In this review, Meng et al. focus on recent developments in our understanding of the molecular actions of the core Hippo kinase cascade and discuss key open questions in Hippo pathway regulation and function.
Keywords: LATS, MAP4K, MST, TAZ, TEAD, YAP
Abstract
The Hippo pathway was initially identified in Drosophila melanogaster screens for tissue growth two decades ago and has been a subject extensively studied in both Drosophila and mammals in the last several years. The core of the Hippo pathway consists of a kinase cascade, transcription coactivators, and DNA-binding partners. Recent studies have expanded the Hippo pathway as a complex signaling network with >30 components. This pathway is regulated by intrinsic cell machineries, such as cell–cell contact, cell polarity, and actin cytoskeleton, as well as a wide range of signals, including cellular energy status, mechanical cues, and hormonal signals that act through G-protein-coupled receptors. The major functions of the Hippo pathway have been defined to restrict tissue growth in adults and modulate cell proliferation, differentiation, and migration in developing organs. Furthermore, dysregulation of the Hippo pathway leads to aberrant cell growth and neoplasia. In this review, we focus on recent developments in our understanding of the molecular actions of the core Hippo kinase cascade and discuss key open questions in the regulation and function of the Hippo pathway.
The Hippo pathway was initially identified in Drosophila; however, most of the recent studies focus on its function and regulation in mammalian cells. Many new regulators of the Hippo pathway have been identified and characterized. We first discuss recent discoveries in the mammalian Hippo pathway and then introduce the Drosophila counterparts to provide a brief history of research in the Hippo pathway. This review mainly focuses on the molecular regulation and function of the core Hippo pathway components.
The core kinase cascade of the Hippo pathway
Core components of the mammalian Hippo pathway
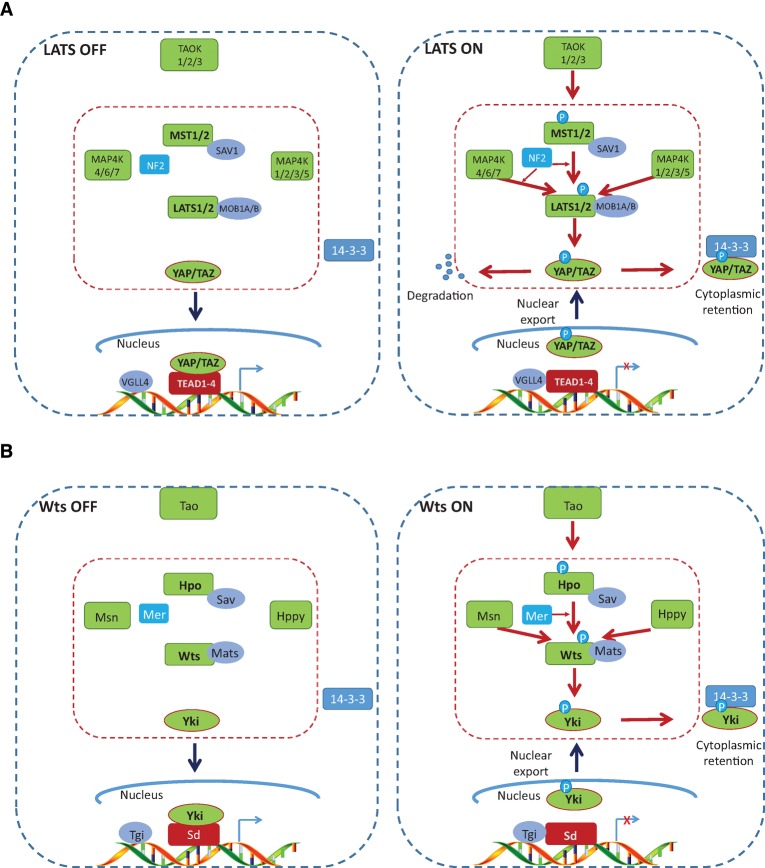
In a classical view, the core of the Hippo pathway in mammals is a kinase cascade in which the mammalian Ste20-like kinases 1/2 (MST1/2; homologs of Drosophila Hippo [Hpo]) phosphorylate and activate large tumor suppressor 1/2 (LATS1/2; homologs of Drosophila Warts [Wts]) (Fig. 1A). The physiological output of this kinase cascade is to restrict the activities of two transcriptional coactivators, Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ; two homologs of Drosophila Yorkie [Yki]). When YAP and TAZ are active, they translocate into the nucleus to bind the TEAD transcription factor family (homologs of Drosophila Scalloped [Sd]) and induce expression of a wide range of genes that are involved in cell proliferation, survival, and migration.
Figure 1.
The core Hippo pathway in mammals and Drosophila. (A) The mammalian Hippo pathway. When the Hippo pathway is inactive, YAP and TAZ are unphosphorylated and localized in the nucleus to compete with VGLL4 for TEAD binding and activation of gene transcription. The Hippo pathway can be activated by TAO kinases, which phosphorylate MST1/2 at its activation loop. MST1/2 in turn phosphorylate LATS1/2, facilitated by scaffold proteins SAV1, MOB1A/B, and NF2. MAP4K4/6/7 and MAP4K1/2/3/5 also phosphorylate and activate LATS1/2. Phosphorylation of LATS1/2 by MAP4K4/6/7 requires NF2 (also known as Mer). Activated LATS1/2 phosphorylate YAP and TAZ, leading to 14-3-3-mediated YAP and TAZ cytoplasmic retention and SCF-mediated YAP and TAZ degradation. (B) The Drosophila Hippo pathway. Active Yki competes Tgi to interact with Sd in the nucleus and activates the transcription of Sd target genes. When Hpo is activated by Tao kinase or dimerization, it phosphorylates and activates Wts with the assistance of the scaffold proteins Sav and Mats as well as Mer. It is unclear whether Msn and Hppy require Mer and Sav to phosphorylate and activate Wts. Active Wts phosphorylates and inactivates Yki, leading to 14-3-3-mediated Yki cytoplasmic retention.
Mechanistically, the Hippo kinase cascade can be initiated by TAO kinases (TAOK1/2/3), which phosphorylate the activation loop of MST1/2 (Thr183 for MST1 and Thr180 for MST2; hereafter, all residues refer to human proteins) and thereby lead to MST1/2 activation (Boggiano et al. 2011; Poon et al. 2011). There is also evidence showing that the activation loop phosphorylation can be achieved by MST1/2 autophosphorylation (Praskova et al. 2004). Consistent with this model, the activation loop phosphorylation is enhanced by MST1/2 dimerization (Glantschnig et al. 2002). Therefore, it is possible that MST1/2 activation can be initiated by dimerization and does not neccesarily require upstream kinases. Active MST1/2 phosphorylate SAV1 (homolog of Drosophila Salvador [Sav]) and MOB1A/B (homologs of Drosophila Mats) (Callus et al. 2006; Praskova et al. 2008), two scaffold proteins that assist MST1/2 in the recruitment and phosphorylation of LATS1/2 at their hydrophobic motifs (T1079 for LATS1 and T1041 for LATS2) (Hergovich et al. 2006; Yin et al. 2013). Another key player in this action is NF2/Merlin, which directly interacts with LATS1/2 and facilitates LATS1/2 phosphorylation by the MST1/2–SAV1 complex (Yin et al. 2013). LATS1/2 subsequently undergo autophosphorylation and are activated (Chan et al. 2005) and in turn phosphorylate and inactivate YAP and TAZ (Zhao et al. 2007). In parallel to MST1/2, two groups of MAP4Ks (mitogen-activated protein kinase kinase kinase kinase), MAP4K1/2/3/5 (homologs of Drosophila Happyhour [Hppy]) and MAP4K4/6/7 (homologs of Drosophila Misshapen [Msn]), can also directly phosphorylate LATS1/2 at their hydrophobic motifs and result in LATS1/2 activation (Meng et al. 2015; Zheng et al. 2015). In HEK293A cells, triple knockout of MAP4K4/6/7 reduces the phosphorylation of YAP/TAZ more dramatically than MST1/2 double knockout under serum deprivation, indicating that MAP4Ks may play a more prominent role than MST in Hippo pathway regulation under certain conditions (Meng et al. 2015). However, deletion of both MST1/2 and MAP4Ks is required to abolish YAP phosphorylation in response to LATS-activating signals, such as contact inhibition, energy stress, serum deprivation, and F-actin disassembly (Meng et al. 2015). Therefore, MST1/2 and MAP4Ks have partially redundant roles in LATS1/2 regulation. Phosphorylation of YAP and TAZ leads to their binding with 14-3-3, and the 14-3-3 binding causes cytoplasmic sequestration of YAP/TAZ (Zhao et al. 2007). Moreover, LATS-induced phosphorylation triggers subsequent phosphorylation of YAP/TAZ by Casein kinase 1δ/ε and recruitment of the SCF E3 ubiquitin ligase, leading to eventual YAP/TAZ ubiquitination and degradation (Liu et al. 2010; Zhao et al. 2010). In addition, YAP protein can also be degraded by autophagy (Liang et al. 2014).
YAP and TAZ are transcriptional coactivators and do not have DNA-binding domains. Rather, when translocated into the nucleus, they regulate gene expression through interaction with TEAD1–4, which are sequence-specific transcription factors that mediate the main transcriptional output of the Hippo pathway in mammlian cells (Zhao et al. 2008). TEAD1–4 can also bind to VGLL4 in the nucleus and thus function as transcriptional repressors. The interaction between YAP/TAZ and TEAD1–4 dissociates VGLL4 from TEAD1–4 and thereby activates TEAD-mediated gene transcription to promote tissue growth and inhibit apoptosis (Koontz et al. 2013). Mouse models with deletion of MST1/2, SAV1, MOB1A/B, NF2, or LATS1/2 or YAP overexpression all exhibit up-regulation of TEAD target gene expression, increased expansion of progenitor cells, and tissue overgrowth (Camargo et al. 2007; Dong et al. 2007; Zhou et al. 2009; Cai et al. 2010; Lee et al. 2010; Lu et al. 2010; Song et al. 2010; Zhang et al. 2010; Nishio et al. 2012; Chen et al. 2015b), supporting the functional roles of these genes in the Hippo pathway.
The Drosophila Hippo core components
The Hippo pathway was named after hpo, a Drosophila kinase gene that was independently identified to restrict tissue growth by several groups more than a decade ago (Fig. 1B; Harvey et al. 2003; Jia et al. 2003; Pantalacci et al. 2003; Udan et al. 2003; Wu et al. 2003). hpo mutants exhibit uncontrolled growth in multiple tissues due to excessive cell proliferation and reduced apoptosis. These phenotypes, together with the elevated transcription of cycE and diap1, are very similar to those previously observed in mutants of salvador (sav) and warts (wts) (Justice et al. 1995; Xu et al. 1995; Kango-Singh et al. 2002; Tapon et al. 2002). Hpo, Sav, and Wts show genetic interactions, and Hpo directly phosphorylates and activates Wts. In fact, the Hippo pathway is also known as the Salvador/Warts/Hippo (SWH) pathway (Harvey and Tapon 2007). Sav serves as an adaptor protein for Hpo to phosphorylate Wts and can also be phosphorylated by Hpo (Pantalacci et al. 2003; Wu et al. 2003). Sav phosphorylation by Hpo promotes its interaction with Hpo, which promotes phosphorylation of Wts and transcriptional repression of cycE and diap1. In addition, the physical binding of Hpo to Sav promotes protein stability of Sav by preventing interaction between Sav and the HECT domain protein Herc4 (HECT and RLD domain-containing E3 ligase), which functions as a Sav E3 ligase and induces Sav ubiquitinylation and degradation (Aerne et al. 2015). Another core component, Mats (Mob as tumor suppressor), was later identified as a Wts-interacting protein that potentiates Wts kinase activity (Lai et al. 2005). Loss of mats also results in uncontrolled tissue growth similar to hpo or wts mutation in Drosophila.
The Hippo pathway effector Yki, which serves as the key link between Wts and the transcriptional regulation of cycE and diap1, was discovered by a yeast two-hybrid screen in 2005 (Huang et al. 2005). Overexpression of Yki recapitulates the hpo, wts, or sav mutant phenotype in cell proliferation, apoptosis, and tissue growth. The underlying biochemical mechanism is that Wts phosphorylates Yki and leads to Yki's interaction with 14-3-3 and cytoplasmic retention (Dong et al. 2007). Yki regulates gene transcription through interacting with the Scalloped (Sd) transcription factor (Goulev et al. 2008; Wu et al. 2008; Zhang et al. 2008). In the absence of Yki binding, Sd binds to Tondu domain-containing growth inhibitor (Tgi) by default and actually represses gene expression. Yki replaces Tgi and converts Sd into a transcriptional activator (Koontz et al. 2013). Therefore, a common molecular mechanism of Hippo pathway regulation is highly conserved between Drosophila and mammals.
The Hippo pathway is regulated by a variety of intrinsic and extrinsic signals. In most scenarios, the central event of the Hippo pathway appears to be phosphorylation-dependent Wts activation and Yki inhibition. The major kinase for Wts is Hpo, which can be phosphorylated and activated by the Tao kinase (Boggiano et al. 2011; Poon et al. 2011). Phosphorylation of Wts by Hpo also requires adaptor proteins such as Mats and Merlin (Mer) to recruit Wts to the plasma membrane (Wei et al. 2007; Yin et al. 2013). Recent studies show that two other kinases, Misshapen (Msn) and Happyhour (Hppy), can activate Wts and repress Yki independently of Hpo (Li et al. 2014a; Zheng et al. 2015). There is evidence that, like Hpo, Hppy also phosphorylates the hydrophobic motif of Wts (Zheng et al. 2015). This study also shows that Msn cannot directly phosphorylate Wts. However, it is worth noting that human MAP4K4/6/7 (the Msn homologs) can directly phosphorylate and activate LATS (Meng et al. 2015). Therefore, future studies are needed to clarify whether Msn can directly phosphorylate and activate Wts. Identifications of Msn, Hppy, and their mammalian homologs, MAP4Ks, have greatly broadened the scope of the Hippo pathway and also revealed the molecular basis of how various signals can activate LATS in MST1/2 knockout cells (Kim et al. 2011; Yu et al. 2012, 2013; Zhao et al. 2012; Meng et al. 2015).
Upstream signals that regulate the Hippo pathway
Studies in the last decade have cemented YAP and TAZ as the major effectors of the Hippo pathway, which regulates the phosphorylation-induced cytoplasmic retention and protein degradation of YAP and TAZ in response to a myriad of intrinsic and extrinsic signals. These signals, in most scenarios, modulate phosphorylation events of the core kinase cascade through peripheral components of the Hippo pathway. In addition, there are a number of proteins that directly regulate YAP localization or transactivation without affecting LATS kinase activity (Yu and Guan 2013). Moreover, the Hippo pathway cross-talks with Wingless/Ints (Wnt), bone morphogenetic proteins (BMPs), Notch, and Hedgehog (Hh), as these signals also modulate the activities of YAP and TAZ (Hansen et al. 2015). In this section, we summarize upstream signals and the peripheral Hippo pathway components that relay signals to the core kinase cascade (Fig. 2).
Figure 2.
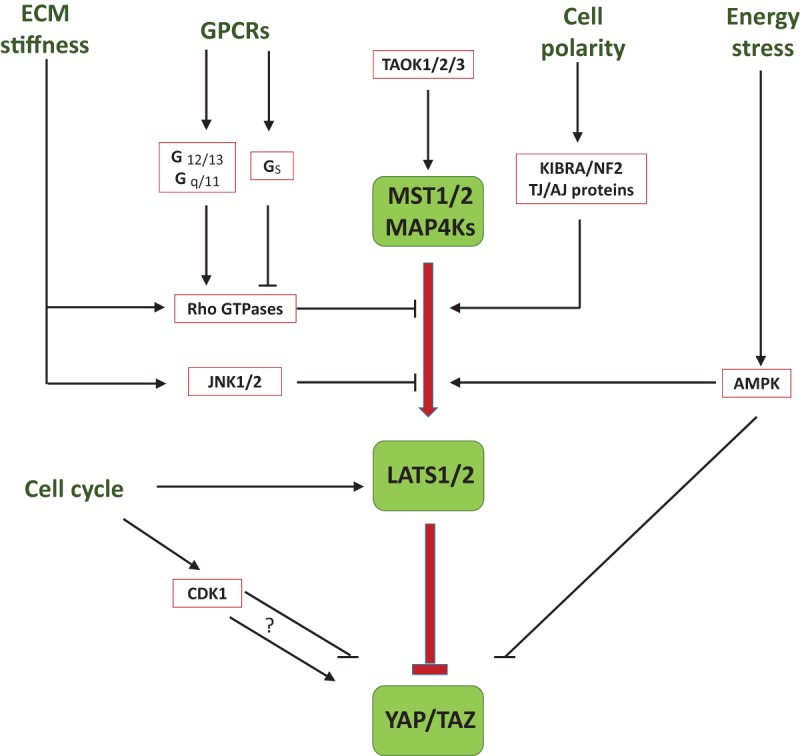
Regulation of the Hippo pathway by upstream signals. Cyclic stretch or high extracellular matrix stiffness inhibits LATS1/2 phosphorylation through Rho-GTPases and JNK1/2. G-protein-coupled receptors (GPCRs) can either activate or suppress LATS1/2 depending on the types of the Gα proteins involved. The LATS1/2 activation is also controlled by cell polarity and architecture through KIBRA/NF2, adherens junctions (AJ), and tight junctions (TJ). Energy status modulates YAP and TAZ activity via AMPK. Cell cycle affects YAP and TAZ through either LATS1/2- or CDK1-mediated protein phosphorylation.
Physical cues: cell contact and mechanical signal
Organ growth and development involve many coordinated actions of cells to adapt to physical restraints and extracellular mechanical cues. Tissue architecture physically restricts cell growth and proliferation and in many cases leads to cell quiescence. For example, cell–cell contact at high cell density produces a growth inhibitory signal that is in large part mediated by the Hippo pathway (Zhao et al. 2007; Ota and Sasaki 2008; Nishioka et al. 2009). As a result, LATS kinase is activated at high cell density, whereas LATS is inactive at low cell density. YAP inactivation is critically important for cell contact inhibition in cell culture. The regulation of the YAP–TEAD transcription program by contact inhibition is also crucial for embryo development (Ota and Sasaki 2008; Nishioka et al. 2009; Gumbiner and Kim 2014). The increased adherens junctions and tight junctions in confluent cells contribute to activation of LATS and inactivation of YAP and TAZ (Zhao et al. 2007; Silvis et al. 2011). Furthermore, loss of cell spreading or decrease of cell size may also be involved, as extracellular matrix (ECM) stiffness regulates YAP and TAZ subcellular localization through changes in cell geometry and cytoskeleton tension (Dupont et al. 2011; Driscoll et al. 2015). Physical attachment of cells to ECM is essential for cells to survive and grow. Attachment of cells to ECM induces YAP nuclear localization through activation of Rho-GTPases or the FAK–Src–PI3K pathway (Zhao et al. 2012; Kim and Gumbiner 2015). Disruption of F-actin blocks the effect of attachment on YAP phosphorylation and nuclear localization. In contrast, detachment of cells inactivates YAP and TAZ and triggers anoikis in a LATS-dependent manner (Zhao et al. 2012). The cell attachment certainly provides mechanical signal to the cell, as the culture plate surface has high stiffness. In addition, YAP and TAZ activities are also modulated by stretching and edge/curvature contouring an epithelial sheet (Aragona et al. 2013). This regulation by mechanical forces similarly requires Rho-GTPases and F-actin capping/severing proteins as mediators and is proposed to function as a physical checkpoint of cell growth and a cell fate determination during stem cell differentiation (Aragona et al. 2013). In fact, activation of YAP and TAZ by increasing substrate rigidity greatly enhances differentiation of human pluripotent stem cells into motor neuron cells, suggesting a potential application of engineered substrates to produce particular types of differentiated cells (Sun et al. 2014).
Recent studies also show that YAP and TAZ are activated by shear stress from fluid flow, indicating a physiological and disease-relevant role of YAP and TAZ in endothelial cell differentiation and vascular homeostasis (Kim et al. 2014; Sabine et al. 2015). The physiological relevance of mechanical forces and cell growth has also been established in a Drosophila study (Rauskolb et al. 2014). Cytoskeleton tension inhibits Wts and subsequently activates Yki and promotes wing growth through recruiting Wts to adherens junctions by α-catenin and Jub. Upon tissue injury, anatomic alternations and emerging space in organs promote cells to exit quiescence and re-enter the cell cycle to expand cell populations and thus maintain tissue homeostasis. From Drosophila to rodent models, genetic inactivation of the Hippo pathway consistently results in overgrowth phenotypes in a variety of organs, whereas inactivation of YAP/TAZ impairs wound healing (Yu et al. 2015).
Most studies have indicated that Rho-GTPases and the actin cytoskeleton play an essential role in regulation of YAP and TAZ by mechanotransduction; however, the involvement of the Hippo core kinase cascade (MST–LATS) is still under debate. Earlier studies exclude MST1/2 and LATS1/2 in the regulation of YAP/TAZ nuclear translocation and transcriptional activation because RNAi targeting LATS1/2 does not block YAP and TAZ regulation by ECM stiffness (Dupont et al. 2011). However, it was recently reported that mechanical strain suppresses YAP phosphorylation and promotes YAP nuclear translocation by inactivating LATS1/2 in a JNK-dependent manner (Codelia et al. 2014). Future studies are required to delineate the mechanosensor/receptor as well as the role of the core Hippo kinase in mechanical signal-induced YAP/TAZ regulation.
Soluble factors and G-protein-coupled receptors (GPCRs)
Tissue growth requires nutrients as well as hormonal signals via autocrine, paracrine, and endocrine mechanisms. Moreover, nutrient uptake is also under the control of growth-stimulating signals. It had long been speculated that extracellular molecules, such as hormones or growth factors, might regulate the Hippo pathway in order to control tissue growth and homeostasis. A major breakthrough in the Hippo pathway came with the discovery that diffusive molecules, such as lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P), activate and stabilize YAP and TAZ through their GPCRs, LPA receptor (LPAR) and S1P receptor (S1PR) (Yu et al. 2012). A series of studies further demonstrate that regulation of the Hippo pathway by GPCRs is indeed a universal response of cells to hormonal cues (Miller et al. 2012; Mo et al. 2012; Yu et al. 2013; Gong et al. 2015; Zhou et al. 2015a). Mechanistically, Rho-GTPases mediate the actions of GPCRs on YAP and TAZ. Gα12/13- and Gαq/11-coupled GPCRs activate Rho-GTPases, which in turn inactivate LATS1/2 by a yet unknown mechanism that is dependent on F-actin assembly (Yu et al. 2012). In contrast, activation of GαS-coupled GPCRs by epinephrine and glucagon increases LATS kinase activities and inactivates YAP and TAZ in a manner dependent on protein kinase A (PKA) (Yu et al. 2013). Therefore, depending on the nature of downstream G proteins, GPCRs can either activate or inhibit the LATS kinase to stimulate or suppress YAP activity. Elevated GPCR expression or mutation of Gα proteins leads to aberrant YAP activation and exhibits strong disease implications (Feng et al. 2014; Yu et al. 2014; Liu et al. 2015; Zhou et al. 2015a). For example, estrogen acts through G protein-coupled estrogen receptor (GPER) to inhibit LATS and activate YAP/TAZ, indicating a possible role of YAP/TAZ activation by estrogen in breast cancer (Zhou et al. 2015a). GPCRs are the largest family of the plasma membrane receptors and mediate the actions of hundreds of extracellular molecules (Lappano and Maggiolini 2011). The regulation of YAP and TAZ by GPCRs implies that the Hippo pathway not only is modulated by a large number of hormonal signals but also contributes to a wide range of physiological regulation and may be targeted for disease intervention with GPCR agonists or antagonists.
Among GPCR ligands, the Wnt proteins, such as Wnt5a/b, are particularly noteworthy. Wnt5a/b activate noncanonical Wnt signaling by binding to the Frizzled receptors, the class F GPCRs (Anastas and Moon 2013). As both the Hippo pathway and canonical Wnt signaling are master regulators of tissue growth and morphogenesis, cross-talk between the two pathways has been extensively studied and thoroughly summarized by recent reviews (Piccolo et al. 2014; Hansen et al. 2015). It was recently reported that the noncanonical Wnt ligands Wnt5a/b activate YAP/TAZ through the Gα12/13–Rho–LATS signaling axis by binding to the Frizzled receptors (Park et al. 2015). This regulation of YAP/TAZ by Wnt5a/b is indeed required for noncanonical Wnt signaling to function in cell differentiation and migration as well as antagonizing the canonical Wnt/β-catenin activation.
Stress signals
The most recognized functional output of YAP and TAZ is to promote cell survival and proliferation (Huang et al. 2005; Camargo et al. 2007; Dong et al. 2007). Therefore, it is not surprising that several stress signals can modulate YAP and TAZ activities. However, the regulation of YAP and TAZ by stress signals, such as energy stress, endoplasmic reticulum stress, and hypoxia, has been characterized only in the last couple of years, although activation of MST1/2 by a high concentration of sodium arsenite or heat shock was observed a long time ago (Taylor et al. 1996). MST1/2 are also activated by hydrogen peroxide and are involved in cellular oxidative stress responses (Lehtinen et al. 2006; Geng et al. 2015). On the other hand, YAP physically interacts with FOXO1 and activates FoxO1-mediated transcription of catalase and MnSOD genes and subsequently reduces oxidative stress and ischaemia/reperfusion (I/R)-induced injury in the heart (Shao et al. 2014), implicating a physiological role of YAP in reactive oxygen species (ROS) scavenging.
Cells rely on carbohydrates as their main energy source. Energy stress caused by glucose deprivation rapidly induces YAP and TAZ phosphorylation due to LATS1/2 activation, which is enhanced by phosphorylation of AMOTL1 at Ser793 by AMPK (DeRan et al. 2014). Furthermore, energy stress-activated AMPK directly phosphorylates YAP at multiple sites, and this phosphorylation interferes with the interaction between YAP and TEAD, thus inhibiting TEAD-mediated gene transcription (Mo et al. 2015; Wang et al. 2015). The additional layer of YAP and TAZ regulation by AMPK is physiologically important in the central brain/ventral nerve cord development in Drosophila neural systems (Gailite et al. 2015). Accessibility of nutrients other than glucose also affects the Hippo pathway. For instance, the nutrient-sensing kinases salt-induced kinase 2 and kinase 3 phosphorylate Sav at Ser413 to promote Yki target gene expression (Wehr et al. 2013). Both mTORC1 and mTORC2 are reported to positively regulate YAP in perivascular epithelioid cell tumors and glioblastomas (Liang et al. 2014; Artinian et al. 2015; Sciarretta et al. 2015). It is worth noting that mTORC1 is highly sensitive to nutrient availability and cellular energy status. In Drosophila, the Tor pathway can regulate Yki's ability to access its target genes in the nucleus (Parker and Struhl 2015). Tor inhibition by nutrient deprivation prevents nuclear Yki from activating its target genes. Besides nutrient stress, inhibition of cholesterol synthesis indirectly inhibits YAP, possibly due to inhibition of the Rho family GTPases, which require C-terminal isoprenylation and membrane localization for their proper biological functions (Sorrentino et al. 2014). Therefore, the Hippo pathway is subjected to regulation by cellular nutrient status.
In contrast to oxidative stress and energy stress, hypoxia seems to induce YAP and TAZ activation by inhibiting LATS. Hypoxia activates an E3 ubiquitin ligase, SIAH2, which destabilizes LATS2. Targeting SIAH2 in tumor cells restores the tumor suppressor function of LATS2 in a xenograft animal model (Ma et al. 2015). The regulation of YAP by the unfolded protein response (UPR) remains convoluted and seems to be more complicated than other stresses. In the initial stage of UPR, YAP is activated by PERK–eIF2α. However, prolonged ER stress suppresses YAP (Wu et al. 2015). In fact, deletion of MST1/2 in mouse livers triggers UPR and induces hepatocellular carcinogenesis, while attenuation of UPR by tauroursodeoxycholic acid causes degradation of YAP and reduces tumor burden.
Cell polarity and architecture
In Drosophila, apical–basal polarity and planar cell polarity provide the intrinsic cues to restrict Yki activity in the epithelium. Many types of polarity machineries, such as adherens junctions, tight junctions, the Mer/Ex/Kibra complex, Crumbs (Crb), the Par complex, and Fat/Dachsous, are involved in this action to maintain the differentiation and morphology of the epithelium in a partially redundant manner. This subject has been comprehensively reviewed elsewhere (Schroeder and Halder 2012; Yu and Guan 2013). Recent studies show that loss of Spectrin, a contractile protein at the cytoskeleton–membrane interface, also generates Hpo mutant-like tissue overgrowth phenotypes in Drosophila wings and eyes, which are likely due to dysregulated cytoskeleton tension (Deng et al. 2015; Fletcher et al. 2015; Wong et al. 2015).
The restriction of Yki activity by cell polarity is caused by either the increased activity and the availability of Hpo/Wts toward Yki or sequestration of Yki at cell junctions (Yin et al. 2013; Yu and Guan 2013; Sun et al. 2015). Mammalian cells have a very similar polarity machinery for YAP/TAZ regulation. For example, PARD3 regulates TAZ activity by promoting the LATS1 and protein phosphatase 1 (PP1) interaction (Lv et al. 2015). Therefore, YAP/TAZ activity is low in terminally differentiated cells in the epithelium and preferentially present in tissue progenitor cells in mammals (Camargo et al. 2007; Cai et al. 2010). In addition, YAP/Yki activity is regulated both autonomously and nonautonomously by apical–basal cell polarity proteins and adherens junctions, respectively (Yang et al. 2015a), indicating that different signal inputs may use different cell polarity complexes and junction proteins to regulate the Hippo pathway.
Cell cycle
LATS1/2 have been considered as regulators of G1/S, G2/M, and mitosis checkpoints and are phosphorylated in a cell cycle-dependent manner in HeLa cells (Tao et al. 1999). However, the intrinsic mechanisms by which LATS1/2 are activated during the cell cycle are still unclear, although a few kinases, such as CDK1 and Aurora A, have been shown to directly phosphorylate LATS1 and LATS2, respectively, during mitosis (Morisaki et al. 2002; Toji et al. 2004; Yabuta et al. 2011; Zhang et al. 2012). It was recently reported that extra centrosomes caused by cytokinesis failure activate LATS2, which in turn stabilizes p53 and inhibits YAP/TAZ transcriptional activity (Ganem et al. 2014). LATS1 also interacts with CDK2 in response to genotoxic stress to restrict CDK2-mediated phosphorylation of BRCA2 and support RAD51 nucleofilaments, thereby maintaining genome fidelity during replication stalling (Pefani et al. 2014). YAP, in complex with the transcription factor PKNOX1, has been shown to control S-phase temporal progression and genomic stability of retinal stem cells (Cabochette et al. 2015).
YAP and TAZ are phosphorylated at multiple sites by CDK1 during the G2/M phase of the cell cycle (Yang et al. 2013, 2015b,c; Zhao et al. 2014; Dent et al. 2015; Zhao and Yang 2015). However, the physiological outcomes of these phosphorylation events are rather perplexing, as their effects on cell growth and migration are not entirely consistent among reports from different groups. While some studies show that CDK1-mediated YAP phosphorylation during the G2–M phase may promote neoplastic transformation via enhancing cell migration and invasion (Yang et al. 2013, 2015c), others suggest that anti-tubulin drugs require YAP phosphorylation by CDK1 to induce cancer cell death (Zhao et al. 2014). This inconsistency may be due to different experimental conditions that differentially affect the coordination between CDK1 and LATS1/2 in the modulation of YAP activity.
Mechanisms of Hippo kinase cascade activation
LATS1/2 belong to the NDR (nuclear Dbf2-related) family of kinases, a subgroup of the protein kinase A/G/C (AGC) family (Pearce et al. 2010). The other two members of the NDR family are NDR1 (STK38) and NDR2 (STK38L). Several recent proteomic studies of the Hippo pathway interactome consistently place NDR1/2 in the Hippo pathway network (Couzens et al. 2013; Kwon et al. 2013; Wang et al. 2014). NDR1/2 might function as a YAP kinase to inhibit YAP-driven tumorigenesis in the intestinal epithelium (Zhang et al. 2015). It is worth noting that NDR1/2 and LATS1/2 share similar phosphorylation motifs. However, the exact role of NDR1/2 in Hippo pathway regulation still needs to be further defined, as deletion of LATS1/2 is sufficient to abolish YAP phosphorylation and cause constitutive YAP nuclear localization under most conditions examined (Meng et al. 2015).
The NDR family kinases require phosphorylation of a conserved Ser/Thr residue within the activation loop and the hydrophobic motif regulatory site for activation. Phosphorylation of the hydrophobic motif is mediated by upstream Ste20-like kinases: MST1/2 for LATS1/2 and NDR1/2 (Chan et al. 2005; Vichalkovski et al. 2008; Hergovich et al. 2009; Tang et al. 2015), and MST3 for NDR1/2 (Stegert et al. 2005). Recent studies have shown that MAP4Ks, also members of the Ste20 family, can phosphorylate the LATS1/2 hydrophobic motif (Meng et al. 2015; Zheng et al. 2015). The Ste20-like kinase-mediated phosphorylation of the hydrophobic motif promotes LATS autophosphorylation in the activation loop, therefore leading to an increase of kinase activity (Tamaskovic et al. 2003; Stegert et al. 2004). Interestingly, the interaction between MOB proteins (the human genome encodes six MOBs: MOB1A/B, MOB2, and MOB3A/B/C) and the N-terminal regulatory domain of the kinases is a common feature of NDR family kinases, although LATS1/2 and NDR1/2 appear to use distinct subsets of MOB proteins. MOB1A/B associate with both LATS1/2 and NDR1/2, while MOB2 mediates an inhibitory interaction with NDR1/2 but not with LATS1/2 (Bichsel et al. 2004; Bothos et al. 2005; Hergovich et al. 2005; Kohler et al. 2010).
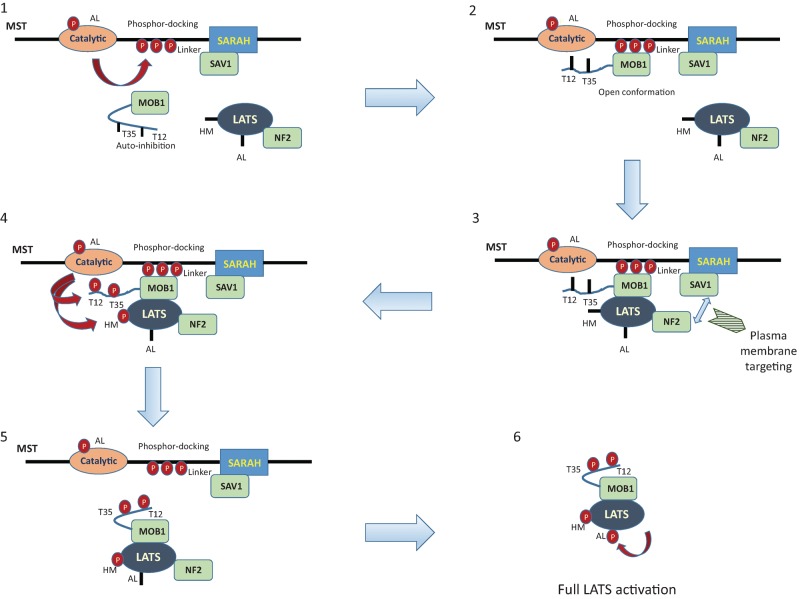
A recent crystal structure study provides new molecular insights into MOB1's roles in LATS1/2 phosphorylation, and activation by MST1/2 as a sequential phosphorylation model is proposed (Fig. 3; Ni et al. 2015). MST2 autophosphorylates its long linker between the kinase domain and the SARA domain to generate a phosphor-docking motif, which can recruit MOB1. The structure of the MOB1–phosphoMST2 complex shows that the binding of MOB1 to the phosphorylated MST2 relieves MOB1 from its autoinhibitory conformation and makes MOB1 accessible to LATS1. Next, LATS1 binds to the MOB1–phosphoMST2 complex to form the MST2–MOB1–LATS1 ternary complex, thereby enhancing the phosphorylation of MOB1 at its N-terminal tail (T35 and T12) and LATS1 at its hydrophobic motif (T1079) by MST2. Phosphorylation of T1079 in LATS1 by MST2 directly contributes to LATS1 activation, while phosphorylation of MOB1 actually triggers the dissociation of phosphorylated LATS1 and MOB1 from MST2. The structure of the phosphoMOB1 and LATS1 complex further reveals that, in addition to mediating the actions of MST2 on LATS1, the phosphorylated MOB1 allosterically promotes LATS1 autophosphorylation at its activation loop (S909), which is required for LATS1 activation after its hydrophobic motif, T1079, has been phosphorylated by MST2. This study reveals structural insights into the molecular mechanism of LATS1/2 activation by MST1/2 and the critical role of MOB1 in this process.
Figure 3.
A proposed sequential phosphorylation model of LATS activation by MST. First, upon activation MST1/2 autophosphorylates its linker region (which is between the catalytic domain and the SARAH domain) at multiple sites to create a phosphor-docking site for MOB1. Second, phosphorylated MST binds to MOB1 and changes MOB1 from an autoinhibitory state to a conformation that is open for LATS binding. Third, LATS binds to MOB1 potentially through recruitment by NF2 to the MST–SAV1 complex at the plasma membrane. Fourth, the formation of the MST–MOB1–LATS complex enables MST to phosphorylate MOB1's N-terminal tail at Thr12 (T12) and Thr35 (T35) and phosphorylate LATS at its hydrophobic motif (HM). Fifth, the phosphorylated N-terminal tail of MOB1 competes with the phosphoMST linker for the same binding site on MOB1 and thus releases MOB1 and LATS from MST. Sixth, phosphoMOB1 allosterically enhances LATS autophosphorylation at its activation loop (AL) and thus promotes LATS activation.
Moreover, another recent study using the crystal structure of the budding yeast homologs of NDR and MOB, Cbk1 and Mob2, shows that Mob2 binding to Cbk1 not only promotes enzymatic activity of Cbk1 but also creates a docking motif for Cbk1 substrates (Gogl et al. 2015). This docking is crucial for robustness and substrate selectivity of Cbk1, which is unique among AGC family kinases, indicating a role of MOB in not only kinase activation but also substrate specificity. One may speculate that a similar mechanism is used in the activation of mammalian MOB and NDR/LATS kinases.
The regulation of LATS-activating kinases MST1/2 and MAP4Ks
The LATS1/2-dependent phosphorylation appears to be the most important event in YAP/TAZ regulation in mammals, as LATS1/2 knockout cells abolish most, if not all, YAP/TAZ phosphorylation in response to many known regulatory signals of the Hippo pathway (Meng et al. 2015).
We and others have recently reported that MST1/2 and MAP4Ks act in parallel to phosphorylate and activate LATS1/2, and deletion of both MST1/2 and MAP4Ks is required to abolish LATS1/2 hydrophobic motif phosphorylation and activation (Li et al. 2014a; Meng et al. 2015; Zheng et al. 2015). Regulation of MST1/2 kinase activity has been extensively studied. MST1/2 requires phosphorylation of the activation loop to be fully active, which can be achieved by transphosphorylation by TAOK1/2/3 or autophosphorylation by MST dimerization (Glantschnig et al. 2002; Praskova et al. 2004; Boggiano et al. 2011; Poon et al. 2011). A few other kinases, such as AKT, ABL, and mTOR, may phosphorylate MST1/2 at multiple sites and modulate kinase activity of MST1/2 by different mechanisms (Jang et al. 2007; Yuan et al. 2010; Collak et al. 2012). However, the role of MST1/2 regulation by these kinases in the Hippo pathway has not been implicated. The STRIPAK complex, the core of which is PP2A, interacts with MST1/2 and may contribute to MST1/2 dephosphorylation in some contexts (Couzens et al. 2013). However, this regulation by STRIPAK still requires further studies because it is unknown whether the interaction or activity of STRIPAK is regulated by signals that are known to control the Hippo pathway.
In addition to interacting with MOB1, MST1/2 also directly interact with SAV1 and RASSFs. SAV1 is also a substrate of MST1/2 and is stabilized by MST1/2 phosphorylation (Callus et al. 2006). It mainly works as a scaffold protein to bridge MST1/2 to LATS1/2. It has not been shown whether SAV1 directly affects MST1/2 kinase activity. The functional role of RASSFs in MST1/2 regulation can be either positive or negative and may depend on the status of MST1/2 (Khokhlatchev et al. 2002; Praskova et al. 2004; Guo et al. 2007, 2011). Nevertheless, it is evident that RASSFs disrupt MST1/2 dimerization and prevent their autophosphorylation. However, interaction of RASSFs with already activated MST1/2 may prevent MST1/2 dephosphorylation and therefore sustain MST1/2 kinase activity (Guo et al. 2011; Ni et al. 2013).
In Drosophila, the Rho-type guanine nucleotide exchange factor Pix (PAK-interacting exchange factor) and GPCR kinase-interacting protein (Git) have also been suggested to influence Hpo kinase activity by facilitating Hpo dimerization and autophosphorylation (Dent et al. 2015). However, a mammalian homolog of Pix, ARHGEF7, may function as a scaffold protein between LATS1/2 and YAP/TAZ to facilitate actions of the Hippo kinase cascade (Heidary Arash et al. 2014). Therefore, the regulation of MST is rather complex, and future studies are needed to provide a clear biochemical understanding of MST activation in response to various upstream signals.
On the other hand, the regulation of MAP4Ks has not been extensively studied. MAP4Ks as well as MST1/2 can be cleaved by Caspase 3/6/7 upon Fas-induced apoptosis. The cleaved kinase domain is active and may activate the JNK pathway (MEKK1–MKK4/7–JNK1/2) or the p38 pathway (MAP3K–MKK3/6–p38MAPK) (Dan et al. 2001). However, the cleaved MAP4Ks and especially MST1/2 have lost certain domains—such as the coiled-coil and SARAH domains—that are essential for their interaction with SAV1 or LATS1/2 (Dan et al. 2001). Therefore, the caspase-dependent MAP4Ks and MST1/2 activation may not be relevant to the Hippo pathway. A few MAP4Ks, including MAP4K1/4/6, are known to interact with NCK1 (noncatalytic region of tyrosine kinase adaptor protein 1), which is an adaptor protein containing Src homology 2 (SH2) and SH3 domains (Su et al. 1997; Ling et al. 1999, 2001; Hu et al. 2004). NCK1 is located in the cytoplasm and is involved in Ras-GTPase activation by receptor tyrosine kinases (Ger et al. 2011) as well as Rho-GTPase activation and actin cytoskeleton remodeling (Ruusala et al. 2008; Buvall et al. 2013). Given the important role of Rho-GTPases and actin cytoskeleton in Hippo regulation, it would be important to investigate whether NCK1 relays the signals of growth factors, cytoskeleton, and mechanotransduction to LATS1/2 through MAP4Ks.
Spatial regulation of MST and LATS
Unlike the large changes in kinase activity of LATS1/2 upon stimulation, kinase activity of MST1/2 does not appear to be dramatically altered under conditions that are known to affect the Hippo pathway. Instead, the accessibility of MST1/2 to LATS1/2 may be a key factor for LATS1/2 activation by MST1/2.
Models of spatial regulation of LATS1/2 kinase activities were proposed a long time ago and have been further refined in the last few years. Early studies have shown that Hpo/Sav interact with Mer/Ex and that Wts associates with Kibra (Genevet et al. 2010; Yu et al. 2010). This interaction suggests that the Mer/Ex/Kibra complex may recruit Hpo and Wts to the apical plasma membrane and results in Wts phosphorylation by Hpo. This model is also supported by evidence that membrane targeting MST1/2 or MOB1 greatly elevates the kinase activities of LATS1/2 in mammalian cells (Khokhlatchev et al. 2002; Hergovich et al. 2006). An updated model proposes that LATS1/2 and MST1/2 are cytoplasmic in their inactive state and are recruited by NF2 and SAV1, respectively, to plasma membrane, where LATS1/2 are phosphorylated and activated by MST1/2 (Yin et al. 2013). However, a recent study in Drosophila suggests that inactive Wts is localized at adherens junctions through Jub, and Wts and Hpo are relocated to Crb–Ex apical junctions to induce Wts phosphorylation and activation (Sun et al. 2015). It is noteworthy that Crb3, a mammalian Crumbs isoform that determines epithelial apical domain identity, also promotes the interaction between YAP and LATS1/2 at apical cell junctions to induce YAP phosphorylation and thereby control airway cell differentiation (Szymaniak et al. 2015). Therefore, an appealing model is that spatial regulation by NF2-dependent recruitment plays a key role in LATS1/2 activation.
Regulation of LATS1/2 protein levels by ubiquitination and beyond
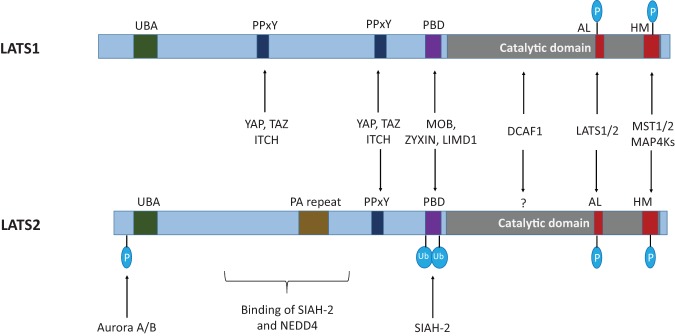
LATS1/2 activities are regulated through various means beyond phosphorylation (Fig. 4). One notable post-translational regulation of LATS1/2 is ubiquitination. A WW domain-containing HECT class E3 ubiquitin ligase, ITCH, ubiquitinates LATS1 and promotes cell growth and survival (Ho et al. 2011; Salah et al. 2011). Another E3 ubiquitin ligase, CRL4 (DCAF1), which is activated in NF2-deficient tumor cells, inhibits LATS1 and LATS2 by ubiquitination in the nucleus (Li et al. 2014b). Ubiquitination of LATS1/2 also plays a role in stress response and differentiation. For instance, a hypoxia-activated E3 ubiquitin ligase, SIAH2, destabilizes LATS2 and promotes YAP activation and tumorigenesis (Ma et al. 2015). NEDD4, another E3 HECT ubiquitin ligase, ubiquitinates and destabilizes both LATS2 and SAV1 and thus activates YAP to enhance intestinal stem cell self-renewal (Bae et al. 2015). In fact, ubiquitination of other Hippo pathway components has also been reported (Wang et al. 2012; Lignitto et al. 2013; Rodrigues-Campos and Thompson 2014), indicating that ubiquitination is a common regulatory mechanism often resulting in degradation of the Hippo pathway components and hyperactivation of YAP and TAZ.
Figure 4.
Domain structure and protein interaction of LATS1/2. Kinase activity of LATS1/2 is primarily regulated by MST1/2 and MAP4Ks through the hydrophobic motif phosphorylation followed by autophosphorylation of the activation loop of LATS1/2. There is a putative ubiquitin-associated domain (UBA) in the N termini of the kinases, and several E3 ubiquitination ligases, such as ITCH, SIAH-2, NEDD4, and DCAF1, are known to regulate LATS1/2 protein stability. The binding of MOB, ZYXIN, LIMD1 with the protein-binding domain (PBD) can also regulate LATS1/2 kinase activity or availability. YAP and TAZ interact with LATS1/2 through the PPxY motifs of LATS1/2. Aurora kinases A/B phosphorylate LATS2 and affect its subcellular locations. (PA repeat) Proline–alanine residue repeat; (AL) activation loop; (HM) hydrophobic motif.
LATS is also regulated at the transcriptional level. LATS2 is a direct target gene of YAP/TAZ, and LATS2 mRNA levels are increased upon YAP/TAZ activation (Chen et al. 2015b; Moroishi et al. 2015b). This LATS2 up-regulation constitutes a negative feedback loop to maintain the homeostasis of the Hippo pathway and prevent overactivation of YAP/TAZ. In addition, LATS1/2 are regulated by aurora kinase-mediated or PKA-mediated phosphorylation (Toji et al. 2004; Kim et al. 2013) or physical interaction with ARHGEF7, ZYXIN, AMOT, and LIMD1 (Hirota et al. 2000; Sun and Irvine 2013; Heidary Arash et al. 2014; Li et al. 2015). These differential regulations of LATS1/2 serve as additional layers of control, in concurrence with MST1/2-mediated and MAP4K-mediated protein phosphorylation, to modulate cell survival and growth.
The YAP/TAZ transcriptional programs and their functional output
Studies of Drosophila and mouse models have established the role of YAP/Yki in regulating tissue progenitor cell self-renewal and expansion, especially in gastrointestinal tissues (Cai et al. 2010; Barry et al. 2013; Gregorieff et al. 2015; Imajo et al. 2015; Taniguchi et al. 2015; Yimlamai et al. 2015). Although earlier transgenic mouse studies have shown striking phenotypes and established a role of the Hippo pathway in development and carcinogenesis (Morin-Kensicki et al. 2006; Camargo et al. 2007; Dong et al. 2007; Yabuta et al. 2007), many more refined transgenic mouse models with tissue-specific deletion and inducible overexpression have been generated in the last few years. These mouse model studies allow for more detailed charaterizations of the physiological contribution of individual Hippo pathway components to tissue growth, cell differentiation, cell competition, and malignant transformation (Zhou et al. 2009; Cai et al. 2010; Barry et al. 2013; Chen et al. 2014, 2015b; Yimlamai et al. 2014; Imajo et al. 2015; Mamada et al. 2015; Shen et al. 2015).
An important function of the Hippo pathway seems to be the inactivation of YAP and TAZ in differentiated cells to maintain cell quiescence (Zhou et al. 2009; Yu et al. 2015). Upon tissue injury, the Hippo pathway is suppressed, and YAP and TAZ are activated to promote stem/progenitor cell self-renewal and tissue repair (Fig. 5; Cai et al. 2010; Schlegelmilch et al. 2011; Gregorieff et al. 2015; Taniguchi et al. 2015). Consistently, wounding of in vitro cultured cells dramatically activates YAP, and the highly nuclear YAP drives cell migration and proliferation to promote wound healing (Zhao et al. 2007, 2008; Lee et al. 2014). Both basic and clinical cancer research has implicated a role of YAP and TAZ in cancer initiation and development through suppressing cell apoptosis and promoting cell prolifieration (Moroishi et al. 2015a). Concordantly, YAP and TAZ have been considered as therapeutic targets for a number of cancers (Liu-Chittenden et al. 2012; Jiao et al. 2014; Yu et al. 2014; Zhou et al. 2015b) as well as several other diseases (Plouffe et al. 2015). Notably, the R331W missense mutation of YAP has recently been linked to a germline risk in lung adenocarcinoma (Chen et al. 2015a). Moreover, almost all epithelioid hemangioendotheliomas contain gene fusions of TAZ–CAMTA1, TAZ–FOSB, or YAP–TFE3 (Tanas et al. 2011; Antonescu et al. 2013; Patel et al. 2015), strongly supporting a role of YAP/TAZ in human tumorigenesis.
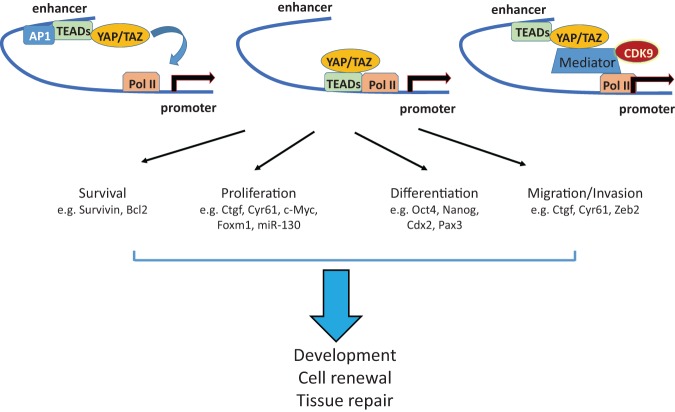
Figure 5.
The transcription program of the Hippo pathway. YAP and TAZ bind to TEAD at promoters or enhancers of the target genes to regulate transcription activation or pause release. The TEAD target genes are involved in a variety of physiological processes, such as cell survival, proliferation, differentiation, migration, and invasion.
The function of YAP and TAZ is believed to be mainly mediated through TEAD1–4, as YAP and TAZ do not bind to DNA directly and act as transcriptional coactivators of TEAD1–4 (Zhao et al. 2008), although YAP and TAZ have been also reported to associate with several other transcription factors (Hansen et al. 2015). Many genes are transcriptionally activated by TEAD complexed with YAP and/or TAZ (Zhu et al. 2015). Mechanistically, YAP and TAZ stimulate TEAD transcriptional activity by recuriting components of the SWI/SNF chromatin remodeling complex or NCOA6 histone methyltransferase complex (Oh et al. 2013, 2014; Qing et al. 2014; Skibinski et al. 2014). Interestingly, the YAP/TAZ–TEAD complex can also operate as transcriptional corepressors by recruiting the NuRD histone deacetylate complex for additional target genes, such as DDIT4 and Trail (Kim et al. 2015) or ΔNp63 (Valencia-Sama et al. 2015). Further studies are needed to show the generality of YAP/TAZ as transcripitional repressors. Nevertheless, nuclear YAP/TAZ can either induce or repress gene expression.
Recent efforts to elucidate the genome-wide action of YAP/TAZ through deep sequencing have led to some unanticipated features of YAP and TAZ in transcriptional regulation. In Drosophila, Yki binds to promoter regions to mediate transcriptional activation (Oh et al. 2013; Ikmi et al. 2014). Similary, the transcriptional functions of YAP/TAZ have been previously associated with the binding of TEADs at the promoters of target genes (Lian et al. 2010). However, ChIP-seq (chromatin immunoprecipitation [ChIP] combined with deep sequencing) studies in cancer cells (breast cancer, glioblastoma, cholangiocarcinoma, and malignant mesothelioma) as well as nontransformed cells (IMR90) have revealed that the majority of YAP/TAZ and TEAD binds to distal enhancer regions to induce gene transcription (Galli et al. 2015; Stein et al. 2015; Zanconato et al. 2015). Through de novo motif analyses at YAP/TAZ peaks, Stein et al. (2015) and Zanconato et al. (2015) confirmed early observations that YAP/TAZ mainly interact with TEAD to bind DNA (Zhao et al. 2008). However, these two independent studies also identified that the consensus motif for AP-1 transcription factors is significantly enriched in the YAP-binding regions (Stein et al. 2015; Zanconato et al. 2015), suggesting that YAP/TAZ–TEAD cooperate with AP-1 to synergistically activate target genes. AP-1 is a heterodimeric protein complex composed of JUN and FOS families of leucine zipper proteins (Eferl and Wagner 2003). TEADs appear to mediate the interaction with AP-1 (Zanconato et al. 2015). Supporting the role of AP-1 in YAP/TAZ/TEAD-mediated transcription, YAP/TAZ-induced MCF10A mammary epithelial cell growth is enhanced by AP-1, while AP-1-driven skin tumorigenesis is blunted by YAP/TAZ depletion (Zanconato et al. 2015). Galli et al. (2015) found that YAP associates with the Mediator complex to recruit CDK9 elongating kinase, mediating transcriptional pause release. Consistently, administration of the CDK9 kinase inhibitor flavopiridol prevented YAP-driven hepatomegary in mice (Galli et al. 2015).
A model for YAP/TAZ in gene expression has emerged. YAP/TAZ bind to DNA mainly via TEAD. However, the majority of YAP/TAZ proteins appear to bind to distal enhancers, although some YAP/TAZ proteins bind to promoters to induce target gene expression. YAP/TAZ coorporate with AP-1 and/or recruit additional regulators to stimulate de novo transcription initiation and enhance transcription elongation, thereby increasing target gene expression. Given the role of YAP and TAZ in controlling stem/progenitor cells in development and tissue homeostasis, it would be informative to perform ChIP-seq analyses of YAP/TAZ and TEAD in stem cells or primary tissue progenitor cells in order to gain new insights into how the YAP/TAZ-mediated transcription program coordinates the expression of multiple downstream genes to control tissue development and homeostasis.
Redefining the Hippo pathway
The Hippo pathway is named after the Drosophila Hpo kinase. The Hpo–Wts kinase cascade constitutes the axis of the Hippo pathway in Drosophila, and the functional outputs are exclusively mediated by Yki. To date, other than Wts, Sav, and Mats, there have been few reports on Hpo substrates in Drosophila. However, in mammalian cells, the functional outputs of MST1/2 are not limited to YAP/TAZ. MST1/2 are known to phosphorylate a number of other “non-Hippo” proteins in addition to LATS1/2, SAV1, and MOB1A/B. For instance, NDR1/2 are reported to be MST1/2 substrates that regulate thymocyte egress and T-cell migration (Vichalkovski et al. 2008; Tang et al. 2015). MST1/2 can phosphorylate FOXO1 to promote its nuclear localization and transcription of genes promoting apoptosis in mammalian neurons (Lehtinen et al. 2006). The apoptotic and functional roles of MST1 in pancreatic β cells also appear to be independent of LATS1/2 but rely on PDX1 phosphorylation by MST1 and JNK (Ardestani et al. 2014). Similarly, LATS1/2 are not involved in PRDX1 phosphorylation and inactivation by MST1 in hydrogen peroxide-treated cells (Rawat et al. 2013). In addition to their roles in cell death and stress responses, MST1/2 affect autophagy by directly phosphorylating Beclin 1 and LC3, although it is still unclear whether MST1 promotes or inhibits autophagy (Maejima et al. 2013; Wilkinson et al. 2015). A few other proteins, such as H2B histone proteins and VASP, are reported as MST1/2 substrates (Cheung et al. 2003; Nishikimi et al. 2014). Therefore, MST1/2 have broad functions in addition to regulating core Hippo pathway components of LATS1/2 and YAP/TAZ.
Conversely, LATS1/2 and YAP/TAZ can be regulated even in the absence of MST1/2. In Drosophila, Hppy can phosphorylate Wts at the hydrophobic motif of Wts. In fact, both Hppy and Msn activate Wts kinase activities and inhibit Yki transcriptional activity, as does Hpo (Li et al. 2014a; Meng et al. 2015; Zheng et al. 2015). Hppy homologs (MAP4K1/2/3/5) and Msn homologs (MAP4K4/6/7) can directly phosphorylate and activate LATS1/2 and appear to have a more important function than MST1/2 in the regulation of LATS1/2 and YAP/TAZ in mammalian cells in response to several upstream signals (Meng et al. 2015). On the contrary, LATS1/2 are essential for regulation of YAP and TAZ under most conditions tested, whereas MST1/2 are not. Therefore, the definition of the Hippo pathway may need to be redefined. Although MST1/2 are the mammalian homologs of the Drosophila Hpo, not all proteins and functions that are regulated by MST1/2 should be defined as the Hippo pathway. On the other hand, the functional output of YAP/TAZ and proteins that specifically regulate LATS1/2 kinase activity and/or YAP/TAZ transcriptional activity should be considered as the Hippo pathway. This definition will more accurately describe the actions of the Hippo pathway in response to extracellular and intracellular stimuli and their physiological outcomes.
Acknowledgments
We apologize for those primary works that are not cited due to the scope of this review and space constraints. We thank Kimberly Lin and Steven Plouffe for critical reading of this manuscript. Research in the laboratory of K.-L.G. is supported by grants from the National Institutes of Health (CA196878, GM51586, and EY22611).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.274027.115.
References
- Aerne BL, Gailite I, Sims D, Tapon N. 2015. Hippo stabilises its adaptor salvador by antagonising the HECT ubiquitin ligase Herc4. PLoS One 10: e0131113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastas JN, Moon RT. 2013. WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13: 11–26. [DOI] [PubMed] [Google Scholar]
- Antonescu CR, Le Loarer F, Mosquera JM, Sboner A, Zhang L, Chen CL, Chen HW, Pathan N, Krausz T, Dickson BC, et al. 2013. Novel YAP1–TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Genes Chromosomes Cancer 52: 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. 2013. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154: 1047–1059. [DOI] [PubMed] [Google Scholar]
- Ardestani A, Paroni F, Azizi Z, Kaur S, Khobragade V, Yuan T, Frogne T, Tao W, Oberholzer J, Pattou F, et al. 2014. MST1 is a key regulator of β cell apoptosis and dysfunction in diabetes. Nat Med 20: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artinian N, Cloninger C, Holmes B, Benavides-Serrato A, Bashir T, Gera J. 2015. Phosphorylation of the Hippo pathway component AMOTL2 by the mTORC2 kinase promotes YAP signaling, resulting in enhanced glioblastoma growth and invasiveness. J Biol Chem 290: 19387–19401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SJ, Kim M, Kim SH, Kwon YE, Lee JH, Kim J, Chung CH, Lee WJ, Seol JH. 2015. NEDD4 controls intestinal stem cell homeostasis by regulating the Hippo signalling pathway. Nat Commun 6: 6314. [DOI] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et al. 2013. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 493: 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichsel SJ, Tamaskovic R, Stegert MR, Hemmings BA. 2004. Mechanism of activation of NDR (nuclear Dbf2-related) protein kinase by the hMOB1 protein. J Biol Chem 279: 35228–35235. [DOI] [PubMed] [Google Scholar]
- Boggiano JC, Vanderzalm PJ, Fehon RG. 2011. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell 21: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothos J, Tuttle RL, Ottey M, Luca FC, Halazonetis TD. 2005. Human LATS1 is a mitotic exit network kinase. Cancer Res 65: 6568–6575. [DOI] [PubMed] [Google Scholar]
- Buvall L, Rashmi P, Lopez-Rivera E, Andreeva S, Weins A, Wallentin H, Greka A, Mundel P. 2013. Proteasomal degradation of Nck1 but not Nck2 regulates RhoA activation and actin dynamics. Nat Commun 4: 2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabochette P, Vega-Lopez G, Bitard J, Parain K, Chemouny R, Masson C, Borday C, Hedderich M, Henningfeld KA, Locker M, et al. 2015. YAP controls retinal stem cell DNA replication timing and genomic stability. Elife 4: e08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. 2010. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev 24: 2383–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callus BA, Verhagen AM, Vaux DL. 2006. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J 273: 4264–4276. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. 2007. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–2060. [DOI] [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. 2005. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24: 2076–2086. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D. 2014. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev 28: 432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Yu SL, Ho BC, Su KY, Hsu YC, Chang CS, Li YC, Yang SY, Hsu PY, Ho H, et al. 2015a. R331W missense mutation of oncogene YAP1 is a germline risk allele for lung adenocarcinoma with medical actionability. J Clin Oncol 33: 2303–2310. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Xie R, Wang W, Cai J, Choi KS, David KK, Huang B, Yabuta N, Nojima H, et al. 2015b. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev 29: 1285–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, Beeser A, Etkin LD, Chernoff J, Earnshaw WC, et al. 2003. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 113: 507–517. [DOI] [PubMed] [Google Scholar]
- Codelia VA, Sun G, Irvine KD. 2014. Regulation of YAP by mechanical strain through Jnk and Hippo signaling. Curr Biol 24: 2012–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collak FK, Yagiz K, Luthringer DJ, Erkaya B, Cinar B. 2012. Threonine-120 phosphorylation regulated by phosphoinositide-3-kinase/Akt and mammalian target of rapamycin pathway signaling limits the antitumor activity of mammalian sterile 20-like kinase 1. J Biol Chem 287: 23698–23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couzens AL, Knight JD, Kean MJ, Teo G, Weiss A, Dunham WH, Lin ZY, Bagshaw RD, Sicheri F, Pawson T, et al. 2013. Protein interaction network of the mammalian Hippo pathway reveals mechanisms of kinase-phosphatase interactions. Sci Signal 6: rs15. [DOI] [PubMed] [Google Scholar]
- Dan I, Watanabe NM, Kusumi A. 2001. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol 11: 220–230. [DOI] [PubMed] [Google Scholar]
- Deng H, Wang W, Yu J, Zheng Y, Qing Y, Pan D. 2015. Spectrin regulates Hippo signaling by modulating cortical actomyosin activity. Elife 4: e06567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent LG, Poon CL, Zhang X, Degoutin JL, Tipping M, Veraksa A, Harvey KF. 2015. The GTPase regulatory proteins Pix and Git control tissue growth via the Hippo pathway. Curr Biol 25: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, et al. 2014. Energy stress regulates hippo–YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep 9: 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. 2007. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130: 1120–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll TP, Cosgrove BD, Heo SJ, Shurden ZE, Mauck RL. 2015. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys J 108: 2783–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. 2011. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183. [DOI] [PubMed] [Google Scholar]
- Eferl R, Wagner EF. 2003. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 3: 859–868. [DOI] [PubMed] [Google Scholar]
- Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, et al. 2014. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell 25: 831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, Tapon N, Thompson BJ. 2015. The Spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J 34: 940–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailite I, Aerne BL, Tapon N. 2015. Differential control of Yorkie activity by LKB1/AMPK and the Hippo/Warts cascade in the central nervous system. Proc Natl Acad Sci 112: E5169–E5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli GG, Carrara M, Yuan WC, Valdes-Quezada C, Gurung B, Pepe-Mooney B, Zhang T, Geeven G, Gray NS, de Laat W, et al. 2015. YAP drives growth by controlling transcriptional pause release from dynamic enhancers. Mol Cell 60: 328–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganem NJ, Cornils H, Chiu SY, O'Rourke KP, Arnaud J, Yimlamai D, Thery M, Camargo FD, Pellman D. 2014. Cytokinesis failure triggers hippo tumor suppressor pathway activation. Cell 158: 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. 2010. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell 18: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng J, Sun X, Wang P, Zhang S, Wang X, Wu H, Hong L, Xie C, Li X, Zhao H, et al. 2015. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat Immunol 16: 1142–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ger M, Zitkus Z, Valius M. 2011. Adaptor protein Nck1 interacts with p120 Ras GTPase-activating protein and regulates its activity. Cell Signal 23: 1651–1658. [DOI] [PubMed] [Google Scholar]
- Glantschnig H, Rodan GA, Reszka AA. 2002. Mapping of MST1 kinase sites of phosphorylation. Activation and autophosphorylation. J Biol Chem 277: 42987–42996. [DOI] [PubMed] [Google Scholar]
- Gogl G, Schneider KD, Yeh BJ, Alam N, Nguyen Ba AN, Moses AM, Hetenyi C, Remenyi A, Weiss EL. 2015. The structure of an NDR/LATS kinase-Mob complex reveals a novel kinase-coactivator system and substrate docking mechanism. PLoS Biol 13: e1002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R, Hong AW, Plouffe SW, Zhao B, Liu G, Yu FX, Xu Y, Guan KL. 2015. Opposing roles of conventional and novel PKC isoforms in Hippo–YAP pathway regulation. Cell Res 25: 985–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. 2008. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol 18: 435–441. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, Wrana JL. 2015. Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 526: 715–718. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM, Kim NG. 2014. The Hippo–YAP signaling pathway and contact inhibition of growth. J Cell Sci 127: 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C, Tommasi S, Liu L, Yee JK, Dammann R, Pfeifer GP. 2007. RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr Biol 17: 700–705. [DOI] [PubMed] [Google Scholar]
- Guo C, Zhang X, Pfeifer GP. 2011. The tumor suppressor RASSF1A prevents dephosphorylation of the mammalian STE20-like kinases MST1 and MST2. J Biol Chem 286: 6253–6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CG, Moroishi T, Guan KL. 2015. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol 25: 499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey K, Tapon N. 2007. The Salvador–Warts–Hippo pathway—an emerging tumour-suppressor network. Nat Rev Cancer 7: 182–191. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK. 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114: 457–467. [DOI] [PubMed] [Google Scholar]
- Heidary Arash E, Song KM, Song S, Shiban A, Attisano L. 2014. Arhgef7 promotes activation of the Hippo pathway core kinase Lats. EMBO J 33: 2997–3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Bichsel SJ, Hemmings BA. 2005. Human NDR kinases are rapidly activated by MOB proteins through recruitment to the plasma membrane and phosphorylation. Mol Cell Biol 25: 8259–8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hergovich A, Schmitz D, Hemmings BA. 2006. The human tumour suppressor LATS1 is activated by human MOB1 at the membrane. Biochem Biophys Res Commun 345: 50–58. [DOI] [PubMed] [Google Scholar]
- Hergovich A, Kohler RS, Schmitz D, Vichalkovski A, Cornils H, Hemmings BA. 2009. The MST1 and hMOB1 tumor suppressors control human centrosome duplication by regulating NDR kinase phosphorylation. Curr Biol 19: 1692–1702. [DOI] [PubMed] [Google Scholar]
- Hirota T, Morisaki T, Nishiyama Y, Marumoto T, Tada K, Hara T, Masuko N, Inagaki M, Hatakeyama K, Saya H. 2000. Zyxin, a regulator of actin filament assembly, targets the mitotic apparatus by interacting with h-warts/LATS1 tumor suppressor. J Cell Biol 149: 1073–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KC, Zhou Z, She YM, Chun A, Cyr TD, Yang X. 2011. Itch E3 ubiquitin ligase regulates large tumor suppressor 1 stability [corrected]. Proc Natl Acad Sci 108: 4870–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Leo C, Yu S, Huang BC, Wang H, Shen M, Luo Y, Daniel-Issakani S, Payan DG, Xu X. 2004. Identification and functional characterization of a novel human misshapen/Nck interacting kinase-related kinase, hMINKβ. J Biol Chem 279: 54387–54397. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122: 421–434. [DOI] [PubMed] [Google Scholar]
- Ikmi A, Gaertner B, Seidel C, Srivastava M, Zeitlinger J, Gibson MC. 2014. Molecular evolution of the Yap/Yorkie proto-oncogene and elucidation of its core transcriptional program. Mol Biol Evol 31: 1375–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imajo M, Ebisuya M, Nishida E. 2015. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol 17: 7–19. [DOI] [PubMed] [Google Scholar]
- Jang SW, Yang SJ, Srinivasan S, Ye K. 2007. Akt phosphorylates MstI and prevents its proteolytic activation, blocking FOXO3 phosphorylation and nuclear translocation. J Biol Chem 282: 30836–30844. [DOI] [PubMed] [Google Scholar]
- Jia J, Zhang W, Wang B, Trinko R, Jiang J. 2003. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev 17: 2514–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W, et al. 2014. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 25: 166–180. [DOI] [PubMed] [Google Scholar]
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. 1995. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev 9: 534–546. [DOI] [PubMed] [Google Scholar]
- Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, Bellen HJ, Halder G. 2002. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development 129: 5719–5730. [DOI] [PubMed] [Google Scholar]
- Khokhlatchev A, Rabizadeh S, Xavier R, Nedwidek M, Chen T, Zhang XF, Seed B, Avruch J. 2002. Identification of a novel Ras-regulated proapoptotic pathway. Curr Biol 12: 253–265. [DOI] [PubMed] [Google Scholar]
- Kim NG, Gumbiner BM. 2015. Adhesion to fibronectin regulates Hippo signaling via the FAK–Src–PI3K pathway. J Cell Biol 210: 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NG, Koh E, Chen X, Gumbiner BM. 2011. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci 108: 11930–11935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim M, Lee S, Kuninaka S, Saya H, Lee H, Lee S, Lim DS. 2013. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J 32: 1543–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Choi YJ, Hwang JH, Kim AR, Cho HJ, Hwang ES, Park JY, Lee SH, Hong JH. 2014. Shear stress induced by an interstitial level of slow flow increases the osteogenic differentiation of mesenchymal stem cells through TAZ activation. PLoS One 9: e92427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim T, Johnson RL, Lim DS. 2015. Transcriptional co-repressor function of the hippo pathway transducers YAP and TAZ. Cell Rep 11: 270–282. [DOI] [PubMed] [Google Scholar]
- Kohler RS, Schmitz D, Cornils H, Hemmings BA, Hergovich A. 2010. Differential NDR/LATS interactions with the human MOB family reveal a negative role for human MOB2 in the regulation of human NDR kinases. Mol Cell Biol 30: 4507–4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz LM, Liu-Chittenden Y, Yin F, Zheng Y, Yu J, Huang B, Chen Q, Wu S, Pan D. 2013. The Hippo effector Yorkie controls normal tissue growth by antagonizing scalloped-mediated default repression. Dev Cell 25: 388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Vinayagam A, Sun X, Dephoure N, Gygi SP, Hong P, Perrimon N. 2013. The Hippo signaling pathway interactome. Science 342: 737–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. 2005. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 120: 675–685. [DOI] [PubMed] [Google Scholar]
- Lappano R, Maggiolini M. 2011. G protein-coupled receptors: novel targets for drug discovery in cancer. Nat Rev Drug Discov 10: 47–60. [DOI] [PubMed] [Google Scholar]
- Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, et al. 2010. The Hippo–Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci 107: 8248–8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Ran Byun M, Furutani-Seiki M, Hong JH, Jung HS. 2014. YAP and TAZ regulate skin wound healing. J Invest Dermatol 134: 518–525. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, et al. 2006. A conserved MST–FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell 125: 987–1001. [DOI] [PubMed] [Google Scholar]
- Li Q, Li S, Mana-Capelli S, Roth Flach RJ, Danai LV, Amcheslavsky A, Nie Y, Kaneko S, Yao X, Chen X, et al. 2014a. The conserved Misshapen–Warts–Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev Cell 31: 291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Cooper J, Zhou L, Yang C, Erdjument-Bromage H, Zagzag D, Snuderl M, Ladanyi M, Hanemann CO, Zhou P, et al. 2014b. Merlin/NF2 loss-driven tumorigenesis linked to CRL4(DCAF1)-mediated inhibition of the hippo pathway kinases Lats1 and 2 in the nucleus. Cancer Cell 26: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhou H, Li F, Chan SW, Lin Z, Wei Z, Yang Z, Guo F, Lim CJ, Xing W, et al. 2015. Angiomotin binding-induced activation of Merlin/NF2 in the Hippo pathway. Cell Res 25: 801–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, et al. 2010. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev 24: 1106–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N, Zhang C, Dill P, Panasyuk G, Pion D, Koka V, Gallazzini M, Olson EN, Lam H, Henske EP, et al. 2014. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med 211: 2249–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lignitto L, Arcella A, Sepe M, Rinaldi L, Delle Donne R, Gallo A, Stefan E, Bachmann VA, Oliva MA, Tiziana Storlazzi C, et al. 2013. Proteolysis of MOB1 by the ubiquitin ligase praja2 attenuates Hippo signalling and supports glioblastoma growth. Nat Commun 4: 1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling P, Yao Z, Meyer CF, Wang XS, Oehrl W, Feller SM, Tan TH. 1999. Interaction of hematopoietic progenitor kinase 1 with adapter proteins Crk and CrkL leads to synergistic activation of c-Jun N-terminal kinase. Mol Cell Biol 19: 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling P, Meyer CF, Redmond LP, Shui JW, Davis B, Rich RR, Hu MC, Wange RL, Tan TH. 2001. Involvement of hematopoietic progenitor kinase 1 in T cell receptor signaling. J Biol Chem 276: 18908–18914. [DOI] [PubMed] [Google Scholar]
- Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, et al. 2010. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCFβ-TrCP E3 ligase. J Biol Chem 285: 37159–37169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yu FX, Kim YC, Meng Z, Naipauer J, Looney DJ, Liu X, Gutkind JS, Mesri EA, Guan KL. 2015. Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway. Oncogene 34: 3536–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. 2012. Genetic and pharmacological disruption of the TEAD–YAP complex suppresses the oncogenic activity of YAP. Genes Dev 26: 1300–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, et al. 2010. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci 107: 1437–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv XB, Liu CY, Wang Z, Sun YP, Xiong Y, Lei QY, Guan KL. 2015. PARD3 induces TAZ activation and cell growth by promoting LATS1 and PP1 interaction. EMBO Rep 16: 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Chen Y, Chen L, Cheng H, Mu C, Li J, Gao R, Zhou C, Cao L, Liu J, et al. 2015. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol 17: 95–103. [DOI] [PubMed] [Google Scholar]
- Maejima Y, Kyoi S, Zhai P, Liu T, Li H, Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, et al. 2013. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat Med 19: 1478–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamada H, Sato T, Ota M, Sasaki H. 2015. Cell competition in mouse NIH3T3 embryonic fibroblasts is controlled by the activity of Tead family proteins and Myc. J Cell Sci 128: 790–803. [DOI] [PubMed] [Google Scholar]
- Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, Park HW, Mo JS, Lu W, Lu S, et al. 2015. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun 6: 8357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM, Liu J, Peters EC, Wu X. 2012. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol 19: 955–962. [DOI] [PubMed] [Google Scholar]
- Mo JS, Yu FX, Gong R, Brown JH, Guan KL. 2012. Regulation of the Hippo–YAP pathway by protease-activated receptors (PARs). Genes Dev 26: 2138–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. 2015. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol 17: 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O'Neal W, Milgram SL. 2006. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol 26: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisaki T, Hirota T, Iida S, Marumoto T, Hara T, Nishiyama Y, Kawasuzi M, Hiraoka T, Mimori T, Araki N, et al. 2002. WARTS tumor suppressor is phosphorylated by Cdc2/cyclin B at spindle poles during mitosis. FEBS Lett 529: 319–324. [DOI] [PubMed] [Google Scholar]
- Moroishi T, Hansen CG, Guan KL. 2015a. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 15: 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Park HW, Qin B, Chen Q, Meng Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D, et al. 2015b. A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes Dev 29: 1271–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Li S, Yu J, Min J, Brautigam CA, Tomchick DR, Pan D, Luo X. 2013. Structural basis for autoactivation of human Mst2 kinase and its regulation by RASSF5. Structure 21: 1757–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni L, Zheng Y, Hara M, Pan D, Luo X. 2015. Structural basis for Mob1-dependent activation of the core Mst-Lats kinase cascade in Hippo signaling. Genes Dev 29: 1416–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikimi A, Ishihara S, Ozawa M, Etoh K, Fukuda M, Kinashi T, Katagiri K. 2014. Rab13 acts downstream of the kinase Mst1 to deliver the integrin LFA-1 to the cell surface for lymphocyte trafficking. Sci Signal 7: ra72. [DOI] [PubMed] [Google Scholar]
- Nishio M, Hamada K, Kawahara K, Sasaki M, Noguchi F, Chiba S, Mizuno K, Suzuki SO, Dong Y, Tokuda M, et al. 2012. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J Clin Invest 122: 4505–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, et al. 2009. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell 16: 398–410. [DOI] [PubMed] [Google Scholar]
- Oh H, Slattery M, Ma L, Crofts A, White KP, Mann RS, Irvine KD. 2013. Genome-wide association of Yorkie with chromatin and chromatin-remodeling complexes. Cell Rep 3: 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Slattery M, Ma L, White KP, Mann RS, Irvine KD. 2014. Yorkie promotes transcription by recruiting a histone methyltransferase complex. Cell Rep 8: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M, Sasaki H. 2008. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development 135: 4059–4069. [DOI] [PubMed] [Google Scholar]
- Pantalacci S, Tapon N, Leopold P. 2003. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol 5: 921–927. [DOI] [PubMed] [Google Scholar]
- Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al. 2015. Alternative Wnt signaling activates YAP/TAZ. Cell 162: 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J, Struhl G. 2015. Scaling the Drosophila wing: TOR-dependent target gene access by the Hippo pathway transducer Yorkie. PLoS Biol 13: e1002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel NR, Salim AA, Sayeed H, Sarabia SF, Hollingsworth F, Warren M, Jakacky J, Tanas M, Oliveira AM, Rubin BP, et al. 2015. Molecular characterization of epithelioid haemangioendotheliomas identifies novel WWTR1–CAMTA1 fusion variants. Histopathology 67: 699–708. [DOI] [PubMed] [Google Scholar]
- Pearce LR, Komander D, Alessi DR. 2010. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9–22. [DOI] [PubMed] [Google Scholar]
- Pefani DE, Latusek R, Pires I, Grawenda AM, Yee KS, Hamilton G, van der Weyden L, Esashi F, Hammond EM, O'Neill E. 2014. RASSF1A–LATS1 signalling stabilizes replication forks by restricting CDK2-mediated phosphorylation of BRCA2. Nat Cell Biol 16: 962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Dupont S, Cordenonsi M. 2014. The biology of YAP/TAZ: hippo signaling and beyond. Physiol Rev 94: 1287–1312. [DOI] [PubMed] [Google Scholar]
- Plouffe SW, Hong AW, Guan KL. 2015. Disease implications of the Hippo/YAP pathway. Trends Mol Med 21: 212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CL, Lin JI, Zhang X, Harvey KF. 2011. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador–Warts–Hippo pathway. Dev Cell 21: 896–906. [DOI] [PubMed] [Google Scholar]
- Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. 2004. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J 381: 453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praskova M, Xia F, Avruch J. 2008. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol 18: 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Y, Yin F, Wang W, Zheng Y, Guo P, Schozer F, Deng H, Pan D. 2014. The Hippo effector Yorkie activates transcription by interacting with a histone methyltransferase complex through Ncoa6. Elife 3: e02564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C, Sun S, Sun G, Pan Y, Irvine KD. 2014. Cytoskeletal tension inhibits Hippo signaling through an Ajuba–Warts complex. Cell 158: 143–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawat SJ, Creasy CL, Peterson JR, Chernoff J. 2013. The tumor suppressor Mst1 promotes changes in the cellular redox state by phosphorylation and inactivation of peroxiredoxin-1 protein. J Biol Chem 288: 8762–8771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues-Campos M, Thompson BJ. 2014. The ubiquitin ligase FbxL7 regulates the Dachsous–Fat–Dachs system in Drosophila. Development 141: 4098–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusala A, Pawson T, Heldin CH, Aspenstrom P. 2008. Nck adapters are involved in the formation of dorsal ruffles, cell migration, and Rho signaling downstream of the platelet-derived growth factor β receptor. J Biol Chem 283: 30034–30044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabine A, Bovay E, Demir CS, Kimura W, Jaquet M, Agalarov Y, Zangger N, Scallan JP, Graber W, Gulpinar E, et al. 2015. FOXC2 and fluid shear stress stabilize postnatal lymphatic vasculature. J Clin Invest 125: 3861–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salah Z, Melino G, Aqeilan RI. 2011. Negative regulation of the Hippo pathway by E3 ubiquitin ligase ITCH is sufficient to promote tumorigenicity. Cancer Res 71: 2010–2020. [DOI] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. 2011. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144: 782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MC, Halder G. 2012. Regulation of the Hippo pathway by cell architecture and mechanical signals. Semin Cell Dev Biol 23: 803–811. [DOI] [PubMed] [Google Scholar]
- Sciarretta S, Zhai P, Maejima Y, Del Re DP, Nagarajan N, Yee D, Liu T, Magnuson MA, Volpe M, Frati G, et al. 2015. mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep 11: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Zhai P, Del Re DP, Sciarretta S, Yabuta N, Nojima H, Lim DS, Pan D, Sadoshima J. 2014. A functional interaction between Hippo–YAP signalling and FoxO1 mediates the oxidative stress response. Nat Commun 5: 3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Guo X, Yan H, Lu Y, Ji X, Li L, Liang T, Zhou D, Feng XH, Zhao JC, et al. 2015. A miR-130a–YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res 25: 997–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvis MR, Kreger BT, Lien WH, Klezovitch O, Rudakova GM, Camargo FD, Lantz DM, Seykora JT, Vasioukhin V. 2011. α-Catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal 4: ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinski A, Breindel JL, Prat A, Galvan P, Smith E, Rolfs A, Gupta PB, Labaer J, Kuperwasser C. 2014. The Hippo transducer TAZ interacts with the SWI/SNF complex to regulate breast epithelial lineage commitment. Cell Rep 6: 1059–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al. 2010. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci 107: 1431–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, et al. 2014. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol 16: 357–366. [DOI] [PubMed] [Google Scholar]
- Stegert MR, Tamaskovic R, Bichsel SJ, Hergovich A, Hemmings BA. 2004. Regulation of NDR2 protein kinase by multi-site phosphorylation and the S100B calcium-binding protein. J Biol Chem 279: 23806–23812. [DOI] [PubMed] [Google Scholar]
- Stegert MR, Hergovich A, Tamaskovic R, Bichsel SJ, Hemmings BA. 2005. Regulation of NDR protein kinase by hydrophobic motif phosphorylation mediated by the mammalian Ste20-like kinase MST3. Mol Cell Biol 25: 11019–11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein C, Bardet AF, Roma G, Bergling S, Clay I, Ruchti A, Agarinis C, Schmelzle T, Bouwmeester T, Schubeler D, et al. 2015. YAP1 exerts its transcriptional control via TEAD-mediated activation of enhancers. PLoS Genet 11: e1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YC, Han J, Xu S, Cobb M, Skolnik EY. 1997. NIK is a new Ste20-related kinase that binds NCK and MEKK1 and activates the SAPK/JNK cascade via a conserved regulatory domain. EMBO J 16: 1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun G, Irvine KD. 2013. Ajuba family proteins link JNK to Hippo signaling. Sci Signal 6: ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Yong KM, Villa-Diaz LG, Zhang X, Chen W, Philson R, Weng S, Xu H, Krebsbach PH, Fu J. 2014. Hippo/YAP-mediated rigidity-dependent motor neuron differentiation of human pluripotent stem cells. Nat Mater 13: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Reddy BV, Irvine KD. 2015. Localization of Hippo signalling complexes and Warts activation in vivo. Nat Commun 6: 8402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. 2015. Crumbs3-mediated polarity directs airway epithelial cell fate through the Hippo pathway effector Yap. Dev Cell 34: 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaskovic R, Bichsel SJ, Rogniaux H, Stegert MR, Hemmings BA. 2003. Mechanism of Ca2+-mediated regulation of NDR protein kinase through autophosphorylation and phosphorylation by an upstream kinase. J Biol Chem 278: 6710–6718. [DOI] [PubMed] [Google Scholar]
- Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, Flanagan J, Luo Y, Fenwick K, Natrajan R, et al. 2011. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med 3: 98ra82. [DOI] [PubMed] [Google Scholar]
- Tang F, Gill J, Ficht X, Barthlott T, Cornils H, Schmitz-Rohmer D, Hynx D, Zhou D, Zhang L, Xue G, et al. 2015. The kinases NDR1/2 act downstream of the Hippo homolog MST1 to mediate both egress of thymocytes from the thymus and lymphocyte motility. Sci Signal 8: ra100. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, et al. 2015. A gp130–Src–YAP module links inflammation to epithelial regeneration. Nature 519: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Zhang S, Turenchalk GS, Stewart RA, St John MA, Chen W, Xu T. 1999. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nat Genet 21: 177–181. [DOI] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D, Hariharan IK. 2002. salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110: 467–478. [DOI] [PubMed] [Google Scholar]
- Taylor LK, Wang HC, Erikson RL. 1996. Newly identified stress-responsive protein kinases, Krs-1 and Krs-2. Proc Natl Acad Sci 93: 10099–10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toji S, Yabuta N, Hosomi T, Nishihara S, Kobayashi T, Suzuki S, Tamai K, Nojima H. 2004. The centrosomal protein Lats2 is a phosphorylation target of Aurora-A kinase. Genes Cells 9: 383–397. [DOI] [PubMed] [Google Scholar]
- Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. 2003. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol 5: 914–920. [DOI] [PubMed] [Google Scholar]
- Valencia-Sama I, Zhao Y, Lai D, Janse van Rensburg HJ, Hao Y, Yang X. 2015. Hippo component TAZ functions as a co-repressor and negatively regulates ΔNp63 transcription through TEA domain (TEAD) transcription factor. J Biol Chem 290: 16906–16917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichalkovski A, Gresko E, Cornils H, Hergovich A, Schmitz D, Hemmings BA. 2008. NDR kinase is activated by RASSF1A/MST1 in response to Fas receptor stimulation and promotes apoptosis. Curr Biol 18: 1889–1895. [DOI] [PubMed] [Google Scholar]
- Wang C, An J, Zhang P, Xu C, Gao K, Wu D, Wang D, Yu H, Liu JO, Yu L. 2012. The Nedd4-like ubiquitin E3 ligases target angiomotin/p130 to ubiquitin-dependent degradation. Biochem J 444: 279–289. [DOI] [PubMed] [Google Scholar]
- Wang W, Li X, Huang J, Feng L, Dolinta KG, Chen J. 2014. Defining the protein–protein interaction network of the human hippo pathway. Mol Cell Proteomics 13: 119–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. 2015. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol 17: 490–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr MC, Holder MV, Gailite I, Saunders RE, Maile TM, Ciirdaeva E, Instrell R, Jiang M, Howell M, Rossner MJ, et al. 2013. Salt-inducible kinases regulate growth through the Hippo signalling pathway in Drosophila. Nat Cell Biol 15: 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Shimizu T, Lai ZC. 2007. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J 26: 1772–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DS, Jariwala JS, Anderson E, Mitra K, Meisenhelder J, Chang JT, Ideker T, Hunter T, Nizet V, Dillin A, et al. 2015. Phosphorylation of LC3 by the Hippo kinases STK3/STK4 is essential for autophagy. Mol Cell 57: 55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KK, Li W, An Y, Duan Y, Li Z, Kang Y, Yan Y. 2015. β-Spectrin regulates the hippo signaling pathway and modulates the basal actin network. J Biol Chem 290: 6397–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D. 2003. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. 2008. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell 14: 388–398. [DOI] [PubMed] [Google Scholar]
- Wu H, Wei L, Fan F, Ji S, Zhang S, Geng J, Hong L, Fan X, Chen Q, Tian J, et al. 2015. Integration of Hippo signalling and the unfolded protein response to restrain liver overgrowth and tumorigenesis. Nat Commun 6: 6239. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. 1995. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121: 1053–1063. [DOI] [PubMed] [Google Scholar]
- Yabuta N, Okada N, Ito A, Hosomi T, Nishihara S, Sasayama Y, Fujimori A, Okuzaki D, Zhao H, Ikawa M, et al. 2007. Lats2 is an essential mitotic regulator required for the coordination of cell division. J Biol Chem 282: 19259–19271. [DOI] [PubMed] [Google Scholar]
- Yabuta N, Mukai S, Okada N, Aylon Y, Nojima H. 2011. The tumor suppressor Lats2 is pivotal in Aurora A and Aurora B signaling during mitosis. Cell Cycle 10: 2724–2736. [DOI] [PubMed] [Google Scholar]
- Yang S, Zhang L, Liu M, Chong R, Ding SJ, Chen Y, Dong J. 2013. CDK1 phosphorylation of YAP promotes mitotic defects and cell motility and is essential for neoplastic transformation. Cancer Res 73: 6722–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CC, Graves HK, Moya IM, Tao C, Hamaratoglu F, Gladden AB, Halder G. 2015a. Differential regulation of the Hippo pathway by adherens junctions and apical–basal cell polarity modules. Proc Natl Acad Sci 112: 1785–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhang L, Chen X, Chen Y, Dong J. 2015b. Oncoprotein YAP regulates the spindle checkpoint activation in a mitotic phosphorylation-dependent manner through up-regulation of BubR1. J Biol Chem 290: 6191–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Zhang L, Purohit V, Shukla SK, Chen X, Yu F, Fu K, Chen Y, Solheim J, Singh PK, et al. 2015c. Active YAP promotes pancreatic cancer cell motility, invasion and tumorigenesis in a mitotic phosphorylation-dependent manner through LPAR3. Oncotarget 6: 36019–36031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. 2014. Hippo pathway activity influences liver cell fate. Cell 157: 1324–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimlamai D, Fowl BH, Camargo FD. 2015. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J Hepatol 63: 1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. 2013. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154: 1342–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Guan KL. 2013. The Hippo pathway: regulators and regulations. Genes Dev 27: 355–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. 2010. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell 18: 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. 2012. Regulation of the Hippo–YAP pathway by G-protein-coupled receptor signaling. Cell 150: 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. 2013. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev 27: 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Luo J, Mo JS, Liu G, Kim YC, Meng Z, Zhao L, Peyman G, Ouyang H, Jiang W, et al. 2014. Mutant Gq/11 promote uveal melanoma tumorigenesis by activating YAP. Cancer Cell 25: 822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Meng Z, Plouffe SW, Guan KL. 2015. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol 77: 201–227. [DOI] [PubMed] [Google Scholar]
- Yuan Z, Kim D, Shu S, Wu J, Guo J, Xiao L, Kaneko S, Coppola D, Cheng JQ. 2010. Phosphoinositide 3-kinase/Akt inhibits MST1-mediated pro-apoptotic signaling through phosphorylation of threonine 120. J Biol Chem 285: 3815–3824. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S. 2015. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol 17: 1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. 2008. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell 14: 377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. 2010. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell 19: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Iyer J, Chowdhury A, Ji M, Xiao L, Yang S, Chen Y, Tsai MY, Dong J. 2012. KIBRA regulates aurora kinase activity and is required for precise chromosome alignment during mitosis. J Biol Chem 287: 34069–34077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Tang F, Terracciano L, Hynx D, Kohler R, Bichet S, Hess D, Cron P, Hemmings BA, Hergovich A, et al. 2015. NDR functions as a physiological YAP1 kinase in the intestinal epithelium. Curr Biol 25: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yang X. 2015. Regulation of sensitivity of tumor cells to antitubulin drugs by Cdk1–TAZ signalling. Oncotarget 6: 21906–21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. 2007. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, et al. 2008. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22: 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. 2010. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(β-TRCP). Genes Dev 24: 72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. 2012. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev 26: 54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Khanal P, Savage P, She YM, Cyr TD, Yang X. 2014. YAP-induced resistance of cancer cells to antitubulin drugs is modulated by a Hippo-independent pathway. Cancer Res 74: 4493–4503. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wang W, Liu B, Deng H, Uster E, Pan D. 2015. Identification of Happyhour/MAP4K as alternative Hpo/Mst-like kinases in the Hippo kinase cascade. Dev Cell 34: 642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, et al. 2009. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16: 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, Li F, Yu FX, Sun Y, Yuan H, et al. 2015a. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest 125: 2123–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hu T, Xu Z, Lin Z, Zhang Z, Feng T, Zhu L, Rong Y, Shen H, Luk JM, et al. 2015b. Targeting Hippo pathway by specific interruption of YAP–TEAD interaction using cyclic YAP-like peptides. FASEB J 29: 724–732. [DOI] [PubMed] [Google Scholar]
- Zhu C, Li L, Zhao B. 2015. The regulation and function of YAP transcription co-activator. Acta Biochim Biophys Sin (Shanghai) 47: 16–28. [DOI] [PubMed] [Google Scholar]