Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a secretory protein that controls cholesterol homeostasis by enhancing endosomal and lysosomal degradation of the low-density lipoprotein receptor (LDL-R). Mutations that cause increased activity of PCSK9 are associated with hypercholesterolemia, atherosclerosis and early cardiovascular disease (CVD), whereas individuals with loss-of-function mutations in PCSK9 are apparently healthy but are hypocholesterolemic and have a dramatically decreased risk of CVD. In this study, we generated Virus-like Particle (VLP)-based vaccines targeting PCSK9. Mice and macaques vaccinated with bacteriophage VLPs displaying PCSK9-derived peptides developed high titer IgG antibodies that bound to circulating PCSK9. Vaccination was associated with significant reductions in total cholesterol, free cholesterol, phospholipids, and triglycerides. A vaccine targeting PCSK9 may, therefore, be an attractive alternative to monoclonal antibody-based therapies.
Keywords: PCSK9, Vaccine, Virus-like Particles, LDL Cholesterol
1. Introduction
Elevated low-density lipoprotein cholesterol (LDL-C) is associated with an increased risk of cardiovascular disease (CVD) [1]. Although lifestyle changes and medication can significantly reduce LDL-C, a substantial percentage of at-risk patients on lipid lowering therapy (>60%) still go on to have a cardiovascular event [2]. Currently, treatment with 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) is the standard of care for hypercholesterolemic patients. Intensive statin therapy has some risks [3] and ~20% of high-risk patients with hypercholesterolemia do not achieve adequate control of LDL-C with just statins [2].
LDL-C in plasma is primarily removed from circulation when it interacts with LDL receptors (LDL-R) that are abundantly expressed on hepatocytes. Upon LDL-R binding, LDL-C is endocytosed and undergoes lysosomal catabolism. Following this process, LDL-R is recycled back to the cell surface. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a hepatic secretory protein that acts as a negative regulator of LDL-R by blocking the recycling of the receptor to the cell surface. PCSK9 in plasma binds to the extracellular domain of LDL-R and mediates its internalization and degradation, thus increasing circulating levels of LDL-C by preventing its uptake [4, 5]. Genetic studies have shown that mutations that modulate PCSK9 activity can have profound effects on LDL-C levels. Gain of function mutations in PCSK9 are associated with autosomal dominant hypercholesterolemia, a disease that is characterized by increased LDL-C levels (>300 mg/dL) and a corresponding increased risk of CVD [6]. In contrast, humans with loss-of-function PCSK9 mutations are hypocholesterolemic (15–25% decrease in LDL-C) and have approximately half the incidence of CVD, most likely because of a lifelong reduction of LDL-C [7]. Strikingly, individuals with compound heterozygote loss-of-function mutations, have exceptionally low serum LDL-C (<20 mg/dL) and appear healthy despite having no detectible circulating PCSK9 [8].
Given the important role of PCSK9 in regulating LDL metabolism and the fact that loss-of-function mutations appear not to be associated with adverse effects, PCSK9 has emerged as an attractive therapeutic target. PCSK9-specific monoclonal antibodies (mAbs), including evolocumab (Amgen), bococizumab (Pfizer), and alirocumab (Aventis/Regeneron) work synergistically with statins, and markedly reduce LDL-C levels by about 60% and, in early stage clinical trials, have been shown to reduce the incidence of cardiovascular events [9–11]. Statin therapy alone increases circulating levels of PCSK9 by as much as 30% as compared to placebo, making them somewhat self-limiting in their ability to further reduce LDL-C [12–14]. This likely occurs because the transcription factor SREBP-2, that is indirectly upregulated by statins, activates the Ldlr and Pcsk9 genes [15]. Indeed, statins are much more effective when there is a deficit of PCSK9 [16]. Therefore, PCSK9-targeted therapeutics may have value in preventing and treating CVD in combination with statins or in vulnerable populations that are either resistant to statin therapy or statin intolerant.
Induction of antibody responses against a self-antigen, such as PCSK9, are seemingly limited by the mechanisms of B cell tolerance, which eliminate, inactivate, or alter the specificity of potentially self-reactive B cells. Yet B cell tolerance is actually highly inefficient and anti-self antibody responses can be readily elicited by immunizing with vaccines that have features that provoke the efficient activation of self-reactive B cells. Vaccines that display self-antigens in a dense, repetitive array and provide a source of foreign T helper epitopes can induce particularly robust, high-titer autoantibody responses [17]. Display of self-antigens in a highly dense, repetitive format on the surface of virus-like particles (VLPs) is one approach for inducing strong antibody responses against self-antigens. VLP display has been successfully used to target self molecules that are involved in the pathogenesis of a variety of chronic diseases, including Alzheimer’s Disease, hypertension, and certain cancers [18]. Many of these vaccines have shown clinical efficacy in animal models and several have been tested in human clinical trials. For example, clinical trials of a VLP-based vaccine targeting angiotensin II, a regulator of blood pressure, showed that this vaccine was highly immunogenic and significantly reduced blood pressure in hypertensive patients [19].
In this study, we used several different approaches to identify a bacteriophage VLP-based vaccine that elicits strong antibody responses against PCSK9. Using both mice and non-human primates, we show that vaccination with VLPs displaying an epitope derived from PCSK9 was associated with significant reductions in pro-atherogenic plasma lipids and lipoproteins.
2. Materials and Methods
2.1. Construction of PCSK9-displaying VLPs
Qβ VLPs were produced in E. coli using methods that we have previously described for the production of MS2 bacteriophage VLPs [20]. Peptides representing huPCSK9 amino acids 68-76, 153-163, and 207-223 were synthesized (GenScript) and modified to include a C-terminal cysteine residue preceded by a 2-glycine-spacer sequence. Peptides were conjugated to VLPs using the bifunctional cross-linker succinimidyl 6-[(β-maleimidopropionamido)hexanoate] (SMPH; ThermoScientific) [21]. Efficiency of conjugation was measured using denaturing polyacrylamide gel electrophoresis.
Recombinant PCSK9-VLP expression vectors were constructed by genetically inserting huPCSK9 sequences (amino acids 153-163, 188-200, 208-222, and 368-381) by PCR at the N-terminus of a single-chain dimer version of the MS2 bacteriophage coat protein [20]. All constructs were sequenced to verify correct location and sequence of PCSK9 insert. Recombinant MS2 VLPs were expressed and purified as described [20].
2.2. Immunizations
All animal studies were performed in accordance with guidelines of the University of New Mexico and NHLBI Animal Care and Use Committees (protocols 12-100827-HSC and H-0059R3). Mouse immunization experiments were performed using four to six-week old male Balb/c mice. Mice were immunized with 5 μg of VLPs three times at 2-week intervals. Vaccines were formulated with incomplete Freund’s adjuvant (Sigma Aldrich) at a 1:1 (volume:volume) ratio in a total volume of 100 μl. Blood plasma was collected prior to the first immunization and 2-weeks following the third immunization.
Macaque studies were performed using nine 9–17 year old rhesus macaques (seven females and two males) that were divided into three experimental groups of three animals each. Groups were vaccinated three times at 2-week intervals with either 50 μg of (i) Qβ-PCSK9207-223 without exogenous adjuvant, or (ii) Qβ-PCSK9207-223 formulated with 2% Alhydrogel adjuvant (Invivogen) at a 1:4 (volume:volume) ratio or, as a control, (iii) wild-type Qβ VLPs plus Alhydrogel. Plasma was obtained prior to immunization and two weeks following each immunization. Approximately 6 months after the initial set of immunizations, groups i and ii were re-boosted with Qβ-PCSK9207-223 formulated with 2% Alhydrogel adjuvant and then treated with simvastatin (at 30 mg/kg/day) for two weeks and these two group were combined for subsequent analyses. Group iii (control group) was reboosted with wild-type Qβ VLPs plus Alhydrogel and treated with simvastatin (at 30 mg/kg/day) for two weeks.
2.3. Characterization of antibody responses
PCSK9-specific IgG titers were determined by end-point dilution ELISA, using either PCSK9 peptide or recombinant huPCSK9 protein (R&D Systems) as the antigen. For peptide ELISAs, Immulon 2 plates (Thermo Scientific) were incubated with 500 ng streptavidin (Invitrogen) in pH 7.4 phosphate-buffered saline (PBS) for 2 hours at 37°C. Following washing, SMPH was added to wells at 1 μg/well and incubated for 2 hours at room temperature. Individual peptides corresponding to the Qβ-conjugated peptides were added to the wells at 1 μg/well and incubated overnight at 4°C. For PSCK9 ELISAs, plates were incubated with recombinant human PCSK9 protein (R&D Systems) at a concentration of 500ng/well in PBS overnight at 4°C. In all ELISAs, plates were blocked with 0.5% milk in PBS for 2 h, and 4-fold dilutions of plasma were added to each well and incubated for 2.5 h. The wells were probed with horseradish peroxidase (HRP)-conjugated secondary antibody [goat anti-mouse-IgG (Jackson ImmunoResearch; 1:5,000) or goat anti-monkey IgG (Fitzgerald Industries; 1:4,000) for 1 h. The reaction was developed using TMB (ThermoScientific) and stopped using 1% HCl. Reactivity of sera for the target antigen was determined by measuring optical density at 450nm (OD450). Wells with twice the OD450 value of background were considered to be positive and the highest dilution with a positive value was considered the end-point dilution titer.
2.4. Plasma lipid and lipoprotein quantification
Plasma lipids were measured enzymatically using a ChemWell instrument and Roche reagents. ApoB was measured nephelometrically on a Dimension analyzer (Siemens). LDL-C was calculated by the Friedewald equation. HDL and LDL particle counts were measured with a Vantera NMR analyzer (LipoScience).
2.5. Plasma PCSK9 quantification
Plasma PCSK9 levels were quantitated by a mouse PCSK9 ELISA kit (R&D Systems) by comparing experimental sera samples diluted 200-fold to an internal standard curve. PCSK9 was quantified in this way both before and after removal of immunoglobulin using Protein G-coated magnetic beads (Life Technologies). Briefly, plasma samples diluted 1:200 were split into equivalent volumes, then either a) incubated for 10 minutes with magnetic Protein G beads or b) set aside at room temperature. The Protein G beads were isolated using a magnet, and the Ig-cleared supernatant was then used directly in the PCSK9 ELISA kit, alongside the untreated plasma sample.
2.6. Statistical analysis
Single comparisons were made with unpaired, two-tailed t-tests using SPSS Statistics software or GraphPad Prism 6. When Levene’s test for equality of variances was found to be a significant factor, the p-values were derived from t-tests performed without equal variances assumed.
3. Results
3.1. Engineering and characterization of VLPs displaying PCSK9 peptides
Antibodies raised against linear peptides derived from PCSK9 have been shown to block PCSK9 binding to LDL-R (PMID: 19196236). Using this data and the crystal structure of PCSK9 bound to LDL-R as a guide, we engineered VLP-based vaccines that targeted five regions of PCSK9 that were predicted to be involved in LDL-R binding (Figure 1). Human PCSK9 peptides were displayed on bacteriophage VLPs either by conjugating synthetic peptides to Qβ bacteriophage VLPs [22] or by constructing recombinant MS2 bacteriophage VLPs in which PCSK9 peptides were displayed on the surface of the MS2 coat protein. For chemical conjugation, peptides representing human PCSK9 amino acids (aa) 68-76, 153-163, or 207-223 were synthesized and conjugated to the surface of Qβ VLPs using a bifunctional cross-linker (SMPH). Approximately 270-360 PCSK9 peptides were linked to each individual VLP (data not shown). Recombinant VLPs were constructed by genetically inserting sequences representing PCSK9-derived peptides (aa153-163, aa188-200, aa208-222, and aa368-381) at the N-terminus of the MS2 bacteriophage single-chain dimer coat protein [23]. In this conformation each recombinant VLP displayed 90 copies of the PCSK9 peptide.
Fig. 1. Three views of the crystal structure of PCSK9 bound to the extracellular domain of LDL-R.
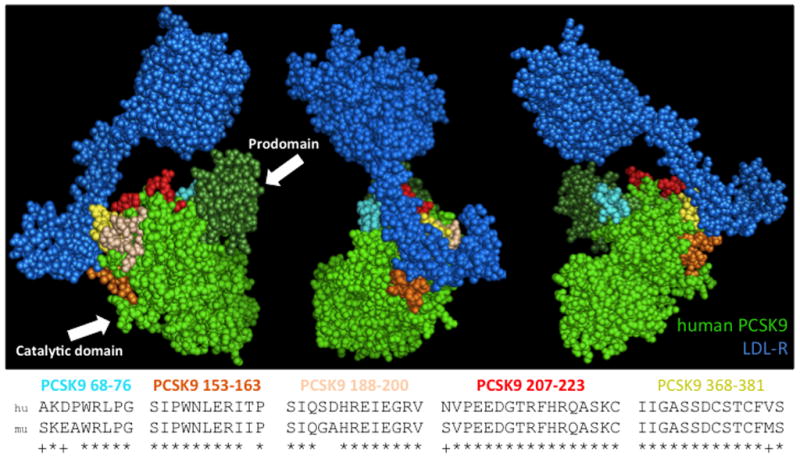
The prodomain and catalytic domain of human PCSK9 are shown in dark and light green, respectively; the extracellular domain of LDL-R is shown in dark blue. The sequences PCSK9-derived peptides investigated in this study are shown. The top sequence shows the human PCSK9 peptide that was displayed on VLPs; the lower sequence shows the homologous mouse PCSK9 sequence. The peptides that were targeted are mapped onto the crystal structure by color. Images were generated using the coordinates provided by the RCSB protein data bank using the program PyMOL.
3.2. PCSK9-VLPs are immunogenic
In order to compare the vaccine candidates, small groups of Balb/c mice (5 per group) were immunized with PCSK9-conjugated Qβ VLPs, recombinant PCSK9-MS2 VLPs, or, as a control, wild-type VLPs. All of the PCSK9-VLPs were highly immunogenic and generated IgG responses against the displayed peptides and also against recombinant human PCSK9, although the antibody titers varied (Fig. 2). In general, the Qβ VLPs displaying conjugated PCSK9 peptides elicited higher titer antibody responses than the recombinant PCSK9-MS2 VLPs, perhaps reflecting the importance of peptide valency in eliciting high-titer antibody responses against self-antigens.
Fig. 2. Peptide- and recombinant hPCSK9-reactive end-point dilution IgG titers in groups of mice given 3 immunizations with VLP-based vaccines targeting PCSK9 peptides.
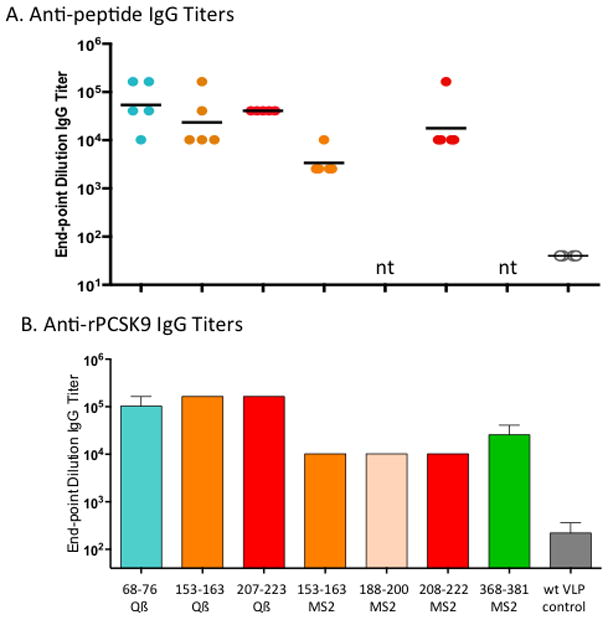
The PCSK9 peptide targeted and the VLP-platform used are denoted along the x-axis of the lower panel. Shown are (A) PCSK9 peptide-specific responses, and (B) hPCSK9-specific responses. In (A) each data point represents an individual mouse, lines denote the geometric mean of each group, and nt means not tested. Data in (B) represents mean end-point dilutions titers for groups of mice (n=5).
3.3. Immunization with PCSK9 VLPs lowers lipid levels in mice
Although mice carry much of their cholesterol on HDL [24], we first assessed the functional effect of immunization against the various PCSK9 epitopes by measuring total cholesterol in the immunized groups Balb/c mice before and after immunization (Fig. 3A). While control mice immunized with wild-type Qβ/MS2 VLPs showed slight increases in total cholesterol levels, all of the groups of mice immunized with PCSK9 peptides conjugated to Qβ VLPs had significantly reduced total cholesterol. Mice immunized with PCSK9207-223-Qβ VLPs had the largest reduction in total cholesterol levels; ~55% relative to the control group. In general, there were not as dramatic reductions in total cholesterol in the groups of mice immunized with recombinant MS2 VLPs displaying PCSK9 peptides. We also measured free cholesterol and triglyceride levels in the mice immunized with Qβ-PCSK9 vaccines (Fig. 3B-C). Free cholesterol levels were also significantly reduced in mice immunized with either the PCSK9207-223-Qβ or the PCSK9153-163-Qβ VLPs, and immunization with each of the Qβ-based vaccines also resulted in significant reductions in plasma triglyceride, which is largely carried in mice by only pro-atherogenic apoB-containing lipoproteins [24].
Fig. 3. Plasma lipid levels in mice vaccinated with various PCSK9 peptides.
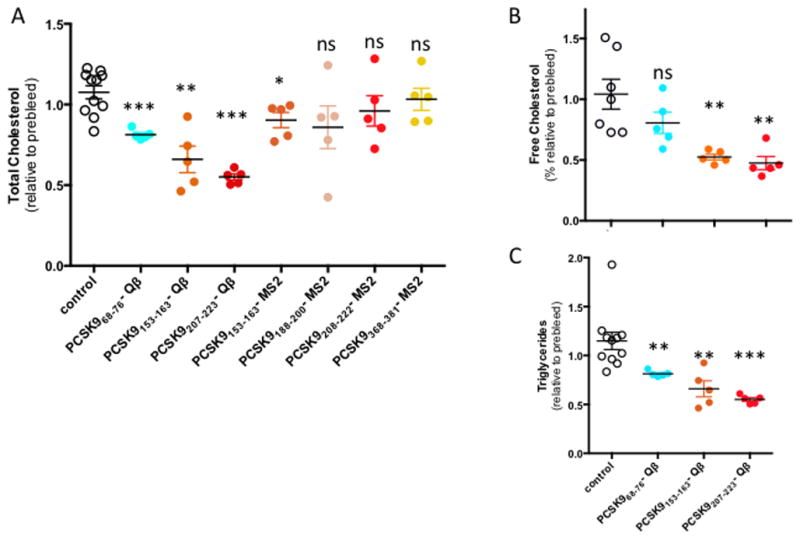
(A) total cholesterol, (B) free cholesterol, and (C) and triglycerides were measured in preimmune plasma samples and after three immunizations, and relative values obtained for each individual sample. Controls are a mixture of mice immunized with either wild-type MS2 or Qβ VLPs. Mean and SEM values are shown for each group. Experimental groups were statistically compared to controls by independent 2-tailed t-test, using Levene’s test to account for unequal variance when necessary. * denotes a significant decrease (p < 0.05), **, p < 0.01, ***, p < 0.001, ns, not significant.
Because immunization with the Qβ-PCSK9207-223 VLPs resulted in the largest decrease in cholesterol levels, our follow-up studies focused on this vaccine. This particular region of the protein is predicted to be in a random coil conformation, which could potentially explain why a synthetic peptide targeting this region was a better immunogen. To better characterize immune responses and their effects on serum lipid levels, a larger group of 20 mice were immunized with Qβ;-PCSK9207-223 VLPs and anti-PCSK9 antibody levels were measured. Consistent with our initial study, vaccination elicited high titer antibody responses against PCSK9 (not shown). Vaccination with Qβ-PCSK9207-223 VLPs also significantly lowered circulating lipids relative to mice immunized with control VLPs. Compared to control mice, triglycerides were decreased by 51%, free cholesterol by 38%, total cholesterol by 28% and phospholipids by 27% (Fig. 4). All of the differences between the vaccinated group and the control group were highly statistically significant.
Fig. 4. Quantification of plasma lipids in mice, before and after immunization with Qβ-PCSK9207-223.
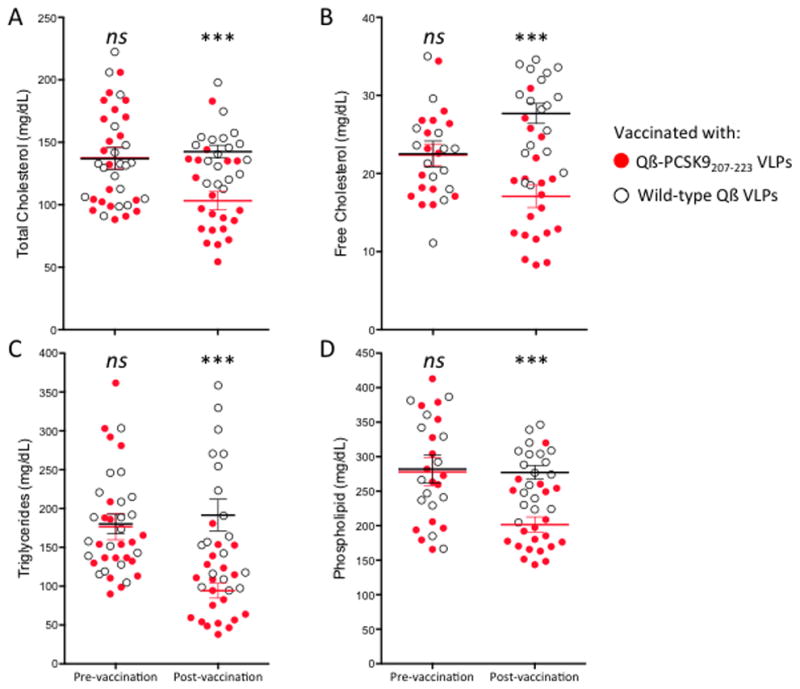
Red/filled circles show Qβ-PCSK9207-223 VLP immunized mouse plasma samples, and blue/empty circles show control-immunized (Qβ VLPs) samples. Mean and SEM are shown for (A) total cholesterol, (B) free cholesterol, (C) triglycerides, and (D) phospholipids. Each data point represents an individual mouse, lines represent means for each group. *** p < 0.001, ns, not significant.
3.4. Immunization with Qβ-PCSK9207-223 VLPs results in increased total serum levels of PCSK9
Previous studies have shown that the presence of anti-PCSK9 antibodies actually raises total soluble PCSK9 levels in the plasma [25, 26], suggesting that anti-PCSK9 antibodies may engage PCSK9 in vivo and form an immune complex. To assess this phenomenon, we measured PCSK9 levels in plasma from mice prior to vaccination and after three immunizations with Qβ-PCSK9207-223 VLPs or wild-type Qβ VLPs. Total PCSK9 levels were indeed significantly elevated in mice that had been vaccinated with Qβ-PCSK9207-223 VLPs compared to control wild-type Qβ VLPs vaccinated mice (Fig. 5A). When plasma was treated with magnetic Protein G-coupled beads to remove immune complexes, free PCSK9 levels were substantially decreased by about 50% (Fig. 5B), similar to what has been observed using anti-PCSK9 mAbs [25]. This suggests that increased detection of total PCSK9 in the experimental group was largely due to the presence of immunoglobulin-bound PCSK9, which likely have decreased plasma clearance compared to free PCSK9 but are ineffective in their interaction with LDL-R. These data also provide evidence that IgG elicited by vaccination binds to PCSK9 in vivo.
Fig. 5. Quantification of plasma PCSK9 in mice, before and after immunization with Qβ-PCSK9207-223.
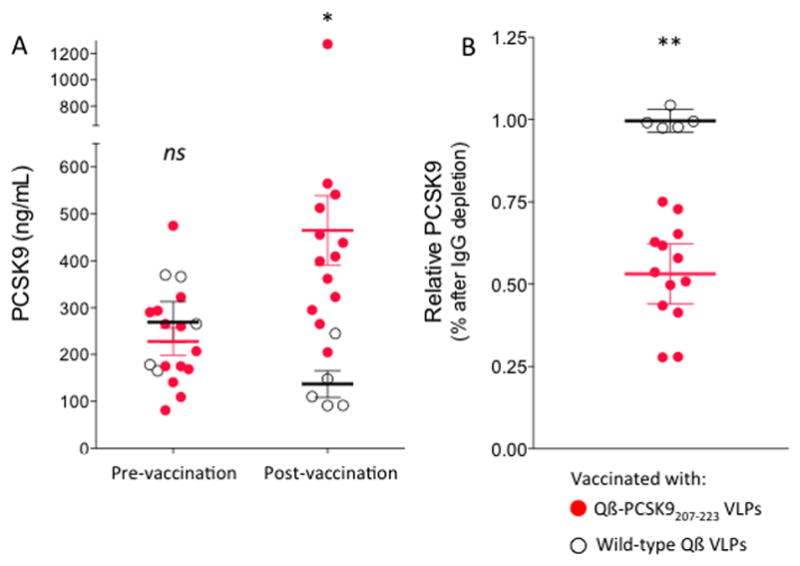
(A) Red/filled circles show Qβ-PCSK9 (207-223)-immunized mouse plasma samples, and black/empty circles show control-immunized (Qβ VLPs) samples. Mean and SEM are shown. (B) Effect of depleting plasma of IgG on PCSK9 detection. Red/filled circles show relative PCSK9 depletion from Qβ-PCSK9 (207-223)-immunized mouse plasma samples, and black/empty circles show no depletion from control-immunized samples. Each data point represents an individual mouse. In all graphs mean and SEM are shown. *, p < 0.05, **, p < 0.01, ns, not significant.
3.5. Immunization with PCSK9 VLPs lowers LDL-C levels in macaques and works synergistically with statins
Non-human primates (NHP) have been used as a pre-clinical model for assessing the LDL-C lowering ability of mAbs that target PCSK9 [25, 27–29]. We, therefore, assessed the lipid lowering capabilities of PCSK9-VLPs by performing a pilot vaccination study in a small number (n=9) of rhesus macaques equally divided into one of 3 groups. One group received three biweekly immunizations of 50 μg of PCSK9207-223-Qβ VLPs, a second group received the same vaccine but with Alum adjuvant, and a control group was immunized with wild-type Qβ VLPs. As shown in Fig. 6A, macaques vaccinated with PCSK9207-223-Qβ VLPs had high-titer anti-PCSK9 antibody levels. Anti-PCSK9 antibody titers were slightly higher in the group that was immunized with VLPs plus Alum adjuvant. LDL-C, LDL-Particles (LDL-P), and ApoB, which are all different measures of pro-atherogenic lipoproteins, were all decreased in the vaccinated groups relative to controls. Total cholesterol was also reduced in macaques given the PCSK9-VLPs plus Alum (Fig. 6B). LDL-C levels were approximately 10–15% lower in the vaccinated groups relative to controls, and LDL-P levels were reduced by 28%. However, in this limited study we did not observe a correlation between higher anti-PCSK9 titers and reductions in pro-atherogenic markers in individual macaques.
Fig. 6. Antibody and Lipid levels in Qβ-PCSK9207-223 vaccinated macaques.
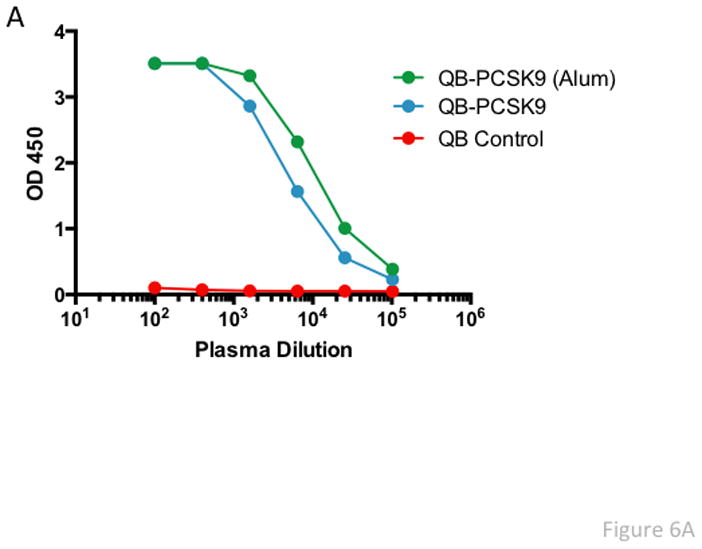
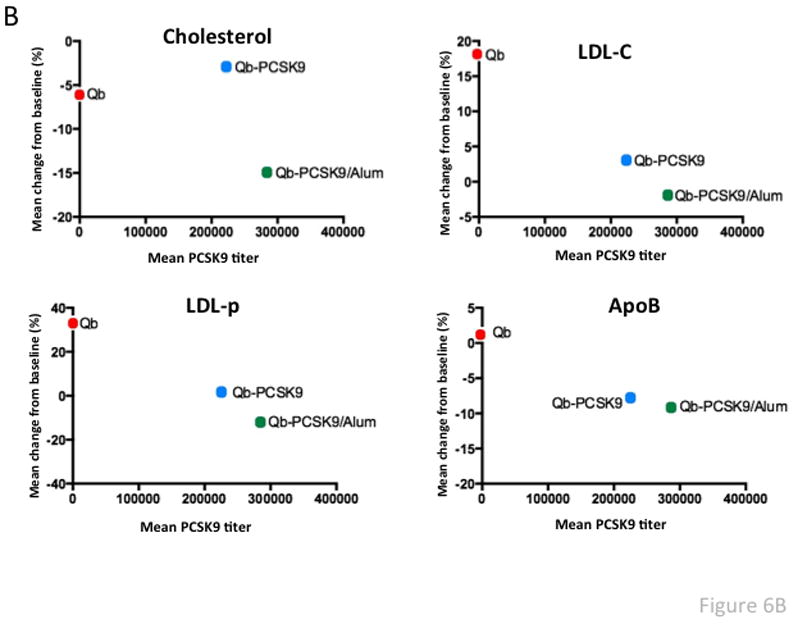
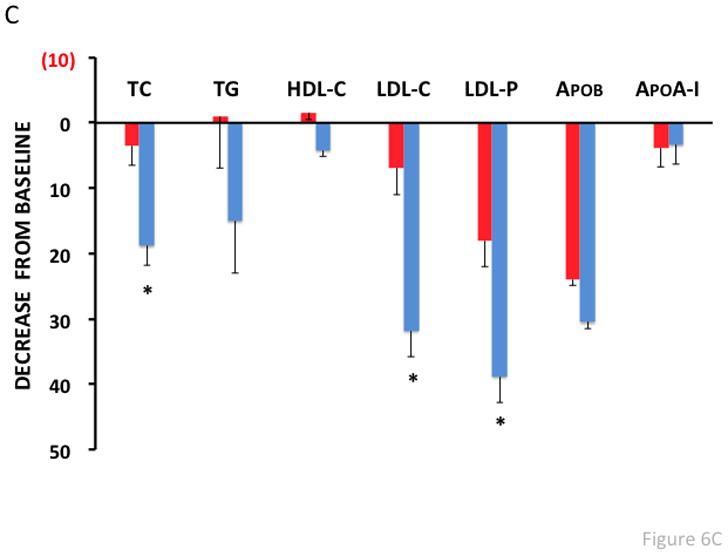
(A) Anti-PCSK9 peptide antibody responses in plasma from vaccinated macaques (taken 2 weeks after the third immunization) were quantitated by ELISA. Shown are average OD450 values for each group of three macaques immunized with vaccine or with control wild-type Qβ VLPs. (B) Lipid measurements in vaccinated macaques. Cholesterol, LDL-C, LDL-p, and ApoB levels were measured and compared to baseline (prior to vaccination) levels. Mean % change in lipid levels from baseline (for each group of macaques) is plotted against the average PCSK9 antibody titer for each group. (C) Lipid measurements in vaccinated macaques that were also treated with simvastatin (30 mg/kg/d). Lipid levels were measured and compared to baseline (i.e. prior to vaccination/statin treatment). There were six macaques in the PCSK9 group (blue bars; the six macaques that received vaccine with or without Alum) and two macaques in the Qβ control group (red bars). Values are averages for each group. TC: Total Cholesterol, TG: Triglycerides, HDL-C: HDL-cholesterol, LDL-C: LDL-cholesterol, LDL-P: LDL-particle number, ApoB: Apolipoprotein B, ApoA-I: Apolipoprotein A-I.
To assess whether vaccination worked synergistically with statins, macaques were revaccinated, treated with simvastatin (at 30 mg/kg/day) for two weeks, and then plasma lipids were remeasured. PCSK9-VLP vaccinated macaques treated with statins had a dramatic and statistically significant further reduction in LDL-C and LDL-P levels (~30–40%) compared to control vaccinated macaques (Fig. 6C). HDL levels, however, were virtually unaffected in both groups.
4. Discussion
Since its discovery in 2003 [30], PCSK9 has become one of the most promising therapeutic targets for cardiovascular disease. Elucidation of the biology of PCSK9 and observations of the metabolic consequences of gain-of-function and loss-of-function mutations have demonstrated that blocking its ability to bind LDL-R is sufficient to neutralize its activity and results in significantly lower circulating LDL-C [31–33]. These features make PCSK9 an ideal candidate for mAb-based therapies and clinical trials of several candidates have been highly promising. Nevertheless, mAbs have some important shortcomings that could hinder widespread implementation. PCSK9 mAbs are expected to be relatively expensive, particularly compared to most other lipid lowering drugs that are now mostly available in generic form. They are estimated to cost patients $7,000 to $12,000 per year, which represents a cost of $16 Billion annually just to treat patients with familial hypercholesterolemia in the United States [34]. While the developed world may be able to sustain these costs, expense is likely to be a major impediment to the use of such drugs in the developing world. In contrast, vaccination for a wide variety of mostly infectious communicable diseases has been proven to be compatible with the health care infrastructure in the developed and developing world. Another limitation is that most mAbs need to be injected frequently (usually one or twice a month) and at high doses (usually 40–100 mg). This can result in tolerability issues and poor compliance, particularly those patients not at very high risk. In fact, approximately half of patients discontinue oral statin therapy within one year of initiation [35]. Moreover, a significant proportion of patients treated with mAb lose responsiveness over time. This is often due to the induction of anti-drug antibodies, a phenomenon that occurs even with fully human mAbs. For example, in a study that followed RA patients treated with adalimumab (a TNF-alpha inhibitor), 28% developed anti-mAb antibodies, and development of these antibodies correlated with treatment failure [36]. Based on these limitations of the passive immunization approach, the development of a vaccine that actively immunizes patients against PCSK9 and effectively and safely lowers LDL-C would be highly desirable.
To develop an active vaccination strategy targeting PCSK9, we displayed linear epitopes that were exposed on the surface of the molecule in proximity to the LDL-R binding site on the surface of bacteriophage VLPs. PCSK9-displaying VLPs elicited high-titer peptide-specific and recombinant human PCSK9-reactive IgG responses. Several vaccine candidates were identified that significantly lowered circulating lipid levels, most notably Qβ-PCSK9207-223 VLPs. In addition to being located near the LDL-R binding site, characteristics of this PCSK9 epitope include a structurally disordered loop, spanning amino acids 213-218, as well as a cleavage site for proprotein convertase furin between residues 218 and 219 [37, 38]. Furin-cleaved PCSK9 is unable to degrade LDL-R, and several naturally occurring gain-of-function mutations in this region may therefore decrease cleavage and hence decrease inactivation of PCSK9 [39, 40]. While these studies would suggest that targeting of this region might prevent physiologic turnover of PCSK9, our data indicate that antibody binding to PCSK9 at this site neutralizes PCSK9 activity.
Although the amino acid sequences of human and mouse PCSK9 are highly similar, there are several differences within some of the regions that we targeted. For example, the mouse and human sequences differ at 3 of 9 amino acids in the PCSK968-76 peptide and 2 of 13 amino acids in the PCSK9188-200 peptide. It is possible that these differences may have affected the anti-PCSK9 activity of antibodies that were elicited by hPCSK9-VLPs, and it may, therefore, not be a coincidence that antibodies targeting huPCSK9207-223 and huPCSK9153-163 mediated the greatest reduction in lipid levels, since the central core of these huPCSK9 epitopes are identical to moPCSK9. The sequence of the macaque 207-223 epitope is identical to the mouse sequence.
Immunization with Qβ-PCSK9207-223 VLPs led to dramatic decreases in all lipid parameters investigated in mice, most notably in triglycerides, which are transported on mostly apoB-contianing lipoproteins in both mice and humans. Although PCSK9 does not appear to modulate HDL-C levels in humans, vaccination of mice against PCSK9 has been previously shown to lower total cholesterol in part by also lowering HDL-C [41], which could have also accounted for our results. Given this limitation and the fact that human and mouse PCSK9 is not completely homologous, we also tested whether PCSK9-VLPs could also lower plasma lipids in non-human primates (macaques). Although the decrease of 10–15% observed in PCSK9-VLPs vaccinated macaques is seemingly small, such changes in humans would be predicted to significantly reduce cardiovascular events [42] and is comparable to several other commonly used lipid-lowering medications, such as bile acid resins and fenofibrates. It is important to note that LDL-C levels at baseline in the macaques are already quite low (~75mg/dL) compared to humans, which could have limited the effect of the treatment. When the vaccinated macaques were co-administered simvastatin much greater reductions of LDL-C and LDL-P were observed, consistent with the known synergy between statins and PCSK9 inhibition. In contrast the level of anti-atherogenic HDL particles, as measured by HDL-P, apoA-I or by HDL-C, did not change with the treatment.
To our knowledge, only one other group (AFFiRiS AG, an Austrian biotechnology company) is actively pursuing an active vaccination approach targeting PCSK9. AFFiRiS uses a peptide-based vaccine in which short peptides with homology (but not identity) to regions in PCSK9 are linked to a KLH-carrier. AFFiRiS has generated interesting data in mice studies [26], but it is unclear whether KLH-peptide conjugates will be adequately immunogenic in humans. Indeed, in the past when we have directly compared VLP and KLH carriers for the induction of anti-self antibody responses against CCR5, we observed higher titer and more functional antibody responses when using VLPs [43]. Our macaque data and clinical trial data by the Swiss biotechnology company Cytos against other self-antigens besides PCSK9 also support the concept that bacteriophage VLP-based vaccines can induce strong anti-self antibody responses in primates [19, 44, 45].
The induction of anti-self antibody responses understandably raises some safety concerns and should be reserved for those targets where the consequences of antibody-mediated inhibition are well known and the benefits of vaccination outweigh potential risks. At this point, PCSK9 appears to meet these criteria. PCSK9 mAb clinical trials have not revealed any major safety concerns and humans with mutations that abolish PCSK9 expression are apparently healthy [8]. Nevertheless, vaccination would presumably elicit longer lasting antibody responses to PCSK9 and, in contrast to mAb-based therapies, it would not be possible to rapidly decrease the serum Ab levels in response to adverse side effects. While it is true that VLP-based vaccines targeting self-antigens can elicit high titer autoantibody responses, these autoantibody responses do wane over time. For example, human clinical trials performed by Cytos have demonstrated that anti-self antibody titers typically decline a half-life of ~3 months [46]. Our laboratory has similar data in non-human primates [47, 48]. There has been no evidence for boosting by endogenous self-antigen, presumably because foreign T cell helper responses are required to stimulate antibody production. Nevertheless, re-immunization with vaccine can boost anti-self antibody levels. In addition, the magnitude of the antibody response to VLP vaccines can be controlled by varying the dose and the number of booster immunizations, and thus titers can wane quite rapidly in the first weeks and months after the initial peak is reached (as the shorter lived plasmablasts expire). Therefore, it should be possible to make an initial assessment of vaccine safety in a dose escalation trial in which the titers in the initial vaccinees would be low and then rapidly drop below the toxic range if any toxicity was observed. National regulators in the U.S. and Europe have approved this type of design for phase I/II trials of other auto-antibody-inducing vaccines [19, 44, 45]. Thus, there is ample precedent for clinical trials of a vaccine targeting PCSK9.
In summary, we have investigated a panel of potential PCSK9-targeting vaccines, and identified at least one candidate for future study. The data reported here, in both mice and macaques, provides proof-of-principle evidence that a vaccine targeting PCSK9 can effectively lower lipid levels and work synergistically with statins. Thus, the use of VLP-based vaccines targeting PCSK9 peptide could serve as a cost-effective alternative to other therapies and could lead to a widely applicable vaccine-based approach for controlling hypercholesteremia and cardiovascular disease. If successful, this approach could obviously have a major impact on human health worldwide.
Highlights.
PCSK9 is a molecule that controls the metabolism of low-density lipoprotein cholesterol (LDL-C).
We have generated Virus-like Particles based vaccines that induce high-titer antibodies against PCSK9.
accination of mice with PCSK9-VLPs resulted in reduced lipid levels.
Macaques vaccinated with PCSK9-VLPs and coadministered statins had significant reduction in LDL-C levels.
Vaccination against PCSK9 may be an alternative to monoclonal antibody-based therapies.
Acknowledgments
This research was funded in part by NIH grant R01 AI083305 (to BC) and by the Intramural Research Program of NHLBI. EC was supported by the University of New Mexico Biology of Infectious Diseases and Inflammation Training Grant (T31-AI007538). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflict of Interest Statement
The authors have submitted a patent application on VLP-based vaccines targeting PCSK9.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cholesterol Treatment Trialists C. Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–90. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan AT, Yan RT, Tan M, Hackam DG, Leblanc KL, Kertland H, et al. Contemporary management of dyslipidemia in high-risk patients: targets still not met. Am J Med. 2006;119:676–83. doi: 10.1016/j.amjmed.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Ballantyne CM, Corsini A, Davidson MH, Holdaas H, Jacobson TA, Leitersdorf E, et al. Risk for myopathy with statin therapy in high-risk patients. Arch Intern Med. 2003;163:553–64. doi: 10.1001/archinte.163.5.553. [DOI] [PubMed] [Google Scholar]
- 4.Qian YW, Schmidt RJ, Zhang Y, Chu S, Lin A, Wang H, et al. Secreted PCSK9 downregulates low density lipoprotein receptor through receptor-mediated endocytosis. J Lipid Res. 2007;48:1488–98. doi: 10.1194/jlr.M700071-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Kwon HJ, Lagace TA, McNutt MC, Horton JD, Deisenhofer J. Molecular basis for LDL receptor recognition by PCSK9. Proc Natl Acad Sci U S A. 2008;105:1820–5. doi: 10.1073/pnas.0712064105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 7.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–23. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballantyne CM, Neutel J, Cropp A, Duggan W, Wang EQ, Plowchalk D, et al. Results of bococizumab, a monoclonal antibody against proprotein convertase subtilisin/kexin type 9, from a randomized, placebo-controlled, dose-ranging study in statin-treated subjects with hypercholesterolemia. Am J Cardiol. 2015;115:1212–21. doi: 10.1016/j.amjcard.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–99. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 12.Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279:48865–75. doi: 10.1074/jbc.M409699200. [DOI] [PubMed] [Google Scholar]
- 13.Careskey HE, Davis RA, Alborn WE, Troutt JS, Cao G, Konrad RJ. Atorvastatin increases human serum levels of proprotein convertase subtilisin/kexin type 9. J Lipid Res. 2008;49:394–8. doi: 10.1194/jlr.M700437-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Awan Z, Seidah NG, MacFadyen JG, Benjannet S, Chasman DI, Ridker PM, et al. Rosuvastatin, proprotein convertase subtilisin/kexin type 9 concentrations, and LDL cholesterol response: the JUPITER trial. Clin Chem. 2012;58:183–9. doi: 10.1373/clinchem.2011.172932. [DOI] [PubMed] [Google Scholar]
- 15.Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, et al. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci U S A. 2003;100:12027–32. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci U S A. 2005;102:5374–9. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chackerian B, Durfee MR, Schiller JT. Virus-like display of a neo-self antigen reverses B cell anergy in a B cell receptor transgenic mouse model. J Immunol. 2008;180:5816–25. doi: 10.4049/jimmunol.180.9.5816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachmann MF, Whitehead P. Active immunotherapy for chronic diseases. Vaccine. 2013;31:1777–84. doi: 10.1016/j.vaccine.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Tissot AC, Maurer P, Nussberger J, Sabat R, Pfister T, Ignatenko S, et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet. 2008;371:821–7. doi: 10.1016/S0140-6736(08)60381-5. [DOI] [PubMed] [Google Scholar]
- 20.Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS ONE. 2011;6:e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chackerian B, Rangel M, Hunter Z, Peabody DS. Virus and virus-like particle-based immunogens for Alzheimer’s disease induce antibody responses against amyloid-beta without concomitant T cell responses. Vaccine. 2006;24:6321–31. doi: 10.1016/j.vaccine.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 22.Jegerlehner A, Tissot A, Lechner F, Sebbel P, Erdmann I, Kundig T, et al. A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses. Vaccine. 2002;20:3104–12. doi: 10.1016/s0264-410x(02)00266-9. [DOI] [PubMed] [Google Scholar]
- 23.Tumban E, Peabody J, Tyler M, Peabody DS, Chackerian B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS ONE. 2012;7:e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol. 2004;55:503–17. [PubMed] [Google Scholar]
- 25.Zhang L, McCabe T, Condra JH, Ni YG, Peterson LB, Wang W, et al. An anti-PCSK9 antibody reduces LDL-cholesterol on top of a statin and suppresses hepatocyte SREBP-regulated genes. Int J Biol Sci. 2012;8:310–27. doi: 10.7150/ijbs.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galabova G, Brunner S, Winsauer G, Juno C, Wanko B, Mairhofer A, et al. Peptide-based anti-PCSK9 vaccines - an approach for long-term LDLc management. PLoS One. 2014;9:e114469. doi: 10.1371/journal.pone.0114469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan JC, Piper DE, Cao Q, Liu D, King C, Wang W, et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci U S A. 2009;106:9820–5. doi: 10.1073/pnas.0903849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang H, Chaparro-Riggers J, Strop P, Geng T, Sutton JE, Tsai D, et al. Proprotein convertase substilisin/kexin type 9 antagonism reduces low-density lipoprotein cholesterol in statin-treated hypercholesterolemic nonhuman primates. J Pharmacol Exp Ther. 2012;340:228–36. doi: 10.1124/jpet.111.187419. [DOI] [PubMed] [Google Scholar]
- 29.Schiele F, Park J, Redemann N, Luippold G, Nar H. An antibody against the C-terminal domain of PCSK9 lowers LDL cholesterol levels in vivo. J Mol Biol. 2014;426:843–52. doi: 10.1016/j.jmb.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 30.Abifadel M, Varret M, Rabes J-P, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nature Genetics. 2003;34:154–6. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 31.Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, et al. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5374–9. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maxwell KN, Fisher EA, Breslow JL. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2069–74. doi: 10.1073/pnas.0409736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:7100–5. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shrank W, Lotvin A, Singh S, Brennan T. In The Debate About Cost And Efficacy, PCSK9 Inhibitors May Be The Biggest Challenge Yet. Health Affairs Blog. http://healthaffairs.org/blog/2015/02/17/in-the-debate-about-cost-and-efficacy-pcsk9-inhibitors-may-be-the-biggest-challenge-yet/2015.
- 35.Maningat P, Gordon BR, Breslow JL. How do we improve patient compliance and adherence to long-term statin therapy? Curr Atheroscler Rep. 2013;15:291. doi: 10.1007/s11883-012-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. 2011;305:1460–8. doi: 10.1001/jama.2011.406. [DOI] [PubMed] [Google Scholar]
- 37.Cunningham D, Danley DE, Geoghegan KF, Griffor MC, Hawkins JL, Subashi TA, et al. Structural and biophysical studies of PCSK9 and its mutants linked to familial hypercholesterolemia. Nat Struct Mol Biol. 2007;14:413–9. doi: 10.1038/nsmb1235. [DOI] [PubMed] [Google Scholar]
- 38.Piper DE, Jackson S, Liu Q, Romanow WG, Shetterly S, Thibault ST, et al. The Crystal Structure of PCSK9: A Regulator of Plasma LDL-Cholesterol. Structure. 2007;15:545–52. doi: 10.1016/j.str.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 39.Allard D, Amsellem S, Abifadel M, Trillard M, Devillers M, Luc G, et al. Novel mutations of the PCSK9 gene cause variable phenotype of autosomal dominant hypercholesterolemia. Human Mutation. 2005;26:497. doi: 10.1002/humu.9383. [DOI] [PubMed] [Google Scholar]
- 40.Cameron J, Holla ØL, Laerdahl JK, Kulseth MA, Ranheim T, Rognes T, et al. Characterization of novel mutations in the catalytic domain of the PCSK9 gene. Journal of Internal Medicine. 2008;263:420–31. doi: 10.1111/j.1365-2796.2007.01915.x. [DOI] [PubMed] [Google Scholar]
- 41.Fattori E, Cappelletti M, Lo Surdo P, Calzetta A, Bendtsen C, Ni YG, et al. Immunization against proprotein convertase subtilisin-like/kexin type 9 lowers plasma LDL-cholesterol levels in mice. J Lipid Res. 2012;53:1654–61. doi: 10.1194/jlr.M028340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 43.Chackerian B, Lowy DR, Schiller JT. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proc Natl Acad Sci USA. 1999;96:2373–8. doi: 10.1073/pnas.96.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ambuhl PM, Tissot AC, Fulurija A, Maurer P, Nussberger J, Sabat R, et al. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J Hypertens. 2007;25:63–72. doi: 10.1097/HJH.0b013e32800ff5d6. [DOI] [PubMed] [Google Scholar]
- 45.Fettelschoss A, Zabel F, Bachmann MF. Vaccination against Alzheimer disease: an update on future strategies. Hum Vaccin Immunother. 2014;10:847–51. doi: 10.4161/hv.28183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bachmann MF, Dyer MR. Therapeutic vaccination for chronic diseases: a new class of drugs in sight. Nat Rev Drug Discov. 2004;3:81–8. doi: 10.1038/nrd1284. [DOI] [PubMed] [Google Scholar]
- 47.Chackerian B, Briglio L, Albert PS, Lowy DR, Schiller JT. Induction of autoantibodies to CCR5 in macaques and subsequent effects upon challenge with an R5-tropic simian/human immunodeficiency virus. J Virol. 2004;78:4037–47. doi: 10.1128/JVI.78.8.4037-4047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Rompay KK, Hunter Z, Jayashankar K, Peabody J, Montefiori D, LaBranche CC, et al. A vaccine against CCR5 protects a subset of macaques upon intravaginal challenge with simian immunodeficiency virus SIVmac251. J Virol. 2014;88:2011–24. doi: 10.1128/JVI.02447-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


