Abstract
Rod photoreceptors are among the most sensitive light detectors in nature. They achieve their remarkable sensitivity across a wide variety of species through a number of essential adaptations: a specialized cellular geometry, a G-protein cascade with an unusually stable receptor molecule, and a low-noise transduction mechanism, a nearly perfect effector enzyme, and highly evolved mechanisms of feedback control and receptor deactivation. Practically any change in protein expression, enzyme activity, or feedback control can be shown to impair photon detection, either by decreasing sensitivity or signal-to-noise ratio, or by reducing temporal resolution. Comparison of mammals to amphibians suggests that rod outer-segment morphology and the molecules and mechanism of transduction may have evolved together to optimize light sensitivity in darkness, which culminates in the extraordinary ability of these cells to respond to single photons at the ultimate limit of visual perception.
Keywords: G-protein receptor, phosphodiesterase, photoreceptors, retina, rhodopsin, rods, vision
Introduction
Sensory receptors combine cell geometry with a mechanism of detection that can signal at the physical limit of perception (see [1]). Antennal receptors in moths sense single molecules of pheromones [2, 3], hair cells in the ear detect vibration nearly at the limit of Brownian motion [4], and rod photoreceptors in the eye reliably detect single photons of light [5]. Rods (together with the photoreceptors of arthropods) are the smallest and most efficient dim-light detectors in nature. Moreover, a whole spectrum of rods exists in different animals having different dimensions and distinct physiological properties. Despite this variation, all rods share a common feature: reliable detection of the absorption of a single photon.
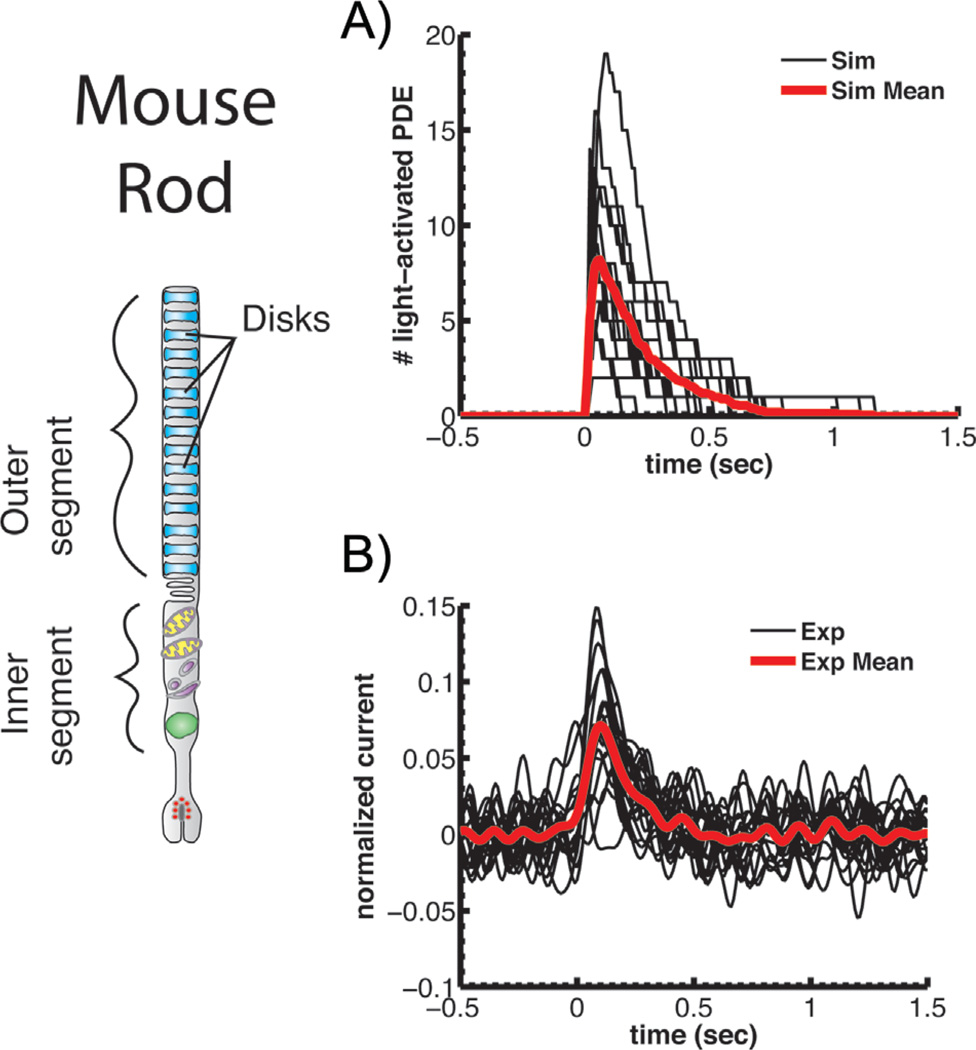
How do these cells achieve this amazing feat? During the preceding decade, many of the mysteries of single-photon detection have been discovered. We now know that, in mammalian retina, a photon is absorbed by a single molecule of rhodopsin which, with high probability, changes conformation to an excited state (Rh*, see [6–8]). Rh* triggers a signal cascade (Fig. 1A) that activates a small number of molecules of the G-protein transducin [9, 10] in the very confined two-dimensional cell membrane array of the rod outer segment (Fig. 1B). These G proteins in turn disinhibit an equal number of molecules of the effector enzyme phosphodiesterase-6 (PDE). Activated PDEs hydrolyze cyclic guanosine monophosphate (cGMP), a diffusible second messenger that gates the opening of ion channels in the plasma membrane (Fig. 1C). A decrease in the cGMP concentration reduces channel opening and produces a brief localized decrease in plasma membrane conductance. Remarkably, only a few light-activated PDEs in a mouse rod (Fig. 2A) can hydrolyze enough cGMP to generate an electrical signal (Fig. 2B) that is large enough to signal detection to the rest of the retina and central nervous system.
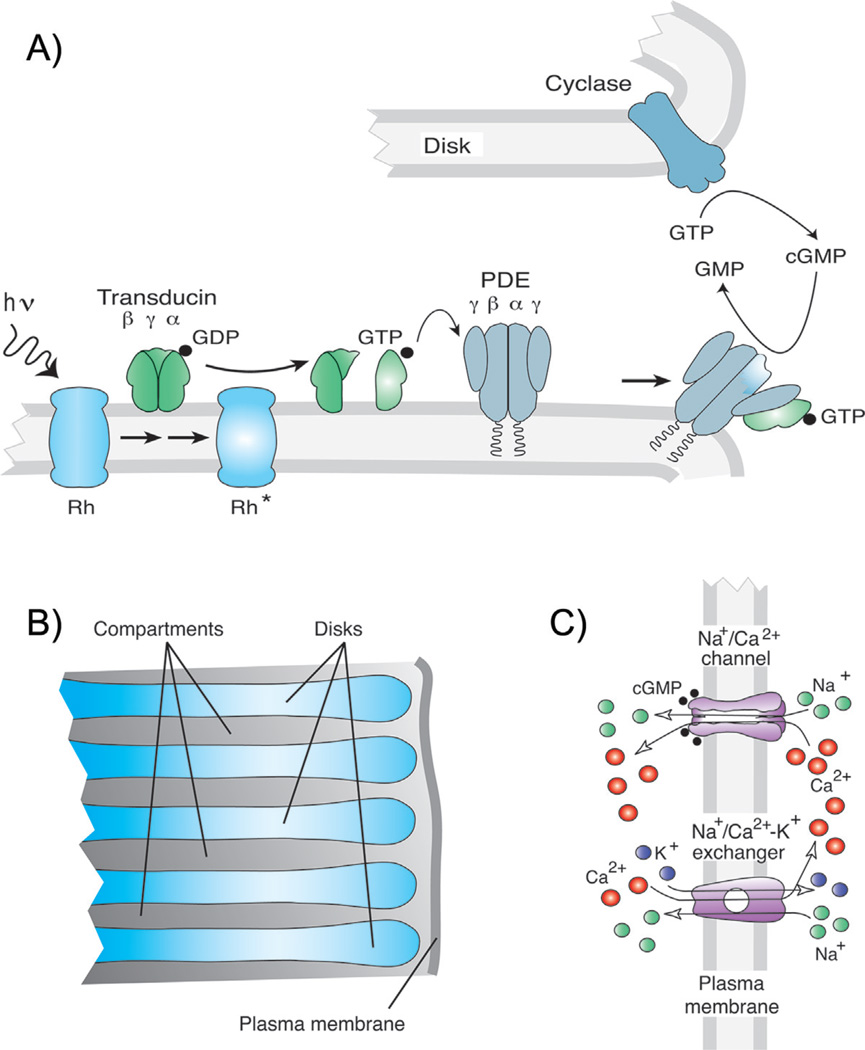
Figure 1.
Anatomy and biochemistry of rod signal transduction. A: Transduction cascade of vertebrate photoreceptors (after [71]). B: Magnified view of part of rod outer segment showing membranous disks containing visual pigment and cytosolic compartments between disks (after [36]). C: Entry and exit of ions in rod outer-segment plasma membrane through cGMP-gated channels and NCKX Ca2+ transporter (after [71]).
Figure 2.
PDE activation and single-photon responses of mouse rods. A: Repeated stochastic simulations of light-activated PDE after absorption of a single photon (black) together with the mean response (red). B: Successive suction-electrode recordings of single-photon responses (black) together with the mean response (red). Currents have been normalized to the circulating current in darkness. Both A and B reprinted from Reingruber et al. [10] with permission of the authors, copyright 2013 National Academy of Sciences, U.S.A.
The design of the cellular mechanism reflects an exquisite balance between reliable photon detection in very dim light and temporal resolution. For example, in a small mouse rod Rh* persists for an average time of only about 40 milliseconds [6–8], which is essential to achieve rapid temporal resolution. If the lifetime of Rh* were longer, the responses of rods would decay slowly and would limit our ability to detect change and motion in dim light. But because the lifetime of Rh* is so short, only a handfull of activated PDE molecules must somehow produce a large enough change in cGMP and outer-segment conductance to be detected against a background of spontaneous fluctuations in signal amplitude.
Noise induced by the molecules of transduction is the greatest problem faced by any sensory receptor at the limit of physical detection. A mouse rod outer segment contains of the order of 108 molecules of rhodopsin, 107 molecules of the G-protein transducin, and 106 molecules of PDE. This very large number of signalling proteins must inevitably produce spontaneous activation. How is it possible for a single rhodopsin molecule and a small number of molecules of transducin and PDE nevertheless to produce a large enough signal to transmit reliable detection?
Considerable evidence indicates that the remarkable ability of the rod to detect single photons in dim light and reliably signal this detection to the rest of the nervous system depends upon an effective combination of cell geometry and biochemistry provided by several key adaptations: (i) outer segment compartmentalization through internal membrane lamellae to achieve a high photopigment density and rapid activation; (ii) exceptionally low spontaneous activity of rhodopsin; (iii) an effector enzyme whose catalytic efficiency is so great that light-dependent hydrolysis of cGMP is limited only by the rate of cGMP binding; (iv) regulation of PDE density and spontaneous PDE activation to limit dark noise without compromising temporal resolution; (v) localized changes in cGMP and Ca2+, which provide spatially confined modulation of membrane conductance and feedback control of cGMP synthesis; and (vi) mechanisms of turn-off that limit variability in the amplitude and waveform of the response.
We describe the role of these adaptations in more detail in the following. One constant theme of our consideration is an apparent co-evolution of rod biochemistry and cell geometry to maintain temporal resolution with a sufficient signal-to-noise ratio to allow rods to detect dim light.
Compartments increase sensitivity and regulate diffusion
The outer segment of a mammalian rod has a highly elaborated membrane structure that parcels the cytoplasmic space into about a thousand nearly separate functional compartments (Fig. 1B). These membrane-delimited compartments organize phototransduction into distinct regions where single-photon absorptions are converted into an electrical signal. This extensive membranous area is necessary because the proteins involved in the early steps of the phototransduction cascade are membrane bound: rhodopsin is an integral membrane protein, and both the G-protein transducin and PDE have lipid attachments that anchor them to the membrane [11]. Attaching the proteins to the membrane increases the amplification and speed of transduction by constraining diffusion to two dimensions instead of three. The high number of internal disks is an adaptation to achieve a high concentration of these membrane-bound proteins in the outer segment. Moreover the tight packing of rhodopsin at an effective concentration in the outer segment of 3 mM increases the probability that a photon is absorbed when it passes through the outer segment, reaching of the order of 60% for a mouse rod only 24µm in length [12]. This high concentration of photopigment is amplified by the orientation of rhodopsin in the disk membrane and the directional and waveguide properties of photoreceptors [13], as well as by other mechanisms that focus light onto the rod photopigment and further enhance the sensitivity of photon detection [14].
The membrane-bound processes of the transduction cascade are confined to the compartment where photon absorption occurs, but there is also diffusional exchange of the second messenger cGMP between compartments. This exchange is absolutely necessary because, as we show in more detail below, single-photon detection requires longitudinal spread of the change in cGMP up and down the outer segment in order to open a sufficient number of ion channels in the plasma membrane. Whereas diffusion within the cytosol of a compartment is relatively unobstructed and rapid, diffusional exchange between compartments along the longitudinal axis of the outer segment is hindered by the internal disks and achieved through narrow gaps between the disks and plasma membrane. Longitudinal diffusion is further enhanced by incisures, which are infoldings of the disks that increase regions of interconnecting cytosol. The small rods of mice have only a single incisure, but the larger rods of amphibians may have as many as 25–30 [15]. Longitudinal diffusion depends on the surface area of the narrow openings through which diffusion can occur (including the incisures), relative to the total disk surface [16, 17]. In summary, outer segment compartmentalization allows for a high density of membrane-bound proteins needed for high light sensitivity. In addition, the highly evolved geometry of the outer segment regulates diffusion and contributes to the amplitude and spread of the signal.
Photon absorption is amplified by the G-protein transducin and by phosphodiesterase activation
The high concentration of rhodopsin and optical properties of the photoreceptors guarantee a high probability of photon absorption, which must then trigger the production of a detectable and reproducible signal. For this task, a sophisticated biochemical signaling pathway functions within the structural constraints imposed by the rod geometry. The first step of the signaling pathway is provided by a G-protein-coupled amplification process leading to activation of a PDE effector enzyme. There are 107 molecules of the G protein transducin in a dark-adapted mouse rod outer segment [18] distributed within about 800 compartments [12], giving somewhat more than 104 transducin molecules per compartment, equally distributed on the two facing disk membranes. Such a high concentration of G protein is unprecedented: photoreceptors may be rivaled only by olfactory receptors, which also use a cyclic-nucleotide-dependent cascade.
Although the concentration of transducin is rather high, it is nevertheless limiting because even small decreases cause fewer transducins to become activated during the short lifetime of Rh*, hence reducing the gain of the cascade [19, 20]. As a consequence, migration of transducin out of the outer segment in very bright light can decrease light sensitivity, reducing ATP consumption and preventing photoreceptor light damage [21, 22]. The concentration of PDE is about 10-fold less than that of transducin – of the order of 106 molecules per rod outer segment or about 103 molecules per compartment; but when PDE expression is decreased by half, there is little change in the rod response [23].
Biochemical and physiological measurements indicate that Rh* can produce a few hundred activated transducin molecules per second [24], but because the mean lifetime of mammalian Rh* is of the order of 40 ms (see [25]), the mean number of activated transducin molecules is only about 5–10. This small number of transducin molecules activates an equal number of phosphodiesterase molecules by binding to the inhibitory γ subunits of the PDE and relieving PDE inhibition (see [9]), all within the membrane of the compartment containing the single Rh*. The activated PDE molecules (Fig. 2A) then hydrolyze cGMP to produce the change in outer segment conductance and current (Fig. 2B). Activated transducin deactivates much more slowly than it binds to and activates PDE. This ensures that transducin accomplishes its task of activating the PDE. As a consequence, the gain of transduction is determined primarily by the gain of transducin activation by Rh*, together with the gain of cGMP hydrolysis during the lifetime of activated PDE.
How can such a small number of activated PDE molecules produce a signal sufficiently large to overcome background noise? A partial explanation is provided by the properties of the enzyme itself: light-activated PDE is an almost perfect enzyme [24, 25], whose rate is limited only by the rate of collision of the enzyme with its substrate cGMP. As a result, the rate of cGMP hydrolysis depends on the encounter rate, which in turn is determined by cGMP diffusion and the geometry of the compartment (primarily its radius, see [26]). Because the compartment of a mouse outer segment is small and cGMP diffusion within a compartment is fast, the hydrolysis rate is so high that the small number of PDE molecules activated by a single photon is sufficient to generate a decrease in the cGMP concentration large enough to produce a detectable response (Fig. 2B).
PDE efficiently hydrolyses cGMP within a compartment, but there must also be spread of the change in cGMP concentration up and down the outer segment produced by longitudinal diffusion. If there were no cGMP spread between compartments, a single photon absorption would rapidly deplete the 40 or so molecules of cGMP freely available within the compartment and would close channels adjacent only to this one compartment. The change in membrane conductance would then be small relative to the conductance of the whole of the outer segment, and the response to a single photon would be lost in the noise [10].
Longitudinal cGMP diffusion ensures that substrate is not depleted for the extremely efficient light-activated PDE, thereby increasing the pool of ion channels affected by photon absorption. Experimental evidence ([27], see also [28, 29]) and theoretical calculation [10, 16] both give a value of the longitudinal diffusion constant of about 40 µm2s−1, which allows the change in cGMP concentration to spread a few micrometers on either side of the compartment absorbing the light [10, 27, 30]. Simulations show [10] that longitudinal diffusion of this order is more than sufficient to allow a single photon to close about 5% of the channels open in darkness. Thus the high efficiency of the PDE enzyme together with diffusion within and between compartments permits a detectable response to a single photon even though very few G proteins and effector enzymes are activated.
Fidelity is limited by background noise
To detect a single photon absorption with fidelity, a rod must generate a signal sufficiently larger than the fluctuations that produce background noise. In darkness, there are two sources of noise in a rod [31]: spontaneous activation of rhodopsin and continuous noise produced by spontaneously active PDE [29]. Because a rod outer segment contains so many rhodopsin molecules, and because spontaneously activated rhodopsin produces a response that is identical to the one produced by rhodopsin activated by light, it is crucial that spontaneous activations occur very infrequently to minimize dark signals that could be confused with photon absorptions. Indeed, on average single rhodopsin molecules spontaneously activate only once every several hundred years [31]. This high stability is an essential feature of rod photodetection and one of the most remarkable properties of the rhodopsin photopigment. Other G-protein receptor molecules activate spontaneously at a much higher rate [32]. Although spontaneous responses are very infrequent, they can be summed together from many rods within the retina and central nervous system and may be ultimately responsible for setting the behavioral threshold of vision [31, 33].
In addition to spontaneous activations of rhodopsin, there is continuous dark noise in the rod produced by random activation and deactivation of PDE [29, 34]. There is also noise due to fast spontaneous openings and closings of the cyclic nucleotide-gated channels, but the amplitude of this noise is small and its frequency range is much higher than that of the rod light response [29, 35]. The PDE noise, on the other hand, is well within the frequency range of the response [29, 31]. Its amplitude depends upon the average number of spontaneously activated PDE molecules in a compartment, which we call ; as well as the rate of cGMP hydrolysis by a spontaneously activated PDE, which we denote as ksp. In darkness, cGMP hydrolysis by spontaneously activated PDE is indispensable to counteract the dark activity of the guanylyl cyclase, the enzyme that synthesizes cGMP. The average number of spontaneously active PDE in a compartment determines the value of the dark turnover rate of cGMP βdark, which is equal to the product of and ksp [36]. The value of βdark sets the steady state cGMP concentration in darkness, and it is also important for temporal resolution and response gain.
In mouse present evidence indicates that is about 1 [10] and βdark approximately 4 per second [27]. Thus on average only a single PDE molecule (out of a total of about 103) is spontaneously active in each compartment at any one time. From and βdark, we can estimate that ksp is of the order 4 s−1. This value for the hydrolysis rate of spontaneously activated PDE is surprising, because it is much smaller than the diffusion-limited rate of light-activated PDE, which is about 60 s−1 [10].
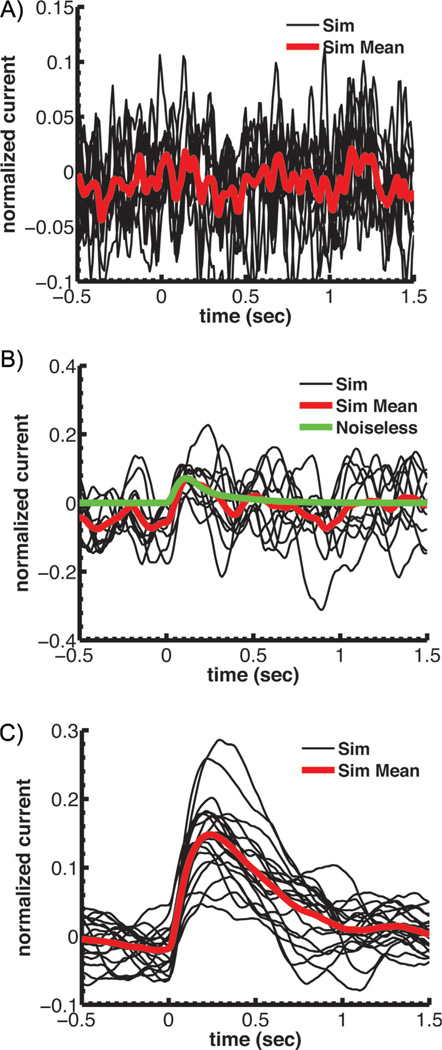
The mechanism responsible for the difference in hydrolysis rates is presently unknown, but it is crucially important. If the rate of spontaneously activated PDE had a value of 60 s−1, a single spontaneously activated PDE per outer-segment compartment would produce a turnover rate βdark of about 60 s−1. Such a high value would drastically reduce the amplitude of the single-photon response (Fig. 3A), because light-activated PDEs would have to compete against a much higher level of spontaneous cGMP hydrolysis. The amplitude of the light response could be maintained only if a greater number of PDEs were activated in the light, but more PDEs could be activated only if the lifetime of Rh* were increased, which would increase the duration of the light response and decrease temporal resolution.
Figure 3.
Simulations of single-photon responses for a mouse rod with modified PDE kinetics or expression. Currents have been normalized to the circulating current in darkness. A: Simulations assuming a rate of spontaneous cGMP hydrolysis set equal to the diffusion-limited rate for light activated PDE. This high spontaneous PDE rate would give a βdark of approximately 55 s−1. B: Simulations of single-photon responses again assuming a rate of spontaneous cGMP hydrolysis set equal to the diffusion-limited rate for light activated PDE as in A but with a PDE density that is 13-fold lower than normal to reduce the amount of spontaneous PDE activations so that βdark is maintained at 4.1 s−1. C: Single-photon simulations with the PDE density reduced by a factor of 5 so as to be the same as in toad. All of A–C reprinted from Reingruber et al. [10] with permission of the authors, copyright 2013 National Academy of Sciences, U.S.A.
A βdark value of around 4 s−1 could be achieved even with a ksp of 60 s−1 by greatly reducing the value of . Reducing would however increase the level of dark noise. Most of the time there would be no spontaneously activated PDE per compartment, and the cGMP concentration would increase. A sudden spontaneous PDE activation would then produce a large reduction in the cGMP concentration because of the high ksp of the PDE. The average cGMP concentration would remain constant, but there would be pronounced fluctuations, which would produce elevated dark noise. The single-photon response would be nearly undetectable (Fig. 3B) unless many more PDEs were activated by light by increasing the lifetime of Rh*, again decreasing temporal resolution. A more detailed analysis suggests that on average around one spontaneously activated PDE per compartment is optimal and needed to restrict the noise to 2–3% of the dark current amplitude, which is at the limit of single-photon detection [10]. These considerations suggest that there must be mechanisms that cause rods to fix the properties of spontaneous PDE activation to optimize background noise, signal gain, and temporal resolution. How this regulation occurs is still unclear.
Of mice and toads
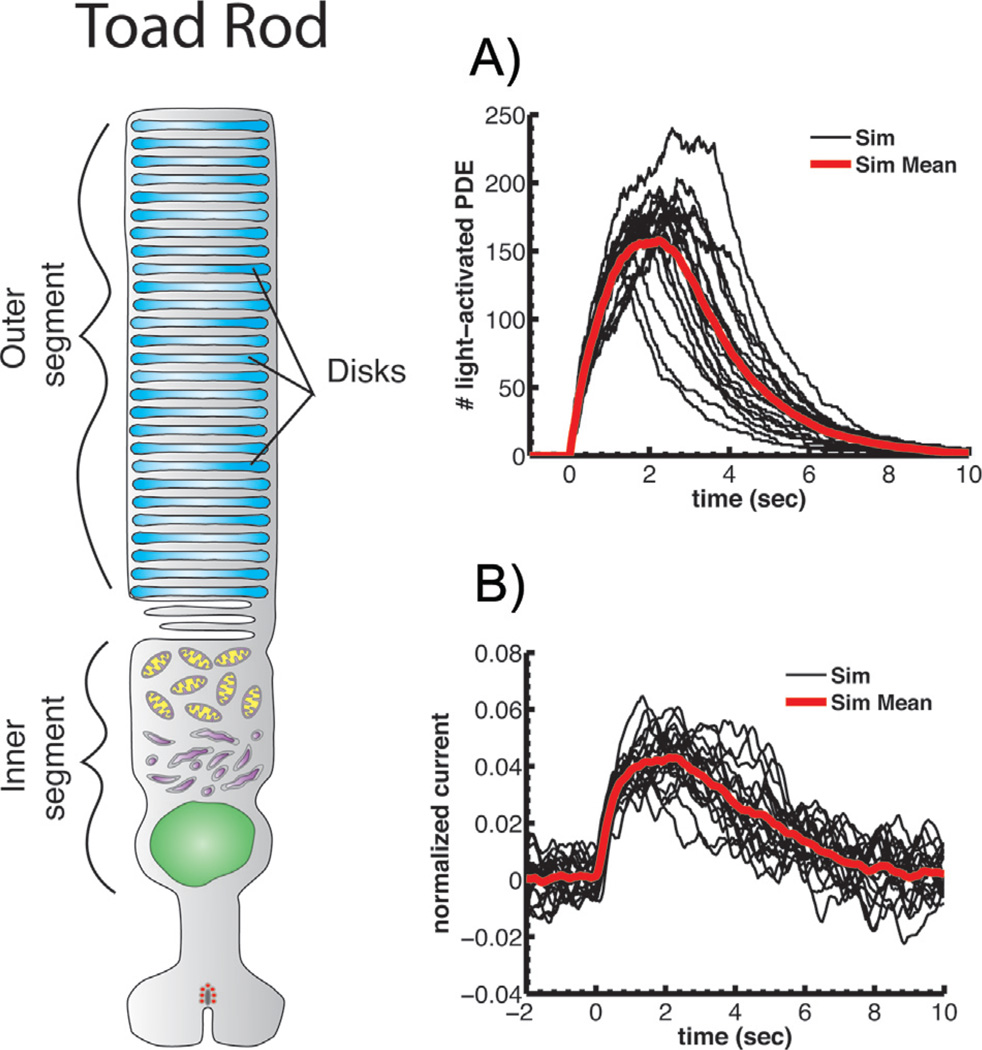
The contrasting effects of signal gain and background noise can be highlighted by comparing mouse rods with the rods of the toad Bufo marinus [10]. A toad rod outer segment is 4–5 times larger in diameter than a mouse rod and has a compartmental volume nearly 20 times greater (see Fig. 4). As a result of this larger diameter, the diffusion-limited hydrolysis rate is much reduced in a toad rod, and many more PDEs must be activated to cause a change in cGMP concentration large enough to produce a detectable response to a single photon (compare Fig. 4A with Fig. 2A). The activation of so many PDEs requires a much longer lifetime of light-activated Rh*, with the consequence that the single-photon response lasts much longer in a toad than in a mammal (compare Fig. 4B with Fig. 2B). Rh* deactivation is so slow in toad that it is probably rate-limiting for the decay of the response [37, 38]. This is in contrast to mouse, where the lifetime of Rh* is much shorter and PDE deactivation limits response decay [6, 7, 39]. Although the temporal resolution of vision is much poorer in a toad than in a mammal in part also as a result of the lower body temperature of amphibians, sensitivity is actually enhanced because rods integrate responses to single photons over a much longer time [40]. This longer integration time makes it possible for a toad to detect a slowly moving worm in much dimmer light than would be possible for a human observer [41, 42].
Figure 4.
PDE activation and single-photon responses of toad rods. Toad rod outer segment drawn to approximately the same scale as mouse rod in Fig. 2. A: Stochastic simulations of light-activated PDE after absorption of a single photon (black) together with the mean response (red), as in Fig. 2A. B: Simulations of single-photon responses with amplitude and time course similar to suction-electrode responses [5, 37]. Currents have been normalized to the circulating current in darkness. Both A and B reprinted from Reingruber et al. [10] with permission of the authors, copyright 2013 National Academy of Sciences, U.S.A.
Although many more PDEs must be activated by light in a toad than in a mouse, a spectral analysis of the dark noise indicates that the number of spontaneously active PDEs per compartment is about the same [10, 29]: has again a value of about one. This finding is unexpected – we would have predicted a higher number from the much larger compartment size in toad compared to mouse. If, however, the number of spontaneously active PDEs were larger in toad, the basal rate of hydrolysis of cGMP in darkness would be higher, and an even larger number of light-activated PDEs would be required to signal the absorption of a photon. More light-activated PDEs would mean yet a longer lifetime of Rh* and a further decrease in temporal resolution. A higher basal rate of cGMP turnover would also waste precious ATP.
How can have the same value in these two species despite the large difference in compartment volume? The answer seems to be that the biochemical concentration of PDE and, therefore, the density of PDE molecules in a compartment is several times lower in toad than in mouse. Put another way, the density of PDE in a mammal is several times higher, about 500 µm−2 of disk membrane versus 100 µm−2 in amphibians (see [43]). If the PDE density were reduced in mouse to be the same as in toad, the value of would also be reduced, leading to greater noise and a commensurate reduction in βdark. As a result, both the amplitude and time course of the mouse single-photon response would be greatly altered (Fig. 3C). The increase in PDE expression in a mammalian rod has no effect on the gain of transduction, because the number of PDEs activated by a photon depends upon the number of transducins produced per Rh*, which is not altered by a change in the density of PDE molecules.
These observations provide a remarkable insight into the evolution of a mammalian rod: one of the key adaptations in a mammal is an increase in the expression level of PDE to compensate for the reduction in outer segment diameter. As a result, a mouse rod can respond to a single photon by closing approximately the same percentage of outer segment channels as in a toad, but it can use many fewer G proteins and effector molecules and achieve higher temporal resolution. The biochemistry of transduction and the geometry of the outer segment may have evolved together so as to ensure the detection of single photons.
Calcium produces feedback control of cGMP synthesis
Calcium enters the rod through cGMP-gated channels in the outer segment (Fig. 1C). These channels are cationic and permeable to monovalent cations such as Na+ and K+, but they are also quite permeable to divalent ions including Ca2+ [44]. As a result, 10–15% of the current entering the outer segment in darkness is carried by Ca2+, and this Ca2+ is continuously expelled by a very active Na+/Ca2+-K+ (or NCKX) exchange protein also located in the outer segment (see [45]). In rods, the NCKX proteins are associated in a complex with the channels, in a ratio of two exchange molecules per channel molecule [46, 47]. The closing of the channels decreases Ca2+ influx into the outer segment, and continued export by the NCKX exchanger produces up to a 10-fold decrease in the free-Ca2+ concentration in a mammalian rod, from about 250nM in darkness to about 25nM in bright light [48]. Because of high exchanger activity and strong Ca2+ buffering [49], the longitudinal distribution of Ca2+ is nearly independent of the diffusion of calcium but simply mirrors the longitudinal distribution of cGMP which controls channel opening [10, 30].
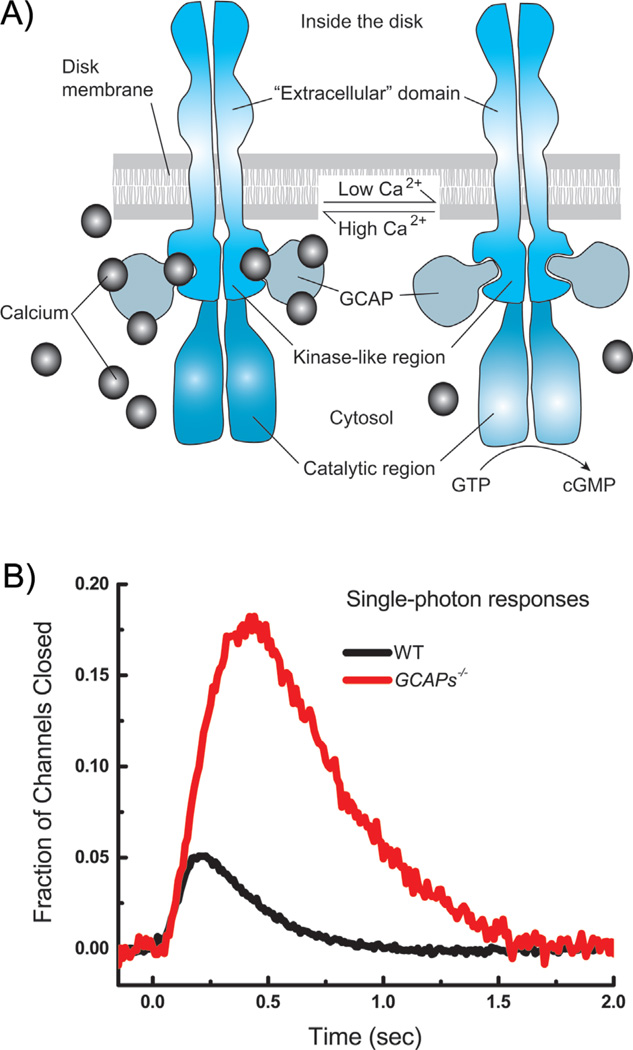
The change in Ca2+ alters the activity of the guanylyl cyclase, the enzyme that synthesizes cGMP. The activity of the cyclase is regulated by Ca2+-binding proteins called guanylyl cyclase-activating proteins (or GCAPs), which control the rate of synthesis of cGMP in the following way (see Fig. 5A and [50]). In darkness, the divalent binding sites of the GCAPs are mostly occupied by Ca2+, and the GCAPs keep the rate of the cyclase rather low and just sufficient to replace the cGMP hydrolyzed by the dark spontaneous rate of the PDE. In the light when PDE is activated, the cGMP concentration falls, channels close, and the free-Ca2+ concentration decreases; Ca2+ falls off the divalent binding sites of the GCAPs and is replaced by Mg2+, and this substitution greatly accelerates cyclase activity. As a consequence, the cyclase makes additional cGMP, opposing the action of the PDE. The GCAPs, therefore, provide negative feedback: activation of PDE decreases cGMP, which closes channels, decreases Ca2+, activates the cyclase, and increases cGMP synthesis to oppose the initial decrease produced by light.
Figure 5.
Regulation of guanylyl cyclase by small molecular-weight Ca2+-binding guanylyl cyclase activating proteins (GCAPs). A: Schematic drawing indicating binding of Ca2+ and small molecular-weight GCAPs to cyclase. B: Comparison of mean responses from 41 wild-type (WT) rods (black) and 21 rods lacking the genes for the GCAP proteins (green). Single-photon responses were calculated from the squared mean and variance (for example [66]). Currents have been normalized to the circulating current in darkness, giving the faction of channels closed as a function of time. The fraction of channels closed is thus the same as the normalized current defined in the legends to Figs. 2–4.
Deletion of the GCAPs causes a large increase in the size of the single-photon response as well as a dramatic slowing of response decay (Fig. 5B). These effects have been extensively investigated [27, 51–53] and are known to be produced in the following way. When the GCAPs are deleted, cyclase activity is no longer modulated, and the single-photon response is larger in amplitude because the change in cGMP concentration is larger: there is no light-dependent increase in cGMP production to counteract the decrease produced by the PDE. Responses decay more slowly because the cyclase rate is not accelerated in the light. The cGMP concentration is no longer able to track the decay rate of PDE but recovers more slowly with the basal rate of the cyclase [52].
Because the amplitude of the single-photon response is so much greater when the GCAPs are deleted, we might wonder whether single-photon detection would be easier without these proteins, notwithstanding the inevitable degradation in temporal resolution produced by slower response decay. But here is the rub: deletion of the GCAPs produces not only an increase in the size of the single-photon response but also an increase in dark noise [10, 52, 54]. Modulation of the cyclase rate produced by the GCAPs acts as negative feedback, as we have said, and in the absence of this feedback, the fluctuations of current in darkness increase as single PDE molecules are spontaneously turned on and off. The signal-to-noise ratio of the rods actually improves somewhat, because the size of the single-photon response increases more than the amplitude of the noise [54]; but the noise is more effectively passed across the synapse between rods and second-order bipolar cells [55, 56]. As a consequence, the signal-to-noise ratio of the single-photon response in bipolar cells is degraded when the GCAPs are deleted, and behaving mice are less able to detect dim illumination [54]. Decreasing GCAP-dependent feedback decreases visual performance.
Increasing GCAP-dependent feedback would also decrease performance, because an increase in cyclase feedback would reduce not only the amplitude of spontaneous noise but also the peak amplitude of the single-photon response. Performance would be increased if GCAP expression were increased while maintaining the amplitude of the single-photon response at a constant value, but the amplitude could be maintained only by increasing the number of light-activated PDEs and the lifetime of Rh*, with a consequent broadening of response time course and reduction in temporal resolution [10]. Thus any alteration in the level of feedback control by the GCAPs and guanylyl cyclase would either reduce signal-to-noise or degrade the ability of the rods to detect change and motion.
Single-photon responses are surprisingly constant in amplitude
One of the remarkable features of the single-photon response is its reproducibility. Denis Baylor and coworkers [5] first showed that the amplitude of the response varies over a surprisingly narrow range, and many later studies have repeated this initial finding [37, 55, 57–59]. How does a rod achieve such a stable amplitude? Most of the amplitude variability reflects differences in the number of light-activated PDEs [38]. The number of light-activated PDEs ultimately determines the amplitude of the response and depends on the lifetime of Rh*. Thus at least part of the explanation of the variability of the response arises from the regulation of Rh* lifetime.
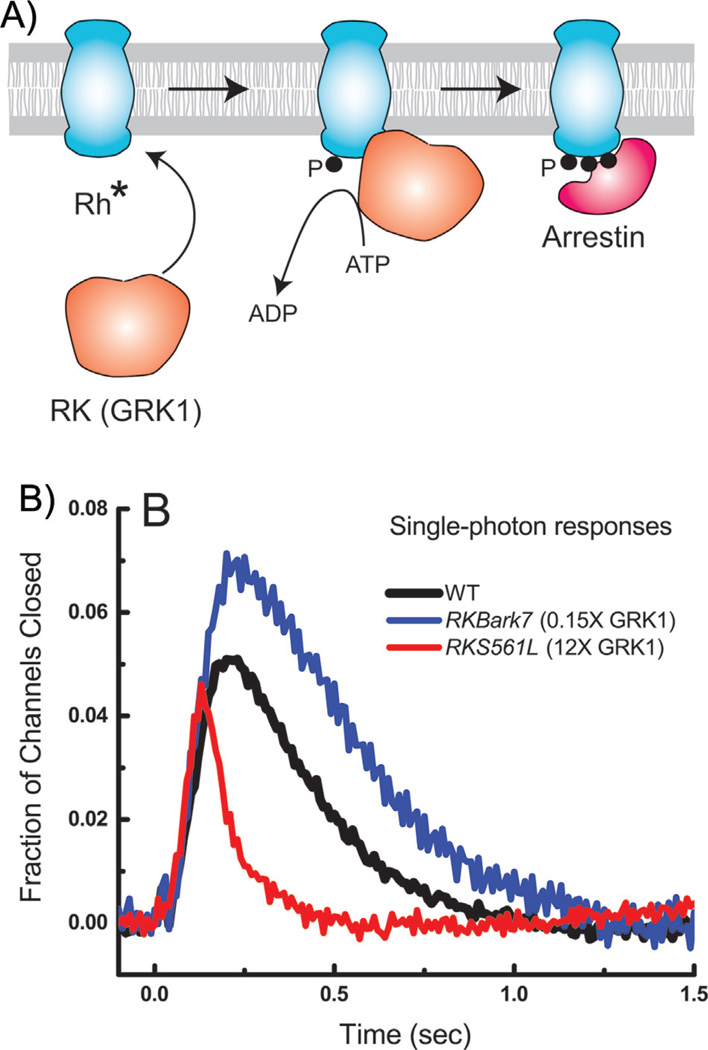
The lifetime of Rh* is controlled at least in part by phosphorylation followed by the binding of arrestin (see Fig. 6A and [1, 60]). Like other G-protein receptors, rhodopsin has carboxyl-terminal serines and threonines (there are six in mouse), and all of them can be phosphorylated by rhodopsin kinase (RK), also known as G-protein-receptor kinase 1 or GRK1 (see for example [61, 62]). How many need to be phosphorylated to extinguish Rh*? Let us suppose for the sake of argument that turning off Rh* requires a single random event like radioactive decay, whose probability is distributed exponentially. We can then quantitate the extent of variability by calculating the coefficient of variation (or CV) of the amplitude of the response, which is the standard deviation divided by the mean. For radioactive decay, the mean and standard deviation are equal, and the CV is one. The CV of the amplitude of the single-photon response, on the other hand, is much less than 1 and of the order of 0.2–0.4 [5, 37, 55, 58, 59]. One widely accepted explanation of the low CV is that rhodopsin inactivation occurs through multiple steps that require several amino acids to be phosphorylated. Many studies have shown that Rh* can be multiply phosphorylated (for example [61, 62, 63]), though there is no agreement about how many and which serines or threonines participate in rhodopsin inactivation and arrestin binding. Some experiments seem to indicate that phosphorylation of all six sites in mouse occurs during Rh* inactivation [64, 65], but it is difficult to conceive how this could happen during the short lifetime of Rh*. The concentration of rhodopsin kinase is at most a few percent of the rhodopsin concentration [61, 66], with a turnover rate (at least in bright light) of only 1–3 phosphorylations per second [61]. Although arrestin binds poorly to singly phosphorylated Rh*, it can bind almost fully when already three of the six sites are phosphorylated [67]. Sites are probably not phosphorylated at random, because Kennedy and coworkers have shown that serines are among the first to be phosphorylated [61], whereas the recent results of Azevedo and coworkers [68] indicate that phosphorylation of threonines is especially important for terminating Rh* activity. Despite clever and determined attempts by many investigators, we still do not know the specificity of the kinase or time course of its action.
Figure 6.
Mechanism of decay of Rh*. A: Schematic drawing indicating phosphorylation of Rh* by rhodopsin kinase (RK or GRK1), followed by binding of arrestin to phosphorylated rhodopsin. B: Comparison of mean responses from 41 WT rods (black), 22 RKBark7 rods (blue) having one-seventh the normal expression level of rhodopsin kinase [7], and 19 RKS561L rods (red) having 12-times the normal expression level of rhodopsin kinase [66]. Single-photon responses were calculated from the squared mean and variance (for example [66]). Currents have been normalized to the circulating current in darkness, giving the faction of channels closed as a function of time. The fraction of channels closed is thus the same as the normalized current defined in the legends to Figs. 2–4.
It is also unclear whether phosphorylation is the only mechanism responsible for Rh* inactivation [37]. If it were, the gain of transduction should depend upon the concentration or activity of the kinase, which is normally such a small percentage of the rhodopsin concentration that it is unlikely to be saturating. Experiments directly varying the expression level of rhodopsin kinase show, however, that a change of over 80-fold from 7-fold smaller to 12-fold larger produces an overall change in the single-photon response amplitude of only about a factor of 2 (Fig. 6B, [7, 66]). The amplitude of the single-photon response is remarkably insensitive to the expression level of the kinase, and the lifetime of Rh* may not be determined by kinase action alone [66].
Other mechanisms have also been proposed to contribute to the small variability of the single-photon response. Ca2+-dependent feedback of the cyclase has been the target of several studies claiming that it does [58, 59] or does not [10, 37, 55, 69, 70] influence response variability. Particularly significant are the recent results of Gross and coworkers [59], which show that the CV of the single-photon response amplitude is increased when the genes for the GCAPs are deleted, from a mean value of 0.34 in WT to 0.42 in GCAPs−/− rods. Contributions have also been suggested for local saturation of cGMP hydrolysis within compartments and a nonlinear relationship between cGMP concentration and outer-segment conductance [70].
Conclusions and prospects
Vertebrate rods detect single photons with a cell geometry that confines transduction to compartments of high lateral diffusion, together with an extremely stable G-protein receptor molecule and an effector enzyme so efficient that its activity is limited only by its collision rate with its cGMP substrate. A single Rh* produced by photon absorption activates only 5–10 PDE molecules in a mammalian rod, but this number is sufficient to produce a detectable change in plasma membrane conductance. Moreover, the number of activated PDEs, the rate of spontaneous PDE activity, and feedback control by the guanylyl cyclase all appear to be optimally adapted to rod geometry: any increase or decrease would make the single-photon response either too small, too long-lasting for mammalian vision, or undetectable above background noise. In amphibians, whose rods have much larger outer segments, the lifetime of Rh* and number of activated PDEs are much greater, and single-photon responses decay more slowly. Amphibian rods have poorer temporal resolution than mammalian rods but integrate photon signals over a longer time, resulting in slower but more sensitive behavioral responses. Considerable progress has been made understanding how responses to single photons are produced, but we still do not fully understand the role of Ca2+ or the surprisingly small variability in single-photon response amplitude. Rh* is extinguished by phosphorylation and arrestin binding, but the identity of the phosphorylated sites and the time course of phosphorylation are still unknown. The biochemistry and geometry of the rod may have adapted together to insure single-photon detection across species, but it is unclear whether these adaptions occurred independently or were coupled together by some other mechanism. These open questions will undoubtedly be the focus of future research for understanding the remarkable sensitivity of rods to single photons.
Acknowledgments
We are grateful to Dr. Margery J. Fain for her assistance in preparing the figures. This work was supported in part by a grant from the National Eye Institute of the NIH to GLF (EY 01844).
Abbreviations
- ATP
adenosine triphosphate
- cGMP
cyclic guanosine monophosphate
- CV
coefficient of variation (standard deviation divided by mean)
- GCAPs
guanylyl cyclase-activating proteins
- GRK1
G-protein-receptor kinase 1 or rhodopsin kinase
- NCKX
sodium/calcium-potassium exchange protein
- PDE
phosphodiesterase 6
- Rh*
photo-excited rhodopsin
- RK
rhodopsin kinase.
Footnotes
The authors have declared no conflict of interest.
References
- 1.Fain GL. Sensory Transduction. Sunderland, MA: Sinauer, Inc.; 2003. [Google Scholar]
- 2.Boeckh J, Kaissling KE, Schneider D. Insect olfactory receptors. Cold Spring Harb Symp Quant Biol. 1965;30:263–280. doi: 10.1101/sqb.1965.030.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Kaissling K-E. Insect olfaction. In: Beidler LM, editor. Olfaction, Handbook of Sensory Physiology. Part I. IV. Berlin: Springer-Verlag; 1971. pp. 351–431. [Google Scholar]
- 4.Hudspeth AJ. Integrating the active process of hair cells with cochlear function. Nat Rev Neurosci. 2014;15:600–614. doi: 10.1038/nrn3786. [DOI] [PubMed] [Google Scholar]
- 5.Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. J Physiol. 1979;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- 6.Krispel CM, Chen D, Melling N, Chen YJ, et al. RGS expression rate-limits recovery of rod photoresponses. Neuron. 2006;51:409–416. doi: 10.1016/j.neuron.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Chen CK, Woodruff ML, Chen FS, Chen D, et al. Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods. J Neurosci. 2010;30:1213–1220. doi: 10.1523/JNEUROSCI.4353-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross OP, Burns ME. Control of rhodopsin’s active lifetime by arrestin-1 expression in mammalian rods. J Neurosci. 2010;30:3450–3457. doi: 10.1523/JNEUROSCI.5391-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arshavsky VY, Burns ME. Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem. 2012;287:1620–1626. doi: 10.1074/jbc.R111.305243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reingruber J, Pahlberg J, Woodruff ML, Sampath AP, et al. Detection of single photons by toad and mouse rods. Proc Natl Acad Sci USA. 2013;110:19378–19383. doi: 10.1073/pnas.1314030110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol. 2008;9:60–71. doi: 10.1038/nrm2299. [DOI] [PubMed] [Google Scholar]
- 12.Nickell S, Park PS, Baumeister W, Palczewski K. Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol. 2007;177:917–925. doi: 10.1083/jcb.200612010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enoch JM. Vertebrate receptor optics and orientation. Documenta Ophthalmologica. 1980;48:373–388. doi: 10.1007/BF00141466. [DOI] [PubMed] [Google Scholar]
- 14.Solovei I, Kreysing M, Lanctot C, Kosem S, et al. Nuclear architecture of rod photoreceptor cells adapts to vision in mammalian evolution. Cell. 2009;137:356–368. doi: 10.1016/j.cell.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 15.Brown PK, Gibbons IR, Wald G. The visual cells and visual pigment of the mudpuppy, Necturus. J Cell Biol. 1963;19:79–106. doi: 10.1083/jcb.19.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holcman D, Korenbrot JI. Longitudinal diffusion in retinal rod and cone outer segment cytoplasm: the consequence of cell structure. Biophys J. 2004;86:2566–2582. doi: 10.1016/S0006-3495(04)74312-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caruso G, Bisegna P, Shen L, Andreucci D, et al. Modeling the role of incisures in vertebrate phototransduction. Biophys J. 2006;91:1192–1212. doi: 10.1529/biophysj.106.083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 19.Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, et al. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;34:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 20.Herrmann R, Lobanova ES, Hammond T, Kessler C, et al. Phosducin regulates transmission at the photoreceptor-to-ON-bipolar cell synapse. J Neurosci. 2010;30:3239–3253. doi: 10.1523/JNEUROSCI.4775-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fain GL. Why photoreceptors die (and why they don’t) BioEssays. 2006;28:344–354. doi: 10.1002/bies.20382. [DOI] [PubMed] [Google Scholar]
- 22.Majumder A, Pahlberg J, Boyd KK, Kerov V, et al. Transducin translocation contributes to rod survival and enhances synaptic transmission from rods to rod bipolar cells. Proc Natl Acad Sci USA. 2013;110:12468–12473. doi: 10.1073/pnas.1222666110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majumder A, Pahlberg J, Muradov H, Boyd KK, et al. Exchange of cone for rod phosphodiesterase 6 catalytic subunits in rod photoreceptors mimics in part features of light adaptation. J Neurosci. 2015;35:9225–9235. doi: 10.1523/JNEUROSCI.3563-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leskov IB, Klenchin VA, Handy JW, Whitlock GG, et al. The gain of rod phototransduction: reconciliation of biochemical and electrophysiological measurements. Neuron. 2000;27:525–537. doi: 10.1016/s0896-6273(00)00063-5. [DOI] [PubMed] [Google Scholar]
- 25.Burns ME, Pugh EN., Jr Lessons from photoreceptors: turning off g-protein signaling in living cells. Physiology. 2011;25:72–84. doi: 10.1152/physiol.00001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reingruber J, Holcman D. Diffusion in narrow domains and application to phototransduction. Phys Rev E, Stat Nonlinear Soft Mat Phys. 2009;79:030904. doi: 10.1103/PhysRevE.79.030904. [DOI] [PubMed] [Google Scholar]
- 27.Gross OP, Pugh EN, Jr, Burns ME. Spatiotemporal cGMP dynamics in living mouse rods. Biophys J. 2012;102:1775–1784. doi: 10.1016/j.bpj.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koutalos Y, Nakatani K, Yau KW. Cyclic GMP diffusion coefficient in rod photoreceptor outer segments. Biophys J. 1995;68:373–382. doi: 10.1016/S0006-3495(95)80198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rieke F, Baylor D. Molecular origin of continuous dark noise in rod photoreceptors. Biophys J. 1996;71:2553–2572. doi: 10.1016/S0006-3495(96)79448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gross OP, Pugh EN, Jr, Burns ME. cGMP in mouse rods: the spatiotemporal dynamics underlying single photon responses. Front Mol Neurosci. 2015;8:6. doi: 10.3389/fnmol.2015.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manglik A, Kobilka B. The role of protein dynamics in GPCR function: insights from the beta2AR and rhodopsin. Curr Opin Cell Biol. 2014;27:136–143. doi: 10.1016/j.ceb.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barlow HB. Retinal noise and absolute threshold. J Opt Soc Am. 1956;46:634–639. doi: 10.1364/josa.46.000634. [DOI] [PubMed] [Google Scholar]
- 34.Holcman D, Korenbrot JI. The limit of photoreceptor sensitivity: molecular mechanisms of dark noise in retinal cones. J Gen Physiol. 2005;125:641–660. doi: 10.1085/jgp.200509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karpen JW, Zimmerman AL, Stryer L, Baylor DA. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci USA. 1988;85:1287–1291. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reingruber J, Holcman D. Estimating the rate constant of cyclic GMP hydrolysis by activated phosphodiesterase in photoreceptors. J Chem Phys. 2008;129:145102. doi: 10.1063/1.2991174. [DOI] [PubMed] [Google Scholar]
- 37.Rieke F, Baylor DA. Origin of reproducibility in the responses of retinal rods to single photons. Biophys J. 1998;75:1836–1857. doi: 10.1016/S0006-3495(98)77625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reingruber J, Holcman D. The dynamics of phosphodiesterase activation in rods and cones. Biophys J. 2008;94:1954–1970. doi: 10.1529/biophysj.107.116202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsang SH, Woodruff ML, Chen CK, Yamashita CY, et al. GAP-Independent termination of photoreceptor light response by excess gamma subunit of the c-GMP-phosphodiesterase. J Neurosci. 2006;26:4472–4480. doi: 10.1523/JNEUROSCI.4775-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nymark S, Heikkinen H, Haldin C, Donner K, et al. Light responses and light adaptation in rat retinal rods at different temperatures. J Physiol. 2005;567:923–938. doi: 10.1113/jphysiol.2005.090662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aho AC, Donner K, Helenius S, Larsen LO, et al. Visual performance of the toad (Bufo bufo) at low light levels: retinal ganglion cell responses and prey-catching accuracy. J Comp Physiol A. 1993;172:671–682. doi: 10.1007/BF00195393. [DOI] [PubMed] [Google Scholar]
- 42.Haldin C, Nymark S, Aho AC, Koskelainen A, et al. Rod phototransduction determines the trade-off of temporal integration and speed of vision in dark-adapted toads. J Neurosci. 2009;29:5716–5725. doi: 10.1523/JNEUROSCI.3888-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pugh EN, Jr, Lamb TD. Handbook of Biological Physics. Amsterdam: Elsevier; 2000. Phototransduction in vertebrate rods and cones: Molecular mechanism of amplification, recovery and light adaptation; pp. 183–255. [Google Scholar]
- 44.Finn JT, Grunwald ME, Yau K-W. Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Ann Rev Physiol. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- 45.McNaughton PA, Cervetto L, Lagnado L, Perry RJ, et al. Control of intracellular calcium in vertebrate photoreceptors. Neurosci Res Suppl. 1989;10:S23–S35. doi: 10.1016/0921-8696(89)90006-6. [DOI] [PubMed] [Google Scholar]
- 46.Schwarzer A, Schauf H, Bauer PJ. Binding of the cGMP-gated channel to the Na/Ca-K exchanger in rod photoreceptors. J Biol Chem. 2000;275:13448–13454. doi: 10.1074/jbc.275.18.13448. [DOI] [PubMed] [Google Scholar]
- 47.Bauer PJ. Binding of the retinal rod Na+/Ca2+-K+ exchanger to the cGMP-gated channel indicates local Ca(2+)-signaling in vertebrate photoreceptors. Ann NY Acad Sci. 2002;976:325–334. doi: 10.1111/j.1749-6632.2002.tb04755.x. [DOI] [PubMed] [Google Scholar]
- 48.Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, et al. Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol. 2002;542:843–854. doi: 10.1113/jphysiol.2001.013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lagnado L, Cervetto L, McNaughton PA. Calcium homeostasis in the outer segments of retinal rods from the tiger salamander. J Physiol. 1992;455:111–142. doi: 10.1113/jphysiol.1992.sp019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wen XH, Dizhoor AM, Makino CL. Membrane guanylyl cyclase complexes shape the photoresponses of retinal rods and cones. Front Mol Neurosci. 2014;7:45. doi: 10.3389/fnmol.2014.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mendez A, Burns ME, Sokal I, Dizhoor AM, et al. Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci USA. 2001;98:9948–9953. doi: 10.1073/pnas.171308998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burns ME, Mendez A, Chen J, Baylor DA. Dynamics of cyclic GMP synthesis in retinal rods. Neuron. 2002;36:81–91. doi: 10.1016/s0896-6273(02)00911-x. [DOI] [PubMed] [Google Scholar]
- 53.Chen J, Woodruff ML, Wang T, Concepcion F, et al. Channel modulation and the mechanism of light adaptation in mouse rods. J Neurosci. 2010;30:16232–16240. doi: 10.1523/JNEUROSCI.2868-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okawa H, Miyagishima KJ, Arman AC, Hurley JB, et al. Optimal processing of photoreceptor signals is required to maximize behavioural sensitivity. J Physiol. 2010;588:1947–1960. doi: 10.1113/jphysiol.2010.188573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Field GD, Rieke F. Mechanisms regulating variability of the single photon responses of mammalian rod photoreceptors. Neuron. 2002;35:733–747. doi: 10.1016/s0896-6273(02)00822-x. [DOI] [PubMed] [Google Scholar]
- 56.Sampath AP, Rieke F. Selective transmission of single photon responses by saturation at the rod-to-rod bipolar synapse. Neuron. 2004;41:431–443. doi: 10.1016/s0896-6273(04)00005-4. [DOI] [PubMed] [Google Scholar]
- 57.Baylor DA, Nunn BJ, Schnapf JL. The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol. 1984;357:575–5607. doi: 10.1113/jphysiol.1984.sp015518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitlock GG, Lamb TD. Variability in the time course of single photon responses from toad rods: termination of rhodopsin’s activity. Neuron. 1999;23:337–351. doi: 10.1016/s0896-6273(00)80784-9. [DOI] [PubMed] [Google Scholar]
- 59.Gross OP, Pugh EN, Jr, Burns ME. Calcium feedback to cGMP synthesis strongly attenuates single-photon responses driven by long rhodopsin lifetimes. Neuron. 2012;76:370–382. doi: 10.1016/j.neuron.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burns ME, Pugh EN., Jr . Visual transduction by rod and cone photoreceptors. In: Werner JS, Chalupa LM, editors. The New Visual Neurosciences. Cambridge, MA: MIT Press; 2014. pp. 7–18. [Google Scholar]
- 61.Kennedy MJ, Lee KA, Niemi GA, Craven KB, et al. Multiple phosphorylation of rhodopsin and the in vivo chemistry underlying rod photoreceptor dark adaptation. Neuron. 2001;31:87–101. doi: 10.1016/s0896-6273(01)00340-3. [DOI] [PubMed] [Google Scholar]
- 62.Ablonczy Z, Darrow RM, Knapp DR, Organisciak DT, et al. Rhodopsin phosphorylation in rats exposed to intense light. Photochem Photobiol. 2005;81:541–547. doi: 10.1562/2004-08-27-RA-294. [DOI] [PubMed] [Google Scholar]
- 63.Wilden U, Kuhn H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 1982;21:3014–3022. doi: 10.1021/bi00541a032. [DOI] [PubMed] [Google Scholar]
- 64.Mendez A, Burns ME, Roca A, Lem J, et al. Rapid and reproducible deactivation of rhodopsin requires multiple phosphorylation sites. Neuron. 2000;28:153–164. doi: 10.1016/s0896-6273(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 65.Doan T, Mendez A, Detwiler PB, Chen J, et al. Multiple phosphorylation sites confer reproducibility of the rod’s single-photon responses. Science. 2006;313:530–533. doi: 10.1126/science.1126612. [DOI] [PubMed] [Google Scholar]
- 66.Chen CK, Woodruff ML, Chen FS, Chen Y, et al. Modulation of mouse rod response decay by rhodopsin kinase and recoverin. J Neurosci. 2012;32:15998–16006. doi: 10.1523/JNEUROSCI.1639-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vishnivetskiy SA, Raman D, Wei J, Kennedy MJ, et al. Regulation of arrestin binding by rhodopsin phosphorylation level. J Biol Chem. 2007;282:32075–32083. doi: 10.1074/jbc.M706057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Azevedo A, Doan T, Moaven H, Sokal I, et al. C-terminal threonines and serines play distinct roles in the desensitization of rhodopsin, a G protein-coupled receptor. eLife. 2015;4:e05981. doi: 10.7554/eLife.05981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamer RD, Nicholas SC, Tranchina D, Liebman PA, et al. Multiple steps of phosphorylation of activated rhodopsin can account for the reproducibility of vertebrate rod single-photon responses. J Gen Physiol. 2003;122:419–444. doi: 10.1085/jgp.200308832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caruso G, Bisegna P, Andreucci D, Lenoci L, et al. Identification of key factors that reduce the variability of the single photon response. Proc Natl Acad Sci USA. 2011;108:7804–7807. doi: 10.1073/pnas.1018960108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fain GL. Molecular and Cellular Physiology of Neurons. 2nd Edn. Cambridge, MA: Harvard University Press; 2014. [Google Scholar]