Abstract
Dust particles lifting and discharge from Africa to Europe is a recurring phenomenon linked to air circulation conditions. The possibility that microorganisms are conveyed across distances entails important consequences in terms of biosafety and pathogens spread. Using culture independent DNA-based analyses via next generation sequencing of the 16 S genes from the airborne metagenome, the atmospheric microbial community was characterized and the hypothesis was tested that shifts in species diversity could be recorded in relation to dust discharge. As sampling ground the island of Sardinia was chosen, being an ideal cornerstone within the Mediterranean and a crossroad of wind circulation amidst Europe and Africa. Samples were collected in two opposite coastal sites and in two different weather conditions comparing dust-conveying winds from Africa with a control situation with winds from Europe. A major conserved core microbiome was evidenced but increases in species richness and presence of specific taxa were nevertheless observed in relation to each wind regime. Taxa which can feature strains with clinical implications were also detected. The approach is reported as a recommended model monitoring procedure for early warning alerts in frameworks of biosafety against natural spread of clinical microbiota across countries as well as to prevent bacteriological warfare.
Global transport of desert dust is believed to play an important role in many geochemical, climatological, and environmental processes1,2,3,4. Dust can convey minerals and nutrients5, but also pollutants6 and viable microorganisms of various species7,8, some of which are capable of harming human, animal, plant, and ecosystem health9,10,11. Given the complex and extended routes of meteorological circulation, airborne dust can transport microorganisms for long distance also across sea and mountain range barriers. Benefits of a deeper awareness of potentially inflowing bacteria, fungi and viruses are envisaged in the fields of human healthcare, veterinary prophylaxis, agricultural crop protection and warfare monitoring. Ground-level technologies have strived to guarantee efficient protection against airborne infections arising in fully air-conditioned buildings, in particular preventive measures for hospitals, and their effectiveness have been long since primary concerns12. Airborne dust contains bacteria, viruses, fungi and their spores but the number of culturable microorganisms in this life-limiting habitat is rather low, scoring values well below 1% of the live cells13. Culture-based studies suggest that the microbial densities can vary across seasons and environmental conditions14. To complement the inefficiency of the culture-dependent census15, direct molecular approaches within the metagenetics framework have been undertaken to unravel the community composition of prokaryotes and eukaryotes.
The emergence of new infectious diseases in many parts of the world16 and the resurgence of old ones like tuberculosis and meningitis17,18, evidenced the global-threat aspect of the consequences related to airborne transportation of microbes across nations and continents.
The atmosphere-dispersed particulate matter, constituted by a mixture of particles, containing variable fractions of natural solids and pollutants19,20,21, represents a carrier able to passively convey an array of different microorganisms. As a consequence, emission of particle-associated bacteria, fungi and viruses determined by the transcontinental displacement via meteoric circulation (water particles and ensuing rainfall), constitutes i) an effective means of spreading for pathogens and ii) a continuous process of expansion of their biogeographical ranges.
After 2000, the U.S. Geological Survey in St. Petersburg, FL, began the USGS Global Dust Program (initially funded by NASA) to investigate whether live microorganisms would be consistently transported in dust masses (initially in Saharan dust22). Authors at USGS using DNA sequencing of the ribosomal gene, were able to isolate and identify over 200 viable bacteria and fungi in samples from St. John in the U.S. Virgin Islands in 2000 during dust air-driven particle transport events23.
Many of the viable microorganisms identified in Saharan dust are known from clinical records to cause respiratory diseases (allergic reactions, asthma, and pulmonary infections), cardiovascular diseases, or skin infections24. Other microbes in airborne dust are known to be pathogenic to humans, including those causing plague, anthrax, tuberculosis23,26, or towards livestock, relatively to foot and mouth disease10 or to plants, causing sugarcane rust, and other pathologies of cotton, peach, rice and beans27,28,29.
Moreover, the association between microorganisms and air pollutants like PM2.5 and PM10 (particulate matter with diameters less than 2.5 and 10 μm, respectively)30 could represent a recently evolved transfer-mechanism supporting and increasing their natural dust-mediated dispersion and opening new ways for their epidemic diffusion.
Facing the African Continent, Europe is subjected to a large-scale dust-transportation. It has been estimated that 80–120 Tg of dust per year are carried across the Mediterranean towards Europe31,32. In particular, dust transported by winds can reach an elevation up to 8 km in the atmosphere over the Mediterranean basin33.
Every dust-carrying event can be very different from others of the same kind; in fact dust transport and concentration in the air can vary remarkably depending on the synoptic situation34. In general the main meteorological scenarios that originate the transport of dust towards the central and western Mediterranean Basin are characterized by the presence of four different synoptic conditions: i) a strong North African thermal low pumps dust till the mid-troposphere, where the western side of a high pressure centered slightly westward transports the plume; ii) the eastern side of an Atlantic trough with the western side of the associated ridge, located between Tunisia and Libya is able to originate an all-level transport; iii) the western side of a well-structured high on the central Mediterranean Basin can supply the flow for low level transport; iv) the north-eastern side of a sea level low centred in south Libya can create the condition for a low level transport35,36.
Its geographic position and seasonal weather conditions make Italy one of the first frontiers subjected to a natural dust-transportation. Satellite data and forecasting models foretell geographical dispersion of African dust, revealing that the main sources are the Sahara and Sahel regions, located in the north-western part of Africa. Dust events that reach Italy are more frequent in the May-November period, but can also take place in the December-April period37.
In the western side of insular Italy, surrounded by the Mediterranean sea, the Sardinia island is located at 130 Nm (240 km) from the North-African coastline and represents a cornerstone for the above phenomena as well as an ideal observation point for monitoring of dust transportation. Dust outbreaks in Sardinia were already described38,39,40 but there is a lack of studies on the microbiological diversity of dust-loaded winds discharging particles from Africa.
The present study addressed the bacterial community of airborne particles in a culture-independent analysis of the 16 S-rRNA genes by high-throughput sequencing. In parallel culturable biota was also analyzed in order to characterize which species remain viable after long-distance atmospheric transport. Two sampling sites were considered, Cagliari and Sassari, covering the whole south-to-north axis aligned with the African winds direction. Two distinct meteorological conditions were chosen; i) a dust outbreak conveying dispersed particles of Saharan origin ii) a baseline situation (negative control) represented by air masses of European origin incoming from the north-western quadrant.
Results
Choice of dust-carrying and control conditions
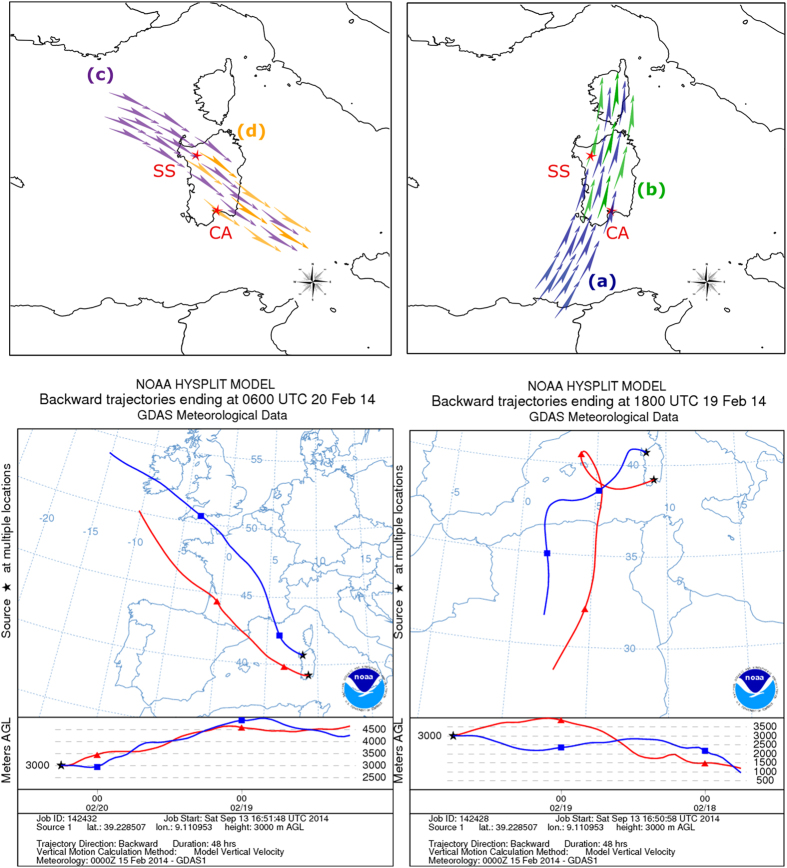
Scheme and conditions are shown in Fig. 1. The sampling was done by considering four-days time laps. Back trajectories plots confirmed the intrusion of African air masses occurring on February 15th through 19th 2014. On the subsequent period from February 20th, the above circulation regime halted, high pressure cells over Europe caused opposite winds blowing from northeastern origin, which passed over Italy and Sardinia and allowed to collect samples representing the dust-free control conditions.
Figure 1. Reconstruction of the wind flows crossing the two sites during the sampling dates.
Top left panel: control conditions event with winds blowing from northwest to south-east, Top right panel: African dust-carrying event with northbound winds. Bottom panels: corresponding particle back-trajectories simulations evaluated by the HYSPLIT model for the two sampling days. SS: Sassari sampling point; CA: Cagliari sampling point. Letters and colored arrows in the two top panels indicate four theoretically distinct pools of putatively airborne bacteria: (a) from Africa and Mediterranean sea (blue arrows); (b) the same plus those raised from inland Sardinia in northbound wind motion from Cagliari to Sassari (green arrows); (c) from continental Europe and Tyrrhenian sea (violet arrows); (d) the same plus those raised from inland Sardinia in southbound wind motion from Sassari to Cagliari (orange arrows). Source of images: top panels: this work; bottom panels: output of the public website service software HYSPLIT (http://ready.arl.noaa.gov/HYSPLIT.php).
The first intrusion of African air masses occurred on the 15th-17th, mainly affecting the PM10 levels in the north of Sardinia. On the 18th and 19th February 2014 air masses loaded with dust crossed the Mediterranean northward and eastward towards Italy and Sardinia, affecting the whole island. The analysis of the PM10 daily pattern registered in February at the sampling sites showed increasing values during the dust event at both sites with a marked decrease on the 20th (Supplementary Figure S1). The arrival of air masses loaded with dust from North Africa was confirmed by satellite imagery (Supplementary Figure S2).
Plate-culturable biota
As regards the results of the culture-dependent approach, Fungi were more abundantly isolated and mostly represented by Aspergillus spp., while among bacteria Bacillus spp. was the leading culturable taxon. Species identified included Bacillus simplex, Paenibacillus amylolyticus, Brevibacillus formosus, Bacillus megaterium, Bacillus cereus, Kocuria rosea.
Culture-independent 16S sequencing analysis
The mean quantitative results of the NGS sequencing in the different samples are shown in Table 1.
Table 1. Total number of sequences and uniquely aligned reads obtained.
| Sample | CA-Dust | SS-Dust | CA-Ctrl | SS-Ctrl |
|---|---|---|---|---|
| n. of paired reads | 1’331’182 | 1’664’672 | 1’270’770 | 1’125’836 |
| n. of uniquely aligned reads | 756’655 | 1’051’021 | 780’713 | 644’623 |
| PRJNA274749 (Bioproject) | SAMN03332112 | SAMN03332148 | SAMN03332147 | SAMN03332149 |
The qualitative results of the sequencing annotation allowed the identification of several hundreds of operational taxonomical units per sample. The data at genus rank level are shown in Table 2 where the number of genera and the corresponding ecological indexes calculated from the community data are reported. The full array of taxa can be browsed as spreadsheet in the supplementary material (Supplementary Table S1).
Table 2. Number of genera detected in the four cases and species diversity indexes.
| Samples | CA-Dust | CA-Ctrl | SS-Dust | SS-Ctrl |
|---|---|---|---|---|
| N. of Genera | 345 | 232 | 293 | 259 |
| Simpson Index | 0.891 | 0.910 | 0.931 | 0.818 |
| Shannon Index | 3.204 | 3.086 | 3.487 | 2.717 |
The most abundant phyla were in all samples Proteobacteria, Firmicutes, Bacteroidetes and Actinobacteria as shown in Fig. 2. A higher amount of orders belonging to Proteobacteria can be observed in the SS-Dust community. Higher percentages of Firmicutes (the Bacillales order in particular), characterize the two Cagliari samples (CA-Dust and CA-Ctrl).
Figure 2. Percentages of sequences assignable to database-identifiable bacterial in each of the two sampling sites at each event (Control or Dust).
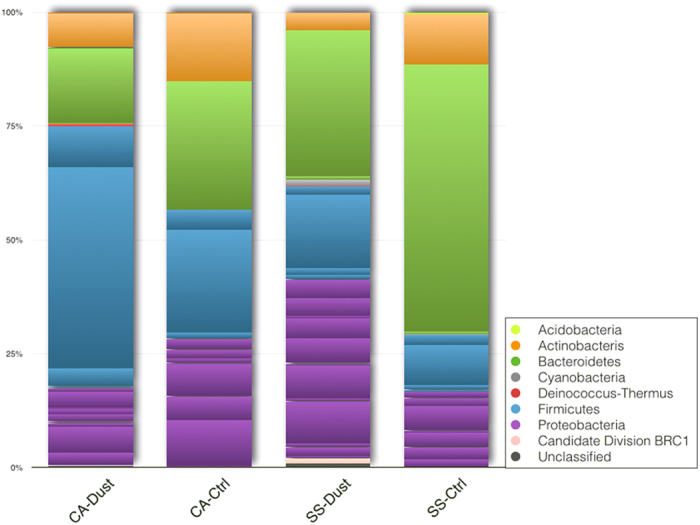
CA: Cagliari sampling site, SS: Sassari sampling site. The slices within each phylum block of the same color indicate the number of orders found within that phylum.
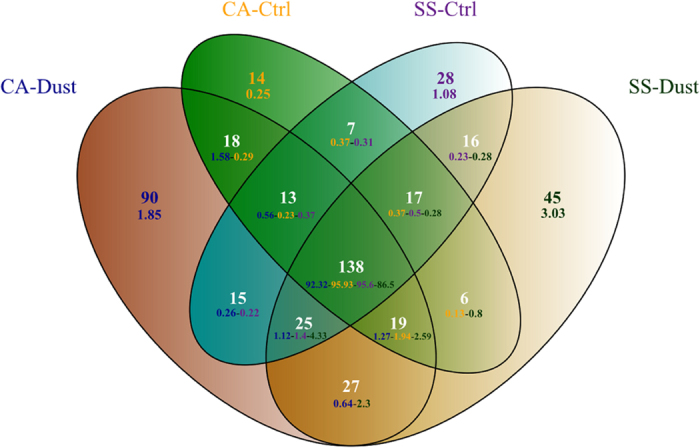
The prevailing occurrence of a common core of shared taxa is shown in detail by the overlapping Venn diagrams representation in Fig. 3. The highest proportions of taxa were those shared by all four, 92.3% and 95.9%, 85.6% and 95.6% for Cagliari-Dust, Cagliari-Control, Sassari-Dust and Sassari-Control, respectively, consisting in 138 genera. Conversely, the non-overlapping occurrences were all within frequencies ranging from 0.25% (Cagliari Control) and 3.03% (Sassari Dust). Although limited in comparison to the constant core higher numbers of event-specific genera occurred for the two dust-exposed samplings, with 90 and 45 for Cagliari and Sassari, respectively.
Figure 3. Venn diagram showing the number of specific or shared genera identified in each of the four locations/conditions and the relative percentages (smaller font digits).
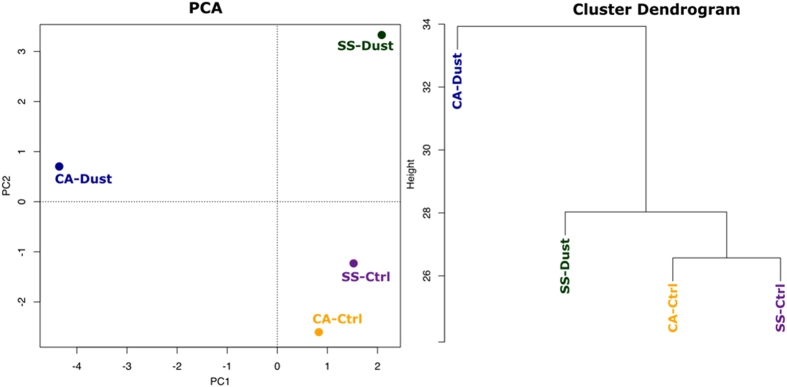
Principal Component Analysis (PCA) and cluster analysis (Fig. 4) define groups where the dust-related samplings occupy distinct positions while the two controls occur in a closer range of the ordination plot. The cluster analysis also shows shorter distances between the two controls and longer branches separating the dust samples, in particular for the one collected in the most southern location (Cagliari).
Figure 4. Principal Component Analysis (left) and Cluster Analysis based on euclidean distance, with average linkage method (right) based on the identified genera.
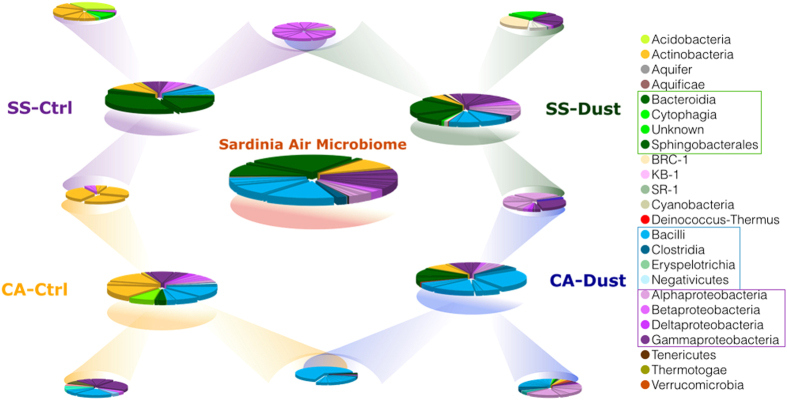
The taxonomical composition of the core bacterial microbiome in comparison with the locality-shared, whether event-shared, or uniquely occurring groups at phylum and class level is shown in Fig. 5.
Figure 5. Proportions of identified phyla and classes in the four sampling cases.
The legend on the right shows the color codes of the phyla, or, for three of them, the occurring classes listed within boxes in the legend as follows: Bacteroidetes (green tints); Firmicutes (blue tints); Proteobacteria (purple tints). The larger central pie chart shows the situation of the constant core of taxa shared by all four cases, constituting the putative Sardinia air microbiome, supposedly independent from place and weather conditions. The four inner-diamond small pies show the taxa shared pairwise by the ‘locality pairs’ (Cagliari or Sassari) or by the ‘whether condition pairs’ (dust or control). The four outer-corners small pies show the unshared (specific) fraction of each case. Pies diameters not drawn in scale with sequences frequency number.
Concerning the identities of specific genera within the relevant phyla, as shown Actinobacteria were identified in all the conditions with lower percentages in the two dust-related samples, 7.5% and 3.8%, for Cagliari and Sassari respectively, and higher for the two controls, 14.8% and 11.0%. Within this major phylum the genus Propionibacterium represented the most abundant. This taxon was previously reported as desert dust particles41 and particles-associated pollutants but our data indicate higher percentages in the two controls. These two samples shared moreover an amount of other exclusive Actinobacteria genera.
On the other hand, interestingly, higher percentages of Corynebacterium, already detected in the Saharan air dispersed-particles10,29 characterized the two dust-related samples.
Members of the Bacteroidetes phylum were identified in all the datasets with percentages varying from 16% (dust) to 28% (control) in the two Cagliari samples while in the Sassari samples, these values were about double as Bacteroidetes-related reads represented from 32% to 59% of the total uniquely-aligned reads in the dust-related and in the control-sample respectively. Several authors observed their environmental abundance42. In terms of taxa variability it is worth noticing in the analyzed datasets, that although different at genus level within each sample the relative percentages of the Bacteroidetes in control vs. dust samplings, remain surprisingly unaltered.
About Firmicutes, the two Cagliari samples showed higher percentages, with a maximum that reaching the 57% for the dust-related samples and 30% for the negative control. Lower values were shown in Sassari, with percentages between 20% and 12% respectively. This phylum was often reported as one of the most abundant in the air-collected samples7,41,43. The Bacillus genus was detected but it does not represent the most abundant. High percentages of Streptococcus and Lactococcus characterized both Cagliari samples and were always detected in lower percentages; these genera had rarely been reported as particle associated in literature. Moreover, several Firmicutes like Anoxybacillus were identified as specific taxa characterizing the Cagliari samples.
A different situation was observed for the Proteobacteria phylum: an opposite trend was evident analyzing the four samples: percentages, from 16% to 28%, characterized the two Cagliari samples, dust and control, respectively, while opposite proportions, from 39% to 16% occurred the two dust-related and control from Sassari. Notwithstanding the phyla disproportions, genera distributions within them did not show evidence of consistent trends or correlations.
Interestingly, several groups were shared between the two dust-related samples. An amount of Alphaproteobacteria and Gammaproteobacteria characterized all the analyzed conditions but within this phylum several differences between dust-events and controls were detected. In particular, Alphaproteobacteria were found as dust-related samples. Taxa previously reported as desert-dust associated as Paracoccus23,29,30,41, Sphingomonas10,23,41,44, Methylobacterium30 or never detected before in these environments, as Caulobacter and Brevundimonas appeared to characterize the samples.
Some exceptions were also encountered: some Gammaproteobacteria like Citrobacter, Cronobacter, Klebsiella, Pantoea and Stenotrophomonas were shared between the Cagliari-control and the Sassari dust-related samples.
These shared genera flanked an amount of other Alpha and Gammaproteobacteria that chaacterize all the analyzed conditions, indicating a broad distribution and their relevance in the Sardinian air-microbiome definition.
Discussion
As a first consideration, the analysis revealed that a wide group of phyla, recurrently consistent in spite of site and meteorology conditions, configures as a global Sardinian air microbiome. Exceptions were related to the intra-phylum biodiversity and the shared taxa between groups of samples.
Notwithstanding the persisting core, it was possible to observe that biodiversity increased in relation to dust-carrying events. As Table 2 shows, Shannon and Simpson richness indexes show upshifts in both localities in coincidence with the particle-carrying winds from the African side.
An enrichment in specific bacterial lineages could be determined by both weather factors and the local geographic distributions as exemplified by the percentages of Firmicutes characterizing the two Cagliari samples. On one hand, a background of bacteria already recognized as air-particles associated (e.g. Propionibacterium) could be representative of taxa that normally use the air-dispersion as mechanism of diffusion. On the other hand, the effects of a dust-event could determine an enrichment concerning specific bacterial populations like Corynebacterium, Paracoccus or Brevundimonas.
Biodiversity indexes confirmed that higher values characterized both the dust-related samples, Sassari and Cagliari (Table 2), underlining that an increasing biodiversity can be transported during a dust event, with a possible influence in modulating the composition of the local microbiome.
PCA and cluster analysis described a correlation between the two control samples while the dust-related taxa occupy different positions (Fig. 4).
About potential clinically-relevant bacteria we detected different cases of Gammaproteobacteria as Citrobacter45,46,47, Cronobacter48,49,50, Klebsiella, Pantoea51,52,53 and Stenotrophomonas. Within these, only Pantoea and Stenotrophomonas54,55,56 had been previously detected as air-particle associated30, but the others do represent warning-worth occurrences. The Klebsiella genus encompasses in this respect a group that can determine nosocomial infections because of its air-dispersion57,58.
The main points arising from the present study can be summarized as follows. A major conserved contingent of bacteria has been identified as representative of a local-air microbiome which appears to characterize at least the Sardinian pool of the atmospheric prokaryotes as it occurred in the two northernmost and southernmost sampling stations of the island irrespective of the wind direction and regime.
Nevertheless, dust outbreaks conveying air from Saharan Africa, do increase the local biodiversity. Such immigrations are testified by upshifts in the richness ecological indicators of the sampled air and by the detectable presence of specific taxa missing from the background resident microbiome.
The culture independent method of analysis has proven 1) sensitive in comparison with the performances attained by culture based plating methods, 2) suitable to reveal differences between localities and weather conditions, and 3) informative to achieve identities of the incoming species via bioinformatics annotation pipeline.
Some of the bacteria encountered do belong to clinically relevant taxa that can possess virulence properties and pathogenic behavior towards humans. The monitoring principle assumes therefore importance in programs of disease prevention against epidemiological outbreaks from affected regions and countries as well as a warning routine in the event of deliberate bacterial warfare and bio-terrorism attacks.
Further investigations are required to refine the composition and the boundaries of the conserved air microbiomes and to put in evidence their seasonal fluctuations.
Materials and Methods
Sampling sites
The Sardinia Island extends from 38.86° N to 41.31° N and 08.14° to 9.84° E.
Sampling sites were located in Cagliari (39.23°N, 9.11°E), located on the southern coast facing the African continent, and Sassari (40.75°N, 8.49°E) which represents, on the opposite side, the first outpost site reached by the north-western winds. The two sampling locations are 174 km apart and the distance covers essentially the whole longitudinal territorial length of the island.
Meteorology forecasts
The predictive evaluation and alert of the Saharan dust discharge events was carried out by continuous monitoring of MODIS satellite data and Meteosat imagery combined with SKIRON59 forecasting model. SKIRON is a version of the ETA/NCEP weather forecasting model developed at the University of Athens with a forecast horizon of 5 days.
The origin and the back-trajectory plots of the dust carried by winds towards Italy were inferred by the NOAA HYSPLIT model (Hybrid Single Particle Lagrangian Integrated Trajectory Model)60,61. In addition, PM10 (particulate matter with a diameter of less than 10 μm) and meteorological data registered by the ARPAS (Regional Environmental Protection Agency of Sardinia) monitoring stations were used to highlight the arrival of African air masses.
Sampling was performed using a Skypost Tecora air-filtering apparatus; filters were collected in sterile boxes and stored at −20 °C until nucleic acids extraction.
PCR and Sequencing
Filters processed for DNA analyses were selected based on the observed weather conditions and the backward trajectories analysis.
Two filters for each specific weather event were considered in each sampling site. Filters were collected in order to represent northbound dust outbreaks reaching Cagliari or Sassari (CA-Dust and SS-Dust, respectively), whereas the dust-negative controls were taken at each of the two above sampling stations under northwesterly wind (CA-Ctrl and SS-Ctrl). Two replicates per site and per condition were taken for a total of eight filters.
DNA was extracted using the E.Z.N.A.® Soil DNA Kit (Omega Bio-Tek Inc.) and following the manufacturer’s protocol. Quality and quantity of the extracted nucleic acids were measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific Inc.).
Amplification of the 16S-rRNA genes was performed using the universal primers 27F-1492R (AGAGTTTGATYMTGGCTCAG and TACGGYTACCTTGTTACGACTT, respectively). PCR was performed using Platinum® Taq DNA Polymerase High Fidelity (Life Technologies) in a PTC-200 Thermal Cycler (MJ Research Inc.) set as follows: 95 °C for 5 min, (95 °C for 0.5 min, 51 °C for 0.5 min, 72 °C for 2 min for 30 cycles), 72 °C for 10 min and 4 °C hold.
Next generation sequencing was done at the Porto Conte Ricerche Srl (Alghero, Italy). Briefly, amplicons were quality-checked on an agarose gel and purified using the Agencourt® Ampure® XP PCR Purification Kit. One ng of DNA was processed using the Nextera XT DNA Sample Preparation Kit (Illumina Inc.) and sequenced using the HiScanSQ (Illumina Inc.) with 93 bp × 2 paired-end reads.
Data Analysis
A bioinformatic pipeline based on a two-step alignment-and-selection strategy was developed in order to classify and quantify each bacterial group.
Illumina reads were quality filtered and aligned against the SILVA reference database62 by using BWA63.
Sequences covered at least for the 10% of the total length by uniquely-aligned reads were firstly selected, obtaining a new dataset of putative subjects. Using these as a reference database, a second BWA alignment was performed and sequences covered at least for the 30% of the total length were then considered to give the final result. The naive 16S-rRNA genes classification attributed by the SILVA database was applied for the subjects taxonomic assignment. The percentage of uniquely-aligned reads on the total number of uniquely-aligned reads for each sample was considered to estimate and quantify each taxa.
Culture-dependent analyses
In order to characterize in parallel the fraction of culturable biota, some of the filters were placed, sample-side up, cut and cleaned with Tween 20. The filters pieces were removed and placed in selective and non-selective agar and incubated at room temperature for 24–72 hours for bacteria and 15–30 days for fungi. The media used were: Blood Agar, Chocolate Agar, Mannitol salt Agar, Mac Conkey agar, Thioglycollate medium usp with resazurine for bacteria and Sabouraud dextrose agar + caf + gent for fungi (Microbiol Diagnostici, Macchiareddu Uta Italy).
Cultured taxa were identified starting from a single colony using Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (MALDI TOF, Microflex LT Bruker), Vitek2 system and optical microscopy for colony morphology assessment.
The MALDI TOF technology is an alternative to classical microbiological identification for bacterial isolates, whose recognition is accomplished through comparison of the peak lists with a library containing the spectra information of species. It has been successfully used for species borne in air samples from Arabia64. Nevertheless it is recommended to couple its use with complementary techniques as the MALDI-TOF database is still incomplete as regards spectra of a number of species, e.g. some of the non-pathogenic Bacillus species. Therefore the Vitek2, providing automated identification based on antibiotic susceptibility profiles, was used as a suitable companion technique which can provide targeted phenotypic tests, in particular those covering aerobic endospore-forming bacteria65.
Additional Information
How to cite this article: Rosselli, R. et al. Microbial immigration across the Mediterranean via airborne dust. Sci. Rep. 5, 16306; doi: 10.1038/srep16306 (2015).
Supplementary Material
Acknowledgments
This work was supported by a grant from Regione Autonoma della Sardegna, Legge 7/2007, Project D.U.S.T. (Desert Upon Sardinian Territory), CRP-17664.
Footnotes
Author Contributions R.R., M.F. and M.D. performed the experiments, R.R. devised and performed the statistical analysis. G.Ps. and A.C. provided data, P.D., R.M. and P.C. obtained the funding and supervised the research, R.R. and A.S. wrote the manuscript.
References
- Foltz G. R. & McPhaden M. J. Impact of Saharan dust on tropical North Atlantic SST. J. Clim. 21, 5048–5060 (2008). [Google Scholar]
- Booth B. B. B., Dunstone N. J., Halloran P. R., Andrews T. & Bellouin N. Aerosols implicated as a prime driver of twentieth-century North Atlantic climate variability. Nature 484, 228–232 (2012). [DOI] [PubMed] [Google Scholar]
- Bangert M. et al. Saharan dust event impacts on cloud formation and radiation over Western Europe. Atmos. Chem. Phys. 12, 4045–4063 (2012). [Google Scholar]
- Gallisai R., Peters F., Volpe G., Basart S. & Baldasano J. M. Saharan Dust Deposition May Affect Phytoplankton Growth in the Mediterranean Sea at Ecological Time Scales. PLoS One 9, e110762 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. et al. The Fertilizing Role of African Dust in the Amazon Rainforest: A First Multiyear Assessment Based on CALIPSO Lidar Observations. Geophys. Res. Lett. 42, 10.1002/2015GL063040 (2015). [DOI] [Google Scholar]
- Bonasoni P. et al. Effect of Saharan Dust Transport on Ozone and Carbon Dioxide Concentration. Environ. Sci. Technol. 11, 313–322 (1996). [Google Scholar]
- Prospero J. M., Blades E., Mathison G. & Naidu R. Interhemispheric transport of viable fungi and bacteria from Africa to the Caribbean with soil dust. Aerobiologia. 21, 1–19 (2005). [Google Scholar]
- Whon T. W. et al. Metagenomic Characterization of Airborne Viral DNA Diversity in the Near-Surface Atmosphere. J. Virol. 86, 8221–8231 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn E. A., Griffin D. W. & Seba D. B. Atmospheric transport of mold spores in clouds of desert dust. Arch. Environ. Health 58, 498–504 (2003). [PubMed] [Google Scholar]
- Griffin D. W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 20, 459–477 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reche I. et al. Effect of Saharan dust inputs on bacterial activity and community composition in Mediterranean lakes and reservoirs. Limnol. Oceanogr. 54, 869–879 (2009). [Google Scholar]
- Giongo A. et al. Microbial hitchhikers on intercontinental dust: high-throughput sequencing to catalogue microbes in small sand samples. Aerobiologia 29, 71–84 (2012). [Google Scholar]
- Foord N. & Lidwell O. M. Airborne infection in a fully air-conditioned hospital. II. Transfer of airborne particles between rooms resulting from the movement of air from one room to another. J. Hyg. 75, 31–44 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovallius A., Bucht B., Roffey R. & Anas P. Three year investigation of the natural airborne bacterial flora at four localities in Sweden. Appl. Environ. Microbiol. 35, 847–852 (1978). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J. & Hernandez M. Incorporating polymerase chain reaction-based identification, population characterization, and quantification of microorganisms into aerosol science: A review. Atmos. Environ. 40, 3941–3961 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyan K. et al. African dust clouds are associated with increased paediatric asthma accident and emergency admissions on the Caribbean island of Trinidad. Int. J. Biometeorol. 49, 371–376 (2005). [DOI] [PubMed] [Google Scholar]
- Cheesbrough J. S., Morse A. P. & Green S. D. Meningococcal meningitis and carriage in western Zaire: a hypoendemic zone related to climate? Epidemiol. Infect. 114, 75–92 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez García-Pando C. et al. Soil dust aerosols and wind as predictors of seasonal meningitis incidence in niger. Environ. Health Perspect. 122, 679–686 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong G. Y. Bulk and single-particle mineralogy of Asian dust and a comparison with its source soils. J. Geophys. Res. 113, D02208, 10.1029/2007JD008606 (2008). [DOI] [Google Scholar]
- Ginoux P., Prospero J. M., Gill T. E., Hsu N. C. & Zhao M. Global-scale attribution of anthropogenic and natural dust sources and their emission rates based on MODIS Deep Blue aerosol products. Rev. Geophys. 50, RG3005, 10.1029/2012RG000388 (2012). [DOI] [Google Scholar]
- Nickovic S., Vukovic A., Vujadinovic M., Djurdjevic V. & Pejanovic G. Technical Note: High-resolution mineralogical database of dust-productive soils for atmospheric dust modeling. Atmos. Chem. Phys. 12, 845–855 (2012). [Google Scholar]
- Shinn E. A. et al. African dust and the demise of Caribbean Coral Reefs. Geophys. Res. Lett. 27, 3029 (2000). [Google Scholar]
- Griffin D. W., Garrison V. H., Herman J. R. & Shinn E. A. African desert dust in the Caribbean atmosphere: Microbiology and public health. Aerobiologia. 17, 203–213 (2001). [Google Scholar]
- Katra I. et al. Richness and diversity in dust stormborne biomes at the southeast mediterranean. Sci Rep Jun 12;4:5265, 10.1038/srep05265. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggs G. F. S. et al. The dynamics and characteristics of aeolian dust in dryland Central Asia: possible impacts on human exposure and respiratory health in the Aral Sea basin. Geogr. J. 169, 142–157 (2003). [Google Scholar]
- Weinhold B. Infectious disease: The human costs of our environmental errors. Environ. Health Perspect. 112, A32–9 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy L. H. Introduction of Sugarcane Rust into the Americas and Its Spread to Florida. Plant Dis. 69, 689–693 (1985). [Google Scholar]
- Taylor D. A. Dust in the wind. Environ. Health Perspect. 110, A80–7 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg C. A. et al. Characterization of aerosolized bacteria and fungi from desert dust events in Mali, West Africa. Aerobiologia. 20, 99–110 (2004). [Google Scholar]
- Cao C. et al. Inhalable Microorganisms in Beijing’s PM 2.5 and PM 10 Pollutants during a Severe Smog Event. Enviromental Sci. Technol. 48, 1499–1507 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Almeida G. A. A Model for Saharan Dust Transport. J. Clim. Appl. Meteorol. 25, 903–916 (1986). [Google Scholar]
- Dulac F. et al. Quantitative remote sensing of African dust transport in the Mediterranean. In The Impact of Desert Dust Across the Mediterranean. (eds Guerzoni S. et al.) 25–49 (Kluwer Academic Publishers, 1996). [Google Scholar]
- Alpert P., Kishcha P., Shtivelman A., Krichak S. O. & Joseph J. H. Vertical distribution of Saharan dust based on 2.5-year model predictions. Atmos. Res. 70, 109–130 (2004). [Google Scholar]
- Moulin C., Lambert C. E., Dulac F. & Dayan U. Control of atmospheric export of dust from North Africa by the North Atlantic Oscillation. Nature 387, 691–694 (1997). [Google Scholar]
- Escudero M. S. et al. Wet and dry African dust episodes over eastern Spain, J. Geophys. Res. 110, D18S08, 10.1029/2004JD004731 (2005). [DOI] [Google Scholar]
- Querol X. J. et al. African dust contributions to mean ambient PM10 mass-levels across the Mediterranean Basin, Atmos. Environ. 43, 4266–4277 (2009). [Google Scholar]
- Israelevich P., Ganor E., Alpert P., Kishcha P. & Stupp A. Predominant transport paths of Saharan dust over the Mediterranean Sea to Europe. J. Geophys. Res. Atmos. 117, D02205, 10.1029/2011JD016482. (2012). [DOI] [Google Scholar]
- Guerzoni S. et al. Mineral atmospheric particulate from south to northwest Mediterranean: seasonal variation and characteristics. Water Pollut. Res. Reports 28, 483–493 (1992). [Google Scholar]
- Guerzoni S. et al. Fluxes of soluble and insoluble metals and nutrients from the atmosphere to the central Mediterranean Sea. Water Pollut. Res. Reports 30, 438–493 (1993). [Google Scholar]
- Guerzoni S., Molinaroli E. & Chester R. Saharan dust inputs to the western Mediterranean Sea: Depositional patterns, geochemistry and sedimentological implications. Deep. Res. Part II Top. Stud. Oceanogr. 44, 631–654 (1997). [Google Scholar]
- Polymenakou P. N., Mandalakis M., Stephanou E. G. & Tselepides A. Particle size distribution of airborne microorganisms and pathogens during an intense African dust event in the eastern Mediterranean. Environ. Health Perspect. 116, 292–296 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F., Hehemann J.-H., Rebuffet E., Czjzek M. & Michel G. Environmental and gut bacteroidetes: the food connection. Front. Microbiol. 2:93. 10.3389/fmicb.2011.00093 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez De La Campa A., García-Salamanca A., Solano J., De La Rosa J. & Ramos J. L. Chemical and microbiological characterization of atmospheric particulate matter during an intense african dust event in Southern Spain. Environ. Sci. Technol. 47, 3630–3638 (2013). [DOI] [PubMed] [Google Scholar]
- Toepfer I. et al. Pathogens as potential hitchhikers on intercontinental dust. Aerobiologia. 28, 221–231 (2012). [Google Scholar]
- Doran T. I. The role of Citrobacter in clinical disease of children: review. Clin. Infect. Dis. 28, 384–394 (1999). [DOI] [PubMed] [Google Scholar]
- Dzeing-Ella A. et al. Infective endocarditis due to Citrobacter koseri in an immunocompetent adult. J. Clin. Microbiol. 47, 4185–4186 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollara G., Savy L., Cropley I. & Hopkins S. Citrobacter koseri meningitis: Another freediving risk? J. Infect. 62, 101–103 (2011). [DOI] [PubMed] [Google Scholar]
- Hunter C. J. & Bean J. F. Cronobacter: an emerging opportunistic pathogen associated with neonatal meningitis, sepsis and necrotizing enterocolitis. J. Perinatol. 33, 581–585 (2013). [DOI] [PubMed] [Google Scholar]
- Holý O. & Forsythe S. Cronobacter spp. as emerging causes of healthcare-associated infection. J. Hosp. Infect. 86, 169–177 (2014). [DOI] [PubMed] [Google Scholar]
- Jaradat Z. W., Al Mousa W., Elbetieha A., Al Nabulsi A. & Tall B. D. Cronobacter spp.–opportunistic food-borne pathogens. A review of their virulence and environmental-adaptive traits. J. Med. Microbiol. 63, 1023–1037 (2014). [DOI] [PubMed] [Google Scholar]
- Flores Popoca E. O. et al. Pantoea agglomerans in immunodeficient patients with different respiratory symptoms. Sci. World J. 2012, 156827 10.1100/2012/156827 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberto M. C. et al. Six cases of sepsis caused by Pantoea agglomerans in a teaching hospital. New Microbiol. 32, 119–123 (2009). [PubMed] [Google Scholar]
- Muresu R., Maddau G., Delogu G., Cappuccinelli P. & Squartini A. Bacteria colonizing root nodules of wild legumes exhibit virulence-associated properties of mammalian pathogens. Antonie van Leeuwenhoek, Int. J. Gen. Mol. Microbiol. 97, 143–153 (2010). [DOI] [PubMed] [Google Scholar]
- Brooke J. S. New strategies against Stenotrophomonas maltophilia: a serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert Rev. Anti. Infect. Ther. 12, 1–4 (2014). [DOI] [PubMed] [Google Scholar]
- Cerezer V. G., Bando S. Y., Pasternak J., Franzolin M. R. & Moreira-Filho C. A. Phylogenetic analysis of Stenotrophomonas spp. isolates contributes to the identification of nosocomial and community-acquired infections. Biomed Res. Int. Article ID 151405, 10.1155/2014/151405 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavi P. et al. Stenotrophomonas comparative genomics reveals genes and functions that differentiate beneficial and pathogenic bacteria. BMC Genomics 15, 482, 10.1186/1471-2164-15-482 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podschun R. & Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11, 589–603 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J. W. The effect of environmental parameters on the survival of airborne infectious agents. J. R. Soc. Interface 6, Suppl 6, S737–S746 10.1098/rsif.2009.0227.focus. (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickovic S., Kallos G., Papadopoulos A. & Kakaliagou O. A model for prediction of desert dust cycle in the atmosphere. J. Geophys. Res., 106(D16), 18113–18129, 10.1029/2000JD900794. (2001). [DOI] [Google Scholar]
- Rolph G. D. Real-time Environmental Applications and Display sYstem (READY) Website (http://ready.arl.noaa.gov). NOAA Air Resources Laboratory (2014) (date of access: 04/10/2014).
- Draxler R. R. & Rolph G. D. HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) Model access via NOAA ARL READY Website (http://www.arl.noaa.gov/HYSPLIT.php). NOAA Air Resources Laboratory, College Park, MD. NOAA Air Resources Laboratory (2014) (date of access: 07/10/2014).
- Quast C. et al. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 41, D590–6 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakis E. et al. MALDI-TOF mass spectrometry and identification of new bacteria species in air samples from Makkah, Saudi Arabia. BMC Res. Notes, 7, 892 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halket G., Dinsdale A. E. & Logan N. A. Evaluation of the VITEK2 BCL card for identification of Bacillus species and other aerobic endospore formers. Lett Appl Microbiol. 50, 120–126 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.