Abstract
Our understanding of the specific genetic lesions in allergy has improved in recent years due to identification of common risk variants from genome-wide association studies (GWAS) and studies of rare, monogenic diseases. Large-scale GWAS have identified novel susceptibility loci and provided information about shared genetics between allergy, related phenotypes and autoimmunity. Studies of monogenic diseases have elucidated critical cellular pathways and protein functions responsible for allergy. These complementary approaches imply genetic mechanisms involved in Th2 immunity, T-cell differentiation, TGFβ signaling, regulatory T-cell function and skin/mucosal function as well as yet unknown mechanisms associated with newly identified genes. Future studies, in combination with data on gene expression and epigenetics, are expected to increase our understanding of the pathogenesis of allergy.
Introduction
Allergy and related diseases are highly heritable, and our understanding of the specific genetic background has improved in recent years due to identification of common risk variants from genome-wide association studies (GWAS) and increased understanding of rare, allergy-related monogenic diseases. Here, we review these two different approaches and their contribution to the field of allergy genetics.
Genome-wide association studies on allergy and allergic sensitization
The identification of susceptibility genes was accelerated by the introduction of microarrays that can determine hundreds of thousands of genetic variants, typically single nucleotide polymorphisms (SNPs), thereby assessing genetic variation across the entire genome. This allowed conduction of GWAS for identification of susceptibility loci without a priori hypotheses about disease mechanisms. The large number of variants tested requires a high level of statistical evidence for positive identification of a risk variant, and typically GWAS findings are based upon association p-values below 5 × 10 −8, so-called ‘genome-wide significance’, as well as replication of disease association in an independent population. For allergy-related diseases this has resulted in identification of relatively few, but robust, loci with more consistency between studies compared to previous candidate gene studies.
Related traits
Most allergy-related GWAS have investigated asthma with more than 35 studies registered to date [1]. A large, consortium-based study identified 6 genome-wide significant loci, all of which have been confirmed in independent studies [2]. The strongest associated asthma locus in this and other GWAS is located on chromosome 17q21. The disease-associated gene at this locus is still unclear with the earliest study pointing toward ORMDL3 [3] while a later study using expression data from lung tissue pointed toward GSDMA [4]. Proposed mechanisms of ORMDL3 include a role in sphingolipid synthesis [5•] and regulation of eosinophils [6•] Two loci spanning IL33 and IL1RL1 (encoding an IL-33 receptor) respectively, imply an important role of IL-33-related airway inflammation in the pathogenesis of asthma [7•,8]. Other loci identified included the HLA region, SMAD3 and IL2RB, and more have been proposed from other GWAS on asthma and other allergy-related diseases, such as eczema (atopic dermatitis) and eosinophilic esophagitis [1]. These highlight a number of potential pathogenetic mechanisms and pathways, mainly related to immune mechanisms and skin/mucosal barrier function (Figure 1).
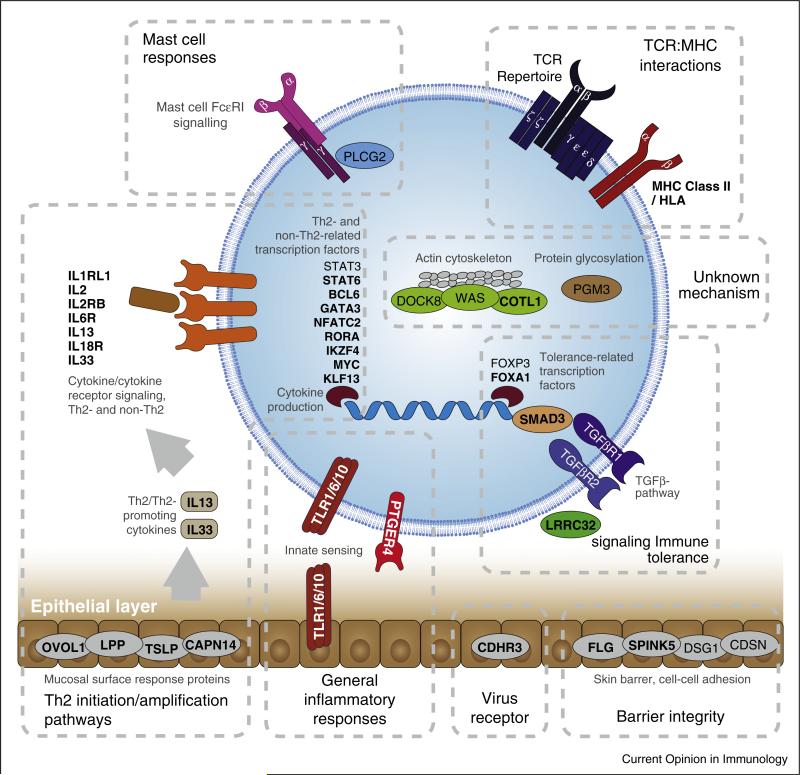
Figure 1.
Genes and pathways identified from GWAS and/or studies of monogenic allergy-related diseases. Genes identified from GWAS are in bold while genes identified from studies of monogenic diseases are in normal font. A generic cell as well as the skin/mucosal barrier are depicted in order to illustrate the cellular location of gene products. For loci with multiple associated genes where one or more genes have a demonstrated function relevant for atopic disease, only these well-established genes are shown for clarity. It should be noted that the genes and pathways named in this figure might not represent the true causal gene or pathway associated with a given locus since these have not been definitively established for many GWAS findings.
GWAS results have been aggregated from the most powerful GWAS (>3 genome-wide significant loci) on allergy/allergic sensitization, eczema, asthma, and eosinophilic esophagitis: Allergy/allergic sensitization: Ramasamy A, 2011, J Allergy Clin Immunol; Hinds DA, 2013, Nat Genet; Ferreira MA, 2013, J Allergy Clin Immunol; Bønnelykke K, 2013, Nat Genet. Eczema: Paternoster L, 2011, Nat Genet; Hirota T, 2012, Nat Genet; Weidinger S, 2013, Hum Mol Genet; Ellinghaus D, 2013, Nat Genet. Asthma: Moffatt MF, 2010, N Engl J Med; Hirota T, 2011, Nat Genet; Torgerson DG, 2011, Nat Genet; Wan YI, 2012, Thorax; Ferreira MA, 2013, J Allergy Clin Immunol. Eosinophilic esophagitis: Rothenberg ME, 2010, Nat Genet; Kottyan LC, 2014, Nat Genet; Sleiman PM, 2014, Nat Commun.
Allergy and allergic sensitization
Asthma and eczema are associated with allergy but still many patients have these diseases without concurrent allergy, the so-called ‘non-atopic’ phenotypes. Therefore susceptibility loci for these diseases are not necessarily associated with allergic mechanisms. Only a few GWAS have specifically addressed allergy or allergic sensitization. The first large-scale study on allergic sensitization, the hallmark of allergic disease, was performed in 2013 by meta-analysis of data from 16 different studies [9••]. Allergic sensitization was assessed objectively and defined by elevated levels of allergen-specific IgE and/or a positive skin prick test. This study identified 10 loci associated with allergic sensitization at the genome-wide significant level and with robust replication. Simultaneously, a large GWAS was performed on allergic symptoms identifying 16 genome-wide significant loci [10••]. This study was based upon self-reported symptoms and a combination of allergic symptoms from different organ systems, including rhinitis, asthma and skin reactions. In spite of these phenotype differences, there was high agreement of results between the two studies (Table 1). Previous GWAS findings on allergic rhinitis and sensitization were confirmed [11] and together these studies increased the number of loci associated with allergy or allergic sensitization to 18. One of the strongest associated loci in both GWAS was on chromosome 11q13. The underlying mechanism is unclear, but this locus was associated with expression of the two nearby genes [9••]: C11orf30, a potential regulator of interferon-stimulated genes and viral immunity [12], and LRRC32, involved in TGFb signaling in regulatory T-cells (Tregs) [13]. The associated loci imply the importance of Th2 promoting/Th2 dominated immune mechanisms (STAT6, TSLP, BCL6, IL1RL1, IL33, GATA3), innate immunity (TLR1/6/10), TGFβ-signaling (LRRC32, SMAD3), T-cell (IL2, PTGER4) and Treg (LRRC32, IL2, NFATC2, FOXA1) differentiation and function in the pathogenesis of allergy.
Table 1.
Genetic loci associated with allergy and/or allergic sensitization in recent GWAS
| Locus | Nearby genes* | Allergic sensitization(1)$ | Allergic symptoms(2)$ | Potential function | Other traits associated with locus§ | |||
|---|---|---|---|---|---|---|---|---|
| Asthma | Eczema | Total IgE | Autoimmune | |||||
| 2q12.1 | IL1RL1/IL18R1 | GWS | GWS | Interleukin receptors, IL33- signaling, Th2-response (IL1RL1); pleiotropic immune responses (IL18R1) |
● | ● | ● | |
| 2q33.1 | PLCL1 | NS | GWS | Phospholipase, intracellular signaling |
● | |||
| 3q28 | LPP/BCL6 | GWS | GWS | Transcription factor, Th2- differentiation (BCL6); cell-cell adhesion (LPP) |
● | |||
| 4p14 | TLR1/6/10 | GWS | GWS | Pattern recognition receptor, innate immunity |
● | |||
| 4q27 | IL2/ADAD1 | GWS | GWS | Interleukin, T-cell differentiation, Treg maturation |
● | |||
| 5p13.1 | PTGER4 | + | GWS | Prostaglandin receptor, T-cell signaling, skin immunity |
● | |||
| 5q22.1 | SLC25A46/TSLP | GWS | GWS | Cytokine, Th2 immune responses | ● | |||
| 6p21.32 | HLA-DQB1 | GWS | GWS | Antigen presenting protein, self tolerance |
● | ● | ● | |
| 6p21.33 | HLA-B/MICA | GWS | GWS | Antigen presenting protein, self tolerance |
● | |||
| 8q24.21 | MYC/PVT1 | GWS | ++ | Transcription factor, B-cell proliferation and differentiation |
● | |||
| 9p24.1 | RANBP6/IL33 | NS | GWS | Interleukin, Th2-signaling, Th2 cytokine production |
● | |||
| 10p14 | GATA3 | + | GWS | Transcription factor, Th2- differentiation. |
||||
| 11q13.5 |
C11orf30/ LRRC32 |
GWS | GWS | Treg expressed, TGFb signaling | ● | ● | ● | |
| 12q13.3 | STAT6 | GWS | ++ | Transcription factor, Th2- differentiation, IL4-response |
● | ● | ||
| 14q21.1 | FOXA1/TTC6 | NS | GWS | Transcription factor, Treg differentiation |
||||
| 15q22.33 | SMAD3 | NS | GWS | Transcriptional factor, TGFb signaling |
● | ● | ||
| 17q12 |
GSDMB/ GSDMA/ ORMDL3 |
NS | GWS | ER† Ca2+ homeostasis and unfolded protein response, sphingolipid metabolism, eosinophil trafficking (ORMDL3); Modulator of mitochondrial oxidative stress (GSDMA) |
● | ● | ||
| 20q13.2 | NFATC2 | NS | GWS | Transcription factor, activated T-cell gene transcription |
||||
Most plausible causal gene(s) in bold if more genes are listed.
GWS: genome-wide significant association (p<5e-8) for the top variant at the locus.
p<0.005 for the top variant in the companion paper
p<0.05, NS: not significantly associated (p>0.05).
Based on NCBI GWAS Catalog SNPs in LD > 0.5 with locus.
ER: Endoplasmic reticulum.
(1) : Bønnelykke et al. Nat Genet. 2013 Aug;45(8):902–6., (2): Hinds et al. Nat Genet. 2013 Aug;45(8):907–11.
Phenotype-specific and shared loci
GWAS studies on different phenotypes allow investigation of shared mechanisms. The relationship between asthma and allergy was investigated in the large GWAS on asthma from the GABRIEL consortium by comparing association results for asthma and total IgE levels [2]. This showed surprisingly little overlap of associated loci indicating that allergy has little relevance in the pathogenesis of asthma. However, the later GWAS on allergic sensitization reached a different conclusion [9••]. This study showed that 9 of the ten loci associated with allergic sensitization were also associated with asthma in the GABRIEL study, in agreement with a potential causal role of allergy in asthma pathogenesis. These results also indicate different genetic backgrounds of total IgE and allergen-specific IgE levels, which is supported by the observation that loci such as FCER1A and HLA-A have been strongly and consistently associated with total IgE levels in GWAS but were weakly associated with allergic sensitization [9••].
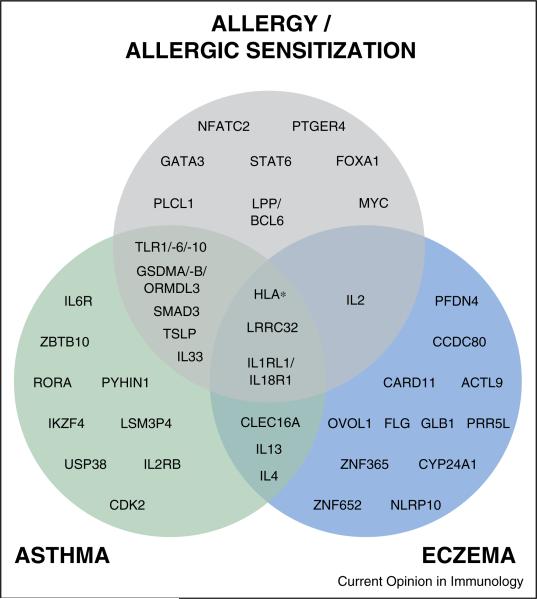
Some susceptibility loci might be phenotype-specific. Age at onset seems to be an important genetic characteristic as demonstrated in the GABRIEL asthma GWAS where most loci were more strongly associated with the childhood onset phenotype [2]. Particularly, the 17q21 locus seems strongly associated with an asthma phenotype characterized by onset in early childhood [14] and recurrent, severe exacerbations [15••] and was more strongly associated with asthma than allergic rhinitis [10••]. Other loci have been associated with multiple allergy-related phenotypes, for example the chromosome 11q13 locus associated with allergic sensitization [9••], allergic symptoms [10••], eczema [16] and asthma [17], suggesting involvement in a central allergy mechanism. The overlap of susceptibility loci for allergy/allergic sensitization, asthma and eczema based upon findings from the largest GWAS are illustrated in Figure 2.
Figure 2.
Overlap of susceptibility genes associated with allergy/allergic sensitization, asthma and/or eczema in GWAS.
GWAS results have been aggregated from the most powerful GWAS (>3 genome-wide significant loci) on allergy/allergic sensitization, eczema, and asthma: Allergy/allergic sensitization: Ramasamy A, 2011, J Allergy Clin Immunol; Hinds DA, 2013, Nat Genet; Ferreira MA, 2013, J Allergy Clin Immunol; Bønnelykke K, 2013, Nat Genet. Eczema: Paternoster L, 2011, Nat Genet; Hirota T, 2012, Nat Genet; Weidinger S, 2013, Hum Mol Genet; Ellinghaus D, 2013, Nat Genet. Asthma: Moffatt MF, 2010, N Engl J Med; Hirota T, 2011, Nat Genet; Torgerson DG, 2011, Nat Genet; Wan YI, 2012, Thorax; Ferreira MA, 2013, J Allergy Clin Immunol. For loci with multiple associated genes where one or more genes have a demonstrated a function relevant for atopic disease, only these genes with relevant function are shown for clarity.
Overlapping susceptibility loci from GWAS should be interpreted with caution, due to potential issues such as diagnostic bias and limited statistical power, but might help understanding the pathogenetic relationship between allergy-related phenotypes and the mechanisms associated with individual loci.
Allergy and autoimmunity
Allergy and autoimmune diseases are classically considered representatives of Th2 and Th1/Th17 driven immune responses, respectively, with counteracting immune mechanisms. Overlapping mechanisms are suggested by some studies of comorbidity suggesting inverse as well as direct relationships [18] and by the parallel epidemic observed for allergy and autoimmune diseases in the last decades [19]. The two recent GWAS on allergy and allergic sensitization demonstrated a large overlap between susceptibility loci for allergy and autoimmune diseases with 12 of the 18 genome-wide significant loci for allergy also encompassing variants associated with autoimmune diseases (Table 1) [9••,10••]. Shared genetics are supported by a recent study showing that ~90% of variants associated to autoimmune diseases are non-coding, mapping to regulatory regions specifically active in immune cells, and that these clustered closely together with asthma and allergy [20••]. Two examples of shared loci are the 11q13 locus, which showed strong association with several allergy-related diseases and was also among the strongest loci for inflammatory bowel disease [21], and the 17q21 asthma locus also associated with several autoimmune diseases, including type 1 diabetes and inflammatory bowel disease. Interestingly, the direction of effect was the same for allergy and autoimmune disease at the 11q13 locus [9••,10••] but opposite at the 17q21 locus [10••]. Further understanding of these shared susceptibility loci may help elucidate the complex relationship and pathogenesis of allergy and autoimmune diseases.
Clinical and research implications
The susceptibility variants identified in GWAS are mainly common variants with relatively small effect sizes (often with odds ratios around 1.1 per risk allele). Such variants have no clinical relevance in terms of predictive capacity, even in combination, as shown for both asthma [2] and allergic sensitization [9••]. On the other hand, the estimated population attributable risk fraction of hay fever for the 10 strongest sensitization loci was estimated to be more than 25% [9••] suggesting that targeting the mechanisms associated with these 10 loci would have a significant impact on disease burden on the population level.
It can be argued that, until now, GWAS findings have contributed little to the basic understanding of disease mechanisms. One limitation is that they often merely identify a susceptibility locus without any clear relationship to a specific gene or functional mechanism. Examples of this are the 17q21 and 11q13 loci, where the associated mechanisms are still poorly understood several years after their discovery, even though these loci are strong and probably central to the pathogenesis of asthma and allergy. One example of a GWAS finding where the functional mechanism might have been identified is CDHR3, a susceptibility locus for childhood asthma with severe exacerbations [15••]. A recent follow-up study suggests that this gene is a rhinovirus C receptor, potentially explaining the underlying mechanism and identifying a target for future asthma and virology research [22•].
Currently, GWAS on allergy and related traits have identified novel and robust susceptibility loci, which need to be followed up in further functional studies. These are likely to increase our understanding of disease mechanisms and may ultimately help identify novel targets for treatment and prevention of disease.
Future GWAS
Larger, consortium-based, studies on allergy-related phenotypes are currently being conducted and are expected to identify many novel susceptibility loci. The resultant more complete picture of the genetic background will increase the possibilities for pathway-based analysis of functionally related loci and also increase the knowledge on shared genetic mechanisms between phenotypes.
An alternative approach to increasing sample size is to perform GWAS on more specific phenotypes. Such phenotypes are likely more closely associated with specific mechanisms and the genetic substrate and might increase study power. This was demonstrated by a GWAS on early childhood asthma with severe exacerbations resulting in similar power for gene identification as previous much larger asthma GWAS, and showing very high effect sizes in the children with the highest number of exacerbations [15••].
The majority of GWAS, including those discussed above, have only included individuals of European descent. Ethnic differences have been demonstrated in allergy-related GWAS [23] and will be the focus of future studies including larger non-European populations. Also, future larger GWAS will have increased power to target gene by environment interactions and directly clinically relevant phenotypes, such as response to medication [24•].
Markers assayed by common GWAS array platforms do not typically include rare variants. For this purpose, approaches using custom arrays and sequencing-based efforts are being used and might uncover some of the genetic variation of allergy and related traits not detected in GWAS. However, a recent study indicated that for asthma, low-frequency variants are not likely to explain the ‘missing heritability’ [25••].
The advent of the era of inexpensive genome-wide nucleotide sequencing applied to gene expression-profiling and epigenome-profiling has brought new possibilities of marrying GWAS data with data from large public ‘omics repositories, inferring additional layers of functionality, regulation and tissue context from associated SNPs. These data will increase the usefulness of GWAS data by providing a shorter path to understanding functional effects of susceptibility loci [26•]. Often, these approaches consider the full range of SNPs, and not just single, genome-wide significant hits. For complex traits, like allergy, such approaches may better estimate the summarized variant burden on cellular regulatory and transcriptional networks, and produce additional mechanistic insight [20••,27]. Future GWAS on allergy (whether chip or sequence-based) will be a part of integrated approaches to discover how other molecular layers, including epigenetics and the immune–microbiome interface, modulate the genetic effect on disease, and will thereby be a central component in the attempt to tailor and improve medical treatment [28].
Monogenic diseases
The molecular complexity of allergic disease is nowhere more apparent than in the diverse group of monogenic diseases that present with a spectrum of atopic phenotypes such as asthma, food allergy, and eczema. Discovery of the underlying genetic defect often leads to better characterization of cellular mechanisms. Remarkably, aberrations in seemingly unrelated pathways can result in similar clinical pictures (Table 2).
Table 2.
Allergy-related monogenic diseases
| Disease | Gene Protein product Type of mutation Inheritance |
Abnormality | Common clinical features | Common laboratory features |
|---|---|---|---|---|
| PLAID PLCΥ2-associated antibody deficiency and immune dysregulation [39,40] |
Gene: PLCG2 Protein: PLCΥ2 (phospholipase CΥ2) Mutation: gain-of-function Inheritance: autosomal dominant |
Decreased receptor-mediated activity in B-cells and NK cells at physiologic temperatures. Spontaneous activation in mast cells, B-cells, neutrophils, monocytes at subphysiologic temperatures. |
Cold urticaria (positive by evaporative cooling test) Recurrent sinopulmonary infections Autoimmune disease Graulomatous skin lesions Allergen-specific atopy |
Elevated IgE Low IgA, IgM Decreased B-cells, including class-switched memory B-cells Decreased NK cells |
| WAS Wiskott-Aldrich syndrome [55,56,74] |
Gene: WAS Protein: WASP (WAS protein) Mutation: loss-of-function (WASP typically truncated or absent) Inheritance: X-linked |
Cytoskeletal abnormalities that affect numerous cell types, including T-cells, B-cells, Treg cells | Moderate to severe eczema Easy bruising and bleeding Recurrent infections Autoimmunity (most commonly autoimmune cytopenias, vasculitis, arthritis) Lymphoma, often EBV-related |
Thrombocytopenia with small platelets Abnormal T-cell and B-cell function with poor response to polysaccharide antigens Diminished T-cell responses to mitogens and anti-CD3 T-cell lymphopenia and oligoclonality that worsen with age Low IgM, elevated IgA & IgE Defective Treg function |
| Autosomal dominant hyper-IgE syndrome [43,75] | Gene: STAT3 Protein: STAT3 (signal transducer and activator of transcription 3) Mutation: dominant negative Inheritance: autosomal dominant |
Aberrant STAT3-dependent signaling | Staphylococcal infections (skin and lung); skin abscesses often “cold” without associated warmth, redness, pain Chronic eczema Musculoskeletal abnormalities (hyperextensibility, osteoporosis, scoliosis) Bacterial pneumonia with resulting pneumatoceles and fungal infection Craniofacial abnormalities (retained primary teeth, craniosynostosis, high-arched palate) Chronic mucocutaneous candidiasis Characteristic facial features (coarse skin, prominent forehead and chin, deep-set eyes, broad nasal bridge, bulbous nose) |
Elevated IgE Eosinophilia Decreased Th17 cells Decreased central memory T-cells Decreased memory B-cells |
| PGM3 deficiency [48••,49••,50••] | Gene: PGM3 Protein: PGM3 (phosphoglucomutase 3) Mutation: hypomorphic protein Inheritance: autosomal recessive |
Aberrant glycosylation | Severe atopic dermatitis Allergen-specific atopy Recurrent viral and bacterial infections Motor and neurocognitive impairment |
Elevated IgE Eosinophilia CD4 lymphopenia |
| DOCK8 deficiency [52•,73] | Gene: DOCK8 Protein: DOCK8 (dedicator of cytokinesis 8) Mutation: loss-of-function Inheritance: autosomal recessive |
Cytoskeletal and immune synapse abnormalities | Viral skin infections with associated malignancies Recurrent bacterial skin and lung infections Atopy (severe atopic dermatitis starting early in life, asthma, food allergy) Mucocutaneous candidiasis Autoimmunity |
T-cell lymphopenia Eosinophilia Elevated IgE Low IgM that declines with age Decreased Treg cell number and function |
| IPEX syndrome Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome [69,70] |
Gene: FOXP3 Protein: FOXP3 (forkhead box protein 3) Mutation: loss-of-function Inheritance: X-linked |
Aberrant development or function of Treg cells | Autoimmune enteropathy (severe, early-onset diarrhea) Endocrinopathy (most commonly type I diabetes, thyroiditis) Dermatitis (eczematiform, ichthyosiform, or psoriasiform) Other autoimmune phenomena (autoimmune cytopenias, autoimmune renal and liver disease) Food allergy |
Elevated IgE Eosinophilia |
| Omenn syndrome [31,32,37,76] | Most common genetic cause: hypomorphic mutations in RAG1, RAG2. Proteins: RAG1, RAG2 (recombination activating gene-1, recombination activating gene-2) Other causative genes include DCLRE1C, ADA, RMRP. |
Impaired V(D)J recombination | Generalized erythroderma Recurrent infections Chronic diarrhea Autoimmunity (especially of skin and gut) Lymphadenopathy Hepatosplenomegaly Thymic dysplasia |
Elevated IgE Eosinophilia Oligoclonal T-cells Decreased T-cell proliferation to antigen and mitogen B-cells low or absent in some forms Defective Treg function |
| Atypical complete DiGeorge syndrome [29,30] | Most commonly due to hemizygous deletion of 22q11.2 | Oligoclonal T-cells | Abnormal facies Congenital heart disease Athymia Eczematous dermatitis with infiltrating T-cells Lymphadenopathy Hypoparathyroidism |
Oligoclonal T-cells with activated phenotype Elevated IgE Eosinophilia Hypocalcemia |
| ADA-SCID Adenosine deaminase deficient severe combined immunodeficiency [33,34] |
Gene: ADA Protein: ADA (adenosine deaminase) Mutation: loss-of-function Inheritance: autosomal recessive |
Defect in purine metabolism | Early-onset & delayed-onset, pre-treatment: Recurrent infections Early-onset, post-treatment: Atopic dermatitis Allergen-specific atopy |
Early-onset, pre-treatment: B-cell, T-cell, NK cell lymphopenia Delayed-onset, pre-treatment & early-onset, post-treatment: Elevated IgE |
| Loeys-Dietz syndrome [41••,42] | Genes: TGFBR1, TGFBR2 Proteins: TGFBR1, TGFBR2 (transforming growth factor β receptor I, transforming growth factor β receptor II) Mutation: gain-of-function Inheritance: autosomal dominant Mutations in SMAD3 are responsible for a minority of cases |
Increased TGFβ signaling skews lymphocytes to Th2 phenotype | Cardiovascular abnormalities Marfanoid habitus with skeletal and craniofacial abnormalities Atopic dermatitis Allergen-specific atopy Eosinophilic gastrointestinal disease |
Elevated IgE Eosinophilia |
| Peeling skin disease (also called peeling skin syndrome type B) [61,65] | Gene: CDSN Protein CDSN (corneodesmosin) Mutation: loss-of-function Inheritance: autosomal recessive |
Abnormal corneodesmosomes | Ichthyosiform erythroderma with peeling skin Severe pruritus Allergen-specific atopy Recurrent skin infections |
Elevated IgE |
| SAM syndrome Severe dermatitis, multiple allergies, metabolic wasting syndrome [59,60] |
Gene: DSG1 Protein: DSG1 (desmoglein-1) Mutation: loss-of-function Inheritance: autosomal recessive Mutation in desmoplakin gene (DSP) reported in one patient [77] |
Abnormal desmosomes in upper epidermis; acantholysis | Severe dermatitis Hypotrichosis Malabsorption Food allergy Recurrent skin and pulmonary infections Metabolic wasting |
Elevated IgE Hypoalbuminemia |
| Netherton syndrome [62,63] | Gene: SPINK5 Protein: LEKTI (lymphoepithelial Kazal-type related inhibitor type 5) Mutation: loss-of-function Inheritance: autosomal recessive |
Defective serine protease inhibitor | Skin manifestations can include congenital ichthyosiform erythroderma and ichthyosis linearis circumflexa Trichorrhexis invaginata (“bamboo hair”) Allergen-specific atopy Urticaria, angioedema Recurrent infections |
Elevated IgE Eosinophilia |
Disorders of lymphocyte repertoire
The emergence of oligoclonal T-cell populations in a background of nearly complete immunodeficiency underlies much of the pathology seen in both atypical complete DiGeorge syndrome and Omenn syndrome. Atypical complete DiGeorge syndrome (which occurs in <1% of those with DiGeorge syndrome) and Omenn syndrome are both characterized by lymphadenopathy, elevated IgE and a severe eczematous skin eruption [29–31]. These patients have very few recent thymic emigrants, consistent with the diagnosis of SCID or athymia; however, they develop an oligoclonal T-cell population with an activated phenotype that infiltrates the skin [29,31]. Omenn syndrome is frequently due to hypomorphic mutations in SCID-causing genes that result in a combination of immunodeficiency and autoimmunity [31]. Patients typically present early in life with infections, diarrhea, and erythroderma. There is decreased or absent humoral immunity with elevated IgE and activated, oligoclonal T-cell populations in the skin and gut [31,32]. Increased IgE in the absence of a severe Omenn-like phenotype is also seen in patients with SCID due to absent adenosine deaminase (ADA), a key enzyme in purine metabolism. Interestingly, patients with both delayed-onset ADA-SCID and treated early-onset disease often have elevated serum IgE, and the latter have increased risk of atopy [33,34]. Whether elevated IgE in ADA-SCID and Omenn syndrome are due to a shared pathway of immune dysregulation is not clear, however disruption of lymphocyte repertoires and cellular tolerance mechanisms are characteristic of both [35–38].
Disorders of antigen or cytokine receptor signaling
Aberrant antigen or cytokine signaling in leukocytes can disrupt homeostasis in a variety of ways leading to allergic phenotypes. Gain-of-function mutations in PLCG2 in PLAID causes paradoxically decreased receptor-mediated intracellular signaling in NK cells and B-cells at physiologic temperatures, leading to autoimmunity and humoral immune deficiency.[39] However, at subphysiologic temperatures, B-cells, neutrophils, monocytes and mast cells have increased cellular activity, even in the absence of receptor crosslinking, leading to granulomatous skin lesions [40], and a form of cold urticaria [39,40]. A tendency toward a Th2 phenotype is also seen in patients with Loeys–Dietz syndrome (LDS), a connective tissue disorder commonly caused by mutations in TGFBR1 or TGFBR2 that result in upregulation of TGFβ signaling [41••,42]; this increased signaling alone appears sufficient to drive naïve T-cells toward a Th2 phenotype [41••]. Interestingly, Tregs in LDS patients have been shown to produce IL-13, which may also contribute to the increased risk of atopic disease [41••]. Signaling abnormalities due to dominant negative mutations in the STAT3 gene lead to autosomal dominant hyper-IgE syndrome (AD-HIES; Job's syndrome) [43]. The classic presentation is recurrent bacterial lung infections, early-onset eczema, skeletal and connective tissue abnormalities, and significantly elevated serum IgE [43,44]. Despite elevated allergen-specific IgE, patients with AD-HIES are at decreased risk of associated food allergies or food-related anaphylaxis compared to atopic individuals without STAT3 mutations [45,46•]. The latteris likely due to altered STAT3-mediated signaling in IgE-dependent mast cell degranulation, which has recently been proposed to occur through a mitochondria-dependent mechanism [46•,47].
Disorders of glycosylation
Several families with hypomorphic mutations in PGM3 have been described [48••,49••,50••]. Common clinical features include atopic dermatitis, recurrent infections, and neurologic abnormalities possibly related to abnormal myelination. The enzyme phosphoglucomutase 3 is a member of the hexose phosphate mutase family and is necessary for production of a key substrate in several glycosylation pathways [48••]. Likely related to the high frequency of atopy in these patients, in vitro stimulation of CD4+ T-cells results in robust production of Th2 cytokines [48••]. The underlying mechanism is not known, but a myriad of immune-related molecules are dependent upon normal glycosylation for function and tolerance [51].
Disorders of cytoskeletal function
The critical role of cytoskeletal rearrangement in lymphocyte function is demonstrated by mutations in DOCK8 and WAS. DOCK8 is part of a family of atypical guanine exchange factors necessary for proper cytoskeletal function. Lack of functional DOCK8 protein results in a hyper-IgE syndrome with early-onset atopic dermatitis, cutaneous viral infections, recurrent bacterial infections, eosinophilia and neoplastic disease [52•,53]. Death from complications typically occurs by early adulthood unless treated with bone marrow transplantation [52•]. WAS protein (WASP) acts in a pathway similar to that of DOCK8; accordingly the two conditions share many clinical characteristics, to include atopic dermatitis with elevated IgE, autoimmunity, and lymphoma risk, while WAS also results in thrombocytopenia [52•,54–56]. Consistent with a shared role in regulating the actin cytoskeleton, DOCK8 or WASP deficiency affects a broad range of cell types, to include T-cells, B-cells, NK cells, and Tregs [55,57]. Although the mechanism by which DOCK8 deficiency results in atopy is not yet clear, it has been demonstrated that WASP can function in the nucleus to participate in transcription of master Th1 transcription factor T-bet [58] which might normally oppose Th2 phenotypes.
Loss of skin barrier
Proper epidermal function is critical for defense against pathogens, maintenance of homeostasis, and as a barrier against foreign substances. Desmosomes are specialized, multi-protein structures that connect keratinocytes; desmogleins are one group of desmosome proteins critical for intercellular adhesion [61]. Desmosomes transform to corneodesmosomes as keratinocytes cornify in the upper stratum granulosum. Both desmoglein-1 (DSG1) and corneodesmosin are two component corneodesmosome proteins. Loss-of-function mutations in the gene encoding DSG1 (DSG1) have been described in two families with SAM syndrome, which presents as a severe, congenital dermatitis with food allergies and recurrent infections [59••]; a third family was recently described with a milder phenotype [60]. Degradation of corneodesmosomes in the stratum corneum causes keratinocyte desquamation, a process facilitated by proteases (kallikreins and cathepsins) in balance with inhibitor proteins, including LEKTI [61]. Netherton syndrome (NS) is an autosomal recessive disease caused by loss-of-function mutations in the gene for LEKTI, SPINK5 [62]. The classic triad of NS is ichthyosis, trichorrhexis invaginata (‘bamboo hair’), and atopy [62,63]. The SPINK5 E420K variant has been implicated in atopic dermatitis in patients without NS [64]. Peeling skin disease is phenotypically similar to NS, with peeling skin, atopy, and recurrent cutaneous infections [61,65]; it is caused by loss-of-function mutations in the corneodesmosin gene, CDSN [61,65]. The fact that multiple different skin barrier protein disruptions lead to atopic inflammation suggests a common pathway by which a Th2 milieu develops after loss of skin integrity [66]. In addition to the structural barrier molecule defects, it is possible that primary genetic lesions causing increased local Th2 cytokine production may impair anti-microbial peptide upregulation [67]. Similarly, in the case of AD-HIES, a failure of STAT3-mediated AMP-upregulation could contribute to the failure to maintain the type of normal skin flora necessary for protection against dermatitis [68].
Impaired Treg function
Treg cells (CD4+CD25+FOXP3+) play a critical role in the maintenance of immune tolerance to self-antigen. Failure to maintain adequate numbers of functional Treg cells results in autoimmunity and inflammation, but quite strikingly and reproducibly, atopic inflammation is a hallmark of the loss of Tregs. The most classic example of this is IPEX syndrome caused by mutations in FOXP3 [69]. These patients have early-onset severe diarrhea, endocrinopathy, and dermatitis. Immune dysregulation with generation of reactive T-cells typically progresses with development of subsequent autoimmune phenomena and food allergy. Other genetic mutations that affect Treg cells and result in an IPEX-like phenotype include loss-of-function (CD25, STAT5b, ITCH, LRBA) and gain-of-function (STAT1 and STAT3) mutations [70–72]. The autoimmune phenomena seen in patients with Omenn syndrome, WAS, and DOCK8 deficiency have also been linked to abnormal Treg function [37,55,73].
Conclusion
GWAS and studies of monogenic diseases have identified a number of susceptibility loci and genes associated with allergy, and more are expected to be identified in the future. These complementary approaches imply genetic mechanisms involved in Th2 immunity, T-cell differentiation, TGFβ signaling, Treg function and skin/mucosal function (Figure 1), and mechanisms shared between allergy and autoimmune diseases. Further studies of the underlying mechanisms, including other omics-based approaches, will increase our understanding of the pathogenesis of allergy, and the hope is that this will provide the basis for improved treatment and prevention of disease.
Acknowledgements
We gratefully express our gratitude to the children and families of the COPSAC studies for all their support and commitment. We acknowledge and appreciate the unique efforts of the Copenhagen Prospective Study on Asthma in Childhood (COPSAC) research team.
COPSAC is funded by private and public research funds all listed on www.copsac.com. The Lundbeck Foundation; The Danish Ministry of Health; Danish Council for Strategic Research; The Danish Council for Independent Research and The Capital Region Research Foundation have provided core support for COPSAC.
This research was further supported in part by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
The funding agencies had no role in the study design; the conduct of the study; data collection and management; data analysis; or the preparation, review, or approval of the manuscript.
Footnotes
Conflicts of interest
The authors report no conflict of interests of relevance to this paper.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Welter D, MacArthur J, Morales J, Burdett T, Hall P, Junkins H, et al. The NHGRI GWAS Catalog, a curated resource of SNP-trait associations. Nucleic Acids Res. 2014;42(Database issue):D1001–D1006. doi: 10.1093/nar/gkt1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448:470–473. doi: 10.1038/nature06014. [DOI] [PubMed] [Google Scholar]
- 4.Hao K, Bossé Y, Nickle DC, Paré PD, Postma DS, Laviolette M, et al. Lung eQTLs to help reveal the molecular underpinnings of asthma. PLoS Genet. 2012;8:e1003029. doi: 10.1371/journal.pgen.1003029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Worgall TS, Veerappan A, Sung B, Kim BI, Weiner E, Bholah R, et al. Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci Transl Med. 2013;5:186ra67. doi: 10.1126/scitranslmed.3005765. [This report suggests ORMDL3-mediated impaired sphingolipid synthesis as a potential mechanism linking the strong chromosome 17q21 locus to bronchial reactivity and asthma.] [DOI] [PubMed] [Google Scholar]
- 6•.Ha SG, Ge XN, Bahaie NS, Kang BN, Rao A, Rao SP, et al. ORMDL3 promotes eosinophil trafficking and activation via regulation of integrins and CD48. Nat Commun. 2013;4:2479. doi: 10.1038/ncomms3479. [This report suggests eosinophil regulation as a potential mechanism underlying the strong asthma locus at chromosome 17q21.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7•.Traister RS, Uvalle CE, Hawkins GA, Meyers DA, Bleecker ER, Wenzel SE. Phenotypic and genotypic association of epithelial IL1RL1 to human TH2-like asthma. J Allergy Clin Immunol. 2015;135:92–99. doi: 10.1016/j.jaci.2014.06.023. [This report investigates the role of the IL-33 receptor ILRL1 in asthmatic airway inflammation suggesting a mechanism underlying the strong asthma locus near IL1RL1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho JE, Chen W-Y, Chen M-H, Larson MG, McCabe EL, Cheng S, et al. Common genetic variation at the IL1RL1 locus regulates IL-33/ST2 signaling. J Clin Invest. 2013;123:4208–4218. doi: 10.1172/JCI67119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Bønnelykke K, Matheson MC, Pers TH, Granell R, Strachan DP, Alves AC, et al. Meta-analysis of genome-wide association studies identifies ten loci influencing allergic sensitization. Nat Genet. 2013;45:902–906. doi: 10.1038/ng.2694. [This report describes the first large-scale GWAS on allergic sensitization identifying robust susceptibility loci and investigating the association to other allergy-related phenotypes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Hinds DA, McMahon G, Kiefer AK, Do CB, Eriksson N, Evans DM, et al. A genome-wide association meta-analysis of self-reported allergy identifies shared and allergy-specific susceptibility loci. Nat Genet. 2013;45:907–911. doi: 10.1038/ng.2686. [This report describes the first large-scale GWAS on allergic symptoms identifying many novel susceptibility loci in high agreement with the above-mentioned paper on allergic sensitization.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramasamy A, Curjuric I, Coin LJ, Kumar A, McArdle WL, Imboden M, et al. A genome-wide meta-analysis of genetic variants associated with allergic rhinitis and grass sensitization and their interaction with birth order. J Allergy Clin Immunol. 2011;128:996–1005. doi: 10.1016/j.jaci.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Ezell SA, Polytarchou C, Hatziapostolou M, Guo A, Sanidas I, Bihani T, et al. The protein kinase Akt1 regulates the interferon response through phosphorylation of the transcriptional repressor EMSY. Proc Natl Acad Sci U S A. 2012;109:E613–E621. doi: 10.1073/pnas.1115029109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stockis J, Colau D, Coulie PG, Lucas S. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur J Immunol. 2009;39:3315–3322. doi: 10.1002/eji.200939684. [DOI] [PubMed] [Google Scholar]
- 14.BouzigninStockis J, Colau D, Coulie PG, Lucas S. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur J Immunol. 2009;39:3315–3322. doi: 10.1002/eji.200939684. [DOI] [PubMed] [Google Scholar]
- 15••.Bønnelykke K, Sleiman P, Nielsen K, Kreiner-Møller E, Mercader JM, Belgrave D, et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat Genet. 2014;46:51–55. doi: 10.1038/ng.2830. [This report presents a GWAS on a specific asthma phenotype with onset in early childhood and recurrent severe exacerbations showing strong association results demonstrating the strength of phenotype specificity in asthma genetics.] [DOI] [PubMed] [Google Scholar]
- 16.Esparza-Gordillo J, Weidinger S, Fölster-Holst R, Bauerfeind A, Ruschendorf F, Patone G, et al. A common variant on chromosome 11q13 is associated with atopic dermatitis. Nat Genet. 2009;41:596–601. doi: 10.1038/ng.347. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira MAR, Matheson MC, Duffy DL, Marks GB, Hui J, Le Souëf P, et al. Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet. 2011;378:1006–1014. doi: 10.1016/S0140-6736(11)60874-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabin RL, Levinson AI. The nexus between atopic disease and autoimmunity: a review of the epidemiological and mechanistic literature. Clin Exp Immunol. 2008;153:19–30. doi: 10.1111/j.1365-2249.2008.03679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bach J-F. The effect ofinfections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347:911–920. doi: 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 20••.Farh KK-H, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518:337–343. doi: 10.1038/nature13835. [This report aims to identify causal variants underlying GWAS findings for autoimmune diseases and investigate their association to regulatory elements and immune cells, including a cluster analysis showing strong clustering with allergy and asthma.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Bochkov YA, Watters K, Ashraf S, Griggs TF, Devries MK, Jackson DJ, et al. Cadherin-related family member 3, a childhood asthma susceptibility gene product, mediates rhinovirus C binding and replication. Proc Natl Acad Sci U S A. 2015;112:5485–5490. doi: 10.1073/pnas.1421178112. [This report suggests that CDHR3, encoded by a susceptibility locus for early childhood asthma, is a rhinovirus C receptor potentially explaining the mechanism underlying this locus.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Israel E, Lasky-Su J, Markezich A, Damask A, Szefler SJ, Schuemann B, et al. Genome-wide association study of short-acting β2-agonists. A novel genome-wide significant locus on chromosome 2 near ASB3. Am J Respir Crit Care Med. 2015;191:530–537. doi: 10.1164/rccm.201408-1426OC. [This report describes a GWAS on response to acute asthma medication identifying a novel susceptibility locus.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Igartua C, Myers RA, Mathias RA, Pino-Yanes M, Eng C, Graves PE, et al. Ethnic-specific associations of rare and low-frequency DNA sequence variants with asthma. Nat Commun. 2015;6:5965. doi: 10.1038/ncomms6965. [This study investigates the role of rare genetic variants for asthma suggesting that these are ethnicity-specific and not likely to explain a significant proportion of the ‘missing heritability’ of asthma.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26•.Albert FW, Kruglyak L. The role of regulatory variation in complex traits and disease. Nat Rev Genet. 2015;16:197–212. doi: 10.1038/nrg3891. [This review describes current possibilities of using gene expression and other data to provide insight into causal links between gene variants, physiological changes and disease.] [DOI] [PubMed] [Google Scholar]
- 27.Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, et al. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashley EA. The precision medicine initiative: a new national effort. JAMA. 2015 Apr; doi: 10.1001/jama.2015.3595. [DOI] [PubMed] [Google Scholar]
- 29.Vu QV, Wada T, Toma T, Tajima H, Maeda M, Tanaka R, et al. Clinical and immunophenotypic features of atypical complete DiGeorge syndrome. Pediatr Int. 2013;55:2–6. doi: 10.1111/j.1442-200X.2012.03722.x. [DOI] [PubMed] [Google Scholar]
- 30.Selim MA, Markert ML, Burchette JL, Herman CM, Turner JW. The cutaneous manifestations of atypical complete DiGeorge syndrome: a histopathologic and immunohistochemical study. J Cutan Pathol. 2008;35:380–385. doi: 10.1111/j.1600-0560.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- 31.Villa A, Notarangelo LD, Roifman CM. Omenn syndrome: inflammation in leaky severe combined immunodeficiency. J Allergy Clin Immunol. 2008;122:1082–1086. doi: 10.1016/j.jaci.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 32.Marrella V, Poliani PL, Sobacchi C, Grassi F, Villa A. Of Omenn and mice. Trends Immunol. 2008;29:133–140. doi: 10.1016/j.it.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Felgentreff K, Perez-Becker R, Speckmann C, Schwarz K, Kalwak K, Markelj G, et al. Clinical and immunological manifestations of patients with atypical severe combined immunodeficiency. Clin Immunol (Orlando, FL) 2011;141:73–82. doi: 10.1016/j.clim.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence MG, Barber JS, Sokolic RA, Garabedian EK, Desai AN, O'Brien M, et al. Elevated IgE and atopy in patients treated for early-onset ADA-SCID. J Allergy Clin Immunol. 2013;132:1444–1446. doi: 10.1016/j.jaci.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu X, Almeida JR, Darko S, van der Burg M, DeRavin SS, Malech H, et al. Human syndromes of immunodeficiency and dysregulation are characterized by distinct defects in T-cell receptor repertoire development. J Allergy Clin Immunol. 2014;133:1109–1115. doi: 10.1016/j.jaci.2013.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sauer AV, Brigida I, Carriglio N, Hernandez RJ, Scaramuzza S, Clavenna D, et al. Alterations in the adenosine metabolism and CD39/CD73 adenosinergic machinery cause loss of Treg cell function and autoimmunity in ADA-deficient SCID. Blood. 2012;119:1428–1439. doi: 10.1182/blood-2011-07-366781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cassani B, Poliani PL, Moratto D, Sobacchi C, Marrella V, Imperatori L, et al. Defect of regulatory T cells in patients with Omenn syndrome. J Allergy Clin Immunol. 2010;125:209–216. doi: 10.1016/j.jaci.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Sauer AV, Brigida I, Carriglio N, Aiuti A. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front Immunol. 2012;3:265. doi: 10.3389/fimmu.2012.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ombrello MJ, Remmers EF, Sun G, Freeman AF, Datta S, Torabi-Parizi P, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med. 2012;366:330–338. doi: 10.1056/NEJMoa1102140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aderibigbe OM, Priel DL, Lee CC, Ombrello MJ, Prajapati VH, Liang MG, et al. Distinct cutaneous manifestations and cold-induced leukocyte activation associated with PLCG2 mutations. JAMA Dermatol. 2015;151:627–634. doi: 10.1001/jamadermatol.2014.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Frischmeyer-Guerrerio PA, Guerrerio AL, Oswald G, Chichester K, Myers L, Halushka MK, et al. TGFbeta receptor mutations impose a strong predisposition for human allergic disease. Sci Transl Med. 2013;5:195ra94. doi: 10.1126/scitranslmed.3006448. [This report describes how altered TGFb signaling results in Th2 skew and Treg cells that produce IL-13.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felgentreff K, Siepe M, Kotthoff S, von Kodolitsch Y, Schachtrup K, Notarangelo LD, et al. Severe eczema and hyper-IgE in Loeys–Dietz-syndrome — contribution to new findings of immune dysregulation in connective tissue disorders. Clin Immunol (Orlando, FL) 2014;150:43–50. doi: 10.1016/j.clim.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Farmand S, Sundin M. Hyper-IgE syndromes: recent advances in pathogenesis, diagnostics and clinical care. Curr Opin Hematol. 2015;22:12–22. doi: 10.1097/MOH.0000000000000104. [DOI] [PubMed] [Google Scholar]
- 44.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 45.Boos AC, Hagl B, Schlesinger A, Halm BE, Ballenberger N, Pinarci M, et al. Atopic dermatitis, STAT3- and DOCK8-hyper-IgE syndromes differ in IgE-based sensitization pattern. Allergy. 2014;69:943–953. doi: 10.1111/all.12416. [DOI] [PubMed] [Google Scholar]
- 46•.Siegel AM, Stone KD, Cruse G, Lawrence MG, Olivera A, Jung MY, et al. Diminished allergic disease in patients with STAT3 mutations reveals a role for STAT3 signaling in mast cell degranulation. J Allergy Clin Immunol. 2013;132:1388–1396. doi: 10.1016/j.jaci.2013.08.045. [This report describes the reduced prevalence of food allergy and food anaphylaxis in patients with AD-HIES, and attributes this to impaired STAT3-mediated mast cell degranulation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erlich TH, Yagil Z, Kay G, Peretz A, Migalovich-Sheikhet H, Tshori S, et al. Mitochondrial STAT3 plays a major role in IgE-antigen-mediated mast cell exocytosis. J Allergy Clin Immunol. 2014;134:460–469. doi: 10.1016/j.jaci.2013.12.1075. [DOI] [PubMed] [Google Scholar]
- 48••.Zhang Y, Yu X, Ichikawa M, Lyons JJ, Datta S, Lamborn IT, et al. Autosomal recessive phosphoglucomutase 3 (PGM3) mutations link glycosylation defects to atopy, immune deficiency, autoimmunity, and neurocognitive impairment. J Allergy Clin Immunol. 2014;133:1400–1409. doi: 10.1016/j.jaci.2014.02.013. [This report, and those by Stray-Pedersen et al. and Sassi et al., describe the clinical and immunologic presentation of PGM3 deficiency.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Stray-Pedersen A, Backe PH, Sorte HS, Morkrid L, Chokshi NY, Erichsen HC, et al. PGM3 mutations cause a congenital disorder of glycosylation with severe immunodeficiency and skeletal dysplasia. Am J Hum Genet. 2014;95:96–107. doi: 10.1016/j.ajhg.2014.05.007. [This report, and those by Zhang et al. and Sassi et al., describe the clinical and immunologic presentation of PGM3 deficiency.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50••.Sassi A, Lazaroski S, Wu G, Haslam SM, Fliegauf M, Mellouli F, et al. Hypomorphic homozygous mutations in phosphoglucomutase 3 (PGM3) impair immunity and increase serum IgE levels. J Allergy Clin Immunol. 2014;133:1410–1419. doi: 10.1016/j.jaci.2014.02.025. [This report, and those by Zhang et al. and Stray-Pedersen et al., describe the clinical and immunologic presentation of PGM3 deficiency.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnaar RL. Glycans and glycan-binding proteins in immune regulation: a concise introduction to glycobiology for the allergist. J Allergy Clin Immunol. 2015;135:609–615. doi: 10.1016/j.jaci.2014.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options — a review of 136 patients. J Clin Immunol. 2015;35:189–198. doi: 10.1007/s10875-014-0126-0. [This report provides a detailed clinical and immunologic description of a large combined cohort of DOCK8 deficiency patients.] [DOI] [PubMed] [Google Scholar]
- 53.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361:2046–2055. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozcan E, Notarangelo LD, Geha RS. Primary immune deficiencies with aberrant IgE production. J Allergy Clin Immunol. 2008;122:1054–1062. doi: 10.1016/j.jaci.2008.10.023. quiz 63-4. [DOI] [PubMed] [Google Scholar]
- 55.Thrasher AJ, Burns SO. WASP: a key immunological multitasker. Nat Rev Immunol. 2010;10:182–192. doi: 10.1038/nri2724. [DOI] [PubMed] [Google Scholar]
- 56.Ochs HD, Thrasher AJ. The Wiskott–Aldrich syndrome. J Allergy Clin Immunol. 2006;117:725–738. doi: 10.1016/j.jaci.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Jing H, Zhang Q, Zhang Y, Hill BJ, Dove CG, Gelfand EW, et al. Somatic reversion in dedicator of cytokinesis 8 immunodeficiency modulates disease phenotype. J Allergy Clin Immunol. 2014;133:1667–1675. doi: 10.1016/j.jaci.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor MD, Sadhukhan S, Kottangada P, Ramgopal A, Sarkar K, D'Silva S, et al. Nuclear role of WASp in the pathogenesis of dysregulated TH1 immunity in human Wiskott–Aldrich syndrome. Sci Transl Med. 2010;2:37ra44. doi: 10.1126/scitranslmed.3000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Samuelov L, Sarig O, Harmon RM, Rapaport D, Ishida-Yamamoto A, Isakov O, et al. Desmoglein 1 deficiency results in severe dermatitis, multiple allergies and metabolic wasting. Nat Genet. 2013;45:1244–1248. doi: 10.1038/ng.2739. [This report is the first description of desmoglein-1 deficiency as a cause of SAM syndrome.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Has C, Jakob T, He Y, Kiritsi D, Hausser I, Bruckner-Tuderman L. Loss of desmoglein 1 associated with palmoplantar keratoderma, dermatitis and multiple allergies. Brit J Dermatol. 2015;172:257–261. doi: 10.1111/bjd.13247. [DOI] [PubMed] [Google Scholar]
- 61.Samuelov L, Sprecher E. Peeling off the genetics of atopic dermatitis-like congenital disorders. J Allergy Clin Immunol. 2014;134:808–815. doi: 10.1016/j.jaci.2014.07.061. [DOI] [PubMed] [Google Scholar]
- 62.Hovnanian A. Netherton syndrome: skin inflammation and allergy by loss of protease inhibition. Cell Tissue Res. 2013;351:289–300. doi: 10.1007/s00441-013-1558-1. [DOI] [PubMed] [Google Scholar]
- 63.Hannula-Jouppi K, Laasanen SL, Heikkila H, Tuomiranta M, Tuomi ML, Hilvo S, et al. IgE allergen component-based profiling and atopic manifestations in patients with Netherton syndrome. J Allergy Clin Immunol. 2014;134:985–988. doi: 10.1016/j.jaci.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Fortugno P, Furio L, Teson M, Berretti M, El Hachem M, Zambruno G, et al. The 420K LEKTI variant alters LEKTI proteolytic activation and results in protease deregulation: implications for atopic dermatitis. Hum Mol Genet. 2012;21:4187–4200. doi: 10.1093/hmg/dds243. [DOI] [PubMed] [Google Scholar]
- 65.Oji V, Eckl KM, Aufenvenne K, Natebus M, Tarinski T, Ackermann K, et al. Loss of corneodesmosin leads to severe skin barrier defect, pruritus, and atopy: unraveling the peeling skin disease. Am J Hum Genet. 2010;87:274–281. doi: 10.1016/j.ajhg.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kubo A, Nagao K, Amagai M. Epidermal barrier dysfunction and cutaneous sensitization in atopic diseases. J Clin Investig. 2012;122:440–447. doi: 10.1172/JCI57416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 68.Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. 2009;206:1291–1301. doi: 10.1084/jem.20082767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. doi: 10.3389/fimmu.2012.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verbsky JW, Chatila TA. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) and IPEX-related disorders: an evolving web of heritable autoimmune diseases. Curr Opin Pediatr. 2013;25:708–714. doi: 10.1097/MOP.0000000000000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125:591–599. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Charbonnier LM, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015;135:217–227. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Janssen E, Morbach H, Ullas S, Bannock JM, Massad C, Menard L, et al. Dedicator of cytokinesis 8-deficient patients have a breakdown in peripheral B-cell tolerance and defective regulatory T cells. J Allergy Clin Immunol. 2014;134:1365–1374. doi: 10.1016/j.jaci.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wada T, Schurman SH, Garabedian EK, Yachie A, Candotti F. Analysis of T-cell repertoire diversity in Wiskott–Aldrich syndrome. Blood. 2005;106:3895–3897. doi: 10.1182/blood-2005-06-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heimall J, Davis J, Shaw PA, Hsu AP, Gu W, Welch P, et al. Paucity of genotype-phenotype correlations in STAT3 mutation positive Hyper IgE Syndrome (HIES). Clin Immunol (Orlando, FL) 2011;139:75–84. doi: 10.1016/j.clim.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shearer WT, Dunn E, Notarangelo LD, Dvorak CC, Puck JM, Logan BR, et al. Establishing diagnostic criteria for severe combined immunodeficiency disease (SCID), leaky SCID, and Omenn syndrome: the Primary Immune Deficiency Treatment Consortium experience. J Allergy Clin Immunol. 2014;133:1092–1098. doi: 10.1016/j.jaci.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McAleer MA, Pohler E, Smith FJ, Wilson NJ, Cole C, MacGowan S, et al. Severe dermatitis, multiple allergies, and metabolic wasting syndrome caused by a novel mutation in the N-terminal plakin domain of desmoplakin. J Allergy Clin Immunol. 2015 doi: 10.1016/j.jaci.2015.05.002. http://dx.doi.org/10.1016/j.jaci.2015.05.002. [in press] [DOI] [PMC free article] [PubMed]