Abstract
Functional overviews of cholinergic mechanisms in the cerebral cortex have traditionally focused on the release of acetylcholine with modulator and transmitter effects. Recently, however, data have emerged that extend the role of acetylcholine and cholinergic innervations to a range of housekeeping and metabolic functions. These include regulation of amyloid precursor protein (APP) processing with production of amyloid β (Aβ) and other APP fragments and control of the phosphorylation of microtubule-associated protein (MAP) tau. Evidence has been also presented for receptor-ligand like interactions of cholinergic receptors with soluble Aβ peptide and MAP tau, with modulator and signaling effects. Moreover, high-affinity binding of Aβ to the neurotrophin receptor p75 (p75NTR) enriched in basalo-cortical cholinergic projections has been implicated in clearance of Aβ and nucleation of amyloid plaques. Here, we critically evaluate these unorthodox cholinergic mechanisms and discuss their role in neuronal physiology and the biology of Alzheimer's disease.
Keywords: acetylcholine, amyloid β, tau protein, p75 neurotrophin receptor, volume transmission, Alzheimer's disease
Background
Cholinergic projections to the cerebral cortex and hippocampal formation arise from the basal forebrain (BF) and form one of the largest modulator systems of the brain. Through distributed innervations, they supply acetylcholine (ACh) to the entire cerebral mantle, modulating the activity of neurons and neural networks. Research over recent years has shed light on the highly complex organization and functionality of cholinergic innervations, in keeping with both the transmitter and neuromodulator roles of ACh (Munoz and Rudy 2014; Zaborszky and others 2015). Ample evidence supporting the major role for cholinergic mechanisms in an array of cognitive processes has been obtained, with progressive depletion of cortical and hippocampal ACh and loss of cholinergic synapses documented in the course of normal aging and especially during Alzheimer's disease (AD) and related dementias (Arendt and others 1983; Mesulam 2004). Released from cholinergic terminals and varicosities, ACh exerts its influence on neurons and synapses via several species of nicotinic (nACh) and muscarinic (mACh) receptors (Cooper and others 2003). Through these, ACh activates membrane currents, finetunes the activity of a range of ion channels and regulates the intracellular Ca2+ dynamics in neurons (Albuquerque and others 2009; Lucas-Meunier and others 2003), influencing their excitability and synaptic functions (Figure 1). Notably, along with its role as a neurotransmitter at specialized cholinergic synapses, ACh is also known to exert slow modulator effects through volume transmission, with a substantial fraction of morphologically identified cholinergic terminals and varicosities in the cerebral cortex and hippocampus lacking postsynaptic specializations (Descarries and others 1997; Sarter and others 2009).
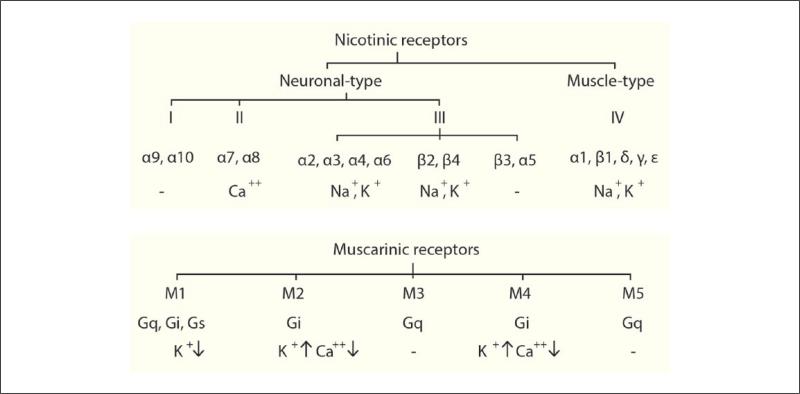
Figure 1.
Overview of cholinergic receptors with their functional effects. Nicotinic and muscarinic receptor trees. Nicotinic receptors (top) are divided into neuronal (I-III) and muscle (IV) types. Neuronal nAChRs are further subdivided into 3 subfamilies (I-III), with subfamily III further subdivided into three tribes. Subunits of nAChR assemble into homo- or hetero-pentamers to form an ionotropic receptor–channel complex. On activation, nAChRs mediate the influx of cations to depolarize the membrane and activate intracellular signaling. Muscarinic receptors (bottom) are encoded by five different genes, which translate into M1-M5 G-protein–coupled receptors. Upon binding to acetylcholine, these stimulate G-protein(s), which activates a cascade of chemical reactions, leading to changes in ion channel activity and synaptic transmission.
Although an evidence-based view is held for the focal transmitter and diffuse neuromodulator effects of ACh in the cortex and hippocampus, its specific roles for these two operational modes in the processing of information processing and integrative brain mechanisms remains elusive (Sarter and others 2014). Reports of potent catalytic activity of acetylcholinesterase (AChE) capable of neutralizing the bulk of synaptic ACh even after large release events (Lawler 1961; Quinn 1987) while call into question the physiological relevance of cholinergic volume transmission, provide little clues towards understanding the physiological role of non-synaptic cholinergic receptors abundantly expressed throughout the brain. Moreover, it remains unclear as to whether the homeostatic and housekeeping functions of ACh such as regulation of the phosphorylation of microtubule-associated protein (MAP) tau or processing of amyloid precursor protein (APP) rely on canonical synaptic transmission or depend on paracrine cholinergic effects. These fascinating questions along with recent evidence regarding the uptake and degradation of Aβ by BF cholinergic neurons highlight serious flaws in the prevalent neurophysiological hypothesis of cortical ACh and suggest that in the brain the role of cholinergic innervations extend beyond the supply of ACh with its neuromodulator and transmitter effects. In this article we overview selective evidence for homeostatic and metabolic functions of the basalo-cortical cholinergic projections and briefly discuss their relevance to neuronal physiology and pathobiology of AD.
The Synaptic Model of Cholinergic Functions: A Brief Overview
Cholinergic projections to the cerebral cortex are primarily formed by axons of large neurons of the nucleus basalis Meynert (NBM), while inputs to the hippocampus and associated structures arise from the medial septum and vertical limb of diagonal band Broca (Mesulam and others 1983a; Mesulam and others 1983b; Zaborszky and others 2015). The topographic organization of basalo-cortical cholinergic projections while evident already in the rodent brain, becomes far more prominent in primates and especially in humans (Mesulam 2013; Zaborszky and others 2015). In order to designate groups of cholinergic neurons within the BF, Mesulam and colleagues introduced the Ch1-4 nomenclature (Mesulam 2013; Mesulam and others 1983a; Mesulam and Geula 1988). According to this description, Ch1/Ch2 mark the group of cholinergic neurons in the medial septum and vertical limb of the diagonal band Broca, which provide cholinergic inputs to the hippocampal complex; cholinergic neurons in the horizontal limb of the diagonal band Broca project to the olfactory bulb (Ch3), while sublenticular cholinergic neurons often referred as neurons of the NBM (Ch4) project to the amygdala and the entire neocortex (Mesulam and others 1983a; Mesulam and others 1986). In keeping with the relative specificity of targets, individual cortical areas receive their major cholinergic innervations from different segments of the Ch4 complex (Mesulam and others 1983a).
Both cortical and hippocampal pyramidal neurons and interneurons receive mono-synaptic inputs from the BF (Freund and Antal 1988; Frotscher and Leranth 1985), where ACh activates a selection of nAChR and mAChR. As a typical ionotropic receptor-channel, nAChR on activation induces fast membrane currents. Produced through pentamerization of α(2-10) and β(2-4) subunits, nAChR widely vary in their composition, subunit stoichiometry and functions (Fenster and others 1997; Nai and others 2003). Among nAChR, α4β2 is the most abundantly expressed receptor in the brain, enriched in cortical and hippocampal neurons, followed by α7 nAChR expressed mainly at glutamate-, GABA-, and cholinergic presynaptic elements (Kawai and Berg 2001; Arroyo and others 2014). Of note, in the majority of cases the physiological state of a single neuron can be affected by three or more subspecies of nAChR. Functionally, all nAChR reveal comparable permeability for K+ and Na+ ions but differ in their permeability to Ca2+, which is highest for α7 nAChR. These special molecular and functional characteristics of nAChR accord with their special role in transmission of fast and temporally discrete cholinergic signals as well as the modulation of functions and plasticity mechanisms at both cholinergic and non-cholinergic synapses (Vizi and Lendvai 1999; Engelman and MacDermott 2004).
Unlike ionotropic nAChR, mAChR represent the first and most thoroughly characterized G-protein–coupled metabotropic receptor, also widely expressed throughout the brain. Based on signal transduction mechanisms, mAChR are generally classified into two subgroups: (1) M1, M3, and M5 linked to phospholipase C via G q/11 and mobilizing intracellular Ca2+ and (2) M2 and M4 coupled negatively to adenylate cyclase through G i/o protein and inhibiting cAMP activity (Cooper and others 2003). M1 AChR is the most abundant muscarinic receptor subtype in the brain, followed by moderately expressed M2 and M4, while M3 and M5 show the lowest expression levels. In the hippocampus and cerebral cortex, M1 and M3 receptors are localized mainly on pyramidal neurons, while M2 and M4 mAChR are enriched on interneurons and pre-synaptic terminals (Volpicelli and Levey 2004). Importantly, and unlike largely presynaptic effects of nAChRs with regulation of the strength and plasticity of synaptic inputs to interneurons and pyramidal cells, the most consistent and well-characterized effects of mAChR are postsynaptic, and lead to slow depolarization of neurons with increase in their membrane resistance. These effects result from inhibition of several hyperpolarizing membrane currents, including IK+, IM, and IAHP, Ca2+ activated IK+ capable of dampening the membrane excitability of neurons and reducing their firing capacities (Cole and Nicoll 1983; Dutar and others 1995; Unal and others 2015). Importantly, ACh also regulates neuronal excitability through inhibition of GABAergic transmission, an effect that is mediated via presynaptic nAChR and mAChR (Brown 2010; Nunez and others 2012; Raiteri and others 1990). Together with the enhancement of membrane excitability, cholinergic inhibition of the GABAergic synaptic drive promotes the activation of all-or-none Ca2+ spikes and bursts of action potentials in cortical neurons in response to strong depolarizing inputs, which facilitate long-lasting synaptic plasticity within the somatosensory cortex (Nunez and others 2012). Finally, stimulation of mAChR receptors has also been shown to enhance the response of NMDA receptors to glutamate and promotes the induction of activity-dependent long-term potentiation (LTP) of glutamatergic synapses (Markram and Segal 1992; Ovsepian and others 2004). These and other related studies demonstrate a major role of mAChR in the regulation of synaptic transmission and plasticity mechanisms at cortical and hippocampal synapses (Ovsepian 2008; Rasmusson 2000). Taken together, the diversity of pre- and postsynaptic AChR with their broad range of effects in neurons and synapses support the dual transmitter and modulator functions of ACh.
Acetylcholine Promotes the Non-Amyloidogenic Cleavage of APP and Inhibits Aβ Production
The first evidence for the regulation of APP cleavage by surface receptors was presented by Roger Nitsch and colleagues (Nitsch and others 1992) in demonstrating that stimulation of mAChR in HEK cells promotes the non-amyloidogenic cleavage of APP with shedding of the sAPPα fragment. Subsequently, a dose-dependent increase in sAPPα production by mAChR was demonstrated also in neurons (Nitsch and others 1993; Nitsch and others 1996). These pioneering findings were later confirmed through the use of selective M1 and M3 mAChR agonists and extensively discussed (Fisher and others 1998; Muller and others 1997; Rossner and others 1998). Importantly, stimulation of sAPPα production by mAChR correlated with activation of G q/11 coupled protein kinase C (PKC) signaling and reduction in secretion of the Aβ peptide (Buxbaum and others 1993; Hung and others 1993) (Figure 2). Stimulation of phosphatidylinositol 3-phosphate (PI3) hydrolysis by bradykinin, interleukin-1, and vasopressin receptor agonists also enhanced the production of sAPPα (Buxbaum and others 1992; Nitsch and others 1992). In contrast, stimulation of M2 and M4 receptors or downstream cAMP had no effects on APP proteolysis (Nitsch and others 1992). Phorbol esters, which mimic the effects of PKC stimulant diacyl-glycerol (DAG) also promoted sAPPα production and reduced the secretion of Aβ (Gandy and Greengard 1994a, 1994b). Recently, molecular events contributing towards the regulation of APP metabolism by cholinergic mechanisms have been revealed in detail, with both α- (ADAM17) and β-secretases (BACE1) proven as primary downstream effectors (Fisher 2012; Thathiah and De Strooper 2011).
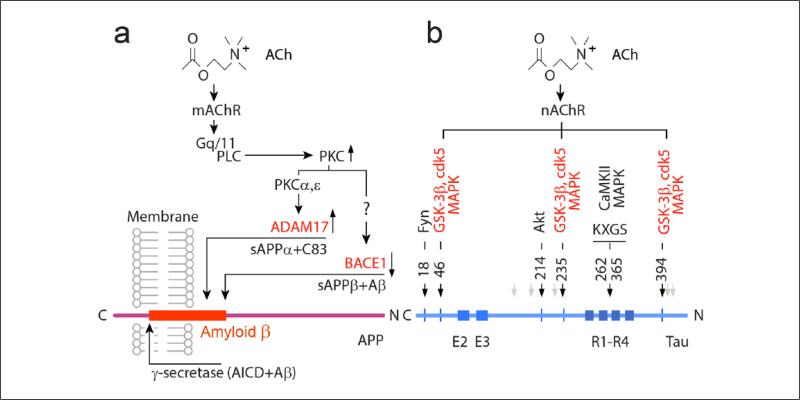
Figure 2.
Biochemical pathways linking muscarinic ACh receptor (mAChR) or nicotinic ACh receptor (nAChR) with amyloid precursor protein (APP) cleavage and phosphorylation of microtubule-associated protein (MAP) tau. (a) On binding to ACh, mAChR stimulates Gq/11 protein, which activates phospholipase C (PLC) and downstream protein kinase C (PKC). These lead to stimulation of ADAM17 and inhibition of BACE1, promoting the non-amyloidogenic cleavage of APP, with shedding of sAPPα. (b) Stimulation of nAChR activates an array of reactions directed to the phosphorylation of MAP tau. Schematic (bottom) illustrates the structure of MAP tau: R1-R4—four repeat sequences, which make up the microtubule-binding domain of tau. On activation, nAChR promote the phosphorylation of tau via stimulation of glycogen synthase kinase 3 (GSK-3β), cyclin-dependent kinase (cdk5) and mitogen-activated protein kinase (MAPK) and potentially other kinases. Black arrows point onto sites of hyperphosphorylation characteristic of Alzheimer's disease. Light brown arrows point to more recently suggested phosphorylation sites (Thr181; Ser202; Thr231; Ser396; Ser404 residues) by nAChR, also relevant to the neurofibrillary pathology in Alzheimer's disease.
In keeping with the up-regulation of the ADAM17 by PKC α/ε and enhancement of the α cleavage of APP, in AD brains, the level and activity of PKC is notably reduced, an effect that correlates with increased deposition of Aβ and related pathological changes in the cerebral cortex and hippocampus (Kurumatani and others 1998). Regulation of APP processing by ACh has also been verified by reports showing that selective ablation of BF cholinergic neurons with immune-toxins (Saporin IgG-192) exacerbates amyloid pathology and promotes the deposition of parenchymal and vascular Aβ in the brains of animals models (Beach and others 1997; Gil-Bea and others 2012; Hartig and others 2014; Laursen and others 2013; Roher and others 2000). Consistent with anti-amyloidogenic effects of M1 mAChR, treatment of AD transgenic mice with selective M1 receptor agonist AF267B or AChE inhibitors lowered the levels of Aβ in their brains and cerebrodpinal fluid (Beach 2002; Beach and others 2001) and improved cognitive functions (Caccamo and others 2006). It is noteworthy that along with promoting the non-amyloidogenic processing of APP, activation of M1 mAChR potently and selectively inhibited the activity of BACE1—the rate-limiting catalyst of the β-cleavage of APP with Aβ production (Davis and others 2010; Zuchner and others 2004) (Fig. 2). Such effect qualifies the agonists of M1 mAChR as potent and selective BACE1 inhibitors, unlike recently developed synthetic BACE1 inhibitors, which demonstrate considerable cross-reactivity with BACE2 protease (Yan and Vassar 2014). In contrast to the anti-amyloidogenic and GSK-3β suppressant effects of AF267B, the M1 mAChR antagonist dicyclomine, in addition to inhibiting ADAM17 activity also stimulates BACE1 and GSK-3β functions, promoting Aβ production and phosphorylation of MAP tau, with detrimental effects on the cognitive functions of AD mice (Fisher 2012).
Regulation of APP cleavage with production of Aβ and other APP fragments by nAChR-dependent mechanisms have also been documented. In primary cell cultures, nicotine at low dosage promoted the shedding of sAPPα without altering the levels of APP mRNA, an effect that was antagonized by a broad-spectrum nAChR antagonist mecamylamine or Ca2+ chelator EGTA. These findings are in agreement with the essential role of nAChR-induced mobilization of intracellular Ca2+ in promoting the non-amyloidogenic cleavage of APP with shedding of the sAPPα fragments (Kim and others 1997). Evidence from in vivo studies remains conflicting and inconclusive; while some reports show nicotine-induced reduction in the deposition of Aβ with amelioration of plaque pathology in AD mouse models (APPsw) (Nordberg and others 2002; Unger and others 2006), others found no effects (Sabbagh and others 2008) or observed exacerbation of the pathology (Oddo and others 2005). In summary, although the details and possible source of controversial findings remain at present unclear, most reports agree on the significance of endogenous ACh in regulating APP processing and its signaling functions. Such effects involve stimulation of both nicotinic and muscarinic ACh receptors with mobilization of intracellular Ca2+ and modulation of the activity of major APP cleaving proteases.
Cholinergic Mechanisms in Control of MAP tau Phosphorylation
Phosphorylation of MAP tau is the major and most thoroughly investigated posttranslational modification for this protein and is of primary relevance to the pathobiology of AD. From a total of 441 tau residues (the longest isoform in the central nervous system), 79 present potential phosphorylation sites catalyzed by an array of kinases, including GSK-3β, cdk5, MAPK (p38), JNK, PKA, PKC, CaM (Avila and others 2004; Querfurth and LaFerla 2010) (Fig. 2). While earlier studies have focused mainly on the serine/threonine residues, recently, tyrosine residues also have become of major interest. Upon hyperphosphorylation, MAP tau fails to bind to microtubules. This leads to destabilization of microtubules with their collapse and formation of paired helical filaments of tau, which oligomerize and provide the raw material for the formation of neurofibrillary tangles during AD and several other neurodegenerative diseases (Fig. 4).
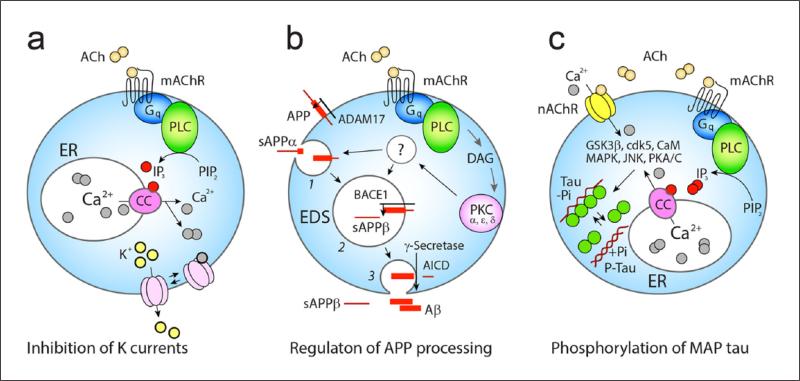
Figure 4.
Dual neuromodulator and homeostatic effects of ACh. (a) Activation of the mAChR M1 receptors stimulates Gq-protein → PLC and downstream events involving IP3 signaling and mobilization of intracellular Ca2+, which inhibits the low-threshold K+ and Ca2+ activated K+ currents. ER, endoplasmic reticular Ca2+ stores; CC, IP3 sensitive calcium channel, Ach, acetylcholine. (b) ACh via the same receptor and stimulation of DAG → PKC α,ε,δ also regulates the activity of BACE1 and ADAM17—two of three key amyloid precursor protein (APP) cleaving proteases. Cleavage of APP by ADAM17 (non-amyloidogenic) with shedding of sAPPα occurs on the surface or within early endosomes (1), while β/γ cleavage takes place in acidifying endosomes (2, 3), a step that is followed by release of sAPPβ and Aβ as well as with the generation of the APP intracellular domain (AICD) involved in nuclear signaling. (c) Stimulation of nAChR and mAChR can increase intracellular Ca2+ and activate an array of kinases (GSK-3b (beta instead of ‘b’), cdk5, CaM, MAPK, JNK, PKA/C) which can promote the phosphorylation of microtubule-associated protein (MAP) tau. This leads to unbinding of MAP tau from microtubules with their collapse and formation of oligomeric and fibrillary tau.
Since the pioneering research led by Arendt and colleagues (Arendt and others 1999) highlighting the close association between hyperphosphorylation of MAP tau and cholinergic depletion in the hippocampus and cortex of subjects affected by AD, several studies have shown that stimulation of nAChR promotes, while activation of mAChR inhibits the phosphorylation of MAP tau (Bencherif and Lippiello 2010; Buckingham and others 2009). Recently, Härtig and colleagues reported that in adult triple transgenic mice mimicking β-amyloidosis and tau hyperphosphorylation, selective lesion of BF cholinergic neurons, in addition to promoting amyloid pathology also enhanced the level of phosphorylated MAP tau and caused the accumulation of its AD specific conformations in the brain (Hartig and others 2014). In SH-SY5Y human neuroblastoma cells overexpressing α3 or α7 nAChR, nicotine or nAChR agonist epibatidine increased the levels of both phosphorylated and non-phosphorylated tau; these effects were antagonized by selective nAChR antagonists mecamylamine or D-tubocurarine (Del Barrio and others 2011; Hellstrom-Lindahl and others 2000). It was also shown that nicotine promotes the phosphorylation of tau at specific amino acid residues (Ser202, Thr231, and Thr 181), which are prone to hyperphosphorylation during AD (Wang and others 2003) (Fig. 2). Treatments of SH-SY5Y cells with AChE inhibitors donepezil and tacrine, similar to nicotine, enhanced the phosphorylation of MAP tau and promoted the expression of nAChR (Hellstrom-Lindahl 2000; Hellstrom-Lindahl and others 2000), with inhibition of these effects by antagonists of nAChR but not mAChR confirming their dependence on the former. Related observations were also made in the SK-N-MC cell line, where hyperphosphorylation of MAP tau by nAChR agonists occurred if cells expressed α7 receptors, with pharmacological blockers or anti-sense oligonucleotides of α7 nAChR preventing the effects of nAChR agonists (Wang and others 2003).
Although the precise molecular events involved in hyperphosphorylation of tau by nAChR are a subject of ongoing research, Ca2+ influx with mobilization of intracellular Ca2+ seem to be sufficient for triggering this process. Indeed, chelating extracellular Ca2+ in cultured medium with EGTA inhibited the phosphorylation of tau by nicotine and other nAChR agonists (Hellstrom-Lindahl and others 2000). These findings accord with reports of increased phosphorylation of MAP tau by the Ca2+ ionophore A23187 (Shea and others 1996), while reduction in phosphorylation of the tau protein by inhibitors of calpain and Ca2+ activated PKC imply essential roles played by these enzymes. It is worth stressing that there is considerable topographical overlap between the brain regions exhibiting the highest levels of neurofibrillary tangles in AD autopsies with those undergoing the most pronounced nicotine-induced hyperphosphorylation (Wang and others 2003). On the other hand, in transgenic AD mice, which develop age dependent amyloidosis with neurofibrillary tangle pathology and LTP deficit (Kitazawa and others 2005; Oddo and others 2003), the expression of nAChR is significantly reduced, an effect which is most pronounced in forebrain structures with the highest levels of intracellular Aβ (Oddo and others 2005). While chronic treatment with nicotine did not change the levels of soluble Aβ in brains of AD mouse models, it caused marked elevation in aggregates of hyperphosphorylated tau, a process that is presumably catalysed by p38-MAP kinase (Oddo and others 2005).
Unlike nicotine, in heterologous expression systems both Carbachol and the M1 receptor agonist AF102B reduced the level of phosphorylated MAP tau in a dose- and time-dependent manner (Kar and others 2004; Sadot and others 1996). Chronic treatment of ApoE-deficient mice with another M1 agonist AF150 also lowered the level of hyperphosphorylated MAP tau in their neurons, an effect associated with improved cognition and recovery of cortical and hippocampal cholinergic markers (Fisher and others 1998). These descriptive reports had been followed by mechanistic studies (Balaraman and others 2006; Caccamo and others 2006) demonstrating that a mAChR-dependent increase in PKC activity with inhibition of GSK-3β could account for both, the reduction in Aβ toxicity and hyperphosphorylation of MAP tau. Interestingly, single cell gene expression profiling with cDNA array analysis of tau in patients affected by mild cognitive impairment and AD revealed a considerable shift from the three-repeat tau (3Rtau) to four-repeat tau (4Rtau) mRNA ratio within individual BF cholinergic neurons as well as in CA1 pyramidal cells; these alterations were AD-specific and could not be detected in the course of normal aging (Ginsberg and others 2006a; Ginsberg and others 2006b). Taken as a whole, these findings support the major regulatory function of ACh in the phosphorylation of MAP tau via both mAChRs and nAChRs and confirm the key importance of the endogenous cholinergic drive in the maintenance of neuronal integrity and synaptic functions.
Clearance of Aβ by Basalo-cortical Cholinergic Projections
In addition to the evidence for the cholinergic regulation of MAP tau phosphorylation and APP processing, recent studies have also suggested the importance of hippocampal and cortical cholinergic innervations in the clearance of Aβ (Ovsepian and Herms 2013a; Ovsepian and others 2014) (Fig. 3). In the nervous system, clearance of Aβ is traditionally viewed in association with three independent processes: (1) gradient driven efflux of Aβ or its ATP-dependent transport into the circulatory systems, (2) local proteolysis of Aβ by amyloid-degrading enzymes, and (3) its phagocytic removal by microglia. Recently, evidence has been obtained for the internalization of Aβ by neurons, with relevance of this process to the pathobiology of AD extensively discussed (Gouras and others 2010; LaFerla and others 2007; Nixon 2013). Depositions of Aβ in catepsin-enriched endosomes within dystrophic axons, which maturate into lysosomes suggest that the uptake and break down of this peptide by neurons is widely represented throughout the brain. The levels of cathepsins (B, D) and the amount of Aβ or APP enriched intra-neuronal endosomes are strongly enhanced in brains affected by AD as well as in cortical neurons of transgenic AD mice (Gouras and others 2000).
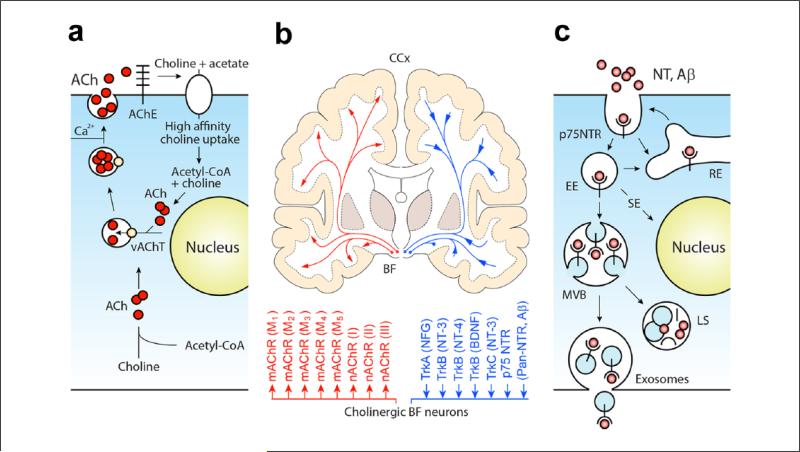
Figure 3.
Functional models of basal forebrain cholinergic neurons. (a) A schematic illustration of the synthesis, storage, and release of ACh followed by its breakdown; the choline transported into pre-synaptic terminals by the high-affinity transporter is reused for de novo synthesis of ACh via a single enzymatic step. The acetyl coenzyme A for ACh synthesis is derived from mitochondria. Synthetized ACh is then loaded into synaptic vesicles by vesicular ACh transporter (yellow circle: vAChT) and released. (b) Schematic illustration of human basalo-cortical projections with dual (1) cholinergic receptor–mediated (via mAChR1-5 and nAChRI-III) (left) and NTR receptor mediated functions and effects. Note, diffuse cholinergic projections internalize and transport trophic factors and other collateral NTR ligands (including Aβ) to their soma (adapted from Ovsepian and Herms 2013a). (c) Graphical illustration of three main intracellular routes taken by p75NTR and its ligands after internalization. Part of the internalized cargo is recycled back to the surface within early and recycling endosomes (EE, RE), while the rest is sorted to signaling endosomes (SE) or multivesicular bodies (MVBs). From here, most of the cargo is sorted for degradation in hybrid compartments formed by fusion of the MVB and late endosomes (LS) with lysosomes, while small amounts of the material escapes from the degradation pathway and becomes available for release.
We proposed that receptor-mediated endocytosis followed by degradation of Aβ within BF cholinergic neurons may be of major importance for maintaining physiological levels of this peptide in forebrain structures innervated by cholinergic inputs (Ovsepian and Herms 2013b). In particular, cholinergic projections enriched with the p75 neurotrophin receptor (NTR) are capable of high-affinity binding to both mono- and oligomeric Aβ (Dechant and Barde 2002; Yaar and others 1997) and appear to be exquisitely suitable for their endocytosis with sorting for degradation in lysosomes (Ovsepian and others 2014). Although Aβ induced death signaling via p75NTR has been documented, the physiological relevance of the receptor-ligand like interactions of Aβ and p75NTR remains elusive (Dechant and Barde 2002). The lack of dystrophic cholinergic axons in Thy1-hAPP-London/Swe-p75NTR−/− mice, which contrasts with the widespread axonal abnormalities and loss of BF cholinergic neurons in the Thy1-hAPP-London/Swe-p75NTR+/+ genotype (Knowles and others 2009) supports the major role of p75NTR in promoting the neurotoxic effects of Aβ. Accordingly, selective ablation of BF cholinergic neurons or deletion of p75NTR accelerated the deposition of Aβ plaques and related histopathological changes in the cerebral cortex and hippocampus in AD mouse models (Gil-Bea and others 2012; Hartig and others 2014; Laursen and others 2013; Wang and others 2011). These observations accord with earlier data from studies conducted in rabbits, which revealed characteristic depositions of perivascular Aβ after targeted lesion of BF cholinergic neurons with immune-toxins (Roher and others 2000). Together with the allegedly unchanged functions of the forebrain cholinergic system in p75NTR−/− mice (Greferath and others 2000), these observations suggest that the functions of BF cholinergic inputs to the hippocampus and cerebral cortex extend beyond the supply of ACh with neurophysiological effects (Figure 3). Indeed, specific binding of Aβ to p75NTR (Kd = 12nM compared with Kd = 7nM reported for NGF) (Yaar and others 1997) would afford the projection fields of basalo-cortical cholinergic axons enriched by the p75NTR with an elaborate molecular ‘drain’ for the sequestration of Aβ followed by its degradation. Such unique functionality of cholinergic innervations accords with results of clinical studies, which revealed a selective decline in the numbers of p75NTR expressing BF cholinergic neurons in plaque laden AD brains (Counts and Mufson 2005).
In the context of increased deposition of Aβ in brains affected by AD, the loss of BF cholinergic neurons with p75NTR dense projections would exacerbate the progression of the amyloidosis and overwhelm the proteolytic machinery of cholinergic cells with Aβ, leading to their lysosomal deficiency with metabolic collapse. It is interesting to note that unlike double transgenic APPSwe/PS1dE9 mice, in which ablation of BF cholinergic neurons leads to exacerbation of amyloid pathology with cognitive decline and memory deficit (Laursen and others 2013), APPSwe/PS1dE9/p75NTR−/− triple transgenic mice revealed no memory deficit in spite of the extensive amyloid pathology in the hippocampus and cerebral cortex (Wang and others 2011). This dissociation of cognitive and homeostatic functions of BF cholinergic system accords with its dual (ACh and p75NTR dependent) functionality (Figure 3) and is in agreement with results of human clinical studies of olivoponto-cerebellar atrophies. Indeed, contrasting to the extensive loss of BF cholinergic axons and synapses in AD brains associated with widespread amyloid pathology in the cerebral cortex and hippocampus, degeneration of tegmentopontine cholinergic neurons while correlate with a strong depletion of cortical ACh is not associated with amyloid pathology (Kish and others 1989; Robitaille and others 1995). Thus, in addition to well recognized neuromodulator and housekeeping functions, the BF cholinergic system appears to play a major homeostatic role in the clearance of Aβ. Through distributed innervations enriched with p75NTR, cholinergic axons afford their projection fields with an elaborate system for the sequestration of Aβ with its removal and degradation in lysosomes (Figure 3).
Aβ and tau Protein as Endogenous Ligands for Cholinergic Receptors
In addition to ACh, cholinergic receptors bind specifically and with high affinity to several collateral ligands, including Aβ and MAP tau. While the physiological relevance of the specific binding of Aβ and tau to cholinergic receptors remains undefined, accumulating data imply their direct neuromodulatory role and possible contribution to AD pathobiology (Auld and others 1998; Avila and others 2014). Accordingly, loss of cholinergic innervations (of 45% to 85% of axons) in AD is most prominent in associative cortical regions, including the temporal, prefrontal, posterior parietal, orbitofrontal, and cingulate cortices, known also to be severely affected by amyloid pathology, whereas cholinergic inputs to primary sensory and motor subsystems remain relatively intact (Mesulam 2004). Even though the density of Aβ plaques in these and associated cortical regions does not directly relate to the amount of degenerated cholinergic axons, it correlates significantly with the number of remaining fibers and with the percentage of lost axons (Geula and others 1998). Strong co-localization of α7 nAChR and Aβ within neurons and amyloid lesions have been found in AD autopsies (Nagele and others 2002). Interestingly, in transgenic AD mouse models, down-regulation of α7 receptor expression appears to be restricted to the brain structures affected most severely with amyloid pathology (Oddo and others 2005). While the mechanisms for the regulation of α7 nAChR expression by Aβ remain elusive, the capability of Aβ to specifically bind and activate the internalization of α7 receptors could be involved in the process. This notion is in line with the data from heterologous expression systems, which demonstrate that in α7 nAChR–transfected neuroblastoma cells, internalization of Aβ strongly exceeds that in non-transfected controls (Nagele and others 2002). In addition to the regulation of nAChR expression, dose-dependent activation or inhibition of nAChR by soluble Aβ has also been reported. Of note, in low amounts Aβ activates α7 nAChR and downstream ERK2-MAPK signaling (Dineley and others 2002; Fodero and others 2004) while at higher concentrations it antagonizes α7 nAChR functions in both human and rat brains and inhibits presynaptic Ca2+ currents (Lee and Wang 2003; Pettit and others 2001). Postsynaptic neuromodulator effects of Aβ have also been reported in septo-hippocampal neurons, with Aβ acting through α7 and α4β2 receptors (Chin and others 2007). Of note, Aβ appears also to modulate the effects of nAChR agonists and antagonists on synaptic functions (Li and others 2011), an effect that varies between different species of nicotinic receptors. For instance, compared with α7 nAChR, α7β2 receptors are more prone to inhibition by Aβ1-42 oligomers. In fact in both, heterologous expression systems and in neurons oligomeric Aβ1-42 specifically and potently antagonizes α7β2 but not α7 receptor mediated whole-cell currents (Liu and others 2009). In contrast with the overtly consistent neurochemical data, results from behavioral studies are sparse and conflicting. While one report showed the enhancement of the oligomerization of Aβ with cognitive decline in α7−/− nAChR (Hernandez and others 2010), another study showed amelioration of AD pathology with improved memory and cognition in the absence of the α7 receptor (Dziewczapolski and others 2009).
Similar to the nAChR, Aβ was found also to interact with mAChR and exerts its effects via activation of M1 receptors (Levey and others 1995; Thathiah and De Strooper 2011). The recent discovery of the activity-dependent release of soluble Aβ and tau from living neurons (Cirrito and others 2005; Pooler and others 2013; Yamada and others 2014) kindled a surge of interest in potential neurophysiological effects of extracellular Aβ and tau on the biology of neurons and synapses. Along with their capacity to induce cytotoxicity via disruption of the integrity of biological membranes and deregulation of neuronal Ca2+ homeostasis, Aβ and tau also disrupt physiological processes and functions through specific interactions with mAChR. Gomez-Ramos and colleagues, for instance, reported that the direct binding of tau to mAChR can mobilize intracellular Ca2+ and alter cellular Ca2+ dynamics (Gomez-Ramos and others 2006). Through combining cDNA transfection and pharmacological screening, M1 and M3 receptors have been implicated in tau induced cytotoxicity and Ca2+ mobilization (Gomez-Ramos and others 2008). Similar observations have also been made in COS-7 cells, where MAP tau coupled to CY5 fluorescence dye activated M1 and M3 receptors, with the estimated binding affinity of MAP tau exceeding that of ACh. It is important to note that while both MAP tau and ACh can mobilize intracellular Ca2+, such an effect although more potent, also inactivates more rapidly when induced with ACh (Rubio and others 2009). In summary, from the brief overview of selected reports, intriguing facets of the regulation of cholinergic functions by Aβ and MAP tau is evident. Further research is warranted for the identification of the physiological relevance of direct interactions of these collateral ligands with cholinergic receptors and defining the effects of these interactions on the biology of neurons and synaptic functions.
Cholinergic Effects Extend beyond Neuromodulation and Synapses
Cholinergic basalo-cortical projections share with other ascending modulator systems of the brain their distributed organization, innervating all regions and layers of the cerebral cortex and hippocampus. Like other cortical modulatory projections, cholinergic axons along with transmission of signals through canonical synaptic wiring also use non-junctional volume transmission, with paracrine activation of extrasynaptic cholinergic receptors (Contant and others 1996; Descarries and others 1997; Umbriaco and others 1995). Ultrastructural analysis of axon terminals and varicosities in the hippocampus and cerebral cortex has demonstrated that the majority of cholinergic presynapses lack specialized postsynaptic elements (Descarries 1998; Descarries and others 1997), with more recent studies however challenging the results of these early reports (Munoz and Rudy 2014). In primates, for instance, only 44% of cholinergic varicosities and axon terminals form morphologically defined synapses (Mrzljak and others 1995). Similar observations have been made also in the human cerebral cortex (Smiley and others 1997), while in the murine hippocampus and neo-striatum the incidents of cholinergic varicosities with postsynaptic specializations appear to be even higher (Contant and others 1996; Umbriaco and others 1995). These special structural arrangements were interpreted in early reports as direct evidence for cholinergic volume transmission, which operates in parallel with communication via synaptic wiring. The non-junctional model of cholinergic effects received also direct support from immuno-cytochemical studies, which demonstrated the prevalence of extra-synaptic cholinergic receptors in the hippocampus and other limbic structures as well as in the neocortex (Lendvai and Vizi 2008; Mrzljak and others 1995). Interestingly, mAChR and nAChR have been identified on both cholinergic and non-cholinergic axon terminals and varicosities, in agreement with auto- and paracrine effects of non-synaptic acetylcholine. Indeed, enrichment of glutamatergic, GABAergic and other non-cholinergic axons with nAChR and mAChR imply that the functions of these afferents can be subject to regulation by ambient ACh while expression nAChR and mAChR on cholinergic terminals accords with their auto-receptor functions. The notion of cholinergic volume transmission is also supported by the dense arrangement of cholinergic terminals and synaptic varicosities in the cerebral cortex and hippocampus (~500 terminals within 10-μm radius, with 5-10 times more undefined terminals and thousands of dendritic spines) (Descarries 1998) along with remarkable extracellular mobility of transmitters and peptides outside of synapses (Jansson and others 2000; Rusakov and others 2011). Moreover, as pointed out earlier, cholinergic axons and synaptic terminals enriched with p75NTR but devoid postsynaptic elements can afford their projection fields with an elaborate system for Aβ clearance. Importantly, unlike temporally discreet and targeted synaptic effects, which rely on highly localized surges of ACh to high micromolar concentrations, cholinergic modulation of APP processing and MAP tau phosphorylation appears to be operational from much lower concentrations of ACh (Mousavi and Hellstrom-Lindahl 2009; Scerri and others 2012). Thus, along with the well-recognized modulation of synaptic and neuronal functions via canonical mechanisms, endogenous ACh evidently plays a major metabolic and homeostatic role (Figure 4), with important implications for regulating an array of important signaling and integrative processes in neurons.
Closing Remarks
In the cerebral cortex and hippocampus, ACh is traditionally viewed as a potent regulator of a range of cellular and molecular processes, through multiple cholinergic receptor subtypes and mechanisms. These are considered to be of prime importance for focal modulation of neuronal and synaptic functions as well as for regulation of the global state and activity of cortical and hippocampal networks implicated in higher brain mechanisms. In this article, we discussed growing evidence for additional homeostatic and housekeeping roles of the BF cholinergic system, which involve the regulation of APP cleavage with Aβ production, control of MAP tau phosphorylation and Aβ clearance (Figure 4). We reviewed selected evidence for the modulation of cortical cholinergic functions by Aβ and MAP tau, which specifically bind and activate cholinergic receptors, and briefly discussed the possible relevance of these processes to neuronal physiology and pathobiology of AD. The heterodox mechanisms reviewed here, while in general agreement with the neurophysiological cholinergic hypothesis, also highlight the limitations of the latter with the need for its careful revision. Future research should extend our understanding of the biology and functions of the BF cholinergic system - one of the key modulator systems of the brain, to facilitate the discovery of novel therapeutic targets for prevention or perhaps treatment of Alzheimer's disease and related disorders.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Zaborszky is supported by NIH/NINDS NS023945.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt T, Bigl V, Arendt A, Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer's disease, paralysis agitans and Korsakoff's disease. Acta Neuropathol. 1983;61:101–8. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- Arendt T, Holzer M, Gertz HJ, Bruckner MK. Cortical load of PHF-tau in Alzheimer's disease is correlated to cholinergic dysfunction. J Neural Transm. 1999;106:513–23. doi: 10.1007/s007020050175. [DOI] [PubMed] [Google Scholar]
- Arroyo S, Bennett C, Hestrin S. Nicotinic modulation of cortical circuits. Front Neural Circuits. 2014;8:30. doi: 10.3389/fncir.2014.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld DS, Kar S, Quirion R. β-Amyloid peptides as direct cholinergic neuromodulators: a missing link? Trends Neurosci. 1998;21:43–9. doi: 10.1016/s0166-2236(97)01144-2. [DOI] [PubMed] [Google Scholar]
- Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84:361–84. doi: 10.1152/physrev.00024.2003. [DOI] [PubMed] [Google Scholar]
- Avila J, Simon D, Diaz-Hernandez M, Pintor J, Hernandez F. Sources of extracellular tau and its signaling. J Alzheimers Dis. 2014;40(Suppl 1):S7–S15. doi: 10.3233/JAD-131832. [DOI] [PubMed] [Google Scholar]
- Balaraman Y, Limaye AR, Levey AI, Srinivasan S. Glycogen synthase kinase 3β and Alzheimer's disease: pathophysiological and therapeutic significance. Cell Mol Life Sci. 2006;63:1226–1235. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG. Muscarinic agonists as preventative therapy for Alzheimer's disease. Curr Opin Investig Drugs. 2002;3:1633–6. [PubMed] [Google Scholar]
- Beach TG, Honer WG, Hughes LH. Cholinergic fibre loss associated with diffuse plaques in the non-demented elderly: the preclinical stage of Alzheimer's disease? Acta Neuropathol. 1997;93:146–53. doi: 10.1007/s004010050595. [DOI] [PubMed] [Google Scholar]
- Beach TG, Kuo YM, Schwab C, Walker DG, Roher AE. Reduction of cortical amyloid β levels in guinea pig brain after systemic administration of physostigmine. Neurosci Lett. 2001;310:21–4. doi: 10.1016/s0304-3940(01)02076-6. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Lippiello PM. α7 Neuronal nicotinic receptors: the missing link to understanding Alzheimer's etiopathology? Med Hypotheses. 2010;74:281–5. doi: 10.1016/j.mehy.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Brown DA. Muscarinic acetylcholine receptors (mAChRs) in the nervous system: some functions and mechanisms. J Mol Neurosci. 2010;41:340–346. doi: 10.1007/s12031-010-9377-2. [DOI] [PubMed] [Google Scholar]
- Buckingham SD, Jones AK, Brown LA, Sattelle DB. Nicotinic acetylcholine receptor signalling: roles in Alzheimer's disease and amyloid neuroprotection. Pharmacol Rev. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Christensen JL, Ruefli AA, Greengard P, Loring JF. Expression of APP in brains of transgenic mice containing the entire human APP gene. Biochem Biophys Res Commun. 1993;197:639–45. doi: 10.1006/bbrc.1993.2527. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci U S A. 1992;89:10075–8. doi: 10.1073/pnas.89.21.10075. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–82. doi: 10.1016/j.neuron.2006.01.020. others. [DOI] [PubMed] [Google Scholar]
- Chin JH, Ma L, MacTavish D, Jhamandas JH. Amyloidβ protein modulates glutamate-mediated neurotransmission in the rat basal forebrain: involvement of presynaptic neuronal nicotinic acetylcholine and metabotropic glutamate receptors. J Neurosci. 2007;27:9262–9. doi: 10.1523/JNEUROSCI.1843-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, et al. Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron. 2005;48:913–22. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983;221:1299–301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Contant C, Umbriaco D, Garcia S, Watkins KC, Descarries L. Ultrastructural characterization of the acetylcholine innervation in adult rat neostriatum. Neuroscience. 1996;71:937–47. doi: 10.1016/0306-4522(95)00507-2. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology. Oxford University Press; Oxford, England: 2003. [Google Scholar]
- Counts SE, Mufson EJ. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J Neuropathol Exp Neurol. 2005;64:263–72. doi: 10.1093/jnen/64.4.263. [DOI] [PubMed] [Google Scholar]
- Davis AA, Fritz JJ, Wess J, Lah JJ, Levey AI. Deletion of M1 muscarinic acetylcholine receptors increases amyloid pathology in vitro and in vivo. J Neurosci. 2010;30:4190–6. doi: 10.1523/JNEUROSCI.6393-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechant G, Barde YA. The neurotrophin receptor p75(NTR): novel functions and implications for diseases of the nervous system. Nat Neurosci. 2002;5:1131–6. doi: 10.1038/nn1102-1131. [DOI] [PubMed] [Google Scholar]
- Del Barrio L, Martin-de-Saavedra MD, Romero A, Parada E, Egea J, Avila J. Neurotoxicity induced by okadaic acid in the human neuroblastoma SH-SY5Y line can be differentially prevented by α7 and β2* nicotinic stimulation. Toxicol Sci. 2011;123:193–205. doi: 10.1093/toxsci/kfr163. others. [DOI] [PubMed] [Google Scholar]
- Descarries L. The hypothesis of an ambient level of acetylcholine in the central nervous system. J Physiol Paris. 1998;92:215–20. doi: 10.1016/s0928-4257(98)80013-2. [DOI] [PubMed] [Google Scholar]
- Descarries L, Gisiger V, Steriade M. Diffuse transmission by acetylcholine in the CNS. Prog Neurobiol. 1997;53:603–25. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- Dineley KT, Bell KA, Bui D, Sweatt JD. β-Amyloid peptide activates α7 nicotinic acetylcholine receptors expressed in Xenopus oocytes. J Biol Chem. 2002;277:25056–61. doi: 10.1074/jbc.M200066200. [DOI] [PubMed] [Google Scholar]
- Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol Rev. 1995;75:393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- Dziewczapolski G, Glogowski CM, Masliah E, Heinemann SF. Deletion of the α7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer's disease. J Neurosci. 2009;29:8805–15. doi: 10.1523/JNEUROSCI.6159-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–45. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J Neurosci. 1997;17:5747–59. doi: 10.1523/JNEUROSCI.17-15-05747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. Cholinergic modulation of amyloid precursor protein processing with emphasis on M1 muscarinic receptor: perspectives and challenges in treatment of Alzheimer's disease. J Neurochem. 2012;120(Suppl 1):22–33. doi: 10.1111/j.1471-4159.2011.07507.x. [DOI] [PubMed] [Google Scholar]
- Fisher A, Brandeis R, Haring R, Eshhar N, Heldman E, Karton Y. Novel m1 muscarinic agonists in treatment and delaying the progression of Alzheimer's disease: a unifying hypothesis. J Physiol Paris. 1998;92:337–40. doi: 10.1016/S0928-4257(99)80001-1. others. [DOI] [PubMed] [Google Scholar]
- Fodero LR, Mok SS, Losic D, Martin LL, Aguilar MI, Barrow CJ. α7-Nicotinic acetylcholine receptors mediate an Aβ(1-42)-induced increase in the level of acetylcholinesterase in primary cortical neurones. J Neurochem. 2004;88:1186–93. doi: 10.1046/j.1471-4159.2003.02296.x. others. [DOI] [PubMed] [Google Scholar]
- Freund TF, Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988;336:170–3. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Leranth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985;239:237–46. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Gandy S, Greengard P. Processing of Alzheimer A β-amyloid precursor protein: cell biology, regulation, and role in Alzheimer disease. Int Rev Neurobiol. 1994a;36:29–50. doi: 10.1016/s0074-7742(08)60302-5. [DOI] [PubMed] [Google Scholar]
- Gandy S, Greengard P. Regulated cleavage of the Alzheimer amyloid precursor protein: molecular and cellular basis. Biochimie. 1994b;76:300–3. doi: 10.1016/0300-9084(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam MM, Saroff DM, Wu CK. Relationship between plaques, tangles, and loss of cortical cholinergic fibers in Alzheimer disease. J Neuropathol Exp Neurol. 1998;57:63–75. doi: 10.1097/00005072-199801000-00008. [DOI] [PubMed] [Google Scholar]
- Gil-Bea FJ, Gerenu G, Aisa B, Kirazov LP, Schliebs R, Ramirez MJ. Cholinergic denervation exacerbates amyloid pathology and induces hippocampal atrophy in Tg2576 mice. Neurobiol Dis. 2012;48:439–46. doi: 10.1016/j.nbd.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Che S, Counts SE, Mufson EJ. Shift in the ratio of three-repeat tau and four-repeat tau mRNAs in individual cholinergic basal forebrain neurons in mild cognitive impairment and Alzheimer's disease. J Neurochem. 2006a;96:1401–8. doi: 10.1111/j.1471-4159.2005.03641.x. [DOI] [PubMed] [Google Scholar]
- Ginsberg SD, Che S, Wuu J, Counts SE, Mufson EJ. Down regulation of trk but not p75NTR gene expression in single cholinergic basal forebrain neurons mark the progression of Alzheimer's disease. J Neurochem. 2006b;97:475–87. doi: 10.1111/j.1471-4159.2006.03764.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Ramos A, Diaz-Hernandez M, Cuadros R, Hernandez F, Avila J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006;580:4842–50. doi: 10.1016/j.febslet.2006.07.078. [DOI] [PubMed] [Google Scholar]
- Gomez-Ramos A, Diaz-Hernandez M, Rubio A, Miras-Portugal MT, Avila J. Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol Cell Neurosci. 2008;37:673–81. doi: 10.1016/j.mcn.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tampellini D, Takahashi RH, Capetillo-Zarate E. Intraneuronal β-amyloid accumulation and synapse pathology in Alzheimer's disease. Acta Neuropathol. 2010;119:523–41. doi: 10.1007/s00401-010-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F. Intraneuronal Aβ42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greferath U, Bennie A, Kourakis A, Bartlett PF, Murphy M, Barrett GL. Enlarged cholinergic forebrain neurons and improved spatial learning in p75 knockout mice. Eur J Neurosci. 2000;12:885–93. doi: 10.1046/j.1460-9568.2000.00976.x. [DOI] [PubMed] [Google Scholar]
- Hartig W, Saul A, Kacza J, Grosche J, Goldhammer S, Michalski D. Immunolesion-induced loss of cholinergic projection neurones promotes beta-amyloidosis and tau hyperphosphorylation in the hippocampus of triple-transgenic mice. Neuropathol Appl Neurobiol. 2014;40:106–20. doi: 10.1111/nan.12050. others. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E. Modulation of beta-amyloid precursor protein processing and tau phosphorylation by acetylcholine receptors. Eur J Pharmacol. 2000;393:255–63. doi: 10.1016/s0014-2999(00)00028-5. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Moore H, Nordberg A. Increased levels of tau protein in SH-SY5Y cells after treatment with cholinesterase inhibitors and nicotinic agonists. J Neurochem. 2000;74:777–84. doi: 10.1046/j.1471-4159.2000.740777.x. [DOI] [PubMed] [Google Scholar]
- Hernandez CM, Kayed R, Zheng H, Sweatt JD, Dineley KT. Loss of α7 nicotinic receptors enhances beta-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer's disease. J Neurosci. 2010;30:2442–53. doi: 10.1523/JNEUROSCI.5038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung AY, Haass C, Nitsch RM, Qiu WQ, Citron M, Wurtman RJ. Activation of protein kinase C inhibits cellular production of the amyloid β-protein. J Biol Chem. 1993;268:22959–62. others. [PubMed] [Google Scholar]
- Jansson A, Lippoldt A, Mazel T, Bartfai T, Ogren SO, Sykova E. Long distance signalling in volume transmission. Focus on clearance mechanisms. Prog Brain Res. 2000;125:399–413. doi: 10.1016/S0079-6123(00)25028-0. others. [DOI] [PubMed] [Google Scholar]
- Kar S, Slowikowski SP, Westaway D, Mount HT. Interactions between β-amyloid and central cholinergic neurons: implications for Alzheimer's disease. J Psychiatry Neurosci. 2004;29:427–41. [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Berg DK. Nicotinic acetylcholine receptors containing α 7 subunits on rat cortical neurons do not undergo long-lasting inactivation even when up-regulated by chronic nicotine exposure. J Neurochem. 2001;78:1367–78. doi: 10.1046/j.1471-4159.2001.00526.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kim YK, Jeong SJ, Haass C, Kim YH, Suh YH. Enhanced release of secreted form of Alzheimer's amyloid precursor protein from PC12 cells by nicotine. Mol Pharmacol. 1997;52:430–6. doi: 10.1124/mol.52.3.430. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Robitaille Y, el-Awar M, Deck JH, Simmons J, Schut L. Non-Alzheimer-type pattern of brain cholineacetyltransferase reduction in dominantly inherited olivopontocerebellar atrophy. Ann Neurol. 1989;26:362–7. doi: 10.1002/ana.410260309. others. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:8843–53. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles JK, Rajadas J, Nguyen TV, Yang T, LeMieux MC, Vander Griend L. The p75 neurotrophin receptor promotes amyloid-β(1-42)-induced neuritic dystrophy in vitro and in vivo. J Neurosci. 2009;29:10627–37. doi: 10.1523/JNEUROSCI.0620-09.2009. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurumatani T, Fastbom J, Bonkale WL, Bogdanovic N, Winblad B, Ohm TG. Loss of inositol 1,4,5-trisphosphate receptor sites and decreased PKC levels correlate with staging of Alzheimer's disease neurofibrillary pathology. Brain Res. 1998;796:209–21. doi: 10.1016/s0006-8993(98)00347-3. others. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Laursen B, Mork A, Plath N, Kristiansen U, Bastlund JF. Cholinergic degeneration is associated with increased plaque deposition and cognitive impairment in APPswe/PS1dE9 mice. Behav Brain Res. 2013;240:146–52. doi: 10.1016/j.bbr.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Lawler HC. Turnover time of acetylcholinesterase. J Biol Chem. 1961;236:2296–301. [PubMed] [Google Scholar]
- Lee DH, Wang HY. Differential physiologic responses of α7 nicotinic acetylcholine receptors to β-amyloid1-40 and β-amyloid1-42. J Neurobiol. 2003;55:25–30. doi: 10.1002/neu.10203. [DOI] [PubMed] [Google Scholar]
- Lendvai B, Vizi ES. Nonsynaptic chemical transmission through nicotinic acetylcholine receptors. Physiol Rev. 2008;88:333–49. doi: 10.1152/physrev.00040.2006. [DOI] [PubMed] [Google Scholar]
- Levey AI, Edmunds SM, Koliatsos V, Wiley RG, Heilman CJ. Expression of m1-m4 muscarinic acetylcholine receptor proteins in rat hippocampus and regulation by cholinergic innervation. J Neurosci. 1995;15:4077–92. doi: 10.1523/JNEUROSCI.15-05-04077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SF, Wu MN, Wang XH, Yuan L, Yang D, Qi JS. Requirement of α7 nicotinic acetylcholine receptors for amyloid β protein-induced depression of hippocampal long-term potentiation in CA1 region of rats in vivo. Synapse. 2011;65:1136–43. doi: 10.1002/syn.20951. [DOI] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci. 2009;29:918–29. doi: 10.1523/JNEUROSCI.3952-08.2009. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Meunier E, Fossier P, Baux G, Amar M. Cholinergic modulation of the cortical neuronal network. Pflugers Arch. 2003;446:17–29. doi: 10.1007/s00424-002-0999-2. [DOI] [PubMed] [Google Scholar]
- Markram H, Segal M. The inositol 1,4,5-trisphosphate pathway mediates cholinergic potentiation of rat hippocampal neuronal responses to NMDA. J Physiol. 1992;447:513–33. doi: 10.1113/jphysiol.1992.sp019015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. The cholinergic lesion of Alzheimer's disease: pivotal factor or side show? Learn Mem. 2004;11:43–9. doi: 10.1101/lm.69204. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease. J Comp Neurol. 2013;521:4124–44. doi: 10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol. 1988;275:216–40. doi: 10.1002/cne.902750205. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Levey AI, Wainer BH. Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connections of the septal area, diagonal band nuclei, nucleus basalis (substantia innominata), and hypothalamus in the rhesus monkey. J Comp Neurol. 1983a;214:170–97. doi: 10.1002/cne.902140206. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983b;10:1185–201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Volicer L, Marquis JK, Mufson EJ, Green RC. Systematic regional differences in the cholinergic innervation of the primate cerebral cortex: distribution of enzyme activities and some behavioral implications. Ann Neurol. 1986;19:144–51. doi: 10.1002/ana.410190206. [DOI] [PubMed] [Google Scholar]
- Mousavi M, Hellstrom-Lindahl E. Nicotinic receptor agonists and antagonists increase sAPPα secretion and decrease Aβ levels in vitro. Neurochem Int. 2009;54:237–44. doi: 10.1016/j.neuint.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Pappy M, Leranth C, Goldman-Rakic PS. Cholinergic synaptic circuitry in the macaque prefrontal cortex. J Comp Neurol. 1995;357:603–17. doi: 10.1002/cne.903570409. [DOI] [PubMed] [Google Scholar]
- Muller DM, Mendla K, Farber SA, Nitsch RM. Muscarinic M1 receptor agonists increase the secretion of the amyloid precursor protein ectodomain. Life Sci. 1997;60:985–91. doi: 10.1016/s0024-3205(97)00038-6. [DOI] [PubMed] [Google Scholar]
- Munoz W, Rudy B. Spatiotemporal specificity in cholinergic control of neocortical function. Curr Opin Neurobiol. 2014;26:149–60. doi: 10.1016/j.conb.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagele RG, D'Andrea MR, Anderson WJ, Wang HY. Intracellular accumulation of β-amyloid(1-42) in neurons is facilitated by the α 7 nicotinic acetylcholine receptor in Alzheimer's disease. Neuroscience. 2002;110:199–211. doi: 10.1016/s0306-4522(01)00460-2. [DOI] [PubMed] [Google Scholar]
- Nai Q, McIntosh JM, Margiotta JF. Relating neuronal nicotinic acetylcholine receptor subtypes defined by subunit composition and channel function. Mol Pharmacol. 2003;63:311–24. doi: 10.1124/mol.63.2.311. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Slack BE, Farber SA, Borghesani PR, Schulz JG, Kim C. Receptor-coupled amyloid precursor protein processing. Ann N Y Acad Sci. 1993;695:122–7. doi: 10.1111/j.1749-6632.1993.tb23039.x. others. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–7. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- Nitsch RM, Wurtman RJ, Growdon JH. Regulation of APP processing. Potential for the therapeutical reduction of brain amyloid burden. Ann N Y Acad Sci. 1996;777:175–82. doi: 10.1111/j.1749-6632.1996.tb34416.x. [DOI] [PubMed] [Google Scholar]
- Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–97. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Hellstrom-Lindahl E, Lee M, Johnson M, Mousavi M, Hall R. Chronic nicotine treatment reduces β-amyloidosis in the brain of a mouse model of Alzheimer's disease (APPsw). J Neurochem. 2002;81:655–8. doi: 10.1046/j.1471-4159.2002.00874.x. others. [DOI] [PubMed] [Google Scholar]
- Nunez A, Dominguez S, Buno W, Fernandez de Sevilla D. Cholinergic-mediated response enhancement in barrel cortex layer V pyramidal neurons. J Neurophysiol. 2012;108:1656–68. doi: 10.1152/jn.00156.2012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Green KN, Liang K, Tran L, Chen Y. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2005;102:3046–51. doi: 10.1073/pnas.0408500102. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–21. doi: 10.1016/s0896-6273(03)00434-3. others. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV. Differential cholinergic modulation of synaptic encoding and gain control mechanisms in rat hippocampus. Neurosci Res. 2008;61:92–8. doi: 10.1016/j.neures.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, Antyborzec I, O'Leary VB, Zaborszky L, Herms J, Oliver Dolly J. Neurotrophin receptor p75 mediates the uptake of the amyloid β (Aβ) peptide, guiding it to lysosomes for degradation in basal forebrain cholinergic neurons. Brain Struct Funct. 2014;219:1527–41. doi: 10.1007/s00429-013-0583-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsepian SV, Anwyl R, Rowan MJ. Endogenous acetylcholine lowers the threshold for long-term potentiation induction in the CA1 area through muscarinic receptor activation: in vivo study. Eur J Neurosci. 2004;20:1267–75. doi: 10.1111/j.1460-9568.2004.03582.x. [DOI] [PubMed] [Google Scholar]
- Ovsepian SV, Herms J. Cholinergic neurons-keeping check on amyloid β in the cerebral cortex. Front Cell Neurosci. 2013a;7:252. doi: 10.3389/fncel.2013.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsepian SV, Herms J. Drain of the brain: low-affinity p75 neurotrophin receptor affords a molecular sink for clearance of cortical amyloid beta by the cholinergic modulator system. Neurobiol Aging. 2013b;34:2517–24. doi: 10.1016/j.neurobiolaging.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Shao Z, Yakel JL. β-Amyloid(1-42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J Neurosci. 2001;21:RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14:389–94. doi: 10.1038/embor.2013.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–44. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Quinn DM. Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition state. Chem Rev. 1987;87:955–79. [Google Scholar]
- Raiteri M, Marchi M, Paudice P, Pittaluga A. Muscarinic receptors mediating inhibition of γ-aminobutyric acid release in rat corpus striatum and their pharmacological characterization. J Pharmacol Exp Ther. 1990;254:496–501. [PubMed] [Google Scholar]
- Rasmusson DD. The role of acetylcholine in cortical synaptic plasticity. Behav Brain Res. 2000;115:205–18. doi: 10.1016/s0166-4328(00)00259-x. [DOI] [PubMed] [Google Scholar]
- Robitaille Y, Schut L, Kish SJ. Structural and immunocytochemical features of olivopontocerebellar atrophy caused by the spinocerebellar ataxia type 1 (SCA-1) mutation define a unique phenotype. Acta Neuropathol. 1995;90:572–81. doi: 10.1007/BF00318569. [DOI] [PubMed] [Google Scholar]
- Roher AE, Kuo YM, Potter PE, Emmerling MR, Durham RA, Walker DG. Cortical cholinergic denervation elicits vascular A β deposition. Ann N Y Acad Sci. 2000;903:366–73. doi: 10.1111/j.1749-6632.2000.tb06388.x. others. [DOI] [PubMed] [Google Scholar]
- Rossner S, Ueberham U, Schliebs R, Perez-Polo JR, Bigl V. The regulation of amyloid precursor protein metabolism by cholinergic mechanisms and neurotrophin receptor signaling. Prog Neurobiol. 1998;56:541–69. doi: 10.1016/s0301-0082(98)00044-6. [DOI] [PubMed] [Google Scholar]
- Rubio A, Gómez-Ramos A, Díaz-Hernández M, Díaz-Hernández JI, Miras-Portugal MT, Avila J. Tau protein interaction with M1 and M3 muscarinic receptors. Alzheimer's & Dementia. 2009;5:P404. [Google Scholar]
- Rusakov DA, Savtchenko LP, Zheng K, Henley JM. Shaping the synaptic signal: molecular mobility inside and outside the cleft. Trends Neurosci. 2011;34:359–69. doi: 10.1016/j.tins.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh MN, Walker DG, Reid RT, Stadnick T, Anand K, Lue LF. Absence of effect of chronic nicotine administration on amyloid β peptide levels in transgenic mice over-expressing mutated human APP (Sw Ind). Neurosci Lett. 2008;448:217–20. doi: 10.1016/j.neulet.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Sadot E, Gurwitz D, Barg J, Behar L, Ginzburg I, Fisher A. Activation of m1 muscarinic acetylcholine receptor regulates tau phosphorylation in transfected PC12 cells. J Neurochem. 1996;66:877–80. doi: 10.1046/j.1471-4159.1996.66020877.x. [DOI] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Howe WM, Gritton H, Berry AS. Deterministic functions of cortical acetylcholine. Eur J Neurosci. 2014;39:1912–20. doi: 10.1111/ejn.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V, Howe WM. Phasic acetylcholine release and the volume transmission hypothesis: time to move on. Nat Rev Neurosci. 2009;10:383–90. doi: 10.1038/nm2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri C, Stewart CA, Balfour DJ, Breen KC. Nicotine modifies in vivo and in vitro rat hippocampal amyloid precursor protein processing in young but not old rats. Neurosci Lett. 2012;514:22–6. doi: 10.1016/j.neulet.2012.02.042. [DOI] [PubMed] [Google Scholar]
- Shea TB, Spencer MJ, Beermann ML, Cressman CM, Nixon RA. Calcium influx into human neuroblastoma cells induces ALZ-50 immunoreactivity: involvement of calpain-mediated hydrolysis of protein kinase C. J Neurochem. 1996;66:1539–49. doi: 10.1046/j.1471-4159.1996.66041539.x. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Morrell F, Mesulam MM. Cholinergic synapses in human cerebral cortex: an ultrastructural study in serial sections. Exp Neurol. 1997;144:361–8. doi: 10.1006/exnr.1997.6413. [DOI] [PubMed] [Google Scholar]
- Thathiah A, De Strooper B. The role of G protein–coupled receptors in the pathology of Alzheimer's disease. Nat Rev Neurosci. 2011;12:73–87. doi: 10.1038/nrn2977. [DOI] [PubMed] [Google Scholar]
- Umbriaco D, Garcia S, Beaulieu C, Descarries L. Relational features of acetylcholine, noradrenaline, serotonin and GABA axon terminals in the stratum radiatum of adult rat hippocampus (CA1). Hippocampus. 1995;5:605–20. doi: 10.1002/hipo.450050611. [DOI] [PubMed] [Google Scholar]
- Unal CT, Pare D, Zaborszky L. Impact of basal forebrain cholinergic inputs on basolateral amygdala neurons. J Neurosci. 2015;35:853–63. doi: 10.1523/JNEUROSCI.2706-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger C, Svedberg MM, Yu WF, Hedberg MM, Nordberg A. Effect of subchronic treatment of memantine, galantamine, and nicotine in the brain of Tg2576 (APPswe) transgenic mice. J Pharmacol Exp Ther. 2006;317:30–6. doi: 10.1124/jpet.105.098566. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Lendvai B. Modulatory role of presynaptic nicotinic receptors in synaptic and non-synaptic chemical communication in the central nervous system. Brain Res Brain Res Rev. 1999;30:219–35. doi: 10.1016/s0165-0173(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- Wang HY, Li W, Benedetti NJ, Lee DH. α7 Nicotinic acetylcholine receptors mediate β-amyloid peptide–induced tau protein phosphorylation. J Biol Chem. 2003;278:31547–53. doi: 10.1074/jbc.M212532200. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Wang X, Lu JJ, Li QX, Gao CY, Liu XH. p75NTR regulates Aβ deposition by increasing Aβ production but inhibiting Aβ aggregation with its extracellular domain. J Neurosci. 2011;31:2292–304. doi: 10.1523/JNEUROSCI.2733-10.2011. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaar M, Zhai S, Pilch PF, Doyle SM, Eisenhauer PB, Fine RE. Binding of β-amyloid to the p75 neurotrophin receptor induces apoptosis. A possible mechanism for Alzheimer's disease. J Clin Invest. 1997;100:2333–40. doi: 10.1172/JCI119772. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–93. doi: 10.1084/jem.20131685. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Vassar R. Targeting the β secretase BACE1 for Alzheimer's disease therapy. Lancet Neurol. 2014;13:319–29. doi: 10.1016/S1474-4422(13)70276-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborszky L, Csordas A, Mosca K, Kim J, Gielow MR, Vadasz C. Neurons in the basal forebrain project to the cortex in a complex topographic organization that reflects corticocortical connectivity patterns: an experimental study based on retrograde tracing and 3D reconstruction. Cereb Cortex. 2015;25:118–37. doi: 10.1093/cercor/bht210. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner T, Perez-Polo JR, Schliebs R. β-Secretase BACE1 is differentially controlled through muscarinic acetylcholine receptor signaling. J Neurosci Res. 2004;77:250–7. doi: 10.1002/jnr.20152. [DOI] [PubMed] [Google Scholar]