Abstract
αA- and αB-crystallins are small heat shock proteins that bind thermodynamically destabilized proteins thereby inhibiting their aggregation. Highly expressed in the mammalian lens, the α-crystallins have been postulated to play a critical role in the maintenance of lens optical properties by sequestering age-damaged proteins prone to aggregation as well as through a multitude of roles in lens epithelial cells. Here, we have examined the role of α-crystallins in the development of the vertebrate zebrafish lens. For this purpose, we have carried out morpholino-mediated knockdown of αA-, αBa- and αBb-crystallin and characterized the gross morphology of the lens. We observed lens abnormalities, including increased reflectance intensity, as a consequence of the interference with expression of these proteins. These abnormalities were less frequent in transgenic zebrafish embryos expressing rat αA-crystallin suggesting a specific role of α-crystallins in embryonic lens development. To extend and confirm these findings, we generated an αA-crystallin knockout zebrafish line. A more consistent and severe lens phenotype was evident in maternal/zygotic αA-crystallin mutants compared to those observed by morpholino knockdown. The penetrance of the lens phenotype was reduced by transgenic expression of rat αA-crystallin and its severity was attenuated by maternal αA-crystallin expression. These findings demonstrate that the role of α-crystallins in lens development is conserved from mammals to zebrafish and set the stage for using the embryonic lens as a model system to test mechanistic aspects of α-crystallin chaperone activity and to develop strategies to fine-tune protein-protein interactions in aging and cataracts.
Keywords: Alpha-crystallin, small heat shock protein, chaperone, lens development, cataract, morpholino, TALEN, maternal transcript, zebrafish
1. Introduction
The development of the ocular lens follows a regulated program that culminates in the terminal differentiation of epithelial cells to fiber cells. During this process, the developing cells shed their organelles and express a group of water soluble proteins, the crystallins, that undergo little if any turnover (Bassnett, 1997; Beebe et al., 2001). The type of crystallins and their relative abundance is species-specific but invariably mammalian lenses have α-, β- and γ-crystallins as the main constituents at unusually high concentrations (Bloemendal et al., 2004). The optical properties of the lens, refractivity and transparency, arise from the glass-like, short-range order packing of the crystallins (Delaye and Tardieu, 1983; Tardieu, 1988). Protein-protein interactions between crystallins are tuned to be consistent with a uniform protein distribution on dimensions comparable with visible light wavelength while preventing long range order or crystallization (Benedek, 1971).
The role of α-crystallin in lens transparency has garnered intense interest owing to its function as a small heat shock protein with chaperone activity towards destabilized and unfolded proteins (Clark and Muchowski, 2000; Claxton et al., 2008; Horwitz, 1992, 2003; Koteiche and McHaourab, 2003, 2006; Kumar et al., 2009; Mchaourab et al., 2002; McHaourab et al., 2007; McHaourab et al., 2009; Sathish et al., 2003; Sathish et al., 2004; Sharma et al., 1997; Sharma and Santhoshkumar, 2009). In the context of long-lived protein solutions, it stands to reason that such an activity may be critical for proteostasis. Constantly assaulted by environmental factors, lens proteins undergo extensive age-related modifications (Hanson et al., 1998; Hanson et al., 2000; Lampi et al., 1998; Lampi et al., 2002; Lampi et al., 2006; Truscott, 2005) that compromise their thermodynamic stability and promote the formation of large aggregates. Large protein aggregates can disturb the short range order of lens proteins and lead to light scattering thereby compromising the optical properties of the lens (Benedek, 1997). We have proposed and experimentally tested a thermodynamic model of α-crystallin chaperone activity using destabilized T4 lysozyme as a model client protein (Claxton et al., 2008; Koteiche and McHaourab, 2003; Mchaourab et al., 2002). Our model implies that once modified lens proteins cross an energetic threshold of destabilization, they become substrates for α-crystallin which sequesters them away. The progressive exhaustion of α-crystallin binding capacity by the binding of age-damaged, destabilized lens proteins eventually leads to the formation of aggregated proteins and the subsequent scattering of light.
There is little doubt that in vitro α-crystallin recognizes and binds destabilized proteins with high capacity. α-crystallin binding affinity and stoichiometry are regulated by changes in its oligomeric structure induced by phosphorylation and by higher temperature (Claxton et al., 2008; Koteiche and McHaourab, 2003; McHaourab et al., 2009; Reddy et al., 2000; Sathish et al., 2003). What lacks direct evidence is the in vivo role of α-crystallin chaperone activity in the maintenance of lens transparency and in delaying its aging. In contrast to the spectrum of model protein substrates that have been investigated, neither αA- nor αB-crystallin display high affinity to thermodynamically destabilized, aggregation-prone, γD-crystallin mutants (Liu et al., 2005; Mishra et al., 2012) shown to induce cataract in mouse models (Liu et al., 2005; Wang et al., 2007). Interpretation of the relevance of these findings is confounded by the unusually high protein concentration of lens fiber cells which shapes protein stability and interactions in vivo as opposed to the dilute solutions where these in vitro studies are typically performed. Molecular crowding leads to excluded volume effects manifested by many-fold difference in the magnitude of equilibrium affinities, rate constants of interactions and free energies of unfolding (van den Berg et al., 1999; van den Berg et al., 2000). Crowding in lens fiber cells, which has been invariably neglected in α-crystallin mechanistic models, is peculiar involving the three molecules whose interactions are to be studied. Therefore, the implications of low affinity between α-crystallin and its putative physiological targets in dilute solution are unclear. To address this unresolved aspect of α-crystallin mechanism, there is a need to develop cell- and organism- based approaches to test the chaperone hypothesis of α-crystallin role in the lens.
Zebrafish has emerged as a powerful model system for eye development and disease (Fadool et al., 1997; Fadool and Dowling, 2008). Zebrafish embryos are extracorporeal and transparent during the first few days of development. Larval embryos have relatively large eyes which become functional 3 days post fertilization (dpf) enabling the examination of lens gross morphology by bright field microscopy. Studies by the Link (Soules and Link, 2005), Clark (Greiling and Clark, 2009; Greiling et al., 2009) and Posner (Runkle et al., 2002; Dahlman et al., 2005; Posner et al., 2008) laboratories lay the morphological and proteomic foundations for zebrafish lens. The structure of the adult zebrafish lens resembles the mature human lens and the development and morphology are similar to those of mammals with few differences. Importantly from the perspective of protein-protein interactions, the zebrafish lens expresses a complement of α-, β- and γ-crystallin orthologs, as well as additional γ-crystallins which are specific to aquatic species. Therefore, it is postulated that similar molecular interactions account for lens transparency.
Similar to their mammalian orthologs to which they have extensive sequence similarity (Runkle et al., 2002; Dahlman et al., 2005); Zebrafish α-crystallins have chaperone activity in vitro (Dahlman et al., 2005). However, their role in embryonic zebrafish lens development and transparency is controversial (Goishi et al., 2006; Posner et al., 2013). Here, we report the results of an investigation into the roles of three α-crystallin genes, cryaa, cryaba, and cryabb, in the development of the zebrafish lens. We report that selective knockdown of each of the α-crystallin genes is associated with lens abnormalities. Lens-specific effects induced by the suppression of αA- and αBa-crystallin, but not αBb-crystallin, can be rescued by transgenic expression of rat αA-crystallin. These findings are confirmed and extended by characterization of the first knockout of αA-crystallin in zebrafish. We find that the lack of αA-crystallin expression is associated with morphological abnormalities and increased intensity of reflectance in zebrafish embryonic lens. That the lens phenotype of the knockout embryos can be partly rescued by the transgenic expression of rat αA-crystallin is consistent with a conserved role of its chaperone activity in lens development across species.
2. Materials and methods
2.1 Zebrafish maintenance and breeding
AB wild-type strain zebrafish (Danio rerio) were used. The embryos were obtained by natural spawning and raised at 28.5°C on a 14/10 hour light/dark cycle in 0.3x Danieau water containing 0.003% PTU (w/v) to prevent pigment formation. Embryos were staged according to their ages (hpf; hours post-fertilization or dpf; days post-fertilization). All animal procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee. The same feeding procedures were performed on all lines, including AB wild-type, cryaa knockout lines, and cryaa:Rno.Cryaa transgenic lines according to their appropriate ages.
2.2 Zebrafish transgenesis
To establish the transgenic zebrafish expressing rat (Rattus norvegicus) Cryaa gene (Rno.Cryaa) specifically in the lens, Tg(cryaa:Rno.Cryaa,myl7:Cerulean) was constructed by inserting Rno.Cryaa cDNA downstream of zebrafish cryaa promoter (0.7 kb; Kurita et al., 2003) in the pT2HBLR vector that was also contains myl7 promoter-driven Cerulean as the selection marker. Tol2 transposase RNA was synthesized in vitro by using mMESSAGE mMACHINE SP6 kit (Ambion). The mixture of 25pg plasmid DNA and 20pg transposase RNA was co-injected into one-cell stage embryos. Injected embryos were screened for Cerulean expression in the heart under Zeiss fluorescence microscope at 3 dpf, and then were raised to adulthood as F0 founder. Each F0 founder fish was out-crossed with AB fish and progenies with Cerulean expression in the heart were raised to establish stable F1 generation. F1s were further confirmed by PCR sequencing and one transgenic line was selected and maintained.
2.3 Morpholino knockdown of zebrafish α-crystallin genes
Translation-blocking morpholino antisense oligos (MOs) against zebrafish cryaa (5′-GTTGGATCGCAATATCCATAATGTC-3′), cryaba (5′-CCATTGTACCTTAGTTTGGAGCTGA-3′), and cryabb (5′-TCCATTTTGAGTCTGGGCCTCTTCT-3′) genes were designed and synthesized by Gene Tools (Philomath, OR). MOs were dissolved in sterile water. Different dosages (2.5ng, 5ng and 10ng) of each MO were injected into the yolk of 1–2 cell stage zygotes, which were the progenies from Tg(cryaa:Rno.Cryaa,myl7:Cerulean) and AB fish. For each MO, a series of dosages were tested to rule out the toxic effects caused by over-injection of MOs.
2.4 Generation of zebrafish cryaa knockout line
Transcription activator-like effector nuclease (TALEN) was used to generate zebrafish cryaa knockout. In brief, TALENs were assembled by using the Golden Gate assembly protocol and library (Cermak et al., 2011). A pair of TALENs with the following repeat variable di-residues (RVDs) was used: HD NN NI NG NG NI NG NN NI HD HD NG NI NG NG HD targeting 5′-CGATTATGACCTATTC-3′, and NI NN NG NI NI NN NN NN HD NG NN NI HD NI NN targeting 5′-CTGTCAGCCCTTACT-3′. TALEN-encoding mRNA was synthesized in vitro using the mMESSAGE mMACHINE T3 Kit (Ambion). The pair of synthesized mRNAs (450pg/mRNA) was co-injected into one-cell stage embryos and all surviving embryos were raised to adulthood. T7 endonuclease I assay (T7EI; Reyon et al., 2012; Jao et al., 2013) was used to identify potential F0 founder and each founder line was subsequently crossed with AB wild-type fish to establish F1 generation. One F1 fish line was found to have an 8-bp deletion (TTCACCAC) in the first exon of cryaa gene by PCR sequencing, which would lead to an early truncation of Cryaa protein. Genotyping was performed by RFLP (restriction fragment length polymorphism) that distinguish wild-type allele and 8bp-deletion mutant allele.
2.5 Western analysis
Western analysis of zebrafish Cryaa expression in MO-cryaa injected embryos was performed to evaluate the knockdown efficiency of MO-cryaa. Embryos from four different post-screening groups (WT with lens defects, WT without lens defects, Rno.Cryaa with lens defects and Rno.Cryaa without lens defects) were sacrificed at 3dpf and lenses were isolated and collected. For each group, 50 lenses were homogenized and extracted in hot SDS lysis buffer containing 63mM Tris, 10% glycerol, 2% SDS, 5% beta-mercaptoethanol and protease inhibitors. Supernatant from each group was loaded to 12% SDS-PAGE gel and later transferred to PVDF membranes. Membranes were blocked with 5% non-fat milk in TBST, incubated with a 1:500 dilution of zebrafish Cryaa antibody overnight (gift from Hyde lab, University of Notre Dame), followed by anti-rabbit horseradish peroxidase bound secondary antibody after three times washing with TBST. The bands were detected by HyGLO™ Quick Spray Chemiluminescent (Denville Scientific Inc.).
2.6 RT-PCR
Total RNAs were isolated from embryos at desired stages by using Trizol Reagent (Invitrogen). The extracted RNAs were analyzed by agarose gel and treated with RNase-free DNase I to get rid of genomic DNA contaminations. To synthesize cDNA, 1μg of total RNA was reversely transcripted using SuperScript™ III First-Strand Synthesis System (Invitrogen, USA), and the resulting cDNAs were used as templates for PCR using KOD Hot Start DNA polymerase (Novagen, USA) with the following primers: 5′-ATCGACACTCACTCTTCCGC-3′ and 5′-CCACTTGTCTTGGGTCCACA-3′ for cryaa; 5′-TGTTCCCAGGCTTCTTCCC-3′ and 5′-GCTTCACATCCAGGTTGATTACA-3′ for cryaba; 5′-AGGACCAATTCTCACTAAGTCTGG-3′ and 5′-CATCCCCGTAACTCGTGATG-3′ for cryabb. PCR of actb2 was used as a loading control.
2.7 Histology
The anaesthetized embryos were washed with 1X PBS, and fixed in 4% paraformaldehyde, 2.5% glutaraldehyde, 3% sucrose, 75mM phosphate buffer (pH 7.4) for 1 h at room temperature and stored at 4 °C overnight (Dahm et al., 2007). The fixed embryos were washed with 1X PBS and dehydrated in a graded series of ethanol (5%, 25% and 50% in 1X PBS; 70%, 90%, 95% aq. and twice with 100%) for 15 min each at room temperature. Subsequently the dehydrated embryos were infiltrated with JB-4 resin (Polysciences, Inc.) overnight at 4 °C. The embryos were embedded in JB-4 resin in molds at room temperature for several days under vacuum. Serial sections of 4 μm thickness were cut with glass knife on microtome. The sections were transferred in water to Superfrost Plus glass slides and following heat fixation stained with hematoxylin and eosin (Melville et al., 2011; Sullivan-Brown et al., 2011).
2.8 Microscopy and image processing
Lenses of live embryos in 0.3x Daneau water with PTU/tricane were analyzed by bright field by stereomicroscopy (Zeiss M2BIO) at 3–4dpf and graded into three classes, WT-like (as shown in Fig. 3C), minor lens defects (similar to Fig. 3E) and major lens defects (similar to Fig. 3D and 3F), depending on the severity of lens defects. Fluorescence screening for Cerulean heart marker was completed by Zeiss Stereo Discovery microscope. Differential interference contrast (DIC) and reflectance analysis were performed by Zeiss LSM510 Inverted Confocal Microscope with λem=488 on 4dpf embryos which were embedded in 2.5% methylcellulose. DIC images were taken with a 20x objective lens and used as a general locator for reflectance analysis. Reflected light was analyzed by digital image analysis program Image J.
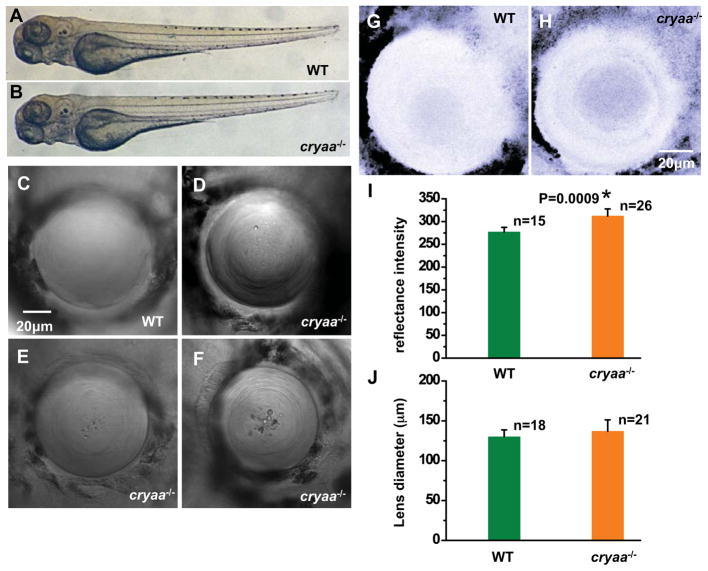
Figure 3. Cryaa mutants show lens defects at 4dpf.
Compared to WT siblings (A), cryaa−/− embryos (B) showed no morphological or anatomical difference at 4dpf. High-magnification representative images from Cryaa−/− lens (D–F) at 4dpf revealed abnormal features, different from WT lens (C). (I) Reflectance analysis at 4dpf showed that the reflectance intensity was higher (t-test; p=0.0009) in cryaa−/− lens (H) than in WT lens (G). Lens diameters were measured and no difference was observed between cryaa−/− and WT embryos (J).
2.9 Statistics
Differences among groups were analyzed by Student’s t-test. Data are shown as means ± standard error (SE). Statistical significance was accepted when p < 0.05.
3. Results
3.1 Transgenic rat αA-crystallin rescues established models of zebrafish lens cataracts
For the purpose of assessing the role of αA-crystallin in lens development, we generated a transgenic zebrafish line that expresses rat αA-crystallin (Rno.Cryaa) under the control of the native zebrafish promoter. As detailed in the methods section, transgenic Rno.Cryaa expression in the lens is associated with the expression of cerulean fluorescence protein in the heart providing a convenient selection marker for transgenic fish. Embryos from the crosses between Tg(cryaa:Rno.Cryaa;myl7:Cerulean) and AB zebrafish were observed at 3–4dpf under a stereoscope equipped with a fluorescence filter for morphological and developmental characteristics. The transgenic progeny, characterized and selected based on Cerulean fluorescence in the heart (Fig. 1F), presented a normal global morphology with no detectable abnormalities in the lens.
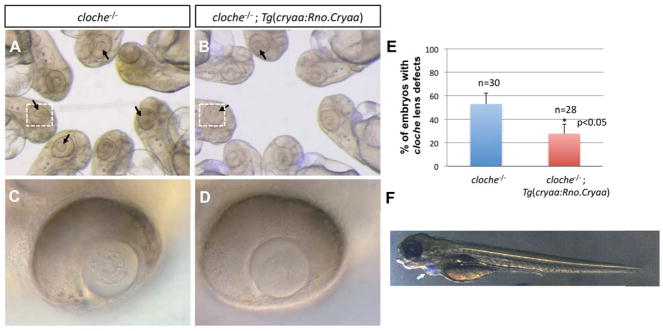
Figure 1. Suppression of cloche lens phenotypes by expression of Rno.Cryaa transgene.
Cloche mutants showed apparent lens defects (A) and transgenic expression of Tg(cryaa:Rno.Cryaa) rescued, at least partially, the lens defects in cloche mutants (B, E). Zoomed-in images (C, D) of the lenses in cloche−/− and cloche−/−; Tg(cryaa:Rno.Cryaa), denoted by white-dotted boxes (A, B). Embryos of lens-specific Rno.Cryaa transgene displayed Cerulean marker in the heart (F; Tg(cryaa:Rno.Cryaa,myl7:Cerulean), white arrow)
To test the functionality of the Rno.Cryaa transgene, we took advantage of cloche, a previously identified zebrafish mutant that develops embryonic lens opacity (Fig. 1A and 1C; Goishi et al., 2006). The cataract/lens opacity phenotype of this mutant is associated with reduced level of cryaa expression. Overexpression of synthetic cryaa mRNA suppressed lens defects in the cloche embryos (Goishi et al., 2006). Therefore, we set to determine if the Rno.Cryaa transgene could similarly suppress embryonic lens defects in the cloche embryos. Consistent with previous results, we found that the expression of Rno.Cryaa transgene effectively suppressed the lens defects in cloche−/− (Fig. 1B and 1D), and significantly reduced the frequency of embryos with the phenotype (Fig. 1E). This result establishes that Rno.Cryaa transgene is functional and must be robustly expressed in the lens. Moreover, this “functional complementation” suggests that the role of α-crystallin is conserved between rodents and zebrafish.
3.2 Knockdown of αA-crystallin compromises lens development and transparency in zebrafish
To interfere with the functions of α-crystallins in zebrafish embryos, synthetic morpholino antisense oligos (MOs) against α-crystallin genes, cryaa, cryaba and cryabb, were injected into one- or two-cell stage zygotes from the crosses between Tg(cryaa:Rno.Cryaa,myl7:Cerulean) and WT lines for the purpose of blocking the translation of α-crystallin mRNAs. Injected embryos were examined at 3dpf by fluorescence microscopy to detect Cerulean expression in the heart, and then sorted accordingly into two groups: a group carrying the Rno.Cryaa transgene (identified by the blue heart marker) and a non-transgenic group that served as a control. Embryos from both groups were further examined by bright field microscopy for abnormal lens morphology and changes in transparency. We observed lens defects spanning a spectrum of severity. Consequently, embryos were classified into three groups that displayed similar coarse levels of lens phenotype upon examination under bright field microscopy. Group 1 consisted of embryos that presented no visible lens defects. Group 2, referred to as the minor lens defect group, showed sporadic mostly spherical droplets in the lens. The third group of embryos was characterized by more frequent droplets or droplets covering a large fraction of the lens. Group 1 lenses were transparent and clear, displaying a homogenous smooth feature, sometimes with concentric layers representing immature fiber cells (Fig. 2A). On the contrary, defective lenses were far from homogenously clear, exhibiting phenotypic features that were either round shiny crystal-like droplet spreading across the lens (Fig. 2D) or big irregular protuberances located in the center of the lens (Fig. 2B–D), both of which could lead to opacity and changes in light scattering. Histological sections of WT and cryaa morphants’ lens at 4dpf were stained with hematoxylin and eosin (H&E). WT lens were evenly stained by eosin, and presented a uniform and transparent character (Fig. 2E), while cryaa morphants’ lens were unevenly stained with detectable small ‘particulates’ (Fig. 2F). The diameter of those lenses that were severely affected was 15–20% smaller than the wild-type ones at 3dpf.
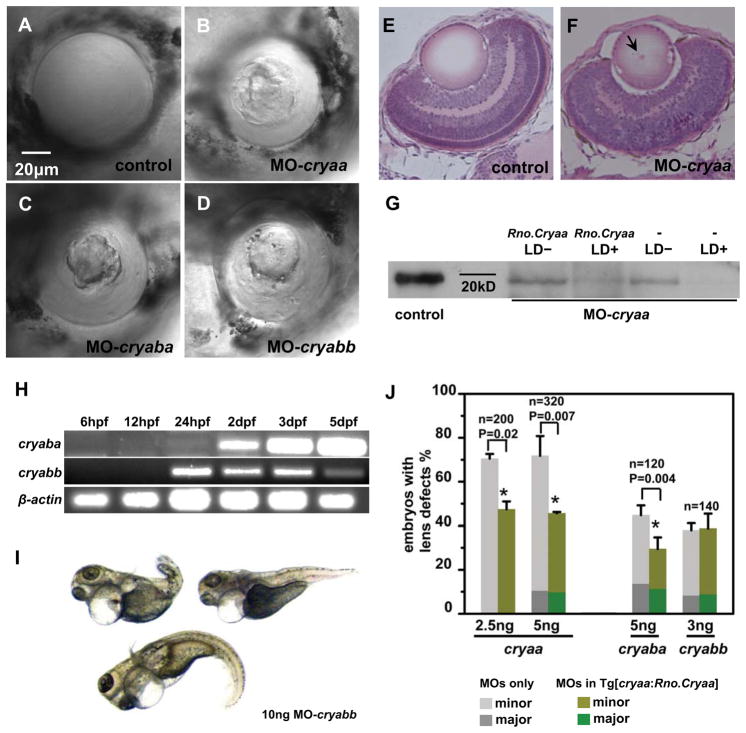
Figure 2. Transgenic expression of rat Cryaa rescues lens phenotypes induced by α-crystallin morpholino knockdown in 4dpf embryos.
DIC images from wild-type (WT) lens (A), MO-cryaa lens (B), MO-cryaba lens (C) and MO-cryabb lens (D) at 4dpf demonstrating the severe lens phenotype observed upon α-crystallin knockdown. The H&E staining on histological sections from WT lens (E) and MO-cryaa lens (F). The slightly immature retina in the MO-cryaa sample might be due to non-specific effects of MOs. Western blot (G) against zebrafish Cryaa protein suggested that MO-cryaa interfered the translation of cryaa gene. RT-PCR analysis (H) of cryaba and cryabb showed that zebrafish 3B-cystallins were expressed relatively early (starting from 24hpf). Significant numbers of MO-cryabb knockdown embryos (10ng) showed heart edema and tail abnormality (I). Statistic analyses of Rno.Cryaa-rescue effect on MO-cryaa, MO-cryaba and MO-cryabb (J).
To establish that the effects observed in the lens are not the results of a non-specific toxicity of MO injection, we compared two doses of MO-cryaa (2.5ng and 5ng) injection on the development and morphological appearance of the embryonic lens. At the lowest dose of 2.5ng, MO-cryaa induced lens abnormalities in 71% of the injected embryos of the non-transgenic group (Fig. 2J). Furthermore transgenic expression of Rno.Cryaa significantly (p=0.016) reduced the percentage of embryos with lens defects to 47% (Fig. 2J). At 5ng, MO-cryaa led to lens abnormalities in 72% of the non-transgenic group and 46% in the Rno.Cryaa transgenic group (p=0.007) (Fig. 2J). Survival ratio was more than 90% and no global morphological deformity was observed. The large percentage of embryos presenting lens defects and the reduction in the frequency of defects by transgenic expression of Rno.Cryaa established that αA-crystallin has a critical role in lens development.
We compared the observed lens defects to those observed as a result of the knockdown of zebrafish cryaba, which is also a small heat shock protein predominantly expressed in the zebrafish lens (Posner et al., 1999). Interference of cryaba expression leads to perturbation in lens morphology similar to those observed for knockdown of cryaa (Fig. 2C). These effects were observed at two doses of MOs in close to 50% of the embryos and were partially rescued by the expression of Rno.Cryaa (Fig. 2J; p=0.004). The survival ratios were 70% for 5ng injection. No other developmental defects were observed besides lens defects, even at the relatively high dose of 10ng (data not shown; 60% survival ratio).
Knockdown of zebrafish cryabb lead to a lens phenotype in a substantial fraction of embryos (Fig. 2D; 39%). In stark contrast, the transgenic expression of Rno.Cryaa did not rescue the phenotype (p=0.55 at 3ng). Moreover, we observed gross morphological defects, predominantly heart edema and shortened curved body length, in a substantial fraction of embryos (Fig. 2I; 20% at 10 ng injection). These results are consistent with the reported expression of cryabb in muscle tissues (Smith et al., 2006). They are also in agreement with mouse knockout mutants of αB-crystallin, which shows degenerations in some skeletal muscles or myopathy (Brady et al., 2001). However, we intepret this result with caution since non-specific effects of morpholinos cannot easily be ruled out (Schulte-Merker and Stainier, 2014) and this overall morphological abnormality in cryabb morphants were observed only when injected with high dose of MO-cryabb, even though the same high doses (10ng) of MO-cryaa and MO-cryaba did not elicit such developmental phenotypes.
We correlated the observation of abnormal lens phenotypes to the down-regulation of Cryaa expression by injection of MO-cryaa. For this purpose, western analysis of homogenized embryonic lenses at 3dpf was performed on four different groups of embryos that were injected with MOs: Rno.Cryaa transgenic groups with and without lens defects; non-transgenic group with and without lens defects. For this purpose, we used a polyclonal antibody raised against zebrafish αA-crystallins but is known to cross react with mammalian αA-crystallins (Vihtelic and Hyde, 2002). In the two groups of embryos without lens defects, the Cryaa protein was expressed whereas it was down-regulated and could barely be detected in the groups with lens defects (Fig. 2G).
Because there were conflicting reports regarding the developmental stages of cryaba expression in zebrafish embryos (Posner et al., 1999; Elicker and Hutson, 2007; Marvin et al., 2008), we carried out RT-PCR to investigate cryaba and cryabb expression at 6hpf, 12hpf, 24hpf, 2dpf, 3dpf and 5dpf (Fig. 2H). For cryaba, a faint band was observed at 24hpf that increased in intensity by 5dpf. The signal of cryabb appeared at 24hpf and the transcription level was fairly consistent at subsequent stages until 5dpf after normalization to the loading control, β-actin. RT-PCR data implies that both cryaba and cryabb are transcribed at least from 24hpf, an early stage of embryonic lens development.
3.3 Knockout of αA-crystallin severely affects zebrafish lens morphology and transparency
In light of the demonstration that cryaa knockdown can be linked to perturbation of lens morphology and transparency, we generated a cryaa knockout mutant line. The flow chart in Figure S1A describes how homozygous cryaa knockout mutant was established. Briefly, one-cell stage zebrafish embryos injected with RNAs encoding the cryaa TALE nuclease pairs (Cermak et al., 2011; Sander et al., 2011) were raised to adulthood as founders (F0), then adult F0 zebrafishes were out-crossed with AB line and their progenies were genotyped by using T7 endonuclease which recognizes mis-matches in DNA (Fig. S1B). Among the F1 progenies, an allele of 8bp fragment deletion, CCACTTCA, in cryaa exon 1, was confirmed by DNA sequencing. Genotyped cryaa heterozygotes (cryaa+/−) were then incrossed to generate homozygous cryaa knockout (cryaa−/−) F2 zebrafish. Adult F2 zebrafish were further genotyped by HphI restriction endonuclease which recognizes the sequence NNNNNNNTCACC. PCR products from WT allele containing CCAC could be digested by HphI into two 200bp fragments whereas PCR products from cryaa mutant allele in the absence of CCAC were resistant to HphI digestion (Fig. S1B). Because a stop codon is generated 60 amino acids into exon 1, we used mass spectrometry analysis of lenses from 3 months old cryaa−/− zebrafish to test and confirm the absence of Cryaa protein expression including any prematurely terminated peptides (data not shown).
The loss of αA-crystallin resulted in lens morphology and transparency defects in zebrafish embryos (Fig. 3). Whereas all embryos appeared morphologically normal (Fig. 3A and 3B), we detected defects in lens appearance that were similar to those observed in the knockdown experiments (Fig. 3C–F). The more severe defects were associated with significant increase in reflectance intensity by the lens (Fig. 3G–I) with relatively little change in the lens diameter.
We examined the genotypes and phenotypes of a set of 99 embryos obtained from an incross between cryaa heterozygotes (cryaa+/−). The embryos were classified into three different groups based on the severity of the observed lens phenotypes which were similar to those induced by MO knockdown (no, minor, or major lens defects). Following DIC imaging of the lens, each embryo was labeled by its lens phenotypic category and saved (n=99). Genomic DNA was extracted and used as templates for PCR, and the PCR products were genotyped using HphI as described above. Genotypes from incross of cryaa+/− segregated as follows (see Table 1): 24% were wild-type (cryaa+/+; 24/99), 26% homozygote (cryaa−/−; 26/99) and 50% heterozygote (cryaa+/−; 49/99), which is consistent with Mendelian inheritance of an allele (¼ homozygote mutant, ¼ homozygote wild-type and ½ heterozygote). Combined data from genotyping and phenotyping showed that 34% of cryaa+/− embryos had lens defects (17 out of 49 cryaa+/− embryos) and more than half of cryaa−/− embryos had lens defects at the stage of 3–4 dpf (15 out of 26 cryaa−/− embryo). This result strongly suggested that Cryaa exerts a gene dosage effect.
Table 1.
Gene dosage effect of zebrafish cryaa gene Phenotype and genotype analysis of cryaa+/− incross
| Phenotyping analysis (number of embryos) | Genotypes | ||
|---|---|---|---|
| cryaa+/+ | cryaa+/− | cryaa−/− | |
| WT-like | 24 | 32 | 11 |
| Lens defects | 0 | 17 | 15 |
| Total number of embryos for each genotype | 24 | 49 | 26 |
| Genotype distribuGons (expected 1:2:1) | 24% | 50% | 26% |
| Lens defects frequency | 0% | 35% | 58% |
By comparison, close to 92% of embryos from incross of cryaa−/− adults showed defects in at least one eye and a larger fraction of the defects were of the major category (53%). We screened close to 230 embryos and we didn’t observe evidence of morphological changes except in the lens (Fig. S2). The lens defects categorized as severe is associated with a substantial increase in reflectance intensity regardless of the embryo’s genotype (Fig. 3I) without a significant change in the lens diameter of the mutant relative to the WT (Fig. 3J).
3.4 Maternal expression of αA-crystallin plays a role in embryonic lens development
Figure 4 reveals a difference in the percentage of cryaa−/− embryos with lens abnormalities depending on the genotype of the parents. A larger percentage of progeny from cryaa homozygote parents have lens defects relative to the progeny from heterozygote parents (Fig. 4). Moreover, the occurrence of major lens defects is more frequent in the former. To determine the origin of the discrepancy in the penetrance of the phenotype, we carried RT-PCR on maternal expression of cryaa mRNA and found transcripts of cryaa in the heterozygote but not in homozygote embryos (Fig. 5). Because Cryaa is not degraded during fiber cell differentiation, its translated protein from maternal mRNA is presumably the protective factor accounting for the reduction in the percentage of homozygote embryos with lens abnormalities. Thus these results represent an intrinsic rescue experiment further demonstrating that the expression of Cryaa is required for normal lens development.
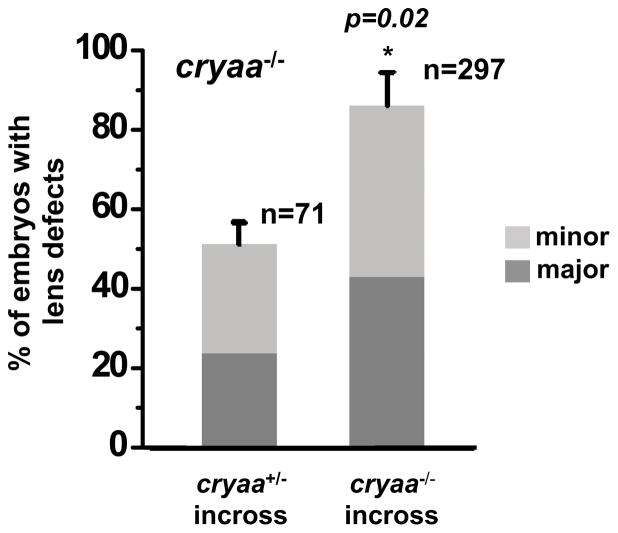
Figure 4. The percentage of cryaa mutant embryos with lens abnormalities depends on the genotype of the parents.
Progenies from the cryaa+/− and cryaa−/− incross showed lens defects, which were graded into three categories based on the severity of the abnormalities: WT-like, minor defects and major defects, in cryaa−/− embryos at 4dpf. In cryaa−/− embryos from cryaa+/− incross, 51% showed lens defects and 24% of which were major defects (left column). When compared to the cryaa−/− embryos from cryaa+/− incross, the resulting cryaa−/− embryos from cryaa−/− incross showed significantly higher percentage of embryos with lens defects (p=0.02), as well as ones with major defects, even though both were the same genotype (right column).
Figure 5. Maternal expression of zebrafish cryaa was detectable in early stage embryos.
Four sets of crossing pairs: WT female x WT male, WT female x cryaa+/− male, cryaa+/− female x cryaa−/− male, cryaa−/− female x cryaa−/− male. Twenty embryos from each cross were pooled together for RT-PCR at 0hpf, 1hpf, 3hpf, 6hpf, and 48hpf.
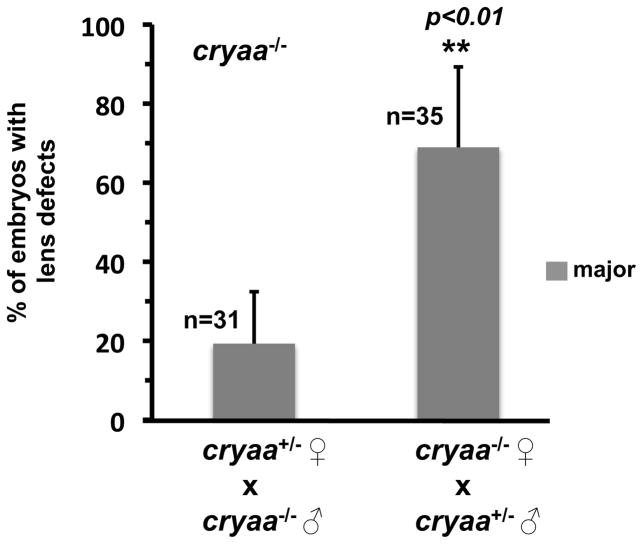
The result above predicts that the difference in the frequency of the phenotype will also depend on the genotype of the female parent. Therefore, we compared the frequency of lens phenotypes in embryos from homozygote and heterozygote females. We performed a reciprocal crossing scheme wherein we obtained embryos from cryaa+/− males X cryaa−/− females and cryaa−/− males X cryaa+/− females (Fig. 6), which would directly test our hypothesis that maternal expression of cryaa plays an essential role in lens development. In all genotyped cryaa−/− embryos, we observed a significantly higher proportion of embryos showing major lens defects produced from the homozygote females (~69%) when compared to heterozygote females (~19%), which strongly supports the conclusion that maternal cryaa contributes to embryonic lens development in the zebrafish.
Figure 6. Maternal expression of cryaa plays a role in embryonic lens development.
A significantly higher proportion of cryaa−/− embryos showed major lens defects produced from the homozygote females (~69%) when compared to those from heterozygote females (~19%), which were crossed to heterozygote and homozygote males, respectively.
3.5 Transgenic expression of rat αA-crystallin rescues the lens phenotypes of cryaa heterozygote embryos
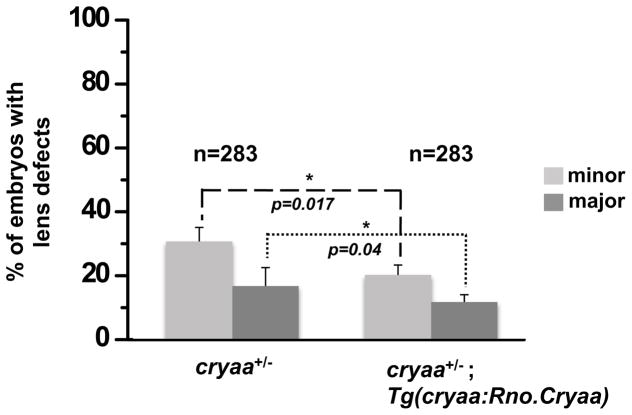
To further confirm that expression of αA-crystallin can rescue the lens phenotypes, we crossed cryaa−/− (either male or female) with Tg(cryaa:Rno.Cryaa,myl7:Cerulean) to generate embryos heterozygous for endogenous cryaa while expressing exogenous Rno.Cryaa (cryaa+/−; Tg(cryaa:Rno.Cryaa)). The embryos were screened for lens defects and separated into three groups based on the severity of lens perturbation as described above. Then each group was subdivided into the transgenic or the non-transgenic groups for Rno.Cryaa, based on Cerulean expression in the heart. We found that embryos with the Rno.Cryaa transgene have lower incidence of lens defects in both minor and major categories (Fig. 7). Because all three groups of embryos are expected to have a uniform genotype with one copy of endogenous cryaa gene, these experiments again demonstrate a critical role of cryaa in lens development that appears to be conserved across species.
Figure 7. Transgenic expression of exogenous Cryaa suppresses the lens defects of cryaa+/− embryos.
Adult cryaa−/− zebrafish was crossed with Tg(cryaa:Rno.Cryaa) zebrafish line. The lens of resulting embryos (cryaa+/−) were examined and classified into three categories as previously mentioned. The proportion of cryaa+/− embryos showing lens defects was significantly lower with the presence of Rno.Cryaa transgene.
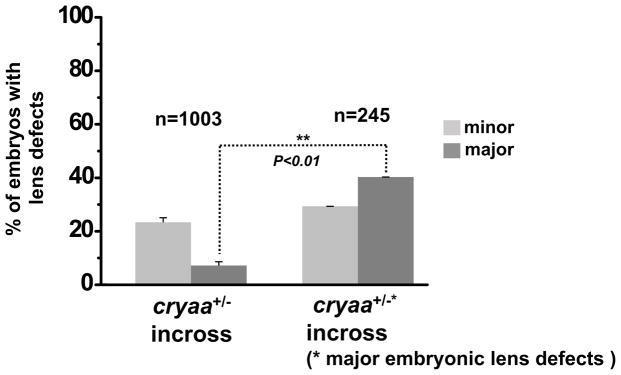
To determine if the differences in the severity of the phenotype reflect modifying genetic factors, we selected embryos that presented severe/major lens defects and raised them to adulthood. Following genotyping, we crossed a pair of cryaa+/− adults and the progeny from this incross was screened for lens defect phenotypes. We found that the embryos exhibited a higher fraction of lens defects, particularly in the category of major lens defect, than the progeny of a randomly selected pair with identical genotype (Fig. 8). More than 60% of embryos at 3–5 dpf presented lens defects, and more than half turned to be the major category. This suggested that the variation in the lens phenotype represents modifying genetic factors.
Figure 8. The influence of genetic modifier(s) on lens phenotype severity.
The embryos from a pair of cryaa+/− adults that presented major embryonic lens defects exhibited a higher fraction of lens defects (right column) than those from a randomly selected pair with the same genotype, cryaa+/− (left column).
4. Discussion
Mouse models were instrumental in pinpointing the contribution of α-crystallins to lens transparency. αA-crystallin null mice progressively develop lens opacity (Brady et al., 1997) and double knockout of α-crystallins (Cryaa−/−; Cryab−/−) is associated with abnormal differentiation of lens fiber cells (Boyle et al., 2003). α-crystallins are involved in an array of physiological roles (Andley, 2007) which include the successful completion of mitosis and cytokinesis, maintenance of genomic stability (Andley et al., 2001), remodeling and protection of the cytoskeleton (Xi et al., 2006), inhibition of apoptosis (Andley et al., 2000), and enhancement of cell resistance to stress (Andley et al., 1998). Thus, it appears that to sustain lens function, not only protein solubility in the fiber cells needs to be maintained, but also the integrity of the epithelial cells (Andley et al., 1998; Andley et al., 2000) and the cortical region.
Despite the many biochemical and biophysical similarities to the mammalian lens, the lower levels of α-crystallins in the zebrafish lens and the higher overall protein concentration brought into question the extension of the conclusions regarding the role of α-crystallins in lens development and transparency to zebrafish. The most compelling evidence supporting a role of αA-crystallin in lens development was described for the zebrafish mutant, cloche (Goishi et al., 2006). In this mutant, αA-crystallin levels are reduced and the embryos have an apparent cataract phenotype. Overexpression of αA-crystallin, achieved by mRNA injection, rescued the phenotype. Posner et al. challenged this conclusion on the basis of morpholino injections that failed to produce visible lens abnormalities (Posner et al., 2013). However, the preliminary nature of their study, manifested by the limited number of embryos screened, calls into question their conclusions.
Our results establish a critical role of αA-crystallin in embryonic lens development and transparency of zebrafish; a finding that reflects the conserved role of this protein in vertebrates. Both knockdown and knockout of this protein led to disruption of lens development with the latter manipulation leading to an almost complete penetrant phenotype. Interfering with expression through morpholino injection led to less penetrant and less severe but nonetheless statistically consistent observation of lens abnormalities. We found that the lack of phenotype in a fraction of the embryos reflect the ineffective interference of MO with αA-crystallin expression. This is not unexpected in morpholino studies and emphasizes the need for genetic manipulation to conclusively assess the role of α-crystallins in lens development. Interestingly, heterozygous cryaa mutant embryos also develop lens abnormalities, although to a lower extent than homozygous mutants, which suggests a gene dosage effect exerted by αA-crystallin (Table 1). This may reflect the overall lower expression of cryaa and higher protein concentration in zebrafish lens compared to that in mammalian lens. Interestingly, a well-documented gene dosage effect has been demonstrated in Pax6 gene during eye development (Glaser et al., 1994; see review in Cvekl et al., 2004), and one of its direct transcriptional targets is cryaa gene (Yang and Cvekl 2005).
Central to our interpretation is the consistent observation of rescue of the lens phenotype by αA-crystallin expression. The coincidental finding that the progeny of cryaa−/− parents (particularly the mother) has more penetrant and severe phenotype than those embryos of identical genotype but from cryaa+/− parents conclusively demonstrates that the expression of inherited maternal αA-crystallin provides a degree of protection against lens abnormalities. Remarkably, phenotype suppression does not depend on species origin of αA-crystallin suggesting that the role of αA-crystallin in lens development is evolutionarily conserved. While speculative at this point, we suggest that this role must be associated with the participation of αA-crystallin in the conserved chaperone machinery that maintains protein homeostasis.
Morpholino experiments provide supporting evidence of an important role of αB-crystallin in the development and transparency of the lens. Genome duplication in zebrafish resulted in two αB-crystallin genes, cryaba and cryabb, and the evidence so far suggests that the cryaba expression is lens-specific. Consistent with this notion, interference with αBa-crystallin expression was associated with abnormalities exclusively in the lens. In contrast, we found that knockdown of cryabb gene, which is also expressed outside the lens, can result in a severe multi-organ developmental phenotype, albeit unresolved concerns for non-specific effects of morpholino injections confound this interpretation. Thus although preliminary, we propose that these two paralogs play certain roles for the development of the embryonic lens.
Together, our results set the stage for using zebrafish as a model system to delineate the cellular roles and reveal the molecular interactions of α-crystallins. Zebrafish possesses several experimental advantages such as faster turnaround for generating transgenic animals, the rapid screening of phenotype and amenability for high throughout screens of small molecules, may prove critical in understanding lens opacity and developing strategies to delay cataract formation.
Supplementary Material
The flow chart described the steps to generate cryaa knockout mutants by TALEN (A). Genotyping of cryaa TALEN mutant allele was performed by PCR and followed with restriction enzyme digestions. The first exon of cryaa gene is shown in red; PCR primers are underlined. The 8bp deletion of cryaa mutant allele is highlighted in gray box; Green box highlights the recognition site of HphI enzyme. The loss of HphI enzyme site offered a reliable genotyping method to distinguish WT and mutant alleles of cryaa gene.
Progenies from cryaa−/− incross were examined for one- or two-lens defects. 13.5% embryos presented only one-lens defects and 86.5% embryos presented two-lens defects.
Highlights.
We generated and characterized the first zebrafish knockout mutant for αA-crystallin
Loss of αA-crystallin severely affects zebrafish embryonic lens morphology
Maternal expression of αA-crystallin plays a role in early zebrafish lens development.
The role of αA-crystallin in lens development is conserved in vertebrates
Our results demonstrate a relevant usage of zebrafish lens to study α-crystallin functions in vivo.
Acknowledgments
The authors thank Drs. Zhen Wang and Kevin Schey at the VUMC Mass Spectrometry Research Center for the helps on proteomic analyses and Dr. Derek Claxton for critical reading of the manuscript. Confocal microscopy was performed in part through the use of the VUMC Cell Imaging Shared Resource (supported by NIH grants CA68485, DK20593, DK58404, DK59637 and EY08126). This work was supported by NIH grants, R01 EY12018 and P30 EY008126 (to HM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andley UP. Crystallins in the eye: Function and pathology. Progress in Retinal & Eye Researcmh. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Bassnett S. The molecular chaperone aA-crystallin enhances lens epithelial cell growth and resistance to UVA stress. Journal of Biological Chemistry. 1998;273:31252–31261. doi: 10.1074/jbc.273.47.31252. [DOI] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Brady JP, Bassnett S, Fleming TP. Lens epithelial cells derived from aB-crystallin knockout mice demonstrate hyperproliferation and genomic instability. FASEB Journal. 2001;15:221–229. doi: 10.1096/fj.00-0296com. [DOI] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Fleming TP, Bassnett S. Differential protective activity of aA- and aB-crystallin in lens epithelial cells. Journal of Biological Chemistry. 2000;275:36823–36831. doi: 10.1074/jbc.M004233200. [DOI] [PubMed] [Google Scholar]
- Bassnett S. Fiber cell denucleation in the primate lens. Investigative Ophthalmology & Visual Science. 1997;38:1678–1687. [PubMed] [Google Scholar]
- Beebe DC, Vasiliev O, Guo J, Shui YB, Bassnett S. Changes in adhesion complexes define stages in the differentiation of lens fiber cells. Investigative Ophthalmology & Visual Science. 2001;42:727–734. [PubMed] [Google Scholar]
- Benedek GB. Theory of transparency of the eyes. Appl Opt. 1971;10:459. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- Benedek GB. Cataract as a protein condensation disease: the Proctor Lecture. Investigative Ophthalmology & Visual Science. 1997;38:1911–1921. [PubMed] [Google Scholar]
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Progress in Biophysics & Molecular Biology. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Boyle DL, Takemoto L, Brady JP, Wawrousek EF. Morphological characterization of the Alpha A- and Alpha B-crystallin double knockout mouse lens. BMC Ophthalmology. 2003;3:3. doi: 10.1186/1471-2415-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland D, Duglas-Tabor Y, Robison WG, Jr, Groome A, Wawrousek EF. Targeted disruption of the mouse aA-crystallin gene induces cataract and cytoplasmic inclusion bodies containing the small heat shock protein aB-crystallin. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:884–889. doi: 10.1073/pnas.94.3.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Investigative ophthalmology & visual science. 2001;42:2924–2934. [PubMed] [Google Scholar]
- Cermak T, Doyle EL, Christian M, Wang L, Zhang Y, Schmidt C, Baller JA, Somia NV, Bogdanove AJ, Voytas DF. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic acids research. 2011;39:e82. doi: 10.1093/nar/gkr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JI, Muchowski PJ. Small heat-shock proteins and their potential role in human disease. Current Opinion in Structural Biology. 2000;10:52–59. doi: 10.1016/s0959-440x(99)00048-2. [DOI] [PubMed] [Google Scholar]
- Claxton DP, Zou P, McHaourab HS. Structure and orientation of T4 lysozyme bound to the small heat shock protein alpha-crystallin. Journal of Molecular Biology. 2008;375:1026–1039. doi: 10.1016/j.jmb.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvekl A, Yang Y, Chauhan BK, Cveklova K. Regulation of gene expression by Pax6 in ocular cells: a case of tissue-preferred expression of crystallins in lens. The International journal of developmental biology. 2004;48:829–844. doi: 10.1387/ijdb.041866ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlman JM, Margot KL, Ding L, Horwitz J, Posner M. Zebrafish alpha-crystallins: protein structure and chaperone-like activity compared to their mammalian orthologs. Molecular Vision. 2005;11:88–96. [PubMed] [Google Scholar]
- Dahm R, Schonthaler HB, Soehn AS, van Marle J, Vrensen GF. Development and adult morphology of the eye lens in the zebrafish. Experimental eye research. 2007;85:74–89. doi: 10.1016/j.exer.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Elicker KS, Hutson LD. Genome-wide analysis and expression profiling of the small heat shock proteins in zebrafish. Gene. 2007;403:60–69. doi: 10.1016/j.gene.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadool JM, Brockerhoff SE, Hyatt GA, Dowling JE. Mutations affecting eye morphology in the developing zebrafish (Danio rerio) Developmental Genetics. 1997;20:288–295. doi: 10.1002/(SICI)1520-6408(1997)20:3<288::AID-DVG11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Fadool JM, Dowling JE. Zebrafish: a model system for the study of eye genetics. Progress in Retinal & Eye Research. 2008;27:89–110. doi: 10.1016/j.preteyeres.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Jepeal L, Edwards JG, Young SR, Favor J, Maas RL. PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nature genetics. 1994;7:463–471. doi: 10.1038/ng0894-463. [DOI] [PubMed] [Google Scholar]
- Goishi K, Shimizu A, Najarro G, Watanabe S, Rogers R, Zon LI, Klagsbrun M. AlphaA-crystallin expression prevents gamma-crystallin insolubility and cataract formation in the zebrafish cloche mutant lens. Development. 2006;133:2585–2593. doi: 10.1242/dev.02424. [DOI] [PubMed] [Google Scholar]
- Greiling TM, Clark JI. Early lens development in the zebrafish: a three-dimensional time-lapse analysis. Developmental Dynamics. 2009;238:2254–2265. doi: 10.1002/dvdy.21997. [DOI] [PubMed] [Google Scholar]
- Greiling TM, Houck SA, Clark JI. The zebrafish lens proteome during development and aging. Molecular Vision. 2009;15:2313–2325. [PMC free article] [PubMed] [Google Scholar]
- Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Experimental eye research. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- Hanson SR, Smith DL, Smith JB. Deamidation and disulfide bonding in human lens gamma-crystallins. Experimental eye research. 1998;67:301–312. doi: 10.1006/exer.1998.0530. [DOI] [PubMed] [Google Scholar]
- Horwitz J. a-crystallin can function as a molecular chaperone. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Experimental Eye Research. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Jao LE, Wente SR, Chen W. Efficient multiplex biallelic zebrafish genome editing using a CRISPR nuclease system. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:13904–13909. doi: 10.1073/pnas.1308335110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koteiche HA, McHaourab HS. Mechanism of chaperone function in small heat-shock proteins. Phosphorylation-induced activation of two-mode binding in alphaB-crystallin. Journal of Biological Chemistry. 2003;278:10361–10367. doi: 10.1074/jbc.M211851200. [DOI] [PubMed] [Google Scholar]
- Koteiche HA, McHaourab HS. Mechanism of a hereditary cataract phenotype. Mutations in alphaA-crystallin activate substrate binding. J Biol Chem. 2006;281:14273–14279. doi: 10.1074/jbc.M512938200. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Koteiche HA, Claxton DP, McHaourab HS. Disulfide cross-links in the interaction of a cataract-linked alphaA-crystallin mutant with betaB1-crystallin. FEBS Lett. 2009;583:175–179. doi: 10.1016/j.febslet.2008.11.047. [DOI] [PubMed] [Google Scholar]
- Kurita R, Sagara H, Aoki Y, Link BA, Arai K, Watanabe S. Suppression of lens growth by alphaA-crystallin promoter-driven expression of diphtheria toxin results in disruption of retinal cell organization in zebrafish. Developmental biology. 2003;255:113–127. doi: 10.1016/s0012-1606(02)00079-9. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Amyx KK, Ahmann P, Steel EA. Deamidation in human lens betaB2-crystallin destabilizes the dimer. Biochemistry. 2006;45:3146–3153. doi: 10.1021/bi052051k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampi KJ, Ma Z, Hanson SR, Azuma M, Shih M, Shearer TR, Smith DL, Smith JB, David LL. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Experimental eye research. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Shih M, Ueda Y, Shearer TR, David LL. Lens proteomics: analysis of rat crystallin sequences and two-dimensional electrophoresis map. Investigative Ophthalmology & Visual Science. 2002;43:216–224. [PubMed] [Google Scholar]
- Liu H, Du X, Wang M, Huang Q, Ding L, McDonald HW, Yates JR, 3rd, Beutler B, Horwitz J, Gong X. Crystallin {gamma}B-I4F mutant protein binds to {alpha}-crystallin and affects lens transparency. Journal of Biological Chemistry. 2005;280:25071–25078. doi: 10.1074/jbc.M502490200. [DOI] [PubMed] [Google Scholar]
- Marvin M, O’Rourke D, Kurihara T, Juliano CE, Harrison KL, Hutson LD. Developmental expression patterns of the zebrafish small heat shock proteins. Developmental dynamics. 2008;237:454–463. doi: 10.1002/dvdy.21414. [DOI] [PubMed] [Google Scholar]
- Mchaourab HS, Dodson EK, Koteiche HA. Mechanism of Chaperone Function in Small Heat-Shock Proteins.Two-Mode Binding of the Excited States of T4 Lysozyme Mutants by aA-Crystallin. Journal of Biological Chemistry. 2002;277:40557–40566. doi: 10.1074/jbc.M206250200. [DOI] [PubMed] [Google Scholar]
- McHaourab HS, Kumar MS, Koteiche HA. Specificity of alphaA-crystallin binding to destabilized mutants of betaB1-crystallin. FEBS Lett. 2007;581:1939–1943. doi: 10.1016/j.febslet.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHaourab HS, Godar JA, Stewart PL. Structure and mechanism of protein stability sensors: chaperone activity of small heat shock proteins. Biochemistry. 2009;48:3828–3837. doi: 10.1021/bi900212j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville DB, Montero-Balaguer M, Levic DS, Bradley K, Smith JR, Hatzopoulos AK, Knapik EW. The feelgood mutation in zebrafish dysregulates COPII-dependent secretion of select extracellular matrix proteins in skeletal morphogenesis. Disease models & mechanisms. 2011;4:763–776. doi: 10.1242/dmm.007625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Stein RA, McHaourab HS. Cataract-linked gammaD-crystallin mutants have weak affinity to lens chaperones alpha-crystallins. FEBS Letters. 2012;586:330–336. doi: 10.1016/j.febslet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner M, Kantorow M, Horwitz J. Cloning, sequencing and differential expression of alphaB-crystallin in the zebrafish, Danio rerio. Biochimica et biophysica acta. 1999;1447:271–277. doi: 10.1016/s0167-4781(99)00155-4. [DOI] [PubMed] [Google Scholar]
- Posner M, Hawke M, Lacava C, Prince CJ, Bellanco NR, Corbin RW. A proteome map of the zebrafish (Danio rerio) lens reveals similarities between zebrafish and mammalian crystallin expression. Molecular Vision. 2008;14:806–814. [PMC free article] [PubMed] [Google Scholar]
- Posner M, Skiba J, Brown M, Liang JO, Nussbaum J, Prior H. Loss of the small heat shock protein alphaA-crystallin does not lead to detectable defects in early zebrafish lens development. Experimental eye research. 2013;116:227–233. doi: 10.1016/j.exer.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GB, Das KP, Petrash JM, Surewicz WK. Temperature-dependent chaperone activity and structural properties of human alphaA- and alphaB-crystallins. Journal of Biological Chemistry. 2000;275:4565–4570. doi: 10.1074/jbc.275.7.4565. [DOI] [PubMed] [Google Scholar]
- Reyon D, Tsai SQ, Khayter C, Foden JA, Sander JD, Joung JK. FLASH assembly of TALENs for high-throughput genome editing. Nature biotechnology. 2012;30:460–465. doi: 10.1038/nbt.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runkle S, Hill J, Kantorow M, Horwitz J, Posner M. Sequence and spatial expression of zebrafish (Danio rerio) alphaA-crystallin. Molecular Vision. 2002;8:45–50. [PMC free article] [PubMed] [Google Scholar]
- Sander JD, Cade L, Khayter C, Reyon D, Peterson RT, Joung JK, Yeh JR. Targeted gene disruption in somatic zebrafish cells using engineered TALENs. Nature biotechnology. 2011;29:697–698. doi: 10.1038/nbt.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish HA, Stein RA, Yang G, McHaourab HS. Mechanism of chaperone function in small heat-shock proteins. Fluorescence studies of the conformations of T4 lysozyme bound to alphaB-crystallin. J Biol Chem. 2003;278:44214–44221. doi: 10.1074/jbc.M307578200. [DOI] [PubMed] [Google Scholar]
- Sathish HA, Koteiche HA, McHaourab HS. Binding of destabilized betaB2-crystallin mutants to alpha-crystallin: the role of a folding intermediate. J Biol Chem. 2004;279:16425–16432. doi: 10.1074/jbc.M313402200. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Stainier DY. Out with the old, in with the new: reassessing morpholino knockdowns in light of genome editing technology. Development. 2014;141:3103–3104. doi: 10.1242/dev.112003. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Kaur H, Kester K. Functional elements in molecular chaperone a-crystallin: identification of binding sites in a B-crystallin. Biochemical & Biophysical Research Communications. 1997;239:217–222. doi: 10.1006/bbrc.1997.7460. [DOI] [PubMed] [Google Scholar]
- Sharma KK, Santhoshkumar P. Lens aging: effects of crystallins. Biochimica et Biophysica Acta. 2009;1790:1095–1108. doi: 10.1016/j.bbagen.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AA, Wyatt K, Vacha J, Vihtelic TS, Zigler JS, Jr, Wistow GJ, Posner M. Gene duplication and separation of functions in alphaB-crystallin from zebrafish (Danio rerio) The FEBS journal. 2006;273:481–490. doi: 10.1111/j.1742-4658.2005.05080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soules KA, Link BA. Morphogenesis of the anterior segment in the zebrafish eye. BMC Developmental Biology. 2005;5:12. doi: 10.1186/1471-213X-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan-Brown J, Bisher ME, Burdine RD. Embedding, serial sectioning and staining of zebrafish embryos using JB-4 resin. Nature protocols. 2011;6:46–55. doi: 10.1038/nprot.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu A. Eye lens proteins and transparency: from light transmission theory to solution X-ray structural analysis. Annual Review of Biophysics & Biophysical Chemistry. 1988;17:47–70. doi: 10.1146/annurev.bb.17.060188.000403. [DOI] [PubMed] [Google Scholar]
- Truscott RJW. Age-related nuclear cataract-oxidation is the key. Experimental eye research. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- van den Berg B, Ellis RJ, Dobson CM. Effects of macromolecular crowding on protein folding and aggregation. EMBO Journal. 1999;18:6927–6933. doi: 10.1093/emboj/18.24.6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg B, Wain R, Dobson CM, Ellis RJ. Macromolecular crowding perturbs protein refolding kinetics: implications for folding inside the cell. EMBO Journal. 2000;19:3870–3875. doi: 10.1093/emboj/19.15.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vihtelic TS, Hyde DR. Zebrafish mutagenesis yields eye morphological mutants with retinal and lens defects. Vision Research. 2002;42:535–540. doi: 10.1016/s0042-6989(01)00261-9. [DOI] [PubMed] [Google Scholar]
- Wang K, Cheng C, Li L, Liu H, Huang Q, Xia CH, Yao K, Sun P, Horwitz J, Gong X. GammaD-crystallin associated protein aggregation and lens fiber cell denucleation. Investigative Ophthalmology & Visual Science. 2007;48:3719–3728. doi: 10.1167/iovs.06-1487. [DOI] [PubMed] [Google Scholar]
- Xi JH, Bai F, McGaha R, Andley UP. Alpha-crystallin expression affects microtubule assembly and prevents their aggregation. FASEB Journal. 2006;20:846–857. doi: 10.1096/fj.05-5532com. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cvekl A. Tissue-specific regulation of the mouse alphaA-crystallin gene in lens via recruitment of Pax6 and c-Maf to its promoter. Journal of molecular biology. 2005;351:453–469. doi: 10.1016/j.jmb.2005.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The flow chart described the steps to generate cryaa knockout mutants by TALEN (A). Genotyping of cryaa TALEN mutant allele was performed by PCR and followed with restriction enzyme digestions. The first exon of cryaa gene is shown in red; PCR primers are underlined. The 8bp deletion of cryaa mutant allele is highlighted in gray box; Green box highlights the recognition site of HphI enzyme. The loss of HphI enzyme site offered a reliable genotyping method to distinguish WT and mutant alleles of cryaa gene.
Progenies from cryaa−/− incross were examined for one- or two-lens defects. 13.5% embryos presented only one-lens defects and 86.5% embryos presented two-lens defects.