Abstract
Mouse embryonic stem cells (ES cells) can proliferate indefinitely. To identify potential signals involved in suppression of self-renewal, we previously screened a kinase/phosphatase expression library in ES cells, and observed that inhibition of Dual Leucine zipper-bearing Kinase (DLK) increased relative cell numbers. DLK protein was detected in both the pluripotent and differentiated states of mouse ES cells while DLK kinase activity increased upon differentiation. Overexpression of DLK in mouse ES cells displayed reductions in relative cell/colony numbers and Nanog expression, suggesting a suppressive role of DLK in self-renewal. By examining protein sequences of DLK, we identified 2 putative Akt phosphorylation sites at S584 and T659. Blocking PI3K/Akt signaling with LY-294002 enhanced DLK kinase activity dramatically. We found that Akt interacts with and phosphorylates DLK. Mutations of DLK amino acid residues at putative Akt phosphorylation sites (S584A, T659A, or S584A and T659A) diminished the level of DLK phosphorylation. While the mutated DLKs (S584A, T659A, or S584A and T659A) were expressed, a further reduction in cell/colony numbers and Nanog expression appeared in mouse ES cells. In addition, these mutant DLKs (S584A, T659A, or S584A and T659A) exhibited more robust kinase activity and cell death compared to wild type DLK or green fluorescence (GFP) controls. In summary, our results show that DLK functions to suppress self-renewal of mouse ES cells and is restrained by Akt phosphorylation.
Keywords: Akt, Dual Leucine zipper-bearing Kinase (DLK), mouse embryonic stem cells, Nanog, self-renewal
Introduction
Embryonic stem cells (ES cells) are derived from the inner cell mass of blastocysts. Once cultured in vitro, ES cells have abilities to proliferate indefinitely and retain pluripotency to differentiate into cells of the 3 germ layers and almost all cell types with the exception of placenta.1-3 A prerequisite for mouse ES cells to proliferate and maintain pluripotency is addition of leukemia inhibitory factor (LIF).4,5 LIF activates phosphoinositide 3-kinase (PI3K)/Akt and Jak1/Stat3 pathways to sustain ES cell growth and expression of pluripotent transcription factors, Nanog, Oct4 and Sox2.6-8 Withdrawal of LIF leads to a decrease of renewal, and mouse ES cells begin to differentiate. As a downstream effector of LIF, Nanog is a homeobox transcription factor that expresses in pluripotent stem cells and diminishes upon differentiation.9,10 Nanog coordinates with Oct4 and Sox2 to form mutual regulatory networks that not only suppress differentiation genes, but also induce self-renewal genes.11-14 Recently, the dependence on LIF was shown to be dispensable for mouse ES cell maintenance upon overexpression of Nanog.9,10 Evidence indicates that constitutive overexpression of Nanog can rescue disruption of self-renewal caused by inactivation of Jak1/Stat3 pathways.9,10 In Nanog knockout mouse ES cells, colony expansion is reduced and cells are susceptible to differentiation signals.15 These findings verified the pivotal roles of these intrinsic transcription factors in mouse ES cells, and demonstrated the significance in regulation of Nanog.
Akt is the major effector that participates in cell metabolism, survival, proliferation and tumorigenicity in somatic cells.16,17 In mouse ES cells, PI3K/Akt signaling is essential for self-renewal and Nanog expression.8,18-22 Inhibition of Akt activity with LY-294002 reduces cellular growth, pluripotency and expression amounts of Nanog protein.18,19,21,22 On the other hand, the increase of Akt activity by overexpression of ERas or knockout of PTEN promotes cell survival and tumorigenic properties of mouse ES cells.23,24 Even in the absence of LIF, Akt overexpression maintains the undifferentiated status of ES cells and Nanog expression levels.20 In addition, Akt is reported to phosphorylate Oct4 directly in embryonic carcinoma cells for maintaining tumorigenicity.25 These studies revealed that Akt may regulate multiple substrates to orchestrate complex signaling within mouse ES cells. The phenomenon is similar to its master regulatory functions in somatic cells.
In this study, we identified DLK as a suppressor of mouse ES cell self-renewal. DLK kinase activity was strongly restrained by Akt phosphorylation in self-renewal state. Disruption of Akt phosphorylation sites in DLK elevated DLK enzyme activity and caused significant reductions in mouse ES cell numbers/colonies and Nanog expression. The modulation of the suppressive activity of DLK is therefore required for the maintenance of ES self-renewal.
Results
Knockdown of DLK increases mouse ES cell numbers and the expression level of Nanog protein
In our previous study, we performed the high-throughput shRNA screen in which 929 individual kinases/phosphatases were knocked-down and we pinpointed dozens of positive regulators of mouse ES cell renewal.26,27 In an effort to find kinases/phosphatases that inhibit self-renewal, we re-examined the screening results and discovered several candidate suppressors. Among these genes, DLK knocked-down by infected with shRNA enhanced cell numbers dramatically. To re-confirm this phenotype, we transfected 2 different sh-DLK plasmids and one sh-Control plasmid separately in D3 mouse ES cells. Consistent with pervious virus infection results, cell numbers of sh-DLK transfectants were increased while measured by Alamar blue assay and trypan blue exclusion assay, and compared to the sh-controls (Fig. 1A and B). These sh-DLK transfectants also exhibited moderate reduction of sub-G1 levels in flow cytometry analysis (Fig. S1A). Alkaline phosphatase activity is a pluripotent marker of ES cells. Compared to control mouse ES cells, sh-DLK transfectants displayed more colonies with alkaline phosphatase activity and the colony sizes were larger (Fig. 1C and D). This suggests that down-regulation of DLK in mouse ES cells enhances ES cell renewal.
Figure 1.
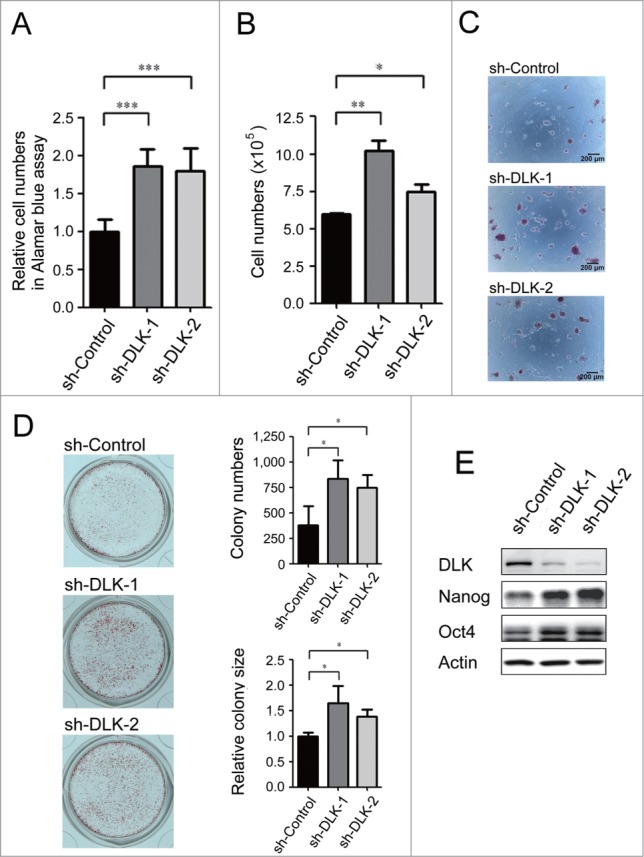
Knockdown of DLK increases mouse ES cell relative cell numbers and expression of Nanog protein. D3 mouse ES cells were transfected with sh-Control, sh-DLK-1 and sh-DLK-2 separately, and selected with 1μg/ml puromycin. (A) Relative cell numbers increased in mouse ES cells transfected with sh-DLKs while compared to sh-control. Relative cell numbers were analyzed by the Alamar blue assay. (B) Absolute cell numbers increased in sh-DLK transfectants while compared to sh-control. Absolute cell numbers were measured by the trypan blue exclusion assay. (C) Mouse ES cells transfected with sh-DLKs had more and larger colonies. The alkaline phosphatase activity was stained and photographed under microscope. (D) Both colony numbers and colony sizes increased in sh-DLK ES cells. Colonies stained with alkaline phosphatase on 24-well plates were photographed and calculated. (E) Nanog and Oct4 proteins were upregulated in sh-DLK ES cells. The expression levels of the indicated proteins were detected by Western blotting. The error bars in the figures represent standard error of the mean (mean±SEM). P values were obtained from 2-tailed Student's t-tests (***, P < 0.0001; **, P < 0.001; *, P < 0.05).
Nanog and Oct4 are well-known for playing critical roles in mouse ES self-renewal.7,9,10 By Western blot analysis, downregulation of DLK increased Nanog protein significantly (Fig. 1E). Thus, the sh-DLKs not only promoted mouse ES cell expansion, but also upregulated Nanog expression in D3 mouse ES cells. This phenomenon was also demonstrated in R1 mouse ES cells (Fig. S1B and C).
DLK kinase activity is upregulated upon differentiation of mouse ES cells
DLK is a mixed-lineage kinase and involves in MAPK pathway.28-31 In developmental studies, it has been reported that DLK is expressed in E10 and involves in late neuronal development.32-36 However, in mouse ES cells or in developmental stages before E10, the functional role(s) of DLK are unknown. DLK proteins are expressed at a low level in undifferentiated mouse ES cells and mouse embryonic fibroblasts (MEFs) (Fig. S2A). To examine DLK expression levels in undifferentiated and differentiated ES cells, embryoid bodies (EBs) which contain ectoderm, mesoderm, and endoderm cells were formed. On day 6 and day 12 of EB formation, DLK RNA expression levels were upregulated to about 2-fold and 10-fold while compared with undifferentiated mouse ES cells (Fig. 2A). DLK protein slightly decreased on 5th or 6th day in mouse EBs, but increased on 10th, 12th, 20th day in EBs (Figs. 2B and S2A). Meanwhile, the expression levels of Nanog and Oct4 protein decreased in EBs (Fig. 2B). Different from the protein expression pattern on 6th day of EBs, DLK kinase activity increased gradually during the EB differentiation process (Fig. 2C).
Figure 2.
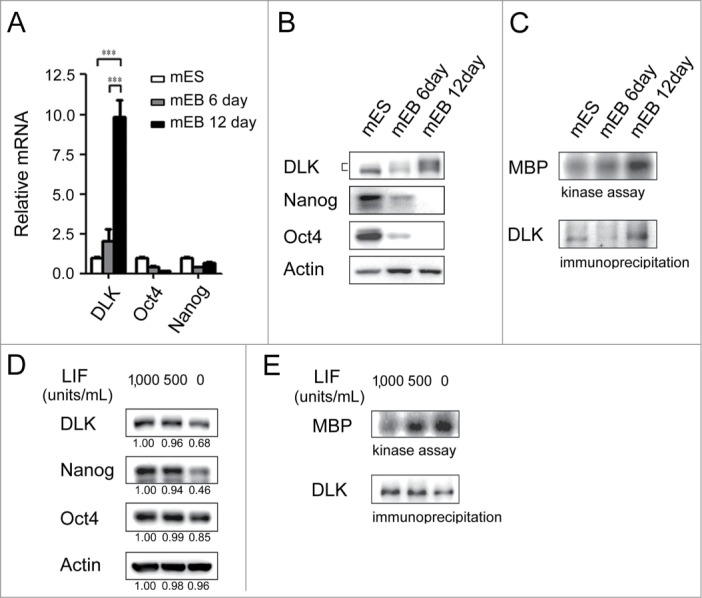
DLK activity is upregulated upon differentiation. (A) The mRNA expression levels of DLK increased upon differentiation. DLK, Nanog, and Oct4 transcripts from D3 mouse ES cells and mouse EBs (6th and 12th days) were quantified by real-time quantitative PCR analyses. Mouse EBs were prepared by suspension method described in the material and methods. (B) The protein expression level of DLK, Nanog, and Oct4 proteins in mouse ES cells and EBs were detected by Western blotting. (C) DLK enzyme activity increased upon differentiation (EB). DLK protein in mouse ES cells and EBs (6th and 12th days) were precipitated with DLK antibody, and aliquoted for DLK kinase activity assays and Western blotting. Myelin Basic Protein (MBP) was used as a DLK substrate to evaluate DLK kinase activity. (D) Nanog protein level decreased upon the removal of LIF. Mouse ES cells maintained in feeder-free conditions were cultured with medium contained 1,000 units/ml of LIF, 500 units/ml of LIF, or without LIF. These cells were harvested for analysis of DLK, Nanog, and Oct4 protein expression by Western blotting. (E) DLK kinase activity increased upon LIF removal or reduction. Mouse ES cells maintained in feeder free conditions were cultured with medium contained 1,000 units/ml of LIF, 500 units/ml of LIF, or without LIF. DLK kinase activity was measured. The error bars in the figures represent standard error of the mean (mean±SEM). P values were obtained from 2-tailed Student's t-tests (***, P < 0.0001; **, P < 0.001; *, P < 0.05).
Once LIF is withdrawal from the culture, inactivation of PI3K/Akt signaling leads mouse ES cells to differentiate.6,8,37 At the same time, cell numbers and expression of self-renewal markers begin to decrease. Consistent with a previous report,38 in the absence of LIF, Nanog protein and phosphorylated Akt were both reduced in D3 mouse ES cells (Fig. 2D and Fig. S2B). Interestingly, upon the reduction or removal of LIF, DLK kinase activity increased significantly (Fig. 2E). An inverse correlation was observed between DLK kinase activity and LIF concentration (Fig. 2E). However, DLK protein decreased while LIF was removed (Fig. 2D). The inconsistency between DLK protein expression amounts and DLK enzyme activity was also observed in the EB formation process (Fig. 2B and C). These findings indicate the activation of DLK kinase activity upon ES cell differentiation and imply the existence of post-translational modification(s) on DLK protein at the onset of differentiation.
Overexpression of DLK reduces mouse ES cell self-renewal
To examine potential suppressive role(s) of DLK in mouse ES cells, the Dlk gene was overexpressed. Expression of DLK protein levels in the transfected mouse cells were close to the protein levels of 15 and 20 day EBs (Fig. 3A). In the transfectants, DLK kinase activity increased robustly (Fig. 3B). By Alamar blue assay, mouse ES cells transfected with DLK had lower relative cell numbers compared to mouse ES cells transfected with GFP (Fig. 3C). Since Nanog protein was up-regulated in sh-DLK mouse ES cells (Fig. 1E), whether induction of DLK also influences Nanog expression was investigated. As expected, down-regulation of Nanog protein was observed in the DLK transfectants (Fig. 3D).
Figure 3.
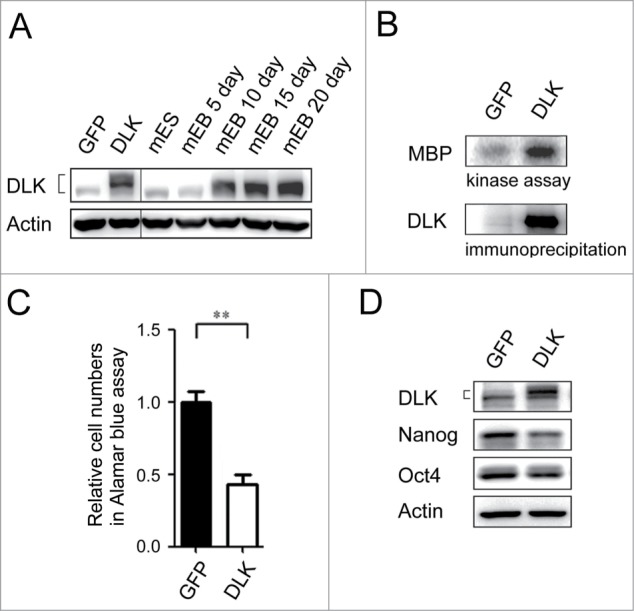
Overexpression of DLK reduces mouse ES cell numbers and the expression of Nanog. (A) The protein expression amounts in DLK overexpression mouse ES cells were similar in Day 15 and Day 20 EBs. DLK protein was detected in DLK overexpressing mouse ES cells (D3) and EBs (5th, 10th, 15th and 20th days) by Western blotting. (B) DLK kinase activity was up-regulated upon the overexpression of DLK. DLK protein precipitated with DLK antibody was aliquoted for DLK kinase activity assays and Western blotting. MBP was used as DLK substrate to evaluate DLK kinase activity. (C) The overexpression of DLK reduced relative cell numbers in ES cells. Relative cell numbers of mouse ES cells transfected with GFP or DLK plasmid were analyzed by the Alamar blue assay in 96-well plates. In the experiment, 2.0 × 104 cells were transfected. (D) Overexpression of DLK slightly down regulated the expression amount of Nanog. The expression of DLK, Nanog and Oct4 proteins were analyzed by Western blotting. The error bars in the figures represent standard error of the mean (mean±SEM). P values were obtained from 2-tailed Student's t-tests (***, P < 0.0001; **, P < 0.001; *, P < 0.05).
Inhibition of PI3K enhances DLK kinase activity and DLK interacts with Akt
DLK activity but not DLK protein was consistently upregulated upon ES differentiation in the withdrawal of LIF or in EB formation (Fig. 2). Thus a post-translational modification mechanism to restrain DLK activity in the self-renewal state of ES cells, or to unbridle DLK activity at the differentiation stage may exist. By analyzing DLK amino acid sequences in mouse, rat, and human, we found 2 conserved putative Akt phosphorylation motifs, R-X-R-X-X-S/T,39,40 in the C-terminal region (Fig. 4A). The potential phosphorylation sites are located at Serine 584 and Threonine 659 (Fig. 4A). In mouse ES cells, Akt is crucial for maintaining self-renewal.20 Inhibition of PI3K/Akt signaling with LY-294002 reduces mouse ES proliferation, pluripotency, and Nanog expression.18,19,21,22 Those effects are similar to the effects of overexpressing DLK in mouse ES cells (Fig. 3C and D). Based on these findings, DLK may be regulated by Akt. To test this hypothesis, we firstly added 0 μM, 0.25 μM and 1.0 μM of LY-294002, the PI3K/Akt inhibitor, into the ES cell culture. Consistent with previous reports, LY-294002 reduced Akt activity and Nanog expression (Figs. 4B and S3A). Nanog protein decreased to 0.67-fold and 0.17-fold respectively upon the treatment of 1μM LY-294002 in D3 and R1 cells (Figs. 4B and S3A). While PI3/Akt signaling was inhibited by LY-294002, DLK activity was dramatically upregulated in the two strains of mouse ES cells (Figs. 4C and S3B). These observations confirm the influence of PI3K/Akt signaling on DLK activity and led us to explore a potential association between DLK and Akt.
Figure 4.
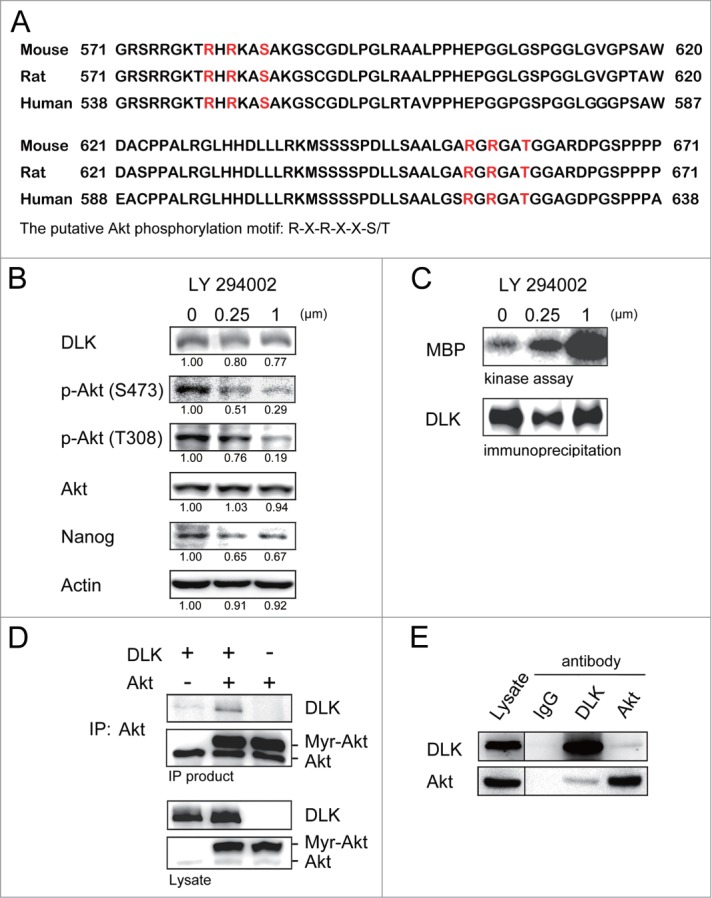
Inhibition of PI3K/Akt signaling increases DLK activity in D3 ES cells. (A) DLK has conserved Akt putative phosphorylation sites in mouse, human, and rat. Mouse, rat and human DLK protein sequences were aligned with CLUSTAL 2.0.12 multiple sequence alignment program.58 Two putative Akt phosphorylation sites in C-terminal of mouse DLK protein are located at Serine 584 and Threonine 659. (B) LY-294002 treatment down-regulated Akt activity and expression of Nanog protein. Western blotting was performed to detect DLK, phospho-Akt at S473, phospho-Akt at T308, total Akt, Nanog and actin after D3 mouse ES cells were treated with 0, 0.25 and 1.0 μM of LY-294002. Under the same condition, (C) DLK activity increased does-dependently upon the treatment of LY-294002. Western blotting and kinase assay using MBP as substrates were carried out as DLK protein was immunoprecipitated. (D) DLK interacted with Akt in the overexpression system. Akt immunoprecipitated products from lysates of mouse ES cells overexpressed DLK, Akt, or DLK plus Akt were hybridized with DLK and Akt antibody in Western blotting analysis. (E) The interaction between endogenous DLK and Akt was demonstrated by co-immunoprecipitation assay. After DLK or Akt protein was immunoprecipitated from D3 mouse ES cells, the co-immunoprecipitated proteins were identified by Western blotting and interaction between endogenous DLK and Akt was demonstrated. The rabbit IgG was used as negative control in the experiment.
To examine if DLK interacts with Akt, we overexpressed Myr-Akt (the constitutive active form of Akt) and DLK in mouse ES cells and performed co-immunoprecipitation assays. By co-immunoprecipitation assay and Western blot analysis, we found DLK was co-precipitated with Akt (Fig. 4D). In addition, the interaction between endogenous DLK and Akt in wild type ES cells was substantiated by reciprocal co-immunoprecipitation assays with either Akt antibody or DLK antibody (Fig. 4E).
Akt phosphorylates DLK
To examine if Akt can phosphorylate DLK in vitro, recombinant DLK 541–888 (including 2 putative Akt phosphorylation sites at S584 and T659) and DLK 668–888 (without S584 and T659) were purified from E.coli and subjected to Akt phosphorylation assay. While Akt was incubated with DLK 541–888 or DLK 668–888, phosphorylation of both Akt and DLK were detected (Fig. 5A and B). But phosphorylation intensity in DLK 668–888 was attenuated substantially (Fig. 5A). In the assay, auto-phosphorylation appeared on Akt protein but not on DLK protein (Fig. 5A and B). To examine if Akt phosphorylates DLK at S584 and T659 sites, the 2 putative Akt phosphorylation sites were separately mutated or both mutated to alanine in DLK 541–888 (referred to as S584A, T659A, and S584A/T659A). After incubated with Akt, the phosphorylation levels of DLK 541–888 S584A and DLK 541–888 T659A slightly decreased (Fig. 5B). Of note, the phosphorylation level in DLK S584A/T659A mutant was a lot less than that in DLK 541–888 or single mutants (Fig. 5B). These results revealed that to disrupt both putative phosphorylation sites of Akt will effectively impair DLK phosphorylation. Therefore, both S584 and T659 residues of DLK can be modified by phosphorylation by Akt.
Figure 5.
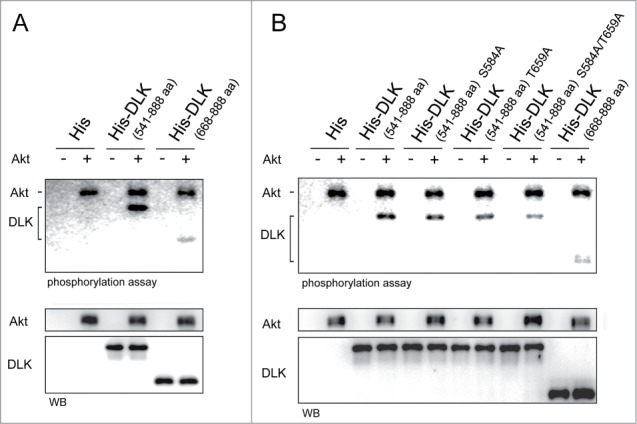
Akt phosphorylates DLK in vitro. (A) Akt phosphorylated both purified His-DLK 541-888, His-DLK 668-888 in vitro. In vitro phosphorylation assay was performed by incubation 400 ng of purified recombinant His-DLK 541-888 and His-DLK 668-888 with or without 15 ng of Akt for 15 minutes. Elutes purified from lysate of E.coli transformed with pGEX-4T vector (modified to expressed 6xHis) were used as negative control. 5 μCi of [γ-32P] ATP was added in each reaction. To quantify these proteins by Western blotting, the [γ-32P]ATP was changed to 20 μM of cold ATP for Western blotting. (B) The mutation of S584A and T659A hampered Akt phosphorylation. In in vitro phosphorylation assay, purified recombinant His-DLK 541-888, His-DLK 541-888 S584A, His-DLK 541-888 T659A, His-DLK 541-888 S584A/T659A or His-DLK 668-888 was incubated with or without of Akt. The experimental conditions were the same as in experiment (A).
DLK S584A, T659A and S584A/T659A mutants significantly reduce self-renewal of mouse ES cells
The linking of PI3K/Akt signaling to DLK revealed a novel potential regulatory pathway. Since S584 and T659 residues of DLK are the putative Akt phosphorylation sites (Fig. 4A), to further evaluate if Akt inhibits DLK activity, DLK amino acid residue(s) at S584, T659, or both S584 and T659 were mutated to alanine and then mutant forms were expressed in mouse ES cells. The cells transfected with DLK mutants showed significant reductions in cell numbers while measured by Alamar blue and cell counting assays (Fig. 6A and B). In addition, colony formation numbers were obviously decreased and most cells went into a sub-G1 phase (Figs. 6C, D and S4A). Like D3 cells (Figs. 6A and 6B), R1 cells overexpressed DLK mutants decreased in cell numbers (Figs. 6A, 6B and S4D). In these remnant cells, a significant downregulation of Nanog and Oct4 protein was observed in both D3 and R1 cell lines (Figs. 6E and S4E). While wild type or mutated DLKs were expressed, there was no discernible difference in Akt activity (Fig. S4B). Consequently, these DLKs were unable to influence Akt functions. Then, a survey of kinase activities in these mutated DLKs (DLK S584A, T659A and S584A/T659A) was undertaken. Compared to wild type DLK, elevated kinase activities appeared in transfectants that expressed the mutated DLKs (Fig. 6F). Additionally, compared to single mutants of DLK, the DLK S584A/T659A mutant has the strongest DLK activity (Fig. 6F). In summary, to dislodge Akt-mediated phosphorylation on DLK will up-regulate DLK kinase activity and enhance its suppressive effects. Akt therefore functions as a rheostat to inhibit DLK activity through phosphorylation in mouse ES cells.
Figure 6.
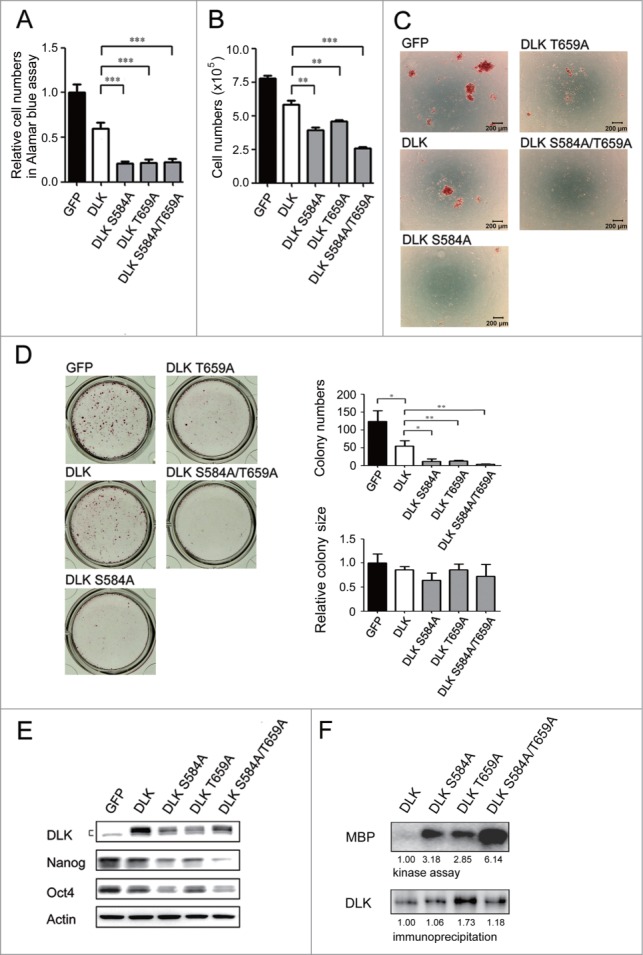
DLK mutated at S584 and T659 significantly reduces mouse ES cell numbers and Nanog expression. D3 mouse ES cells were transfected with GFP, DLK, DLK S584A, DLK T659A, or DLK S584A/T659A. (A) The transfection of DLK S584A, DLK T659A, and DLK S584A/T659A significantly reduced relative cell numbers measured by the Alamar blue assay. (B) Mouse ES cells expressed DLK S584A, DLK T659A, or DLK S584A/T659A displayed significantly reduced cell numbers after counting by trypan blue exclusion assay. (C) Mouse ES cells expressed DLK S584A, DLK T659A, or DLK S584A/T659A dramatically reduced numbers of undifferentiated cell colonies. The alkaline phosphatase activity was stained and photographed under microscope. (D) Colony numbers decreased obviously in mouse ES cells expressed DLK S584A, DLK T659A, or DLK S584A/T659A. Colonies stained with alkaline phosphatase on 24-well were photographed and calculated. (E) Transfection of DLK S584A, DLK T659A, and DLK S584A/T659A DLK downregulated expression amount of Nanog and Oct4. The protein expression levels were detected by Western blotting. (F) DLK mutant at S584A, DLK T659A, or DLK S584A/T659A had higher DLK kinase activity compared to wild type. Wild type DLK and different mutation forms of DLK (DLK S584A, DLK T659A, and DLK S584A/T659A) were expressed, precipitated and aliquoted for Western blotting and kinase assay. In the kinase assay, MBP was used as substrates and the acquired signals were normalized to each precipitated DLKs. The error bars in the figures represent standard error of the mean (mean±SEM). P values were obtained from 2-tailed Student's t-tests (***, P < 0.0001; **, P < 0.001; *, P < 0.05).
Discussion
Using previous functional screening data that analyzed kinases and phosphatases in mouse ES cells as a starting point,26,27 we have discovered that DLK plays suppressive roles in ES cell self-renewal. Manipulation of DLK expression by knockdown or overexpression in mouse ES cells affects cell numbers, colony formation and the expression of Nanog (Fig. 1 and 3). The phenomenon that DLK activity was induced in mouse EBs and in the withdrawal of LIF further indicates a negative relationship between DLK and ES self-renewal (Fig. 2). Because DLK has not been investigated before in ES cells, our results establish functional roles of DLK in mouse ES cells.
Activation of PI3K/Akt signaling is essential for self-renewal of mouse ES cells.18-22 Many proteins and signaling pathways involved in proliferation or pluripotency are modulated by PI3K/Akt including Gsk3β/Myc, FoxO, mTOR and Tbx3/Nanog pathways.8,21,22,41-43, In this study, for the first time, DLK was demonstrated to be an Akt substrate. Akt interacts with and phosphorylates DLK (Figs. 4 and 5). The strength of PI3K/Akt signaling determines DLK kinase activity in undifferentiated mouse ES cells. Blocking PI3K/Akt signaling with LY-294002 or mutating Akt phosphorylation sites at S584 or T659 leads to activation of DLK kinase activity (Figs. 4 and 6). These results not only emphasize the ‘master regulator’ characteristics of Akt in mouse ES cells, but also suggest the existence of an inhibitory phosphorylation mechanism on DLK.
Recently, some kinases expressed in mouse ES cells were found to decline ‘stemness’.44 Addition of ERK and Gsk3β kinase inhibitors (2i condition) with LIF in the culture medium promotes cell viability and prevents differentiation of mouse ES cells even in the absence of serum.44,45 The usage of these chemical kinase inhibitors therefore provides a new strategy to keep mouse ES cells in the self-renewal state. Some DLK inhibitors have been shown to suppress DLK functions in neurons and retinal ganglion cells.46,47 It will be interesting to examine effects of these DLK inhibitors in 2i plus LIF medium, or to develop a new cocktail for the expansion of mouse ES cells.
In an in vitro phosphorylation assay, we confirmed Akt phosphorylates DLK (Fig. 5). Disruption of the predicted phosphorylation sites at S584 and T659 residues on DLK, or deletion of a protein segment with S584 and T659 from DLK protein, led to the decrease in Akt-mediated phosphorylation (Fig. 5). However, there remained a weak phosphorylation signal in the double mutated or truncated DLKs (Fig. 5). Based on previous reports that many Akt substrates have non-typical Akt phosphorylation motifs,48-50 there might be additional non-typical motif(s) on DLK protein. The importance of this weak phosphorylation in DLK is still unknown. Determining the precise location(s) of this additional phosphorylation site(s) by mass spectrometry may help to reveal the detailed regulatory mechanisms between DLK and Akt.
Because DLK had been found to promote apoptosis of neuroblastoma cells and breast cancer epithelial cells,51,52 the un-phosphorylated over-active DLK discovered in our experiments may act as a powerful suppressor in these cells or other cell types. To find DLK activator proteins or a chemical inducer that specifically sustains active DLK may be a feasible way to destroy cancer cells or cancer stem cells. On the other hand, phosphorylation status of DLK at S584 and T659 may provide growth potential information in tested cells. Cells with high levels of phosphorylated DLK or low levels of un-phosphorylated active DLK at S584 and T659 may promote tumorigenesis. In addition, DLK play crucial roles in neuronal development, injure response and adult keratinocyte differentiation.32-36,53-56 It will be interesting to examine if Akt also modulates DLK activity in these cells.
Nanog is one of core transcription factors that govern self-renewal of ES cells.7-10 Expression level of Nanog is regulated by PI3K/Akt signaling,8,18,22 and DLK (Figs. 1, 3 and 6). When Nanog is knocked-out, ES cells maintain their pluripotency, but colony numbers and cell growth are reduced.15 This phenotype is similar to that which occurred in the DLK transfectants, displaying down-regulation of cell/colony numbers and Nanog expression but not influencing alkaline phosphatase activity per cell (ALP/AB value) (Figs. 6 and S4C).
In summary, our results establish a role for an Akt-DLK-Nanog pathway in maintaining ES cell renewal. For the function of Akt as a master regulator and for the multiple established functions of DLK in diverse cell types, our finding that Akt regulates DLK activity may yield new clues to resolving complicated signaling network in ES cells and other cell types.
Materials and Methods
Cell culture and EB formation
D3 and R1 mouse ES cells (ATCC) were cultured on inactivated mouse embryonic fibroblasts STO in Dulbecco's modified Eagle's medium (DMEM) supplemented with 15% fetal bovine serum (FBS; HyClone), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 1 mM glutamine, 0.1 mM β-mercaptoethanol and 1,000 units/ml LIF (Millipore). In feeder free culture, FBS in the medium was replaced by Knockout Serum Replacement (Invitrogen), and mouse ES cells were grown on culture dishes coated with 0.2% bovine gelatin (Sigma-Aldrich). To inhibit PI3K/Akt signaling, mouse ES cells were incubated with medium containing LY-294002 (Tocris Bioscience) for 1 day.
For embryoid body (EB) formation, suspension and hanging drop methods were used.57 In the suspension method, mouse ES cells (5 × 104) were suspended in 10 ml of culture medium without LIF and transferred onto petri dishes for 6 d and 12 d In the hanging drop method, 20 μl of cells (5,000 cells per milliliter) were put onto lids of Petri dishes and the lids were placed back on petri dishes to let the drops hang down. The differentiated EB cells were harvested after 5 days, 10 days, 15 d and 20 d
Plasmids
The pLKO.1 shNRA plasmids targeting mouse DLK (sh-DLK-1 is clone TRCN0000022570 and sh-DLK-2 is clone TRCN0000022569) and a control shNRA plasmids (sh-Control; targeting sequence: 5’- CCGGTCCTAAGGTTAAGTCGCCC TCGCTCGAGCGAGGGCGACTTAACCTTAGGTTTTTG.) were obtained from National RNAi Core Facility (Taiwan). To generate DLK overexpression plasmid, cDNA encoding DLK was amplified from D3 mouse ES cells and cloned into pCDNA4A plasmid (Invitrogen). The control plasmid was constructed by insertion of eGFP gene into pCDNA4A. Site-directed mutagenesis was performed to generate mutated DLK constructs at S584, T659, or both S584 and T659. Point mutations were created by PCR with primers designed to change serine or threonine codon to an alanine codon. The pCDNA3-HA vector and pCDNA3-HA-Myr-Akt that expressed active Akt were kindly provided by Dr. Jong-Young Yen (Academic Sinica, Taipei, Taiwan).
Transfections
Plasmids were transfected into mouse ES cells with Lipofectamine 2000 (Invitrogen). Sixteen hours later, mouse ES cells transfected with shRNAs were selected with 1 μg/ml puromycin, and mouse ES cells transfected with pCDNA4 backbone plasmids were selected with 30 μg/ml zeocin.
Alamar blue assay and trypan blue exclusion assay
Mouse ES cells were incubated with medium containing 10% Alamar blue (AbD Serotec) at the 4th day after transfection. Within 6 hours, Alamar blue activities were measured by OD570 and OD600 in testing medium. To count cell numbers, transfected mouse ES cells were trypsinized and resuspended with serum. The dissociated cells were mixed with trypan blue (0.04%) and counted with a hemocytometer.
Alkaline phosphatase staining and colony formation assay
Mouse ES cells (1 × 105 cells in 24 well plates) were transfected with shRNA or overexpression plasmids. On 4th day after selection with puromycin or zeocin, cells were washed with PBS twice, fixed with methanol for 5 minutes, and stained with an Alkaline Phosphatase detection kit (Millipore). Cell colonies were kept in PBS and photographed under the microscope. To count colony numbers and to obtain the relative area per colony (colony size), cell colonies on each whole well were first photographed with a digital camera and then measured by Image-Pro Plus software (Media Cybernetics).
Western blotting
Cells were lysed with lysis buffer (20 mM Tris-HCl pH 7.5, 100 mM NaCl, 2 mM EDTA, 0.5% Triton X-100, 100 mM sodium orthovanadate, 50 mM sodium fluoride, 12.5 mM β-glycerophosphate and complete protease inhibitor (Roche)), and centrifuged at 16,000 xg for 30 minutes to eliminate cell debris. The supernatants (protein lysates) were quantified using Bradford reagent (Bio-Rad). After boiling in 1x sample buffer (50 mM Tris-HCl pH 6.8, 100 mM DTT, 2% SDS, 10% glycerol and 0.04% bromophenol blue), equal amounts of protein were separated through SDS-polyacrylamide gels and transferred onto PVDF membranes. Membranes were probed with antibodies against DLK (GTX124127; generated by GeneTex, Inc..), Nanog (MLC-51; eBioscience), Oct4 (H-134; Santa Cruz), phospho-Akt S473 (#4060; Cell Signaling), phospho-Akt T308 (#2695; Cell Signaling), Akt (#4691; Cell Signaling) and actin (AC-15; Sigma-Aldrich). After washing with PBS, membranes with antibodies binding to targeting proteins were incubated with an HRP conjugated secondary antibody and detected by Immobilon™ Western Chemiluminescent HRP substrate (Millipore). The signals were acquired using a LAS-4000 image system (Fujifilm). Finally, brightness and contrast of a whole image was adjusted linearly by Multi Gauge version 3.0 (Fujifilm).
Quantitative real-time PCR analysis
Total RNA was extracted with MaestroZol™ Plus RNA Extraction Reagent (Omics Biotechnology). To synthesize cDNA, RNA was treated with DNase I (Promega), and reverse transcription was performed using random hexamers and the Superscript III first strand synthesis kit (Invitrogen). Quantitative real-time PCR was performed with cDNA, gene-specific primers (supplemental Table 1) and KAPA SYBR® FAST qPCR Master Mix (Kapa Biosystems). The threshold cycle (Ct) that reflects relative expression level of an amplified gene was analyzed and normalized against the Ct value of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Immunoprecipitation and kinase assays
Cell lysates isolated from mouse ES cells were incubated with antibodies (anti-DLK) overnight at 4°C. Protein G-sepharose beads (GE Healthcare) were added to capture immune complexes for 3 hours and samples were spun down at 3,000 ×g for 5 minutes. The precipitated products were washed 3 times with lithium chloride buffer (500 mM lithium chloride, 100 mM Tris-HCl, pH 7.6, 0.1% Triton X-100 and 1 mM DTT) and boiled in 1x sample buffer for 5 minutes. For cross-linked immunoprecipitations, cells were lysed in lysis buffer without Tris-base and EDTA, and immune complexes bound to protein G-sepharose were incubated with 2mM of dithiobis succinimidyl propionate (DSP; Thermo Scientific) crosslinking reagent for 30 minutes. The remaining unreacted DSP was quenched with 20 mM of Tris-HCl, pH 7.5.
In kinase assays, precipitated proteins were aliquoted into 2 tubes after washing. One aliquot used for kinase reactions was re-washed 3 times with kinase buffer (20 mM MOPS, pH 7.2, 2 mM EGTA, 10 mM MgCl2, 0.1% Triton X-100, 1 mM DTT and 0.1 mM sodium orthovandate). Then reactions were carried out in kinase buffer with 1 μg of myelin basic protein (MBP; Sigma-Aldrich) and 5 μCi of [γ-32P]ATP for 15 minutes. After boiled in 1× sample buffer to stop reactions, samples were subjected to SDS-PAGE. Gels were dried on a gel dryer and emission signals of labeled MBP were visualized by scanning with a phospho-screen (Typhoon 9400; GE Healthcare). Finally, the other aliquot of precipitated protein was used for quantification of protein amounts by Western blotting
Recombinant protein purification and in vitro phosphorylation assays
DNA coded for C-terminal fragments of DLK from 541 to 888 amino acids (aa) residues and from 668 to 888 aa residues were cloned into pGEX-4T vector whose GST gene was replaced by 6xHis. The plasmids were transformed into E. coli BL21(DE3)pLysS, and recombinant DLK proteins were induced by IPTG and purified with His-Select Nickel Affinity Gel (Sigma-Aldrich). The bound proteins were eluted with buffer containing 100 mM sodium phosphate, pH 8.0, 5 M NaCl and 250 mM imidazole. To express mutated His-DLK, nucleotides coding for serine at 584 and threonine at 659 were both or separately mutated to alanine with PCR based site-directed mutagenesis. The expression and purification protocols of these proteins were the same as that of wild type His-DLK. Eluted proteins were quantified by comparing with BSA on SDS-PAGE and adjusted to the same concentration.
In phosphorylation assays, equal amounts of recombinant DLK were incubated with recombinant Akt (Millipore) and 5 μCi of [γ-32P]ATP for 15 minutes. Detection of DLK phosphorylation intensity was carried out by SDS-PAGE and phospho-image scanning (Typhoon 9400; GE Healthcare).
Statistics
The error bars in figures represent standard error of the mean (mean ± SEM). P values were obtained from 2-tailed Student's t-tests (***, P < 0.0001; **, P < 0.001; *, P < 0.05).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Funding
We thank the funding support from Academia Sinica, Taiwan Ministry of Science and Technology (NSC102-2321-B-001-013-, NSC103-2311-B-182-005-, NSC103-2321-B-001-064), National Health Research Institutes (EX102-10025S, EX104-10415SI), the Taiwan Ministry of Education (EMRP-D1D0771) and the Chang Gung Memorial Hospital (CMRP-D1B0313; CMRP-D1C0612).
References
- 1. Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 2008; 132:661-80; PMID:18295582; http://dx.doi.org/ 10.1016/j.cell.2008.02.008 [DOI] [PubMed] [Google Scholar]
- 2. Spagnoli FM, Hemmati-Brivanlou A. Guiding embryonic stem cells towards differentiation: lessons from molecular embryology. Curr Opin Genet Dev 2006; 16:469-75; PMID:16919445; http://dx.doi.org/ 10.1016/j.gde.2006.08.004 [DOI] [PubMed] [Google Scholar]
- 3. Niwa H. How is pluripotency determined and maintained? Development 2007; 134:635-46; PMID:17215298; http://dx.doi.org/ 10.1242/dev.02787 [DOI] [PubMed] [Google Scholar]
- 4. Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature 1988; 336:684-7; PMID:3143916; http://dx.doi.org/ 10.1038/336684a0 [DOI] [PubMed] [Google Scholar]
- 5. Davis S, Aldrich TH, Stahl N, Pan L, Taga T, Kishimoto T, Ip NY, Yancopoulos GD. LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science 1993; 260:1805-8; PMID:8390097; http://dx.doi.org/ 10.1126/science.8390097 [DOI] [PubMed] [Google Scholar]
- 6. Anneren C. Tyrosine kinase signalling in embryonic stem cells. Clin Sci 2008; 115:43-55; PMID:18547167; http://dx.doi.org/ 10.1042/CS20070388 [DOI] [PubMed] [Google Scholar]
- 7. He S, Nakada D, Morrison SJ. Mechanisms of stem cell self-renewal. Annu Rev Cell Dev Biol 2009; 25:377-406; PMID:19575646; http://dx.doi.org/ 10.1146/annurev.cellbio.042308.113248 [DOI] [PubMed] [Google Scholar]
- 8. Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature 2009; 460:118-22; PMID:19571885; http://dx.doi.org/ 10.1038/nature08113 [DOI] [PubMed] [Google Scholar]
- 9. Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 2003; 113:631-42; PMID:12787504; http://dx.doi.org/ 10.1016/S0092-8674(03)00393-3 [DOI] [PubMed] [Google Scholar]
- 10. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003; 113:643-55; PMID:12787505; http://dx.doi.org/ 10.1016/S0092-8674(03)00392-1 [DOI] [PubMed] [Google Scholar]
- 11. Young RA. Control of the embryonic stem cell state. Cell 2011; 144:940-54; PMID:21414485; http://dx.doi.org/ 10.1016/j.cell.2011.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marson A, Levine SS, Cole MF, Frampton GM, Brambrink T, Johnstone S, Guenther MG, Johnston WK, Wernig M, Newman J, et al. Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell 2008; 134:521-33; PMID:18692474; http://dx.doi.org/ 10.1016/j.cell.2008.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, Okochi H, Okuda A, Matoba R, Sharov AA, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol 2007; 9:625-35; PMID:17515932; http://dx.doi.org/ 10.1038/ncb1589 [DOI] [PubMed] [Google Scholar]
- 14. Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet 2006; 38:431-40; PMID:16518401; http://dx.doi.org/ 10.1038/ng1760 [DOI] [PubMed] [Google Scholar]
- 15. Chambers I, Silva J, Colby D, Nichols J, Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L, Smith A. Nanog safeguards pluripotency and mediates germline development. Nature 2007; 450:1230-4; PMID:18097409; http://dx.doi.org/ 10.1038/nature06403 [DOI] [PubMed] [Google Scholar]
- 16. Hawkins PT, Anderson KE, Davidson K, Stephens LR. Signalling through Class I PI3Ks in mammalian cells. Biochem Soc Trans 2006; 34:647-62; PMID:17052169; http://dx.doi.org/ 10.1042/BST0340647 [DOI] [PubMed] [Google Scholar]
- 17. Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 2006; 7:606-19; PMID:16847462; http://dx.doi.org/ 10.1038/nrg1879 [DOI] [PubMed] [Google Scholar]
- 18. Storm MP, Kumpfmueller B, Thompson B, Kolde R, Vilo J, Hummel O, Schulz H, Welham MJ. Characterization of the phosphoinositide 3-kinase-dependent transcriptome in murine embryonic stem cells: identification of novel regulators of pluripotency. Stem Cells 2009; 27:764-75; PMID:19350676; http://dx.doi.org/ 10.1002/stem.3 [DOI] [PubMed] [Google Scholar]
- 19. Lianguzova MS, Chuykin IA, Nordheim A, Pospelov VA. Phosphoinositide 3-kinase inhibitor LY294002 but not serum withdrawal suppresses proliferation of murine embryonic stem cells. Cell Biol Int 2007; 31:330-7; PMID:17321171; http://dx.doi.org/ 10.1016/j.cellbi.2007.01.019 [DOI] [PubMed] [Google Scholar]
- 20. Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene 2006; 25:2697-707; PMID:16407845; http://dx.doi.org/ 10.1038/sj.onc.1209307 [DOI] [PubMed] [Google Scholar]
- 21. Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem 2004; 279:48063-70; PMID:15328362; http://dx.doi.org/ 10.1074/jbc.M406467200 [DOI] [PubMed] [Google Scholar]
- 22. Storm MP, Bone HK, Beck CG, Bourillot PY, Schreiber V, Damiano T, Nelson A, Savatier P, Welham MJ. Regulation of Nanog expression by phosphoinositide 3-kinase-dependent signaling in murine embryonic stem cells. J Biol Chem 2007; 282:6265-73; PMID:17204467; http://dx.doi.org/ 10.1074/jbc.M610906200 [DOI] [PubMed] [Google Scholar]
- 23. Takahashi K, Matsui K, Yamanaka S. Role of ERas in promoting tumor-like properties in mouse embryonic stem cells. Nature 2003; 423:541-45; PMID:12774123; http://dx.doi.org/ 10.1038/nature01646 [DOI] [PubMed] [Google Scholar]
- 24. Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci U S A 1999; 96:6199-204; PMID:10339565; http://dx.doi.org/ 10.1073/pnas.96.11.6199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin Y, Yang Y, Li W, Chen Q, Li J, Pan X, Zhou L, Liu C, Chen C, He J, et al. Reciprocal regulation of Akt and Oct4 promotes the self-renewal and survival of embryonal carcinoma cells. Mol Cell 2012; 48:627-40; PMID:23041284; http://dx.doi.org/ 10.1016/j.molcel.2012.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang CH, Ma N, Lin YT, Wu CC, Hsiao M, Lu FL, Yu CC, Chen SY, Lu J. A shRNA functional screen reveals Nme6 and Nme7 are crucial for embryonic stem cell renewal. Stem Cells 2012; 30:2199-211; PMID:22899353; http://dx.doi.org/ 10.1002/stem.1203 [DOI] [PubMed] [Google Scholar]
- 27. Wang CH, Ma N, Lin YT, Wu CC, Wu HJ, Yu CC, Hsiao M, Lu FL, Schuyler SC, Lu J. Array-based high-throughput screening in mouse embryonic stem cells with shRNAs. Curr Protoc Stem Cell Biol 2013; 26:Unit 5C 3; PMID:24510793; http://dx.doi.org/ 10.1002/9780470151808.sc05c03s26 [DOI] [PubMed] [Google Scholar]
- 28. Fan G, mERRITT SE, Kortenjann M, Shaw PE, Holzman LB. Dual leucine zipper-bearing kinase (DLK) activates p46SAPK and p38mapk but not ERK2. J Biol Chem 1996; 271:24778-93 [DOI] [PubMed] [Google Scholar]
- 29. Merritt SE, Mata M, Nihalani D, Zhu C, Hu X, Holzman LB. The mixed lineage kinase DLK utilizes MKK7 and not MKK4 as substrate. J Biol Chem 1999; 274:10195-202; PMID:10187804; http://dx.doi.org/ 10.1074/jbc.274.15.10195 [DOI] [PubMed] [Google Scholar]
- 30. Nihalani D, Merritt S, Holzman LB. Identification of structural and functional domains in mixed lineage kinase dual leucine zipper-bearing kinase required for complex formation and stress-activated protein kinase activation. J Biol Chem 2000; 275:7273-9; PMID:10702297; http://dx.doi.org/ 10.1074/jbc.275.10.7273 [DOI] [PubMed] [Google Scholar]
- 31. Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 2002; 3:663-72; PMID:12209126; http://dx.doi.org/ 10.1038/nrm906 [DOI] [PubMed] [Google Scholar]
- 32. Nadeau A, Grondin G, Blouin R. In situ hybridization analysis of ZPK gene expression during murine embryogenesis. J Histochem Cytochem 1997; 45:107-18; PMID:9010475; http://dx.doi.org/ 10.1177/002215549704500114 [DOI] [PubMed] [Google Scholar]
- 33. Hirai S, Kawaguchi A, Hirasawa R, Baba M, Ohnishi T, Ohno S. MAPK-upstream protein kinase (MUK) regulates the radial migration of immature neurons in telencephalon of mouse embryo. Development 2002; 129:4483-95; PMID:12223406 [DOI] [PubMed] [Google Scholar]
- 34. Hirai S, Kawaguchi A, Suenaga J, Ono M, Cui DF, Ohno S. Expression of MUK/DLK/ZPK, an activator of the JNK pathway, in the nervous systems of the developing mouse embryo. Gene Expr Patterns: GEP 2005; 5:517-23; PMID:15749080; http://dx.doi.org/ 10.1016/j.modgep.2004.12.002 [DOI] [PubMed] [Google Scholar]
- 35. Hirai S, Cui de F, Miyata T, Ogawa M, Kiyonari H, Suda Y, Aizawa S, Banba Y, Ohno S. The c-Jun N-terminal kinase activator dual leucine zipper kinase regulates axon growth and neuronal migration in the developing cerebral cortex. J Neurosci: Off J Soc Neurosci 2006; 26:11992-2002; PMID:17108173; http://dx.doi.org/ 10.1523/JNEUROSCI.2272-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eto K, Kawauchi T, Osawa M, Tabata H, Nakajima K. Role of dual leucine zipper-bearing kinase (DLK/MUK/ZPK) in axonal growth. Neurosci Res 2010; 66:37-45; PMID:19808064; http://dx.doi.org/ 10.1016/j.neures.2009.09.1708 [DOI] [PubMed] [Google Scholar]
- 37. Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol 2002; 12:432-8; PMID:12220864; http://dx.doi.org/ 10.1016/S0962-8924(02)02352-8 [DOI] [PubMed] [Google Scholar]
- 38. Bechard M, Dalton S. Subcellular localization of glycogen synthase kinase 3beta controls embryonic stem cell self-renewal. Mol Cell Biol 2009; 29:2092-104; PMID:19223464; http://dx.doi.org/ 10.1128/MCB.01405-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P. Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett 1996; 399:333-8; PMID:8985174; http://dx.doi.org/ 10.1016/S0014-5793(96)01370-1 [DOI] [PubMed] [Google Scholar]
- 40. Obata T, Yaffe MB, Leparc GG, Piro ET, Maegawa H, Kashiwagi A, Kikkawa R, Cantley LC. Peptide and protein library screening defines optimal substrate motifs for AKT/PKB. J Biol Chem 2000; 275:36108-15; PMID:10945990; http://dx.doi.org/ 10.1074/jbc.M005497200 [DOI] [PubMed] [Google Scholar]
- 41. Zhang X, Yalcin S, Lee DF, Yeh TY, Lee SM, Su J, Mungamuri SK, Rimmelé P, Kennedy M, Sellers R, et al. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol 2011; 13:1092-9; PMID:21804543; http://dx.doi.org/ 10.1038/ncb2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu Y, Liang D, Tian Q, Chen X, Jiang B, Chou BK, Hu P, Cheng L, Gao P, Li J, et al. Stimulation of somatic cell reprogramming by ERas-Akt-FoxO1 signaling axis. Stem Cells 2014; 32:349-63; PMID:23765875; http://dx.doi.org/ 10.1002/stem.1447 [DOI] [PubMed] [Google Scholar]
- 43. Ryu JM, Han HJ. L-threonine regulates G1/S phase transition of mouse embryonic stem cells via PI3K/Akt, MAPKs, and mTORC pathways. J Biol Chem 2011; 286:23667-78; PMID:21550972; http://dx.doi.org/ 10.1074/jbc.M110.216283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature 2008; 453:519-23; PMID:18497825; http://dx.doi.org/ 10.1038/nature06968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Martello G, Sugimoto T, Diamanti E, Joshi A, Hannah R, Ohtsuka S, Göttgens B, Niwa H, Smith A. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell 2012; 11:491-504; PMID:23040478; http://dx.doi.org/ 10.1016/j.stem.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maroney AC, Finn JP, Connors TJ, Durkin JT, Angeles T, Gessner G, Xu Z, Meyer SL, Savage MJ, Greene LA, et al. Cep-1347 (KT7515), a semisynthetic inhibitor of the mixed lineage kinase family. J Biol Chem 2001; 276:25302-8; PMID:11325962; http://dx.doi.org/ 10.1074/jbc.M011601200 [DOI] [PubMed] [Google Scholar]
- 47. Welsbie DS, Yang Z, Ge Y, Mitchell KL, Zhou X, Martin SE, Berlinicke CA, Hackler L, Jr, Fuller J, Fu J, et al. Functional genomic screening identifies dual leucine zipper kinase as a key mediator of retinal ganglion cell death. Proc Natl Acad Sci U S A 2013; 110:4045-50; PMID:23431148; http://dx.doi.org/ 10.1073/pnas.1211284110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vandermoere F, El Yazidi-Belkoura I, Slomianny C, Demont Y, Bidaux G, Adriaenssens E, Lemoine J, Hondermarck H. The valosin-containing protein (VCP) is a target of Akt signaling required for cell survival. J Biol Chem 2006; 281:14307-13; PMID:16551632; http://dx.doi.org/ 10.1074/jbc.M510003200 [DOI] [PubMed] [Google Scholar]
- 49. Vandermoere F, El Yazidi-Belkoura I, Demont Y, Slomianny C, Antol J, Lemoine J, Hondermarck H. Proteomics exploration reveals that actin is a signaling target of the kinase Akt. Mol Cell Proteomics 2007; 6:114-24; PMID:17088265; http://dx.doi.org/ 10.1074/mcp.M600335-MCP200 [DOI] [PubMed] [Google Scholar]
- 50. Huang Q, Lan F, Zheng Z, Xie F, Han J, Dong L, Xie Y, Zheng F. Akt2 kinase suppresses glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-mediated apoptosis in ovarian cancer cells via phosphorylating GAPDH at threonine 237 and decreasing its nuclear translocation. J Biol Chem 2011; 286:42211-20; PMID:21979951; http://dx.doi.org/ 10.1074/jbc.M111.296905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Robitaille K, Daviau A, Lachance G, Couture JP, Blouin R. Calphostin C-induced apoptosis is mediated by a tissue transglutaminase-dependent mechanism involving the DLK/JNK signaling pathway. Cell Death Differ 2008; 15:1522-31; PMID:18497756; http://dx.doi.org/ 10.1038/cdd.2008.77 [DOI] [PubMed] [Google Scholar]
- 52. Robitaille K, Daviau A, Tucholski J, Johnson GV, Rancourt C, Blouin R. Tissue transglutaminase triggers oligomerization and activation of dual leucine zipper-bearing kinase in calphostin C-treated cells to facilitate apoptosis. Cell Death Differ 2004; 11:542-9; PMID:14739943; http://dx.doi.org/ 10.1038/sj.cdd.4401392 [DOI] [PubMed] [Google Scholar]
- 53. Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 2012; 74:1015-22; PMID:22726832; http://dx.doi.org/ 10.1016/j.neuron.2012.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Watkins TA, Wang B, Huntwork-Rodriguez S, Yang J, Jiang Z, Eastham-Anderson J, Modrusan Z, Kaminker JS, Tessier-Lavigne M, Lewcock JW. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci U S A 2013; 110:4039-44; PMID:23431164; http://dx.doi.org/ 10.1073/pnas.1211074110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Robitaille H, Simard-Bisson C, Larouche D, Tanguay RM, Blouin R, Germain L. The small heat-shock protein Hsp27 undergoes ERK-dependent phosphorylation and redistribution to the cytoskeleton in response to dual leucine zipper-bearing kinase expression. J Invest Dermatol 2010; 130:74-85; PMID:19675578; http://dx.doi.org/ 10.1038/jid.2009.185 [DOI] [PubMed] [Google Scholar]
- 56. Robitaille H, Proulx R, Robitaille K, Blouin R, Germain L. The mitogen-activated protein kinase kinase kinase dual leucine zipper-bearing kinase (DLK) acts as a key regulator of keratinocyte terminal differentiation. J Biol Chem 2005; 280:12732-41; PMID:15695824; http://dx.doi.org/ 10.1074/jbc.M411619200 [DOI] [PubMed] [Google Scholar]
- 57. Proetzel G, Wiles MV. The use of a chemically defined media for the analyses of early development in ES cells and mouse embryos. Methods Mol Biol 2002; 185:17-26; PMID:11768988 [DOI] [PubMed] [Google Scholar]
- 58. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. Clustal W and clustal X version 2.0. Bioinformatics 2007; 23:2947-8; PMID:17846036; http://dx.doi.org/ 10.1093/bioinformatics/btm404 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


