production, as jasmonate biosynthesis involves the synthesis of oxylipins, through lipid oxidation. Particularly LOX6, whose transcript levels were significantly induced in our conditions (Supplemental Table S2), is responsible for stress-induced jasmonate accumulation in roots (Grebner et al., 2013). Jasmonates are involved in stomatal closure (Suhita et al., 2004) during drought stress and negatively regulate cell cycle progression, keeping the cells in the G1 stage (Noir et al., 2013). Our microarray data showed significant changes in the transcripts of three other key regulatory enzymes of jasmonate-biosynthesis (12-OXO-PHYTODIENOIC ACID REDUCTASE5, 12-OXO-PHYTODIENOIC ACID REDUCTASE6, and ALLENE OXIDE CYCLASE1; Supplemental Table S2). The transcription pattern of 12-OXO-PHYTODIENOIC ACID REDUCTASE5 followed the one of LOX. Isoform 6 of the same reductase was down-regulated in the stress conditions, but to a much smaller extent compared with the up-regulation of isoform 5 transcription, suggesting that the latter plays a more important role during water stress. The transcription of the enzyme ALLENE OXIDE CYCLASE1, shown to be linked to ROS regulation during salinity stress (Hazman et al., 2015), was only induced in the elongation zone of severely stressed plants.
The existence of a transgenic line overexpressing an Arabidopsis gene for FeSOD (Van Breusegem et al., 1999) allowed a direct investigation of the link between antioxidant activity in the meristem and the inhibitory effect of drought on cell division rates. Several studies have already demonstrated the enhanced performance of plants with increased antioxidant levels under stress conditions (McKersie et al., 1993; Van Camp et al., 1996; Van Breusegem et al., 1999), but the cellular basis for this was never determined. We demonstrated that the better growth of the FeSOD-overexpressing line was due to increased cell production rates. In the Arabidopsis root tip, a correlation between levels of glutathione (a nonenzymatic antioxidant metabolite) and cell cycle regulation was established (Vernoux et al., 2000). Our results are consistent with this and, to our knowledge, show for the first time that increased enzymatic antioxidant levels in the leaf meristem can positively regulate cell division and thereby improve growth. We demonstrate that combining molecular genetic insights from Arabidopsis with studies of the maize growth zone not only validates results in a crop species but also increases our knowledge of plant growth regulation in general.
MATERIALS AND METHODS
Maize Lines
All measurements were performed using the maize (Zea mays) inbred line B73 (Iowa Stiff Stalk Synthetic). A transgenic line, overexpressing the FeSOD gene from Arabidopsis (Arabidopsis thaliana) under the control of the Cauliflower mosaic virus 35S promoter (P35S:ATFeSOD), and its wild type, a backcross of Pa91 × H99 to the H99 parent, were used to test the impact of increased antioxidant capacity on plant growth. Seeds from the transgenic and wild-type lines were obtained from Frank Van Breusegem (Van Breusegem et al., 1999).
Growth Experiment
Maize seedlings were grown in a growth chamber under controlled conditions (16-h day/8-h night, 25°C/18°C day/night, 300–400 μE m−2 s−1 photosynthetically active radiation, provided by high-pressure sodium lamps). For control plants, the pots were rewatered daily to an SWC of 54%. For drought treatments, water contents were allowed to drop after sowing to 43% SWC (mild stress, no wilting) and 34% SWC (severe stress, leaves wilting during the day), where they were maintained. Three days after emergence of the fifth leaf, randomly chosen plants were harvested and the growth zone (the first 10 cm from the leaf base) of leaf 5 of each plant was cut in 10 segments of 1 cm, and the samples were stored at −80°C for further measurements (total RNA extraction, pigment and antioxidant quantification, and enzyme activities). The remaining plants were used to determine the length of the fifth leaf until it reached maturity. Five independently reproducible drought experiments were conducted, each one of them on a batch of at least 20 plants for each condition.
Kinematic Analysis
The kinematic analysis was done according to an established protocol (Rymen et al., 2010). It entails leaf elongation rates and final leaf length measurements, measurements of the cell length profile along the axis of the leaf, and estimation of the size of the leaf basal meristem. Leaf length was measured daily with a ruler on the fifth leaf from the moment it was visible among the older leaves until the moment it reached its final leaf size. LER was calculated during the first 3 d of leaf growth as the difference in length divided by the time difference between successive measurements (day 3 − day 2/24 h). For meristem measurements, samples harvested 3 d after leaf emergence were analyzed by fluorescence microscopy (AxioScope A1, Axiocam ICm1; Zeiss) at 20× magnification. The size of the meristematic zone of the leaves was estimated by locating the most distal mitosis in the cell files. Cell length was measured by light microscopy (Scope A1 Axiocam ICm1; Zeiss) using differential interference contrast at 40× magnification and the online measurement module in the Axiovision (Zeiss) software. Measurement was carried out at four locations on each segment: at the tip (0 cm), at one-third of the segment (0.3 cm), at two-thirds (0.6 cm), and at the end of the segment (0.99 cm). Around 20 cells were measured at each location. The raw data obtained for individual leaves were smoothed and interpolated at an interval of 50 mm using the kernel-smoothing function locpoly of the KernSmooth (Wand and Jones, 1995) package for the R statistical package (R Foundation for Statistical Computing), which allowed averaging between leaves and comparison between treatments. The calculations of all other parameters (Tables I and II) were done based on these data as described earlier (Fiorani et al., 2000).
RNA Extraction, Labeling, and Hybridization
Total RNA was extracted from each zone (meristem, elongation, and mature zones) of the fifth leaf on day 3 after its appearance on control plants and plants subjected to mild and severe drought stress. Four biological replicates (each one a pool of four plants) were used for each zone/treatment combination. The total RNA was extracted using the TRIZOL reagent (Invitrogen) and purified using the RNeasy Plant Mini Kit (Qiagen). Probe concentrations and purity were determined using the NanoDrop ND-1000 UV-VIS spectrophotometer (Thermo Scientific), and the quality was assessed using a gel cartridge on the QIAxcel platform (Qiagen). Samples were labeled using the Quick Amp Labeling Kit (Two Color; Agilent). The labeled samples were purified (using the RNeasy Mini Kit), and complimentary RNA yield and the relative amount of incorporated labeled dCTPs were determined on the NanoDrop ND-1000 UV-VIS spectrophotometer (Thermo Scientific). The microarray analysis was conducted using the Agilent 44K maize chips (Ma et al., 2008). Three separate hybridization loops (Supplemental Fig. S3), each consisting of six arrays, were used on five four-pack formatted microarray slides. Labeling, hybridization, and washing were performed as described in the Agilent Two-Color Microarray-Based Gene Expression Analysis Protocol.
Microarray Analysis
Microarrays were scanned using the Genepix Personal 4100A confocal scanner (Axon Instruments) at a resolution of 5 µm and excitation wavelengths of 635 and 532 nm. All spots were identified and quantified by GenePix Pro 6.0 software (Axon Instruments). The R packages arrayQualityMetrics and arrayQuality were used to perform a quality control: spot filtering was done for each array, and spots that did not pass the criterion of FG > BG + 2 sd (Sclep et al., 2007) were excluded from the analysis. A variance stabilization (Huber et al., 2002), which is a between-array normalization, was used, following the function normalizeBetweenArrays (x, method = “vsn”, lts.quantile = 0.5), contained in the package LIMMA (Smyth, 2005). Statistical analysis for differences between the stress conditions and the developmental zones was conducted using a two-way ANOVA on the software MultiExperiment Viewer (Saeed et al., 2003). After a stringent cutoff (Bonferroni multiple testing correction for the stress effect, FDR correction for the zone effect, and a cutoff of P > 0.05 and log2FC > 0.75), differentially expressed genes were visualized and clustered using quality threshold clustering (Pearson correlation measure; cluster diameter = 0.5; minimum cluster population = 20) in MultiExperiment Viewer. For the comparison of the stress effect in each developmental zone, three separate one-way ANOVAs (for meristem, elongation, and mature zones) were done, and FDR was used as a multiple testing correction. Only significant values (FDR < 0.05 and log2FC > 0.75) were taken into account. Data from four different databases (Ware et al., 2002; Thimm et al., 2004; Coetzer et al., 2011; Van Bel et al., 2012) were combined in order to functionally annotate the differentially expressed genes in the analysis. Gene enrichment studies were carried out by PageMan (Usadel et al., 2006). MapMan (Thimm et al., 2004) was employed to show the differences in gene expression in different cellular and metabolic processes.
Photosynthesis Measurements
Net photosynthesis rate and stomatal resistance were measured on the exposed/mature part of the fifth leaf using a portable photosynthesis system (LI-6400; LI-COR). The CO2 concentration and temperature in the leaf chamber were kept at 400 μmol mol–1 and 25°C ± 0.5°C, respectively. The measurements were conducted at photon flux density (1,500 μmol m−2 s−1) by a red/blue light-emitting diode light source (LI-6400-02B; LI-COR) and at ambient relative humidity. All parameters were measured at noon inside the growth room. Measurements were done once during the stress treatment and four times after recovery using five plants for each treatment.
Biochemical Measurements
Photosynthetic Pigments
Photosynthetic pigments were extracted and determined according to the method described by Markwell et al. (1986). The contents of chlorophylls a and b were calculated using the formulas described previously (Porra et al., 1989) and expressed as μg pigment g–1 fresh weight.
Determination of H2O2 and MDA
For H2O2 determination, four independent assays were used. (1) A total of 100 mg of the samples was homogenized in 1 mL of 5% (w/v) TCA (Velikova et al., 2000) using a MagNALyser (Roche). Homogenates were centrifuged (14,000 rpm for 30 min), and Xylenol Orange dye reagent (Bellincampi et al., 2000) was added to the supernatant. After 45 min of incubation, the Fe3+-Xylenol Orange complex was measured at 595 nm. (2) Extraction in 50 mm phosphate buffer (pH 6.5) containing the catalase inhibitor hydroxylamine (1 mm) followed by quantification with Xylenol Orange reagent. (3) Extraction in the phosphate buffer with hydroxylamine as above, followed by quantification with the Amplex Red H2O2/peroxidase assay (Molecular Probes; Shin and Schachtman, 2004). (4) Localization along the leaf axis using DAB staining (Thordal-Christensen et al., 1997).
MDA was extracted in 2 mL of 80% (v/v) ethanol and measured using a thiobarbituric acid-MDA assay (Hodges et al., 1999). The quantity of MDA (µm) was calculated by the formula [6.45 × (A532 − A600) − 0.56 × A440]/0.478).
Enzyme Extraction and Enzyme Activity Assays
Around 100 mg of frozen leaf tissue was homogenized in 1 mL of potassium phosphate buffer (0.05 m, pH 7), containing 2% (w/v) polyvinylpyrrolidone, EDTA (0.4 mm), phenylmethylsulfonyl fluoride (0.2 mm), and ascorbic acid (1 mm). POX activity was measured by monitoring the production of purpurogallin at 430 nm (Kumar and Khan, 1982). CAT activity was calculated out of the decrease in H2O2 concentration, measured at 240 nm (Aebi, 1984). Measuring the inhibition of nitroblue tetrazolium reduction at 550 nm was used to assay SOD activity (Dhindsa et al., 1981). The activity of LOX was assayed according to Axelrod et al. (1981) by monitoring the production of conjugate diene at 234 nm. NOX was assayed according to Sarath et al. (2007), where NADPH-dependent superoxide generation was measured by the reduction rate of nitroblue tetrazolium into monoformazan at 530 nm.
Soluble Protein Content
Soluble protein was determined according to the method of Lowry et al. (1951).
Statistical Analysis
For all of the biochemical measurements, a two-way ANOVA was performed (factor 1, the segment of the growth zone; factor 2, the stress treatment) using the statistical package SPSS (version 20; IBM). Data are presented as means of three biological replicates ± se. Effects were considered significant at P < 0.05.
Raw microarray data have been deposited with the National Center for Biotechnology Information’s Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo) and are accessible through the Gene Expression Omnibus series accession number GSE55592.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. SWC during the growth experiment.
Supplemental Figure S2. The effect of drought on the cell length profile.
Supplemental Figure S3. Hybridization design.
Supplemental Figure S4. Gene enrichment analysis.
Supplemental Figure S5. Comparison between different methods of determination of the H2O2 concentration across the growth zone of maize leaves, subjected to mild and severe drought stress.
Supplemental Table S1. Overview of core cell cycle genes in maize.
Supplemental Table S2. Overview of the expression levels of genes coding different isoforms of the key redox enzymes in response to mild and severe drought stress in different positions of the leaf growth zone.
Supplemental Text S1. Table with microarray data, including the expression values of the genes, statistical analysis, and the presence in clusters illustrated in Figure 1C.
Supplementary Material
Acknowledgments
We thank Frank Van Breusegem, who provided the seeds of the FeSOD-overexpressing maize line.
Glossary
- ROS
reactive oxygen species
- SWC
soil water content
- LER
leaf elongation rate
- FDR
false discovery rate
- H2O2
hydrogen peroxide
- DAB
3′,3-diaminobenzidine
- MDA
malondialdehyde
Footnotes
This work was supported by the Interuniversity Attraction Poles Program (Belgian Network Maize Arabidopsis Root and Shoot growth (MARS): Growth and Development of Higher Plants; grant no. IUAP VII/29), initiated by the Science Policy Office of the Belgian State, by a Ph.D. fellowship of the University of Antwerp to V.A., by an Exchange Grant from Fonds Wetenschappelijk Onderzoek and Ministerio de Ciencia, Tecnología e Innovación Productiva (FWO-MINCyT; to G.T.S.B.and E.T.), and by the National Natural Science Foundation of China (grant no. 31000116 to Z.Z.).
Articles can be viewed without a subscription.
References
- Aebi H. (1984) Catalase in vitro. Methods Enzymol 105: 121–126 [DOI] [PubMed] [Google Scholar]
- Aparicio OM, Weinstein DM, Bell SP (1997) Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell 91: 59–69 [DOI] [PubMed] [Google Scholar]
- Ashghizadeh HR, Ehsanzadeh P (2008) Maize (Zea maize L.) performance under drought: decreased photosynthetic area vs. decreased efficiency of PSII. In Allen JF, Gantt E, Golbeck JH, Osmond B, eds, Photosynthesis: Energy from the Sun: 14th International Congress on Photosynthesis. Springer, Dordrecht, The Netherlands, pp 1445–1449 [Google Scholar]
- Axelrod B, Cheesbrough TM, Laakso S (1981) Lipoxygenase from soybeans: EC 1.13.11.12 linoleate:oxygen oxidoreductase. Methods Enzymol 71: 441–451 [Google Scholar]
- Beemster GTS, De Veylder L, Vercruysse S, West G, Rombaut D, Van Hummelen P, Galichet A, Gruissem W, Inzé D, Vuylsteke M (2005) Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol 138: 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellincampi D, Dipierro N, Salvi G Cervone F, De Lorenzo G (2000) Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol 122: 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme L, Valot B, Tardieu F, Zivy M (2012) Phosphoproteome dynamics upon changes in plant water status reveal early events associated with rapid growth adjustment in maize Leaves. Mol Cell Proteomics 11: 957–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudolf V, Inzé D, De Veylder L (2006) What if higher plants lack a CDC25 phosphatase? Trends Plant Sci 11: 474–479 [DOI] [PubMed] [Google Scholar]
- Boyer JS. (1982) Plant productivity and environment. Science 218: 443–448 [DOI] [PubMed] [Google Scholar]
- Carpin S, Crèvecoeur M, Greppin H, Penel C (1999) Molecular cloning and tissue-specific expression of an anionic peroxidase in zucchini. Plant Physiol 120: 799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo FJ. (1992) Peroxidase and stress. In Penel C, Gaspar T, Greppin H, eds, Plant Peroxidases 1980-1990: Topics and Detailed Literature on Molecular, Biochemical and Physiological Aspects. University of Geneva, Geneva, pp 87–203 [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot (Lond) 103: 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman JM. (2006) Hydrogen peroxide concentrations in leaves under natural conditions. J Exp Bot 57: 2435–2444 [DOI] [PubMed] [Google Scholar]
- Chen Z, Gallie DR (2006) Dehydroascorbate reductase affects leaf growth, development, and function. Plant Physiol 142: 775–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JPJ, Thömmes P, Blow JJ (1996) The role of MCM/P1 proteins in the licensing of DNA replication. Trends Biochem Sci 21: 102–106 [PubMed] [Google Scholar]
- Coetzer N, Myburg AA, Berger DK (2011) Maize microarray annotation database. Plant Methods 7: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine MJ, Foyer CH (2014) Redox regulation of plant development. Antioxid Redox Signal 21: 1305–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz de Carvalho MH. (2008) Drought stress and reactive oxygen species: production, scavenging and signaling. Plant Signal Behav 3: 156–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GTS, Krols L, Terras F, Landrieu I, van der Schueren E, Maes S, Naudts M, Inzé D (2001) Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13: 1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Inzé D (2007) The ins and outs of the plant cell cycle. Nat Rev Mol Cell Biol 8: 655–665 [DOI] [PubMed] [Google Scholar]
- Dhindsa RS, Plumbdhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane-permeability and lipid-peroxidation, and decreased levels of superoxide-dismutase and catalase. J Exp Bot 32: 93–101 [Google Scholar]
- Doerner P, Jørgensen JE, You R, Steppuhn J, Lamb C (1996) Control of root growth and development by cyclin expression. Nature 380: 520–523 [DOI] [PubMed] [Google Scholar]
- Dwyer LM, Stewart DW, Tollenaar M (1992) Analysis of maize leaf photosynthesis under drought stress. Can J Plant Sci 72: 477–481 [Google Scholar]
- Eloy NB, Coppens F, Beemster GTS, Hemerly AS, Ferreira PC (2006) The Arabidopsis anaphase promoting complex (APC): regulation through subunit availability in plant tissues. Cell Cycle 5: 1957–1965 [DOI] [PubMed] [Google Scholar]
- Eloy NB, de Freitas Lima M, Van Damme D, Vanhaeren H, Gonzalez N, De Milde L, Hemerly AS, Beemster GTS, Inzé D, Ferreira PCG (2011) The APC/C subunit 10 plays an essential role in cell proliferation during leaf development. Plant J 68: 351–363 [DOI] [PubMed] [Google Scholar]
- Fehér A, Otvös K, Pasternak TP, Szandtner AP (2008) The involvement of reactive oxygen species (ROS) in the cell cycle activation (G(0)-to-G(1) transition) of plant cells. Plant Signal Behav 3: 823–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorani F, Beemster GTS, Bultynck L, Lambers H (2000) Can meristematic activity determine variation in leaf size and elongation rate among four Poa species? A kinematic study. Plant Physiol 124: 845–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al. (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Granier C, Inzé D, Tardieu F (2000) Spatial distribution of cell division rate can be deduced from that of p34cdc2 kinase activity in maize leaves grown at contrasting temperatures and soil water conditions. Plant Physiol 124: 1393–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebner W, Stingl NE, Oenel A, Mueller MJ, Berger S (2013) Lipoxygenase6-dependent oxylipin synthesis in roots is required for abiotic and biotic stress resistance of Arabidopsis. Plant Physiol 161: 2159–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayano-Kanashiro C, Calderón-Vázquez C, Ibarra-Laclette E, Herrera-Estrella L, Simpson J (2009) Analysis of gene expression and physiological responses in three Mexican maize landraces under drought stress and recovery irrigation. PLoS One 4: e7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazman M, Hause B, Eiche E, Nick P, Riemann M (2015) Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J Exp Bot 66: 3339–3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer LJ, Kruglyak S, Yooseph S (1999) Exploring expression data: identification and analysis of coexpressed genes. Genome Res 9: 1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207: 604–611 [DOI] [PubMed] [Google Scholar]
- Hohl M, Greiner H, Schopfer P (1995) The cryptic-growth response of maize coleoptiles and its relationship to H2O2-dependent cell-wall stiffening. Physiol Plant 94: 491–498 [Google Scholar]
- Huber W, von Heydebreck A, Sültmann H, Poustka A, Vingron M (2002) Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics (Suppl 1) 18: S96–S104 [DOI] [PubMed] [Google Scholar]
- Humbert S, Subedi S, Cohn J, Zeng B, Bi YM, Chen X, Zhu T, McNicholas PD, Rothstein SJ (2013) Genome-wide expression profiling of maize in response to individual and combined water and nitrogen stresses. BMC Genomics 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakumanu A, Ambavaram MMR, Klumas C, Krishnan A, Batlang U, Myers E, Grene R, Pereira A (2012) Effects of drought on gene expression in maize reproductive and leaf meristem tissue revealed by RNA-Seq. Plant Physiol 160: 846–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar RK. (2011) Plant responses to water stress: role of reactive oxygen species. Plant Signal Behav 6: 1741–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50: 347–363 [DOI] [PubMed] [Google Scholar]
- Klomsiri C, Karplus PA, Poole LB (2011) Cysteine-based redox switches in enzymes. Antioxid Redox Signal 14: 1065–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravchik M, Bernstein N (2013) Effects of salinity on the transcriptome of growing maize leaf cells point at cell-age specificity in the involvement of the antioxidative response in cell growth restriction. BMC Genomics 14: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KB, Khan PA (1982) Peroxidase and polyphenol oxidase in excised ragi (Eleusine corcocana cv Pr 202) leaves during senescence. Indian J Exp Biol 20: 412–416 [PubMed] [Google Scholar]
- Li P, Ponnala L, Gandotra N, Wang L, Si Y, Tausta SL, Kebrom TH, Provart N, Patel R, Myers CR, et al. (2010) The developmental dynamics of the maize leaf transcriptome. Nat Genet 42: 1060–1067 [DOI] [PubMed] [Google Scholar]
- Lobell DB, Roberts MJ, Schlenker W, Braun N, Little BB, Rejesus RM, Hammer GL (2014) Greater sensitivity to drought accompanies maize yield increase in the U.S. Midwest. Science 344: 516–519 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275 [PubMed] [Google Scholar]
- Ma J, Skibbe DS, Fernandes J, Walbot V (2008) Male reproductive development: gene expression profiling of maize anther and pollen ontogeny. Genome Biol 9: R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeran W, Friso G, Ponnala L, Connolly B, Huang M, Reidel E, Zhang C, Asakura Y, Bhuiyan NH, Sun Q, et al. (2010) Structural and metabolic transitions of C4 leaf development and differentiation defined by microscopy and quantitative proteomics in maize. Plant Cell 22: 3509–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell JP, Danko SJ, Bauwe H, Osterman J, Gorz HJ, Haskins FA (1986) A temperature-sensitive chlorophyll b-deficient mutant of sweetclover (Melilotus alba). Plant Physiol 81: 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazars C, Thuleau P, Lamotte O, Bourque S (2010) Cross-talk between ROS and calcium in regulation of nuclear activities. Mol Plant 3: 706–718 [DOI] [PubMed] [Google Scholar]
- McKersie BD, Chen Y, de Beus M, Bowley SR, Bowler C, Inzé D, D’Halluin K, Botterman J (1993) Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.). Plant Physiol 103: 1155–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges M, de Jager SM, Gruissem W, Murray JAH (2005) Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J 41: 546–566 [DOI] [PubMed] [Google Scholar]
- Menon SG, Goswami PC (2007) A redox cycle within the cell cycle: ring in the old with the new. Oncogene 26: 1101–1109 [DOI] [PubMed] [Google Scholar]
- Nelissen H, Rymen B, Jikumaru Y, Demuynck K, Van Lijsebettens M, Kamiya Y, Inzé D, Beemster GTS (2012) A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Curr Biol 22: 1183–1187 [DOI] [PubMed] [Google Scholar]
- Noir S, Bömer M, Takahashi N, Ishida T, Tsui TL, Balbi V, Shanahan H, Sugimoto K, Devoto A (2013) Jasmonate controls leaf growth by repressing cell proliferation and the onset of endoreduplication while maintaining a potential stand-by mode. Plant Physiol 161: 1930–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents: verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim Biophys Acta 975: 384–394 [Google Scholar]
- Queval G, Hager J, Gakière B, Noctor G (2008) Why are literature data for H2O2 contents so variable? A discussion of potential difficulties in the quantitative assay of leaf extracts. J Exp Bot 59: 135–146 [DOI] [PubMed] [Google Scholar]
- Reichheld JP, Vernoux T, Lardon F, Van Montagu M, Inze D (1999) Specific checkpoints regulate plant cell cycle progression in response to oxidative stress. Plant J 17: 647–656 [Google Scholar]
- Repetto MG, Ferrarotti NF, Boveris A (2010) The involvement of transition metal ions on iron-dependent lipid peroxidation. Arch Toxicol 84: 255–262 [DOI] [PubMed] [Google Scholar]
- Ribaut JM, Jiang C, Gonzalez-de-Leon D, Edmeades GO, Hoisington DA (1997) Identification of quantitative trait loci under drought conditions in tropical maize. 2. Yield components and marker-assisted selection strategies. Theor Appl Genet 94: 887–896 [Google Scholar]
- Riccardi F, Gazeau P, Vienne Dd, Zivy M (1998) Protein changes in response to progressive water deficit in maize. Plant Physiol 117: 1253–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez AA, Grunberg KA, Taleisnik EL (2002) Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol 129: 1627–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymen B, Coppens F, Dhondt S, Fiorani F, Beemster GTS (2010) Kinematic analysis of cell division and expansion. Methods Mol Biol 655: 203–227 [DOI] [PubMed] [Google Scholar]
- Rymen B, Fiorani F, Kartal F, Vandepoele K, Inzé D, Beemster GTS (2007) Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol 143: 1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelli PA, Liu Y, Dante RA, Lizarraga LE, Nguyen HN, Brown SW, Klingler JP, Yu J, LaBrant E, Layton TM, et al. (2013) Control of cell proliferation, endoreduplication, cell size, and cell death by the retinoblastoma-related pathway in maize endosperm. Proc Natl Acad Sci USA 110: E1827–E1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. (2003) TM4: a free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378 [DOI] [PubMed] [Google Scholar]
- Sarath G, Hou G, Baird LM, Mitchell RB (2007) Reactive oxygen species, ABA and nitric oxide interactions on the germination of warm-season C4-grasses. Planta 226: 697–708 [DOI] [PubMed] [Google Scholar]
- Sauer H, Wartenberg M, Hescheler J (2001) Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem 11: 173–186 [DOI] [PubMed] [Google Scholar]
- Schopfer P. (1996) Hydrogen peroxide-mediated cell-wall stiffening in vitro in maize coleoptiles. Planta 199: 43–49 [Google Scholar]
- Sclep G, Allemeersch J, Liechti R, De Meyer B, Beynon J, Bhalerao R, Moreau Y, Nietfeld W, Renou JP, Reymond P, et al. (2007) CATMA, a comprehensive genome-scale resource for silencing and transcript profiling of Arabidopsis genes. BMC Bioinformatics 8: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin R, Schachtman DP (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci USA 101: 8827–8832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk WK, Erickson RO (1979) Kinematics of plant growth. J Theor Biol 76: 481–501 [DOI] [PubMed] [Google Scholar]
- Skirycz A, Inzé D (2010) More from less: plant growth under limited water. Curr Opin Biotechnol 21: 197–203 [DOI] [PubMed] [Google Scholar]
- Skirycz A, Vandenbroucke K, Clauw P, Maleux K, De Meyer B, Dhondt S, Pucci A, Gonzalez N, Hoeberichts F, Tognetti VB, et al. (2011) Survival and growth of Arabidopsis plants given limited water are not equal. Nat Biotechnol 29: 212–214 [DOI] [PubMed] [Google Scholar]
- Smyth GK. (2005) Limma: linear models for microarray data. In Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, eds, Bioinformatics and Computational Biology Solutions using R and Bioconductor. Springer, New York, pp 397–420 [Google Scholar]
- Spollen WG, Tao W, Valliyodan B, Chen K, Hejlek LG, Kim JJ, Lenoble ME, Zhu J, Bohnert HJ, Henderson D, et al. (2008) Spatial distribution of transcript changes in the maize primary root elongation zone at low water potential. BMC Plant Biol 8: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A (2004) Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiol 134: 1536–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M (2004) MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37: 914–939 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang ZG, Wei YD, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187–1194 [Google Scholar]
- Tsukagoshi H, Busch W, Benfey PN (2010) Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143: 606–616 [DOI] [PubMed] [Google Scholar]
- Usadel B, Nagel A, Steinhauser D, Gibon Y, Bläsing OE, Redestig H, Sreenivasulu N, Krall L, Hannah MA, Poree F, et al. (2006) PageMan: an interactive ontology tool to generate, display, and annotate overview graphs for profiling experiments. BMC Bioinformatics 7: 535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bel M, Proost S, Wischnitzki E, Movahedi S, Scheerlinck C, Van de Peer Y, Vandepoele K (2012) Dissecting plant genomes with the PLAZA comparative genomics platform. Plant Physiol 158: 590–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Slooten L, Stassart JM, Moens T, Botterman J, Van Montagu M, Inzé D (1999) Overproduction of Arabidopsis thaliana FeSOD confers oxidative stress tolerance to transgenic maize. Plant Cell Physiol 40: 515–523 [DOI] [PubMed] [Google Scholar]
- Van Camp W, Capiau K, Van Montagu M, Inzé D, Slooten L (1996) Enhancement of oxidative stress tolerance in transgenic tobacco plants overproducing Fe-superoxide dismutase in chloroplasts. Plant Physiol 112: 1703–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandepoele K, Vlieghe K, Florquin K, Hennig L, Beemster GTS, Gruissem W, Van de Peer Y, Inzé D, De Veylder L (2005) Genome-wide identification of potential plant E2F target genes. Plant Physiol 139: 316–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Coppens F, Lee E, Donner T, Xie Z, Isterdael GV, D’Hondt S, Winter FD, Rybel BD, Veylder LD, et al. (2011) Developmental regulation of CYCA2s contributes to tissue-specific proliferation in Arabidopsis. EMBO J 30: 3430–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci 151: 59–66 [Google Scholar]
- Vernoux T, Wilson RC, Seeley KA, Reichheld JP, Muroy S, Brown S, Maughan SC, Cobbett CS, Van Montagu M, Inzé D, et al. (2000) The ROOT MERISTEMLESS1/CADMIUM SENSITIVE2 gene defines a glutathione-dependent pathway involved in initiation and maintenance of cell division during postembryonic root development. Plant Cell 12: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK (2006) Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J 45: 523–539; erratum Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK (2006) Plant J 46: 1092 [DOI] [PubMed] [Google Scholar]
- Wallace JS. (2000) Increasing agricultural water use efficiency to meet future food production. Agric Ecosyst Environ 82: 105–119 [Google Scholar]
- Walter A, Silk WK, Schurr U (2009) Environmental effects on spatial and temporal patterns of leaf and root growth. Annu Rev Plant Biol 60: 279–304 [DOI] [PubMed] [Google Scholar]
- Wand MP, Jones MC (1995) Kernel Smoothing. Chapman and Hall, London [Google Scholar]
- Ware D, Jaiswal P, Ni J, Pan X, Chang K, Clark K, Teytelman L, Schmidt S, Zhao W, Cartinhour S, et al. (2002) Gramene: a resource for comparative grass genomics. Nucleic Acids Res 30: 103–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H, Chételat A, Reymond P, Farmer EE (2004) Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J 37: 877–888 [DOI] [PubMed] [Google Scholar]
- West G, Inzé D, Beemster GTS (2004) Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol 135: 1050–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willekens H, Inze D, Vanmontagu M, Van Camp W (1995) Catalases in plants. Mol Breed 1: 207–228 [Google Scholar]
- Xu Z, Zhou G, Shimizu H (2009) Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J Exp Bot 60: 3737–3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Sharp RE (2010) Complexity and coordination of root growth at low water potentials: recent advances from transcriptomic and proteomic analyses. Plant Cell Environ 33: 590–603 [DOI] [PubMed] [Google Scholar]
- Zhu J, Alvarez S, Marsh EL, Lenoble ME, Cho IJ, Sivaguru M, Chen S, Nguyen HT, Wu Y, Schachtman DP, et al. (2007) Cell wall proteome in the maize primary root elongation zone: II. Region-specific changes in water soluble and lightly ionically bound proteins under water deficit. Plant Physiol 145: 1533–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.