Abstract
The mammalian BAD protein belongs to the BH3-only subgroup of the BCL-2 family. In contrast to its known pro-apoptotic function, we found that endogenous and overexpressed BADL can inhibit cell death in neurons and other cell types. Several mechanisms regulate the conversion of BAD from an anti-death to a pro-death factor, including alternative splicing that produces the N-terminally truncated BADS. In addition, caspases convert BADL into a pro-death fragment that resembles the short splice variant. The caspase site that is selectively cleaved during cell death following growth factor (inter-leukin-3) withdrawal is conserved between human and murine BAD. A second cleavage site that is required for murine BAD to promote death following Sindbis virus infection, γ-irradiation, and staurosporine treatment is not conserved in human BAD, consistent with the inability of human BAD to promote death with these stimuli. However, loss of the BAD N terminus by any mechanism is not always sufficient to activate its pro-death activity, suggesting that the N terminus is a regulatory domain rather than an anti-death domain. These findings suggest that BAD is more than an inert death factor in healthy cells; it is also a pro-survival factor, prior to its role in promoting cell death.
Healthy mammalian cells constitutively express pro-apoptotic BCL-2 family members such as the BH3-only protein BAD and the multidomain proteins BAX and BAK. These proteins are generally regarded as latent death factors that are normally held in check and must be activated to exhibit their death-inducing functions. However, it is possible that pro-death BCL-2 family proteins are not only latent death factors but also carry out important functions in healthy cells. Consistent with this idea, BAX and BAK can function as potent anti-death factors in cultured neurons and in mouse models (1–4). This dual function of BAK may be explained by its ability to decrease neuronal excitability in healthy neurons because this function is consistent with both its ability to promote death during development (based on the phenotype of double Bax/Bak knock-out mice) and to inhibit death induced by excitotoxic stimuli in postnatal animals (4, 5). Consistent with this dual role for BAX and BAK, the anti-apoptotic family members human BCL-2 and BCL-xL and Caenorhabditis elegans CED-9 can function as pro-death factors (6–8). Several mechanisms appear to contribute to the activation of the pro-death function of Bcl-2 family proteins. BAX undergoes conformational changes, relocalizes to mitochondria, may oligomerize with other BAX molecules in the mitochondrial membrane, and can be cleaved by calpain to enhance its pro-death activity (9–12). Cleavage of BCL-2 and BCL-xL by caspases produces C-terminal fragments that behave similarly to pro-apoptotic BAX in that they localize to mitochondria, induce cytochrome c release, and can form pores in synthetic membranes that are sufficient in size to pass cytochrome c and larger molecules (7, 13, 14); however, non-proteolytic events can also explain the pro-death effects of BCL-2 (15).
Several mechanisms have been proposed to explain how BAD and other BH3-only proteins facilitate cell death by inhibiting anti-apoptotic BCL-2 proteins, by activating pro-death BAX and BAK, or by regulating mitochondrial structure (16–18). BAD plays an important role in connecting the growth factor signaling pathway with the cell death pathway. Recent genetic evidence confirms that when a variety of cell types are deprived of their extracellular survival signals, BAD is dephosphorylated to assume its pro-death function (19, 20). Although it is logical that the pro-death function of BAD must be inactivated or suppressed in healthy cells, this does not preclude the possibility that BAD and other pro-death BCL-2 family proteins have anti-death activity or alternative biochemical functions that benefit healthy cells prior to receiving a death stimulus. Analogously, cytochrome c has a key function in the mitochondrial respiratory chain of healthy cells but promotes caspase activation in the cytosol following a death stimulus (21).
BAD-deficient mice are developmentally normal by most criteria, including normal B and T cell development (20). However, isolated thymocytes, fibroblasts, and epithelial cells from BAD-deficient mice were found to be variably resistant to cell death induced by growth factor withdrawal, consistent with a pro-death function of BAD under these conditions (20). Furthermore, BAD-deficient mice develop B cell lymphomas and other malignancies, and this propensity is dramatically increased by exposure to limited γ-irradiation (20). Again, this tumor suppressor phenotype is consistent with a pro-death function for BAD; however, seemingly inconsistent with a pro-death function, BAD was reported to promote glycolysis, a pathway induced by the same growth factors that “inactivate” the pro-death function of BAD (22). That is, BAD-deficient mice and derived hepatocytes have a reduced capacity to utilize glucose apparently because of the requirement for BAD to assemble active glucokinase (liver hexokinase) complexes (22). Thus, it is paradoxical that a pro-death factor facilitates utilization of glucose in the first step of the glycolytic pathway. This paradox could be resolved if, prior to its conversion to a pro-death factor, BAD has a normal cellular function that promotes maintenance/survival of the cell, but BAD and other BH3-only proteins have not previously been found to play a physiological role in promoting cell survival.
Here we show that BAD can function as a potent inhibitor of cell death and is converted into a pro-death factor by multiple mechanisms that alter or inactivate the BAD N terminus. These include splicing, dephosphorylation, and caspase cleavage at death stimulus-specific sites. The N terminus of BAD is important but not essential for anti-death function in all paradigms, suggesting that the N terminus is a guard against activation of pro-death function rather than a direct anti-death domain. These findings are consistent with the possibility that other “pro-death” Bcl-2 family proteins may have normal cell functions that promote survival.
MATERIALS AND METHODS
Plasmids and Transfections
Murine BADL (Stratagene) and human BADS (Science Reagents) cDNAs were inserted into pSG5 (Stratagene) or derivative pDB59 (containing an N-terminal HA1 tag) for expression in mammalian cells or in vitro transcription/translation. Point mutants of BAD were generated by two-step PCR, and those clones with a perfect sequence were selected for further study. Cell lines were transfected with Fugene 6 (Roche Applied Science). Primary cortical neurons were transfected with calcium phosphate as described previously (23, 24). Briefly, the media of cortical neurons were replaced with Dulbecco's modified Eagle's medium and saved. DNA/calcium phosphate precipitate was prepared by mixing 1 μg of GFP plasmid and 5 μg of the expression vector for wild type or D61A mBAD in 250 mm CaCl2 with 2× Hepes-buffered saline solution (274 mm NaCl, 10 mm KCl, 1.4 mm Na2HPO4, 15 mm d-glucose, 42 mm Hepes, pH 7.05). After 25–30 min, 120 μl of the DNA/calcium phosphate precipitate were added dropwise to each 60-mm-diameter dish and incubated in a 5% CO2 incubator. Twenty-five min after the first precipitate formed, cells were washed three times with Dulbecco's modified Eagle's medium and returned to the saved medium.
Viruses and Animals
Coding sequences for wild type and mutant BAD were subcloned from pSG5/pDB59 into the Sindbis virus vectors dsTE12Q (for infection of newborn mice) and dsNSV (for infection of 5-week-old mice) (3, 25) or into the retrovirus construct pLXSN (26) for generating recombinant viruses. Control Sindbis viruses contain the reverse (non-coding) orientations of mBADL, viral KSBcl-2, or other irrelevant cDNA of similar size to ensure equivalent replication rates between viruses by maintaining the same genome size in experimental and control viruses. The data obtained with various control viruses were indistinguishable (thereby eliminating concerns about antisense effects on mBAD). Data presented here were generated with Sindbis viruses prepared from at least two independently derived constructs. Mice were inoculated by intracranial injection with 5000 plaque-forming units of Sindbis virus or with buffer diluent alone. Cultured cell lines and dissociated rat or mouse primary cortical neurons were infected with 5 plaque-forming units/cell. Organotypic cultures were infected with 5 × 106 plaque-forming units/tissue slice. BAD knock-out mice and genotyping strategy were described previously (20). The BAD knock-out mice were backcrossed once to a C57BL/6 mouse, and the colony was interbred over a 2-year period. All experiments were performed on littermates from heterozygous crosses and subsequently correlated to the genotype.
Cell Lines, Viability Assays, and Immunoblots
COS-1 (African green monkey kidney), BHK (baby hamster kidney), and HEK293 (human embryonic kidney) cells were grown in Dulbecco's modified Eagle's medium with 10% heat-inactivated fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. Ba/F3 (murine hematopoietic) cells were grown in RPMI 1640 medium supplemented with IL-3 (26). Stable BHK cell lines were established by co-transfection with a neomycin-resistant plasmid (at a 1:10 ratio with BAD-expressing plasmids) and selected in 0.5 μg/ml G418. Individual clones were separated, maintained in G418, and analyzed only at low passage numbers. Cell lysates were harvested in radioimmune precipitation assay buffer containing protease inhibitors (aprotinin, benzamidine, chymostatin, leupeptin, pepstatin A, and phenylmethylsulfonyl fluoride) and separated by SDS-PAGE for immunoblot analysis. Viability of various cell lines was determined by trypan blue dye exclusion, by propidium iodide (PI) staining and flow cytometry or fluorescence microscopy, or by co-transfection or [H9252]-galactosidase assay as described previously (27). IL-3 withdrawal-induced cell death was performed as described previously (7).
Primary Neurons
Dissociated cultures of (postmitotic) cortical neurons were prepared from embryonic day 15-16 Sprague-Dawley rats or embryonic day 13-14 mice as described previously (23) and plated in 24-well dishes (1 × 105/well) with 5-fluoro-2’-deoxyuridine from days 3 to 6 after plating to inhibit expansion of non-neuronal cells that constitute <10% of the cultures (data not shown). Death of the infected cortical neurons was assessed by determining the percentage of neurons that were stained with PI relative to the total number of Hoechst-stained nuclei. Randomly selected fields were imaged and printed for counting. N-methyl-d-aspartate and staurosporine treatments were performed (4). Transfected cortical neurons were scored for viability or death based on morphology of GFP-positive cells. Those cells with no processes or with two or more fragmented processes were scored as non-viable. Hippocampal and spinal cord tissues were dissected from newborn mice, and four 200-μm slices were cultured on one Millipore membrane per well with basal medium Eagle (Invitrogen) plus 25% horse serum in 6-well dishes (28). The viability of organotypic hippocampal or spinal cord cultures was assessed by incubating cultures for 16 h in 0.1 mg/ml PI. Relative PI intensity was determined by computer-based image analysis (4).
In Vitro Caspase Cleavage
35S-Labeled, in vitro transcribed and translated proteins were digested with recombinant purified caspase-3 (2000 units) or caspase-7 (750 units) as described previously (29).
RESULTS
BAD Switches from Anti-death to Pro-death Function during Neuron Maturation
Sindbis virus can induce classic morphological and biochemical characteristics of apoptotic cell death in a variety of cell lines and in neurons (30, 31). The use of Sindbis virus as a vector to express both anti- and pro-death BCL-2 family proteins in cell cultures and in animals has faithfully revealed the endogenous function of these proteins (1, 4, 32). Therefore, the function of BAD was quantified by inserting a cDNA encoding the widely studied long form of murine BAD into the Sindbis virus genome and measuring the ability of mBADL to modulate the outcome of a Sindbis virus infection. Infection of COS-1 cells with recombinant Sindbis virus encoding murine BADL (SV-mBADL) confirmed the expected pro-death function of transiently overexpressed mBADL. That is, BAD accelerated Sindbis virus-induced cell death compared with control viruses (SV-control) (Figs. 1A and 4B). In contrast, BCL-xL inhibited Sindbis virus-induced cell death as reported previously (33).
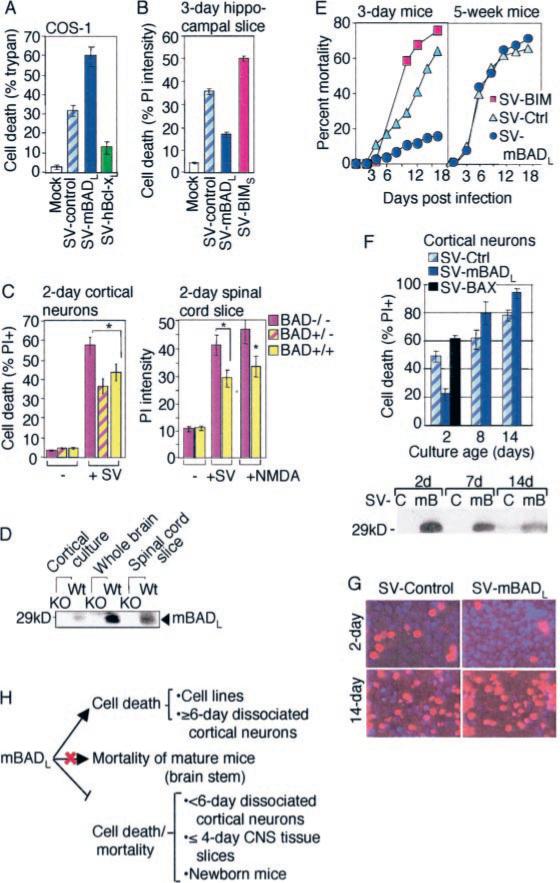
Fig. 1. Cell type and age-dependent regulation of cell death by mBAD.
A, cell death of COS-1 cells was determined by trypan blue staining at 24 h after infection with the indicated recombinant SV encoding HA-tagged murine BADL or HA-tagged human BCL-xL proteins (mean ± S.E. of three independent experiments). B, neuronal death in tissue slices cultured for 3 days prior to infection was determined by fluorescence-based computer imaging of PI staining at 68 h after infection with recombinant viruses expressing mBADL or mBIMS (mean ± S.E. of three independent experiments). C, cell death of 2-day-old cultures of dissociated cortical neurons prepared from individual bad+/+, bad+/−, and bad−/− embryos was determined by counting >3000 dual stained (PI-Hoechst) cells in 15 randomly selected fields per sample at 0 and 24 h after infection with Sindbis virus (SV-control) and subsequently correlated to genotypes. Neuronal death in 2-day-old cultures of spinal cord slices prepared from genotyped newborn littermates was determined as described for B (bad+/− cultures were not tested). Data shown are the mean ± S.E. for three independent experiments (*, p < 0.05 compared with bad−/−). D, immunoblot of lysates from neuronal cultures described in C or brain tissue from bad+/+ (Wt) and bad−/− (KO) mice using anti-BAD antibody C-20 (Santa Cruz Biotechnology). E, percentage of mortality following intracranial injection of the indicated recombinant Sindbis viruses in 3-day-old CD-1 mice (n > 65/group) or 5-week-old C57BL/6 mice (n > 30/group). F, cell death of rat embryonic cortical neurons was determined at 48 h after infection with the indicated recombinant viruses as described for C (counting > 1000 cells/sample in each of three independent experiments). An immunoblot for BAD in SV-control (SV-C) and SV-mBADL (mB)-infected cortical neuron cultures is shown (lower panel). G, representative fields of PI-Hoechst-stained cortical neurons from F are shown. H, summary of the age-dependent function of BAD. The precise neuron population responsible for virus-induced mortality is not known but is likely to involve the brain stem that regulates vital functions.
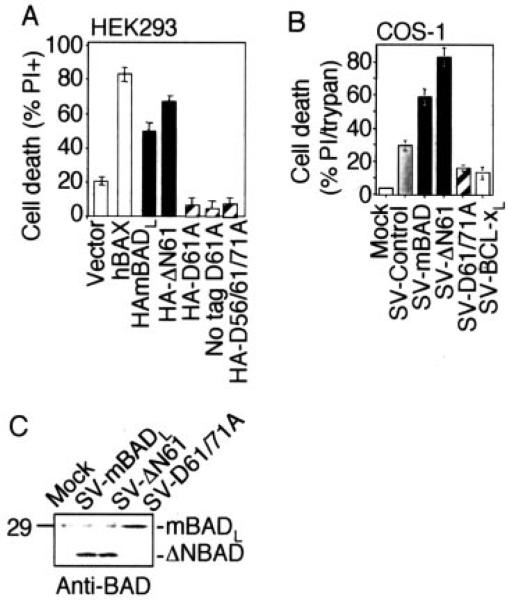
Fig. 4. Murine BAD enhances cell death, whereas uncleavable BAD suppresses cell death in transient assays.
A, percentage of death of HEK293 cells at 48 h after transfection of the indicated plasmids was determined by morphology. B, percentage of cell death of COS-1 cells was determined at 48 h after infection with the indicated recombinant Sindbis viruses. Data were compiled from three independent experiments that were analyzed by PI staining and flow cytometry or by trypan blue staining counting >200 cells/sample. C, immunoblot of BHK cell lysates at 24 h after infection using anti-BAD antibody C-20 (Santa Cruz Biotechnology).
Because Sindbis virus is neuronotropic, the same recombinant viruses were used to determine the function of overex-pressed BAD in neurons of organotypic hippocampal slice cultures prepared from 3-day-old mice (4, 34). Contrary to expectation, mBADL significantly reduced Sindbis virus-induced neuronal death compared with control virus-infected cultures, suggesting that mBADL protects hippocampal neurons from virus-induced death (Figs. 1B and 7B). In contrast, another pro-death BH3-only family member, BIMS, was a potent pro-death factor in this model (Fig. 1B). This finding with BIM strongly argues against the possibility that the anti-death function of BAD results from the indirect effects of its pro-death function.
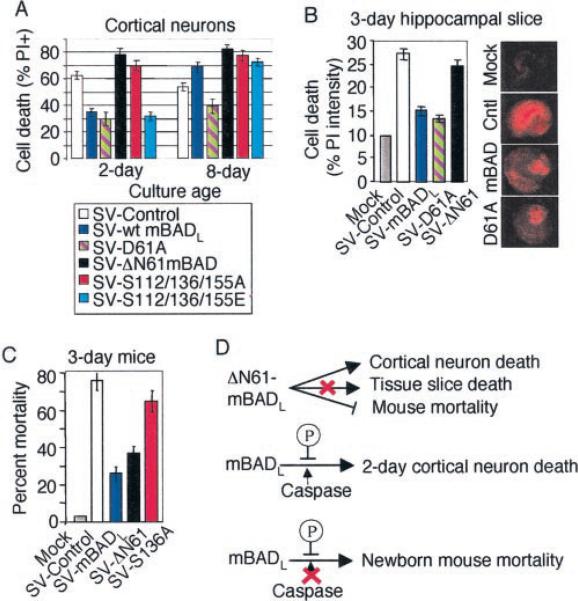
Fig. 7. Caspases and phosphorylation regulate mBAD function in immature neurons.
A, cell viability of 2- and 8-day-old embryonic rat cortical neuron cultures following infection with the indicated recombinant viruses was determined by PI-Hoechst double staining as described for Fig. 1, F and G. B, percentage of cell death of infected hippocampal slice cultures was determined as described for Fig. 1B. Data presented are the mean ± S.E. for three independent experiments. Representative images of PI-stained hippocampal slice cultures show a pattern of cell death consistent with the neurotropism of Sindbis virus. C, percentage of mortality of 3-day-old CD-1 mice (n > 100/group) infected with the indicated viruses was determined as described for Fig. 1E. D, summary of the roles of caspases and phosphorylation in regulating BAD function in immature neurons.
To determine whether endogenous BAD is an anti-death factor in neurons, dissociated embryonic cortical neurons were prepared from bad-deficient mice and infected with Sindbis virus as a death stimulus. Consistent with the overexpression model, more cell death occurred in bad−/− cortical neurons and spinal cord slices compared with cultures prepared from bad+/+ or bad+/− control littermates, indicating that endogenous BAD is protective in these immature neurons (Fig. 1C). Spinal cord slice cultures prepared from newborn bad+/+ and bad−/− mice yielded similar results when exposed to Sindbis virus or N-methyl-d-aspartate to induce an excitotoxic death. Thus, endogenous BAD also protects neurons against a nonviral death stimulus. Confidence in these findings is enhanced because they were generated in primary cells derived from several different animals per genotype. Genotypes of cultures and mice were confirmed by immunoblot analysis (Fig. 1D).
The tropism of Sindbis virus for central nervous system neurons in mice results in neuronal death that strongly correlates with mouse mortality (4, 31). Furthermore, endogenous and overexpressed BCL-2 (via the Sindbis virus vector) protects newborn mice from neuronal apoptosis (32, 34). Therefore, BAD was evaluated by a similar approach. Infection of newborn bad-deficient mice with Sindbis virus (SV-control) caused increased mouse mortality compared with control bad+/+ and bad−/− littermates, consistent with a protective function of BAD (data not shown). However, the interpretation of this result is complicated by the fact that these mice are defective for production of antigen-stimulated antibody (20), which is critical for clearing Sindbis virus from infected mouse brains (35). Therefore, to confirm that BAD protects in animals, 3-day-old wild type mice were infected with recombinant Sindbis virus expressing mBADL. These mice had increased survival compared with those infected with control virus, consistent with the protective function of mBADL (Fig. 1E). In contrast, the pro-death BH3-only protein BIMS significantly accelerated mouse mortality, arguing that the protective effect is specific to mBADL (Fig. 1E).
In contrast to newborn mice, mBADL failed to significantly alter the rate or extent of mortality in mature (5-week-old) mice (Fig. 1E). To confirm this age-dependent shift in BAD function using an alternate model, BAD was evaluated in dissociated embryonic rat cortical neurons that were allowed to mature in culture 2–14 days prior to infection. Overexpression of mBADL (SV-mBADL) protected 2 day-old cultures, similar to the function of endogenous BAD (compare Fig. 1, C and F). Unlike BAD, BAX was modestly pro-death in these cells as expected. In contrast to the younger cultures, mBADL exhibited consistent pro-death activity in 8–14-day-old cultures (Fig. 1F). Viability of cortical neurons was determined by dual staining with Hoechst to detect nuclei and PI to detect dead cells as shown for representative fields (Fig. 1G). These findings indicate that BAD is converted from a pro-survival to a pro-death or neutral factor as postmitotic cortical neurons mature in culture and in vivo (Fig. 1H).
Caspase Cleavage of mBAD Promotes Age-dependent Neuronal Death
To pursue the mechanisms responsible for converting BAD into a pro-death factor as neurons mature, a role for proteolysis was examined based on the observation that other anti-apoptotic BCL-2 family members can be converted into killer proteins when cleaved by caspases (7). In addition, the pro-death function of murine BAD was reported to be enhanced when BAD is cleaved by caspases at Asp-56 and Asp-61 (36), positions roughly analogous to the caspase cleavage sites in BCL-2, BCL-xL, and the BH3-only protein BID. Using in vitro translated 35S-labeled mBADL, we failed to detect cleavage at Asp-56 because the only cleavage site detected with recombinant purified caspase-3 and caspase-7 was at Asp-61 (Fig. 2A). Mutation of the only other potential caspase cleavage site in this region, Asp-71, also did not appear to alter cleavage patterns (Fig. 2B).
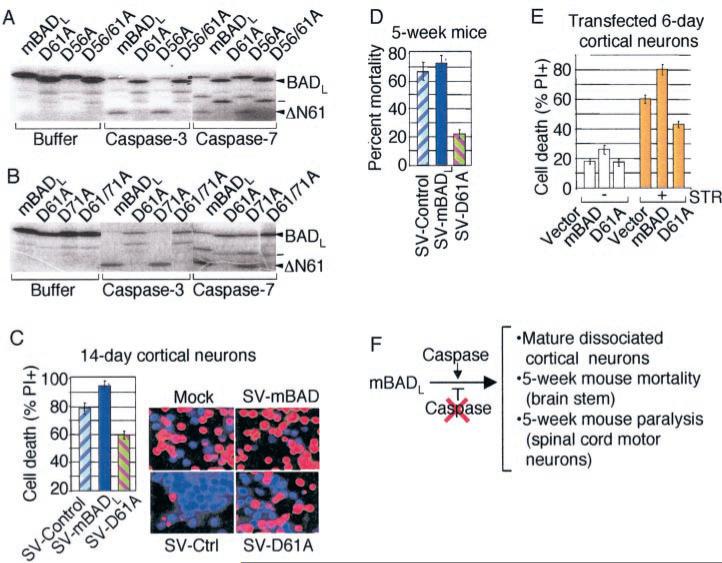
Fig. 2. Caspase cleavage of mBAD promotes death in mature neurons.
A and B, in vitro translated 35S-labeled wild type mBADL and derived point mutants were treated with buffer only, caspase-3 or -7 and analyzed by SDS-PAGE and autoradiography. (A potential unidentified caspase-7 cleavage product is marked with a dash.). C, cell death of infected rat cortical neurons was determined as described for Fig. 1, F and G. D, mortality of 5-week-old C57BL/6 mice (n > 20/group from three independent experiments) was determined as described for Fig. 1E. E, viability was determined by morphology of GFP-positive cells at 48 h after cotransfection of 6–7-day-old rat cortical neuron cultures with the indicated plasmids (5 μg) plus GFP (1 μg) in the absence or presence of 150 nm staurosporine (STR) during the final 24 h. F, summary of the role of caspases in converting BAD into a pro-death factor in mature neurons.
To determine whether caspase cleavage contributes to the pro-death function of BAD in mature neurons, 14-day rat cortical cultures and 5-week-old mice were infected with Sindbis virus expressing a point mutant of mBADL in which the aspar-tate 61 cleavage site was changed to alanine (D61A). Compared with wild type BAD and controls, this mutant significantly inhibited death of mature cortical neurons (Fig. 2C), reduced mortality in mature mice (Fig. 2D), and decreased the extent of hind limb paralysis (reflecting spinal cord motor neuron function, data not shown). Thus, the neutral or pro-death function of wild type mBADL in mature neurons was converted to anti-death activity by a point mutation in the Asp-61 cleavage site. Uncleavable BAD also protected against a non-viral death stimulus because cortical neurons transfected with mBADL (D61A) were protected from cell death induced by staurosporine (Fig. 2E). These results suggest that caspase cleavage of BAD at position 61 is required to activate its pro-death function in these models (Fig. 2F).
Caspases Convert mBAD from an Anti- to a Pro-death Factor in Cell Lines
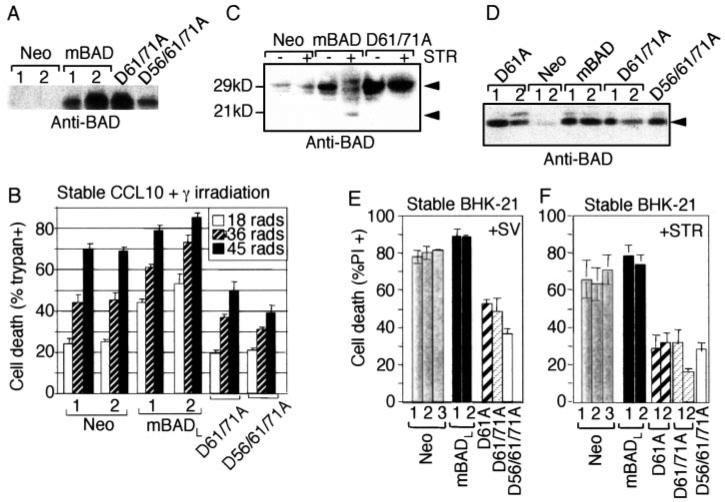
To determine whether caspase cleavage serves to regulate mBAD function in non-neuronal cells, stable CCL10 BHK cell lines were generated that overexpress wild type mBADL or uncleavable mutants in which Asp-56, Asp-61, and Asp-71 were changed to Ala (Fig. 3A). Consistent with the established pro-death function of BAD, cells expressing wild type mBADL were more susceptible to cell death induced by γ-irradiation in a dose-dependent manner compared with control cells stably transfected with the neo vector alone. However, double or triple point mutations in potential caspase cleavage sites caused mBADL to exhibit a gain of anti-death activity (Fig. 3B). Similar results were obtained with the same cell lines when cell death was induced by Sindbis virus infection or staurosporine treatment (data not shown). Mutation of the caspase cleavage site impaired cleavage of BAD in these cells because immunoblot analysis of staurosporine-treated cells revealed the predicted 21-kD cleavage product of wild type mBADL, whereas the full-length D61A/D71A mutant was stabilized and cleavage-resistant (Fig. 3C). Another series of stably transfected cell clones were generated in the related BHK-21 cell line, including this time the single point mutant D61A (Fig. 3D). Single, double, or triple cleavage site mutants of mBADL significantly inhibited cell death induced by virus infection (Fig. 3E) and staurosporine treatment (Fig. 3F). Like cortical neurons, the Asp-61 site appears to be primarily responsible for activating the pro-death function of mBADL, consistent with the cleavage site specificity of recombinant caspases in vitro.
Fig. 3. Uncleavable mBAD protects stable cell lines from apoptotic stimuli.
A, immunoblot analysis of the stably transfected CCL10-BHK cells expressing the indicated HA-tagged constructs (individual cell clones are numbered, 1 and 2), loading 20–25 μg/lane and blotting with anti-BAD antibody C-20 (Santa Cruz Bio-technology). B, death of the cells shown in A was determined by trypan blue staining at 48 h after exposure to the indicated doses of γ-irradiation (mean ± S.E. for three independent experiments counting > 150 cells/sample). C, immunoblot analysis of the indicated stable CCL10-BHK cells with or without treatment with 1 μm staurosporine (STR) for 3 h was performed as described for A. Arrows mark full-length and cleaved BAD. D, immunoblot analysis (anti-BAD C-20) of individual BHK-21 cell clones (numbered, 1 and 2) stably transfected with the indicated constructs. E and F, cell death of BHK-21 cell line from D was determined by PI staining, flow cytometry, and/or manual counting at 24 h after infection with control Sindbis virus or 3 h after treatment with 1 μm staurosporine. Data presented are the mean ± S.E. from duplicate determinations in each of three independent experiments.
Results obtained with these stable cell lines could be potentially misleading because only those cells that are resistant to the pro-death functions of overexpressed BAD will survive the selection process to produce clonal cell lines. Although this is unlikely to explain the anti-death function of uncleavable mBADL, we returned to transient assays using either trans- fected plasmids or Sindbis virus vectors. Consistent with the observations of many laboratories, transfection of mBADL into HEK293 cells induced cell death in the absence of an additional death stimulus, albeit less efficiently than BAX (Fig. 4A). Also consistent with the findings of others (36, 37), the pro-death function of BAD increased when the N terminus (amino acids 2–61) was deleted to mimic caspase-cleaved mBADL (ΔN61). However, the pro-death function of mBADL was completely dependent on the Asp-61 cleavage site because the D61A and triple D56A/D61A/D71A mutants had no killing activity and even reduced the slight toxic effects of transfection compared with vector control (Fig. 4A). Similar results were obtained in transiently transfected COS-1 cells (data not shown). To verify that uncleavable BAD inhibits death in cell lines, an additional death stimulus was applied. Transiently overexpressed caspase-resistant BAD (via the Sindbis virus vector) protected COS-1 cells (Fig. 4B) and BHK cells (data not shown) from Sindbis virus-induced death as efficiently as human BCL-xL. The generally enhanced cell death by truncated ΔN61 over wild type mBADL in the same assays (Fig. 4, A and B) supports the hypothesis that mutation of Asp-61 serves to inhibit proteolytic cleavage of BAD rather than by ablating some other intrinsic pro-death function of BAD. Immunoblot analysis of infected COS-1 and BHK cells confirmed that wild type mBADL was cleaved in apoptotic cells to produce a fragment that co-migrates with an engineered ΔN61-BAD, whereas uncleavable D61A/ D71A mBADL remained stable (Fig. 4C, and data not shown).
Splicing Regulates Activation of hBAD Pro-death Activity
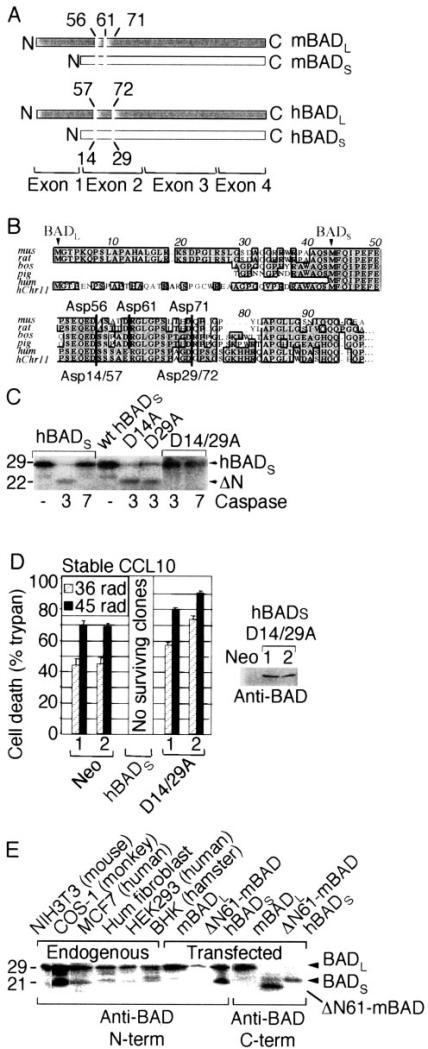
There are two prominent forms of murine BAD proteins expressed in cell lines and tissues, the widely studied mBADL and the shorter mBADS that is produced by alternative splicing and lacks an exon 1-encoded N-terminal sequence (20) (Fig. 5A). In contrast, only one form of human BAD, corresponding to mBADS, has been identified, and current annotation of the human genome indicates only three exons that correspond to exons 2–4 in the mouse genome. Another striking difference between mouse and human BAD is that human BAD lacks the critical Asp-61 caspase cleavage site of mBADL. This aspartate is conserved between mouse and other mammalian BAD proteins, but human BAD has a Glu at this position. Although caspases from other species may also cleave following Glu (38), the absence of Asp-61 in hBAD casts doubt on the significance of this cleavage event, at least in humans (Fig. 5B).
Fig. 5. Regulation of human BAD function by splicing.
A, diagram of the long and short mouse and human BAD proteins produced by alternative splicing. Caspase cleavage site positions are numbered. B, alignment of predicted N-terminal amino acid sequences of BAD from mouse (mus), rat (rat), cow (bos) (Bos taurus, BF043486), pig (pig) (Sus scrofa, BF441436), and human (hum) chromosome 11 (hChr11). Expressed sequence tags for human slice variants 2 (NM_032989) and 1 (NM_004322) also lack the long N terminus. The predicted N termini of long and short splice variants (arrowheads) and potential caspase cleavage sites (thick vertical lines with amino acid numbers) are indicated. C, autoradiograph of in vitro translated (untagged) hBADS and derived point mutants as described for Fig. 2A. D, cell death of the indicated stably transfected CCL10-BHK cell clones (numbered) following exposure to the indicated doses of γ-irradiation was determined as described for Fig. 3B. (No cells transfected with wild type human BADS survived selection.) An immunoblot of untreated cell extracts is shown. E, immunoblot of endogenous or transfected BAD. Cell lysates were prepared from the indicated cell lines (left) or from HEK293 cells transfected with plasmids (2 μg) encoding the indicated untagged BAD constructs (right) and detected by immunoblot with anti-BAD antibodies H-168 (N terminus) or C-20 (C terminus).
To determine whether hBADS is susceptible to caspase cleavage at Asp-14 and Asp-29 (corresponding to Asp-56 and -71 of mBADL), in vitro translated 35S-labeled human BADS was incubated with purified caspases. In contrast to murine BAD, recombinant caspase-3 cleaved hBADS at both Asp-14 and Asp-29 because both sites must be mutated to block in vitro cleavage of human BADS (Fig. 5C). Unlike murine BAD, no caspase-7 cleavage products of human BADS were observed (lane 3). To determine whether caspases also regulate the func- tion of hBADS, stable CCL10-BHK cell lines were generated. However, hBADS was found to be toxic based on the observation that no cell clones expressing hBADS survived selection in several experiments in which numerous cell colonies were produced with transfected uncleavable hBADS mutant or control vector. Nevertheless, cells expressing uncleavable human BADS (D14A/D29A) were not protected from γ-irradiation but instead had 10–30% increased cell death (Fig. 5D). Thus, although caspase cleavage enhanced the pro-death activity of hBADS, cleavage of this short form of hBAD was not required for pro-death function. In this way, the shorter splice variant of human BAD resembles the caspase cleavage product of mBADL (Fig. 5B).
These observations suggested that humans have only the pro-death version of BAD and lack the longer anti-death form of BAD found in mice. However, some commercially available antibodies to human BAD were advertised to detect a larger ~29-kD BADL species in human cells that corresponds in size to mBADL (Santa Cruz Biotechnology and Upstate Biotechnology). Similarly, immunoblot analysis for endogenous BAD in cell lines derived from human and other species revealed two endogenous BAD proteins corresponding in size to transfected murine BADL and transfected human BADS (Fig. 5E, and data not shown). The short form of endogenous BAD is unlikely to be a cleavage product because it is recognized by a BAD antibody that fails to detect the engineered caspase cleavage product ΔN61-mBAD.
To explore the possibility that humans have an antiapoptotic form of BAD with additional N-terminal sequences, the amino acid sequence from exon 1 of murine BAD was used in a BLAST search of the human genome. A single hit was found on chromosome 11 starting at ~500 nucleotides upstream of the known human BAD gene. By joining the putative human exon 1 sequences at predicted splice sites, the predicted human BAD protein has a 43-amino acid N-terminal extension (Fig. 5B); however, concerted effort failed to produce a cDNA for hBADL. This may be caused in part by the predicted extensive secondary structure in the 5’ region of hBAD (using Mfold).
Death Stimulus-specific Cleavage Site Specificity Is Conserved in Mouse and Human BAD
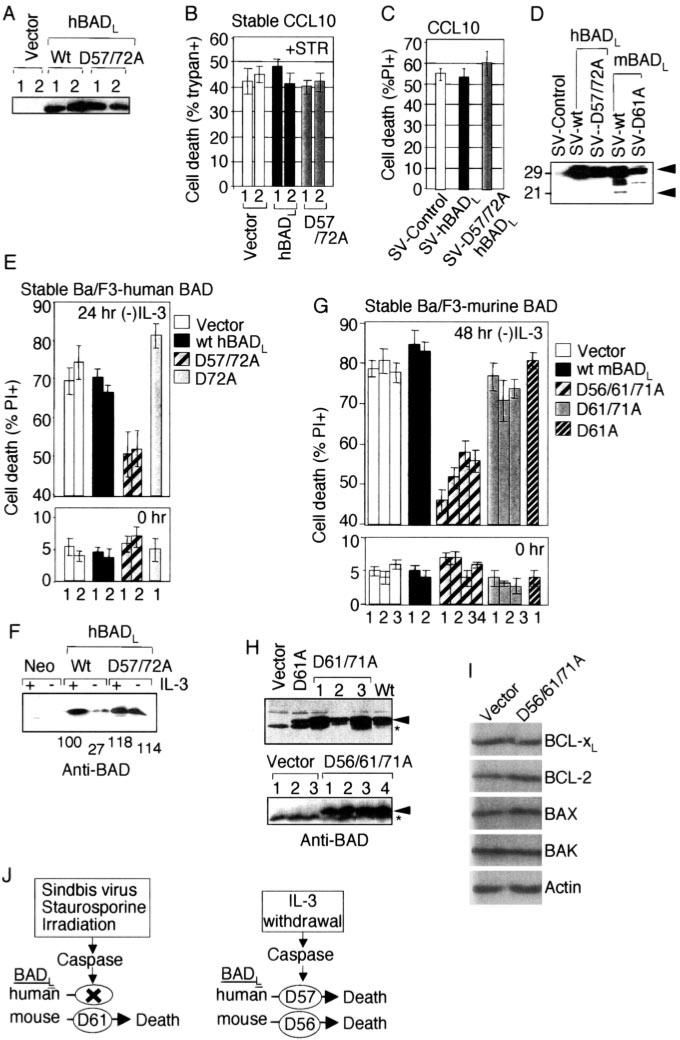
To determine whether the predicted human BADL protein has anti-death activity, an engineered cDNA (with alternate codon usage to avoid potential hairpins) was tested in stably transfected CCL10-BHK cell clones (Fig. 6A). Both the wild type and the double cleavage site mutant (corresponding to amino acids Asp-57 and −72 in hBADL) failed to alter cell death triggered by staurosporine treatment (Fig. 6B). Similarly, neither protein exhibited pro- or anti-death activity in a transient assay of Sindbis virus-induced death (Fig. 6C). Immunoblot analysis also failed to detect a stable cleavage product of hBADL in virus-infected cells, whereas mBADL was processed under the same conditions (Fig. 6D). Thus, human BADL may fail to kill cells because it lacks the critical Asp-61 cleavage site required to convert murine BADL into a pro-death factor by these death stimuli.
Fig. 6. A death stimulus-specific cleavage site is conserved in human and mouse BAD.
A, immunoblot analysis (anti-BAD C-20) of human BAD in untreated CCL10-BHK cell clones (numbered) stably transfected with the indicated constructs. B, percentage of cell death in the cell lines shown in A was determined by counting fluorescence microscopy images of PI-stained samples following treatment with 1 μm staurosporine (+STR). Data presented are the mean ± S.E. from duplicate determinations in each of three independent experiments. C, percentage of death of CCL10-BHK cells at 24 h after infection with the indicated recombinant viruses was determined by PI staining and flow cytometry in three independent experiments (mean ± S.E.). D, immunoblot analysis of the indicated stable cell lines from C and Fig. 3, D and E (anti-BAD C-20). E, death of individual stably transfected Ba/F3 cell clones (numbered) expressing the indicated human BADL proteins was determined by PI staining and flow cytometry at the indicated time points following IL-3 withdrawal. F, immunoblot analysis of the cells shown in E using anti-BAD antibody (R&D Systems). Relative densito-metric values of BAD normalized against actin blots are indicated. G, the function of murine BADL and derived mutants was assessed as described in E. Data presented are the average ± ranges of two independent experiments for each of four clonally derived cell lines of mBADL (D56A/D61A/D71A) and the mean ± S.E. of three independent experiments for all other constructs in E and G. H, immuno-blot analysis of untreated Ba/F3 cell lines used in G (anti-BAD antibody) (R&D Systems). I, immunoblot analysis of a Ba/F3 cell line (in the presence of IL-3) expressing mBAD (D56A/D61A/D71A) using antibodies against BCL-xL (BioCarta), BCL-2 (BD Biosciences), BAX (Upstate Biotechnology), BAK (Upstate Biotechnology), and actin (MP Biomedicals). J, summary of the role of conserved and un-conserved caspase cleavage sites in human and mouse BADL.
We sought a death paradigm involving the Asp-56 or Asp-71 cleavage site that is conserved between humans and mice. In stably transfected Ba/F3 cell lines that are dependent on IL-3, human BADL, lacking both cleavage sites (D57A/D72A) but not the single mutant (D72A) or the wild type, suppressed cell death induced by IL-3 withdrawal (Fig. 6E). Consistent with this observation, wild type hBADL protein levels were reduced ~70% following IL-3 withdrawal, whereas the uncleavable mutant was stabilized (Fig. 6F). The hBADL cleavage fragment was either unstable or not efficiently recognized by the antibody. Thus, cleavage at hBADL Asp-57 (alone or in combination with Asp-71) appears to abolish the anti-death activity of human BAD during growth factor withdrawal. Analogous results were obtained with murine BADL in a series of stably transfected Ba/F3 cell clones (Fig. 6H) in which mutation of Asp-61 (or Asp-61 and −71) in mBADL provided minimal or no protection compared with vector controls. However, mutation of all three potential cleavage sites (Asp-56/Asp-61/Asp-71) provided significant protection (Fig. 6G). The somewhat variable expression levels of various BAD proteins in different clonally derived cell lines (Fig. 6H, and also see Figs. 3, A and D, and 6A) prior to induction of cell death were apparently inconsequential and did not alter the conclusions. Further analysis of one of these cell lines indicates that there are no obvious compensatory changes in the endogenous expression levels of other anti- and pro-death BCL-2 proteins that could explain the protective effect of BAD (Fig. 6I). Thus, Asp-56 of mBADL and Asp-57 of hBADL make a critical contribution to the conversion of BAD function in growth factor withdrawal in Ba/F3 cells. This is in sharp contrast to the cell death paradigms described above (irradiation, virus infection, and staurosporine treatment) that were tested in one or more cell types (COS-1, HEK293, BHK, primary neurons, and/or mice), in which Asp-61 was apparently the only cleavage site required to convert murine BAD into a pro-death factor (Fig. 6J).
Caspase Cleavage of mBADL Is Not Sufficient to Kill Immature Neurons
Of all the experiments described above, the only example in which mutation of the caspase cleavage site was not required for BAD to exhibit death was in immature neurons, either in culture or in newborn mice. This suggests that in immature neurons mBADL is either not cleaved or does not require its N terminus for anti-death activity. To address this issue, an engineered cleavage fragment of mBADL (ΔN61) was expressed via the Sindbis virus vector. In 2-day-old dissociated neuron cultures, ΔN61-BAD increased cell death compared with the control (Fig. 7A). Therefore, removal of the BAD N terminus was sufficient to activate its pro-death function in immature cortical cultures. In contrast, ΔN61-BAD failed to enhance neuronal death in brain slices, although this deletion largely abolished its protective function (Fig. 7B). Furthermore, ΔN61-BAD protected newborn mice almost as efficiently as wild type mBADL (Fig. 7C). Therefore, other mechanisms are apparently required to activate the pro-death function of BAD when neuronal connections are maintained in the immature cultured tissues and in animals.
Dephosphorylation of BAD in response to growth factor deprivation promotes cell death in thymocytes, cerebellar granule neurons, and fibroblasts (19). Therefore, to determine whether dephosphorylation could impair the anti-death function of BAD, Ser residues at known phosphorylation sites (Ser-112, −136, and −155) were changed to Ala to mimic the unphosphorylated protein or to Glu to mimic phosphorylated BAD. In 2-day cortical neurons, the triple Ser/Ala mutant (Fig. 7A) and the single S136A mutant (not shown) failed to protect against Sindbis virus-induced death, although the glutamate mutant was indistinguishable from wild type mBADL (Fig. 7A). Consistent with this observation, mutation of S136A largely abolished the anti-death function of mBADL in the whole animal model (Fig. 7C). In contrast, the Ser mutations had no signifi cant effect on BAD function in 8-day cultures in which cleavage of mBADL is the primary regulator of its function (Fig. 7A). It appears that dephosphorylation of mBADL at Ser-136 and perhaps other sites is also required to abolish anti-death function in immature neurons. Thus, as one compares dissociated neurons to neurons in tissue slices and finally to neurons in the whole animals, the pro-death function of BAD becomes progressively more difficult to activate as the model approaches physiological conditions (Fig. 7D).
DISCUSSION
Here we demonstrate that the BH3-only protein BAD can inhibit cell death induced by different death stimuli in a variety of cell types. Depending on the model system, the anti-death function of BAD can be abolished or converted into a pro-death function by multiple mechanisms including proteolysis, splicing, and dephosphorylation. The general assumption has been that BAD is a latent death factor in healthy cells and is activated to kill cells by dephosphorylation (39) or by caspase cleavage during IL-3 withdrawal (36), herpesvirus infections (40), exposure to transforming growth factor-[H9252]1 (37), and raloxifene treatment (41). A revised viewpoint consistent with these earlier findings is that BADL is not simply an inert factor in healthy cells but has an active pro-survival function prior to initiation of programmed cell death. The question remains as to whether the anti-death function of BAD is biochemically related or unrelated to its pro-death function. That is, the interactions between anti- and pro-death BCL-2 family members, which are widely thought to modulate cell death or survival, may be distinct from their normal cellular pro-survival mechanisms. The available evidence does not definitively distinguish between these possibilities, and both mechanisms could co-exist. Nevertheless, the hypothesis that BAD has a normal cellular function that is not necessarily related to its pro-death activity is supported by the recent report (22) that BAD is a regulator of cellular metabolism by facilitating utilization of glucose in the glycolytic pathway. Although the function of BAD in glycolysis could conceivably contribute to cell death through increased exposure of cells to damaging byproducts of respiration, the role of BAD in glycolysis appears to be beneficial to cells and their host. This is based on the observation that glucose induces phosphorylation of BAD and that BAD-deficient mice exhibit characteristics of diabetes (22). Furthermore, dephosphorylated (pro-death) BAD also decreases glucokinase activity and suppresses glycolysis in hepatocytes. Similarly, BAK and BCL-xL have alternative biochemical functions in healthy cells where they regulate neuronal excitability (4, 42).
It is inherently challenging to distinguish between the lack of a pro-death function and the presence of a pro-survival function. Results derived from a knock-in mouse expressing S112A/S136A/S155A mutant BAD indicate that endogenous unphosphorylated BAD causes decreased cell viability, consistent with the long-standing interpretation that phosphorylated BAD is an inactivate pro-death protein (19). However, these data are simultaneously compatible with a second conclusion that phosphorylated BAD actively inhibits programmed cell death prior to a death stimulus. This conclusion is supported by the observation that knock-in unphosphorylated BAD causes decreased cell viability with or without a death stimulus (19). Our observation that BAD inhibits cell death could appear to contradict the observation that BAD-deficient primary hepatocytes are resistant to cell death induced by glucose deprivation or other stimuli (20, 22). To the contrary, knock-out mice, by definition, lack both the anti- and pro-death functions of BAD. Therefore, decreased death in the absence of BAD would be expected under conditions where BAD is normally converted into a killer protein, particularly if this occurs by an irreversible event such as proteolysis.
Analogous to BAD, other anti-apoptotic proteins can be converted into pro-apoptotic factors when cleaved by caspases or other proteases. Cell lines overexpressing uncleavable BCL-2 and BCL-xL proteins have increased resistance to cell death induced by growth factor withdrawal and virus infections compared with wild type proteins (7, 8). Cleavage of the C. elegans BCL-2 homologue CED-9 by the pro-death C. elegans caspase CED-3 testifies to the conservation of this proteolytic event, although the function of this event in C. elegans is not understood (38). The striking reversal of pro-death to anti-death function by mutation of the caspase cleavage sites in BAD is likely because of both consequences of a two-pronged mechanism, preserving full-length BADL to carry out its pro-survival function and blocking the release of a pro-death fragment. It is also possible that the cleavage site itself is involved in the inhibitory process as suggested for CED-9 and BCL-xL (8, 38). Caspase cleavage of the unrelated proteins c-IAP1 (cellular inhibitor of apoptosis) and Rb (retinoblastoma protein) can also promote cell death (43–45). A knock-in mouse in which the caspase cleavage site near the C terminus of retinoblastoma protein was abolished confirms the importance of proteolysis in regulating retinoblastoma protein function (45). Thus, a single caspase cleavage site can have profound biological consequences. However, we found that mechanisms other than caspase cleavage can convert BAD from an anti-death to a pro-death protein. Similarly, BCL-2 can promote cell death without being cleaved when it binds to Nur-77 (15).
Different caspases appear to cleave BAD at different sites in response to different death stimuli. The Asp-56/57 site in murine or human BADL plays a significant role in cell death induced by growth factor deprivation in Ba/F3 cells, whereas Asp-61 is the critical site for a variety of other cell types and death stimuli. This idea is further supported by our observation that recombinant caspase-3 cleaves mBADL at Asp-61 but not at Asp-56 (Fig. 2), and caspase-7 cleaves murine but not human BAD (Fig. 5). Furthermore, recombinant caspase-2, −4, −6, and −9 failed to cleave murine or human BAD (data not shown). However, if BAD is a sentinel that monitors glycolysis or other functions and serves to activate the death pathway on loss of growth factor stimulation, then one would expect BAD to be cleaved in cells by initiator caspases (e.g. caspase-2, −8, −9) rather than effector caspases (e.g. caspase-3, −7). Indeed, the specificities of recombinant caspases (especially initiator caspases that are normally bound to regulatory subunits) are not necessarily expected to faithfully mimic apoptotic cells. Consistent with this idea, BCL-xL is readily cleaved by caspase-3 in vitro to produce a pro-death fragment, yet in COS-1 cells where BAD is converted to a pro-death factor by caspase cleavage, BCL-xL remains anti-apoptotic (see Fig. 1A) and is not detectably cleaved (data not shown).
Posttranslational modifications that occur in dying cells could also determine which cleavage sites are utilized in BAD, as demonstrated for BID (46). However, we observed that the triple Ser/Glu mutations at positions 112, 136, and 155 in murine BAD unexpectedly increased susceptibility of in vitro translated BAD to recombinant caspase-3 (data not shown). This enhanced susceptibility to caspase-3 might be inconsequential where “cleaved” ΔN61-BAD still protects (Fig. 7) but was reversed and further suppressed by addition of the BAD binding partner 14-3-3ξ in the same experiments. Although we did not detect age-dependent changes in 14-3-3ξ expression that could potentially explain the age-dependent conversion of BAD function in neurons (data not shown), a switch in protein function could easily be overlooked by simply measuring expression levels. Similarly, caution should be used when interpreting the significance of altered levels of anti- and pro-death BCL-2 family members in patient samples and elsewhere.
Proteolytic cleavage and dephosphorylation of BAD are not the only mechanisms that govern the activation of pro-death function, because the short splice variant of BAD lacking exon 1 structurally and functionally resembles its proteolytic cleavage product (Fig. 5). Furthermore, the mouse and human genomes have yet another conserved initiation codon and reading frame further upstream of exon 1 (not shown), suggesting the existence of an extra long version of BAD, perhaps analogous to the three N-terminal splice variants of another BH3-only protein, BIM (47). In addition to splicing, the presence of extensive predicted secondary structure in exon 1 and upstream regions raises the possibility that an internal ribosome entry site-like element is involved in selection of alternative translation initiation sites, providing yet another caspase-independent mechanism. Selective use of 5’-7-methyl-G-dependent and internal ribosome entry site-dependent translation can be differentially regulated by physiological stimuli including apoptosis (48). Thus, there are potentially multiple mechanisms for regulating the pro-survival and pro-death functions BAD.
Because removal of the BAD N terminus enhances cell death in most (but not all) circumstances, we might be led to think that the N terminus encodes an essential anti-death function. However, neither BAD nor BAK requires its N terminus for anti-death activity in intact tissue (hippocampal tissues slices and whole animals) (Fig. 7) (4). Also, murine BADS, which lacks its N terminus, was the predominant form of BAD identified in glucokinase complexes (22). Similarly, CED-9 lacking its N-terminal CED-3 cleavage fragment partially retains its anti-death activity in C. elegans (38). These observations suggest that the N termini of BCL-2 family proteins may not contribute directly to their anti-death or alternate biochemical functions but instead are required under some conditions to block the activation of their own pro-death functions. Thus, the N terminus may serve as a safety cap.
Acknowledgment
We thank Shifa Zou for expert technical contributions.
Footnotes
This work was supported by National Institutes of Health Grants NS34175 and NS37402. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The abbreviations used are: HA, hemagglutinin; GFP, green fluorescent protein; IL, interleukin; PI, propidium iodide; mBAD, murine BAD; hBAD, human BAD; SV, Sindbis virus; BHK, baby hamster kidney; HEK, human embryonic kidney.
REFERENCES
- 1.Lewis J, Oyler GA, Ueno K, Fannjiang Y, Chau BN, Vornov J, Korsmeyer SJ, Zou S, Hardwick JM. Nat. Med. 1999;5:832–835. doi: 10.1038/10556. [DOI] [PubMed] [Google Scholar]
- 2.Middleton G, Davies AM. Development (Camb.) 2001;128:4715–4728. doi: 10.1242/dev.128.23.4715. [DOI] [PubMed] [Google Scholar]
- 3.Kerr DA, Larsen T, Cook SH, Fannjiang YR, Choi E, Griffin DE, Hardwick JM, Irani DN. J. Virol. 2002;76:10393–10400. doi: 10.1128/JVI.76.20.10393-10400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fannjiang Y, Kim C-H, Huganir RL, Zou RL, S., Lindsten T, Thompson CB, Mito T, Traystman RJ, Larsen T, Griffin DE, Mandir AS, Dawson TM, Dike S, Sappington AL, Kerr DA, Jonas EA, Kaczmarek LK, Hardwick JM. Dev. Cell. 2003;4:575–585. doi: 10.1016/s1534-5807(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 5.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, Chen Y, Wei M, Eng VM, Adelman DM, Simon MC, Ma A, Golden JA, Evan G, Korsmeyer SJ, MacGregor GR, Thompson CB. Mol. Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hengartner MO, Horvitz HR. Nature. 1994;369:318–320. doi: 10.1038/369318a0. [DOI] [PubMed] [Google Scholar]
- 7.Cheng EHY, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 8.Clem RJ, Cheng EHY, Karp CL, Kirsch DG, Ueno K, Takahashi A, Kastan MB, Griffin DE, Earnshaw WC, Veliuona MA, Hardwick JM. Proc. Natl. Acad. Sci. U. S. A. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu Y-T, Wolter KG, Youle RJ. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Biochem. J. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- 11.Wood DE, Thomas A, Devi LA, Berman Y, Beavis RC, Reed JC, Newcomb EW. Oncogene. 1998;17:1069–1078. doi: 10.1038/sj.onc.1202034. [DOI] [PubMed] [Google Scholar]
- 12.Wood DE, Newcomb EW. Exp. Cell Res. 2000;256:375–382. doi: 10.1006/excr.2000.4859. [DOI] [PubMed] [Google Scholar]
- 13.Kirsch DG, Doseff A, Chau BN, Lin D-S, de Souza-Pinto NC, Hansford R, Kastan MB, Lazebnik YA, Hardwick JM. J. Biol. Chem. 1999;274:21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- 14.Basañez G, Sharpe JC, Galanis J, Brandt TA, Hardwick JM, Zimmerberg J. J. Biol. Chem. 2002;277:49360–49365. doi: 10.1074/jbc.M206069200. [DOI] [PubMed] [Google Scholar]
- 15.Lin B, Kolluri KS, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 16.Cheng EHY, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 17.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Cancer Cells. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 18.Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. Dev. Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 19.Datta SR, Ranger AM, Lin MZ, Sturgill JF, Ma YC, Cowan CW, Dikkes P, Korsmeyer SJ, Greenberg ME. Dev. Cell. 2002;3:631–643. doi: 10.1016/s1534-5807(02)00326-x. [DOI] [PubMed] [Google Scholar]
- 20.Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, Kutok JL, Le Beau MM, Greenberg ME, Korsmeyer SJ. Proc. Natl. Acad. Sci. U. S. A. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Kin CN, Yang J, Jemmerson R, Wang X. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 22.Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, Gygi SP, Korsmeyer SJ. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh A, Greenberg ME. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 24.Xia Z, Dudek H, Miranti CK, Greenberg ME. J. Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardwick JM, Levine B. Methods Enzymol. 2000;322:492–508. doi: 10.1016/s0076-6879(00)22045-4. [DOI] [PubMed] [Google Scholar]
- 26.Irusta PM, DiMaio D. EMBO J. 1998;17:6912–6923. doi: 10.1093/emboj/17.23.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellows DS, Howell M, Pearson C, Hazlewood SA, Hardwick JM. J. Virol. 2002;76:2469–2479. doi: 10.1128/jvi.76.5.2469-2479.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vornov JJ, Tasker RC, Park J. Exp. Neurol. 1995;133:7–17. doi: 10.1006/exnr.1995.1002. [DOI] [PubMed] [Google Scholar]
- 29.Bellows DS, Chau BN, Lee P, Lazebnik Y, Burns WH, Hardwick JM. J. Virol. 2000;74:5024–5031. doi: 10.1128/jvi.74.11.5024-5031.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levine B, Huang Q, Isaacs JT, Reed JC, Griffin DE, Hardwick JM. Nature. 1993;361:739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- 31.Lewis J, Wesselingh SL, Griffin DE, Hardwick JM. J. Virol. 1996;70:1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hardwick JM, Lewis J, Zou S, Cheng EHY, Kirsch DG, Bellows DS, Chau N, Oyler GA, Ueno K, Zhang J, Clem RJ. In: Apoptosis in Health and Disease. Ruffolo RR, Walsh F, editors. Harwood Academic Publishers; Amsterdam: 2000. pp. 195–213. [Google Scholar]
- 33.Cheng EHY, Levine B, Boise LH, Thompson CB, Hardwick JM. Nature. 1996;379:554–556. doi: 10.1038/379554a0. [DOI] [PubMed] [Google Scholar]
- 34.Levine B, Goldman JE, Jiang HH, Griffin DE, Hardwick JM. Proc. Natl. Acad. Sci. U. S. A. 1996;93:4810–4815. doi: 10.1073/pnas.93.10.4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine B, Hardwick JM, Trapp BD, Crawford TO, Bollinger RC, Griffin DE. Science. 1991;254:856–860. doi: 10.1126/science.1658936. [DOI] [PubMed] [Google Scholar]
- 36.Condorelli F, Salomoni P, Cotteret S, Cesi V, Srinivasula SM, Elnemri ES, Calabretta B. Mol. Cell. Biol. 2001;21:3025–3036. doi: 10.1128/MCB.21.9.3025-3036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim BC, Mamura M, Choi KS, Calabretta B, Kim SJ. Mol. Cell. Biol. 2002;22:1369–1378. doi: 10.1128/mcb.22.5.1369-1378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue D, Horvitz HR. Nature. 1997;390:305–308. doi: 10.1038/36889. [DOI] [PubMed] [Google Scholar]
- 39.Datta SR, Brunet A, Greenberg ME. Genes Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 40.Munger J, Roizman B. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10410–10415. doi: 10.1073/pnas.181344498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim HT, Kim BC, Kim IY, Mamura M, Seong do H, Jang JJ, Kim SJ. J. Biol. Chem. 2002;277:32510–32515. doi: 10.1074/jbc.M202852200. [DOI] [PubMed] [Google Scholar]
- 42.Jonas EA, Hoit D, Hickman JA, Brandt TA, Polster BM, Fannjiang Y, McCarthy E, Hardwick JM, Kaczmarek LK. J. Neurosci. 2003;23:8423–8431. doi: 10.1523/JNEUROSCI.23-23-08423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clem RJ, Sheu T-T, Richter BWM, He W-W, Thornberry NA, Duckett CS, Hardwick JM. J. Biol. Chem. 2001;276:7602–7608. doi: 10.1074/jbc.M010259200. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Yang Y, Ashwell JD. Nature. 2002;416:345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 45.Chau BN, Borges HL, Chen TT, Masselli A, Hunton IC, Wang JY. Nat. Cell Biol. 2002;4:757–765. doi: 10.1038/ncb853. [DOI] [PubMed] [Google Scholar]
- 46.Degli Esposti M, Ferry G, Masdehors P, Boutin JA, Hickman JA, Dive C. J. Biol. Chem. 2003;278:15749–15757. doi: 10.1074/jbc.M209208200. [DOI] [PubMed] [Google Scholar]
- 47.O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stoneley M, Chappell SA, Jopling CL, Dickens M, MacFarlane M, Willis AE. Mol. Cell. Biol. 2000;20:1162–1169. doi: 10.1128/mcb.20.4.1162-1169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]