Abstract
BACKGROUND:
Pulmonary nodules (PNs) are a common reason for referral to pulmonologists. The majority of data for the evaluation and management of PNs is derived from studies performed in academic medical centers. Little is known about the prevalence and diagnosis of PNs, the use of diagnostic testing, or the management of PNs by community pulmonologists.
METHODS:
This multicenter observational record review evaluated 377 patients aged 40 to 89 years referred to 18 geographically diverse community pulmonary practices for intermediate PNs (8-20 mm). Study measures included the prevalence of malignancy, procedure/test use, and nodule pretest probability of malignancy as calculated by two previously validated models. The relationship between calculated pretest probability and management decisions was evaluated.
RESULTS:
The prevalence of malignancy was 25% (n = 94). Nearly one-half of the patients (46%, n = 175) had surveillance alone. Biopsy was performed on 125 patients (33.2%). A total of 77 patients (20.4%) underwent surgery, of whom 35% (n = 27) had benign disease. PET scan was used in 141 patients (37%). The false-positive rate for PET scan was 39% (95% CI, 27.1%-52.1%). Pretest probability of malignancy calculations showed that 9.5% (n = 36) were at a low risk, 79.6% (n = 300) were at a moderate risk, and 10.8% (n = 41) were at a high risk of malignancy. The rate of surgical resection was similar among the three groups (17%, 21%, 17%, respectively; P = .69).
CONCLUSIONS:
A substantial fraction of intermediate-sized nodules referred to pulmonologists ultimately prove to be lung cancer. Despite advances in imaging and nonsurgical biopsy techniques, invasive sampling of low-risk nodules and surgical resection of benign nodules remain common, suggesting a lack of adherence to guidelines for the management of PNs.
A pulmonary nodule (PN) is defined as a radiographic opacity ≤ 3 cm in diameter surrounded by lung parenchyma.1 The prevalence of PNs is unknown, with estimates ranging from 150,000 to 1 million per year in the United States.2 The identification of PNs may increase over the next decade for several reasons. First, chest CT scans are ordered frequently for a myriad of clinical indications, including dyspnea or chest pain. Smith-Bindman et al3 estimate the rate of chest CT scanning as 23 per 1,000, which, extrapolated to the US population, equates to approximately 7 million chest CT scans performed annually. Second, the National Lung Screening Trial found three fewer deaths from lung cancer per 1,000 in those screened annually for 3 years with low-dose chest CT scans, leading to the B recommendation by the US Preventive Services Task Force4 to screen high-risk individuals with yearly low-dose CT scans. However, the National Lung Screening Trial also found a 25% rate of screen-detected nodules, 96% of which were benign. Currently, 8.6 million Americans meet the criteria for screening.5 Depending on the penetration of screening, the incidence of detected PNs could increase dramatically.6
The American College of Chest Physicians (CHEST) and the Fleischner Society have published guidelines for management based on pretest probability of malignancy (pCA).7,8 Management decisions fall into three general categories based on the physician’s pCA. For those in whom the pCA is low, serial imaging is recommended. When the pCA is intermediate, functional imaging (PET scan), biopsy, or both is warranted. When the pCA is high, surgery is recommended.8 Although these recommendations appear straightforward, other factors such as patient comorbidities and preferences often influence the choice of management strategy.8 Clinical prediction models have been developed and validated to assess the pCA in PNs, which can be used to help guide decisions about selection and interpretation of additional diagnostic testing.9‐12
Outside clinical practice guidelines, there remain significant challenges in interpretation of the literature. First, the prevalence of malignancy in a PN varies by the context within which it is studied, from a low of 2% in lung cancer screening trials to a high of 83% in surgical series.13‐15 Unfortunately, most of these studies are from single-center academic institutions focused on a single aspect of nodule management (eg, PET scan use) and do not always combine modalities to give a cohesive picture of how nodules are managed from presentation to final outcome. We undertook this study to better understand the management patterns of physicians faced with the evaluation of intermediate-sized nodules, which most often present a diagnostic dilemma,16 in community settings across the United States.
Materials and Methods
This was a multicenter, community-based, retrospective observational study of patients with PNs, ranging from 8 to 20 mm in diameter, presenting to 18 geographically representative outpatient pulmonary clinics across the United States. The study was approved at 15 sites by a central institutional review board and at three sites by local institutional review board approval.
Site Selection
Four hundred forty sites were identified based on investigator databases and claims data from a large insurance carrier whose coverage population was representative of the overall US population. Of these, 77 sites expressed interest in participating, and 48 sites went on to sign confidentiality agreements. Of these, 17 did not request additional information, leaving 31 sites undergoing qualification review. Eighteen outpatient pulmonary clinics were chosen to participate based on the following criteria: (1) management of patients with PNs, (2) availability of medical records, and (3) ability to perform data abstraction. In addition, investigators targeted enrollment of geographically diverse patients to limit the potential bias associated with differences in practice patterns and to account for variation in disease prevalence (eg, endemic mycoses) that could alter management decisions.
Patient Selection
Patients were identified by querying databases (eg, billing and scheduling systems) using five International Classification of Diseases, Ninth Revision, Clinical Modification codes for PN (793.1, 786.6, 518.89, 519.8, 519.9) to ensure homogeneity in patient identification and inclusion.17 Manual chart abstraction was then used to identify those who met the criteria. To minimize selection bias, the sites were not permitted to use additional codes during database query to identify patients. To ensure a systematic sample, patient eligibility was determined by examining consecutively referred patients to the site.
Inclusion criteria included age ≥ 40 years and ≤ 89 years at the time of nodule finding, presentation to a pulmonologist, nodule size 8 to 20 mm, and definitive diagnosis ascertained by tissue diagnosis or radiographic follow-up for 2 years. Exclusion criteria included chest CT scan performed > 60 days prior to the initial visit, prior diagnosis of any cancer within 2 years of nodule detection, or incomplete chart data.
Patients were categorized into three groups by the most invasive procedure performed during management, as follows: surveillance (serial imaging), biopsy (CT scan-guided transthoracic needle aspiration [TTNA] or bronchoscopy), or surgery (including mediastinoscopy, video-assisted thorascopic surgery, and/or thoracotomy).
Data Collection
Clinical data were abstracted retrospectively by designated study staff into an electronic data capture system from initial consultation through establishment of a definitive diagnosis (ie, pathology results) or a minimum 2-year follow-up. Data included patient demographic and clinical characteristics, PN characteristics, imaging tests, invasive testing, and surgery. PET scan reports were reviewed and abstracted where available for a subset of patients. To adjudicate a PET scan report as positive or negative, an algorithm was developed that prioritized the following components of the report from highest to lowest: final radiology impression, description of findings, and standard uptake values (SUVs) (e-Fig 1 (608KB, pdf) ). PET scanning was defined as negative if the report included any of the following statements: no evidence of malignancy, no 18F-fluorodeoxyglucose uptake or hypermetabolic activity, or an SUV of < 2.5. A positive PET scan was defined as a report that included any of the following statements: concern or suggestion of malignancy, findings that described increased 18F-fluorodeoxyglucose uptake or hypermetabolic activity, or an SUV ≥ 2.5. In the 27 cases in which the findings were discordant with the final impression, adjudication was performed by two independent pulmonologists and agreement was reached in all cases. Data quality was ensured through ongoing site monitoring. Programmed edit checks were built into the electronic data capture system and at the conclusion of chart abstraction, each site provided access to a random 10% sample of deidentified patient records for review.
Pretest Probability of Malignancy
Two previously developed and validated models9,10 were used to estimate the pCA in each patient. Model accuracy was determined by comparing the pCA with the final diagnosis. Receiver operating characteristic curves and the area under the curve were generated with 95% CIs. The pCA was calculated for each patient and categorized into three groups (< 5%, 5% to < 65%, and ≥ 65%). Procedure use by group was examined.
Data Analysis
χ2 or analysis of variance tests were used to compare subgroups, and P values < .05 were considered significant. A nodule was classified as benign based on confirmed benign pathology or the absence of radiographic change as determined by the managing physician during surveillance for at least 2 years. Multivariate logistic regression was performed to identify factors associated with the use of an invasive diagnostic procedure. All statistical analyses were performed using SAS/STAT, version 9.3 (SAS Institute inc).
Results
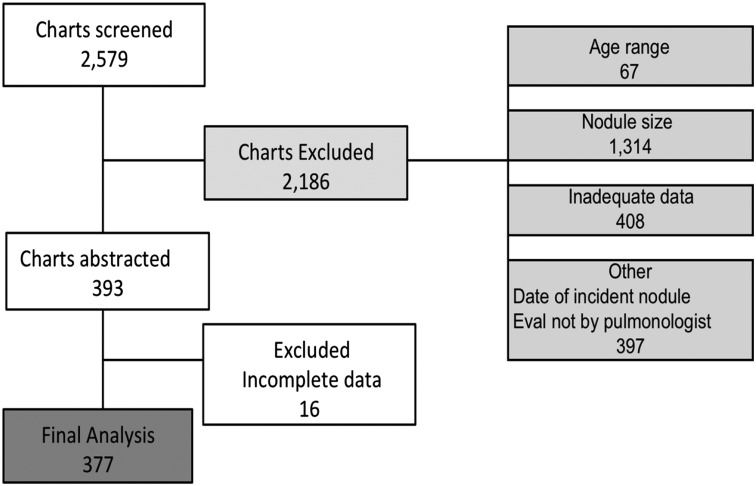
Eighteen sites were chosen based on geographic location and ability to meet the inclusion criteria (e-Fig 2 (608KB, pdf) ). A total of 2,579 patients were screened for eligibility. Following review, 377 patients were included in the final analysis (Fig 1). Most of the exclusions were based on nodule size (< 8 mm or > 20 mm) (n = 1,314), missing clinical information (n = 424), or insufficient follow-up time (n = 303). There was some variation at both the site level and regionally in the numbers of patients excluded because of missing data (e-Tables 1, 2 (608KB, pdf) ).
Figure 1 –
Inclusion and exclusion. Eval = evaluation.
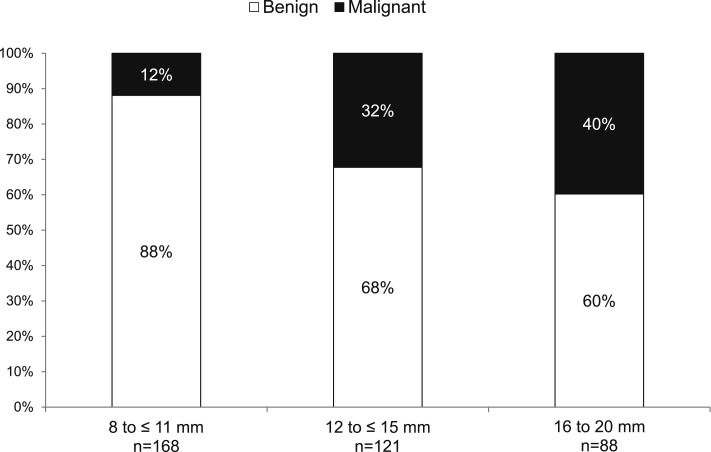
Of the 377 patients included, 283 (75%) had a nodule that was benign, and 94 (25%) had a malignant nodule (Table 1). The average age was 64.5 years, although patients with malignancy were slightly older (P < .02). There was an equal sex distribution. Those with cancer were more likely to be current or former smokers (P < .0001). Although the number of pack-years smoked was significantly higher in those with malignancy (45 vs 27, P < .0001), a substantial proportion of those with benign nodules smoked (67%). Malignant nodules were larger than benign nodules (P < .0001), although there did not appear to be a reliable cutoff above which malignancy could be predicted. Figure 2 demonstrates the diagnostic outcome by nodule size. Twelve percent of nodules ≤ 11 mm in size were malignant, whereas 40% of nodules between 16 and 20 mm were malignant.
TABLE 1 ] .
Characteristics of Patients and Lung Nodules
| Characteristics | All Patients | Cancer | Benign | P Value |
| Patients, No. | 377 | 94 | 283 | … |
| Age, y | 65 (40-88) | 67 (42-83) | 64 (40-88) | .0186 |
| Sex | .9308 | |||
| Male | 171 (45) | 43 (46) | 128 (45) | |
| Female | 206 (55) | 51 (54) | 155 (55) | |
| Smoking history | ||||
| Status | < .0001 | |||
| Never | 102 (27) | 8 (9) | 94 (33) | |
| Former | 169 (45) | 50 (53) | 119 (42) | |
| Current | 106 (28) | 36 (38) | 70 (25) | |
| Pack-y, mean (range) | 32 (0-165) | 45 (0-125) | 27 (0-165) | < .0001 |
| Known family history of lung cancer | 46 (12) | 19 (20) | 27 (10) | .0062 |
| Geographic region | .1087 | |||
| Midwest | 118 (31) | 25 (27) | 93 (33) | |
| Northeast | 88 (23) | 17 (18) | 71 (25) | |
| South | 88 (23) | 24 (26) | 64 (23) | |
| West | 83 (22) | 28 (30) | 55 (19) | |
| Lung nodules | ||||
| Size, mm | 13 (8-20) | 15 (8-20) | 12 (8-20) | < .0001 |
| Location | .0262 | |||
| Left lower lobe | 76 (20) | 14 (15) | 62 (22) | |
| Left upper lobe | 91 (24) | 29 (31) | 62 (22) | |
| Right lower lobe | 77 (20) | 16 (17) | 61 (22) | |
| Right middle lobe | 45 (12) | 6 (6) | 39 (14) | |
| Right upper lobe | 88 (23) | 29 (31) | 59 (21) | |
| Spiculated | 118 (31) | 45 (48) | 73 (26) | < .0001 |
| Diagnosis | ||||
| Clinical benign | 175 (46) | … | 175 (62) | … |
| Histopathology | ||||
| Benign diagnosis | … | |||
| No malignancy | 21 (6) | … | 21 (7) | |
| Infection/inflammation | 22 (6) | … | 22 (8) | |
| Granuloma | 7 (2) | … | 7 (2) | |
| Scar | 1 (0) | … | 1 (0) | |
| Nondiagnostic biopsy/radiographically stable or resolved at 2 y | 57 (15) | … | 57 (20) | |
| Cancer diagnosis | … | |||
| NSCLC (type not specified) | 57 (15) | 57 (61) | … | |
| NSCLC (bronchial alveolar carcinoma) | 2 (1) | 2 (2) | … | |
| Adenocarcinoma | 14 (4) | 14 (15) | … | |
| Squamous cell | 10 (3) | 10 (11) | … | |
| Small cell | 4 (1) | 4 (4) | … | |
| Othera | 7 (2) | 7 (7) | … | |
| Procedure use | ||||
| Surgery | 77 (20) | 50 (53) | 27 (10) | < .0001 |
| Biopsy | 144 (38) | 55 (59) | 89 (32) | < .0001 |
| Surgery or biopsy | 202 (54) | 94 (100) | 108 (38) | < .0001 |
| PET scan | 141 (37) | 56 (60) | 85 (30) | < .0001 |
| CT scan/radiograph only | 133 (35) | 0 (0) | 133 (47) | < .0001 |
| Procedure use category | ||||
| Most invasive = surgery | 77 (20) | 50 (53) | 27 (10) | < .0001 |
| Most invasive = biopsy | 125 (33) | 44 (47) | 81 (29) | .0012 |
| Surveillance | 175 (46) | 0 (0) | 175 (62) | < .0001 |
Data are given as No. (%) or mean (range) unless otherwise indicated. NSCLC = non-small cell lung cancer.
Other cancer diagnoses include spindle cell, mesothelioma, and metastatic cancer.
Figure 2 –
Diagnostic outcome by nodule size (N = 377).
Current smokers (OR = 3.29; 95% CI, 1.36-7.98) and patients with larger nodules (16-20 mm) were more likely (OR = 4.77; 95% CI, 2.95-7.72) to have a procedure (biopsy or surgery) performed. There was no difference in receipt of procedure based on age, family history of cancer, or geographic location (Table 2).
TABLE 2 ] .
Factors Associated With the Decision to Pursue Invasive Procedures in the Management of PNs
| Predictor Variable | Multivariable Model | |
| OR | 95% CI | |
| Age | ||
| ≤ 50 y | Reference | … |
| 50-64 y | 0.617 | 0.196-1.941 |
| 64-79 y | 0.623 | 0.226-1.714 |
| ≥ 80 y | 0.854 | 0.244-2.993 |
| Smoking history | ||
| Never | Reference | … |
| Former | 1.343 | 0.627-2.875 |
| Current | 3.290 | 1.357-7.978 |
| Nodule size | ||
| 8-11 mm | Reference | … |
| 12-15 mm | 3.487 | 2.338-5.200 |
| 16-20 mm | 4.773 | 2.950-7.722 |
| Family history of lung cancer | ||
| No | Reference | … |
| Yes | 2.042 | 0.668-6.238 |
| Region | ||
| Northeast | Reference | … |
| West | 1.222 | 0.365-4.089 |
| South | 0.620 | 0.263-1.460 |
| Midwest | 0.714 | 0.330-1.545 |
PN = pulmonary nodule.
To further understand nodule management decisions, we used two prediction models to determine the pCA for each individual in the study.9,10 These models had very good accuracy in this cohort of patients, with an area under the curve of 0.77 (95% CI, 0.72-0.81) and 0.74 (95% CI, 0.69-0.80), respectively (e-Fig 3 (608KB, pdf) ). Because the results did not vary significantly between models, we report the results from the Veteran Affairs model here and results from the Mayo model in e-Table 3 (608KB, pdf) . Table 3 lists the pretest probability for cancer by three ranges as defined by the CHEST guidelines for PN management: low risk (pCA < 0.05), intermediate risk (pCA 0.05 to < 0.65), and high risk (> 0.65).8 The average pCA for all patients as calculated by the model was 0.33, which is only slightly higher than the cancer prevalence of 25% in this population. Thirty-six patients had nodules that had a low risk of malignancy, and all were benign. Slightly more than one-half were followed by serial imaging alone (n = 20, 56%). However, despite a pCA of < 0.05, 10 (28%) underwent biopsy, and six (17%) had surgical resection. The majority of nodules (n = 300) fell into the intermediate-risk category, and 75% of these were benign. Forty-one patients (11%) had high-risk nodules, and 23 (58%) were benign. The rate of surgical resection was similar among the three groups (17%, 21%, 17%, respectively; P = .69). Alternative cut points for the low-risk category were investigated. All nodules with a pCA of < 0.10 were found to be benign, whereas 98% of those with a pCA of < 0.15 were benign (Table 4).
TABLE 3 ] .
Calculated Pretest Probability and Procedure Use
| Variables | Clinical Probability of Cancer | P Value | ||
| < 0.05 (n = 36) | 0.05 to < 0.65 (n = 300) | ≥ 0.65 (n = 41) | ||
| VA model probability, pCA | 0.03 ± 0.013 | 0.32 ± 0.162 | 0.74 ± 0.056 | … |
| Outcome | ||||
| Benign | 36 (100) | 224 (75) | 23 (58) | < .0001 |
| Malignant | 0 (0) | 76 (25) | 18 (45) | < .0001 |
| Age | 55.1 ± 12.74 | 64.2 ± 10.10 | 74.6 ± 6.66 | < .0001 |
| Smoking status | ||||
| Ever | 11 (31) | 223 (74) | 41 (100) | < .0001 |
| Never | 25 (69) | 77 (26) | 0 (0) | < .0001 |
| Years of abstinencea | 27.1 ± 42.79 | 7.8 ± 13.07 | 3.9 ± 6.60 | < .0001 |
| Pack-y | 6.4 ± 13.92 | 32.6 ± 32.42 | 46.8 ± 21.44 | < .0001 |
| Nodule size | 10.6 ± 2.99 | 12.6 ± 3.63 | 16.1 ± 3.32 | < .0001 |
| Procedure useb | ||||
| Surgery | 6 (17) | 64 (21) | 7 (17) | .6878 |
| Biopsy | 11 (31) | 112 (37) | 21 (51) | .1401 |
| Surgery or biopsy | 16 (44) | 159 (53) | 27 (66) | .1548 |
| PET scan | 13 (36) | 113 (38) | 15 (37) | .9771 |
| CT scan/radiograph only | 15 (42) | 107 (36) | 11 (27) | .3781 |
| Procedure use categoryb | ||||
| Most invasive = surgery | 6 (17) | 64 (21) | 7 (17) | .6878 |
| Most invasive = biopsy | 10 (28) | 95 (32) | 20 (49) | .0711 |
| Surveillance | 20 (56) | 141 (47) | 14 (34) | .1548 |
Data are given as No. (%) or mean ± SD. pCA = pretest probability of malignancy; VA = Veterans Affairs.
Both never smokers and current smokers are included.
Differences are not statistically significant, P > .05.
TABLE 4 ] .
Alternative Pretest Probabilities for Low-Risk Category
| Variables | Clinical Probability for Cancer | ||||
| < 0.05 (n = 36) | < 0.10 (n = 61) | < 0.15 (n = 93) | < 0.20 (n = 127) | < 0.25 (n = 160) | |
| VA model probability, pCA | 0.03 ± 0.013 | 0.05 ± 0.030 | 0.07 ± 0.045 | 0.10 ± 0.059 | 0.13 ± 0.073 |
| Outcome | |||||
| Benign | 36 (100) | 61 (100) | 91 (98) | 121 (95) | 150 (94) |
| Malignant | 0 (0) | 0 (0) | 2 (2) | 6 (5) | 10 (6) |
Data are given as No. (%) or mean ± SD. See Table 3 for expansion of abbreviations.
Nearly one-half of all patients (46%, n = 175) were followed with serial imaging only. The median number of scans performed was three (range, one to seven). Although the majority (62%) had three to five follow-up imaging studies, 4% underwent seven repeat scans. All nodules in this group were benign by resolution or 2 years of radiographic stability. Biopsy (TTNA or bronchoscopy) was performed as the most invasive procedure in 125 patients (33%) and was positive for malignancy in 44 (35%). In more than one-half (n = 71, 57%), a specific benign diagnosis was obtained, whereas 10 (8%) were nondiagnostic and subsequently followed for 2 years.
A total of 77 patients (20%) had surgery as their most invasive test. Prior to surgery, 19 patients (25%) underwent a TTNA or bronchoscopy; 11 had a nondiagnostic biopsy (seven malignant, four benign at surgery), and eight had a benign biopsy result (three malignant, five benign at surgery). Overall, malignancy was diagnosed in 65% (n = 50; 95% CI, 53.2%-75.5%) of those who received surgery, whereas 35% (n = 27; 95% CI, 24.5%-46.8%) underwent surgery for benign disease.
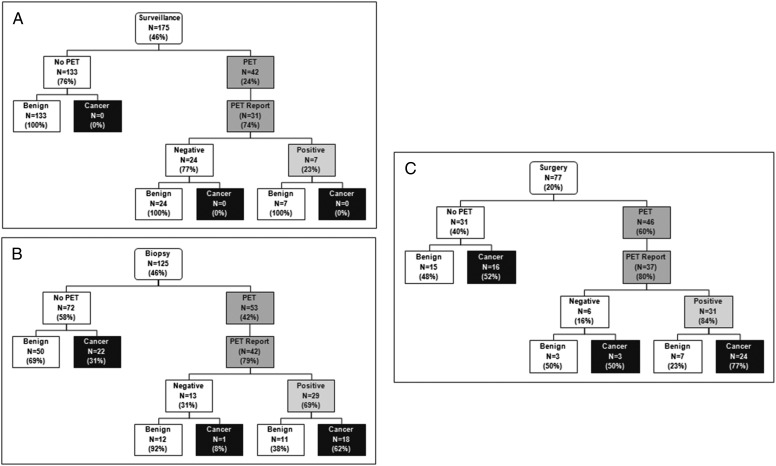
A PET scan was performed in 141 patients (37%). Overall, PET scan use increased with the intensity of testing (surveillance 24%, biopsy 42%, surgery 60%, P < .0001). Results were available for 78% (n = 110) of those who underwent PET scanning (Fig 3). The overall accuracy of PET scanning was 74%, with a false-positive (FP) rate of 39% and a false-negative (FN) rate of 9%. In 8- to 11-mm nodules with PET scan data (n = 38), the FN rate was 10% and the FP rate was 36%. Nodules measuring > 11 to 15 mm (n = 48) had FN and FP rates of 9% and 36%, respectively. The FN rate for PET scans in nodules > 15 mm (n = 24) was 8%, and the FP rate was 55%.
Figure 3 –
PET scan use grouped based on most invasive procedure. A, Surveillance. B, Biopsy. C, Surgery.
In the surveillance group (n = 175), 42 (24%) underwent PET scanning, for whom 31 reports were available. Seven (23%) had PET scan positive results and were followed with serial imaging. Among the 125 patients who underwent biopsy as the most invasive procedure, 53 (42%) underwent PET scan prior to the biopsy. Results were available in 42 cases. Twenty-nine (69%) were PET scan positive and 13 (31%) were negative, yet all went on to biopsy. In the 77 patients who underwent surgery, 46 (60%) underwent a preoperative PET scan, for which 37 reports were available. Thirty-one (84%) had PET scan positive results. Among the 19 patients who underwent preoperative biopsy, 16 had a PET scan, with results available in 15. The majority (n = 14) were positive.
Discussion
This study describing the management of intermediate PNs referred to community pulmonologists has several important findings. First, a quarter of patients referred for intermediate-sized PN evaluation were found to have cancer, emphasizing the importance of the diagnostic evaluation. Second, 35% of patients who underwent surgery had benign nodules. Third, despite guideline recommendations for surveillance of PNs with < 5% pCA, 44% of low-risk patients underwent one or more invasive procedures for a benign nodule. Finally, the rate of surgical resection for nodules with various pretest probabilities of malignancy was similar. This suggests that pulmonologists do not routinely consider pCA, are unaware that guidelines exist for nodule management, or do not to follow them.
To our knowledge, this is the first study that documents the prevalence of cancer (25%) in patients with intermediate-sized nodules who are referred to community-based pulmonologists. This finding has implications for managing PNs in the community; because the risk of cancer is substantial, patients should be managed based on their individualized risk of malignancy.
We found that a high proportion of patients who underwent surgery had benign disease without variation when stratified by the pretest probability of cancer in the nodule. The rates of resection for benign disease varied from 9% to 23% in screening trials and surgical series.14,18 Guidelines recommend surgery if the pretest probability of cancer is high (pCA > 65%) because a negative biopsy result would not dissuade the physician from pursuing a definitive diagnosis and a positive biopsy result would lead to surgery anyway.
The risk models we evaluated were very good at identifying those patients who did not have cancer (100% nodules with < 5% pCA were benign), yet surgical resection was performed at rates similar to those with a high probability of cancer. Similarly, there was no difference in the proportion of patients undergoing surveillance when stratified by pCA. This suggests that pulmonologists did not readily follow the guidelines because they were unaware of them, did not believe them useful, or overestimated the pCA. This study does not allow us to explore the reasons why pulmonologists aggressively manage some nodules that turn out to be benign. There are several possible explanations as to what may have led to biopsy or surgery, and they include being misled by PET scan results, patient preferences for diagnostic certainty, an overestimation of the pCA, or incentives for procedure referral.19 Further research is needed to determine the exact drivers of physician decision-making in PN management.
Although physicians did not appear to use pCA in making decisions about surgery or surveillance, there was a trend toward an increasing use of biopsy as the pCA increased (P = .07). This finding suggests that pulmonologists may use clinical intuition and experience to distinguish benign from malignant disease, although, admittedly, data on provider years in practice and the proportion who were board certified were not available. A significant proportion of patients in this study with benign nodules were older and had significant smoking histories and larger nodules, all which typically suggest malignancy and may have led physicians to overestimate pCA.
In the setting of a positive or ambiguous PET scan interpretation, it is possible that the pulmonologists felt compelled to confirm the abnormal findings with a definitive tissue diagnosis. The FP rate for PET scans in this study was 39%, as compared with the 5% to 28% rate20‐26 reported in the literature. Conversely, in some instances, PET scan results did not seem to be taken into account when making management decisions. For instance, in the surveillance-only group, eight patients with positive PET scans did not undergo further diagnostic procedures. Similarly, in the biopsy group, 13 patients with negative PET scan results still underwent an invasive procedure. Another consideration is that patient preferences influenced the decision toward more aggressive care. The guidelines advocate a patient-centered approach to decision-making after patients are informed of the pCA.8 Thus, some patients may request surgery despite a low pCA because of anxiety about the unknown and an unwillingness to continue with surveillance.27 The cornerstone of management for PNs has been calculating pCA and acting on those calculations.8 This study provides evidence that physicians may not use pCA to guide decision-making and also suggests that an alternative low-risk-category pCA higher than the current CHEST guidelines would shift more patients into the surveillance management strategy without missing malignancy.
Although the exact reason for management decisions cannot be elicited, it is clear that there is wide variation in the management of PNs by pulmonologists. The goal for nodule management is to efficiently detect lung cancer while decreasing the number of unnecessary invasive procedures. A number of radiographic improvements, novel tests, and biomarkers have been developed with the potential to help determine the likelihood of malignancy in a nodule. The use of semiautomated volumetric software to measure the diameter and volume doubling time of a nodule between scans has been used successfully in screening trials to improve the sensitivity, specificity, and negative predictive value of CT scans.28 Similarly, volumetric measurements added to traditional nodule malignancy prediction models have also been shown to improve the classification of malignancy from 60% to 80%, with better performance than that of the traditional model.29 Identification of aberrations in airway epithelial cell gene expression, circulating tumor antibodies, plasma proteomics, exhaled breath analysis, and microRNA are all being studied actively for use in diagnosing lung cancer.30‐35 These novel tests have the potential to further refine nodule management and alleviate the ambiguity of current management strategies.
This study has several limitations. First, the retrospective design precluded a complete understanding of the rationale for test ordering. However this design did allow for a definitive final diagnosis to be established with biopsy, surgery, or 2 years of radiographic surveillance. Second, incomplete PET scan data coupled with difficulties in scan interpretation resulted in variations in the definition of a “positive” PET scan. To overcome this limitation, we developed a hierarchical algorithm for determining a definitive PET scan result. Finally, excluding patients who had had a CT scan > 60 days prior to initial consultation may have selectively excluded some patients with low probability nodules.
This study also has several strengths. The findings are generalizable because of the large sample size, geographic distribution, and multicenter design. The study also focuses on a size range of nodules (8-20 mm) that most often presents a diagnostic dilemma for physicians.16 Nodules smaller than 8 mm are followed with surveillance because the pCA is low, PET scans do not reliably characterize small nodules, and biopsy of small nodules is difficult; by contrast, nodules > 20 mm are likely to undergo biopsy or surgery because the pCA is high. As opposed to other published series, this study allowed for all three management strategies to be studied in detail.
Conclusions
In conclusion, one in four patients referred with a nodule to a community pulmonologist has cancer, making management decisions critical to patient outcomes. To spare patients unnecessary testing, use of a higher pCA to define a nodule as low risk seems reasonable. There is a wide variation in how nodules are managed and the choice of management may be influenced by a variety of factors in addition to pretest probability. Future research should focus on what influences decision-making in PN management so that interventions may be developed to promote proper guideline adherence and avoidance of unnecessary invasive procedures.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: N. T. T., J. A., and G. A. S. take responsibility for the content of the manuscript, for the integrity of the data, and the accuracy of the data analysis, including and especially any adverse effects. N. T. T., J. A., and G. A. S. contributed to the data analysis and interpretation, writing of the manuscript, and components of the study design; M. K. G. contributed to the data interpretation and writing of the manuscript; P. K. contributed to the study design and editing of the manuscript; G. D. and A. V. contributed to the data interpretation and editing of the manuscript; and K. C. F. contributed to the study design, data analysis and interpretation, and writing of the manuscript.
Conflict of interest: N. T. T., J. A., G. D., and A. V. have received consulting fees from Integrated Diagnostics, Inc. P. K. is employed by Integrated Diagnostics, Inc. K. C. F. has stock options in Integrated Diagnostics, Inc. G. A. S. has received grant funding from Integrated Diagnostics, Inc. None declared (M. K. G.).
Role of sponsors: The sponsor had a role in the design of the study. The sponsor had no role in the collection and analysis of the data, or in the preparation of the manuscript.
Additional information: The e-Figures and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- CHEST
American College of Chest Physicians
- FN
false negative
- FP
false positive
- pCA
pretest probability of malignancy
- PN
pulmonary nodule
- SUV
standard uptake value
- TTNA
transthoracic needle aspiration
Footnotes
References
- 1.Kanne JP, Jensen LE, Mohammed TL, et al. ; Expert Panel on Thoracic Imaging. ACR appropriateness Criteria® radiographically detected solitary pulmonary nodule. J Thorac Imaging. 2013;28(1):W1-3. [DOI] [PubMed] [Google Scholar]
- 2.Ost D, Fein AM, Feinsilver SH. Clinical practice. The solitary pulmonary nodule. N Engl J Med. 2003;348(25):2535-2542. [DOI] [PubMed] [Google Scholar]
- 3.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehto RH. Identifying primary concerns in patients newly diagnosed with lung cancer. Oncol Nurs Forum. 2011;38(4):440-447. [DOI] [PubMed] [Google Scholar]
- 5.Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381-1385. [DOI] [PubMed] [Google Scholar]
- 6.Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235(1):259-265. [DOI] [PubMed] [Google Scholar]
- 7.MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. [DOI] [PubMed] [Google Scholar]
- 8.Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5_suppl):e93S-120S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swensen SJ, Silverstein MD, Ilstrup DM, Schleck CD, Edell ES. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157(8):849-855. [PubMed] [Google Scholar]
- 10.Gould MK, Ananth L, Barnett PG; Veterans Affairs SNAP Cooperative Study Group. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131(2):383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz EM, Sanders GD, Trotter PR, et al. Validation of two models to estimate the probability of malignancy in patients with solitary pulmonary nodules. Thorax. 2008;63(4):335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369(10):910-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith MA, Battafarano RJ, Meyers BF, Zoole JB, Cooper JD, Patterson GA.Prevalence of benign disease in patients undergoing resection for suspected lung cancer. Ann Thorac Surg. 2006;81(5):1824-1828. [DOI] [PubMed] [Google Scholar]
- 15.Grogan EL, Deppen SA, Ballman KV, et al. Accuracy of fluorodeoxyglucose-positron emission tomography within the clinical practice of the American College of Surgeons Oncology Group Z4031 trial to diagnose clinical stage I non-small cell lung cancer. Ann Thorac Surg. 2014;97(4):1142-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ost DE, Gould MK. Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med. 2012;185(4):363-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danforth KN, Early MI, Ngan S, Kosco AE, Zheng C, Gould MK. Automated identification of patients with pulmonary nodules in an integrated health system using administrative health plan data, radiology reports, and natural language processing. J Thorac Oncol. 2012;7(8):1257-1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grogan EL, Weinstein JJ, Deppen SA, et al. Thoracic operations for pulmonary nodules are frequently not futile in patients with benign disease. J Thorac Oncol. 2011;6(10):1720-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cronin P, Dwamena BA, Kelly AM, Carlos RC. Solitary pulmonary nodules: meta-analytic comparison of cross-sectional imaging modalities for diagnosis of malignancy. Radiology. 2008;246(3):772-782. [DOI] [PubMed] [Google Scholar]
- 21.Grgic A, Yüksel Y, Gröschel A, et al. Risk stratification of solitary pulmonary nodules by means of PET using (18)F-fluorodeoxyglucose and SUV quantification. Eur J Nucl Med Mol Imaging. 2010;37(6):1087-1094. [DOI] [PubMed] [Google Scholar]
- 22.Kagna O, Solomonov A, Keidar Z, et al. The value of FDG-PET/CT in assessing single pulmonary nodules in patients at high risk of lung cancer. Eur J Nucl Med Mol Imaging. 2009;36(6):997-1004. [DOI] [PubMed] [Google Scholar]
- 23.Mizugaki H, Shinagawa N, Kanegae K, et al. Combining transbronchial biopsy using endobronchial ultrasonography with a guide sheath and positron emission tomography for the diagnosis of small peripheral pulmonary lesions. Lung Cancer. 2010;68(2):211-215. [DOI] [PubMed] [Google Scholar]
- 24.Mori T, Nomori H, Ikeda K, et al. Diffusion-weighted magnetic resonance imaging for diagnosing malignant pulmonary nodules/masses: comparison with positron emission tomography. J Thorac Oncol. 2008;3(4):358-364. [DOI] [PubMed] [Google Scholar]
- 25.Ohba Y, Nomori H, Mori T, et al. Is diffusion-weighted magnetic resonance imaging superior to positron emission tomography with fludeoxyglucose F 18 in imaging non-small cell lung cancer? J Thorac Cardiovasc Surg. 2009;138(2):439-445. [DOI] [PubMed] [Google Scholar]
- 26.Ohba Y, Nomori H, Mori T, Shiraishi K, Namimoto T, Katahira K. Diffusion-weighted magnetic resonance for pulmonary nodules: 1.5 vs. 3 Tesla. Asian Cardiovasc Thorac Ann. 2011;19(2):108-114. [DOI] [PubMed] [Google Scholar]
- 27.Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. What do you mean, a spot? A qualitative analysis of patients’ reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143(3):672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361(23):2221-2229. [DOI] [PubMed] [Google Scholar]
- 29.Mehta HJ, Ravenel JG, Shaftman SR, et al. The utility of nodule volume in the context of malignancy prediction for small pulmonary nodules. Chest. 2014;145(3):464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XJ, Hayward C, Fong PY, et al. A blood-based proteomic classifier for the molecular characterization of pulmonary nodules. Sci Transl Med. 2013;5(207):207ra142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pecot CV, Li M, Zhang XJ, et al. Added value of a serum proteomic signature in the diagnostic evaluation of lung nodules. Cancer Epidemiol Biomarkers Prev. 2012;21(5):786-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazzone PJ. Analysis of volatile organic compounds in the exhaled breath for the diagnosis of lung cancer. J Thorac Oncol. 2008;3(7):774-780. [DOI] [PubMed] [Google Scholar]
- 33.Spira A, Beane JE, Shah V, et al. Airway epithelial gene expression in the diagnostic evaluation of smokers with suspect lung cancer. Nat Med. 2007;13(3):361-366. [DOI] [PubMed] [Google Scholar]
- 34.Bianchi F, Nicassio F, Marzi M, et al. A serum circulating miRNA diagnostic test to identify asymptomatic high-risk individuals with early stage lung cancer. EMBO Mol Med. 2011;3(8):495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapman CJ, Healey GF, Murray A, et al. EarlyCDT®-Lung test: improved clinical utility through additional autoantibody assays. Tumour Biol. 2012;33(5):1319-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement