Abstract
Alcoholic hepatitis affects up to one-third of individuals who abuse alcohol and can be associated with high mortality. Although this disorder is characterized by hepatocellular damage, steatosis and neutrophil infiltration, recent evidence suggests that cholestasis or impaired bile secretion may be a frequent occurrence as well. Bile secretion results from the concerted activity of hepatocytes and cholangiocytes, the epithelial cells that line the bile ducts. Hepatocytes secrete bile acids and conjugated products into the bile canaliculi, which then are modified by cholangiocytes through secretion of bicarbonate and water to give rise to the final secreted bile. Here the molecular mechanisms regulating bile secretion in cholangiocytes are reviewed. Moreover, we discuss how the expression of intracellular Ca2+ channels might be regulated in cholangiocytes, plus evidence that components of the Ca2+ signaling machinery are altered in a range of cholestatic diseases of the bile ducts.
Keywords: Bile ducts, alcoholic hepatitis, cholestasis, InsP3 receptors
Introduction
Bile secretion in the liver involves the combined activities of hepatocytes and cholangiocytes1. Bile acids and conjugated excretion products along with cholesterol and other lipids are secreted by hepatocytes through specific transporters localized at the apical (canalicular) membrane and modified by the secretory activity of cholangiocytes, mostly through bicarbonate (HCO3−) and water secretion to the lumen of bile ducts. A range of liver diseases that are characterized by cholestasis, or impaired bile secretion, result from defective secretory activity of cholangiocytes rather than hepatocytes. These disorders include primary biliary cirrhosis (PBC), primary sclerosing cholangitis (PSC), biliary atresia, bile duct obstruction, and cystic fibrosis liver disease (CFLD)2. Although jaundice is commonly observed in alcoholic liver disease, it has typically been attributed to hepatocellular damage rather than to defective secretion. However, several recent lines of evidence suggest that cholestasis can occur in alcoholic hepatitis and may in fact be associated with a worse outcome3, 4. In this review we discuss the basic mechanisms that regulate fluid secretion in cholangiocytes, plus recent evidence implicating novel regulators of Ca2+ signaling in the pathogenesis of ductular cholestasis.
Signaling mechanisms in biliary secretion
The secretory activity of cholangiocytes is regulated by both cAMP and Ca2+i signaling pathways5. The prototypical example of cAMP-dependent fluid secretion in cholangiocytes is the secretin receptor pathway. These receptors are localized at the basolateral membrane of cholangiocytes and upon binding of secretin, trigger formation of intracellular cAMP. This second messenger activates protein kinase A (PKA), which phosphorylates and activates the cystic fibrosis transmembrane conductance regulator (CFTR) at the apical membrane, promoting Cl− secretion into the lumen of the bile ducts6. This Cl− efflux creates the driving force for the secretion of HCO3− via activation of the anion exchanger 2 (AE2), the protein encoded by the SLC4A2 gene, the net result of which is the alkalinization of bile7. An additional contribution stems from the activation of apical Ca2+-activated Cl− channels. These channels have been recently identified as the product of the TMEM16A gene and promote efflux of Cl− in response to elevations of intracellular Ca+2 and reach their maximum current at [Ca2+i] of about 5 μM8.
Multiple mechanisms contribute to increases in intracellular Ca2+ in cholangiocytes. First, experiments on primary mouse cholangiocytes, isolated intrahepatic bile duct units (IBDU) and microdissected intrahepatic bile duct segments have shown that muscarinic receptor activation promotes fluid secretion9–11. Acetylcholine or its analog carbachol binding to M3 muscarinic receptors on the basolateral membranes promotes formation of inositol 1,4,5-trisphosphate (InsP3) and diacylglycerol (DAG) from the breakdown of phosphatidylinositol 4,5-bisphosphate (PIP2) by phospholipase C (PLC). InsP3 diffuses through the cytoplasm and binds to the InsP3 receptors (InsP3R) on the surface of the endoplasmic reticulum (ER) leading to the release of Ca2+ from the ER lumen to the cytosol. Second, activation of purinergic nucleotide receptors on the apical membrane of cholangiocytes represents a major driver of bile duct secretion. Similar to muscarinic receptors, binding of ATP or UTP to P2Y receptors (P2YR) leads to an InsP3-dependent Ca2+ release from intracellular stores10, 12. This unique mechanism requires the presence of ATP in the lumen of bile ducts, the source of which is still unclear13. Experimental evidence has established two potential mechanisms to explain the origin of luminal ATP in bile ducts, which are not mutually exclusive. One of the proposed pathways suggests that CFTR, either directly or indirectly, mediates the secretion of ATP from cholangiocytes to the lumen of the ducts10, 11. Once in the lumen ATP will act in autocrine/paracrine fashion to activate apical P2YR. A second proposed mechanism is that ATP is released via classical exocytosis of vesicles. In this scenario, ATP is co-released alongside the contents of exocytic vesicles and then activates the apical P2YR14. Third, there is also evidence that hepatocytes secrete ATP into bile, which is then detected by the cholangiocytes that are downstream15, 16.
An additional aspect to be considered here is the morpho-functional distinction between small and large cholangiocytes. Small cholangiocytes are cuboidal epithelial cells which on average have a diameter ≤ 15 μm and line the lumen of intrahepatic bile ducts of 20 – 100 μm cross-section17. Functionally, these cholangiocytes do no express CFTR and their secretory activity is mainly driven by the ATP-Ca2+-TMEM16A axis. Conversely, large cholangiocytes (diameter ≥ 15 μm) are columnar cells with a higher cytoplasm to nucleus ratio and form the walls of large intra-hepatic bile ducts (≥ 100 μm). Fluid secretion in this subtype of cholangiocytes is dependent on both Ca2+ and cAMP since both basolateral secretin receptors and apical CFTR are expressed in large cholangiocytes18.
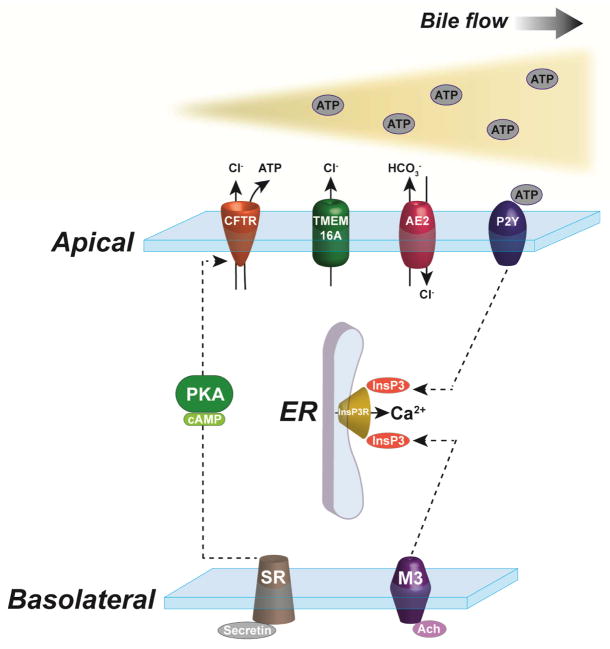
Water secretion via aquaporins (AQP) constitutes an additional component of the secretory activity of cholangiocytes. Aquaporins 1 and 4 (AQP1 and AQP4, respectively) are expressed in rat cholangiocytes and secretin activation of cAMP synthesis induces insertion of subapical vesicles containing AQP1 with the plasma membrane19. This increased expression of AQP1 on the apical surface of cholangiocytes contributes to the overall choleretic effect of secretin. AQP4 instead is mainly localized to the basolateral membrane and its localization does not change after secretin treatment. The expression profile of the 12 known AQPs is less well described in human and mouse cholangiocytes. A recent report identified mRNAs for all the known AQP isoforms in adult murine bile duct cells but the functional relevance of such findings has not been investigated20. Interestingly, the absence of AQP1 in mice has no impact on fluid secretion by cholangiocytes21. A general overview of the regulatory mechanisms of fluid secretion in cholangiocytes is presented in Figure 1.
Figure 1. Regulatory mechanisms of fluid secretion in cholangiocytes.
Activation of secretin receptors (SR) on the basolateral membrane leads to the formation of cAMP and PKA phosphorylation of CFTR, which promotes apical Cl− efflux. Also originating at the basolateral domain, InsP3 formation due to M3 acetylcholine activation induces Ca2+ release from the ER, which activates Cl− secretion through TMEM16A on the apical membrane. ATP, released by upstream hepatocytes or secreted by cholangiocytes in association with CFTR, binds apical P2Y receptors and stimulates further intracellular Ca2+ release. The Cl− gradient across the apical membrane drives the AE2 exchanger, resulting in HCO3− secretion and alkalization of the bile.
InsP3 receptors in cholangiocytes
Ca2+ signaling in epithelial cells is dependent on both release from intracellular stores of the ER and Ca+2 influx through plasma membrane channels. Ca2+ release from the ER occurs via activation of members of two families of intracellular ER Ca2+ channels, the InsP3R and Ryanodine receptors (RyR)22. Each of these families has 3 known mammalian isoforms, which can be expressed in a variety of combinations and subcellular localizations in different cells types. Hepatocytes for example express the type I and type II InsP3R (InsP3R1 and InsP3R2, respectively), but not the type III InsP3R (InsP3R3)23. Full length RyRs are not present in hepatocytes, however a functional truncated form has been described24. InsP3R have been shown to modulate diverse cellular functions in hepatocytes ranging from cellular proliferation and energy metabolism to bile secretion25–27.
Cholangiocytes in turn have all three InsP3R isoforms albeit at different levels of expression. InsP3R3 constitutes about 90% of the total InsP3R pool and InsP3R1 and InsP3R2 combined account for the remaining 10%28. Beyond being the predominant InsP3R isoform, InsP3R3 is also most concentrated along the subapical membrane space as opposed to the diffuse distribution of both InsP3R1 and InsP3R228. Furthermore, this peri-apical concentration of InsP3R3 is seen in both small and large cholangiocytes (Figure 2). Silencing of each of the InsP3Rs in microdissected rat bile duct segments by siRNA demonstrated that InsP3R3, but not InsP3R1 and InsP3R2, is essential for both forskolin-mediate fluid secretion and alkalization of bile by cholangiocytes10. Although whole body and tissue specific InsP3R3 knock out mice have been generated, no studies related to bile secretion or cholangiocytes function have been performed in these mice to date29, 30.
Figure 2. InsP3R3 is expressed in both small and large cholangiocytes.
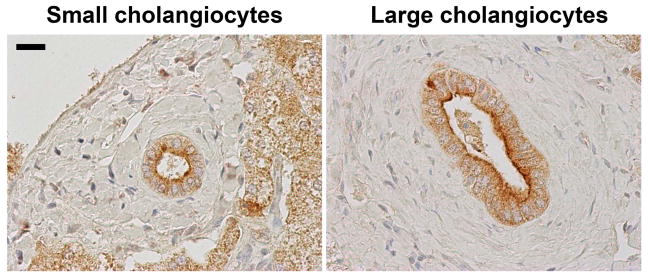
Immunohistochemistry staining of histologically normal human liver biopsies shows the presence of InsP3R3 (brown) concentrated near the apical membrane in both small (left) and large (right) cholangiocytes. Scale bar represents 20 μm.
Loss of InsP3R3 from cholangiocytes is a common event in a number of human cholestatic disorders as well as in animal models of cholestasis2. InsP3R3 was significantly reduced in cholangiocytes of liver samples from patients diagnosed with PSC, PBC, biliary atresia and secondary biliary obstruction. InsP3R3 is similarly reduced in three models of cholestasis in rats: 1) Two-week bile duct ligation (BDL); 2) acute lipopolysaccharide (LPS) treatment; 3) feeding with α-naphthylisothiocyanate (ANIT). These results provide evidence that InsP3R3 and its Ca2+ release properties are mechanistically linked to the pathogenesis of cholestasis.
Cholestasis in alcoholic liver disease (ALD)
ALD comprises a number of histological and clinical features ranging from hepatic steatosis to alcoholic hepatitis and liver cirrhosis, and is the result of excessive alcohol intake for prolonged periods of time31. Jaundice is commonly associated with alcoholic hepatitis (AH) and is thought to be caused primarily by hepatocellular damage. However, no clear cellular and molecular mechanism has been established as the underlying cause of jaundice in AH32. Recent observations that AH can include increased serum levels of alkaline phosphatase and histological evidence of cholestasis in liver biopsies, suggests that cholangiocyte damage and cholestasis may be present in AH as well, and may contribute to the jaundice seen in this disorder3, 4. Although studies employing rodent models of alcoholic liver injury have implicated a number of molecular mechanisms in the pathogenesis of alcoholic hepatitis including activation of tumor necrosis factor alpha (TNFα), formation of reactive oxygen species (ROS) and mitochondrial dysfunction and decreased hepatocyte regeneration, no significant recent advancement has been made in terms of targeted treatments of alcoholic liver diseases32. Our preliminary work in a small cohort of liver specimens from patients diagnosed with alcoholic hepatitis indicates that InsP3R3 is either reduced or mis-localized in bile ducts in the majority of patients (unpublished data). These results in association with the elevated serum alkaline phosphatase levels sometimes seen in AH patients raises the possibility that a dysfunction in secretory activity of cholangiocytes may contribute to cholestasis in AH and that InsP3R3 expression/activity mighty constitute an underlying molecular mechanism.
Regulation of InsP3R3 expression in cholestasis
Several mechanisms contribute to regulation of InsP3R expression and activity. Post-translational modifications such as serine phosphorylation by PKA and PKC were shown to modulate channel activity33. Protein interaction with both ER-lumen proteins (chromogranin-B) and cytoplasmic proteins (IRBIT and protein 4.1N) also regulate Ca2+ flow and InsP3R targeting34–36. Also InsP3R protein degradation by ubiquitination was shown to attenuate Ca2+ signaling in in vitro experiments37. However little is known regarding the transcriptional regulation of InsP3Rs, especially in terms of specificity for each of the three InsP3R isoforms. Studies in neurons revealed that InsP3R1 gene expression is regulated by TNF-α via specific protein 1 (SP1) binding elements on the InsP3R1 promoter. InsP3R2 expression was shown to be regulated by microRNA-133 (miR-133) in cardiomyocytes in the context of cardiac hypertrophy. Our group has recently reported that miR-506 mediates the downregulation of InsP3R3 expression in cholangiocyte cell lines38. This microRNA binds to seed sequences on the InsP3R3 mRNA 3′ untranslated region (UTR) and promotes the reduction of both InsP3R3 mRNA and protein expression. Additionally, treatment of isolated rat bile duct units with miR-506 mimics decreased forskolin-mediated fluid secretion. Moreover, increases in miR-506 correlated with reduced expression of InP3R3 in bile ducts of PBC patients. Together, these results suggest that miR-506 regulation of InsP3R3 expression might be relevant to the pathogenesis of specific forms of cholestatic diseases. Our preliminary work also indicates that additional mechanisms operate in the transcriptional regulation of InsP3R3 in bile ducts. One such mechanism is the down regulation of InsP3R3 expression by the nuclear factor erythroid 2-related factor 2 (Nrf2; data not shown). This transcription factor typically is activated by reactive oxygen species (ROS) and promotes the expression of a range of anti-oxidant enzymes39. Activated Nrf2 classically binds to a 41 base pair consensus sequence in promoter region of genes called ARE (anti oxidant response element)40. Work in progress is examining whether this regulates expression of InsP3R3 in cholangiocytes (unpublished data). Interestingly, Nrf2 was shown to be overexpressed in the liver after excessive alcohol use41. Together, these results show that multiple mechanisms are involved in the regulation of InsP3R expression and can be explored as potential mediators of cholestasis, including those that are associated with alcoholic hepatitis.
Acknowledgments
This work was supported by NIH grants DK045710, DK057751, DK61747 and DK034989 to Michael H. Nathanson
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boyer JL. Bile formation and secretion. Comprehensive Physiology. 2013;3:1035–1078. doi: 10.1002/cphy.c120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasztelan-Szczerbinska B, Slomka M, Celinski K, Szczerbinski M. Alkaline phosphatase: The next independent predictor of the poor 90-day outcome in alcoholic hepatitis. Bio Med research international. 2013;2013:614081. doi: 10.1155/2013/614081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231–1239. e1231–1236. doi: 10.1053/j.gastro.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaya MJ, Nathanson MH. Calcium signaling and the secretory activity of bile duct epithelia. Cell Calcium. 2014;55:317–324. doi: 10.1016/j.ceca.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Anso E, Castillo JE, Diez J, Medina JF, Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–1406. [PubMed] [Google Scholar]
- 8.Dutta AK, Khimji AK, Kresge C, Bugde A, Dougherty M, Esser V, et al. Identification and functional characterization of tmem16a, a ca2+-activated cl- channel activated by extracellular nucleotides, in biliary epithelium. J Biol Chem. 2011;286:766–776. doi: 10.1074/jbc.M110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shibao K, Fiedler MJ, Nagata J, Minagawa N, Hirata K, Nakayama Y, et al. The type iii inositol 1,4,5-trisphosphate receptor is associated with aggressiveness of colorectal carcinoma. Cell Calcium. 2010;48:315–323. doi: 10.1016/j.ceca.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minagawa N, Nagata J, Shibao K, Masyuk AI, Gomes DA, Rodrigues MA, et al. Cyclic amp regulates bicarbonate secretion in cholangiocytes through release of atp into bile. Gastroenterology. 2007;133:1592–1602. doi: 10.1053/j.gastro.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorotto R, Spirli C, Fabris L, Cadamuro M, Okolicsanyi L, Strazzabosco M. Ursodeoxycholic acid stimulates cholangiocyte fluid secretion in mice via cftr-dependent atp secretion. Gastroenterology. 2007;133:1603–1613. doi: 10.1053/j.gastro.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 12.Cadamuro M, Nardo G, Indraccolo S, Dall’olmo L, Sambado L, Moserle L, et al. Platelet-derived growth factor-d and rho gtpases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013;58:1042–1053. doi: 10.1002/hep.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feranchak AP, Lewis MA, Kresge C, Sathe M, Bugde A, Luby-Phelps K, et al. Initiation of purinergic signaling by exocytosis of atp-containing vesicles in liver epithelium. J Biol Chem. 2010;285:8138–8147. doi: 10.1074/jbc.M109.065482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sathe MN, Woo K, Kresge C, Bugde A, Luby-Phelps K, Lewis MA, et al. Regulation of purinergic signaling in biliary epithelial cells by exocytosis of slc17a9-dependent atp-enriched vesicles. J Biol Chem. 2011;286:25363–25376. doi: 10.1074/jbc.M111.232868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathanson MH, Burgstahler AD, Masyuk AI, LaRusso NF. Stimulation of atp secretion in the liver by therapeutic bile acids. Biochem J. 2001;358:1–5. doi: 10.1042/0264-6021:3580001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to nearby hepatocytes and bile duct cells by secretion of nucleotides. Gastroenterology. 1996;110:A1315. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 18.Glaser SS, Gaudio E, Rao A, Pierce LM, Onori P, Franchitto A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Laboratory investigation; a journal of technical methods and pathology. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marinelli RA, Pham L, Agre P, LaRusso NF. Secretin promotes osmotic water transport in rat cholangiocytes by increasing aquaporin-1 water channels in plasma membrane. Evidence for a secretin-induced vesicular translocation of aquaporin-1. J Biol Chem. 1997;272:12984–12988. doi: 10.1074/jbc.272.20.12984. [DOI] [PubMed] [Google Scholar]
- 20.Poling HM, Mohanty SK, Tiao GM, Huppert SS. A comprehensive analysis of aquaporin and secretory related gene expression in neonate and adult cholangiocytes. Gene expression patterns: GEP. 2014;15:96–103. doi: 10.1016/j.gep.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mennone A, Verkman AS, Boyer JL. Unimpaired osmotic water permeability and fluid secretion in bile duct epithelia of aqp1 null mice. Am J Physiol Gastrointest Liver Physiol. 2002;283:G739–746. doi: 10.1152/ajpgi.00540.2001. [DOI] [PubMed] [Google Scholar]
- 22.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: Dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 23.Hirata K, Pusl T, O’Neill AF, Dranoff JA, Nathanson MH. The type ii inositol 1,4,5-trisphosphate receptor can trigger ca2+ waves in rat hepatocytes. Gastroenterology. 2002;122:1088–1100. doi: 10.1053/gast.2002.32363. [DOI] [PubMed] [Google Scholar]
- 24.Pierobon N, Renard-Rooney DC, Gaspers LD, Thomas AP. Ryanodine receptors in liver. J Biol Chem. 2006;281:34086–34095. doi: 10.1074/jbc.M607788200. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W, et al. Nucleoplasmic calcium is required for cell proliferation. J Biol Chem. 2007;282:17061–17068. doi: 10.1074/jbc.M700490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cruz LN, Guerra MT, Kruglov E, Mennone A, Garcia CR, Chen J, et al. Regulation of multidrug resistance-associated protein 2 by calcium signaling in mouse liver. Hepatology. 2010;52:327–337. doi: 10.1002/hep.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozcan L, Wong CC, Li G, Xu T, Pajvani U, Park SK, et al. Calcium signaling through camkii regulates hepatic glucose production in fasting and obesity. Cell metabolism. 2012;15:739–751. doi: 10.1016/j.cmet.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirata K, Dufour JF, Shibao K, Knickelbein R, O’Neill AF, Bode HP, et al. Regulation of ca(2+) signaling in rat bile duct epithelia by inositol 1,4,5-trisphosphate receptor isoforms. Hepatology. 2002;36:284–296. doi: 10.1053/jhep.2002.34432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Futatsugi A, Nakamura T, Yamada MK, Ebisui E, Nakamura K, Uchida K, et al. Ip3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 30.Ouyang K, Leandro Gomez-Amaro R, Stachura DL, Tang H, Peng X, Fang X, et al. Loss of ip3r-dependent ca2+ signalling in thymocytes leads to aberrant development and acute lymphoblastic leukemia. Nature communications. 2014;5:4814. doi: 10.1038/ncomms5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 32.Orman ES, Odena G, Bataller R. Alcoholic liver disease: Pathogenesis, management, and novel targets for therapy. Journal of gastroenterology and hepatology. 2013;28 (Suppl 1):77–84. doi: 10.1111/jgh.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yule DI, Straub SV, Bruce JI. Modulation of ca2+ oscillations by phosphorylation of ins(1,4,5)p3 receptors. Biochem Soc Trans. 2003;31:954–957. doi: 10.1042/bst0310954. [DOI] [PubMed] [Google Scholar]
- 34.Thrower EC, Choe CU, So SH, Jeon SH, Ehrlich BE, Yoo SH. A functional interaction between chromogranin b and the inositol 1,4,5-trisphosphate receptor/ca2+ channel. J Biol Chem. 2003;278:49699–49706. doi: 10.1074/jbc.M309307200. [DOI] [PubMed] [Google Scholar]
- 35.Ando H, Mizutani A, Kiefer H, Tsuzurugi D, Michikawa T, Mikoshiba K. Irbit suppresses ip3 receptor activity by competing with ip3 for the common binding site on the ip3 receptor. Mol Cell. 2006;22:795–806. doi: 10.1016/j.molcel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Zhang S, Mizutani A, Hisatsune C, Higo T, Bannai H, Nakayama T, et al. Protein 4. 1n is required for translocation of inositol 1,4,5-trisphosphate receptor type 1 to the basolateral membrane domain in polarized madin-darby canine kidney cells. J Biol Chem. 2003;278:4048–4056. doi: 10.1074/jbc.M209960200. [DOI] [PubMed] [Google Scholar]
- 37.Wojcikiewicz RJ. Type i, ii, and iii inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J Biol Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- 38.Ananthanarayanan M, Banales JM, Guerra MT, Spirli C, Munoz-Garrido P, Mitchell-Richards K, et al. Posttranslational regulation of the type iii inositol 1,4,5-trisphosphate receptor by mirna-506. J Biol Chem. 2014 doi: 10.1074/jbc.M114.587030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rushmore TH, Pickett CB. Transcriptional regulation of the rat glutathione s-transferase ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990;265:14648–14653. [PubMed] [Google Scholar]
- 41.Bae SH, Sung SH, Cho EJ, Lee SK, Lee HE, Woo HA, et al. Concerted action of sulfiredoxin and peroxiredoxin i protects against alcohol-induced oxidative injury in mouse liver. Hepatology. 2011;53:945–953. doi: 10.1002/hep.24104. [DOI] [PubMed] [Google Scholar]