Abstract
Background
Crohn’s disease (CD) is a chronic inflammatory disease with increasing incidence in children. Current medications have potentially serious side effects, hence increasing interest in alternative therapies. We previously developed an herbal formula, FAHF-2, based on a classical traditional Chinese herbal formula Wu Mei Wan that has long been used in China to treat colitis. We investigated FAHF-2’s potential anti-inflammatory effects.
Methods
FAHF-2 efficacy was tested in vivo in the CD45RbhiRAG1−/− transfer colitis model. Weight loss, colonic histology, and cytokine production from mesenteric lymph nodes were assessed. Human peripheral blood mononuclear cells (PBMCs) and colonic biopsies were obtained from children newly diagnosed with CD and controls and cultured with or without FAHF-2. Cytokine levels were measured by multiplex immunoassay. The effect of FAHF-2 on TNF-α-producing cells was determined by flow cytometry. NF-κB signaling was investigated in human lamina propria mononuclear cells upon FAHF-2 treatment by In-Cell Western.
Results
FAHF-2–treated mice had decreased weight loss, improved histology, and reduced TNF-α, IL-17, IL-6, and IFN-γ production. In vitro treated PBMCs produced less TNF-α, IFN-γ, and IL-12. FAHF-2 reduced the TNF-α–producing monocytes and T cells. Inflamed CD biopsies produced less TNF-α, IL-17, IL-6, and IL-1β. These effects are because of decreased NF-κB activation.
Conclusions
FAHF-2 inhibited both adaptive and innate immune proinflammatory cytokine responses in PBMCs and inflamed CD mucosa due in part to blockage of NF-κB activation. FAHF-2 was effective in halting progression of colitis in a murine model. This study shows that FAHF-2 has potential as a novel treatment of CD.
Keywords: Crohn’s disease, herbal therapy, pediatric, murine colitis model
Inflammatory bowel disease (IBD) is characterized by chronic and relapsingin flammation of the gastrointestinal tract. Crohn’s disease (CD) and ulcerative colitis (UC) represent the major forms of IBD.1 CD is a growing health concern, affecting 241 per 100,000 adults and 58 per 100,000 children in the United States. The incidence in both adults and children has increased in the past 60 years,2,3 especially in children younger than 10 years.4
CD is a multifactorial disease driven by disturbances in the innate and adaptive immune responses.5 CD pathophysiology is believed to be the result of aberrant immune responses to bacterial antigens in a genetically predisposed individual.6 It is associated with a Th1/Th17 signature, which accounts for the upregulation of effector T-cell responses and defective Treg cells.7 Multiple cytokines are known to play roles in the pathogenesis of CD including TNF-α, IFN-γ, IL-1β, IL-2, IL-6, IL-12, and IL-17. Of these, TNF-α has been shown to play a major role in inflammation, and multiple treatments have targeted this cytokine.
Although there are numerous IBD treatment options, many have serious side effects, especially in children whose growth and development can be permanently affected. There is an increasing interest in complementary and alternative medicine (CAM). CAM use in children with IBD from North America, Europe, and Australia ranges from 6.7% to 72%.8 A study comparing 236 children with IBD to children with constipation showed that almost half of the IBD group had used CAM.9 In another study, an estimated 40% of IBD patients used some form of megavitamin or herbal supplement.10 At Mount Sinai Children’s IBD Center, 34.8% of children with IBD had used CAM, whereas 18.8% were currently using CAM.11 In addition, more than 96% of our patients’ families reported that they were supportive of CAM use even if they were not past or current users of CAM.
Herbal medicines may have the potential to treat CD with fewer side effects than traditional medications; however, reliable mechanistic data about safety and efficacy are only beginning to be evaluated. Promising studies have examined potential therapies including resveratrol, curcumin, bromelain, green tea polyphenols, pomegranate, rutin, and Andrographis paniculata.12–19 Food Allergy Herbal Formula-2 (FAHF-2)20 is derived from Wu Mei Wan and consists of 9 Chinese herbal medicines. FAHF-2 has received Food and Drug Administration investigational new drug approval (FDA IND#77,468) under the botanical drug title for treating patients (including children) with multiple food allergies. A recently completed phase I study showed that FAHF-2 is safe and well tolerated.21 Studies of FAHF-2 for the treatment of food allergy have shown immunomodulatory effects of Th2 suppression and enhancement of IFN-γ.20 Interestingly, the herbal components of FAHF-2 have long been used in traditional Chinese medicine to treat gastrointestinal disorders, including colitis.22
The aim of this study was to investigate the immunomodulatory effect of FAHF-2 in CD, by first examining the effectiveness in a murine model, and then by examining the effect on cytokines involved in CD by human peripheral blood mononuclear cells (PBMCs) and inflamed colonic mucosa from pediatric CD patients naive to immunomodulatory medications. We determined the target cell population and mechanism by which FAHF-2 inhibits proinflammatory cytokine secretion in human PBMCs and lamina propria mononuclear cells (LPMCs), respectively.
MATERIAL AND METHODS
FAHF-2 Production and Quality Control
FAHF-2 was obtained from Xiyuan Chinese Medicine Research and Pharmaceutical Manufacturer, Chinese Academy of Chinese Medicine Sciences, Beijing, China. Herbal components of FAHF-2 were identified as Prunus mume, Zanthoxylum schinifolium, Angelica sinensis, Zingiber officinalis, Cinnamomum cassiae, Phellodendron chinense, Coptis chinensis, Panax ginseng, and Ganoderma lucidum. Extensive data on quality control and analytical chemistry and batch consistency pertaining to the formula have been published previously.21,23 We also identified chemical markers by high-pressure liquid chromatography and liquid chromatography–mass spectrometry (Fig., Supplemental Digital Content 1, http://links.lww.com/IBD/A343). Endotoxin levels in FAHF-2 were measured using the Pyrogent Plus assay kit (Lonza, MA) and found to be below 0.03 EU/mL, which is the limit of sensitivity for this kit.
Macrophage Cell Line Stimulation and Treatment with FAHF-2
The RAW264.7 macrophage cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA). To establish the optimal dose of FAHF-2, cells were cultured with various doses of FAHF-2 (20–750 μg/mL) for 1 hour, and then stimulated with 1 μg/mL of lipopolysaccharide (LPS) for 24 hours. TNF-α levels were measured in the supernatants by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (BD Bioscience, San Jose, CA).
CD45RB T Cell Transfer Model of Colitis
Animal studies were approved by the Institutional Animal Care and Use Committee at Mount Sinai. Cells were obtained from C57BL/6 WT mice and enriched using a MACS microbead system (Miltenyi Biotec, Auburn, CA) to deplete CD8+, CD11b+, CD11c+, CD19+, and B220+ cells by negative selection. The resulting CD4+ enriched population was labeled with FITC-conjugated anti-CD4 Ab and PE-conjugated anti-CD45RB Ab (eBiosciences, San Diego, CA). Subpopulations of CD4+ cells were sorted by flow cytometry. CD4+CD45RBhi T cells (3.5 × 105) were adoptively transferred by intraperitoneal injection into recipient RAG1−/− mice. Transfer was confirmed by flow cytometry of peripheral blood in all mice after 2 to 4 weeks. To prevent the progression to colitis, the recipient mice were fed FAHF-2 (50 mg/d) by gavage 24 hours after CD4+CD45RBhi T cells transfer. Control mice received water by gavage. Weights were recorded semi-weekly. All mice were sacrificed once any mouse lost 20% of their initial weight. Colonic histology was scored for inflammatory infiltrates and epithelial damage by a pathologist blinded to the treatment group.24,25 Lymphocytes from mesenteric lymph nodes were isolated and stimulated with anti-CD3/CD28 antibodies for 48 hours. Cytokine secretion was measured in the supernatants (IL-4, IL-6, IL-10, IL-12, TNF-α, IFN-γ, and IL-17) by cytometric bead array (BD Biosciences) according to the manufacturer’s instructions.
Subjects
Human studies were approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai. Blood samples (n = 26) and inflamed and/or noninflamed colonic biopsy specimens (n = 15) were collected from newly diagnosed pediatric CD patients (8–19 yr). Additionally, blood samples (n=18) and colonic biopsy specimens (n = 9) were obtained from non-IBD pediatric patients (controls) (4–18 yr).
PBMC Separation, Cell Culture, and Cytokine Measurements
PBMCs were isolated by Ficoll Hypaque (Pharmacia, Piscataway, NJ) with density gradient centrifugation. Purified PBMCs were incubated with or without FAHF-2 (125 or 250 μg/mL) for 24 hours followed by LPS (2 μg/mL) stimulation for additional 24 hours to determine the appropriate concentration to be used in this study.20 Cell viability was determined by trypan blue dye exclusion. Cytokine levels (IL-1β, IL-4, IL-5, IL-6, IL-8, IL-12, IL-13, TNF-α, IFN-γ, and IL-17) in the culture supernatants were determined by enzyme-linked immunosorbent assay (BD Biosciences) or by multiplex immunoassay. Multiplex assays were performed according to the manufacturer’s instructions (Luminex Human assay, Invitrogen, Grand Island, NY).26
Flow Cytometry
PBMCs were cultured with or without FAHF-2 (250 μg/mL) for 24 hours. Then, RPMI complete medium containing LPS (2 μg/mL) or anti-CD3/28 Dynabeads (1 μL) (Invitrogen) and protein transport inhibitor brefeldin A (10 μg/mL) (BD Pharmingen, San Jose, CA) was added to the cells and incubated for 4 hours at 378°C. Cells were then harvested, washed, and incubated with fluorochrome-labeled anti-CD14+ (monocyte marker), anti-CD3+ (T-cell marker) and anti-CD19+ (B-cell marker) along with adequate standards as previously described.27 Cells were fixed, permeabilized and stained with fluorochrome-labeled anti-TNF-α with isotype controls. Data were acquired in 4-color mode on an LSR II flow cytometer (BD Biosciences). Software analysis was performed using FlowJo (TreeStar, Ashland, OR).
Biopsy Preparation and Culture
Colonic biopsies were cultured with or without FAHF-2 (250 μg/mL) in complete RPMI for 24 hours. Supernatants were filtered and cytokines (IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, TNF-α, IFN-γ, and IL-17A) were assessed by cytometric bead array (BD Biosciences) according to the manufacturer’s instructions.
Macrophage Cell Line Stimulation and Western Blot Analysis
The RAW264.7 macrophage cells were cultured with FAHF-2 (250 μg/mL) and stimulated with LPS for various time points (5, 10, 30, and 60 min). The cells cultured in medium alone or FAHF-2 without LPS stimulation served as negative controls. Whole-cell protein lysates were prepared using the Active Motif protein isolation kit (Active Motif, Carlsbad, CA). Protein concentration was determined using a Bio-Rad DC kit (Bio-Rad, Hercules, CA). Whole-cell lysates were resolved onto a 12% sodium dodecyl sulfate polyacrylamide gel by electrophoresis. After transfer, nitrocellulose membranes were blotted against anti-IκB-α, anti-phospho-IκB-α (Cell Signaling, Danvers, MA), anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies and the appropriate horseradish peroxidase-conjugated secondary antibodies (Invitrogen). The presence of antibodies was revealed with a chemiluminescence detection system (Amer-sham Biosciences, Pittsburgh, PA). Equal protein loading was confirmed by measurement of β-actin. Signal/band intensity was quantified by densitometric analysis using the National Institutes of Health J Image program for Macintosh.
Human Lamina Propria Mononuclear Cell Culture and In-Cell Western Blot
LPMCs were isolated from inflamed surgical specimens from patients with CD undergoing bowel resection at the Mount Sinai Medical Center. LPMCs were isolated according to an established protocol using Dispase II (Roche Diagnostics, Indianapolis, IN) and collagenase (Sigma, St. Louis, MO) treatment.28,29 LPMCs were cultured overnight in serum-free medium. FAHF-2 (250 μg/mL) was then added for 1 hour, and cells were stimulated with 0.1 μg/ mL of TNF-α for various durations (0, 5 and 10 min). In-Cell Western Assay (Li-Cor, Lincoln, NE) was performed according to the manufacturer’s instructions using antibodies against phospho-IκB-α (Cell Signaling) and β-actin (Santa Cruz Biotechnology) and secondary antibodies IRDye800CW donkey anti-goat and IR-Dye680RD donkey anti-rabbit (Li-Cor). Plates were scanned, florescence detected at 700 and 800 nm, and data normalized using an Odyssey CLx Infrared Imaging System.
Statistical Analysis
SigmaStat software 3.5 was used for statistical analysis. The values from PBMC, biopsy, RAW264.7 culture, and mouse data were expressed as the mean ± SD. Statistical differences between groups from PBMC and biopsy culture were evaluated by the paired t-test for normal distribution, but Wilcoxon signed-rank test was applied for non-normally distributed data. Statistical differences between groups from RAW264.7 culture and LPMCs were evaluated by 1-way analysis of variance followed by Bonferroni test. Statistical differences between treatment groups for the colitis model were determined by Mann–Whitney t-test assuming non-normally distributed data. The results were considered statistically significant for P-values <0.05.
RESULTS
FAHF-2 Decreased TNF-α Production from a Murine Macrophage Cell Line
It is known that macrophages produce TNF-α when stimulated by LPS. We therefore tested the production of TNF-α after stimulation with LPS and treatment with varying doses of FAHF-2 (750 μg/mL, 500 μg/mL, 250 μg/mL, 100 μg/mL, and 20 μg/ mL). FAHF-2 inhibited the production of TNF-α in a noncytotoxic dose-dependent manner (P< 0.01, Fig. 1).
FIGURE 1.
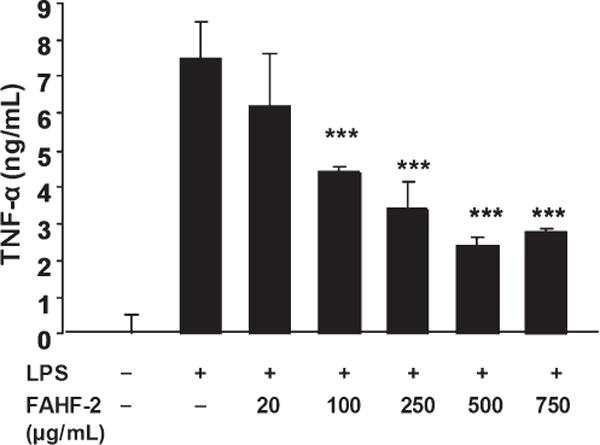
RAW264.7 cells were cultured with various doses of FAHF-2 (20–750 μg/mL) for 1 hour and then stimulated with 1 μg/mL of LPS for 24 hours. TNF-α levels were measured in the supernatants by enzyme-linked immunosorbent assay according to the manufacturer’s instructions (BD Bioscience). ***p< 0.001 vs. LPS alone.
FAHF-2 Decreased Colitis Progression in a Murine Model
Next, we assessed the effect of FAHF-2 in vivo using the CD45RBhi T cell transfer model of colitis. This model exhibits features similar to those found in CD including transmural colitis and elevated levels of TNF-α and IL-17. FAHF-2 was administered 1 day after transfer of T cells into recipient mice. FAHF-2–treated mice lost significantly less weight than untreated controls (P< 0.05, Fig. 2A), and significantly decreased histological inflammatory infiltrates and epithelial damage (P< 0.05 and P< 0.01) (Fig. 2B–D). Inflammatory cytokines in FAHF-2–treated mice were reduced in the mesenteric lymph nodes: TNF-α, IFN-γ, IL-6, and IL-17A (P< 0.01, P< 0.05, P< 0.01, P< 0.05, respectively) (Fig. 2E). Levels of IL-4, IL-10, and IL-12 were similar in both groups (data not shown).
FIGURE 2.
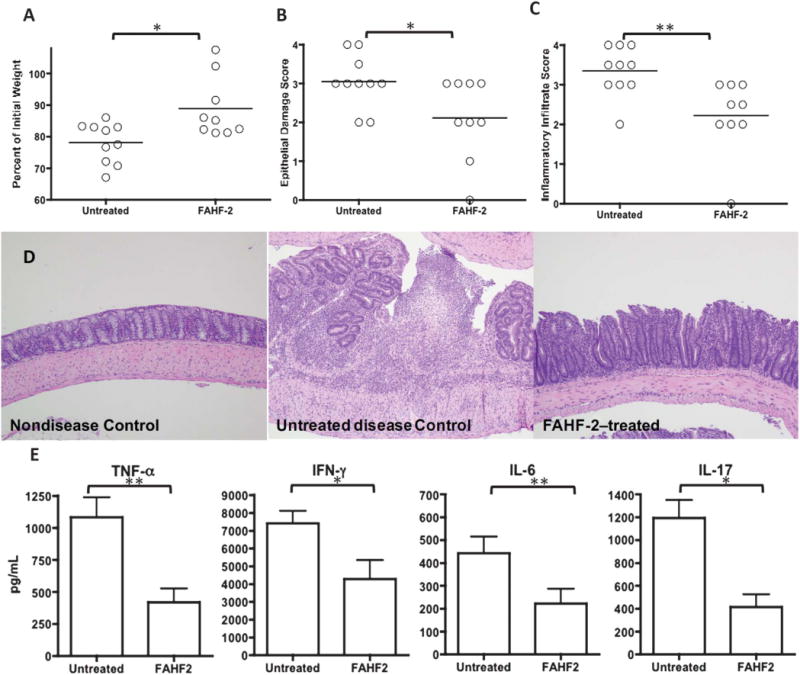
The effects of FAHF-2 on weight loss, histology, and cytokine secretion from mesenteric lymph nodes in the CD45RBhi transfer colitis model. The final weight measurement as a percentage of initial weight (A), histological score for epithelial damage (B), and inflammatory infiltrate (C). Representative histological hematoxylin–eosin slides (D) at 10× from: nondiseased control (left): normal mucosa with no inflammatory infiltrate, untreated disease control (middle): mucosal ulceration with underlying mixed inflammatory infiltrate and disordered gland architecture, FAHF-2–treated (right): no ulceration, mixed inflammatory infiltrate, and architectural distortion improved from untreated disease control. MLN cytokine secretion in untreated and FAHF-2–treated mice with colitis: TNF-α, IFN-γ, IL-17, and IL-6 (E). *P< 0.05, ** P< 0.01.
These results demonstrate that FAHF-2: decreases susceptibility to colitis progression, reduces colitis symptoms, and inhibits the inflammatory milieu associated with colitis.
FAHF-2 Suppressed Production of TNF-α from PBMCs from Children with Crohn’s Disease but Not from Control Subjects
Given the importance that TNF-α plays in the pathogenesis of CD and the results seen in our murine model, we first tested the effect of FAHF-2 on TNF-α secretion by PBMCs from CD subjects at 125 and 250 μg/mL, based on previously published data.20 FAHF-2 significantly suppressed TNF-α production at both doses (Fig. 3A) with 250 μg/mL generating greater inhibition and without cytotoxicity (Fig. 3B).
FIGURE 3.
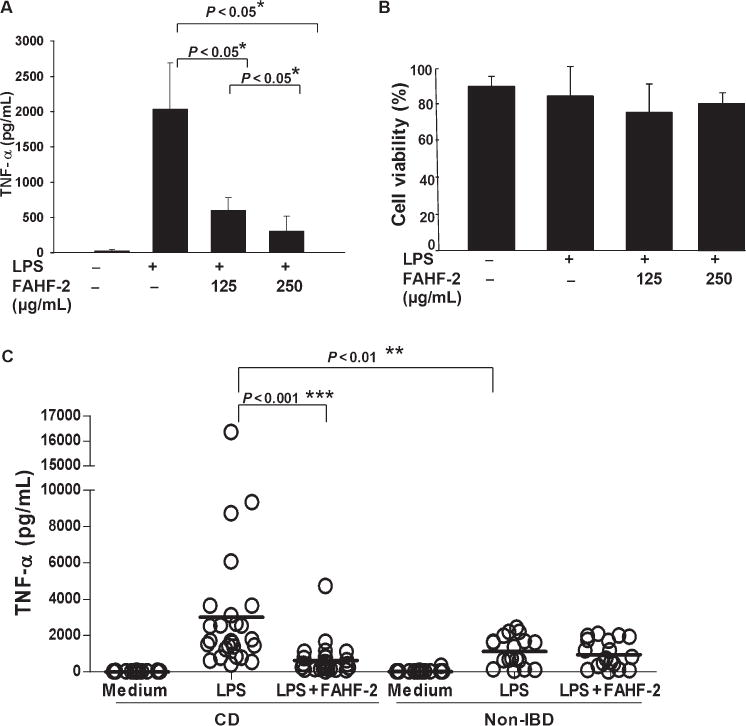
Effect of FAHF-2 on TNF-α levels from PBMCs from CD subjects and controls. A. TNF-α levels as measured by enzyme-linked immunosorbent assay from 3 individual CD subject’s PBMCs cultured in medium alone, LPS alone, or LPS with FAHF-2 treatment (125 or 250 μg/mL). B. Cell viability after the cells were cultured. C. TNF-α levels from PBMCs from 26 CD and 18 controls. Symbols indicate individual subjects. Lines indicate the mean values for each condition. *P< 0.05, ** P< 0.01, *** P< 0.001.
We then sought to determine the response to FAHF-2 at 250 μg/mL on PBMCs from 26 CD subjects and 18 controls. Subjects were naive to immunomodulating medications including steroids, thiopurines, and biologicals. CD patients were diagnosed based on standard clinical, radiographic, and endoscopic criteria. Most CD patients had mild or moderate disease based on Pediatric Crohn’s Disease Activity Index30 and Physician Global Assessment (Pediatric Crohn’s Disease Activity Index: 28.7 ± 11.1; Physician Global Assessment [mild/moderate/severe]: 12/12/2) (Table 1). All non-IBD gastrointestinal disease patients presented for evaluation of abdominal pain and were determined not to have IBD as the cause of their pain.
TABLE 1.
CD Subject and Control Subject Characteristics
| CD (n = 26) | Controls (n = 18) | |
|---|---|---|
| Gender (male/female) | 20/6 | 11/7 |
| Age, mean ± SD | 13.2 ± 2.0 | 11.4 ± 4.7 |
| PCDAI, mean ± SD | 28.7 ± 11.1 | N/A |
| PGA (mild/moderate/severe) | 12/12/2 | N/A |
PCDAI, Pediatric Crohn’s Disease Activity Index; PGA, Physician Global Assessment; N/A, not applicable.
PBMCs from CD subjects secreted significantly more TNF-α than normal PBMCs upon LPS stimulation (P< 0.01, Fig. 3C). Levels were significantly reduced when CD PBMCs were cultured with FAHF-2 (Fig. 3C) and became comparable to non-IBD PBMCs. Importantly, FAHF-2 did not affect TNF-α secretion from non-IBD PBMCs (Fig. 3C). No cytotoxicity was detected (data not shown).
FAHF-2 significantly suppressed the TNF-α production from CD subjects (P< 0.001) whereas not affecting production from non-IBD PBMCs (Fig. 3C).
FAHF-2 Reduced Production of TNF-α by Acting on Monocytes and T Cells from Children with Crohn’s Disease
To determine which cells were affected by FAHF-2, we evaluated the effect of FAHF-2 on TNF-α–producing CD14+ monocytes in response to LPS by flow cytometry. The percentage of TNF-α–producing CD14+ monocytes from PBMCs significantly increased after LPS stimulation (Fig. 4A), and the percentage of TNF-α–producing CD3+ T cells and CD19+ B cells remained unchanged (data not shown). However, FAHF-2 in vitro treatment significantly reduced the TNF-α–producing CD14+ monocytes from PBMCs stimulated by LPS (P < 0.05) (Fig. 4A, B). Although TNF-α is produced mainly by monocytes and macrophages, it is also produced by T lymphocytes.31 Using anti-CD3/28 Dynabeads, we specifically stimulated T cells and determined that FAHF-2 treatment significantly reduced TNF-α–producing CD3+ T cells (P < 0.05) (Fig. 4C, D). Thus, FAHF-2’s suppression of TNF-α is because of effects on both monocytes and T cells.
FIGURE 4.
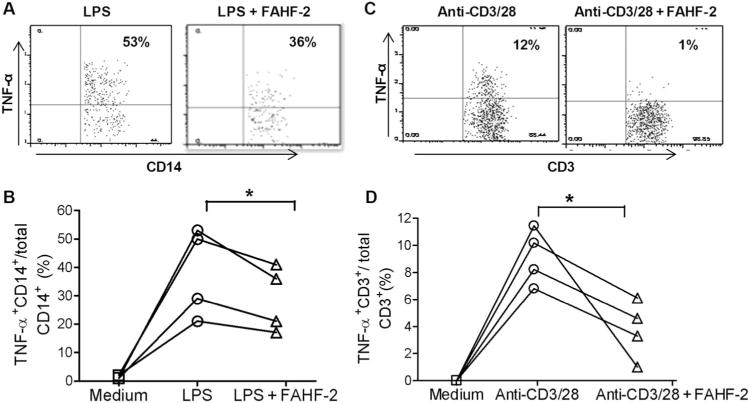
Effect of FAHF-2 on TNF-α–producing cell populations measured by flow cytometry: changes in the ratio of TNF-α+CD14+/CD14+ cells with or without FAHF-2 treatment in an individual subject (A) and 4 subjects (B). Changes in the ratio of TNF-α+CD3+/CD3+ cells with or without FAHF-2 treatment in vitro in an individual subject (C) and 4 subjects (D). * P< 0.05.
FAHF-2 Altered Cytokine Profiles of PBMCs from Children with Crohn’s Disease
Because FAHF-2 had no affect on control subjects PBMCs, we examined other proinflammatory cytokines involved in the pathogenesis of CD in CD subjects only with a focus on those that were altered in our murine model (TNF-α, IFN-γ, IL-6, and IL-17A) and in previous studies in food-allergic patients (IFN-γ and Th2 cytokines).32
The cytokine profiles of PBMCs from 14 CD subjects were determined by immunoassay with or without FAHF-2 treatment. FAHF-2–treated CD PBMCs produced significantly less IL-12 and IFN-γ than untreated CD PBMCs (P=0.004, P = 0.03, respectively) (Table 2). IL-17, IL-1RA, IL-1β, IL-4, IL-5, IL-6, and IL-13 levels were low after stimulation and/or not significantly affected by FAHF-2 treatment (Table 2).
TABLE 2.
Cytokine Levels in Culture Supernatants of PBMCs Cultured with or Without FAHF-2 Under LPS Stimulation from 14 CD Subjects Measured by Multiplex Bead-based Immunoassay
| pg/mL | Culture Conditions
|
P (LPS Versus LPS + FAHF-2) | |
|---|---|---|---|
| LPS | LPS + FAHF-2 | ||
| IL-12 | 1008.23 ± 408.39 | 224.06 ± 47.46 | 0.004 |
| IFN-γ | 32.87 ± 6.13 | 19.93 ± 2.24 | 0.03 |
| IL-17 | 28.87 ± 2.79 | 25.79 ± 2.69 | 0.34 |
| IL-1RA | 4785.33 ± 1157.34 | 5754.97 ± 1462.99 | 0.061 |
| IL-1β | 273.55 ± 130.069 | 193.62 ± 52.42 | 0.839 |
| IL-4 | 44.52 ± 5.55 | 43.24 ± 5.61 | 0.565 |
| IL-5 | 5.07 ± 0.92 | 5.39 ± 1.02 | 0.525 |
| IL-6 | 5769.01 ± 1508.93 | 4902.03 ± 1649.74 | 0.326 |
| IL-13 | 9.51 ± 2.08 | 10.51 ± 2.24 | 0.499 |
The bold text indicates significant difference between LPS and LPS+ FAHF-2.
These results demonstrate that FAHF-2 is able to suppress multiple other inflammatory cytokines (in addition to TNF-α) produced by LPS-stimulated PBMCs from CD subjects.
FAHF-2 Suppressed Inflammatory Cytokine Production from Mucosa from Children with CD
To assess the effects of FAHF-2 on colonic mucosa where any medication for the treatment of CD is likely to have the largest effect, we incubated healthy and CD biopsies with and without FAHF-2 and quantified cytokines from the supernatant. Biopsies from inflamed areas from CD subjects had significantly more production of TNF-α, IL-1β, IL-6, IL-17A, and IL-8 compared with non-IBD controls and noninflamed areas from CD subjects (Fig. 5). FAHF-2 significantly suppressed the production of TNF-α, IL-1β, IL-6, and IL-17A (P< 0.01, P< 0.05, P< 0.001, P< 0.001, respectively) (Fig. 5A–D) but not IL-8 (data not shown) from the inflamed CD biopsies. Healthy and CD biopsies showed negligible production of other tested cytokines including IFN-γ and IL-12 (data not shown).
FIGURE 5.
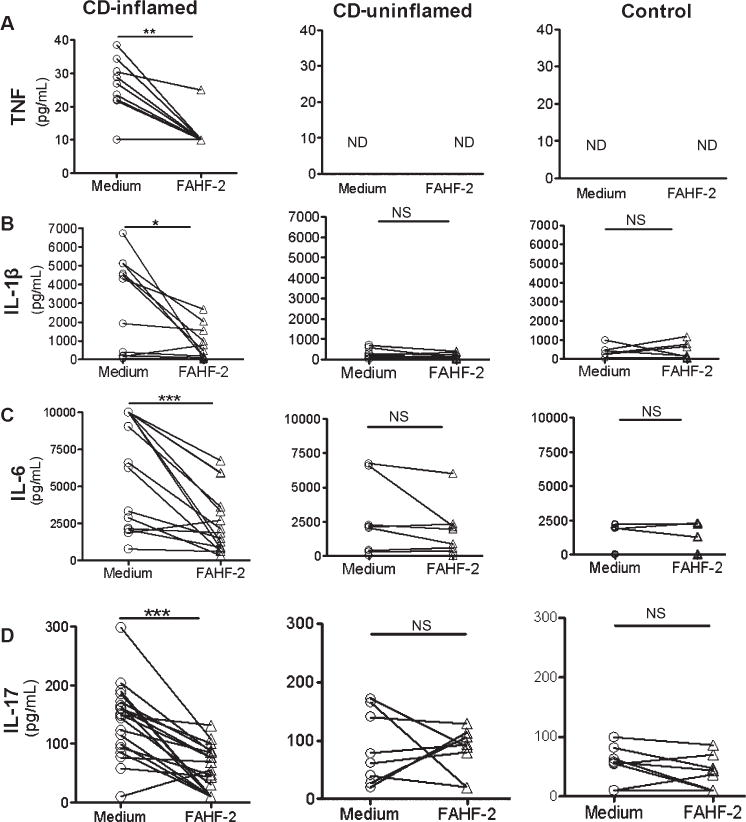
Effect of FAHF-2 on cytokine levels from colonic biopsies from CD subjects and controls. Inflamed and noninflamed biopsies from CD and control subjects were cultured with or without 250 μg/mL of FAHF-2 for 24 hours. Cytokine levels were measured from the supernatants by cytometric bead array. Cytokine levels without (medium) and with treatment with FAHF-2 are shown for each cytokine: TNF-α, IL-1β, IL-6, and IL-17A. CD inflamed: n = 15; CD uninflamed: n = 8; non-IBD control (control): n = 9. ND, not detectable; NS, not significant. *P< 0.05, **P< 0.01, ***P< 0.001.
FAHF-2 Altered the NF-κB Signaling Pathway
LPS stimulation of cells induces the secretion of proinflammatory cytokines through activation of NF-κB signaling pathway.33 FAHF-2 pretreatment of LPS-stimulated CD PBMCs and of CD biopsy specimens reduced proinflammatory cytokine secretion. Because FAHF-2 also showed consistent inhibition of TNF-α production from RAW264.7 cells in a noncytotoxic dose-dependent manner (Fig. 1A), we investigated the effects of FAHF-2 on NF-κB activation in RAW264.7 cells as an initial tool. Translocation of NF-κB requires phosphorylation of IκB-α and subsequent degradation of IκB-α by the 26S proteasome. FAHF-2 significantly inhibited both IκB-α degradation and phos-phorylation (P< 0.001 and P< 0.001) (Fig., Supplemental Digital Content 2, http://links.lww.com/IBD/A344).
Given these results and to elucidate the mechanism of FAHF-2 on the mucosa, we examined human LPMCs from CD subjects by looking at phosphorylation of IκB-α by In-Cell Western. Phosphorylation of IκB-α occurred by 10 minutes and FAHF-2 significantly inhibited this phosphorylation (P< 0.01) (Fig. 6A, B).
FIGURE 6.
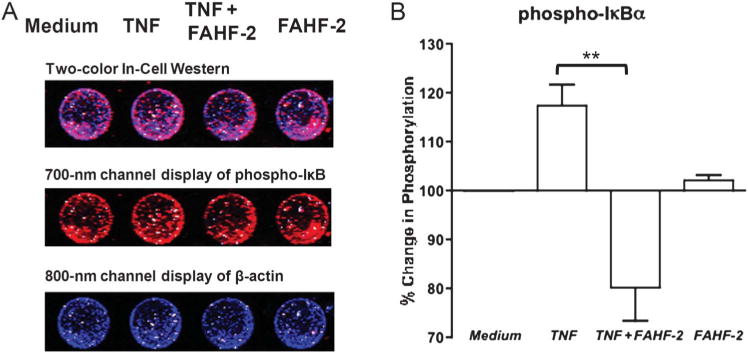
A. Representative In-Cell Western with 700 and 800 nm channels detecting phospho-IκB and β-actin, respectively, in LPMCs cultured in medium, stimulated with TNF, stimulated and treated with FAHF-2, or with FAHF-2 alone. B. Percentage of phosphorylated IκB measured by In-Cell Western after TNF stimulation and with FAHF-2 treatment as compared with medium alone (n = 3). All values normalized to β-actin. **P< 0.01.
These results demonstrate that FAHF-2 disrupts the NF-κB pathway in the mucosa leading to decreased production of inflammatory cytokines.
DISCUSSION
In this study, we demonstrate that FAHF-2 has immunoregulatory effects on human PBMCs and mucosal tissue from CD subjects and in a murine model of colitis by affecting both the adaptive and innate immune systems. FAHF-2 inhibited TNF-α production by PBMCs and mucosa of CD subjects by affecting both monocytes and T cells. These results suggest that FAHF-2 may specifically aim at pathogenic cells secreting inflammatory cytokines. Besides targeting specific pathogenic cells producing TNF-α, FAHF-2 has immunomodulatory effects on many of the inflammatory cytokines shown to be elevated in CD. Innate and adaptive responses occurring in IBD involve many cytokines including: IFN-γ, IL-1β, IL-2, IL-6, IL-12, and IL-17. FAHF-2 in vitro treatment suppressed IFN-γ and IL-12 production from PBMCs; and TNF-α, IL-1β, IL-17, and IL-6 production from biopsies from CD subjects without suppression of Th2 cytokines. This was in contrast to the effects seen in food-allergic patients where FAHF-2 suppressed Th2 cytokines and increased IFN-γ.21 Thus, this indicates that FAHF-2 has an immunomodulatory effect that varies depending on the initial pathologic alteration in cytokine profile. Given the multiple effects seen here, FAHF-2 may generate superior therapeutic effects when compared with single-target medications like infliximab without the side effect profile seen with corticosteroids and other broad-reaching immunosuppressants.
The anti-inflammatory properties of FAHF-2 may be at least partially attributed to its blocking of the nuclear factor (NF-κB) pathway which plays a crucial role in proinflammatory cytokine production. The inhibition of NF-κB is considered a putative target for intervention in IBD.34 FAHF-2 led to a decrease in IκB-α phosphorylation and IκB-α degradation concomitantly in a murine macrophage cell line and inhibition of IκB-α phosphorylation in inflamed LPMCs. IκB-α phosphorylation and IκB-α degradation might be directly inhibited by FAHF-2 or the consequence of interference upstream by FAHF-2. However, our study also suggests that FAHF-2 may be operating through additional mechanisms given the lack of broad-reaching immuno-suppression. Further investigation can better hone in on the exact mechanisms of FAHF-2 by using individual active compounds once they are isolated.
To determine whether FAHF-2 would work in vivo, we used the CD45Rbhi transfer model, which has been shown to be a reliable model of colitis.25,35,36 This model has been found to have multiple similarities to human IBD: chronic, progressive disease with diarrhea and weight loss, heavily inflamed colon, and a Th1/Th17-dominated cytokine profile. It also lends itself well to testing of medications that are commonly used in the treatment of IBD.36 Our findings in this model demonstrate FAHF-2 in vivo effectiveness that correlates with our findings in PBMCs and colonic mucosa. We tested FAHF-2’s ability to prevent progression of colitis given that we envision use of this medication in mild disease or for maintenance of remission.
Complementary and alternative medicine treatments are increasingly used in Western countries because of their reputed effectiveness, low cost, and favorable safety profiles. In addition, conventional IBD therapy has potentially serious side effects, especially in children whose growth and development can be permanently affected.37,38 Studies have suggested the utility of Chinese herbal medicine for the treatment of a variety of inflammatory and infectious diseases,8,9 including CD and ulcerative colitis. Promising studies have examined potential therapies including resveratrol, curcumin, bromelain, tea polyphenols, pomegranate, and rutin.12–18 Among these, one recently studied herbal therapy, HMPL-004, an aqueous ethanol herbal extract of Andrographis paniculata, has been shown to prevent colitis in animal models.19,39 A pilot human clinical trial further confirmed the efficacy and safety of HMPL-004 in patients with ulcerative colitis.40 Similar to HMPL-004, our work on FAHF-2 uses a traditional Chinese medicine, Wu Mei Wan that has been used safely for thousands of years to treat gastrointestinal disorders, including colitis.22 Unlike HMPL-004, our extensive preclinical FAHF-2 studies focus on CD therapy as opposed to ulcerative colitis. Additionally, FAHF-2 does not contain the same herbs or active compounds as HMPL-004 and FAHF-2 contains multiple active compounds, which may function synergistically. Our preliminary studies suggest that compounds in FAHF-2 are likely from various phytochemical classes and target different components in the immune system (unpublished data), which may explain FAHF-2’s immunomodulatory effect and the fact that it does not cause global immunosuppression. Some of the known compounds from the 9 herbs likely contribute to the anti-inflammatory effects we observed. One of the herbs in FAHF-2 is Panax ginseng, which contains the major compound ginsenoside Rg1. This compound possesses glucocorticoid activities, acts through NF-κB, and can effectively inhibit TNF-α and IL-6 production but does not cause hyperglycemia or osteoporosis as seen with glucocorticoids.41 One individual compound isolated from FAHF-2, ganoderic acid C1, can also suppress TNF-α production by inhibiting NF-κB, AP-1, and MAPK (unpublished data). Thus, multiple compounds in FAHF-2 are likely to have some role in modulating the immune response: their combined effects may be greater than their individual effects. These compounds are in the process of being isolated and characterized.
In summary, FAHF-2 had an immunomodulatory effect on PBMCs and inflamed colonic mucosa from pediatric CD patients. It suppressed proinflammatory cytokines by blocking signal transduction through the NF-κB pathway. These results suggest that FAHF-2 may have a beneficial effect on the immune profile associated with CD. This study provides preliminary evidence of beneficial immunomodulatory effects of FAHF-2 on PBMCs and mucosa from CD subjects and in a murine colitis model, thus warranting future clinical studies. FAHF-2 may be a safe and effective herbal therapy for CD to induce and maintain remission and avoid the need for pharmacologic escalation in therapy with medications that have potentially severe side effects.
Supplementary Material
Acknowledgments
The authors thank Dr. Nanci Pittman for collecting specimens, Dr. M. Cecilia Berin and Dr. J.F. Colombel for scientific advice.
Supported by Crohn’s and Colitis Foundation of America Career Development Award and Molly’s Research Fund (D.D) and the National Institutes of Health/National Center for Complementary and Alternative Medicine grants 1R01AT001495-01A1 and 2R01AT001495-05A1 (X.-M.L.).
Footnotes
X.-M. Li is a consultant for the FAI and has shares of US Patent PCT/US 05/08600 on FAHF-2 and Herbal Springs, LLC. The other authors have no conflicts of interest to disclose.
References
- 1.Kappelman MD, Moore KR, Allen JK, et al. Recent trends in the prevalence of Crohn’s disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58:519–525. doi: 10.1007/s10620-012-2371-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Zaag-Loonen HJ, Casparie M, Taminiau JA, et al. The incidence of pediatric inflammatory bowel disease in the Netherlands: 1999–2001. J Pediatr Gastroenterol Nutr. 2004;38:302–307. doi: 10.1097/00005176-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Benchimol EI, Fortinsky KJ, Gozdyra P, et al. Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis. 2011;17:423–439. doi: 10.1002/ibd.21349. [DOI] [PubMed] [Google Scholar]
- 4.Benchimol EI, Guttmann A, Griffiths AM, et al. Increasing incidence of paediatric inflammatory bowel disease in Ontario, Canada: evidence from health administrative data. Gut. 2009;58:1490–1497. doi: 10.1136/gut.2009.188383. [DOI] [PubMed] [Google Scholar]
- 5.Shih DQ, Targan SR, McGovern D. Recent advances in IBD pathogenesis: genetics and immunobiology. Curr Gastroenterol Rep. 2008;10:568–575. doi: 10.1007/s11894-008-0104-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maul J, Loddenkemper C, Mundt P, et al. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868–1878. doi: 10.1053/j.gastro.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Munoz F, Dominguez-Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilsden RJ, Verhoef MJ, Rasmussen H, et al. Use of complementary and alternative medicine by patients with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:655–662. doi: 10.1002/ibd.21360. [DOI] [PubMed] [Google Scholar]
- 9.Wong AP, Clark AL, Garnett EA, et al. Use of complementary medicine in pediatric patients with inflammatory bowel disease: results from a multicenter survey. J Pediatr Gastroenterol Nutr. 2009;48:55–60. doi: 10.1097/MPG.0b013e318169330f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Head K, Jurenka JS. Inflammatory bowel disease. Part II: Crohn’s disease–pathophysiology and conventional and alternative treatment options. Altern Med Rev. 2004;9:360–401. [PubMed] [Google Scholar]
- 11.Ceballos CBK, Dunkin DS, Song Y, et al. Complementary and alternative medicine use at a single pediatric inflammatory bowel disease center. Gastroenterol Nurs. 2014 doi: 10.1097/SGA.0000000000000050. in press. [DOI] [PubMed] [Google Scholar]
- 12.Martin AR, Villegas I, La Casa C, et al. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol. 2004;67:1399–1410. doi: 10.1016/j.bcp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 13.Hale LP, Greer PK, Trinh CT, et al. Treatment with oral bromelain decreases colonic inflammation in the IL-10-deficient murine model of inflammatory bowel disease. Clin Immunol. 2005;116:135–142. doi: 10.1016/j.clim.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Holt PR, Katz S, Kirshoff R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50:2191–2193. doi: 10.1007/s10620-005-3032-8. [DOI] [PubMed] [Google Scholar]
- 15.Kwon KH, Murakami A, Tanaka T, et al. Dietary rutin, but not its aglycone quercetin, ameliorates dextran sulfate sodium-induced experimental colitis in mice: attenuation of pro-inflammatory gene expression. Biochem Pharmacol. 2005;69:395–406. doi: 10.1016/j.bcp.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 16.Mazzon E, Muia C, Paola RD, et al. Green tea polyphenol extract attenuates colon injury induced by experimental colitis. Free Radic Res. 2005;39:1017–1025. doi: 10.1080/10715760500197177. [DOI] [PubMed] [Google Scholar]
- 17.Hale LP, Chichlowski M, Trinh CT, et al. Dietary supplementation with fresh pineapple juice decreases inflammation and colonic neoplasia in IL-10-deficient mice with colitis. Inflamm Bowel Dis. 2010;16:2012–2021. doi: 10.1002/ibd.21320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larrosa M, Gonzalez-Sarrias A, Yanez-Gascon MJ, et al. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J Nutr Biochem. 2010;21:717–725. doi: 10.1016/j.jnutbio.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Sandborn WJ, Targan SR, Byers VS, et al. Andrographis paniculata extract (HMPL-004) for active ulcerative colitis. Am J Gastroenterol. 2013;108:90–98. doi: 10.1038/ajg.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava KD, Kattan JD, Zou ZM, et al. The Chinese herbal medicine formula FAHF-2 completely blocks anaphylactic reactions in a murine model of peanut allergy. J Allergy Clin Immunol. 2005;115:171–178. doi: 10.1016/j.jaci.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Patil SP, Yang N, et al. Safety, tolerability, and immunologic effects of a food allergy herbal formula in food allergic individuals: a ran-domized, double-blinded, placebo-controlled, dose escalation, phase 1 study. Ann Allergy Asthma Immunol. 2010;105:75–84. doi: 10.1016/j.anai.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bensky D, Clavey S, Stöger E. Chinese Herbal Medicine: Materia Medica. 3rd. Seattle, WA: Eastland Press; 2004. [Google Scholar]
- 23.Patil SP, Wang J, Song Y, et al. Clinical safety of Food Allergy Herbal Formula-2 (FAHF-2) and inhibitory effect on basophils from patients with food allergy: extended phase I study. J Allergy Clin Immunol. 2011;128:1259–1265. doi: 10.1016/j.jaci.2011.06.015. e1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 25.Ostanin DV, Bao J, Koboziev I, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patil SP, Wisnivesky JP, Busse PJ, et al. Detection of immunological biomarkers correlated with asthma control and quality of life measure ments in sera from chronic asthmatic patients. Ann Allergy Asthma Immunol. 2011;106:205–213. doi: 10.1016/j.anai.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster B, Prussin C, Liu F, et al. Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol. 2007;78:6.24.1–6.24.21. doi: 10.1002/0471142735.im0624s78. [DOI] [PubMed] [Google Scholar]
- 28.Hovhannisyan Z, Treatman J, Littman DR, et al. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957–965. doi: 10.1053/j.gastro.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu QT, Saruta M, Avanesyan A, et al. Expression and functional characterization of FOXP3+ CD4+ regulatory T cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:191–199. doi: 10.1002/ibd.20053. [DOI] [PubMed] [Google Scholar]
- 30.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. [PubMed] [Google Scholar]
- 31.Shetty A, Forbes A. Pharmacogenomics of response to anti-tumor necrosis factor therapy in patients with Crohn’s disease. Am J Pharmacogenomics. 2002;2:215–221. doi: 10.2165/00129785-200202040-00001. [DOI] [PubMed] [Google Scholar]
- 32.Brown SJ, Mayer L. The immune response in inflammatory bowel disease. Am J Gastroenterol. 2007;102:2058–2069. doi: 10.1111/j.1572-0241.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 33.Kawai T, Adachi O, Ogawa T, et al. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/s1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 34.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 35.Kim N, Kunisawa J, Kweon MN, et al. Oral feeding of Bifidobacterium bifidum (BGN4) prevents CD4(+) CD45RB(high) T cell-mediated inflammatory bowel disease by inhibition of disordered T cell activation. Clin Immunol. 2007;123:30–39. doi: 10.1016/j.clim.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 36.Lindebo Holm T, Poulsen SS, Markholst H, et al. Pharmacological Evaluation of the SCID T Cell Transfer Model of Colitis: as a Model of Crohn’s Disease. Int J Inflam. 2012;2012:412178. doi: 10.1155/2012/412178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Booth IW, Harries JT. Inflammatory bowel disease in childhood. Gut. 1984;25:188–202. doi: 10.1136/gut.25.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmerman MJ, Jewell DP. Cytokines and mechanisms of action of glucocorticoids and aminosalicylates in the treatment of ulcerative colitis and Crohn’s disease. Aliment Pharmacol Ther. 1996;10(suppl 2):93–98. doi: 10.1046/j.1365-2036.1996.22164026.x. discussion 99. [DOI] [PubMed] [Google Scholar]
- 39.Michelsen KS, Wong MH, Ko B, et al. HMPL-004 (Andrographis paniculata extract) prevents development of murine colitis by inhibiting T-cell proliferation and TH1/TH17 responses. Inflamm Bowel Dis. 2013;19:151–164. doi: 10.1002/ibd.22983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang T, Targan SR, Li ZS, et al. Randomised clinical trial: herbal extract HMPL-004 in active ulcerative colitis—a double-blind comparison with sustained release mesalazine. Aliment Pharmacol Ther. 2011;33:194–202. doi: 10.1111/j.1365-2036.2010.04515.x. [DOI] [PubMed] [Google Scholar]
- 41.Du J, Cheng B, Zhu X, et al. Ginsenoside Rg1, a novel glucocorticoid receptor agonist of plant origin, maintains glucocorticoid efficacy with reduced side effects. J Immunol. 2011;187:942–950. doi: 10.4049/jimmunol.1002579. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


