Abstract
Schizophrenia (SZ) is a devastating psychiatric condition affecting numerous brain systems. Recent studies have identified genetic factors that confer an increased risk of SZ and participate in the disease etiopathogenesis. In parallel to such bottom-up approaches, other studies have extensively reported biological changes in patients by brain imaging, neurochemical and pharmacological approaches. This review highlights the molecular substrates identified through studies with SZ patients, namely those using top-down approaches, while also referring to the fruitful outcomes of recent genetic studies. We have sub-classified the molecular substrates by system, focusing on elements of neurotransmission, targets in white matter-associated connectivity, immune/inflammatory and oxidative stress-related substrates, and molecules in endocrine and metabolic cascades. We further touch on crosstalk among these systems and comment on the utility of animal models in charting the developmental progression and interaction of these substrates. Based on this comprehensive information, we propose a framework for SZ research based on the hypothesis of an imbalance in homeostatic signaling from immune/inflammatory, oxidative stress, endocrine and metabolic cascades that, at least in part, underlies deficits in neural connectivity relevant to SZ. Thus, this review aims to provide information that is translationally useful and complementary to pathogenic hypotheses that have emerged from genetic studies. Based on such advances in SZ research, it is highly expected that we will discover biomarkers that may help in the early intervention, diagnosis or treatment of SZ.
Keywords: Schizophrenia, neurotransmission, white matter, inflammation, oxidative stress, endocrine and metabolism
Introduction
Schizophrenia (SZ) is one of the most devastating mental conditions with a lifetime prevalence of about 0.7-1%.1 If we include the prevalence of subclinical psychotic experiences and symptoms, which are likely to share similar biological mechanisms with SZ, the prevalence increases to about 8%.2 The cross-sectional clinical manifestations and course of SZ are heterogeneous with a complex phenomenology. However, even among clinical psychiatrists, a more simplified sub-classification of clinical symptoms is frequently used to facilitate biological and translational studies3: positive symptoms (e.g., hallucinations, delusions, and disorganized thoughts and speech), negative symptoms (e.g., apathy, affective flattening, social withdrawal), and cognitive dysfunction (e.g., deficits in working memory, verbal memory). Although SZ typically manifests in the second decade of life, abnormal neurodevelopmental processes are thought to begin during prenatal and perinatal stages.4
Several ‘biological’ hypotheses of SZ were originally proposed because of the effects of drugs. The dopamine hypothesis of SZ evolved from multiple observations and studies including findings that antipsychotic drugs used for SZ block dopamine D2 receptors.5-7 Years later the glutamatergic hypothesis of SZ was proposed based on observations that phencyclidine or ketamine mimic SZ-like manifestations, even including some aspects of negative symptoms and cognitive dysfunction, by blocking N-methyl-D-aspartate receptors (NMDAR).8, 9
SZ is now considered a polygenic condition;10-12 many candidate susceptibility genes have been identified, however, individual effect sizes are modest in sporadic cases. Single nucleotide polymorphisms (SNP), copy number variations, and de novo mutations have been implicated in conferring risk of SZ.13, 14 In addition, in the contexts of both common and rare variants, susceptibility factors that have been suggested for SZ confer risk for other mental conditions, such as bipolar disorder and autism.10, 15-18 This is reasonable given that the current diagnostic criteria, such as the Diagnostic and Statistical Manual of Mental Disorders (DSM), emphasize clinical reliability and utility rather than etiological validity.19
In addition to genetic studies (bottom-up approach), years of research with clinical subjects and biospecimens have implicated multiple molecular targets of SZ. In this review, we discuss the different ‘molecular’ substrates of SZ that have been identified primarily through human (patient) studies, namely those using top-down approaches, and sub-classify them by biological system (Table 1): neurotransmission, white matter-associated connectivity, immune/inflammatory response and oxidative stress, endocrine system, and metabolic cascades. For each system, we focus on evidence from brain imaging, neurochemical, postmortem, genetic, and clinicopharmacological studies (Table 2). Lastly, we describe the possible integration of these systems and additional evidence from animal models of SZ under an overall perspective of an in-depth understanding of the disease pathology and translational application.
Table 1.
Molecular and Cellular1 Substrates of Schizophrenia, Organized by System
| Neurotransmission |
| Dopamine |
| D2 Receptor |
| D1 Receptor |
| Homovanillic acid |
| Tyrosine hydroxylase |
| 5HT2A receptor |
| Glutamate |
| NMDA Receptor |
| Glutamate receptor, ionotropic, NMDAR 2A (GRIN2A) |
| Glutamate receptor, ionotropic, AMPA 1 (GRIA1) |
| Activity-regulated cytoskeleton-associated protein (ARC) signaling complex |
| Glutamine |
| GABA |
| GAD67 |
| Parvalbumin (PV) |
| Kv3.1 |
| KCNS3 |
| Somatostatin |
| Cholecystokinin |
| Nicotinic acetylcholine receptors (nAChRs) |
| Potassium channel tetramerization domain containing 13 (KCTD13) |
| Contactin 4 (CNTN4) |
| P21 protein-activated kinase 6 (PAK6) |
| Neuroligin 4, X-linked (NLGN4X) |
| Disrupted in schizophrenia 1 (DISCI) |
| P21 protein-activated kinase 7 (PAK7) |
| N-thylmaleimide sensitive factor (NSF) |
| Synapsin II (SYN2) |
| Dendritic Spines |
| White matter-associated connectivity |
| Proteolipid protein 1 (PLP1) |
| Myelin-associated glycoprotein (MAG) |
| Myelin oligodendrocyte glycoprotein (MOG) |
| oligodendrocyte transcription factor 2 (OLIG2) |
| Neuregulin-1 (NRG1) |
| Receptor tyrosine-protein kinase erbB4 (ERBB4) |
| reticulon 4 (RTN4/NOGO) |
| Cyclic-nucleotide-3′-phosphodiesterase (CNP1) |
| Akyrin-3 (ANK3) |
| Zinc finger binding protein 804A (ZNF804A) |
| L-type voltage-dependent calcium channel CAV1.2 (CACNA1C) |
| MicroRNA-137 (MIR137) |
| Disrupted in schizophrenia 1 (DISC1) |
| Myelin |
| Node of Ranvier |
| Oligodendrocytes |
| Immune/inflammatory response and oxidative stress |
| IL-1β |
| IL-2 |
| IL-6 |
| TNF-α |
| soluble IL-2 receptor |
| IL-12 |
| IFN-γ |
| IL-8 |
| Translocator protein (TSPO) |
| Major histocompability complex (MHC) |
| Glutathione (GSH) |
| Microsomal glutathione S -transferase 1 (MGST1) |
| Superoxide dismutase (SOD) |
| Catalase |
| Inflammation |
| Oxidative stress |
| Microglia |
| T Cells |
| Macrophages |
| Endocrine system |
| Adrenocorticotropic hormone (ACTH); proACTH |
| Corticosterone; Cortisol |
| Glucocorticoids; Glucocorticoid receptors |
| Estradiol |
| Hypothalamic-pituitary-adrenal (HPA) axis |
| Metabolic cascades |
| Glucose |
| Insulin; proinsulin |
| Akt |
| Leptin |
| Ghrelin |
| Apolipoprotein1 |
| Melanocortin 4 receptor |
| 5HT2C receptor |
Cellular substrates appear in italics
Table 2.
Summary of Clinical Evidence by System
| System | Brain Imaging | Neurochemical | Postmortem | Genetic | Clinicopharmacological |
|---|---|---|---|---|---|
| Neurotransmission | +++ | +++ | ++ | +++ | ++ |
| White matter-associated connectivity | +++ | + | ++ | ++ | ++ |
| Immune/Inflammatory response and oxidative stress | + | ++ | + | ++ | + |
| Endocrine system | ++ | +++ | ++ | +/− | ++ |
| Metabolic cascades | ++ | ++ | ++ | ++ | ++ |
+++, strong evidence; ++ sufficient evidence; + additional evidence needed; +/− some evidence
The goal of this review article is to provide comprehensive information that is translationally useful and complementary to pathogenic hypotheses that have recently emerged from genetic studies. To address this goal, we propose a framework for SZ research based on the hypothesis of an imbalance in homeostatic signaling that, at least in part, underlies deficits in neural connectivity relevant to SZ. More concretely, we describe how inflammatory, oxidative stress, endocrine, and metabolic homeostatic signaling processes mediate and pathologically modulate neurotransmission and myelinated tracks. Given that many comprehensive review articles on psychiatric genetics and animal models have been published recently,20-24 we only touch on the critical conceptual viewpoints in these areas.
By referring to the information from genetic studies, we can address the question of whether molecular substrates identified through human patient studies are primary or secondary. In particular, molecular studies in first episode psychosis and individuals with high genetic risk of SZ combined with convergent evidence from genetic and animal models can help determine the central disease processes. The successful integration of pathogenic-oriented (bottom-up) and patient phenotype-oriented (top-down) research has precedence in many other diseases, such as cancer, metabolic syndrome and Alzheimer's disease.25-29
Neurotransmission
Dopamine
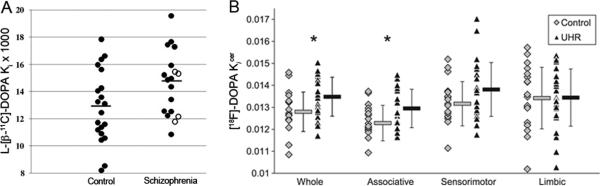
Molecular brain imaging studies have provided useful insights into dopamine, glutamate, and γ-aminobutyric acid (GABA) neurotransmission in SZ. The majority of the molecular imaging studies using positron emission tomography (PET) and single photon emission computerized tomography (SPECT) have suggested that presynaptic striatal dopamine is elevated and dopamine release is increased in subjects with SZ. PET studies have found that striatal L-[β-11C]DOPA or [18F]DOPA uptake is elevated in subjects with SZ as well as subjects showing prodromal symptoms of SZ, suggesting an elevation in presynaptic striatal dopamine in SZ (Figure 1).30-34 A PET study using [11C]raclopride and SPECT studies using [123I]IBZM found significantly greater amphetamine-induced reductions in the binding potential of the radiotracers in the striatum of subjects with SZ compared to controls, suggesting that there is enhanced dopamine release in SZ.35-37 However, it has been suggested that the elevated dopamine observed in SZ is linked to psychosis rather than the disease itself.38
Figure 1.
L-[β-11C]-DOPA and [18F]-DOPA positron emission tomography (PET) imaging. (A) Individual ki values in the left caudate nucleus. Horizontal lines represent mean values of the groups. In the schizophrenia group, closed circles indicate antipsychotic drug-naïve patients while open circles indicate drug-free patients. There was a significant group difference in ki values for the left caudate nucleus. (reproduced with permission from Nozaki et al.33) (B) Individual and group mean±SD [18F]-DOPA uptake (kicer) (min–1) values in the whole, associative, sensorimotor, and limbic striatum. Data in the healthy control group are represented by grey diamonds and data in the ultra-high risk group (UHR) by black triangles. kicer values were significantly higher in the UHR than control group in the whole and associative striatum (*p<.05), indicating elevated dopamine synthesis capacity. (reproduced with permission from Egerton et al.34)
It has been further shown that subjects with SZ have a significant increase in dopamine D2 receptor (D2R) availability in the associative striatum, specifically in the precommissural dorsal caudate, but not in the ventral or the sensorimotor striatum, after dopamine depletion.39 A meta-analysis of 13 studies using PET and SPECT found a significant, but small, elevation in striatal D2R density in subjects with SZ.40 Studies analyzing D2R availability in the thalamus, anterior cingulate cortex and temporal cortex have found both decreased and unaltered D2R availability in SZ.41 Additionally, a recent genome-wide association study (GWAS) found that the D2R gene is within a SZ-associated locus.42
Results of PET studies using [11C]SCH23390 and [11C]NNC112 to image prefrontal cortex dopamine D1 receptor (D1R) binding are contradictory, reporting reduced, unaltered and increased receptor binding in SZ patients.43-45 A meta-analysis of seven postmortem studies and one PET study did not find an elevation in D1R in SZ.46 Studies measuring levels of homovanillic acid, a metabolite of dopamine, in the cerebrospinal fluid (CSF) of subjects with SZ are inconsistent; some report no difference between SZ and control subjects but others report lower levels in SZ.47, 48
Abnormalities in dopamine neurotransmission have also been suggested by postmortem studies. Changes in the density and mRNA levels of some classes of dopamine receptors have been suggested in the prefrontal cortex, striatum, and hippocampus of SZ subjects.49-51 Reductions in the density of tyrosine hydroxylase, an enzyme involved in dopamine synthesis, and the density of dopamine membrane transporter immunoreactive axons in the prefrontal cortex have been reported in SZ.52 A reduced density of tyrosine hydroxylase immunoreactive axons in the entorhinal cortex has also been reported.53
In summary, the main finding on dopaminergic neurotransmission is that a state of overstimulation of D2R in the associative striatum exists in SZ starting early in the prodromal phase and corresponds with psychosis as well as with the therapeutic response to antipsychotics.31, 39, 54 Other symptom domains are thought to relate to deficits in dopamine transmission in cortical and extrastriatal regions, but conclusive evidence for this has not emerged yet.55
Serotonin
Most typical antipsychotics are D2R antagonists, whereas several atypical antipsychotic drugs also target serotonin receptors, in particular the 5HT2A receptor, for antipsychotic efficacy.56 Furthermore, indoleamine hallucinogens such as psilocybin, can elicit SZ-like symptoms that can be blocked by 5HT2 antagonists.57 Postmortem studies have shown a reduction in the density of 5HT2A receptors in the prefrontal and frontal cortex in SZ.58 These results suggest involvement of serotonin in the pathophysiology of SZ, or at least in psychotic manifestations.
Glutamate
Pharmacological evidence has also implicated glutamate neurotransmission in SZ. Acute administration of NMDAR antagonists, such as phencyclidine (PCP) or ketamine, transiently induces symptoms typically associated with psychosis, including positive, negative and cognitive deficits.59, 60 This observation led to a hypothesis for NMDAR glutamate dysregulation as a prominent pathophysiological feature of SZ. Studies using autopsied brains have shown reduced numbers of dendritic spines on pyramidal neurons in SZ.61, 62 Furthermore, analysis of GWAS and genetic and gene expression studies have indicated the significance of genes involved in glutamate receptor signaling.12, 14, 42, 63 These include the NMDAR subunit gene GRIN2A, glutamate receptor, ionotropic, AMPA 1 (GRIA1), and genes involved in the activity-regulated cytoskeleton-associated protein (ARC) signaling complex.
Many 1H magnetic resonance spectroscopy (MRS) studies have measured glutamate, glutamine or the sum of glutamate and glutamine (Glx) in subjects with SZ; some studies also include GABA in the measurement.64 Several reports have indicated that glutamatergic levels in the medial prefrontal cortex and anterior cingulate cortex are elevated in medication-naïve and unmedicated SZ subjects; however, studies also report that glutamatergic levels tend to decrease during the disease course, possibly reflecting treatment response and/or disease progression (Table 3).8, 64-68 A recent review summarizes the findings of many MRS studies with an emphasis on regional specificity: medication-naïve and unmedicated SZ subjects have elevated glutamatergic levels in the medial prefrontal cortex, but not in the dorsolateral prefrontal cortex and hippocampus, while studies on the thalamus report more variable effects with unaltered, increased or decreased glutamatergic levels (Table 3).64, 69 Meanwhile, studies with CSF have shown that the levels of glutamate are similar between control and medicationnaïve or unmedicated SZ subjects.70-72 Lastly, some studies have explored a role for glutamate in SZ pathophysiology:73, 74 ketamine administration to rodents elicited hypermetabolism in the CA1 sub-region of the hippocampus, while repeated exposure shifted the hippocampus to a hypermetabolic basal state with concurrent atrophy. Similarly, hypermetabolism of the hippocampus, in particular the CA1 sub-region, has also been seen in SZ patients, which may be a predictive factor of hippocampal atrophy.73, 74
Table 3.
Summary of glutamate and GABA 1H MRS studies
| Glutamatergic level (subject vs control) | Brain Region | Disease stage | Antipsychotic Status | References |
|---|---|---|---|---|
| ↑ | MPFC, ACC | E, C | - | Poels et al.64 |
| ↓ | MPFC, ACC | Marsman et al.65 | ||
| ARMS | - | |||
| E | - | |||
| E | + | |||
| C | NR, + | |||
| - | MPFC, ACC | C | + | Poels et al.64 |
| - | DLPFC | E, C | -, + | Poels et al.64 |
| - | Hippocampus | Poels et al.64, Marsman et al.65 | ||
| ARMS | - | |||
| C | NR | |||
| E, C | + | |||
| ↓ | Hippocampus | NR | -, + | Stan et al.69 |
| ↓ | Thalamus | ARMS | -, + | Poels et al.64 |
| ↑ | Thalamus | E | - | Poels et al.64 |
| - | Thalamus | Poels et al.64, Marsman et al.65 | ||
| ARMS | - | |||
| E | - | |||
| C | + |
| GABA level (subject vs control) | Brain Region | Disease stage | Antipsychotic Status | References |
|---|---|---|---|---|
| - | Frontal Lobe | E | + | Goto et al.81 |
| ↑ | ACC | C | + | Ongur et al.84 |
| - | ACC | C | + | Tayoshi et al.82 |
| ↓ | ACC | C, older age | + | Rowland et al.85 |
| ↑ | MPFC | C | - | Kegeles et al.66 |
| - | MPFC | C | + | Kegeles et al.66 |
| - | DLPFC | C | -, + | Kegeles et al.66 |
| ↓ | Basal Ganglia | E | + | Goto et al.81 |
| - | Basal Ganglia | C | + | Tayoshi et al.82 |
| ↑ | Parieto-Occipital Cortex | C | + | Ongur et al.84 |
| ↓ | Visual Cortex | E, C | -, + | Yoon et al.83 |
Abbreviations: MPFC, medial prefrontal cortex; ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; E, first-episode or early; C, chronic; ARMS, at-risk mental state; -, unmedicated; +, medicated; NR, not reported
In addition, reports of patients who have autoantibodies to the NMDAR (anti-NMDAR encephalitis) and show psychotic symptoms including visual or auditory hallucinations and paranoid thoughts have supported the involvement of glutamate and NMDAR in the pathophysiology of SZ and psychosis.75-78 The causality of the autoantibody to psychosis, at least in this specific disease condition, has been demonstrated in cases where an ovarian teratoma is present: tumor removal and intravenous immunoglobulin or intravenous steroids can reverse the psychiatric symptoms within weeks.79, 80 Collectively, these pharmacological, clinical, genetic, and autoimmune studies have repeatedly implicated glutamate dysfunction in SZ.
GABA
There is still a technical debate about measuring GABA consistently in different scanners and institutions. However, one group found reduced GABA concentrations in the basal ganglia, but not in the frontal lobe, in early stage SZ subjects.81 In contrast, another group has reported that GABA levels were elevated in the medial prefrontal cortex in unmedicated SZ subjects.66 As for chronic SZ, studies have reported unaltered GABA concentrations in the basal ganglia, reduced concentrations in the visual cortex, and elevated levels in the parieto-occipital cortex.82-84 Unaltered and elevated GABA concentrations in the anterior cingulate cortex have been reported in chronic SZ, while a more recent study found reduced GABA concentrations in the anterior cingulate of older subjects with chronic SZ (Table 3).82, 84, 85 Recently, a study using MRS and magnetoencephalography (MEG) to measure resting GABA and glutamate concentrations and stimulus-induced gamma oscillations in the occipital cortex of healthy subjects, reported that there was no correlation between GABA, glutamate, or the GABA/glutamate ratio and gamma peak frequency or gamma amplitude.86 In addition, several studies have measured GABA levels in the plasma and CSF of unmedicated SZ patients and reported no major differences between SZ and controls, however, one report found a significant decrease in mean CSF GABA levels after neuroleptic treatment.87-89 One study reported significantly lower CSF GABA levels in patients whose duration of illness was 4 years or less compared to subjects whose duration of illness was greater than 4 years.90 However, another study reported similar GABA levels between acute SZ subjects and controls but increased CSF GABA levels in chronic SZ subjects.91 In general, MRS studies on GABA in SZ are scarce and show inconclusive results at this time.
Postmortem studies have provided convincing evidence of GABAergic changes in SZ.92, 93 These studies have consistently reported alterations in GABAergic interneurons in the prefrontal cortex. These alterations include decreased glutamic acid decarboxylase 67 (GAD67) and parvalbumin (PV).94-98 Additionally, some potassium channels (Kv3.1 and KCNS3) that are predominately expressed in PV positive neurons, are decreased in the prefrontal cortex in SZ.99, 100 PV positive neurons are fast spiking, synchronize pyramidal neuron firing and play a role in the generation of gamma oscillations.101 Thus, deficits in this population of neurons may contribute to the cognitive deficits observed in SZ.101 Decreased somatostatin and cholecystokinin mRNA levels have also been reported in SZ.102
α7-nicotinic receptor
A high percentage of SZ patients smoke cigarettes. It has been shown that cigarette smoking improves sensory gating, P50 inhibition, and prepulse inhibition in SZ.103-105 The P50 sensory deficit observed in SZ might be genetically linked to the locus of the α7-nicotinic acetylcholine receptor gene, CHRNA7, on chromosome 15q14.106 Multiple linkage studies have also supported this evidence.107, 108 Postmortem studies using [125I]alpha-bungarotoxin have shown fewer nicotinic receptors in the hippocampus, the reticular nucleus of the thalamus, and the cingulate cortex of subjects with SZ.109-111 Brain imaging studies to validate this hypothesis using [123I]5-IA-85380 and 2-[18F]FA, radiotracers for high affinity nicotinic acetylcholine receptors, are under way.112, 113
Synaptic assembly
As described above in the context of glutamate, some genetic susceptibility factors for SZ have distinct synaptic functions in a broader sense.12, 14, 42, 114 Some of the genes involved in synaptic function and plasticity that have been identified within SZ-associated loci include potassium channel tetramerization domain containing 13 (KCTD13), contactin 4 (CNTN4), p21 protein-activated kinase 6 (PAK6), neuroligin 4, X-linked (NLGN4X), and genes related to the postsynaptic ARC complex.12, 42 Functional studies of such candidates, including disrupted in schizophrenia 1 (DISC1) and p21 protein-activated kinase 7 (PAK7), also support synaptic roles.115-117 Microarray analysis of the prefrontal cortex found that the expression of genes related to the presynaptic secretory machinery [for example, N-thylmaleimide sensitive factor (NSF) and synapsin II (SYN2)] were decreased in SZ.118 Although brain imaging studies of synaptic changes in SZ are lacking, the field awaits the development of new techniques to assess synaptic changes in SZ.
As described, multiple lines of evidence suggest that multiple types of neurotransmission, in particular dopaminergic, glutamatergic, and GABAergic neurotransmission, are altered in the brains of SZ patients. However, at this time it is unclear if the reported changes are causal or secondary, but in either case, the changes are critical for the pathophysiology.
White matter-associated connectivity
Neurotransmitters play a role in cell-cell communication in the brain, but distant communication is also dependent on proper transduction of action potentials along axon tracts. White matter regions contain long-range axonal connections insulated by myelin, a lipid-dense material produced by oligodendrocytes. Signaling between oligodendroyctes and axons mediates the formation of nodes of Ranvier (NoR) and internodal myelin sheathes.119 The myelin sheathes increase electrical resistance, allowing for faster propagation of electrical signals along axons. Dysmyelination can affect neuronal connectivity; decreased white matter integrity has been correlated with functional deficits in demyelinating diseases.120 Although there is no robust demyelination in the brain in SZ, many reports have indicated a deficit in white matter associated connectivity.121
Diffusion tensor imaging (DTI) with MRI can measure the diffusion of water molecules along white matter bundles. Fractional anisotropy (FA), a metric of the rate of diffusion along axons and a measure of myelin integrity, is reduced in SZ patients.121-123 Deep white matter tracts in the frontal and temporal lobes interconnecting to the thalamus, cingulate gyrus, amygdala, insula, hippocampus, and occipital lobe are reportedly those most affected in SZ.124 Changes are observed in chronic, first episode, and adolescents with early onset SZ as well as subjects in prodromal stages and adolescents at high clinical risk for developing SZ.125, 126 A longitudinal study in SZ patients also found heterogeneous decreases in white matter volume (total cerebral, frontal, temporal, parietal) over the disease course, primarily at the onset of the disorder.127 Notably, the regions that are prominently affected are those still undergoing myelination during late adolescence. Furthermore, functional MRI (fMRI) studies now suggest altered connectivity in SZ patients. Recently, it was reported that the global brain signal (the average signal across all voxels in the brain derived from fMRI) is altered in SZ subjects, but not in subjects with bipolar disorder.128 Time series of resting-state fMRI suggest that patients with SZ have reduced functional network strength and altered brain network topology.129-131 Also, recent investigations have documented robust and replicable dysconnectivity in thalamocortical systems in chronic SZ.132, 133 Patients with SZ also show impaired task-dependent communication between brain regions including reduced suppression of default network activity and weaker activation of task-associated regions.134-136 Several recent studies combining functional and structural imaging have found correlations between reduced FA and altered brain activity in specific neural circuits.137 Collectively, these structural and functional investigations repeatedly point to profound alterations in major neural systems related to executive control.138, 139
Postmortem tissue analyses of SZ brains have found abnormalities in oligodendrocytes, the brain's source of myelin.140 For example, a report indicated a decrease in the number and density of oligodendrocytes in the grey and white matter of Brodmann area 9.141 Additionally, microarray and real-time quantitative polymerase chain reaction (qPCR) studies identified changes in the expression of oligodendrocyte and myelin-related genes such as proteolipid protein 1 (PLP1), myelin-associated glycoprotein (MAG), myelin oligodendrocyte glycoprotein (MOG), and oligodendrocyte transcription factor 2 (OLIG2) in the prefrontal cortex.142, 143 Similar studies have noted expression changes in myelination-related genes in additional brain areas including the temporal cortex, cingulate cortex, and the hippocampus.144-146
Furthermore, a number of myelin-related genes have been genetically linked to an increased risk of SZ.147 These include genes encoding neuregulin-1 (NRG1), receptor tyrosine-protein kinase erbB-4 (ERBB4), reticulon 4 (RTN4/NOGO), PLP1, MAG, MOG, OLIG2, 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNP1) and ankyrin-3 (ANK3).147-156 Several of the molecules that are genetically associated with SZ also have altered expression in the SZ brain, such as PLP1, MAG, MOG and OLIG2. While no myelin-related genes reached significance in a large GWAS set for SZ,11 a few (NRG1, ERBB4, ANK3) had sub-threshold associations (p-value < 5 × 10−5), and broader pathway analysis revealed a significant association for genes in myelination-related pathways and those with specific glial cell-type functions.157, 158 NRG1 and its erbB receptors are important signaling molecules for axon guidance and myelination.150 Unaffected subjects carrying a risk allele for NRG1 have reduced white matter density.159 ANK3 is an axonal protein that helps form the scaffolding for NoR and has also been identified as one of the most significant genetic risk factors shared between SZ and bipolar disorder.156 Another recent study identified several NoR proteins affected in SZ, including ANK3 and its interactors.160 These results highlight the importance of neuron-glia interactions at the NoR in psychiatric illness.161 In addition, while not traditionally identified as myelin/oligodendrocyte-associated genes, several susceptibility genes for SZ that encode the zinc finger binding protein 804A (ZNF804A), L-type voltage-dependent calcium channel CAV1.2 (CACNA1C), DISC1, and microRNA-137 (MIR137) have been associated with white matter phenotypes in SZ patients, as well as, control subjects that carry risk alleles.161, 162
Lastly, we refer to some interesting aspects of clinicopharmacology. Studies have suggested that treatment with antipsychotics, lithium, antidepressants, and electroconvulsive therapy may share common myelin-protective signaling mechanisms.163 One study found that higher white matter integrity in first episode SZ was predictive of responsiveness to antipsychotics.164
In summary, data from imaging and postmortem studies have suggested the presence of abnormal myelination in SZ. Genetic studies have suggested the importance of neuron-oligodendrocyte interactions. Given that the typical onset of SZ coincides with the final stages of myelination in the frontal and temporal cortices, aberrant myelin development may contribute to the presentation of symptoms in patients.
Immune/inflammatory response and oxidative stress
Epidemiological studies have suggested that prenatal infections with viruses, bacteria, or parasites increases the risk of their offspring developing SZ in adulthood.165 Given that the full onset of SZ is frequently in late adolescence and young adulthood, addressing mechanisms and mediators of the long pathological trajectory from initial predisposition to disease onset remains a central question of SZ research.4, 166
Cytokines
An imbalance in the cytokine/chemokine network may be an explanation for why multiple infections can increase the risk of SZ; the disturbance of this network during the neonatal period may alter fetal brain development.165 In accordance with this hypothesis, studies on serum levels of cytokines in SZ suggest that IL-1β, IL-6, TNF-α and IL-2 are increased in SZ.167-171 However, it should be noted that there are also studies that report unaltered serum levels of IL-1β, IL-6, TNF-α and IL-2 in SZ.167, 169, 172, 173 A recent meta-analysis also found that serum levels of IL-1β, the soluble IL-2 receptor (sIL-2R), IL-6 and TNF-α were increased in drug-naïve first episode psychosis (SZ, schizophreniform disorder, delusional disorder and brief reactive psychosis), however, IL-2, IL-4 and IFN-γ levels were not altered.174 A meta-analysis of antipsychotic-induced cytokine changes in SZ found that antipsychotic treatment increased IL-12 and sIL-2R and reduced IL-1β and IFN-γ plasma levels, with treatment duration having no effect.175 Additionally, two studies found an association between maternal cytokine levels (TNF-α and IL-8) and increased risk of SZ in the offspring.176, 177 Studies that compare such inflammatory mediators in the CSF of controls and SZ subjects (and prodromal subjects) are also available. In particular, a recent study that purely utilized unmedicated first onset SZ and prodromal subjects supports alterations of inflammatory mediators in psychotic subjects, including the IL-6 cascade and IL-8.178
Microglia
Chronically activated T cells, macrophages and microglia produce cytokines that may impact brain development.167, 179, 180 The potential involvement of microglia is of particular interest from a mechanistic viewpoint since recent studies have indicated a role for microglia in synaptic pruning and maintenance.181-183 Aberrant synaptic pruning in adolescence is one of the important working hypotheses in SZ pathology.184 Furthermore, a study using in vivo two-photon imaging has shown that blockade of excess synaptic pruning in late adolescence ameliorates deficits in prepulse inhibition, at least in an animal model that may model some SZ manifestations.116 Taken together, reactivation during adolescence of microglia pathologically primed in early development may be a likely scenario to account for the mechanism. Some postmortem studies have found an increase in the density or activation of microglia in SZ: however, other studies found no difference between controls and subjects with SZ.179, 185-189 A more recent study that used next generation sequencing and Western blotting has shown that microglial markers are upregulated in the dorsolateral prefrontal cortex of SZ.190 Furthermore, immunohistochemistry for the human leukocyte antigen (HLA) class II molecules (HLA-DP, -DQ and –DR), which are expressed on antigen-presenting cells, revealed more positive cells in the white matter of subjects with SZ; the authors also indicated that these positive cells morphologically resembled microglia.190, 191
Although these studies provide promising evidence for the involvement of microglia and the inflammatory cascade in SZ, we also need to be prudent in interpreting data from the autopsied brain because confounding factors exist and the plausibility of detecting adolescent pathology from typically aged autopsied brains is unknown. Validation of ‘brain’ inflammation in SZ at the time of psychosis has recently been pursued using PET imaging with tracers that target molecules changed in neuroinflammation. In particular, radioligands that target the translocator protein (TSPO) (previously known as the peripheral benzodiazepine receptor) have been frequently used.192 Activation of microglia results in an increase in the number of mitochondria per cell and an increase in the density of the peripheral benzodiazepine receptor in the outer mitochondrial membrane.193, 194 Two PET studies found an increase in the regional binding potential of [11C]PK11195, a classic TSPO tracer, in subjects with a psychotic disorder or SZ.195, 196 Given the limitations in the [11C]PK11195 tracer, such as high nonspecific binding, high lipophilicity and poor signal-to-noise ratio, recently new PET tracers (e.g., [11C]PBR28 and [11C]DPA713) have been developed and are beginning to be used.197, 198 A general limitation of TSPO brain imaging to test neuroinflammation is that this protein can also be influenced by mitochondrial deficits and oxidative stress that may also occur in SZ.199 Assessment of brain inflammation in SZ using radioligands that target other inflammatory markers will shed further light on the role of inflammation in this disease.
Autoimmunity
Multiple combinations of environmental stressors and genetic factors contribute to SZ pathology.165, 200-202 Aberrant activation and an imbalance of immune/inflammatory cascades are likely due to both environmental influences and host susceptibility. Epidemiological data linking autoimmune diseases and SZ has recently been reviewed: celiac disease, autoimmune thyroiditis, Graves’ disease, type 1 diabetes, multiple sclerosis, autoimmune hepatitis, psoriasis, and Sjögren's syndrome are all associated with SZ, whereas, there is a very unique and negative association with rheumatoid arthritis.203, 204 The increased co-morbidity of SZ with many autoimmune diseases supports the idea that SZ has an intrinsic susceptibility to the immune/inflammatory response. This notion is further supported by GWAS, which found an association of SZ with the major histocompatibility complex (MHC) region.11, 42, 205, 206 Additionally, as discussed in the neurotransmission section, in some cases of anti-NMDAR encephalitis the presence of the autoantibody appears to be related to the psychiatric symptoms.79, 80
Oxidative Stress
The inflammatory response is interconnected with oxidative stress.207 The significance of oxidative stress in SZ pathology is that the stress can lead to synaptic deterioration, abnormal myelination and interneuron deficits.207, 208 Oxidative stress occurs when antioxidant defense mechanisms fail to counterbalance reactive oxygen species (hydrogen peroxide, superoxide radicals, and hydroxyl radicals). Imbalances in the oxidative and antioxidant defense systems have been reported in SZ.208, 209 Studies with biospecimens (autopsied brain, olfactory cells, CSF and blood) have indicated changes in glutathione (GSH), microsomal glutathione S-transferase 1 (MGST1), superoxide dismutase (SOD), and catalase.209-214 A recent report indicates a significant reduction in CSF SOD-1 from recent onset SZ compared with matched controls, however, there was no difference in the level of SOD-1 in chronic SZ.212 The most reproducible evidence may be a reduction of GSH in the blood. Because access to biospecimens from unmedicated first onset SZ and prodromal subjects have become more frequently available, the validation of peripheral signs of oxidative stress with such subjects is awaited.
Several studies have investigated ‘brain’ oxidative stress by directly measuring GSH in the brain of living SZ patients. Although the measurement of GSH via MRS is a promising approach, the data thus far are inconsistent.208 An initial study found a 52% reduction in the GSH level in the medial prefrontal cortex of drug-free subjects with SZ.215 Two subsequent studies, focusing on the anterior cingulate and the posterior medial frontal cortex, did not find a difference in the GSH level in medicated subjects with SZ.216, 217 Finally, another study reported an increase in GSH levels in the medial temporal lobe of subjects with first episode psychosis.218
In summary, many clinical and epidemiological studies have supported immunological deficits in SZ, which are mediated, at least in part, by microglia. Such immuno-inflammatory changes are interconnected with deficits in antioxidant cascades and may affect neural connectivity.
Endocrine system
Although there are contradictory reports, multiple types of studies have suggested that the hypothalamic-pituitary-adrenal (HPA) axis is affected in first episode psychosis and SZ.219 An MRI study comparing first episode and chronic SZ subjects to controls found increased and reduced pituitary volumes in first episode and chronic SZ, respectively.220 Additional MRI studies support the finding of an increased pituitary volume in first episode SZ, as well as, an increased pituitary volume in subjects at high risk for developing psychosis (i.e., at-risk mental state).221, 222 However, other studies have not found a change in pituitary volume in first episode or chronic SZ.223-225 A smaller pituitary volume in unmedicated SZ has also been reported.226 Findings from studies analyzing the volume of the hypothalamus in SZ are inconsistent and report both an increased volume as well as no change.227-230
Consistent with some brain imaging reports, a fraction of unmedicated, medicated, or chronic SZ patients have displayed elevated baseline adrenocorticotropic hormone (ACTH) and cortisol levels.231-234 ACTH is secreted from the anterior pituitary gland in response to the hypothalamus releasing corticotropin-releasing hormone (CRH). Higher mean ACTH and cortisol levels have also been shown in first episode unmedicated SZ.219, 235 Furthermore, the North American prodrome longitudinal study reported higher baseline cortisol levels in prodromal (i.e., at-risk mental state) subjects and found that baseline cortisol levels correlated with symptom progression: prodromal subjects who transitioned to psychotic level symptoms had significantly higher baseline cortisol levels compared to controls and prodromal subjects in remission.236 However, other studies have not found elevated baseline cortisol levels in first episode psychosis and first episode SZ.219, 237 Interestingly, antipsychotics (risperidone, haloperidol, olanzapine or flupenthixol) have been shown to significantly reduce cortisol levels.168, 238 Consistent with some peripheral measures of ACTH, a postmortem analysis of pituitary glands found that the level of proACTH was elevated in pituitaries from subjects with SZ compared to controls.239 Postmortem studies have also suggested a reduction in glucocorticoid receptors: glucocorticoid receptor mRNA expression was reduced in the basolateral/lateral nuclei of the amygdala, the frontal and temporal cortex and hippocampus.240, 241 Overall, the data are promising, however, additional studies assessing the relation of ACTH and cortisol with other biomarkers will improve our understanding of the role of the HPA axis in SZ.
Psychosis can occur as a result of elevated endogenous steroid levels (e.g., Cushing syndrome) or with exposure to exogenous steroids.242 Steroid psychosis, a potential side effect of exogenous glucocorticoids, can present with sensory flooding, delusions, depression, and auditory and visual hallucinations.243 Delusions and hallucinations, primarily auditory, can also occur in subjects with psychotic depression; some studies have shown that glucocorticoid antagonists, such as mifepristone, can have therapeutic effects on psychotic depression.244, 245 Given that the effects of glucocorticoids on the brain include synaptic regulation and epigenetic control of key molecules for dopamine neurons (e.g., tyrosine hydroxylase),246, 247 involvement of the HPA axis in SZ and psychosis is an important subject that should be studied to a greater extent. However, it may be important to sub-classify these conditions to increase the homogeneity of the study subjects.
SZ typically manifests later in life in females than men. This difference in age at onset might be partially attributed to the protective effect of estrogen. Estradiol has been found to be lower in subjects with SZ, males and females, compared to controls.248, 249 Furthermore, an association study found that a polymorphism in an intron of the estrogen receptor alpha gene occurred more frequently in subjects with SZ and was related to lower mRNA levels in the prefrontal cortex.250 The molecular targets of estrogen are diverse, but animal studies have shown that it can modulate the dopaminergic, serotonergic and glutamatergic pathways: for example, estrogen has been shown to alter D2R density, decrease monoamine oxidase activity, increase tryptophan hydroxylase activity, downregulate 5HT1A receptors, upregulate 5HT2A receptors, and alter NMDA receptor density.251-253
Taken together, hormonal measurements and studies with patient tissue suggest that the endocrine system is altered in SZ.
Metabolic cascades
Metabolic disturbances are highly prevalent in SZ patients. Obesity and diabetes are twice as common in SZ compared to the general population.254, 255 These disturbances create an increased metabolic load, eventually leading to cardiovascular issues and other physical conditions that possibly account for the higher mortality rate and shorter life span in SZ.256 It has also been shown that diabetic SZ patients have worse overall cognitive performance compared to SZ patients without diabetes mellitus. Furthermore, patients with untreated diabetes mellitus showed poorer overall cognitive performance compared to SZ patients without diabetes mellitus.257 Major metabolic disturbance in SZ patients is likely elicited by neuroleptic medication,258-260 however, first onset SZ patients, including unmedicated patients, also show higher fasting blood insulin levels, insulin resistance, and impaired glucose tolerance.261-264 While lifestyle and dietary habits may contribute to this difference, it is also likely that an inherent metabolic dysregulation underlies the pathology of SZ. Although this concept may not have a scientific consensus yet, we discuss observed changes in several peripheral factors and their effects on the brain in SZ.
Studies using blood samples indicate functional deficits in insulin signaling in SZ,261-264 while a separate study reported elevated glucose levels in the CSF, but not in serum, of first onset drug naïve SZ subjects.265 In unmedicated SZ subjects, PET studies have shown that glucose metabolism is reduced in several brain areas, in particular the frontal cortex.266, 267 A recent review has summarized microarray studies and found that several genes associated with metabolic cascades are differentially expressed in postmortem brains from SZ patients.268 Postmortem studies have also found disturbances in the insulin-Akt pathway, a signaling pathway linked with several types of cognitive deficits.269-272 Although data derived from postmortem brain tissue may be confounded by medication-induced metabolic changes, further analysis is warranted.273 Some genetic studies have provided suggestive support for the involvement of glucose metabolism cascades in the genetic risk of SZ.274, 275 The gene coding for Akt is also a putative risk factor for SZ, at least in part disturbing the signaling cascade involving the insulin-Akt pathway.276
In addition to glucose and insulin, other metabolic mediators (e.g., leptin and ghrelin) may play key roles in SZ pathology. Weight gain is associated with increased leptin and leptin resistance.277, 278 Leptin inhibits the effects of cortisol signaling in the hypothalamus and predisposes to mental manifestations, in particular depression.279, 280 The ghrelin receptor GHS-R1a forms functional heterodimers with the D2R.281 Antipsychotics have differential effects on these two molecules: fasting morning leptin levels are increased by atypical antipsychotics (e.g., olanzapine and clozapine) but are unaffected by conventional antipsychotics (e.g., haloperidol).282 On the other hand, fasting morning ghrelin levels are decreased in the first 2 weeks after atypical antipsychotic treatment but increase in the long-term.282 Neurochemical studies suggest lipid metabolism may also be altered in SZ. Both chronic and unmedicated first onset SZ have significantly decreased apolipoprotein A1 (apoA1) in the brain, liver, red blood cells, sera, and CSF.283, 284 However, another group found that apoA1 was increased in the CSF of medicated first onset SZ.285 Vitamin D deficiency is commonly observed in SZ patients and is associated with clinical features of the disease.286 Vitamin D deficiency is also observed in first-episode psychosis patients and developmental vitamin D deficiency has been shown to contribute to the risk of SZ, suggesting that neurodevelopment may be affected by vitamin D deficiency.287, 288
Pharmacogenetic studies, primarily focusing on olanzapine and clozapine, have identified SNP in several genes associated with antipsychotic-induced weight gain in SZ patients.289 SNP within the genes encoding the serotonin 2C receptor (5-HT2CR), melanocortin 4 receptor (MC4R), and leptin are those with the most consistently strong evidence, implicating serotonergic and feeding-regulation systems in antipsychotic-induced weight gain.290-293 Although these molecules have been identified as genetic modifiers of adverse metabolic effects in SZ patients, elicited by medication, the question remains whether the cascades involving these factors play roles in the intrinsic abnormalities of both metabolic and mental dysfunction found in SZ patients.
In summary, metabolic cascades are altered in SZ patients, which include changes in the levels of glucose, insulin, leptin, ghrelin, and apoA1. Antipsychotics affect these metabolic cascades, yet there is also evidence that some metabolic disturbances precede antipsychotic exposure. Genetic factors are known to modulate these changes, but it remains elusive whether and how a genetic predisposition to metabolic disturbance plays a primary role in SZ pathology.
Modeling and future perspectives: towards understanding integrative systems
For this review article we chose to organize the molecular targets of SZ by biological system. However, there is obviously tremendous crosstalk among these biological systems (Figure 2). Oxidative stress, inflammation, the HPA axis, and metabolic signaling are tightly interconnected as homeostatic and stress cascades.294-296 Neural networks, the foundation of brain function and dysfunction, are finely regulated by neurotransmitters and white matter-associated connectivity (Figure 3): recently, the significance of neuron-glia interactions in these fine regulations has been particularly recognized.297 As described above, deviations in homeostatic and stress cascades affect key elements of neuronal circuitry, such as synaptic maintenance, interneuron functions, and myelination. Thus, the molecular changes described in the neurotransmission and white matter-associated connectivity subsections may be outcomes of the molecular disturbances in stress signaling described in the second half of this article. Alternately, disturbances in neural connectivity may also activate stress signaling, which in turn further alters the connectivity. While integration of evidence across biological systems remains elusive, this promising working hypothesis suggests a potential framework through which they could be linked.
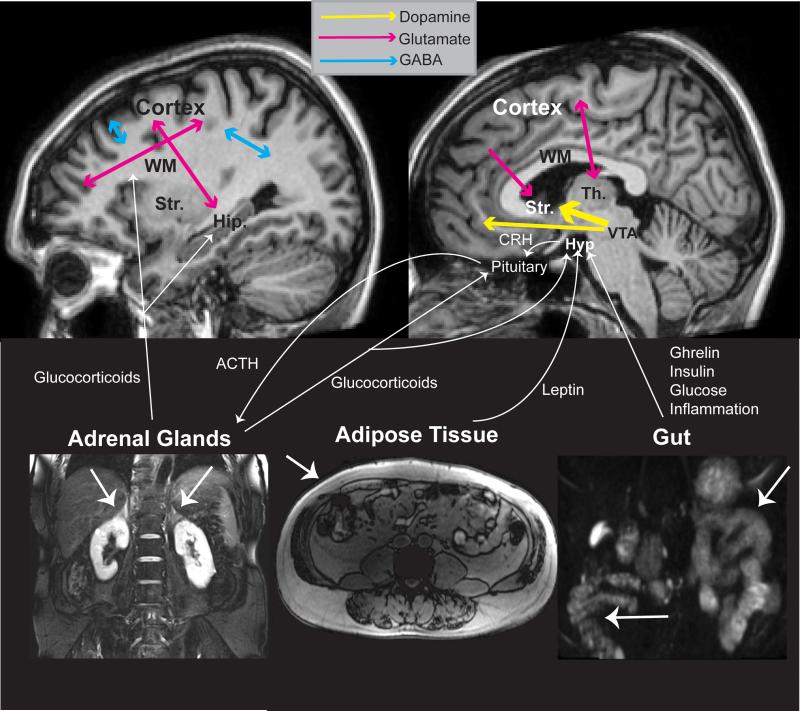
Figure 2.
Molecular crosstalk between the biological systems in schizophrenia. Mesolimbic dopaminergic projections (yellow) between the ventral tegmental area (VTA) and striatum (Str.) are significantly increased. The dopaminergic mesocortical pathway is also affected. Multiple glutamatergic (pink) pathways are affected. GABAergic (blue) pathways are also affected. Glucocorticoids released from the adrenal glands affect numerous brain targets including the cortex, hippocampus (Hip.), hypothalamus (Hyp) and pituitary gland. Molecular substrates of adipose tissue and the gut interact with the hypothalamic-pituitary-adrenal axis via the hypothalamus. Th, thalamus; WM, white matter; CRH, corticotropin releasing hormone; ACTH, adrenocorticotropic hormone.
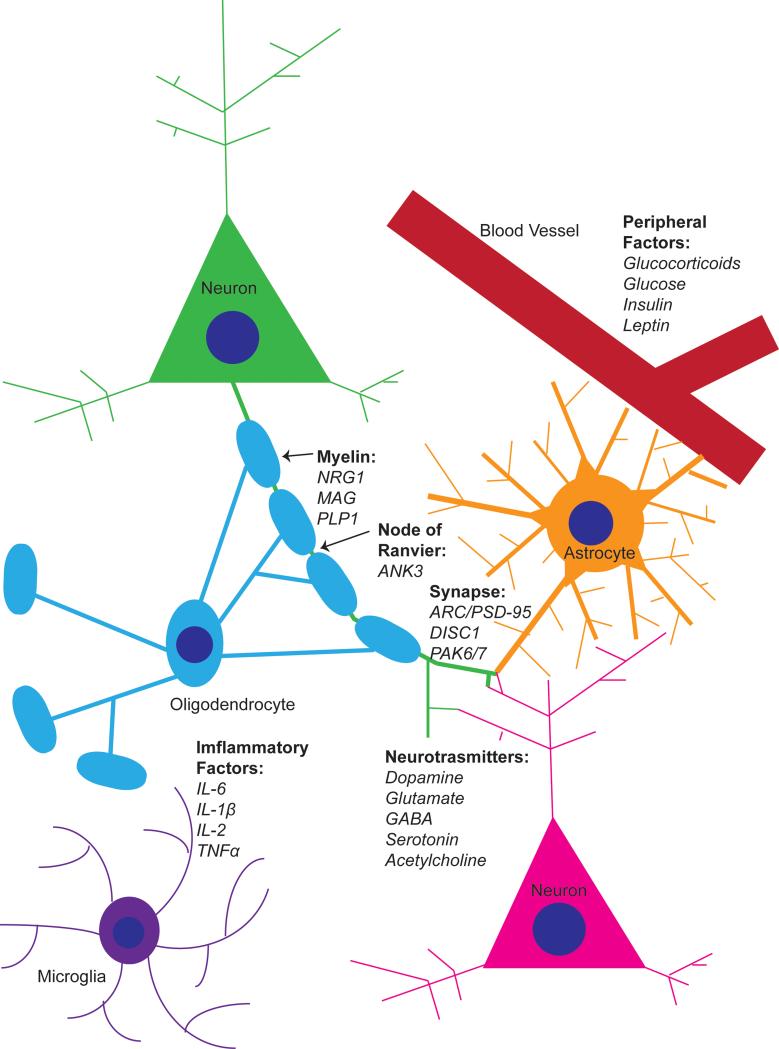
Figure 3.
Inter-cellular interactions of some important molecular substrates affected in schizophrenia. A glutamatergic synapse with contact from a nearby astrocyte represents changes in neuronal signaling. Changes in myelination and formation of nodes of Ranvier along the axon are represented by a single oligodendrocyte. Peripheral factors enter the brain through the blood-brain barrier formed between blood vessels and astrocyte end feet. Inflammatory factors are released by microglia and astrocytes. NRG1, neuregulin-1; MAG, myelin-associated glycoprotein; PLP1, proteolipid protein 1; ANK3, ankyrin-3; ARC, activity-regulated cytoskeleton-associated protein; PSD-95, postsynaptic density protein 95; DISC1, disrupted in schizophrenia 1; PAK6, p21 protein-activated kinase 6; PAK7, p21 protein-activated kinase 7.
Although human studies with patients and matched controls are essential to identify molecular substrates of SZ, there are many barriers to extending an in-depth understanding of the disease mechanisms exclusively through human studies. For example, the current spatial resolution of human brain imaging at both structural and functional levels cannot depict direct entities such as synapses and NoR where we can directly observe the functional outcome of the molecular substrates described above. In addition, while it is very important to grasp the overall molecular changes in the disease trajectory over 20 years, longitudinal human studies on this time scale are challenging and time-consuming.
Given the limitations of human studies, many people have supported the use of animal models. One of the major historical debates in regard to SZ animal models is whether we can reconstruct models for ‘human’ disease. It is impossible to recapitulate the human condition per se in animals. However, by targeting a particular system(s) and/or clinical endophenotype(s) in SZ, animal models can become very useful for understanding biological mechanisms. In addition to analyzing cross-sectional phenotypes in adult animals, it is even more crucial to analyze the disease trajectory from early development to disease onset in preclinical studies.4 Given the diversity of SZ molecular substrates, ranging from homeostatic and stress cascades to neural circuitry components, a major working question in the field of animal models is whether and how stress cascades may affect neural connectivity along a neurodevelopmental trajectory leading to SZ.
The selection of animal models and their validity in SZ research is very important to consider. After the recent introduction of new genetic manipulation technologies,298-300 simultaneous modulation of multiple genes is now technically feasible, but only a few studies have been successful thus far. Thus, instead of reconstructing genetic validity from multiple common variants with mild biological impact on the pathology, we propose that models stemming from the modulation of rare genetic variants with a stronger biological impact (e.g., chromosomal micro-deletion or duplication, and genetic modulation identified from cytogenetic approaches) may be more widely used in SZ research.4, 18, 301 To test the functional outcome of multiple common variants for SZ, human stem cell biology can be used as a complementary approach to animal models.302 For example, CRISPR/Cas9 genetic manipulation of induced pluripotent stem cells (iPSCs) originally derived from patients carrying multiple disease-associated common genetic variants and subsequently differentiated into neurons and glia can be used to assess the contribution of each individual genetic variant.
Gene environment interaction plays a key role in the etiology of SZ;165, 200-202 therefore, consideration of proper environmental stressors, possibly in combination with a genetic factor(s), is crucial when building animal models. Some successful examples include combining adolescent social isolation or prenatal immune activation with a genetic risk factor.202, 247, 303-305 Although an advantage of animal models includes their potential to trace the possible developmental trajectory to full onset of disease,306-308 it is also useful to build models by administering relevant drugs to recapitulate specific pathophysiologies and phenotypes associated with SZ.
Taken together, several animal models have been proposed to understand the biology relevant to SZ. Regardless of whether these animal models are genetic or non-genetic, many of them are generated by perturbing biological processes of neurotransmission, white matter-associated connectivity, inflammatory and oxidative stress, or endocrinology/metabolisms (see in Table 4 where representative models are introduced).304, 309-370 Most of the models were studied from the viewpoints of neurotransmission, neuropsychopharmacology, and behavioral neuroscience.371, 372 However, recent studies have revisited these models beyond these classic paradigms and also elucidated deficits in white matter and stress-associated cascades, such as inflammation, oxidative stress, and the HPA axis.323, 324, 328, 333, 359, 366, 373 Furthermore, models in which stress-associated cascades are primarily perturbed have also displayed abnormalities in neurotransmission and behavior: for example, mice with glutathione deficiency show impairments in parvalbumin-positive interneurons, myelination, and behavior.340, 341, 343, 344, 374, 375 Several animal models have been designed to test the effect of stress cascades on neurodevelopment, including both prenatal and juvenile models of stress and immune activation.322, 376, 377 These developmental stressors have been shown to affect neurotransmission, myelination, and even metabolism. The integrative biological concept of “disturbed homeostatic signaling to connectivity deficits” can be tested, at least in part, with such animal models.
Table 4.
Animal models relevant to schizophrenia classified by molecular system
| Molecular Substrates Affected | ||||||
|---|---|---|---|---|---|---|
| Target System | Animal Model1 | Neurotransmission2 | White Matter3 | Immune/Oxidative Stress4 | Endocrine/Metabolic5 | Misc. |
| NT | NMDAR antagonists (PCP, MK-801, ketamine) | Glu, GABA, DA, 5HT, spines | WM tracts | cytokines, nitrosative/oxidative stress | HPA, glucose/insulin | BDNF |
| NT | NR1 haplomorph | Glu, DA | ||||
| White Matter | NRG1 and ErbB4 knockout | Glu, GABA, DA, 5HT, spines, | WM tracts, oligodendrocytes | HPA | ||
| Immune/Oxidative Stress | Maternal immune activation | Glu, GABA, DA, 5HT, spines, | WM tracts | cytokines, oxidative stress | HPA, glucose/insulin | |
| Immune/Oxidative Stress | Short-term cuprizone | Glu, DA, | WM tracts | cytokines | ||
| Immune/Oxidative Stress | Genetic glutathione depletion (GCLM knockout) | Glu, GABA, DA | WM tracts, oligodendrocytes | GSH | ||
| Endocrine/Metabolic | Vitamin D deficiency | Glu, GABA, DA, | redox | |||
| Endocrine/Metabolic | AKT1 knockout | Spines | ||||
| Misc. | Social isolation | Glu, GABA, DA, 5HT, CB1, spines | oligodendrocytes | cytokines | HPA, metabolic | BDNF |
| Misc. | Neonatal ventral hippocampal lesion | Glu, GABA, DA, ACh, spines, | WM tracts | oxidative stress | HPA, glucose/insulin | |
| Misc. | MAM | Glu, GABA, DA, spines | WM tracts | GSH | HPA | BDNF, NGF |
Abbreviations: NT, neurotransmission; PCP, phencyclidine; Glu, glutamate; GABA, γ-aminobutyric acid; DA, Dopamine; 5HT, serotonin; WM, white matter; HPA, hypothalamus-pituitary-adrenal axis; BDNF, brain-derived neurotrophic factor; NR1, NMDA receptor subunit NR1; NRG1, neuregulin-1; ErbB4, receptor tyrosine kinase erbB4; GCLM, glutamate-cysteine ligase modulatory subunit; GSH, glutathione; AKT1, RAC-alpha serine/threonine-protein kinase; CB1, cannabinoid receptor type 1; ACh, Acetylcholine; MAM, methylazoxymethanol; NGF, nerve
A selection of representative genetic and non-genetic animal models targeting particular systems discussed in the text are listed. These animal models have already been validated from behavioral viewpoints.
Molecular substrates affecting neurotransmission are grouped into glutamate (Glu), GABA, dopamine (DA), serotonin (5HT), acetylcholine (Ach), and dendritic spine (spines) systems.
White matter is divided between tissue level analysis (WM tracts) and cellular-level studies (oligodendrocytes).
Molecular substrates affecting inflammation (cytokines) and oxidative stress cascades (oxidative stress) are grouped. We make special note of redox and glutathione abnormalities (GSH).
Molecular substrates affecting the hypothalamic-pituitary-adrenal axis (HPA) and glucose/insulin pathways (glucose/insulin) are broadly grouped. Broader metabolic abnormalities including lipid metabolism are labeled “metabolic.”
In summary, this review highlights the molecular substrates discovered through studies with SZ patients that are implicated in SZ pathophysiology. We sub-classify the substrates by system, focusing on neurotransmission, targets in white matter-associated connectivity, immune/inflammatory and oxidative stress-related substrates, and molecules in endocrine and metabolic cascades, taking special note where there is convergence with genetic data. Crosstalk among these systems may be important in understanding disease progression and animal models should prove important in charting the developmental progression and interaction of these substrates. As a working hypothesis, we propose the molecular changes in neurotransmission and white matter-associated connectivity may be outcomes of molecular disturbances in stress signaling. Alternately, disturbances in neural connectivity may also activate stress signaling, in turn further altering the connectivity. We optimistically propose that the identified molecular markers, together with further continuous efforts, will aid in discovering biomarkers that may help in the diagnosis, treatment, or early intervention of SZ.
Acknowledgements
We thank Alan Anticevic, Anissa Abi-Dargham, Jennifer Coughlin and Anouk Marsman for scientific discussion. We thank Yukiko Lema for organizing the figures, in particular for contributing to the formatting process. MRI images were kindly provided by Susumu Mori and Michael Jacobs. This work was supported by USPHS grants MH-084018 (A.S.), MH-094268 Silvo O. Conte center (A.S.), MH-069853 (A.S.), MH-085226 (A.S.), MH-088753 (A.S.), MH-092443 (A.S.), Stanley (A.S.), RUSK (A.S.), S-R foundations (A.S.), NARSAD (A.S.) and the Maryland Stem Cell Research Fund (A.S.).
Footnotes
Conflict of Interest
A.S. currently receives research funding from Johnson and Johnson, Astellas, Takeda, Tanabe-Mitsubishi, Dainippon-Sumitomo, and Sucampo; has served as an advisory board and consultant for Amgen, Asubio, Eli Lilly, Pfizer, Sucampo, Taisho, and Takeda, and collaborated with Afraxis, Astrazeneca, and Sanofi-Aventis. However, none of these relationships affected the contents and statements of this review article. In addition, M.A.L-S and T.E.F. declare no potential conflict of interest.
References
- 1.Perala J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsa E, Pirkola S, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Archives of general psychiatry. 2007;64(1):19–28. doi: 10.1001/archpsyc.64.1.19. Epub 2007/01/03. [DOI] [PubMed] [Google Scholar]
- 2.van Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychological medicine. 2009;39(2):179–95. doi: 10.1017/S0033291708003814. Epub 2008/07/09. [DOI] [PubMed] [Google Scholar]
- 3.van Os J, Kapur S. Schizophrenia. Lancet. 2009;374(9690):635–45. doi: 10.1016/S0140-6736(09)60995-8. Epub 2009/08/25. [DOI] [PubMed] [Google Scholar]
- 4.Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends in neurosciences. 2009;32(9):485–95. doi: 10.1016/j.tins.2009.05.007. Epub 2009/08/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seeman P, Lee T. Antipsychotic drugs: direct correlation between clinical potency and presynaptic action on dopamine neurons. Science (New York, NY) 1975;188(4194):1217–9. doi: 10.1126/science.1145194. Epub 1975/06/20. [DOI] [PubMed] [Google Scholar]
- 6.Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science (New York, NY) 1976;192(4238):481–3. doi: 10.1126/science.3854. Epub 1976/04/30. [DOI] [PubMed] [Google Scholar]
- 7.Snyder SH. The dopamine hypothesis of schizophrenia: focus on the dopamine receptor. The American journal of psychiatry. 1976;133(2):197–202. doi: 10.1176/ajp.133.2.197. Epub 1976/02/01. [DOI] [PubMed] [Google Scholar]
- 8.Merritt K, McGuire P, Egerton A. Relationship between Glutamate Dysfunction and Symptoms and Cognitive Function in Psychosis. Frontiers in psychiatry. 2013;4:151. doi: 10.3389/fpsyt.2013.00151. Epub 2013/12/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. The American journal of psychiatry. 1991;148(10):1301–8. doi: 10.1176/ajp.148.10.1301. Epub 1991/10/01. [DOI] [PubMed] [Google Scholar]
- 10.Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460(7256):748–52. doi: 10.1038/nature08185. Epub 2009/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ripke S, O'Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nature genetics. 2013;45(10):1150–9. doi: 10.1038/ng.2742. Epub 2013/08/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506(7487):185–90. doi: 10.1038/nature12975. Epub 2014/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doherty JL, O'Donovan MC, Owen MJ. Recent genomic advances in schizophrenia. Clinical genetics. 2012;81(2):103–9. doi: 10.1111/j.1399-0004.2011.01773.x. Epub 2011/09/08. [DOI] [PubMed] [Google Scholar]
- 14.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506(7487):179–84. doi: 10.1038/nature12929. Epub 2014/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carroll LS, Owen MJ. Genetic overlap between autism, schizophrenia and bipolar disorder. Genome medicine. 2009;1(10):102. doi: 10.1186/gm102. Epub 2009/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nature genetics. 2013;45(9):984–94. doi: 10.1038/ng.2711. Epub 2013/08/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet. 2013;381(9875):1371–9. doi: 10.1016/S0140-6736(12)62129-1. Epub 2013/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nature reviews Neuroscience. 2011;12(12):707–22. doi: 10.1038/nrn3120. Epub 2011/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. The American journal of psychiatry. 2010;167(7):748–51. doi: 10.1176/appi.ajp.2010.09091379. Epub 2010/07/03. [DOI] [PubMed] [Google Scholar]
- 20.Malhotra D, Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148(6):1223–41. doi: 10.1016/j.cell.2012.02.039. Epub 2012/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nature reviews Genetics. 2012;13(8):537–51. doi: 10.1038/nrg3240. Epub 2012/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratt J, Winchester C, Dawson N, Morris B. Advancing schizophrenia drug discovery: optimizing rodent models to bridge the translational gap. Nature reviews Drug discovery. 2012;11(7):560–79. doi: 10.1038/nrd3649. Epub 2012/06/23. [DOI] [PubMed] [Google Scholar]
- 23.Jaaro-Peled H, Ayhan Y, Pletnikov MV, Sawa A. Review of pathological hallmarks of schizophrenia: comparison of genetic models with patients and nongenetic models. Schizophrenia bulletin. 2010;36(2):301–13. doi: 10.1093/schbul/sbp133. Epub 2009/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kvajo M, McKellar H, Gogos JA. Avoiding mouse traps in schizophrenia genetics: lessons and promises from current and emerging mouse models. Neuroscience. 2012;211:136–64. doi: 10.1016/j.neuroscience.2011.07.051. Epub 2011/08/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hartwell L, Mankoff D, Paulovich A, Ramsey S, Swisher E. Cancer biomarkers: a systems approach. Nature biotechnology. 2006;24(8):905–8. doi: 10.1038/nbt0806-905. Epub 2006/08/11. [DOI] [PubMed] [Google Scholar]
- 26.Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nature reviews Genetics. 2008;9(11):819–30. doi: 10.1038/nrg2468. Epub 2008/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oresic M, Vidal-Puig A, editors. A Systems Biology Approach to Study Metabolic Syndrome. Springer International Publishing; 2014. p. 384. [Google Scholar]
- 28.Zhang B, Gaiteri C, Bodea LG, Wang Z, McElwee J, Podtelezhnikov AA, et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell. 2013;153(3):707–20. doi: 10.1016/j.cell.2013.03.030. Epub 2013/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. Epub 2011/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]fluorodopa study. Archives of general psychiatry. 2004;61(2):134–42. doi: 10.1001/archpsyc.61.2.134. Epub 2004/02/06. [DOI] [PubMed] [Google Scholar]
- 31.Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Archives of general psychiatry. 2009;66(1):13–20. doi: 10.1001/archgenpsychiatry.2008.514. Epub 2009/01/07. [DOI] [PubMed] [Google Scholar]
- 32.Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nature neuroscience. 2002;5(3):267–71. doi: 10.1038/nn804. Epub 2002/02/28. [DOI] [PubMed] [Google Scholar]
- 33.Nozaki S, Kato M, Takano H, Ito H, Takahashi H, Arakawa R, et al. Regional dopamine synthesis in patients with schizophrenia using L-[beta-11C]DOPA PET. Schizophrenia research. 2009;108(1-3):78–84. doi: 10.1016/j.schres.2008.11.006. Epub 2008/12/06. [DOI] [PubMed] [Google Scholar]
- 34.Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MA, Bhattacharyya S, Allen P, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biological psychiatry. 2013;74(2):106–12. doi: 10.1016/j.biopsych.2012.11.017. Epub 2013/01/15. [DOI] [PubMed] [Google Scholar]
- 35.Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, et al. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(6):2569–74. doi: 10.1073/pnas.94.6.2569. Epub 1997/03/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laruelle M, Abi-Dargham A, van Dyck CH, Gil R, D'Souza CD, Erdos J, et al. Single photon emission computerized tomography imaging of amphetamine-induced dopamine release in drug-free schizophrenic subjects. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(17):9235–40. doi: 10.1073/pnas.93.17.9235. Epub 1996/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. The American journal of psychiatry. 1998;155(6):761–7. doi: 10.1176/ajp.155.6.761. Epub 1998/06/10. [DOI] [PubMed] [Google Scholar]
- 38.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophrenia bulletin. 2009;35(3):549–62. doi: 10.1093/schbul/sbp006. Epub 2009/03/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Archives of general psychiatry. 2010;67(3):231–9. doi: 10.1001/archgenpsychiatry.2010.10. Epub 2010/03/03. [DOI] [PubMed] [Google Scholar]
- 40.Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. The quarterly journal of nuclear medicine : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR) 1998;42(3):211–21. Epub 1998/10/31. [PubMed] [Google Scholar]
- 41.Kuepper R, Skinbjerg M, Abi-Dargham A. The dopamine dysfunction in schizophrenia revisited: new insights into topography and course. Handbook of experimental pharmacology. 2012;(212):1–26. doi: 10.1007/978-3-642-25761-2_1. Epub 2012/11/07. [DOI] [PubMed] [Google Scholar]
- 42.Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–7. doi: 10.1038/nature13595. Epub 2014/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385(6617):634–6. doi: 10.1038/385634a0. Epub 1997/02/13. [DOI] [PubMed] [Google Scholar]
- 44.Karlsson P, Farde L, Halldin C, Sedvall G. PET study of D(1) dopamine receptor binding in neuroleptic-naive patients with schizophrenia. The American journal of psychiatry. 2002;159(5):761–7. doi: 10.1176/appi.ajp.159.5.761. Epub 2002/05/03. [DOI] [PubMed] [Google Scholar]
- 45.Abi-Dargham A, Xu X, Thompson JL, Gil R, Kegeles LS, Urban N, et al. Increased prefrontal cortical D(1) receptors in drug naive patients with schizophrenia: a PET study with [(1)(1)C]NNC112. Journal of psychopharmacology (Oxford, England) 2012;26(6):794–805. doi: 10.1177/0269881111409265. Epub 2011/07/20. [DOI] [PubMed] [Google Scholar]
- 46.Kestler LP, Walker E, Vega EM. Dopamine receptors in the brains of schizophrenia patients: a meta-analysis of the findings. Behavioural pharmacology. 2001;12(5):355–71. doi: 10.1097/00008877-200109000-00007. Epub 2001/11/17. [DOI] [PubMed] [Google Scholar]
- 47.Beuger M, van Kammen DP, Kelley ME, Yao J. Dopamine turnover in schizophrenia before and after haloperidol withdrawal. CSF, plasma, and urine studies. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1996;15(1):75–86. doi: 10.1016/0893-133X(95)00158-A. Epub 1996/07/01. [DOI] [PubMed] [Google Scholar]
- 48.Wieselgren IM, Lindstrom LH. CSF levels of HVA and 5-HIAA in drug-free schizophrenic patients and healthy controls: a prospective study focused on their predictive value for outcome in schizophrenia. Psychiatry research. 1998;81(2):101–10. doi: 10.1016/s0165-1781(98)00090-0. Epub 1998/12/19. [DOI] [PubMed] [Google Scholar]
- 49.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. The American journal of psychiatry. 1991;148(11):1474–86. doi: 10.1176/ajp.148.11.1474. Epub 1991/11/01. [DOI] [PubMed] [Google Scholar]
- 50.Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ. Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex. Focal abnormalities in orbitofrontal cortex in schizophrenia. Archives of general psychiatry. 1997;54(12):1089–95. doi: 10.1001/archpsyc.1997.01830240045007. Epub 1997/12/24. [DOI] [PubMed] [Google Scholar]
- 51.Pantazopoulos H, Stone D, Walsh J, Benes FM. Differences in the cellular distribution of D1 receptor mRNA in the hippocampus of bipolars and schizophrenics. Synapse (New York, NY) 2004;54(3):147–55. doi: 10.1002/syn.20076. Epub 2004/09/29. [DOI] [PubMed] [Google Scholar]
- 52.Akil M, Pierri JN, Whitehead RE, Edgar CL, Mohila C, Sampson AR, et al. Lamina-specific alterations in the dopamine innervation of the prefrontal cortex in schizophrenic subjects. The American journal of psychiatry. 1999;156(10):1580–9. doi: 10.1176/ajp.156.10.1580. Epub 1999/10/13. [DOI] [PubMed] [Google Scholar]
- 53.Akil M, Edgar CL, Pierri JN, Casali S, Lewis DA. Decreased density of tyrosine hydroxylase-immunoreactive axons in the entorhinal cortex of schizophrenic subjects. Biological psychiatry. 2000;47(5):361–70. doi: 10.1016/s0006-3223(99)00282-6. Epub 2000/03/08. [DOI] [PubMed] [Google Scholar]
- 54.Abi-Dargham A, Rodenhiser J, Printz D, Zea-Ponce Y, Gil R, Kegeles LS, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(14):8104–9. doi: 10.1073/pnas.97.14.8104. Epub 2000/07/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinberger DR, Berman KF, Daniel DG. Mesoprefrontal cortical dopaminergic activity and prefrontal hypofunction in schizophrenia. Clinical neuropharmacology. 1992;15(Suppl 1 Pt A):568A–9A. doi: 10.1097/00002826-199201001-00296. Epub 1992/01/01. [DOI] [PubMed] [Google Scholar]
- 56.Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. The Journal of pharmacology and experimental therapeutics. 1989;251(1):238–46. Epub 1989/10/01. [PubMed] [Google Scholar]
- 57.Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport. 1998;9(17):3897–902. doi: 10.1097/00001756-199812010-00024. Epub 1999/01/06. [DOI] [PubMed] [Google Scholar]
- 58.Burnet PW, Eastwood SL, Harrison PJ. 5-HT1A and 5-HT2A receptor mRNAs and binding site densities are differentially altered in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1996;15(5):442–55. doi: 10.1016/S0893-133X(96)00053-X. Epub 1996/11/01. [DOI] [PubMed] [Google Scholar]
- 59.Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, et al. NMDA receptor function in large-scale anticorrelated neural systems with implications for cognition and schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(41):16720–5. doi: 10.1073/pnas.1208494109. Epub 2012/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of general psychiatry. 1994;51(3):199–214. doi: 10.1001/archpsyc.1994.03950030035004. Epub 1994/03/01. [DOI] [PubMed] [Google Scholar]
- 61.Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Archives of general psychiatry. 2000;57(1):65–73. doi: 10.1001/archpsyc.57.1.65. Epub 2000/01/13. [DOI] [PubMed] [Google Scholar]
- 62.Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. Journal of neurology, neurosurgery, and psychiatry. 1998;65(4):446–53. doi: 10.1136/jnnp.65.4.446. Epub 1998/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ayalew M, Le-Niculescu H, Levey DF, Jain N, Changala B, Patel SD, et al. Convergent functional genomics of schizophrenia: from comprehensive understanding to genetic risk prediction. Molecular psychiatry. 2012;17(9):887–905. doi: 10.1038/mp.2012.37. Epub 2012/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poels EM, Kegeles LS, Kantrowitz JT, Slifstein M, Javitt DC, Lieberman JA, et al. Imaging glutamate in schizophrenia: review of findings and implications for drug discovery. Molecular psychiatry. 2014;19(1):20–9. doi: 10.1038/mp.2013.136. Epub 2013/10/30. [DOI] [PubMed] [Google Scholar]
- 65.Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophrenia bulletin. 2013;39(1):120–9. doi: 10.1093/schbul/sbr069. Epub 2011/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kegeles LS, Mao X, Stanford AD, Girgis R, Ojeil N, Xu X, et al. Elevated prefrontal cortex gamma-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Archives of general psychiatry. 2012;69(5):449–59. doi: 10.1001/archgenpsychiatry.2011.1519. Epub 2012/01/04. [DOI] [PubMed] [Google Scholar]
- 67.Egerton A, Brugger S, Raffin M, Barker GJ, Lythgoe DJ, McGuire PK, et al. Anterior cingulate glutamate levels related to clinical status following treatment in first-episode schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(11):2515–21. doi: 10.1038/npp.2012.113. Epub 2012/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Demjaha A, Egerton A, Murray RM, Kapur S, Howes OD, Stone JM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biological psychiatry. 2014;75(5):e11–3. doi: 10.1016/j.biopsych.2013.06.011. Epub 2013/07/31. [DOI] [PubMed] [Google Scholar]
- 69.Stan AD, Ghose S, Zhao C, Hulsey K, Mihalakos P, Yanagi M, et al. Magnetic resonance spectroscopy and tissue protein concentrations together suggest lower glutamate signaling in dentate gyrus in schizophrenia. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.54. Epub 2014/06/11. [DOI] [PubMed] [Google Scholar]
- 70.Tsai G, van Kammen DP, Chen S, Kelley ME, Grier A, Coyle JT. Glutamatergic neurotransmission involves structural and clinical deficits of schizophrenia. Biological psychiatry. 1998;44(8):667–74. doi: 10.1016/s0006-3223(98)00151-6. Epub 1998/11/03. [DOI] [PubMed] [Google Scholar]
- 71.Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindstrom LH, Iyo M. Elevated glutamine/glutamate ratio in cerebrospinal fluid of first episode and drug naive schizophrenic patients. BMC psychiatry. 2005;5:6. doi: 10.1186/1471-244X-5-6. Epub 2005/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Do KQ, Lauer CJ, Schreiber W, Zollinger M, Gutteck-Amsler U, Cuenod M, et al. gamma-Glutamylglutamine and taurine concentrations are decreased in the cerebrospinal fluid of drug-naive patients with schizophrenic disorders. Journal of neurochemistry. 1995;65(6):2652–62. doi: 10.1046/j.1471-4159.1995.65062652.x. Epub 1995/12/01. [DOI] [PubMed] [Google Scholar]
- 73.Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Archives of general psychiatry. 2009;66(9):938–46. doi: 10.1001/archgenpsychiatry.2009.115. Epub 2009/09/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. doi: 10.1016/j.neuron.2013.02.011. Epub 2013/04/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wandinger KP, Saschenbrecker S, Stoecker W, Dalmau J. Anti-NMDA-receptor encephalitis: a severe, multistage, treatable disorder presenting with psychosis. Journal of neuroimmunology. 2011;231(1-2):86–91. doi: 10.1016/j.jneuroim.2010.09.012. Epub 2010/10/19. [DOI] [PubMed] [Google Scholar]
- 76.Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet neurology. 2008;7(12):1091–8. doi: 10.1016/S1474-4422(08)70224-2. Epub 2008/10/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet neurology. 2011;10(1):63–74. doi: 10.1016/S1474-4422(10)70253-2. Epub 2010/12/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet neurology. 2011;10(8):759–72. doi: 10.1016/S1474-4422(11)70096-5. Epub 2011/07/23. [DOI] [PubMed] [Google Scholar]
- 79.Lesher AP, Myers TJ, Tecklenburg F, Streck CJ. Anti-N-methyl-D-aspartate receptor encephalitis associated with an ovarian teratoma in an adolescent female. Journal of pediatric surgery. 2010;45(7):1550–3. doi: 10.1016/j.jpedsurg.2010.04.002. Epub 2010/07/20. [DOI] [PubMed] [Google Scholar]
- 80.Seki M, Suzuki S, Iizuka T, Shimizu T, Nihei Y, Suzuki N, et al. Neurological response to early removal of ovarian teratoma in anti-NMDAR encephalitis. Journal of neurology, neurosurgery, and psychiatry. 2008;79(3):324–6. doi: 10.1136/jnnp.2007.136473. Epub 2007/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goto N, Yoshimura R, Moriya J, Kakeda S, Ueda N, Ikenouchi-Sugita A, et al. Reduction of brain gamma-aminobutyric acid (GABA) concentrations in early-stage schizophrenia patients: 3T Proton MRS study. Schizophrenia research. 2009;112(1-3):192–3. doi: 10.1016/j.schres.2009.04.026. Epub 2009/05/26. [DOI] [PubMed] [Google Scholar]
- 82.Tayoshi S, Nakataki M, Sumitani S, Taniguchi K, Shibuya-Tayoshi S, Numata S, et al. GABA concentration in schizophrenia patients and the effects of antipsychotic medication: a proton magnetic resonance spectroscopy study. Schizophrenia research. 2010;117(1):83–91. doi: 10.1016/j.schres.2009.11.011. Epub 2009/12/22. [DOI] [PubMed] [Google Scholar]
- 83.Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, et al. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(10):3777–81. doi: 10.1523/JNEUROSCI.6158-09.2010. Epub 2010/03/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ongur D, Prescot AP, McCarthy J, Cohen BM, Renshaw PF. Elevated gamma-aminobutyric acid levels in chronic schizophrenia. Biological psychiatry. 2010;68(7):667–70. doi: 10.1016/j.biopsych.2010.05.016. Epub 2010/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rowland LM, Kontson K, West J, Edden RA, Zhu H, Wijtenburg SA, et al. In vivo measurements of glutamate, GABA, and NAAG in schizophrenia. Schizophrenia bulletin. 2013;39(5):1096–104. doi: 10.1093/schbul/sbs092. Epub 2012/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cousijn H, Haegens S, Wallis G, Near J, Stokes MG, Harrison PJ, et al. Resting GABA and glutamate concentrations do not predict visual gamma frequency or amplitude. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(25):9301–6. doi: 10.1073/pnas.1321072111. Epub 2014/06/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gerner RH, Hare TA. CSF GABA in normal subjects and patients with depression, schizophrenia, mania, and anorexia nervosa. The American journal of psychiatry. 1981;138(8):1098–101. doi: 10.1176/ajp.138.8.1098. Epub 1981/08/01. [DOI] [PubMed] [Google Scholar]
- 88.Lichtshtein D, Dobkin J, Ebstein RP, Biederman J, Rimon R, Belmaker RH. Gamma-aminobutyric acid (GABA) in the CSF of schizophrenic patients before and after neuroleptic treatment. The British journal of psychiatry : the journal of mental science. 1978;132:145–8. doi: 10.1192/bjp.132.2.145. Epub 1978/02/01. [DOI] [PubMed] [Google Scholar]
- 89.Petty F, Sherman AD. Plasma GABA levels in psychiatric illness. Journal of affective disorders. 1984;6(2):131–8. doi: 10.1016/0165-0327(84)90018-1. Epub 1984/04/01. [DOI] [PubMed] [Google Scholar]
- 90.van Kammen DP, Sternberg DE, Hare TA, Waters RN, Bunney WE., Jr. CSF levels of gamma-aminobutyric acid in schizophrenia. Low values in recently ill patients. Archives of general psychiatry. 1982;39(1):91–7. doi: 10.1001/archpsyc.1982.04290010065012. Epub 1982/01/01. [DOI] [PubMed] [Google Scholar]
- 91.McCarthy BW, Gomes UR, Neethling AC, Shanley BC, Taljaard JJ, Potgieter L, et al. gamma-Aminobutyric acid concentration in cerebrospinal fluid in schizophrenia. Journal of neurochemistry. 1981;36(4):1406–8. doi: 10.1111/j.1471-4159.1981.tb00579.x. Epub 1981/04/01. [DOI] [PubMed] [Google Scholar]
- 92.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2001;25(1):1–27. doi: 10.1016/S0893-133X(01)00225-1. Epub 2001/05/30. [DOI] [PubMed] [Google Scholar]
- 93.Volk DW, Lewis DA. Early developmental disturbances of cortical inhibitory neurons: contribution to cognitive deficits in schizophrenia. Schizophrenia bulletin. 2014;40(5):952–7. doi: 10.1093/schbul/sbu111. Epub 2014/07/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(15):6315–26. doi: 10.1523/JNEUROSCI.23-15-06315.2003. Epub 2003/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Volk DW, Matsubara T, Li S, Sengupta EJ, Georgiev D, Minabe Y, et al. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. The American journal of psychiatry. 2012;169(10):1082–91. doi: 10.1176/appi.ajp.2012.12030305. Epub 2012/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr., et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of general psychiatry. 1995;52(4):258–66. doi: 10.1001/archpsyc.1995.03950160008002. Epub 1995/04/01. [DOI] [PubMed] [Google Scholar]
- 97.Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Archives of general psychiatry. 2000;57(11):1061–9. doi: 10.1001/archpsyc.57.11.1061. Epub 2000/11/14. [DOI] [PubMed] [Google Scholar]
- 98.Curley AA, Arion D, Volk DW, Asafu-Adjei JK, Sampson AR, Fish KN, et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. The American journal of psychiatry. 2011;168(9):921–9. doi: 10.1176/appi.ajp.2011.11010052. Epub 2011/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yanagi M, Joho RH, Southcott SA, Shukla AA, Ghose S, Tamminga CA. Kv3.1-containing K(+) channels are reduced in untreated schizophrenia and normalized with antipsychotic drugs. Molecular psychiatry. 2014;19(5):573–9. doi: 10.1038/mp.2013.49. Epub 2013/05/01. [DOI] [PubMed] [Google Scholar]
- 100.Georgiev D, Arion D, Enwright JF, Kikuchi M, Minabe Y, Corradi JP, et al. Lower gene expression for KCNS3 potassium channel subunit in parvalbumin-containing neurons in the prefrontal cortex in schizophrenia. The American journal of psychiatry. 2014;171(1):62–71. doi: 10.1176/appi.ajp.2013.13040468. Epub 2013/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature reviews Neuroscience. 2005;6(4):312–24. doi: 10.1038/nrn1648. Epub 2005/04/02. [DOI] [PubMed] [Google Scholar]
- 102.Hashimoto T, Arion D, Unger T, Maldonado-Aviles JG, Morris HM, Volk DW, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Molecular psychiatry. 2008;13(2):147–61. doi: 10.1038/sj.mp.4002011. Epub 2007/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. The American journal of psychiatry. 1993;150(12):1856–61. doi: 10.1176/ajp.150.12.1856. Epub 1993/12/01. [DOI] [PubMed] [Google Scholar]
- 104.Chen XS, Li CB, Smith RC, Xiao ZP, Wang JJ. Differential sensory gating functions between smokers and non-smokers among drug-naive first episode schizophrenic patients. Psychiatry research. 2011;188(3):327–33. doi: 10.1016/j.psychres.2010.12.009. Epub 2011/01/11. [DOI] [PubMed] [Google Scholar]
- 105.Kumari V, Soni W, Sharma T. Influence of cigarette smoking on prepulse inhibition of the acoustic startle response in schizophrenia. Human psychopharmacology. 2001;16(4):321–6. doi: 10.1002/hup.286. Epub 2002/10/31. [DOI] [PubMed] [Google Scholar]
- 106.Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(2):587–92. doi: 10.1073/pnas.94.2.587. Epub 1997/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaufmann CA, Suarez B, Malaspina D, Pepple J, Svrakic D, Markel PD, et al. NIMH Genetics Initiative Millenium Schizophrenia Consortium: linkage analysis of African-American pedigrees. American journal of medical genetics. 1998;81(4):282–9. Epub 1998/07/23. [PubMed] [Google Scholar]
- 108.Riley BP, Makoff A, Mogudi-Carter M, Jenkins T, Williamson R, Collier D, et al. Haplotype transmission disequilibrium and evidence for linkage of the CHRNA7 gene region to schizophrenia in Southern African Bantu families. American journal of medical genetics. 2000;96(2):196–201. doi: 10.1002/(sici)1096-8628(20000403)96:2<196::aid-ajmg15>3.0.co;2-4. Epub 2000/07/14. [DOI] [PubMed] [Google Scholar]
- 109.Freedman R, Hall M, Adler LE, Leonard S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biological psychiatry. 1995;38(1):22–33. doi: 10.1016/0006-3223(94)00252-X. Epub 1995/07/01. [DOI] [PubMed] [Google Scholar]
- 110.Court J, Spurden D, Lloyd S, McKeith I, Ballard C, Cairns N, et al. Neuronal nicotinic receptors in dementia with Lewy bodies and schizophrenia: alpha-bungarotoxin and nicotine binding in the thalamus. Journal of neurochemistry. 1999;73(4):1590–7. doi: 10.1046/j.1471-4159.1999.0731590.x. Epub 1999/09/29. [DOI] [PubMed] [Google Scholar]
- 111.Marutle A, Zhang X, Court J, Piggott M, Johnson M, Perry R, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. Journal of chemical neuroanatomy. 2001;22(1-2):115–26. doi: 10.1016/s0891-0618(01)00117-x. Epub 2001/07/27. [DOI] [PubMed] [Google Scholar]
- 112.D'Souza DC, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F, et al. Lower ss2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. The American journal of psychiatry. 2012;169(3):326–34. doi: 10.1176/appi.ajp.2011.11020189. Epub 2011/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brasic JR, Cascella N, Kumar A, Zhou Y, Hilton J, Raymont V, et al. Positron emission tomography experience with 2-[(1)(8)F]fluoro-3-(2(S)-azetidinylmethoxy)pyridine (2-[(1)(8)F]FA) in the living human brain of smokers with paranoid schizophrenia. Synapse (New York, NY) 2012;66(4):352–68. doi: 10.1002/syn.21520. Epub 2011/12/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seshadri S, Zeledon M, Sawa A. Synapse-specific contributions in the cortical pathology of schizophrenia. Neurobiology of disease. 2013;53:26–35. doi: 10.1016/j.nbd.2013.01.009. Epub 2013/01/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nature neuroscience. 2010;13(3):327–32. doi: 10.1038/nn.2487. Epub 2010/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hayashi-Takagi A, Araki Y, Nakamura M, Vollrath B, Duron SG, Yan Z, et al. PAKs inhibitors ameliorate schizophrenia-associated dendritic spine deterioration in vitro and in vivo during late adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(17):6461–6. doi: 10.1073/pnas.1321109111. Epub 2014/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morris DW, Pearson RD, Cormican P, Kenny EM, O'Dushlaine CT, Perreault LP, et al. An inherited duplication at the gene p21 Protein-Activated Kinase 7 (PAK7) is a risk factor for psychosis. Human molecular genetics. 2014;23(12):3316–26. doi: 10.1093/hmg/ddu025. Epub 2014/01/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28(1):53–67. doi: 10.1016/s0896-6273(00)00085-4. Epub 2000/11/22. [DOI] [PubMed] [Google Scholar]
- 119.Arancibia-Carcamo IL, Attwell D. The node of Ranvier in CNS pathology. Acta neuropathologica. 2014 doi: 10.1007/s00401-014-1305-z. Epub 2014/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bjartmar C, Yin X, Trapp BD. Axonal pathology in myelin disorders. Journal of neurocytology. 1999;28(4-5):383–95. doi: 10.1023/a:1007010205037. Epub 2000/03/30. [DOI] [PubMed] [Google Scholar]
- 121.Shenton ME, Whitford TJ, Kubicki M. Structural neuroimaging in schizophrenia: from methods to insights to treatments. Dialogues in clinical neuroscience. 2010;12(3):317–32. doi: 10.31887/DCNS.2010.12.3/mshenton. Epub 2010/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kanaan R, Barker G, Brammer M, Giampietro V, Shergill S, Woolley J, et al. White matter microstructure in schizophrenia: effects of disorder, duration and medication. The British journal of psychiatry : the journal of mental science. 2009;194(3):236–42. doi: 10.1192/bjp.bp.108.054320. Epub 2009/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.White T, Nelson M, Lim KO. Diffusion tensor imaging in psychiatric disorders. Topics in magnetic resonance imaging : TMRI. 2008;19(2):97–109. doi: 10.1097/RMR.0b013e3181809f1e. Epub 2009/04/14. [DOI] [PubMed] [Google Scholar]
- 124.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia research. 2009;108(1-3):3–10. doi: 10.1016/j.schres.2008.11.021. Epub 2009/01/09. [DOI] [PubMed] [Google Scholar]
- 125.Witthaus H, Brune M, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, et al. White matter abnormalities in subjects at ultra high-risk for schizophrenia and first-episode schizophrenic patients. Schizophrenia research. 2008;102(1-3):141–9. doi: 10.1016/j.schres.2008.03.022. Epub 2008/06/03. [DOI] [PubMed] [Google Scholar]
- 126.Epstein KA, Cullen KR, Mueller BA, Robinson P, Lee S, Kumra S. White matter abnormalities and cognitive impairment in early-onset schizophrenia-spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53(3):362–72. e1–2. doi: 10.1016/j.jaac.2013.12.007. Epub 2014/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biological psychiatry. 2011;70(7):672–9. doi: 10.1016/j.biopsych.2011.05.017. Epub 2011/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang GJ, Murray JD, Repovs G, Cole MW, Savic A, Glasser MF, et al. Altered global brain signal in schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(20):7438–43. doi: 10.1073/pnas.1405289111. Epub 2014/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, et al. Functional connectivity and brain networks in schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(28):9477–87. doi: 10.1523/JNEUROSCI.0333-10.2010. Epub 2010/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biological psychiatry. 2011;70(1):43–50. doi: 10.1016/j.biopsych.2011.02.010. Epub 2011/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Baker JT, Holmes AJ, Masters GA, Yeo BT, Krienen F, Buckner RL, et al. Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA psychiatry. 2014;71(2):109–18. doi: 10.1001/jamapsychiatry.2013.3469. Epub 2013/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. The American journal of psychiatry. 2012;169(10):1092–9. doi: 10.1176/appi.ajp.2012.12010056. Epub 2012/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, et al. Characterizing Thalamo-Cortical Disturbances in Schizophrenia and Bipolar Illness. Cerebral cortex (New York, NY : 1991) 2013 doi: 10.1093/cercor/bht165. Epub 2013/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jeong B, Kubicki M. Reduced task-related suppression during semantic repetition priming in schizophrenia. Psychiatry research. 2010;181(2):114–20. doi: 10.1016/j.pscychresns.2009.09.005. Epub 2010/01/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36(10):2009–17. doi: 10.1038/npp.2011.88. Epub 2011/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in cognitive sciences. 2012;16(12):584–92. doi: 10.1016/j.tics.2012.10.008. Epub 2012/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fitzsimmons J, Kubicki M, Shenton ME. Review of functional and anatomical brain connectivity findings in schizophrenia. Current opinion in psychiatry. 2013;26(2):172–87. doi: 10.1097/YCO.0b013e32835d9e6a. Epub 2013/01/18. [DOI] [PubMed] [Google Scholar]
- 138.Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. NeuroImage. 2012;62(4):2296–314. doi: 10.1016/j.neuroimage.2011.12.090. Epub 2012/03/06. [DOI] [PubMed] [Google Scholar]
- 139.Anticevic A, Cole MW, Repovs G, Savic A, Driesen NR, Yang G, et al. Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Frontiers in psychiatry. 2013;4:169. doi: 10.3389/fpsyt.2013.00169. Epub 2014/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sequeira PA, Martin MV, Vawter MP. The first decade and beyond of transcriptional profiling in schizophrenia. Neurobiology of disease. 2012;45(1):23–36. doi: 10.1016/j.nbd.2011.03.001. Epub 2011/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hof PR, Haroutunian V, Friedrich VL, Jr., Byne W, Buitron C, Perl DP, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biological psychiatry. 2003;53(12):1075–85. doi: 10.1016/s0006-3223(03)00237-3. Epub 2003/06/20. [DOI] [PubMed] [Google Scholar]
- 142.Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4746–51. doi: 10.1073/pnas.081071198. Epub 2001/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, et al. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362(9386):798–805. doi: 10.1016/S0140-6736(03)14289-4. Epub 2003/09/19. [DOI] [PubMed] [Google Scholar]
- 144.Aston C, Jiang L, Sokolov BP. Microarray analysis of postmortem temporal cortex from patients with schizophrenia. Journal of neuroscience research. 2004;77(6):858–66. doi: 10.1002/jnr.20208. Epub 2004/08/31. [DOI] [PubMed] [Google Scholar]
- 145.Katsel P, Davis KL, Haroutunian V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophrenia research. 2005;79(2-3):157–73. doi: 10.1016/j.schres.2005.06.007. Epub 2005/09/06. [DOI] [PubMed] [Google Scholar]
- 146.Haroutunian V, Katsel P, Dracheva S, Stewart DG, Davis KL. Variations in oligodendrocyte-related gene expression across multiple cortical regions: implications for the pathophysiology of schizophrenia. Int J Neuropsychopharmacol. 2007;10(4):565–73. doi: 10.1017/S1461145706007310. Epub 2007/02/13. [DOI] [PubMed] [Google Scholar]
- 147.Takahashi N, Sakurai T, Davis KL, Buxbaum JD. Linking oligodendrocyte and myelin dysfunction to neurocircuitry abnormalities in schizophrenia. Progress in neurobiology. 2011;93(1):13–24. doi: 10.1016/j.pneurobio.2010.09.004. Epub 2010/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. American journal of human genetics. 2002;71(4):877–92. doi: 10.1086/342734. Epub 2002/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Williams NM, Preece A, Spurlock G, Norton N, Williams HJ, Zammit S, et al. Support for genetic variation in neuregulin 1 and susceptibility to schizophrenia. Molecular psychiatry. 2003;8(5):485–7. doi: 10.1038/sj.mp.4001348. Epub 2003/06/17. [DOI] [PubMed] [Google Scholar]
- 150.Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nature neuroscience. 2004;7(6):575–80. doi: 10.1038/nn1258. Epub 2004/05/27. [DOI] [PubMed] [Google Scholar]
- 151.Novak G, Kim D, Seeman P, Tallerico T. Schizophrenia and Nogo: elevated mRNA in cortex, and high prevalence of a homozygous CAA insert. Brain research Molecular brain research. 2002;107(2):183–9. doi: 10.1016/s0169-328x(02)00492-8. Epub 2002/11/12. [DOI] [PubMed] [Google Scholar]
- 152.Tan EC, Chong SA, Wang H, Chew-Ping Lim E, Teo YY. Gender-specific association of insertion/deletion polymorphisms in the nogo gene and chronic schizophrenia. Brain research Molecular brain research. 2005;139(2):212–6. doi: 10.1016/j.molbrainres.2005.05.010. Epub 2005/06/15. [DOI] [PubMed] [Google Scholar]
- 153.Sinibaldi L, De Luca A, Bellacchio E, Conti E, Pasini A, Paloscia C, et al. Mutations of the Nogo-66 receptor (RTN4R) gene in schizophrenia. Human mutation. 2004;24(6):534–5. doi: 10.1002/humu.9292. Epub 2004/11/09. [DOI] [PubMed] [Google Scholar]
- 154.Prata DP, Kanaan RA, Barker GJ, Shergill S, Woolley J, Georgieva L, et al. Risk variant of oligodendrocyte lineage transcription factor 2 is associated with reduced white matter integrity. Human brain mapping. 2013;34(9):2025–31. doi: 10.1002/hbm.22045. Epub 2012/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Felsky D, Voineskos AN, Lerch JP, Nazeri A, Shaikh SA, Rajji TK, et al. Myelin-associated glycoprotein gene and brain morphometry in schizophrenia. Frontiers in psychiatry. 2012;3:40. doi: 10.3389/fpsyt.2012.00040. Epub 2012/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Genome-wide association study identifies five new schizophrenia loci. Nature genetics. 2011;43(10):969–76. doi: 10.1038/ng.940. Epub 2011/09/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu H, Bi W, Liu C, Zhao Y, Zhang JF, Zhang D, et al. A hypothesis-driven pathway analysis reveals myelin-related pathways that contribute to the risk of schizophrenia and bipolar disorder. Progress in neuro-psychopharmacology & biological psychiatry. 2014 doi: 10.1016/j.pnpbp.2014.01.006. Epub 2014/01/23. [DOI] [PubMed] [Google Scholar]
- 158.Goudriaan A, de Leeuw C, Ripke S, Hultman CM, Sklar P, Sullivan PF, et al. Specific Glial Functions Contribute to Schizophrenia Susceptibility. Schizophrenia bulletin. 2013 doi: 10.1093/schbul/sbt109. Epub 2013/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.McIntosh AM, Moorhead TW, Job D, Lymer GK, Munoz Maniega S, McKirdy J, et al. The effects of a neuregulin 1 variant on white matter density and integrity. Molecular psychiatry. 2008;13(11):1054–9. doi: 10.1038/sj.mp.4002103. Epub 2007/10/11. [DOI] [PubMed] [Google Scholar]
- 160.Roussos P, Katsel P, Davis KL, Bitsios P, Giakoumaki SG, Jogia J, et al. Molecular and genetic evidence for abnormalities in the nodes of Ranvier in schizophrenia. Archives of general psychiatry. 2012;69(1):7–15. doi: 10.1001/archgenpsychiatry.2011.110. Epub 2011/09/07. [DOI] [PubMed] [Google Scholar]
- 161.Roussos P, Haroutunian V. Schizophrenia: susceptibility genes and oligodendroglial and myelin related abnormalities. Frontiers in cellular neuroscience. 2014;8:5. doi: 10.3389/fncel.2014.00005. Epub 2014/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Voineskos AN. Genetic underpinnings of white matter ‘connectivity’: Heritability, risk, and heterogeneity in schizophrenia. Schizophrenia research. 2014 doi: 10.1016/j.schres.2014.03.034. Epub 2014/06/05. [DOI] [PubMed] [Google Scholar]
- 163.Bartzokis G. Neuroglialpharmacology: myelination as a shared mechanism of action of psychotropic treatments. Neuropharmacology. 2012;62(7):2137–53. doi: 10.1016/j.neuropharm.2012.01.015. Epub 2012/02/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reis Marques T, Taylor H, Chaddock C, Dell'acqua F, Handley R, Reinders AA, et al. White matter integrity as a predictor of response to treatment in first episode psychosis. Brain : a journal of neurology. 2014;137(Pt 1):172–82. doi: 10.1093/brain/awt310. Epub 2013/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brown AS. The environment and susceptibility to schizophrenia. Progress in neurobiology. 2011;93(1):23–58. doi: 10.1016/j.pneurobio.2010.09.003. Epub 2010/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Insel TR. Rethinking schizophrenia. Nature. 2010;468(7321):187–93. doi: 10.1038/nature09552. Epub 2010/11/12. [DOI] [PubMed] [Google Scholar]
- 167.Drexhage RC, Weigelt K, van Beveren N, Cohen D, Versnel MA, Nolen WA, et al. Immune and neuroimmune alterations in mood disorders and schizophrenia. International review of neurobiology. 2011;101:169–201. doi: 10.1016/B978-0-12-387718-5.00007-9. Epub 2011/11/05. [DOI] [PubMed] [Google Scholar]
- 168.Zhang XY, Zhou DF, Cao LY, Wu GY, Shen YC. Cortisol and cytokines in chronic and treatment-resistant patients with schizophrenia: association with psychopathology and response to antipsychotics. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30(8):1532–8. doi: 10.1038/sj.npp.1300756. Epub 2005/05/12. [DOI] [PubMed] [Google Scholar]
- 169.Ebrinc S, Top C, Oncul O, Basoglu C, Cavuslu S, Cetin M. Serum interleukin 1 alpha and interleukin 2 levels in patients with schizophrenia. The Journal of international medical research. 2002;30(3):314–7. doi: 10.1177/147323000203000313. Epub 2002/08/09. [DOI] [PubMed] [Google Scholar]
- 170.Monteleone P, Fabrazzo M, Tortorella A, Maj M. Plasma levels of interleukin-6 and tumor necrosis factor alpha in chronic schizophrenia: effects of clozapine treatment. Psychiatry research. 1997;71(1):11–7. doi: 10.1016/s0165-1781(97)00036-x. Epub 1997/06/16. [DOI] [PubMed] [Google Scholar]
- 171.Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biological psychiatry. 2009;65(6):481–8. doi: 10.1016/j.biopsych.2008.10.018. Epub 2008/12/09. [DOI] [PubMed] [Google Scholar]
- 172.Erbagci AB, Herken H, Koyluoglu O, Yilmaz N, Tarakcioglu M. Serum IL-1beta, sIL-2R, IL-6, IL-8 and TNF-alpha in schizophrenic patients, relation with symptomatology and responsiveness to risperidone treatment. Mediators of inflammation. 2001;10(3):109–15. doi: 10.1080/09629350123895. Epub 2001/09/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Theodoropoulou S, Spanakos G, Baxevanis CN, Economou M, Gritzapis AD, Papamichail MP, et al. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophrenia research. 2001;47(1):13–25. doi: 10.1016/s0920-9964(00)00007-4. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 174.Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophrenia research. 2014;155(1-3):101–8. doi: 10.1016/j.schres.2014.03.005. Epub 2014/04/08. [DOI] [PubMed] [Google Scholar]
- 175.Tourjman V, Kouassi E, Koue ME, Rocchetti M, Fortin-Fournier S, Fusar-Poli P, et al. Antipsychotics' effects on blood levels of cytokines in schizophrenia: a meta-analysis. Schizophrenia research. 2013;151(1-3):43–7. doi: 10.1016/j.schres.2013.10.011. Epub 2013/11/10. [DOI] [PubMed] [Google Scholar]
- 176.Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, et al. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. The American journal of psychiatry. 2004;161(5):889–95. doi: 10.1176/appi.ajp.161.5.889. Epub 2004/05/04. [DOI] [PubMed] [Google Scholar]
- 177.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain, behavior, and immunity. 2001;15(4):411–20. doi: 10.1006/brbi.2001.0644. Epub 2002/01/10. [DOI] [PubMed] [Google Scholar]
- 178.Hayes LN, Severance EG, Leek JT, Gressitt KL, Rohleder C, Coughlin JM, et al. Inflammatory Molecular Signature Associated With Infectious Agents in Psychosis. Schizophrenia bulletin. 2014 doi: 10.1093/schbul/sbu052. Epub 2014/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Beumer W, Gibney SM, Drexhage RC, Pont-Lezica L, Doorduin J, Klein HC, et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. Journal of leukocyte biology. 2012;92(5):959–75. doi: 10.1189/jlb.0212100. Epub 2012/08/10. [DOI] [PubMed] [Google Scholar]
- 180.Nikkila HV, Muller K, Ahokas A, Miettinen K, Rimon R, Andersson LC. Accumulation of macrophages in the CSF of schizophrenic patients during acute psychotic episodes. The American journal of psychiatry. 1999;156(11):1725–9. doi: 10.1176/ajp.156.11.1725. Epub 1999/11/30. [DOI] [PubMed] [Google Scholar]
- 181.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74(4):691–705. doi: 10.1016/j.neuron.2012.03.026. Epub 2012/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science (New York, NY) 2011;333(6048):1456–8. doi: 10.1126/science.1202529. Epub 2011/07/23. [DOI] [PubMed] [Google Scholar]
- 183.Ji K, Miyauchi J, Tsirka SE. Microglia: an active player in the regulation of synaptic activity. Neural plasticity. 2013;2013:627325. doi: 10.1155/2013/627325. Epub 2013/12/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? Journal of psychiatric research. 1982;17(4):319–34. doi: 10.1016/0022-3956(82)90038-3. Epub 1982/01/01. [DOI] [PubMed] [Google Scholar]
- 185.Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepien T, Pasennik E. Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia neuropathologica / Association of Polish Neuropathologists and Medical Research Centre, Polish Academy of Sciences. 2005;43(2):81–9. Epub 2005/07/14. [PubMed] [Google Scholar]
- 186.Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. Journal of neuropathology and experimental neurology. 2000;59(2):137–50. doi: 10.1093/jnen/59.2.137. Epub 2000/04/05. [DOI] [PubMed] [Google Scholar]
- 187.Arnold SE, Trojanowski JQ, Gur RE, Blackwell P, Han LY, Choi C. Absence of neurodegeneration and neural injury in the cerebral cortex in a sample of elderly patients with schizophrenia. Archives of general psychiatry. 1998;55(3):225–32. doi: 10.1001/archpsyc.55.3.225. Epub 1998/03/24. [DOI] [PubMed] [Google Scholar]
- 188.Falke E, Han LY, Arnold SE. Absence of neurodegeneration in the thalamus and caudate of elderly patients with schizophrenia. Psychiatry research. 2000;93(2):103–10. doi: 10.1016/s0165-1781(00)00104-9. Epub 2000/03/22. [DOI] [PubMed] [Google Scholar]
- 189.Kahn RS, Sommer IE. The neurobiology and treatment of first-episode schizophrenia. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.66. Epub 2014/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fillman SG, Cloonan N, Catts VS, Miller LC, Wong J, McCrossin T, et al. Increased inflammatory markers identified in the dorsolateral prefrontal cortex of individuals with schizophrenia. Molecular psychiatry. 2013;18(2):206–14. doi: 10.1038/mp.2012.110. Epub 2012/08/08. [DOI] [PubMed] [Google Scholar]
- 191.Fillman SG, Cloonan N, Miller LC, Weickert CS. Markers of inflammation in the prefrontal cortex of individuals with schizophrenia. Molecular psychiatry. 2013;18(2):133. doi: 10.1038/mp.2012.199. Epub 2013/01/25. [DOI] [PubMed] [Google Scholar]
- 192.Rupprecht R, Papadopoulos V, Rammes G, Baghai TC, Fan J, Akula N, et al. Translocator protein (18 kDa) (TSPO) as a therapeutic target for neurological and psychiatric disorders. Nature reviews Drug discovery. 2010;9(12):971–88. doi: 10.1038/nrd3295. Epub 2010/12/02. [DOI] [PubMed] [Google Scholar]
- 193.Banati RB, Newcombe J, Gunn RN, Cagnin A, Turkheimer F, Heppner F, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain : a journal of neurology. 2000;123(Pt 11):2321–37. doi: 10.1093/brain/123.11.2321. Epub 2000/10/26. [DOI] [PubMed] [Google Scholar]
- 194.Banati RB, Egensperger R, Maassen A, Hager G, Kreutzberg GW, Graeber MB. Mitochondria in activated microglia in vitro. Journal of neurocytology. 2004;33(5):535–41. doi: 10.1007/s11068-004-0515-7. Epub 2005/05/21. [DOI] [PubMed] [Google Scholar]
- 195.Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50(11):1801–7. doi: 10.2967/jnumed.109.066647. Epub 2009/10/20. [DOI] [PubMed] [Google Scholar]
- 196.van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biological psychiatry. 2008;64(9):820–2. doi: 10.1016/j.biopsych.2008.04.025. Epub 2008/06/07. [DOI] [PubMed] [Google Scholar]
- 197.Doorduin J, de Vries EF, Dierckx RA, Klein HC. PET imaging of the peripheral benzodiazepine receptor: monitoring disease progression and therapy response in neurodegenerative disorders. Current pharmaceutical design. 2008;14(31):3297–315. doi: 10.2174/138161208786549443. Epub 2008/12/17. [DOI] [PubMed] [Google Scholar]
- 198.Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32(1):1–5. doi: 10.1038/jcbfm.2011.147. Epub 2011/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, et al. Impaired mitochondrial function in psychiatric disorders. Nature reviews Neuroscience. 2012;13(5):293–307. doi: 10.1038/nrn3229. Epub 2012/04/19. [DOI] [PubMed] [Google Scholar]
- 200.McGrath JJ, Mortensen PB, Visscher PM, Wray NR. Where GWAS and epidemiology meet: opportunities for the simultaneous study of genetic and environmental risk factors in schizophrenia. Schizophrenia bulletin. 2013;39(5):955–9. doi: 10.1093/schbul/sbt108. Epub 2013/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ayhan Y, Sawa A, Ross CA, Pletnikov MV. Animal models of gene-environment interactions in schizophrenia. Behavioural brain research. 2009;204(2):274–81. doi: 10.1016/j.bbr.2009.04.010. Epub 2009/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Kannan G, Sawa A, Pletnikov MV. Mouse models of gene-environment interactions in schizophrenia. Neurobiology of disease. 2013;57:5–11. doi: 10.1016/j.nbd.2013.05.012. Epub 2013/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Benros ME, Eaton WW, Mortensen PB. The Epidemiologic Evidence Linking Autoimmune Diseases and Psychosis. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.09.023. Epub 2013/11/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Eaton WW, Byrne M, Ewald H, Mors O, Chen CY, Agerbo E, et al. Association of schizophrenia and autoimmune diseases: linkage of Danish national registers. The American journal of psychiatry. 2006;163(3):521–8. doi: 10.1176/appi.ajp.163.3.521. Epub 2006/03/04. [DOI] [PubMed] [Google Scholar]
- 205.Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460(7256):744–7. doi: 10.1038/nature08186. Epub 2009/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jia P, Wang L, Fanous AH, Chen X, Kendler KS, Zhao Z. A bias-reducing pathway enrichment analysis of genome-wide association data confirmed association of the MHC region with schizophrenia. Journal of medical genetics. 2012;49(2):96–103. doi: 10.1136/jmedgenet-2011-100397. Epub 2011/12/22. [DOI] [PubMed] [Google Scholar]
- 207.Bitanihirwe BK, Woo TU. Oxidative stress in schizophrenia: an integrated approach. Neuroscience and biobehavioral reviews. 2011;35(3):878–93. doi: 10.1016/j.neubiorev.2010.10.008. Epub 2010/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Emiliani FE, Sedlak TW, Sawa A. Oxidative stress and schizophrenia: recent breakthroughs from an old story. Current opinion in psychiatry. 2014;27(3):185–90. doi: 10.1097/YCO.0000000000000054. Epub 2014/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biological psychiatry. 2013;74(6):400–9. doi: 10.1016/j.biopsych.2013.03.018. Epub 2013/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2011;14(1):123–30. doi: 10.1017/S1461145710000805. Epub 2010/07/17. [DOI] [PubMed] [Google Scholar]
- 211.Evans DR, Parikh VV, Khan MM, Coussons C, Buckley PF, Mahadik SP. Red blood cell membrane essential fatty acid metabolism in early psychotic patients following antipsychotic drug treatment. Prostaglandins, leukotrienes, and essential fatty acids. 2003;69(6):393–9. doi: 10.1016/j.plefa.2003.08.010. Epub 2003/11/19. [DOI] [PubMed] [Google Scholar]
- 212.Coughlin JM, Ishizuka K, Kano SI, Edwards JA, Seifuddin FT, Shimano MA, et al. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Molecular psychiatry. 2013;18(1):10–1. doi: 10.1038/mp.2012.6. Epub 2012/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Raffa M, Atig F, Mhalla A, Kerkeni A, Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC psychiatry. 2011;11:124. doi: 10.1186/1471-244X-11-124. Epub 2011/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kano S, Colantuoni C, Han F, Zhou Z, Yuan Q, Wilson A, et al. Genome-wide profiling of multiple histone methylations in olfactory cells: further implications for cellular susceptibility to oxidative stress in schizophrenia. Molecular psychiatry. 2013;18(7):740–2. doi: 10.1038/mp.2012.120. Epub 2012/08/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Do KQ, Trabesinger AH, Kirsten-Kruger M, Lauer CJ, Dydak U, Hell D, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. The European journal of neuroscience. 2000;12(10):3721–8. doi: 10.1046/j.1460-9568.2000.00229.x. Epub 2000/10/13. [DOI] [PubMed] [Google Scholar]
- 216.Terpstra M, Vaughan TJ, Ugurbil K, Lim KO, Schulz SC, Gruetter R. Validation of glutathione quantitation from STEAM spectra against edited 1H NMR spectroscopy at 4T: application to schizophrenia. Magma (New York, NY) 2005;18(5):276–82. doi: 10.1007/s10334-005-0012-0. Epub 2005/12/02. [DOI] [PubMed] [Google Scholar]
- 217.Matsuzawa D, Obata T, Shirayama Y, Nonaka H, Kanazawa Y, Yoshitome E, et al. Negative correlation between brain glutathione level and negative symptoms in schizophrenia: a 3T 1H-MRS study. PloS one. 2008;3(4):e1944. doi: 10.1371/journal.pone.0001944. Epub 2008/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wood SJ, Berger GE, Wellard RM, Proffitt TM, McConchie M, Berk M, et al. Medial temporal lobe glutathione concentration in first episode psychosis: a 1H-MRS investigation. Neurobiology of disease. 2009;33(3):354–7. doi: 10.1016/j.nbd.2008.11.018. Epub 2009/01/03. [DOI] [PubMed] [Google Scholar]
- 219.Borges S, Gayer-Anderson C, Mondelli V. A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology. 2013;38(5):603–11. doi: 10.1016/j.psyneuen.2012.12.025. Epub 2013/02/02. [DOI] [PubMed] [Google Scholar]
- 220.Pariante CM, Vassilopoulou K, Velakoulis D, Phillips L, Soulsby B, Wood SJ, et al. Pituitary volume in psychosis. The British journal of psychiatry : the journal of mental science. 2004;185:5–10. doi: 10.1192/bjp.185.1.5. Epub 2004/07/03. [DOI] [PubMed] [Google Scholar]
- 221.Takahashi T, Nakamura K, Nishiyama S, Furuichi A, Ikeda E, Kido M, et al. Increased pituitary volume in subjects at risk for psychosis and patients with first-episode schizophrenia. Psychiatry and clinical neurosciences. 2013;67(7):540–8. doi: 10.1111/pcn.12093. Epub 2013/10/10. [DOI] [PubMed] [Google Scholar]
- 222.Buschlen J, Berger GE, Borgwardt SJ, Aston J, Gschwandtner U, Pflueger MO, et al. Pituitary volume increase during emerging psychosis. Schizophrenia research. 2011;125(1):41–8. doi: 10.1016/j.schres.2010.09.022. Epub 2010/11/16. [DOI] [PubMed] [Google Scholar]
- 223.Romo-Nava F, Hoogenboom WS, Pelavin PE, Alvarado JL, Bobrow LH, Macmaster FP, et al. Pituitary volume in schizophrenia spectrum disorders. Schizophrenia research. 2013;146(1-3):301–7. doi: 10.1016/j.schres.2013.02.024. Epub 2013/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gruner P, Christian C, Robinson DG, Sevy S, Gunduz-Bruce H, Napolitano B, et al. Pituitary volume in first-episode schizophrenia. Psychiatry research. 2012;203(1):100–2. doi: 10.1016/j.pscychresns.2011.09.017. Epub 2012/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tournikioti K, Tansella M, Perlini C, Rambaldelli G, Cerini R, Versace A, et al. Normal pituitary volumes in chronic schizophrenia. Psychiatry research. 2007;154(1):41–8. doi: 10.1016/j.pscychresns.2006.04.004. Epub 2006/12/23. [DOI] [PubMed] [Google Scholar]
- 226.Upadhyaya AR, El-Sheikh R, MacMaster FP, Diwadkar VA, Keshavan MS. Pituitary volume in neuroleptic-naive schizophrenia: a structural MRI study. Schizophrenia research. 2007;90(1-3):266–73. doi: 10.1016/j.schres.2006.09.033. Epub 2006/12/26. [DOI] [PubMed] [Google Scholar]
- 227.Tognin S, Rambaldelli G, Perlini C, Bellani M, Marinelli V, Zoccatelli G, et al. Enlarged hypothalamic volumes in schizophrenia. Psychiatry research. 2012;204(2-3):75–81. doi: 10.1016/j.pscychresns.2012.10.006. Epub 2012/12/12. [DOI] [PubMed] [Google Scholar]
- 228.Goldstein JM, Seidman LJ, Makris N, Ahern T, O'Brien LM, Caviness VS, Jr., et al. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biological psychiatry. 2007;61(8):935–45. doi: 10.1016/j.biopsych.2006.06.027. Epub 2006/10/19. [DOI] [PubMed] [Google Scholar]
- 229.Koolschijn PC, van Haren NE, Hulshoff Pol HE, Kahn RS. Hypothalamus volume in twin pairs discordant for schizophrenia. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2008;18(4):312–5. doi: 10.1016/j.euroneuro.2007.12.004. Epub 2008/01/29. [DOI] [PubMed] [Google Scholar]
- 230.Klomp A, Koolschijn PC, Hulshoff Pol HE, Kahn RS, Haren NE. Hypothalamus and pituitary volume in schizophrenia: a structural MRI study. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2012;15(2):281–8. doi: 10.1017/S1461145711000794. Epub 2011/07/08. [DOI] [PubMed] [Google Scholar]
- 231.Walsh P, Spelman L, Sharifi N, Thakore JH. Male patients with paranoid schizophrenia have greater ACTH and cortisol secretion in response to metoclopramide-induced AVP release. Psychoneuroendocrinology. 2005;30(5):431–7. doi: 10.1016/j.psyneuen.2004.11.003. Epub 2005/02/22. [DOI] [PubMed] [Google Scholar]
- 232.Ritsner M, Gibel A, Maayan R, Ratner Y, Ram E, Modai I, et al. State and trait related predictors of serum cortisol to DHEA(S) molar ratios and hormone concentrations in schizophrenia patients. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2007;17(4):257–64. doi: 10.1016/j.euroneuro.2006.09.001. Epub 2006/11/17. [DOI] [PubMed] [Google Scholar]
- 233.Gallagher P, Watson S, Smith MS, Young AH, Ferrier IN. Plasma cortisol-dehydroepiandrosterone (DHEA) ratios in schizophrenia and bipolar disorder. Schizophrenia research. 2007;90(1-3):258–65. doi: 10.1016/j.schres.2006.11.020. Epub 2007/01/16. [DOI] [PubMed] [Google Scholar]
- 234.Yilmaz N, Herken H, Cicek HK, Celik A, Yurekli M, Akyol O. Increased levels of nitric oxide, cortisol and adrenomedullin in patients with chronic schizophrenia. Medical principles and practice : international journal of the Kuwait University, Health Science Centre. 2007;16(2):137–41. doi: 10.1159/000098367. Epub 2007/02/17. [DOI] [PubMed] [Google Scholar]
- 235.Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary-adrenal overactivity in first episode, drug naive patients with schizophrenia. Psychoneuroendocrinology. 2004;29(8):1065–70. doi: 10.1016/j.psyneuen.2003.08.011. Epub 2004/06/29. [DOI] [PubMed] [Google Scholar]
- 236.Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA, et al. Cortisol levels and risk for psychosis: initial findings from the North American prodrome longitudinal study. Biological psychiatry. 2013;74(6):410–7. doi: 10.1016/j.biopsych.2013.02.016. Epub 2013/04/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Strous RD, Maayan R, Lapidus R, Goredetsky L, Zeldich E, Kotler M, et al. Increased circulatory dehydroepiandrosterone and dehydroepiandrosterone-sulphate in first-episode schizophrenia: relationship to gender, aggression and symptomatology. Schizophrenia research. 2004;71(2-3):427–34. doi: 10.1016/j.schres.2004.03.005. Epub 2004/10/12. [DOI] [PubMed] [Google Scholar]
- 238.Venkatasubramanian G, Chittiprol S, Neelakantachar N, Shetty T, Gangadhar BN. Effect of antipsychotic treatment on Insulin-like Growth Factor-1 and cortisol in schizophrenia: a longitudinal study. Schizophrenia research. 2010;119(1-3):131–7. doi: 10.1016/j.schres.2010.01.033. Epub 2010/03/17. [DOI] [PubMed] [Google Scholar]
- 239.Krishnamurthy D, Harris LW, Levin Y, Koutroukides TA, Rahmoune H, Pietsch S, et al. Metabolic, hormonal and stress-related molecular changes in post-mortem pituitary glands from schizophrenia subjects. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2013;14(7):478–89. doi: 10.3109/15622975.2011.601759. Epub 2012/01/18. [DOI] [PubMed] [Google Scholar]
- 240.Perlman WR, Webster MJ, Kleinman JE, Weickert CS. Reduced glucocorticoid and estrogen receptor alpha messenger ribonucleic acid levels in the amygdala of patients with major mental illness. Biological psychiatry. 2004;56(11):844–52. doi: 10.1016/j.biopsych.2004.09.006. Epub 2004/12/04. [DOI] [PubMed] [Google Scholar]
- 241.Webster MJ, Knable MB, O'Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Molecular psychiatry. 2002;7(9):985–94, 24. doi: 10.1038/sj.mp.4001139. Epub 2002/10/26. [DOI] [PubMed] [Google Scholar]
- 242.Hirsch D, Orr G, Kantarovich V, Hermesh H, Stern E, Blum I. Cushing's syndrome presenting as a schizophrenia-like psychotic state. The Israel journal of psychiatry and related sciences. 2000;37(1):46–50. Epub 2000/06/17. [PubMed] [Google Scholar]
- 243.Hall RC, Popkin MK, Stickney SK, Gardner ER. Presentation of the steroid psychoses. The Journal of nervous and mental disease. 1979;167(4):229–36. doi: 10.1097/00005053-197904000-00006. Epub 1979/04/01. [DOI] [PubMed] [Google Scholar]
- 244.Flores BH, Kenna H, Keller J, Solvason HB, Schatzberg AF. Clinical and biological effects of mifepristone treatment for psychotic depression. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31(3):628–36. doi: 10.1038/sj.npp.1300884. Epub 2005/09/15. [DOI] [PubMed] [Google Scholar]
- 245.Schatzberg AF, Lindley S. Glucocorticoid antagonists in neuropsychiatric [corrected] disorders. European journal of pharmacology. 2008;583(2-3):358–64. doi: 10.1016/j.ejphar.2008.01.001. Epub 2008/03/15. [DOI] [PubMed] [Google Scholar]
- 246.Myers B, McKlveen JM, Herman JP. Glucocorticoid actions on synapses, circuits, and behavior: implications for the energetics of stress. Frontiers in neuroendocrinology. 2014;35(2):180–96. doi: 10.1016/j.yfrne.2013.12.003. Epub 2013/12/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Niwa M, Jaaro-Peled H, Tankou S, Seshadri S, Hikida T, Matsumoto Y, et al. Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science (New York, NY) 2013;339(6117):335–9. doi: 10.1126/science.1226931. Epub 2013/01/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Huber TJ, Tettenborn C, Leifke E, Emrich HM. Sex hormones in psychotic men. Psychoneuroendocrinology. 2005;30(1):111–4. doi: 10.1016/j.psyneuen.2004.05.010. Epub 2004/09/11. [DOI] [PubMed] [Google Scholar]
- 249.Bergemann N, Mundt C, Parzer P, Jannakos I, Nagl I, Salbach B, et al. Plasma concentrations of estradiol in women suffering from schizophrenia treated with conventional versus atypical antipsychotics. Schizophrenia research. 2005;73(2-3):357–66. doi: 10.1016/j.schres.2004.06.013. Epub 2005/01/18. [DOI] [PubMed] [Google Scholar]
- 250.Weickert CS, Miranda-Angulo AL, Wong J, Perlman WR, Ward SE, Radhakrishna V, et al. Variants in the estrogen receptor alpha gene and its mRNA contribute to risk for schizophrenia. Human molecular genetics. 2008;17(15):2293–309. doi: 10.1093/hmg/ddn130. Epub 2008/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Kulkarni J, Hayes E, Gavrilidis E. Hormones and schizophrenia. Current opinion in psychiatry. 2012;25(2):89–95. doi: 10.1097/YCO.0b013e328350360e. Epub 2012/01/18. [DOI] [PubMed] [Google Scholar]
- 252.Lokuge S, Frey BN, Foster JA, Soares CN, Steiner M. The rapid effects of estrogen: a mini-review. Behavioural pharmacology. 2010;21(5-6):465–72. doi: 10.1097/FBP.0b013e32833da5c3. Epub 2010/07/28. [DOI] [PubMed] [Google Scholar]
- 253.Cyr M, Ghribi O, Di Paolo T. Regional and selective effects of oestradiol and progesterone on NMDA and AMPA receptors in the rat brain. Journal of neuroendocrinology. 2000;12(5):445–52. doi: 10.1046/j.1365-2826.2000.00471.x. Epub 2000/05/03. [DOI] [PubMed] [Google Scholar]
- 254.Allison DB, Fontaine KR, Heo M, Mentore JL, Cappelleri JC, Chandler LP, et al. The distribution of body mass index among individuals with and without schizophrenia. The Journal of clinical psychiatry. 1999;60(4):215–20. doi: 10.4088/jcp.v60n0402. Epub 1999/04/30. [DOI] [PubMed] [Google Scholar]
- 255.Consensus development conference on antipsychotic drugs and obesity and diabetes. The Journal of clinical psychiatry. 2004;65(2):267–72. doi: 10.4088/jcp.v65n0219. Epub 2004/03/09. [DOI] [PubMed] [Google Scholar]
- 256.Boke O, Aker S, Sarisoy G, Saricicek EB, Sahin AR. Prevalence of metabolic syndrome among inpatients with schizophrenia. International journal of psychiatry in medicine. 2008;38(1):103–12. doi: 10.2190/PM.38.1.j. Epub 2008/07/16. [DOI] [PubMed] [Google Scholar]
- 257.Takayanagi Y, Cascella NG, Sawa A, Eaton WW. Diabetes is associated with lower global cognitive function in schizophrenia. Schizophrenia research. 2012;142(1-3):183–7. doi: 10.1016/j.schres.2012.08.034. Epub 2012/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Popovic V, Doknic M, Maric N, Pekic S, Damjanovic A, Miljic D, et al. Changes in neuroendocrine and metabolic hormones induced by atypical antipsychotics in normal-weight patients with schizophrenia. Neuroendocrinology. 2007;85(4):249–56. doi: 10.1159/000103868. Epub 2007/06/16. [DOI] [PubMed] [Google Scholar]
- 259.Doknic M, Maric NP, Britvic D, Pekic S, Damjanovic A, Miljic D, et al. Bone remodeling, bone mass and weight gain in patients with stabilized schizophrenia in real-life conditions treated with long-acting injectable risperidone. Neuroendocrinology. 2011;94(3):246–54. doi: 10.1159/000329391. Epub 2011/10/12. [DOI] [PubMed] [Google Scholar]
- 260.De Hert M, Peuskens J, van Winkel R. Mortality in patients with schizophrenia. Lancet. 2009;374(9701):1591. doi: 10.1016/S0140-6736(09)61942-5. author reply 2-3. Epub 2009/11/10. [DOI] [PubMed] [Google Scholar]
- 261.Spelman LM, Walsh PI, Sharifi N, Collins P, Thakore JH. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia. Diabetic medicine : a journal of the British Diabetic Association. 2007;24(5):481–5. doi: 10.1111/j.1464-5491.2007.02092.x. Epub 2007/03/27. [DOI] [PubMed] [Google Scholar]
- 262.Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naive patients with schizophrenia. The American journal of psychiatry. 2003;160(2):284–9. doi: 10.1176/appi.ajp.160.2.284. Epub 2003/02/04. [DOI] [PubMed] [Google Scholar]
- 263.Kirkpatrick B. Schizophrenia as a systemic disease. Schizophrenia bulletin. 2009;35(2):381–2. doi: 10.1093/schbul/sbn183. Epub 2009/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Guest PC, Martins-de-Souza D, Vanattou-Saifoudine N, Harris LW, Bahn S. Abnormalities in metabolism and hypothalamic-pituitary-adrenal axis function in schizophrenia. International review of neurobiology. 2011;101:145–68. doi: 10.1016/B978-0-12-387718-5.00006-7. Epub 2011/11/05. [DOI] [PubMed] [Google Scholar]
- 265.Holmes E, Tsang TM, Huang JT, Leweke FM, Koethe D, Gerth CW, et al. Metabolic profiling of CSF: evidence that early intervention may impact on disease progression and outcome in schizophrenia. PLoS medicine. 2006;3(8):e327. doi: 10.1371/journal.pmed.0030327. Epub 2006/08/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Archives of general psychiatry. 1992;49(7):522–30. doi: 10.1001/archpsyc.1992.01820070016003. Epub 1992/07/01. [DOI] [PubMed] [Google Scholar]
- 267.Buchsbaum MS, Buchsbaum BR, Hazlett EA, Haznedar MM, Newmark R, Tang CY, et al. Relative glucose metabolic rate higher in white matter in patients with schizophrenia. The American journal of psychiatry. 2007;164(7):1072–81. doi: 10.1176/ajp.2007.164.7.1072. Epub 2007/07/04. [DOI] [PubMed] [Google Scholar]
- 268.Lin CY, Sawa A, Jaaro-Peled H. Better understanding of mechanisms of schizophrenia and bipolar disorder: from human gene expression profiles to mouse models. Neurobiology of disease. 2012;45(1):48–56. doi: 10.1016/j.nbd.2011.08.025. Epub 2011/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhao Z, Ksiezak-Reding H, Riggio S, Haroutunian V, Pasinetti GM. Insulin receptor deficits in schizophrenia and in cellular and animal models of insulin receptor dysfunction. Schizophrenia research. 2006;84(1):1–14. doi: 10.1016/j.schres.2006.02.009. Epub 2006/04/04. [DOI] [PubMed] [Google Scholar]
- 270.Bernstein HG, Ernst T, Lendeckel U, Bukowska A, Ansorge S, Stauch R, et al. Reduced neuronal expression of insulin-degrading enzyme in the dorsolateral prefrontal cortex of patients with haloperidol-treated, chronic schizophrenia. Journal of psychiatric research. 2009;43(13):1095–105. doi: 10.1016/j.jpsychires.2009.03.006. Epub 2009/04/28. [DOI] [PubMed] [Google Scholar]
- 271.O'Neill C, Kiely AP, Coakley MF, Manning S, Long-Smith CM. Insulin and IGF-1 signalling: longevity, protein homoeostasis and Alzheimer's disease. Biochemical Society transactions. 2012;40(4):721–7. doi: 10.1042/BST20120080. Epub 2012/07/24. [DOI] [PubMed] [Google Scholar]
- 272.C ON. PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer's disease. Experimental gerontology. 2013;48(7):647–53. doi: 10.1016/j.exger.2013.02.025. Epub 2013/03/09. [DOI] [PubMed] [Google Scholar]
- 273.McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophrenia research. 2005;80(1):19–32. doi: 10.1016/j.schres.2005.07.014. Epub 2005/09/03. [DOI] [PubMed] [Google Scholar]
- 274.Stone WS, Faraone SV, Su J, Tarbox SI, Van Eerdewegh P, Tsuang MT. Evidence for linkage between regulatory enzymes in glycolysis and schizophrenia in a multiplex sample. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2004;127B(1):5–10. doi: 10.1002/ajmg.b.20132. Epub 2004/04/27. [DOI] [PubMed] [Google Scholar]
- 275.Olsen L, Hansen T, Jakobsen KD, Djurovic S, Melle I, Agartz I, et al. The estrogen hypothesis of schizophrenia implicates glucose metabolism: association study in three independent samples. BMC medical genetics. 2008;9:39. doi: 10.1186/1471-2350-9-39. Epub 2008/05/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. The American journal of psychiatry. 2010;167(4):388–96. doi: 10.1176/appi.ajp.2009.08121873. Epub 2009/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Sinha MK, Opentanova I, Ohannesian JP, Kolaczynski JW, Heiman ML, Hale J, et al. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. The Journal of clinical investigation. 1996;98(6):1277–82. doi: 10.1172/JCI118913. Epub 1996/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. The New England journal of medicine. 1996;334(5):292–5. doi: 10.1056/NEJM199602013340503. Epub 1996/02/01. [DOI] [PubMed] [Google Scholar]
- 279.Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, et al. Leptin in depressed women: cross-sectional and longitudinal data from an epidemiologic study. Journal of affective disorders. 2008;107(1-3):221–5. doi: 10.1016/j.jad.2007.07.024. Epub 2007/08/31. [DOI] [PubMed] [Google Scholar]
- 280.Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG, et al. Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(24):6643–50. doi: 10.1523/JNEUROSCI.5126-05.2006. Epub 2006/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kern A, Albarran-Zeckler R, Walsh HE, Smith RG. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73(2):317–32. doi: 10.1016/j.neuron.2011.10.038. Epub 2012/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Sentissi O, Epelbaum J, Olie JP, Poirier MF. Leptin and ghrelin levels in patients with schizophrenia during different antipsychotics treatment: a review. Schizophrenia bulletin. 2008;34(6):1189–99. doi: 10.1093/schbul/sbm141. Epub 2008/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Huang JT, Wang L, Prabakaran S, Wengenroth M, Lockstone HE, Koethe D, et al. Independent protein-profiling studies show a decrease in apolipoprotein A1 levels in schizophrenia CSF, brain and peripheral tissues. Molecular psychiatry. 2008;13(12):1118–28. doi: 10.1038/sj.mp.4002108. Epub 2007/10/17. [DOI] [PubMed] [Google Scholar]
- 284.Wu X, Huang Z, Wu R, Zhong Z, Wei Q, Wang H, et al. The comparison of glycometabolism parameters and lipid profiles between drug-naive, first-episode schizophrenia patients and healthy controls. Schizophrenia research. 2013;150(1):157–62. doi: 10.1016/j.schres.2013.07.051. Epub 2013/09/04. [DOI] [PubMed] [Google Scholar]
- 285.Martins-De-Souza D, Wobrock T, Zerr I, Schmitt A, Gawinecka J, Schneider-Axmann T, et al. Different apolipoprotein E, apolipoprotein A1 and prostaglandin-H2 D-isomerase levels in cerebrospinal fluid of schizophrenia patients and healthy controls. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2010;11(5):719–28. doi: 10.3109/15622971003758748. Epub 2010/05/08. [DOI] [PubMed] [Google Scholar]
- 286.Cieslak K, Feingold J, Antonius D, Walsh-Messinger J, Dracxler R, Rosedale M, et al. Low Vitamin D levels predict clinical features of schizophrenia. Schizophrenia research. 2014;159(2-3):543–5. doi: 10.1016/j.schres.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Crews M, Lally J, Gardner-Sood P, Howes O, Bonaccorso S, Smith S, et al. Vitamin D deficiency in first episode psychosis: a case-control study. Schizophrenia research. 2013;150(2-3):533–7. doi: 10.1016/j.schres.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 288.McGrath JJ, Burne TH, Feron F, Mackay-Sim A, Eyles DW. Developmental vitamin D deficiency and risk of schizophrenia: a 10-year update. Schizophrenia bulletin. 2010;36(6):1073–8. doi: 10.1093/schbul/sbq101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kao AC, Muller DJ. Genetics of antipsychotic-induced weight gain: update and current perspectives. Pharmacogenomics. 2013;14(16):2067–83. doi: 10.2217/pgs.13.207. Epub 2013/11/28. [DOI] [PubMed] [Google Scholar]
- 290.Wallace TJ, Zai CC, Brandl EJ, Muller DJ. Role of 5-HT(2C) receptor gene variants in antipsychotic-induced weight gain. Pharmacogenomics and personalized medicine. 2011;4:83–93. doi: 10.2147/PGPM.S11866. Epub 2011/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Sicard MN, Zai CC, Tiwari AK, Souza RP, Meltzer HY, Lieberman JA, et al. Polymorphisms of the HTR2C gene and antipsychotic-induced weight gain: an update and meta-analysis. Pharmacogenomics. 2010;11(11):1561–71. doi: 10.2217/pgs.10.123. Epub 2010/12/03. [DOI] [PubMed] [Google Scholar]
- 292.Malhotra AK, Correll CU, Chowdhury NI, Muller DJ, Gregersen PK, Lee AT, et al. Association between common variants near the melanocortin 4 receptor gene and severe antipsychotic drug-induced weight gain. Archives of general psychiatry. 2012;69(9):904–12. doi: 10.1001/archgenpsychiatry.2012.191. Epub 2012/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Brandl EJ, Frydrychowicz C, Tiwari AK, Lett TA, Kitzrow W, Buttner S, et al. Association study of polymorphisms in leptin and leptin receptor genes with antipsychotic-induced body weight gain. Progress in neuro-psychopharmacology & biological psychiatry. 2012;38(2):134–41. doi: 10.1016/j.pnpbp.2012.03.001. Epub 2012/03/20. [DOI] [PubMed] [Google Scholar]
- 294.Bellavance MA, Rivest S. The HPA - Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Frontiers in immunology. 2014;5:136. doi: 10.3389/fimmu.2014.00136. Epub 2014/04/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Valleau JC, Sullivan EL. The impact of leptin on perinatal development and psychopathology. Journal of chemical neuroanatomy. 2014 doi: 10.1016/j.jchemneu.2014.05.001. Epub 2014/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cai D, Liu T. Inflammatory cause of metabolic syndrome via brain stress and NF-kappaB. Aging. 2012;4(2):98–115. doi: 10.18632/aging.100431. Epub 2012/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Bernstein HG, Steiner J, Guest PC, Dobrowolny H, Bogerts B. Glial cells as key players in schizophrenia pathology: recent insights and concepts of therapy. Schizophrenia research. 2014 doi: 10.1016/j.schres.2014.03.035. Epub 2014/06/21. [DOI] [PubMed] [Google Scholar]
- 298.Inui M, Miyado M, Igarashi M, Tamano M, Kubo A, Yamashita S, et al. Rapid generation of mouse models with defined point mutations by the CRISPR/Cas9 system. Scientific reports. 2014;4:5396. doi: 10.1038/srep05396. Epub 2014/06/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Shen B, Zhang W, Zhang J, Zhou J, Wang J, Chen L, et al. Efficient genome modification by CRISPR-Cas9 nickase with minimal off-target effects. Nature methods. 2014;11(4):399–402. doi: 10.1038/nmeth.2857. Epub 2014/03/04. [DOI] [PubMed] [Google Scholar]
- 300.Wijshake T, Baker DJ, van de Sluis B. Endonucleases: new tools to edit the mouse genome. Biochimica et biophysica acta. 2014 doi: 10.1016/j.bbadis.2014.04.020. Epub 2014/05/06. [DOI] [PubMed] [Google Scholar]
- 301.Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T. Copy number variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Molecular psychiatry. 2013;18(11):1153–65. doi: 10.1038/mp.2013.92. Epub 2013/08/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, et al. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.22. Epub 2014/04/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, et al. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biological psychiatry. 2010;68(12):1172–81. doi: 10.1016/j.biopsych.2010.09.022. Epub 2010/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Desbonnet L, O'Tuathaigh C, Clarke G, O'Leary C, Petit E, Clarke N, et al. Phenotypic effects of repeated psychosocial stress during adolescence in mice mutant for the schizophrenia risk gene neuregulin-1: a putative model of gene x environment interaction. Brain, behavior, and immunity. 2012;26(4):660–71. doi: 10.1016/j.bbi.2012.02.010. Epub 2012/03/20. [DOI] [PubMed] [Google Scholar]
- 305.Vuillermot S, Joodmardi E, Perlmann T, Ogren SO, Feldon J, Meyer U. Prenatal immune activation interacts with genetic Nurr1 deficiency in the development of attentional impairments. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(2):436–51. doi: 10.1523/JNEUROSCI.4831-11.2012. Epub 2012/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Niwa M, Kamiya A, Murai R, Kubo K, Gruber AJ, Tomita K, et al. Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron. 2010;65(4):480–9. doi: 10.1016/j.neuron.2010.01.019. Epub 2010/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Goto Y, O'Donnell P. Delayed mesolimbic system alteration in a developmental animal model of schizophrenia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22(20):9070–7. doi: 10.1523/JNEUROSCI.22-20-09070.2002. Epub 2002/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Feleder C, Tseng KY, Calhoon GG, O'Donnell P. Neonatal intrahippocampal immune challenge alters dopamine modulation of prefrontal cortical interneurons in adult rats. Biological psychiatry. 2010;67(4):386–92. doi: 10.1016/j.biopsych.2009.09.028. Epub 2009/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Kluge W, Alsaif M, Guest PC, Schwarz E, Bahn S. Translating potential biomarker candidates for schizophrenia and depression to animal models of psychiatric disorders. Expert review of molecular diagnostics. 2011;11(7):721–33. doi: 10.1586/erm.11.61. Epub 2011/09/10. [DOI] [PubMed] [Google Scholar]
- 310.Pechnick RN, George R, Poland RE, Hiramatsu M, Cho AK. Characterization of the effects of the acute and chronic administration of phencyclidine on the release of adrenocorticotropin, corticosterone and prolactin in the rat: evidence for the differential development of tolerance. The Journal of pharmacology and experimental therapeutics. 1989;250(2):534–40. Epub 1989/08/01. [PubMed] [Google Scholar]
- 311.Fattorini G, Melone M, Bragina L, Candiracci C, Cozzi A, Pellegrini Giampietro DE, et al. GLT-1 expression and Glu uptake in rat cerebral cortex are increased by phencyclidine. Glia. 2008;56(12):1320–7. doi: 10.1002/glia.20700. [DOI] [PubMed] [Google Scholar]
- 312.Murai R, Noda Y, Matsui K, Kamei H, Mouri A, Matsuba K, et al. Hypofunctional glutamatergic neurotransmission in the prefrontal cortex is involved in the emotional deficit induced by repeated treatment with phencyclidine in mice: implications for abnormalities of glutamate release and NMDA-CaMKII signaling. Behavioural brain research. 2007;180(2):152–60. doi: 10.1016/j.bbr.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 313.Jenkins TA, Harte MK, McKibben CE, Elliott JJ, Reynolds GP. Disturbances in social interaction occur along with pathophysiological deficits following sub-chronic phencyclidine administration in the rat. Behavioural brain research. 2008;194(2):230–5. doi: 10.1016/j.bbr.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 314.McKibben CE, Jenkins TA, Adams HN, Harte MK, Reynolds GP. Effect of pretreatment with risperidone on phencyclidine-induced disruptions in object recognition memory and prefrontal cortex parvalbumin immunoreactivity in the rat. Behavioural brain research. 2010;208(1):132–6. doi: 10.1016/j.bbr.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 315.Braun I, Genius J, Grunze H, Bender A, Moller HJ, Rujescu D. Alterations of hippocampal and prefrontal GABAergic interneurons in an animal model of psychosis induced by NMDA receptor antagonism. Schizophrenia research. 2007;97(1-3):254–63. doi: 10.1016/j.schres.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 316.Bullock WM, Bolognani F, Botta P, Valenzuela CF, Perrone-Bizzozero NI. Schizophrenia-like GABAergic gene expression deficits in cerebellar Golgi cells from rats chronically exposed to low-dose phencyclidine. Neurochemistry international. 2009;55(8):775–82. doi: 10.1016/j.neuint.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Choi YK, Snigdha S, Shahid M, Neill JC, Tarazi FI. Subchronic effects of phencyclidine on dopamine and serotonin receptors: implications for schizophrenia. Journal of molecular neuroscience : MN. 2009;38(3):227–35. doi: 10.1007/s12031-009-9204-9. [DOI] [PubMed] [Google Scholar]
- 318.Beninger RJ, Beuk J, Banasikowski TJ, van Adel M, Boivin GA, Reynolds JN. Subchronic phencyclidine in rats: alterations in locomotor activity, maze performance, and GABA(A) receptor binding. Behavioural pharmacology. 2010;21(1):1–10. doi: 10.1097/FBP.0b013e3283347091. [DOI] [PubMed] [Google Scholar]
- 319.Hyde JF. Effects of phencyclidine on 5-hydroxytryptophan- and suckling-induced prolactin release. Brain research. 1992;573(2):204–8. doi: 10.1016/0006-8993(92)90764-z. [DOI] [PubMed] [Google Scholar]
- 320.Takahashi M, Kakita A, Futamura T, Watanabe Y, Mizuno M, Sakimura K, et al. Sustained brain-derived neurotrophic factor up-regulation and sensorimotor gating abnormality induced by postnatal exposure to phencyclidine: comparison with adult treatment. Journal of neurochemistry. 2006;99(3):770–80. doi: 10.1111/j.1471-4159.2006.04106.x. [DOI] [PubMed] [Google Scholar]
- 321.Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain, behavior, and immunity. 2012;26(4):623–34. doi: 10.1016/j.bbi.2012.01.015. Epub 2012/02/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biological psychiatry. 2014;75(4):307–15. doi: 10.1016/j.biopsych.2013.07.011. Epub 2013/08/14. [DOI] [PubMed] [Google Scholar]
- 323.Xiu Y, Kong XR, Zhang L, Qiu X, Chao FL, Peng C, et al. White matter injuries induced by MK-801 in a mouse model of schizophrenia based on NMDA antagonism. Anatomical record. 2014;297(8):1498–507. doi: 10.1002/ar.22942. [DOI] [PubMed] [Google Scholar]
- 324.Zhu S, Wang H, Shi R, Zhang R, Wang J, Kong L, et al. Chronic Phencyclidine Induces Inflammatory Responses and Activates GSK3beta in Mice. Neurochemical research. 2014;39(12):2385–93. doi: 10.1007/s11064-014-1441-9. [DOI] [PubMed] [Google Scholar]
- 325.Venancio C, Felix L, Almeida V, Coutinho J, Antunes L, Peixoto F, et al. Acute Ketamine Impairs Mitochondrial Function and Promotes Superoxide Dismutase Activity in the Rat Brain. Anesthesia and analgesia. 2014 doi: 10.1213/ANE.0000000000000539. [DOI] [PubMed] [Google Scholar]
- 326.Dean B, Karl T, Pavey G, Boer S, Duffy L, Scarr E. Increased levels of serotonin 2A receptors and serotonin transporter in the CNS of neuregulin 1 hypomorphic/mutant mice. Schizophrenia research. 2008;99(1-3):341–9. doi: 10.1016/j.schres.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 327.Iltis I, Koski DM, Eberly LE, Nelson CD, Deelchand DK, Valette J, et al. Neurochemical changes in the rat prefrontal cortex following acute phencyclidine treatment: an in vivo localized (1)H MRS study. NMR in biomedicine. 2009;22(7):737–44. doi: 10.1002/nbm.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Ernst A, Ma D, Garcia-Perez I, Tsang TM, Kluge W, Schwarz E, et al. Molecular validation of the acute phencyclidine rat model for schizophrenia: identification of translational changes in energy metabolism and neurotransmission. Journal of proteome research. 2012;11(7):3704–14. doi: 10.1021/pr300197d. [DOI] [PubMed] [Google Scholar]
- 329.Hou XJ, Ni KM, Yang JM, Li XM. Neuregulin 1/ErbB4 enhances synchronized oscillations of prefrontal cortex neurons via inhibitory synapses. Neuroscience. 2014;261:107–17. doi: 10.1016/j.neuroscience.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 330.Ginsberg Y, Lotan P, Khatib N, Awad N, Errison S, Weiner Z, et al. Maternal lipopolysaccharide alters the newborn oxidative stress and C-reactive protein levels in response to an inflammatory stress. Journal of developmental origins of health and disease. 2012;3(5):358–63. doi: 10.1017/S204017441200027X. [DOI] [PubMed] [Google Scholar]
- 331.Kirsten TB, Lippi LL, Bevilacqua E, Bernardi MM. LPS exposure increases maternal corticosterone levels, causes placental injury and increases IL-1Beta levels in adult rat offspring: relevance to autism. PloS one. 2013;8(12):e82244. doi: 10.1371/journal.pone.0082244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Burt MA, Tse YC, Boksa P, Wong TP. Prenatal immune activation interacts with stress and corticosterone exposure later in life to modulate N-methyl-D-aspartate receptor synaptic function and plasticity. Int J Neuropsychopharmacol. 2013;16(8):1835–48. doi: 10.1017/S1461145713000229. [DOI] [PubMed] [Google Scholar]
- 333.Pacheco-Lopez G, Giovanoli S, Langhans W, Meyer U. Priming of metabolic dysfunctions by prenatal immune activation in mice: relevance to schizophrenia. Schizophrenia bulletin. 2013;39(2):319–29. doi: 10.1093/schbul/sbr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Cole TB, Giordano G, Co AL, Mohar I, Kavanagh TJ, Costa LG. Behavioral Characterization of GCLM-Knockout Mice, a Model for Enhanced Susceptibility to Oxidative Stress. Journal of toxicology. 2011;2011:157687. doi: 10.1155/2011/157687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Flores C, Wen X, Labelle-Dumais C, Kolb B. Chronic phencyclidine treatment increases dendritic spine density in prefrontal cortex and nucleus accumbens neurons. Synapse (New York, NY) 2007;61(12):978–84. doi: 10.1002/syn.20452. [DOI] [PubMed] [Google Scholar]
- 336.Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98(4):427–36. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 337.Fradley RL, O'Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behavioural brain research. 2005;163(2):257–64. doi: 10.1016/j.bbr.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 338.Holloway T, Moreno JL, Umali A, Rayannavar V, Hodes GE, Russo SJ, et al. Prenatal stress induces schizophrenia-like alterations of serotonin 2A and metabotropic glutamate 2 receptors in the adult offspring: role of maternal immune system. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(3):1088–98. doi: 10.1523/JNEUROSCI.2331-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Tezuka T, Tamura M, Kondo MA, Sakaue M, Okada K, Takemoto K, et al. Cuprizone short-term exposure: astrocytic IL-6 activation and behavioral changes relevant to psychosis. Neurobiology of disease. 2013;59:63–8. doi: 10.1016/j.nbd.2013.07.003. Epub 2013/07/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, et al. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(22):9130–5. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Monin A, Baumann PS, Griffa A, Xin L, Mekle R, Fournier M, et al. Glutathione deficit impairs myelin maturation: relevance for white matter integrity in schizophrenia patients. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.88. [DOI] [PubMed] [Google Scholar]
- 342.Kendig EL, Chen Y, Krishan M, Johansson E, Schneider SN, Genter MB, et al. Lipid metabolism and body composition in Gclm(−/−) mice. Toxicology and applied pharmacology. 2011;257(3):338–48. doi: 10.1016/j.taap.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Kulak A, Cuenod M, Do KQ. Behavioral phenotyping of glutathione-deficient mice: relevance to schizophrenia and bipolar disorder. Behavioural brain research. 2012;226(2):563–70. doi: 10.1016/j.bbr.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 344.Chen Y, Curran CP, Nebert DW, Patel KV, Williams MT, Vorhees CV. Effect of chronic glutathione deficiency on the behavioral phenotype of Gclm−/− knockout mice. Neurotoxicology and teratology. 2012;34(4):450–7. doi: 10.1016/j.ntt.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Eyles D, Almeras L, Benech P, Patatian A, Mackay-Sim A, McGrath J, et al. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. The Journal of steroid biochemistry and molecular biology. 2007;103(3-5):538–45. doi: 10.1016/j.jsbmb.2006.12.096. [DOI] [PubMed] [Google Scholar]
- 346.Kesby JP, Burne TH, McGrath JJ, Eyles DW. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: An animal model of schizophrenia. Biological psychiatry. 2006;60(6):591–6. doi: 10.1016/j.biopsych.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 347.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Frontiers in neuroendocrinology. 2013;34(1):47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 348.Lai WS, Xu B, Westphal KG, Paterlini M, Olivier B, Pavlidis P, et al. Akt1 deficiency affects neuronal morphology and predisposes to abnormalities in prefrontal cortex functioning. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(45):16906–11. doi: 10.1073/pnas.0604994103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Balu DT, Carlson GC, Talbot K, Kazi H, Hill-Smith TE, Easton RM, et al. Akt1 deficiency in schizophrenia and impairment of hippocampal plasticity and function. Hippocampus. 2012;22(2):230–40. doi: 10.1002/hipo.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. The Journal of biological chemistry. 2001;276(42):38349–52. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 351.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochemical Society transactions. 2007;35(Pt 2):231–5. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 352.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science (New York, NY) 2012;337(6100):1357–60. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Toua C, Brand L, Moller M, Emsley RA, Harvey BH. The effects of sub-chronic clozapine and haloperidol administration on isolation rearing induced changes in frontal cortical N-methyl-D-aspartate and D1 receptor binding in rats. Neuroscience. 2010;165(2):492–9. doi: 10.1016/j.neuroscience.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 354.Malone DT, Kearn CS, Chongue L, Mackie K, Taylor DA. Effect of social isolation on CB1 and D2 receptor and fatty acid amide hydrolase expression in rats. Neuroscience. 2008;152(1):265–72. doi: 10.1016/j.neuroscience.2007.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Cassidy AW, Mulvany SK, Pangalos MN, Murphy KJ, Regan CM. Developmental emergence of reelin deficits in the prefrontal cortex of Wistar rats reared in social isolation. Neuroscience. 2010;166(2):377–85. doi: 10.1016/j.neuroscience.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 356.Lipska BK, Lerman DN, Khaing ZZ, Weinberger DR. The neonatal ventral hippocampal lesion model of schizophrenia: effects on dopamine and GABA mRNA markers in the rat midbrain. The European journal of neuroscience. 2003;18(11):3097–104. doi: 10.1111/j.1460-9568.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- 357.Wan RQ, Giovanni A, Kafka SH, Corbett R. Neonatal hippocampal lesions induced hyperresponsiveness to amphetamine: behavioral and in vivo microdialysis studies. Behavioural brain research. 1996;78(2):211–23. doi: 10.1016/0166-4328(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 358.Mitchell CP, Goldman MB. Neonatal lesions of the ventral hippocampal formation disrupt neuroendocrine responses to auditory stress in the adult rat. Psychoneuroendocrinology. 2004;29(10):1317–25. doi: 10.1016/j.psyneuen.2004.04.002. Epub 2004/08/04. [DOI] [PubMed] [Google Scholar]
- 359.Francois J, Koning E, Ferrandon A, Sandner G, Nehlig A. Metabolic activity in the brain of juvenile and adult rats with a neonatal ventral hippocampal lesion. Hippocampus. 2010;20(7):841–51. doi: 10.1002/hipo.20686. [DOI] [PubMed] [Google Scholar]
- 360.Meng Y, Payne C, Li L, Hu X, Zhang X, Bachevalier J. Alterations of hippocampal projections in adult macaques with neonatal hippocampal lesions: A Diffusion Tensor Imaging study. NeuroImage. 2014;102(Pt 2):828–37. doi: 10.1016/j.neuroimage.2014.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Roy K, Murtie JC, El-Khodor BF, Edgar N, Sardi SP, Hooks BM, et al. Loss of erbB signaling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(19):8131–6. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, et al. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59(4):581–95. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Flagstad P, Mork A, Glenthoj BY, van Beek J, Michael-Titus AT, Didriksen M. Disruption of neurogenesis on gestational day 17 in the rat causes behavioral changes relevant to positive and negative schizophrenia symptoms and alters amphetamine-induced dopamine release in nucleus accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29(11):2052–64. doi: 10.1038/sj.npp.1300516. [DOI] [PubMed] [Google Scholar]
- 364.Penschuck S, Flagstad P, Didriksen M, Leist M, Michael-Titus AT. Decrease in parvalbumin-expressing neurons in the hippocampus and increased phencyclidine-induced locomotor activity in the rat methylazoxymethanol (MAM) model of schizophrenia. The European journal of neuroscience. 2006;23(1):279–84. doi: 10.1111/j.1460-9568.2005.04536.x. [DOI] [PubMed] [Google Scholar]
- 365.Matsutani T. A neurochemical study of experimental microencephalic rat. The Journal of toxicological sciences. 1984;9(3):205–18. doi: 10.2131/jts.9.205. [DOI] [PubMed] [Google Scholar]
- 366.Chin CL, Curzon P, Schwartz AJ, O'Connor EM, Rueter LE, Fox GB, et al. Structural abnormalities revealed by magnetic resonance imaging in rats prenatally exposed to methylazoxymethanol acetate parallel cerebral pathology in schizophrenia. Synapse (New York, NY) 2011;65(5):393–403. doi: 10.1002/syn.20857. [DOI] [PubMed] [Google Scholar]
- 367.Fonnum F, Lock EA. The contributions of excitotoxicity, glutathione depletion and DNA repair in chemically induced injury to neurones: exemplified with toxic effects on cerebellar granule cells. Journal of neurochemistry. 2004;88(3):513–31. doi: 10.1046/j.1471-4159.2003.02211.x. [DOI] [PubMed] [Google Scholar]
- 368.Zimmerman EC, Bellaire M, Ewing SG, Grace AA. Abnormal stress responsivity in a rodent developmental disruption model of schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013;38(11):2131–9. doi: 10.1038/npp.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Fiore M, Grace AA, Korf J, Stampachiacchiere B, Aloe L. Impaired brain development in the rat following prenatal exposure to methylazoxymethanol acetate at gestational day 17 and neurotrophin distribution. Neuroreport. 2004;15(11):1791–5. doi: 10.1097/01.wnr.0000135934.03635.6a. [DOI] [PubMed] [Google Scholar]
- 370.Makinodan M, Tatsumi K, Manabe T, Yamauchi T, Makinodan E, Matsuyoshi H, et al. Maternal immune activation in mice delays myelination and axonal development in the hippocampus of the offspring. Journal of neuroscience research. 2008;86(10):2190–200. doi: 10.1002/jnr.21673. [DOI] [PubMed] [Google Scholar]
- 371.Marcotte ER, Pearson DM, Srivastava LK. Animal models of schizophrenia: a critical review. Journal of psychiatry & neuroscience : JPN. 2001;26(5):395–410. [PMC free article] [PubMed] [Google Scholar]
- 372.Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;23(3):223–39. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 373.Johnson AW, Jaaro-Peled H, Shahani N, Sedlak TW, Zoubovsky S, Burruss D, et al. Cognitive and motivational deficits together with prefrontal oxidative stress in a mouse model for neuropsychiatric illness. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(30):12462–7. doi: 10.1073/pnas.1307925110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Yang Y, Dieter MZ, Chen Y, Shertzer HG, Nebert DW, Dalton TP. Initial characterization of the glutamate-cysteine ligase modifier subunit Gclm(−/−) knockout mouse. Novel model system for a severely compromised oxidative stress response. The Journal of biological chemistry. 2002;277(51):49446–52. doi: 10.1074/jbc.M209372200. [DOI] [PubMed] [Google Scholar]
- 375.Steullet P, Cabungcal JH, Kulak A, Kraftsik R, Chen Y, Dalton TP, et al. Redox dysregulation affects the ventral but not dorsal hippocampus: impairment of parvalbumin neurons, gamma oscillations, and related behaviors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(7):2547–58. doi: 10.1523/JNEUROSCI.3857-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Matrisciano F, Tueting P, Dalal I, Kadriu B, Grayson DR, Davis JM, et al. Epigenetic modifications of GABAergic interneurons are associated with the schizophrenia-like phenotype induced by prenatal stress in mice. Neuropharmacology. 2013;68:184–94. doi: 10.1016/j.neuropharm.2012.04.013. Epub 2012/05/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Ferdman N, Murmu RP, Bock J, Braun K, Leshem M. Weaning age, social isolation, and gender, interact to determine adult explorative and social behavior, and dendritic and spine morphology in prefrontal cortex of rats. Behavioural brain research. 2007;180(2):174–82. doi: 10.1016/j.bbr.2007.03.011. Epub 2007/05/05. [DOI] [PubMed] [Google Scholar]