Abstract
Norovirus is increasingly recognized as a major cause of acute gastroenteritis among children <5 years of age. We searched for publications that reported detailed age distributions of pediatric norovirus cases, and assessed associations between age distribution and socio-demographic factors to identify the most critical age periods to prevent norovirus cases among young children. Approximately 70% of pediatric norovirus cases occurred between 6 and 23 months of age. A younger age distribution was found in lower income countries and inpatient settings. These findings suggest that a norovirus immunization schedule completed by 6 months could have the potential to prevent about 85% of pediatric cases, while a vaccine delivered at 12 months of age would only have the potential to prevent about 50% of pediatric cases. With a younger age distribution in lower income settings, early prevention would be even more critical.
Keywords: Norovirus, Gastroenteritis, Age distribution, Vaccines, Vaccine, Socioeconomic factors
1. Introduction
With the substantial decline of rotavirus-associated diarrhea in countries that have introduced rotavirus vaccines, norovirus is increasingly recognized as a main cause of acute gastroenteritis (AGE) [1,2]. Norovirus vaccines are under development and have shown promise in safety and immunogenicity studies, as well as protection against infection and disease in experimental challenge studies [3,4]. Young children have the highest incidence of norovirus gastroenteritis [5,6], so stand to benefit from a vaccine. However all vaccine studies have been performed among adults. For development of a pediatric vaccine, a number of specific questions will arise, including the number of doses required, the acceptability of an adjuvant, and the appropriate age to vaccinate. To identify the most critical age periods to prevent norovirus among young children, we conducted meta-analysis to understand the detailed age distribution of pediatric norovirus AGE cases (defined as children aged <5 years).
2. Methods
We used the database described in a previous systematic review [7] that included studies published between January 1997 and March 2014; we updated the literature search to include studies published between March 1, 2014, and August 31, 2014 in the Medline database. We searched for studies using the search term “norovirus.” Two individuals reviewed titles and abstracts and obtained full articles if studies were deemed relevant to our research. Studies that met all four of the following criteria were included: (1) recruited patients with AGE symptoms from a specific geographic area or group of people; (2) used PCR to detect norovirus in stool; (3) done continuously for ≥1 year; and (4) reported the age distribution among laboratory-confirmed pediatric norovirus cases.
2.1. Summary of age distributions
Data on the total number of cases and the number of cases in each age group were extracted from each study. After trying various categorizations (Supplementary Table/Figs. 1 and 2), the following four age groups were used for analyses: <6, 6–11, 12–23, and 24–59 months.
The cumulative proportion of pediatric norovirus cases by age was calculated for each study. We pooled this cumulative proportion of cases and calculated a weighted average. The following formula shows the weighted average of the cumulative proportion of pediatric norovirus cases by the age of 6 months:
where P(Cases 0 − 5m)i is the proportion of pediatric norovirus cases that were among the <6 month age group and Casesi is the total number of norovirus cases aged 0–59 months in study i. ∑iCasesi is the sum of all norovirus cases 0–59 months of age for all included studies. Similar formulae were used to calculate weighted average for <12 and <24 month age groups.
2.2. Association of age distribution with socio-demographic factors
Per capita gross domestic product (GDP) of each country from the year of publication was used as an indicator of income level [8]. Countries were additionally categorized into three levels of development according to World Health Organization (WHO) mortality strata [9]: high-mortality developing (HMD), low-mortality developing (LMD), and developed. The study settings were grouped into inpatient (including emergency departments), outpatient, community, and other. The “other” category included studies in which setting was not described or a mixture of settings was included but stratified data were not reported.
We used meta-regression analyses to evaluate associations between potential predictors (i.e., income level and development index of each country and study setting) with the proportion of pediatric cases that occurred in the <12 month age group. We selected this age limit as the outcome because the largest variation in cumulative proportion of cases across socio-demographic factors was observed at 12 months. Mixed-effects models were fitted using restricted maximum-likelihood estimation, with the number of pediatric norovirus AGE cases in each study as weights and “study” as a random effect. We assumed that per capita GDP and WHO mortality strata would indicate similar levels of development; thus we fit a multivariate mixed-effects model with per capita GDP and study setting. All analyses were performed using the package “metafor” in R [10,11].
3. Results
We identified 78 studies about pediatric norovirus from the previously developed database [7]. We also identified 261 publications published from March to August 2014 from the Medline database search. Of these, 225 articles were excluded after screening titles and abstracts. We assessed the eligibility of 114 full-text articles in total. Of these, 79 were excluded for various reasons (Supplementary Fig. 3). In total, 35 articles met the inclusion criteria.
There were 23 countries represented in these 35 papers. Twenty (57%) studies were conducted in LMD countries, representing a total of 2420 pediatric norovirus cases, eight (23%) were in developed countries, representing 2173 cases, and seven (20%) were in HMD countries, representing 607 cases. In total, there were 2347 pediatric norovirus cases in the inpatient setting, 1329 in outpatient, 530 in community, and 994 in other settings.
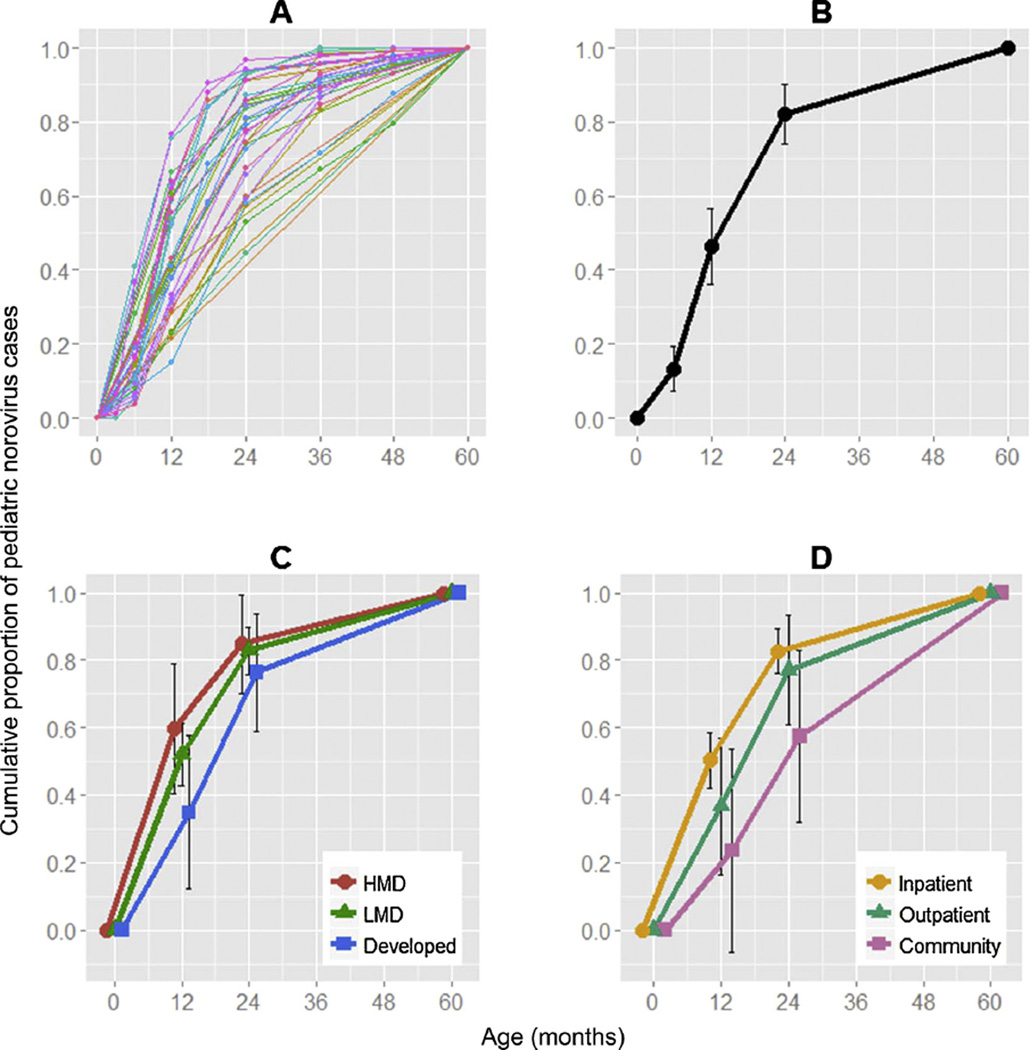
After weighting by the number of pediatric norovirus cases in each study, the percentage of cases in each age group was as follows: 0–5 months: 13% (95% confidence interval (CI): 7–19%); 6–11 months: 33% (46% cumulative; 95% CI: 36–57%); 12–23 months: 36% (82% cumulative; 95% CI: 74–90%); and 24–59 months: 18% (100% cumulative) (Fig. 1A and B).
Fig. 1.
Cumulative proportion of pediatric norovirus cases (A: 35 individual studies, B: weighted average of cumulative proportion, C: weighted average of cumulative proportion stratified by country development level, D: weighted average of cumulative proportion stratified by study settings). Abbreviations: HMD high mortality developing countries; LMD low mortality developing countries.
A younger age distribution of pediatric norovirus cases was observed in studies pooled from developing countries, with 60% (95% CI: 41–79%), 52% (95% CI: 43–61%), and 35% (95% CI: 12–57%) of cases among children <12 months in HMD, LMD, and developed countries, respectively (p = 0.09, Table 1 and Fig. 1C). A younger age distribution was observed in low income countries, with a decrease of 5.9% (95% CI: 1.6–10.1%) in cumulative cases <12 months for every 10,000 USD increase in per capita GDP (Table and Supplementary Fig. 4). The age distribution was younger in inpatient settings with 50% (95% CI: 42–59%) of cases aged <12 months, compared to 37% (95% CI: 16–57%) in outpatient settings and 24% (95% CI: −0.6–54%) in community settings (p = 0.01, Table 1 and Fig. 1D).
Table 1.
Bivariate and multivariate associations between the predictors and the cumulative proportion of pediatric cases by 12 month of age.a
| Predictors | Bivariate association | Multivariate association | ||||
|---|---|---|---|---|---|---|
| Parameter estimate (se) | p-value | Parameter estimate (se) | p-value | |||
| WHO mortality strata | ||||||
| HMD | 0.27 | (0.13) | 0.04 | – | – | |
| LMD | 0.19 | (0.10) | 0.06 | – | – | |
| Developed | Ref | – | – | |||
| Settings | ||||||
| Outpatient | −0.14 | (0.11) | 0.21 | −0.05 | (0.10) | 0.6 |
| Community | −0.25 | (0.11) | 0.02 | −0.16 | (0.10) | 0.1 |
| Otherb | 0.14 | (0.09) | 0.13 | 0.13 | (0.09) | 0.1 |
| Inpatient | Ref | Ref | ||||
| Per capita GDP (per 10,000 USD) | −0.06 | (0.02) | 0.007 | −0.04 | (0.02) | 0.02 |
Abbreviations: se, standard error; HMD, high-mortality developing countries; LMD, low-mortality developing countries; Ref, reference group; GDP, gross domestic product; USD, United States Dollars.
Note that a negative coefficient denotes a lower proportion of cases less than 12 month of age in a given category compared to the reference group.
The “other” settings category included studies in which setting was not described or in which a mixture of settings was included but stratified data were not reported.
After controlling for study setting in the multivariate model, per capita GDP remained significant, with the cumulative proportion of cases by 12 months reduced by 4.1% (95% CI: 0.8–7.5%) for every 10,000 USD increase in per capita GDP (Table 1). Although not statistically significant, the cumulative proportion of cases aged <12 months in inpatient settings was 14% higher than that in outpatient settings in developed countries, while there was no difference in developing countries (Supplementary Fig. 5).
4. Discussion
Approximately 70% of pediatric norovirus-associated AGE cases occurred within the 6–23 month age range, with less than 15% occurring before 6 months. We found a younger age distribution of pediatric norovirus cases in lower income settings. This might be attributable to a higher force of norovirus infection/basic reproduction number (R0) in low income settings due to poorer hygiene and sanitation and/or higher levels of contact between individuals [12]. These findings suggest that a norovirus immunization schedule completed by 6 months could have the potential to prevent about 85% of pediatric cases, while a vaccine delivered at 12 months of age would only have the potential to prevent about 50%. With a younger age distribution in lower income settings, early prevention is even more critical.
The proportion of cases among children aged <12 months increased from community to outpatient to inpatient settings. Assuming setting correlates with severity of illness, this suggests that the age distribution of severe norovirus cases is younger than mild cases and that younger cases are more likely to be hospitalized or seek medical care, consistent with previous studies [13,14]. This trend was apparent (though non-significant) in developed countries but not in developing countries. This could reflect more access to care for mild cases in developed countries, or a clearer triage of cases in inpatient versus outpatient settings. Also, there may be biases in surveillance in the individual studies that affected case ascertainment. For example, if older children are less likely to be hospitalized in developing countries, either because they do not seek care, or because they are triaged as outpatients, then we would observe an older distribution in developed country settings. If true, our observed differences could be a result of care-seeking and case detection rather than disease severity.
An important limitation was that few included studies were conducted in HMD countries or community settings, which may limit the representativeness of age distribution of these subgroups in the aggregated results. Also, we could not assess the age distribution by genogroup due to limitations in the published data, and this may be important since routes of GI and GII differ somewhat, and, therefore, may be associated with different age at first infection. Additionally, we only included studies which used PCR for norovirus detection. While our inclusion criteria also required cases to demonstrate AGE symptoms, the detection of norovirus by PCR does not necessarily imply that AGE in every case was caused by norovirus.
In conclusion, we found that a majority of pediatric norovirus AGE cases occurred between 6 and 23 months. Norovirus vaccines are progressing through the development pipeline; our review makes clear that a pediatric formulation would need to be delivered by 6 months of age to prevent the majority of pediatric norovirus cases.
Supplementary Material
Acknowledgements
This research was supported by (a) an appointment to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and CDC (to K.S. and A.K.) and (b) the Foodborne Disease Burden Epidemiology Reference Group (FERG) of the World Health Organization (WHO). We thank Marion Koopmans and Linda Verhoef at the National Institute for Public Health and the Environment in the Netherlands for their previous contribution to developing the database.
Footnotes
Conflicts of interest
None. The authors do not have commercial or other associations that might pose a conflict of interest.
Disclaimer
The findings and conclusions presented in this paper are of the authors and do not necessarily represent the Centers for Disease Control and Prevention.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.05. 051
Contributor Information
Kayoko Shioda, Email: yji4@cdc.gov.
Anita Kambhampati, Email: wyc4@cdc.gov.
Aron J. Hall, Email: esg3@cdc.gov.
Ben A. Lopman, Email: iow4@cdc.gov.
References
- 1.Payne DC, Vinje J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013 Mar;368(12):1121–1130. doi: 10.1056/NEJMsa1206589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemming M, Rasanen S, Huhti L, Paloniemi M, Salminen M, Vesikari T. Major reduction of rotavirus, but not norovirus, gastroenteritis in children seen in hospital after the introduction of RotaTeq vaccine into the National Immunization Programme in Finland. Eur J Pediatr. 2013 Jun;172(6):739–746. doi: 10.1007/s00431-013-1945-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, et al. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011 Dec;365(23):2178–2187. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010 Dec;202(11):1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips G, Tam CC, Conti S, Rodrigues LC, Brown D, Iturriza-Gomara M, et al. Community incidence of norovirus-associated infectious intestinal disease in England: improved estimates using viral load for norovirus diagnosis. Am J Epidemiol. 2010 May;171(9):1014–1022. doi: 10.1093/aje/kwq021. [DOI] [PubMed] [Google Scholar]
- 6.Hall AJ, Lopman BA, Payne DC, Patel MM, Gastanaduy PA, Vinje J, et al. Norovirus disease in the United States. Emerg Infect Dis. 2013 Aug;19(8):1198–1205. doi: 10.3201/eid1908.130465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014 Aug;14(8):725–730. doi: 10.1016/S1473-3099(14)70767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Bank GDP per capita (current US$) 2014 Available from: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD. [Google Scholar]
- 9.World Health Organization. List of Member States by WHO region and mortality stratum. 2007 Available from: http://www.who.int/mental_health/neurology/annexes_neuro_disorders_public_h_challenges.pdf.
- 10.R Development Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 11.Viechtbauer W. Conducting meta analysis in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 12.Fine PE, Carneiro IA. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am J Epidemiol. 1999 Nov;150(10):1001–1021. doi: 10.1093/oxfordjournals.aje.a009924. [DOI] [PubMed] [Google Scholar]
- 13.Patel AB, Ovung R, Badhoniya NB, Dibley MJ. Risk factors for predicting diarrheal duration and morbidity in children with acute diarrhea. Indian J Pediatr. 2012 Apr;79(4):472–477. doi: 10.1007/s12098-011-0561-3. [DOI] [PubMed] [Google Scholar]
- 14.Rocha MC, Carminate DL, Tibirica SH, Carvalho IP, Silva ML, Chebli JM. Acute diarrhea in hospitalized children of the municipality of Juiz de Fora MG, Brazil: prevalence and risk factors associated with disease severity. Arq gastroenterol. 2012 Dec;49(4):259–265. doi: 10.1590/s0004-28032012000400006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.