Abstract
Background
Recovery after surgery is highly variable. Risk-stratifying patients based on their predicted recovery profile will afford individualized perioperative management strategies. Recently, application of mass cytometry in patients undergoing hip arthroplasty revealed strong immune correlates of surgical recovery in blood samples collected shortly after surgery. However, the ability to interrogate a patient’s immune state before surgery and predict recovery is highly desirable in perioperative medicine.
Methods
To evaluate a patient’s pre-surgical immune state, cell-type specific intracellular signaling responses to ex-vivo ligands (LPS, IL-6, IL-10, IL-2/GM-CSF) were quantified by mass cytometry in pre-surgical blood samples. Selected ligands modulate signaling processes perturbed by surgery. Twenty-three cell surface and 11 intracellular markers were used for the phenotypic and functional characterization of major immune cell subsets. Evoked immune responses were regressed against patient-centered outcomes contributing to protracted recovery including functional impairment, postoperative pain, and fatigue.
Results
Evoked signaling responses varied significantly and defined patient-specific pre-surgical immune states. Eighteen signaling responses correlated significantly with surgical recovery parameters (|R|=0.37–0.70; FDR<0.01). Signaling responses downstream of the TLR4 receptor in CD14+ monocytes were particularly strong correlates, accounting for 50% of observed variance. Pre-surgical immune correlates mirrored correlates previously described in post-surgical samples.
Conclusion
Convergent findings in pre- and post-surgical analyses provide validation of reported immune correlates and suggest a critical role of the TLR4 signaling pathway in monocytes for the clinical recovery process. The comprehensive assessment of patients’ preoperative immune state is promising for predicting important recovery parameters and may lead to clinical tests using standard flow cytometry.
INTRODUCTION
Anesthesiologists play a sentinel role in multi-disciplinary efforts to improve perioperative care by reducing the incidence of postoperative complications, shortening the recovery period, and optimizing the allocation of health care resources.1 Outcomes that are meaningful to patients are at the very core of such efforts as ultimately the aim is to provide the best possible value to patients.2,3 Time to recovery and return to normal activities are patient-centered outcomes of high priority in the perioperative context.4 Major factors that determine the speed of recovery include fatigue, pain, and functional impairment.5,6
Postoperative recovery is highly variable among patients undergoing similar surgical interventions.7,8 The ability to risk-stratify patients based on their predicted recovery profile is of significant interest as it would enable patient-tailored and cost-conscious approaches to perioperative management. For example, patients would be better able to make appropriate arrangements for postoperative needs, and patients at risk for protracted recovery could be stratified to resource-intense interventions such as prehabilitation programs to accelerate their recovery.9
A recent application of single-cell mass cytometry at the “bedside” revealed strong immune correlates of surgical recovery in patients undergoing primary hip arthroplasty.8 Mass cytometry allows for the simultaneous phenotypic and functional characterization of all major immune cell subsets in peripheral blood at unparalleled single-cell resolution.10,11 Surgery-induced signaling changes in monocyte subsets within 24 hours after surgery were strongly associated with the speed of recovery from fatigue, pain, and functional impairment, and accounted for 40–60% of observed variance. The identification of strong immune correlates shortly after surgery is an important advancement in understanding the biology that drives recovery. The ability to interrogate a patient’s immune state before surgery and accurately predict recovery would have a major impact on the practice of perioperative medicine.
This study was built on the premise that patients undergoing surgery differ in their pre-surgical immune state, which then affects their immune response to surgery and determines speed of their clinical recovery. To evaluate a patient’s pre-surgical immune state, cell type-specific intracellular signaling responses to ex vivo ligands were quantified by mass cytometry in whole blood samples collected before surgery. This experiment was designed to mimic the surgical stress on a patient’s immune system by use of ligands known to modulate signaling processes in specific immune cells that are perturbed by surgery.8 The major hypothesis tested in this study was that patient-specific pre-surgical immune states predict the speed of recovery from fatigue, pain, and functional impairment in patients undergoing primary hip arthroplasty.
MATERIALS AND METHODS
Study design
This study was registered at ClinicalTrials.gov on March 23, 2012 (NCT01578798) and was conducted from March 2012 through July 2013. The study produced two distinct and large molecular data sets in patients undergoing hip arthroplasty. An initial analysis addressed the question of whether specific immune responses to surgery correlated with the clinical recovery profile of individual patients. Strong immune correlates were identified in post-surgical blood samples, and results have been published in a separate manuscript.8 The analysis described here addressed whether evoked immune responses in pre-surgical blood samples correlate with the clinical recovery profile of individual patients. The workflow of this study is illustrated in Figure 1. Some portions of the methods and results sections, including the description of subjects, aspects of the study protocol, and clinical outcomes, are included in both manuscripts to ensure completeness and accessibility for the reader.
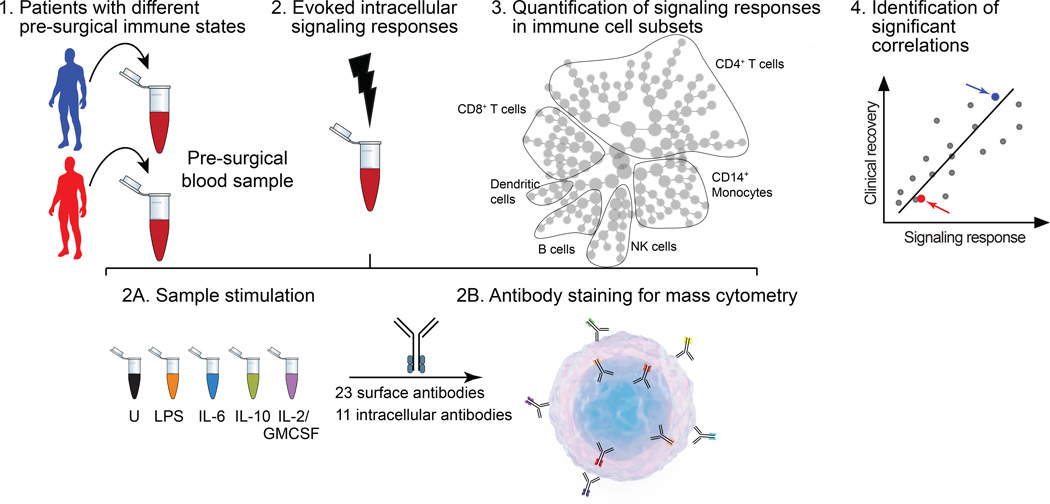
Figure 1. Flow-chart summarizing the experimental approach.
Whole blood was obtained one hour before surgery from patients undergoing primary hip arthroplasty (PANEL 1) Separate whole blood aliquots were stimulated ex vivo with extracellular ligands (LPS, lipopolysaccharide; IL-6, interleukin 6; IL-10, interleukin 10; or a combination of IL-2 and GMCSF, interleukin 2 and granulocyte monocyte colony-stimulating factor) or left untreated (U) (PANEL 2a). Using mass cytometry, the expression of 23 cell surface markers and the phosphorylation states of 11 intracellular signaling proteins were measured in single cells from blood samples (PANEL 2b). Unsupervised hierarchical clustering and manual gating strategies were applied to visualize and quantify patient-specific signaling responses in immune cell subsets spanning the entire immune system. Shown is a visual representation of a cluster hierarchy plot (PANAL 3). Signaling responses that correlated significantly with clinical recovery parameters were identified by significance analysis of microarrays (PANAL 4). Arrows indicate results for the two hypothetical patients (PANAL 1).
Subjects
Patients scheduled for primary hip arthroplasty for non-traumatic osteoarthritis were recruited from the Arthritis and Joint Replacement Clinic in the Department of Orthopedic Surgery at Stanford University School of Medicine. The study was approved by the Institutional Review Board of Stanford University School of Medicine (Stanford, CA, USA). All patients gave written informed consent before being enrolled in the study. Inclusion criteria were: 1) age between 18–90 years, 2) fluent in English, and 3) willing and able to sign informed consent and the Health Insurance Portability and Accountability Act (HIPAA) authorization. Exclusion criteria were: 1) any systemic disease or medication that might compromise the immune system, 2) diagnosis of cancer within the last 5 years, 3) auto-immune, psychiatric, or neurological conditions interfering with data collection and interpretation, 4) pregnancy, and 5) other conditions that, in the opinion of the investigators, may have compromised a participant’s safety or the integrity of the study, such as a history of substance abuse, chronic opioid therapy (> 30 mg/day), infectious disease within 1 month, or renal, hepatic, cardiovascular, or respiratory disease resulting in clinically significant functional impairment.
Surgery and anesthesia
Hip arthroplasties were performed by one of three surgeons using a standard lateral approach, wound drains, and compression dressings. Patients were mobilized on the day of surgery, their bladder catheter was removed on postoperative day 1 or 2, and hospital discharge was planned for postoperative day 3 or 4. Captured surgery-related data included duration of surgery, intraoperative blood loss, intravenous fluid administration, and time to hospital discharge.
Patients were premedicated with midazolam (NOVAPLUS, Irvine, Texas, USA). General anesthesia was induced with propofol (NOVAPLUS), fentanyl (Hospira, Lake Forest, Illinois, USA), and rocuronium (NOVAPLUS) and was maintained with the volatile anesthetics sevoflurane (AbbVie, North Chicago, IL, USA) or desflurane (Baxter, Deerfield, IL, USA). Use of nitrous oxide (Praxair Technology, Danbury, CT, USA) was at the discretion of the anesthesiologist as was the choice of using either fentanyl (Hospira) or hydromorphone (Perdue, Stamford, CT, USA) for subsequent dosing of an opioid. Towards the end of surgery muscle relaxation was reversed with neostigmine (Éclat, Chesterfield, MO, USA) and glycopyrrolate (American Regent, Shirley, NY, USA). Patients received ondansetron (NOVAPLUS) for prophylaxis of postoperative nausea and vomiting. All patients were offered adjuvant spinal anesthesia, which was performed in the sitting position using a midline approach at the lumbar level (L2/3 or L3/4). Typically, a 25 Gauge Whitacre spinal needle was used (Portex, Smiths Medical, Norwell, MA, USA) but on rare occasion a 22 Gauge Quincke needle (Becton-Dickinson, Franklin Lakes, New Jersey, USA) was chosen instead. The intrathecal space was identified by the return of cerebrospinal fluid through the spinal needle. Single shot administration of 1.2–1.4 mL of hyperbaric 0.75% bupivacaine in dextrose (Portex) established spinal anesthesia. Addition of 100–200 µg morphine (Astramorph, AstraZeneca, Wilmington, NC, USA) was at the discretion of the anesthesiologist. Postoperative pain was treated with intravenous PCA using bolus administration of hydromorphone (Perdue). Adjuvant analgesic included intravenous or per-os acetaminophen (Cadence, San Diego, CA, USA, or Gemini, Commack, NY, USA) according to the surgeons’ preference. Captured anesthesia-related data included average alveolar concentrations of the volatile anesthetic and nitrous oxide, intraoperative opioid dose, intrathecal dose of bupivacaine and morphine in patients with adjuvant spinal anesthesia, cumulative postoperative opioid dose during in-hospital stay, and postoperative opioid dose after hospital discharge (assessed every third day for up to six weeks).
Clinical outcomes
The three major clinical outcomes—fatigue and resulting functional impairment, function of the operated hip, and pain—were evaluated before surgery, daily during hospitalization, and every third day for up to six weeks after hospital discharge. Fatigue and resulting global functional impairment were assessed with aid of the surgical recovery scale (SRS).12 The SRS is a categorical questionnaire that 1) assesses how energetic, lively, vigorous, worn-out, fatigued, and physically tired a patient feels and 2) quantifies the level of daily activities, restrictions in daily activity, and the ability to read/watch TV, dress, socialize, perform leisurely or recreational activities, and shop or do errands. A score of 100 indicates no fatigue and typical daily functioning; 17 is the worst possible score. Function of the operated hip was assessed with a modified Western Ontario and McMaster Universities Arthritis Index scale (WOMAC) that evaluates impairment when lying in bed, rising from bed, sitting, raising from sitting, standing, and walking on a flat surface on six separate 11-point numerical rating scales.8 A score of 60 indicates maximum impairment, and a score of zero indicates no impairment. Postoperative pain was captured on a modified WOMAC scale assessing pain at rest, during the night, when bearing weight, and when walking on a flat surface on four separate 11-point numerical rating scales. A score of 40 indicates maximum pain, whereas a score of zero indicates no pain. In addition, daily opioid consumption was captured and expressed as intravenous hydromorphone equivalents using an opioid conversion table13.
Parameterization of clinical data
Parameterization was performed as previously described.8 Recovery from postoperative fatigue and resulting functional impairment was quantified as the time required to half-maximum recovery (SRS-t1/2), where maximum recovery is defined by the pre-surgical SRS-score. SRS-t1/2 was chosen as the outcome because the time to full recovery was affected by ceiling effects. Recovery of hip function was quantified as the time required to regress to a score ≤18 on the WOMAC scale. A score of 18 is indicative for the transition from moderate to mild impairment. Recovery from pain was quantified as the time required to regress to a score ≤12 on the WOMAC scale. A score of 12 is indicative of the transition from moderate to mild pain.
Specimen collection and stimulation with external ligands
Whole blood was collected in heparin containing tubes one hour before surgery (Figure 1). Whole blood samples were processed within 30 minutes of collection. Samples were divided into 1-mL aliquots and incubated at 37 °C for 15 minutes with PBS (control), 100 ng/mL IL-6 (BD Biosciences, San Jose, CA, USA), 100 ng/mL IL-10 (BD Biosciences), a combination of 100 ng/mL IL-2 (BD Biosciences) and 2 ng/mL GMCSF (PeproTech, Rocky Hill, NJ, USA), or 1 µg/mL LPS (LPS-EK Ultra Pure, InvivoGen, San Diego, CA, USA). Blood samples were resuspended in 1.4 mL stabilizing buffer (Smarttube Inc., Palo Alto, CA, USA), incubated for 10 minutes at room temperature for fixation, cooled to 4 °C, and stored at −80 °C until further processing for barcoding and antibody staining.
Selection of stimulation conditions
Previous work identified cell-type specific signaling responses that were significantly modulated by surgery.8 Three of these signaling responses strongly correlated with clinical recovery parameters. External ligands were selected to specifically target pathways containing these signaling proteins (Table 1).11,14 LPS was chosen to activate downstream effectors of the TLR4 signaling pathway in CD14+ monocytes, IL-6 and IL-10 were chosen to evoke STAT1 and STAT3 phosphorylation in CD14+ monocytes and T cell subsets, and a combination of GMCSF and IL-2 was chosen to stimulate STAT5 phosphorylation in CD14+ monocytes and T cell subsets.
Table 1.
Ligand Selection and Evoked Signaling Responses
| Ligand | Cell type | Signaling protein(1) | Arcsinh ratio (mean ± SD) |
p value |
|---|---|---|---|---|
| IL-6 | CD14+ monocyte | pStat1 | 0.171±0.092 | 2.25×10−09 |
| IL-6 | CD14+ monocyte | pStat3(2) | 0.437±0.136 | 2.40×10−14 |
| IL-10 | CD14+ monocyte | pStat3(2) | 0.502±0.173 | 2.27×10−13 |
| GMCSF | CD14+ monocyte | pStat5 | 1.855±0.253 | 1.41×10−22 |
| LPS | CD14+ monocyte | pNFkB(2) | 0.249±0.089 | 4.95×10−13 |
| LPS | CD14+ monocyte | pCREB(2) | 0.463±0.126 | 1.22×10−15 |
| LPS | CD14+ monocyte | pP38 | 2.001±0.221 | 8.96×10−25 |
| LPS | CD14+ monocyte | pMAPKAPK2 | 1.552±0.241 | 3.03×10−21 |
| LPS | CD14+ monocyte | pP90RSK | 0.515±0.192 | 1.21×10−12 |
| LPS | CD14+ monocyte | prpS6 | 0.625±0.235 | 1.45×10−12 |
| LPS | CD14+ monocyte | pERK | 0.970±0.205 | 3.95×10−18 |
| IL-6 | CD4+ T cell | pStat3 | 0.224±0.095 | 2.05×10−11 |
| IL-10 | CD4+ T cell | pStat3 | 0.264±0.128 | 2.46×10−10 |
| IL-2 | CD4+ T cell | pStat5 | 1.606±0.284 | 5.93×10−20 |
| IL-6 | CD8+ T cell | pStat3 | 0.069±0.045 | 5.36×10−08 |
| IL-10 | CD8+ T cell | pStat3 | 0.293±0.138 | 1.41×10−10 |
| IL-2 | CD8+ T cell | pStat5 | 1.677±0.262 | 3.50×10−21 |
Signaling responses previously shown to be significantly modulated by surgery.8
Signaling response to surgery previously shown to correlate significantly with clinical recovery parameters.8
Abbreviations: GMCSF, Granulocyte macrophage colony-stimulating factor; IL-2, interleukin 2; IL-6, interleukin 6; IL-10, interleukin 10; LPS, lipopolysaccharide. pCREB, phosphorylated cAMP response element-binding protein; pERK1/2, phosphorylated extracellular regulated kinase 1/2; pMAPKAPK2, phosphorylated mitogen activated protein kinase-activated protein kinase-2; pNFkB, phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells; pP38, phosphorylated P38 map kinase; pp90RSK, phosphorylated ribosomal S6 kinase; prpS6, phosphorylated ribosomal protein S6; pSTAT1/3/5, phosphorylated signal transducer and activator of transcription 1/3/5.
Sample barcoding
Reagents for barcoding were prepared as previously described.15 Two molar equivalents of maleimido-mono-amide-DOTA (Macrocyclics, Inc., Dallas, TX) were added to palladium 102, 104,105, 106, 108, and 110 solutions in 20 mM ammonium acetate, pH 6.0. Solutions were immediately lyophilized, and solids were dissolved in dimethyl sulfoxide (DMSO) to 10 mM for storage at −20 °C. Each well of a barcoding plate contained a distinct combination of three palladium isotopes at 200 nM in DMSO.
After thawing and red blood cell lysis in a hypotonic buffer, cells were barcoded as previously described.16 Briefly, cells were transferred into a deep-well block and washed once with cell staining media (CSM, PBS with 0.5% BSA, 0.02% NaN3), once with PBS, and once with 0.02% saponin in PBS. The barcoding plate was thawed, and each well of barcode reagent was diluted in 1 mL 0.02% saponin in PBS. Diluted barcode reagent was transferred to cells, and samples were incubated at room temperature for 15 minutes, washed twice with CSM, and then pooled for staining.
Antibody staining
The panel included 23 antibodies for the comprehensive phenotypic characterization of immune cells, and 11 antibodies (pCREB, pERK, pMAPKAPK2, pP38, pNFκB [pP65], pPLCg2, pP90RSK, prpS6, pSTAT1, pSTAT3, and pSTAT5) directed toward phosphorylated forms of intracellular signaling proteins for the functional characterization of each immune cell (see Table 1, Supplementary Digital Content 1, which is a table that lists the antibodies used in the study). Panel development and validation have previously been described in detail.8
Cells were washed once with CSM and then incubated for 10 minutes at room temperature with one test of FcX block (Biolegend, San Diego, CA) to block non-specific Fc binding. Cells were stained with all surface antibodies for 30 minutes and washed once with CSM. Cells were permeabilized with 1 mL of methanol for 10 minutes on ice. Cells were then washed twice with PBS and once with CSM and incubated with the intracellular antibody cocktail for 30 minutes at room temperature. Cells were washed once with CSM then incubated overnight at 4 °C with an iridium-containing intercalator (Fluidigm, South San Francisco, CA) in PBS with 1.6% formaldehyde. Cells were then washed twice with CSM, once with water, and resuspended in a solution of normalization beads as previously described.17 Cells were filtered through a 35-µm membrane prior to analysis by mass cytometry.
Mass cytometry
Barcoded and antibody-stained cells were analyzed on a mass cytometer (CyTOF, Fluidigm) at an event rate of 400–500 cells per second. The data were normalized using Normalizer v0.1 MCR.17 Files were de-barcoded using a single-cell Matlab Debarcoder Tool.18 Gating was performed using the Cytobank platform as previously described.8
Phenotyping of immune cells
Major immune cell subsets including granulocytes, CD14+ monocytes, natural killer cells, classical and plasmacytoid dendritic cells, CD4+ and CD8+ T cells, and B cells were identified with a manual gating strategy as previously described.8 Hierarchical clustering using Ward’s linkage and Euclidean distance in R was used as a second phenotyping strategy for the detailed characterization and visual representation of CD45+CD66− cells. Cells were clustered based on the expression of CD7, CD19, CD11b, CD4, CD8, CD127, CCR7, CD123, CD45RA, CD33, CD11c, CD14, CD16, FoxP3, CD25, CD3, HLA-DR, and CD56. Ten thousand events were sampled from each patient sample. The cluster hierarchy plots and histograms were created in R. Clusters containing at least 1% of all clustered cells are graphically displayed. Cell clusters are colored based on the median arcsinh ratio of the phosphorylated protein level in a stimulated condition (IL-6, IL-10, IL-2/GMCSF, and LPS) relative to the control condition (PBS). The arcsinh ratio is the median difference between the median arcsinh value in control and stimulated cell subsets.8,11 Protein phosphorylation data were transformed to arcsinh values by taking the inverse hyperbolic sine of the raw data.
Statistical analysis
Data are expressed as means plus or minus standard deviations or as medians with interquartile ranges if inter-individual differences were of particular interest. Alternatively, the mean or median and corresponding 95% confidence intervals are reported if confidence in the mean or median are of particular interest.
The sample size was based on a power calculation pertinent to the detection of surgery-induced alterations in cell signaling responses rather than the detection of immune correlates of surgical recovery.8 Considering all signaling responses with arcsinh ratios equal or greater than 0.2, the response associated with the highest variance (pp38 in CD14+ monocytes) was used to determine power. A sample size of 24 patients allows detecting signaling response with a power of 90% at an alpha-level of 0.001.
Cell-type specific signaling responses to stimulation with external ligands
To verify that selected ligands changed the phosphorylation state of signaling proteins altered by surgery, a two-class paired t-test was used to compare arcsinh transformed raw data between control and stimulated conditions at a Bonferroni-adjusted p value < 8.92 × 10−4 (56 conditions: 14 signaling proteins in specific cell subsets and four stimulations).8
Correlation analyses between molecular and clinical parameters
Significant correlations between the three major clinical recovery parameters and ligand-evoked changes in the phosphorylation state of intracellular signaling proteins in hand-gated cell subsets were detected by Significance Analysis of Microarrays (SAM) using the “samr” package in R.19 SAM is a non-parametric test specifically designed for multiple comparison analysis of high-dimensional data sets. Two approaches were used to infer statistical significance. The first approach was most stringent and used a Bonferroni corrected p value < 1.42 × 10−4 (352 conditions: four stimulations, 11 signaling proteins, and eight cell subsets). This approach may miss biologically important results in high-dimensional data sets.19 Therefore, a more inclusive approach was used by setting the statistical threshold for inferring significance at a false discovery rate <1% (FDR, q<0.01). Statistically significant correlations were further filtered by applying two effect-size criteria. Results were only considered significant if the arcsinh ratio passed the threshold of 0.5 in at least one patient and the 95% interval of the arcsinh ratio did not include zero.
Additional correlational analysis
Previously reported post-surgical immune correlates (pMAPKAPK2, pCREB, prpS6) were correlated with the same pre-surgical immune correlates, and functional recovery outcomes using R (Bonferroni-adjusted p value < 1.16 × 10−2; three conditions). Similarly, correlational analysis was performed for the three major clinical outcome variables (p value < 1.16 × 10−2).
Covariate analysis
Partial correlation analysis was performed to test whether a correlation between an immune parameter and a clinical recovery parameter remained significant when controlling for a particular covariate. This analysis correlated residuals that resulted from correlating clinical covariates and immune parameters with residuals resulting from correlating clinical covariates and clinical recovery parameters. Clinical covariates included age, sex, body mass index, estimated intraoperative blood loss, duration of surgery, use of spinal anesthesia, and pre-surgical values for major clinical outcomes. Analysis was performed in R. Correlations of residuals were considered significant for a Bonferroni-adjusted p value < 2.5 × 10−3 (20 conditions: 10 covariates and two immune parameters). A significant partial correlation indicated that the correlation between an immune parameter and a clinical recovery parameter remained significant when adjusting for a particular covariate.
Multi-linear regression analysis
The analysis was performed in SPSS (IBM SPSS Statistics version 20, Armonk, New York, USA) to determine whether age contributed to the correlation between immune parameters and clinical outcomes that passed Bonferroni correction. The age range of patients was wide enough to warrant such analysis.
RESULTS
Subjects
Two hundred and fifty-one patients from the Arthritis and Joint Replacement Clinic in the Department of Orthopedic Surgery at Stanford University School of Medicine were screened. One hundred and five patients did not meet inclusion criteria, 31 declined to participate, 65 could not be enrolled due to other reasons (e.g., scheduling conflicts), 11 withdrew consent before undergoing surgery, and seven were lost to follow up (e.g., second surgery, illness).
Data from six patients were used for validation and refinement of the molecular analysis. One patient’s sample was lost due to failed barcoding. The analysis set included complete data sets of 25 patients: 24 Caucasian and one African American, 16 men and nine women, median age of 59 years (interquartile range (IQR) 54–67), and median body mass index of 24.4 kg/m2 (IQR 26.4–28.0). Comorbidities included controlled arterial hypertension (8 subjects), stable coronary artery disease (2 subjects), hyperlipidemia (8 subjects), controlled asthma (2 subjects), controlled diabetes mellitus type 2 (1 subject), controlled hypothyroidism without evidence for an autoimmune disease (2 subjects), and asymptomatic hyperparathyroidism (1 subject). Over the course of the 6-week study period none of the patients suffered from cardiac, pulmonary, neurological, infectious, thromboembolic, or bleeding complications, or required a second operation for surgical complications.
Surgery and anesthesia
The median duration of surgery was 97 min (IQR 85–113), median blood loss was 225 mL (IQR 200–315), and median intraoperative crystalloid load was 1,500 mL (IQR 1,000–2,000). Nineteen of 25 patients received an adjuvant spinal anesthetic with a median bupivacaine dose of 11.3 mg (IQR 10.5–12.0) and a median morphine dose of 0.1 mg (IQR 0.1–0.2). The median minimal alveolar concentration (MAC) of the volatile anesthetic was 0.5% (IQR 0.5–0.7), and the median MAC of nitrous oxide was 0.4% (IQR 0.4–0.6) in the 10 patients who were exposed to nitrous oxide. The median opioid doses were 2.6 mg (IQR 1.5–3.8) intravenous hydromorphone equivalents during surgery, 16.5 mg (IQR 13.1–27.4) after surgery and during hospital stay, and 9.0 mg (IQR 5.3–16.9) for up to six weeks after hospital discharge. The median time to hospital discharge was 3.1 days (IQR 3.0–3.8).
Clinical outcomes
Clinical outcomes were previously reported in detail.8 The median time required to half-maximum recovery from postoperative fatigue and resulting functional impairment was 10 days (IQR 6–15); this time varied widely among patients with a range of 0 to 36 days. The median time required to regress to mild functional impairment of the operated hip was 15 days (IQR 11–21); this time also varied widely from 2 to 42 days. The median time required to regress to mild pain was 10 days (IQR 6–18) with a range from 2 to 36 days. The median time required to half-maximum recovery from postoperative fatigue and resulting functional impairment did not correlate significantly with the time required to regress to mild functional impairment of the hip or the time required to regress to mild pain. The time required to regress to mild functional impairment of the hip and the time required to regress to mild pain were significantly correlated (R=0.54, p=5.00×10−3).
Evoked signaling responses in pre-surgical blood samples
Ligands selected for ex vivo stimulation of pre-surgical blood samples were those that were previously shown to evoke the cell-type specific signaling responses that were modulated by surgery (Table 1).8 All ligand-evoked signaling responses in pre-surgical samples are listed (see Table 2, Supplementary Digital Content 1, which is a table with the complete list of ligand-evoked signaling responses). Signal induction was highly significant with p-values ranging between 8.96 × 10−25 and 5.36 × 10−08. Of particular interest were the evoked signaling responses in pre-surgical samples that correlated significantly with clinical recovery parameters when measured in whole blood samples collected after surgery. In post-surgical samples, pSTAT3 signaling correlated with recovery from postoperative fatigue and resulting functional impairment, pCREB signaling correlated with recovery from functional impairment of the operated hip, and pNFκB signaling correlated with recovery from postoperative pain.8 All these responses were measured in subsets of CD14+ monocytes. Results of the ex vivo stimulation of STAT3, CREB, and NFκB in pre-surgical samples with IL-6 or LPS are graphically displayed in Figure 2. The results confirm that stimulation of pre-surgical samples with the selected ligands interrogated signaling pathways that were altered by surgery and correlated with clinical recovery parameters.
Table 2.
Partial Covariate Analysis
| Covariate | Classical dendritic cells | CD14+ Monocytes | ||
|---|---|---|---|---|
| R | p value | R | p value | |
|
Pre-surgical values for major clinical outcomes |
||||
| Surgical recovery scale | 0.675 | 1.81×10−5 | 0.652 | 5.38×10−5 |
| WOMAC function | 0.701 | 4.09×10−6 | 0.688 | 8.87×10−6 |
| WOMAC pain | 0.695 | 5.95×10−6 | 0.681 | 1.28×10−5 |
| Demographics | ||||
| Age | 0.731 | 5.07×10−7 | 0.706 | 3.00×10−6 |
| Sex | 0.684 | 1.11×10−5 | 0.650 | 6.10×10−5 |
| Body mass index | 0.701 | 4.05×10−6 | 0.688 | 8.76×10−6 |
| Anesthesia | ||||
| Adjuvant spinal anesthetic | 0.702 | 1.10×10−6 | 0.701 | 4.00×10−6 |
| Nitrous oxide | 0.697 | 8.35×10−6 | 0.681 | 2.04×10−5 |
| Surgery | ||||
| Duration | 0.700 | 4.29×10−6 | 0.723 | 9.07×10−7 |
| Blood loss | 0.654 | 7.39×10−5 | 0.657 | 6.41×10−5 |
Abbreviations: R, correlation coefficient; WOMAC, Western Ontario and McMaster Universities Arthritis Index scale.
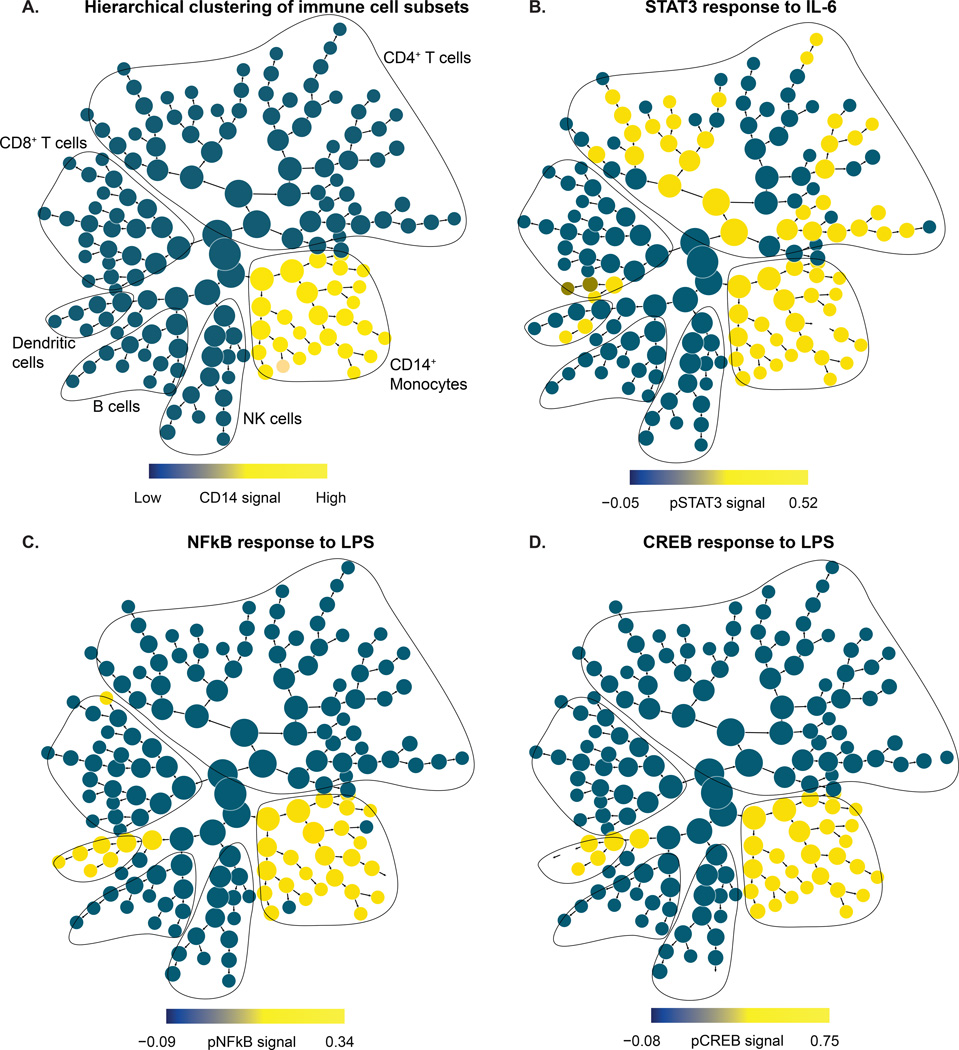
Figure 2. Evoked signaling responses in pre-surgical whole blood samples.
(A) The cluster hierarchy plot represents immune cell subsets across the entire peripheral immune system. The cluster hierarchy plot is annotated and contoured based on the expression of canonical lineage markers indicative of major immune cell subsets. As an example, the monocyte branch is highlighted in yellow based on the expression of the cell surface marker CD14. (B) STAT3 (phosphorylated signal transducer and activator of transcription 3) signaling response to IL-6 (interleukin 6): Individual clusters are colored based on the median signal induction of pSTAT3. A significant response was evoked in CD14+ monocytes, dendritic cells, CD4+ T cells, and a small fraction of CD8+ T cells. (C) NFκB (phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells) signaling response to LPS (lipopolysaccharide): Individual clusters are colored based on the median signal induction of pNFκB. A significant response was evoked in CD14+ monocytes and dendritic cells. (D) CREB (phosphorylated cAMP response element-binding protein) signaling response to LPS: Individual clusters are colored based on the median signal induction of pCREB. A significant response was evoked in CD14+ monocytes and dendritic cells.
Variability of evoked signaling responses in pre-surgical blood samples
Although evoked signaling responses in samples collected before surgery were directionally consistent, the magnitude of these signaling responses varied 3- to 5-fold among patients (Table 1 and Table 2, Supplementary Digital Content 1). This is illustrated in Figure 3 in plots of the arcsinh ratios of CD14+ monocyte responses to stimulation in individual patient samples. The results are reminiscent of the variability reported for pSTAT3, pCREB, and pNFκB signaling responses in CD14+ monocytes 1 hour to 24 hours after surgery; in the previous study, these three responses accounted for 40–60% of variability in clinical recovery.8 These results raise the question whether patient-specific evoked signaling responses in whole blood samples collected before surgery reflect differences in patients’ pre-surgical immune states that predict clinical recovery.
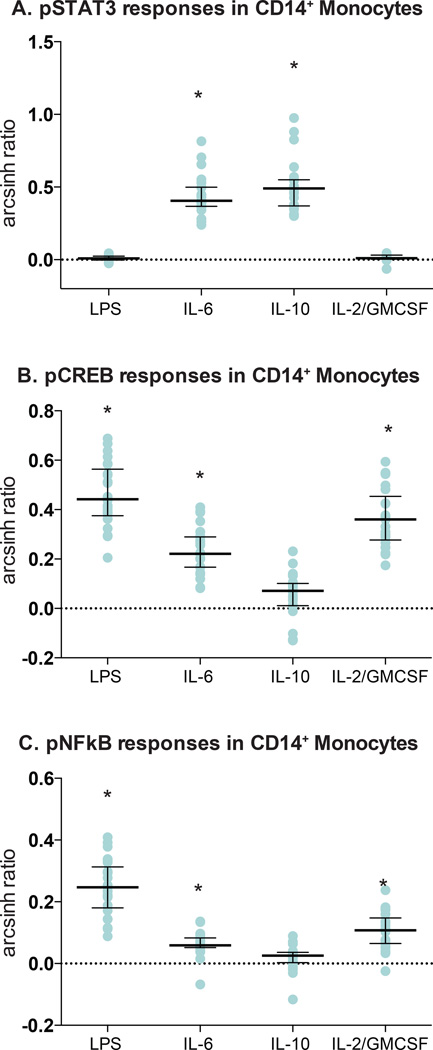
Figure 3. Variability of evoked signaling responses in pre-surgical whole blood samples.
Cell-type specific signaling responses were quantified in manually gated cell subsets. Values reflect the arcsinh ratio, i.e., the difference between arcsinh transformed values (inverse hyperbolic sine) obtained in unstimulated and stimulated conditions. Asterisks (*) denote statistical significance based on the Bonferroni-adjusted p value (p=8.92×10−4). (A) The pSTAT3 (phosphorylated signal transducer and activator of transcription 3) signal in CD14+ monocytes increased significantly in response to IL-6 (interleukin6; p=2.40×10−14) and IL-10 (interleukin 10; p=2.27×10−13), but not in response to LPS (lipopolysaccharide) or the combination of IL-2 (interleukin 2) and GMCSF (granulocyte monocyte colony-stimulating factor). The signaling response varied 3.5-fold among patients. (B) The pCREB (phosphorylated cAMP response element-binding protein) signal in CD14+ monocytes increased in response to LPS (p=1.22×10−15), the combination of IL-2 and GMCSF (p=4.80×10−14), and IL-6 (p=3.03×10−12), but not in response to IL-10. The signaling responses varied by 3.5- to 5.5-fold among patients. (C) The pNFκB (phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells) signal in CD14+ monocytes increased in response to LPS (p=4.95×10−13), the combination of IL-2 and GMCSF (p=1.65×10−9), and IL-6 (p=1.87×10−8), but not in response to IL-10. The signaling responses varied 5-fold among patients.
Evoked signaling responses in pre-surgical blood samples correlate with clinical recovery
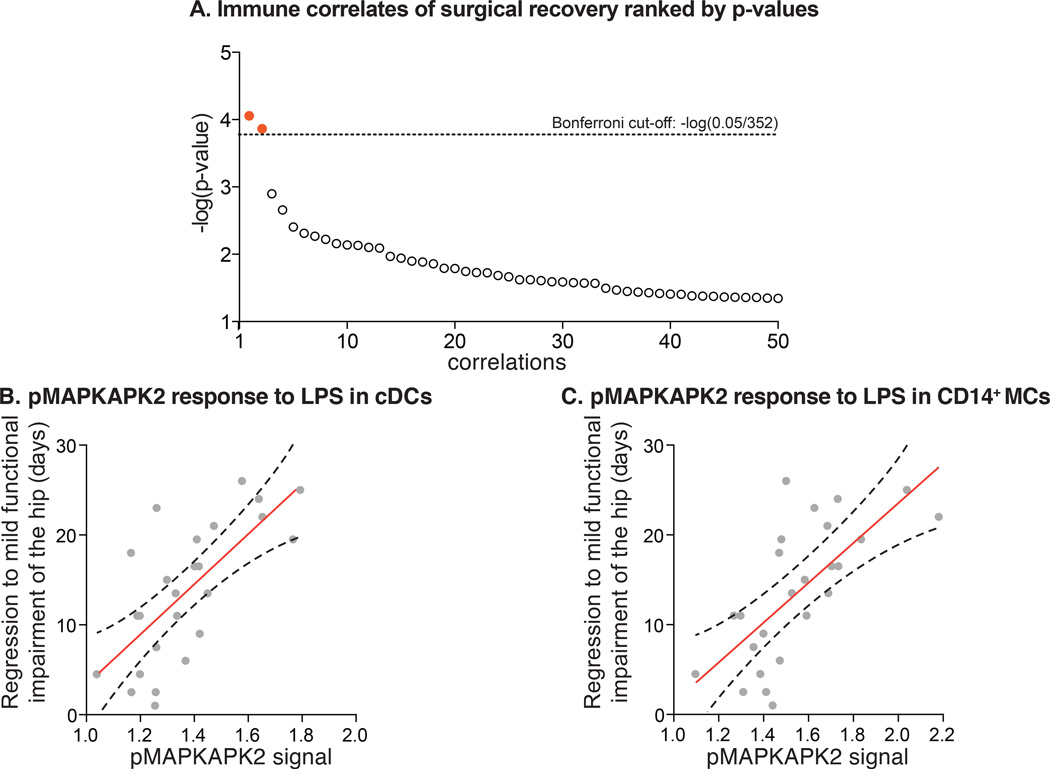
Patient-specific immune states before surgery were characterized by measuring 352 different immune parameters, i.e., response of 11 intracellular signaling proteins to 4 different ex vivo ligands separately assessed for 8 different immune cell types (Figure 4). Each immune parameter was regressed against the three clinical recovery parameters. Two correlates passed the stringent Bonferroni correction (Figure 5a). The pMAPKAPK2 signaling responses to LPS in classical dendritic cells (R=0.70) and in CD14+ monocytes (R=0.69) strongly correlated with the clinical recovery parameter “time to regress to mild functional impairment of the operated hip” (Figure 5b and 5c). A more pronounced signaling response was associated with prolonged recovery. These correlations remained significant when accounting for clinical covariates (Table 2) and explained about 50% of observed inter-patient variability. Multi-linear regression revealed that pMAPKAPK2 signaling in dendritic cells (p<0.001) and age (p=0.032) both correlated significantly with the clinical recovery parameter “time to regress to mild functional impairment of the operated hip”. pMAPKAPK2 signaling in CD14+ monocytes (p<0.001) but not age (p=0.053) was significantly correlated with this clinical outcome. The overall contribution of age relative to the pMAPKAPK2 signaling was small as indicated by beta-coefficients of 0.2 and 27.3 for dendritic cells, and 0.2 and 21.3 for CD14+ monocytes.
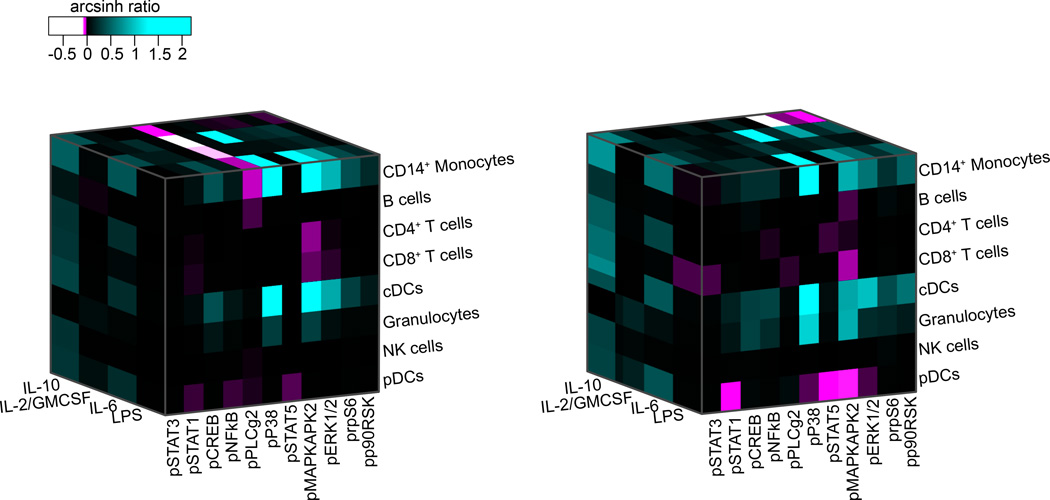
Figure 4. Patient-specific evoked signaling responses in myeloid cell subsets.
Patient-specific immune states before surgery were characterized along three axes including eight cell types, eleven signaling proteins, and the activity of these signaling proteins in response to four different extracellular ligands. Signaling activity is indicated by the color-coded arcsinh ratio, i.e., the difference between arcsinh transformed values (inverse hyperbolic sine) obtained in unstimulated and stimulated conditions. A total of 352 parameters described each patient’s immune state. Shown are the three-dimensional heat maps characterizing and differentiating the immune states of two exemplary patients. Abbreviations: IL-10, interleukin 10; IL-2, interleukin 2; GMCSF, granulocyte monocyte colony-stimulating factor; IL-6, interleukin 6; LPS, lipopolysaccharide; pSTAT1/3/5, phosphorylated signal transducer and activator of transcription 1/3/5; pCREB, phosphorylated cAMP response element-binding protein; pNFkB, phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells; pPLCg2, phosphorylated phospholipase C gamma 2; pP38, phosphorylated P38 map kinase; pMAPKAPK2, phosphorylated mitogen activated protein kinase-activated protein kinase-2; pERK1/2, phosphorylated extracellular regulated kinase 1/2; prpS6, phosphorylated ribosomal protein S6; pp90RSK, phosphorylated ribosomal S6 kinase; pDCs, plasmacytoid dendritic cells; cDCs, classical dendritic cells.
Figure 5. Immune correlates of functional impairment of the hip.
(A) P-values are plotted as the negative of the log base 10 for correlations between ligand-evoked intracellular signaling responses and clinical recovery parameters. The lowest 50 p-values are shown. The pMAPKAPK2 (phosphorylated mitogen activated protein kinase-activated protein kinase-2) response to LPS (lipopolysaccharide) in CD14+ monocytes (MCs) and classical dendritic cells (cDCs) passed the Bonferroni correction as depicted in red. (B) pMAPKAPK2 response to LPS in cDCs strongly correlated with functional recovery of the hip (R=0.70, p=9.17×10−5). (C) pMAPKAPK2 response to LPS in CD14+ MCs strongly correlated with functional recovery of the hip (R=0.69, p=1.39×10−4). Shown in red are the regression lines with adjacent 95% confidence intervals.
Eighteen significant correlations were identified when using a false discovery rate < 1%, a less stringent approach commonly applied to large data sets (|R| = 0.36–0.70, q<0.01; Table 3). Significant findings were limited to specific immune cell subsets, signaling proteins and stimulation conditions. Importantly, these correlations were restricted to innate immune cells (CD14+ monocytes, dendritic cells, and granulocytes) and concerned only five of the 11 examined signaling proteins. Significant correlations were identified between signaling activities in CD14+ monocytes, dendritic cells, and granulocytes and the two clinical recovery parameters 1) time required to regress to mild functional impairment of the operated hip, and 2) time required to regress to mild pain (Table 3). Two themes evolved. First, LPS-evoked signaling responses in CD14+ monocytes and classical dendritic cells, including pMAPKAPK2, pCREB, and prpS6, were correlated with a prolonged functional recovery of the operated hip and a slower resolution of postoperative pain. These findings are supported by intermediate to strong correlations (|R|=0.39–70). Second, IL-2/GMCSF-evoked signaling in plasmacytoid dendritic cells and granulocytes, including pERK, p90RSK and pMAPKAPK2, were correlated with shortened functional recovery of the operated hip. These findings are supported by correlations of intermediate strength (|R|=0.36–0.54). Taken together these data are consistent with the view that the interrogation of a patient’s immune state before surgery has significant predictive potential regarding a patient’s clinical recovery after surgery.
Table 3.
Significant Immune Correlates
| Cell type | Ligand | Signaling protein |
Arcsinh ratio (median and 95%-CI) |
Clinical outcome |
R |
|---|---|---|---|---|---|
| cDCs | LPS | pMAPKAPK2 | 1.336 (1.293 – 1.453) | Impaired hip function | 0.702 |
| CD14+ monocytes | LPS | pMAPKAPK2 | 1.500 (1.453 – 1.652) | Impaired hip function | 0.689 |
| pDCs | IL-2/GMCSF | pERK | 1.159 (0.947 – 1.203) | Impaired hip function | −0.544 |
| pDCs | IL-2/GMCSF | pP90RSK | 0.485 (0.388 – 0.569) | Impaired hip function | −0.517 |
| Granulocytes | IL-2/GMCSF | pMAPKAPK2 | 0.574 (0.516 – 0.647) | Impaired hip function | −0.498 |
| CD14+ monocytes | LPS | prpS6 | 0.576 (0.528 – 0.722) | Impaired hip function | 0.467 |
| CD14+ monocytes | LPS | pCREB | 0.442 (0.411 – 0.514) | Impaired hip function | 0.445 |
| Granulocytes | IL-2/GMCSF | pERK | 1.053 (0.886 – 1.114) | Impaired hip function | −0.442 |
| CD14+ monocytes | LPS | pMAPKAPK2 | 1.500 (1.453 – 1.652) | Pain | 0.415 |
| pDCs | IL-2/GMCSF | prpS6 | 0.875 (0.725 – 0.924) | Impaired hip function | −0.410 |
| CD14+ monocytes | LPS | pCREB | 0.442 (0.411 – 0.514) | Pain | 0.409 |
| Granulocytes | IL-2/GMCSF | pP90RSK | 0.269 (0.211 – 0.306) | Impaired hip function | −0.408 |
| pDCs | IL-2/GMCSF | pMAPKAPK2 | 0.361 (0.292 – 0.389) | Impaired hip function | −0.400 |
| CD14+ monocytes | LPS | prpS6 | 0.576 (0.528 – 0.722) | Pain | 0.392 |
| pDCs | IL-2/GMCSF | pCREB | 0.635 (0.552 – 0.735) | Impaired hip function | −0.381 |
| CD14+ monocytes | IL-2/GMCSF | pP90RSK | 0.397 (0.301 – 0.432) | Impaired hip function | −0.376 |
| cDCs | IL-2/GMCSF | pP90RSK | 0.172 (0.119 – 0.212) | Impaired hip function | −0.372 |
| cDCs | IL-2/GMCSF | pERK | 0.467 (0.392 – 0.623) | Impaired hip function | −0.367 |
Abbreviations: cDCs, classical dendritic cells; pDCs, plasmacytoid dendritic cells. GMCSF, Granulocyte macrophage colony-stimulating factor; IL-2, interleukin 2; LPS, lipopolysaccharide; pCREB, phosphorylated cAMP response element-binding protein; pERK1/2, phosphorylated extracellular regulated kinase 1/2; pMAPKAPK2, phosphorylated mitogen activated protein kinase-activated protein kinase-2; pp90RSK, phosphorylated ribosomal S6 kinase; prpS6, phosphorylated ribosomal protein S6. R, correlation coefficient.
DISCUSSION
The results of this study in patients undergoing primary hip arthroplasty suggest that patient-specific immune states before surgery are a key determinant of surgical recovery. A patient’s pre-surgical immune state was interrogated in peripheral blood samples by assessing ligand-evoked signaling responses across all major immune cell subsets with mass cytometry at single-cell resolution.10 The analysis revealed patient-specific immune states that contained strong correlates of surgical recovery. Immune correlates accounted for 15–50% of observed patient variability in functional recovery and regression of pain.
The study interrogated cell-specific intracellular signaling pathways implicated in the endogenous immune response to surgery, a subset of which—namely pSTAT3, pCREB, and pNFkB measured in CD14+ monocytes shortly after surgery—have previously been shown to correlate strongly with surgical recovery.8 In the current analysis, eighteen pre-surgical signaling responses correlated significantly with clinical recovery parameters; two particularly strong correlates were the pMAPKAPK2 response to LPS in CD14+ monocytes and classical dendritic cells (Table 3). Each correlate accounted for almost 50% of the variability associated with the speed at which patients recovered from functional impairment of the operated hip. Thus, among all measured immune responses in adaptive and innate immune cells, evoked signaling responses in innate cells of myeloid lineage were the strongest indicators of the recovery process. In post-surgical samples, the strongest immune correlates of surgical recovery were also signaling events in monocyte subsets.8 Current results dovetail with a large body of work implicating a critical role of monocyte subsets and dendritic cells in the immune response to traumatic injury and surgical recovery.20–27
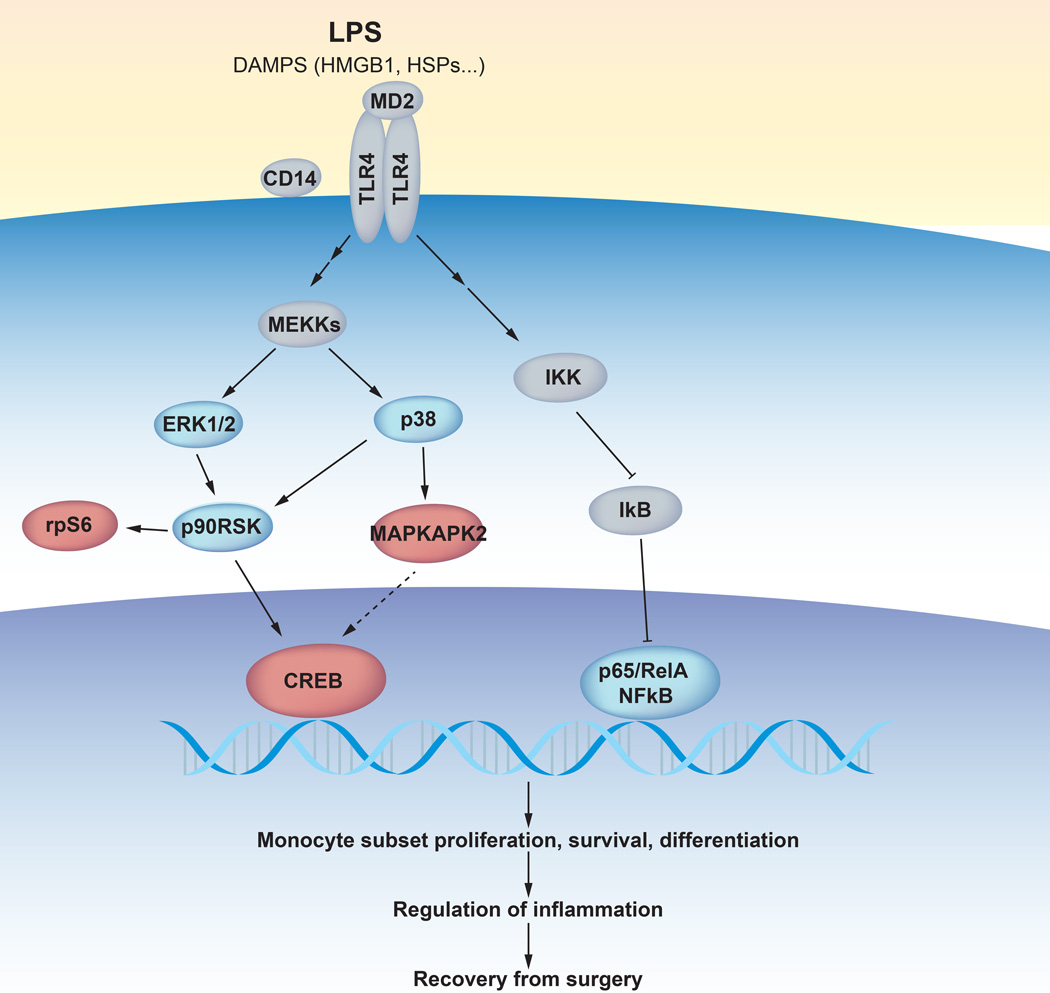
The findings integrate with the well-established role of the TLR4 signaling pathway in the innate immune response to traumatic injury.28 MAPKAPK2 is a component of the p38 MAP kinase pathway, which mediates TLR4 priming in injury.29,30 Injured cells release danger-associated molecular pattern molecules (DAMPs) that alert the immune system to the presence of tissue damage.31 Certain DAMPs, including high mobility group protein B1 (HMGB1) and heat shock proteins (HSPs), act principally on innate immune cells by binding to TLR4, the primary receptor for LPS.32–35 Upon TLR4 activation, p38 phosphorylates MAPKAPK2, mitogen and stress-activated kinases (MSK1/2), and ribosomal S6 kinase (RSK).30,36 This signaling cascade converges on the phosphorylation of CREB and ribosomal protein S6, which regulate transcriptional and translational mechanisms that are essential for the survival, proliferation and differentiation of innate immune cells.36,37
Recovery from functional impairment of the operated hip was not only correlated with the pMAPKAPK2 response to LPS in CD14+ monocytes but also with the prpS6 and pCREB responses in CD14+ monocytes (Table 3). These findings are consistent with previous results documenting that levels of pMAPKAPK2, prpS6, and pCREB in CD14+ monocytes at 1 hour after surgery correlated with recovery from functional impairment of the hip (Table 4).8 Furthermore, the pMAPKAPK2, prpS6, and pCREB responses to LPS in pre-surgical samples correlated with respective pMAPKAPK2, pCREB, and prpS6 signals measured 1 hour after surgery (Table 4).These results suggest that the LPS-evoked activation of a TLR4-dependent signaling network engaging MAPKAPK2, CREB, and rpS6 in CD14+ monocytes recapitulates aspects of surgery-evoked mobilization of the same signaling network. As pMAPKAPK2, pCREB, and prpS6 in interrogated pre-surgical and post-surgical samples correlated with the same clinical functional parameter of recovery, the activation of an entire signaling network downstream of TLR4, rather than isolated signaling activities, may determine functional recovery (Figure 6).
Table 4.
Immune Correlates Pre- and Post-surgery
| Signaling protein(1) |
Pre-surgery signaling versus hip function |
Post-surgery signaling versus hip function |
Pre-surgery versus post- surgery signaling |
|||
|---|---|---|---|---|---|---|
| R | p value | R | p value | R | p value | |
| pMAPKAPK2 | 0.689 | 1.39×10−4 | 0.465 | 1.91×10−2 | 0.570 | 2.87×10−3 |
| pCREB | 0.445 | 2.59×10−2 | 0.569 | 2.99×10−3 | 0.589 | 1.93×10−3 |
| prpS6 | 0.467 | 1.86×10−2 | 0.591 | 1.88×10−3 | 0.622 | 8.87×10−4 |
Values for pre-surgery signaling are arcsinh ratios in CD14+ monocytes for LPS-stimulated and control samples, and values for post-surgery signaling are arcsinh ratios in CD14+ monocytes for samples collected before surgery and 1 hour after surgery.
Abbreviations: pCREB, phosphorylated cAMP response element-binding protein; pMAPKAPK2, phosphorylated mitogen activated protein kinase-activated protein kinase-2; prpS6, phosphorylated ribosomal protein S6. R, correlation coefficient.
Figure 6. A model summarizing the TLR4-dependent signaling responses implicated in surgical recovery.
In response to tissue damage, damage-associated molecular patterns (DAMPs) molecules including alarmins such as HMGB1 (high-mobility group protein B1) and HSPs (heat shock proteins) bind to and activate the TLR4 receptor (toll-like receptor 4) on the surface of innate immune cells. The TLR4 signaling pathway is also induced by binding of LPS (lipopolysaccharide) to the TLR4 receptor and to co-receptors CD14 (cluster of differentiation 14) and MD2 (myeloid differentiation factor 2). The TLR4 receptor engages a complex signaling cascade (only partially represented) leading to the activation of the p38 (phosphorylated P38 mitogen-activated protein kinase) and ERK/MAP (extracellular regulated kinase and mitogen activated protein kinase) and the NFκB (phosphorylated nuclear factor kappa-light-chain-enhancer of activated B cells) signaling pathways. Signaling proteins that changed significantly in response to surgery are indicated by blue circles. A subset of signaling proteins modulated after surgery (MAPKAPK2, mitogen-activated protein kinase-activated protein kinase 2; rpS6, ribosomal protein S6; CREB, cAMP response element-binding protein) overlapped with pre-surgical signaling responses that correlated with surgical recovery parameters and are indicated by red circles. Other abbreviations: MEKKs, mitogen activated protein kinase kinase kinase; IKK, IκB kinase; IκB, inhibitor of NFκB.
The analysis revealed other interesting, though somewhat less robust immune correlates. In particular, the pMAPKAPK2, pCREB, and prpS6 responses to LPS in CD14+ monocytes correlated with the speed at which postoperative pain resolved and accounted for 16% of observed variance. Such association may be expected, as the resolution of pain and the recovery from functional impairment of the operated hip are interdependent outcomes. This finding is also reminiscent of the strong relationship between the pNFκB signal measured 1 hour after surgery in CD14+ monocytes and the resolution of pain as all signaling molecules are components of the TLR4 signaling pathway (Figure 6).8 The pERK, p90RSK, and pMAPKAPK2 responses to IL-2/GMCSF stimulation in plasmacytoid dendritic cells (pDCs) and granulocytes were correlated with the speed at which patients recovered from functional impairment of the operated hip. GMCSF promotes survival and proliferation in pDCs and granulocytes via several interconnected pathways including the ERK/MAPK, PI3K, NFκB, and JAK2/STAT5 pathways.38 The suggestion that a predisposition for pDCs and granulocytes to mount a robust response to GMCSF may hasten the recovery process warrants further investigation.
Identified immune correlates provide the mechanistic framework for developing a diagnostic test that will predict the speed of functional recovery after hip arthroplasty. In a next step, present findings will need to be validated in a larger and independent patient cohort and the response elements within the TLR4 signaling pathway in CD14+ monocytes that best predict the speed of functional recovery will need to be defined. This will then allow reducing the number of parameters required in a clinical test by only including the most predictive parameters, and permit switching from the complex 50-parameter mass cytometry platform to a standard 4–6 parameter fluorescence flow cytometry platform readily available in clinical laboratories. A clinical test could then be performed fairly quickly in freshly collected whole blood without further requirements for sample freezing, storing or barcoding. While samples in this study were collected 1 hour prior to surgery, in a clinical setting sampling would likely occur during a pre-operative assessment visit days before surgery to guide perioperative management.
The study has certain limitations. First, the clinical indices of surgical recovery are derived from the same patient cohort as in our first study.8 Second, this patient cohort suffered from minimal comorbidities and underwent the same surgical procedure to eliminate many confounding variables and enable the identification of immune correlates. As such results may not extrapolate to other types of surgeries or patient cohorts with complex comorbidities. Third, while convergent findings in the pre- and post-surgical analyses provide validation for reported immune correlates of functional recovery and resolution of pain, all clinical outcomes were questionnaire-based. Future studies will need to broaden clinical assessments and include more objective outcome tools to better understand the overall impact of the current findings on short and long-term function and pain, as well as other outcomes domains including cognitive function and sleep. For example, WOMAC-based based functional results reported in this study should be examined in the context of results obtained with standardized performance-based measures of physical activity and the continuous monitoring of physical activity with wearable technology.39,40 Similarly, quantification of pain during standardized physical tasks may provide more robust outcomes data that could actually strengthen the correlation between immune parameters and clinical outcomes. Forth, none of the evoked signaling responses correlated significantly with recovery from fatigue and resulting functional impairment. In our previous study, changes in STAT3 signaling between 1 hour and 24 hours after surgery strongly correlated with recovery from fatigue. The signaling differences between time points after surgery may be more challenging to recapitulate in a pre-surgical in vitro assay. Finally, while mass cytometry currently enables the simultaneous detection of up to 50 antigens per cell, the technology nonetheless requires an a priori selection of a subset of intracellular signaling proteins. Only a fraction of the downstream effectors of TLR4 signaling were therefore analyzed in the present study. There is the possibility that relevant intracellular signals remained undetected. LPS or trauma-induced TLR4 agonists such as HMGB1 modulate the function of over 30 intracellular proteins in innate immune cells.41 Future studies will expand on this first report and comprehensively assess the predictive value of the entire TLR4 signaling network on parameters of surgical recovery.
Despite significant interest in identifying predictors of surgical recovery ahead of surgery, current clinical and molecular variables with predictive potential typically account for less than 10–15% of observed patient variability.22,42–49 Most studies have focused on metrics that may predict hospital length of stay and the incidence of postoperative complications and mortality; patient-centered outcomes, including functional impairment, pain, and fatigue, have received less attention.2 Our findings suggest that the comprehensive assessment of a patient’s preoperative immune state may add significant value to predict important surgical recovery parameters as reported immune correlates accounted for up to 50% of observed patient variability. The a priori identification of patients at risk for a protracted and/or complicated recovery will allow for patient-tailored and cost-effective strategies to mitigate such risks. Mitigation might include postponing procedures to coincide with a more favorable recovery profile and tailoring intervention to improve the “readiness” to recover from surgical trauma to the individual patient. Examples of interventions requiring further validation include preoperative exercise and/or stress-reduction programs, dietary supplementation, and administration of immune-modulating medications.9,50–55
The ability to detect strong immune correlates was due to the application of a recently developed mass cytometry protocol, which enabled functional interrogation of precisely phenotyped cells with single-cell resolution.10 Previous studies probing for molecular markers of recovery may have missed strong correlates as these studies relied on bulk analysis, precluded detailed identification of cell subsets, or could not measure functional attributes of cell subsets.22,42,43 While a high-dimensional mass cytometry assay enabled the detection of strong correlates of surgical recovery, identified immune correlates can readily be assessed with traditional and widely available clinical flow cytometry platforms. This prospect holds significant promise for the development and validation of a diagnostic test that will help predict surgical recovery.
Supplementary Material
Final Box Summary Statement.
What we already know about this topic
Recovery from functional impairment, pain, and fatigue following surgery is highly variable
Examination of immune cell type-specific states and activation after surgery accounted for much of the inter-individual differences in these outcomes, but whether preoperative samples would also be predictive is unknown
What this article tells us that is new
In an analysis of the same group of orthopedic surgery patients from the postoperative sample study, preoperative immune state as assessed by mass cytometry of blood samples was predictive of recovery across several domains, with TLR-4 signaling in CD14+ monocytes accounted for 50% of observed variance
Acknowledgements
Funding disclosure:
G.K.F. is supported by the Stanford Bio-X graduate research fellowship (Stanford, CA, USA) and the US National Institute of Health (T32GM007276; Bethesda, MD, USA). B.G. is supported by the US National Institute of Health (1K23GM111657-01,T32GM089626). N.A. is supported by an Ann Schreiber Mentored Investigator Award from the Ovarian Cancer Research Fund (OCRF 292495; New York, NY, USA), a Canadian Institute of Health Research Postdoctoral Fellowship (CIHR 321510; Ottawa, ON, Canada), and an International Society for Advancement of Cytometry Scholarship (Bethesda, MD, USA). This work was supported by grants from the US National Institutes of Health (NIH) (U19 AI057229, U54CA149145, N01-HV-00242, 1U19AI100627, 5R01AI07372405, R01CA184968, 1 R33 CA183654, R33 CA183692, 1R01GM10983601, 201303028, 1R01NS08953301), NIH-the Baylor Research Institute (41000411217; Dallas, TX, USA), the NIH-Northrop Grumman Corp. (7500108142; Falls Church, CA, USA), the California Institute for Regenerative Medicine (CIRM; San Francisco, CA, USA) (DR1-01477), the US Department of Defense (OC110674; Washington, DC, USA), the European Commission (Health.2010.1.2-1; Brussels, Belgium), the US Food and Drug Administration (HHSF223201210194C; Silver Spring, MD, USA), the Bill and Melinda Gates Foundation (OPP 1017093, OPP1113682; Seattle, WA, USA), the Alliance for Lupus Research (New York, NY, USA), the Lymphoma Research Foundation (New York, NY, USA), the Entertainment Industry Foundation (National Women’s Cancer Research Alliance grant; Los Angeles, CA, USA) and the Stanford Department of Anesthesiology, Perioperative and Pain Medicine (Stanford, CA, USA).
We would like to acknowledge the surgeons Dr. William Maloney (M.D., Department of Orthopedic Surgery, Stanford University, Stanford, CA, USA), Dr. James Huddleston (M.D., Department of Orthopedic Surgery, Stanford University, Stanford, CA, USA), and Dr. Stuart Goodman (M.D., Ph.D., Department of Orthopedic Surgery, Stanford University, Stanford, CA, USA) for their assistance in the conduct of the clinical study. We would like to acknowledge Erica Savig (M.A., B.S., Department of Cancer Biology, Stanford University, Stanford, CA, USA) for figure design contributions.
Footnotes
Conflicts of Interest: G.P.N. has a personal financial interest in Fluidigm (South San Francisco, CA, USA), the manufacturer of the mass cytometer used in this manuscript. All other authors have no conflict of interest.
References
- 1.Vetter TR, Ivankova NV, Goeddel LA, McGwin G, Jr, Pittet JF, Group UABPSH. An analysis of methodologies that can be used to validate if a perioperative surgical home improves the patient-centeredness, evidence-based practice, quality, safety, and value of patient care. Anesthesiology. 2013;119:1261–1274. doi: 10.1097/ALN.0b013e3182a8e9e6. [DOI] [PubMed] [Google Scholar]
- 2.Neville A, Lee L, Antonescu I, Mayo NE, Vassiliou MC, Fried GM, Feldman LS. Systematic review of outcomes used to evaluate enhanced recovery after surgery. Br J Surg. 2014;101:159–170. doi: 10.1002/bjs.9324. [DOI] [PubMed] [Google Scholar]
- 3.Vetter TR, Boudreaux AM, Jones KA, Hunter JM, Jr, Pittet JF. The perioperative surgical home: how anesthesiology can collaboratively achieve and leverage the triple aim in health care. Anesth Analg. 2014;118:1131–1136. doi: 10.1213/ANE.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 4.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–2481. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 5.Bisgaard T, Klarskov B, Rosenberg J, Kehlet H. Factors determining convalescence after uncomplicated laparoscopic cholecystectomy. Arch Surg. 2001;136:917–921. doi: 10.1001/archsurg.136.8.917. [DOI] [PubMed] [Google Scholar]
- 6.Bisgaard T, Stockel M, Klarskov B, Kehlet H, Rosenberg J. Prospective analysis of convalescence and early pain after uncomplicated laparoscopic fundoplication. Br J Surg. 2004;91:1473–1478. doi: 10.1002/bjs.4720. [DOI] [PubMed] [Google Scholar]
- 7.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–1928. doi: 10.1016/S0140-6736(03)14966-5. [DOI] [PubMed] [Google Scholar]
- 8.Gaudilliere B, Fragiadakis GK, Bruggner RV, Nicolau M, Finck R, Tingle M, Silva J, Ganio EA, Yeh CG, Maloney WJ, Huddleston JI, Goodman SB, Davis MM, Bendall SC, Fantl WJ, Angst MS, Nolan GP. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med. 2014;6:255ra131. doi: 10.1126/scitranslmed.3009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, Liberman AS, Stein B, Charlebois P, Feldman LS, Carli F. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937–947. doi: 10.1097/ALN.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 10.Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK. A deep profiler’s guide to cytometry. Trends Immunol. 2012;33:323–332. doi: 10.1016/j.it.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendall SC, Simonds EF, Qiu P, Amir el AD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe’er D, Tanner SD, Nolan GP. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paddison JS, Sammour T, Kahokehr A, Zargar-Shoshtari K, Hill AG. Development and validation of the Surgical Recovery Scale (SRS) J Surg Res. 2011;167:e85–e91. doi: 10.1016/j.jss.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 13.McAuley DF. GlobalrpH - Opioid Analgesic Converter. http://www.globalrph.com/narcoticonv.htm (last accessed 06/05/2015).
- 14.Krutzik PO, Crane JM, Clutter MR, Nolan GP. High-content single-cell drug screening with phosphospecific flow cytometry. Nat Chem Biol. 2008;4:132–142. doi: 10.1038/nchembio.2007.59. [DOI] [PubMed] [Google Scholar]
- 15.Bodenmiller B, Zunder ER, Finck R, Chen TJ, Savig ES, Bruggner RV, Simonds EF, Bendall SC, Sachs K, Krutzik PO, Nolan GP. Multiplexed mass cytometry profiling of cellular states perturbed by small-molecule regulators. Nat Biotechnol. 2012;30:858–867. doi: 10.1038/nbt.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behbehani GK, Thom C, Zunder ER, Finck R, Gaudilliere B, Fragiadakis GK, Fantl WJ, Nolan GP. Transient partial permeabilization with saponin enables cellular barcoding prior to surface marker staining. Cytometry A. 2014;85:1011–1019. doi: 10.1002/cyto.a.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finck R, Simonds EF, Jager A, Krishnaswamy S, Sachs K, Fantl W, Pe’er D, Nolan GP, Bendall SC. Normalization of mass cytometry data with bead standards. Cytometry A. 2013;83:483–494. doi: 10.1002/cyto.a.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zunder ER, Finck R, Behbehani GK, Amir el AD, Krishnaswamy S, Gonzalez VD, Lorang CG, Bjornson Z, Spitzer MH, Bodenmiller B, Fantl WJ, Pe’er D, Nolan GP. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat Protoc. 2015;10:316–333. doi: 10.1038/nprot.2015.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy TJ, Paterson HM, Mannick JA, Lederer JA. Injury, sepsis, and the regulation of Toll-like receptor responses. J Leukoc Biol. 2004;75:400–407. doi: 10.1189/jlb.0503233. [DOI] [PubMed] [Google Scholar]
- 21.Wakefield CH, Carey PD, Foulds S, Monson JR, Guillou PJ. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br J Surg. 1993;80:205–209. doi: 10.1002/bjs.1800800224. [DOI] [PubMed] [Google Scholar]
- 22.Handy JM, Scott AJ, Cross AM, Sinha P, O’Dea KP, Takata M. HLA-DR expression and differential trafficking of monocyte subsets following low to intermediate risk surgery. Anaesthesia. 2010;65:27–35. doi: 10.1111/j.1365-2044.2009.06161.x. [DOI] [PubMed] [Google Scholar]
- 23.Ditschkowski M, Kreuzfelder E, Rebmann V, Ferencik S, Majetschak M, Schmid EN, Obertacke U, Hirche H, Schade UF, Grosse-Wilde H. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg. 1999;229:246–254. doi: 10.1097/00000658-199902000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hershman MJ, Cheadle WG, Wellhausen SR, Davidson PF, Polk HC., Jr Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77:204–207. doi: 10.1002/bjs.1800770225. [DOI] [PubMed] [Google Scholar]
- 25.Tarnok A, Bocsi J, Pipek M, Osmancik P, Valet G, Schneider P, Hambsch J. Preoperative prediction of postoperative edema and effusion in pediatric cardiac surgery by altered antigen expression patterns on granulocytes and monocytes. Cytometry. 2001;46:247–253. doi: 10.1002/cyto.1135. [DOI] [PubMed] [Google Scholar]
- 26.Degos V, Vacas S, Han Z, van Rooijen N, Gressens P, Su H, Young WL, Maze M. Depletion of bone marrow-derived macrophages perturbs the innate immune response to surgery and reduces postoperative memory dysfunction. Anesthesiology. 2013;118:527–536. doi: 10.1097/ALN.0b013e3182834d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumitriu IE, Baruah P, Valentinis B, Voll RE, Herrmann M, Nawroth PP, Arnold B, Bianchi ME, Manfredi AA, Rovere-Querini P. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–7515. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 28.Paterson HM, Murphy TJ, Purcell EJ, Shelley O, Kriynovich SJ, Lien E, Mannick JA, Lederer JA. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol. 2003;171:1473–1483. doi: 10.4049/jimmunol.171.3.1473. [DOI] [PubMed] [Google Scholar]
- 29.Maung AA, Fujimi S, Miller ML, MacConmara MP, Mannick JA, Lederer JA. Enhanced TLR4 reactivity following injury is mediated by increased p38 activation. J Leukoc Biol. 2005;78:565–573. doi: 10.1189/jlb.1204698. [DOI] [PubMed] [Google Scholar]
- 30.Arthur JS, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- 31.Chan JK, Roth J, Oppenheim JJ, Tracey KJ, Vogl T, Feldmann M, Horwood N, Nanchahal J. Alarmins: awaiting a clinical response. J Clin Invest. 2012;122:2711–2719. doi: 10.1172/JCI62423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker TA, Romero J, Bach HHt, Strom JA, Gamelli RL, Majetschak M. Systemic release of cytokines and heat shock proteins in porcine models of polytrauma and hemorrhage*. Crit Care Med. 2012;40:876–885. doi: 10.1097/CCM.0b013e318232e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin C, Cleveland JC, Ao L, Li J, Zeng Q, Fullerton DA, Meng X. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: the proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Mol Med. 2014;20:280–289. doi: 10.2119/molmed.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen MJ, Brohi K, Calfee CS, Rahn P, Chesebro BB, Christiaans SC, Carles M, Howard M, Pittet JF. Early release of high mobility group box nuclear protein 1 after severe trauma in humans: role of injury severity and tissue hypoperfusion. Crit Care. 2009;13:R174. doi: 10.1186/cc8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 37.Park JM, Greten FR, Wong A, Westrick RJ, Arthur JS, Otsu K, Hoffmann A, Montminy M, Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis--CREB and NF-kappaB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 38.van de Laar L, van den Bosch A, Boonstra A, Binda RS, Buitenhuis M, Janssen HL, Coffer PJ, Woltman AM. PI3K–PKB hyperactivation augments human plasmacytoid dendritic cell development and function. Blood. 2012;120:4982–4991. doi: 10.1182/blood-2012-02-413229. [DOI] [PubMed] [Google Scholar]
- 39.Dobson F, Hinman RS, Roos EM, Abbott JH, Stratford P, Davis AM, Buchbinder R, Snyder-Mackler L, Henrotin Y, Thumboo J, Hansen P, Bennell KL. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042–1052. doi: 10.1016/j.joca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 40.Cook DJ, Thompson JE, Prinsen SK, Dearani JA, Deschamps C. Functional recovery in the elderly after major surgery: assessment of mobility recovery using wireless technology. Ann Thorac Surg. 2013;96:1057–1061. doi: 10.1016/j.athoracsur.2013.05.092. [DOI] [PubMed] [Google Scholar]
- 41.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 42.Rosenberger PH, Ickovics JR, Epel E, Nadler E, Jokl P, Fulkerson JP, Tillie JM, Dhabhar FS. Surgical stress-induced immune cell redistribution profiles predict short-term and long-term postsurgical recovery. A prospective study. J Bone Joint Surg Am. 2009;91:2783–2794. doi: 10.2106/JBJS.H.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall GM, Peerbhoy D, Shenkin A, Parker CJ, Salmon P. Relationship of the functional recovery after hip arthroplasty to the neuroendocrine and inflammatory responses. Br J Anaesth. 2001;87:537–542. doi: 10.1093/bja/87.4.537. [DOI] [PubMed] [Google Scholar]
- 44.Shin JH, Worni M, Castleberry AW, Pietrobon R, Omotosho PA, Silberberg M, Ostbye T. The application of comorbidity indices to predict early postoperative outcomes after laparoscopic Roux-en-Y gastric bypass: a nationwide comparative analysis of over 70,000 cases. Obes Surg. 2013;23:638–649. doi: 10.1007/s11695-012-0853-3. [DOI] [PubMed] [Google Scholar]
- 45.Contrada RJ, Goyal TM, Cather C, Rafalson L, Idler EL, Krause TJ. Psychosocial factors in outcomes of heart surgery: the impact of religious involvement and depressive symptoms. Health Psychol. 2004;23:227–238. doi: 10.1037/0278-6133.23.3.227. [DOI] [PubMed] [Google Scholar]
- 46.Kim SW, Han HS, Jung HW, Kim KI, Hwang DW, Kang SB, Kim CH. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg. 2014;149:633–640. doi: 10.1001/jamasurg.2014.241. [DOI] [PubMed] [Google Scholar]
- 47.Visnjevac O, Lee J, Pourafkari L, Dosluoglu HH, Nader ND. Functional capacity as a significant independent predictor of postoperative mortality for octogenarian ASA-III patients. J Gerontol A Biol Sci Med Sci. 2014;69:1229–1235. doi: 10.1093/gerona/glu062. [DOI] [PubMed] [Google Scholar]
- 48.Zhang H, Zhang L, Zhu K, Shi B, Yin Y, Zhu J, Yue D, Zhang B, Wang C. Prognostic Significance of Combination of Preoperative Platelet Count and Neutrophil-Lymphocyte Ratio (COP-NLR) in Patients with Non-Small Cell Lung Cancer: Based on a Large Cohort Study. PLoS One. 2015;10:e0126496. doi: 10.1371/journal.pone.0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ackland GL, Scollay JM, Parks RW, de Beaux I, Mythen MG. Pre-operative high sensitivity C-reactive protein and postoperative outcome in patients undergoing elective orthopaedic surgery. Anaesthesia. 2007;62:888–894. doi: 10.1111/j.1365-2044.2007.05176.x. [DOI] [PubMed] [Google Scholar]
- 50.de la Motte L, Kehlet H, Vogt K, Nielsen CH, Groenvall JB, Nielsen HB, Andersen A, Schroeder TV, Lonn L. Preoperative methylprednisolone enhances recovery after endovascular aortic repair: a randomized, double-blind, placebo-controlled clinical trial. Ann Surg. 2014;260:540–548. doi: 10.1097/SLA.0000000000000895. discussion 548-9. [DOI] [PubMed] [Google Scholar]
- 51.Lunn TH, Andersen LO, Kristensen BB, Husted H, Gaarn-Larsen L, Bandholm T, Ladelund S, Kehlet H. Effect of high-dose preoperative methylprednisolone on recovery after total hip arthroplasty: a randomized, double-blind, placebo-controlled trial. Br J Anaesth. 2013;110:66–73. doi: 10.1093/bja/aes345. [DOI] [PubMed] [Google Scholar]
- 52.Evans DC, Martindale RG, Kiraly LN, Jones CM. Nutrition optimization prior to surgery. Nutr Clin Pract. 2014;29:10–21. doi: 10.1177/0884533613517006. [DOI] [PubMed] [Google Scholar]
- 53.Plank LD, Mathur S, Gane EJ, Peng SL, Gillanders LK, McIlroy K, Chavez CP, Calder PC, McCall JL. Perioperative immunonutrition in patients undergoing liver transplantation: a randomized double-blind trial. Hepatology. 2015;61:639–647. doi: 10.1002/hep.27433. [DOI] [PubMed] [Google Scholar]
- 54.Kahokehr A, Broadbent E, Wheeler BR, Sammour T, Hill AG. The effect of perioperative psychological intervention on fatigue after laparoscopic cholecystectomy: a randomized controlled trial. Surg Endosc. 2012;26:1730–1736. doi: 10.1007/s00464-011-2101-7. [DOI] [PubMed] [Google Scholar]
- 55.Fassl SK, Austermann J, Papantonopoulou O, Riemenschneider M, Xue J, Bertheloot D, Freise N, Spiekermann C, Witten A, Viemann D, Kirschnek S, Stoll M, Latz E, Schultze JL, Roth J, Vogl T. Transcriptome assessment reveals a dominant role for TLR4 in the activation of human monocytes by the alarmin MRP8. J Immunol. 2015;194:575–583. doi: 10.4049/jimmunol.1401085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.