Abstract
The tetrapod vertebral column has become increasingly complex during evolution as an adaptation to a terrestrial life. At the same time, the evolution of the vertebral formula became subject to developmental constraints acting on the size of the cervical and thoraco-lumbar regions. In the course of our studies concerning the evolution of Hox gene regulation, we produced a transgenic mouse model expressing fish Hox genes, which displayed a reduced number of thoraco-lumbar vertebrae and concurrent sacral homeotic transformations. Here, we analyze this mutant stock and conclude that the ancestral, pre-tetrapodial Hox code already possessed the capacity to induce vertebrae with sacral characteristics. This suggests that alterations in the interpretation of the Hox code may have participated to the evolution of this region in tetrapods, along with potential modifications of the HOX proteins themselves. With its reduced vertebral number, this mouse stock violates a previously described developmental constraint, which applies to the thoraco-lumbar region. The resulting offset between motor neuron morphology, vertebral patterning and the relative positioning of hind limbs illustrates that the precise orchestration of the Hox-clock in parallel with other ontogenetic pathways places constraints on the evolvability of the body plan.
Keywords: Hox genes, Sacrum, Hind limb, Homeotic transformation, Tetrapod evolution, Developmental constraint
Highlights
-
•
A transgenic mouse line expressing fish Hox genes has anterior homeotic transformations.
-
•
Fish Hox genes are capable of inducing tetrapod specific vertebral characters.
-
•
A sacral Hox-code influences adult hindlimb position, yet not the position of limb budding.
‘The whole subject of homology and segmentation is very complex, imperfectly understood, and well worthy of further study’
Edwin E. Goodrich, 1913
1. Introduction
The vertebrate spine is built as a sequence of serial homologous elements, the vertebrae, which develop with different morphologies at different positions along the anterior–posterior axis. Various vertebral formulae reflect both the requirements and the constraints associated with a skeleton that needs to accommodate protective, respiratory and locomotor functions (Romer, 1956, Woltering, 2012). The differentiation of initially similar somites into distinct type of vertebrae (i.e. cervical, thoracic, lumbar, sacral and caudal in mammals) is established early on during embryogenesis, mainly due to a collinear pattern of Hox gene expression (Kmita and Duboule, 2003) along the antero-posterior axis (Casaca et al., 2014, Deschamps and van Nes, 2005, Mallo et al., 2010, Wellik, 2009). These coordinated expression patterns indeed generate various combinations of HOX proteins at distinct body levels (or a ‘Hox code’ (Kessel and Gruss, 1991)), which genetically instruct somites about their fates in terms of morphology. In addition, the correspondence between particular combinations of HOX proteins and critical morphological transitions are maintained throughout tetrapods, suggesting an instructive role for these proteins in setting up these boundaries (Burke et al., 1995, Gaunt, 1994). However, the fact that various HOX proteins display some functional hierarchies in these processes makes a pure combinatorial system unlikely (see Duboule and Morata, 1994).
The evolution of land vertebrates was paralleled by an increasing complexity of axial regionalization, as an adaptation to a terrestrial lifestyle: the sacrum evolved during the fish–tetrapod transition as a connection between the pelvic girdle and the axial skeleton (Carroll and Holmes, 2007), whereas the lumbar region first appeared in mammals as an adaptation to sagittal flexion during locomotion as well as to accommodate the diaphragm (Carroll, 1988). In mammals, the sacral and lumbar regions are genetically characterized by the transcription of Hox genes belonging to paralogy groups 10 and 11 (Hox10 and Hox11 genes), evolutionary related to the insect posterior gene Abd-B (Izpisua-Belmonte et al., 1991). Functional approaches have revealed that HOX10 proteins suppress rib formation whereas HOX11 proteins can induce sacral processes (Carapuco et al., 2005, Wellik and Capecchi, 2003). Interestingly, this collinear distribution of Hox transcripts predates the origin of vertebrates and fish for instance already express Hox10 and Hox11 genes in paraxial mesoderm, in spite of the absence of both sacral and lumbar regions (Oulion et al., 2011, Prince et al., 1998, van der Hoeven et al., 1996). Therefore, it is likely that these particular region-specific morphologies did not arise through mere changes in Hox gene expression domains. Instead, they may have involved concomitant alterations in the activation of downstream target genes, for example via modification in the interpretation of the ‘code’ either following changes in the cis-regulatory modules controlling these targets, or due to changes in the HOX proteins themselves. Altogether, it is currently unknown whether the emergence of these particular body regions involved a simple exaptation of a pre-existing Hox pattern, or if it was accompanied by essential structural changes in HOX proteins leading to novel functions.
Regardless of which evolutionary mechanism leads to such critical modifications of the tetrapod spine, its realm of action was likely reduced due to the strong developmental constraints applied to the axial formula. The existence of developmental constraints applied to the organization of segmental patterns in animals was recognized more than a century ago, by the mere observations of natural ‘rules’ governing the formation of metamerized body plans (see for example the work of Lankester, described in Jeffs and Keynes, 1990). Nowadays, such constraints or limitations in the evolution of otherwise potentially adaptive traits are thought to derive in part from the way the underlying regulatory processes are implemented and shared between various developmental contexts, leading to severe pleiotropic effects at least in vertebrates (Duboule and Wilkins, 1998, Kirschner et al., 2005).
The comparative analysis of vertebral columns provides many instances of such canalized processes (Asher et al., 2011), as for example the well-known constraint that fixes the number of cervical vertebrae to seven in all mammals but manatees and sloths (see e.g. Galis, 1999, Galis and Metz, 2007, Varela-Lasheras et al., 2011), even though natural selection favored in some instances either the increase or the decrease in neck length, as in giraffes and whales, respectively. In such cases however, variations occurred through changes in the sizes of vertebrae rather than in their number. It was suggested that this constraint was generated by a potential interference with the migration of the diaphragm muscles, thus leading to an impaired respiration (Buchholtz et al., 2012, Hirasawa and Kuratani, 2013). Likewise, in the thoraco-lumbar region, a constraint seems to restrict the overall number of vertebrae to 19 or 20 in most mammals (Narita and Kuratani, 2005), perhaps associated with the proper implementation of locomotor mechanisms (Buchholtz, 2014, Galis et al., 2014).
Unfortunately, even though the mechanisms underlying both the time-sequenced production of somites (Pourquie, 2003) and the concurrent progressive activation of Hox genes (Noordermeer et al., 2014) start to be understood, evolutionary scenarios accounting for the macroevolution of the axial skeleton remain complex to address experimentally and lack empirical support. In this study, we investigate the phenotypic abnormalities in a transgenic mouse containing a HoxAa BAC from the pufferfish (Tetraodon nigroviridis) genome. This transgenic line was produced in the aim of studying inter-species regulatory controls (Woltering et al., 2014) and expresses fish HoxAa genes at slightly ectopic positions during the developing mouse body axis. The resulting morphological transformations indicate that pre-tetrapodial HOX proteins can induce tetrapod-specific anatomical features. Also, the number of thoracic and lumbar vertebrae obtained scores below the developmental constraint identified by Narita and Kuratani (2005). The paraplegia observed in these mice suggests that the origin of the constraint on the evolutionary bias in TL vertebral number may lie in the mechanistic independence between axial patterning by Hox genes, on the one hand, and hind limb positioning, on the other hand.
2. Results
2.1. A mouse stock with a reduced number of TL vertebrae
In the course of our studies of Hox gene regulation during the fin to limb transition, a transgenic mouse stock was generated by using a Pufferfish (T. nigroviridis) BAC covering part of the posterior HoxAa cluster and containing from HoxA9a through HoxA13a (Woltering et al., 2014). Three F0 males were obtained, which all showed locomotory incapacitation of the hind limbs (paraplegia), as well as a trunk shortened along the anterior to posterior axis (Fig. 1A, Supplementary movies 1–2). One male died shortly after weaning and the cadaver was lost; a second male died without any apparent pathological cause, at approximately two years of age, but never reproduced; the third male proved capable of reproducing and was used to establish a line through natural mating. As further paternal transmission of this transgenic condition was never achieved in this line, it was maintained through hemizygous maternal crosses. In addition to the male reproductive defects, the problems in hind limb coordination with an abnormal gate caused by (partial) hind-limb paralysis persisted in this line. Adult animals improved in this respect after 5 months of age.
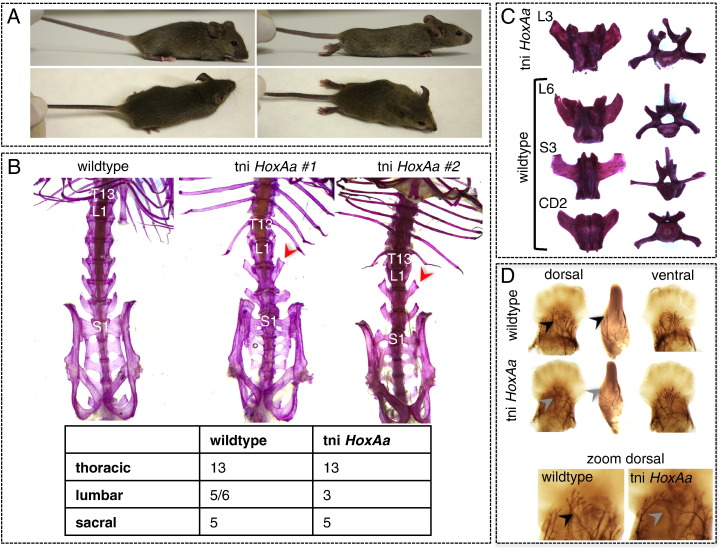
Fig. 1.
Paralysis, sacral transformations and absence of the peroneal nerve in the hind limbs of the tni HoxAa transgenic line. A) Habitus of wild type and tni HoxAa transgenic mice. Note the hind limb paralysis, as visible from a dorsal view. B) Skeletal staining using alizarin red on either wild type specimen, tni HoxAa established line (labeled #1) and an independently obtained F0 transgenic male who never gave rise to offspring (labeled #2). Both transgenic animals displayed a shortening of the lumbar region, from the normal L6 (sometimes L5) formula observed in wild type mice (a wild type specimen with L6 phenotype is shown) to a L3 formula. Partial sacral transformations in the 2nd lumbar vertebra are indicated with red arrowheads (also present but not indicated for the L3). The observed changes in the axial formula are indicated in the table shown underneath the skeletal preparations. C) Comparison between vertebral morphologies shows that the posterior most lumbar vertebrae (L3) in the tni HoxAa skeleton have partial transformations into sacral and/or caudal vertebrae, as indicated by the broader and more horizontally oriented lateral processes. D) Neurofilament staining on E12.5 hind limb buds shows the absence of the peroneal nerve in tni HoxAa embryos. The peroneal nerve innervates the dorsal aspect of the limbs and is indicated with a black arrowhead in the dorsal and the lateral view of the wild type limb bud. Its vacant position is indicated using a gray arrowhead in the tni HoxAa transgenic limb buds. The ventral view shows no apparent mis-specification of the tibial nerve, which innervates the ventral aspect of the hind limbs. A zoom on the peroneal nerve and its vacant position in the transgenic limb bud is shown in the lower panel. T13: 13th thoracic vertebra; L1: 1st lumbar vertebra; S1: 1st sacral vertebra; L3: 3rd lumbar vertebra; L6: sixth lumbar vertebra; S3: 3rd sacral vertebra; CD2: 2nd caudal vertebra.
To try and understand the etiology of these various phenotypes, we initially analyzed these mice for potential skeletal and/or neural abnormalities. Alizarin red-alcian blue staining in newborns and adults revealed major homeotic transformations in the posterior trunk, including a large anterior shift of the sacrum leading to a reduction of the lumbar region from usually six (sometimes five) lumbar vertebrae in wild-type mice, to only three lumbar vertebrae in the transgenic condition. In addition, the second and third lumbar vertebrae (L2 and L3) showed partial sacral transformation, as shown by clearly broadened lateral processes (Fig. 1B, C, Supplementary Fig. 1). This phenotype was also scored in the skeleton of the second F0 male, for which no line could be established (Fig. 1B [tni HoxAa#2]). The early innervation pattern of the hind limbs was investigated in 12.5 days old fetuses (E12.5), using immune-staining of neurofilaments (Fig. 1D). In transgenic mice, an abnormal truncation of the peroneal nerve, which innervates the dorsal aspect of the hind limbs, was observed consistent with the locomotory abnormalities detected in these mice. Both the neuronal and reproductive phenotypes proved very similar to those observed for the loss of function of Hox10 group genes. These latter mutants indeed display a misspecification of the sciatic part of the lumbosacral plexus, which normally innervates the hind limbs (Carpenter et al., 1997, Tarchini et al., 2005, Wu et al., 2008).
2.2. Gain of function of Tetraodon Hoxa11a
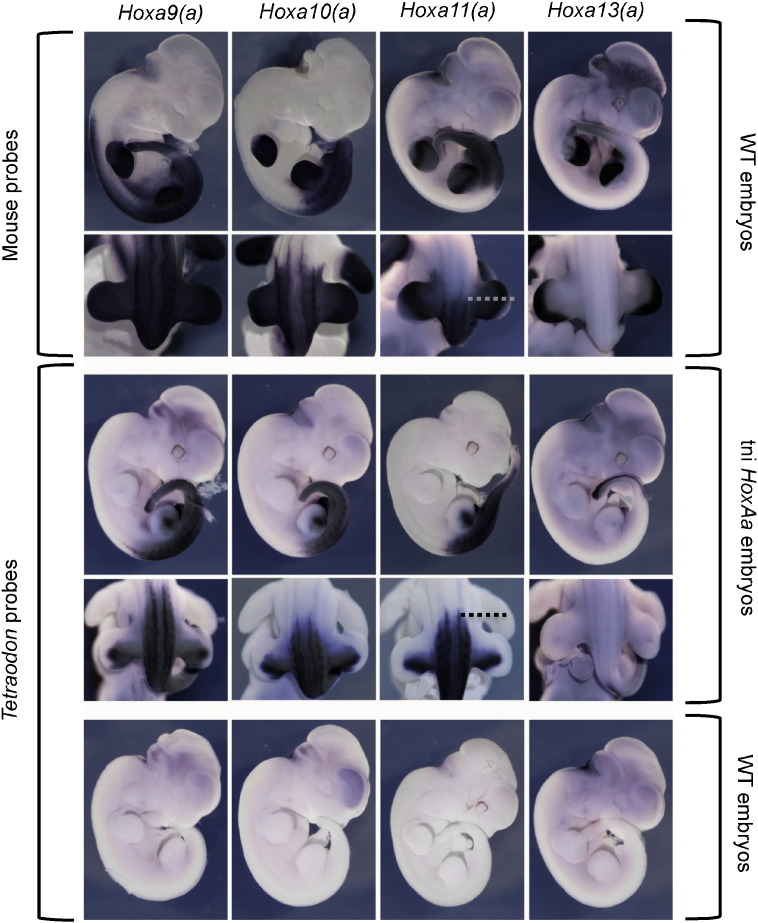
In order to associate the phenotypic abnormalities observed in the transgenic line with the potential expression of the transgenic Tetraodon Hox genes present in the BAC, we performed in situ hybridization for the Tetraodon Hoxa9a to Hoxa13a genes (Fig. 2). Interestingly, these genes were expressed during mouse development with the expected spatial collinear pattern along the main body axis, with Hoxa9a being expressed most anteriorly and Hoxa13a being confined to the posterior tail region. Comparison with the expression of the endogenous mouse Hoxa genes however, indicated the presence of transgenic Hoxa11a transcripts at a too anterior position, i.e. about three to four somites more anterior than the corresponding pattern for the mouse Hoxa11 gene. Such an anteriorized transcriptional pattern associated with a Hox11 gene was previously reported, associated with the replacement in vivo of an endogenous Hoxd11 enhancer by its teleost counterpart. Mice carrying this fish enhancer at the correct endogenous position expressed their own Hoxd11 gene too anteriorly (Gerard et al., 1997). Therefore, an anterior gain of function of the Tetraodon Hoxa11a gene as reported here may reflect a specific cis-regulatory difference between fish and mammals, as somewhat supported by the high divergence in non-coding DNA sequences between the fish and tetrapods posterior HoxA clusters (Supplementary Fig. 2A). This anteriorized expression was equally observed at earlier stages (E10.5). In such embryos, the transgenic Hoxa11a gene was expressed more anteriorly than the three mouse paralogous genes Hoxa11, Hoxc11 and Hoxd11 (Fig. 3A).
Fig. 2.
Expression of mouse HoxA and Tetraodon HoxAa genes in either wild type or tni HoxAa mice. In situ hybridization was performed on E11.5 wild type and transgenic embryos (genotypes are indicated on the right hand side). Wild type embryos were analyzed with mouse specific probes to visualize the expression of the endogenous Hoxa genes and with Tetraodon specific probes to exclude potential cross reactivity with the endogenous mouse genes. Tni HoxAa transgenic embryos were processed for Tetraodon specific probes. Probe names are indicated above as well as whether the probe used was for a wild type or transgenic specimen (indicated on the left hand side). The expression pattern of the Tetraodon probes in the transgenic context shows the expected collinear pattern with Hoxa9a being expressed most anteriorly (although not as far as the endogenous mouse Hoxa9) and Hoxa13a restricted to the posterior most tail. There is however marginal differentiation, if any, between the anterior expression limits of Hoxa10a and Hoxa11a. A clear difference is observed in expression between mouse Hoxa11, which has an anterior expression limit close to the posterior limit of the hind limb buds, and the Tetraodon Hoxa11a, that has a limit around three to four somites more anterior, coinciding with the anterior limit of the hind limb buds (the anterior level of axial expression in both wild type and tni HoxAa panels is indicated with a dotted line). This particular area where the difference is observed is the part of the body where sacral transformations are scored (Fig. 1). The lower row shows that there is no cross reactivity in the in situ hybridization between the Tetraodon probes and the endogenous mouse genes.
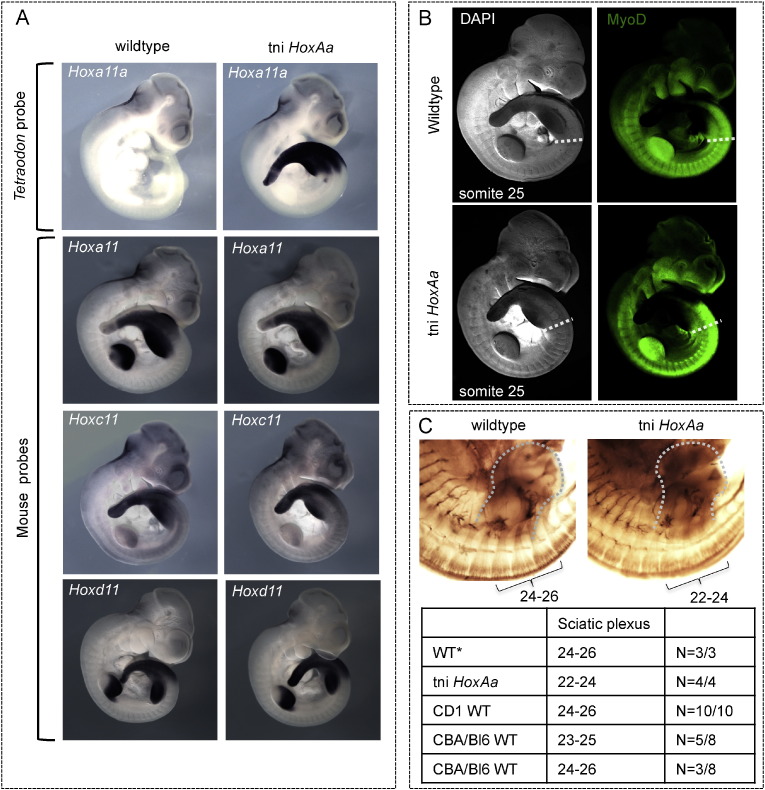
Fig. 3.
Expression of endogenous Hox11 genes and lack of a direct effect on hindlimb bud induction. A) Expression patterns of both the Tetraodon Hoxa11a gene and the three mouse Hox11 genes (Hoxa11, Hoxc11 and Hoxd11), either in wild type or in transgenic E10.5 embryos. As in older stages (see Fig. 2), Hoxa11a is expressed more anteriorly than the mouse Hox11 genes, i.e. up to the anterior border of the hind limb bud, in tni HoxAa embryos. Analysis of the expression of endogenous genes in transgenic embryos shows no alteration in expression, indicating no major ectopic cross activation, which could explain the observed homeotic transformations as a result of up-regulating endogenous Hox genes. B) The visualization of somites using DAPI staining and fluorescent in situ hybridization for MyoD shows no detectable difference in the anterior position of the hind limb buds between wild type and transgenic embryos. In both genetic backgrounds, these buds emerge with an anterior boundary around somite 25 (indicated with dotted lines). This indicates that the observed anterior positioning of hind limbs in the tni HoxAa line (see also Supplementary Fig. 1) is likely the result of the anterior shift of the sacrum and not of a more anterior induction of hind limb buds induced by an altered Hox code. C) Neurofilament staining of E11.5 embryos shows a change in the position of the sciatic plexus, i.e. the part of the lumbosacral plexus innervating hind limbs. In wild type mice, this plexus encompasses spinal nerves 24 to 26 (in CD1 and some CBA/Bl6 backgrounds) or 23 to 25 (in other CBA/Bl6 backgrounds). In wild type littermates obtained from transgenic crosses (indicated ‘WT*’), the plexus derives from spinal nerves 24 to 26. In transgenic embryos however, the neural branches innervating hind limbs belong to spinal nerves 22 to 24. As the transgenic hind limb buds (dotted lines) are in register with the innervating nerves, this indicates both an anterior shift of the plexus and a more anterior position of the hind limb buds at this stage.
Expression of endogenous Hox11 genes and lack of a direct effect on hindlimb bud induction. A) Expression patterns of both the Tetraodon Hoxa11a gene and the three mouse Hox11 genes (Hoxa11, Hoxc11 and Hoxd11), either in wild type or in transgenic E10.5 embryos. As in older stages (see Fig. 2), Hoxa11a is expressed more anteriorly than the mouse Hox11 genes, i.e. up to the anterior border of the hind limb bud, in tni HoxAa embryos. Analysis of the expression of endogenous genes in transgenic embryos shows no alteration in expression, indicating no major ectopic cross activation, which could explain the observed homeotic transformations as a result of up-regulating endogenous Hox genes. B) The visualization of somites using DAPI staining and fluorescent in situ hybridization for MyoD shows no detectable difference in the anterior position of the hind limb buds between wild type and transgenic embryos. In both genetic backgrounds, these buds emerge with an anterior boundary around somite 25 (indicated with dotted lines). This indicates that the observed anterior positioning of hind limbs in the tni HoxAa line (see also Supplementary Fig. 1) is likely the result of the anterior shift of the sacrum and not of a more anterior induction of hind limb buds induced by an altered Hox code. C) Neurofilament staining of E11.5 embryos shows a change in the position of the sciatic plexus, i.e. the part of the lumbosacral plexus innervating hind limbs. In wild type mice, this plexus encompasses spinal nerves 24 to 26 (in CD1 and some CBA/Bl6 backgrounds) or 23 to 25 (in other CBA/Bl6 backgrounds). In wild type littermates obtained from transgenic crosses (indicated ‘WT*’), the plexus derives from spinal nerves 24 to 26. In transgenic embryos however, the neural branches innervating hind limbs belong to spinal nerves 22 to 24. As the transgenic hind limb buds (dotted lines) are in register with the innervating nerves, this indicates both an anterior shift of the plexus and a more anterior position of the hind limb buds at this stage.
This ‘anteriorized’ Hoxa11a transcript domain coincided in space with the observed sacral homeotic transformations of the lumbar region. In addition, the morphological transformations, which were scored precisely within this very region where Hoxa11a was gained, gave the expected phenotype for an ectopic expression of a tetrapod Hox11 group gene. Hox11 genes indeed determine sacral vertebral identity (Wellik and Capecchi, 2003) and their expression at ectopic anterior positions was shown to produce sacral transformations (Carapuco et al., 2005, Gerard et al., 1997). Likewise, the ectopic expression of Hoxd11 induced the mis-specification of the motor neurons innervating the dorsal aspects of hind limbs (Misra et al., 2009), a nerve pattern normally specified by Hox10 genes (Carpenter et al., 1997). Noteworthy, the spatial expression patterns of the endogenous Hox11 group genes were not noticeably modified in transgenic embryos (Fig. 3A), ruling out a potential auto-regulatory interaction between HOX11 proteins and their own promoters as a cause of the observed phenotypic alterations. Therefore, we concluded that the mis-expression of the Tetraodon Hoxa11a gene was directly responsible for the severe abnormalities detected in these transgenic animals. In this view, the anteriorization of the axial Hox11 domain, due to the expression of the transgenic Tetraodon Hoxa11a gene, induced both neuronal and vertebral homeosis.
2.3. Hind-limb bud formation and Hox-mediated axial patterning
The anterior shift of the sacrum observed in our Tetraodon HoxAa transgenic line was accompanied by a more anterior positioning of the hind-limbs along the body axis, as illustrated by the positioning of the hip joint (Supplementary Fig. 1). In newborn mice, an evaluation of the approximate hind-limb position indicated that a two to three somites offset when compared to wild type specimen, consistent with the extent of the shift observed for the sacrum and thus suggesting that the entire posterior part of the animal had been shifted anteriorly. The position of forelimb buds seems to be determined by the combination of HOX proteins found in the lateral plate mesoderm (Nishimoto et al., 2014). Also, hind limb buds were shifted two somites backwards in mice mutant for multiple group 9 Hox genes (McIntyre et al., 2007). Despite these reports, however, our observation was unexpected since the determination of hind limb positioning along the trunk was recently suggested to be a Hox independent process (Jurberg et al., 2013), as assessed by both loss- and gain of function approaches. In this latter case, even the Hoxa11 gene was unable to shift hind limb buds anteriorly when overexpressed at an early stage (Jurberg et al., 2013). Potential reasons for this apparent discrepancy such as differences in the strength of transgene expression in the lateral plate mesoderm (Fig. 3A) are discussed below.
We further investigated if any alteration in hind limb positioning could already be observed at the stage of limb bud formation (E10.5) and hence whether additional evidence could be found to document a direct link between hind limb induction on the one hand, and the sacral ‘Hox code’, on the other hand. To better evaluate this parameter, somites were visualized by using DAPI staining in combination with fluorescent in situ hybridization for the MyoD transcripts (Hebrok et al., 1997). Analysis of E10.5 transgenic embryos showed that the hind limb buds developed at an axial position comparable to the wild type situation, i.e. at around somite level 25 (Fig. 3B). Therefore, the anteriorized positioning of hind limbs in adult mice was likely the mere manifestation of an anterior shift of the entire sacral region, rather than of a local change in the positioning of the limb field, the territory from where limbs bud out of the lateral plate mesoderm.
It has been noticed (e.g. Burke et al., 1995) that hind limbs initially emerge at a body level corresponding to lumbar somites. Subsequently, these buds adopt a more caudal position, at the level of the sacral somites, where they will become attached through the pelvic-sacral connection. This relative shift of the buds with respect to the somites may reflect a more general offset between paraxial and lateral plate mesoderm in the growing embryo. The possibility thus exists that the sacrum itself provides a cue for an arrest of this relative posterior shift of the hind limbs. In such a case, animals displaying a genetically ‘sacralized’ lumbar region may concomitantly show anteriorized hind limbs, due to a premature termination of this relative transition. In this view, the more anterior location of hind limbs in adult mice would be an indirect result of repositioning the sacrum, rather than be caused by the direct influence of ectopic Hoxa11 upon hind limb bud induction.
This anteriorization of the hind limbs becomes apparent at E11.5 already, when neuro-filament staining revealed an axial position corresponding to the innervation of spinal nerves 22 to 24 in transgenic mice (Fig. 3C), whereas spinal nerves 24 to 26 are involved in wild type littermates. This observation also illustrated a shift in the positioning of the sciatic plexus along the axis. This shift was accompanied by the absence of the peroneal branches of the newly specified sciatic nerve, similar to the effect of Hoxd10 loss of function (Carpenter et al., 1997, Tarchini et al., 2005). In this case, it is possible that, due to its new anterior position, the sacrum became located outside the territory of Hoxd10-dependent motoneurons thus leading to a position-dependent loss of function within those neurons entering the limb bud.
3. Discussion
3.1. Exaptation of the Hox code; ancestral versus novel regulation
Even though the concept of biological complexity is delicate to handle in many respects (Carroll, 2001, Hall, 1999), vertebrate evolution tends to show a trend towards an increasingly complex body plan. In this context, modifications in Hox gene regulation and function have been invoked as potential causes to the origin of morphological novelties (e.g. Head and Polly, 2015, Rijli et al., 1993, Sordino et al., 1995, Wagner et al., 2003). Within such a conceptual framework, two distinct regulatory levels can be identified where important changes in the function of Hox genes may have occurred. The first case is well illustrated by neomorphic structures such as digits and external genitals, which evolved concomitantly partly via the recruitment of novel Hox expression domains (Dolle et al., 1991, Montavon et al., 2008). These regulatory specificities were acquired through both the emergence of new enhancer sequences and the co-optation of regulatory sequences already used for another purpose (Lonfat et al., 2014, Montavon et al., 2011). In such cases, morphological novelties coincide with regulatory innovations, even when the latter are based upon pre-existing modules (Lonfat et al., 2014, Woltering et al., 2014). Likewise, modifications in the organization of the spine may have relied upon the mere displacement of Hox expression boundaries, as illustrated by the different positions of the cervico–thoracic transitions in mammals and birds (Burke et al., 1995, Gaunt, 1994).
In an alternative – yet not exclusive – scenario, morphological novelties can evolve not only as the result of new regulatory modalities but, rather, through different responses from the systems downstream of Hox control, for example via the loss or gain of target genes. For instance a modification in the response of a target gene following changes in binding sequence can lead to Hox-derived phenotypic alterations (Guerreiro et al., 2013). Also, variations in the HOX protein structures could potentially lead to important quantitative and/or qualitative modifications in large sets of target loci, as shown for example in insects where a modification of the Ubx protein is thought to have accompanied the emergence of the hexapod body plan (Ronshaugen et al., 2002). Interestingly, changes in the Hoxa11 coding sequence were shown to have paralleled the evolution of pregnancy in mammals (Lynch et al., 2008). In addition, sequence analyses of this gene in various species showed potential signatures of adaptive sequence change across the fin-to-limb transition (Chiu et al., 2000). The comparison between the mouse HOXA11 and Tetraodon HOXA11A sequences indeed shows a very strong conservation of the homeodomain peptide sequence, whereas the N-terminal parts of the proteins are much more divergent (Supplementary Fig. 2B). However, despite this divergence in protein sequences outside the homeodomain, the pufferfish Hoxa11a protein was clearly capable of inducing the sacrum, a well-defined tetrapod novelty and hence this protein already had the capacity to control sacral characteristics well before the evolution of a sacrum. Consequently, modifications in its coding sequence were likely not necessary for this functional exaptation.
How other features associated with the vertebrates evolved or disappeared, and to which degree the above patterning concepts may provide explanatory frameworks for these key events remains to be investigated. Structures that would be of particular interest in this context would be for example the occipital–synarcual complex found in cartilaginous fishes and extinct placoderms (Davis et al., 2012, Soshnikova et al., 2013), the ostariophysan Weberian vertebrae (Bird and Mabee, 2003), the teleost specific homocercal caudal fin vertebrae (Moriyama and Takeda, 2013), the anuran urostyle (Rockova and Rocek, 2005), the snake's forked lympapophyses (Woltering, 2012) or the enigmatic interlocking lumbar vertebrae of hero shrew (Stanley et al., 2013).
It is also noteworthy that the general principle of collinear distribution of Hox expression domains along the rostro-caudal body axis observed in vertebrates (Gaunt et al., 1988) largely predates their appearances (Duboule and Dolle, 1989, Graham et al., 1989, Lewis, 1978) and thus could not be initially associated with the evolution of a complex axial skeleton. It is possible that the Hox system was originally linked to the organization of either neural (Deutsch and Le Guyader, 1998) or endodermal (Kondo et al., 1996) structures along the AP axis, and was subsequently recruited by mesodermal derivatives due to its capacity to specialize particular body segments. From there onwards, modifications in either the regulation and structures of these genes, or of their specific targets (either at the regulatory or at the functional level) may have produced the variety of vertebral formulae known today in extant vertebrates and in the fossil record.
3.2. The Hox constraint on the body plan
This diversity in vertebral formulae is however not infinite and the observed anatomical bias towards certain prototypes at the detriment of others, is hard to explain on pure adaptive grounds. Therefore, it is likely that developmental constraints restrict the realm of possibilities for a vertebral column to combine and associate various vertebral types, both in their number and qualities (Asher et al., 2011). The identification of the developmental processes associated with these constrained morphologies has been problematic and without empirical support. The mouse line we describe in this study displays a lumbar region with only three vertebrae without a concomitant increase in the number of thoracic vertebrae. As such, it ‘violates’ the thoracolumbar constraint identified by Narita and Kuratani (2005) and may help thinking about both the nature of the underlying constraints and the mechanisms involved.
In our mutant mice, the reduction in the number of thoracolumbar vertebrae is caused by the anterior expression of the transgenic Hoxa11a gene, presumably as a result of a different interpretation of cis-regulatory information between the fish and the mouse contexts as previously noted (Gerard et al., 1997). The resulting transformation of the posterior lumbar region into a sacrum likely induced a morphological offset between structures and/or cell types, leading to the severe phenotypic condition observed. In the case of hind limb positioning, this offset seems to be compensated for, to some extent, since the final position of the hind limbs is in register with the anteriorized sacrum. This could be due to an instructive relationship between both structures, the sacrum helping to position the hind limbs. Alternatively, the strong gain of function of Hoxa11a observed in the lateral plate mesoderm may directly participate in the rostral positioning of the hind limbs, even though Hoxa11 was apparently not able to achieve a similar result in another experimental context (Jurberg et al., 2013).
In contrast, the differentiation of the appropriate columns of motor neurons innervating the limbs, which is greatly influenced by Hox gene expression in the developing spinal cord (Jung et al., 2014), may not have been equally influenced by our gain of function. As a result, the motor neurons innervating the mutant hind limbs may originate from a territory of the spinal cord lacking the proper combination of HOX proteins, in particular HOXD10, the targeted or spontaneous mutation of which generates similar paraplegia phenotypes (Carpenter et al., 1997, Misra et al., 2009, Tarchini et al., 2005, Wahba et al., 2001). This case potentially illustrates the result of interfering with the collinear distribution of HOX proteins, having Hoxa11a transcripts abruptly produced more anteriorly (or at the same body level; see Fig. 2) than the native Hoxd10 mRNAs. The resulting compromised locomotion and accompanying reproductive incapacitation would of course be strongly selected against under natural conditions.
3.3. Mechanisms and constraints underlying the phylotypic progression
In vertebrates, the general body architecture (Bauplan) materializes during late gastrulation, where all vertebrate embryos tend to share important morphological features (the zootype). This ‘phylotypic stage’ was associated with the expression of particular transcription factors, including Hox genes (Slack et al., 1993). It was subsequently argued that early embryos, regardless of their various modes of gastrulation had to ‘converge’ towards this particular body plan to set the general ground from which different adult morphological traits can be subsequently derived. This ‘Hourglass model’ (Duboule, 1994) implies that embryos progress through a short period (the phylotypic progression) where the underlying developmental mechanisms must be maximally constrained, thus making alternative solutions impossible. While the existence of the developmental hourglass has been recently documented in a variety of contexts (see e.g. Irie and Kuratani, 2014) but also (Richardson, 1995) the nature of the constraints responsible for the phylotypic progression (into the bottleneck of the hourglass) is elusive.
Initially, two kinds of constraints were proposed: On the one hand, constraints based on meta-trans regulations (Duboule, 1994), i.e. due to particular interactions between networks of genes, necessary at this stage to properly set up the body plan (Raff, 1996). On the other hand, a single mechanism may underlie the passage through the hourglass bottleneck, provided that this mechanism is invariable and requires a particular context to be implemented. In this view, early meta-trans regulations are necessary to bring the developing system into a point where this invariable mechanism can now operate, during the phylotypic period. The fact that this period covers the extension and patterning of the rostro-caudal axis suggested that genetic mechanisms at work to orchestrate these critical steps might be particularly constrained, for some reasons. Hox genes are the major players in the patterning of the body axis (see Mallo et al., 2010) and possibly in its extension (Denans et al., 2015, Di-Poi et al., 2010, Young et al., 2009) and their sequential activation (temporal collinearity) relies upon a meta-cis mechanism (the Hox clock) that appears difficult to evolve as it relies upon a process that reads the linearity of DNA at the Hox loci (see Noordermeer and Duboule, 2013). On this ground, the Hox clock was proposed as the major constraint acting during the phylotypic progression (Duboule, 1994). Subsequently, the Hox clock was reported to closely interact with the segmentation clock, the mechanism whereby the vertebrate body becomes segmented (Palmeirim et al., 1997). Hox genes indeed can be regulated as a read out of the segmentation clock (Zakany et al., 2001) and, conversely, the amount of caudal non-segmented mesoderm available as substrate for the segmentation clock may be regulated by the Hox clock (Denans et al., 2015). Therefore, a perfect coordination between these two precise mechanisms must be secured and hence the vertebrate embryo may have to converge towards this point where both clocks will click in concert for about two days, before the system relaxes, giving more opportunities again for variable interactions between gene networks.
Offsets between these two clocks can naturally lead to the variety of vertebral formulae found in vertebrates. Here, we show an example of how the anteriorization of a single Hox11 group gene can lead to a complete reorganization of the spine. However, this gain of function did not respect the constraint applied to the system, as it was not accompanied by a gain of function of the entire set of Hox genes, bringing Hoxa11a expression at the rostral position of Hox10 genes. While the effect on the spine may not have been in itself a cause of counter selection, the interactions of body parts with the spine was affected in spite of some intrinsic re-organization, such as the anteriorized position of the hind limbs. This suggests the existence of developmental check-points where the relative connections between various structures can be adjusted. The hind legs were nevertheless not properly innervated, likely due to the absence of Hox10-positive/Hox11-negative motor neurons, which are required for the peroneal branch of the sciatic nerve (Carpenter et al., 1997, Tarchini et al., 2005).
It has also been argued that high developmental constraints may apply at the phylotypic stage due to the necessity to coordinate interactions between the various nascent tissue types (Galis and Metz, 2001, Raff, 1994, Sander, 1983). In other words, to produce a coherent organism, tissues that differentiate within spatially segregated embryonic domains during gastrulation (such as for instance neurons, vascular progenitors or muscles) need to be integrated at this stage. However, a common landmark to the differentiation of these tissues or cell types, as well as of the origin of all major internal organs, is their original position along the developing rostral to caudal axis, suggesting that this latter information may be the critical determinant. Accordingly, the mechanism(s) underlying the organization of the major body axis may be more constrained than others. In this context, our mouse line illustrates this subtle equilibrium whereby several patterning processes must act in concert during the phylotypic stage. The fact that motor neuron specification, vertebral patterning and hind limb positioning are out of register shows that these processes are not naturally interconnected with one another, which may re-enforce the constraints acting at this stage upon the evolvability of the body plan.
Clades outside mammals can show high diversity in both the number of pre-caudal vertebra and the positioning of the posterior paired appendages along the body. In this regard, the teleost fish are particularly interesting as they can have their pelvic fins positioned as far anterior as their pectoral fins (Murakami and Tanaka, 2011, Murata et al., 2010, Tanaka, 2011). In fishes the initial position of the pelvic fins is specified by GDF11, as in vertebrates (see Jurberg et al., 2013), generally at the position of the trunk to tail transition (Murata et al., 2010). In some species, however, the pelvic fin buds subsequently migrate towards the anterior, along the trunk. The appropriate innervation of these anteriorly displaced pelvic fins occurs through locally exiting motor neurons, which are thus apparently rather independent from the combination of Hox genes they express (Murakami and Tanaka, 2011, Murata et al., 2010, Tanaka, 2011), perhaps due to a lesser complexity in the realm of movements implemented by these fins. This great flexibility in the fish body plan may be accounted for by a lower interdependence between specific motor neurons and hind limbs such that fishes may not need to tightly orchestrate the connection between a specific set of neurons and the pelvic fins to secure a proper functional outcome. The evolution of the sacrum and of a more generic connection between the posterior appendages and the spine, may have introduced yet another strong constraint, thus further decreasing the evolvability of the body plan.
4. Materials and methods
4.1. Mouse strains
The mouse tni HoxAa stock (Woltering et al., 2014) was generated by pronuclear injection following well-established procedures. All experiments were performed in agreement with the Swiss law on animal protection (LPA) with the appropriate legal authorization to D.D. Because of the severity of the phenotype, this transgenic line is no longer maintained as living animals.
4.2. In situ hybridizations
Whole mount in situ hybridizations using mouse and Tetraodon probes were performed as described previously, using 1.3 × SSC concentration in the hybridization buffer to prevent cross reactivity between mouse RNAs and Tetraodon probes (Woltering et al., 2009, Woltering et al., 2014). Unpublished probes for Tetraodon Hoxa9a and Hoxa10a correspond to sequences within exon 1 and were cloned from BAC DNA using the following primers:
a9a-FW; ATGTCGACATCCGGAACGCTG
a9a-RV; TTGGATCGAGGCCTGGTTTCTC
a10a-FW; ATGGCATGTTCGGACACCC,
a10a-RV CTTTGGGGCCCTTGGCTGCAC.
The mouse Hoxc11 probe was cloned from genomic DNA using the following primers:
FW; AACCGGACGAGCTGGGATTC
RV; AGACTAAGACGGATAACGCG.
Fluorescent in situ hybridization for the MyoD probe (Hebrok et al., 1997) was performed using staining with Fluorescein Thyramid Amplification System (Perkin-Elmer). Antibody staining for neurofilaments was carried out as described previously (Tarchini et al., 2005) using anti-NF160 (clone NN18, Sigma N-5264) and anti-mouse Ig Fab HRP conjugate (Sigma A-3882). For DAPI staining, embryos were incubated with 0.1 mM DAPI in TBS-T after in situ hybridization. Whole mount in situ images in Fig. 2, Fig. 3 were constructed as overlays of gray-scale DAPI fluorescent images and bright field images using Adobe Photoshop.
4.3. In silico sequence analysis
Analysis of non-coding regions in the Hoxa clusters was done using LAGAN-VISTA (http://genome.lbl.gov/vista/lagan/submit.shtml) (Frazer et al., 2004). Alignment of HOXA11 and HOXA11A proteins was done at EMBL (http://www.ebi.ac.uk/Tools/psa/emboss_needle/). Conserved domains were predicted using NCBI's conserved domain database (CDD) (http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (Marchler-Bauer et al., 2015).
The following are the supplementary data related to this article.
Axial skeleton of newborn, wild type and tni HoxAa mice. Skeletons were stained with alizarin red for bone and alcian blue for cartilage. The same lumbar to sacral transformation are visible in Fig. 1B. Wild type mice have either 5 or 6 lumbar vertebra (TL18 and TL19 as indicated by the total of thoraco-lumbar vertebrae), while transgenic mice have 3 lumbar vertebrae (TL16), as the result of an anterior shift of the sacrum. The black arrowheads indicate the posterior most thoracic rib (T13). In newborns, this rib looks underdeveloped in some transgenic mice, although this phenotype is largely recovered in adults. Red arrowheads indicate the lateral processes on L2 that show partial sacral transformation (also present on L3 but not indicated) as shown in Fig. 3C. The axial position of the hind limbs, as determined by the position of the hip joint, is indicated with a dotted line and vertebral count is given to the right of the skeletons (indicated ‘HL’). Transgenic mice show an anterior displacement of the hind limbs of 2 vertebrae, consistent with the shift of the sacrum.
Comparative analysis of Hoxa11 coding and non-coding sequences. A) LAGAN-VISTA plot of Tetraodon sequences spanning from Hoxa10a to Hoxa13a compared with orthologous DNA regions from either the mouse (Mus musculus), the spotted gar (Lepiosteus oculatus) or the Tilapia (Oreochromis niloticus) (sequence information is given below the species name in format ‘assembly:chromosome/linkage group:base start:base end:strand’.) Both the 3′ and the 5′Hoxa11a flanking regions show relatively low sequence conservation between Tetraodon and mouse. Four peaks of sequence conservation representing putative regulatory elements (red peaks) are indicated using arrowheads. The elements located in the Hoxa10a–Hoxa11a and Hoxa11a–Hoxa13a interval are conserved with the spotted gar (red peaks), but appear highly divergent in mouse (white peaks). Peaks of coding sequence conservation are indicated in purple. B) Protein alignment between the mouse HOXA11 and the Tetraodon HOXA11A sequences. The C-terminal homeodomain peptide is nearly 100% conserved at the amino acid level. The N-terminus of the protein is much less conserved indicating possible changes in function during evolution. Despite this divergence, the N-terminus constitutes a conserved domain (DUF3528, indicated in blue) as recognized by the NCBI Conserved Domain Database. This domain appears specific for Hox11 proteins as a similar domain is recognized in Hoxc11 and Hoxd11 (not shown).
Hind leg paralysis in HoxAa transgenic mice. Movie 1 shows the normal locomotory behavior of a wild type littermate of the transgenic mice shown in Movie 2. Movie 2 demonstrates the locomotory abnormalities present in tni HoxAa transgenic mouse. Note the dragging of the hind limbs.
Acknowledgments
We would like to thank all members of the Duboule laboratories. In particular Joska Zakany for useful discussion and sharing of reagents and Fabienne Chabaud and Bénédicte Mascrez for help with animal care and implementation of legal guidelines. We also thank Moisés Mallo for providing constructs. This work was supported by funds (to D.D.) from the University of Geneva, the EPFL, the Swiss National Research Fund (SNSF) (No. 310030B_138662), the European Research Council (ERC; SystemsHox.ch, No. 232790) and the Claraz Foundation.
References
- Asher R.J., Lin K.H., Kardjilov N., Hautier L. Variability and constraint in the mammalian vertebral column. J. Evol. Biol. 2011;24:1080–1090. doi: 10.1111/j.1420-9101.2011.02240.x. [DOI] [PubMed] [Google Scholar]
- Bird N.C., Mabee P.M. Developmental morphology of the axial skeleton of the zebrafish, Danio rerio (Ostariophysi: Cyprinidae) Dev. Dyn. 2003;228:337–357. doi: 10.1002/dvdy.10387. [DOI] [PubMed] [Google Scholar]
- Buchholtz E.A. Crossing the frontier: a hypothesis for the origins of meristic constraint in mammalian axial patterning. Zoology (Jena) 2014;117:64–69. doi: 10.1016/j.zool.2013.09.001. [DOI] [PubMed] [Google Scholar]
- Buchholtz E.A., Bailin H.G., Laves S.A., Yang J.T., Chan M.Y., Drozd L.E. Fixed cervical count and the origin of the mammalian diaphragm. Evol. Dev. 2012;14:399–411. doi: 10.1111/j.1525-142X.2012.00560.x. [DOI] [PubMed] [Google Scholar]
- Burke A.C., Nelson C.E., Morgan B.A., Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–346. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- Carapuco M., Novoa A., Bobola N., Mallo M. Hox genes specify vertebral types in the presomitic mesoderm. Genes Dev. 2005;19:2116–2121. doi: 10.1101/gad.338705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter E.M., Goddard J.M., Davis A.P., Nguyen T.P., Capecchi M.R. Targeted disruption of Hoxd-10 affects mouse hindlimb development. Development. 1997;124:4505–4514. doi: 10.1242/dev.124.22.4505. [DOI] [PubMed] [Google Scholar]
- Carroll R. W.H. Freeman and company; New York: 1988. Vertebrate Paleontology and Evolution; pp. 361–400. [Google Scholar]
- Carroll S.B. Chance and necessity: the evolution of morphological complexity and diversity. Nature. 2001;409:1102–1109. doi: 10.1038/35059227. [DOI] [PubMed] [Google Scholar]
- Carroll R., Holmes R. Evolution of the appendicular skeleton of amphibians. In: Hall B., editor. Fins Into Limbs. The University of Chicago Press; Chicago: 2007. pp. 185–224. [Google Scholar]
- Casaca A., Santos A.C., Mallo M. Controlling Hox gene expression and activity to build the vertebrate axial skeleton. Dev. Dyn. 2014;243:24–36. doi: 10.1002/dvdy.24007. [DOI] [PubMed] [Google Scholar]
- Chiu C.H., Nonaka D., Xue L., Amemiya C.T., Wagner G.P. Evolution of Hoxa-11 in lineages phylogenetically positioned along the fin-limb transition. Mol. Phylogenet. Evol. 2000;17:305–316. doi: 10.1006/mpev.2000.0837. [DOI] [PubMed] [Google Scholar]
- Davis S.P., Finarelli J.A., Coates M.I. Acanthodes and shark-like conditions in the last common ancestor of modern gnathostomes. Nature. 2012;486:247–250. doi: 10.1038/nature11080. [DOI] [PubMed] [Google Scholar]
- Denans N., Iimura T., Pourquie O. Hox genes control vertebrate body elongation by collinear Wnt repression. Elife. 2015;4 doi: 10.7554/eLife.04379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps J., van Nes J. Developmental regulation of the Hox genes during axial morphogenesis in the mouse. Development. 2005;132:2931–2942. doi: 10.1242/dev.01897. [DOI] [PubMed] [Google Scholar]
- Deutsch J., Le Guyader H. The neuronal zootype. An hypothesis. C. R. Acad. Sci. III. 1998;321:713–719. doi: 10.1016/s0764-4469(98)80012-7. [DOI] [PubMed] [Google Scholar]
- Di-Poi N., Montoya-Burgos J.I., Miller H., Pourquie O., Milinkovitch M.C., Duboule D. Changes in Hox genes' structure and function during the evolution of the squamate body plan. Nature. 2010;464:99–103. doi: 10.1038/nature08789. [DOI] [PubMed] [Google Scholar]
- Dolle P., Izpisua-Belmonte J.C., Brown J.M., Tickle C., Duboule D. HOX-4 genes and the morphogenesis of mammalian genitalia. Genes Dev. 1991;5:1767–1776. doi: 10.1101/gad.5.10.1767. [DOI] [PubMed] [Google Scholar]
- Duboule D. Temporal colinearity and the phylotypic progression: a basis for the stability of a vertebrate Bauplan and the evolution of morphologies through heterochrony. Development. 1994:135–142. [PubMed] [Google Scholar]
- Duboule D., Dolle P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989;8:1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D., Morata G. Colinearity and functional hierarchy among genes of the homeotic complexes. Trends Genet. 1994;10:358–364. doi: 10.1016/0168-9525(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Duboule D., Wilkins A.S. The evolution of ‘bricolage’. Trends Genet. 1998;14:54–59. doi: 10.1016/s0168-9525(97)01358-9. [DOI] [PubMed] [Google Scholar]
- Frazer K.A., Pachter L., Poliakov A., Rubin E.M., Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis F. Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. J. Exp. Zool. 1999;285:19–26. [PubMed] [Google Scholar]
- Galis F., Metz J.A. Testing the vulnerability of the phylotypic stage: on modularity and evolutionary conservation. J. Exp. Zool. 2001;291:195–204. doi: 10.1002/jez.1069. [DOI] [PubMed] [Google Scholar]
- Galis F., Metz J.A. Evolutionary novelties: the making and breaking of pleiotropic constraints. Integr. Comp. Biol. 2007;47:409–419. doi: 10.1093/icb/icm081. [DOI] [PubMed] [Google Scholar]
- Galis F., Carrier D.R., van Alphen J., van der Mije S.D., Van Dooren T.J., Metz J.A., ten Broek C.M. Fast running restricts evolutionary change of the vertebral column in mammals. Proc. Natl. Acad. Sci. U. S. A. 2014;111:11401–11406. doi: 10.1073/pnas.1401392111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt S.J. Conservation in the Hox code during morphological evolution. Int. J. Dev. Biol. 1994;38:549–552. [PubMed] [Google Scholar]
- Gaunt S.J., Sharpe P.T., Duboule D. Spatially restricted domains of homeo-gene transcripts in mouse embryos: relation to a segmented body plan. Development. 1988;104:169–179. [Google Scholar]
- Gerard M., Zakany J., Duboule D. Interspecies exchange of a Hoxd enhancer in vivo induces premature transcription and anterior shift of the sacrum. Dev. Biol. 1997;190:32–40. doi: 10.1006/dbio.1997.8679. [DOI] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989;57:367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Guerreiro I., Nunes A., Woltering J.M., Casaca A., Novoa A., Vinagre T., Hunter M.E., Duboule D., Mallo M. Role of a polymorphism in a Hox/Pax-responsive enhancer in the evolution of the vertebrate spine. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10682–10686. doi: 10.1073/pnas.1300592110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B.K. Kluwer Academic Publishers; Dordrecht, Netherlands: 1999. Evolutionary Developmental Biology. [Google Scholar]
- Head J.J., Polly P.D. Evolution of the snake body form reveals homoplasy in amniote Hox gene function. Nature. 2015;520:86–89. doi: 10.1038/nature14042. [DOI] [PubMed] [Google Scholar]
- Hebrok M., Fuchtbauer A., Fuchtbauer E.M. Repression of muscle-specific gene activation by the murine Twist protein. Exp. Cell Res. 1997;232:295–303. doi: 10.1006/excr.1997.3541. [DOI] [PubMed] [Google Scholar]
- Hirasawa T., Kuratani S. A new scenario of the evolutionary derivation of the mammalian diaphragm from shoulder muscles. J. Anat. 2013;222:504–517. doi: 10.1111/joa.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie N., Kuratani S. The developmental hourglass model: a predictor of the basic body plan? Development. 2014;141:4649–4655. doi: 10.1242/dev.107318. [DOI] [PubMed] [Google Scholar]
- Izpisua-Belmonte J.C., Falkenstein H., Dolle P., Renucci A., Duboule D. Murine genes related to the Drosophila AbdB homeotic genes are sequentially expressed during development of the posterior part of the body. EMBO J. 1991;10:2279–2289. doi: 10.1002/j.1460-2075.1991.tb07764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffs P.S., Keynes R.J. A brief history of segmentation. Semin. Dev. Biol. 1990;1:77–87. [Google Scholar]
- Jung H., Mazzoni E.O., Soshnikova N., Hanley O., Venkatesh B., Duboule D., Dasen J.S. Evolving Hox activity profiles govern diversity in locomotor systems. Dev. Cell. 2014;29:171–187. doi: 10.1016/j.devcel.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurberg A.D., Aires R., Varela-Lasheras I., Novoa A., Mallo M. Switching axial progenitors from producing trunk to tail tissues in vertebrate embryos. Dev. Cell. 2013;25:451–462. doi: 10.1016/j.devcel.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Kessel M., Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kirschner M., Gerhart J., ebrary Inc. Yale University Press; New Haven: 2005. The Plausibility of Life Resolving Darwin's Dilemma; p. xiii. (314 pp. ill. 24 cm) [Google Scholar]
- Kmita M., Duboule D. Organizing axes in time and space; 25 years of colinear tinkering. Science. 2003;301:331–333. doi: 10.1126/science.1085753. [DOI] [PubMed] [Google Scholar]
- Kondo T., Dolle P., Zakany J., Duboule D. Function of posterior HoxD genes in the morphogenesis of the anal sphincter. Development. 1996;122:2651–2659. doi: 10.1242/dev.122.9.2651. [DOI] [PubMed] [Google Scholar]
- Lewis E.B. A gene complex controlling segmentation in Drosophila. Nature. 1978;276:565–570. doi: 10.1038/276565a0. [DOI] [PubMed] [Google Scholar]
- Lonfat N., Montavon T., Darbellay F., Gitto S., Duboule D. Convergent evolution of complex regulatory landscapes and pleiotropy at Hox loci. Science. 2014;346:1004–1006. doi: 10.1126/science.1257493. [DOI] [PubMed] [Google Scholar]
- Lynch V.J., Tanzer A., Wang Y., Leung F.C., Gellersen B., Emera D., Wagner G.P. Adaptive changes in the transcription factor HoxA-11 are essential for the evolution of pregnancy in mammals. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14928–14933. doi: 10.1073/pnas.0802355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo M., Wellik D.M., Deschamps J. Hox genes and regional patterning of the vertebrate body plan. Dev. Biol. 2010;344:7–15. doi: 10.1016/j.ydbio.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A., Derbyshire M.K., Gonzales N.R., Lu S., Chitsaz F., Geer L.Y., Geer R.C., He J., Gwadz M., Hurwitz D.I., Lanczycki C.J., Lu F., Marchler G.H., Song J.S., Thanki N., Wang Z., Yamashita R.A., Zhang D., Zheng C., Bryant S.H. CDD: NCBI's conserved domain database. Nucleic Acids Res. 2015;43:D222–D226. doi: 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre D.C., Rakshit S., Yallowitz A.R., Loken L., Jeannotte L., Capecchi M.R., Wellik D.M. Hox patterning of the vertebrate rib cage. Development. 2007;134:2981–2989. doi: 10.1242/dev.007567. [DOI] [PubMed] [Google Scholar]
- Misra M., Shah V., Carpenter E., McCaffery P., Lance-Jones C. Restricted patterns of Hoxd10 and Hoxd11 set segmental differences in motoneuron subtype complement in the lumbosacral spinal cord. Dev. Biol. 2009;330:54–72. doi: 10.1016/j.ydbio.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montavon T., Le Garrec J.-F., Kerszberg M., Duboule D. Modeling Hox gene regulation in digits: reverse collinearity and the molecular origin of thumbness. Genes Dev. 2008;22:346–359. doi: 10.1101/gad.1631708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montavon T., Soshnikova N., Mascrez B., Joye E., Thevenet L., Splinter E., de Laat W., Spitz F., Duboule D. A regulatory archipelago controls Hox genes transcription in digits. Cell. 2011;147:1132–1145. doi: 10.1016/j.cell.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Moriyama Y., Takeda H. Evolution and development of the homocercal caudal fin in teleosts. Develop. Growth Differ. 2013;55:687–698. doi: 10.1111/dgd.12088. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Tanaka M. Evolution of motor innervation to vertebrate fins and limbs. Dev. Biol. 2011;355:164–172. doi: 10.1016/j.ydbio.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Murata Y., Tamura M., Aita Y., Fujimura K., Murakami Y., Okabe M., Okada N., Tanaka M. Allometric growth of the trunk leads to the rostral shift of the pelvic fin in teleost fishes. Dev. Biol. 2010;347:236–245. doi: 10.1016/j.ydbio.2010.07.034. [DOI] [PubMed] [Google Scholar]
- Narita Y., Kuratani S. Evolution of the vertebral formulae in mammals: a perspective on developmental constraints. J. Exp. Zool. B Mol. Dev. Evol. 2005;304:91–106. doi: 10.1002/jez.b.21029. [DOI] [PubMed] [Google Scholar]
- Nishimoto S., Minguillon C., Wood S., Logan M.P. A combination of activation and repression by a colinear Hox code controls forelimb-restricted expression of Tbx5 and reveals Hox protein specificity. PLoS Genet. 2014;10:e1004245. doi: 10.1371/journal.pgen.1004245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer D., Duboule D. Chromatin architectures and Hox gene collinearity. Curr. Top. Dev. Biol. 2013;104:113–148. doi: 10.1016/B978-0-12-416027-9.00004-8. [DOI] [PubMed] [Google Scholar]
- Noordermeer D., Leleu M., Schorderet P., Joye E., Chabaud F., Duboule D. Temporal dynamics and developmental memory of 3D chromatin architecture at Hox gene loci. Elife. 2014;3:e02557. doi: 10.7554/eLife.02557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oulion S., Borday-Birraux V., Debiais-Thibaud M., Mazan S., Laurenti P., Casane D. Evolution of repeated structures along the body axis of jawed vertebrates, insights from the Scyliorhinus canicula Hox code. Evol. Dev. 2011;13:247–259. doi: 10.1111/j.1525-142X.2011.00477.x. [DOI] [PubMed] [Google Scholar]
- Palmeirim I., Henrique D., Ish-Horowicz D., Pourquie O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–648. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- Pourquie O. The segmentation clock: converting embryonic time into spatial pattern. Science. 2003;301:328–330. doi: 10.1126/science.1085887. [DOI] [PubMed] [Google Scholar]
- Prince V.E., Joly L., Ekker M., Ho R.K. Zebrafish hox genes: genomic organization and modified colinear expression patterns in the trunk. Development. 1998;125:407–420. doi: 10.1242/dev.125.3.407. [DOI] [PubMed] [Google Scholar]
- Raff R.A. Developmental mechanisms in the evolution of animal form: origins and evolvability of body plans. In: Bengston S., editor. Early Life on Earth. Columbia University Press; New York: 1994. pp. 489–500. [Google Scholar]
- Raff R.A. University of Chicago Press; Form: 1996. The Shape of Life: Genes, Development and the Evolution of Animal. [Google Scholar]
- Richardson M.K. Heterochrony and the phylotypic period. Dev. Biol. 1995;172:412–421. doi: 10.1006/dbio.1995.8041. [DOI] [PubMed] [Google Scholar]
- Rijli F.M., Mark M., Lakkaraju S., Dierich A., Dolle P., Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75:1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Rockova H., Rocek Z. Development of the pelvis and posterior part of the vertebral column in the Anura. J. Anat. 2005;206:17–35. doi: 10.1111/j.0021-8782.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer S. Krieger publishing company; Malabar, Florida: 1956. Osteology of the Reptiles; pp. 218–297. [Google Scholar]
- Ronshaugen M., McGinnis N., McGinnis W. Hox protein mutation and macroevolution of the insect body plan. Nature. 2002;415:914–917. doi: 10.1038/nature716. [DOI] [PubMed] [Google Scholar]
- Sander K. The evolution of patterning mechanisms: gleanings from insect embryogenesis and spermatogenesis. In: Goodwin B.C., editor. Development and Evolution. Cambridge University press; Cambridge: 1983. pp. 137–159. [Google Scholar]
- Slack J.M., Holland P.W., Graham C.F. The zootype and the phylotypic stage. Nature. 1993;361:490–492. doi: 10.1038/361490a0. [DOI] [PubMed] [Google Scholar]
- Sordino P., van der Hoeven F., Duboule D. Hox gene expression in teleost fins and the origin of vertebrate digits. Nature. 1995;375:678–681. doi: 10.1038/375678a0. [DOI] [PubMed] [Google Scholar]
- Soshnikova N., Dewaele R., Janvier P., Krumlauf R., Duboule D. Duplications of hox gene clusters and the emergence of vertebrates. Dev. Biol. 2013;378:194–199. doi: 10.1016/j.ydbio.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Stanley W.T., Robbins L.W., Malekani J.M., Mbalitini S.G., Migurimu D.A., Mukinzi J.C., Hulselmans J., Prevot V., Verheyen E., Hutterer R., Doty J.B., Monroe B.P., Nakazawa Y.J., Braden Z., Carroll D., Peterhans J.C., Bates J.M., Esselstyn J.A. A new hero emerges: another exceptional mammalian spine and its potential adaptive significance. Biol. Lett. 2013;9:20130486. doi: 10.1098/rsbl.2013.0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M. Revealing the mechanisms of the rostral shift of pelvic fins among teleost fishes. Evol. Dev. 2011;13:382–390. doi: 10.1111/j.1525-142X.2011.00493.x. [DOI] [PubMed] [Google Scholar]
- Tarchini B., Huynh T.H., Cox G.A., Duboule D. HoxD cluster scanning deletions identify multiple defects leading to paralysis in the mouse mutant Ironside. Genes Dev. 2005;19:2862–2876. doi: 10.1101/gad.351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hoeven F., Sordino P., Fraudeau N., Izpisua-Belmonte J.C., Duboule D. Teleost HoxD and HoxA genes: comparison with tetrapods and functional evolution of the HOXD complex. Mech. Dev. 1996;54:9–21. doi: 10.1016/0925-4773(95)00455-6. [DOI] [PubMed] [Google Scholar]
- Varela-Lasheras I., Bakker A.J., van der Mije S.D., Metz J.A., van Alphen J., Galis F. Breaking evolutionary and pleiotropic constraints in mammals: on sloths, manatees and homeotic mutations. Evodevo. 2011;2:11. doi: 10.1186/2041-9139-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner G.P., Amemiya C., Ruddle F. Hox cluster duplications and the opportunity for evolutionary novelties. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14603–14606. doi: 10.1073/pnas.2536656100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba G.M., Hostikka S.L., Carpenter E.M. The paralogous Hox genes Hoxa10 and Hoxd10 interact to pattern the mouse hindlimb peripheral nervous system and skeleton. Dev. Biol. 2001;231:87–102. doi: 10.1006/dbio.2000.0130. [DOI] [PubMed] [Google Scholar]
- Wellik D.M. Hox genes and vertebrate axial pattern. Curr Top Dev Biol. 2009;88:257–258. doi: 10.1016/S0070-2153(09)88009-5. [DOI] [PubMed] [Google Scholar]
- Wellik D.M., Capecchi M.R. Hox10 and Hox11 genes are required to globally pattern the mammalian skeleton. Science. 2003;301:363–367. doi: 10.1126/science.1085672. [DOI] [PubMed] [Google Scholar]
- Woltering J.M. From lizard to snake; behind the evolution of an extreme body plan. Curr. Genom. 2012;13:289–299. doi: 10.2174/138920212800793302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltering J.M., Vonk F.J., Muller H., Bardine N., Tuduce I.L., de Bakker M.A., Knochel W., Sirbu I.O., Durston A.J., Richardson M.K. Axial patterning in snakes and caecilians: evidence for an alternative interpretation of the Hox code. Dev. Biol. 2009;332:82–89. doi: 10.1016/j.ydbio.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Woltering J.M., Noordermeer D., Leleu M., Duboule D. Conservation and divergence of regulatory strategies at Hox Loci and the origin of tetrapod digits. PLoS Biol. 2014;12:e1001773. doi: 10.1371/journal.pbio.1001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Wang G., Scott S.A., Capecchi M.R. Hoxc10 and Hoxd10 regulate mouse columnar, divisional and motor pool identity of lumbar motoneurons. Development. 2008;135:171–182. doi: 10.1242/dev.009225. [DOI] [PubMed] [Google Scholar]
- Young T., Rowland J.E., van de Ven C., Bialecka M., Novoa A., Carapuco M., van Nes J., de Graaff W., Duluc I., Freund J.N., Beck F., Mallo M., Deschamps J. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev. Cell. 2009;17:516–526. doi: 10.1016/j.devcel.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Zakany J., Kmita M., Alarcon P., de la Pompa J.L., Duboule D. Localized and transient transcription of Hox genes suggests a link between patterning and the segmentation clock. Cell. 2001;106:207–217. doi: 10.1016/s0092-8674(01)00436-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Axial skeleton of newborn, wild type and tni HoxAa mice. Skeletons were stained with alizarin red for bone and alcian blue for cartilage. The same lumbar to sacral transformation are visible in Fig. 1B. Wild type mice have either 5 or 6 lumbar vertebra (TL18 and TL19 as indicated by the total of thoraco-lumbar vertebrae), while transgenic mice have 3 lumbar vertebrae (TL16), as the result of an anterior shift of the sacrum. The black arrowheads indicate the posterior most thoracic rib (T13). In newborns, this rib looks underdeveloped in some transgenic mice, although this phenotype is largely recovered in adults. Red arrowheads indicate the lateral processes on L2 that show partial sacral transformation (also present on L3 but not indicated) as shown in Fig. 3C. The axial position of the hind limbs, as determined by the position of the hip joint, is indicated with a dotted line and vertebral count is given to the right of the skeletons (indicated ‘HL’). Transgenic mice show an anterior displacement of the hind limbs of 2 vertebrae, consistent with the shift of the sacrum.
Comparative analysis of Hoxa11 coding and non-coding sequences. A) LAGAN-VISTA plot of Tetraodon sequences spanning from Hoxa10a to Hoxa13a compared with orthologous DNA regions from either the mouse (Mus musculus), the spotted gar (Lepiosteus oculatus) or the Tilapia (Oreochromis niloticus) (sequence information is given below the species name in format ‘assembly:chromosome/linkage group:base start:base end:strand’.) Both the 3′ and the 5′Hoxa11a flanking regions show relatively low sequence conservation between Tetraodon and mouse. Four peaks of sequence conservation representing putative regulatory elements (red peaks) are indicated using arrowheads. The elements located in the Hoxa10a–Hoxa11a and Hoxa11a–Hoxa13a interval are conserved with the spotted gar (red peaks), but appear highly divergent in mouse (white peaks). Peaks of coding sequence conservation are indicated in purple. B) Protein alignment between the mouse HOXA11 and the Tetraodon HOXA11A sequences. The C-terminal homeodomain peptide is nearly 100% conserved at the amino acid level. The N-terminus of the protein is much less conserved indicating possible changes in function during evolution. Despite this divergence, the N-terminus constitutes a conserved domain (DUF3528, indicated in blue) as recognized by the NCBI Conserved Domain Database. This domain appears specific for Hox11 proteins as a similar domain is recognized in Hoxc11 and Hoxd11 (not shown).
Hind leg paralysis in HoxAa transgenic mice. Movie 1 shows the normal locomotory behavior of a wild type littermate of the transgenic mice shown in Movie 2. Movie 2 demonstrates the locomotory abnormalities present in tni HoxAa transgenic mouse. Note the dragging of the hind limbs.