Abstract
Mitochondria has an essential role in myocardial tissue homeostasis; thus deterioration in mitochondrial function eventually leads to cardiomyocyte and endothelial cell death and consequent cardiovascular dysfunction. Several chemical compounds and drugs have been known to directly or indirectly modulate cardiac mitochondrial function, which can account both for the toxicological and pharmacological properties of these substances. In many cases, toxicity problems appear only in the presence of additional cardiovascular disease conditions or develop months/years following the exposure, making the diagnosis difficult. Cardiotoxic agents affecting mitochondria include several widely used anticancer drugs [anthracyclines (Doxorubicin/Adriamycin), cisplatin, trastuzumab (Herceptin), arsenic trioxide (Trisenox), mitoxantrone (Novantrone), imatinib (Gleevec), bevacizumab (Avastin), sunitinib (Sutent), and sorafenib (Nevaxar)], antiviral compound azidothymidine (AZT, Zidovudine) and several oral antidiabetics [e.g., rosiglitazone (Avandia)]. Illicit drugs such as alcohol, cocaine, methamphetamine, ecstasy, and synthetic cannabinoids (spice, K2) may also induce mitochondria-related cardiotoxicity. Mitochondrial toxicity develops due to various mechanisms involving interference with the mitochondrial respiratory chain (e.g., uncoupling) or inhibition of the important mitochondrial enzymes (oxidative phosphorylation, Szent-Györgyi-Krebs cycle, mitochondrial DNA replication, ADP/ATP translocator). The final phase of mitochondrial dysfunction induces loss of mitochondrial membrane potential and an increase in mitochondrial oxidative/nitrative stress, eventually culminating into cell death. This review aims to discuss the mechanisms of mitochondrion-mediated cardiotoxicity of commonly used drugs and some potential cardioprotective strategies to prevent these toxicities.
Keywords: heart, heart failure, cardiomyopathy, toxicology, drug development, reactive oxygen species
Cardiotoxicity from Drug Developmental Perspective
adverse cardiac effects are the leading cause of drug discontinuation and failure of clinical trials. Cardiotoxicity accounted for 45% of all drugs withdrawn between 1994 and 2006, which was due mainly to cardiac ischemia-related and arrhythmogenic side effects (Table 1) (28). Primarily, cardiotoxic drugs may induce cardiovascular adverse effects in a predictable dose- and time-dependent manner (e.g., doxorubicin). In contrast, secondarily cardiotoxic drugs promote adverse consequences in an unpredictable manner, often in patients with cardiovascular comorbidities (e.g., rosiglitazone). Although the above-mentioned adverse effects of numerous widely used drugs have been recognized recently, the cellular mechanisms of their cardiotoxicities are poorly understood. Moreover, the predictive value of currently available toxicity screening methods is very poor, particularly in subjects with cardiovascular comorbidities.
Table 1.
Latest drug discontinuations due to cardiotoxicity issues
| Drug Name | Drug Classification | Year of Recall |
|---|---|---|
| Fenfluramine | Anorectic | 1997 |
| Terfenadine | Antihistamine | 1998 |
| Sertindole | Antipsychotic | 1998 |
| Astemizole | Antihistamine | 1999 |
| Grepafloxacin | Antibiotic | 1999 |
| Cisapride | Prokinetic | 2000 |
| Droperidol | Tranquilizer | 2001 |
| Levomethadyl | Treatment of opiate dependance | 2003 |
| Rofecoxib | Nonstreoidal anti-inflammatory agent | 2004 |
| Tegaserod | Prokinetic | 2007 |
| Benfluorex | Anorectic | 2009 |
| Sibutramine | Anorectic | 2010 |
| Rosiglitazone | Antidiabetic | 2010 |
Strikingly, almost 10% of drugs in the last four decades have been recalled from the clinical market worldwide due to cardiovascular safety concerns. Recently, there have been major cases when already marketed drugs were withdrawn or their clinical indications were heavily restricted due to cardiovascular safety concerns, i.e., significantly increased risk of acute myocardial infarction or cardiac fibrosis revealed in phase IV postmarketing clinical studies [e.g., selective COX2 inhibitor rofecoxib (Vioxx) used for the treatment of inflammatory conditions (2004); a serotonin 4 receptor agonist, tegaserod (Zelnorm/Zelmac), used in irritable bowel syndrome (2007); an anti-obesity drug, sibutramine (Meridia; 2010); and peroxisome proliferator-activated receptor-γ (PPARγ) agonist antidiabetic drug rosiglitazone (Avandia; 2011)] (50, 62, 108). A surprising result of the SAVOR-TIMI 53 randomized trial showed that the selective dipeptidyl peptidase-4 inhibitor antidiabetic drug saxagliptin (Onglyza) aggravated heart failure morbidity in diabetic patients (132). Despite the great efforts to reveal cardiotoxicity in the preclinical phase of development of medicinal products, cardiotoxicity continues to lead safety concerns (87). This is obviously due to the lack of sufficient knowledge of the mechanisms of cardiotoxicity (54). Accumulating, recent evidence suggests that cardiotoxic drugs commonly induce mitochondrial dysfunction, which will be overviewed here.
Mitochondrial Oxidative Stress and Dysfunction is a Common Mechanism in Cardiotoxic Effects
Cardiomyocytes utilize an enormous amount of adenosine triphosphate (ATP), being in a constant energy-consuming contractile state. To maintain constant ATP production, malfunctioning mitochondria are constantly replaced by newly synthetized organelles by processes involving mitochondrial biogenesis and replication and autophagy/mitophagy (34, 153). These processes work in a tightly regulated manner, with mitochondrial fusion and fission allowing the dynamic formation and remodeling of a reticulated mitochondrial network (2). Since mitochondria are responsible for the production of ATP, agents that interfere with the physiological myocardial mitochondrial function are expected to induce depletion of ATP pool. Eventually, these processes may lead to subsequent myocardial dysfunction. There are several potential ways how drugs may induce mitochondrial dysfunction. Mitochondrial replication is a specific process that is required to maintain a “healthy” mitochondrial population. The antiviral nucleotide reverse transcriptase inhibitors are interfering with the action of the polymerase of the mitochondrial DNA, thereby inhibiting mitochondrial replication. This gradually reduces mitochondrial function in various tissues that will be apparent first in metabolically active organs such as the heart and the liver, resulting in cardiotoxicity and hepatotoxicity. Other drugs may directly interact with the electron transport chain (antidiabetic thiazolidinediones/glitazones, nonsteroidal anti-inflammatory drugs), resulting in uncoupling of electron transport from ATP production or directly induce oxidative stress in the mitochondria by redox cycling or by promoting iron accumulation and gluthation depletion [doxorubicin (Adriamycin); ethanol and acetaminophen (Tylenol); Fig. 1].
Fig. 1.
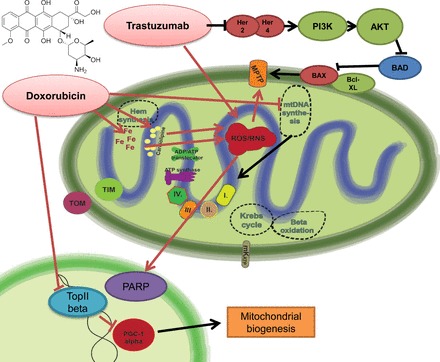
Doxorubicin-induced mitochondrial dysfunction and the effect of trastuzumab on mitochondria-related survival pathways. Doxorubicin leads to marked induction of mitochondrial reactive oxygen species (ROS) production. It shows specific binding activity to the mitochondrial abundant cardiolipin, leading to selective mitochondrial accumulation. Doxorubicin is prone to redox cycling, thereby promoting ROS and reactive nitrogen species (RNS) production. There is also increased intramitochondrial free iron accumulation after doxorubicin exposition, giving rise to additional nonenzymatic ROS production by the Haber-Weiss reaction. The major consequences of uncontrolled ROS/RNS production are mitochondrial permeability transition pore (MPTP) opening and poly(ADP-ribose) polymerase (PARP) activation, converging to the propagation to cell death signaling mechanisms. In parallel with ROS/RNS induction, doxorubicin also interferes with the action of topoisomerase-2β, being involved in the regulation of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) and thereby mitochondrial biogenesis- and metabolism-related pathways. Altogether, these doxorubicin-induced alterations profoundly alter both mitochondrial structure and function. The release of neuregulin-1 by the coronary endothelium activates human epidermal growth factor receptor (Her)4 (ErbB4) to dimerize with Her2 (ErbB2). The Her4/Her2 (ErbB4/ErbB2) dimer activates cardioprotective signaling pathways, including phosphatidyinositol 3-kinase (PI3K)/protein kinase B (Akt), ERK1/2, and focal adhesion kinase (FAK), which promote cell survival upon cellular stress. Trastuzumab blocks Her2 signaling and disrupts this cardioprotective machinery, resulting in loss of cytoprotective mechanisms. Furthermore, trastuzumab may trigger cellular oxidative stress and induce the expression and activation of proapoptotic proteins [e.g., Bcl-2-associated X protein (BAX)]. These events result in mitochondrial defects, leading to the opening of the MPTP and the activation of cell death pathways that precipitate myocardial dysfunction. BAD, Bcl-2-associated death promoter; mKATP, mitochondrial ATP-sensitive potassium channel.
Oxidative/nitrative modifications of mitochondrial proteins might play a crucial role in the development of myocardial dysfunction (see Table 2). Oxidative/nitrative modification may trigger potentially harmful events, including dissociation of catalytic subunits of enzymes, local or global unfolding, aggregation, or fragmentation, all promoting degradation of modified proteins leading to autophagy/mitophagy and endoplasmic reticulum stress (10, 17, 153). Oxidation/nitration might be directly triggered by reactive oxygen species (ROS)/reactive nitrogen species (RNS) or by products of secondary oxidation reactions formed during lipid peroxidation (e.g., malondialdehyde or 4-hydroxynonenal) (89, 174). The free radicals produced intramitochondrially can directly inactivate the electron transport complexes by interacting with the iron-sulfur cluster, or they may lead to activation of apoptosis-initiating pathways by inducing mitochondrial transition pore opening.
Table 2.
Oxidative and nitrative modifications of key mitochondrial proteins in various forms of cardiomyopathy
| Modified Protein | Modification | Function | Ref. No(s). |
|---|---|---|---|
| 3-Ketoacyl-CoA thiolase | Oxidation | Fatty acid β-oxidation | 23 |
| Acetyl-CoA acetyl transferase | Oxidation | Fatty acid β-oxidation | 23 |
| Acyl-CoA dehydrogenase | Oxidation | Fatty acid β-oxidation | 23 |
| ATP synthase subunits | Nitration | ATP synthesis | 20, 82 |
| BNIP3 | Oxidation | Mitophagy, oxidative stress sensor | 65 |
| CaMK II | Oxidation | Mitochondrial stress response | 44, 51 |
| Carnitine palmitoyltransferase-1 | Nitration | Fatty acid transport | 29 |
| Complex I, III, and V | Oxidation | ATP synthesis | 9, 18, 147 |
| Complex I (24-kDa subunit) | Nitration | ATP synthesis | 149 |
| Complex II | Oxidation | ATP synthesis | 141 |
| Oxidation | 158, 159 | ||
| Connexin43 | Nitration | Mitochondrial potassium uptake | 38, 52, 138 |
| Creatine kinase | Nitration | Maintenance of ATP pool | 93, 94 |
| Oxidation | 162 | ||
| Cytochrome c | Nitration | Mitochondrial apoptosis | 16 |
| Oxidation | 58 | ||
| Enoyl-CoA hydratase | Oxidation | Fatty acid β-oxidation | 23 |
| mtHSP70, mitofilin | Oxidation | Mitochondrial protein import | 9 |
| Peroxiredoxin 3 | Nitration | Antioxidant defense | 149 |
| Oxidation | 23, 67 | ||
| Prohibitin | Nitration | Unknown | 85 |
| Succinyl-CoA:3-oxoacid CoA transferase | Nitration | Ketone body metabolism | 149, 160 |
| Superoxide dismutase-2 | Oxidation | Antioxidant defense | 23, 63 |
| Nitration | 41 | ||
| VDAC1 | Nitration | Mitochondrial apoptosis | 150 |
BNIP3, Bcl-2/adenovirus E1B 19-kDa-interacting protein 3; VDAC1, voltage-dependent anion channel.
Inhibition of the tricarboxylic acid (TCA) cycle (also known as the citric acid cycle or Szent-Györgyi-Krebs cycle) also occurs due to excessive mitochondrial oxidants production by the oxidation of aconitase. Increased ROS generation in cardiomyocytes may trigger the activation of various mitochondrial-dependent and -independent cell death pathways involved in apoptotic and necrotic cell death [e.g., activation of caspases and poly(ADP-ribose) polymerases (PARP)] (115). Furthermore, superoxide in the mitochondria may react with nitric oxide to generate a highly reactive oxidant, peroxynitrite (110, 111), which may impair cellular function and lead to cell death (100) and/or dysfunction (114) in cardiomyocytes and endothelial cells via multiple interrelated mechanisms involving PARP (112) and matrix metalloprotease (MMP) activation (5). Mitochondrial proteins are particularly vulnerable to peroxynitrite-induced nitration, leading to irreversible functional loss (110, 119, 142).
Cardiotoxicity of Anticancer Drugs
Doxorubicin-induced cardiotoxicity: is mitochondrial oxidative stress a cause or consequence?
Doxorubicin was discovered in the late 1960s. It is an anticancer antibiotic belonging to the anthracycline family and was isolated from a culture of the Streptomyces peucetius (1).
Although the early results of doxorubicin clinical trials achieved great success (12) a few years later in patients who underwent doxorubicin treatment, development of cardiac toxicity and cardiomyopathy has been reported (74, 139). Despite this serious side effect, doxorubicin is clinically widely used and is still probably one of the most potent anti-cancer agents available for the clinical practice (47). Doxorubicin is commonly used to treat several types of tumors, such as in different forms of leukemia and lymphomas, soft-tissue sarcomas, and solid tumors. Patients subjected to doxorubicin usually exhibit typical symptoms of cytotoxic chemotherapy (nausea, vomiting, alopecia, myelosuppression, stomatitis, and gastrointestinal disturbances); nevertheless, the cardiotoxicity is completely different, being probably the most hazardous side effect associated with doxorubicin treatment. In the worst case it may reach 50% mortality for the highest cumulative dosages. This dose-dependent chronic doxorubicin-induced cardiotoxicity can develop within 1 mo or even years after the treatment initiation (dosages >500 mg pro 1 m2 body surface have 5% probability of inducing cardiac heart failure) (97). Recent studies also highlight the occurrence of late-onset cardiac dysfunction in adults who were treated with doxorubicin during their childhood; 5.8% of this population had severely reduced ejection fraction. However, systolic and diastolic dysfunction by sensitive modalities (strain rate imaging) was more prevalent (apparent in >30% of patients). Interestingly, survivors with metabolic syndrome were more prone to develop contractile dysfunction, suggesting a common molecular link in doxorubicin- and metabolic syndrome-induced contractile dysfunction (3).
The mechanism for doxorubicin-induced cardiotoxicity is controversial, and numerous hypotheses have been proposed in past decades. Initially, it was widely accepted that doxorubicin-induced cardiotoxicity is completely independent from its anticancer activity. This concept was in agreement with the fact that cardiomyocytes, as terminal, differentiated, nondividing cells, should not be sensitive to the primary antineoplastic activity, which is related the blockade of DNA transcription and replication.
Therefore, the majority of studies focused on the involvement of overt mitochondrial abnormalities and components. It has been shown that doxorubicin forms adducts with mitochondrial DNA and binds to other biomolecules like the mitochondrial abundant phospholipid cardiolipine. Mitochondrial DNA oxidation is cardioselective and cumulative (133) and might be a major contributor of heart failure development (71, 84). Once doxorubicin accumulates in mitochondria, it can initiate intramitochondrial ROS and RNS production (102) by various, mainly nonenzymatic mechanisms, leading to activation of cell death pathways (e.g., PARP-dependent cell death; see Refs. 112 and 113). One major hypothesis of doxorubicin-induced ROS formation implies the redox cycling-dependent production of superoxide anion described first by Davies and Doroshow (21; also see Ref. 26) and confirmed by others (66, 121, 135). Increased ROS production subsequently induces activation of proinflammatory transcription factor NF-κB and inducible nitric oxide synthase (100). The diffusion-limited reaction of superoxide and nitric oxide forms peroxynitrite, a potent oxidant that further aggravates initiation of cell death (111). It has also been suggested that doxorubicin induces an alternative iron-mediated increase in ROS production (11, 103). According to this hypothesis, it is likely that mitochondrial accumulation of iron is detrimental, since doxorubicin-derived superoxide that will be eventually converted to H2O2 will form highly toxic hydroxyl radicals in the presence of iron by a reaction described as Haber-Weiss reaction. In addition, doxorubicin can interact with iron directly to form a complex that will result in iron cycling between the ferro [Fe(II)] and ferri [Fe(III)] forms and will lead to additional ROS production (49, 165). Doxorubicin-induced oxidative stress is further aggravated by the infectivity and/or inhibition of antioxidant mechanisms by doxorubicin. In line with this, overexpression of antioxidant enzyme systems [manganese superoxide dismutase (166), catalase (55), and metallothionein (39, 56)] alleviates doxorubicin cardiotoxicity.
Recently, the doxorubicin-related DNA transcription blockade has been also linked to mitochondrial dysfunction. The primary target of doxorubicin is the topoisomerase IIα expressed in many cancerous tissues (117). Detailed investigations shed light on the fact that cardiomyocytes express the other isoenzyme of topoisomerase 2, the Top2β (15). Accordingly, recent results suggest that doxorubicin-induced cardiotoxicity is not due solely to the ROS producing redox cycling reactions of doxorubicin. In the presence of Top2β (like in case of cardiac tissue), doxorubicin activates DNA response genes and consequently apoptosis pathways and further triggers marked alterations in the transcriptome, which selectively affects oxidative phosphorylation and mitochondrial biogenesis [downregulation of peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α and PGC-1β] in cardiomyocytes, leading to mitochondrial oxidative stress and metabolic failure (42, 172) (Fig. 1). This explains the classical observation that doxorubicin causes both structural and functional mitochondrial abnormalities.
Targeting doxorubicin-induced cardiac pathological alterations in the clinical setting is really challenging. Although several drug candidates have been positively evaluated in animal models, only a few of them have been tested so far in clinical studies. It is also important to keep in mind that the drug used to treat/prevent doxorubicin-induced cardiomyopathy should not interfere with its antitumor activity.
Dexrazoxane is the most studied cardioprotective adjuvant for doxorubicin chemotherapy, which itself has antineoplastic properties. Due to a metabolite that chelates free iron (131), dexrazoxane alleviates doxorubicin-induced mitochondrial oxidative stress and the subsequent depletion of mitochondrial DNA (72). The clinical efficacy of dexrazoxane has been confirmed by Lipshultz et al. (81) in children with acute lymphoblastic leukemia undergoing doxorubicin treatment. They found that dexrazoxane attenuated doxorubicin-induced cardiac injury without compromising its antileukemic efficacy (81).
Since β-adrenergic receptor antagonists along with angiotensin-converting enzyme inhibitors are the most widely used drugs in the treatment of heart failure, their applicability in doxorubicin-treated patients is plausible. Accordingly, in patients taking carvedilol, a combined α- and β-receptor antagonist with antioxidant properties, left ventricle size remained constant, and diastolic function was preserved after doxorubicin treatment (53).
Consistently with the importance of mitochondrial ROS generation in the cardiotoxicity of doxorubicin, mitochondria-targeted antioxidants mito-tempol and MitoQ exerted cardioprotective effects in rodents without interfering with doxorubicin's antitumor effect (24). Activation of the nuclear enzyme PARP due to doxorubicin-induced oxidative DNA injury and the consequent cell death are key events in doxorubicin-induced cardiotoxicity (112). Consequently, PARP inhibitors (e.g., olaparib), novel FDA-approved anticancer medications often combined with doxorubicin or cisplatin, are promising cardioprotective agents against doxorubicin-induced cardiomyopathy based on preclinical results (112).
Cisplatin.
Cisplatin belongs to the alkylating group of broad-spectrum chemotherapeutic drugs used against various types of tumors [sarcomas, carcinomas (e.g., small cell lung cancer, ovarian), lymphomas, and germ cell tumors]. A significant factor limiting its applicability is acute and cumulative cardiotoxicity and nephrotoxicity (116), sharing similar cellular and molecular mechanisms (86, 99, 177). Cisplatin-induced cardiac dysfunction is associated with mitochondrial membrane depolarization along with ultrastructural abnormalities of the mitochondria. Following cisplatin treatment, cardiomyocytes also show signs of activation of the endoplasmic reticulum stress response, increased caspase 3 activity, and increased rate of apoptosis (86). It is well documented that cisplatin promotes kidney injury by triggering mitochondrial ROS generation in renal tubular cells (177). It is highly plausible that cisplatin induces cardiotoxicity by similar mechanisms (involving increased mitochondrial oxidative stress). In accord with this, mitochondria targeted antioxidants might represent a promising approach to alleviate both cardiac and kidney injury in patients undergoing cisplatin chemotherapy (99).
Trastuzumab.
Trastuzumab is a monoclonal antibody that inhibits the activation of the receptor tyrosine-protein kinase erbB-2/proto-oncogene neu (HER2/neu), thereby interfering with the growth of certain breast cancers. Extensive data have shown recently that HER2 has also an important role in embryonic heart development, and in the adult heart it is involved in cardiac protection (107). HER2-initiated signaling is essential for growth, survival, and inhibition of apoptosis of cardiomyocytes; therefore, in cases where the heart is subjected to biomechanical stress (e.g., hypoxia, myocardial injury, or concomitant anthracycline use), neuroregulin binds to HER2/HER4 heterodimers, thereby promoting cardiomyocyte survival via the activation of the phosphatidylinositide 3-kinase (PI3K) and MAPK pathways (106, 170). In addition, a decrease in HER2 and HER4 protein expression has been shown to be associated with the transition of compensated cardiac hypertrophy to heart failure (124). Furthermore, trastuzumab triggers cellular oxidative stress and induces the expression and activation of proapoptotic proteins. These events result in mitochondrial defects, leading to the opening of the mitochondrial permeability transition pore (MPTP) and the activation of cell death pathways, which precipitate myocardial dysfunction (Fig. 1) (6, 176).
Anthracyclines induce a variable degree of myocardial damage even when administered at safe doses. The anthracyclin-induced myocardial injury is associated with the concomitant activation of HER2 survival pathways within the cardiomyocytes as an attempt to prevent cardiomyopathy. Several in vitro, in vivo, and clinical studies have clearly shown that trastuzumab significantly aggravates anthracycline-induced cardiac damage, resulting in an extremely high incidence of symptomatic heart failure that reached 27% of treated patients (137). Although the incidence of symptomatic heart failure of trastuzumab-treated patients in monotherapy is significantly lower (∼4%) (152), even trastuzumab alone exhibits inherent cardiac toxicity that is associated with significant alterations in the expression of myocardial genes essential for DNA repair and cardiac and mitochondrial function (31).
Arsenic trioxide.
Arsenic trioxide is an antineoplastic drug, inducing changes in apoptotic signaling in cancer cells. It also appears that arsenic trioxide inhibits the PML-RAR fusion protein often detected in acute promyelocytic leukaemia. Although it induces dramatic remissions in patients with acute promyelocytic leukaemia, several clinical reports have shown that the treatment is associated with significant cardiotoxicity (QT prolongation, torsade de pointes, sudden death) (105, 151). Exposure of H9C2 cardiomyocytes to clinically relevant concentrations of the drug (2–10 μM) induced apoptosis, ROS formation, intracellular calcium overload, and caspase-3 activation (173). The negative impact of arsenic trioxide is further confirmed by in vivo studies showing a significant decrease in the maximum rate of rise in intraventricular pressure during ventricular contraction (maximal dP/dt) and significant increases in the end diastolic pressure. There is also impaired response to β-adrenergic stimulation (isoproterenol) (77). In a recent study, the proapoptotic effect of arsenic trioxide was confirmed, and the role of Parkin-dependent ubiquitin proteasome activation was described to be associated with arsenic trioxide-induced loss of mitochondrial membrane potentials (161).
Mitoxantrone.
Mitoxantrone is a DNA topoisomerase inhibitor and nonanthracycline antineoplastic agent used for the treatment of various cancers (metastatic breast cancer, acute myeloid leukemia, non-Hodgkin's lymphoma, acute lymphoblastic leukemia in children, and metastatic hormone-refractory prostate cancer). In addition to cancer therapy, it is widely used in multiple sclerosis, effectively slowing the progression and preventing the relapses in relapsing remitting and the progressive-relapsing form of the disease. Cardiomyopathy is a particularly concerning side effect of long-term mitoxantrone therapy, as it is usually severe, may occur years after treatment, and is irreversible (128, 140). Mitoxantrone induces striking energetic imbalance, as evidenced by decreased ATP levels, hyperpolarization of the mitochondrial membrane potential, and significant rise in the intracellular calcium levels in vitro. This is further complicated by late inhibition of ATP-synthase expression and activity with concomitant increase in ROS formation (126). It is now evident also that cardiac functional alterations are due to the presence of aberrant mitochondria, changes in mitochondrial complex IV and V activities, and depletion of cardiac ATP levels (125). It cannot be also excluded that mitoxantrone as a DNA topoisomerase inhibitor may interact with the mitochondrial topoisomerase enzyme being critically involved in maintenance of mitochondrial integrity and cellular energy metabolism (27).
Imatinib mesylate.
Imatinib mesylate is one of the first marketed drugs that has been developed for tyrosine kinase inhibition (inhibitor of the BCR/Abl fusion protein). Despite its cardiotoxic effect, imatinib mesylate represents a revolution in the management of patients with Philadelphia chromosome-positive chronic myelogenous leukemia, increasing the survival of patients by inducing complete cytogenetic response in more than 70% of patients treated (22, 57). In 2001 the FDA approved its clinical use, and the cardiotoxic effect of imatinib was described first only 5 years later, in 2006, by Kerkelä et al. (60). In several patients, sudden development of NYHA class 3–4 heart failure has been reported after a few months (7.2 ± 5.4) of imatinib therapy. The ultrastructural analysis revealed prominent membrane whorls in myocytes and an increased number of mitochondria and pleomorphic mitochondria with effaced cristae, which is indicative of increased mitochondrial biogenesis that is typically detectable in hearts with impaired mitochondrial energy production. In vitro studies on isolated cardiomyocytes revealed that imatinib produces a dose-dependent collapse in mitochondrial membrane potential. The inhibition of Abl kinase by imatinib resulted in increased endoplasmic reticulum stress, as detected by increased PRKR-like endoplasmic reticulum kinase (PERK) activation and phosphorylation of eukaryotic initiation factor-2α eIF2α. PERK also influences mitochondrial proteostasis through translational attenuation of translocase of the inner membrane (the 23-kDa form TIM23), a major component of the mitochondrial protein import machinery. TIM23 is degraded after eIF2α phosphorylation, and thus unfolded proteins may accumulate in mitochondrial intermembrane space, challenging the mitochondrial proteostasis pathways and inducing mitochondrial death pathways (120). Therefore, alterations in ER stress pathways leading to mitochondrial function impairment seem to be essential in the cause of imatinib-induced cardiotoxicity (Fig. 2). Recent studies also confirm that the action of imatinib on the heart targets cardiomyocytes and involves mitochondrial impairment and cell death that can be aggravated further by the presence of oxidative stress (88).
Fig. 2.
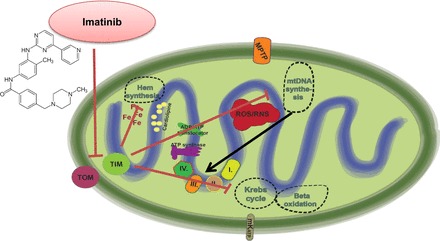
Mechanisms of imatinib cardiotoxicity. The small molecule inhibitor of the Bcr/Abl (fusion kinase of the break point cluster region of chromosome 22 and the Abelson1 gene of chromosome 9) kinase imatinib induces mitochondrial dysfunction by interfering with mitochondrial protein import machinery. Increased PRKR-like endoplasmic reticulum kinase (PERK) activation in the endoplasmic reticulum leads to the phosphorylation eukaryotic initiation factor 2α (eIF2α) and to translational attenuation of translocase of the inner membrane (TIM) 23-kDa form that is essential for protein import into the mitochondrial matrix. Impaired protein import will negatively affect major mitochondrial metabolic pathways, including mitochondrial DNA synthesis, the Krebs cycle, β-oxidation, and hem synthesis. TOM, translocase of the outer membrane.
Cardiotoxic effect of antiangiogenic drugs.
Antiangiogenic drugs inhibiting vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1-related signaling have been approved for the treatment of advanced carcinomas of the lung, breast, colon, and rectum (59). After their initiation in the clinical practice, several cardiovascular side effects involving left ventricular dysfunction and subsequent heart failure have been reported (13, 19, 129). Myocardial dysfunction develops partially on the basis of the heart's dependence on adequate angiogenesis; however, other mitochondria-related signaling pathways also play important roles in the observed pathology (164).
Bevacizumab (Avastin) is a recombinant humanized monoclonal antibody that blocks angiogenesis by inhibiting VEGF-A. The development of cardiomyopathy and heart failure, however, is a rare event in bevacizumab-treated patients (∼2%; see Ref. 95). Small-molecule, multitargeted receptor tyrosine kinase inhibitors have been developed to inhibit divergent pathways involved in tumor cell survival (VEGF, platelet-derived growth factor, c-kit, RET proto-oncogene, RAF proto-oncogene serine/threonine-protein kinase-1, and Fms-like tyrosine kinase 3). Sunitinib (Sutent) and sorafenib (Nevaxar) are the most widely used drugs with potent antiangiogenic activity that are currently approved for the treatment of metastatic renal cell carcinoma and for imatinib-resistant gastrointestinal stromal cell tumors. Sunitinib is reported to be cardiotoxic, deteriorating myocardial contractility in up to 28% of treated patients (19). The mechanism of sunitinib cardiotoxicity can be explained by inhibition of off-target pathways such as the ribosomal S6 kinase and AMP-activated protein kinase (43, 61), both of which are involved in mitochondrial energy homeostasis and quality control (175).
Cardiotoxicity of Antiviral Drugs
In attempt to control HIV infection, multiple antiviral drug combinations have been developed. The majority of highly active antiretroviral therapy regimens involve nucleoside analogs that inhibit the reverse transcriptase of the virus, such as zidovudine [azidothymidine (AZT)]. However, long-term treatment with AZT may cause cardiomyopathy as a result of mitochondrial toxicity. AZT-triphosphate interferes with the mitochondrial DNA polymerase-γ, the enzyme responsible for mitochondrial DNA replication (75) (Fig. 3). It has also been suggested that AZT may directly inhibit important mitochondrial transport mechanisms such as the mitochondrial ADP/ATP tranlocator (8) and the mitochondrial deoxynucleotide carrier (25). Although energy depletion by both direct inhibitions of ADP/ATP translocation and mitochondrial replication contributes to cardiac dysfunction, it is now evident that AZT induces increased mitochondrial ROS production as well (36). It is supported by the fact that AZT-induced cardiomyopathy is prevented in mitochondrial superoxide dismutase transgenic mice. In addition, catalase targeted directly into the mitochondria also prevents the AZT-induced oxidative stress and cardiomyopathy development (64). In line with these results, we have reported a sudden increase in mitochondrial ROS production in AZT-treated human cardiomyocytes that was associated with subsequent activation of major cell death pathways (caspase-3 and -7 as well as PARP) (36) being involved in cardiomyopathy development.
Fig. 3.
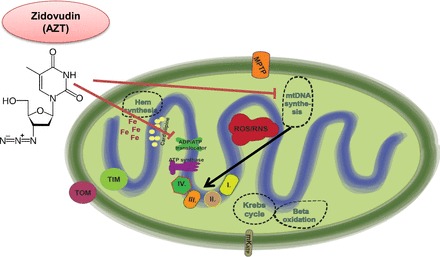
Mechanisms of zidovudin [azidothymidine (AZT)] cardiotoxicity. AZT interferes with mitochondrial DNA polymerase-γ, the enzyme responsible for mtDNA replication. Since the mitochondrial DNA (mtDNA) codes the proteins of the electron transport chain (ETC), inhibition of mtDNA replication will result in mitochondrial energetic imbalance. AZT may directly inhibit the mitochondrial ADP/ATP tranlocator, inhibiting the transport of ATP produced from oxidative phosphorylation and the substrate ADP to be transported from the cytoplasm to the mitochondrial matrix.
Cardiotoxicity of Antidiabetics
Diabetes is a major risk factor and comorbidity for heart diseases, including ischemic heart disease and diabetic cardiomyopathy (32, 153). Therefore, many of the cardiac disease patients are treated with oral antidiabetics on top of general medications for ischemic heart disease. However, some of the antidiabetic drugs, especially glitazones and sulfonylureas, have been shown to exert potential cardiotoxicity.
Rosiglitazone, a thiazolidinedione class of antidiabetic compound acting as an insulin sensitizer via activation of PPAR receptors (122), has been shown by meta-analyses to increase cardiovascular risk. In 2007, a meta-analysis of four randomized controlled trials of rosiglitazone used for at least 12 mo for prevention or treatment of type 2 diabetes showed that rosiglitazone was associated with a significantly increased risk of myocardial infarction and heart failure, although no increase in risk of cardiovascular mortality was observed (136). A recent metaanalysis of the cardiovascular outcomes in 16 studies, including 810,000 thiazolidinedione users, confirmed that the use of rosiglitazone is associated with significantly higher odds of congestive heart failure, myocardial infarction, and death relative to pioglitazone users (83).
The mechanism by which thiazolidinedione antidiabetics, especially rosiglitazone, may exert cardiotoxocity is still not exactly known, but several mechanisms were suspected in preclinical studies (Fig. 4). Both rosiglitazone and pioglitazone have been shown to block KATP, thereby leading to increased incidence of ventricular fibrillation during ischemia in pigs (127). Adverse electophysiological changes in mice, rats, and canine cardiac myocytes have been also shown due to rosiglitazone treatment in vitro (143, 144). Inhibition of mitochondrial respiration has been shown by PPAR agonists troglitazone and darglitazone in isolated mitochondria of the rat liver (104). By a toxico-proteomics approach, other mitochondrial off-targets of troglitazone that may lead to impaired mitochondrial glutathione import and increased oxidative stress have been found (73). In another toxico-proteomics study, off-target affinity of glitazones was identified with various targets, including components of mitochondrial energy metabolism (46). Troglitazone has also been shown to induce cytotoxicity and mitochondrial toxicity at least in part by promoting the degradation of PPARγ coactivator-1α (PGC-1α) (78). More interestingly, troglitazone has been shown to activate mitochondrial permeability transitions pore (MPTP) in isolated rat liver mitochondria (109). Since MPTP inhibition is a common downstream target of most cardioprotective signals, activation of MPTP by troglitazone may lead to deteriorated ischemic tolerance of the heart, i.e., hidden cardiotoxicity (32).
Fig. 4.
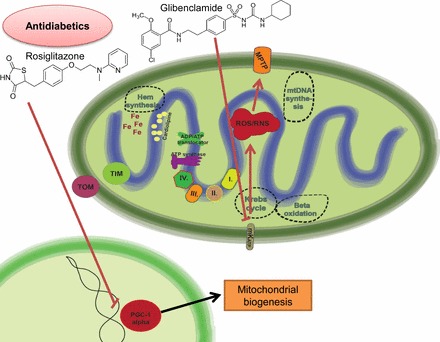
Mechanisms of antidiabetic-related cardiotoxicity. The insulin sensitizer “glitazone” may promote the degradation of PGC-1α, a master regulator of mitochondrial biogenesis and metabolism. Sulfanylureas (e.g., glibenclamide) directly inhibit the mKATPs that are involved in cardioprotective mechanisms. Inhibition of the mKATP leads to increased ROS production and MPTP opening.
The KATP blocker sulfonylureas show potential cardiotoxicity. A meta-analysis of all trials with a duration of at least 6 mo comparing a sulfonylurea with a nonsulfonylurea agent in type 2 diabetes concluded that the use of sulfonylureas was associated with increased mortality and a higher risk of stroke, whereas the overall incidence of major cardiovascular events was not affected (96). Other meta-analyses showed that patients receiving sulfonylureas had increased all-cause and cardiovascular mortality risks (35, 118). The mechanism by which sulfonylureas may exert cardiotoxicity is the inhibition of both sarcolemmal and mitochondrial KATP channels. Sarcolemmal KATP blockade may lead to action potential shortening causing tachyarrhythmias. Mitochondrial KATP blockade may lead to oxidative stress, thereby leading to mitochondrial dysfunction (32, 33).
Cardiotoxicity of Recreational Drugs: Alcohol, Cocaine, Methamphetamine, Ecstasy, and Cannabinoids
Cardiotoxicity in alcohol abuse.
Alcohol abuse impairs myocardial contractility, leading to systolic dysfunction and dilation of the ventricles, which is known as alcoholic cardiomyopathy (30, 37).
The toxic effect is explained by three closely related hypotheses (Fig. 5). Alcohol may exert cardiotoxic effect by directly damaging cardiac mitochondria since the metabolizing enzyme, the alcohol dehydrogenase, is almost absent from cardiomyocytes (4). In addition, acetaldehyde, the metabolic by-product synthetized in the liver during oxidative alcohol catabolism, may further trigger the damage of cardiomyocytes by reducing the synthesis of myocardial proteins (68, 130) and thereby disturbing calcium homeostasis and inducing endoplasmic reticulum stress (40, 76). Nonoxidative metabolism of alcohol usually results the production of fatty acid ethyl esters that have been shown to be produced in the heart and to induce mitochondrial dysfunction through uncoupling of mitochondrial oxidative phosphorylation (69). In addition, alcohol-induced cardiac toxicity may also involve the inhibition of the mitochondrial respiratory chain by impairing the function of tricarboxylic acid cycle (Szent-Györgyi-Krebs cycle) enzymes (92). Alcohol induces oxidative stress mainly in the mitochondria due to its metabolism by cytochrome p450 2E1 isoenzyme (45, 171). Mitochondrial oxidative damage in ethanol-treated rats also contributes to myocardial fibrotic changes (155). Alcohol-induced oxidative stress is further enhanced by aggravated angiotensin II signaling, resulting in myocardial NADPH oxidase upregulation, oxidative stress, inflammation, and fibrosis (146), which are known contributors of myocardial dysfunction (154). Ethanol also may activate inducible nitric oxide synthase (partially by increasing endotoxin load from the gastrointestinal tract; see Ref. 7) to produce nitric oxide, which when reacting with superoxide forms the highly reactive substance peroxynitrite, which can further impair the function of mitochondrial function by posttranslational protein modifications (110, 153). There is also evidence in animal models of chronic alcoholism for decreased number of mitochondria and impaired expression of pivotal mitochondrial components like the master regulator of mitochondrial biogenesis, PGC-1α (48, 90). The combination of these different toxic effects both contributes to the pathology and potentially culminates in the decrease of myocardial contractility and function.
Fig. 5.
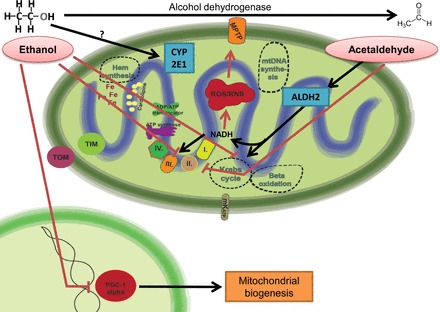
Mechanisms of ethanol cardiotoxicity. Ethanol will increase mitochondrial ROS production by complex mechanisms. On the one hand, ethanol and acetaldehyde will inhibit the function of the Krebs cycle and the ETC, whereas in parallel there is increased intramitochondrial NADH production by aldehyde dehydrogenase 2 (ALDH2). To bypass the inhibited ETC, NADH may be used for ROS/RNS production, leading to MPTP opening. Inhibition of PGC-1α by ethanol leads to deteriorated mitochondrial biogenesis and oxidative metabolism. CYP2E1, cytochrome P450 2E1.
Cardiotoxicity in cocaine abuse.
Cocaine abuse causes irreversible structural and functional abnormalities in the heart, resulting in chronic reduction in left ventricular contractility and increased incidence of arrhythmias. Additionally, coronary vasoconstriction and atherosclerosis develops, which makes cocaine users more susceptible to myocardial infarction (123).
The main cause of cardiac side effects in cocaine abuse is overstimulation of the adrenergic system. Most of the toxic effects of cocaine on the molecular level are mediated by oxidative stress or mitochondrial dysfunction caused by metabolism of the excess of catecholamines (79). The transformation of catecholamines into “aminochromes” occurs, which may undergo redox cycling after entering the mitochondria. This leads to the generation of significant amounts of oxygen-derived free radicals. There is also evidence for inhibition of complex I by cocaine (169) and for xanthine oxidase-dependent increase in mitochondrial ROS production after cocaine exposure (157). In turn, calcium overload and oxidative stress promote mitochondrial permeability transition and cardiomyocyte cell death via activation of both the apoptotic and necrotic pathways (70). The central role of mitochondrial oxidative stress in cocaine-induced cardiotoxicity is also supported by the results of Vergeade et al. (156) showing the attenuation of cardiotoxicity by MitoQ, a mitochondrial-targeted antioxidant.
Cardiotoxicity in methamphetamine and ecstasy abuse.
Methamphetamine abuse is a significant problem with a steeply increasing frequency of use worldwide. Chronic methamphetamine use is associated with focal contraction band necrosis in association with cellular degeneration and myocytolysis in the heart. After chronic administration, cardiac hypertrophy, intracellular vacuolization, and fibrosis can be also observed (167). The direct mechanisms by which methamphetamine exerts its harmful effects on the heart are not known in detail. Nevertheless, increased mitochondrial superoxide production (91), increased mitochondrial protein tyrosine residue nitration (see Table 2) (85), and induction of Fas- and mitochondria-dependent apoptosis have been reported recently (80).
Ecstasy is a substituted amphetamine, producing structural and functional alterations in the myocardium that are associated with increased oxidative stress. It has been reported that ecstasy significantly increases nitrotyrosine content in the heart. In addition, a detailed proteomic analysis revealed increased nitration of contractile proteins (troponin-T, tropomyosin-α1 chain, myosin light polypeptide, and myosin regulatory light chain), mitochondrial proteins (ubiquinon-cytochrome c reductase and ATP synthase), and sarcoplasmic reticulum calcium ATPase (134).
Cardiotoxicity in cannabinoid abuse.
Synthetic or designer cannabinoid compounds are getting popular, especially among young people. These illicit drugs are among the most frequently used ones, since these mixtures can still be purchased easily due to the lack of legal restrictions (manufacturers are constantly changing and substituting different chemicals in their mixtures). Several case reports were published recently, showing occurrence of life-threatening complications induced by synthetic cannabinoids involving cardiotoxicity (168), acute kidney injury (14), or cerebral ischemia (145). The majority of studies suggest cannabinoid receptor 1 (CB1)-dependent toxicity (148, 163), which is in line with our recent results showing the role of increased endocannabinoid levels (98) and overactivated CB1 signaling (101) in doxorubicin-treated hearts. Our results also suggest that peripherally restricted CB1 receptor antagonism might be a promising strategy to alleviate cardiac dysfunction and reduce doxorubicin-induced apoptosis in the myocardium (97).
Conclusions and Therapeutic Perspectives
Collectively, multiple lines of evidence briefly discussed in this synopsis strongly suggest that the cardiotoxicity of multiple commonly used anticancer drugs, antiviral compounds, and antidiabetic or illicit drugs of abuse such as alcohol, cocaine, methamphetamine, ecstasy, and synthetic cannabinoids involves direct or indirect mitochondria-related toxicity, which is comprised of interference with the mitochondrial respiratory chain (e.g., uncoupling) or inhibition of the important mitochondrial enzymes (oxidative phosphorylation, Szent-Györgyi-Krebs cycle, mitochondrial DNA replication, ADP/ATP translocator), eventually leading to loss of mitochondrial membrane potential, an increase in mitochondrial oxidative/nitrative stress, and cell demise. More thorough understanding of the common mechanisms of mitochondrial cardiovascular toxicities is required to develop sensitive and high-throughput mitochondrial toxicity screening methods and in vivo models to better predict the unforeseen cardiotoxicity issues with novel compounds as well as devise novel cardioprotective strategies based on more selective targeting of specific mitochondrial processes for prevention of the above-discussed severe cardiovascular adverse consequences of common drugs.
GRANTS
This study was supported by the Intramural Research Program of the of National Institutes of Health/NIAAA (to P. Pacher). Z. V. Varga was supported by the Rosztoczy Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.V.V. prepared figures; Z.V.V., P.F., L.L., and P.P. drafted manuscript; Z.V.V., P.F., L.L., and P.P. edited and revised manuscript; Z.V.V., P.F., L.L., and P.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We are indebted to Dr. George Kunos, the Scientific Director of the National Institute on Alcohol Abuse and Alcoholism (NIAAA), for continuous support.
REFERENCES
- 1.Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C, Spalla C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol Bioeng 11: 1101–1110, 1969. [DOI] [PubMed] [Google Scholar]
- 2.Archer SL. Mitochondrial dynamics—mitochondrial fission and fusion in human diseases. N Engl J Med 369: 2236–2251, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GT, Joshi VM, Ness KK, Marwick TH, Zhang N, Srivastava D, Griffin BP, Grimm RA, Thomas J, Phelan D, Collier P, Krull KR, Mulrooney DA, Green DM, Hudson MM, Robison LL, Plana JC. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac Dysfunction in Adult Survivors of Childhood Cancer: Results From the St. Jude Lifetime Cohort Study. J Am Coll Cardiol 65: 2511–2522, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Awtry EH, Philippides GJ. Alcoholic and cocaine-associated cardiomyopathies. Prog Cardiovasc Dis 52: 289–299, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Bai P, Mabley JG, Liaudet L, Virag L, Szabo C, Pacher P. Matrix metalloproteinase activation is an early event in doxorubicin-induced cardiotoxicity. Oncol Rep 11: 505–508, 2004. [PubMed] [Google Scholar]
- 6.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434: 658–662, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One 9: e96864, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barile M, Valenti D, Passarella S, Quagliariello E. 3′-Azido-3′-deoxythmidine uptake into isolated rat liver mitochondria and impairment of ADP/ATP translocator. Biochem Pharmacol 53: 913–920, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, Razunguzwa TT, Hollander JM. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol 300: R186–R200, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bayeva M, Ardehali H. Mitochondrial dysfunction and oxidative damage to sarcomeric proteins. Curr Hypertens Rep 12: 426–432, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol 23: 15–25, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Bonadonna G, Monfardini S, De Lena M, Fossati-Bellani F, Beretta G. Phase I and preliminary phase II evaluation of adriamycin (NSC 123127). Cancer Res 30: 2572–2582, 1970. [PubMed] [Google Scholar]
- 13.Bordun KA, Premecz S, daSilva M, Mandal S, Goyal V, Glavinovic T, Cheung M, Cheung D, White CW, Chaudhary R, Freed DH, Villarraga HR, Herrmann J, Kohli M, Ravandi A, Thliveris J, Pitz M, Singal PK, Mulvagh S, Jassal DS. The utility of cardiac biomarkers and echocardiography for the early detection of bevacizumab- and sunitinib-mediated cardiotoxicity. Am J Physiol Heart Circ Physiol 309: H692–H701, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Buser GL, Gerona RR, Horowitz BZ, Vian KP, Troxell ML, Hendrickson RG, Houghton DC, Rozansky D, Su SW, Leman RF. Acute kidney injury associated with smoking synthetic cannabinoid. Clin Toxicol (Phila) 52: 664–673, 2014. [DOI] [PubMed] [Google Scholar]
- 15.Capranico G, Tinelli S, Austin CA, Fisher ML, Zunino F. Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim Biophys Acta 1132: 43–48, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem 275: 21409–21415, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Castro L, Demicheli V, Tortora V, Radi R. Mitochondrial protein tyrosine nitration. Free Radic Res 45: 37–52, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Choksi KB, Boylston WH, Rabek JP, Widger WR, Papaconstantinou J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim Biophys Acta 1688: 95–101, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Morgan JA, Harris DM, Ismail NS, Chen JH, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 370: 2011–2019, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cong W, Zhao T, Zhu Z, Huang B, Ma W, Wang Y, Tan Y, Chakrabarti S, Li X, Jin L, Cai L. Metallothionein prevents cardiac pathological changes in diabetes by modulating nitration and inactivation of cardiac ATP synthase. J Nutr Biochem 25: 463–474, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem 261: 3060–3067, 1986. [PubMed] [Google Scholar]
- 22.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood 105: 2640–2653, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Dennis KE, Hill S, Rose KL, Sampson UK, Hill MF. Augmented cardiac formation of oxidatively-induced carbonylated proteins accompanies the increased functional severity of post-myocardial infarction heart failure in the setting of type 1 diabetes mellitus. Cardiovasc Pathol 22: 473–480, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dickey JS, Gonzalez Y, Aryal B, Mog S, Nakamura AJ, Redon CE, Baxa U, Rosen E, Cheng G, Zielonka J, Parekh P, Mason KP, Joseph J, Kalyanaraman B, Bonner W, Herman E, Shacter E, Rao VA. Mito-tempol and dexrazoxane exhibit cardioprotective and chemotherapeutic effects through specific protein oxidation and autophagy in a syngeneic breast tumor preclinical model. PLoS One 8: e70575, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dolce V, Fiermonte G, Runswick MJ, Palmieri F, Walker JE. The human mitochondrial deoxynucleotide carrier and its role in the toxicity of nucleoside antivirals. Proc Natl Acad Sci USA 98: 2284–2288, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem 261: 3068–3074, 1986. [PubMed] [Google Scholar]
- 27.Douarre C, Sourbier C, Dalla Rosa I, Brata Das B, Redon CE, Zhang H, Neckers L, Pommier Y. Mitochondrial topoisomerase I is critical for mitochondrial integrity and cellular energy metabolism. PLoS One 7: e41094, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dykens JA, Will Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov Today 12: 777–785, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Eaton S, Fukumoto K, Stefanutti G, Spitz L, Zammit VA, Pierro A. Myocardial carnitine palmitoyltransferase I as a target for oxidative modification in inflammation and sepsis. Biochem Soc Trans 31: 1133–1136, 2003. [DOI] [PubMed] [Google Scholar]
- 30.El Hajj EC, El Hajj MC, Voloshenyuk TG, Mouton AJ, Khoutorova E, Molina PE, Gilpin NW, Gardner JD. Alcohol modulation of cardiac matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs favors collagen accumulation. Alcohol Clin Exp Res 38: 448–456, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ElZarrad MK, Mukhopadhyay P, Mohan N, Hao E, Dokmanovic M, Hirsch DS, Shen Y, Pacher P, Wu WJ. Trastuzumab alters the expression of genes essential for cardiac function and induces ultrastructural changes of cardiomyocytes in mice. PLoS One 8: e79543, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 66: 1142–1174, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Ferdinandy P, Schulz R, Baxter GF. Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol Rev 59: 418–458, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Fischer F, Hamann A, Osiewacz HD. Mitochondrial quality control: an integrated network of pathways. Trends Biochem Sci 37: 284–292, 2012. [DOI] [PubMed] [Google Scholar]
- 35.Forst T, Hanefeld M, Jacob S, Moeser G, Schwenk G, Pfutzner A, Haupt A. Association of sulphonylurea treatment with all-cause and cardiovascular mortality: a systematic review and meta-analysis of observational studies. Diab Vasc Dis Res 10: 302–314, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Gao RY, Mukhopadhyay P, Mohanraj R, Wang H, Horvath B, Yin S, Pacher P. Resveratrol attenuates azidothymidine-induced cardiotoxicity by decreasing mitochondrial reactive oxygen species generation in human cardiomyocytes. Mol Med Rep 4: 151–155, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner JD, Mouton AJ. Alcohol effects on cardiac function. Compr Physiol 5: 791–802, 2015. [DOI] [PubMed] [Google Scholar]
- 38.Gorbe A, Varga ZV, Kupai K, Bencsik P, Kocsis GF, Csont T, Boengler K, Schulz R, Ferdinandy P. Cholesterol diet leads to attenuation of ischemic preconditioning-induced cardiac protection: the role of connexin 43. Am J Physiol Heart Circ Physiol 300: H1907–H1913, 2011. [DOI] [PubMed] [Google Scholar]
- 39.Guo J, Guo Q, Fang H, Lei L, Zhang T, Zhao J, Peng S. Cardioprotection against doxorubicin by metallothionein Is associated with preservation of mitochondrial biogenesis involving PGC-1α pathway. Eur J Pharmacol 737: 117–124, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Guo KK, Ren J. Cardiac overexpression of alcohol dehydrogenase (ADH) alleviates aging-associated cardiomyocyte contractile dysfunction: role of intracellular Ca2+ cycling proteins. Aging Cell 5: 259–265, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Guo W, Adachi T, Matsui R, Xu S, Jiang B, Zou MH, Kirber M, Lieberthal W, Cohen RA. Quantitative assessment of tyrosine nitration of manganese superoxide dismutase in angiotensin II-infused rat kidney. Am J Physiol Heart Circ Physiol 285: H1396–H1403, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Hao E, Mukhopadhyay P, Cao Z, Erdelyi K, Holovac E, Liaudet L, Lee WS, Hasko G, Mechoulam R, Pacher P. Cannabidiol Protects against Doxorubicin-Induced Cardiomyopathy by Modulating Mitochondrial Function and Biogenesis. Mol Med 21: 38–45, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hasinoff BB, Patel D, O'Hara KA. Mechanisms of myocyte cytotoxicity induced by the multiple receptor tyrosine kinase inhibitor sunitinib. Mol Pharmacol 74: 1722–1728, 2008. [DOI] [PubMed] [Google Scholar]
- 44.He BJ, Joiner ML, Singh MV, Luczak ED, Swaminathan PD, Koval OM, Kutschke W, Allamargot C, Yang J, Guan X, Zimmerman K, Grumbach IM, Weiss RM, Spitz DR, Sigmund CD, Blankesteijn WM, Heymans S, Mohler PJ, andAnderson ME. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med 17: 1610–1618, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology 122: 2049–2063, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoffmann BR, El-Mansy MF, Sem DS, Greene AS. Chemical proteomics-based analysis of off-target binding profiles for rosiglitazone and pioglitazone: clues for assessing potential for cardiotoxicity. J Med Chem 55: 8260–8271, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hortobagyi GN. Anthracyclines in the treatment of cancer. An overview. Drugs 54, Suppl 4: 1–7, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Hu C, Ge F, Hyodo E, Arai K, Iwata S, Lobdell H 4th, Walewski JL, Zhou S, Clugston RD, Jiang H, Zizola CP, Bharadwaj KG, Blaner WS, Homma S, Schulze PC, Goldberg IJ, Berk PD. Chronic ethanol consumption increases cardiomyocyte fatty acid uptake and decreases ventricular contractile function in C57BL/6J mice. J Mol Cell Cardiol 59: 30–40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ichikawa Y, Ghanefar M, Bayeva M, Wu R, Khechaduri A, Naga Prasad SV, Mutharasan RK, Naik TJ, Ardehali H. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124: 617–630, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, Torp-Pedersen C, Sharma AM, Shepherd GM, Rode RA, Renz CL SCOUT. Investigators. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med 363: 905–917, 2010. [DOI] [PubMed] [Google Scholar]
- 51.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, Luczak ED, Hall DD, Fink BD, Chen B, Yang J, Moore SA, Scholz TD, Strack S, Mohler PJ, Sivitz WI, Song LS, Anderson ME. CaMKII determines mitochondrial stress responses in heart. Nature 491: 269–273, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Joshi MS, Mihm MJ, Cook AC, Schanbacher BL, Bauer JA. Alterations in connexin 43 during diabetic cardiomyopathy: competition of tyrosine nitration versus phosphorylation. J Diabetes 7: 250–259, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, Inanc T, Oguzhan A, Eryol NK, Topsakal R, Ergin A. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol 48: 2258–2262, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Kang YJ. Molecular and cellular mechanisms of cardiotoxicity. Environ Health Perspect 109, Suppl 1: 27–34, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem 271: 12610–12616, 1996. [DOI] [PubMed] [Google Scholar]
- 56.Kang YJ, Chen Y, Yu A, Voss-McCowan M, Epstein PN. Overexpression of metallothionein in the heart of transgenic mice suppresses doxorubicin cardiotoxicity. J Clin Invest 100: 1501–1506, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kantarjian HM, O'Brien S, Cortes J, Giles FJ, Rios MB, Shan J, Faderl S, Garcia-Manero G, Ferrajoli A, Verstovsek S, Wierda W, Keating M, Talpaz M. Imatinib mesylate therapy improves survival in patients with newly diagnosed Philadelphia chromosome-positive chronic myelogenous leukemia in the chronic phase: comparison with historic data. Cancer 98: 2636–2642, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Kaplan P, Tatarkova Z, Racay P, Lehotsky J, Pavlikova M, Dobrota D. Oxidative modifications of cardiac mitochondria and inhibition of cytochrome c oxidase activity by 4-hydroxynonenal. Redox Rep 12: 211–218, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science 312: 1171–1175, 2006. [DOI] [PubMed] [Google Scholar]
- 60.Kerkelä R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A., Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 12: 908–916, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Kerkela R, Woulfe KC, Durand JB, Vagnozzi R, Kramer D, Chu TF, Beahm C, Chen MH, Force T. Sunitinib-induced cardiotoxicity is mediated by off-target inhibition of AMP-activated protein kinase. Clin Transl Sci 2: 15–25, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kerr DJ, Dunn JA, Langman MJ, Smith JL, Midgley RS, Stanley A, Stokes JC, Julier P, Iveson C, Duvvuri R, McConkey CC, Group VT. Rofecoxib and cardiovascular adverse events in adjuvant treatment of colorectal cancer. N Engl J Med 357: 360–369, 2007. [DOI] [PubMed] [Google Scholar]
- 63.Knight-Lozano CA, Young CG, Burow DL, Hu ZY, Uyeminami D, Pinkerton KE, Ischiropoulos H, Ballinger SW. Cigarette smoke exposure and hypercholesterolemia increase mitochondrial damage in cardiovascular tissues. Circulation 105: 849–854, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Kohler JJ, Cucoranu I, Fields E, Green E, He S, Hoying A, Russ R, Abuin A, Johnson D, Hosseini SH, Raper CM, Lewis W. Transgenic mitochondrial superoxide dismutase and mitochondrially targeted catalase prevent antiretroviral-induced oxidative stress and cardiomyopathy. Lab Invest 89: 782–790, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kubli DA, Quinsay MN, Huang C, Lee Y, Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 295: H2025–H2031, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kumar D, Lou H, Singal PK. Oxidative stress and apoptosis in heart dysfunction. Herz 27: 662–668, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Kumar V, Kitaeff N, Hampton MB, Cannell MB, Winterbourn CC. Reversible oxidation of mitochondrial peroxiredoxin 3 in mouse heart subjected to ischemia and reperfusion. FEBS Lett 583: 997–1000, 2009. [DOI] [PubMed] [Google Scholar]
- 68.Lang CH, Korzick DH. Chronic alcohol consumption disrupts myocardial protein balance and function in aged, but not adult, female F344 rats. Am J Physiol Regul Integr Comp Physiol 306: R23–R33, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lange LG, Sobel BE. Mitochondrial dysfunction induced by fatty acid ethyl esters, myocardial metabolites of ethanol. J Clin Invest 72: 724–731, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lattanzio FA Jr. Tiangco D, Osgood C, Beebe S, Kerry J, Hargrave BY. Cocaine increases intracellular calcium and reactive oxygen species, depolarizes mitochondria, and activates genes associated with heart failure and remodeling. Cardiovasc Toxicol 5: 377–390, 2005. [DOI] [PubMed] [Google Scholar]
- 71.Lauritzen KH, Kleppa L, Aronsen JM, Eide L, Carlsen H, Haugen OP, Sjaastad I, Klungland A, Rasmussen LJ, Attramadal H, Storm-Mathisen J, Bergersen LH. Impaired dynamics and function of mitochondria caused by mtDNA toxicity leads to heart failure. Am J Physiol Heart Circ Physiol 309: H434–H449, 2015. [DOI] [PubMed] [Google Scholar]
- 72.Lebrecht D, Geist A, Ketelsen UP, Haberstroh J, Setzer B, Walker UA. Dexrazoxane prevents doxorubicin-induced long-term cardiotoxicity and protects myocardial mitochondria from genetic and functional lesions in rats. Br J Pharmacol 151: 771–778, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee YH, Goh WW, Ng CK, Raida M, Wong L, Lin Q, Boelsterli UA, Chung MC. Integrative toxicoproteomics implicates impaired mitochondrial glutathione import as an off-target effect of troglitazone. J Proteome Res 12: 2933–2945, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 32: 302–314, 1973. [DOI] [PubMed] [Google Scholar]
- 75.Lewis W, Simpson JF, Meyer RR. Cardiac mitochondrial DNA polymerase-gamma is inhibited competitively and noncompetitively by phosphorylated zidovudine. Circ Res 74: 344–348, 1994. [DOI] [PubMed] [Google Scholar]
- 76.Li SY, Ren J. Cardiac overexpression of alcohol dehydrogenase exacerbates chronic ethanol ingestion-induced myocardial dysfunction and hypertrophy: role of insulin signaling and ER stress. J Mol Cell Cardiol 44: 992–1001, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Myocardial toxicity of arsenic trioxide in a mouse model. Cardiovasc Toxicol 2: 63–73, 2002. [DOI] [PubMed] [Google Scholar]
- 78.Liao X, Wang Y, Wong CW. Troglitazone induces cytotoxicity in part by promoting the degradation of peroxisome proliferator-activated receptor gamma co-activator-1alpha protein. Br J Pharmacol 161: 771–781, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liaudet L, Calderari B, Pacher P. Pathophysiological mechanisms of catecholamine and cocaine-mediated cardiotoxicity. Heart Fail Rev 19: 815–824, 2014. [DOI] [PubMed] [Google Scholar]
- 80.Liou CM, Tsai SC, Kuo CH, Williams T, Ting H, Lee SD. Chronic methamphetamine exposure induces cardiac fas-dependent and mitochondria-dependent apoptosis. Cardiovasc Toxicol 14: 134–144, 2014. [DOI] [PubMed] [Google Scholar]
- 81.Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, Colan SD, Asselin BL, Barr RD, Clavell LA, Hurwitz CA, Moghrabi A, Samson Y, Schorin MA, Gelber RD, Sallan SE. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med 351: 145–153, 2004. [DOI] [PubMed] [Google Scholar]
- 82.Liu B, Tewari AK, Zhang L, Green-Church KB, Zweier JL, Chen YR, He G. Proteomic analysis of protein tyrosine nitration after ischemia reperfusion injury: mitochondria as the major target. Biochim Biophys Acta 1794: 476–485, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loke YK, Kwok CS, Singh S. Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ 342: d1309, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lombardi AA, Elrod JW. mtDNA damage in the development of heart failure. Am J Physiol Heart Circ Physiol 309: H393–H395, 2015. [DOI] [PubMed] [Google Scholar]
- 85.Lord KC, Shenouda SK, McIlwain E, Charalampidis D, Lucchesi PA, Varner KJ. Oxidative stress contributes to methamphetamine-induced left ventricular dysfunction. Cardiovasc Res 87: 111–118, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma H, Jones KR, Guo R, Xu P, Shen Y, Ren J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin Exp Pharmacol Physiol 37: 460–465, 2010. [DOI] [PubMed] [Google Scholar]
- 87.Madonna R, Cadeddu C, Deidda M, Mele D, Monte I, Novo G, Pagliaro P, Pepe A, Spallarossa P, Tocchetti CG, Zito C, Mercuro G. Improving the preclinical models for the study of chemotherapy-induced cardiotoxicity: a Position Paper of the Italian Working Group on Drug Cardiotoxicity and Cardioprotection. Heart Fail Rev 20: 621–631, 2015. [DOI] [PubMed] [Google Scholar]
- 88.Maharsy W, Aries A, Mansour O, Komati H, Nemer M. Ageing is a risk factor in imatinib mesylate cardiotoxicity. Eur J Heart Fail 16: 367–376, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marin-Corral J, Fontes CC, Pascual-Guardia S, Sanchez F, Olivan M, Argiles JM, Busquets S, Lopez-Soriano FJ, Barreiro E. Redox balance and carbonylated proteins in limb and heart muscles of cachectic rats. Antioxid Redox Signal 12: 365–380, 2010. [DOI] [PubMed] [Google Scholar]
- 90.Marín-García J, Goldenthal MJ. Mitochondria play a critical role in cardioprotection. J Card Fail 10: 55–66, 2004. [DOI] [PubMed] [Google Scholar]
- 91.Mashayekhi V, Eskandari MR, Kobarfard F, Khajeamiri A, Hosseini MJ. Induction of mitochondrial permeability transition (MPT) pore opening and ROS formation as a mechanism for methamphetamine-induced mitochondrial toxicity. Naunyn Schmiedebergs Arch Pharmacol 387: 47–58, 2014. [DOI] [PubMed] [Google Scholar]
- 92.Mihailovic D, Nikolic J, Bjelakovic BB, Stankovic BN, Bjelakovic G. Morphometric and biochemical characteristics of short-term effects of ethanol on rat cardiac muscle. Exp Toxicol Pathol 51: 545–547, 1999. [DOI] [PubMed] [Google Scholar]
- 93.Mihm MJ, Bauer JA. Peroxynitrite-induced inhibition and nitration of cardiac myofibrillar creatine kinase. Biochimie 84: 1013–1019, 2002. [DOI] [PubMed] [Google Scholar]
- 94.Mihm MJ, Yu F, Weinstein DM, Reiser PJ, Bauer JA. Intracellular distribution of peroxynitrite during doxorubicin cardiomyopathy: evidence for selective impairment of myofibrillar creatine kinase. Br J Pharmacol 135: 581–588, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23: 792–799, 2005. [DOI] [PubMed] [Google Scholar]
- 96.Monami M, Genovese S, Mannucci E. Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials. Diabetes Obes Metab 15: 938–953, 2013. [DOI] [PubMed] [Google Scholar]
- 97.Mukhopadhyay P, Batkai S, Rajesh M, Czifra N, Harvey-White J, Hasko G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol 50: 528–536, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mukhopadhyay P, Horvath B, Rajesh M, Matsumoto S, Saito K, Batkai S, Patel V, Tanchian G, Gao RY, Cravatt BF, Hasko G, Pacher P. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med 50: 179–195, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mukhopadhyay P, Horvath B, Zsengeller Z, Zielonka J, Tanchian G, Holovac E, Kechrid M, Patel V, Stillman IE, Parikh SM, Joseph J, Kalyanaraman B, Pacher P. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med 52: 497–506, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, Szabo C, Pacher P. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol 296: H1466–H1483, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mukhopadhyay P, Rajesh M, Batkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Hasko G, Pacher P. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res 85: 773–784, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mukhopadhyay P, Rajesh M, Yoshihiro K, Hasko G, Pacher P. Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem Biophys Res Commun 358: 203–208, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Myers C. The role of iron in doxorubicin-induced cardiomyopathy. Semin Oncol 25: 10–14, 1998. [PubMed] [Google Scholar]
- 104.Nadanaciva S, Dykens JA, Bernal A, Capaldi RA, Will Y. Mitochondrial impairment by PPAR agonists and statins identified via immunocaptured OXPHOS complex activities and respiration. Toxicol Appl Pharmacol 223: 277–287, 2007. [DOI] [PubMed] [Google Scholar]
- 105.Naito K, Kobayashi M, Sahara N, Shigeno K, Nakamura S, Shinjo K, Tobita T, Takeshita A, Ohno R, Ohnishi K. Two cases of acute promyelocytic leukemia complicated by torsade de pointes during arsenic trioxide therapy. Int J Hematol 83: 318–323, 2006. [DOI] [PubMed] [Google Scholar]
- 106.Negro A, Brar BK, Gu Y, Peterson KL, Vale W, Lee KF. erbB2 is required for G protein-coupled receptor signaling in the heart. Proc Natl Acad Sci USA 103: 15889–15893, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Negro A, Brar BK, Lee KF. Essential roles of Her2/erbB2 in cardiac development and function. Recent Prog Horm Res 59: 1–12, 2004. [DOI] [PubMed] [Google Scholar]
- 108.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356: 2457–2471, 2007. [DOI] [PubMed] [Google Scholar]
- 109.Okuda T, Norioka M, Shitara Y, Horie T. Multiple mechanisms underlying troglitazone-induced mitochondrial permeability transition. Toxicol Appl Pharmacol 248: 242–248, 2010. [DOI] [PubMed] [Google Scholar]
- 110.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev 87: 315–424, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation 107: 896–904, 2003. [DOI] [PubMed] [Google Scholar]
- 112.Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther 300: 862–867, 2002. [DOI] [PubMed] [Google Scholar]
- 113.Pacher P, Liaudet L, Mabley JG, Cziraki A, Hasko G, Szabo C. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int J Mol Med 17: 369–375, 2006. [PMC free article] [PubMed] [Google Scholar]
- 114.Pacher P, Schulz R, Liaudet L, Szabo C. Nitrosative stress and pharmacological modulation of heart failure. Trends Pharmacol Sci 26: 302–310, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev 25: 235–260, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patane S. Cardiotoxicity: cisplatin and long-term cancer survivors. Int J Cardiol 175: 201–202, 2014. [DOI] [PubMed] [Google Scholar]
- 117.Petit T, Wilt M, Velten M, Millon R, Rodier JF, Borel C, Mors R, Haegele P, Eber M, Ghnassia JP. Comparative value of tumour grade, hormonal receptors, Ki-67, HER-2 and topoisomerase II alpha status as predictive markers in breast cancer patients treated with neoadjuvant anthracycline-based chemotherapy. Eur J Cancer 40: 205–211, 2004. [DOI] [PubMed] [Google Scholar]
- 118.Phung OJ, Schwartzman E, Allen RW, Engel SS, Rajpathak SN. Sulphonylureas and risk of cardiovascular disease: systematic review and meta-analysis. Diabet Med 30: 1160–1171, 2013. [DOI] [PubMed] [Google Scholar]
- 119.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med 33: 1451–1464, 2002. [DOI] [PubMed] [Google Scholar]
- 120.Rainbolt TK, Saunders JM, Wiseman RL. Stress-responsive regulation of mitochondria through the ER unfolded protein response. Trends Endocrinol Metab 25: 528–537, 2014. [DOI] [PubMed] [Google Scholar]
- 121.Ravi D, Das KC. Redox-cycling of anthracyclines by thioredoxin system: increased superoxide generation and DNA damage. Cancer Chemother Pharmacol 54: 449–458, 2004. [DOI] [PubMed] [Google Scholar]
- 122.Richter B, Bandeira-Echtler E, Bergerhoff K, Clar C, Ebrahim SH. Rosiglitazone for type 2 diabetes mellitus. Cochrane Database Syst Rev 18: CD006063, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Riezzo I, Fiore C, De Carlo D, Pascale N, Neri M, Turillazzi E, Fineschi V. Side effects of cocaine abuse: multiorgan toxicity and pathological consequences. Curr Med Chem 19: 5624–5646, 2012. [DOI] [PubMed] [Google Scholar]
- 124.Rohrbach S, Yan X, Weinberg EO, Hasan F, Bartunek J, Marchionni MA, Lorell BH. Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation 100: 407–412, 1999. [DOI] [PubMed] [Google Scholar]
- 125.Rossato LG, Costa VM, Dallegrave E, Arbo M, Silva R, Ferreira R, Amado F, Dinis-Oliveira RJ, Duarte JA, de Lourdes Bastos M, Palmeira C, Remiao F. Mitochondrial cumulative damage induced by mitoxantrone: late onset cardiac energetic impairment. Cardiovasc Toxicol 14: 30–40, 2014. [DOI] [PubMed] [Google Scholar]
- 126.Rossato LG, Costa VM, Vilas-Boas V, de Lourdes Bastos M, Rolo A, Palmeira C, Remiao F. Therapeutic concentrations of mitoxantrone elicit energetic imbalance in H9c2 cells as an earlier event. Cardiovasc Toxicol 13: 413–425, 2013. [DOI] [PubMed] [Google Scholar]
- 127.Sarraf M, Lu L, Ye S, Reiter MJ, Greyson CR, Schwartz GG. Thiazolidinedione drugs promote onset, alter characteristics, and increase mortality of ischemic ventricular fibrillation in pigs. Cardiovasc Drugs Ther 26: 195–204, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schell FC, Yap HY, Blumenschein G, Valdivieso M, Bodey G. Potential cardiotoxicity with mitoxantrone. Cancer Treat Rep 66: 1641–1643, 1982. [PubMed] [Google Scholar]
- 129.Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M, Schmidinger H. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 26: 5204–5212, 2008. [DOI] [PubMed] [Google Scholar]
- 130.Schreiber SS, Oratz M, Rothschild MA. Alcoholic cardiomyopathy: the effect of ethanol and acetaldehyde on cardiac protein synthesis. Recent Adv Stud Card Struct Metab 7: 431–442, 1975. [PubMed] [Google Scholar]
- 131.Schroeder PE, Hasinoff BB. Metabolism of the one-ring open metabolites of the cardioprotective drug dexrazoxane to its active metal-chelating form in the rat. Drug Metab Dispos 33: 1367–1372, 2005. [DOI] [PubMed] [Google Scholar]
- 132.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL; SAVOR-TIMI 53 Steering Committee and Investigators*. Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation 130: 1579–1588, 2014. [DOI] [PubMed] [Google Scholar]
- 133.Serrano J, Palmeira CM, Kuehl DW, Wallace KB. Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochim Biophys Acta 1411: 201–205, 1999. [DOI] [PubMed] [Google Scholar]
- 134.Shenouda SK, Lord KC, McIlwain E, Lucchesi PA, Varner KJ. Ecstasy produces left ventricular dysfunction and oxidative stress in rats. Cardiovasc Res 79: 662–670, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med 339: 900–905, 1998. [DOI] [PubMed] [Google Scholar]
- 136.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 298: 1189–1195, 2007. [DOI] [PubMed] [Google Scholar]
- 137.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344: 783–792, 2001. [DOI] [PubMed] [Google Scholar]
- 138.Soetkamp D, Nguyen TT, Menazza S, Hirschhäuser C, Hendgen-Cotta UB, Rassaf T, Schlüter KD, Boengler K, Murphy E, Schulz R. S-nitrosation of mitochondrial connexin 43 regulates mitochondrial function. Basic Res Cardiol 109: 433, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 266: 1672–1677, 1991. [PubMed] [Google Scholar]
- 140.Stuart-Harris R, Pearson M, Smith IE, Olsen EG. Cardiotoxicity associated with mitoxantrone. Lancet 2: 219–220, 1984. [DOI] [PubMed] [Google Scholar]
- 141.Sverdlov AL, Elezaby A, Behring JB, Bachschmid MM, Luptak I, Tu VH, Siwik DA, Miller EJ, Liesa M, Shirihai OS, Pimentel DR, Cohen RA, Colucci WS. High fat, high sucrose diet causes cardiac mitochondrial dysfunction due in part to oxidative post-translational modification of mitochondrial complex II. J Mol Cell Cardiol 78: 165–173, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov 6: 662–680, 2007. [DOI] [PubMed] [Google Scholar]
- 143.Szebeni A, Szentandrassy N, Pacher P, Simko J, Nanasi PP, Kecskemeti V. Can the electrophysiological action of rosiglitazone explain its cardiac side effects? Curr Med Chem 18: 3720–3728, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Szentandrassy N, Harmati G, Barandi L, Simko J, Horvath B, Magyar J, Banyasz T, Lorincz I, Szebeni A, Kecskemeti V, Nanasi PP. Effects of rosiglitazone on the configuration of action potentials and ion currents in canine ventricular cells. Br J Pharmacol 163: 499–509, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Takematsu M, Hoffman RS, Nelson LS, Schechter JM, Moran JH, Wiener SW. A case of acute cerebral ischemia following inhalation of a synthetic cannabinoid. Clin Toxicol (Phila) 52: 973–975, 2014. [DOI] [PubMed] [Google Scholar]
- 146.Tan Y, Li X, Prabhu SD, Brittian KR, Chen Q, Yin X, McClain CJ, Zhou Z, Cai L. Angiotensin II plays a critical role in alcohol-induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase-dependent manner. J Am Coll Cardiol 59: 1477–1486, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taylor SW, Fahy E, Murray J, Capaldi RA, Ghosh SS. Oxidative post-translational modification of tryptophan residues in cardiac mitochondrial proteins. J Biol Chem 278: 19587–19590, 2003. [DOI] [PubMed] [Google Scholar]
- 148.Tomiyama K, Funada M. Cytotoxicity of synthetic cannabinoids on primary neuronal cells of the forebrain: the involvement of cannabinoid CB1 receptors and apoptotic cell death. Toxicol Appl Pharmacol 274: 17–23, 2014. [DOI] [PubMed] [Google Scholar]
- 149.Turko IV, Li L, Aulak KS, Stuehr DJ, Chang JY, Murad F. Protein tyrosine nitration in the mitochondria from diabetic mouse heart. Implications to dysfunctional mitochondria in diabetes. J Biol Chem 278: 33972–33977, 2003. [DOI] [PubMed] [Google Scholar]
- 150.Turko IV, Murad F. Quantitative protein profiling in heart mitochondria from diabetic rats. J Biol Chem 278: 35844–35849, 2003. [DOI] [PubMed] [Google Scholar]
- 151.Unnikrishnan D, Dutcher JP, Varshneya N, Lucariello R, Api M, Garl S, Wiernik PH, Chiaramida S. Torsades de pointes in 3 patients with leukemia treated with arsenic trioxide. Blood 97: 1514–1516, 2001. [DOI] [PubMed] [Google Scholar]
- 152.Untch M, Himsl I, Kahlert S, Lueck HJ, Eidtmann H, Du Bois A, Meerpohl HG, Thomssen C, Harbeck N, Jackisch C, Kreienberg R, Emons G, Wallwiener D, Wiese W, Schaller G, Kuhn W, Muscholl M, Pauschinger M, Langer B. Anthracycline and trastuzumab in breast cancer treatment. Oncology (Williston Park) 18: 59–64, 2004. [PubMed] [Google Scholar]
- 153.Varga ZV, Giricz Z, Liaudet L, Hasko G, Ferdinandy P, Pacher P. Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy. Biochim Biophys Acta 1852: 232–242, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Varga ZV, Kupai K, Szucs G, Gaspar R, Paloczi J, Farago N, Zvara A, Puskas LG, Razga Z, Tiszlavicz L, Bencsik P, Gorbe A, Csonka C, Ferdinandy P, Csont T. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol 62: 111–121, 2013. [DOI] [PubMed] [Google Scholar]
- 155.Vendemiale G, Grattagliano I, Altomare E, Serviddio G, Portincasa P, Prigigallo F, Palasciano G. Mitochondrial oxidative damage and myocardial fibrosis in rats chronically intoxicated with moderate doses of ethanol. Toxicol Lett 123: 209–216, 2001. [DOI] [PubMed] [Google Scholar]
- 156.Vergeade A, Mulder P, Vendeville-Dehaudt C, Estour F, Fortin D, Ventura-Clapier R, Thuillez C, Monteil C. Mitochondrial impairment contributes to cocaine-induced cardiac dysfunction: Prevention by the targeted antioxidant MitoQ. Free Radic Biol Med 49: 748–756, 2010. [DOI] [PubMed] [Google Scholar]
- 157.Vergeade A, Mulder P, Vendeville C, Ventura-Clapier R, Thuillez C, Monteil C. Xanthine oxidase contributes to mitochondrial ROS generation in an experimental model of cocaine-induced diastolic dysfunction. J Cardiovasc Pharmacol 60: 538–543, 2012. [DOI] [PubMed] [Google Scholar]
- 158.Wang SB, Foster DB, Rucker J, O'Rourke B, Kass DA, Van Eyk JE. Redox regulation of mitochondrial ATP synthase: implications for cardiac resynchronization therapy. Circ Res 109: 750–757, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang SB, Murray CI, Chung HS, Van Eyk JE. Redox regulation of mitochondrial ATP synthase. Trends Cardiovasc Med 23: 14–18, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang Y, Peng F, Tong W, Sun H, Xu N, Liu S. The nitrated proteome in heart mitochondria of the db/db mouse model: characterization of nitrated tyrosine residues in SCOT. J Proteome Res 9: 4254–4263, 2010. [DOI] [PubMed] [Google Scholar]
- 161.Watanabe M, Funakoshi T, Unuma K, Aki T, Uemura K. Activation of the ubiquitin-proteasome system against arsenic trioxide cardiotoxicity involves ubiquitin ligase Parkin for mitochondrial homeostasis. Toxicology 322: 43–50, 2014. [DOI] [PubMed] [Google Scholar]
- 162.Wendt S, Schlattner U, Wallimann T. Differential effects of peroxynitrite on human mitochondrial creatine kinase isoenzymes. Inactivation, octamer destabilization, and identification of involved residues. J Biol Chem 278: 1125–1130, 2003. [DOI] [PubMed] [Google Scholar]
- 163.Wiebelhaus JM, Poklis JL, Poklis A, Vann RE, Lichtman AH, Wise LE. Inhalation exposure to smoke from synthetic “marijuana” produces potent cannabimimetic effects in mice. Drug Alcohol Depend 126: 316–323, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Will Y, Dykens JA, Nadanaciva S, Hirakawa B, Jamieson J, Marroquin LD, Hynes J, Patyna S, Jessen BA. Effect of the multitargeted tyrosine kinase inhibitors imatinib, dasatinib, sunitinib, and sorafenib on mitochondrial function in isolated rat heart mitochondria and H9c2 cells. Toxicol Sci 106: 153–161, 2008. [DOI] [PubMed] [Google Scholar]
- 165.Xu X, Persson HL, Richardson DR. Molecular pharmacology of the interaction of anthracyclines with iron. Mol Pharmacol 68: 261–271, 2005. [DOI] [PubMed] [Google Scholar]
- 166.Yen HC, Oberley TD, Vichitbandha S, Ho YS, St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest 98: 1253–1260, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yi SH, Ren L, Yang TT, Liu L, Wang H, Liu Q. Myocardial lesions after long-term administration of methamphetamine in rats. Chin Med Sci J 23: 239–243, 2008. [DOI] [PubMed] [Google Scholar]
- 168.Young AC, Schwarz E, Medina G, Obafemi A, Feng SY, Kane C, Kleinschmidt K. Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med 30: 1320.e5–1320.e7, 2012. [DOI] [PubMed] [Google Scholar]
- 169.Yuan C, Acosta D Jr. Effect of cocaine on mitochondrial electron transport chain evaluated in primary cultures of neonatal rat myocardial cells and in isolated mitochondrial preparations. Drug Chem Toxicol 23: 339–348, 2000. [DOI] [PubMed] [Google Scholar]
- 170.Zeglinski M, Ludke A, Jassal DS, Singal PK. Trastuzumab-induced cardiac dysfunction: A “dual-hit”. Exp Clin Cardiol 16: 70–74, 2011. [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang RH, Gao JY, Guo HT, Scott GI, Eason AR, Wang XM, Ren J. Inhibition of CYP2E1 attenuates chronic alcohol intake-induced myocardial contractile dysfunction and apoptosis. Biochim Biophys Acta 1832: 128–141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 18: 1639–1642, 2012. [DOI] [PubMed] [Google Scholar]
- 173.Zhao X, Feng T, Chen H, Shan H, Zhang Y, Lu Y, Yang B. Arsenic trioxide-induced apoptosis in H9c2 cardiomyocytes: implications in cardiotoxicity. Basic Clin Pharmacol Toxicol 102: 419–425, 2008. [DOI] [PubMed] [Google Scholar]
- 174.Zhao Y, Miriyala S, Miao L, Mitov M, Schnell D, Dhar SK, Cai J, Klein JB, Sultana R, Butterfield DA, Vore M, Batinic-Haberle I, Bondada S, St Clair DK. Redox proteomic identification of HNE-bound mitochondrial proteins in cardiac tissues reveals a systemic effect on energy metabolism after doxorubicin treatment. Free Radic Biol Med 72: 55–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao Y, Xue T, Yang X, Zhu H, Ding X, Lou L, Lu W, Yang B, He Q. Autophagy plays an important role in sunitinib-mediated cell death in H9c2 cardiac muscle cells. Toxicol Appl Pharmacol 248: 20–27, 2010. [DOI] [PubMed] [Google Scholar]
- 176.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med 192: 1001–1014, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zsengeller ZK, Ellezian L, Brown D, Horvath B, Mukhopadhyay P, Kalyanaraman B, Parikh SM, Karumanchi SA, Stillman IE, Pacher P. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem 60: 521–529, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


