Abstract
Interferon-Stimulated Gene 15 (ISG15) transcript is aberrantly expressed in most human malignancies, suggesting that it has a protumor function. However, at the protein level ISG15 has both protumor and immunomodulatory antitumor functions. Therapeutic strategies to maximize the latter may benefit cancer patients overexpressing the ISG15 pathway.
Keywords: cancer, immune system, immunostimulatory cytokine, ISG15, ubiquitin/26S proteasome
Abbreviations
- ATG8 and ATG12
autophagy-related 8 and 12
- ISG15
interferon-stimulatory gene 15
- MHC Class I
major histocompatibility complex class I
- NEDD8
neural precursor cell expressed developmentally downregulated 8
- NK cells
natural killer cells
- SUMO1-4
small ubiquitin-like modifier1-4 UBL, ubiquitin-like protein.
Interferon-Stimulated Gene 15 (ISG15), an ubiquitin-like protein (UBL),1 is emerging as an important oncoprotein and a potential diagnostic2 and therapeutic3 target for cancer. Although discovered in 1979, ISG15 research has only recently begun to gain momentum because of its deregulated expression in most human malignancies and its newly recognized novel function of inhibiting the canonical ubiquitin pathway, a master regulator of cell survival and death.4
It is well understood that the ubiquitin/26S proteasome pathway for protein turnover regulates cell homeostasis through vital cellular processes including cell proliferation, apoptosis, and signal transduction among others.5 However, it is also becoming increasingly evident that these cellular processes are not exclusively regulated by ubiquitin but frequently require collateral signaling by other proteins called ubiquitin-like proteins (UBLs), including NEDD8, SUMO1–4, ATG8 and ATG12, and ISG15.6 All UBLs show structural and mechanistic similarity with ubiquitin. Each harbors a conserved β-grasp fold and C-terminal Gly-Gly motif through which distinct sets of conjugating enzymes, covalently append them to a range of cellular protein targets. However, the biological consequences of their conjugation to the cellular proteins are distinct from ubiquitin. For example, NEDD8 conjugation to cullins, a component of SCF-E3 ubiquitin ligases, activates the functional activity of the E3 ligases and facilitates ubiquitin conjugation to their cellular targets. In contrast, ISG15 conjugation to specific E3 ubiquitin ligases is thought to inhibit their ligase function, thereby impinging on the consequences of ubiquitin conjugation. Hence, it is not surprising that deregulation of the ISG15 pathway has direct impact on the pleiotropic cellular functions of ubiquitin, as demonstrated by our group and others, thus leading to several human diseases including cancer.
Using ISG15-specific antibodies in Western blotting analysis, our group initially demonstrated that free ISG15 and its protein conjugated form (ISGylation) are increased in human solid tumors and tumor cell lines relative to their respective normal counterparts.4 Using gene silencing, we further demonstrated that expression of ISG15 is regulated by oncogenic K-RAS and that the conjugated form of ISG15 but not free ISG15 contributes to malignant transformation of breast cells in culture.7,8 Since then, a number of studies have revealed that the ISG15 pathway is constitutively elevated in most human malignancies and that elevated ISG15 contributes to malignant transformation of cells in culture. These two corroborating observations, namely that ISG15 gene is overexpressed in cancers and that ISG15 conjugates confers malignant phenotypes led us and others to conclude that ISG15 has protumor functions (Fig. 1).
Contrasting with the above observations, early studies demonstrated that recombinant purified free ISG15 has an immunomodulatory cytokine-like activity suggesting that ISG15 may have antitumor cancer immunosurveillance functions in vivo.9 These 2 contradictory findings implicating both protumor and antitumor ISG15 functional properties raised the obvious question of whether amplification of the ISG15 pathway in most cancers would be a friend or foe in a clinical setting. We therefore sought to clarify the role of ISG15 in tumorigenesis in vivo using nude mice and cell culture models. We found that ISG15 inhibits tumor growth when added extracellularly and induces infiltration of NK cells in tumors grown in nude mice and intracellular free ISG15 enhances 26S proteasome-dependent surface expression of MHC Class I complexes on breast cancer cells.10 Thus, our results reveal that free ISG15 exerts an antitumor effect by activating the innate and adaptive arms of the immune system in vivo, therefore supporting the conclusions of early in vitro cell culture studies that free ISG15 has an immunocytokine-like function (Fig. 1).
To date, it is very clear that ISG15 gene expression is elevated in most cancers. However, very little information is available on the expression levels of free ISG15 protein and its conjugates in human cancers. In a small study, we have demonstrated that expression of free ISG15 and ISG15 conjugates is heterogeneous in various human tumors. We found that some tumors displayed very high levels of ISG15 conjugates and low levels of free ISG15, while others displayed high levels of free ISG15 and low levels of ISG15 conjugates. Whether high levels of free ISG15 and low levels of conjugates correlate with better outcome of patients and vice versa is currently unknown. However, our findings that free ISG15 has anti-tumor functions and that ISG15 conjugates have pro-tumor functions have led to our working hypothesis that tumor cells inhibit secretion of free ISG15 by conjugating it to the cellular proteins consequently escaping immune surveillance and fostering tumor cell survival. If our hypothesis is proven correct, future development of therapeutic strategies to decrease conjugation and increase the systemic levels of free ISG15 would be beneficial for treating cancer patients overexpressing the ISG15 pathway.
Of interest, ISG15 has been also identified as a tumor biomarker as its expression is exclusively elevated in neoplasms,4 and its presence is also a biomarker for drug sensitivity as tumors overexpressing ISG15 are sensitive to the clinically used anticancer drug camptothecin (Topotecan).3 Hence, elevated ISG15 may be beneficial for diagnostic purposes, predicting patients' responses to anticancer drugs, and as an immunostimulant in cancer patients. Conversely, empirical evidence suggests that elevated ISG15 conjugates could also harm patients by stabilizing cellular proteins that promote cancer in patients. Thus, ISG15 clearly has double-edged functions in malignant disease and proper consideration must be given in order to assess risk-benefit prior to administering ISG15-targeted cancer therapy.
Figure 1.
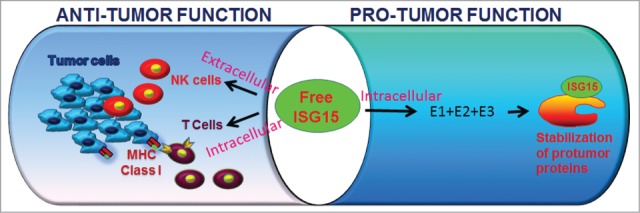
The intracellular and extracellular functions of free ISG15.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Haas AL, Ahrens P, Bright PM, Ankel H. Interferon induces a 15-kilodalton protein exhibiting marked homology to ubiquitin. J Biol Chem 1987; 262:11315-23; PMID:2440890 [PubMed] [Google Scholar]
- 2.Bektas N, Noetzel E, Veeck J, Press MF, Kristiansen G, Naami A, Hartmann A, Dimmler A, Beckmann MW, Knüchel R, et al.. The ubiquitin-like molecule interferon-stimulated gene 15 (ISG15) is a potential prognostic marker in human breast cancer. Breast Cancer Res 2008; 10:R58; PMID:18627608; http://dx.doi.org/ 10.1186/bcr2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desai SD, Wood LM, Tsai YC, Hsieh TS, Marks JR, Scott GL, Giovanella BC, Liu LF. ISG15 as a novel tumor biomarker for drug sensitivity. Mol Cancer Ther 2008; 7:1430-9; PMID:18566215; http://dx.doi.org/ 10.1158/1535-7163.MCT-07-2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai SD, Haas AL, Wood LM, Tsai YC, Pestka S, Rubin EH, Saleem A, Nur-E-Kamal A, Liu LF. Elevated expression of ISG15 in tumor cells interferes with the ubiquitin/26S proteasome pathway. Cancer Res. 2006; 66:921-8; PMID:16424026; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1123 [DOI] [PubMed] [Google Scholar]
- 5.Hershko A, Ciechanover A, Varshavsky A. Basic medical research award. The ubiquitin system. Nat Med 2000; 6:1073-81; PMID:11017125; http://dx.doi.org/ 10.1038/80384 [DOI] [PubMed] [Google Scholar]
- 6.van der Veen AG, Ploegh HL. Ubiquitin-like proteins. Annu Rev Biochem 2012; 81:323-57; PMID:22404627; http://dx.doi.org/ 10.1146/annurev-biochem-093010-153308 [DOI] [PubMed] [Google Scholar]
- 7.Burks J, Reed RE, Desai SD. ISGylation governs the oncogenic function of Ki-Ras in breast cancer. Oncogene 2014. 33:794-803; PMID:23318454; http://dx.doi.org/ 10.1038/onc.2012.633 [DOI] [PubMed] [Google Scholar]
- 8.Desai SD, Reed RE, Burks J, Wood LM, Pullikuth AK, Haas AL, Liu LF, Breslin JW, Meiners S, Sankar S. ISG15 disrupts cytoskeletal architecture and promotes motility in human breast cancer cells. Exp Biol Med (Maywood) 2012; 237:38-49; PMID:22185919; http://dx.doi.org/ 10.1258/ebm.2011.011236 [DOI] [PubMed] [Google Scholar]
- 9.D'Cunha J, Knight E Jr., Haas AL, Truitt RL, Borden EC. Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc Natl Acad Sci U S A 1996; 93:211-5; PMID:8552607; http://dx.doi.org/ 10.1073/pnas.93.1.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burks J, Reed RE, Desai SD. Free ISG15 triggers an antitumor immune response against breast cancer: a new perspective. Oncotarget 2015; 6:7221-31; PMID:25749047 [DOI] [PMC free article] [PubMed] [Google Scholar]


