Abstract
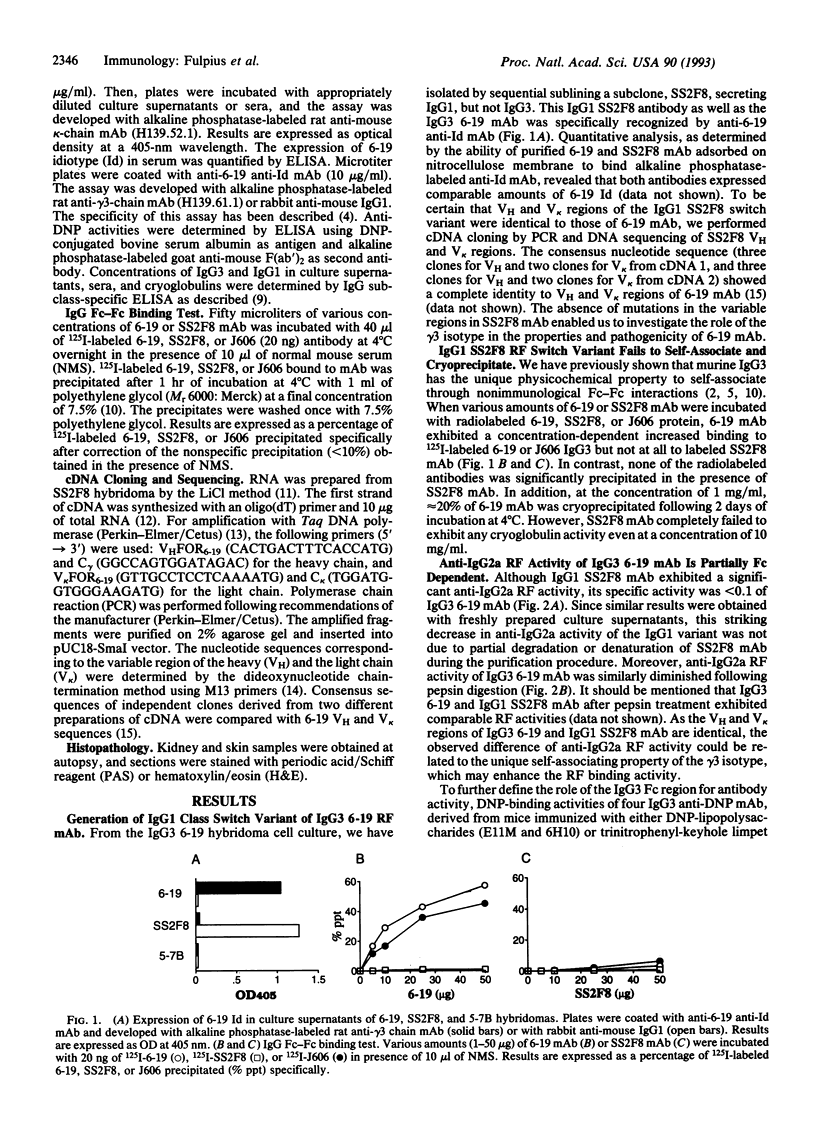
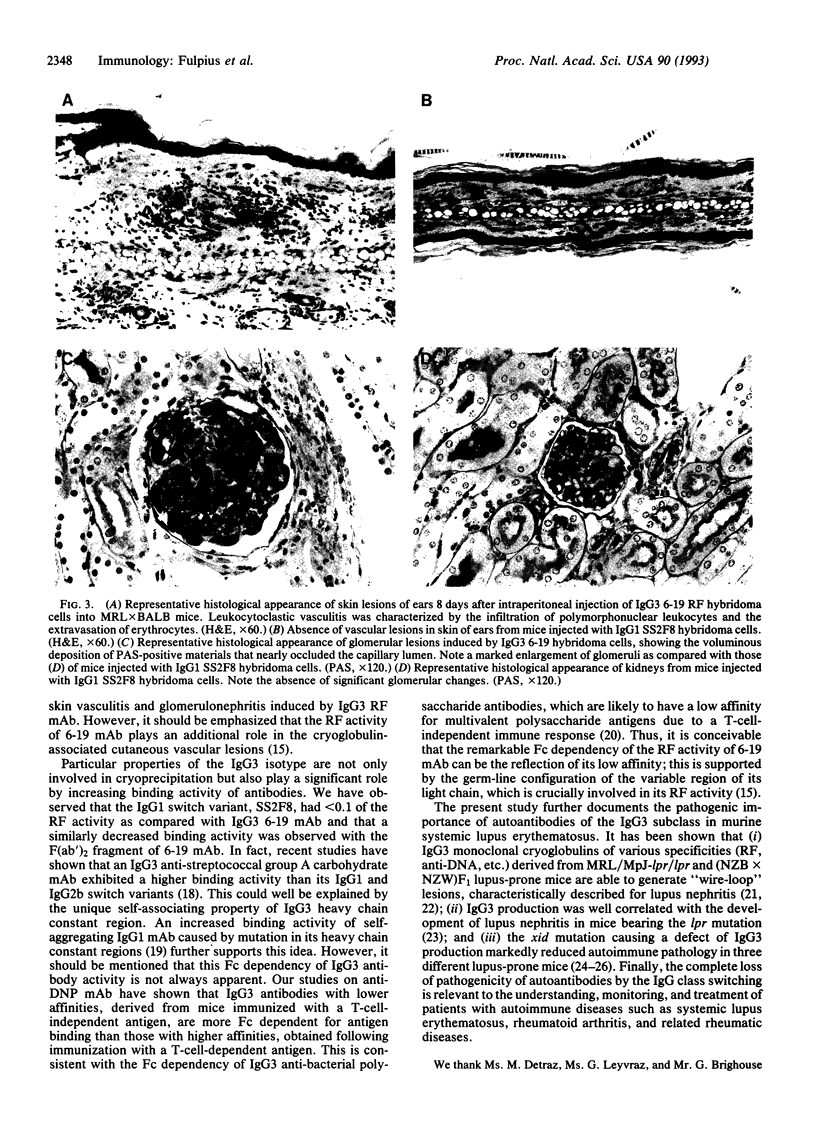
An IgG3 monoclonal antibody, 6-19, derived from unmanipulated MRL/MpJ-lpr/lpr mice, exhibiting cryoglobulin and anti-IgG2a rheumatoid factor activities, induces skin leukocytoclastic vasculitis and glomerulonephritis when injected into normal mice. To determine the role of the gamma 3 heavy chain constant region in the generation of cryoglobulins and associated tissue lesions, we have established an IgG1 class switch variant, clone SS2F8, from the 6-19 hybridoma by sequential sublining. Here we report that the SS2F8 monoclonal antibody, which loses the cryoglobulin activity but retains the rheumatoid factor activity, fails to generate skin and glomerular lesions. The lack of pathogenicity of the IgG1 SS2F8 switch variant is not due to mutations in variable regions, since nucleotide sequence analysis shows no differences between both clones. In addition, we have observed that the IgG1 SS2F8 switch variant exhibits < 10% of the rheumatoid factor activity, as compared with the IgG3 6-19 monoclonal antibody, suggesting that the self-associating property of the gamma 3 isotype promotes antibody-binding activity. The present study indicates that the cryoglobulin activity associated with the gamma 3 isotype is critically involved in the pathogenicity of 6-19 anti-IgG2a rheumatoid factor monoclonal antibody and highlights the pathogenic relevance of autoantibodies of the IgG3 subclass in murine systemic lupus erythematosus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelmoula M., Spertini F., Shibata T., Gyotoku Y., Luzuy S., Lambert P. H., Izui S. IgG3 is the major source of cryoglobulins in mice. J Immunol. 1989 Jul 15;143(2):526–532. [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Bazin H., Cormont F., De Clercq L. Purification of rat monoclonal antibodies. Methods Enzymol. 1986;121:638–652. doi: 10.1016/0076-6879(86)21063-0. [DOI] [PubMed] [Google Scholar]
- Berney T., Fulpius T., Shibata T., Reininger L., Van Snick J., Shan H., Weigert M., Marshak-Rothstein A., Izui S. Selective pathogenicity of murine rheumatoid factors of the cryoprecipitable IgG3 subclass. Int Immunol. 1992 Jan;4(1):93–99. doi: 10.1093/intimm/4.1.93. [DOI] [PubMed] [Google Scholar]
- Cooper L. J., Schimenti J. C., Glass D. D., Greenspan N. S. H chain C domains influence the strength of binding of IgG for streptococcal group A carbohydrate. J Immunol. 1991 Apr 15;146(8):2659–2663. [PubMed] [Google Scholar]
- Davies A. J., Carter R. L., Leuchars E., Wallis V., Dietrich F. M. The morphology of immune reactions in normal, thymectomized and reconstituted mice. 3. Response to bacterial antigens: salmonellar flagellar antigen and pneumococcal plysaccharide. Immunology. 1970 Dec;19(6):945–957. [PMC free article] [PubMed] [Google Scholar]
- French D. L., Pollock R. R., Aguila H. L., Scharff M. D. The molecular and biochemical characterization of mutant monoclonal antibodies with increased antigen binding. J Immunol. 1991 Mar 15;146(6):2010–2016. [PubMed] [Google Scholar]
- Gallo G. R., Caulin-Glaser T., Lamm M. E. Charge of circulating immune complexes as a factor in glomerular basement membrane localization in mice. J Clin Invest. 1981 May;67(5):1305–1313. doi: 10.1172/JCI110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyotoku Y., Abdelmoula M., Spertini F., Izui S., Lambert P. H. Cryoglobulinemia induced by monoclonal immunoglobulin G rheumatoid factors derived from autoimmune MRL/MpJ-lpr/lpr mice. J Immunol. 1987 Jun 1;138(11):3785–3792. [PubMed] [Google Scholar]
- Labit C., Pierres M. Rat monoclonal antibodies to mouse IgG1, IgG2a, IgG2b, and IgG3 subclasses, and kappa chain isotypic determinants. Hybridoma. 1984 Summer;3(2):163–169. doi: 10.1089/hyb.1984.3.163. [DOI] [PubMed] [Google Scholar]
- Lemoine R., Berney T., Shibata T., Fulpius T., Gyotoku Y., Shimada H., Sawada S., Izui S. Induction of "wire-loop" lesions by murine monoclonal IgG3 cryoglobulins. Kidney Int. 1992 Jan;41(1):65–72. doi: 10.1038/ki.1992.9. [DOI] [PubMed] [Google Scholar]
- Luzuy S., Merino J., Engers H., Izui S., Lambert P. H. Autoimmunity after induction of neonatal tolerance to alloantigens: role of B cell chimerism and F1 donor B cell activation. J Immunol. 1986 Jun 15;136(12):4420–4426. [PubMed] [Google Scholar]
- Müller C. E., Rajewsky K. Isolation of immunoglobulin class switch variants from hybridoma lines secreting anti-idiotope antibodies by sequential sublining. J Immunol. 1983 Aug;131(2):877–881. [PubMed] [Google Scholar]
- Reininger L., Berney T., Shibata T., Spertini F., Merino R., Izui S. Cryoglobulinemia induced by a murine IgG3 rheumatoid factor: skin vasculitis and glomerulonephritis arise from distinct pathogenic mechanisms. Proc Natl Acad Sci U S A. 1990 Dec;87(24):10038–10042. doi: 10.1073/pnas.87.24.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomchik M., Mascelli M., Shan H., Radic M. Z., Pisetsky D., Marshak-Rothstein A., Weigert M. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990 Jan 1;171(1):265–292. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. R., Chused T. M., Steinberg A. D. The effect of the X-linked immune deficiency gene (xid) upon the Y chromosome-related disease of BXSB mice. J Immunol. 1983 Sep;131(3):1257–1262. [PubMed] [Google Scholar]
- Spertini F., Coulie P. G., Van Snick J., Davidson E., Lambert P. H., Izui S. Inhibition of cryoprecipitation of murine IgG3 anti-dinitrophenyl (DNP) monoclonal antibodies by anionic DNP-amino acid conjugates. Eur J Immunol. 1989 Feb;19(2):273–278. doi: 10.1002/eji.1830190209. [DOI] [PubMed] [Google Scholar]
- Spertini F., Donati Y., Welle I., Izui S., Lambert P. H. Prevention of murine cryoglobulinemia and associated pathology by monoclonal anti-idiotypic antibody. J Immunol. 1989 Oct 15;143(8):2508–2513. [PubMed] [Google Scholar]
- Steinberg B. J., Smathers P. A., Frederiksen K., Steinberg A. D. Ability of the xid gene to prevent autoimmunity in (NZB X NZW)F1 mice during the course of their natural history, after polyclonal stimulation, or following immunization with DNA. J Clin Invest. 1982 Sep;70(3):587–597. doi: 10.1172/JCI110651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg E. B., Santoro T. J., Chused T. M., Smathers P. A., Steinberg A. D. Studies of congenic MRL-Ipr/Ipr.xid mice. J Immunol. 1983 Dec;131(6):2789–2795. [PubMed] [Google Scholar]
- Takahashi S., Itoh J., Nose M., Ono M., Yamamoto T., Kyogoku M. Cloning and cDNA sequence analysis of nephritogenic monoclonal antibodies derived from an MRL/lpr lupus mouse. Mol Immunol. 1993 Feb;30(2):177–182. doi: 10.1016/0161-5890(93)90089-t. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nose M., Sasaki J., Yamamoto T., Kyogoku M. IgG3 production in MRL/lpr mice is responsible for development of lupus nephritis. J Immunol. 1991 Jul 15;147(2):515–519. [PubMed] [Google Scholar]
- Wolfowicz C. B., Sakorafas P., Rothstein T. L., Marshak-Rothstein A. Oligoclonality of rheumatoid factors arising spontaneously in lpr/lpr mice. Clin Immunol Immunopathol. 1988 Mar;46(3):382–395. doi: 10.1016/0090-1229(88)90057-8. [DOI] [PubMed] [Google Scholar]



