Abstract
Numerous studies in the past years provided definite evidence that the nuclear envelope is much more than just a simple barrier. It rather constitutes a multifunctional platform combining structural and dynamic features to fulfill many fundamental functions such as chromatin organization, regulation of transcription, signaling, but also structural duties like maintaining general nuclear architecture and shape. One additional and, without doubt, highly impressive aspect is the recently identified key function of selected nuclear envelope components in driving meiotic chromosome dynamics, which in turn is essential for accurate recombination and segregation of the homologous chromosomes. Here, we summarize the recent work identifying new key players in meiotic telomere attachment and movement and discuss the latest advances in our understanding of the actual function of the meiotic nuclear envelope.
Keywords: germ cells, KASH domain proteins, LINC complex, meiosis, nuclear envelope, SUN domain proteins, telomeres
Introduction
Recent years have significantly advanced our understanding of the nuclear envelope (NE) from a mere barrier between nucleus and cytoplasm to an active platform regulating a broad variety of cellular functions, mediated by selected components. As such, particular components of the NE have been implicated in the regulation and maintenance of nuclear structure, in directional nuclear movement and anchorage, as well as in signaling, general nuclear and chromatin organization and gene regulation (for review see).1 Several studies in the field further established a key function for the NE in meiotic chromosome alignment and synaptic pairing of the homologs.2 Though the NE turned out to be a crucial determinant for reproduction and fertility, how the NE actually contributes to meiotic chromosome behavior and accurate genome haploidization until recently was not that clear. Within this Extra View we now summarize important recent work identifying new players in NE attachment and movement of meiotic telomeres and discuss the latest advances providing novel insights into the actual function of the meiotic NE.
Structurally, the NE can be subdivided into four main components: the outer nuclear membrane (ONM), the inner nuclear membrane (INM), the nuclear pore complexes and the nuclear lamina. The ONM and INM are two distinct membrane systems, merging only at sites were the nuclear pore complexes are inserted into the NE. The ONM is in continuity with the membrane of the endoplasmic reticulum, making the lumen between ONM and INM, the perinuclear space (PNS), a continuous lumen with that of the endoplasmic reticulum. The composition of proteins associated with and inserted into the INM and ONM strictly differ from each other. The conserved KASH domain proteins (Klarsicht ANC-1 Syne-homology) are one example of a class of proteins predominantly associated with the ONM.3,4 On the other hand, there are numerous prominent examples of membrane proteins restricted to the INM, such as the SUN domain (Sad-1 UNC-84 homology) or LEM-domain proteins (LAP2, emerin, MAN1 homology).5 The SUN domain proteins of the INM on the one hand and the KASH domain proteins of the ONM on the other hand are of particular interest as these proteins together form a membrane-spanning protein complex by directly interacting with each other within the PNS. This SUN-KASH complex (the so-called LINC complex; linker of nucleoskeleton and cytoskeleton)6 enables a direct communication of the INM and the ONM and their associated components, thereby mediating a connection between nuclear and cytoplasmic content. The LINC complex is also responsible for the transduction of cytoskeletal forces to the NE and into the nucleus through the demonstrated interactions of KASH domain proteins with components of the cytoskeleton or associated motorproteins. The cytoskeletal forces have been shown to be essential for nuclear positioning and anchorage as well as for the directed movement of meiotic chromosomes within the nucleus.2,7
The nuclear lamina is a dense protein network intimately associated with the nucleoplasmic side of the INM. The main structural components of the nuclear lamina are the lamin proteins, which directly or indirectly interact with numerous membrane proteins of the INM. Lamins are a class of intermediate filament proteins, competent to self-assemble into the highly structured and organized network of the nuclear lamina. This self-assembly is mediated by the functional domains at the N-terminal and C-terminal ends of the central rod domain.8 Lamin proteins can be classified into A- and B-type lamins, differing in their expression patterns, biochemical features and polymerisation characteristics during mitotic nuclear envelope breakdown and reassembly.9,10 In mammals, the B-type lamins B1, B2 and B3 are encoded by two genes, LMNB1 and LMNB2,14-17 whereas all A-type lamins, the lamins A, AΔ10, C, and C2, are isoforms expressed from the single LMNA gene.14-17 While at least one B-type lamin seems to be present in all nucleated cell types, the expression of A-type lamins is rather developmentally regulated and most prominent in differentiated cells. The tight and highly ordered lamina network, which in most mammalian cell types is composed of two A- and two B-type lamins, is known to be essential for the maintenance of nuclear stability and shape. Furthermore, the nuclear lamina is involved in chromatin organization and gene regulation through both direct and indirect interactions with chromatin.18,19
Adaptations of the Meiotic Nuclear Envelope are Related to its Specific Function
Although the basic features of the NE are highly conserved between all eukaryotic cells, numerous cell type specific adaptations regarding its structure and composition have been described in the recent years. A striking example for cell type specific modulation of the NE concerns the central LINC complex components and their interaction partners. Mammals contain at least five different SUN domain protein coding genes and five genes coding for KASH proteins. This renders the possibility for the assembly of diverse LINC complexes with distinct functions, which in turn connect different nuclear structures to specific cytoskeletal elements. Recent studies show clear evidence that individual SUN and KASH proteins or derived splice isoforms are differentially expressed, strongly pointing toward cell type and tissue specific adaptations of LINC complex composition.20-24
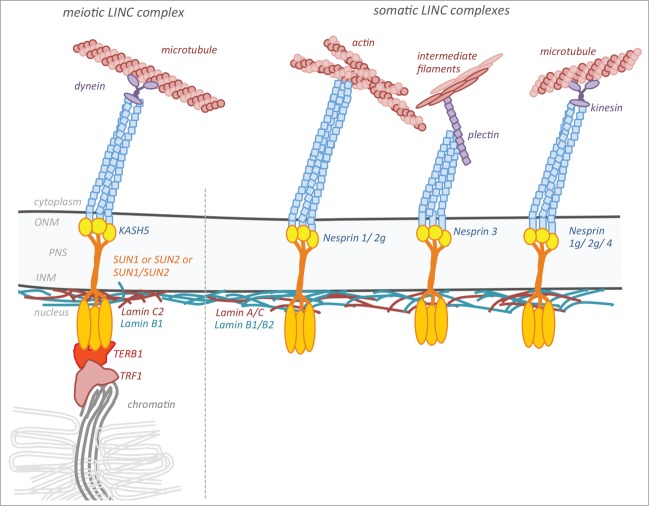
Cell type specific expression and assembly of LINC components is a prominent feature of mammalian meiosis.2 Meiosis is a specialized cell division resulting in haploid cells capable to differentiate into fertilisation competent gametes. A conserved hallmark of early meiotic stages is the tethering and intimate attachment of chromosome ends to the NE during early prophase I. The attached chromosome ends are actively repositioned within the NE during the progress of prophase I and transiently cluster within a restricted area of the NE adjacent to the microtubule organizing center (MTOC).25,26 Attachment and movement of the meiotic chromosomes are proposed to facilitate the pairing and synapsis of the homologous chromosomes as well as efficient meiotic homologous recombination, both being essential for the successful completion of meiosis and thus fertility.27 Telomere tethering and movement during meiosis require a uniquely remodeled NE and are mediated by meiosis-specific LINC complexes.28 In mice, these are composed of the conventional, ubiquitously expressed SUN domain proteins, which assemble with KASH5, a meiosis-specific KASH domain protein, to form a unique meiosis-specific nucleocytoplasmatic bridge29-31 (Fig. 1).
Figure 1.
Mammalian meiotic and somatic LINC complexes. The SUN-domain proteins SUN1 and/or SUN2 are central INM components of mammalian LINC complexes, both in meiotic and somatic cells. The meiotic LINC complex further consists of the ONM residing meiosis-specific KASH5 protein, which via dynein/dynactin likely connects to the microtubule cytoskeleton. In somatic cells, versatile, cell-type specific KASH-domain proteins interact with SUN1 and/or 2 to form several distinct LINC complexes. The different KASH proteins in turn specifically connect to different components of the cytoskeleton, allowing the LINC complexes to fulfill specialized cellular functions.
Concomitant with the conversion of the LINC complexes, also the nuclear lamina undergoes remarkable germ cell specific rearrangements. In meiotic cells, it consists only of one B-type lamin, lamin B1, and the unconventional A-type lamin isoform, lamin C2. Lamin C2 is exclusively found in the lamina of meiotic cells, implicating an additional essential adaptation of the NE to the requirements of meiotic cell function.15,32 These remarkable modifications within the composition and structure of the NE clearly emphasize the active role of the NE in meiotic progression, successful completion of recombination and genome haploidization, which represent most central aspects of fertility and reproduction.
Components of the Meiotic LINC Complex Involved in Tethering Telomeres to the NE
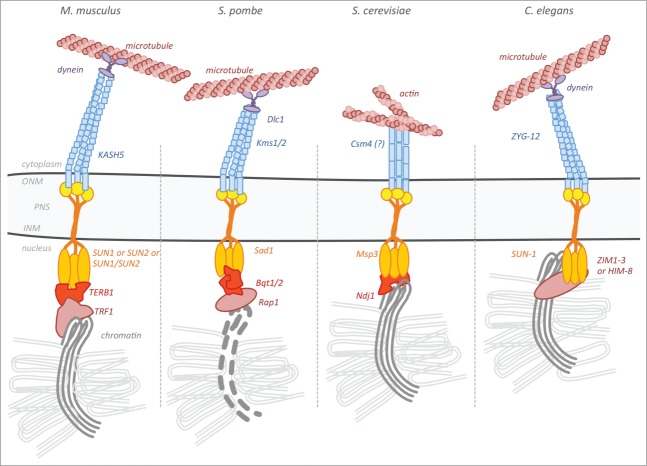
A most conserved feature of meiosis is the tethering and stable attachment of chromosome ends to the NE. This prerequisite for successful completion of meiosis has been found to be mediated by SUN and KASH domain proteins in all meiotic model organisms studied so far (Fig. 2). Particularly with regard to mammals, the continuous work over the past years finally disclosed the composition of the anchors connecting the meiotic telomeres to the NE and some research groups set out to elucidate the precise functions of the individual components. The first identified mammalian constituents of these highly specialized telomere anchors were the ubiquitously expressed LINC complex components SUN1 and SUN2.29,30 At the onset of meiosis both, SUN1 and SUN2, show a unique and quite remarkable redistribution. In contrast to the homogenous distribution of the two SUN domain proteins in the NE of somatic cells, in meiotic prophase I cells they feature a peculiar punctuated pattern within the NE, located only at the sites of telomere attachment. Interestingly, the redistribution of ubiquitously expressed SUN domain proteins to the sites of telomere attachment upon the induction of meiosis is not specific for mammals, but also observed in all other meiotic model organisms studied so far. With initiation of meiosis in the fission yeast, for example, the ubiquitiously expressed SUN domain protein Sad1 transiently relocates from its somatic localization at the spindle pole body (SPB) into smaller foci within the NE to the sites, where the telomeres are attached.33,34
Figure 2.
Meiotic LINC complexes of common model organisms. Meiotic LINC complexes of the different model organisms are all composed of specialized SUN-KASH protein bridges that are used to connect meiotic telomeres to the cytoskeleton. It requires unique adaptor proteins responsible to connect the chromosome ends to the nuclear domain of the SUN protein. These adaptor proteins are highly diverged, species-specific, most likely analogous proteins with similar function. The KASH component of meiotic LINC complexes in most cases interact with microtubule-associated motor proteins. The only described exception refers to S. cerevisiae, in which Csm4 likely links to the actin cytoskeleton.
With Kms1, a KASH domain protein was identified that directly interacts with Sad1 to form a functional meiotic LINC complex in S. pombe.35 Through the connection of Kms1 to the microtubule motor system, this complex facilitates bouquet arrangement of chromosomes with all telomeres tightly clustered at the SPB.36,37 Similar to Kms1 in the fission yeast, the KASH domain partner within the meiotic LINC complex of C. elegans, ZYG-12, which was also among the earliest identified meiotic KASH domain proteins,38 also connects the NE-tethered meiotic telomeres to the microtubule cytoskeleton. Although in the case of mammals the existence of a protein capable to connect meiotic telomeres to a cytoplasmic motor system was postulated as well,30,39 the real meiotic KASH partner of SUN1 and/or SUN2 remained elusive for substantial time. But finally, Morimoto and colleagues could in fact identify a new KASH protein specific for mammalian meiotic cells, which they named KASH531. Following this initial work defining KASH5 as a SUN1 and SUN2 interacting meiotic LINC component, which actually mediates the linkage of meiotic telomeres to the microtubule cytoskeleton, a second, independent study could demonstrate the essential function of KASH5 for meiotic chromosome dynamics and meiotic progression.40 Hence, these two studies provided clear evidence that similar to the situation in lower eukaryotes telomere movement in mammals is also mediated by the microtubule cytoskeleton through the interaction of KASH5 with the dynein-dynactin complex (Fig. 1, Fig. 2).
During meiotic prophase I chromosome ends must attach to the NE stably enough to withstand the vigorous movements during bouquet formation and release, which in turn is required for accurate chromosome pairing and recombination.27 To ensure a mechanically robust connection between telomeres and meiotic LINC complexes, different organisms employed unique meiotic adaptor proteins (Fig. 2), which are not well conserved between the different species. Very prominent examples of such adaptor proteins are the meiosis-specific Bqt proteins 1–4 of fission yeast. These proteins form a complex essential for the stable attachment of telomeres to the NE and their subsequent movement within the NE during prophase I.41-43 In budding yeast meiosis, this important task of tethering chromosome ends to the SUN nucleoplasmic domain appears to be taken by Ndj1.44,45 How meiotic telomeres actually bind to the nucleoplasmic side of the LINC complexes in mammals has remained uncertain for a long time. Very recent work, however, was finally able to identify the meiosis specific adaptor protein TERB1. Like the Bqt-complex in the fission yeast and Ndj1 in the budding yeast, mammalian TERB1 was also shown to connect meiotic telomeres to the SUN domain proteins.46,47 With this, the existence of a meiotic LINC complex with all its necessary components to connect chromosome ends through the NE to the cytoskeletal motor system can now be postulated not only for lower organisms, but also for higher organisms such as mammals (Fig. 2). The fact that there is no sequence similarity between TERB1, Ndj1 and any of the Bqt proteins is quite surprising and raises the question, whether the adaptor mechanisms mediating the anchorage of the meiotic telomeres to the nucleoplasmic compartment of the LINC complexes have evolved more than once during evolution.
Analyses of mammalian meiotic LINC complex function have mostly been conducted through the investigation of meiotic phenotypes caused by the deficiency of its respective components. Mice deficient for either SUN1, KASH5 or TERB1 show a strong meiotic phenotype with homologous pairing being severely affected, leading to a dramatic increase in apoptosis by mid-pachytene stage and, as a consequence, to infertility. Regarding TERB1, mice deficient for this meiosis-specific telomere adaptor protein, show an almost entire loss of telomere attachment to the NE, abolishment of telomere movement and a severely defective homologous pairing.47 This phenotype is assumed to be the consequence of the loss of the connection between chromosome ends and the INM located SUN domain proteins.46 Consistent with the perception of cytoplasmic driven meiotic chromosome movements, which are assumed to be required for chromosome pairing and recombination,27 mice deficient for KASH5 also show almost complete absence of homologous pairing. In addition, KASH5 deficient mice also exhibit a reduction in telomere-NE associations.31 Whether the loss of telomere attachment is due to reduced attachment of telomeres or premature loss of telomere attachment is, however, unclear. Nonetheless, it is postulated that the absence of KASH5 leads to a misorganization of SUN1 within the NE, possibly reducing the stability or efficiency of telomere attachment within the NE.40 Functional analyses of the SUN domain proteins involved in this meiotic LINC complex have proven to be slightly more complex, due to the ubiquitous expression of both SUN1 and SUN2, on the one hand, and due to their demonstrated functional redundancy in somatic cells, on the other hand.48 Mice deficient for SUN1 were shown to be infertile.29,49 They show increased apoptosis of the germ cells, yet the meiotic phenotype is significantly less severe than that observed in KASH5 or TERB1 deficient mice. Homologous pairing, for example, though impaired and incomplete in SUN1 deficient mice, to some extend still occurs leading to at least partial synapsis of homologous chromosomes in spermatocytes.50 Interestingly, even though SUN1 was absent, a significant progression of recombination could be observed during oogenesis. This suggests, that, although critical for completion of meiosis per se, SUN1 may be to some extent dispensable for the attachment of telomeres to the meiotic NE. Taking into account the redundant functions of SUN1 and SUN2 in somatic nuclear anchorage and positioning as well as the shared localization of SUN1 and SUN2 in meiotic cells, it seemed plausible that in the absence of SUN1 some telomeres may still manage to attach to the NE, likely through the contribution of SUN2.50 Indeed, recent cytological and electron microscopy analyses of SUN1 deficient meiocytes were able to demonstrate that in the absence of SUN1 a major fraction of meiotic telomeres is still able to attach to the NE forming attachment plates similar to those observed in wildtype meiocytes. Furthermore, in the absence of SUN1, SUN2 and KASH5 localize to the telomeres that are still associated with the NE. This suggests that the remaining components of the mammalian meiotic LINC complex, in principle, are still capable to connect telomeres to the NE. The functionality of the LINC complex in attaching telomeres in the absence of SUN1 is also reflected in the ability of these telomeres to move within the NE, forming wildtype-like bouquet clusters.50 These observations suggest that, similar to the situation described for somatic cells, SUN1 and SUN2 may fulfill at least to some extent redundant functions within the meiotic LINC complex as well. This is well in accordance with the less severe meiotic phenotype in the SUN1 deficient mice compared to the phenotypes caused by KASH5 or TERB1 deficiency, respectively. However, how the meiosis-specific functions of the ubiquitously expressed SUN1 and SUN2 are regulated in mice, still remains to be elucidated.
With regard to this, studies in C. elegans have demonstrated that the SUN domain protein implicated in chromosome end attachment, SUN-1, is subjected to substantial post-translational modifications, regulating its meiotic functions and mediating its role in meiotic checkpoint mechanisms. In the case of SUN-1, N-terminal phosphorylations lead to increased mobility of the SUN-1 aggregates within the NE during early meiotic stages, thus mediating the observed rapid movements of the attached chromosome ends typical for these meiotic stages. Upon progression of homologous pairing, subsequent dephosphorylation of specific residues within SUN-1 at later meiotic stages are required to monitor the regulated progression of meiosis into pachytene, demonstrating the involvement of SUN-1 in a feedback checkpoint mechanism.51,52 It appears plausible, but yet to be determined, whether similar modifications may also occur on mammalian SUN domain proteins to regulate their redistribution within the NE and their meiosis-specific interactions with other LINC complex components. Interestingly, a recent study reported that in human cells during mitosis the SUN1 nucleoplasmic domain becomes phosphorylated at different sites, most likely by Cdk1 and/or Plk1. This phosphorylation in turn accounts for a significant weakening of SUN1 binding to other NE components.53 During mammalian meiotic prophase I the mitotic kinase Cdk2, which in somatic cells is partially redundant with Cdk1, can be found highly accumulated at the telomeres of meiotic chromosomes. This suggests a critical role of Cdk2 in meiotic telomere maintenance, attachment and/or movement.54,55 Consistent with this, Cdk2 deficient mice show severely defective meiotic telomeres, with a significant number of telomeres being not accurately attached at the NE.55-57 Recent more detailed analysis of the Cdk2 deficient meiotic phenotype confirmed a crucial role of Cdk2 in telomere attachment and, furthermore, in regulating general meiotic NE integrity and dynamics, which particularly also concerns SUN1, a bona fide target of Cdk2.58
Adaptations of the Nuclear Envelope to Facilitate Meiotic Chromosome Movements
Not only is the attachment of telomeres within the NE an essential feature for meiotic success, but also their directed and strongly regulated movement within the NE. These movements are considered to be operated by cytoskeletal forces that are transferred to the chromosomes via the meiotic LINC complexes. During this choreography, chromosome ends transiently cluster within a restricted region, forming the so called bouquet-stage. The actual function of the formation and release of the meiotic bouquet is still not entirely clear. However, it seems likely that bringing chromosome ends together to a more confined space within the nuclear periphery supports homologous pairing, synapsis formation, double strand break (DSB) repair and recombination. Furthermore, the active release of the meiotic bouquet is implicated to prevent and dissolve unwanted, heterologous associations between non-homologous chromosomes to avoid failures in homologous pairing.27,59 Thus, the tightly regulated movement of the attached chromosomes appears to be an essential prerequisite for the efficient and successful completion of meiosis in numerous model organisms, from yeast to mammals.
From several studies it is evident that the NE of meiotic cells serves as a platform for the movement of the attached chromosomes and must therefore be stable enough to withstand the forces generated by the cytoskeleton and transferred by the LINC complex. On the other hand, the NE also has to be flexible enough to allow the rapid movement of the LINC complexes and attached telomeres within it. In somatic cells, the nuclear lamina is a rigid protein network, renowned for its function in maintaining nuclear stability and shape. In mammals, the nuclear lamina, like many other components of the NE, undergoes remarkable remodeling at the onset of meiosis. This remodeling is reflected in the composition of the meiotic lamina, where the meiosis specific A-type lamin C2 is the only A-type lamin expressed besides the ubiquitously expressed B-type lamin, lamin B1.32,60,61 Compared to its somatic counterparts, lamin C2 is an N-terminally truncated isoform, missing the globular head and the N-terminal part of the central coiled-coil domain. These domains are replaced by a unique hexapeptide sequence required for its targeting to the NE.62 Hence, meiotic lamin C2 is lacking protein domains, which in somatic lamins have been demonstrated to be critical for the longitudinal and lateral higher ordered polymerisation of the lamina network. Consistent with this, lamin C2 shows a significantly increased mobility within the nuclear lamina compared to the somatic A-type lamin variants.63 The general localization of lamin C2 within the NE is also unique in that it does not localize homogenously within the meiotic lamina, but rather enriches within domains at sites were telomeres are attached to the NE. Investigating mice specifically deficient for this meiosis-specific lamin variant demonstrated that lamin C2 is critical for the correct and efficient movement of attached telomeres.64 In the absence of lamin C2 the release of the meiotic bouquet is significantly delayed, leading to failures in synapsis of the homologs and a high incidence of heterologous associations. Furthermore, lamin C2 deficient mice show defective DSB repair and recombination, culminating in infertility of the male mice. Taking into account the peculiar characteristics of lamin C2, it is assumed that lamin C2 locally increases the flexibility of the meiotic nuclear lamina to allow efficient movement of the attached telomeres within the NE. Therefore, this remarkable meiotic nuclear lamina adaptation appears to be another critical feature of the mammalian meiotic NE essential for correct and proper meiotic progression and fertility.64
Studies on the function of the meiotic nuclear lamina in mammals clearly indicate that besides bouquet formation, the release of the meiotic bouquet may be critical for efficient repair of DSB and for resolving heterologous associations between non-homologous chromosomes. This is essential to prevent defects in synaptic pairing of the homologs and required to avoid mistakes in chromosome segregation. Recently it was hypothesized, that homolog recognition and synaptic pairing in mice is mainly mediated by searching for homology in chromosome architecture. This could be defined by meiotic cohesion, rather than being supported by more general dynamic chromosome reorganization, SPO11 dependent DSB inductions or telomere driven chromosome movements.65 That homolog pairing in mammals may occur independent from telomere movements was inferred from the fact that in SUN1 deficient mice a significant number of chromosomes retain the ability to pair and synapse. This conclusion was based on the supposition that in the absence of SUN1 all telomeres are detached from the NE. However, in a recent study we provided definite evidence that in SUN1 deficient mice up to 70% of the telomeres are still connected to the NE and to the cytoskeletal motor system. This telomere attachment in the absence of SUN1 is likely accomplished due to a partial redundant function of a SUN2-KASH5 bridging complex.50 Since the attached telomeres in SUN1 deficient meiocytes are still capable to accomplish a bouquet-like conformation, a significant influence of gathering these NE attached telomeres on homolog recognition and synaptic pairing seems to be plausible. In fact, it could well explain for partial synapsis and the significant progression of recombination, which is observed in the SUN1 deficient mouse model system.29,49,50 This perception receives further support by the results obtained with KASH5 and TERB1 deficient mice. KASH5 or TERB1 deficiency both lead to functionally disrupted telomere-cytoskeletal connection, which compared to SUN1 deficient mice actually lead to more severe phenotypes.40,47 Interestingly, two recent studies described that during medaka and zebrafish meiosis synaptonemal complex formation and synaptic pairing of the homologs not only timely correlate with bouquet formation, but are initiated near NE attached and tightly clustered chromosomal ends, from where synapsis then disperses over the entire homologs. This indicates that at least in the fish synaptic pairing is mediated by telomere driven chromosome gathering.66,67
In any case, chromosome cohesion without doubt has a very central function in initial meiotic chromosome pairing and further meiotic progression as evidenced by mice showing defective meiotic cohesion.68-74 Meiotic cohesion is established very early in meiosis, giving it a predisposition for mediating primary homolog interactions. Additionally, intermediate meiotic processes such as the assembly of the synaptonemal complexes and the efficient repair of DSBs imperatively depend on proper meiotic cohesion. Nonetheless, recent results from mouse models, where early and intermediate meiotic events are still intact, yet later meiotic events are defective, clearly point toward the importance of other mechanisms for homologous pairing and meiotic progression as well. In lamin C2 deficient meiocytes, for example, several normal appearing synaptonemal complexes are still assembled and early steps of DSB formation and repair occur. However, in these mice the release of the meiotic bouquet is significantly impaired, leading to an accumulation of heterologous associations and defects in late recombination events.64 At present it seems evident that the mechanisms required for the establishment of correct homologous associations rely on combination of both, the internal nuclear cohesion-dependent chromosome reorganization and the telomere driven chromosome rearrangements, which mainly occur during the formation and release of the meiotic bouquet. Directed telomere movements appear to be particularly critical for avoiding incorrect heterologous pairing, efficient DSB repair and meiotic recombination.
Concluding Remarks
Both telomere attachment and their regulated movement within the NE are critical parameters for meiosis and thus are pivotal for fertility. Telomere tethering and attachment is mediated by specialized components of the NE, fulfilling meiosis-specific functions. Meiosis-specific adaptations and features of the NE and lamina have evolved to facilitate the efficient movement of the attached telomeres within the NE. Recent studies investigating mammalian meiotic NE components have now provided detailed insights into a number of specialized meiotic adaptations of the NE required to facilitate meiotic functions, highlighting the active role of the NE as a regulatory platform for nuclear processes. Nonetheless, many question, in particular regarding the cell type specific regulation of ubiquitously expressed NE components, such as the two core LINC constituents SUN1 and SUN2, remain to be investigated and answered in the future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
Our work on the meiotic nuclear envelope was supported by the Priority Program 1384 “Mechanisms of genome haploidization” of the German Research Foundation (grant Al 1090/2–1) and the Graduate School GK1048 of the University of Würzburg.
References
- 1. Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Dev Cell 2009; 17:626-38; PMID:19922868; http://dx.doi.org/ 10.1016/j.devcel.2009.10.016 [DOI] [PubMed] [Google Scholar]
- 2. Kracklauer MP, Link J, Alsheimer M. LINCing the nuclear envelope to gametogenesis. Curr Top Dev Biol 2013; 102:127-57; PMID:23287032; http://dx.doi.org/ 10.1016/B978-0-12-416024-8.00005-2 [DOI] [PubMed] [Google Scholar]
- 3. Wilhelmsen K, Ketema M, Truong H, Sonnenberg A. KASH-domain proteins in nuclear migration, anchorage and other processes. J Cell Sci 2006; 119:5021-9; PMID:17158909; http://dx.doi.org/ 10.1242/jcs.03295 [DOI] [PubMed] [Google Scholar]
- 4. Rothballer A, Kutay U. The diverse functional LINCs of the nuclear envelope to the cytoskeleton and chromatin. Chromosoma 2013; 122:415-29; PMID:23736899; http://dx.doi.org/ 10.1007/s00412-013-0417-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gomez-Cavazos JS, Hetzer MW. Outfits for different occasions: tissue-specific roles of Nuclear Envelope proteins. Curr Opin Cell Biol 2012; 24:775-83; PMID:22995343; http://dx.doi.org/ 10.1016/j.ceb.2012.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol 2006; 172:41-53; PMID:16380439; http://dx.doi.org/ 10.1083/jcb.200509124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Razafsky D, Hodzic D. Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J Cell Biol 2009; 186:461-72; PMID:19687252; http://dx.doi.org/ 10.1083/jcb.200906068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu Rev Biochem 2004; 73:749-89; PMID:15189158; http://dx.doi.org/ 10.1146/annurev.biochem.73.011303.073823 [DOI] [PubMed] [Google Scholar]
- 9. Stuurman N, Heins S, Aebi U. Nuclear lamins: their structure, assembly, and interactions. J Struct Biol 1998; 122:42-66; PMID:9724605; http://dx.doi.org/ 10.1006/jsbi.1998.3987 [DOI] [PubMed] [Google Scholar]
- 10. Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol 2005; 6:21-31; PMID:15688064; http://dx.doi.org/ 10.1038/nrm1550 [DOI] [PubMed] [Google Scholar]
- 11. Hoger TH, Krohne G, Franke WW. Amino acid sequence and molecular characterization of murine lamin B as deduced from cDNA clones. Eur J Cell Biol 1988; 47:283-90; PMID:3243285 [PubMed] [Google Scholar]
- 12. Zewe M, Hoger TH, Fink T, Lichter P, Krohne G, Franke WW. Gene structure and chromosomal localization of the murine lamin B2 gene. Eur J Cell Biol 1991; 56:342-50; PMID:1802718 [PubMed] [Google Scholar]
- 13. Furukawa K, Hotta Y. cDNA cloning of a germ cell specific lamin B3 from mouse spermatocytes and analysis of its function by ectopic expression in somatic cells. Embo J 1993; 12:97-106; PMID:8094052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisher DZ, Chaudhary N, Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci U S A 1986; 83:6450-4; PMID:3462705; http://dx.doi.org/ 10.1073/pnas.83.17.6450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Furukawa K, Inagaki H, Hotta Y. Identification and cloning of an mRNA coding for a germ cell-specific A-type lamin in mice. Exp Cell Res 1994; 212:426-30; PMID:8187835; http://dx.doi.org/ 10.1006/excr.1994.1164 [DOI] [PubMed] [Google Scholar]
- 16. Nakajima N, Abe K. Genomic structure of the mouse A-type lamin gene locus encoding somatic and germ cell-specific lamins. FEBS Lett 1995; 365:108-14; PMID:7781761; http://dx.doi.org/ 10.1016/0014-5793(95)00453-G [DOI] [PubMed] [Google Scholar]
- 17. Machiels BM, Zorenc AH, Endert JM, Kuijpers HJ, van Eys GJ, Ramaekers FC, Broers JL. An alternative splicing product of the lamin A/C gene lacks exon 10. J Biol Chem 1996; 271:9249-53; PMID:8621584; http://dx.doi.org/ 10.1074/jbc.271.16.9249 [DOI] [PubMed] [Google Scholar]
- 18. Butin-Israeli V, Adam SA, Goldman AE, Goldman RD. Nuclear lamin functions and disease. Trends Genet 2012; 28:464-71; PMID:22795640; http://dx.doi.org/ 10.1016/j.tig.2012.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burke B, Stewart CL. The nuclear lamins: flexibility in function. Nat Rev Mol Cell Biol 2013; 14:13-24; PMID:23212477; http://dx.doi.org/ 10.1038/nrm3488 [DOI] [PubMed] [Google Scholar]
- 20. Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, Burke B. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci U S A 2009; 106:2194-9; PMID:19164528; http://dx.doi.org/ 10.1073/pnas.0808602106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frohnert C, Schweizer S, Hoyer-Fender S. SPAG4L/SPAG4L-2 are testis-specific SUN domain proteins restricted to the apical nuclear envelope of round spermatids facing the acrosome. Mol Hum Reprod 2010; 17:207-18; PMID:21159740 [DOI] [PubMed] [Google Scholar]
- 22. Gob E, Schmitt J, Benavente R, Alsheimer M. Mammalian Sperm Head Formation Involves Different Polarization of Two Novel LINC Complexes. PLoS One 2010; 5:e12072; PMID:20711465; http://dx.doi.org/ 10.1371/journal.pone.0012072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gob E, Meyer-Natus E, Benavente R, Alsheimer M. Expression of individual mammalian Sun1 isoforms depends on the cell type. Commun Integr Biol 2011; 4:440-2; PMID:21966565; http://dx.doi.org/ 10.4161/cib.15369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duong NT, Morris GE, Lam le T, Zhang Q, Sewry CA, Shanahan CM, Holt I. Nesprins: tissue-specific expression of epsilon and other short isoforms. PLoS One 2014; 9:e94380; PMID:24718612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scherthan H. A bouquet makes ends meet. Nat Rev Mol Cell Biol 2001; 2:621-7; PMID:11483995; http://dx.doi.org/ 10.1038/35085086 [DOI] [PubMed] [Google Scholar]
- 26. Bass HW. Telomere dynamics unique to meiotic prophase: formation and significance of the bouquet. Cell Mol Life Sci 2003; 60:2319-24; PMID:14625678; http://dx.doi.org/ 10.1007/s00018-003-3312-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zickler D, Kleckner N. The leptotene-zygotene transition of meiosis. Annu Rev Genet 1998; 32:619-97; PMID:9928494; http://dx.doi.org/ 10.1146/annurev.genet.32.1.619 [DOI] [PubMed] [Google Scholar]
- 28. Hiraoka Y, Dernburg AF. The SUN rises on meiotic chromosome dynamics. Dev Cell 2009; 17:598-605; PMID:19922865; http://dx.doi.org/ 10.1016/j.devcel.2009.10.014 [DOI] [PubMed] [Google Scholar]
- 29. Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell 2007; 12:863-72; PMID:17543860; http://dx.doi.org/ 10.1016/j.devcel.2007.03.018 [DOI] [PubMed] [Google Scholar]
- 30. Schmitt J, Benavente R, Hodzic D, Hoog C, Stewart CL, Alsheimer M. Transmembrane protein Sun2 is involved in tethering mammalian meiotic telomeres to the nuclear envelope. Proc Natl Acad Sci U S A 2007; 104:7426-31; PMID:17452644; http://dx.doi.org/ 10.1073/pnas.0609198104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morimoto A, Shibuya H, Zhu X, Kim J, Ishiguro K, Han M, Watanabe Y. A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J Cell Biol 2012; 198:165-72; PMID:22826121; http://dx.doi.org/ 10.1083/jcb.201204085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alsheimer M, Benavente R. Change of karyoskeleton during mammalian spermatogenesis: Expression pattern of nuclear lamin C2 and its regulation. Exp Cell Res 1996; 228:181-8; PMID:8912709; http://dx.doi.org/ 10.1006/excr.1996.0315 [DOI] [PubMed] [Google Scholar]
- 33. Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol 1995; 129:1033-47; PMID:7744953; http://dx.doi.org/ 10.1083/jcb.129.4.1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jin Y, Uzawa S, Cande WZ. Fission yeast mutants affecting telomere clustering and meiosis-specific spindle pole body integrity. Genetics 2002; 160:861-76; PMID:11901107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miki F, Kurabayashi A, Tange Y, Okazaki K, Shimanuki M, Niwa O. Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol Genet Genomics 2004; 270:449-61; PMID:14655046; http://dx.doi.org/ 10.1007/s00438-003-0938-8 [DOI] [PubMed] [Google Scholar]
- 36. Niwa O, Shimanuki M, Miki F. Telomere-led bouquet formation facilitates homologous chromosome pairing and restricts ectopic interaction in fission yeast meiosis. Embo J 2000; 19:3831-40; PMID:10899136; http://dx.doi.org/ 10.1093/emboj/19.14.3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miki F, Okazaki K, Shimanuki M, Yamamoto A, Hiraoka Y, Niwa O. The 14-kDa dynein light chain-family protein Dlc1 is required for regular oscillatory nuclear movement and efficient recombination during meiotic prophase in fission yeast. Mol Biol Cell 2002; 13:930-46; PMID:11907273; http://dx.doi.org/ 10.1091/mbc.01-11-0543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Malone CJ, Misner L, Le Bot N, Tsai MC, Campbell JM, Ahringer J, White JG. The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell 2003; 115:825-36; PMID:14697201; http://dx.doi.org/ 10.1016/S0092-8674(03)00985-1 [DOI] [PubMed] [Google Scholar]
- 39. Alsheimer M. The dance floor of meiosis: evolutionary conservation of nuclear envelope attachment and dynamics of meiotic telomeres. Genome Dyn 2009; 5:81-93; PMID:18948709; http://dx.doi.org/ 10.1159/000166621 [DOI] [PubMed] [Google Scholar]
- 40. Horn HF, Kim DI, Wright GD, Wong ES, Stewart CL, Burke B, Roux KJ. A mammalian KASH domain protein coupling meiotic chromosomes to the cytoskeleton. J Cell Biol 2013; 202:1023-39; PMID:24062341; http://dx.doi.org/ 10.1083/jcb.201304004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y. Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell 2006; 125:59-69; PMID:16615890; http://dx.doi.org/ 10.1016/j.cell.2006.01.048 [DOI] [PubMed] [Google Scholar]
- 42. Chikashige Y, Yamane M, Okamasa K, Tsutsumi C, Kojidani T, Sato M, Haraguchi T, Hiraoka Y. Membrane proteins Bqt3 and -4 anchor telomeres to the nuclear envelope to ensure chromosomal bouquet formation. J Cell Biol 2009; 187:413-27; PMID:19948484; http://dx.doi.org/ 10.1083/jcb.200902122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ichikawa Y, Kagawa W, Saito K, Chikashige Y, Haraguchi T, Hiraoka Y, Kurumizaka H. Purification and characterization of the fission yeast telomere clustering factors, Bqt1 and Bqt2. Protein Expr Purif 2013; 88:207-13; PMID:23337086; http://dx.doi.org/ 10.1016/j.pep.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 44. Conrad MN, Lee CY, Wilkerson JL, Dresser ME. MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 2007; 104:8863-8; PMID:17495028; http://dx.doi.org/ 10.1073/pnas.0606165104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wanat JJ, Kim KP, Koszul R, Zanders S, Weiner B, Kleckner N, Alani E. Csm4, in collaboration with Ndj1, mediates telomere-led chromosome dynamics and recombination during yeast meiosis. PLoS Genet 2008; 4:e1000188; PMID:18818741; http://dx.doi.org/ 10.1371/journal.pgen.1000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Daniel K, Trankner D, Wojtasz L, Shibuya H, Watanabe Y, Alsheimer M, Toth A. Mouse CCDC79 (TERB1) is a meiosis-specific telomere associated protein. BMC Cell Biol 2014; 15:17; PMID:24885367; http://dx.doi.org/ 10.1186/1471-2121-15-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shibuya H, Ishiguro K, Watanabe Y. The TRF1-binding protein TERB1 promotes chromosome movement and telomere rigidity in meiosis. Nat Cell Biol 2014; 16:145-56; PMID:24413433; http://dx.doi.org/ 10.1038/ncb2896 [DOI] [PubMed] [Google Scholar]
- 48. Lei K, Zhang X, Ding X, Guo X, Chen M, Zhu B, Xu T, Zhuang Y, Xu R, Han M. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc Natl Acad Sci U S A 2009; 106:10207-12; PMID:19509342; http://dx.doi.org/ 10.1073/pnas.0812037106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chi YH, Cheng LI, Myers T, Ward JM, Williams E, Su Q, Faucette L, Wang JY, Jeang KT. Requirement for Sun1 in the expression of meiotic reproductive genes and piRNA. Development 2009; 136:965-73; PMID:19211677; http://dx.doi.org/ 10.1242/dev.029868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Link J, Leubner M, Schmitt J, Gob E, Benavente R, Jeang KT, Xu R, Alsheimer M. Analysis of meiosis in SUN1 deficient mice reveals a distinct role of SUN2 in mammalian meiotic LINC complex formation and function. PLoS Genet 2014; 10:e1004099; PMID:24586178; http://dx.doi.org/ 10.1371/journal.pgen.1004099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Penkner AM, Fridkin A, Gloggnitzer J, Baudrimont A, Machacek T, Woglar A, Csaszar E, Pasierbek P, Ammerer G, Gruenbaum Y, et al. . Meiotic chromosome homology search involves modifications of the nuclear envelope protein Matefin/SUN-1. Cell 2009; 139:920-33; PMID:19913286; http://dx.doi.org/ 10.1016/j.cell.2009.10.045 [DOI] [PubMed] [Google Scholar]
- 52. Woglar A, Daryabeigi A, Adamo A, Habacher C, Machacek T, La Volpe A, Jantsch V. Matefin/SUN-1 phosphorylation is part of a surveillance mechanism to coordinate chromosome synapsis and recombination with meiotic progression and chromosome movement. PLoS Genet 2013; 9:e1003335; PMID:23505384; http://dx.doi.org/ 10.1371/journal.pgen.1003335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patel JT, Bottrill A, Prosser SL, Jayaraman S, Straatman K, Fry AM, Shackleton S. Mitotic phosphorylation of SUN1 loosens its connection with the nuclear lamina while the LINC complex remains intact. Nucleus 2014; 5:462-73; PMID:25482198; http://dx.doi.org/ 10.4161/nucl.36232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ashley T, Walpita D, de Rooij DG. Localization of two mammalian cyclin dependent kinases during mammalian meiosis. J Cell Sci 2001; 114:685-93; PMID:11171374 [DOI] [PubMed] [Google Scholar]
- 55. Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet 2003; 35:25-31; PMID:12923533; http://dx.doi.org/ 10.1038/ng1232 [DOI] [PubMed] [Google Scholar]
- 56. Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P. Cdk2 knockout mice are viable. Curr Biol 2003; 13:1775-85; PMID:14561402; http://dx.doi.org/ 10.1016/j.cub.2003.09.024 [DOI] [PubMed] [Google Scholar]
- 57. Viera A, Rufas JS, Martinez I, Barbero JL, Ortega S, Suja JA. CDK2 is required for proper homologous pairing, recombination and sex-body formation during male mouse meiosis. J Cell Sci 2009; 122:2149-59; PMID:19494131; http://dx.doi.org/ 10.1242/jcs.046706 [DOI] [PubMed] [Google Scholar]
- 58. Viera A, Alsheimer M., Gómez R, Berenguer I, Ortega S, Symonds CE, Santamaría D, Benavente R, Suja JA. CDK2 regulates nuclear envelope protein dynamics and telomere attachment in mouse meiotic prophase. J Cell Sci accepted 128(1):88-99; PMID:25380821 [DOI] [PubMed] [Google Scholar]
- 59. Moens PB, Pearlman RE, Heng HH, Traut W. Chromosome cores and chromatin at meiotic prophase. Curr Top Dev Biol 1998; 37:241-62; PMID:9352188; http://dx.doi.org/ 10.1016/S0070-2153(08)60176-3 [DOI] [PubMed] [Google Scholar]
- 60. Alsheimer M, von Glasenapp E, Hock R, Benavente R. Architecture of the nuclear periphery of rat pachytene spermatocytes: distribution of nuclear envelope proteins in relation to synaptonemal complex attachment sites. Mol Biol Cell 1999; 10:1235-45; PMID:10198069; http://dx.doi.org/ 10.1091/mbc.10.4.1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Alsheimer M, Jahn D, Schramm S, Benavente R. Nuclear lamins in mammalian spermatogenesis. In: Rousseaux S, Khochbin S, eds. Epigenetics and Human Reproduction: Springer, New York, 2011:279-88 [Google Scholar]
- 62. Alsheimer M, von Glasenapp E, Schnolzer M, Heid H, Benavente R. Meiotic lamin C2: the unique amino-terminal hexapeptide GNAEGR is essential for nuclear envelope association. Proc Natl Acad Sci U S A 2000; 97:13120-5; PMID:11078531; http://dx.doi.org/ 10.1073/pnas.240466597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jahn D, Schramm S, Benavente R, Alsheimer M. Dynamic properties of meiosis-specific lamin C2 and its impact on nuclear envelope integrity. Nucleus 2010; 1:273-83; PMID:21327075; http://dx.doi.org/ 10.4161/nucl.11800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Link J, Jahn D, Schmitt J, Gob E, Baar J, Ortega S, Benavente R, Alsheimer M. The meiotic nuclear lamina regulates chromosome dynamics and promotes efficient homologous recombination in the mouse. PLoS Genet 2013; 9:e1003261; PMID:23382700; http://dx.doi.org/ 10.1371/journal.pgen.1003261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ishiguro K, Kim J, Shibuya H, Hernandez-Hernandez A, Suzuki A, Fukagawa T, Shioi G, Kiyonari H, Li XC, Schimenti J, et al. . Meiosis-specific cohesin mediates homolog recognition in mouse spermatocytes. Genes Dev 2014; 28:594-607; PMID:24589552; http://dx.doi.org/ 10.1101/gad.237313.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Iwai T, Yoshii A, Yokota T, Sakai C, Hori H, Kanamori A, Yamashita M. Structural components of the synaptonemal complex, SYCP1 and SYCP3, in the medaka fish Oryzias latipes. Exp Cell Res 2006; 312:2528-37; PMID:16764855; http://dx.doi.org/ 10.1016/j.yexcr.2006.04.015 [DOI] [PubMed] [Google Scholar]
- 67. Saito K, Sakai C, Kawasaki T, Sakai N. Telomere distribution pattern and synapsis initiation during spermatogenesis in zebrafish. Dev Dyn 2014; 243:1448-56; PMID:25044979; http://dx.doi.org/ 10.1002/dvdy.24166 [DOI] [PubMed] [Google Scholar]
- 68. Bannister LA, Reinholdt LG, Munroe RJ, Schimenti JC. Positional cloning and characterization of mouse mei8, a disrupted allelle of the meiotic cohesin Rec8. Genesis 2004; 40:184-94; PMID:15515002; http://dx.doi.org/ 10.1002/gene.20085 [DOI] [PubMed] [Google Scholar]
- 69. Xu H, Beasley MD, Warren WD, van der Horst GT, McKay MJ. Absence of mouse REC8 cohesin promotes synapsis of sister chromatids in meiosis. Dev Cell 2005; 8:949-61; PMID:15935783; http://dx.doi.org/ 10.1016/j.devcel.2005.03.018 [DOI] [PubMed] [Google Scholar]
- 70. Herran Y, Gutierrez-Caballero C, Sanchez-Martin M, Hernandez T, Viera A, Barbero JL, de Alava E, de Rooij DG, Suja JA, Llano E, et al. . The cohesin subunit RAD21L functions in meiotic synapsis and exhibits sexual dimorphism in fertility. Embo J 2011; 30:3091-105; PMID:21743440; http://dx.doi.org/ 10.1038/emboj.2011.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Llano E, Herran Y, Garcia-Tunon I, Gutierrez-Caballero C, de Alava E, Barbero JL, Schimenti J, de Rooij DG, Sanchez-Martin M, Pendas AM. Meiotic cohesin complexes are essential for the formation of the axial element in mice. J Cell Biol 2012; 197:877-85; PMID:22711701; http://dx.doi.org/ 10.1083/jcb.201201100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fukuda T, Fukuda N, Agostinho A, Hernandez-Hernandez A, Kouznetsova A, Hoog C. STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. Embo J 2014; 33:1243-55; PMID:24797475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hopkins J, Hwang G, Jacob J, Sapp N, Bedigian R, Oka K, Overbeek P, Murray S, Jordan PW. Meiosis-specific cohesin component, Stag3 is essential for maintaining centromere chromatid cohesion, and required for DNA repair and synapsis between homologous chromosomes. PLoS Genet 2014; 10:e1004413; PMID:24992337; http://dx.doi.org/ 10.1371/journal.pgen.1004413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Winters T, McNicoll F, Jessberger R. Meiotic cohesin STAG3 is required for chromosome axis formation and sister chromatid cohesion. Embo J 2014; 33:1256-70; PMID:24797474 [DOI] [PMC free article] [PubMed] [Google Scholar]