Abstract
The Conserved Oligomeric Golgi (COG) complex is an evolutionarily conserved hetero-octameric protein complex that has been proposed to organize vesicle tethering at the Golgi apparatus. Defects in seven of the eight COG subunits are linked to Congenital Disorders of Glycosylation (CDG)-type II, a family of rare diseases involving misregulation of protein glycosylation, alterations in Golgi structure, variations in retrograde trafficking through the Golgi and system-wide clinical pathologies. A troublesome aspect of these diseases are the neurological pathologies such as low IQ, microcephaly, and cerebellar atrophy. The essential function of the COG complex is dependent upon interactions with other components of trafficking machinery, such as Rab-GTPases and SNAREs. COG-interacting Rabs and SNAREs have been implicated in neurodegenerative diseases like Alzheimer's disease and Parkinson's disease. Defects in Golgi maintenance disrupts trafficking and processing of essential proteins, frequently associated with and contributing to compromised neuron function and human disease. Despite the recent advances in molecular neuroscience, the subcellular bases for most neurodegenerative diseases are poorly understood. This article gives an overview of the potential contributions of the COG complex and its Rab and SNARE partners in the pathogenesis of different neurodegenerative disorders.
Keywords: conserved oligomeric Golgi complex, COG, congenital disorders of glycosylation, neurodegeneration, glycosylation, vesicular trafficking, Rab, SNARE
Introduction
The Conserved Oligomeric Golgi (COG) complex is an evolutionarily conserved hetero-octameric protein complex that is a proposed membrane tether during vesicular trafficking at the Golgi apparatus (Lupashin and Ungar, 2008; Reynders et al., 2011; Miller and Ungar, 2012; Willett et al., 2013b). COG is composed of two functionally distinct sub-complexes lobe A (COG1-4) and lobe B (COG5-8) (Fotso et al., 2005; Ungar et al., 2005). Secretory and transmembrane proteins make up 30–50% of all cellular proteins, and are trafficked through the endoplasmic reticulum (ER) to the Golgi for folding and modifications before delivery to their final destination. Secretory cargo molecules are thought to travel through the Golgi complex mostly inside flat cisternae that are constantly maturing in a cis-to-trans (anterograde) fashion via the so called cisternal maturation mechanism (Glick and Malhotra, 1998). However, resident Golgi proteins and Soluble NSF Attachment protein Receptors (SNAREs) are constantly recycled back in vesicular carriers to replenish the content of newly formed cis-cisternae. COG regulates the recycling of vesicles containing glycosylation enzymes and other resident Golgi proteins in a trans-to-cis (retrograde) direction. An intricate assortment of trafficking machineries including small Rab-GTPases, SNAREs, Sec1/Munc18 (SM) proteins, vesicular coat proteins, and tethering proteins are required for vesicular transport (Bonifacino and Glick, 2004). Intracellular pathways rely on these protein families at each step of vesicular transport. Though the functional interaction between the tethers and other trafficking regulators is not completely understood, a multi-subunit tethering complex (MTC), like the COG complex, may coordinate the interactions between all other components of the trafficking machinery at the site of vesicle docking on the target membrane for efficient fusion of the two membranes (Cottam and Ungar, 2012; Willett et al., 2013b).
SNAREs are an essential COG partner. SNAREs catalyze the fusion of the vesicle membrane with the target membrane by the assembly of a quaternary SNARE complex that functions as a zipper to coalesce the opposing lipid bilayers While SNAREs alone have an innate ability to fuse membranes, the SNARE regulatory proteins and tethering complexes are thought to be necessary for physiologically relevant fusion events (Rizo and Südhof, 2012). The COG complex interacts with at least two Golgi SNARE complexes: the cis-Golgi STX5/GOSR1(GS28)/YKT6/BET1L(GS15) complex; and the trans-Golgi STX16/STX6/VTI1a/VAMP4 complex (Shestakova et al., 2007; Laufman et al., 2009, 2013b). Additionally, SNARE complexes that contain Sec22b, GOSR2(GS27), or SNAP29 are evidenced to interact with COG (Kudlyk et al., 2013; Willett et al., 2013a). SNARE partner transitions connect all compartments of the endocytic and secretory pathways. Thus, it is likely that a defect in one trafficking step may have a cascading effect on all other cargo trafficking steps. In this review we will focus on those SNAREs where deficiency was shown to be associated with neuronal abnormalities, i.e., Ykt6, Sec22b, STX5, SNAP29, GS27, GS28, and Vti1a/b (Table 1).
Table 1.
Neurological phenotypes associated with COG and COG-interacting Rabs and SNAREs.
| Protein | Disorder | Associated neurological manifestation | References |
|---|---|---|---|
| COG PROTEINS | |||
| COG1 | CDG-IIg (COG1-CDG) | Cerebral atrophy, developmental delay, hypotonia | Foulquier et al., 2006 |
| COG2 | CDG-II (COG2-CDG) | Developmental delay, epilepsy | Kodera et al., 2015 |
| COG4 | CDG-IIj (COG4-CDG) | Developmental delay, epilepsy, hypotonia, lack of speech, nystagmus | Reynders et al., 2009; Ng et al., 2011 |
| COG5 | CDG-IIi (COG5-CDG) | Ataxia, cerebral atrophy, developmental delay, epilepsy, hypotonia | Paesold-Burda et al., 2009; Fung et al., 2012; Rymen et al., 2012 |
| COG6 | CDG-IIl (COG6-CDG) Shaheen syndrome (SHNS) | Ataxia, cerebral atrophy, developmental delay, epilepsy, hypotonia, optic nerve atrophy, sensoneural hearing loss Intellectual disability | Lübbehusen et al., 2010; Huybrechts et al., 2012; Shaheen et al., 2013; Rymen et al., 2015 |
| COG7 | CDG-IIe (COG7-CDG) | Cerebral atrophy, developmental delay, epilepsy, hypotonia | Wu et al., 2004; Morava et al., 2007; Ng et al., 2007; Zeevaert et al., 2009a |
| COG8 | CDG-IIh (COG8-CDG) | Cerebral atrophy, developmental delay, hypotonia | Foulquier et al., 2007; Kranz et al., 2007 |
| SNARE PROTEINS | |||
| Ykt6 | Parkinson's Disease | Trafficking defects and cytotoxicity in vitro in NRK and PC12 cell lines | Hasegawa et al., 2003, 2004 |
| Sec22b | Parkinson's Disease | Trafficking defects and cytotoxicity in vitro in NRK and PC12 cell lines | Hasegawa et al., 2003, 2004 |
| STX5 | Parkinson's Disease Alzheimer's Disease | Trafficking defects and cytotoxicity in vitro in NRK and PC12 cell lines Regulates processing of APP in PC12, HeLa, COS-7, and NG108-15 cell lines and primary cultures of rat hippocampal neurons | Suga et al., 2005b; Thayanidhi et al., 2010; Rendón et al., 2013; Suga et al., 2015 |
| SNAP29 | CEDNIK-Neuro-cutaneous syndrome | Cerebral Dysgenesis, Neuropathy, Ichthyosis, and Keratoderma | Sprecher et al., 2005; Fuchs-Telem et al., 2011 |
| GS27 | Myoclonus epilepsy/early ataxia Parkinson's Disease | Action myoclonus, mild cerebral atrophy, and early ataxia Trafficking defects and cytotoxicity in vitro in NRK and PC12 cell lines | Thayanidhi et al., 2010; Corbett et al., 2011 |
| GS28 | Neurodegeneration | Retinal degeneration in in vivo Drosophila photoreceptors | Rosenbaum et al., 2014 |
| Vti1a/b | Neurodegeneration | Perinatal lethality in double knockouts in an in vivo mouse model. Neuronal axon tracks missing, reduced in size or misrouted | Kunwar et al., 2011; Walter et al., 2014 |
| RAB PROTEINS | |||
| Rab1a | Parkinson's Disease | Neuroprotective in C. elegans, D. melanogaster and primary rat neuron cultures. Rescue from the neurotoxic effects of α-synuclein | Cooper et al., 2006; Gitler et al., 2008 |
| Rab1b | Alzheimer's Disease | Dominant negative mutant of Rab1b blocks trafficking of APP and decreases the secretion of Aβ | Dugan et al., 1995 |
| Rab2 | Parkinson's Disease | Reduced expression of Rab2 can rescue Golgi fragmentation in PD models | Rendón et al., 2013 |
| Rab4a | Neumann-Pick disease Alzheimer's Disease Down's syndrome | Reduced Rab4-dependent recycling in vitro in Neumann-Pick type A and type C fibroblasts. Postmortem samples: increased Rab4 in patients with AD and mild cognitive disorder Aβ partially co-localizes with Rab4 in a mouse model of Down Syndrome | Cataldo et al., 2000; Choudhury et al., 2004; Arriagada et al., 2010; Ginsberg et al., 2010 |
| Rab6a | Alzheimer's Disease | Dominant negative mutant of Rab6 increases the secretion of soluble APP and decreased Aβ secretion | McConlogue et al., 1996 |
The essential function of the COG complex has been proposed to depend not only upon SNAREs interactions, but also interactions with Rabs (Miller et al., 2013; Willett et al., 2013a; Figure 1). Within this context, Rabs are believed to act as molecular switches that cycle between GDP-bound (inactive) and GTP-bound (active) states and regulate cargo trafficking by acting in an intracellular compartment-specific manner. Active (GTP-bound) Rabs regulate trafficking by binding effector molecules like tethering factors and motor proteins (D'Adamo et al., 2014). Binding to some effectors leads to the activation of other Rabs, in a sequence known as the Rab Cascade (Pfeffer, 2013). Nine distinct, Golgi-localized Rabs are known to interact with COG (Miller et al., 2013; Willett et al., 2013b, 2014). The compartment-specific nature of these different Rabs makes them a potential landmark for COG membrane localization and interaction. As with the SNAREs, this review will limit the discussion to COG partners Rab1a, Rab1b, Rab2, Rab4a, and Rab6a that are implicated in neuronal abnormalities (Table 1).
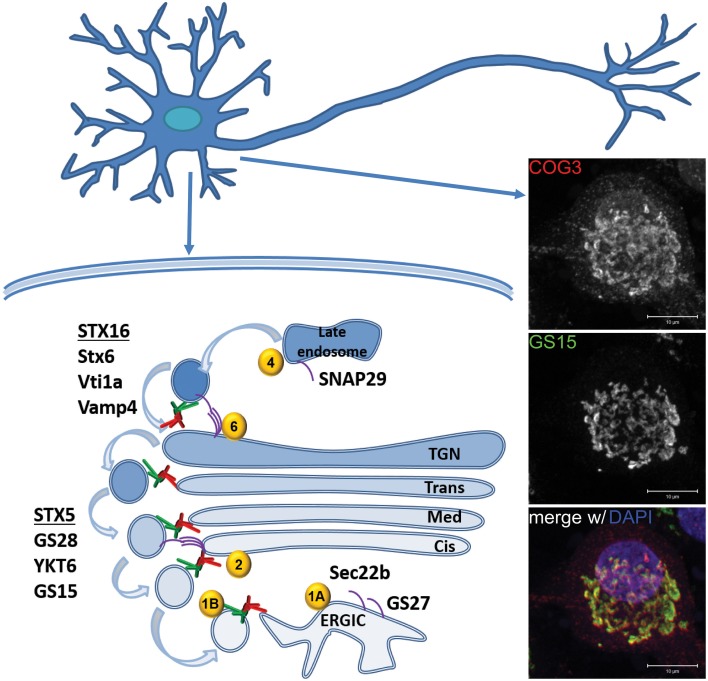
Figure 1.
Model of COG trafficking within neurons. A cartoon depicting the proposed roles for the COG complex (depicted as red shapes for lobe A and green shapes for lobe B subcomplexes) and its interacting protein partners in Golgi trafficking: Rabs (yellow circles), and SNAREs (purple lines). Right panel: Immunofluorescence images of COG complex subunit COG3 (top-red) and Golgi SNARE GS15 (middle-green) in rat dorsal root ganglion. Perinuclear (DAPI) co-localization is indicated by yellow in the merged image (bottom). Scale Bar = 10 μm.
Neurophysiological abnormalities and COG defects
Since 2004, defects in seven out of the eight COG subunits have been associated with human Congenital Disorders of Glycosylation (CDG)-type II, a growing family of diseases involving malfunctions in the processing of N- and O-linked glycans and resulting from mutations in proteins involved with glycosylation (Wu et al., 2004; Spaapen et al., 2005; Foulquier et al., 2006, 2007; Kranz et al., 2007; Ng et al., 2007, 2011; Paesold-Burda et al., 2009; Reynders et al., 2009; Richardson et al., 2009; Lübbehusen et al., 2010; Fung et al., 2012; Huybrechts et al., 2012; Rymen et al., 2012, 2015; Kodera et al., 2015; Table 1). Glycosylation is a highly dynamic process that occurs in the ER and Golgi which requires an estimated two percent of the human genome to encode the enzymes and trafficking components for the proper maturation of newly formed glycan chains (Freeze et al., 2014). COG deficiency can cause a redistribution of COG-dependent Golgi resident proteins, including glycosylation enzymes. Most COG-CDG patients have defects in sialylation and galactosylation, as indicated by fluorescent lectin staining of plasma membrane glycoconjugates from patient fibroblasts and MALDI-TOF mass spectrometry of serum glycoproteins (Foulquier et al., 2007; Kranz et al., 2007; Paesold-Burda et al., 2009; Reynders et al., 2009; Zeevaert et al., 2009a,b). Along with other multi-system pathologies, COG-CDG patients display mild to severe neurological defects including hypotonia, intellectual disability, developmental delays, epilepsy, and ataxia (Table 1). Specific symptoms and the severity of condition appears to relate to the COG subunit that is deficient with COG6 and COG7 patients demonstrating the most severe phenotypes (Rymen et al., 2015). Additionaly, COG defects have not yet been attributed to any other subtype of CDG.
Several CDGs result from mutated COG subunits that are either severely truncated or rapidly degraded. Loss of one COG subunit can destabilize the remaining subunits and reduce their expression and association with the Golgi. Early studies of the COG3 subunit invoked participation of the COG complex in the proper distribution of Golgi enzymes. COG3 depletion by siRNA in HeLa cells causes extensive Golgi fragmentation and destabilization of the COG complex (Zolov and Lupashin, 2005). COG3 and COG7 knockdown generates an accumulation of COG complex-dependent (CCD) vesicles carrying the SNAREs GS15 and GS28, and Golgi enzymes MAN2A1 and GALNT2 (Zolov and Lupashin, 2005; Shestakova et al., 2006). The accumulation of CCD vesicles suggests that in COG deficient cells a significant fraction of Golgi glycosylation enzymes are separated from the proteins they need to modify. COG8-CDG patient fibroblasts have decreased levels of the other lobe B subunits (COG5, COG6, and COG7) all of which have lost their association with the Golgi (Foulquier et al., 2007; Kranz et al., 2007). COG lobe B destabilization was also seen in COG7-CDG patient fibroblasts, resulting in the loss of COG6 association with the Golgi (Kudlyk et al., 2013). The loss of COG also challenges the function of interacting SNAREs. The endosome-to-trans-Golgi Network (TGN) SNARE protein STX16 was mislocalized in COG8-CDG patient fibroblasts (Willett et al., 2013a), and the STX5/GS28/Ykt6/GS15 and STX6/STX16/Vti1a/VAMP4 SNARE complexes were destabilized in both COG7- and COG8-CDG patient fibroblasts (Laufman et al., 2013a). In a non-CDG patient presenting intellectual disability, Shaheen et al. identified a mutation in COG6 which resulted in reduced COG6 and STX6 protein expression (Shaheen et al., 2013). Anterograde trafficking does not appear to be affected in cells with COG mutations, but retrograde trafficking is affected as indicated by a partial resistance to treatment with the transport inhibitor brefeldin A (Steet and Kornfeld, 2006; Foulquier et al., 2007; Kranz et al., 2007; Ng et al., 2007; Paesold-Burda et al., 2009; Reynders et al., 2009) and by endosome-to-TGN trafficking defects elucidated by application of Shiga toxin and SubAB toxin (Zolov and Lupashin, 2005; Smith et al., 2009). Therefore, retrograde intra-Golgi and endosome-to-TGN sorting are particularly impaired by COG deficiency.
Neuropathology and defects in COG-associated proteins
Extensive in vitro analyses in control and disease models demonstrate that genetic deficiency in SNARE and Rab COG partners may also result in disintegration of the Golgi apparatus, thereby potentially influencing neurological impairment (Table 1). Interestingly, the therapeutic implications of studying neurodegeneration associated with defective COG function is broader than the COG-CDG patient population (D'Adamo et al., 2014; Rymen et al., 2015). Golgi fragmentation is a common feature of neurodegenerative diseases (Gonatas et al., 2006). Current theories argue that the Golgi fragmentation seen in Alzheimer's Disease (AD) and Parkinson's Disease (PD) is either a result of misfolded or aggregated proteins, or that fragmented Golgi causes etiologically important proteins to aggregate and misfold leading to further progression of these diseases (Gonatas et al., 2006; Nakagomi et al., 2008; Bellouze et al., 2014; Joshi and Wang, 2015). However, Golgi fragmentation has also been linked to SNARE and Rab proteins making it difficult to pinpoint a single disease progression. Thus, the COG-Rab-SNARE dynamic is important for understanding neurodegenerative phenotypes.
SNAREs
Genetic deficiency in SNAREs was shown to be associated with the progression of neurodegenerative diseases like AD and PD. PD is marked by the presence of Lewy bodies which are principally composed of aggregated α-synuclein. Under physiological conditions, α-synuclein may regulate vesicle trafficking and promote synaptic transmission by binding directly to SNAREs and stimulating SNARE complex formation (Burré et al., 2010; Thayanidhi et al., 2010). Overexpression of wild type or the PD-associated mutant of α-synuclein (A53T) leads to cytotoxicity and inhibition of ER-to-Golgi trafficking in in vitro models which can be partially suppressed by co-overexpression of SNAREs Ykt6 or Sec22. Ykt6—a protein enriched in neurons—was more protective than Sec22 and suggests a specialized role in mammals (Hasegawa et al., 2003, 2004). In vitro binding experiments also point toward the direct interaction of α-synuclein with STX5 and GS27 along with mutant α-synuclein which destabilizes the STX5-GS27-rbet1-sec22b SNARE complex (Thayanidhi et al., 2010). This line of evidence further implicates trafficking defects in bringing about neurodegenerative cytotoxicity.
Although the exact molecular mechanisms connecting Golgi fragmentation and disease mutations is still much under investigation, in vitro models can be used to recapitulate Golgi fragmentation seen in neurodegenerative disorders (Suga et al., 2005a). Fragmentation has been recreated in vitro and can be rescued by regulating levels of SNAREs. A STX5 knockdown is known to induce Golgi fragmentation in HeLa cells and cultured neurons (Suga et al., 2005a; Amessou et al., 2007). On the other hand, in PC12 cells treated with 6-hydroxydopamine or methamphetamine (an established in vitro PD model), a decrease in the level of STX5 rescues Golgi fragmentation (Rendón et al., 2013). This same study also demonstrated that Golgi fragmentation could intensify disease progression by inducing α-synuclein aggregation and the formation of Lewy bodies.
STX5 defects have also been shown to affect processing of AD-related proteins. In AD, amyloid precursor protein (APP) undergoes a series of proteolytic events by β- and γ-secretases to create the amyloidogenic variants of β-amyloid (Aβ) that are longer and more likely to form aggregates (Peric and Annaert, 2015). Presenilins form a complex with γ-secretase, and mutations in presenilin 1 (PS1) are the most frequently associated mutations found in AD which result in increased production of Aβ, or altered ratios of amyloid peptide species (Hardy, 2006; Saito et al., 2011; De Strooper et al., 2012). Overexpressed STX5 was shown to co-localize with and directly bind to PS1. Further, STX5 overexpression increased the accumulation of APP in the ER and cis-Golgi and inhibited Aβ secretion in a neuroblastoma cell line (NG108-15)(Suga et al., 2005b). In NG108-15 cells expressing the familial AD mutation PS1ΔE9, STX5 was shown to have a decreased association with presenilin. A study of ER stress in an AD model demonstrated that ER stressors can increase synthesis of STX5 and its accumulation in the ER-Golgi intermediate compartment (ERGIC) and transport vesicles. Thus, upregulation of trafficking machinery induced by ER stress could be a cellular mechanism for correcting the accumulation of the amyloidogenic cleavage products of APP (Suga et al., 2015).
As stated above, the cis-Golgi SNARE GS27 (GOSR2) binds to α-synuclein and is part of a SNARE complex that is destabilized by mutant α-synuclein. GS27 has also been shown to be associated with a neurological disorder in humans. Six patient were identified baring a mutation that results in improper subcellular localization and loss of function of GS27 leading to symptoms common in COG-CDG patients such as cerebral atrophy, epilepsy, and early ataxia (Corbett et al., 2011; Table 1).
GS28 (GOSR1) is a Golgi SNARE involved in both ER-to-Golgi and intra-Golgi transport (Nagahama et al., 1996; Subramaniam et al., 1996), and accordingly has been shown to be associated with three SNARE complexes (Zhang and Hong, 2001; Parlati et al., 2002; Xu et al., 2002; Siddiqi et al., 2010). GS28 mutants have been used to study retinal degeneration in Drosophila photoreceptors (Rosenbaum et al., 2014). Lack of expression of GS28 in mutant flies alters trafficking and glycosylation of rhodopsin (Rh1). The photoreceptors in these mutants also exhibit enlarged ER and Golgi membranes and retinal degeneration over time.
Vti1a is a TGN-localized SNARE that functions in vesicle generation and Ca2+ channel trafficking (von Mollard et al., 1997; Lupashin et al., 1997; Walter et al., 2014). A double knockout of Vti1a and Vti1b genes results in progressive neurodegeneration and perinatal lethality in a mouse model (Kunwar et al., 2011). Single knockout of Vti1a or Vti1b does not result in a lethal phenotype indicating overlapping functions of these proteins. The death-after-birth clearly demonstrates that these SNAREs are not required during organismal development, indicating a specialized requirement for Vti1a and Vti1b in neurons leading to neurodegeneration in the double-knockout animals (Walter et al., 2014). Vti1a SNARE partners, STX6 and STX16, are also required for neurite outgrowth (Chua and Tang, 2008; Kabayama et al., 2008).
In humans, a disease known as CEDNIK (Cerebral Dysgenesis, Neuropathy, Ichthyosis, and Keratoderma) syndrome has been linked with loss of function mutations in SNAP29 (Sprecher et al., 2005; Fuchs-Telem et al., 2011). SNAP29 is a member of the SNAP25 family that localizes to the Golgi, endosomal, and lysosomal compartments (Steegmaier et al., 1998). CEDNIK patients exhibit severe neuropathy likely due to the loss of SNAP29 functional involvement in neurotransmission (Pan et al., 2005) and trafficking within neuroglia during active myelination (Schardt et al., 2009).
Rabs
As for SNAREs, regulating levels of Rabs has been shown to rescue Golgi fragmentation in multiple in vitro models of AD and PD. For example, overexpression of Rab1 can rescue Golgi fragmentation while reduced expression of Rab2 has the same affect in PD models which demonstrates the delicate balance in the regulatory functions of Golgi-associated Rabs (Rendón et al., 2013). Overexpression of Rab1 was shown to be neuroprotective in Caenorhabditis elegans, Drosophila melanogaster and primary rat neuron cultures (Cooper et al., 2006; Gitler et al., 2008). Rab1 is a key protein in maintaining Golgi architecture and function (Haas et al., 2007). It can also promote the restoration of ER-to-Golgi trafficking and thus afford rescue from the neurotoxic effects of α-synuclein (Cooper et al., 2006; Gitler et al., 2008).
Multiple Rabs are associated with processing of APP. For example, ERGIC and cis-Golgi Rab1b-dependent trafficking could modulate the processing of APP as demonstrated in an in vitro system in which a dominant-negative mutant of Rab1b blocked trafficking of APP and decreased the secretion of Aβ (Dugan et al., 1995). trans-Golgi Rab6A is also implicated in APP trafficking. The dominant negative mutant of Rab6 increased the secretion of soluble APP and decreased Aβ secretion (McConlogue et al., 1996). Rab6 membrane association is dependent upon PS1 based on studies using PS1 knockout fibroblasts, thus implicating PS1 in vesicular trafficking (Scheper et al., 2004).
Golgi-associated and endosomal Rab4 is important for a specific pool of constitutively recycling endosomes which are apparently critical for dendritic spine size. Rab4-dependent recycling is greatly reduced in fibroblasts of patients with type A/B Niemann-Pick disease, a sphingolipid storage disorder which commonly manifests with neurological symptoms such as developmental delay and dementia (Choudhury et al., 2004). Postmortem samples revealed that Rab4 is upregulated in patients with AD and mild cognitive disorder (Cataldo et al., 2000; Ginsberg et al., 2010), and Aβ is known to partially co-localize within Rab4 positive compartments in a mouse model of Down Syndrome (Arriagada et al., 2010) indicating that defects in endosomal sorting may underpin these disorders (Peric and Annaert, 2015).
Conclusions and perspectives
The COG complex and its Rab and SNARE partners are evolutionarily conserved and ubiquitously expressed across multiple tissues and organ systems in humans. Yet, neurological symptoms are the most debilitating and troublesome clinical manifestations of COG-associated disorders. Why is this the case? We propose that brain-specific manifestations of COG defects result from either COG-dependent (a) glycosylation defects and/or, (b) trafficking defects and/or, (c) a yet unknown neuron/neuroglia-specific function of the COG complex and its partners.
Neuronal function critically depends on coordinated delivery of properly modified ion channels, transporters and components of the synaptic apparatus at the appropriate rates and over long distances, to specific subcellular compartments. Remarkably, the localization of the synaptobrevin homolog Snc1 is altered in COG deficient yeast cells (Whyte and Munro, 2002). In addition, underglycosylated low density lipoprotein receptor is severely destabilized in CHO cells deficient for COG1 or COG2 proteins (Kingsley et al., 1986). Since glycosylation of channels, transporters and transport regulators is essential for their correct delivery, stability and/or activity (Gong et al., 2002; Watanabe et al., 2004; Scott and Panin, 2014), it is reasonable to predict that a majority of underglycosylated ion channels and transporters may similarly be destabilized, thus altering the functionality of COG-CDG patient neurons.
Smooth transport of cargo by the trafficking machinery is very important during development and synaptic transmission. Defective glycosylation of proteins and lipids disrupts development pathways and alters brain function (Freeze et al., 2012). COG deficient cells also display altered glycosphingolipid biosynthesis. Complex gangliosides are sialic acid containing glycosphingolipids synthesized sequentially, beginning with GM3 and then extended by glycosyltransferases to the more elaborate GM1 gangliosides. Biochemical studies revealed decreased levels of sphingomyelin and GM3 gangliosides in COG2 deficient CHO cells (Spessott et al., 2010a,b). Gangliosides are ubiquitously expressed, but in the brain the expression of gangliosides and their glycosyltransferases change dramatically during development, from an abundance in of the precursor GM3 to a greater abundance of GM1 (Yu et al., 1988; Kracun et al., 1991, 1992; Ngamukote et al., 2007). Therefore, altered glycosphingolipid biosynthesis could be another reason for the specific neurological manifestation in COG-related congenital disorders.
An additional factor that may add to the apparently higher neuronal vulnerability to COG deficiency is that unlike many other cells, neurons are non-dividing cells and hence cannot easily dilute toxic proteins, peptides, and organelles. Another possibility is that COG is playing a specific and yet uncovered role in neuronal cells. Although the COG complex has been largely detected at the established perinuclear Golgi location in neurons (Figure 1) this does not exclude some sort of moonlighting function for COG subunits at other neuron-specific locations where it could be involved in tethering of specialized vesicles.
Considering the systemic and complex character of COG-related diseases, multiple questions regarding the associated neurological symptoms remain to be addressed. Thus, more in vivo studies are needed to dissect the role of COG and its Rab and SNARE partners in these symptoms. Input from COG deficiencies within neuroglia and subtypes of neuroglia presents yet another poorly studied field and avenue requiring further studies. Further research is also needed to establish in which diseases COG-deficiency-associated neurological syndromes are secondary manifestations of some other primary disease state.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful to Rachel D. Hendrix and Wezley C. Griffin for critical reading of the manuscript. This work was supported by the NIH grants GM083144 and U54 GM105814, and by the Pilot grant from the Arkansas Biosciences Institute.
References
- Amessou M., Fradagrada A., Falguières T., Lord J. M., Smith D. C., Roberts L. M., et al. (2007). Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins. J. Cell Sci. 120, 1457–1468. 10.1242/jcs.03436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada C., Bustamante M., Atwater I., Rojas E., Caviedes R., Caviedes P. (2010). Apoptosis is directly related to intracellular amyloid accumulation in a cell line derived from the cerebral cortex of a trisomy 16 mouse, an animal model of Down syndrome. Neurosci. Lett. 470, 81–85. 10.1016/j.neulet.2009.12.062 [DOI] [PubMed] [Google Scholar]
- Bellouze S., Schäfer M. K., Buttigieg D., Baillat G., Rabouille C., Haase G. (2014). Golgi fragmentation in pmn mice is due to a defective ARF1/TBCE cross-talk that coordinates COPI vesicle formation and tubulin polymerization. Hum. Mol. Genet. 23, 5961–5975. 10.1093/hmg/ddu320 [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. (2004). The mechanisms of vesicle budding and fusion. Cell 116, 153–166. 10.1016/S0092-8674(03)01079-1 [DOI] [PubMed] [Google Scholar]
- Burré J., Sharma M., Tsetsenis T., Buchman V., Etherton M. R., Südhof T. C. (2010). Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329, 1663–1667. 10.1126/science.1195227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo A. M., Peterhoff C. M., Troncoso J. C., Gomez-Isla T., Hyman B. T., Nixon R. A. (2000). Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and down syndrome: differential effects of APOE genotype and presenilin mutations. Am. J. Pathol. 157, 277–286. 10.1016/S0002-9440(10)64538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A., Sharma D. K., Marks D. L., Pagano R. E. (2004). Elevated endosomal cholesterol levels in Niemann-Pick cells inhibit rab4 and perturb membrane recycling. Mol. Biol. Cell 15, 4500–4511. 10.1091/mbc.E04-05-0432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua C. E., Tang B. L. (2008). Syntaxin 16 is enriched in neuronal dendrites and may have a role in neurite outgrowth. Mol. Membr. Biol. 25, 35–45. 10.1080/09687680701504649 [DOI] [PubMed] [Google Scholar]
- Cooper A. A., Gitler A. D., Cashikar A., Haynes C. M., Hill K. J., Bhullar B., et al. (2006). Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson's models. Science 313, 324–328. 10.1126/science.1129462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett M. A., Schwake M., Bahlo M., Dibbens L. M., Lin M., Gandolfo L. C., et al. (2011). A mutation in the Golgi Qb-SNARE gene GOSR2 causes progressive myoclonus epilepsy with early ataxia. Am. J. Hum. Genet. 88, 657–663. 10.1016/j.ajhg.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam N. P., Ungar D. (2012). Retrograde vesicle transport in the Golgi. Protoplasma 249, 943–955. 10.1007/s00709-011-0361-7 [DOI] [PubMed] [Google Scholar]
- D'Adamo P., Masetti M., Bianchi V., Morè L., Mignogna M. L., Giannandrea M., et al. (2014). RAB GTPases and RAB-interacting proteins and their role in the control of cognitive functions. Neurosci. Biobehav. Rev. 46(Pt 2), 302–314. 10.1016/j.neubiorev.2013.12.009 [DOI] [PubMed] [Google Scholar]
- De Strooper B., Iwatsubo T., Wolfe M. S. (2012). Presenilins and gamma-secretase: structure, function, and role in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2:a006304. 10.1101/cshperspect.a006304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan J. M., deWit C., McConlogue L., Maltese W. A. (1995). The Ras-related GTP-binding protein, Rab1B, regulates early steps in exocytic transport and processing of beta-amyloid precursor protein. J. Biol. Chem. 270, 10982–10989. 10.1074/jbc.270.18.10982 [DOI] [PubMed] [Google Scholar]
- Fotso P., Koryakina Y., Pavliv O., Tsiomenko A. B., Lupashin V. V. (2005). Cog1p plays a central role in the organization of the yeast conserved oligomeric Golgi complex. J. Biol. Chem. 280, 27613–27623. 10.1074/jbc.M504597200 [DOI] [PubMed] [Google Scholar]
- Foulquier F., Ungar D., Reynders E., Zeevaert R., Mills P., García-Silva M. T., et al. (2007). A new inborn error of glycosylation due to a Cog8 deficiency reveals a critical role for the Cog1-Cog8 interaction in COG complex formation. Hum. Mol. Genet. 16, 717–730. 10.1093/hmg/ddl476 [DOI] [PubMed] [Google Scholar]
- Foulquier F., Vasile E., Schollen E., Callewaert N., Raemaekers T., Quelhas D., et al. (2006). Conserved oligomeric Golgi complex subunit 1 deficiency reveals a previously uncharacterized congenital disorder of glycosylation type II. Proc. Natl. Acad. Sci. U.S.A. 103, 3764–3769. 10.1073/pnas.0507685103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze H. H., Chong J. X., Bamshad M. J., Ng B. G. (2014). Solving glycosylation disorders: fundamental approaches reveal complicated pathways. Am. J. Hum. Genet. 94, 161–175. 10.1016/j.ajhg.2013.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze H. H., Eklund E. A., Ng B. G., Patterson M. C. (2012). Neurology of inherited glycosylation disorders. Lancet Neurol. 11, 453–466. 10.1016/S1474-4422(12)70040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs-Telem D., Stewart H., Rapaport D., Nousbeck J., Gat A., Gini M., et al. (2011). CEDNIK syndrome results from loss-of-function mutations in SNAP29. Br. J. Dermatol. 164, 610–616. 10.1111/j.1365-2133.2010.10133.x [DOI] [PubMed] [Google Scholar]
- Fung C. W., Matthijs G., Sturiale L., Garozzo D., Wong K. Y., Wong R., et al. (2012). COG5-CDG with a mild neurohepatic presentation. JIMD Rep. 3, 67–70. 10.1007/8904_2011_61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg S. D., Alldred M. J., Counts S. E., Cataldo A. M., Neve R. L., Jiang Y., et al. (2010). Microarray analysis of hippocampal CA1 neurons implicates early endosomal dysfunction during Alzheimer's disease progression. Biol. Psychiatry 68, 885–893. 10.1016/j.biopsych.2010.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler A. D., Bevis B. J., Shorter J., Strathearn K. E., Hamamichi S., Su L. J., et al. (2008). The Parkinson's disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc. Natl. Acad. Sci. U.S.A. 105, 145–150. 10.1073/pnas.0710685105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B. S., Malhotra V. (1998). The curious status of the Golgi apparatus. Cell 95, 883–889. 10.1016/S0092-8674(00)81713-4 [DOI] [PubMed] [Google Scholar]
- Gonatas N. K., Stieber A., Gonatas J. O. (2006). Fragmentation of the Golgi apparatus in neurodegenerative diseases and cell death. J. Neurol. Sci. 246, 21–30. 10.1016/j.jns.2006.01.019 [DOI] [PubMed] [Google Scholar]
- Gong Q., Anderson C. L., January C. T., Zhou Z. (2002). Role of glycosylation in cell surface expression and stability of HERG potassium channels. Am. J. Physiol. Heart Circ. Physiol. 283, H77–H84. 10.1152/ajpheart.00008.2002 [DOI] [PubMed] [Google Scholar]
- Haas A. K., Yoshimura S., Stephens D. J., Preisinger C., Fuchs E., Barr F. A. (2007). Analysis of GTPase-activating proteins: Rab1 and Rab43 are key Rabs required to maintain a functional Golgi complex in human cells. J. Cell Sci. 120, 2997–3010. 10.1242/jcs.014225 [DOI] [PubMed] [Google Scholar]
- Hardy J. (2006). A hundred years of Alzheimer's disease research. Neuron 52, 3–13. 10.1016/j.neuron.2006.09.016 [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Yang Z., Oltedal L., Davanger S., Hay J. C. (2004). Intramolecular protein-protein and protein-lipid interactions control the conformation and subcellular targeting of neuronal Ykt6. J. Cell Sci. 117, 4495–4508. 10.1242/jcs.01314 [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Zinsser S., Rhee Y., Vik-Mo E. O., Davanger S., Hay J. C. (2003). Mammalian ykt6 is a neuronal SNARE targeted to a specialized compartment by its profilin-like amino terminal domain. Mol. Biol. Cell 14, 698–720. 10.1091/mbc.E02-09-0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts S., De Laet C., Bontems P., Rooze S., Souayah H., Sznajer Y., et al. (2012). Deficiency of subunit 6 of the conserved oligomeric Golgi complex (COG6-CDG): second patient, different phenotype. JIMD Rep. 4, 103–108. 10.1007/8904_2011_79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi G., Wang Y. (2015). Golgi defects enhance APP amyloidogenic processing in Alzheimer's disease. Bioessays 37, 240–247. 10.1002/bies.201400116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabayama H., Tokushige N., Takeuchi M., Mikoshiba K. (2008). Syntaxin 6 regulates nerve growth factor-dependent neurite outgrowth. Neurosci. Lett. 436, 340–344. 10.1016/j.neulet.2008.03.061 [DOI] [PubMed] [Google Scholar]
- Kingsley D. M., Kozarsky K. F., Segal M., Krieger M. (1986). Three types of low density lipoprotein receptor-deficient mutant have pleiotropic defects in the synthesis of N-linked, O-linked, and lipid- linked carbohydrate chains. J. Cell Biol. 102, 1576–1585. 10.1083/jcb.102.5.1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodera H., Ando N., Yuasa I., Wada Y., Tsurusaki Y., Nakashima M., et al. (2015). Mutations in COG2 encoding a subunit of the conserved oligomeric golgi complex cause a congenital disorder of glycosylation. Clin. Genet. 87, 455–460. 10.1111/cge.12417 [DOI] [PubMed] [Google Scholar]
- Kracun I., Rosner H., Drnovsek V., Heffer-Lauc M., Cosovic C., Lauc G. (1991). Human brain gangliosides in development, aging and disease. Int. J. Dev. Biol. 35, 289–295. [PubMed] [Google Scholar]
- Kracun I., Rosner H., Drnovsek V., Vukelic Z., Cosovic C., Trbojevic-Cepe M., et al. (1992). Gangliosides in the human brain development and aging. Neurochem. Int. 20, 421–431. 10.1016/0197-0186(92)90057-X [DOI] [PubMed] [Google Scholar]
- Kranz C., Ng B. G., Sun L., Sharma V., Eklund E. A., Miura Y., et al. (2007). COG8 deficiency causes new congenital disorder of glycosylation type IIh. Hum. Mol. Genet. 16, 731–741. 10.1093/hmg/ddm028 [DOI] [PubMed] [Google Scholar]
- Kudlyk T., Willett R., Pokrovskaya I. D., Lupashin V. (2013). COG6 interacts with a subset of the Golgi SNAREs and is important for the Golgi complex integrity. Traffic 14, 194–204. 10.1111/tra.12020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar A. J., Rickmann M., Backofen B., Browski S. M., Rosenbusch J., Schöning S., et al. (2011). Lack of the endosomal SNAREs vti1a and vti1b led to significant impairments in neuronal development. Proc. Natl. Acad. Sci. U.S.A. 108, 2575–2580. 10.1073/pnas.1013891108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufman O., Freeze H. H., Hong W., Lev S. (2013a). Deficiency of the Cog8 subunit in normal and CDG-derived cells impairs the assembly of the COG and Golgi SNARE complexes. Traffic 14, 1065–1077. 10.1111/tra.12093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufman O., Hong W., Lev S. (2013b). The COG complex interacts with multiple Golgi SNAREs and enhances fusogenic assembly of SNARE complexes. J. Cell Sci. 126, 1506–1516. 10.1242/jcs.122101 [DOI] [PubMed] [Google Scholar]
- Laufman O., Kedan A., Hong W., Lev S. (2009). Direct interaction between the COG complex and the SM protein, Sly1, is required for Golgi SNARE pairing. EMBO J. 28, 2006–2017. 10.1038/emboj.2009.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbehusen J., Thiel C., Rind N., Ungar D., Prinsen B. H., de Koning T. J., et al. (2010). Fatal outcome due to deficiency of subunit 6 of the conserved oligomeric Golgi complex leading to a new type of congenital disorders of glycosylation. Hum. Mol. Genet. 19, 3623–3633. 10.1093/hmg/ddq278 [DOI] [PubMed] [Google Scholar]
- Lupashin V., Ungar D. (2008). COG complex, in The Golgi Apparatus, eds Mironov A., Pavelka M. (Vienna: Springer; ), 120–129. 10.1007/978-3-211-76310-0_9 [DOI] [Google Scholar]
- Lupashin V. V., Pokrovskaya I. D., McNew J. A., Waters M. G. (1997). Characterization of a novel yeast SNARE protein implicated in Golgi retrograde traffic. Mol. Biol. Cell 8, 2659–2676. 10.1091/mbc.8.12.2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConlogue L., Castellano F., deWit C., Schenk D., Maltese W. A. (1996). Differential effects of a Rab6 mutant on secretory versus amyloidogenic processing of Alzheimer's beta-amyloid precursor protein. J. Biol. Chem. 271, 1343–1348. 10.1074/jbc.271.3.1343 [DOI] [PubMed] [Google Scholar]
- Miller V. J., Sharma P., Kudlyk T. A., Frost L., Rofe A. P., Watson I. J., et al. (2013). Molecular insights into vesicle tethering at the Golgi by the conserved oligomeric Golgi (COG) complex and the golgin TATA element modulatory factor (TMF). J. Biol. Chem. 288, 4229–4240. 10.1074/jbc.M112.426767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. J., Ungar D. (2012). Re'COG'nition at the Golgi. Traffic 13, 891–897. 10.1111/j.1600-0854.2012.01338.x [DOI] [PubMed] [Google Scholar]
- Morava E., Zeevaert R., Korsch E., Huijben K., Wopereis S., Matthijs G., et al. (2007). A common mutation in the COG7 gene with a consistent phenotype including microcephaly, adducted thumbs, growth retardation, VSD and episodes of hyperthermia. Eur. J. Hum. Genet. 15, 638–645. 10.1038/sj.ejhg.5201813 [DOI] [PubMed] [Google Scholar]
- Nagahama M., Orci L., Ravazzola M., Amherdt M., Lacomis L., Tempst P., et al. (1996). A v-SNARE implicated in intra-Golgi transport. J. Cell Biol. 133, 507–516. 10.1083/jcb.133.3.507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi S., Barsoum M. J., Bossy-Wetzel E., Sütterlin C., Malhotra V., Lipton S. A. (2008). A Golgi fragmentation pathway in neurodegeneration. Neurobiol. Dis. 29, 221–231. 10.1016/j.nbd.2007.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng B. G., Kranz C., Hagebeuk E. E., Duran M., Abeling N. G., Wuyts B., et al. (2007). Molecular and clinical characterization of a Moroccan Cog7 deficient patient. Mol. Genet. Metab. 91, 201–204. 10.1016/j.ymgme.2007.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng B. G., Sharma V., Sun L., Loh E., Hong W., Tay S. K., et al. (2011). Identification of the first COG-CDG patient of Indian origin. Mol. Genet. Metab. 102, 364–367. 10.1016/j.ymgme.2010.11.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngamukote S., Yanagisawa M., Ariga T., Ando S., Yu R. K. (2007). Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J. Neurochem. 103, 2327–2341. 10.1111/j.1471-4159.2007.04910.x [DOI] [PubMed] [Google Scholar]
- Paesold-Burda P., Maag C., Troxler H., Foulquier F., Kleinert P., Schnabel S., et al. (2009). Deficiency in COG5 causes a moderate form of congenital disorders of glycosylation. Hum. Mol. Genet. 18, 4350–4356. 10.1093/hmg/ddp389 [DOI] [PubMed] [Google Scholar]
- Pan P. Y., Cai Q., Lin L., Lu P. H., Duan S., Sheng Z. H. (2005). SNAP-29-mediated modulation of synaptic transmission in cultured hippocampal neurons. J. Biol. Chem. 280, 25769–25779. 10.1074/jbc.M502356200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F., Varlamov O., Paz K., McNew J. A., Hurtado D., Söllner T. H., et al. (2002). Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc. Natl. Acad. Sci. U.S.A. 99, 5424–5429. 10.1073/pnas.082100899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peric A., Annaert W. (2015). Early etiology of Alzheimer's disease: tipping the balance toward autophagy or endosomal dysfunction? Acta Neuropathol. 129, 363–381. 10.1007/s00401-014-1379-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S. R. (2013). Rab GTPase regulation of membrane identity. Curr. Opin. Cell Biol. 25, 414–419. 10.1016/j.ceb.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendón W. O., Martínez-Alonso E., Tomás M., Martínez-Martínez N., Martínez-Menárguez J. A. (2013). Golgi fragmentation is Rab and SNARE dependent in cellular models of Parkinson's disease. Histochem. Cell Biol. 139, 671–684. 10.1007/s00418-012-1059-4 [DOI] [PubMed] [Google Scholar]
- Reynders E., Foulquier F., Annaert W., Matthijs G. (2011). How Golgi glycosylation meets and needs trafficking: the case of the COG complex. Glycobiology 21, 853–863. 10.1093/glycob/cwq179 [DOI] [PubMed] [Google Scholar]
- Reynders E., Foulquier F., Leão Teles E., Quelhas D., Morelle W., Rabouille C., et al. (2009). Golgi function and dysfunction in the first COG4-deficient CDG type II patient. Hum. Mol. Genet. 18, 3244–3256. 10.1093/hmg/ddp262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson B. C., Smith R. D., Ungar D., Nakamura A., Jeffrey P. D., Lupashin V. V., et al. (2009). Structural basis for a human glycosylation disorder caused by mutation of the COG4 gene. Proc. Natl. Acad. Sci. U.S.A. 106, 13329–13334. 10.1073/pnas.0901966106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J., Südhof T. C. (2012). The membrane fusion enigma: SNAREs, Sec1/Munc18 proteins, and their accomplices–guilty as charged? Annu. Rev. Cell Dev. Biol. 28, 279–308. 10.1146/annurev-cellbio-101011-155818 [DOI] [PubMed] [Google Scholar]
- Rosenbaum E. E., Vasiljevic E., Cleland S. C., Flores C., Colley N. J. (2014). The Gos28 SNARE protein mediates intra-Golgi transport of rhodopsin and is required for photoreceptor survival. J. Biol. Chem. 289, 32392–32409. 10.1074/jbc.M114.585166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymen D., Keldermans L., Race V., Régal L., Deconinck N., Dionisi-Vici C., et al. (2012). COG5-CDG: expanding the clinical spectrum. Orphanet J. Rare Dis. 7:94. 10.1186/1750-1172-7-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymen D., Winter J., van Hasselt P. M., Jaeken J., Kasapkara C., Gokcay G., et al. (2015). Key features and clinical variability of COG6-CDG. Mol. Genet. Metab.. [Epub ahead of print]. 10.1016/j.ymgme.2015.07.003 [DOI] [PubMed] [Google Scholar]
- Saito T., Suemoto T., Brouwers N., Sleegers K., Funamoto S., Mihira N., et al. (2011). Potent amyloidogenicity and pathogenicity of Abeta43. Nat. Neurosci. 14, 1023–1032. 10.1038/nn.2858 [DOI] [PubMed] [Google Scholar]
- Schardt A., Brinkmann B. G., Mitkovski M., Sereda M. W., Werner H. B., Nave K. A. (2009). The SNARE protein SNAP-29 interacts with the GTPase Rab3A: implications for membrane trafficking in myelinating glia. J. Neurosci. Res. 87, 3465–3479. 10.1002/jnr.22005 [DOI] [PubMed] [Google Scholar]
- Scheper W., Zwart R., Baas F. (2004). Rab6 membrane association is dependent of Presenilin 1 and cellular phosphorylation events. Brain Res. Mol. Brain Res. 122, 17–23. 10.1016/j.molbrainres.2003.11.013 [DOI] [PubMed] [Google Scholar]
- Scott H., Panin V. M. (2014). N-glycosylation in regulation of the nervous system. Adv. Neurobiol. 9, 367–394. 10.1007/978-1-4939-1154-7_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaheen R., Ansari S., Alshammari M. J., Alkhalidi H., Alrukban H., Eyaid W., et al. (2013). A novel syndrome of hypohidrosis and intellectual disability is linked to COG6 deficiency. J. Med. Genet. 50, 431–436. 10.1136/jmedgenet-2013-101527 [DOI] [PubMed] [Google Scholar]
- Shestakova A., Suvorova E., Pavliv O., Khaidakova G., Lupashin V. (2007). Interaction of the conserved oligomeric Golgi complex with t-SNARE Syntaxin5a/Sed5 enhances intra-Golgi SNARE complex stability. J. Cell Biol. 179, 1179–1192. 10.1083/jcb.200705145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestakova A., Zolov S., Lupashin V. (2006). COG complex-mediated recycling of Golgi glycosyltransferases is essential for normal protein glycosylation. Traffic 7, 191–204. 10.1111/j.1600-0854.2005.00376.x [DOI] [PubMed] [Google Scholar]
- Siddiqi S., Mani A. M., Siddiqi S. A. (2010). The identification of the SNARE complex required for the fusion of VLDL-transport vesicle with hepatic cis-Golgi. Biochem. J. 429, 391–401. 10.1042/BJ20100336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. D., Willett R., Kudlyk T., Pokrovskaya I., Paton A. W., Paton J. C., et al. (2009). The COG complex, Rab6 and COPI define a novel Golgi retrograde trafficking pathway that is exploited by SubAB toxin. Traffic 10, 1502–1517. 10.1111/j.1600-0854.2009.00965.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaapen L. J. M., Bakker J. A., van der Meer S. B., Sijstermans H. J., Steet R. A., Wevers R. A., et al. (2005). Clinical and biochemical presentation of siblings with COG-7 deficiency, a lethal multiple O- and N-glycosylation disorder. J. Inherit. Metab. Dis. 28, 707–714. 10.1007/s10545-005-0015-z [DOI] [PubMed] [Google Scholar]
- Spessott W., Uliana A., Maccioni H. J. (2010a). Cog2 null mutant CHO cells show defective sphingomyelin synthesis. J. Biol. Chem. 285, 41472–41482. 10.1074/jbc.M110.150011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spessott W., Uliana A., MacCioni H. J. (2010b). Defective GM3 synthesis in Cog2 null mutant CHO cells associates to mislocalization of lactosylceramide sialyltransferase in the Golgi complex. Neurochem. Res. 35, 2161–2167. 10.1007/s11064-010-0319-8 [DOI] [PubMed] [Google Scholar]
- Sprecher E., Ishida-Yamamoto A., Mizrahi-Koren M., Rapaport D., Goldsher D., Indelman M., et al. (2005). A mutation in SNAP29, coding for a SNARE protein involved in intracellular trafficking, causes a novel neurocutaneous syndrome characterized by cerebral dysgenesis, neuropathy, ichthyosis, and palmoplantar keratoderma. Am. J. Hum. Genet. 77, 242–251. 10.1086/432556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegmaier M., Yang B., Yoo J. S., Huang B., Shen M., Yu S., et al. (1998). Three novel proteins of the syntaxin/SNAP-25 family. J. Biol. Chem. 273, 34171–34179. 10.1074/jbc.273.51.34171 [DOI] [PubMed] [Google Scholar]
- Steet R., Kornfeld S. (2006). COG-7-deficient human fibroblasts exhibit altered recycling of golgi proteins. Mol. Biol. Cell 17, 2312–2321. 10.1091/mbc.E05-08-0822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam V. N., Peter F., Philp R., Wong S. H., Hong W. (1996). GS28, a 28-kilodalton Golgi SNARE that participates in ER-Golgi transport. Science 272, 1161–1163. 10.1126/science.272.5265.1161 [DOI] [PubMed] [Google Scholar]
- Suga K., Hattori H., Saito A., Akagawa K. (2005a). RNA interference-mediated silencing of the syntaxin 5 gene induces Golgi fragmentation but capable of transporting vesicles. FEBS Lett. 579, 4226–4234. 10.1016/j.febslet.2005.06.053 [DOI] [PubMed] [Google Scholar]
- Suga K., Saito A., Akagawa K. (2015). ER stress response in NG108-15 cells involves upregulation of syntaxin 5 expression and reduced amyloid beta peptide secretion. Exp. Cell Res. 332, 11–23. 10.1016/j.yexcr.2015.01.001 [DOI] [PubMed] [Google Scholar]
- Suga K., Saito A., Tomiyama T., Mori H., Akagawa K. (2005b). Syntaxin 5 interacts specifically with presenilin holoproteins and affects processing of betaAPP in neuronal cells. J. Neurochem. 94, 425–439. 10.1111/j.1471-4159.2005.03210.x [DOI] [PubMed] [Google Scholar]
- Thayanidhi N., Helm J. R., Nycz D. C., Bentley M., Liang Y., Hay J. C. (2010). Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs. Mol. Biol. Cell 21, 1850–1863. 10.1091/mbc.E09-09-0801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar D., Oka T., Vasile E., Krieger M., Hughson F. M. (2005). Subunit architecture of the conserved oligomeric Golgi complex. J. Biol. Chem. 280, 32729–32735. 10.1074/jbc.M504590200 [DOI] [PubMed] [Google Scholar]
- von Mollard G. F., Nothwehr S. F., Stevens T. H. (1997). The yeast v-SNARE Vti1p mediates two vesicle transport pathways through interactions with the t-SNAREs Sed5p and Pep12p. J. Cell Biol. 137, 1511–1524. 10.1083/jcb.137.7.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A. M., Kurps J., de Wit H., Schoning S., Toft-Bertelsen T. L., Lauks J., et al. (2014). The SNARE protein vti1a functions in dense-core vesicle biogenesis. EMBO J. 33, 1681–1697. 10.15252/embj.201387549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I., Zhu J., Recio-Pinto E., Thornhill W. B. (2004). Glycosylation affects the protein stability and cell surface expression of Kv1.4 but Not Kv1.1 potassium channels. A pore region determinant dictates the effect of glycosylation on trafficking. J. Biol. Chem. 279, 8879–8885. 10.1074/jbc.M309802200 [DOI] [PubMed] [Google Scholar]
- Whyte J. R., Munro S. (2002). Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627–2637. [DOI] [PubMed] [Google Scholar]
- Willett R., Kudlyk T., Pokrovskaya I., Schönherr R., Ungar D., Duden R., et al. (2013a). COG complexes form spatial landmarks for distinct SNARE complexes. Nat. Commun. 4, 1553. 10.1038/ncomms2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett R., Pokrovskaya I., Kudlyk T., Lupashin V. (2014). Multipronged interaction of the COG complex with intracellular membranes. Cell. Logist. 4:e27888. 10.4161/cl.27888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett R., Ungar D., Lupashin V. (2013b). The Golgi puppet master: COG complex at center stage of membrane trafficking interactions. Histochem. Cell Biol. 140, 271–283. 10.1007/s00418-013-1117-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Steet R. A., Bohorov O., Bakker J., Newell J., Krieger M., et al. (2004). Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat. Med. 10, 518–523. 10.1038/nm1041 [DOI] [PubMed] [Google Scholar]
- Xu Y., Martin S., James D. E., Hong W. (2002). GS15 forms a SNARE complex with syntaxin 5, GS28, and Ykt6 and is implicated in traffic in the early cisternae of the Golgi apparatus. Mol. Biol. Cell 13, 3493–3507. 10.1091/mbc.E02-01-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R. K., Macala L. J., Taki T., Weinfield H. M., Yu F. S. (1988). Developmental changes in ganglioside composition and synthesis in embryonic rat brain. J. Neurochem. 50, 1825–1829. 10.1111/j.1471-4159.1988.tb02484.x [DOI] [PubMed] [Google Scholar]
- Zeevaert R., Foulquier F., Cheillan D., Cloix I., Guffon N., Sturiale L., et al. (2009a). A new mutation in COG7 extends the spectrum of COG subunit deficiencies. Eur. J. Med. Genet. 52, 303–305. 10.1016/j.ejmg.2009.06.006 [DOI] [PubMed] [Google Scholar]
- Zeevaert R., Foulquier F., Dimitrov B., Reynders E., Van Damme-Lombaerts R., Simeonov E., et al. (2009b). Cerebrocostomandibular-like syndrome and a mutation in the conserved oligomeric Golgi complex, subunit 1. Hum. Mol. Genet. 18, 517–524. 10.1093/hmg/ddn379 [DOI] [PubMed] [Google Scholar]
- Zhang T., Hong W. (2001). Ykt6 forms a SNARE complex with syntaxin 5, GS28, and Bet1 and participates in a late stage in endoplasmic reticulum-Golgi transport. J. Biol. Chem. 276, 27480–27487. 10.1074/jbc.M102786200 [DOI] [PubMed] [Google Scholar]
- Zolov S. N., Lupashin V. V. (2005). Cog3p depletion blocks vesicle-mediated Golgi retrograde trafficking in HeLa cells. J. Cell Biol. 168, 747–759. 10.1083/jcb.200412003 [DOI] [PMC free article] [PubMed] [Google Scholar]