Abstract
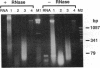
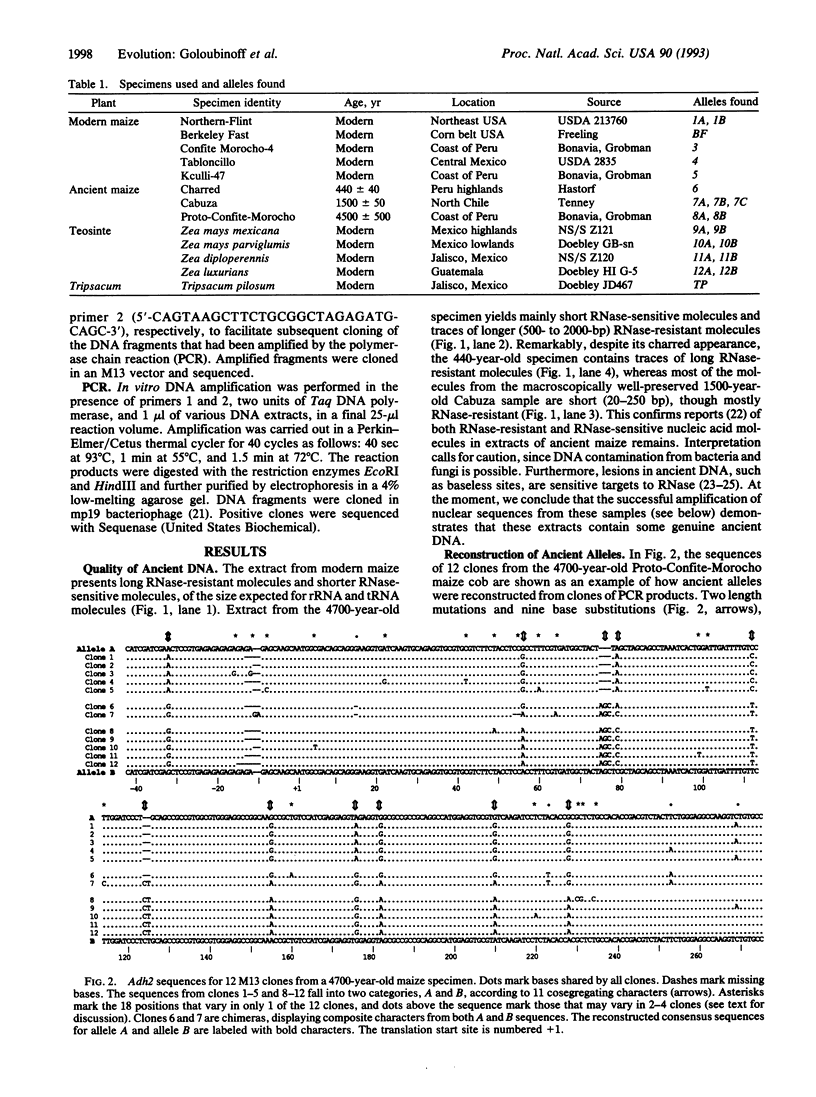
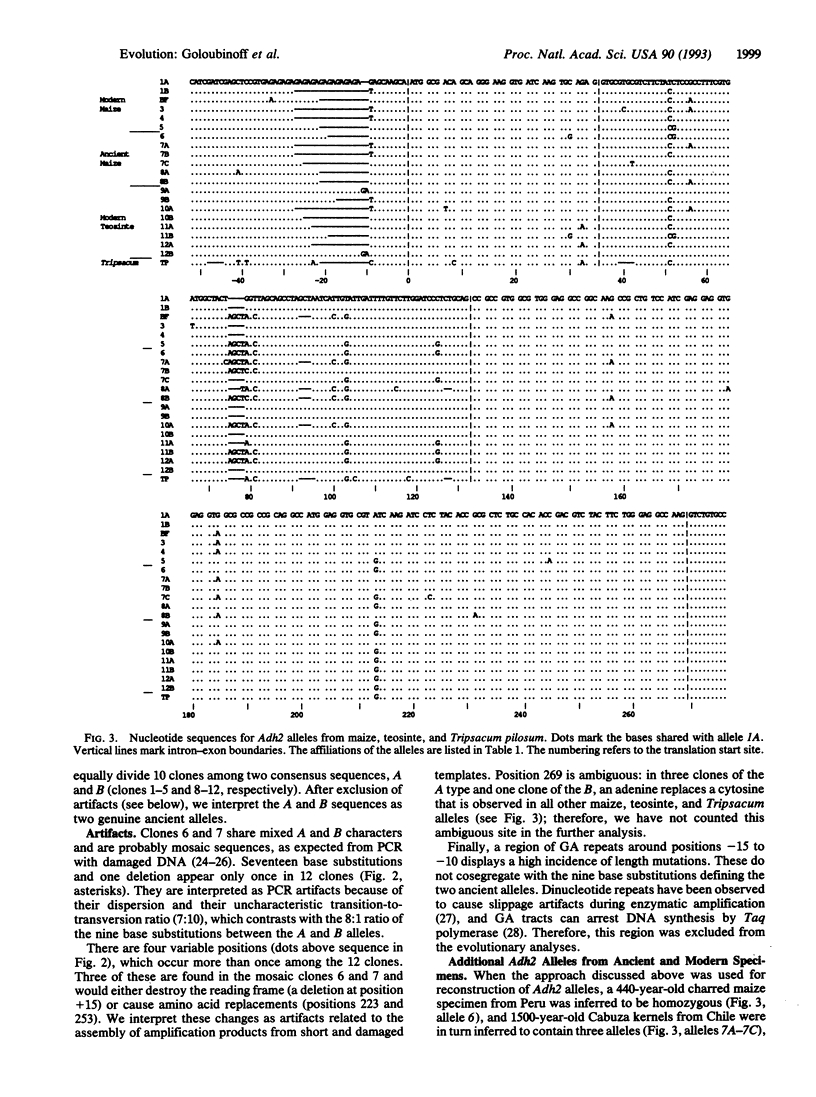
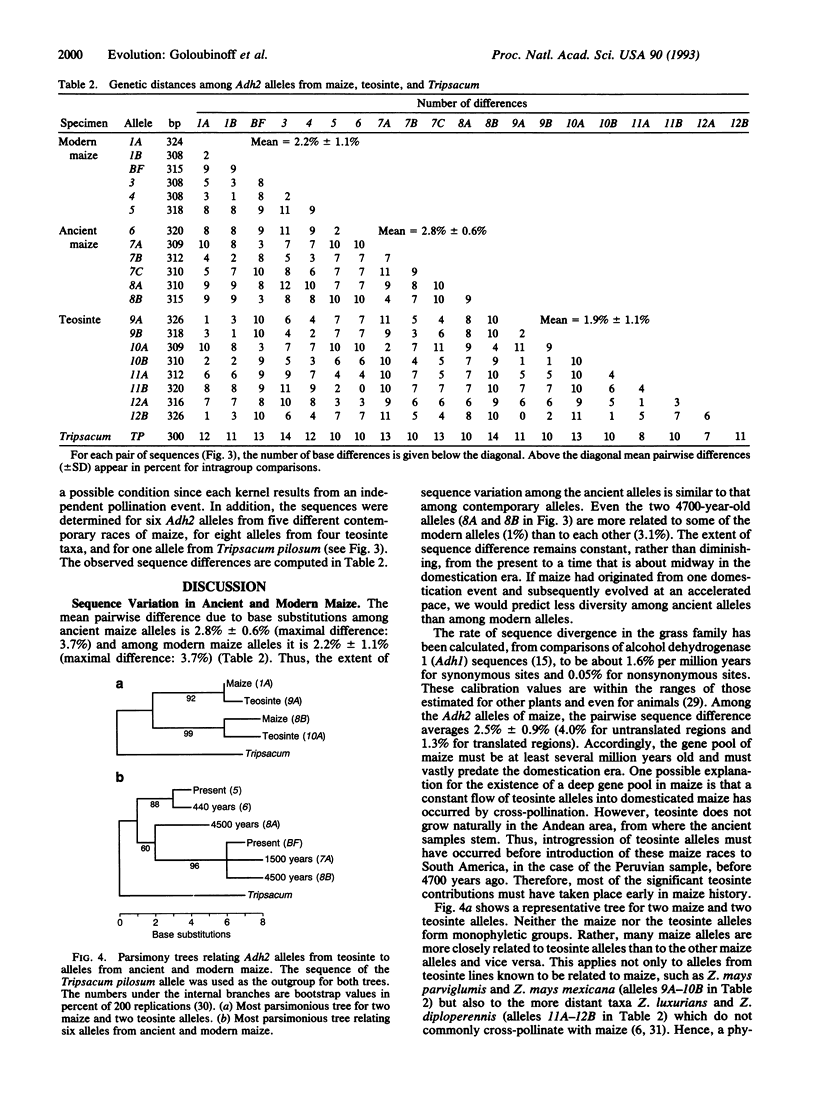
A segment of the nuclear gene encoding alcohol dehydrogenase 2 (Adh2) was amplified and sequenced from extracts of archaeological maize specimens up to 4700 years old and from contemporary samples. Sequence diversity in ancient maize equals that of contemporary maize. Some ancient Adh2 alleles are identical or closely related to contemporary alleles. The data suggest that the gene pool of maize is millions of years old and that domestic races of maize stem from several wild ancestral populations.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baran N., Lapidot A., Manor H. Formation of DNA triplexes accounts for arrests of DNA synthesis at d(TC)n and d(GA)n tracts. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):507–511. doi: 10.1073/pnas.88.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. S., Sachs M. M., Gerlach W. L., Finnegan E. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase 2 (Adh2) gene of maize. Nucleic Acids Res. 1985 Feb 11;13(3):727–743. doi: 10.1093/nar/13.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley J., Renfroe W., Blanton A. Restriction site variation in the zea chloroplast genome. Genetics. 1987 Sep;117(1):139–147. doi: 10.1093/genetics/117.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doebley J., Stec A., Wendel J., Edwards M. Genetic and morphological analysis of a maize-teosinte F2 population: implications for the origin of maize. Proc Natl Acad Sci U S A. 1990 Dec 15;87(24):9888–9892. doi: 10.1073/pnas.87.24.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling M., Bennett D. C. Maize Adh1. Annu Rev Genet. 1985;19:297–323. doi: 10.1146/annurev.ge.19.120185.001501. [DOI] [PubMed] [Google Scholar]
- Galinat W. C. The origin of maize. Annu Rev Genet. 1971;5:447–478. doi: 10.1146/annurev.ge.05.120171.002311. [DOI] [PubMed] [Google Scholar]
- Gaut B. S., Clegg M. T. Molecular evolution of alcohol dehydrogenase 1 in members of the grass family. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2060–2064. doi: 10.1073/pnas.88.6.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor D. A., Dickel C. D., Hauswirth W. W., Parham P. Ancient HLA genes from 7,500-year-old archaeological remains. Nature. 1991 Feb 28;349(6312):785–788. doi: 10.1038/349785a0. [DOI] [PubMed] [Google Scholar]
- Litt M., Luty J. A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am J Hum Genet. 1989 Mar;44(3):397–401. [PMC free article] [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984 Nov 16;226(4676):792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Päbo S. Ancient DNA: extraction, characterization, molecular cloning, and enzymatic amplification. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1939–1943. doi: 10.1073/pnas.86.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Päbo S., Higuchi R. G., Wilson A. C. Ancient DNA and the polymerase chain reaction. The emerging field of molecular archaeology. J Biol Chem. 1989 Jun 15;264(17):9709–9712. [PubMed] [Google Scholar]
- Päbo S., Irwin D. M., Wilson A. C. DNA damage promotes jumping between templates during enzymatic amplification. J Biol Chem. 1990 Mar 15;265(8):4718–4721. [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Generation time and genomic evolution in primates. Science. 1973 Mar 16;179(4078):1144–1147. doi: 10.1126/science.179.4078.1144. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gierl A., Cuypers H., Peterson P. A., Saedler H. Plant transposable elements generate the DNA sequence diversity needed in evolution. EMBO J. 1985 Mar;4(3):591–597. doi: 10.1002/j.1460-2075.1985.tb03671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck-Eidens D. M., Bell R. N., Neuhausen S. L., Helentjaris T. DNA sequence variation within maize and melon: observations from polymerase chain reaction amplification and direct sequencing. Genetics. 1990 Sep;126(1):207–217. doi: 10.1093/genetics/126.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. D. Origins of agriculture in eastern north america. Science. 1989 Dec 22;246(4937):1566–1571. doi: 10.1126/science.246.4937.1566. [DOI] [PubMed] [Google Scholar]
- Werr W., Frommer W. B., Maas C., Starlinger P. Structure of the sucrose synthase gene on chromosome 9 of Zea mays L. EMBO J. 1985 Jun;4(6):1373–1380. doi: 10.1002/j.1460-2075.1985.tb03789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe K. H., Li W. H., Sharp P. M. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9054–9058. doi: 10.1073/pnas.84.24.9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer E. A., Jupe E. R., Walbot V. Ribosomal gene structure, variation and inheritance in maize and its ancestors. Genetics. 1988 Dec;120(4):1125–1136. doi: 10.1093/genetics/120.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]