Abstract
Fucose, the monosaccharide frequent in N- and O-glycans, is a part of Lewis-type antigens that are known to mediate direct sperm binding to the zona pellucida. Such interaction was found to be inhibited in vitro by fucose-containing oligo- and polysaccharides, as well as neoglycoproteins. The objective of this study was to screen seminal plasma proteins of infertile/subfertile men for the content and density of fucosylated glycoepitopes, and compare them to samples of fertile normozoospermic subjects. Seminal proteins were separated in polyacrylamide gel electrophoresis and blotted onto nitrocellulose membrane and probed with fucose-specific Aleuria aurantia lectin (AAL). Twelve electrophoretic bands were selected for quantitative densitometric analysis. It was found that the content, and especially the density of fucosylated glycans, were higher in glycoproteins present in seminal plasma of subfertile men. No profound differences in fucosylation density were found among the groups of normozoospermic, oligozoospermic, asthenozoospermic, and oligoasthenozoospermic subfertile men. According to the antibody probing, AAL-reactive bands can be attributed to male reproductive tract glycoproteins, including prostate-specific antigen, prostatic acid phosphatase, glycodelin and chorionic gonadotropin. Fibronectin, α1-acid glycoprotein, α1-antitrypsin, immunoglobulin G and antithrombin III may also contribute to this high fucosylation. It is suggested that the abundant fucosylated glycans in the sperm environment could interfere with the sperm surface and disturb the normal course of the fertilization cascade.
Keywords: fucose, glycosylation, infertility, seminal plasma
INTRODUCTION
The current knowledge of the etiology of male infertility is still insufficient. Most of the studies are focused on the count, motility, vitality and structure of sperm cells, features obviously involved in successful fertilization.1,2 Less attention has been paid for many years to the composition of seminal plasma, the microenvironment “designed” to maintain the spermatozoa in an appropriate condition. As this field has attracted more interest recently, an increasing number of studies on the seminal plasma proteome have become available.3,4,5,6,7
Apart from proteome composition, posttranslational modifications of proteins are of crucial interest, with glycosylation at the first line.8 Oligosaccharide structures that decorate cell surfaces and proteins present in the secretions mediate numerous cell-cell, cell-protein and protein-protein interactions. The contribution of carbohydrates to fertilization has been postulated ever since fucoidan (a sialylated fucose polymer) was found to inhibit gamete fusion (see the comment of Wassarman9). Only recently, matrix-assisted laser desorption/ionization time-of-flight profiling of human egg zona pellucida glycans revealed the unusual density of sialyl-Lewisx (sLex) epitopes and their critical role in sperm-egg binding.10 Moreover, this interaction has been inhibited in vitro with sLex containing oligosaccharides and neoglycoproteins. Therefore, the egg-binding ligands on the sperm surface must be sLex specific.
The impact of a carbohydrate-protein interaction was also demonstrated for the regulatory role of male and female glycodelin isoforms in time control of the acrosomal reaction.11,12 Glycodelin S (GdS) binds to the glycocalyx of a spermatozoon after ejaculation, and is replaced with female isoforms in the female reproductive tract. As these isoforms differ only in their oligosaccharide structure, the sperm head receptors must be able to distinguish between specific carbohydrate structures. Out of the two GdS oligosaccharides, one is known to contain numerous fucose residues in both the core and the antennary regions,12,13 the latter in the form of Lex and bifucosylated Ley epitopes.14
Detailed data on glycosylation in seminal plasma are limited to a small number of glycoproteins, e.g. glycodelin, prostate-specific antigen (PSA), α1-acid glycoprotein (AGP) and fibronectin (Fn), as we have reviewed recently.15 Some other glycoproteins, such as prostatic acid phosphatase (PAP), chorionic gonadotropin (CG) and prolactin-inducible protein, are less investigated, although the role of glycan structures for their function has been postulated.16,17,18,19 It seems possible that also other proteins present in seminal plasma are decorated with oligosaccharides able to mediate cell-cell or protein-protein interactions and contribute to this complex issue.
In this study, we compared general fucosylation in seminal plasma of fertile men with samples obtained from male partners living in childless couples suspected of male factor caused infertility, with respect to their spermiogram patterns. Our aim was to find out if fucose expression in glycoproteins of seminal plasma of subfertile men is altered and to indicate proteins/protein bands in which the alterations of fucose content and its accessibility for ligands allow one to distinguish fertile from infertile/subfertile subjects.
MATERIALS AND METHODS
Clinical material
Semen samples were collected after obtaining the patients’ informed consent, in accordance with the Declaration of Helsinki. The study was approved by the Medical University Bioethics Council (approval number KB-504/2012). Patients attending the 2nd Clinic of Gynecology and Obstetrics, Wrocław Medical University for intrauterine insemination were enrolled in the study. Only the male partners from couples in which there was no suspicion of female fertility problems (correct structure of the reproductive tract evaluated through ultrasound examination, normal ovulation) were included. The semen samples obtained by masturbation were liquefied, supplemented with buffered saline of Earle's solution and centrifuged (400 g) to obtain sperm for the insemination procedure. The supernatant containing all the components of seminal plasma, routinely discarded in the procedure, was collected and used as a material in the study. According to the earlier routine semen analysis, performed according to World Health Organization (WHO) directives,20 the samples were grouped into the following classes: normozoospermia (n = 67), oligozoospermia (n = 14), asthenozoospermia (n = 25) and oligoasthenozoospermia (n = 20). Brief characteristics of these groups are given in Table 1.
Table 1.
Characteristics of semen samples
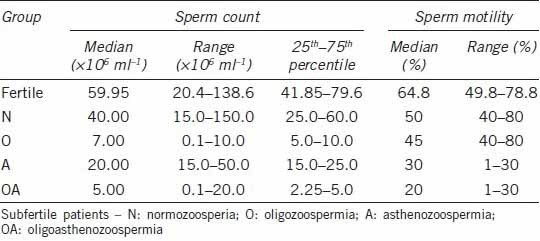
The control group comprised semen samples obtained from healthy volunteers with proven fertility (at least one child fathered), also after informed consent of the subjects (n = 12). In this group, semen parameters were compatible with the normal range in WHO-approved analysis (Table 1).
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
Seminal plasma proteins were separated in 12.5% gel in sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).21 Samples were denatured for 5 min with 2.5% beta mercaptoethanol and 1% SDS before the electrophoresis, 1.5 μg of protein (determined according to Bradford22) was loaded on the gel lane for protein staining and 2.5 μg per lane for lectin probing. In each gel one lane was loaded with 0.1 μg of bovine serum albumin, to calculate protein amounts in individual bands. After electrophoretic separation the gel was either stained with SyproRuby to visualize the protein bands or transferred onto a nitrocellulose membrane for lectin probing. Stained gels were digitalized with a CCD camera.
Western blotting and lectin probes
The nitrocellulose transferred gels were blocked overnight with 1% Tween-20 solution in 50 mmol l−1 Tris-HCl buffer, pH 7.5, 150 mmol l−1 NaCl (Tris-buffered saline (TBS)). The membranes were then incubated with biotinylated fucose-specific Aleuria aurantia lectin (AAL) in TBS-Tween 0.1%, and next with ExtrAvidin-Alkaline Phosphatase conjugate. The color reaction was developed using bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium salt as substrates, in 100 mmol l−1 Tris-HCl buffer pH = 9.5 containing 100 mmol l−1 NaCl and 50 mmol l−1MgCl2.
To avoid inter-experiment scattering and standardize the procedure of lectin probing, slot-blotting of pooled seminal plasma was used (30 normozoospermic specimens pooled by equal protein amount). Samples containing 2.5 μg of protein and their serial two-fold dilutions up to 19.5 ng of protein were loaded directly on the nitrocellulose membrane in the slot-blotting device. This strip was developed simultaneously with the western-blot, with the same blocking/lectin/conjugate/stain solutions. No saturation of bands up to 2.5 μg of seminal plasma protein, the amount loaded on the gel lane in SDS-PAGE, was observed. The band containing 0.31 μg of protein was taken as a reference for the calculation of the lectin reactivity in electrophoretically separated bands (Figure 1e and 1f).
Figure 1.
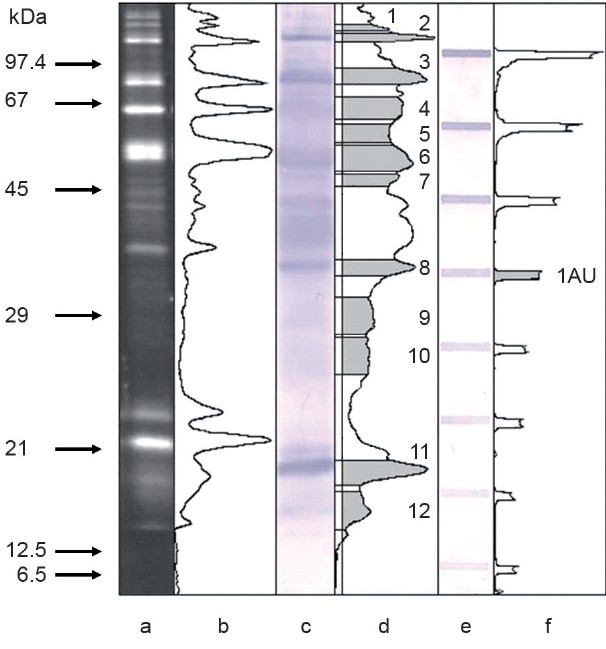
Electrophoretic analysis of seminal plasma glycoproteins. (a) SyproRuby stained gel, 1.5 μg of protein. (b) Densitometric profile of gel lane a. (c) Aleuria aurantia probed blot, 2.5 μg of protein. (d) Densitometric profile of lane c, background line and the area of measured bands are indicated. (e) Slot-blot of pooled seminal plasma sample probed with a. aurantia lectin. (f) Densitometric profile of lane e, the band considered as lectin reactivity equal to 1 arbitrary unit is indicated. Arrows on the left indicate molar mass calibrators.
Densitometric analysis
Digitalized gels and blots were subjected to densitometric analysis with ImageJ Image Processing and analysis software (National Institutes of Health, U.S. Department of Health and Human Services, Washington, DC, USA). Lectin reactivity was expressed in arbitrary units, defined as the optical density (OD) of an AAL-stained band of pooled seminal plasma containing 0.31 μg of protein. Thus, the AAL reactivity of band “x” was calculated as ODx/OD0.31 μg ref.
To see whether the possible alterations in the lectin reactivity are related to the increased/decreased protein amount or to the intensity of the glycosylation process itself, the density of fucosylated epitopes was calculated as the ratio of AAL reactivity to protein content in each individual electrophoretic band. The latter was calculated from SyproRuby stained gels.
Reactivity of seminal plasma with antibodies
Pooled seminal plasma, described in the previous paragraph, was used for SDS-PAGE. Five micrograms of protein were loaded onto gel lanes, except for glycodelin, requiring 20 μg. After Western blotting the nitrocellulose membranes were incubated with the following primary antibodies: polyclonal goat anti-transferrin (Tf), anti AGP, anti-α1-antitrypsin (α1-AT), anti-PAP, anti-immunoglobulin G (anti-IgG) and rabbit anti-Fn, anti-human CG (hCG), anti-PSA, anti-GdA. As glycodelin isoforms share an identical protein component, commercially available anti-GdA antibodies were used to detect the seminal plasma isoform. Secondary antibodies were horseradish peroxidase-labeled anti-goat or anti-rabbit immunoglobulins respectively. Blots were stained with H2O2 and diaminobenzidine.
Statistical analysis
Statistica 10.0 software (StatSoft Inc., Tulsa, OK, USA) was used for the data analysis. The distribution of the results was presented as whisker-box diagrams, showing median values (central point) and 25%–75% quartiles (boxes). Mann–Whitney nonparametric U-test was used to verify statistically significant differences between fertile (F) and subfertile/infertile (IFtot) groups. To evaluate differences among the groups of seminal plasma samples distinguished by means of routine semen analysis (normozoospermic subfertile, oligozoospermic, asthenozoospermic, oligoasthenozoospermic) data, Kruskal–Wallis ANOVA rank test was applied.
RESULTS
Examples of electrophoretic patterns of seminal plasma proteins are shown in Figure 1. Up to 20 well-separated bands were visualized with SyproRuby (Figure 1a) and detected with ImageJ software (Figure 1b). Most of them were fucosylated as shown by their binding of AAL in lectin blotting (Figure 1c). Both the gels and the blots were submitted to quantitative densitometric analysis (Figure 1b and 1d). Peaks were detected manually after the background correction. The area under the peak was used to calculate the amount of protein in each particular band, compared with OD of bovine serum albumin (100 ng per lane). Proportionality of lectin staining on Western blots was controlled by plotting of OD versus protein content in AAL-stained dot-blot (Figure 1e). Twelve AAL-binding bands were selected arbitrarily for a more detailed analysis, their molar masses ranging from 14 to 186 kDa (Table 2). Their ODs were compared against the groups of samples representing fertile and subfertile/infertile men. Further comparison also regarded the subgroups of subfertile subjects with different spermiogram patterns. The results are presented in Figure 2.
Table 2.
Molar masses of AAL-reactive bands subjected to quantitative analysis and their reactivity with antibodies
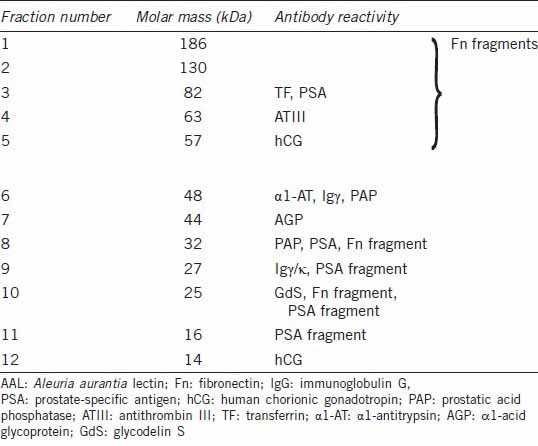
Figure 2.
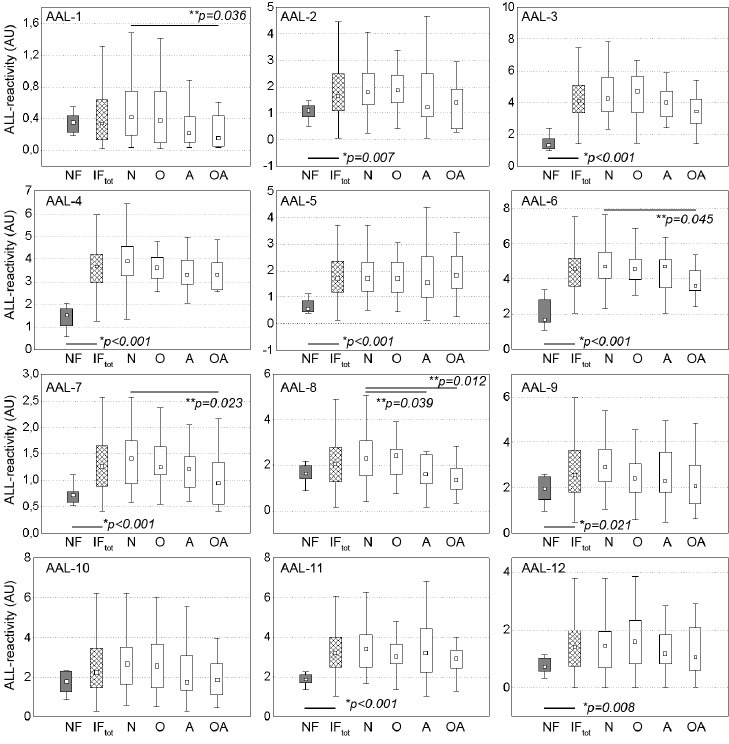
Lectin reactivity of seminal plasma glycoprotein bands. F: normozoospermic fertile; IFtot: infertile/subfertile, whole group, subgroups; N: normozoospermic; O: oligozoospermic; A: asthenozoospermic; OA: oligoasthenozoospermic. ■ : median; boxes: 25%–75% quartiles, whiskers: nonoutliers, *Statistical significance of the difference between fertile and subfertile groups, calculated with Mann–Whitney U-test; **Statistical significance of the difference among subgroups of subfertile subjects, calculated with Kruskal–Wallis ANOVA test.
In 9 out of 12 analyzed bands, increased (P < 0.05) AAL reactivity was observed in the infertile/subfertile group when compared to male subjects with confirmed fatherhood. Much higher inter-individual spread of values was also observed in the subfertile group as a whole, as well as in the subgroups distinguished by the semen analysis data. Statistically significant difference among the pathological groups (P < 0.05) was limited to OA versus N groups in 4 out of 12 bands and A versus N group in 1 band (Figure 2).
The question arises, what is the background of the described abundance of fucosylated structures: is it the result of increased content of proteins, with the same/similar rate of fucosylation as in the group of fertile men, or fucosylation itself is increased, leading to an elevated density of fucose-containing glycans, decorating the protein surface? To answer this question, the density of fucosylated epitopes has been estimated as the ratio of lectin binding to protein content in the individual bands and is shown in Figure 3. The density of fucosylated epitopes was increased in 11 out of 12 analyzed bands, and the only exception was the AAL-12 band. There were no statistically significant differences among the subgroups of subfertile subjects (P > 0.05).
Figure 3.
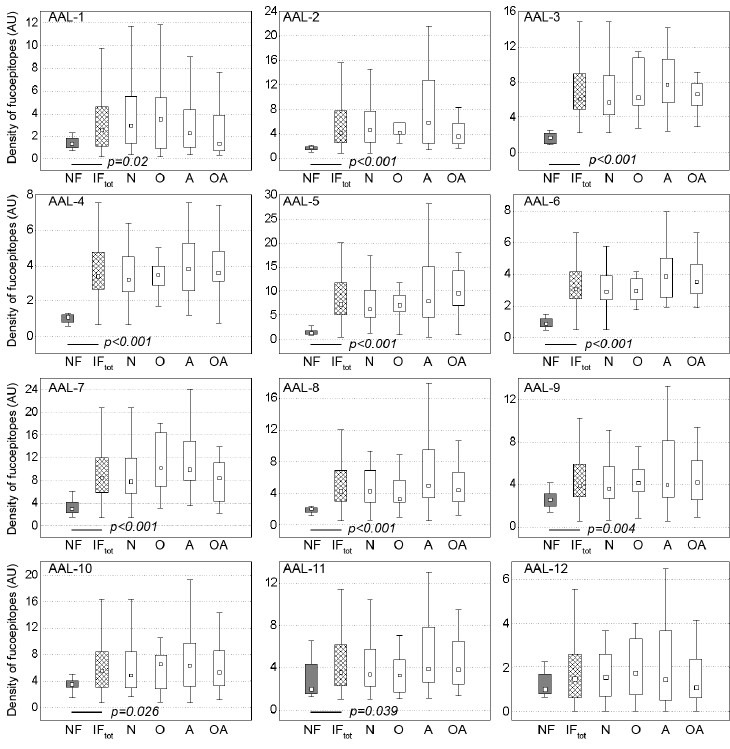
Density of fucosylated glycoepitopes in seminal plasma glycoproteins.F: normozoospermic fertile; IFtot: infertile/subfertile, whole group, subgroups; N: normozoospermic; O: oligozoospermic; A: asthenozoospermic; OA: oligoasthenozoospermic. ■ : median; boxes: 25%–75% quartiles, whiskers: nonoutliers. Mann–Whitney U-test was applied to evaluate statistical significance between F and IFtot groups. Only P < 0.05 values are shown. No statistically significant difference was found among subgroups of subfertile subjects with Kruskal–Wallis ANOVA test (all P > 0.05).
Identification of seminal plasma glycoproteins
In an attempt to identify glycoproteins apparently affected by increased fucosylation, a normozoospermic pooled seminal plasma sample was separated with SDS-PAGE, transferred onto nitrocellulose and probed with a wide panel of anti-human antibodies. First, we were looking for glycoproteins commonly considered as carriers of disease-related glycosylation changes, confirmed to be present in seminal plasma. Anti-AGP, Fn, α1-AT, antithrombin III (ATIII), IgG and Tf antibodies were thus applied. The panel of antibodies was subsequently completed with those directed at glycoproteins synthesized in the male reproductive tract and known (or suggested) to participate in the regulation of the fertilization cascade: anti-PSA, PAP, GdS and hCG. The blotting patterns are shown in Figure 4, and antibody-reactivity concomitant to AAL binding in Table 2.
Figure 4.
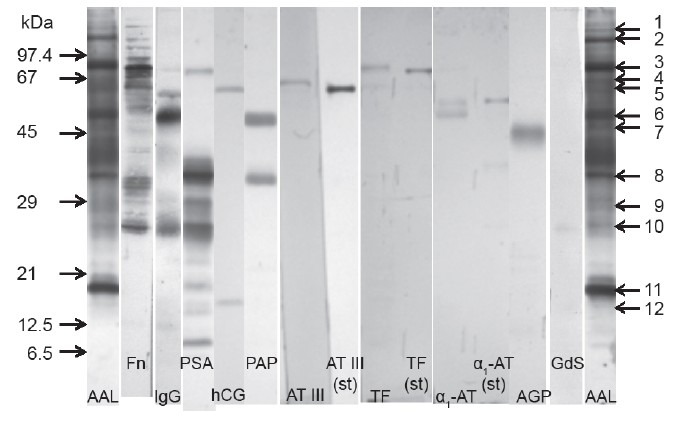
Immunoreactivity of seminal plasma glycoproteins compared to their AAL reactivity. AAL: Aleuria aurantia lectin; Fn: fibronectin; IgG: immunoglobulin G; PSA: prostate-specific antigen; HCG: human chorionic gonadotropin; PAP: prostatic acid phosphatase; ATIII: antithrombin III; TF: transferrin; α1-AT: α1-antitrypsin; AGP: α1-acid glycoprotein; GdS: glycodelin S. Arrows on the left indicate molar mass calibrators. Arrows on the right indicate consecutive numbers of AAL-reactive bands.
Single bands were observed in antibody-probed seminal plasma for ATIII, Tf and AGP. In ATIII and Tf electrophoretic mobility was lowered when compared with commercial, serum-derived standards, with a mass difference estimated as 1–3 kDa. A molar mass difference of this order is often related to altered glycosylation. Tf, ATIII and AGP bands corresponded to AAL-reactive bands 3, 4 and 7, respectively. Anti α1-AT antibodies detected two protein bands, one of them identical to the 52 kDa standard, and the other 4 kDa lighter. This band corresponded to AAL-6. Anti-IgG reactivity suggested fucosylation of both heavy (AAL-6) and light (AAL-9) chains.
The intact form of Fn was absent in the sample, and the main fragments identified with antibodies corresponded to AAL-2, -3, -8 and -10. Prominent fragmentation was also observed in PSA. Out of antibody reactive PSA fragments, 3 were also fucosylated and identical to AAL-9, -10 and -11. Apart from intact and fragmented PSA forms, antibodies were also bound by 82 kDa glycoprotein, intensive in AAL staining (AAL-3). The dominant PAP form corresponded to the AAL-6 band with molar mass of 48 kDa. Another form with molar mass of 32 kDa was also detected, representing the same mobility as PSA (and AAL-8). Anti-hCG antibodies, described by the manufacturer as both α and β subunit specific, also detected two bands, estimated as 57 (AAL-5) and 14 kDa (AAL-12). Anti-Gd antibodies detected a 25 kDa band (AAL-10).
DISCUSSION
Proteomic studies established the presence of over 900 proteins in the seminal fluid and prostatic secretions.4,17 One-third of them have been found to express binding activities,4 and some are involved in carbohydrate-protein interactions.23 Although various functions are being suggested for seminal plasma proteins, for most of them their role at the molecular level has not been elucidated in detail yet.24
Seminal plasma proteins, like most of the secretory ones, are decorated with N- and O-linked oligosaccharide chains, frequently terminated with fucose residues. This monosaccharide may be linked with α1,6 bond located in the core part of the glycan, and also with α1,3,α1,2 (rarely α1,4) bonds in glycan antennae, as part of Lewis-type antigens.
Lewis-type glycoepitopes are well known to mediate interactions of cells and proteins, but the role of core fucose is less understood so far. Remarkable amounts of fucose have been found in the seminal plasma glycome. Pang et al.25 reported the presence of several, up to 9, fucose residues in a single glycan in samples obtained from healthy fertile men. Next to core fucose, antennary Lex and Ley epitopes were present even in each of the three/four antennae. In spite of the relevant inter-subject variability of glycans, presented in that paper, high content of fucose seemed to be a common feature. Core and antennary fucosylation have also been reported in the majority of seminal plasma glycoproteins in which glycosylation has been studied, such as Fn,26,27 GdS11,12 and PSA.16,28 It seems that for sperm interactions the antennary location of fucose residues, especially of Lex type, is more important (or better explained) than the location in the glycan core. Antennary fucose is known to be recognized by Lotus tetragonolobus lectin, but only in the absence of sialic acid in the oligosaccharide. As the latter monosaccharide is also indispensable for sperm-egg binding,9 and abundant in seminal plasma secretory glycoproteins,17 in our investigations we decided to use AAL, a lectin much less selective in fucose binding. Although this lectin binds preferentially the core α1,6 bound fucose, it is also sufficiently effective in recognition of the antennary monosaccharide, and, moreover, the presence of sialic acid does not disturb the binding.
The investigations presented here have shown that an increase in AAL reactivity is observed in glycoproteins of seminal plasma of subfertile men, independently of their spermiogram pattern. The increase in the total fucose content may be related to some extent to increased expression of particular proteins, decorated with fucosylated glycans. Still, in most cases, increased density of fucosylated glycoepitopes is clearly visible, indicating intensification of the fucosylation pathway itself, independently from protein synthesis. Fucosylation of seminal plasma glycoproteins has been previously elucidated, including in our group. The pattern of fucosylation proved to be rather complex: in leukocytospermic samples an increase in antennary fucosylation was found in Fn, AGP26 and IgG.29 However, in these cases AAL reactivity was diminished. Fucosylation changes in AGP were also found to be related more to the protein level than to fertility status.30 In this study, we found that the abundant fucosylation seems to be a common feature of seminal plasma glycoproteins. The question of how such glycans could interfere with the interactions taking place in vivo needs further serious reflection. Lectin-like, carbohydrate-binding receptors, are present at the sperm surface.31 Such proteins are responsible, for example, for GdS binding. It might be possible that the excess of fucosylated glycans may disturb normal sperm interactions when the ejaculate is being formed or probably at the initial phase of capacitation, when PSA removes semenogelin and Fn, uncovering the sperm surface. It is also possible that the hyperfucosylation of numerous glycoproteins is simply the result of increased fucosylation pathway/fucosyltransferase activity in the secretory cells of the reproductive tract, but the biological consequence is limited to glycodelin or probably some other, still unidentified, glycoproteins, in which the altered glycan structure also alters their affinity to the receptors and kinetics of binding. Thus, the identification of the most effective carriers of fucosylated glycans seems to be interesting.
In our attempt to identify hyperfucosylated glycoproteins, we faced some surprising phenomena concerning electrophoretic mobility and location of antibody-reactive bands. In two glycoproteins synthesized in the male reproductive tract (PSA, hCG) we observed bands of higher than commonly reported molar masses. It seems that stable complexes are present in the sample, not dissociating in the experimental conditions. A similar pattern of PSA was earlier observed by Zhang et al.32 and of hCG by Berger et al.33 Still, the possible molecular structure of such isoforms is not established for sure. It seems important, however, that we found all these forms to be intensively AAL-reactive. In two other glycoproteins, the bands were slightly shifted towards higher molar masses than serum-derived standards (Tf, ATIII). Fragmentation of Fn and PSA is not surprising, as Fn is one of the main substrates for proteolytic PSA activity, and partial autolysis of the enzyme during sperm capacitation is also possible.32 The glycodelin fraction corresponded to the predicted molar mass.13 The structure of the 32 kDa form of PAP is also unresolved. There are also some controversies concerning the predicted mass of seminal CG. According to Zenzmaier et al.19 the dominant form in semen is a 24 kDa free α subunit. On the other hand, de Medeiros and Norman34 reports the α-subunit mass as 14 kDa, consistent with our AAL- and antibody-reactive fraction. This value is also consistent with the β subunit core region (hCGβcr), present in semen according to de Medeiros and Norman,34 though undetected by Zenzmaier et al.19
All the proteins examined with antibodies were found to correspond to AAL-reactive bands, supporting the hypothesis that fucosylation may be a common and important trait of seminal plasma glycoproteins, and hyperfucosylated glycans may disturb their natural course of mutual interactions. Those proteins which express the probable alterations to the highest extent, when identified, may serve as helpful glycomarkers in the examination of semen status. In SDS-PAGE, single bands can contain more than one protein; thus, it is not surprising that some AAL-reactive bands also react with different antibodies. It is not possible now to conclude which of them is responsible for the observed increased fucosylation. Although it could be suggested that the altered fucosylation pathway is attributable to the male reproductive tract, we have observed that AAL-reactive bands are also concomitant to the glycoproteins that may be regarded as serum-derived. On the other hand, it was well documented that in AGP the isoforms expressing altered glycosylation are synthesized in the prostate.30 The origin and contribution of the other proteins in total expression of fucosylated glycans will be an area of further studies, focused on confirmation of their identity in liquid chromatography-mass spectrometry/mass spectrometry. Fucose expression in these proteins will next be quantitatively analyzed in individual samples by means of lectin-ELISA to decide whether this parameter may be used as a kind of glyco-biomarker in elucidation of limited male fertility.
AUTHOR CONTRIBUTIONS
BO carried out the experimental work, densitometric and statistical analysis, discussed the results, and participated in data interpretation and drafting the manuscript. EMK verified routine semen analysis, assigned patients to appropriate groups, discussed the results and contributed in critical revision of the manuscript. MZ qualified patients for intrauterine insemination and the study, and assisted in critical revision of the manuscript. MFS conceived and designed the study, supervised the experimental work, participated in data analysis and interpretation, and prepared the manuscript. All authors read and approved the final manuscript.
COMPETING INTEREST
All authors declare no competing interests.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Ricardo Faundez from Warsaw Embryology Laboratory InviMed – European Center of Motherhood for his help in the recruitment of fertile normozoospermic volunteers for the study. The study was supported by Wrocław Medical University project Pbmn81.
REFERENCES
- 1.Barratt CL, Mansell S, Beaton C, Tardif S, Oxenham SK. Diagnostic tools in male infertility-the question of sperm dysfunction. Asian J Androl. 2011;13:53–8. doi: 10.1038/aja.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franken DR, Oehninger S. Semen analysis and sperm function testing. Asian J Androl. 2012;14:6–13. doi: 10.1038/aja.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fung KY, Glode LM, Green S, Duncan MW. A comprehensive characterization of the peptide and protein constituents of human seminal fluid. Prostate. 2004;61:171–81. doi: 10.1002/pros.20089. [DOI] [PubMed] [Google Scholar]
- 4.Pilch B, Mann M. Large-scale and high-confidence proteomic analysis of human seminal plasma. Genome Biol. 2006;7:R40. doi: 10.1186/gb-2006-7-5-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davalieva K, Kiprijanovska S, Noveski P, Plaseski T, Kocevska B, et al. Proteomic analysis of seminal plasma in men with different spermatogenic impairment. Andrologia. 2012;44:256–64. doi: 10.1111/j.1439-0272.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- 6.Tomar AK, Sooch BS, Singh S, Yadav S. Differential proteomics of human seminal plasma: a potential target for searching male infertility marker proteins. Proteomics Clin Appl. 2012;6:147–51. doi: 10.1002/prca.201100084. [DOI] [PubMed] [Google Scholar]
- 7.Milardi D, Grande G, Vincenzoni F, Messana I, Pontecorvi A, et al. Proteomic approach in the identification of fertility pattern in seminal plasma of fertile men. Fertil Steril. 2012;97:67–73.e1. doi: 10.1016/j.fertnstert.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Drake PM, Cho W, Li B, Prakobphol A, Johansen E, et al. Sweetening the pot: adding glycosylation to the biomarker discovery equation. Clin Chem. 2010;56:223–36. doi: 10.1373/clinchem.2009.136333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wassarman PM. Development. The sperm's sweet tooth. Science. 2011;333:1708–9. doi: 10.1126/science.1212841. [DOI] [PubMed] [Google Scholar]
- 10.Pang PC, Chiu PC, Lee CL, Chang LY, Panico M, et al. Human sperm binding is mediated by the sialyl-Lewis (x) oligosaccharide on the zona pellucida. Science. 2011;333:1761–4. doi: 10.1126/science.1207438. [DOI] [PubMed] [Google Scholar]
- 11.Seppälä M, Koistinen H, Koistinen R, Chiu PC, Yeung WS. Glycosylation related actions of glycodelin: gamete, cumulus cell, immune cell and clinical associations. Hum Reprod Update. 2007;13:275–87. doi: 10.1093/humupd/dmm004. [DOI] [PubMed] [Google Scholar]
- 12.Morris HR, Dell A, Easton RL, Panico M, Koistinen H, et al. Gender-specific glycosylation of human glycodelin affects its contraceptive activity. J Biol Chem. 1996;271:32159–67. doi: 10.1074/jbc.271.50.32159. [DOI] [PubMed] [Google Scholar]
- 13.Koistinen H, Koistinen R, Dell A, Morris HR, Easton RL, et al. Glycodelin from seminal plasma is a differentially glycosylated form of contraceptive glycodelin-A. Mol Hum Reprod. 1996;2:759–65. doi: 10.1093/molehr/2.10.759. [DOI] [PubMed] [Google Scholar]
- 14.Piludu M, Cossu M, De Lisa A, Piras M, Lantini MS. Ultrastructural localization of glycodelin oligosaccharides Le-x and Le-y in human seminal vesicles by immunogold staining. J Anat. 2007;210:352–6. doi: 10.1111/j.1469-7580.2007.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferens-Sieczkowska M, Kowalska B, Kratz EM. Seminal plasma glycoproteins in male infertility and prostate diseases: is there a chance for glyco-biomarkers? Biomarkers. 2013;18:10–22. doi: 10.3109/1354750X.2012.719035. [DOI] [PubMed] [Google Scholar]
- 16.White KY, Rodemich L, Nyalwidhe JO, Comunale MA, Clements MA, et al. Glycomic characterization of prostate-specific antigen and prostatic acid phosphatase in prostate cancer and benign disease seminal plasma fluids. J Proteome Res. 2009;8:620–30. doi: 10.1021/pr8007545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake RR, White KY, Fuller TW, Igwe E, Clements MA, et al. Clinical collection and protein properties of expressed prostatic secretions as a source for biomarkers of prostatic disease. J Proteomics. 2009;72:907–17. doi: 10.1016/j.jprot.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan MI, Bilgrami S, Kumar V, Singh N, Yadav S, et al. Crystal structure of the novel complex formed between zinc alpha2-glycoprotein (ZAG) and prolactin-inducible protein (PIP) from human seminal plasma. J Mol Biol. 2008;384:663–72. doi: 10.1016/j.jmb.2008.09.072. [DOI] [PubMed] [Google Scholar]
- 19.Zenzmaier C, Gerth R, Gruschwitz M, Lindner H, Plas E, et al. Decreased levels of genuine large free hCG alpha in men presenting with abnormal semen analysis. Reprod Biol Endocrinol. 2011;9:114. doi: 10.1186/1477-7827-9-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.5th ed. Geneva: WHO; 2010. World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen. [Google Scholar]
- 21.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 23.Kumar V, Hassan MI, Tomar AK, Kashav T, Nautiyal J, et al. Proteomic analysis of heparin-binding proteins from human seminal plasma: a step towards identification of molecular markers of male fertility. J Biosci. 2009;34:899–908. doi: 10.1007/s12038-009-0104-5. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez-Martínez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal plasma proteins: what role do they play? Am J Reprod Immunol. 2011;66(Suppl 1):11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 25.Pang PC, Tissot B, Drobnis EZ, Morris HR, Dell A, et al. Analysis of the human seminal plasma glycome reveals the presence of immunomodulatory carbohydrate functional groups. J Proteome Res. 2009;8:4906–15. doi: 10.1021/pr9001756. [DOI] [PubMed] [Google Scholar]
- 26.Kratz EM, Faundez R, Katnik-Prastowska I. Fucose and sialic acid expressions in human seminal fibronectin and α1-acid glycoprotein associated with leukocytospermia of infertile men. Dis Markers. 2011;31:317–25. doi: 10.3233/DMA-2011-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosanovic MM, Jankovic MM. Molecular heterogeneity of gelatin-binding proteins from human seminal plasma. Asian J Androl. 2010;12:363–75. doi: 10.1038/aja.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada T, Sato Y, Kobayashi N, Sumida K, Satomura S, et al. Structural characteristics of the N-glycans of two isoforms of prostate-specific antigens purified from human seminal fluid. Biochim Biophys Acta. 2001;1525:149–60. doi: 10.1016/s0304-4165(00)00182-3. [DOI] [PubMed] [Google Scholar]
- 29.Kratz EM, Ferens-Sieczkowska M, Faundez R, Katnik-Prastowska I. Changes in fucosylation of human seminal IgG and secretory component of IgA in leukocytospermic patients. Glycoconj J. 2014;31:51–60. doi: 10.1007/s10719-013-9501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poland DC, Kratz E, Vermeiden JP, De Groot SM, Bruyneel B, et al. High level of alpha1-acid glycoprotein in human seminal plasma is associated with high branching and expression of Lewis (a) groups on its glycans: supporting evidence for a prostatic origin. Prostate. 2002;52:34–42. doi: 10.1002/pros.10085. [DOI] [PubMed] [Google Scholar]
- 31.Wassarman PM. Towards molecular mechanisms for gamete adhesion and fusion during mammalian fertilization. Curr Opin Cell Biol. 1995;7:658–64. doi: 10.1016/0955-0674(95)80107-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhang WM, Leinonen J, Kalkkinen N, Dowell B, Stenman UH. Purification and characterization of different molecular forms of prostate-specific antigen in human seminal fluid. Clin Chem. 1995;41:1567–73. [PubMed] [Google Scholar]
- 33.Berger P, Gruschwitz M, Spoettl G, Dirnhofer S, Madersbacher S, et al. Human chorionic gonadotropin (hCG) in the male reproductive tract. Mol Cell Endocrinol. 2007;260-262:190–6. doi: 10.1016/j.mce.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 34.de Medeiros SF, Norman RJ. Human choriogonadotrophin protein core and sugar branches heterogeneity: basic and clinical insights. Hum Reprod Update. 2009;15:69–95. doi: 10.1093/humupd/dmn036. [DOI] [PubMed] [Google Scholar]


