Abstract
The goal of this study was to define profiles of secreted neuropeptide and catecholamine neurotransmitters that undergo co-release from sympathoadrenal chromaffin cells upon stimulation by distinct secretagogues. Chromaffin cells of the adrenal medulla participate in the dynamic responses to stress, especially that of ‘fight and flight’, and, thus, analyses of the co-release of multiple neurotransmitters is necessary to gain knowledge of how the stress response regulates cell-cell communication among physiological systems. Results of this study demonstrated that six different secretagogues stimulated the co-release of the neuropeptides Met-enkephalin, galanin, NPY, and VIP with the catecholamines dopamine, norepinephrine, and epinephrine. Importantly, the quantitative profiles of the secreted neurotransmitters showed similarities and differences upon stimulation by the different secretagogues evaluated, composed of KCl depolarization, nicotine, carbachol, PACAP, bradykinin, and histamine. The rank-orders of the secreted profiles of the neurotransmitters were generally similar among these secretagogues, but differences in the secreted amounts of each neurotransmitter occurred with different secretagogues. Epinephrine among the catecholamines showed the highest level of secretion. (Met)enkephalin showed the largest levels of secretion compared to the other neuropeptides examined. Levels of secreted catecholamines were greater than that of the neuropeptides. These data support the hypothesis that profiles of secreted neuropeptide and catecholamine neurotransmitters show similarities and differences upon stimulation by distinct secretagogues. These results illustrate the co-release of concerted neurotransmitter profiles that participate in the stress response of the sympathoadrenal nervous system.
Keywords: neurotransmitter profiles, secretion, catecholamines, neuropeptides, secretagogues, chromaffin cells, stress response
Introduction
Neurotransmitters are released from neurons to mediate cell-cell communication in the nervous system for the regulation of brain and peripheral physiological functions. Neurons have been classically viewed to secrete a predominant neurotransmitter that defined the transmitter phenotype of the indicated neuron (e.g., adrenergic, cholinergic, neuropeptidergic, and others). Subsequently, the dual release of two classical small molecule neurotransmitters has been demonstrated in numerous neuronal systems (Hnsako and Edwards, 2012; Gutierrez, 2009). Importantly, findings of multiple neurotransmitters within secretory vesicles, such as that of the large dense core secretory vesicles (LDCSV) of neuronal-like chromaffin cells (Njus et al., 1985; Laslop and Mahata, 2002; Gupta et al., 2010), predict the corelease of multiple neurotransmitters in an activity-dependent, regulated manner. While many of these neurotransmitters have been studied individually with respect to their secretory properties, analyses of the ‘profile’ of multiple neurotransmitters released simultaneously has not been extensively studied. Therefore, this study evaluated the co-release of quantitative profiles neuropeptides and catecholamines from adrenal medullary chromaffin cells to assess similarities and/or differences in secreted profiles of these neurotransmitters when stimulated by different secretagogue agents.
Chromaffin cells of the sympathoadrenal medullary system are key regulators of the body's responses to emergency ‘fight or flight’ reactions and mediate stress responses by releasing neurotransmitter and neurohumoral chemical molecules targeted at regulating multiple physiological systems (Burgoyne, 1995; Aunis 1998; Arun, 2004; Eisenhofer et al., 2004;; Kvetnansky et al., 2009; Purves et al., 2001). Epinephrine is a key stress neurotransmitter released from the adrenal medulla for the regulation of of metabolic, cardiac, lung, and related physiological functions, as well as emotions, memory, and related behaviors (Joels et al., 2011; Shannsky et al., 2013). Stress responses involve sympathetic nerve activation of the adrenal medulla via multiple secretagogues (Arun et al., 2004; Carmichael and Winkler, 1985; Fulop et al., 2005; Smith and Eiden, 2012; Stroth et al., 2013) that stimulate the secretion of epinephrine with peptide neurotransmitters (neuropeptides) and other catecholamines in an activity-dependent manner (Holman et al., 1994).
Epinephrine is present in adrenal medulla together with the catecholamines dopamine and norepinephrine, as well as with numerous neuropeptides (Eisenhofer et al., 2004; Kvetnansky et al., 2009, Purves et al., 2001). These neurotransmitters are secreted from chromaffin cells in a regulated manner from secretory vesicles upon stimulation by sympathetic activation. Secretion of these catecholamine and neuropeptide transmitters (ie., enkephalin, NPY, galanin, and others) have been studied individually from chromaffin cells (Zhang et al., 2006; Hook et al., 2008; Smith and Eiden, 2012; Ges et al., 2013) and other neuronal cell types (Eiden, 2013). But characterization of the secreted profiles of co-released neurotransmitters in response to stimulation by different secretagogues has not yet been fully defined. Therefore, the goal of this study was to assess the quantitative profiles of neuropeptides and catecholamines co-secreted from chromaffin cells upon stimulation by different secretagogues that were composed of KCl depolarization, nicotine, carbachol, PACAP, bradykinin, and histamine (Toneff et al., 2013; Taylor et al., 2000; Stroth et al., 2013; Kuwashima et al., 2000; Wallace et al., 2002).
Results showed that quantitative profiles of secreted neuropeptide and catecholamine neurotransmitters show similarities and differences upon stimulation by distinct secretagogues. These findings demonstrate that specific profiles of secreted neurotransmitters, with similarities and differences, are released from chromaffin cells by distinct secretagogues. These data illustrate the co-release of multiple neurotransmitter profiles from adrenal medullary chromaffin cells related to stress responses.
Materials and Methods
Chromaffin cells of the adrenal medulla in primary culture
Primary cultures of bovine adrenal chromaffin cells were prepared using a modification of a method described previously (O'Connor et al., 2007; Hook et al., 2008). Ten fresh bovine adrenal glands received from a local slaughterhouse were placed onto ice and trimmed of excess fat. The glands were sterilized by brief immersion in 70% ethanol prior to washing of the glands’ lumen by washing with standard release medium (SRM was composed of 188 mM NaCl, 4.6 mM KCl, 10 mM D-Glucose, 25 mM HEPES pH 7.3, 2.2 mM CaCl2, 1.2 mM MgSO4, 100 units/ml penicillin, 100 μg/ml streptomycin). The chromaffin cells were dissociated by washing the gland lumens with collagenase (1.5 mg/ml) at 37°C for 20 minutes, repeated three times. Adrenal glands were dissected to obtain the adrenal medulla tissue, placed into SRM, and dissociated cells were collected as the supernatant after centrifugation (500 × g, 30 sec). The pellet of undissociated tissue was subject to another incubation with collagenase solution at 37°C for 30 minutes, and dissociated cells were collected as the supernatant after centrifugation (500 × g, 30 sec). The resultant pellet was washed with SRM another two times and the supernatants (obtained by centrifugation) pooled with the previous supernatant fractions. The collagenase activity of the supernatant was neutralized by the addition of SRM containing 1% BSA. The cell suspension was passed through filters with progressively smaller pore sizes (surgical gauze of 1 sheet, 2 sheets, and 4 sheets followed by cell strainer (Falcon) of 100 μm and then 70 μm). The filtered cell suspension was subjected to centrifugation (550 × g, 5 mins) and the pellet was washed with SRM/1%BSA three times. The final pellet was resuspended in cell culture medium (Dulbecco's Modified Eagle's Medium containing 10% FCS, cytosine arabinofuranoside (10 μM) and antibiotics) and seeded onto fibronectin-coated (2.5 μg/cm2) 6 well plates at a density of 1.5 million cells/well. Cells were kept at 37°C in 95%humidified air/5% CO2. Chromaffin cells in primary culture have been shown to be stable in neurotransmitter content up to 2-3 weeks in culture (Kilpatrick et al., 1980).
Secretagogue stimulation of neurotransmitter secretion from chromaffin cells
Chromaffin cells were maintained in culture for 7 days prior to stimulation with a variety of secretagogues. Culture media was aspirated from the cells and replaced with 1ml SRM-B (SRM plus 0.5 μg/ml BSA). Following incubation for 15 min at 37°C the SRM-B was aspirated and replaced with 1 ml incubation media (i.e. SRM-B containing secretagogues). Secretagogues evaluated were high KCl (50 mM) that produces depolarization, nicotine (up to 10 μM), carbachol (1 mM), PACAP (100 nM), bradykinin (300 nM), and histamine 10 μM). Cells were incubated with each secretagogue for 15 min at 37°C, and the secretion media was collected and was centrifuged (500 × g, 5 min) to remove residual cells. The collected media was stored at - 70° C for measurement of secreted neurotransmitters.
Isolation of chromaffin granules (large dense core secretory vesicles, LDCSV)
Chromaffin granules (large dense core secretory vesicles, LDCSV) were purified from fresh bovine adrenal medulla by differential sucrose density gradient centrifugation, as described previously (O'Connor et al., 2007; Wegrzyn et al., 2010), involving extensive wash steps (five washes) to obtain purified chromaffin granules. We have documented the high purity of this preparation of isolated secretory vesicles by electron microscopy (Wegrzyn et al., 2010) and biochemical markers (Wegrzyn et al., 2010). The sucrose gradient purification results in a preparation of purified, intact chromaffin secretory vesicles that lack biochemical markers for the subcellular organelles of lysosomes (acid phosphatase marker), cytoplasm (lactate dehydrogenase marker), mitochondria (fumarase and glutamate dehydrogenase markers), and endoplasmic reticulum (glucose-6-phosphatase marker) (Smith and Winkler, 1967; Gratzl et al., 1981; Wegrzyn et al., 2010). These results have established the high purity of the chromaffin granule preparation.
Neuropeptide and catecholamine assays
The secretion media was analyzed for levels of the neuropeptides (Met)enkephalin, galanin, NPY, and VIP were measured by radioimmunoassays (Peninsula Laboratories, CA). The catecholamines dopamine, norepinephrine, and epinephrine were each assayed as described previously (Kennedy and Ziegler, 1990). Measurements were conducted in triplicate for each sample, and each experiment utilized triplicate wells of cells treated under identical conditions. Further, experiments were repeated at least three times. Statistical significance of data was assessed by student's t-test and one-way ANOVA analyses (p < 0.05) (using Prism Graphpad program).
Analyses of the purified chromaffin granules measured levels of the catecholamine and neuropeptide neurotransmitters. Protein content of the purified chromaffin granules was measured by the Bio-Rad DC protein assay kit (Biorad, Hercules, CA). The content of catecholamines and peptide neurotransmitters were expressed as fmol per μg protein.
Results
Regulated secretion of neuropeptides and catecholamines from chromaffin cells was evaluated for several different secretagogues. This study assessed six types of secretagogues (KCl, nicotine, carbachol, PACAP, bradykinin, and histamine) for their abilities to stimulate the release of several neuropeptides ((Met)enkephalin galanin, NPY, and VIP) and the catecholamines (dopamine, norepinephrine, and epinephrine). These experiments used secretagogues at concentrations known to stimulate secretion of catecholamines (dopamine, norepinephrine, and epinephrine). The distinct secretagogues and resultant profiles of secreted neurotransmitters were quantitatively compared to assess their similarities and/or differences.
Regulated co-secretion of enkephalin, galanin, NPY, and VIP neuropeptides from chromaffin
cells
The co-release of these neuropeptides was assessed by stimulation with nicotine (appendix, figure A.1). Secretion of these neuropeptides was stimulated by nicotine in a concentration-dependent manner. The EC50 values for nicotine stimulation of enkephalin, galanin, VIP, and NPY was 4.3, 2.4, 3.5, and 5.2 μM nicotine, respectively (appendix, table A.1). These EC50 values are similar to those reported for nicotine-stimulated secretion of epinephrine and norepinephrine from chromaffin cells (Molloy et al., 1995; Mizobe et al., 1979), indicating that neuropeptides undergo stimulated secretion by nicotine at concentrations similar to those for stimulating catecholamine secretion.
To assess the levels of neuropeptides secreted above basal levels upon stimulation of chromaffin cells by different secretagogues, secretagogues of different mechanisms for stimulation of regulated secretion were evaluated. High KCl (50 mM KCl in the media) represents a model of activity-dependent depolarization-mediated stimulation of neurotransmitter secretion (Hwang et al., 2007; Toneff et al., 2013). Nicotine and carbachol were assessed as nicotine cholinergic receptor agonists (Efthimiopoulos et al., 1996; Evinger et al., 1994). PACAP (pituitary adenylyl cyclase activating polypeptide), a co-transmitter of acetylcholine, controls adrenal medullary catecholamine secretion and stress responses (Smith and Eiden, 2012; Stroth et al., 2013). Bradykinin and histamine both evoke catecholamine release from chromaffin cells (Nostramo et al., 2013; Choi et al., 1993).
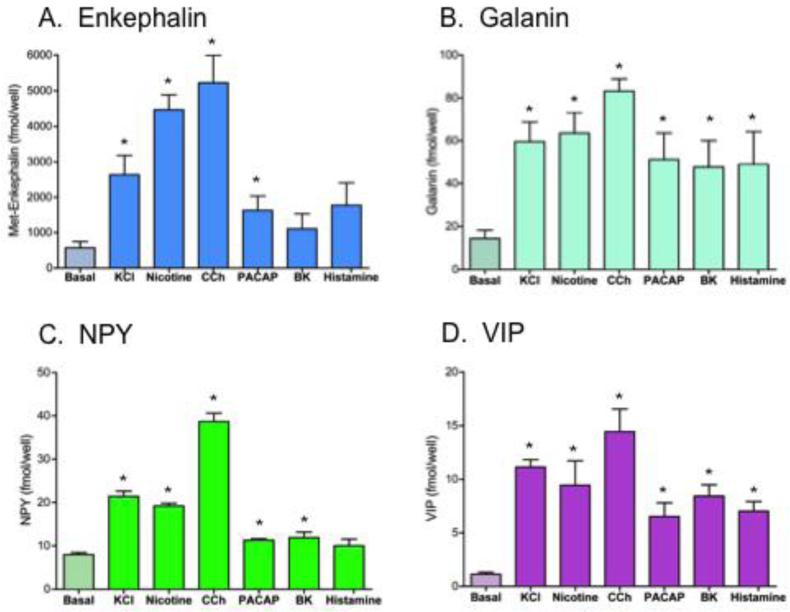
Results showed that the profiles of secreted neuropeptides – enkephalin, galanin, NPY, and VIP – showed generally similar patterns of relative amounts released by the different secretagogues (figure 1). The KCl, nicotine, and carbachol secretagogues stimulated secretion of the four neuropeptides above basal levels (figure 1). Carbachol (1 mM), a non-hydrolysable analogue of acetylcholine, stimulated the greatest secretion of the four neuropeptides. Nicotine and KCl also stimulated secretion of the neuropeptides. Nicotine stimulation of release was similar to that of KCl for galanin, NPY, and VIP; somewhat greater stimulation of enkephalin release was observed for nicotine compared to KCl secretagogues.
Figure 1. Co-release of neuropeptides stimulated by secretagogues: basal and stimulated secretion.
Chromaffin cells were treated with (and without) the secretagogues of high KCl (50 mM), nicotine (10 μM), carbachol (1 mM), PACAP (0.1 μM), bradykinin (0.3 μM), and histamine (10 μM) for 15 minutes and the media was collected for measurements of Met-enkephalin, galanin, NPY, and VIP neuropeptides (panels A, B, C, and D, respectively). Graphs show plots of average secreted neuropeptide values among replicate cellular samples (x ± s.e.m., replicates of n=3). Secretagogue stimulation of released neuropeptides was compared to control (no secretagogue) conditions (*statistically significant, p < 0.05).
But differences in amounts of secreted neuropeptides were also observed among the secretagogues. PACAP was effective at inducing significant secretion above basal for (Met)enkephalin, galanin, and VIP, but not for NPY. Bradykinin and histamine stimulated the secretion of galanin and VIP above basal levels; and these secretagoguges did not substantially increase the release of (Met)enkephalin or NPY above basal levels.
Differences and similarities in secreted profiles of neuropeptides induced by secretagogues
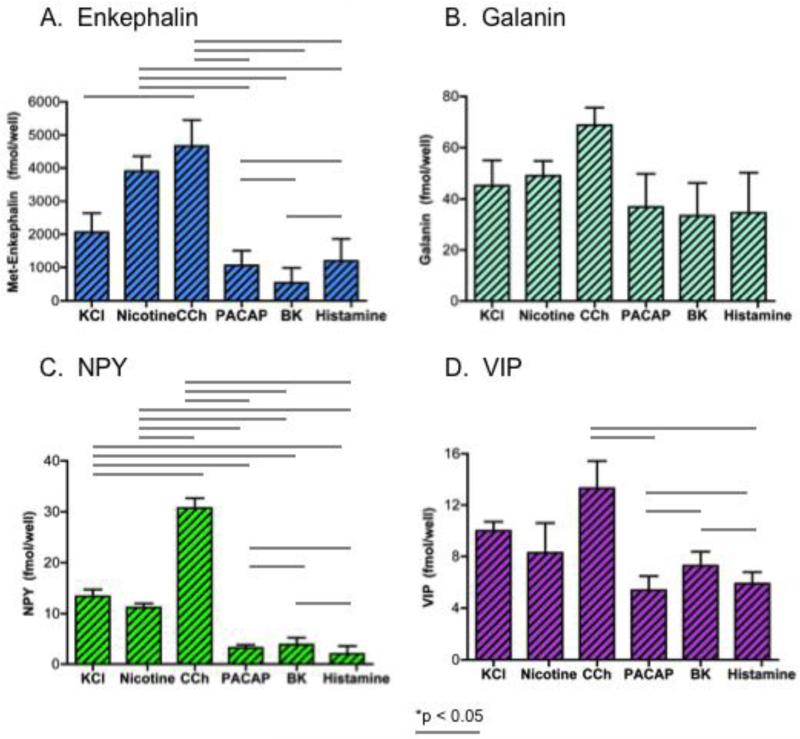
The amounts of secreted neuropeptides caused by secretagogue stimulation was evaluated by subtraction of the basal levels of secretion measured in the media samples collected after secretagogue treatment of cells. The data show similarities and differences among the profiles of neuropeptides secreted (figure 2). Carbachol and nicotine stimulated the greatest amounts of secreted neuropeptides; but NPY was an exception since carbachol, but not nicotine, stimulated the greatest levels of secreted NPY. PACAP, bradykinin, and histamine similarly stimulated secretion of galanin and VIP at levels less than that of carbachol. Compared to nicotine and carbachol, PACAP, bradykinin, and histamine stimulated lower levels of of secreted enkephalin and NPY.
Figure 2. Secretagogue differences in stimulated amounts of neuropeptides secreted.
Differences between pairs of secretagogues for stimulation of different amounts of secreted neuropeptides are shown for (Met)enkephalin, galanin, NPY, and VIP in panels A, B, C, and D, respectively. Secretagogue-stimulated neuropeptide amounts were calculated from the measured levels in the media of secretagogue treated chromaffin cells, with subtraction of the basal levels of neuropeptide secretion occurring in the absence of secretagogue. One-way ANOVA analyses indicated significant differences among pairs of secretagogues for stimulated neuropeptide secretion, shown by the lines with *p < 0.05.
Comparison of differences and similarities among pairs of secretagogues was conducted (by one-way ANOVA statistics), which indicated significant differences among the pairs of secretagogues shown (figure 2). The pattern of secretagogue differences in amounts of neuropeptides secreted appeared similar for (Met)enkephalin and NPY, which differed from that of galanin and VIP. Furthermore, the pattern of secretagoguge differences in secreted amounts of neuropeptides appeared similar for galanin and VIP, but these differed from that of enkephalin and NPY.
Regulated co-secretion of dopamine, norepinephrine, and epinephrine from chromaffin cells
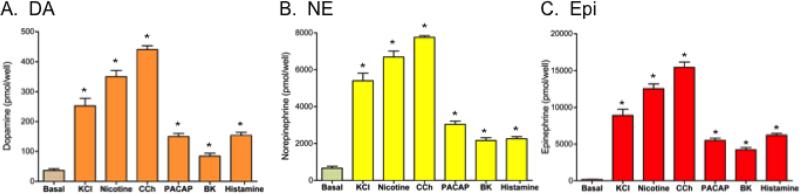
The effects of the secretagogues to stimulate secretion of the catecholamines dopamine (DA), norepinephrine (NE), and epinephrine (Epi) from chromaffin cells were assessed (figure 3). The relative increases in stimulated secretion (above basal control) for each of these catecholamines was similar for the different secretagogues. High KCl, nicotine, and carbachol secretagogues prominently stimulated corelease of the catecholamines well above basal control secretion. The other secretagogues – PACAP, bradykinin, and histamine - stimulated more moderate increases in co-release of the three catecholamines.
Figure 3. Co-release of dopamine, norepinephrine, and epinephrine catecholamines stimulated by secretagogues: basal and stimulated secretion.
Chromaffin cells were treated with (and without) the secretagogues of high KCl (50 mM), nicotine (10 μM), carbachol (1 mM), PACAP (0.1 μM), bradykinin (0.3 μM), and histamine (10 μM) for 15 minutes and the media was collected for measurements of dopamine (DA), norepinephrine (NE), and epinephrine (Epi) (panels A, B, and C, respectively). Graphs show average secreted neuropeptide values among replicate cellular samples (x ± s.e.m., replicates of n=3). Secretagogue stimulation of released catecholamines was compared to control (no secretagogue) conditions (*statistically significant, p < 0.05).
Differences and similarities in secreted profiles of catecholamines induced by secretagogues
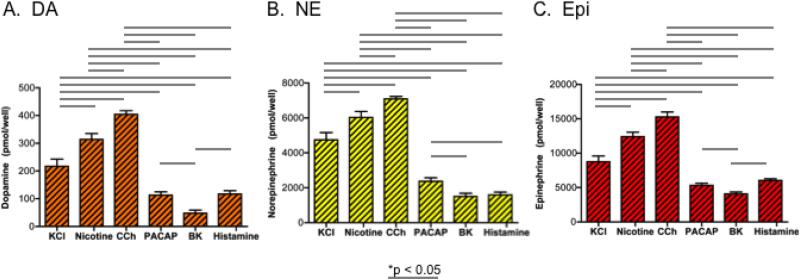
The amounts of secreted catecholamines caused by secretagogue stimulation was evaluated by substraction of the basal levels measured in the experimental media samples collected after secretagogue treatment of cells. The data show similarities and differences among the profiles of catecholamines secreted (figure 4). Carbachol and nicotine similarly stimulated the greatest amounts of the three catecholamines. KCl was the next best stimulator of catecholamine secretion. Lower levels of the catecholamines were secreted by PACAP, bradykinin, and histamine secretagogues.
Figure 4. Secretagogue differences in stimulated amounts of catecholamines secreted.
Differences between different secretagogues for stimulation of different amounts of secreted catecholamines are shown for dopamine (DA), norepinephrine (NE), and epinephrine (Epi) in panels A, B, and C, respectively. Secretagogue-stimulated catecholamine amounts were calculated from the measured catecholamine levels in the media of secretagogue-treated chromaffin cells, with subtraction of the basal levels of catecholamines secreted in the absence of secretagogue. One-way ANOVA analyses indicated significant differences among pairs of secretagogues for stimulated catecholamine secretion, shown by the lines with *p < 0.05.
Comparison of differences and similarities among pairs of secretagogues was conducted (by one-way ANOVA statistics, shown in figure 4). The pattern of secretagogue differences in amounts of the catecholamines secreted appeared quite similar. But differences among PACAP-, bradykinin-, and histamine-stimulated secretion of the catecholamines were smaller, due to the lower levels of catecholamine secretion induced by these three secretagogues.
Comparison of the patterns of secretagogue differences for catecholamine and neuropeptide
secretion
Evaluation of paired secretagogue differences in stimulated secretion of the catecholamines (figure 4) compared to neuropeptides (figure 2) showed similarities and differences (shown by the asterisks of paired comparisons in figures 2 and 4). The pattern of secretagogue differences in secretion of the three catecholamines (figure 4) appeared similar to that of the enkephalin and NPY neuropeptides, but not to that of galanin and VIP (figure 2).
Profiles of neuropeptide and catecholamine neurotransmitters co-released from chromaffin cells by different secretagogue agents
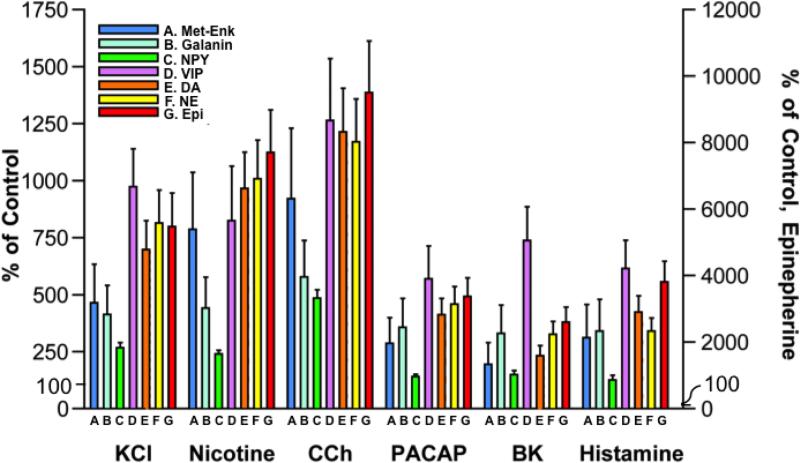
Evaluation of the quantitative profiles of neurotransmitters co-released upon stimulation by each of the secretagogues showed similarities in the rank-orders of the profiles of neurotransmitters co-released by each secretagogue (figure 5). Comparison among the neurotransmitters showed that epinephrine showed the greatest release upon stimulation by KCl, nicotine, carbachol, PACAP, bradykinin, and histamine compared to basal release (shown as relative 100% compared to stimulated secretion of neurotransmitters above the basal, normalized 100% level). Epinephrine secretion was stimulated by 25-fold to 95-fold above basal levels by the six secretagogues. Dopamine and norepinephrine secretion were stimulated by up to ~10-fold above basal secretion. Secretagogue stimulation of the neuropeptides (above basal levels) was greatest for VIP, followed by (Met)-enkephalin and galanin, and NPY.
Figure 5. Profiles of neurotransmitters co-released by each secretagogue.
Experiments assessed profiles of neuropeptide and catecholamine neurotransmitters co-released upon stimulation of chromaffin cells by each of the different secretagogues composed of KCl, nicotine, carbachol, PACAP, bradykinin, and histamine. Data are shown as percent of control basal release of neurotransmitter (without secretagogue control) in the left y-axis for the neuropeptides, DA, and NE. The right y-axis represents secretion for Epi. All experiments were conducted in triplicate and the mean ± s.e.m. are graphed. *Statistically significant compared to basal control secretion (p < 0.05 by student's t-test).
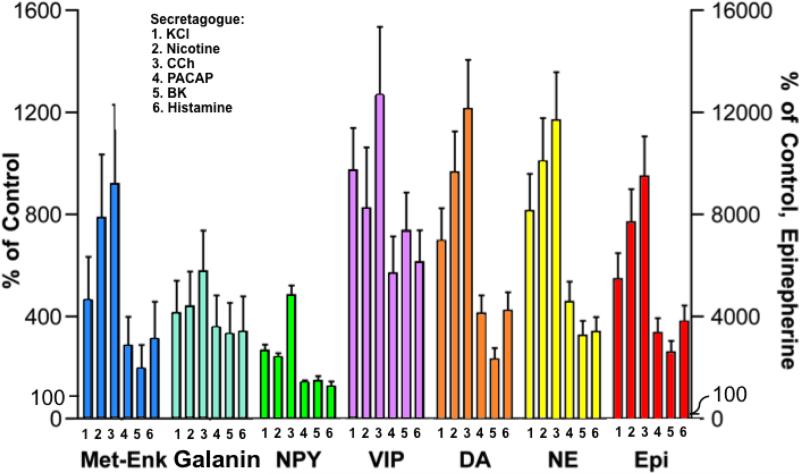
The secreted levels of each neurotransmitter induced by the different secretagogues (above basal secretion levels) was also assessed (figure 6). The extent of the stimulated secretion of each neurotransmitter induced by the different secretagogues were generally similar. Carbachol stimulated the greatest increase in the co-release of the neuropeptide and catecholamine neurotransmitters. Nicotine and KCl were also excellent stimulators of neurotransmitter co-release. The other secretagagues (PACAP, bradykinin, and histamine) induced more moderate increases in the secretion of these neurotransmitters.
Figure 6. Effects of secretagogues on stimulated secretion of each neuropeptide and catecholamine from chromaffin cells.
Co-release of neuropeptides and catecholamines stimulated by secretagogues was evaluated. Data are shown as percent of control release of neurotransmitter (without secretagogue control) in the left y-axis for the neuropeptides, DA, and NE; the right y-axis represents that for Epi. All experiments were conducted in triplicate and the mean ± s.e.m. are graphed. *Statistically significant compared to basal control secretion (p < 0.05 by student's t-test).
Molar ratios of secreted neurotransmitter profiles among the different secretagogues
Based on the amounts of each neurotransmitter secreted, normalized molar ratios of secreted neuropeptide and catecholamine neurotransmitters were computed for each of the secretagogues (tables 1 and 2). For the neuropeptides, the molar ratios of secreted VIP/NPY/Galanin/Enkephalin (normalized to VIP as ‘1’) displayed similar rank orders for each of the secretagogues (table 1). For the six secretagogues, the rank order from lowest to highest amount of neuropeptide secreted was VIP/NPY/Galanin/Enkephalin with average molar ratios of 1.0/1.02/5.7/250.
Table 1.
Molar Ratios of Neuropeptides Released by Secretagogue Stimulation
| Secretagogue | (Met)enkephalin | Galanin | NPY | VIP | Molar Ratio. |
|---|---|---|---|---|---|
| fmol/well | VIP/NPY/Gal/Enk | ||||
| KCl | 2064.9 ± 577.9 | 45.2 ± 9.9 | 13.4 ± 1.3 | 10.0 ± 0.7 | 1.0/1.3/5.0/207 |
| Nicotine | 3899.8 ± 451.6 | 49.1 ± 5.8 | 11.2 ± 0.8 | 8.3 ± 2.3 | 1.0/1.3/6.0/471 |
| Carbachol | 4655.8 ± 780.7 | 68.8 ± 6.8 | 30.7 ± 2.0 | 13.3 ± 2.1 | 1.0/2.0/5.0/350 |
| PACAP | 1055.1 ± 447.0 | 36.9 ± 12.9 | 3.3 ± 0.6 | 5.4 ± 1.1 | 1.0/0.6/7.0/196 |
| Bradykinin | 538.3 ± 450.7 | 33.3 ± 13.0 | 3.9 ± 1.4 | 7.3 ± 1.1 | 1.0/0.5/5.0/74 |
| Histamine | 1196.8 ± 664.4 | 34.6 ± 15.7 | 2.0 ± 1.6 | 5.9 ± 0.9 | 1.0/0.4/6.0/204 |
Secretagogue stimulation of neuropeptide secretion from chromaffin cells was measured for (Met)enkephalin, galanin, NPY and VIP (stimulated amounts of neuropeptides released into the culture media minus basal secretion of each of these neuropeptides). Results are shown as mean ± s.e.m. (n = 3). Molar ratios of secreted neuropeptides are illustrated for VIP/NPY/Gal/Enk (Gal, galanin; Enk, (Met)enkephalin). The average molar ratio for all secretagogues was 1.0/1.02/5.7/250.
Table 2.
Molar Ratios of Catecholamines Released by Secretagogue Stimulation
| Secretagogue | Dopamine | Norepinephrine | Epinephrine | Molar Ratio |
|---|---|---|---|---|
| . | pmol/well | DA/NE/Epi | ||
| KCl | 216.8 ± 25.5 | 4736.0 ± 424.7 | 8 753.5 ± 846.4 | 1/22/40 |
| Nicotine | 314.2 ± 21.1 | 6026.6 ± 338.4 | 12373.5 ± 666.5 | 1/19/40 |
| Carbachol | 404.5 ± 13.6 | 7092.2 ± 141.4 | 15294.6 ± 696.5 | 1/17/38 |
| PACAP | 113.5 ± 11.9 | 2373.9 ± 207.7 | 5324.6 ± 311.1 | 1/20/50 |
| Bradykinin | 48.1 ± 10.8 | 1497.0 ± 185.9 | 4074.3 ± 295.1 | 1/31/84 |
| Histamine | 117.4 ± 11.9 | 1592.5 ± 159.3 | 6039.9 ± 252.3 | 1/14/51 |
Secretagogue stimulation of catecholamine secretion from chromaffin cells was measured for dopamine (DA), norepinephrine (NE), and epinephrine (Epi) (stimulated amounts of neuropeptides released into the culture media minus basal secretion of each of these catecholamines). Results are shown as mean ± s.e.m. (n = 3). Molar ratios of secreted catecholamines are illustrated for DA/NE/Epi. The average molar ratio for all secretagogues tested was 1.0/20.5/50.5.
For the catecholamines, the molar ratios of secreted dopamine/norepinephrine/epinephrine (DA/NE/Epi) for each of the secretagogues are shown in table 2. The molar ratios of secreted DA/NE/Epi displayed similar rank orders of reach of the secretagogues. For the six secretagogues, the rank order from lowest to highest amount of catecholamine secreted was DA/NE/Epi with average molar ratios of 1.0/20.5/50.5.
Large dense core secretory vesicles (LDCSV) of chromaffin cells: content of neuropeptide and catecholamine neurotransmitters
The presence of catecholamines with neuropeptide transmitters in LDCSV isolated from chromaffin cells was measured (table 3). The LDCSV were found to contain the enkephalin, galanin, NPY, and VIP neuropeptides and the catecholamines dopamine, norepinephrine, and epinephrine. Their concentrations (fmol neurotransmitter per μg protein) showed that the neuropeptide levels from lowest to highest were indicated by VIP, galanin, NPY and (Met)enkephalin (highest level). The catecholamine levels from lowest to highest were found to be DA, NE, and Epi. These findings illustrated the co-storage of different levels of neuropeptides and catecholamines in the LDCSV, which provides secretion in stress responses.
Table 3.
Content of neuropeptide and catecholamine neurotransmitters in large dense core secretory vesicles (LDCSV) isolated from bovine adrenal medulla.
| A. Neuropeptides | ||
|---|---|---|
| Neuropeptide | Concentration (fmol/μg) | Molar Ratio, Normalized |
| Met-enkephalin | 375 ± 30.7 | 123,333 |
| Galanin | 0.13 ± 0.006 | 43 |
| NPY | 63 ± 9.3 | 27,000 |
| VIP | 0.004 ± 0.0009 | 1 |
| B. Catecholamines | ||
|---|---|---|
| Catecholamine | Concentration (fmol/μg) | Molar Ratio, Normalized |
| Dopamine | 7.5 ± 8.9 | 1.0 |
| Norepinephrine | 27.0 ± 2.0 | 3.6 |
| Epinephrine | 35.5 ± 5.6 | 4.7 |
LDCSV were isolated from chromaffin cells of bovine adrenal medulla and neurotransmitters were assayed as described in the methods, for neuropeptides (panel A) and catecholmines (panel B). Neurotransmitter concentrations were determined in three preparations of isolated LDCSV, and averages ± s.e.m. are expressed as fmol neurotransmitter per μg protein. Molar ratios of neuropeptides were calculated by normalization to VIP as ‘1’. Molar ratios of catecholamines were calculated by normalization to dopamine as ‘1.0.’
Overall, results of this chromaffin cell study demonstrate similarities and differences in quantitative profiles of secreted neurotransmitters upon stimulation by different secretagogues.
Discussion
This study evaluated the profiles of catecholamine and neuropeptide neurotransmitters secreted from sympathoadrenal chromaffin cells upon stimulation by distinct secretagogues that induce regulated secretion. Results demonstrated that six different secretagogues stimulated the co-release of the neuropeptides (Met)-enkephalin, galanin, NPY, and VIP with the catecholamines dopamine, norepinephrine, and epinephrine. Importantly, the quantitative profiles of the secreted neurotransmitters showed similarities and differences upon stimulation by the different secretagogues evaluated, composed of KCl depolarization, nicotine, carbachol, PACAP, bradykinin, and histamine. The rank orders of the secreted profiles of the neurotransmitters were generally similar among these secretagogues, but differences in the secreted amounts of each neurotransmitter occurred with different secretagogues. These data support the hypothesis that profiles of secreted neuropeptide and catecholamine neurotransmitters show similarities and differences upon stimulation by distinct secretagogues.
More specifically, the different secretagogues stimulated similar rank orders of relative levels of secreted neuropeptides and catecholamine neurotransmitters that were above basal unstimulated secretion. The three catecholamines of dopamine, norepinephrine, and epinephrine were secreted at the greatest levels, with epinephrine secreted at the highest level among the catecholamines. Amounts of secreted catecholamines for each secretagogue were greater than the neuropeptides enkephalin, galalnin and NPY; the VIP neuropeptide, however, was secreted at similar or greater levels compared to dopamine and norepinephrine.
Furthermore, for each neurotransmitter investigated, the six secretagogues stimulated similar profiles of secreted neurotransmitters. Nicotine and carbachol provided the largest degrees of stimulated neurotransmitter release. KCl was similar or a more modest stimulator of neurotransmitters secreted compared to nicotine and carbachol, depending on the particular neurotransmitter. The stimulated secretion by PACAP, bradykinin, and histamine occurred at lower levels than nicotine, caraboachol, or KCl secretagogues.
The co-release of multiple neurotransmitters from chromaffin cells of the adrenal medulla indicates that responses to stress and emergency ‘fight or flight’ situations, involve the co-release of profiles of neurotransmitters, leading to regulation of multiple target physiological systems in stress responses. Secreted epinephrine controls metabolic processes and cardiovascular functions, epinephrine and NPY regulate vasoconstriction, enkephalin functions as an endogenous opioid for analgesia, PACAP regulates neuroendocrine stress circuits (Smith and Eiden, 2012; Stroth et al., 2013), galanin controls metabolism (Lang et al., 2015; Fang et al., 2012; Steiner et al., 2001), and VIP regulates metabolism and neuroendocrine regulation. The response to stress by the adrenal medulla involves the co-release of multiple neurotransmitters and, thus, the profiles of co-released neurotransmitters together coordinate the multiple physiological functions participating in stress responses.
The release of neurotransmitter profiles provides opportunities for fine controls of targeted cellular and physiological functions. Moreover, changes in the quantitative composition of profiles of neurotransmitters can provide fine-tuning of controls among physiological systems. As example, nicotine and carbachol, nicotinic cholinergic receptor agonists, induced the co-release of similar profiles of the catecholamine and neuropeptides examined. In comparison, the secretagogues PACAP, bradykinin, and histamine stimulated the co-release of lower levels of the neurotransmitters (compared to nicotine and carboachol). These findings show that differences in relative quantities of co-released neurotransmitter profiles occur with different secretagogues. In vivo, the spectrum of endogenous secretagogue stimulators of adrenal chromaffin cell secretion are predicted to participate in regulating various stress responses. It will be of interest in future investigations to examine the dynamics of potential changes in neurotransmitter profiles co-released in a variety of stress conditions.
It has been viewed traditionally that neurons release one neurotransmitter, but more recent investigations, including this study, demonstrate the co-release of neurotransmitters from neuronal systems. Several examples of the co-release of classical neurotransmitters have been illustrated in the field, as reviewed recently (Hnasko and Edwards, 2012; Gutierrez, 2009). Evidence for co-release of neurotransmitter profiles leads to the hypothesis that multiple secreted neurotransmitters function together as chemical regulators of neuronal and cell-cell communication for the regulation of target systems.
The hypothesis that profiles of secreted neurotransmitters are regulated in sympathoadrenal chromaffin cells may involve the different epinephrine and norepinephrine cell types in the adrenal medulla (Moro et al., 1990; Ciolkowski et al., 1992; Koval et al., 2001; O'Connor et al., 2007). The majority of adrenomedullary chromaffin cells store and release primarily epinephrine (~50% of the cells), and a smaller portion of cells contain norepinephrine (~30%). Evidence in the field also suggests the presence of chromaffin cells containing both epinephrine and norepinephrine (~20%). Enrichment of these three different cell populations has been described in the literature (Moro et al., 1990; O'Connor et al., 2007) but it seems that isolation to homogeneity has not yet been achieved. Specific analyses of the neurotransmitter profiles in each of these three different cell types will be important in the future to understand the cellular source of neurotransmitters released from the adrenal medulla in stress responses. However, the analyses in this study of endogenous chromaffin cells of the adrenal medulla represents the in vivo cell composition and, therefore, illustrates how the adrenal medulla can be regulated by different secretagogues for co-release of multiple neurotransmitters.
The finding of similar and different profiles of neurotransmitters secreted from chromaffin cells implicates the presence of subpopulations of secretory vesicles that produce and store neurotransmitters for their release. Indeed, studies in chromaffin cells (bovine) show that secretory vesicles segregate into distinct populations of new and old vesicles (Duncan et al., 2003). The newly formed secretory vesicles are docked at the plasma membrane and undergo regulated release of its contents, known as the readily releasable pool (RRP). But the older vesicles are located within the cytoplasm and are less accessible for secretion. Furthermore, different secretagogues may selectively induce secretion of the RRP or from the deeper cytoplasmic pool (Duncan et al., 2003).
These findings about chromaffin cell secretion of neuropeptides and catecholamines involve the large dense core secretory vesicle (LDCSV) within these cells, because the LDCSV participate in the biosynthesis, storage, and regulated secretion of neurotransmitters and neurohumoral factors. Neurons of the brain and other regions of the nervous system contain LDCSV, and also contain synaptic vesicles for the regulated secretion of small classical neurotransmitters (Kim et al., 2006; Wegrzyn et al., 2010; Hnansko and Edwards, 2012; Sudhof 2013). It will be of interest in future studies to compare profiles of neurotransmitters secreted from neurons that are released from LDCSV and synaptic vesicles.
Studies in the field illustrate the plasticity of neurons to possess the ability to switch neurotransmitters utilized in an activity-dependent manner (Spitzer, 2012; Guemez-Gamboa et al., 2014). Opportunities for switching of neurotransmitter phenotypes may readily be achieved with alterations in profiles of neurotransmitters released in an activity-dependent manner. It will be of interest to examine whether profiles of neurotransmitters undergo switching, providing new mechanisms for plasticity and modified neurotransmitter-mediated responses.
Future analyses of neurotransmitter profiles will benefit from unbiased profiling by mass spectrometry (MS/MS) approaches to provide quantitative profiling. Profiling by MS/MS approaches have the advantage over focused assays in being able to identify multiple transmitters in an objective manner, and being able to identify multiple neurotransmitters in a single experiment. Ongoing research in the field is developing neuropeptidomics mass spectrometry technology for defining the structural profiles of endogenous neuropeptides (Amare et al., 2006; Gupta et al., 2010; Svensson et al., 2007; Yin et al., 2011; Zhang et al., 2012), and metabolomics approaches are being applied for profiling the classical small molecule neurotransmitters including the catecholamines (Falus, 2003). These emerging neurotechnologies will greatly facilitate investigations of the neurotransmitters profiles mediating cell-cell communication in the nervous and endocrine systems.
Conclusions
Findings of this study demonstrate that profiles of secreted neuropeptide and catecholamine neurotransmitters show similarities and differences upon stimulation by distinct secretagogues. The profiles of secreted neurotransmitters has been illustrated in this study by the co-release of the catecholamines dopamine, norepinephrine and epinephrine, together with several neuropeptide transmitters of (Met)-enkephalin, galanin, NPY, and VIP from chromaffin cells of the adrenal medulla. A generally similar profile of these neurotransmitters were secreted when stimulated by different secretagogues, along with some differences in quantities of co-released neurotransmitter profiles.
More specifically, with respect to neurotransmitter profiles secreted upon induction by the different secretagogues, the results showed that (1) epinephrine among the catecholamines was released at the highest levels upon secretagogue stimulation, (2) enkephalin was secreted in larger amounts than the other neuropeptides studied, and (3) catecholamines were generally secreted in greater amounts than neuropeptides. With respect to the effects of different secretatogues on neurotransmitters secreted, (1) nicotine and carbachol stimulated high levels of the neurotransmitters compared to more modest levels of secretion induced by PACAP, bradykinin, and histamine, and (2) KCl stimulated levels of neurotransmitters that were generally intermediate in amounts compared to the other secretagogues.
These findings show that profiles of secreted neurotransmitters may be controlled for participation in the adrenal medulla's response to stress.
Supplementary Material
Highlights.
Quantitative profiles of secreted neuropeptide and catecholamine neurotransmitters.
Distinct secretagogues show similarities and differences in secreted profiles of neurotransmitters.
Secretagogues stimulated epinephrine secretion at greater levels than other catecholamines
Stimulated enkephalin secretion occurred at higher levels than other neuropeptides
Co-release of neurotransmitter profiles related to the adrenal medullary stress response.
Acknowledgements
This research was supported by grants to V. Hook (R01MH077305, R01DA04275, P01HL58120, and T32DA07315) and M. Ziegler (P01HL58120). S. Podvin was a postdoctoral scholar supported by T32DA7315. Technical assistance by B. Kennedy and J. Pottenger was appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amare A, Hummon AB, Southey BR, Zimmerman TA, Rodriguez-Zas SL, Sweedler JV. Bridging neuropeptidomics and genomics with bioinformatics: prediction of mammalian neuropeptide prohormone processing. J. Proteome Res. 2006;5:1162–1167. doi: 10.1021/pr0504541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arun CP. Fight or flight, forbearance and fortitude: the spectrum of actions of the catecholamines and their cousins. Ann. N. Y. Acad. Sci. 2004;1018:137–140. doi: 10.1196/annals.1296.016. [DOI] [PubMed] [Google Scholar]
- Aunis D. Exocytosis in chromaffin cells of the adrenal medulla. Int. Rev. Cytol. 1998;181:213–320. doi: 10.1016/s0074-7696(08)60419-2. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD. Mechanisms of catecholamine secretion from adrenal chromaffin cells. J. Physiol. Pharmacol. 1995;46:273–83. [PubMed] [Google Scholar]
- Carmichael SW, Winkler H. The adrenal chromaffin cell. Sci. Am. 1985;253:40–49. doi: 10.1038/scientificamerican0885-40. [DOI] [PubMed] [Google Scholar]
- Choi AY, Cahill AL, Perry BD, Perlman RL. Histamine evokes greater increases in phosphatidylinositol metabolism and catecholamine secretion in epinephrine-containing than in norepinephrine-containing chromaffin cells. J Neurochem. 1993;61:541–549. doi: 10.1111/j.1471-4159.1993.tb02157.x. [DOI] [PubMed] [Google Scholar]
- Ciolkowski EL, Cooper BR, Jankowski JA, Jorgenson JW, Wightman RM. Direct observation of epinephrine and norepinephrine cosecretion from individual adrenal medllary chromaffin cells. J. Am. Chem. Soc. 1992;114:2815–2821. [Google Scholar]
- Duncan RR, Greaves J, Wiegand UK, Matskevich I, Bodammer G, Apps DK, Shipston MJ, Chow RH. Functional and spatial segregation of secretory vesicle pools according to vesicle age. Nature. 2003;422:176–180. doi: 10.1038/nature01389. [DOI] [PubMed] [Google Scholar]
- Efthimiopoulos S, Vassilacopoulou D, Ripellino JA, Tezapsidis N, Robakis NK. Cholinergic agonists stimulate secretion of soluble full-length amyloid precursor protein in neuroendocrine cells. Proc. Natl. Acad. Sci. USA. 1996;93:8046–8050. doi: 10.1073/pnas.93.15.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden LE. Neuropeptide-catecholamine interactions in stress. Adv. Pharmacol. 2013;68:399–404. doi: 10.1016/B978-0-12-411512-5.00018-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- Evinger MJ, Ernsberger P, Regunathan S, Joh TH, Reis DJ. A single transmitter regulates gene expression through two separate mechanisms: cholinergic regulation of phenylethanolamine N-methyltransferase mRNA via nicotinic and muscarinic pathways. J. Neurosci. 1994;14:2106–2116. doi: 10.1523/JNEUROSCI.14-04-02106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falus A. Histamine, part of the metabolome. Acta Biol. Hung. 2003;54:27–34. doi: 10.1556/ABiol.54.2003.1.3. [DOI] [PubMed] [Google Scholar]
- Fang P, Yu M, Shi M, Zhang Z, Sui Y, Guo L, Bo P. Galanin peptide family as a modulating target for contribution to metabolic syndrome. Gen. Comp. Endocrinol. 2005;179:115–120. doi: 10.1016/j.ygcen.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Fulop T, Radabaugh S, Smith C. Activity-dependent differential transmitter release in mouse adrenal chromaffin cells. J. Neurosci. 2005;25:7324–7332. doi: 10.1523/JNEUROSCI.2042-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ges IA, Brindley RL, Currie KP, Baudenbacher FJ. A microfluidic platform for chemical stimulation and real time analysis of catecholamine secretion from neuroendocrine cells. Lab Chip. 2013;13:4663–4673. doi: 10.1039/c3lc50779c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratzl M, Krieger-Brauer H, Ekerdt R. Latent acetylcholinesterase in secretory vesicles isolated from adrenal medulla. Biochim. Biophys. Acta. 1981;649:355–366. doi: 10.1016/0005-2736(81)90425-9. [DOI] [PubMed] [Google Scholar]
- Guemez-Gamboa A, Xu L, Meng D, Spitzer NC. Non-cell-autonomous mechanism of activity-dependent neurotransmitter switching. Neuron. 2014;82:1004–1016. doi: 10.1016/j.neuron.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Bark SJ, Lu WD, Taupenot L, O'Connor DT, Pevzner P, Hook V. Mass spectrometry-based neuropeptidomics of secretory vesicles from human adrenal medullary pheochromocytoma reveals novel peptide products of prohormone processing. J. Proteome Res. 2010;9:5065–5075. doi: 10.1021/pr100358b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R. Co-existence and co-release of classical neurotransmitters. Springer; New York: 2009. [Google Scholar]
- Holman ME, Coleman HA, Tonta MA, Parkington HC. Synaptic transmission from splanchnic nerves to the adrenal medulla of guinea pigs. J. Physiol. 1994;478:115–124. doi: 10.1113/jphysiol.1994.sp020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook V, Toneff T, Baylon S, Sei C. Differential activation of enkephalin, galanin, somatostatin, NPY, and VIP neuropeptide production by stimulators of protein kinases A and C in neuroendocrine chromaffin cells. Neuropeptides. 2008;42:503–511. doi: 10.1016/j.npep.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SR, Garza C, Mosier C, Toneff T, Wunderlich E, Goldsmith P, Hook V. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretion. J. Biol. Chem. 2007;282:9556–9563. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Edwards RH. Neurotransmitter corelease: mechanism and physiological role. Annu. Rev. Physiol. 2012;74:225–243. doi: 10.1146/annurev-physiol-020911-153315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M, Fernandez G, Roozendaal B. Stress and emotional memory: a matter of timing. Trends Cogn. Sci. 2011;15:280–288. doi: 10.1016/j.tics.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–53. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DL, Ledbetter FH, Carson KA, Kirshner AG, Slepetis R, Kirshner N. Stability of bovine adrenal medulla cells in culture. J Neurochem. 1980;35:679–92. doi: 10.1111/j.1471-4159.1980.tb03707.x. [DOI] [PubMed] [Google Scholar]
- Kim T, Gondré-Lewis MC, Arnaoutova I, Loh YP. Dense-core secretory granule biogenesis. Physiology. 2006;2:124–33. doi: 10.1152/physiol.00043.2005. [DOI] [PubMed] [Google Scholar]
- Koval LM, Yavorskaya EN, Lukyanetz EA. Ultrastructural features of medullary chromaffin cell cultures. Neuroscience. 2000;96:639–49. doi: 10.1016/s0306-4522(99)00563-1. [DOI] [PubMed] [Google Scholar]
- Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress; structural and molecular genetic approaches. Physiol Rev. 2009;89:535–606. doi: 10.1152/physrev.00042.2006. [DOI] [PubMed] [Google Scholar]
- Kuwashima H, Matsumura C, Kimura T. Differential secretion of adrenaline and noradrenaline in response to various secretagogues from bovine chromaffin cells. Clin. Exp. Pharmacol. Physiol. 2009;27:494–499. doi: 10.1046/j.1440-1681.2000.03284.x. [DOI] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Holmes FE, Hobson SA, Wynick D, Hökfelt T, Kofler B. Physiology, Signaling, and Pharmacology of Galanin Peptides and Receptors: Three Decades of Emerging Diversity. Pharmacol. Rev. 2015;67:118–175. doi: 10.1124/pr.112.006536. [DOI] [PubMed] [Google Scholar]
- Laslop A, Mahata SK. Neuropeptides and chromogranins: session overview. Ann. N Y Acad. Sci. 2002;971:294–299. doi: 10.1111/j.1749-6632.2002.tb04483.x. [DOI] [PubMed] [Google Scholar]
- Mizobe F, Kozousek V, Dean DM, Livett BG. Pharmacological characterization of adrenal paraneurons: substance P and somatostatin as inhibitory modulators of the nicotinic response. Brain Res. 1979;178:555–66. doi: 10.1016/0006-8993(79)90714-5. [DOI] [PubMed] [Google Scholar]
- Molloy L, Wonnacott S, Gallagher T, Brough PA, Livett BG. Anatoxin-a is a potent agonist of the nicotinic acetylcholine receptor of bovine adrenal chromaffin cells. Eur J Pharmacol. 1995;289:447–53. doi: 10.1016/0922-4106(95)90153-1. [DOI] [PubMed] [Google Scholar]
- Moro MA, López MG, Gandía L, Michelena P, García AG. Separation and culture of living medullae. Anal Biochem. 1990;185:243–8. doi: 10.1016/0003-2697(90)90287-j. [DOI] [PubMed] [Google Scholar]
- Njus D, Kelley PM, Harnadek GJ. The chromaffin vesicle: a model secretory organelle. Physiologist. 1985;28:235–241. [PubMed] [Google Scholar]
- O'Connor DT, Mahata SK, Mahata M, Jiang Q, Hook VY, Taupenot L. Primary culture of bovine chromaffin cells. Nat. Protoc. 2007;2:1248–1253. doi: 10.1038/nprot.2007.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Augustine GJ, Fitzpatrick D, Katz LC, LaMantia AS, McNamara JO, Williams SM. Neuroscience. 2nd edition. Sinauer Associates; 2001. pp. 443–467. chapter 21. [Google Scholar]
- Shansky RM, Lipps J. Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Front. Hum. Neurosci. 2013;7:123. doi: 10.3389/fnhum.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CB, Eiden LE. Is PACAP the major neurotransmitter for stress transduction at the adrenomedullary synapse? J. Mol. Neurosci. 2012;48:403–412. doi: 10.1007/s12031-012-9749-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem. J. 1967;103:480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer NC. Activity-dependent neurotransmitter respecification. Nat Rev Neurosci. 2012;13:94–106. doi: 10.1038/nrn3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner RA, Hohmann JG, Holmes A, Wrenn CC, Cadd G, Juréus A, Clifton DK, Luo M, Gutshall M, Ma SY, Mufson EJ, Crawley JN. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroth N, Kuri BA, Mustafa T, Chan SA, Smith CB, Eiden LE. PACAP controls adrenomedullary catecholamine secretion and expression of catecholamine biosynthetic enzymes at high splanchnic nerve firing rates characteristic of stress transduction in male mice. Endocrinology. 2013;154:330–339. doi: 10.1210/en.2012-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–90. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M, Sköld K, Nilsson A, Fälth M, Svenningsson P, Andrén PE. Neuropeptidomics: expanding proteomics downwards. Biochem. Soc. Trans. 2007;35:588–593. doi: 10.1042/BST0350588. [DOI] [PubMed] [Google Scholar]
- Taylor CV, Taupenot L, Mahata SK, Mahata M, Wu H, Yasothornsrikul S, Toneff T, Caporale C, Jiang Q, Parmer RJ, Hook VY, O'Connor DT. Formation of the catecholamine release-inhibitory peptide catestatin from chromogranin A. Determination of proteolytic cleavage sites in hormone storage granules. J. Biol. Chem. 2000;275:22905–22915. doi: 10.1074/jbc.M001232200. [DOI] [PubMed] [Google Scholar]
- Toneff T, Funkelstein L, Mosier C, Abagyan A, Ziegler M, Hook V. Beta-amyloid peptides undergo regulated co-secretion with neuropeptide and catecholamine neurotransmitters. Peptides. 2013;46:126–135. doi: 10.1016/j.peptides.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DJ, Chen C, Marley PD. Histamine promotes excitability in bovine adrenal chromaffin cells by inhibiting an M-current. J. Physiol. 2002;540:921–939. doi: 10.1113/jphysiol.2001.013370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzyn JL, Bark SJ, Funkelstein L, Mosier CA, Yap A, Kazemi-Esfarjani P, La Spada JL, Sigurdson C, O'Connor DT, Hook V. Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication. J. Proteome Res. 2010;9:5002–5024. doi: 10.1021/pr1003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin P, Hou X, Romanova EV, Sweedler JV. Neuropeptidomics: mass spectrometry-based qualitative and quantitative analysis. Methods Mol. Biol. 2011;789:223–236. doi: 10.1007/978-1-61779-310-3_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Rao F, Wen G, Salem R, Vaingankar S, Mahata M, Mahapatra NR, Lillie EO, Cadman PE, Friese RS, Hamilton BA, Hook VY, Mahata SK, Taupenot L, O'Connor DT. Catecholamine storage vesicles and the metabolic syndrome: The role of the chromogranin A fragment pancreastatin. Diabetes Obesity and Metabolism. 2006;8:621–633. doi: 10.1111/j.1463-1326.2006.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Ye H, Wang J, Hui L, Li L. Pressure-assisted capillary electrophoresis coupling with matrix-assisted laser desorption/ionization-mass spectrometric imaging for quantitative analysis of complex peptide mixtures. Anal. Chem. 84:7684–7691. doi: 10.1021/ac300628s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.