Abstract
IMPORTANCE
The prevalence of cardiometabolic multimorbidity is increasing.
OBJECTIVE
To estimate reductions in life expectancy associated with cardiometabolic multimorbidity.
DESIGN, SETTING, AND PARTICIPANTS
Age- and sex-adjusted mortality rates and hazard ratios (HRs) were calculated using individual participant data from the Emerging Risk Factors Collaboration (689 300 participants; 91 cohorts; years of baseline surveys: 1960–2007; latest mortality follow-up: April 2013; 128 843 deaths). The HRs from the Emerging Risk Factors Collaboration were compared with those from the UK Biobank (499 808 participants; years of baseline surveys: 2006–2010; latest mortality follow-up: November 2013; 7995 deaths). Cumulative survival was estimated by applying calculated age-specific HRs for mortality to contemporary US age-specific death rates.
EXPOSURES
A history of 2 or more of the following: diabetes mellitus, stroke, myocardial infarction (MI).
MAIN OUTCOMES AND MEASURES
All-cause mortality and estimated reductions in life expectancy.
RESULTS
In participants in the Emerging Risk Factors Collaboration without a history of diabetes, stroke, or MI at baseline (reference group), the all-cause mortality rate adjusted to the age of 60 years was 6.8 per 1000 person-years. Mortality rates per 1000 person-years were 15.6 in participants with a history of diabetes, 16.1 in those with stroke, 16.8 in those with MI, 32.0 in those with both diabetes and MI, 32.5 in those with both diabetes and stroke, 32.8 in those with both stroke and MI, and 59.5 in those with diabetes, stroke, and MI. Compared with the reference group, the HRs for all-cause mortality were 1.9 (95%CI, 1.8–2.0) in participants with a history of diabetes, 2.1 (95%CI, 2.0–2.2) in those with stroke, 2.0 (95%CI, 1.9–2.2) in those with MI, 3.7 (95%CI, 3.3–4.1) in those with both diabetes and MI, 3.8 (95%CI, 3.5–4.2) in those with both diabetes and stroke, 3.5 (95%CI, 3.1–4.0) in those with both stroke and MI, and 6.9 (95%CI, 5.7–8.3) in those with diabetes, stroke, and MI. The HRs from the Emerging Risk Factors Collaboration were similar to those from the more recently recruited UK Biobank. The HRs were little changed after further adjustment for markers of established intermediate pathways (eg, levels of lipids and blood pressure) and lifestyle factors (eg, smoking, diet). At the age of 60 years, a history of any 2 of these conditions was associated with 12 years of reduced life expectancy and a history of all 3 of these conditions was associated with 15 years of reduced life expectancy.
CONCLUSIONS AND RELEVANCE
Mortality associated with a history of diabetes, stroke, or MI was similar for each condition. Because any combination of these conditions was associated with multiplicative mortality risk, life expectancy was substantially lower in people with multimorbidity.
The prevalence of cardiometabolic multimorbidity (defined herein as a history of ≥2 of the following: diabetes mellitus, stroke, myocardial infarction [MI]) is increasing rapidly.1–3 Considerable evidence exists about the mortality risk of having any 1 of these conditions alone.4–7 However, evidence is sparse about life expectancy among people who have 2 or 3 cardiometabolic conditions concomitantly. Valid estimation of the associations of cardiometabolic multimorbidity with mortality requires comparison of people with multimorbidity with participants within the same cohorts who did not have any of the conditions at baseline. However, few population cohorts have had sufficient power, detail, and longevity to enable such comparisons.8–14
We aimed to provide reliable estimates of the associations of cardiometabolic multimorbidity with mortality and reductions in life expectancy. We analyzed individual participant data in the Emerging Risk Factors Collaboration (ERFC) from 689 300 participants recruited during 1960 through 2007 into 91 prospective cohorts that have recorded mortality during prolonged follow-up. We compared the ERFC results with those from the UK Biobank, a prospective cohort study of 499 808 participants recruited during 2006 through 2010.
Methods
Overall Design
Our analysis involved several interrelated components (eFigure 1 in the Supplement). First, we quantified associations of cardiometabolic multimorbidity with all-cause mortality. To maximize power, we analyzed data from the ERFC in which a total of about 129 000 deaths have accrued. Second, we compared results from the ERFC with those from the UK Biobank. The UK Biobank recruited participants more recently than the ERFC and it had accrued about 8000 deaths at the time of this analysis. Third, we estimated reductions in life expectancy associated with cardiometabolic multimorbidity by applying results from the ERFC to contemporary US age-specific death rates. Fourth, we placed our findings in the context of previous relevant studies identified through a systematic review.
Data Sources
Both the ERFC and the UK Biobank have been described.15–17 Prospective cohort studies contributing to the ERFC were included in this analysis if they met all the following criteria: (1) had recruited participants on the basis of informed consent, (2) had recorded information about the diagnosis of diabetes, stroke, and MI at the baseline survey, (3) did not select participants on the basis of having previous chronic disease (including cardiovascular disease and diabetes), (4) had recorded cause-specific deaths, and (5) had accrued more than 1 year of follow-up. Details of the contributing studies in the ERFC are presented in eTable 1 and eAppendix 2 in the Supplement. Information on the methods used to characterize diagnosis of diabetes, stroke, and MI at the baseline survey are presented in eTable 2. The contributing studies classified deaths according to the primary cause (or, in its absence, the underlying cause), on the basis of coding from the International Classification of Diseases, Eighth-Tenth Revisions, to at least 3 digits, or according to study-specific classification systems. Classification of deaths was based on death certificates, which was supplemented in 53 studies by medical records, findings on autopsy, and other sources. The date of the latest mortality follow-up was April 2013.
In the UK Biobank, information on a baseline history of diabetes, stroke, and MI was available for 499 808 participants recruited from 22 centers throughout the United Kingdom (eAppendix 3 in the Supplement). After giving consent, participants provided biological samples and completed a touch-screen questionnaire, a computer-assisted interview, and a physical examination. Participants have been linked with the death records of the UK Office for National Statistics through National Health Service identification numbers. Deaths were classified according to the primary cause (or, in its absence, the underlying cause), or on the basis of coding from the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, to at least 3 digits. The date of the latest mortality follow-up was November 2013.
Details of our systematic review of population-based prospective studies reported between January 1970 and April 2015 appear in eAppendix 4 in the Supplement. No language restrictions were applied to the publications. Studies were not eligible for the review if they had contributed data to the ERFC.8,13,18 Two authors (P.W. and L.M.O.K.) extracted and cross-checked information from publications according to a prespecified protocol and disagreements were resolved by a third author (E.D.A.).
Approval was provided by the Cambridgeshire Ethics Review Committee.
Statistical Analysis
For both the ERFC and the UK Biobank, we categorized participants into the following 8 mutually exclusive groups according to baseline disease: (1) diabetes, (2) stroke, (3) MI, (4) diabetes and MI, (5) diabetes and stroke, (6) stroke and MI, (7) diabetes, stroke, and MI, (8) none of these (reference group). We assessed associations of these baseline groups with the risk of death from any cause.
Hazard ratios (HRs) were calculated using Cox proportional hazards regression models. The principal objective of our study was to estimate reductions in life expectancy associated with having different combinations of cardiometabolic multimorbidity. To this end, our primary analysis calculated HRs stratified by sex and adjusted for age only. A secondary objective was to explore the extent to which markers of some established intermediate pathways (ie, total and high-density lipoprotein cholesterol, blood pressure, body mass index) and lifestyle factors (ie, smoking, diet, socioeconomic status) could explain associations between cardiometabolic multimorbidity and mortality. To this end, subsidiary analyses calculated HRs adjusted for these additional factors. The HRs in the ERFC were calculated using a 2-stage approach, with estimates calculated separately within each study before pooling across studies by random-effects meta-analysis using an extension of the DerSimonian and Laird procedure.16,19
Participants were included in the analyses irrespective of previous nonfatal events. For each specific cause of death, outcomes were censored if a participant was lost to follow-up, died of other causes, or reached the end of the follow-up period. The proportional hazards assumption was satisfied for all-cause mortality (eFigure 2 in the Supplement). We used the I2 statistic to quantify between-study heterogeneity and the Wald test to assess interactions.
Because age-specific mortality rates cannot be directly obtained from a 2-stage approach using Cox regression models (ie, these models estimate instantaneous probability of death), we used a 2-level mixed-effects Poisson regression model with random study intercept adjusted for baseline disease status, sex and age at risk (linear and quadratic terms), and interactions of age at risk with the preceding variables. This Poisson regression model was used to obtain mortality rates adjusted to the age of 60 years (ie, marginal effects).
Detail of the methods used to estimate reductions in life expectancy is in eAppendix 5 in the Supplement. Briefly, estimates of cumulative survival from 40 years of age onward among the 8 baseline disease groups were calculated by applying the HRs for cause-specific mortality from the ERFC (specific to age at risk and sex) to the detailed mortality component of the US Centers for Disease Control and Prevention’s CDC WONDER database, which recorded almost 10 million deaths among more than 305 million individuals during 2007 through 2010.20,21 We modeled results throughout middle age and old age, giving specific consideration to the HRs with cardiometabolic multimorbidity recorded by the age of 60 years, the period of life when multimorbidity becomes increasingly common.22 Analyses involved Stata version 12.0 (StataCorp), 2-sided P values, and used a significance level of P < .05.
Results
Emerging Risk Factors Collaboration
At baseline, the mean (SD) age was 53 (9) years and 51% were women (Table 1). The large majority of participants were enrolled in Europe (69%) or North America (24%) (eTable 1 in the Supplement). Of 689 300 participants, 24 677 (3.6%) had a history of diabetes at enrollment, 8583 (1.2%) had stroke, 21 591 (3.1%) had MI, 3233 (0.5%) had a history of both diabetes and MI, 1321 (0.2%) had both diabetes and stroke, 1836 (0.3%) had both stroke and MI, and 541 (0.1%) had diabetes, stroke, and MI. There were 128 843 deaths (50 595 due to vascular causes; 39 266, cancer; 30 664, other causes; and 8318, unknown or ill-defined causes) during8.83 million person-years at risk (median follow-up, 12.8 years; 5th–95th percentile, 4.0–29.5 years) (eTable 1).
Table 1.
Baseline Characteristics of Participants by Disease Status at Baseline
| Disease Status at Baseline | ||||||||
|---|---|---|---|---|---|---|---|---|
| None | Diabetes | Stroke | MI | Diabetes and MI |
Diabetes and Stroke |
Stroke and MI |
Diabetes, Stroke, and MI |
|
| Emerging Risk Factors Collaboration (91 Studies; 689 300 Participants) | ||||||||
| No. (%) of participants | 627 518 (91.0) | 24 677 (3.6) | 8583 (1.2) | 21 591 (3.1) | 3233 (0.5) | 1321 (0.2) | 1836 (0.3) | 541 (0.1) |
| Age at survey, mean (SD), y | 52.1 (8.9) | 57.3 (8.1) | 50.9 (7.8) | 60.5 (7.0) | 69.4 (6.5) | 67.7 (6.8) | 69.8 (6.9) | 63.8 (6.9) |
| Male sex, No. (%)a | 305 031 (49) | 12 347 (50) | 4496 (52) | 14 643 (68) | 2121 (66) | 738 (56) | 1232 (67) | 322 (60) |
| Current smoker, No. (%)a | 197 335 (31) | 5343 (22) | 2086 (24) | 5759 (27) | 515 (16) | 224 (17) | 412 (22) | 82 (15) |
| Systolic blood pressure, mean (SD), mm Hg | 132 (19) | 141 (21) | 142 (22) | 139 (22) | 142 (22) | 150 (22) | 144 (23) | 146 (22) |
| Body mass index, mean (SD)b | 25.6 (4.2) | 27.9 (5.3) | 26.3 (4.5) | 26.6 (4.3) | 30.5 (4.8) | 29.0 (5.2) | 27.3 (4.5) | 28.1 (5.1) |
| Cholesterol, mean (SD), mmol/L | ||||||||
| Total | 5.84 (1.12) | 5.67 (1.18) | 5.85 (1.12) | 5.87 (1.15) | 5.93 (1.14) | 5.70 (1.18) | 5.76 (1.14) | 5.30 (1.15) |
| High-density lipoprotein | 1.37 (0.39) | 1.24 (0.37) | 1.33 (0.40) | 1.22 (0.36) | 1.10 (0.34) | 1.15 (0.34) | 1.12 (0.37) | 1.06 (0.33) |
| UK Biobank (499 808 Participants) | ||||||||
| No. (%) of participants | 461 754 (92.4) | 18 549 (3.7) | 6835 (1.4) | 8770 (1.8) | 2036 (0.4) | 966 (0.2) | 668 (0.1) | 230 (0.05) |
| Age at survey, mean (SD), y | 56.7 (8.1) | 59.6 (7.2) | 60.8 (7.0) | 62.1 (6.3) | 62.7 (5.7) | 62.2 (6.2) | 62.5 (6.1) | 61.7 (6.5) |
| Male sex, No. (%)c | 202 816 (44) | 11 184 (60) | 3683 (54) | 6981 (80) | 1709 (84) | 627 (65) | 500 (75) | 178 (77) |
| Current smoker, No. (%)c | 47 771 (10) | 1983 (11) | 1057 (15) | 1249 (14) | 277 (14) | 131 (14) | 145 (22) | 55 (24) |
| Systolic blood pressure, mean (SD), mm Hg | 137 (19) | 141 (17) | 140 (19) | 136 (19) | 138 (19) | 141 (19) | 137 (20) | 137 (18) |
| Body mass index, mean (SD)b | 27.2 (4.7) | 31.2 (5.9) | 28.3 (4.9) | 28.8 (4.6) | 31.8 (5.4) | 31.8 (5.9) | 29.3 (5.1) | 31.9 (5.3) |
| Education (vocational or university), No./Total (%) | 278 419/457 263 (61) | 9813/18 162 (54) | 3344/6746 (50) | 4127/8636 (48) | 851/1989 (43) | 409/945 (43) | 281/657 (43) | 89/224 (40) |
| Food consumption | ||||||||
| Meat (≥2/wk), No. (%)c | 301 797 (65) | 13 006 (70) | 4555 (67) | 6154 (70) | 1479 (73) | 672 (70) | 474 (71) | 158 (69) |
| Fruit (≥3/d)c | 165 676 (36) | 7915 (43) | 2393 (35) | 2966 (34) | 824 (41) | 433 (45) | 224 (34) | 99 (43) |
Abbreviation: MI, myocardial infarction.
SI conversion factors: To convert high-density lipoprotein and total cholesterol to mg/dL, divide by 0.0259.
The denominators used to calculate the percentages are in row 2 of this Table.
Calculated as weight in kilograms divided by height in meters squared.
The denominators used to calculate the percentages are in row 12 of this Table.
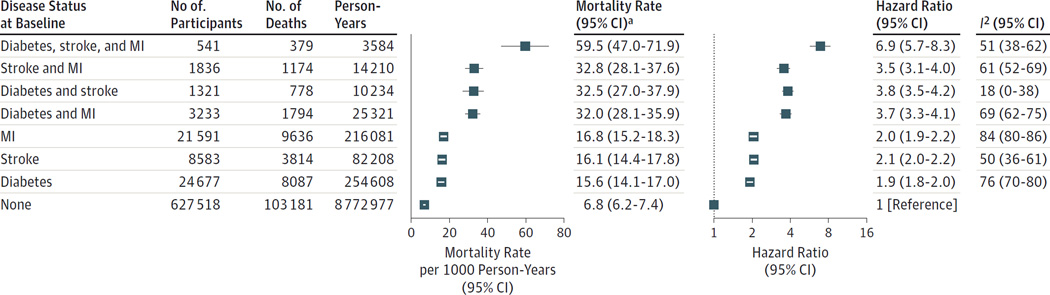
In the reference group, the sex-adjusted mortality rate at the age of 60 years was 6.8 (95% CI, 6.2–7.4) per 1000 person-years at risk. By contrast, the age- and sex-adjusted mortality rates were 15.6 (95% CI, 14.1–17.0) in participants with a history of diabetes, 16.1 (95% CI, 14.4–17.8) in those with stroke, 16.8 (95% CI, 15.2–18.3) in those with MI, 32.0 (95% CI, 28.1–35.9) in those with a history of both diabetes and MI, 32.5 (95% CI, 27.0–37.9) in those with both diabetes and stroke, 32.8 (95% CI, 28.1–37.6) in those with both stroke and MI, and 59.5 (95% CI, 47.0–71.9) in those with diabetes, stroke, and MI (Figure 1).
Figure 1.
All-Cause Mortality for the Emerging Risk Factors Collaboration by Disease Status of Participants at Baseline
The mortality rates were calculated using a Poisson regression model and are sex-adjusted rates to the age of 60 years. The hazard ratios were calculated using a Cox proportional hazards regression model and are stratified by sex and adjusted by age at baseline. Analyses were based on participants from 91 studies.
MI indicates myocardial infarction.
a Mortality rate is per 1000 person-years.
Compared with the reference group, the age- and sex-adjusted HRs for mortality were 1.9 (95% CI, 1.8–2.0) for participants with a history of diabetes, 2.1 (95% CI, 2.0–2.2) in those with stroke, 2.0 (95% CI, 1.9–2.2) in those with MI, 3.7 (95% CI, 3.3–4.1) in those with a history of both diabetes and MI, 3.8 (95% CI, 3.5–4.2) in those with both diabetes and stroke, 3.5 (95% CI, 3.1–4.0) in those with both stroke and MI, and 6.9 (95% CI, 5.7–8.3) in those with diabetes, stroke, and MI (Figure 1).
The HRs for participants with a history of 2 or more conditions were generally consistent with multiplicative effects (P > .05 for deviation from multiplicative effects), with the exception of the HR for those with a history of both stroke and MI (P < .001). The HRs were stronger among women than men for participants with diabetes only, stroke only, and those with both diabetes and MI (P < .001; eFigure 3 in the Supplement). The HRs were little changed after additional adjustment for smoking (Table 2). The HRs attenuated slightly after further adjustment for total and high-density lipoprotein cholesterol, systolic blood pressure, and body mass index. In participants with all 3 conditions at baseline, the age- and sex-adjusted HRs were 11.8 (95% CI, 9.6–14.6) for cardiovascular mortality, 2.1 (95%CI, 1.5–2.9) for cancer mortality, and 7.9(95% CI, 6.6–9.6) for the aggregate of nonvascular, noncancer deaths (eFigure 4).
Table 2.
All-Cause Mortality in Participants With Information on Cardiovascular Risk Factors and Other Characteristics
| Disease Status at Baseline | No. of Participants | No. of Deaths | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|---|---|
| Age and Sex | Age, Sex, and Smoking |
Age, Sex, Smoking, and Intermediate Risk Factors |
Age, Sex, Smoking, Intermediate Risk Factors, and Other Lifestyle Factors |
|||
| Emerging Risk Factors Collaboration (68 Studies, 355 639 Participants, 47 067 Deaths)a | ||||||
| Diabetes, stroke, and MI | 260 | 165 | 6.2 (5.1–7.4) | 6.3 (5.2–7.5) | 6.0 (5.0–7.1) | |
| Stroke and MI | 921 | 517 | 3.7 (3.1–4.3) | 3.8 (3.2–4.4) | 3.7 (3.2–4.4) | |
| Diabetes and stroke | 654 | 334 | 3.7 (3.3–4.2) | 3.9 (3.4–4.4) | 3.6 (3.2–4.1) | |
| Diabetes and MI | 1827 | 930 | 3.6 (3.1–4.0) | 3.8 (3.3–4.4) | 3.6 (3.2–4.1) | |
| MI | 12 141 | 4270 | 2.0 (1.9–2.1) | 2.0 (1.9–2.2) | 2.0 (1.9–2.2) | |
| Stroke | 4357 | 1530 | 2.1 (1.9–2.2) | 2.0 (1.9–2.2) | 2.0 (1.8–2.1) | |
| Diabetes | 12 887 | 3629 | 1.9 (1.7–2.0) | 1.9 (1.8–2.0) | 1.8 (1.7–1.9) | |
| None | 322 592 | 35 692 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |
| UK Biobank (491 424 Participants, 7688 Deaths)b | ||||||
| Diabetes, stroke, and MI | 218 | 26 | 5.8 (3.9–8.5) | 5.2 (3.5–7.7) | 4.9 (3.3–7.2) | 4.9 (3.3–7.2) |
| Stroke and MI | 638 | 51 | 3.6 (2.7–4.7) | 3.2 (2.5–4.3) | 3.1 (2.4–4.1) | 3.1 (2.3–4.0) |
| Diabetes and stroke | 919 | 75 | 3.9 (3.1–4.9) | 3.8 (3.0–4.8) | 3.6 (2.9–4.5) | 3.6 (2.8–4.5) |
| Diabetes and MI | 1943 | 190 | 4.3 (3.7–5.0) | 4.2 (3.6–4.8) | 4.0 (3.4–4.6) | 3.9 (3.4–4.5) |
| MI | 8572 | 407 | 2.1 (1.9–2.3) | 2.0 (1.8–2.3) | 2.0 (1.8–2.2) | 2.0 (1.8–2.2) |
| Stroke | 6632 | 259 | 2.1 (1.8–2.4) | 2.0 (1.8–2.3) | 2.0 (1.7–2.2) | 1.9 (1.7–2.2) |
| Diabetes | 17 928 | 504 | 1.6 (1.5–1.8) | 1.6 (1.5–1.8) | 1.5 (1.4–1.7) | 1.5 (1.4–1.7) |
| None | 454 574 | 6176 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
Abbreviation: MI, myocardial infarction.
The intermediate risk factors available were body mass index; systolic blood pressure; and high-density lipoprotein and total cholesterol.
The intermediate risk factors available were body mass index and systolic blood pressure. Other lifestyle factors available were socioeconomic status (defined as education level) and diet (defined as self-reported consumption of meat and fruit).
Broadly similar HRs to those noted above were observed in analyses that (1) used alternative definitions of baseline disease (eFigure 5 in the Supplement), (2)were restricted to studies that supplemented death certificates with additional information (eFigure 6), (3) excluded the initial 5 years of follow-up (eFigure 7), or (4) used fixed-effect meta-analysis (eFigure 8). The HRs for mortality appeared to decline somewhat with increasing calendar year of baseline study enrollment (eFigure 9).
UK Biobank
At baseline, the mean (SD) age was 57 (8) years and 55%were women (Table 1). Of 499 808 participants, 18 549 (3.7%) had a history of diabetes at enrollment,6835 (1.4%)hadstroke,8770 (1.8%) had MI, 2036 (0.4%) had a history of both diabetes and MI, 966 (0.2%) had both diabetes and stroke, 688 (0.1%) had both stroke and MI, and 230 (0.05%) had diabetes, stroke, and MI. There were 7995 deaths during 2.39 million person-years at risk (median follow-up, 4.8 years; interquartile range, 4.1–5.5 years).
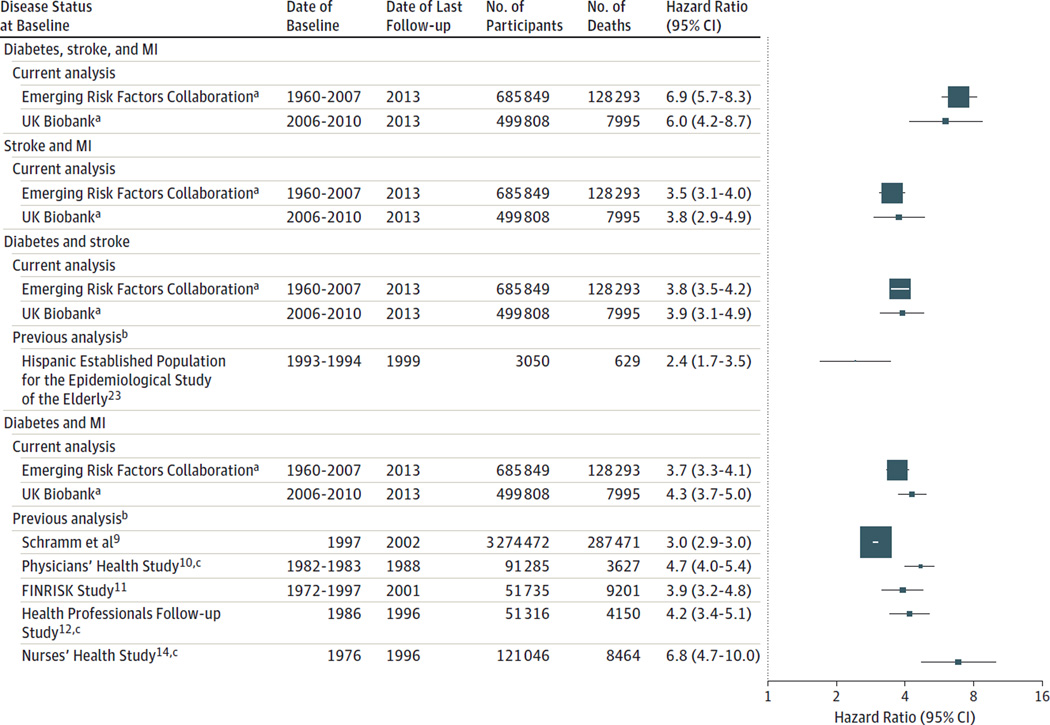
Compared with the reference group, the age- and sex-adjusted HRs for mortality were 1.6 (95% CI, 1.5–1.8) for participants with diabetes, 2.1 (95% CI, 1.9–2.4) for those with stroke, 2.1 (95% CI, 1.9–2.3) for those with MI, 4.3 (95% CI, 3.7–5.0) for those with both diabetes and MI, 3.9 (95% CI, 3.1–4.9) for those with both diabetes and stroke, 3.8 (95% CI, 2.9–4.9) for those with both stroke and MI, and 6.0 (95% CI, 4.2–8.7) for those with diabetes, stroke, and MI (Figure 2 and eTable 3 in the Supplement). The HRs were little changed after additional adjustment for smoking, systolic blood pressure, body mass index, diet, and socioeconomic status (Table 2).
Figure 2.
All-Cause Mortality From the Emerging Risk Factors Collaboration Compared With the UK Biobank and Previous Reports
The hazard ratios were adjusted for sex when appropriate and age, except those for the Hispanic Established Population for the Epidemiological Study of the Elderly, which were adjusted for additional variables. MI indicates myocardial infarction. The size of the data markers is proportional to the information content in each study.
a For participant-level analyses in the Emerging Risk Factors Collaboration and the UK Biobank, participants with the disease status indicated at baseline have been compared with participants within the same cohorts without diabetes, stroke, or myocardial infarction at baseline.
b For previously published studies, participants with cardiometabolic multimorbidity at baseline were compared with participants without any such conditions.
c Used history of coronary heart disease instead of history of MI.
Estimated Reductions in Life Expectancy
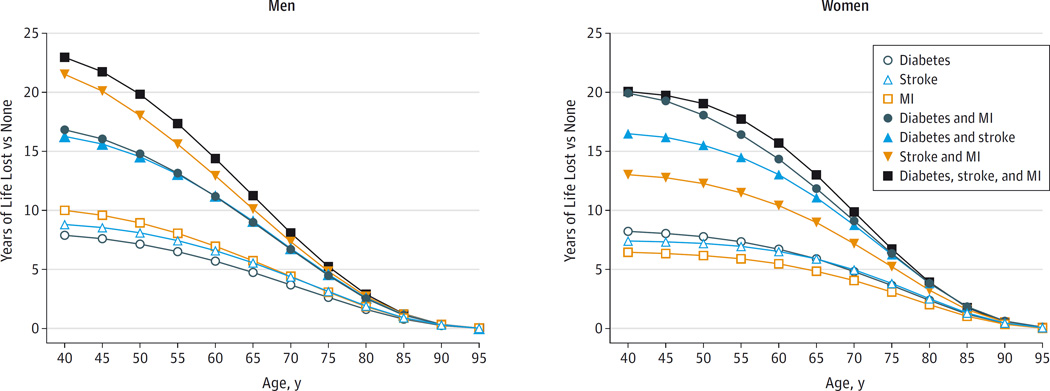
We estimated that at the age of 60 years, men with any 2 of the cardiometabolic conditions we studied would on average have 12 years of reduced life expectancy, and men with all 3 conditions would have 14 years of reduced life expectancy (Figure 3 and eTable 4 in the Supplement). For women at the age of 60 years, the corresponding estimates were 13 years and 16 years of life lost. When calculated for patients at younger ages, estimated reductions in life expectancy were greater than for older patients (eg, 23 years of life were estimated to be lost for men at age 40 years with 3 conditions compared with 20 years of life lost for men at age 50 years with 3 conditions). Estimated reductions in life expectancy in patients with MI only were greater for men than women; estimated reductions in life expectancy in patients with diabetes only were greater for women (Figure 3 and eTable 4).
Figure 3.
Modeling of Years of Life Lost by Disease Status of Participants at Baseline Compared With Those Free of Diabetes, Stroke, and Myocardial Infarction (MI)
The estimates of cumulative survival from 40 years of age onward among the 8 baseline disease groups were calculated by applying hazard ratios (specific to age at risk and sex) for all-cause mortality associated with baseline disease status to US cause-specific death rates at the age of 40 years or older.
On average, about 59% of the survival difference associated with cardiometabolic multimorbidity in men was attributed to excess cardiovascular deaths, and the remainder to excess nonvascular, noncancer deaths (36%), cancer deaths (4%), and unclassified deaths (1%). By contrast, for women, 45% of the estimated survival difference was attributed to excess cardiovascular deaths, and the remainder by nonvascular, noncancer deaths (49%), excess cancer deaths (5%), and unclassified deaths (2%) (eFigure 10 in the Supplement). Broadly similar results were observed when modeling involved cause-specific death rates from the European Union (eFigure 11).
Systematic Review
We could not identify any previous relevant reports of all-cause mortality that had investigated participants having the combination of diabetes, stroke, and MI, or any previous relevant reports of participants having the combination of stroke and MI. We identified only 1 previous relevant report on the combination of diabetes and stroke, albeit of limited statistical power.23 By contrast, we identified 5 previous reports on the combination of diabetes and MI, which generally yielded similar HRs as in the current analysis (Figure 2 and eTable 5 in the Supplement), although none estimated reductions in life expectancy associated with such multimorbidity.9–12,14
Discussion
Our analysis of more than 135 000 deaths accrued during prolonged follow-up of almost 1.2 million participants in population cohorts has provided estimates of reductions in life expectancy associated with different combinations of cardiometabolic multimorbidity (ie, a history of diabetes, stroke, and/or MI). Each of our 3 main findings has potential implications.
First, in patients who had only 1 condition that we studied, we observed an HR for mortality of about 2; for a combination of any 2 conditions, the HR was about 4; and for a combination of all 3 conditions, the HR was about 8. These results suggest that associations of cardiovascular disease and diabetes with mortality are multiplicative and essentially nonoverlapping. This finding is consistent with previous observations that associations of diabetes with chronic disease outcomes are largely independent of major cardiovascular risk factors.5,24 Consequently, our results emphasize the importance of measures to prevent cardiovascular disease in people who already have diabetes, and, conversely, to avert diabetes in people who already have cardiovascular disease.25,26
Second, our results suggest that estimated reductions in life expectancy associated with cardiometabolic multimorbidity are of similar magnitude to those previously noted for exposures of major concern to public health, such as lifelong smoking (10 years of reduced life expectancy27) and infection with the human immunodeficiency virus (11 years of reduced life expectancy28,29). For example, cardiometabolic multimorbidity at the age of 60 years was associated with an average reduction in life expectancy of about 15 years. We estimated even greater reductions in life expectancy in patients with multimorbidity at younger ages, such as 23 years of life lost in patients with 3 conditions at the age of 40 years.
Third, we noted modification by sex of associations between cardiometabolic multimorbidity and mortality. For men, the association between baseline cardiovascular disease (ie, a history of stroke or MI) and reduced survival was stronger than for women, whereas the association between baseline diabetes and reduced survival was stronger for women. Consequently, about 60% of the years of life lost from cardiometabolic multimorbidity can be attributed to cardiovascular deaths for men compared with only about 45% for women. Nevertheless, for both men and women, our findings indicate that associations of cardiometabolic multimorbidity extend beyond cardiovascular mortality. Future work will seek to elucidate explanations for these interactions by sex.
Our results highlight the need to balance the primary prevention and secondary prevention of cardiovascular disease. About 1% of the participants in the cohorts we studied had cardiometabolic multimorbidity compared with an estimate of 3% from recent surveys in the United States.30,31 There are currently an estimated 10 million adults in the United States and the European Union with cardiometabolic multimorbidity.1,3,20,21 Nevertheless, an overemphasis on the substantial reductions in life expectancy estimated for the subpopulation with multimorbidity could divert attention and resources away from population-wide strategies that aim to improve health for the large majority of the population.32
Our study had potential limitations. Our definition of cardiometabolic multimorbidity was both pragmatically motivated (we had information available on a history of diabetes, stroke, and MI) and biologically motivated (we purposefully focused on binary disease states). However, we did not include a history of hypertension in our definition of multimorbidity because categorizing elevated blood pressure as a binary variable would necessarily underestimate the true effect of blood pressure on chronic disease because blood pressure has a continuous log-linear relationship with the risk of cardiovascular diseases throughout its range of values.33 Furthermore, inclusion of hypertension in our definition would have created 16 possible disease combinations, which are too many for stable analyses even in the ERFC. We did not have access to time-varying exposure information to enable updating of multimorbidity status during follow-up. Only subsets of participants had information on some covariates, such as medication use, and dates of diagnosis of baseline conditions.
The generalizability of our results was enhanced by involvement in the ERFC of individual participant data from 91 cohorts in 18 different countries that recruited participants during 1960 through 2007. To what extent do the HRs from the ERFC reflect the contemporary situation? Our study addressed this concern in several ways. We analyzed data in the ERFC by calendar decade, and we did not find evidence of large differences in the HRs by calendar period of recruitment. We noted broadly similar findings between the ERFC and the UK Biobank, which recruited participants during 2006 through 2010. Our systematic review found that the HRs reported in previous relevant publications were compatible with those in the ERFC, although previous data were sparse. In addition, for the survival modeling, we applied the HRs observed in the ERFC to the death rates derived from the contemporary US population and secondarily to the European Union population.
Conclusions
Mortality associated with a history of diabetes, stroke, or MI was similar for each condition. Because any combination of these conditions was associated with multiplicative mortality risk, life expectancy was substantially lower in people with multimorbidity.
Supplementary Material
Acknowledgments
Dr Di Angelantonio reported receiving personal fees from Elsevier (France). Dr Wormser reported being employed by F. Hoffmann-La Roche; and receiving personal fees and holding shares in F. Hoffmann-La Roche. Dr Butterworth reported receiving grants from Pfizer, Merck Sharp and Dohme, and Novartis. Dr Hart reported receiving grant funding from the National Health Service for Scotland. Dr Psaty reported serving on a data and safety monitoring board for a clinical trial of a device funded by the manufacturer (Zoll LifeCor) and on the steering committee of the Yale Open Data Access Project funded by Johnson & Johnson. Dr Nietert reported receiving grant UL1TR000062 from the National Institutes of Health; and providing expert testimony to clients of the TASA Group. Dr Amouyel reported receiving personal fees from Servier and F. Hoffmann-La Roche. Dr Roussel reported receiving personal fees from Johnson & Johnson, sanofi, Novo Nordisk, AbbVie, AstraZeneca, Novartis, and Merck Sharp and Dohme; and receiving grants from sanofi. Dr Leening reported receiving grants from Prins Bernhard Cultuurfonds, De Drie Lichten Foundation, Erasmus University Trustfonds, the American Heart Association, the European Society of Cardiology, and the Netherlands Epidemiology Society. Dr Kiechl reported receiving a grant funded by Bundesministerium für Verkehr, the Innovation und Technologie, Federal Minister of Science, Research and Economy, Wirtschaftsagentur Wien, and Standortagentur Tirol. Dr Franco reported receiving grants from Nestle and Metagenics. Dr Sundström reported serving on advisory boards for Itrim and AstraZeneca. Dr Woodward reported serving on a data and safety monitoring board for Novartis; and receiving personal fees from Amgen and sanofi. Dr Whitsel reported receiving grants from the National Heart, Lung, and Blood Institute, the National Institute of Environmental Health Sciences, the National Institute of Child Health and Human Development, the National Institute on Aging, the Federal Aviation Administration, and the American Heart Association. Dr Selvin reported serving on an advisory board and receiving personal fees from Roche Diagnostics. Dr Danesh reported receiving personal fees and nonfinancial support for serving on advisory boards for Merck Sharp and Dohme, Novartis, Pfizer, and sanofi; receiving grants from the BUPA Foundation, diaDexus, Evelyn Trust, Fogarty International Centre, GlaxoSmithKline, Merck, National Heart, Lung and Blood Institute, National Institute for Health Research, National Institute of Neurological Disorders and Stroke, NHS Blood and Transplant, Novartis, Pfizer, UK Medical Research Council, University of British Columbia, University of Sheffield, Wellcome Trust, and UK Biobank; and receiving nonfinancial support from Roche. Drs Kaptoge, Willeit, Bansal, O’Keeffe, Gao, Wood, Burgess, Freitag, Pennells, S. A. Peters, Håheim, Gillum, Nordestgaard, Yeap, Knuiman, Kauhanen, Salonen, Kuller, Simons, van der Schouw, Barrett- Connor, Selmer, Crespo, Rodriguez, Verschuren, Salomaa, Svärdsudd, van der Harst, Björkelund, Wilhelmsen, Wallace, Brenner, Barr, Iso, Onat, Trevisan, D’Agostino, Cooper, Kavousi, Welin, Hu, Sato, Davidson, Howard, Rosengren, Dörr, Deeg, Stehouwer, Nissinen, Giampaoli, Donfrancesco, Kromhout, Price, A. Peters, Meade, Casiglia, Lawlor, Gallacher, Nagel, Assmann, Dagenais, Jukema, Brunner, Khaw, Wareham, Njølstad, Hedblad, Wassertheil-Smoller, Engström, Rosamond, Sattar, and Thompson reported having no disclosures.
Funding/Support: The work of the coordinating center was funded by the UK Medical Research Council (grant G0800270), the British Heart Foundation (grant SP/09/002), the British Heart Foundation Cambridge Cardiovascular Centre of Excellence, UK National Institute for Health Research Cambridge Biomedical Research Centre, European Research Council (grant 268834), and the European Commission Framework Programme 7 (grant HEALTH-F2-2012-279233). This research has been conducted using the UK Biobank resource. The Emerging Risk Factor Collaboration’s website http://www.phpc.cam.ac.uk/ceu/research/erfc/studies/ has a compiled list of some of the funders of the component studies in this analysis.
Role of the Funder/Sponsor: None of the funding organizations were involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The Emerging Risk Factors Collaboration Authors/Members
Emanuele Di Angelantonio, MD; Stephen Kaptoge, PhD; David Wormser, PhD; Peter Willeit, MD; Adam S. Butterworth, PhD; Narinder Bansal, PhD; Linda M. O’Keeffe, PhD; Pei Gao, PhD; Angela M. Wood, PhD; Stephen Burgess, PhD; Daniel F. Freitag, PhD; Lisa Pennells, PhD; Sanne A. Peters, PhD; Carole L. Hart, PhD; Lise Lund Håheim, PhD; Richard F. Gillum, MD; Børge G. Nordestgaard, MD; Bruce M. Psaty, MD; Bu B. Yeap, FRACP; Matthew W. Knuiman, PhD; Paul J. Nietert, PhD; Jussi Kauhanen, MD; Jukka T. Salonen, MD; Lewis H. Kuller, MD; Leon A. Simons, MD; Yvonne T. van der Schouw, PhD; Elizabeth Barrett-Connor, MD; Randi Selmer, PhD; Carlos J. Crespo, DrPH; Beatriz Rodriguez, MD; W. M. Monique Verschuren, PhD; Veikko Salomaa, MD; Kurt Svärdsudd, MD; Pim van der Harst, MD; Cecilia Björkelund, MD; Lars Wilhelmsen, MD; Robert B. Wallace, MD; Hermann Brenner, MD; Philippe Amouyel, MD; Elizabeth L. M. Barr, PhD; Hiroyasu Iso, MD; Altan Onat, MD; Maurizio Trevisan, MD; Ralph B. D'Agostino Sr, PhD; Cyrus Cooper, FRCP; Maryam Kavousi, MD; Lennart Welin, MD; Ronan Roussel, MD, PhD; Frank B. Hu, MD; Shinichi Sato, MD; Karina W. Davidson, PhD; Barbara V. Howard, PhD; Maarten J.G. Leening, MD, MSc; Annika Rosengren, MD; Marcus Dörr, MD; Dorly J. H. Deeg, PhD; Stefan Kiechl, MD; Coen D. A. Stehouwer, MD; Aulikki Nissinen, MD; Simona Giampaoli, MD; Chiara Donfrancesco, DrStat; Daan Kromhout, PhD; Jackie F. Price, MD; Annette Peters, PhD; Tom W. Meade, FRS; Edoardo Casiglia, MD; Debbie A. Lawlor, PhD; John Gallacher, PhD; Dorothea Nagel, PhD; Oscar H. Franco, MD; Gerd Assmann, FRCP; Gilles R. Dagenais, MD; J. Wouter Jukema, MD; Johan Sundström, MD; Mark Woodward, PhD; Eric J. Brunner, PhD; Kay-Tee Khaw, FMedSci; Nicholas J. Wareham, FRCP; Eric A. Whitsel, MD, MPH; Inger Njølstad, MD, PhD; Bo Hedblad, MD; Sylvia Wassertheil-Smoller, PhD; Gunnar Engström, MD; Wayne D. Rosamond, PhD, MSc; Elizabeth Selvin, PhD; Naveed Sattar, FRCP; Simon G. Thompson, FMedSci; John Danesh, FMedSci.
Affiliations of The Emerging Risk Factors Collaboration Authors/Members: University of Cambridge, Cambridge, England (Di Angelantonio, Kaptoge, Wormser, Willeit, Butterworth, Bansal, O’Keeffe, Gao, Wood, Burgess, Freitag, Pennells, Khaw, Thompson, Danesh); University Medical Center Utrecht, Utrecht, the Netherlands (S. A. Peters, van der Schouw); University of Glasgow, Glasgow, Scotland (Hart, Sattar); University of Oslo, Oslo, Norway (Håheim); Howard University College of Medicine, Washington, DC (Gillum); Copenhagen University Hospital, University of Copenhagen, Copenhagen, Denmark (Nordestgaard); University of Washington, Seattle (Psaty); University of Western Australia, Perth (Yeap, Knuiman); Department of Public Health Sciences, Medical University of South Carolina, Charleston (Nietert); University of Eastern Finland, Kuopio (Kauhanen);Metabolic Analytical Services Inc, Helsinki, Finland (Salonen); University of Pittsburgh, Pittsburgh, Pennsylvania (Kuller); University of New South Wales, New South Wales, Australia (Simons); University of California–San Diego, La Jolla (Barrett-Connor); Norwegian Institute of Public Health, Oslo, Norway (Selmer); Portland State University, Portland, Oregon (Crespo); University of Hawaii, Honolulu (Rodriguez); National Institute for Public Health and the Environment, Bilthoven, the Netherlands (Verschuren); National Institute for Health and Welfare, Helsinki, Finland (Salomaa, Nissinen); Uppsala University, Uppsala, Sweden (Svärdsudd, Sundström); University Medical Center Groningen, University of Groningen, Groningen, the Netherlands (van der Harst); University of Gothenburg, Gothenburg, Sweden (Björkelund, Wilhelmsen); Department of Epidemiology, University of Iowa, Iowa City (Wallace); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (Brenner); Institut Pasteur de Lille, Lille, France (Amouyel); Baker IDI Heart and Diabetes Institute, Victoria, Australia (Barr); Osaka University, Suita, Japan (Iso); Istanbul University, Istanbul, Turkey (Onat); City College of New York, New York, New York (Trevisan); Boston University, Boston, Massachusetts (D'Agostino); University of Southampton, Southampton, England (Cooper); University of Oxford, Oxford, England (Cooper); Erasmus Medical Center, Rotterdam, the Netherlands (Kavousi, Leening, Franco); Lidköping Hospital, Lidköping, Sweden (Welin); INSERM, Centre de Recherche des Cordeliers, Paris, France (Roussel); Université Paris Diderot, Paris, France (Roussel); Diabétologie, AP-HP, Département Hospitalo-Universitaire FIRE, Hôpital Bichat, Paris, France (Roussel); Harvard School of Public Health, Boston, Massachusetts (Hu); Osaka Medical Center for Health Science and Promotion/Chiba Prefectural Institute of Public Health, Suita, Japan (Sato); Columbia University Medical Center, New York, New York (Davidson); MedStar Health Research Institute, Hyattsville, Maryland (Howard); Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden (Rosengren); University Medicine Greifswald, Greifswald, Germany (Dörr); DZHK (German Centre for Cardiovascular Research), Partner Site Greifswald, Greifswald, Germany (Dörr); Vrije Universiteit Medical Center, Amsterdam, the Netherlands (Deeg); Medical University Innsbruck, Innsbruck, Austria (Kiechl); Maastricht University Medical Centre, Maastricht, the Netherlands (Stehouwer); Istituto Superiore di Sanità, Rome, Italy (Giampaoli, Donfrancesco); Wageningen University, Wageningen, the Netherlands (Kromhout); Centre for Population Health Sciences, University of Edinburgh, Edinburgh, Scotland (Price); Institute of Epidemiology II, Helmholtz Zentrum München- German Research Center for Environmental Health, Neuherberg, Germany (A. Peters); German Research Center for Cardiovascular Research (DZHK eV), Partner-Site Munich, Munich, Germany (A. Peters); London School of Hygiene and Tropical Medicine, London, England (Meade); University of Padova, Padova, Italy (Casiglia); University of Bristol, Bristol, England (Lawlor); Cardiff University, Cardiff, England (Gallacher); Klinikum der Universität München LMU, München, Germany (Nagel); Assmann-Stiftung für Prävention, Munster, Germany (Assmann); Institut Universitaire de Cardiologie et Pneumologie de Québec, Quebec, Canada (Dagenais); Leiden University Medical Center, Leiden, the Netherlands (Jukema); University of Sydney, Sydney, Australia (Woodward); University College London, London, England (Brunner); Medical Research Council Epidemiology Unit, Cambridge, England (Wareham); Department of Medicine, University of North Carolina, Chapel Hill (Whitsel); Department of Epidemiology, University of North Carolina, Chapel Hill (Whitsel); University of Tromsø, Tromsø, Norway (Njølstad); Lund University, Lund, Sweden (Hedblad, Engström); Albert Einstein College of Medicine, New York, New York (Wassertheil-Smoller); University of North Carolina, Chapel Hill (Rosamond); Johns Hopkins University, Baltimore, Maryland (Selvin).
Footnotes
Author Contributions: Drs Di Angelantonio and Kaptoge had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Di Angelantonio, Kaptoge, Wormser, Gao, Wood, Nietert, Kauhanen, Wallace, Trevisan, Cooper, Howard, Stehouwer, Casiglia, Jukema, Woodward, Brunner, Sattar, Danesh.
Acquisition, analysis, or interpretation of data: Di Angelantonio, Kaptoge, Wormser, Willeit, Butterworth, Bansal, O’Keeffe, Gao, Wood, Burgess, Freitag, Pennells, S. A. Peters, Hart, Håheim, Gillum, Nordestgaard, Psaty, Yeap, Knuiman, Nietert, Kauhanen, Salonen, Kuller, Simons, van der Schouw, Barrett-Connor, Selmer, Crespo, Rodriguez, Verschuren, Salomaa, Svärdsudd, van der Harst, Björkelund, Wilhelmsen, Wallace, Brenner, Amouyel, Barr, Iso, Onat, Trevisan, D’Agostino, Cooper, Kavousi, Welin, Roussel, Hu, Sato, Davidson, Leening, Rosengren, Dörr, Deeg, Kiechl, Stehouwer, Nissinen, Giampaoli, Donfrancesco, Kromhout, Price, A. Peters, Meade, Casiglia, Lawlor, Gallacher, Nagel, Franco, Assmann, Dagenais, Jukema, Sundström, Woodward, Khaw, Wareham, Whitsel, Njølstad, Hedblad, Wassertheil-Smoller, Engström, Rosamond, Selvin, Sattar, Thompson, Danesh.
Drafting of the manuscript: Di Angelantonio, Kaptoge, Wormser, Bansal, Burgess, D’Agostino, Cooper, Brunner, Wareham, Sattar, Danesh.
Critical revision of the manuscript for important intellectual content: Di Angelantonio, Kaptoge, Wormser, Willeit, Butterworth, Bansal, O’Keeffe, Gao, Wood, Burgess, Freitag, Pennells, S. A. Peters, Hart, Håheim, Gillum, Nordestgaard, Psaty, Yeap, Knuiman, Nietert, Kauhanen, Salonen, Kuller, Simons, van der Schouw, Barrett-Connor, Selmer, Crespo, Rodriguez, Verschuren, Salomaa, Svärdsudd, van der Harst, Björkelund, Wilhelmsen, Wallace, Brenner, Amouyel, Barr, Iso, Onat, Trevisan, Cooper, Kavousi, Welin, Roussel, Hu, Sato, Davidson, Howard, Leening, Rosengren, Dörr, Deeg, Kiechl, Stehouwer, Nissinen, Giampaoli, Donfrancesco, Kromhout, Price, A. Peters, Meade, Casiglia, Lawlor, Gallacher, Nagel, Franco, Assmann, Dagenais, Jukema, Sundström, Woodward, Khaw, Whitsel, Njølstad, Hedblad, Wassertheil-Smoller, Engström, Rosamond, Selvin, Sattar, Thompson, Danesh.
Statistical analysis: Di Angelantonio, Kaptoge, Wormser, Willeit, Bansal, Gao, Wood, D’Agostino, Cooper, Woodward, Thompson, Danesh.
Obtained funding: Nordestgaard, Salonen, Simons, Verschuren, van der Harst, Amouyel, Welin, Davidson, Howard, Rosengren, Dörr, Kiechl, Kromhout, Casiglia, Lawlor, Brunner, Khaw, Wareham, Wassertheil-Smoller, Thompson, Danesh.
Administrative, technical, or material support: Bansal, O’Keeffe, Freitag, Håheim, Gillum, Yeap, Kauhanen, Salonen, Simons, Crespo, Verschuren, Salomaa, Svärdsudd, van der Harst, Björkelund, Wilhelmsen, Barr, Iso, Trevisan, Kavousi, Hu, Leening, Deeg, Kiechl, Kromhout, A. Peters, Casiglia, Lawlor, Franco, Woodward, Khaw, Engström, Rosamond.
Study supervision: Di Angelantonio, Kaptoge, Wood, Salonen, Kuller, Simons, Salomaa, Wallace, Howard, Donfrancesco, Kromhout, A. Peters, Casiglia, Franco, Sattar, Thompson, Danesh.
Conflict of Interest Disclosures: The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
REFERENCES
- 1.Glynn LG. Multimorbidity: another key issue for cardiovascular medicine. Lancet. 2009;374(9699):1421–1422. doi: 10.1016/S0140-6736(09)61863-8. [DOI] [PubMed] [Google Scholar]
- 2.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition—multimorbidity. JAMA. 2012;307(23):2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss CO, Boyd CM, Yu Q, et al. Patterns of prevalent major chronic disease among older adults in the United States. JAMA. 2007;298(10):1160–1162. doi: 10.1001/jama.298.10.1160-b. [DOI] [PubMed] [Google Scholar]
- 4.Booth GL, Kapral MK, Fung K, Tu JV. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people. Lancet. 2006;368(9529):29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- 5.Seshasai SR, Kaptoge S, Thompson A, et al. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364(9):829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases. Lancet Diabetes Endocrinol. 2015;3(2):105–113. doi: 10.1016/S2213-8587(14)70219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judd SE, Kleindorfer DO, McClure LA, et al. Self-report of stroke, transient ischemic attack, or stroke symptoms and risk of future stroke in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Stroke. 2013;44(1):55–60. doi: 10.1161/STROKEAHA.112.675033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carnethon MR, Biggs ML, Barzilay J, et al. Diabetes and coronary heart disease as risk factors for mortality in older adults. Am J Med. 2010;123(6):556.e1–556.e9. doi: 10.1016/j.amjmed.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schramm TK, Gislason GH, Køber L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk. Circulation. 2008;117(15):1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 10.Lotufo PA, Gaziano JM, Chae CU, et al. Diabetes and all-cause and coronary heart disease mortality among US male physicians. Arch Intern Med. 2001;161(2):242–247. doi: 10.1001/archinte.161.2.242. [DOI] [PubMed] [Google Scholar]
- 11.Hu G, Jousilahti P, Qiao Q, et al. Sex differences in cardiovascular and total mortality among diabetic and non-diabetic individuals with or without history of myocardial infarction. Diabetologia. 2005;48(5):856–861. doi: 10.1007/s00125-005-1730-6. [DOI] [PubMed] [Google Scholar]
- 12.Cho E, Rimm EB, Stampfer MJ, et al. The impact of diabetes mellitus and prior myocardial infarction on mortality from all causes and from coronary heart disease in men. J Am Coll Cardiol. 2002;40(5):954–960. doi: 10.1016/s0735-1097(02)02044-2. [DOI] [PubMed] [Google Scholar]
- 13.Whiteley L, Padmanabhan S, Hole D, Isles C. Should diabetes be considered a coronary heart disease risk equivalent? Diabetes Care. 2005;28(7):1588–1593. doi: 10.2337/diacare.28.7.1588. [DOI] [PubMed] [Google Scholar]
- 14.Hu FB, Stampfer MJ, Solomon CG, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women. Arch Intern Med. 2001;161(14):1717–1723. doi: 10.1001/archinte.161.14.1717. [DOI] [PubMed] [Google Scholar]
- 15.Danesh J, Erqou S, Walker M, et al. Emerging Risk Factors Collaboration. The Emerging Risk Factors Collaboration. Eur J Epidemiol. 2007;22(12):839–869. doi: 10.1007/s10654-007-9165-7. [DOI] [PubMed] [Google Scholar]
- 16.Thompson S, Kaptoge S, White I, et al. Emerging Risk Factors Collaboration. Statistical methods for the time-to-event analysis of individual participant data from multiple epidemiological studies. Int J Epidemiol. 2010;39(5):1345–1359. doi: 10.1093/ije/dyq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wannamethee SG, Shaper AG, Lennon L. Cardiovascular disease incidence and mortality in older men with diabetes and in men with coronary heart disease. Heart. 2004;90(12):1398–1403. doi: 10.1136/hrt.2003.026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med. 2010;29(12):1282–1297. doi: 10.1002/sim.3602. [DOI] [PubMed] [Google Scholar]
- 20.World Population Prospects. New York, NY: United Nations; 2005. UN Population Division. [Google Scholar]
- 21.World Health Organization. WHO Statistical Information System(WHOSIS) Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 22.Rocca WA, Boyd CM, Grossardt BR, et al. Prevalence of multimorbidity in a geographically defined American population. Mayo Clin Proc. 2014;89(10):1336–1349. doi: 10.1016/j.mayocp.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otiniano ME, Du XL, Ottenbacher K, Markides KS. The effect of diabetes combined with stroke on disability, self-rated health, and mortality in older Mexican Americans. Arch Phys Med Rehabil. 2003;84(5):725–730. doi: 10.1016/s0003-9993(02)04941-9. [DOI] [PubMed] [Google Scholar]
- 24.Sarwar N, Gao P, Seshasai SR, et al. Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [published correction appears in Lancet. 2010;376(9745):958]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mozaffarian D, Marfisi R, Levantesi G, et al. Incidence of new-onset diabetes and impaired fasting glucose in patients with recent myocardial infarction and the effect of clinical and lifestyle risk factors. Lancet. 2007;370(9588):667–675. doi: 10.1016/S0140-6736(07)61343-9. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association. Cardiovascular disease and risk management. Diabetes Care. 2015;38(suppl):S49–S57. doi: 10.2337/dc15-S011. [DOI] [PubMed] [Google Scholar]
- 27.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May M, Gompels M, Delpech V, et al. Impact of late diagnosis and treatment on life expectancy in people with HIV-1. BMJ. 2011;343:d6016. doi: 10.1136/bmj.d6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146(2):87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 30.National Center for Health Statistics. About the National Health and Nutrition Examination Survey. [Accessed April 30, 2015]; http://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
- 31.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 32.Rose G. Sick individuals and sick populations. Int J Epidemiol. 1985;14(1):32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 33.Lewington S, Clarke R, Qizilbash N, et al. Age-specific relevance of usual blood pressure to vascular mortality. Lancet. 2002;360(9349):1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.