Abstract
While the pace of discovery of human genetic variants in tumors, patients, and diverse populations has rapidly accelerated, deciphering their functional consequence has become rate-limiting. Using cross-species complementation, model organisms like the budding yeast, Saccharomyces cerevisiae, can be utilized to fill this gap and serve as a platform for testing human genetic variants. To this end, we performed two parallel screens, a one-to-one complementation screen for essential yeast genes implicated in chromosome instability and a pool-to-pool screen that queried all possible essential yeast genes for rescue of lethality by all possible human homologs. Our work identified 65 human cDNAs that can replace the null allele of essential yeast genes, including the nonorthologous pair yRFT1/hSEC61A1. We chose four human cDNAs (hLIG1, hSSRP1, hPPP1CA, and hPPP1CC) for which their yeast gene counterparts function in chromosome stability and assayed in yeast 35 tumor-specific missense mutations for growth defects and sensitivity to DNA-damaging agents. This resulted in a set of human–yeast gene complementation pairs that allow human genetic variants to be readily characterized in yeast, and a prioritized list of somatic mutations that could contribute to chromosome instability in human tumors. These data establish the utility of this cross-species experimental approach.
Keywords: human–yeast cross-species complementation, chromosome instability, tumor-specific missense mutations, human genetic variants, model organisms and human disease
DIFFERENTIATING passenger from driver mutations in tumor cell genomes and linking somatic variants to cancer phenotypes are major challenges. A hallmark of cancer is chromosome instability (CIN), which is an important predisposing factor in the progression and heterogeneity of tumors because it increases the likelihood of loss of tumor-suppressor genes and mutation/amplification/rearrangement of oncogenes (Lengauer et al. 1997; Charames and Bapat 2003; Schvartzman et al. 2010; Hanahan and Weinberg 2011). The budding yeast, Saccharomyces cerevisiae, has been used to define cellular pathways and catalog a comprehensive list of yeast genes required for the maintenance of chromosome stability (Yuen et al. 2007; Stirling et al. 2011). The yeast CIN gene list facilitates identification of candidate human CIN genes whose somatic variants may contribute to chromosome instability and tumorigenesis (Barber et al. 2008). Budding yeast can be exploited to screen these human genetic variants for prioritizing and directing functional studies in mammalian models (reviewed in Dunham and Fowler 2013).
There are several approaches for studying human genetic variants in yeast. Based on sequence conservation, human variants can be tested by introducing homologous mutations in the yeast ortholog. However, such sequence-based efforts restrict the number of variants that can be studied, and inferences based on this approach can be misleading as function can be maintained despite sequence divergence. For example, the impact of a nonsynonymous substitution in the context of the yeast protein may not accurately predict the same effect in the context of the human protein (Marini et al. 2010). A more desirable approach is functionally testing variants directly in the context of the human protein sequence by utilizing cross-species complementation to “humanize” the yeast strain. This refers to the ability of a human gene (or set of genes) to complement the loss-of-function phenotype of its yeast ortholog (or set of orthologs). Cross-species complementation has previously been used in yeast to isolate human orthologs, elucidate human gene function, and characterize gene variants (reviewed in Osborn and Miller 2007; Dunham and Fowler 2013). Expression of human cDNAs in wild-type yeast cells has also been used as means to study genetic variants and characterize protein domains (Kato et al. 2003; Tarnowski et al. 2012).
While cross-species complementation can be scored by any measurable phenotype, the most straight-forward phenotype to assay and quantify is the rescue of growth defects. Complementation of essential yeast genes by candidate human gene orthologs can be tested by the ability of a human cDNA to rescue lethality caused by (i) a null allele (deletion in a haploid strain), (ii) a conditional allele under restrictive conditions (e.g., temperature-sensitive strain), or (iii) downregulation by a repressible promoter (e.g., Tet system) (Kachroo et al. 2015). Nonessential yeast gene mutants can be assayed when they present a phenotype (e.g., drug sensitivity) or the nonessential yeast gene can be converted to an essential gene by disrupting a synthetic lethal partner (Greene et al. 1999). Hundreds of studies testing individual genes have revealed >200 successful human–yeast complementation pairs (Dunham and Fowler 2013). Two studies systematically tested for human–yeast complementation pairs. One screened for rescue of lethality caused by inducible loss-of-function of 25 essential yeast genes (repressible promoter) following transformation of a human cDNA library (i.e., pool-to-one screens) and identified six essential genes that were rescued by a human ortholog (Zhang et al. 2003). More recently, 176/414 essential yeast genes (as null and/or Ts mutations and/or using a repressible promoter) were found to be replaceable by their 1:1 human ortholog (i.e., one-to-one screens) (Kachroo et al. 2015).
We report the screening of 621 essential yeast gene null mutants for complementation by all potential human homologs in two parallel screens (“one-to-one” screens for 199 essential CIN genes corresponding to 322 candidate complementation pairs, and a “pool-to-pool” screen for all possible essential yeast genes). In addition, we demonstrate the feasibility of using yeast to test human gene variants by extending cross-species complementation to 35 tumor-specific mutations in genes functioning in chromosome stability.
Materials and Methods
Generating expression vectors for the complementation screens
One-to-one screen:
Human cDNAs in Gateway-compatible entry clones were obtained from hORFeome V8.1 (Yang et al. 2011) and shuttled into yeast destination vectors (Alberti et al. 2007) using LR clonase II (Invitrogen) to generate expression clones. The destination vector used was pAG416GPD-ccdB-HA (URA3, CEN, constitutive GPD promoter, C-terminal HA tag) with a stop codon contributed by the vector backbone resulting in a 55-amino-acid C-terminal extension. The identity of the human cDNA was confirmed by sequencing the expression vector using a common primer that hybridizes to the vector backbone (CAGGAAACAGCTATGAC).
Pool-to-pool screen:
The same experimental outline was followed as the one-to-one screen with the following modifications and additional steps. Human cDNAs were randomly grouped into 13 pools with each pool comprising up to 96 unique entry clones. Each of the 13 pools of entry clones were shuttled en masse into a yeast destination vector to generate 13 pools of expression vectors (Arnoldo et al. 2014). The destination vector used was pAG416GPD-ccdB (URA3, CEN, constitutive GPD promoter) with a stop codon contributed by the vector backbone resulting in a 50-amino-acid C-terminal extension. To ensure sufficient coverage of each expression vector within a pool, the bulk LR reaction was repeated three times to obtain a minimum of 10,000 transformants for 100-fold coverage (∼96 entry clones × 100).
Yeast strains and media used in complementation screens
One-to-one screen:
Generated expression vectors were transformed into the corresponding haploid-convertible heterozygous diploid knockout yeast strain (Pan et al. 2004) and transformants were selected on −Ura media. Transformants were then inoculated in liquid sporulation media (1% w/v potassium acetate, 0.005% w/v zinc acetate, and 0.3 mM histidine) (Pan et al. 2007) to a cell density of ∼1–2 OD600 at 25° with shaking for 5 days and sporulation efficiency was assessed using microscopy. Following sporulation, 50 µl of cells were resuspended in 1 ml water of which 100 µl was plated on the haploid selection media MM −Ura (−Leu −His −Arg −Ura +canavanine +G418) (Pan et al. 2007) and incubated at 30°. To confirm that the generated yeast essential haploid knockout is dependent on the expression vector, cells were then replica plated on MM +5-FOA (0.1%) (−Leu −His −Arg +canavanine +G418 +5-FOA) and incubated at 30°.
Pool-to-pool screen:
The same experimental outline was followed as the one-to-one screen except with the following modifications. Heterozygous yeast strains were pinned in a 96-well array format on YPD +G418 (200 µg/ml) agar plates and incubated at 30°. Colonies were then scraped and pooled (by suspension in 1 ml YPD), and inoculated in 250 ml YPD and allowed to grow for only two generations to prevent competitive outgrowth before proceeding with the transformation (Pan et al. 2007) for protocol on high-efficiency transformation). The 250 ml yeast culture was then divided into 13 equal aliquots into which 13 pools of expression vector DNAs were transformed. Creating pools of transforming DNA and cells to be transformed was done to ensure equal representation of yeast strains across all pools. To ensure sufficient coverage of each yeast strain/vector combination in sufficient numbers, the transformation was repeated a second time for each pool to obtain a minimum of 6 million transformants for 100-fold coverage (621 yeast strains × ∼96 expression vectors × 100). Transformed diploid colonies were then scraped, pooled, and inoculated in 13 separate 50 ml sporulation cultures as described. Sporulated cultures were then pelleted, resuspended in water, and plated on MM −Ura for haploid selection, after which the haploid-converted cells were scraped, pooled, and re-plated again on MM −Ura to obtain single colonies, which were confirmed by replica plating to MM +5-FOA. For each pool, ∼20–50 5-FOA-sensitive colonies were isolated. To determine the identity of the yeast strain, genomic DNA was prepped and the yeast barcode was amplified using the U1/D1 primers to allow sequencing of the UPTAG/DNTAG using the kanB or kanC primers (Giaever et al. 2002). To determine the identity of the rescuing human cDNA, expression vectors were isolated and sequenced using a common primer that hybridizes to the vector backbone (CAGGAAACAGCTATGAC). Each potential hit (a rescued yeast colony) was reconfirmed by retransformation of the extracted plasmid into the corresponding heterozygous diploid as described for the one-to-one screen. To confirm the nonorthologous hits, both extracted pAG416GPD-hSEC61A1 and newly generated pAG416GPD-hSEC61A1-HA expression vectors along with pAG416GPD-hRFT1-HA and GAL-inducible ySEC61 from the FLEX array (Hu et al. 2007) were separately transformed into the RFT1/rft1∆ heterozygous diploid yeast strain and the experiment was carried out as described for the one-to-one screen.
Generating lists and analysis for the complementation screens
A comprehensive list of essential yeast CIN genes was obtained from Stirling et al. (2011), while a list of essential yeast genes was obtained from the Yeast Deletion Project (Giaever et al. 2002). A list of human genes was generated from two sources: Yeastmine (Balakrishnan et al. 2012) and Ensembl BioMart (Kinsella et al. 2011) databases. Gene Ontology (GO) processes and other features used to analyze the complementation set were obtained from Yeastmine and each feature was represented as a proportion of the total number of genes input for each gene set. Significance for each feature was calculated using the hypergeometric distribution and subjected to the Bonferroni correction to obtain the adjusted P-value (critical value: 0.05; number of tests: 24 for features analyzed in Figure 3, A–F). Sequence identity (%) in relation to the yeast gene was calculated for all possible human–yeast gene pairs using the Ensembl BioMart database. Significance between the % sequence identity of complementation pairs and % sequence identity of all human–yeast pairs included in this study was calculated using a Mann–Whitney test. For some manually curated orthologs and others generated from Yeastmine, sequence identity was determined using NWalign (Y. Zhang, http://zhanglab.ccmb.med.umich.edu/NW-align).
Figure 3.
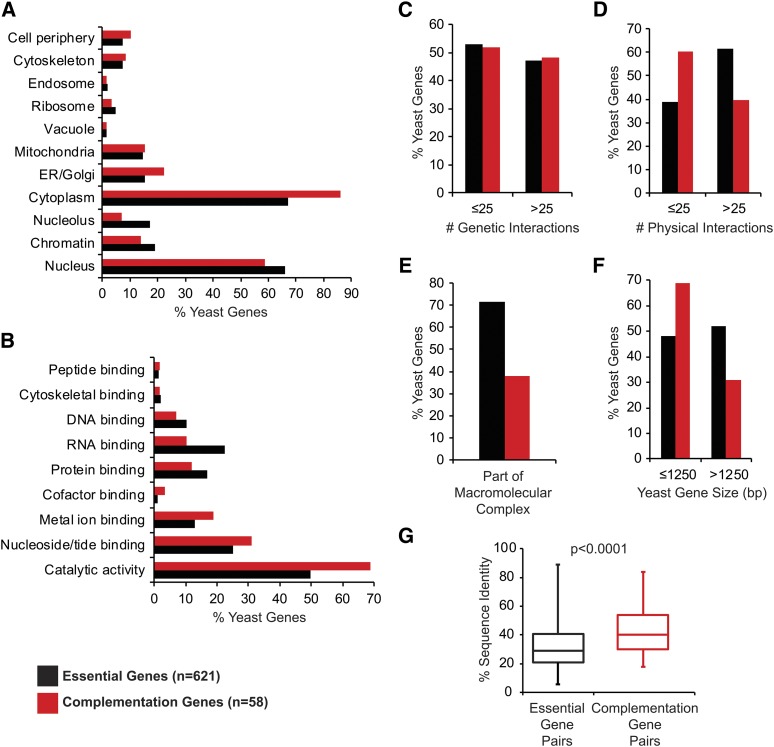
Analyzing features of essential yeast genes that predict replaceability including (A) localization patterns, (B) molecular function, (C) no. of genetic interactions, (D) no. of physical interactions, (E) part of macromolecular complexes, (F) yeast gene size, and (G) human–yeast sequence identity. Localization data, Gene Ontology (GO) terms, no. of genetic/physical interactions, and gene size for each yeast gene were obtained from Yeastmine and each feature is represented as a proportion of the total number of genes input for each set (n = 621 for all essential genes included in both screens and n = 58 for the complementation genes). Overall, the complementation set was enriched for yeast proteins that localize to the cytoplasm (P = 8.18E-03), have catalytic activity (P = 2.28E-02), less physical interactions (P = 5.77E-03), are less likely to be part of macromolecular complexes (P = 3.36E-07), and have smaller gene size (P = 9.16E-03). For sequence identity, “essential gene pairs” refers to the 1076 human–yeast pairs included in this study corresponding to 621 yeast genes and “complementation gene pairs” refers to the 65 complementation pairs corresponding to 58 yeast genes. The box plot highlights the median and range of sequence identity for each set of gene pairs.
Generating variants and yeast strains for growth curves
Expression vectors and yeast strain construction:
As described, hLIG1, hSSRP1, hPPP1CA, and hPPP1CC in entry clones were shuttled into the yeast destination vectors pAG416GPD-ccdB-HA (URA3) and pAG415GPD-ccdB-HA (LEU2) to generate expression clones (Supporting Information, Table S1). Missense mutations were introduced in vector pAG415GPD-hORF-HA (LEU2) using the QuikChange Site-Directed Mutagenesis Kit (Agilent), and verified by Sanger sequencing. To generate the yeast haploid knockout strains, tetrads were dissected following transformation of the expression vector pAG416GPD-hORF-HA (URA3) to the corresponding heterozygous diploid deletion strain and then haploids were maintained on −Ura media (Table S1). To generate the strains used for growth curve analysis, expression clones containing wild-type and mutant human cDNA on pAG415GPD-hORF-HA (LEU2) were first transformed into both the generated haploid knockouts (covered by wild-type human cDNA marked by URA3) and wild-type strain (BY4742) (Brachmann et al. 1998) and maintained on −Ura −Leu or −Leu media, respectively. For the plasmid shuffle, strains were then plated on −Leu +Ura +5-FOA media and individual colonies were picked and thereafter maintained on −Leu media. To confirm that the URA3-marked plasmid was lost, strains were streaked on −Ura media to observe lack of growth.
Growth curves:
Unless otherwise indicated, all growth conditions were carried out in −Leu media at 30°. Strains were inoculated overnight, diluted to a cell density of 0.1 OD600, and then grown to mid-log phase. Strains were then diluted to OD600 = 0.1 in 200 µl −Leu media with no drug, 0.01% MMS, or 100 mM HU in 96-well plates. OD600 readings were measured every 30 min over a period of 48 hr in a TECAN M200 plate reader. Prior to each reading, plates were shaken for 10 min. Each strain was tested in three to four replicates per plate per condition and area under the curve (AUC) value was calculated for each replicate independently. For growth curves in −Leu and −Leu +100 mM HU, AUC values were calculated from 0–24 hr corresponding to when most strains reached saturation. For growth curves in −Leu +0.01% MMS, AUC values were calculated from 0 to 48 hr. For each mutant, strain fitness was defined as the AUC of the mutant curves relative to the AUC of the wild-type allele grown on the same plate in the same media condition. Significant differences in growth in the “no drug” condition was determined against the wild-type allele in the same condition, while significant differences in growth in the “drug” condition was determined against the same allele in the no drug condition using a Student’s t-test. Sets of all individual growth curves are shown in Figure S1, Figure S2, Figure S3, and Figure S4.
Data availability
Plasmids and strains are available upon request. Plasmids and strains used for screening tumor-specific variants in yeast are listed in Table S1.
Results
Systematic identification of human–yeast cross-species complementation pairs
A comprehensive list of yeast CIN genes revealed that many essential genes are mutable to a CIN phenotype (323 CIN genes/∼1100 essential genes, or ∼29%) (Stirling et al. 2011). To establish an experimental platform for testing tumor-specific somatic mutations in candidate human CIN genes, we tested all possible yeast CIN gene null mutants for complementation by all potential human homologs in a series of one-to-one screens. While one-to-one screening reduces the number of false negatives that can arise as an artifact of the skewed representation associated with pooled screening, it does not allow identification of unexpected or nonorthologous complementation pairs. Therefore, we set up a pool-to-pool screen to test all possible essential (CIN and non-CIN) yeast genes for complementation by all sequence-related human homologs that are available as full-length cDNA clones. For the purposes of this study, we use “homolog” as an umbrella term that encompasses orthologs (genes derived from speciation and that typically perform equivalent functions) and paralogs (genes related by duplication and that generally perform biologically distinct yet mechanistically related functions) (reviewed in Koonin 2005).
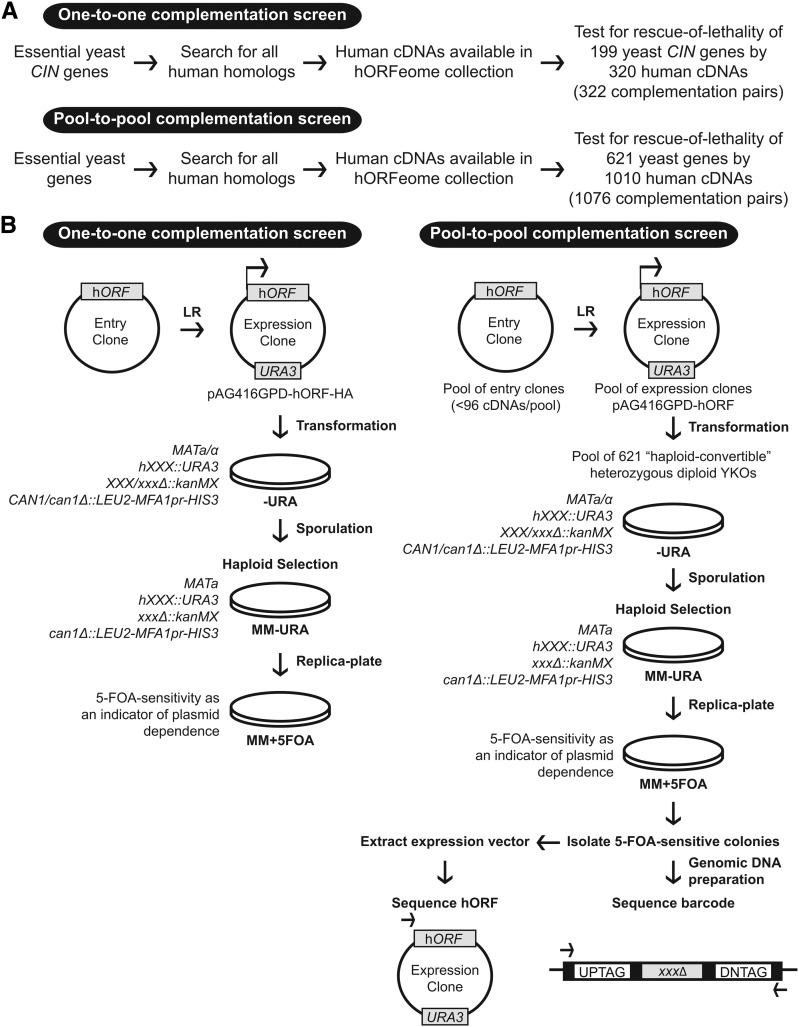
To set up both screens (Figure 1), we used Gateway cloning to enable the systematic shuttling of DNA fragments between cloning vectors (Hartley et al. 2000). Briefly, a human cDNA of interest flanked by recombination sites on an entry clone can be transferred to Gateway-compatible destination vectors in a single-step reaction to create yeast expression vectors. We used the human ORFeome V8.1, which is a Gateway-compatible clone library of sequence-confirmed human cDNAs (Yang et al. 2011) as the source of human ORFs. We also used the Gateway-compatible library of yeast expression vectors, which offers a selection of promoters (constitutive GPD vs. inducible GAL), plasmid copy number (2µ vs. CEN backbone), N- or C-terminal tags, and auxotrophic markers (Alberti et al. 2007). To express human cDNAs in yeast, we selected a centromere-based backbone and a constitutive promoter to reduce plasmid copy-number variation and minimize potential toxicity that can arise from overexpression of human cDNAs in yeast (Tugendreich et al. 2001). To test for complementation of essential gene null mutations, we assayed for rescue of lethality of the haploid yeast gene deletion following sporulation of the “haploid-convertible” heterozygous diploids (Pan et al. 2004).
Figure 1.
Overview of the experimental design for the complementation screens. (A) Pipeline outlining which human–yeast complementation pairs were included in both screens. (B) Flowchart for the complementation screens. Human cDNAs were shuttled from entry clones to indicated yeast destination vectors to generate yeast expression vectors. Single or pooled expression vectors were then transformed to matched or pooled haploid convertible heterozygous diploids and maintained on −Ura media. Following sporulation, heterozygous diploids were plated on haploid selection media (MM −Ura). “Rescued” haploids were tested for plasmid dependency by replica plating on MM +5-FOA. For the pooled screen, 5-FOA-sensitive colonies were isolated for sequencing of yeast barcode and expression vectors.
To design and execute the complementation assay, we compiled all sequence homologs of essential yeast genes generated from multiple sources using the Yeastmine database (Balakrishnan et al. 2012), Ensembl BioMart database (Kinsella et al. 2011) and to a lesser extent through manual curation. Each candidate complementation pair (human cDNA and cognate yeast gene) was included in our screens based on the availability and quality control of both the human cDNA in the hORFeome collection and the corresponding yeast strain in the heterozygous collection. In an effort to be as comprehensive as possible, our screens tested all possible complementation pairs even when the least diverged candidate ortholog was not available in the hORFeome collection. For instance, yCDC42 is a rho-like GTPase required for the establishment of cell polarity (Johnson 1999). The list of homologs for yCDC42 included genes such as hCDC42, hRHOJ, hRHOV, hRHOU, and other related members of the RHO family of small GTPases. Of these homologs, the least diverged ortholog, hCDC42 (80% sequence identity, previously shown to complement the yeast deletion mutant (Kachroo et al. 2015), was not available in the human ORFeome V8.1 collection and therefore not tested in this study. We did test hRHOJ (60% sequence identity) and determined that it does not complement the null allele of yCDC42. In total, we tested 199 essential CIN deletion mutants for rescue of lethality by 320 human cDNAs one-by-one (322 candidate complementation pairs) and 621 essential gene deletion mutants for rescue of lethality by 1010 human cDNAs pool-to-pool (1076 candidate complementation pairs) (Figure 1A, Table S2 and Table S3). Our screens identified 65 human cDNAs that complement the null allele of 58 essential yeast genes, including a complementation pair of nonorthologous proteins (yeast Rft1 and human Sec61A1) (Table 1). When compared to a curated list of complementation pairs available from the Saccharomyces Genome Database (SGD) that includes the recent results of Kachroo et al. (2015) (http://downloads.yeastgenome.org/curation/literature/functional_complementation.tab (June 2015) and other published reports, our work identified 20 novel complementation pairs in which the human cDNA rescues lethality of the yeast null allele (Table S4).
Table 1. Human cDNAs that complement essential yeast deletion mutants.
| Yeast systematic name | Yeast standard name | Human Entrez Gene ID | Human standard name | Yeast gene brief descriptiond |
|---|---|---|---|---|
| YBL020W | RFT1a | 29927 | SEC61A1 | Membrane protein required for translocation of Man5GlcNac2-PP-Dol |
| YBR002C | RER2 | 79947 | DHDDS | Cis-prenyltransferase involved in dolichol synthesis |
| YBR029C | CDS1 | 8760 | CDS2 | Phosphatidate cytidylyltransferase (CDP-diglyceride synthetase) |
| YBR109C | CMD1b | 801; 805; 808 | CALM1; CALM2; CALM3 | Calmodulin |
| YBR160W | CDC28b | 983; 1017 | CDK1; CDK2 | Cyclin-dependent kinase (CDK) catalytic subunit |
| YBR252W | DUT1 | 1854 | DUT | Deoxyuridine triphosphate diphosphatase (dUTPase) |
| YCR012W | PGK1b | 5230; 5232 | PGK1c; PGK2 | 3-Phosphoglycerate kinase |
| YDL045C | FAD1b | 80308 | FLAD1c | Flavin adenine dinucleotide (FAD) synthetase |
| YDL064W | UBC9b | 7329 | UBE2I | SUMO-conjugating enzyme involved in the Smt3p conjugation pathway |
| YDL120W | YFH1 | 2395 | FXN | Mitochondrial matrix iron chaperone |
| YDL147W | RPN5b | 5718 | PSMD12c | Subunit of the CSN and 26S proteasome lid complexes |
| YDL164C | CDC9b | 3978 | LIG1c | DNA ligase found in the nucleus and mitochondria |
| YDL205C | HEM3 | 3145 | HMBS | Porphobilinogen deaminase |
| YDR050C | TPI1b | 7167 | TPI1 | Triose phosphate isomerase, abundant glycolytic enzyme |
| YDR086C | SSS1 | 23480 | SEC61G | Subunit of the Sec61p translocation complex (Sec61p-Sss1p-Sbh1p) |
| YDR208W | MSS4b | 8394; 8395 | PIP5K1A; PIP5K1Bc | Phosphatidylinositol-4-phosphate 5-kinase |
| YDR236C | FMN1 | 55312 | RFK | Riboflavin kinase, produces riboflavin monophosphate (FMN) |
| YDR404C | RPB7b | 5436 | POLR2G | RNA polymerase II subunit B16 |
| YDR454C | GUK1 | 2987 | GUK1 | Guanylate kinase |
| YDR510W | SMT3b | 7341 | SUMO1c | Ubiquitin-like protein of the SUMO family |
| YEL026W | SNU13b | 4809 | NHP2L1 | RNA binding protein |
| YEL058W | PCM1 | 5238 | PGM3 | Essential N-acetylglucosamine-phosphate mutase |
| YER094C | PUP3b | 5691 | PSMB3 | Beta 3 subunit of the 20S proteasome |
| YER112W | LSM4 | 25804 | LSM4 | Lsm (Like Sm) protein |
| YER133W | GLC7b | 5499; 5501 | PPP1CA; PPP1CC | Type 1 serine/threonine protein phosphatase catalytic subunit |
| YER136W | GDI1 | 2665 | GDI2 | GDP dissociation inhibitor |
| YFL017C | GNA1b | 64841 | GNPNAT1c | Glucosamine-6-phosphate acetyltransferase |
| YGL001C | ERG26 | 50814 | NSDHL | C-3 sterol dehydrogenase |
| YGL030W | RPL30b | 6156 | RPL30 | Ribosomal 60S subunit protein L30 |
| YGL048C | RPT6 | 5705 | PSMC5 | ATPase of the 19S regulatory particle of the 26S proteasome |
| YGR024C | THG1 | 54974 | THG1L | tRNAHis guanylyltransferase |
| YGR075C | PRP38 | 55119 | PRPF38B | Unique component of the U4/U6.U5 tri-snRNP particle |
| YGR175C | ERG1 | 6713 | SQLE | Squalene epoxidase |
| YGR185C | TYS1 | 8565 | YARS | Cytoplasmic tyrosyl-tRNA synthetase |
| YGR277C | CAB4 | 80347 | COASY | Subunit of the CoA-synthesizing protein complex (CoA-SPC) |
| YGR280C | PXR1 | 54984 | PINX1 | Essential protein involved in rRNA and snoRNA maturation |
| YIL083C | CAB2 | 79717 | PPCS | Subunit of the CoA-synthesizing protein complex (CoA-SPC) |
| YJL097W | PHS1 | 201562 | PTPLB | Essential 3-hydroxyacyl-CoA dehydratase of the ER membrane |
| YJR006W | POL31b | 5425 | POLD2 | Subunit of DNA polymerase delta (polymerase III) |
| YKL013C | ARC19b | 10093 | ARPC4c | Subunit of the ARP2/3 complex |
| YKL024C | URA6b | 51727 | CMPK1c | Uridylate kinase |
| YKL033W | TTI1b | 9675 | TTI1 | Subunit of the ASTRA complex, involved in chromatin remodeling |
| YKL035W | UGP1b | 7360 | UGP2c | UDP-glucose pyrophosphorylase (UGPase) |
| YKL145W | RPT1 | 5701 | PSMC2 | ATPase of the 19S regulatory particle of the 26S proteasome |
| YKL189W | HYM1 | 51719; 81617 | CAB39; CAB39L | Component of the RAM signaling network |
| YML069W | POB3b | 6749 | SSRP1 | Subunit of the heterodimeric FACT complex (Spt16p-Pob3p) |
| YML077W | BET5b | 58485 | TRAPPC1 | Core component of transport protein particle (TRAPP) complexes I-III |
| YMR208W | ERG12 | 4598 | MVK | Mevalonate kinase |
| YMR308C | PSE1b | 3843 | IPO5 | Karyopherin/importin that interacts with the nuclear pore complex |
| YMR314W | PRE5b | 5682 | PSMA1 | Alpha 6 subunit of the 20S proteasome |
| YOL133W | HRT1 | 9978 | RBX1 | RING-H2 domain core subunit of multiple ubiquitin ligase complexes |
| YOR143C | THI80 | 27010 | TPK1 | Thiamine pyrophosphokinase |
| YOR149C | SMP3b | 80235 | PIGZ | Alpha 1,2-mannosyltransferase |
| YOR176W | HEM15 | 2235 | FECH | Ferrochelatase |
| YOR236W | DFR1 | 1719 | DHFR | Dihydrofolate reductase involved in tetrahydrofolate biosynthesis |
| YPL117C | IDI1b | 3422 | IDI1c | Isopentenyl diphosphate:dimethylallyl diphosphate isomerase |
| YPR082C | DIB1b | 10907 | TXNL4Ac | 17-kDa component of the U4/U6aU5 tri-snRNP |
| YPR113W | PIS1 | 10423 | CDIPT | Phosphatidylinositol synthase |
Complementation by nonorthologous gene.
Yeast CIN gene.
Complementation identified by both screens.
Brief description obtained from Yeastmine.
Assessing features of essential yeast genes that predict replaceability
We used Yeastmine to assess GO terms and looked for features that predict replaceability of essential yeast genes. Our yeast complementation set (n = 58) is composed predominantly of metabolic genes (Figure 2), a feature also observed in a recent large-scale complementation study (Kachroo et al. 2015). In general, the complemented yeast genes encode proteins that localize to the cytoplasm and associated organelles rather than the nucleus or other nuclear-associated regions (Figure 3A). When grouped by molecular function, the complementation set is enriched for proteins that display catalytic activity and other enzymatic features such as nucleotide, metal ion, and cofactor binding (Figure 3B).
Figure 2.
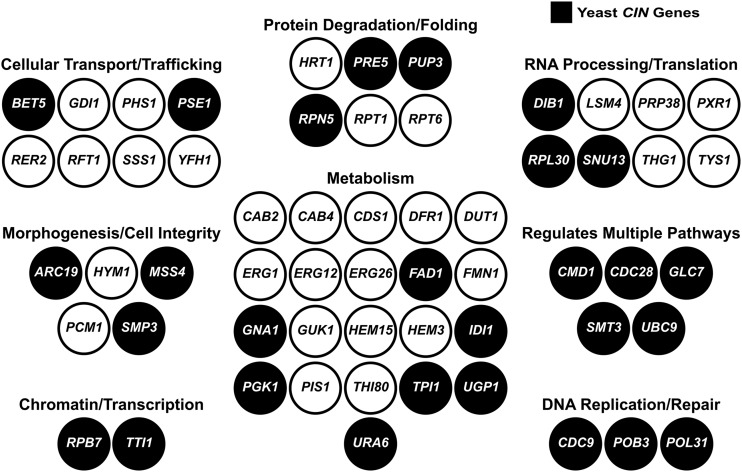
Essential yeast genes that are replaceable by human genes. A total of 58 yeast genes are represented by nodes and grouped according to cellular processes (Yeastmine). Yeast CIN genes are represented by black nodes.
Even though the complemented yeast genes display no difference in the number of genetic interactions (Figure 3C), there is a marked difference in the number of physical interactions in this set: replaceable yeast proteins tend to have fewer physical interactions (Figure 3D) and are less likely to be part of macromolecular complexes (Figure 3E). We further show that replaceable yeast genes tend to be short (Figure 3F), corroborating the observation of Kachroo et al. (2015).
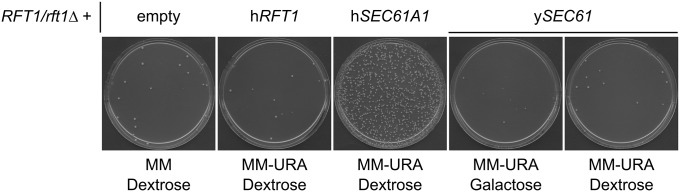
Complementation pairs tend to have higher than average sequence identity/similarity, but sequence identity alone is a poor predictor of replaceability as evidenced by the observations that high sequence similarity does not guarantee replaceability and complementation pairs can have low sequence similarity (Figure 3G). We assessed whether replaceability by one human homolog predicts the same outcome for additional human homologs. In one case, we found that the glycolytic enzyme yPGK1 can be replaced by either hPGK1 (66% sequence identity) or hPGK2 (63% sequence identity), human proteins that share 87% sequence identity and catalyze the same reaction but are differentially expressed (McCarrey and Thomas 1987). In the only other example, the phosphatase yGLC7 can be replaced by the isozymes hPPP1CA (84% sequence identity) and hPPP1CC (84% sequence identity), human proteins that are 91% identical and part of the highly conserved PP1 subfamily of protein phosphatases (Ceulemans and Bollen 2004). In contrast, some yeast genes such as yDIB1, yIDI1, and ySMT3 are only replaceable by one of multiple human homologs, whereas others like yCMD1, yMSS4, and yCDC28 are replaceable by several (but not all) human homologs. For these cases and the previously mentioned yCDC42, we observed that replaceable human proteins share higher sequence identity with the yeast protein than the nonreplaceable ones (Table S5), suggesting that the least diverged homologs were the most likely to complement. The only contradiction of this observation is hSEC61A1, which can replace the nonorthologous yRFT1 and rescue lethality of rft1∆. Sec61 forms an ER membrane channel and is required for cotranslational and post-translational translocation of proteins into the ER (Osborne et al. 2005), while Rft1 also functions at the ER membrane and is implicated in the translocation of lipid-linked oligosaccharides into the ER (Helenius et al. 2002). We further demonstrated that hRFT1 cannot complement yRFT1 (also shown by Kachroo et al. 2015), and that overexpression of the yeast ortholog of hSEC61A1, ySEC61, also fails to rescue lethality of rft1∆ (Figure 4). The fact that hSEC61A1 also fails to complement ySEC61 (Table S2) highlights the unexpected and complex manner in which the human protein acts to functionally substitute for the nonorthologous yeast protein. Overall, our observations and those of Kachroo et al. (2015) suggest that complementation cannot be predicted by sequence similarity.
Figure 4.
hSEC61A1 complements yRFT1. Expression vectors (hRFT1 and hSEC61A1 under the control of the GPD constitutive promoter and ySEC61 under the control of the GAL-inducible promoter) were transformed into the RFT1/rft1∆ heterozygous diploid yeast strain and plated on haploid selection media (MM −Ura) following sporulation. Complementation was scored by higher than background growth on MM −Ura as shown and confirmed by testing for plasmid dependency on MM +5-FOA and tetrad dissection.
Characterizing tumor-specific variants using cross-species complementation
Human–yeast complementation enables direct testing of human gene variants for function. Genetic variants can be screened rapidly and are characterized in the context of the human protein. For example, complementation of yeast cys4∆ by its human ortholog CBS gene enabled the testing of 84 variants from patients with homocystinuria for functionality and cofactor dependence (Mayfield et al. 2012). One resource from our human–yeast complementation screen is a list of human genes whose variants can be characterized in a yeast deletion strain. We focused on testing tumor-specific mutations found in human orthologs of yeast CIN genes. Our complementation set (Table 1) includes 28 essential yeast CIN genes rescued by 34 candidate human CIN genes. We observe that the replaceable yeast CIN genes function across diverse biological processes, including those pathways predicted to protect genome integrity (e.g., DNA replication/repair and chromatin-related processes) and more peripheral pathways (e.g., metabolism and cellular trafficking) (Figure 2).
We selected three yeast CIN genes corresponding to four complementation pairs (yCDC9:hLIG1, yPOB3:hSSRP1, yGLC7:hPPP1CA, and yGLC7:hPPP1CC) and assessed the impact of single amino acid substitutions in the human protein. Briefly, yCDC9 encodes DNA ligase, an essential enzyme that joins Okazaki fragments during DNA replication and also functions in DNA repair pathways (Lindahl and Barnes 1992); yPOB3 is a subunit of the heterodimeric FACT complex (yPOB3-ySPT16) that functions in nucleosome reorganization to facilitate transcription and replication processes (Formosa 2008); yGLC7 is the catalytic subunit of protein phosphatase which, depending on its bound regulatory subunit, regulates numerous cellular processes (Cannon 2010). We selected one cancer type (colorectal cancer) and screened all reported missense mutations compiled in two databases: Catalogue of Somatic Mutations in Cancer (COSMIC) (Forbes et al. 2015) and cBIOPORTAL for Cancer Genomics (Cerami et al. 2012; Gao et al. 2013). In total, 35 single amino acid substitutions were constructed corresponding to 16 in hLIG1, 12 in hSSRP1, 2 in hPPP1CA, and 5 in hPPP1CC. As a control, we expressed each human gene variant in a wild-type yeast strain and compared the growth of these yeast strains to the growth of the same yeast strain expressing the wild-type human allele. This step is required to identify any mutants whose ectopic expression may impact the growth phenotype of the yeast strain either by causing toxicity to the yeast cell or increasing the growth rate. Of the 35 mutants analyzed, the K228E mutation in hSSRP1 is the only amino acid substitution whose ectopic expression decreased fitness of wild-type yeast (Table S6, Figure S1).
We used a plasmid shuffle strategy to introduce the human gene variants in the corresponding yeast gene deletion mutants. Haploid yeast gene knockout strains carrying the rescuing human cDNA on a URA3-marked plasmid were transformed with wild-type or mutated human cDNA on a LEU2-marked plasmid and plated on media containing 5-FOA (Figure 5), which selects for cells that have lost the URA3 plasmid. Growth on 5-FOA plates indicates that the mutant human cDNA is able to complement the essential gene. Lack of growth on 5-FOA indicates that the mutation in the human cDNA results in a nonfunctional protein. We observed that 4 of the 35 mutant alleles [2 in hSSRP1 (K228E, S481P), 1 in hPPP1CA (Y272C), and 1 in hPPP1CC (L289R)] are nonfunctional (Table 2). Although hSSRP1-K228E was found to be detrimental in the context of the human gene, the same substitution in the conserved site of the yeast protein (yPob3-K271E) is tolerated (VanDemark et al. 2006). This corroborates previous observations that amino acid substitutions in the context of the yeast gene (or other orthologs) do not necessarily have the same effect in the context of the human gene (Marini et al. 2010), emphasizing the importance of characterizing human variants in their native gene context. To determine the accuracy of our experimental readout, we surveyed the literature and found the conserved Y272 and L289 in PP1 phosphatases are essential for substrate binding (Egloff et al. 1995, 1997; Zhang et al. 1996).
Figure 5.
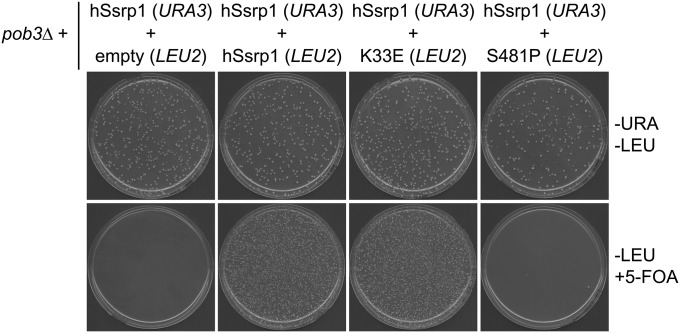
Plasmid shuffle used to generate strains expressing human cDNAs with missense mutations. Yeast haploid knockout strains covered by wild-type human cDNAs on URA3-marked vectors were transformed with the following LEU2-marked vectors (empty, wild-type human cDNA, and human cDNA with missense mutation) and maintained on −Ura −Leu media. Strains were plated on −Leu +5-FOA media to generate haploid yeast knockouts covered by LEU2-marked vectors. Strains were confirmed to have lost the URA3-marked plasmid by streaking on −Ura media to observe no growth. In the presented example, hSsrp1-K33E is able to complement pob3∆, but S481P results in a nonfunctional hSsrp1 protein.
Table 2. Tumor-specific variants constructed and analyzed in a yeast deletion background.
| Nucleotide mutation | Amino acid mutation | Zygosity | Amino acid conserved in yeast | PredictSNP (confidence) | No drug | 0.01% MMS | 100 mM HU | |
|---|---|---|---|---|---|---|---|---|
| hLIG1 | 179C > T | A60V | Unknown | No | Neutral (83%) | 0.56 ± 0.06*** | 0.50 ± 0.05 | 0.38 ± 0.03* |
| 421A > G | S141G | Heterozygous | Yes | Neutral (75%) | 0.45 ± 0.03*** | 0.50 ± 0.07 | 0.25 ± 0.05* | |
| 454A > G | K152E | Heterozygous | Yes | Neutral (83%) | 0.52 ± 0.06*** | 0.98 ± 0.06*** | 0.46 ± 0.03 | |
| 455A > G | K152R | Heterozygous | Yes | Neutral (83%) | 0.50 ± 0.05*** | 0.50 ± 0.06 | 0.23 ± 0.01*** | |
| 457G > A | E153K | Heterozygous | No | Neutral (83%) | 0.60 ± 0.12*** | 0.44 ± 0.06 | 0.30 ± 0.07** | |
| 488G > A | S163N | Unknown | No | Neutral (83%) | 0.60 ± 0.12*** | 0.49 ± 0.05 | 0.23 ± 0.06*** | |
| 664C > T | R222C | Heterozygous | No | Neutral (63%) | 0.35 ± 0.01*** | 0.23 ± 0.01*** | 0.21 ± 0.02*** | |
| 1045G > A | V349M | Unknown | No | Neutral (60%) | 0.47 ± 0.03*** | 0.59 ± 0.04* | 0.29 ± 0.05* | |
| 1120G > A | A374T | Unknown | No | Neutral (83%) | 0.71 ± 0.03*** | 0.71 ± 0.06 | 0.51 ± 0.06** | |
| 1184C > A | P395Q | Unknown | No | Neutral (83%) | 0.63 ± 0.05*** | 0.48 ± 0.04** | 0.44 ± 0.04* | |
| 1502T > C | M501T | Unknown | No | Neutral (83%) | 0.61 ± 0.06*** | 0.34 ± 0.01*** | 0.46 ± 0.03* | |
| 1835C > T | S612L | Heterozygous | No | Neutral (74%) | 0.49 ± 0.04*** | 0.33 ± 0.02*** | 0.42 ± 0.04 | |
| 1969T > G | L657V | Heterozygous | Yes | Neutral (83%) | 0.71 ± 0.08*** | 0.70 ± 0.10 | 0.44 ± 0.04** | |
| 2290G > A | A764T | Unknown | Yes | Deleterious (87%) | 0.73 ± 0.09*** | 0.30 ± 0.02*** | 0.61 ± 0.04 | |
| 2353G > A | E785K | Unknown | No | Neutral (75%) | 0.59 ± 0.07*** | 0.52 ± 0.05 | 0.56 ± 0.09 | |
| 2446G > A | V816M | Unknown | No | Neutral (74%) | 0.70 ± 0.06*** | 0.36 ± 0.01*** | 0.47 ± 0.04* | |
| hSSRP1 | 97A > G | K33E | Heterozygous | Yes | Deleterious (87%) | 1.02 ± 0.12 | 0.70 ± 0.08 | 0.95 ± 0.12 |
| 566C > T | A189V | Heterozygous | Yes | Deleterious (72%) | 0.69 ± 0.11** | 0.35 ± 0.03 | 0.79 ± 0.05 | |
| 626C > T | T209I | Unknown | Yes | Deleterious (61%) | 1.09 ± 0.14 | 0.68 ± 0.07* | 1.00 ± 0.12 | |
| 682A > G | K228E | Heterozygous | Yes | Deleterious (87%) | Non-functional | n/d | n/d | |
| 970C > T | R324C | Heterozygous | Yes | Neutral (60%) | 1.00 ± 0.06 | 0.80 ± 0.18 | 0.97 ± 0.10 | |
| 1108C > T | R370C | Heterozygous | No | Deleterious (65%) | 1.05 ± 0.11 | 0.67 ± 0.06* | 0.97 ± 0.17 | |
| 1306C > T | P436S | Heterozygous | No | Neutral (83%) | 0.94 ± 0.05 | 0.81 ± 0.10 | 1.01 ± 0.11 | |
| 1441T > C | S481P | Unknown | No | Deleterious (61%) | Non-functional | n/d | n/d | |
| 1493A > G | N498S | Heterozygous | No | Neutral (74%) | 1.05 ± 0.06 | 0.70 ± 0.04* | 1.08 ± 0.08 | |
| 1723A > G | T575A | Heterozygous | No | Neutral (83%) | 1.02 ± 0.06 | 0.76 ± 0.04* | 1.22 ± 0.13 | |
| 1724C > T | T575M | Unknown | No | Neutral (60%) | 1.02 ± 0.05 | 0.78 ± 0.08* | 1.07 ± 0.10 | |
| 1950G > T | K650N | Unknown | No | Neutral (83%) | 1.07 ± 0.16 | 0.82 ± 0.03 | 1.22 ± 0.03 | |
| hPPP1CA | 428G > A | R143H | Heterozygous | Yes | Deleterious (87%) | 0.69 ± 0.08 | 0.72 ± 0.09 | 0.67 ± 0.03 |
| 815A > G | Y272C | Heterozygous | Yes | Deleterious (87%) | Non-functional | n/d | n/d | |
| hPPP1CC | 348G > T | E116D | Unknown | Yes | Neutral (75%) | 0.57 ± 0.11** | 0.24 ± 0.03* | 0.33 ± 0.01* |
| 559C > T | R187W | Unknown | Yes | Deleterious (87%) | 0.05 ± 0.01*** | 0.05 ± 0.01 | 0.02 ± 0.01* | |
| 601C > T | L201F | Heterozygous | Yes | Deleterious (51%) | 1.48 ± 0.18* | 1.09 ± 0.24 | 1.10 ± 0.11 | |
| 610C > A | L204I | Unknown | Yes | Neutral (63%) | 0.97 ± 0.10 | 0.89 ± 0.22 | 1.07 ± 0.14 | |
| 866T > G | L289R | Heterozygous | Yes | Deleterious (87%) | Nonfunctional | n/d | n/d |
Strain fitness for each allele is expressed as a ratio relative to the yeast strain expressing the wild-type allele grown in the same plate in the same media condition. For “no drug” condition, significance was calculated compared to the wild-type allele grown in the same plate to assess impact of missense mutation on strain fitness. For “drug” condition, significance was calculated compared to the same allele in the no drug condition grown in the same plate to assess impact of drug on strain fitness. Alleles were defined as nonfunctional based on inability to complement the corresponding deletion mutant yeast strain. Strain fitness values that were not determined are indicated (n/d). *P < 0.01, **P < 0.001, ***P < 0.0001.
The remaining 31 mutant alleles were assessed for their ability to rescue growth of the null mutant (Table 2, Figure S2, Figure S3, and Figure S4). We observed that 19 of the 31 mutations cause a significant decrease in strain fitness relative to the wild-type allele, while 1 mutant allele (hPPP1CC-L201F) grows considerably better than the corresponding wild-type allele. Notably, 16 of the 19 “slow growers” are substitutions in hLIG1, with the mutations dispersed throughout the protein. Given that mutants with defects in chromosome stability are often sensitive to DNA-damaging agents, we assayed our 31 mutants for growth in the presence of methyl methane sulfonate (MMS) and hydroxyurea (HU). MMS is an alkylating agent (Beranek 1990) while HU slows DNA replication by decreasing the rate of dNTP synthesis (Koc et al. 2004), and haploid viable mutant alleles of yeast CDC9, POB3, and GLC7 have been shown to cause sensitivity to sublethal doses of MMS and/or HU (Prakash and Prakash 1977; Schlesinger and Formosa 2000; Bazzi et al. 2010). Twelve mutant alleles cause sensitivity to MMS, while two cause increased resistance to MMS. The majority of the mutants sensitive to HU are in hLIG1 (12/14), which is expected given the major role of that protein in DNA replication. To assess whether computational predictions match empirical results, we used PredictSNP (a consensus classifier grouping six computational tools) (Bendl et al. 2014) to predict the effects of the mutations on protein function. Our results demonstrate that predicted effects do not always match observed results. While some substitutions predicted to be neutral with high confidence displayed detrimental growth phenotypes (e.g., hPPP1CC-E116D), others predicted to be deleterious with high confidence exhibited no growth defects (e.g., hSSRP1-R370C). Moreover, amino acid conservation alone or in combination with computational predictions did not accurately predict observable phenotypes. For instance, an amino acid that is conserved between yeast and human proteins might be expected to be important for protein function. However, even a substitution predicted to be deleterious by computational tools may not result in any growth phenotypes (e.g., hSSRP1-T209I). Overall, these results underscore the requirement for direct functional testing of variants in their native gene context.
Discussion
The work presented in this study extends the growing list of human–yeast complementation pairs that will serve as an important resource for model organism and human biology. Many factors impact replaceability of yeast genes by human genes. Different types of yeast strains permit different complementation readouts; therefore, choosing the appropriate yeast strain depends on what is required for downstream applications. We restricted our complementation assays to testing rescue of lethality of the haploid yeast gene knockout because rescue of a yeast conditional allele or downregulated yeast gene can represent partial or indirect complementation. This is evident in the results of Kachroo et al. (2015), which indicate that <60% (32/56) of the 69 human orthologs identified by rescue of temperature-sensitive alleles are able to rescue the null allele. Similarly, ∼60% (24/39) of the 44 human orthologs identified by rescue of downregulated yeast genes are able to rescue the null allele. If the main objective is to use yeast as a platform to characterize the functional consequence of human gene variants, we believe that complementation of the null allele is more desirable mainly for two reasons. First, expressing human cDNAs in a yeast deletion mutant diminishes the possibility of misleading phenotypic readouts by eliminating unwanted side effects of the residual yeast protein that is present in partial loss-of-function alleles. Second, the use of null alleles distinguishes those human proteins that are truly substituting for the essential function of the yeast protein from those human proteins that are suppressing a growth phenotype by a secondary mechanism that may or may not relate to the conserved function. Other factors that impact replaceability include the conditions used to express the human cDNA in yeast to account for dosage, toxicity, or timing of expression. Examples of what can be manipulated include picking a suitable promoter (endogenous yeast promoter vs. constitutive vs. inducible), integration of human cDNA vs. episomal vector-based expression, and inclusion of epitope tags. For instance, in this study we used yeast expression vectors that introduce short C-terminal extensions, which can interfere with replaceability by some human proteins (Kachroo et al. 2015). In general, there are no sets of conditions that satisfy the replaceability requirements of all candidate complementation pairs, as each human–yeast gene pair is unique. Even when no complementation is observed, partial fusions to create chimeric human–yeast proteins may allow complementation and provide a useful resource for specific applications (Zhou and Reed 1998).
The experimental design of systematic complementation assays impacts the scope of the results. Testing complementation pairs one-by-one reduces the number of false negatives inherent in pooled screening. This was observed in our study: our one-to-one screen identified 34 complementation pairs corresponding to 28 yeast strains, while our pool-to-pool screen identified 12 of these complementation pairs corresponding to 12 yeast strains, resulting in a 35% recovery rate from the pooled screen (Table 1). This modest recovery rate may be due to several factors: (i) even with 100-fold coverage, shorter cDNAs are shuttled more efficiently from entry clones to destination vectors in Gateway’s LR reaction, resulting in a potentially skewed representation of some expression vectors within a pool; (ii) competitive outgrowth can result in a skewed representation of yeast strains pre- and post-transformation; (iii) even with 100-fold coverage, pool-to-pool transformation reduces the chances of a particular human cDNA complementing the matched yeast strain, especially given the possibility of skewed representation of expression vectors and yeast strains; (iv) toxicity from some human cDNAs can skew representation of yeast strains within a pool; (v) sporulation efficiencies of different yeast strains skew haploid selection; and (vi) sequence coverage of rescued haploids will impact the scope of the results. Nevertheless, the advantage of pooled screening is the potential for identification of nonorthologous complementation pairs, as demonstrated in this study. With the development of better screening methods and updated collections, the potential for discovery of additional nonorthologous complementation pairs will aid in deciphering biological mechanisms that would otherwise be overlooked.
We demonstrated a robust approach to determining the functional consequence of human gene variants in yeast. Because the functional effects of missense mutations are difficult to predict, a quick yet systematic approach to screen and prioritize human gene variants for subsequent testing in mammalian models is of great value. As we have picked mutants of genes implicated in chromosome stability, mutant alleles that result in a nonfunctional protein, or reduction of function as displayed by decreased strain fitness, or increased sensitivity to DNA-damaging agents, are candidate mutations that might contribute to chromosome instability in tumor cells. While we have focused on screening somatic mutations found in tumors, this approach is also applicable to rare-disease variants that are being discovered at an unprecedented rate through next-generation sequencing (Hieter and Boycott 2014). Of course, there are limitations to complementation in model organisms, including representation of multicellular and developmental pathways that are absent in single-celled yeast. Nevertheless, advances in sequencing technologies have led to the discovery of an overwhelming number of human genetic variants whose empirical assessment of their biological effects would be impractical without the support of model organisms.
Supplementary Material
Acknowledgments
We thank Peter Stirling and Nigel O’Neil for helpful discussion and critical reading of the manuscript. This work was supported by grants to P.H. from the Canadian Institutes of Health Research (CIHR) (MOP 38096) and the National Institutes of Health (R01CA158162). A.H. is supported by doctoral awards from CIHR and the University of British Columbia. C.N. and G.G. are funded by the US Department of Agriculture and the Canada Foundation for Innovation. P.H. is a senior fellow in the Genetic Networks program at the Canadian Institute for Advanced Research.
Footnotes
Communicating editor: M. Johnston
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.181099/-/DC1.
Literature Cited
- Alberti S., Gitler A. D., Lindquist S., 2007. A suite of Gateway cloning vectors for high-throughput genetic analysis in Saccharomyces cerevisiae. Yeast 24: 913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnoldo A., Kittanakom S., Heisler L. E., Mak A. B., Shukalyuk A. I., et al. , 2014. A genome scale overexpression screen to reveal drug activity in human cells. Genome Med. 6: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan R., Park J., Karra K., Hitz B. C., Binkley G., et al. , 2012. YeastMine–an integrated data warehouse for Saccharomyces cerevisiae data as a multipurpose tool-kit. Database (Oxford) 2012: bar062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber T. D., McManus K., Yuen K. W., Reis M., Parmigiani G., et al. , 2008. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc. Natl. Acad. Sci. USA 105: 3443–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi M., Mantiero D., Trovesi C., Lucchini G., Longhese M. P., 2010. Dephosphorylation of gamma H2A by Glc7/protein phosphatase 1 promotes recovery from inhibition of DNA replication. Mol. Cell. Biol. 30: 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendl J., Stourac J., Salanda O., Pavelka A., Wieben E. D., et al. , 2014. PredictSNP: robust and accurate consensus classifier for prediction of disease-related mutations. PLOS Comput. Biol. 10: e1003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beranek D. T., 1990. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat. Res. 231: 11–30. [DOI] [PubMed] [Google Scholar]
- Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., et al. , 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14: 115–132. [DOI] [PubMed] [Google Scholar]
- Cannon J. F., 2010. Function of protein phosphatase-1, Glc7, in Saccharomyces cerevisiae. Adv. Appl. Microbiol. 73: 27–59. [DOI] [PubMed] [Google Scholar]
- Cerami E., Gao J., Dogrusoz U., Gross B. E., Sumer S. O., et al. , 2012. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2: 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans H., Bollen M., 2004. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol. Rev. 84: 1–39. [DOI] [PubMed] [Google Scholar]
- Charames G. S., Bapat B., 2003. Genomic instability and cancer. Curr. Mol. Med. 3: 589–596. [DOI] [PubMed] [Google Scholar]
- Dunham M. J., Fowler D. M., 2013. Contemporary, yeast-based approaches to understanding human genetic variation. Curr. Opin. Genet. Dev. 23: 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff M. P., Cohen P. T., Reinemer P., Barford D., 1995. Crystal structure of the catalytic subunit of human protein phosphatase 1 and its complex with tungstate. J. Mol. Biol. 254: 942–959. [DOI] [PubMed] [Google Scholar]
- Egloff M. P., Johnson D. F., Moorhead G., Cohen P. T., Cohen P., et al. , 1997. Structural basis for the recognition of regulatory subunits by the catalytic subunit of protein phosphatase 1. EMBO J. 16: 1876–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes S. A., Beare D., Gunasekaran P., Leung K., Bindal N., et al. , 2015. COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 43: D805–D811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa T., 2008. FACT and the reorganized nucleosome. Mol. Biosyst. 4: 1085–1093. [DOI] [PubMed] [Google Scholar]
- Gao J., Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., et al. , 2013. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6: pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaever G., Chu A. M., Ni L., Connelly C., Riles L., et al. , 2002. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418: 387–391. [DOI] [PubMed] [Google Scholar]
- Greene A. L., Snipe J. R., Gordenin D. A., Resnick M. A., 1999. Functional analysis of human FEN1 in Saccharomyces cerevisiae and its role in genome stability. Hum. Mol. Genet. 8: 2263–2273. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R. A., 2011. Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Hartley J. L., Temple G. F., Brasch M. A., 2000. DNA cloning using in vitro site-specific recombination. Genome Res. 10: 1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius J., Ng D. T., Marolda C. L., Walter P., Valvano M. A., et al. , 2002. Translocation of lipid-linked oligosaccharides across the ER membrane requires Rft1 protein. Nature 415: 447–450. [DOI] [PubMed] [Google Scholar]
- Hieter P., Boycott K. M., 2014. Understanding rare disease pathogenesis: a grand challenge for model organisms. Genetics 198: 443–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Rolfs A., Bhullar B., Murthy T. V., Zhu C., et al. , 2007. Approaching a complete repository of sequence-verified protein-encoding clones for Saccharomyces cerevisiae. Genome Res. 17: 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. I., 1999. Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63: 54–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachroo A. H., Laurent J. M., Yellman C. M., Meyer A. G., Wilke C. O., et al. , 2015. Evolution. Systematic humanization of yeast genes reveals conserved functions and genetic modularity. Science 348: 921–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S., Han S. Y., Liu W., Otsuka K., Shibata H., et al. , 2003. Understanding the function-structure and function-mutation relationships of p53 tumor suppressor protein by high-resolution missense mutation analysis. Proc. Natl. Acad. Sci. USA 100: 8424–8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella R. J., Kahari A., Haider S., Zamora J., Proctor G., et al. , 2011. Ensembl BioMarts: a hub for data retrieval across taxonomic space. Database (Oxford) 2011: bar030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc A., Wheeler L. J., Mathews C. K., Merrill G. F., 2004. Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. J. Biol. Chem. 279: 223–230. [DOI] [PubMed] [Google Scholar]
- Koonin E. V., 2005. Orthologs, paralogs, and evolutionary genomics. Annu. Rev. Genet. 39: 309–338. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Kinzler K. W., Vogelstein B., 1997. Genetic instability in colorectal cancers. Nature 386: 623–627. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Barnes D. E., 1992. Mammalian DNA ligases. Annu. Rev. Biochem. 61: 251–281. [DOI] [PubMed] [Google Scholar]
- Marini N. J., Thomas P. D., Rine J., 2010. The use of orthologous sequences to predict the impact of amino acid substitutions on protein function. PLoS Genet. 6: e1000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J. A., Davies M. W., Dimster-Denk D., Pleskac N., McCarthy S., et al. , 2012. Surrogate genetics and metabolic profiling for characterization of human disease alleles. Genetics 190: 1309–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey J. R., Thomas K., 1987. Human testis-specific PGK gene lacks introns and possesses characteristics of a processed gene. Nature 326: 501–505. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Miller J. R., 2007. Rescuing yeast mutants with human genes. Brief. Funct. Genomics Proteomics 6: 104–111. [DOI] [PubMed] [Google Scholar]
- Osborne A. R., Rapoport T. A., van den Berg B., 2005. Protein translocation by the Sec61/SecY channel. Annu. Rev. Cell Dev. Biol. 21: 529–550. [DOI] [PubMed] [Google Scholar]
- Pan X., Yuan D. S., Xiang D., Wang X., Sookhai-Mahadeo S., et al. , 2004. A robust toolkit for functional profiling of the yeast genome. Mol. Cell 16: 487–496. [DOI] [PubMed] [Google Scholar]
- Pan X., Yuan D. S., Ooi S. L., Wang X., Sookhai-Mahadeo S., et al. , 2007. dSLAM analysis of genome-wide genetic interactions in Saccharomyces cerevisiae. Methods 41: 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Prakash S., 1977. Isolation and characterization of MMS-sensitive mutants of Saccharomyces cerevisiae. Genetics 86: 33–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. B., Formosa T., 2000. POB3 is required for both transcription and replication in the yeast Saccharomyces cerevisiae. Genetics 155: 1593–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartzman J. M., Sotillo R., Benezra R., 2010. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nat. Rev. Cancer 10: 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling P. C., Bloom M. S., Solanki-Patil T., Smith S., Sipahimalani P., et al. , 2011. The complete spectrum of yeast chromosome instability genes identifies candidate CIN cancer genes and functional roles for ASTRA complex components. PLoS Genet. 7: e1002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnowski L. J., Kowalec P., Milewski M., Jurek M., Plochocka D., et al. , 2012. Nuclear import and export signals of human cohesins SA1/STAG1 and SA2/STAG2 expressed in Saccharomyces cerevisiae. PLoS One 7: e38740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugendreich S., Perkins E., Couto J., Barthmaier P., Sun D., et al. , 2001. A streamlined process to phenotypically profile heterologous cDNAs in parallel using yeast cell-based assays. Genome Res. 11: 1899–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDemark A. P., Blanksma M., Ferris E., Heroux A., Hill C. P., et al. , 2006. The structure of the yFACT Pob3-M domain, its interaction with the DNA replication factor RPA, and a potential role in nucleosome deposition. Mol. Cell 22: 363–374. [DOI] [PubMed] [Google Scholar]
- Yang X., Boehm J. S., Yang X., Salehi-Ashtiani K., Hao T., et al. , 2011. A public genome-scale lentiviral expression library of human ORFs. Nat. Methods 8: 659–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen K. W., Warren C. D., Chen O., Kwok T., Hieter P., et al. , 2007. Systematic genome instability screens in yeast and their potential relevance to cancer. Proc. Natl. Acad. Sci. USA 104: 3925–3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhang Z., Long F., Lee E. Y., 1996. Tyrosine-272 is involved in the inhibition of protein phosphatase-1 by multiple toxins. Biochemistry 35: 1606–1611. [DOI] [PubMed] [Google Scholar]
- Zhang N., Osborn M., Gitsham P., Yen K., Miller J. R., et al. , 2003. Using yeast to place human genes in functional categories. Gene 303: 121–129. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Reed R., 1998. Human homologs of yeast prp16 and prp17 reveal conservation of the mechanism for catalytic step II of pre-mRNA splicing. EMBO J. 17: 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Plasmids and strains are available upon request. Plasmids and strains used for screening tumor-specific variants in yeast are listed in Table S1.