Abstract
The evolutionarily conserved transmembrane anterior posterior transformation 1 protein, encoded by TAPT1, is involved in murine axial skeletal patterning, but its cellular function remains unknown. Our study demonstrates that TAPT1 mutations underlie a complex congenital syndrome, showing clinical overlap between lethal skeletal dysplasias and ciliopathies. This syndrome is characterized by fetal lethality, severe hypomineralization of the entire skeleton and intra-uterine fractures, and multiple congenital developmental anomalies affecting the brain, lungs, and kidneys. We establish that wild-type TAPT1 localizes to the centrosome and/or ciliary basal body, whereas defective TAPT1 mislocalizes to the cytoplasm and disrupts Golgi morphology and trafficking and normal primary cilium formation. Knockdown of tapt1b in zebrafish induces severe craniofacial cartilage malformations and delayed ossification, which is shown to be associated with aberrant differentiation of cranial neural crest cells.
Introduction
The primary cilium is a highly specialized organelle that projects from the cell surface, senses extracellular signals through various receptors on the ciliary membrane, and transduces these signals to the nucleus.1 It is crucial for proliferation and differentiation of many cell types and for embryonic and postnatal development of multiple organs. The importance of the primary cilium is further illustrated by the occurrence of a heterogeneous group of heritable multisystemic disorders, collectively called the ciliopathies, that are linked to defective ciliogenesis and are caused by a multitude of genes that affect ciliary structure and/or function.2 Clinical phenotypes include, among others, retinal degeneration, polycystic kidneys, cerebral anomalies, infertility, diabetes, and obesity.1,2 Recent research in mice has also revealed the medical relevance of primary cilia to vertebrate skeletal development, as illustrated by their importance in, among others, mesenchymal stem cell, chondrocyte, and osteoblast differentiation.1
This study describes two consanguineous families in which several pregnancies were terminated due to the presence of multiple bone fractures and deformities, undermineralization of the skull and axial skeleton, and congenital anomalies, including ascites, pleural effusion, and intracranial ventriculomegaly (Figures 1A–1F and Figure S1). Because of the presence of multiple intra-uterine fractures, the diagnosis of lethal osteogenesis imperfecta (OI [MIM: 166210]) was suspected at referral.3 Molecular analysis of the type I procollagen-encoding genes4 and more recently described genes linked to OI5 did not reveal a pathogenic variant.
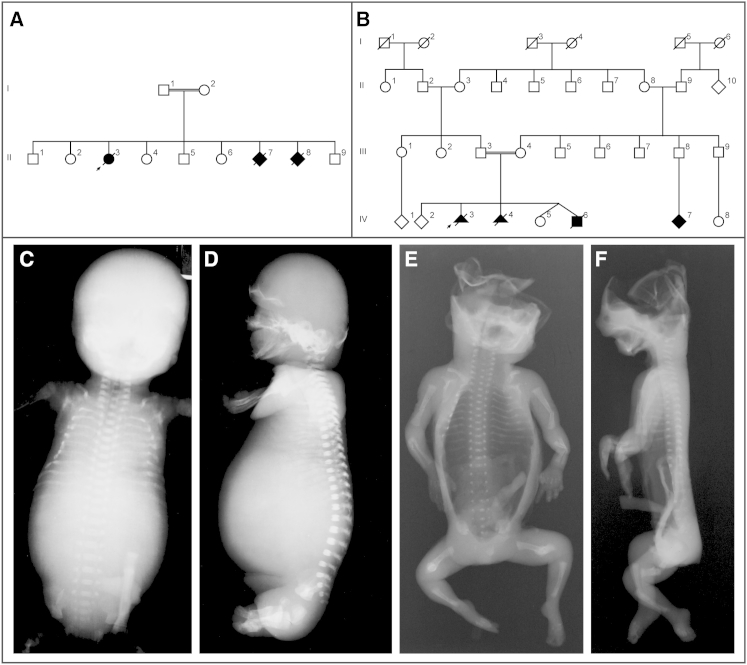
Figure 1.
Pedigrees of Affected Families and Clinical Description
(A–B) Pedigrees of family 1 (F1) and family 2 (F2). Black symbols, affected individuals carrying a homozygous TAPT1 mutation; strikethrough symbols, deceased individuals.
(C–D) Radiographs of fetus II-7 from family 1. Frontal (C) and lateral (D) views show generalized osteopenia of the skull, a barrel-shaped thorax with short ribs, and multiple posterior and axillary rib fractures. Upper and lower extremities are undermineralized.
(E–F) Radiographs of fetus IV-3 from family 2. Frontal (E) and lateral (F) views how generalized radiolucency of the entire skeleton, thin ribs with multiple fractures, and fractures of upper and lower extremities.
Combined whole-genome homozygosity mapping and exome sequencing for the first family (family 1) and direct Sanger sequencing for a second family (family 2) led to the identification of pathogenic homozygous mutations in TAPT1, encoding the evolutionary highly conserved transmembrane anterior posterior transformation 1 protein. Immunocytochemical staining experiments demonstrated that the fetal fibroblasts from both families have defective cilium formation and organization of the Golgi apparatus, including delayed intracellular protein trafficking. Knock-down of zebrafish tapt1b resulted in severe malformations of the craniofacial skeleton and delayed ossification, for which tapt1b was shown to be linked to disturbed differentiation of cranial neural crest (CNC) cells.
Material and Methods
Family Ascertainment
Family 1 (F1; Figure 1A) is a consanguineous Moroccan family with three affected fetuses (II-3, II-7, and II-8) born after interruption of pregnancy (IOP) due to multiple fractures and severe bone deformities. Family 2 (F2; Figure 1B) is a Syrian consanguineous family with three affected fetuses (IV-3, IV-4, and IV-6); two affected fetuses were delivered after IOP and the third affected sibling was part of a non-identical dichorial-diamniotic twin pregnancy. The major clinical features in both families included generalized severe skeletal osteopenia, microcephaly, multiple fractures, and congenital anomalies, including ascites, pleural effusion, and intracranial ventriculomegaly.
Skin biopsies were obtained from the affected fetuses of both families and the unaffected parents of family 1. Blood samples were collected from the unaffected siblings of family 1 and of the unaffected parents of family 2. No bone specimens of the affected fetuses of either family were available.
Because of the generalized radiolucency of the skeleton and the presence of multiple fractures, a clinical diagnosis of lethal OI was suspected. Molecular analysis of the type I procollagen-encoding genes (COL1A1 [MIM: 120150] and COL1A2 [MIM: 120160]),5 as well as other genes, in which mutations are associated with severe-to-lethal OI (LEPRE1 [MIM: 610339],6–8 CRTAP [MIM: 605497],8,9 PPIB [MIM: 123841],10,11 BMP1 [MIM: 112264],12,13 SERPINH1 [MIM: 600943],14,15 SERPINF1 [MIM: 172860],16 and CREB3L1 [MIM: 616215]17), did not reveal a causal mutation in either family. To completely exclude mutations in OI-related genes, molecular analysis of all other genes known to be linked to OI (FKBP10 [MIM: 607063],18 SP7 [MIM: 606633],19 PLOD2 [MIM: 601865],20 IFITM5 [MIM: 614757],21,22 WNT1 [MIM: 164820],23–25 TMEM38B [MIM: 611236], 26,27 and the PLS3 [MIM 300131] 28 gene involved in X-linked osteoporosis) was performed, but no causal mutations could be detected.
Study Approval
The study was approved by the ethics committee of the Ghent University Hospital, the National Institute of Child Health and Human Development institutional review board, and Universität zu Lübeck Schleswig-Holstein. Informed consent was obtained from both families.
Molecular Studies
Whole-Genome Homozygosity Linkage Mapping and Whole-Exome Sequencing for Family 1
Genome-wide homozygosity linkage mapping in family 1 was performed (ABI 400 marker set) for the three affected fetuses (II-3, II-7, and II-8) of family 1 after pooling of their DNA prior to linkage analysis. Homozygous regions were confirmed at the gDNA level separately for each fetus, and segregation analysis of the homozygous SNP markers was performed for each family member. Subsequently, exome sequencing on gDNA from fetus II-3 from family 1 was performed by Aros Applied Biotechnology AS. The TruSeq Exome Enrichment Kit (Illumina) was used for exome capture, and sequencing was carried out on the Illumina HiSeq 2000 platform with paired-end 100-bp reads. The CLC Genomics Workbench v.5.1 (CLCBio) software was used for duplicate-read removal, read mapping against the human genome reference sequence (NCBI Genome browser GRCh37.p5), coverage analysis, and variant calling. Variant annotation was performed with the Genomics Gateway beta plug-in (CLCBio). Resulting variants were compared with 27 in-house exomes from individuals with unrelated genetic disorders. Non-synonymous variants, variants located at acceptor and donor splice sites, and small coding insertions or deletions with at least 10-fold coverage, a relative allele frequency of at least 90% (in order to detect homozygous sequence changes), and that were not present in the Single Nucleotide Polymorphism database (dbSNP135) were further considered. In addition, genes previously linked with ciliary chondrodysplasias were analyzed (filter settings were as follows: all coding exons including 20 base pairs from flanking introns, coverage ≥ 3, forward and reverse read counts ≥ 1, and population frequency < 10%; Tables S3 and S4). No causal mutations were identified.
TAPT1 [MIM: 612758] was sequenced at the gDNA level by direct Sanger sequencing (ABI3730XL automated sequencer, Applied Biosystems). The segregation of the TAPT1 mutation was investigated in all family members of family 1. PCR amplification and direct Sanger sequencing of all exons and flanking introns of the entire TAPT1 gene were performed for family 2 (primer sequences available upon request). Mutation nomenclature is in accordance to the Human Genome Variation Society guidelines (TAPT1 reference sequence [GenBank: NM_153365.2]). The mutation was analyzed with the Alamut Visual 2.5 software (includes prediction algorithms SIFT, MutationTaster, and PolyPhen-2), Splice Site Prediction by Neural Network,29 and Human Splicing Finder30 software, and its absence was checked in 105 control samples31 and in the 1000 Genomes database. RT-PCR was performed on total mRNA to determine the splicing outcome. Multiple sequence alignment of the TAPT1 protein sequence was consulted at the NCBI website (HomoloGene). In silico analysis of the TAPT1 protein sequence was performed with the algorithms TopPred II, TMpred, Phobius, and PSORT II (Expasy).
In Vitro Studies
Generation of TAPT1 Expression Constructs
Using total fibroblast RNA of two fetuses from families 1 (fetus II-7) and 2 (fetus IV-4), as well as of control cells, the entire coding sequence of TAPT1 was amplified by PCR. Primer sequences were complemented with additional sequences specific for the restriction enzymes HindIII and XhoI at their 5′ end in order to allow for unidirectional cloning into the pcDNA3 eukaryotic expression vector (5′-AAGCTTCCAGTTTGTTGTGCTCGGAAC-3′ TAPT1_F_HindIII and 5′-CTCGAGGAAGCCACACAGATTCAGTCAAT-3′ TAPT1_R_XhoI). After PCR amplification, amplicons were cloned into the pCR2Topo vector (Invitrogen), digested with the restriction enzymes HindIII and XhoI, and ligated into the pcDNA3 eukaryotic expression vector (Life Technologies). Sequences were checked by bidirectional Sanger sequencing (ABI3730XL automated sequencer, Applied Biosystems).
Immunoprecipitation of TAPT1
In order to investigate the production and secretion of the wild-type and defective TAPT1 proteins, dermal control fibroblasts, and dermal fibroblasts of fetus II-3 from family 1 and fetus IV-6 from family 2 were lysed with RIPA buffer (Sigma). Cell lysates were incubated with Dynabeads Protein A, to which TAPT1 primary antibody (HPA042567, Sigma) was bound. Subsequently, bound proteins were eluted and subjected to western blot analysis.
siRNA and shRNA Experiment
For the siRNA experiment, the efficiency of transfection and knockdown was determined according to the manufacturer’s protocol (Thermo Scientific DharmaFECT transfection reagents, siRNA Transfection Protocol) in an initial pilot study. In brief, 30,000 MG-63 cells were seeded per well in an eight-well chamber slide format with varying DharmaFECT 1 siRNA Transfection Reagents (Thermo Fisher Scientific Biosciences, T-2001-01) and varying ON-TARGETplus human TAPT1 siRNA - SMARTpool (Thermo Fisher Scientific Biosciences, L-015826-02-0005) or ON-TARGETplus siCONTROL Non-Targeting Pool (Thermo Fisher Scientific Biosciences, D-001810-10-05). On the basis of this pilot study, the 1/666 dilution for DharmaFECT 1 siRNA Transfection Reagent and the 50 nM siRNA pool were selected because they generated the best TAPT1 knockdown percentage (determined by qPCR: 77.38% TAPT1 knockdown when comparing TAPT1 siRNA treated cells to negative control cells).
Subsequently, bone osteosarcoma cells (MG-63 cells) were seeded and grown in 8-chamber glass slides (30,000 cells/well) for immunocytochemistry and RNA isolation. 24 hr post-seeding, cells were transfected with 50 nM ON-TARGETplus Human TAPT1 siRNA - SMART pool (Thermo Fisher Scientific Biosciences, L-015826-02-0005) or 50 nM ON-TARGETplus siCONTROL Non-Targeting Pool (Thermo Fisher Scientific Biosciences, D-001810-10-05) and DharmaFECT 1 siRNA Transfection Reagent (1/666 dilution, Thermo Fisher Scientific Biosciences, T-2001-01). 24 hr post-transfection cells were starved for 24 hr and harvested. The efficiency of TAPT1 knockdown was determined by qPCR (knockdown of TAPT1 expression: 72%).
In order to get stable shRNA MG-63 cell lines, a virus for TAPT1 (Sigma, TRCN0000136711, TRCN0000136644, and TRCN0000134168), a positive control LMNA (Sigma, TRCN0000061835), and a negative control (Sigma, SHC002) were generated with Trans-Lentiviral Packaging Mix (Thermo, catTLP4606). Subsequently, 250,000 MG-63 cells were seeded in a six-well format, transduced in a 1/8 ratio, and selected with 2 μg/ml puromycin (Sigma, 250 mg of P7255). Finally, knockdown percentage was determined by qPCR (calculated in comparison to the negative control): 32.12% TAPT1 knockdown for TRCN0000136711, –5.70% TAPT1 knockdown for TRCN0000136644, 29.40% TAPT1 knockdown for TRCN0000134168, and 84.45% LMNA knockdown for TRCN0000061835. For TRCN0000136711, an immunocytochemical staining experiment, in which cells were starved for 48 hr, was performed for TAPT1 and acetylated tubulin. To quantify the staining for TAPT1, the size of the centrosomes was measured (blindly, by two independent investigators; 150 centrosomes were measured per sample).
Immunocytochemistry
Transfected HEK293T cells, TAPT1 siRNA-treated MG-63 cells, and dermal fibroblasts from fetus II-3 from family 1, fetuses IV-4 and IV-6 from family 2, and a control individual were grown on glass chamber slides and at confluency, starved for 24 hr. Cells were fixed with 3.7% paraformaldehyde, blocked in 10% BSA in PBS, and incubated with primary antibodies against TAPT1 (Sigma, HPA042567), α-acetylated tubulin (Sigma, T6793), Golph4 (Abcam, ab280489), and γ-tubulin (Abcam, ab11316). After washing, cells were incubated with secondary antibodies (goat anti-mouse Alexa Fluor 488 and donkey anti-rabbit Alexa Fluor 594) and mounted with coverslips. Images were captured with a Zeiss AxioObserved-Z1 microscope. Cilium formation was independently evaluated by three investigators by counting the number of cells that formed a cilium and by assessing cilium abnormalities.
Centrosome Purification, Sucrose Gradient Ultracentrifugation, and Western Blotting
Centrosome preparation was performed as previously described.32 Ten million exponentially growing HEK293T or MG-63 cells were incubated with culture medium containing 1 μg/ml cytochalasin D and 0.2 μM nocodazole for 1 hr at 37°C to depolymerize actin and microtubule filaments. Cells were harvested by trypsinization and lysed in 5 ml solution of 1 mM HEPES (pH 7.2), 0.5% Nonidet P-40, 0.5 mM MgCl2, and 0.1% 2-mercaptoethanol with EDTA-free proteinase inhibitors. Swollen nuclei and chromatin aggregates were removed by centrifugation at 2,500 × g for 10 min, and the supernatant was filtered through a 40-μm nylon mesh. HEPES was adjusted to 10 mM, DNase I was added to 2 U/ml, and the mixture was incubated for 30 min on ice. The lysate was underlaid with 500 μl 60% sucrose solution (60% wt/wt sucrose in 10 mM Pipes, pH 7.2 in 0.1% Triton X-100 in 0.1% 2-mercaptoethanol) and centrosomes were sedimented into the sucrose cushion by centrifugation at 10,000 × g for 30 min. 1.5 ml of the bottom layer was pipetted onto a discontinuous sucrose gradient (500 μl of 70%, 300 μl of 50%, and 300 μl of 40% sucrose solutions) and centrifuged at 120,000 × g for 1 hr. Fractions were collected from the top: 1,200 μl for the first fraction and 200 μl for fractions two–eight. Each fraction was diluted in 1 ml of 10 mM Pipes buffer, pH 7.2. Centrosomes were recovered by centrifugation at 13,200 rpm for 15 min and dissolved in 100 μl 2× Laemmli buffer. Samples were reduced and proteins were separated on NU-PAGE 4%–12% Bis-Tris gels, transferred to nitrocellulose membranes, blocked for 1 hr with 2% blocking agent in 1× tris-buffered saline with Tween-20 (TBST), and developed with primary antibodies anti-TAPT1 (Sigma, HPA042567, 1/1,000) or anti-γ-tubulin (Abcam, ab11316, 1/1,000) and secondary antibodies anti-rabbit horseradish peroxidase (HRP) linked (Bioké, 7074S, 1/1,500) or anti-mouse HRP linked (Bioké, 7076S, 1/1,500).
Steady-State Collagen Analysis
Control cells and fibroblasts from affected members from family 1 and family 2 were grown to confluence in six-well culture dishes. Steady-state collagen analysis was conducted as previously described.33 In brief, fibroblasts were labeled overnight with [3H]-proline. Medium and cell layer collagens were ammonium sulfate precipitated and pepsin-digested before electrophoresis on a 6% SDS-urea-PAGE.
Folding Assay of Type I Collagen
Collagen folding assays were performed as previously described.34 In brief, confluent fibroblasts were pulsed with [14C]-proline for 15 min and cell layer procollagens were collected every 5 min. Samples were digested with trypsin and chymotrypsin for 2 min and halted with soybean trypsin inhibitor. Collagens were precipitated with 4 M NaCl in 1 M acetic acid overnight, washed in 70% EtOH, and electrophoresed on a 3%–8% tris-acetate gel (Life Technologies). Densitometry of the α1(I)-chains was measured with ImageJ software and normalized to control measurements.
Pulse-Chase Secretion Kinetics
As described previously,35 confluent fibroblasts were labeled for 4 hr with [14C]-proline and chased with fresh medium containing 2 mM cold proline. Medium and cell layer procollagens were harvested at the indicated times and pepsin-digested. Two wells for each assay were left unlabeled to provide an accurate cell count for normalization between cell lines. Normalized samples were run on a 3%–8% tris-acetate gel (Life Technologies), and α1(I)-collagen bands were measured by densitometry with ImageJ for calculation of the percent secretion.
Amino Acid Analysis
Amino acid analysis to quantify hydroxylysine and lysine was performed by high-pressure liquid chromatography (AIBiotech).9
Differential Scanning Calorimetry and Differential Scanning Circular Dichroism
Differential scanning calorimetry and differential scanning circular dichroism of collagen solutions in 0.2 M sodium phosphate and 0.5 M glycerol at pH 7.4 were performed as previously described.36
Trafficking Assay and Immunofluorescence Microscopy
Control and fetal (fetus II-3 from family 1 and fetus IV-4 from family 2) fibroblasts were plated on two-well chamber slides. Cells were washed and incubated on ice with cholera toxin subunit B (CTx-B)-488 (LifeTechnologies) for 15 min, then incubated for 1 hr at 37°C in serum-free media before fixing in 4% paraformaldehyde.37 Cells were permeabilized in 1% serum plus 0.2% Triton X-100, washed, and blocked in 1% BSA in PBS. Cells were incubated with Golgin-97 (Life Technologies), washed, incubated with secondary antibody, and stained with DAPI (Vector Labs). Cells were imaged on a Zeiss LSM510 confocal microscope, with the supplied software.
Zebrafish Studies
Zebrafish Maintenance
Wild-type AB and transgenic Tg(Fli1:EGFP) and Tg(Col2:mcherry) zebrafish were reared at a constant temperature of∼28°C and maintained by standard protocols. Transgenic lines were reported previously.38,39
In Situ Hybridization
High-resolution whole-mount in situ hybridization (WISH) of zebrafish embryos was carried out as previously described.40 Dioxygenin uridine-5′-triphosphate (DIG)-labeled RNA probes (sequences available upon request) targeting the zebrafish tapt1b, barx1,41 and sox9a42 genes were used to study expression at 24, 48, 60, 72, and 96 hr post fertilization (hpf). Whole-mount embryos were observed and photographed with a Leica M165 FC Fluorescent Stereo Microscope (Leica Microsystems). Hybridized specimens were processed for embedding in epon and serial cross sectioning (4 μm).43 Photomicrographs were taken with a Zeiss Axioimager Z1 equipped with differential interference contrast (DIC) optics and an Axiocam MRc camera. Morphants (96 hpf) were compared to 72 hpf uninjected zebrafish to account for a possible developmental delay.
Morpholino Injections
Antisense splice-blocking morpholinos (MOs; Gene Tools) were designed against the intron-1-exon-2 (i1e2) acceptor splice site (5′-GAGATCTGCACACAGACATACAAAT-3′) and the exon-2-intron-2 (e2i2) donor splice site (5′-AGCTGTGTTTGGTACTGTACCTTCT-3′) of tapt1b. Routinely, MOs were microinjected in 1–5 nl of volume into one- to two-cell stage wild-type embryos at 2 ng for both tapt1b i1e2 MOs and tapt1b e2i2 MOs. To control for possible non-specificity effects of MO injection, (1) MO injections were performed in parallel with a standard control MO, and (2) MOs were injected in p53 mutant zebrafish embryos to check for p53-induced apoptosis.44 All MOs were dissolved in 0.5% phenol red and 1× Danieu’s buffer [58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM HEPES (pH 7.6)]. MO-injected specimens were processed in epon followed by serial cross sectioning (1 μm and staining with toluidine blue [0.5% in 1% borax]). Photomicrographs were taken with a Zeiss Axioimager Z1 and an AxiocamMRc camera.
CRISPR/Cas Injections
Tapt1b CRISPR target sequences (available on request) were identified with CRISPRdirect. Cas9-NLS protein (250 pg; LabOmics) and gRNA (25 pg) were co-injected into one-cell-stage wild-type embryos.
Alcian Blue Staining
Cartilage was stained with Alcian blue, via a modified protocol of Neuhauss et al., 1996.45 Uninjected, tapt1b MO- and tapt1b CRISPR/Cas (F0 tapt1bCas9/gRNA)-injected larvae were collected at 4 days post fertilization (dpf) and fixed overnight in 4% paraformaldehyde and washed several times in PBS with 0.1% Tween-20 (PBST). In order to enhance their optical clarity, the specimens were bleached in a solution containing 1.5% hydrogen peroxide and 1% potassium hydroxide for 2 hr or until the embryos were sufficiently translucent. The embryos were rinsed in PBST and transferred into an Alcian blue solution (1% concentrated hydrochloric acid, 70% ethanol, 0.1% Alcian blue) and stained overnight. Specimens were cleared in acidic ethanol (5% concentrated hydrochloric acid, 70% ethanol) for 4 hr and rehydrated in an ethanol series. To further reduce background staining and interference of surrounding tissues, a digest was done with a 1 mg/ml Trypsine solution in 60% saturated borax for 20 min. Finally, the specimens were rinsed with demineralized water and gradually transferred to pure glycerol for documentation and storage. Analysis was done using a Leica M165 FC Fluorescent Stereo Microscope.
Alizarin Red Staining
Fixed specimens were stained via a modified protocol.46 Embryos were fixed in 4% paraformaldehyde in 0.1 M sodium phosphate buffer for 1 hr, washed with demineralized water, and subsequently bleached in 1% hydrogen peroxide in 1% potassium hydroxide for 30 min. After bleaching, embryos were stained with 0.5% Alizarin Red S (Sigma) in a 1% potassium hydroxide in 2% Triton X-100 solution. Clearing was carried out in a solution containing 20% glycerol and 0.25% potassium hydroxide. Specimens were stored at 4°C in 100% glycerol.
Whole-Mount Immunofluorescence
Immunofluorescence staining was performed as previously described.47 Embryos were digested in 10 μg/ml proteinase K for 5 min at room temperature after fixation in 4% paraformaldehyde in PBS. The embryos were subsequently washed in PBS + 0.8% Triton X-100 and blocked in 10% normal goat serum: 1% dimethyl sulphoxide in PBS + 0.8% Triton X-100 blocking solution for 1 hr at room temperature. This was followed by incubation with primary antibody at 4°C overnight (anti-acetylated mouse alpha-tubulin, 1:1,000, Sigma, T6793) in blocking solution. Following several washes, the embryos were incubated at 4°C overnight in an appropriate fluorescent secondary antibody (goat anti-rabbit Alexa Fluor 488, 1:200, Invitrogen, A10034). Fluorescently labeled embryos were imaged on a Leica TCS LSI Super Zoom confocal microscope (Leica Microsystems). Cilial length in the otic vesicle of three morphants and three uninjected controls was measured on maximal intensity projections with the Leica Application Suite Advanced Fluorescence software (Leica Microsystems).
Statistics
Statistical analysis for the measurements of the cilial length in the otic vesicle of the zebrafish was carried out with R software. Continuous outcome variables exhibiting a skewed distribution were transformed, using the natural logarithms, before a two-way ANOVA was conducted to satisfy the prerequisite assumptions of normality. To assess the difference in cilia length between the morphants and the control group, the non-parametric Mann-Whitney U test was performed in non-transformed data.
Results
Clinical Phenotype
Family 1
Family 1 (Figure 1A) is a consanguineous family of Moroccan origin with three affected fetuses (individuals II-3, II-7, and II-8) delivered after IOP due to multiple fractures and severe bone deformities. Both parents are healthy, have no signs of increased bone fragility, and have six living healthy children.
The first fetus (II-3; Figures S1A–S1D) was a female (karyotype 46, XX) who, at antenatal ultrasound examination at 26 weeks of gestation, revealed multiple fractures, hydramnios with ascites, and dilated third and fourth cerebral ventricles. Post-mortem examination showed a fetus, small for gestational age, with a prominent forehead, hypertelorism, short nose, broad nasal bridge, anteverted nostrils, low-set ears, and a short and broad neck. Some bony fragments were palpable over the skull. In addition, short limbs with flexion contractures were noticed and lower extremities were in abduction. Radiologic examination showed generalized radiolucency of the skeleton and an incompletely ossified skull with wormian bones. The thorax was small and thin with discontinuously beaded ribs and platyspondyly of the vertebrae. The upper limbs showed fractures of the humerus and metaphyseal flaring, and the femora were very short, poorly modeled, and angulated. The tibiae were relatively thin and bowed with some metaphyseal flaring. The fibulae were extremely thin and deformed.
The second fetus (II-7; Figures 1C and 1D) was delivered at 18.5 weeks of gestation after IOP because ultrasonographic examination revealed undermineralization of the skull, short ribs, a barrel-shaped thorax, and bilateral femur fractures. Post-mortem radiological examination confirmed the presence of a barrel-shaped thorax with multiple rib fractures, bilateral femur shaft fractures, fractures of the radius and ulna, and fracture of the right tibia shaft with mild bowing of the tibia. Also, decreased mineralization of the upper and lower extremities was noticed.
The third fetus (II-8) died in utero at 16 weeks of gestation. Post-mortem radiographs showed almost absent ossification of the skull, poor mineralization of the ribs with multiple rib fractures, and fractures of the left and right femur and right tibia shafts.
Family 2
Family 2 (Figure 1B) is a complex Syrian consanguineous family with two affected fetuses (individuals IV-3 and IV-4) who were delivered after IOP. A previous pregnancy was terminated due to extrauterine gravidity. The third affected sibling was part of a non-identical dichorial-diamniotic twin pregnancy (individual IV-6). The parents are healthy and have no signs of increased bone fragility or any other congenital anomaly.
The first fetus (IV-3; Figures 1E and 1F) was a male (karyotype 46, XY) in whom ultrasound examination at 19 weeks of gestation revealed brachycephaly of the skull (occipital frontal circumference [OFC] was 144 mm), a high prominent forehead, ventriculomegaly of the brain, and a hypoplastic cerebellum. He had a flat face, micrognathia, and a hypoplastic nose. Skeletal investigation showed the presence of multiple fractures of the ribs and long bones and generalized radioluceny of the entire skeleton. Other developmental anomalies included cardiomegaly, ascites, hygroma colli, a small thorax, and a single umbilical artery.
The second fetus (IV-4; Figures S1E and S1F) was a female (karyotype 46, XX) who also showed intra-uterine growth retardation (weight of the fetus was 190 g, whereas comparable gestational fetuses weigh ∼359 g). Ultrasound examination at 19 weeks of gestation revealed a fetus with microbrachycephaly of the skull (OFC was 12.2 cm, −12.8 SD), a flat cerebellum, micrognathia, and a hypoplastic nose. In addition, multiple fractures of the ribs and long bones and generalized osteopenia of the entire skeleton were noticed. Other developmental anomalies included cardiomegaly, pleural effusion with mild ascites, hydrops fetalis, lung hypoplasia, and a single umbilical artery.
The third affected sibling (IV-6; Figures S1G–S1K) is a male sibling who was part of a dichorial-diamniotic twin pregnancy. Whereas the female fetus developed normally, the male fetus showed intrauterine growth retardation at 17 and 20 weeks of gestation. Moreover, brachymicrocephaly of the skull and agenesis of the brain vermis was noticed. He was microcephalic and he had a unilateral cleft lip and palate. Additionally, multiple fractures of the ribs and the long bones and generalized undermineralization of the entire skeleton were noticed. Other observed anomalies included a cardiac ventricular septal defect, hypertrophic cardiomyopathy and bilateral hydronephrosis, and a single umbilical artery. Ultrasound investigation at 31 weeks of gestation revealed severe microcephaly (OFC was 14.4 cm, −8.4 SD). In addition, the skull was brachycephalic. Fetus IV-6 also showed a high prominent forehead, intracranial ventriculomegaly, and a hypoplastic cerebellum. Birth took place after 37 weeks of gestation. The affected sibling had a birth weight of 1,140 g (–4.4 SD), length of 43 cm (–3 SD), and OFC of 21 cm (–14 SD). Clinical examination after birth revealed the following findings in addition to the anomalies seen by ultrasound examination: large and fleshy posteriorly rotated ears, hypertelorism, telecanthus, adducted thumbs, a micropenis, and hypospadias.
Molecular Studies
Defects in TAPT1 Are the Underlying Genetic Cause
We used both genome-wide linkage analysis and whole-exome sequencing to find the underlying genetic defect for family 1 (individual II-3). A common homozygous region was identified on chromosome 4 (Figure S2, Table S1) and contained two variants: a missense variant, c.688C>T (p.His230Tyr), in the pseudogene FAM90A26P (GenBank: XM_002342428.2) and an acceptor-splice-site variant, c.1108-1G>C in TAPT1 (GenBank: NM_153365.2), which segregated with the phenotype (Figure 2A). We considered the predicted transmembrane protein TAPT1 to be the better candidate given that TAPT1 is involved in murine skeletal patterning.48,49 At the mRNA level, the c.1108-1G>C splice-site variant resulted in skipping of the highly conserved exon 10 (p.Val370_Asn389del, Figure 2B, Figure S3A) and the production of a truncated TAPT1 protein (Figure 2C).
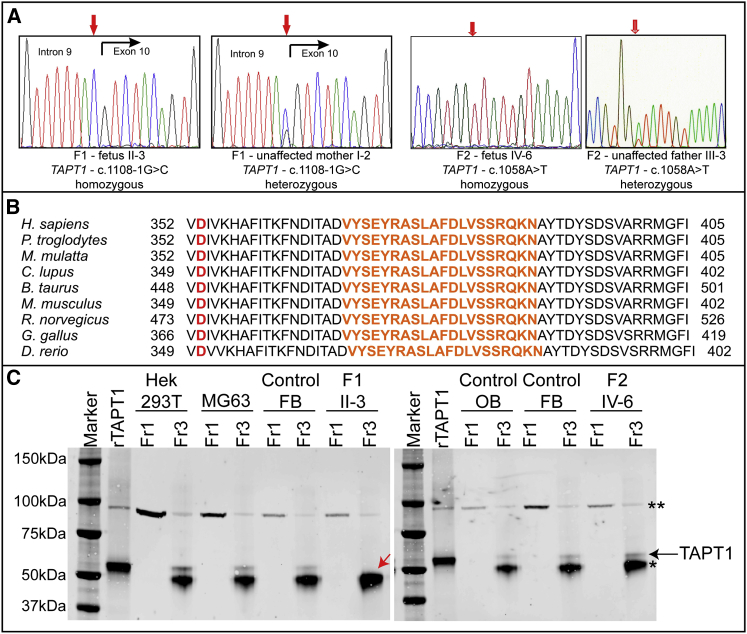
Figure 2.
Molecular Studies
(A) Electropherogram of TAPT1 mutations. The left two panels delineate the acceptor-splice-site mutation c.1108-1G>C in F1; the right two panels delineate the missense mutation c.1058A>T in F2. For each mutation, one affected fetus and one unaffected carrier parent is shown.
(B) Multiple sequence alignment across species of the TAPT1 mutated region reveals that the deleted and substituted amino acids are highly conserved. The orange text represents the highly conserved exon 10. The amino acid residue in red is the affected amino acid residue in family 2.
(C) Immunoprecipitation of TAPT1 in multiple cell types revealed that the protein migrates around 60 kDa. For the sample of fetus II-3 from family 1 a smaller molecular weight is observed, which is in line with the production of a shorter TAPT1 protein as a result of the removal of exon 10 (deletion of 20 highly conserved amino acids and shorter protein band marked with a red arrow). As a positive control, cell lysates of HEK293T cells, which were transfected with the wild-type TAPT1 expression construct, were loaded. Black arrow, TAPT1 protein; asterisks, heavy chain of the antibody used for immunoprecipitation; double asterisks, aspecific band; rTAPT, recombinant TAPT1; FB, fibroblasts; OB, osteoblasts; Fr1, fraction 1 = cell lysate before immunoprecipitation; Fr3, fraction 3 = eluate.
Direct Sanger sequencing of TAPT1 in 58 probands with a clinical diagnosis of severe-to-lethal congenital OI identified a homozygous missense variant (c.1058A>T, p.Asp353Val), which is predicted by the Alamut Visual software to be damaging and disease causing, in a second family (F2, Figure 2A-C). Although little is known about TAPT1 protein topology, both mutations are considered to affect the second extracellular/luminal loop of this transmembrane protein (Figures S3B and S3C).
Functional Studies
TAPT1 Is Localized at the Centrosome and/or Ciliary Basal Body Region
We determined the subcellular localization of TAPT1. Immunocytochemical staining showed that, in human fibroblasts, TAPT1 is present as an intense perinuclear spot, which co-localizes with the centrosomal protein γ-tubulin (Figure 3A). The centrosomal localization of TAPT1 in HEK293T and MG-63 cells was confirmed by centrosome isolation and western blotting (Figure S5).
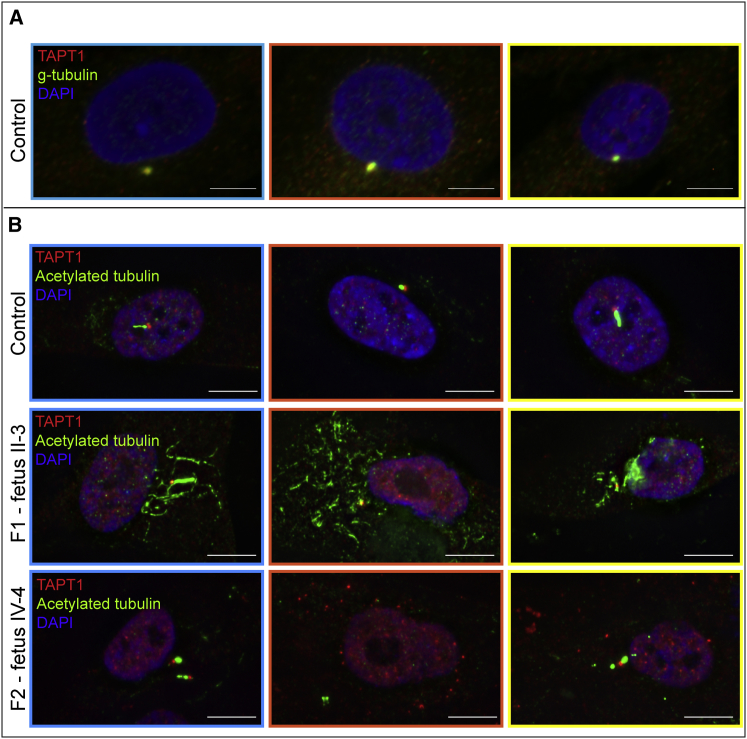
Figure 3.
TAPT1 Localizes to the Centrosome and/or Base of the Cilium and Is Important for Correct Ciliogenesis
(A) Immunostaining for TAPT1 (red), the centrosomal protein γ-tubulin (green), and nuclei (DAPI, blue) in control fibroblasts revealed co-localization of TAPT1 and γ-tubulin, implicating a centrosomal localization of TAPT1.
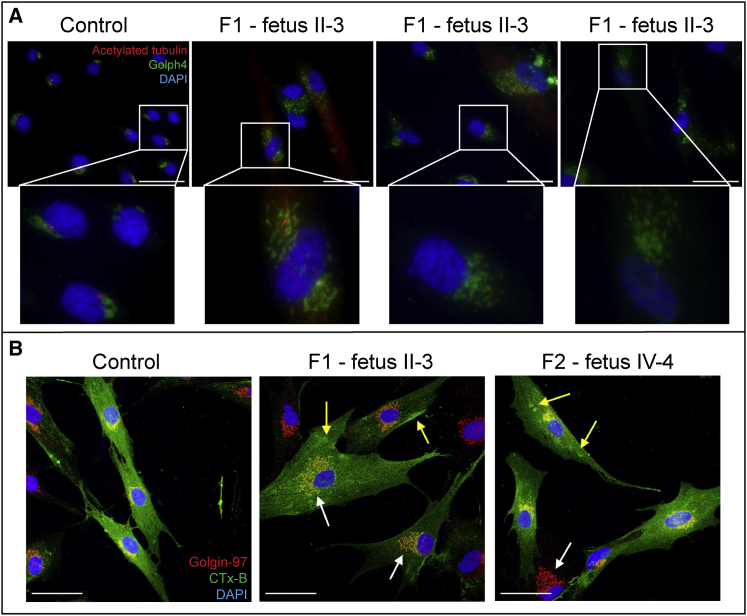
(B) Immunostaining of control and proband (II-3 from family 1 and IV-4 from family 2) dermal fibroblasts for TAPT1 (red), primary cilium (acetylated α-tubulin, green), and nuclei (DAPI, blue) showed that control fibroblasts were capable of inducing cilium formation after serum-starvation, whereas proband-derived fibroblasts were severely deficient for normal ciliogenesis. Fetus II-3 from family 1 failed to organize acetylated α-tubulin into a ciliary structure; instead, acetylated α-tubulin was present throughout the entire cell. On cells from fetus IV-4 from family 2, cilia were short and broadened. Additionally, multiple spots for TAPT1 were detected, implying that TAPT1 is mislocalized within the entire cell. For panels (A) and (B), close-ups are shown of the color-merged pictures: colored boundaries (blue, orange-red, and yellow borders) around the pictures denote the area selected from the original pictures (see Figure S4).
Because centrosomal components play a role in ciliogenesis,50 human fibroblasts were serum-starved to induce cilium formation and co-stained for acetylated α-tubulin. In control fibroblasts, TAPT1 localized at the ciliary basal body (Figure 3B upper panel, Figures S4 and S5). In contrast, in cells from fetus II-3 from family 1, the primary cilium was nearly absent and aberrant acetylated α-tubulin was present throughout the cell (Figure 3B middle panel, Figures S4 middle panel and S5), whereas for fetus IV-4 from family 2, the primary cilia formation was disturbed, and TAPT1 was scattered throughout the cytoplasm (Figure 3B lower panel, Figures S4 lower panel and S6). Transfection of TAPT1 expression constructs into HEK293T cells showed that wild-type TAPT1 localized to the ciliary basal body, whereas both defective TAPT1 proteins were spread throughout the cytoplasm (Figure S7). Additionally, TAPT1-siRNA-treated MG-63 cells displayed fewer and shorter primary cilia (Figure S8), and TAPT1-shRNA-treated MG-63 cells revealed that centrosomes varied more in size (Figure S9).
TAPT1 Defects Alter Collagen Folding and Secretion
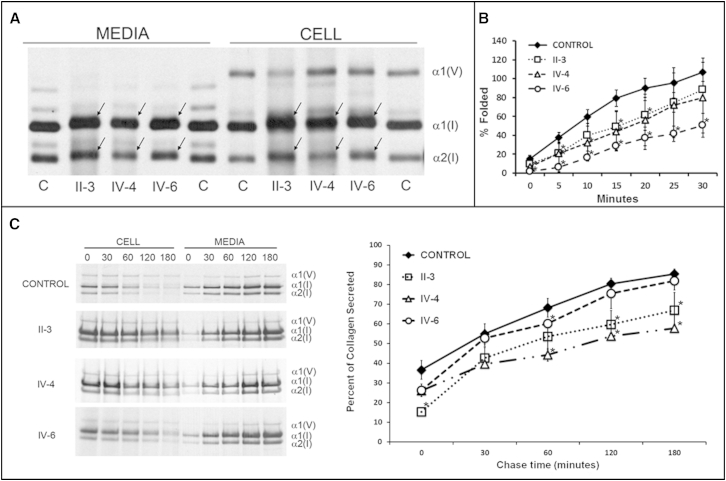
Because both families were initially diagnosed with lethal OI, several parameters of type I collagen biosynthesis were evaluated. Biochemical analysis revealed that type I collagen folding was moderately but significantly slower in proband cells (Figure 4A), allowing longer exposure to post-translational modifying enzymes. As a result, hydroxylysine (Hyl) content of collagen synthesized by TAPT1 proband cells was increased (Hyl/total lysine content: control, 22.1% versus fetus IV-4, 39.0%) and the collagen α-chains were moderately broadened and migrated more slowly on gel electrophoresis (Figure 4B). In addition, the secretion of type I collagen from the cells was slower (Figure 4C), albeit the collagen stability was not altered (Figure S10).
Figure 4.
Type I Collagen Studies
(A) Intracellular collagen folding in proband fibroblasts is moderately delayed. n = 3 for each cell line. ∗ = p < 0.05.
(B) Compared to controls, collagen biochemistry of proband fibroblasts showed broadened bands and delayed migration of the α1(I)- and α2(I)-collagen chains.
(C) Collagen secretion assay. Left panel shows SDS-PAGE analysis of the intracellular and secreted fibrillar types I and V collagen during a 180 min time range (time points are indicated above the gel). Right panel shows measurement of the intensities of the type I collagen bands (ImageJ). This revealed that type I collagen is more slowly secreted from the proband fibroblasts. n = 2–3 for each cell line. ∗p < 0.05.
TAPT1 Defects Alter Golgi Morphology and Trafficking
To investigate the mechanism by which TAPT1 defects contributed to the observed collagen abnormalities, we used CTx-B to examine the Golgi morphology (Figure 5A) and plasma-membrane-to-Golgi protein trafficking, (Figure 5B), a trafficking system that depends on centrosome-derived microtubules.37 Whereas control cells showed normal perinuclear, stacked Golgi morphology, TAPT1 mutant cells had a more disperse, not-polarized, and distended Golgi morphology (Figure 5A). Also, the CTx-B experiment revealed altered trafficking of CTx-B from the plasma membrane to the Golgi apparatus in TAPT1 mutant cells, which was most obvious for fetus II-3 from family 1 in comparison to fetus IV-4 from family 2 (Figure 5B, Table S2). The disturbed trafficking in proband cells most likely accounts for the slower collagen folding and overmodification (Figures 4A and 4B), as well as a slightly delayed collagen secretion (Figure 4C).
Figure 5.
Golgi Morphology and Trafficking
(A) Dermal fibroblasts were stained for the cilium (acetylated α-tubulin) and the Golgi apparatus (Golph4). Whereas control cells showed normal, perinuclear, stacked Golgi morphology, fetal dermal fibroblasts (fetus II-3 from family 1) showed altered and vesicular Golgi morphology. Scale bars represent 50 μm.
(B) Dermal fibroblasts were labeled with cholera toxin subunit B (CTx-B, green) and stained for Golgi (red). Control cells with normal Golgi morphology showed internalization of CTx-B as visualized by intense overlap of the Golgi apparatus with CTx-B. Cells from fetus II-3 from family 1 and fetus IV-4 from family 2 with a more disperse and distended Golgi (white arrows) showed less intense Golgi overlap and some CTx-B mislocalization (yellow arrows).
Zebrafish Studies
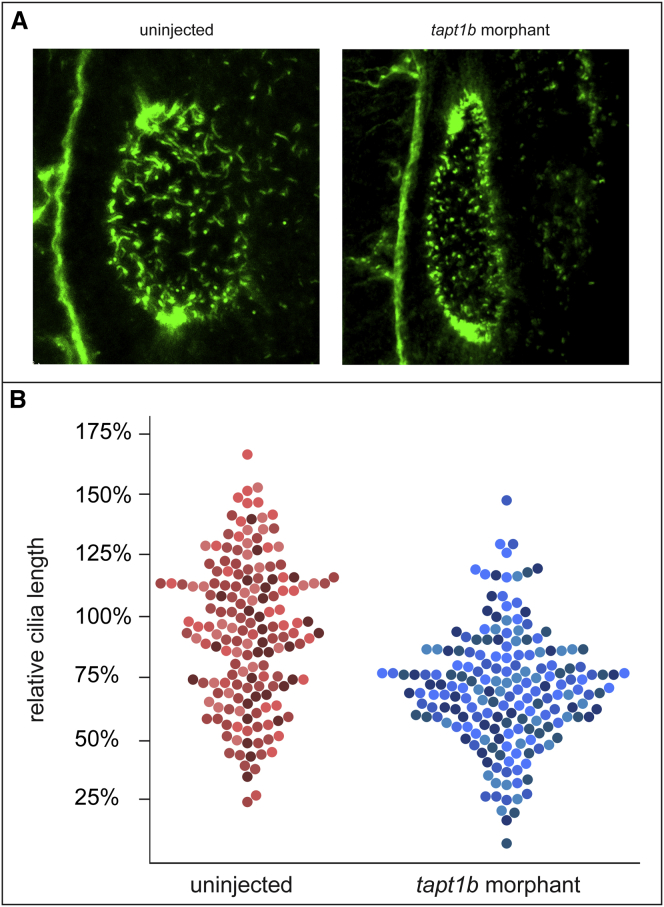
The function of TAPT1 was further examined by studies in zebrafish, which harbor two orthologs, tapt1a and tapt1b. Because tapt1b is most homologous to human TAPT1, we focused on this ortholog. WISH for tapt1b in embryos at 3 dpf showed strong expression in the mesenchyme of the pectoral fins and the epithelial lining of the oropharyngeal cavity and weaker expression in the pronephric duct and the liver (Figure S11). Ciliogenesis was investigated in the otic vesicle, which shows high tapt1b expression at 1 dpf and which is easily accessible for confocal imaging. In embryos in which tapt1b was depleted, via either of two independent splice-blocking MOs, the cilia in the otic vesicle showed decreased length, supporting a ciliary function for tapt1b and TAPT1 (Figures 6A and 6B).
Figure 6.
tapt1b Knockdown in Zebrafish
(A) Cilia in zebrafish otic vesicle at 1 dpf, visualized by WISH, revealed decreased cilial length in tapt1b morphants.
(B) Quantification of cilial length in three tapt1b morphants and three uninjected controls. On average, cilia were 24% smaller in the tapt1b morphant group when compared to those in the uninjected group (p < 0.001). Measurements in the graph represent relative cilial length in percentage when compared to the mean cilial length of the uninjected group (100%). Each dot in the graph represents a single measurement; dots with the same color shade represent measurements for the same sample.
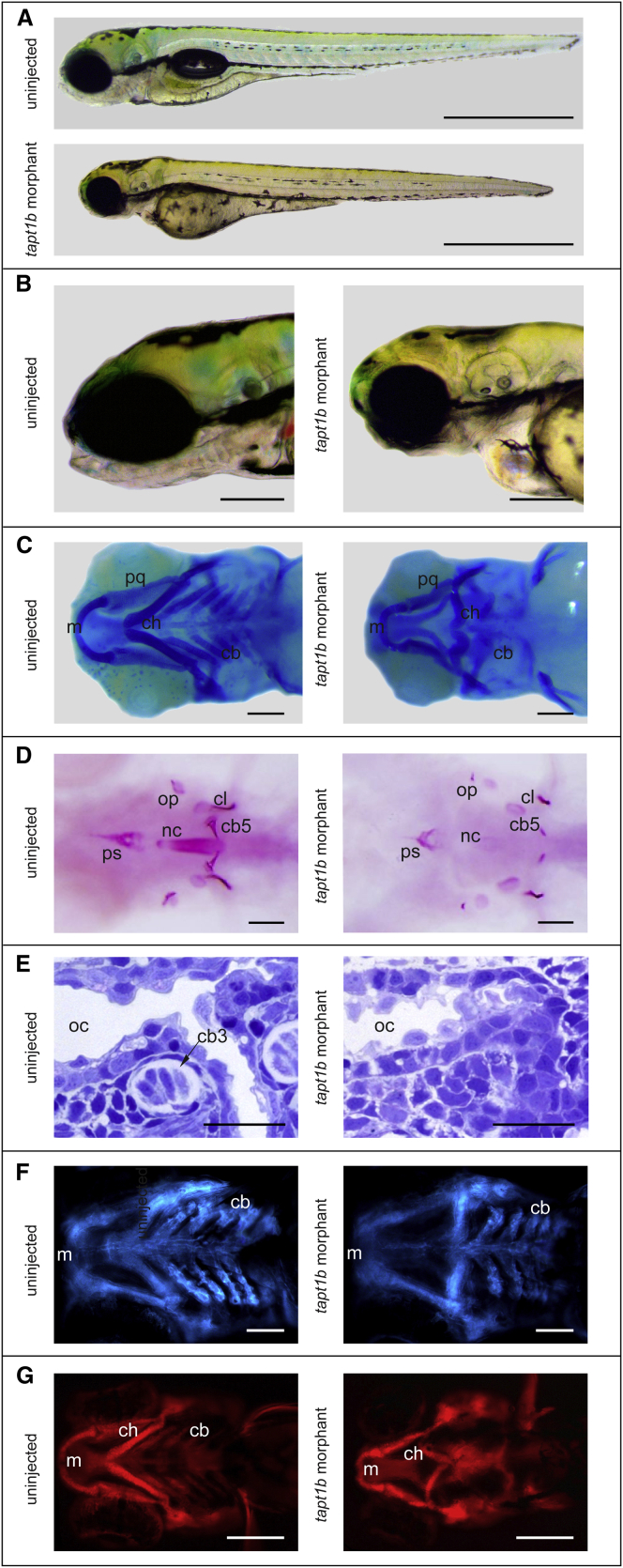
Tapt1b-depleted embryos (Figures 7A and 7B, Figures S12A and S12B) further resulted in severe craniofacial distortions (Figure 7C, Figure S12C), microcephaly, malformation of the pectoral fins (evolutionarily related to limbs), pericardial edema, and generally delayed ossification (Figure 7D, Figure S12D), both in a wild-type and p53 mutant44 background. Alcian blue staining for cartilage revealed a complete absence of ceratobranchial cartilages in tapt1b morphant embryos and an abnormal polarity of the ceratohyal cartilage (Figure 7C, Figure S12C). To further confirm the specificity of our MO assay, we utilized CRISPR/Cas9-mediated gene editing to induce aberrations in tapt1b.51,52 In F0 tapt1bCas9/gRNA embryos, we observed malformation of the cranial cartilage and disorganization of chondrocytes in the ceratohyal and ceratobranchial cartilage (Figure S13). These zebrafish MO tapt1b knock-down and CRISPR/Cas9-induced tapt1b knock-out phenotypes are concordant with the phenotypic abnormalities in the affected fetuses in both families.
Figure 7.
Phenotypic Characterization of tapt1b Morphants
(A–B) Phenotype of the tapt1b morphants at 4 dpf. Shows craniofacial distortions, underdevelopment of the splanchnocranium, microcephaly, malformation of the pectoral fins, and pericardial edema.
(C) Alcian blue staining for cartilage at 4 dpf. tapt1b morphant embryos lacked ceratobranchial cartilages (cb) and showed abnormal polarity of the ceratohyal cartilage (ch), whereas the palatoquadrate (pq) and Meckel’s cartilage (m) were largely unaffected.
(D) Alizarin red staining for bone at 4 dpf. tapt1b morphants showed delayed ossification of the cranial skeleton.
(E) Histological examination of the pharyngeal region in the 3 dpf tapt1b morphants showed abnormal cellular morphology in the oral epithelium and the neighboring ectomesenchyme. In control embryos (3 dpf), cartilage condensations were clearly visible in the pharyngeal arches, whereas undifferentiated and misorganized mesenchymal cells were present in tapt1b morphants (Figure S12).
(F) tapt1b morphants in a fli1:GFP background revealed normal migration of cranial neural crest cells, although they are less abundant and more disorganized when compared to control embryos.
(G) tapt1b morphants in a col2a1:mCherry background display an absence of differentiated chondrocytes in the pharyngeal arch region (cb). cb3, ceratobranchial 3; cb5, ceratobranchial 5; cl, cleithrum; ps, parasphenoid; nc, notochord; op, opercle; oc, oral cavity. Scale bars represent 1 mm in (A), 200 μm in (B), (E), and (G) and 100 μm in (C), (D) and (F).
The defects in the morphant zebrafish craniofacial cartilage, which is predominantly derived from the CNC, were analyzed in more detail. Histological staining showed abnormal morphologies of cells in the oral epithelium and the neighboring CNC-derived mesenchyme of tapt1b morphants (Figure 7E, Figure S14). Whereas cartilage condensations were clearly visible in the pharyngeal arches of control embryos, only undifferentiated loosely organized mesenchymal cells were observed in tapt1b morphants (Figure 7E), thereby suggesting that the defect is downstream of CNC cell migration. In line with this, tapt1b depletion in fli1:GFP transgenic embryos, expressing GFP in the CNC,39 also indicated the presence of post-migratory CNC cells in the pharyngeal arches (Figure 7F). However, the presence of differentiated chondrocytes could not be detected in the pharyngeal arches after tapt1b depletion in col2a1:mCherry transgenic embryos, expressing mCherry under the control of the promotor of col2a1,38 which is a marker gene for differentiated chondrocytes (Figure 7G).
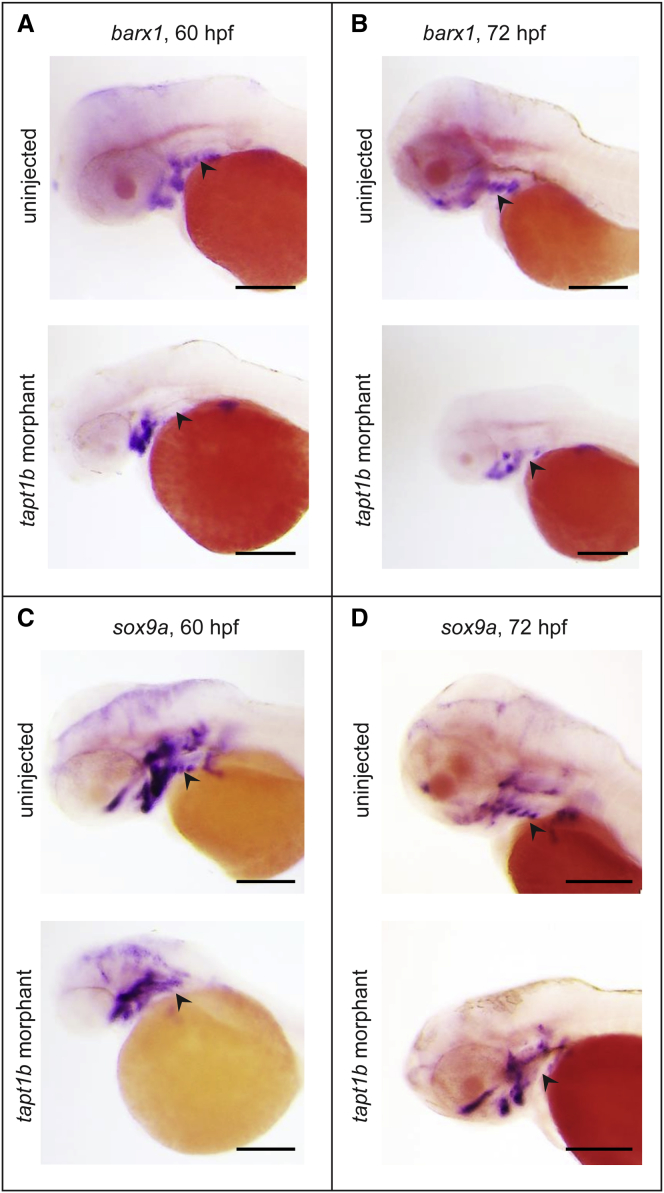
Next, we performed WISH for barx1, a gene regulating proliferation and differentiation of post-migratory CNC,41 and sox9a, a transcription factor essential for chondrocyte differentiation.42 Expression of both markers was unaffected in the precursors of the Meckel’s and ceratohyal cartilage but was lost in the more posterior ceratobranchial region (Figure 8). Together, our findings indicate that the cartilage defect in the tapt1b morphants derived primarily from disturbed condensation or differentiation of the CNC cells after their migration into the branchial arches.
Figure 8.
WISH for sox9a and barx1
(A–B) Expression pattern of barx1, a gene regulating proliferation and differentiation of post-migratory CNC,41 at 60 hpf (A) and 72 hpf (B) showed clear expression of barx1 in the region of the pharyngeal arches in the uninjected controls (upper panel), whereas no expression of barx1 in that region (arrowhead) was detected in the tapt1b morphants (lower panel).
(C–D) Expression pattern of sox9a, a transcription factor essential for chondrocyte differentiation,42 at 60 hpf (C) and 72 hpf (D) showed normal expression of sox9a in the pharyngeal arch region in the uninjected controls (upper panel), whereas no expression of sox9a in the pharyngeal arch region (arrowhead) was detected in the tapt1b morphants. Expression of both barx1 and sox9a was not affected in the Meckel’s and ceratohyal cartilage. Scale bars represent 200 μm.
Discussion
With our study, we established that genetic defects in TAPT1 are the underlying cause of a lethal and complex congenital disorder that is primarily characterized by severe undermineralization of the skeleton with intra-uterine fractures and that thus clinically overlaps with the presentation of severe-to-lethal OI.3 However, additional phenotypic manifestations, including microcephaly, hydramnios with ascites, intracranial ventriculomegaly, hydronephrosis, and other anomalies classify this disorder within the complex developmental skeletal dysplasias. Additionally, the observed dysfunction of the primary cilium may also link this disorder to the ciliopathies. Some variation in disease severity in our families was observed; the observed phenotypic congenital abnormalities were more severe in family 2. This might be correlated with the ultimate effect of TAPT1 mutations on primary cilium formation, in that cells from fetuses of family 1 almost failed to induce ciliogenesis, whereas cells from fetuses of family 2 produced a short and stubby cilium.
Although classic ciliopathies are known as multisystemic disorders, ciliary chondrodyplasias have been described that represent a group of genetically and phenotypically related disorders in which mainly the ribs, limbs, and craniofacial skeleton are affected, but also sometimes involve extraskeletal symptoms affecting the kidneys, liver, heart, eyes, and other organs and tissues.53 The underlying pathogenetic basis is found within malfunctioning cilia, causing imbalances in the ciliary hedgehog signaling pathway in chondrocytes. In 2010 and 2012,37,54–56 two additional ciliary chondrodysplasias were described; both are characterized by primordial dwarfism with microcephaly, and/or skeletal defects, and are caused by defects in the centrosomal protein CENPJ (encoding centromeric protein J)54,56 or the centrosome-associated protein POC1A (encoding POC1 centriolar protein A).37,55 These proteins are among several centrosomal components known to play a crucial role in the growth of the primary cilium50 while also affecting intracellular transport.57 In particular, POC1A is important for proper assembly of the Golgi stacks and normal trafficking through the Golgi apparatus,37 a finding that also seems to hold true for TAPT1, as illustrated by disturbed Golgi morphology and trafficking and alterations of type I collagen biosynthesis. Also, it is possible that the altered acetylated α-tubulin organization in the proband cells influences the pericentrosomal Golgi ribbon,58 thereby contributing to the observed altered trafficking as illustrated by the CTx-B experiment. In analogy to the observed disturbances of type I collagen in our families, a recent study of a transgenic mouse model, allowing the conditional expression of fluorescently labeled wild-type or mutant (R992C) type II collagen, demonstrated a distorted polarity of the Golgi apparatus to the nucleus axis in mutant chondrocytes of the growth plate in combination with altered biosynthesis and secretion of mutant type II collagen.59 Interestingly, in these transgenic mutant chondrocytes, changes were also observed in length and spatial orientation of the primary cilia. Hence, it is was proposed that altered polarity of the chondrocytes (expressing the mutant type II collagen) contributes to alterations in primary cilium organization, which in turn can influence secretion and deposition of structural elements, such as collagens, in the extracellular matrix.59,60 This overlap between Golgi and cilium alterations fits well with the group of complex skeletal dysplasias, some of which are associated with defects in primary-cilia elements and some with defects in the structural proteins of the Golgi apparatus.61–63 In line with this, defective cartilage development might also underlie the bone fragility in these families, resulting in the observed chondroosseous dysplasia. Our findings in the tapt1b morphant zebrafish revealed delayed ossification of cranial bones and aberrant differentiation of the ceratobranchial cartilage precursors, thus perturbing subsequent peri- and endochondral bone formation. This implies that both dermal and chondral bone development are distorted. This is supported by a recent study that reported on the crucial role of the primary cilium to direct differentiation of mesenchymal stem cells to chondrocytes and osteoblasts.64 One of the best described pathways impacted by the primary cilium is the Hedgehog (Hh) pathway, which is also centrally involved in osteoblast commitment and differentiation.65 Interestingly, the craniofacial phenotype of the tapt1b morphants is very similar to that of the con/disp1 zebrafish mutant, a hypomorphic disp1 zebrafish model that was developed to characterize the role of Hh signaling in the pharyngeal arches skeleton.66 Although the phenotypic overlap between both zebrafish models suggests a direct involvement of the Hh-pathway in the tapt1b morphant phenotype, alternative mechanisms cannot be excluded given that primary cilia are also important for alternative signaling pathways (e.g., Wnt or PDGF) and also display mechanosensory functions, all of which can be critical for cartilage and bone formation.1
In conclusion, TAPT1 is an, until now, uncharacterized centrosome and/or ciliary basal body protein that could have a dual role in cartilage and bone development. First, as a centrosomal protein, TAPT1 affects intracellular protein trafficking and organization of cellular organelles, such as the Golgi apparatus. Second, because TAPT1 could play a role in ciliary assembly and signaling, it might affect osteogenesis by influencing osteoblast and/or chondrocyte differentiation. These insights contribute to the ongoing unravelling of the pathways and proteins involved in bone formation.
Acknowledgments
We wish to thank P. Tapaneeyaphan, J. Weytens, P. Vermassen, L. Demuynck, P. Simoens, H. De Saffel and M. Soenens for excellent technical assistance. We are grateful to E. Parthoens and Dr. C. Guérin for access to the BioImaging Core, Ghent Platform, VIB. A.M.B. and J.C.M. acknowledge the NICHD Microscopy and Imaging Core. F.M. is a senior clinical investigator at the Fund for Scientific Research-Flanders. Contract grants include FWO grant number G.0171.05 to A.D.P. and Methusalem grant number 08/01M01108 to A.D.P. and NICHD, NIH Intramural Funding went to J.C.M. This work was also supported by funding from the Belgian Science Policy Office Interuniversity Attraction Poles (BELSPO-IAP) program through the project IAP P7/43-BeMGI. The Leica TCS LSI Super Zoom confocal microscope is supported by the Hercules Foundation, Flanders (grant AUGE/11/14).
Published: September 10, 2015
Footnotes
Supplemental Data include 14 supplemental figures and four supplemental tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2015.08.009.
Web Resources
The URLs for data presented herein are as follows:
1000 Genomes Database, http://www.1000genomes.org/
CRISPRdirect, http://crispr.dbcls.jp/
Expasy, www.expasy.org/proteomics
GoNL (Genome of the Netherlands), http://www.nlgenome.nl/search/
Human Splicing Finder (HSF), http://umd.be/HSF/
MutationTaster, http://www.mutationtaster.org/
NCBI Gene, http://www.ncbi.nlm.nih.gov/gene
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
SIFT, http://sift.jcvi.org/
Supplemental Data
References
- 1.Yuan X., Serra R.A., Yang S. Function and regulation of primary cilia and intraflagellar transport proteins in the skeleton. Ann. N Y Acad. Sci. 2015;1335:78–99. doi: 10.1111/nyas.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hildebrandt F., Benzing T., Katsanis N. Ciliopathies. N. Engl. J. Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sillence D.O., Rimoin D.L. Classification of osteogenesis imperfect. Lancet. 1978;1:1041–1042. doi: 10.1016/s0140-6736(78)90763-8. [DOI] [PubMed] [Google Scholar]
- 4.Marini J.C., Forlino A., Cabral W.A., Barnes A.M., San Antonio J.D., Milgrom S., Hyland J.C., Körkkö J., Prockop D.J., De Paepe A. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum. Mutat. 2007;28:209–221. doi: 10.1002/humu.20429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marini J.C., Blissett A.R. New genes in bone development: what’s new in osteogenesis imperfecta. J. Clin. Endocrinol. Metab. 2013;98:3095–3103. doi: 10.1210/jc.2013-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabral W.A., Chang W., Barnes A.M., Weis M., Scott M.A., Leikin S., Makareeva E., Kuznetsova N.V., Rosenbaum K.N., Tifft C.J. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat. Genet. 2007;39:359–365. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk F., Nikkels P.G., den Hollander N.S., Nesbitt I.M., van Rijn R.R., Cobben J.M., Pals G. Lethal/severe osteogenesis imperfecta in a large Family: a novel homozygous LEPRE1 mutation and bone histological findings. Pediatr. Dev. Pathol. 2011;14:228–234. doi: 10.2350/10-03-0806-CR.1. [DOI] [PubMed] [Google Scholar]
- 8.Baldridge D., Schwarze U., Morello R., Lennington J., Bertin T.K., Pace J.M., Pepin M.G., Weis M., Eyre D.R., Walsh J. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum. Mutat. 2008;29:1435–1442. doi: 10.1002/humu.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes A.M., Chang W., Morello R., Cabral W.A., Weis M., Eyre D.R., Leikin S., Makareeva E., Kuznetsova N., Uveges T.E. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N. Engl. J. Med. 2006;355:2757–2764. doi: 10.1056/NEJMoa063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes A.M., Carter E.M., Cabral W.A., Weis M., Chang W., Makareeva E., Leikin S., Rotimi C.N., Eyre D.R., Raggio C.L., Marini J.C. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. N. Engl. J. Med. 2010;362:521–528. doi: 10.1056/NEJMoa0907705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Dijk F.S., Nesbitt I.M., Zwikstra E.H., Nikkels P.G., Piersma S.R., Fratantoni S.A., Jimenez C.R., Huizer M., Morsman A.C., Cobben J.M. PPIB mutations cause severe osteogenesis imperfecta. Am. J. Hum. Genet. 2009;85:521–527. doi: 10.1016/j.ajhg.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Glez V., Valencia M., Caparrós-Martín J.A., Aglan M., Temtamy S., Tenorio J., Pulido V., Lindert U., Rohrbach M., Eyre D. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Hum. Mutat. 2012;33:343–350. doi: 10.1002/humu.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asharani P.V., Keupp K., Semler O., Wang W., Li Y., Thiele H., Yigit G., Pohl E., Becker J., Frommolt P. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am. J. Hum. Genet. 2012;90:661–674. doi: 10.1016/j.ajhg.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christiansen H.E., Schwarze U., Pyott S.M., AlSwaid A., Al Balwi M., Alrasheed S., Pepin M.G., Weis M.A., Eyre D.R., Byers P.H. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. Am. J. Hum. Genet. 2010;86:389–398. doi: 10.1016/j.ajhg.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drögemüller C., Becker D., Brunner A., Haase B., Kircher P., Seeliger F., Fehr M., Baumann U., Lindblad-Toh K., Leeb T. A missense mutation in the SERPINH1 gene in Dachshunds with osteogenesis imperfecta. PLoS Genet. 2009;5:e1000579. doi: 10.1371/journal.pgen.1000579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Becker J., Semler O., Gilissen C., Li Y., Bolz H.J., Giunta C., Bergmann C., Rohrbach M., Koerber F., Zimmermann K. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. Am. J. Hum. Genet. 2011;88:362–371. doi: 10.1016/j.ajhg.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Symoens S., Malfait F., D’hondt S., Callewaert B., Dheedene A., Steyaert W., Bächinger H.P., De Paepe A., Kayserili H., Coucke P.J. Deficiency for the ER-stress transducer OASIS causes severe recessive osteogenesis imperfecta in humans. Orphanet J. Rare Dis. 2013;8:154. doi: 10.1186/1750-1172-8-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alanay Y., Avaygan H., Camacho N., Utine G.E., Boduroglu K., Aktas D., Alikasifoglu M., Tuncbilek E., Orhan D., Bakar F.T. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. Am. J. Hum. Genet. 2010;86:551–559. doi: 10.1016/j.ajhg.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lapunzina P., Aglan M., Temtamy S., Caparrós-Martín J.A., Valencia M., Letón R., Martínez-Glez V., Elhossini R., Amr K., Vilaboa N., Ruiz-Perez V.L. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. Am. J. Hum. Genet. 2010;87:110–114. doi: 10.1016/j.ajhg.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Slot A.J., Zuurmond A.M., Bardoel A.F., Wijmenga C., Pruijs H.E., Sillence D.O., Brinckmann J., Abraham D.J., Black C.M., Verzijl N. Identification of PLOD2 as telopeptide lysyl hydroxylase, an important enzyme in fibrosis. J. Biol. Chem. 2003;278:40967–40972. doi: 10.1074/jbc.M307380200. [DOI] [PubMed] [Google Scholar]
- 21.Cho T.J., Lee K.E., Lee S.K., Song S.J., Kim K.J., Jeon D., Lee G., Kim H.N., Lee H.R., Eom H.H. A single recurrent mutation in the 5′-UTR of IFITM5 causes osteogenesis imperfecta type V. Am. J. Hum. Genet. 2012;91:343–348. doi: 10.1016/j.ajhg.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semler O., Garbes L., Keupp K., Swan D., Zimmermann K., Becker J., Iden S., Wirth B., Eysel P., Koerber F. A mutation in the 5′-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type V with hyperplastic callus. Am. J. Hum. Genet. 2012;91:349–357. doi: 10.1016/j.ajhg.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fahiminiya S., Majewski J., Mort J., Moffatt P., Glorieux F.H., Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J. Med. Genet. 2013;50:345–348. doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- 24.Keupp K., Beleggia F., Kayserili H., Barnes A.M., Steiner M., Semler O., Fischer B., Yigit G., Janda C.Y., Becker J. Mutations in WNT1 cause different forms of bone fragility. Am. J. Hum. Genet. 2013;92:565–574. doi: 10.1016/j.ajhg.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pyott S.M., Tran T.T., Leistritz D.F., Pepin M.G., Mendelsohn N.J., Temme R.T., Fernandez B.A., Elsayed S.M., Elsobky E., Verma I. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. Am. J. Hum. Genet. 2013;92:590–597. doi: 10.1016/j.ajhg.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaheen R., Alazami A.M., Alshammari M.J., Faqeih E., Alhashmi N., Mousa N., Alsinani A., Ansari S., Alzahrani F., Al-Owain M. Study of autosomal recessive osteogenesis imperfecta in Arabia reveals a novel locus defined by TMEM38B mutation. J. Med. Genet. 2012;49:630–635. doi: 10.1136/jmedgenet-2012-101142. [DOI] [PubMed] [Google Scholar]
- 27.Volodarsky M., Markus B., Cohen I., Staretz-Chacham O., Flusser H., Landau D., Shelef I., Langer Y., Birk O.S. A deletion mutation in TMEM38B associated with autosomal recessive osteogenesis imperfecta. Hum. Mutat. 2013;34:582–586. doi: 10.1002/humu.22274. [DOI] [PubMed] [Google Scholar]
- 28.van Dijk F.S., Zillikens M.C., Micha D., Riessland M., Marcelis C.L., de Die-Smulders C.E., Milbradt J., Franken A.A., Harsevoort A.J., Lichtenbelt K.D. PLS3 mutations in X-linked osteoporosis with fractures. N. Engl. J. Med. 2013;369:1529–1536. doi: 10.1056/NEJMoa1308223. [DOI] [PubMed] [Google Scholar]
- 29.Reese M.G., Eeckman F.H., Kulp D., Haussler D. Improved splice site detection in Genie. J. Comput. Biol. 1997;4:311–323. doi: 10.1089/cmb.1997.4.311. [DOI] [PubMed] [Google Scholar]
- 30.Desmet F.O., Hamroun D., Lalande M., Collod-Béroud G., Claustres M., Béroud C. Human Splicing Finder: an online bioinformatics tool to predict splicing signals. Nucleic Acids Res. 2009;37:e67. doi: 10.1093/nar/gkp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Czika W., Berry J.J. Using all alleles in the multiallelic versions of the SDT and combined SDT/TDT. Am. J. Hum. Genet. 2002;71:1235–1236. doi: 10.1086/344290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu L.C., White R.L. BRCA1 is associated with the centrosome during mitosis. Proc. Natl. Acad. Sci. USA. 1998;95:12983–12988. doi: 10.1073/pnas.95.22.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabral W.A., Makareeva E., Colige A., Letocha A.D., Ty J.M., Yeowell H.N., Pals G., Leikin S., Marini J.C. Mutations near amino end of alpha1(I) collagen cause combined osteogenesis imperfecta/Ehlers-Danlos syndrome by interference with N-propeptide processing. J. Biol. Chem. 2005;280:19259–19269. doi: 10.1074/jbc.M414698200. [DOI] [PubMed] [Google Scholar]
- 34.Barnes A.M., Cabral W.A., Weis M., Makareeva E., Mertz E.L., Leikin S., Eyre D., Trujillo C., Marini J.C. Absence of FKBP10 in recessive type XI osteogenesis imperfecta leads to diminished collagen cross-linking and reduced collagen deposition in extracellular matrix. Hum. Mutat. 2012;33:1589–1598. doi: 10.1002/humu.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forlino A., D’amato E., Valli M., Camera G., Hopkins E., Marini J.C., Cetta G., Coviello D.A. Phenotypic comparison of an osteogenesis imperfecta type IV proband with a de novo alpha2(I) Gly922--> Ser substitution in type I collagen and an unrelated patient with an identical mutation. Biochem. Mol. Med. 1997;62:26–35. doi: 10.1006/bmme.1997.2620. [DOI] [PubMed] [Google Scholar]
- 36.Makareeva E., Mertz E.L., Kuznetsova N.V., Sutter M.B., DeRidder A.M., Cabral W.A., Barnes A.M., McBride D.J., Marini J.C., Leikin S. Structural heterogeneity of type I collagen triple helix and its role in osteogenesis imperfecta. J. Biol. Chem. 2008;283:4787–4798. doi: 10.1074/jbc.M705773200. [DOI] [PubMed] [Google Scholar]
- 37.Sarig O., Nahum S., Rapaport D., Ishida-Yamamoto A., Fuchs-Telem D., Qiaoli L., Cohen-Katsenelson K., Spiegel R., Nousbeck J., Israeli S. Short stature, onychodysplasia, facial dysmorphism, and hypotrichosis syndrome is caused by a POC1A mutation. Am. J. Hum. Genet. 2012;91:337–342. doi: 10.1016/j.ajhg.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitchell R.E., Huitema L.F., Skinner R.E., Brunt L.H., Severn C., Schulte-Merker S., Hammond C.L. New tools for studying osteoarthritis genetics in zebrafish. Osteoarthritis Cartilage. 2013;21:269–278. doi: 10.1016/j.joca.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lawson N.D., Weinstein B.M. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev. Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 40.Thisse C., Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat. Protoc. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- 41.Sperber S.M., Dawid I.B. barx1 is necessary for ectomesenchyme proliferation and osteochondroprogenitor condensation in the zebrafish pharyngeal arches. Dev. Biol. 2008;321:101–110. doi: 10.1016/j.ydbio.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan Y.L., Willoughby J., Liu D., Crump J.G., Wilson C., Miller C.T., Singer A., Kimmel C., Westerfield M., Postlethwait J.H. A pair of Sox: distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development. 2005;132:1069–1083. doi: 10.1242/dev.01674. [DOI] [PubMed] [Google Scholar]
- 43.Verstraeten B., Sanders E., Huysseune A. Whole mount immunohistochemistry and in situ hybridization of larval and adult zebrafish dental tissues. In: Kiouss, editor. Odontogenesis Methods and Protocols Methods in Molecular Biology. Humana Press; USA: 2012. pp. 179–191. [DOI] [PubMed] [Google Scholar]
- 44.Robu M.E., Larson J.D., Nasevicius A., Beiraghi S., Brenner C., Farber S.A., Ekker S.C. p53 activation by knockdown technologies. PLoS Genet. 2007;3:e78. doi: 10.1371/journal.pgen.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neuhauss S.C., Solnica-Krezel L., Schier A.F., Zwartkruis F., Stemple D.L., Malicki J., Abdelilah S., Stainier D.Y., Driever W. Mutations affecting craniofacial development in zebrafish. Development. 1996;123:357–367. doi: 10.1242/dev.123.1.357. [DOI] [PubMed] [Google Scholar]
- 46.Spoorendonk K.M., Peterson-Maduro J., Renn J., Trowe T., Kranenbarg S., Winkler C., Schulte-Merker S. Retinoic acid and Cyp26b1 are critical regulators of osteogenesis in the axial skeleton. Development. 2008;135:3765–3774. doi: 10.1242/dev.024034. [DOI] [PubMed] [Google Scholar]
- 47.Mahmood F., Fu S., Cooke J., Wilson S.W., Cooper J.D., Russell C. A zebrafish model of CLN2 disease is deficient in tripeptidyl peptidase 1 and displays progressive neurodegeneration accompanied by a reduction in proliferation. Brain. 2013;136:1488–1507. doi: 10.1093/brain/awt043. [DOI] [PubMed] [Google Scholar]
- 48.Howell G.R., Shindo M., Murray S., Gridley T., Wilson L.A., Schimenti J.C. Mutation of a ubiquitously expressed mouse transmembrane protein (Tapt1) causes specific skeletal homeotic transformations. Genetics. 2007;175:699–707. doi: 10.1534/genetics.106.065177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ching Y.H., Munroe R.J., Moran J.L., Barker A.K., Mauceli E., Fennell T., Dipalma F., Lindblad-Toh K., Abcunas L.M., Gilmour J.F. High resolution mapping and positional cloning of ENU-induced mutations in the Rw region of mouse chromosome 5. BMC Genet. 2010;11:106. doi: 10.1186/1471-2156-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi T., Dynlacht B.D. Regulating the transition from centriole to basal body. J. Cell Biol. 2011;193:435–444. doi: 10.1083/jcb.201101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borck G., Hög F., Dentici M.L., Tan P.L., Sowada N., Medeira A., Gueneau L., Holger T., Kousi M., Lepri F. BRF1 mutations alter RNA polymerase III-dependent transcription and cause neurodevelopmental anomalies. Genome Res. 2015;25:609. [PMC free article] [PubMed] [Google Scholar]
- 52.Jao L.E., Wente S.R., Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proc. Natl. Acad. Sci. USA. 2013;110:13904–13909. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidts M. Clinical genetics and pathobiology of ciliary chondrodysplasias. J. Pediatr. Genet. 2014;3:46–94. doi: 10.3233/PGE-14089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Al-Dosari M.S., Shaheen R., Colak D., Alkuraya F.S. Novel CENPJ mutation causes Seckel syndrome. J. Med. Genet. 2010;47:411–414. doi: 10.1136/jmg.2009.076646. [DOI] [PubMed] [Google Scholar]
- 55.Shaheen R., Faqeih E., Shamseldin H.E., Noche R.R., Sunker A., Alshammari M.J., Al-Sheddi T., Adly N., Al-Dosari M.S., Megason S.G. POC1A truncation mutation causes a ciliopathy in humans characterized by primordial dwarfism. Am. J. Hum. Genet. 2012;91:330–336. doi: 10.1016/j.ajhg.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu K.S., Tang T.K. CPAP is required for cilia formation in neuronal cells. Biol. Open. 2012;1:559–565. doi: 10.1242/bio.20121388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rusan N.M., Rogers G.C. Centrosome function: sometimes less is more. Traffic. 2009;10:472–481. doi: 10.1111/j.1600-0854.2009.00880.x. [DOI] [PubMed] [Google Scholar]
- 58.Vinogradova T., Paul R., Grimaldi A.D., Loncarek J., Miller P.M., Yampolsky D., Magidson V., Khodjakov A., Mogilner A., Kaverina I. Concerted effort of centrosomal and Golgi-derived microtubules is required for proper Golgi complex assembly but not for maintenance. Mol. Biol. Cell. 2012;23:820–833. doi: 10.1091/mbc.E11-06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arita M., Fertala J., Hou C., Steplewski A., Fertala A. Mechanisms of aberrant organization of growth plates in conditional transgenic mouse model of spondyloepiphyseal dysplasia associated with the R992C substitution in collagen II. Am. J. Pathol. 2015;185:214–229. doi: 10.1016/j.ajpath.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Poole C.A., Jensen C.G., Snyder J.A., Gray C.G., Hermanutz V.L., Wheatley D.N. Confocal analysis of primary cilia structure and colocalization with the Golgi apparatus in chondrocytes and aortic smooth muscle cells. Cell Biol. Int. 1997;21:483–494. doi: 10.1006/cbir.1997.0177. [DOI] [PubMed] [Google Scholar]
- 61.Huber C., Cormier-Daire V. Ciliary disorder of the skeleton. Am. J. Med. Genet. C. Semin. Med. Genet. 2012;160C:165–174. doi: 10.1002/ajmg.c.31336. [DOI] [PubMed] [Google Scholar]
- 62.Smits P., Bolton A.D., Funari V., Hong M., Boyden E.D., Lu L., Manning D.K., Dwyer N.D., Moran J.L., Prysak M. Lethal skeletal dysplasia in mice and humans lacking the golgin GMAP-210. N. Engl. J. Med. 2010;362:206–216. doi: 10.1056/NEJMoa0900158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haycraft C.J., Serra R. Cilia involvement in patterning and maintenance of the skeleton. Curr. Top. Dev. Biol. 2008;85:303–332. doi: 10.1016/S0070-2153(08)00811-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tummala P., Arnsdorf E.J., Jacobs C.R. The Role of Primary Cilia in Mesenchymal Stem Cell Differentiation: A Pivotal Switch in Guiding Lineage Commitment. Cell. Mol. Bioeng. 2010;3:207–212. doi: 10.1007/s12195-010-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soltanoff C.S., Yang S., Chen W., Li Y.P. Signaling networks that control the lineage commitment and differentiation of bone cells. Crit. Rev. Eukaryot. Gene Expr. 2009;19:1–46. doi: 10.1615/critreveukargeneexpr.v19.i1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schwend T., Ahlgren S.C. Zebrafish con/disp1 reveals multiple spatiotemporal requirements for Hedgehog-signaling in craniofacial development. BMC Dev. Biol. 2009;9:59. doi: 10.1186/1471-213X-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.