Abstract
Calcium and protons exert control over the formation and activity of the cytoskeleton, usually by modulating an associated motor protein or one that affects the structural organization of the polymer.
The cytoskeleton is well recognized as an ever-present component of all eukaryotic cells; more recently, it has been identified in prokaryotic cells as well (van den Ent et al., 2001; Wickstead and Gull, 2011). The cytoskeleton gives order to a cell, and in large cells, where diffusion may become limiting, it provides a means to move components around, thus facilitating reactions (Verchot-Lubicz and Goldstein, 2010). Increasingly, we also see that the cytoskeleton in eukaryotic cells participates in the uptake of material from outside of the cell (e.g. endocytosis and phagocytosis; Yutin et al., 2009). And inside the cell, where organelles, membrane systems, and macromolecular complexes are exquisitely and dynamically organized in minute detail, it is largely the cytoskeleton that is responsible for this organization. Because the cytoskeleton participates in so many processes, it becomes a matter of consequence to understand how it is controlled. Indeed, this is a topic of major interest at present and one whose solution will contribute fundamentally to our understanding of many aspects of cell growth and development.
Among several possible control elements, it has been widely known for many years that ions, in particular calcium, can exert a profound effect on the structure and activity of both the actomyosin and tubulin cytoskeletons (Hepler, 2005; Hepler and Winship, 2015). One of the best examples is the stimulation of the contraction of striated muscle by calcium, where, through its binding to troponin C, tropomyosin is displaced along the actin filament, exposing myosin-binding sites and permitting contraction to occur (Alberts et al., 2008). There are numerous other examples found in both plant and animal cells involving calcium regulation of the actin cytoskeleton. In addition, it is also well established that calcium can have profound effects on microtubules (MTs). Indeed, the demonstration in 1972 by Richard Weisenberg that elevated calcium caused MT depolymerization was transformative (Weisenberg, 1972). Whereas biochemists until that time had been unsuccessful in obtaining in vitro polymerization of MTs, this now became possible. But for me, it raised intriguing possibilities concerning a role for calcium in the control of cellular processes such as mitosis and cytokinesis (Hepler and Wayne, 1985; Hepler, 2005). It also got me to think more widely about the role of calcium as a general signaling agent. In the 30 years since Randy Wayne and I reviewed this topic (Hepler and Wayne, 1985), it is now apparent that calcium reaches into countless events and processes and can be viewed as a universal signaling agent in plant cell growth and development (Edel and Kudla, 2015).
In advance, I must tell you that I will not review the broad scope of calcium research today, which is vast; there are many reviews to which the interested reader is directed (Hetherington and Brownlee, 2004; Kudla et al., 2010; Verret et al., 2010; Hashimoto and Kudla, 2011; Hamel et al., 2014; Edel and Kudla, 2015). I will also not discuss the role of small GTPase proteins, even though they can have profound effects on calcium and the cytoskeleton in plant cells. Again, the interested reader is directed to pertinent reviews on this topic (Gu et al., 2005; Nibau et al., 2006; Craddock et al., 2012; Oda and Fukuda, 2013; Li et al., 2015). Rather, in this article, I will focus on the role of ions in the control of the cytoskeleton in plant cells, giving attention to those actin- and tubulin-binding proteins that are modulated by calcium and/or protons. Before discussing ion regulation, I briefly consider, first, how calcium and protons emerged as signaling agents and, second, aspects of cytoskeleton evolution in the progression from prokaryotic to eukaryotic cells.
CALCIUM AND PROTONS AS SIGNALING AGENTS
Calcium
Calcium is an abundant element in the earth’s crust; however, high concentrations are harmful and indeed fatal (Case et al., 2007; Kazmierczak et al., 2013; Blackstone, 2015). Most important is the reaction of calcium with phosphate, forming a highly insoluble precipitate, thus incapacitating phosphate-based energy metabolism. The importance of this reaction is made apparent by the realization that all forms of life use phosphate energy metabolism in one form or another (Harold, 2014). But beyond this, high calcium condenses chromatin, aggregates proteins, and in general impairs a host of intracellular activities such as mitochondrial function and chromosome motion (Case et al., 2007; Blackstone, 2015). Indeed, calcium is so abundant and cellular chemistry so sensitive that one wonders how the first protocell arose. For a cell living in today’s ocean, the calcium concentration within the cell is 5 orders of magnitude lower than that in the ocean (e.g. from 0.1 to 10,000 µm; Blackstone, 2015). Energetically, it would seem to be a tall order to imagine that the first protocell would have been able to reduce the concentration of free calcium by 5 orders of magnitude. A possible way around this apparent impasse emerges from the evidence indicating that the early oceans or bodies of water in which life arose were more alkaline than the oceans of today (Kazmierczak et al., 2013). Alkalinity would favor the formation of calcium carbonate precipitates and thus reduce the free concentration of the ion, possibly by a few orders of magnitude. Over geological time, as the oceans became less alkaline, cells could progressively improve their ability to exclude calcium. Through the evolution of pumps and carriers that remove calcium from the cell, and of an impermeable plasma membrane that minimizes leakage, cells became able to establish and maintain a 100,000-fold gradient in the concentration of calcium between the low level in the cytosol and the high level in the extracellular milieu.
Although not as well studied as in eukaryotic cells, it appears that prokaryotic cells, including both bacteria and archea, possess calcium pumps and exchangers that extrude calcium from the cytoplasm either to the outside or to internal storage bodies (e.g. acidocalcisomes; Dominguez, 2004; Docampo and Moreno 2011; Dominguez et al., 2015). As a result, these prokaryotic cells, similar to eukaryotic cells, possess a low internal concentration of calcium, in the vicinity of 0.1 to 0.3 µm (Tisa and Adler, 1995; Watkins et al., 1995; Dominguez, 2004; Dominguez et al., 2015). Some of the calcium pumps in prokaryotes are similar to the phosphorylation or P-type ATPases of eukaryotic cells. These include the plasma membrane proton pump, the calcium pump, and the sodium/potassium pump. Other pumps may be more closely related to the F-type ATPases, such as those found in mitochondria (Dominguez, 2004; Dominguez et al., 2015). Additionally, prokaryotic cells have several proteins that possess binding pockets with a high affinity for calcium. The best known are those proteins with the canonical calcium-binding EF-hands. Here, a loop of a dozen amino acids, which is set off by two α-helices, provides an environment with neutral oxygen, carbonyl, and carboxyl residues that permit the loop to bind calcium with enormous selectivity over magnesium (Kawasaki et al., 1998; Nelson and Chazin, 1998; Zhou et al., 2006; Dominguez et al., 2015). Thus, despite the presence of 1 mm (1,000 µm) magnesium, the flexible coordination in the loop allows it to preferentially respond to a change in calcium in the physiological range (0.1–1 µm). The best known EF-hand protein is calmodulin, which occurs ubiquitously in plants and animals. Typically, as in calmodulin, in which there are four EF-hands organized in two pairs, the binding of calcium induces a conformational change exposing a hydrophobic core that then permits its interaction with another protein, the presumed response element (Nelson and Chazin, 1998). This subsequent reaction can be either positive or negative. For muscle contraction, the binding of calcium to troponin C stimulates contraction and is thus positive (Alberts et al., 2008). However, for the KINESIN-LIKE CALCIUM-BINDING PROTEIN (KCBP), which participates in several different processes in plants, the binding of calcium to calmodulin renders the motor inactive; here, calcium is a negative regulator (Deavours et al., 1998; Narasimhulu and Reddy, 1998). To summarize, it seems evident that several important aspects of calcium regulation, which originated in prokaryotes, have been maintained in eukaryotic cells, using protein complexes that arose early and that have been retained through evolution from prokaryotes to eukaryotes.
Given the enormous concentration gradient (100,000-fold) across the cell membrane, the situation was poised for exploitation. Thus, the evolution of a calcium-specific channel, which would allow a relatively small number of atoms to enter the cell, could elevate the internal ion concentration in ways that would have little or no effect on phosphate-based energy metabolism but would still be sufficient to activate an EF-hand protein such as calmodulin (Hepler and Wayne, 1985; Hepler, 2005). To give an idea about what is involved, imagine a cubic micrometer of cytoplasm (e.g. an Escherichia coli cell) in which the free calcium is 0.1 µm; this would amount to only 60 free atoms of calcium. If the concentration then increases 10-fold (to 1 µm), that would still only be 600 calcium atoms. Small changes such as these could occur and be sufficient to trigger a developmental event despite the constant surveillance by phosphate.
In addition to its selectivity over magnesium, calcium possesses remarkably faster on/off rates than magnesium (Hepler and Wayne, 1985). This property arises because magnesium has a much smaller atomic radius than calcium (65 versus 99 pm) and because the electrostatic field is inversely proportional to its atomic radius. Magnesium, therefore, will bind water much more strongly than will calcium, but it will shed water much more slowly than calcium. These properties mean that, in any given association, calcium will react much more rapidly than magnesium, explaining its participation in muscle contraction and many other events where speed is important. Speed of reaction and signal propagation may also be important in plants. A recent example comes from the study of Choi et al. (2014), who report that salt stress in Arabidopsis (Arabidopsis thaliana) roots induced calcium waves that traveled relatively long distances and at rapid rates (approximately 400 µm s−1). In this example, the calcium wave moved through the cortex and endodermal layers of the root, where its propagation was dependent on the activity of a vacuole-localized two-pore channel. When considered as a whole, we see that calcium signaling takes different forms. However, all of these different forms derive from the element’s unique chemical properties.
Protons
A great deal of attention is given to the changes in calcium concentration, or the presence of localized gradients in calcium, and the role that these concentration differences play in controlling a host of activities; this is referred to as amplitude modulation. It is also important to recognize the existence of changes in proton concentration and localized proton gradients and their contribution to cell growth and development (Felle, 2001). Because water, which is the solvent for life, weakly ionizes, it yields a solution containing 0.1 µm protons and hydroxyl ions. At pH 7, the proton concentration approximately equals the basal level of calcium and, thus, might seem poised to serve as a second messenger. However, we should be cautious about extrapolating from these data (Felle, 2001). For example, although the extracellular environment of the cell wall is usually acidic (pH 5), giving a proton concentration around 10 µm, the resulting gradient with the inside is 100-fold, whereas for calcium it is 100,000-fold. For calcium, when a selective channel in the plasma membrane opens, a huge influx ensues, whereas for protons, the driving force is much reduced. With regard to protons, while selective channels are found widely in animals (Decoursey, 2003; Beg et al., 2008) and in some marine and freshwater algae (Taylor et al., 2012), an inward-directed proton channel has not been found in flowering plants (Decoursey, 2003; Taylor et al., 2012). Nevertheless, protons can permeate cell membranes.
Another major difference between these ions is the substantially greater mobility for protons than for calcium (Feijó et al., 1999; Decoursey, 2003). The early, classic work of Hodgkin and Keynes (1957) showed that calcium is largely bound within the cytoplasm, exhibiting limited and slow mobility. By contrast, protons move quite freely, indeed much faster than expected for a monovalent cation in solution (Decoursey, 2003). One explanation may be prototropic transfer or proton hopping, where a proton within an electric field binds to one side of the water molecule, while another proton on the other side leaves the water molecule (for review, see Decoursey, 2003). This process can occur along a file of water molecules, thus allowing for the rapid displacement of the proton independent of diffusion. As a consequence, a localized proton gradient will dissipate faster than a similarly localized calcium gradient.
Finally, the way in which calcium and protons affect a response element in any signal transduction scheme is likely to be different. For calcium, as noted earlier, there are specific binding proteins, notably those with EF-hands, such as calmodulin, which can transmit the calcium signal to the response element. Such a mechanism is thus far unknown for protons. Rather, it appears that changes in proton concentration modify the protonation state of amino acids and, thereby, affect the overall charge of a protein and its interaction with other proteins and substrates. Dumetz et al. (2008) report that, in general, protein interactions increase with increasing acidity (i.e. decreasing pH).
Despite these issues, proton gradients do exist and emerge as potential signaling factors (Feijó et al., 1999). Through the activity of the plasma membrane proton ATPase, plant cells establish proton gradients, where the gradient emerges as the single most important factor in creating the membrane potential and in driving ion and nutrient transport (Sze et al., 1999; Palmgren, 2001). Proton-pumping enzymes are already well established in prokaryotes, including both bacteria and archea, with the most prevalent enzyme being the F1F0 ATPase of bacteria or the A1A0 ATPase of archea (Harold, 2014). The former is particularly well known in oxidative phosphorylation in mitochondria and photophosphorylation in plastids. This enzyme either uses ATP to pump out protons, thus creating a proton motive force that can be used to drive other transport reactions, or can use the gradient to synthesize ATP. Curiously, and in contrast to that of mitochondria and plastids, the plasma membrane proton ATPase on plant cells is not closely related to the F1F0 of bacteria (Harold, 2014); rather, it belongs to the P-type ATPase, being more closely related to the sarcoplasmic/endoplasmic reticulum (ER) calcium pump and the sodium/potassium ATPase (Dominguez et al., 2015). Importantly, the P-type ATPase also occurs in prokaryotes, where it participates in pumping calcium and other metals (Dominguez et al., 2015); these enzymes would appear to be the evolutionary ancestor of the eukaryotic proton pump.
EVOLUTION OF THE CYTOSKELETON
Actin and Tubulin Ancestors
For some time, it seemed plausible that a cytoskeleton, such as we know it in eukaryotic cells, did not exist in prokaryotic cells. The argument supporting this conclusion asserted that the prokaryotic cells were very small, and therefore that activities requiring the movement of components within the cell could be satisfied simply by diffusion. However, this notion has been changed by the relatively recent discovery that bacteria and archea possess a cytoskeleton (van den Ent et al., 2001; Doolittle and York, 2002; Carballido-Lopez, 2006; Erickson, 2007; Derman et al., 2009; Erickson et al., 2010; Bernander et al., 2011; Ettema et al., 2011; Wickstead and Gull, 2011; Ingerson-Mahar and Gitai, 2012). Briefly, proteins have been found, notably FtsZ and MreB (also ParM and FtsA), that are members of the tubulin and actin families, respectively, common to virtually all eukaryotic cells. Although far diverged in overall sequence, these ancestral cytoskeletal proteins share similarities with their descendants in eukaryotic cells, not only in their three-dimensional shape but in the amino acid sequences necessary for binding nucleotides and for protein-protein interactions (Carballido-Lopez, 2006; Erickson, 2007). FtsZ and MreB, while not forming structures resembling MTs and microfilaments (MFs), nevertheless do form extended filaments and appear to undergird a system of transport and polarity analogous to that of the eukaryotic cytoskeleton.
The prokaryotic cytoskeleton thus participates in processes similar to those with which the eukaryotic cytoskeleton is involved. For example, FtsZ contributes to cell division by forming the Z ring that physically separates the cell into two daughter cells (Erickson et al., 2010). The Z ring consists of curved filaments lying in the plane of the plasma membrane but oriented perpendicular to the long axis of the cell. The constriction of these filaments appears to be driven by the hydrolysis of GTP, which causes a bending in the FtsZ subunit (Li et al., 2013). These events, occurring at a midpoint normal to the longitudinal axis of the filament, generate a curvature that powers the constriction force. It is important to note that FtsZ-generated constrictions and divisions occur commonly in eukaryotic cells, specifically in mitochondria and plastids, organelles that are derived from endosymbiosis (Osteryoung and Pyke, 2014).
The early actin-related proteins also take part in activities in prokaryotic cells that are manifestly cytoskeletal. For example, MreB contributes to cell shape formation, where it assists in the formation of the peptidoglycan cell wall (Carballido-Lopez, 2006). Another actin-related protein, ParM, spans the length of an E. coli cell and appears to participate in the separation of genetic elements (plasmids; Garner et al., 2004; Bharat et al., 2015). These polarized ParM filaments grow but then can undergo rapid shortening (dynamic instability; Garner et al., 2004). During a growth phase, the plasmids, which are attached at their ends by FtsA, are maximally pushed apart. Thus, when the FtsZ ring completes cytokinesis, these plasmids, which are maximally separated by ParM, will be segregated to the daughter cells.
Despite the clear similarity between prokaryotic and eukaryotic cytoskeletons, and despite the fact that they are involved in operations that are similar, the specific mechanisms by which these activities are achieved are different. For example, thus far, none of the canonical actin- or tubulin-binding proteins has been identified in the prokaryotic cytoskeleton, including notably the motor proteins (i.e. myosin, kinesin, and dynein). Thus, motility or cell shape changes induced in prokaryotes by their cytoskeleton are caused by polymerization/depolymerization, lateral association of the elements themselves, or nucleotide-dependent changes. While, presumably, there are ancillary proteins involved, to the best of current knowledge, they do not fall into the families of actin- and tubulin-binding proteins well known in studies of the eukaryotic cytoskeleton (Wickstead and Gull, 2011).
Calcium Regulation of the Prokaryotic Cytoskeleton
With regard to regulating the cytoskeleton, calcium seems far less involved in prokaryotes compared with eukaryotes. Nevertheless, calcium has been implicated as a potential general regulator in chemotaxis and motility in E. coli and Bacillus subtilis cells (Tisa and Adler, 1995; Tisa et al., 2000). Thus, agents that repel bacteria cause a spike in calcium, which then causes the cell to tumble and change direction. However, cells challenged with an attractant maintain their basal level of calcium, with a concomitant suppression of tumbling. Calcium also affects the polymerization of FtsZ (Chatterjee and Chakrabarti, 2014). In E. coli, the effect requires millimolar levels of calcium and thus is not likely physiological. However, in Vibrio cholerae, an enhancement of FtsZ polymerization starts at 0.4 µm and may emerge as an in vivo regulatory mechanism (Chatterjee and Chakrabarti, 2014). Comparing with studies on eukaryotic tubulin, I note that the ability of calcium to stimulate the polymerization of FtsZ is nearly the opposite of its effect on eukaryotic tubulin, where 0.6 µm calcium initiates depolymerization (Weisenberg, 1972). Therefore, it appears that some aspects of prokaryotic motility are regulated by calcium; future studies may uncover more examples.
Cytoskeletal Changes in the Evolution of the Eukaryotic Cell
The evolution of the eukaryotic cell, with its marked increase in size over the prokaryotic cell, with its elaboration on internal membranes and organelles, and with its ability to phagocytose and endocytose, demanded substantial modifications in its cytoskeleton, the cellular component best suited to support such a significant change in cell development and organization (Yutin et al., 2009). Among transitional organisms, the recent report by Spang et al. (2015) provides evidence for an archean organism (Lokiarchaeum) that possesses several eukaryotic signature proteins, including notable actin, which bears considerable similarity to that of eukaryotic cells. In addition, they note the presence of proteins with gelsolin-like domains, with the implication that the villin/gelsolin family of proteins might be present (Spang et al., 2015). This is interesting because, as I will note in detail below, some members of the villin/gelsolin family are calcium regulated and play a pivotal role in controlling actin dynamics in plant cells. However, caution is warranted until more work is done. Even in primitive eukaryotic genera such as Giardia, none of the canonical actin-binding proteins has been identified, despite that fact that the organism has actin and produces obvious filaments that bear a structural relationship to the typical actin filaments of multicellular organisms (Paredez et al., 2011).
The evolution of the eukaryotic cell brought with it a marked change in the extent of the cytoskeleton. With large size, diffusion becomes limiting, and thus ways to transport macromolecules and organelles become necessary. A good example of an enlarged eukaryotic cell is found in Nitella, in which the internode cells are several centimeters long and within which the cytoplasm exhibits extremely fast cytoplasmic streaming (approximately 70 µm s−1; Hepler and Palevitz, 1974; Shimmen and Yokota, 1994; Verchot-Lubicz and Goldstein, 2010). Very long bundles of actin, bound to the immobile chloroplasts in the stationary ectoplasm, extend the length of the cell (Kersey and Wessells, 1976). Myosin XI, the fastest myosin examined thus far, drives the circulatory streaming pattern (Kashiyama et al., 2000; Tominaga et al., 2003).
The radiation of cytoskeletal structures accompanying the evolution of the eukaryotic cell occurred with a remarkable conservation of the cytoskeleton itself. In prokaryotes, there are several actin analogs that differ not only from eukaryotic actin but substantially from one another. By contrast, eukaryotic actins are highly conserved (Meagher et al., 1999; McCurdy et al., 2001; Galkin et al., 2002; Yutin et al., 2009); between birds and mammals, the actin sequence conservation is nearly 100%, and between some widely spaced organisms such as yeast and mammals, the sequence conservation is approximately 90%. The strength of this conservation was brought home to us in a study spearheaded by Barry Palevitz, who was then a postdoctoral fellow in my laboratory. We were attempting to determine the identity of filaments in the alga Nitella using the binding of heavy meromyosin from rabbit skeletal muscle as the assay. Strikingly, the results showed that muscle myosin bound to the filaments of Nitella, producing the well-known arrowhead pattern (Palevitz et al., 1974). The binding pattern plus the further observation that the heavy meromyosin arrowheads were removed with the addition of ATP provided convincing evidence that the filaments were actin (for a historical account, see Dietrich, 2015). From an evolutionary point of view, these studies indicated that there must be a considerable degree of conservation in plant actin relative to its mammalian counterpart.
Similarly, eukaryotic tubulin is also highly conserved between different taxa. Although demonstrating cross-phylum conservation of tubulin was not an objective of mine, research in my laboratory nevertheless benefited from this fact. Before the advent of expressed protein markers, we microinjected Tradescantia stamen hair cells with fluorescently labeled pig brain tubulin and were able to see that it incorporated into the plant MT arrays, including the preprophase band, the spindle apparatus, and the phragmoplast (Zhang et al., 1990a). These observations underscore the depth of tubulin conservation.
The extraordinary conservation of actin and tubulin likely reflects the need to interact with multiple binding proteins, which themselves are also quite well conserved (Meagher et al., 1999; McCurdy et al., 2001; Yutin et al., 2009). Any modification in the actin or tubulin sequence itself that compromises its association with these several binding proteins may be harmful in ways that ensure its negative selection. By comparison, the apparent lack of conservation in prokaryotic actin and tubulin, perhaps somewhat indirectly, suggests that these organisms do not possess a cohort of specific binding proteins that regulate their function. Although future work could turn up contrary evidence, at the moment, there appears to be a substantial difference in how prokaryotic and eukaryotic cytoskeletons are regulated.
CALCIUM AND PROTON REGULATION OF ACTOMYOSIN
Calcium Regulation of Myosin
Earlier, I made reference to the well-known role of calcium in the control of muscle contraction. In plants, calcium also plays a central role in the regulation of intracellular motility; cytoplasmic streaming, which occurs in nearly all plant cells, is permitted by basal levels (approximately 0.1 µm) and inhibited by elevated levels (approximately 1 µm) of calcium. In rapidly streaming characean internode cells, elicitation of an action potential causes streaming to abruptly stop, in a process that is reversible (Tazawa and Kishimoto, 1968). Studies in the 1960s using ion replacement methods both on Nitella internode cells and those of angiosperms directed attention to calcium, rather than potassium or magnesium, as a potential streaming regulator (for review, see Hepler, 2005). The landmark study by Williamson and Ashley (1982), using internode cells that had been microinjected with the calcium-sensitive photoprotein aequorin, was pivotal and conclusive; it showed a sharp and significant rise in the intracellular calcium concentration virtually simultaneously with the elicitation of the action potential. This study, quickly confirmed by Kikuyama and Tazawa (1983), provided compelling evidence that the rise in calcium concentration from submicromolar to several micromolar inhibited cytoplasmic streaming.
More recent studies have provided insight about how this inhibition is achieved. First, I note that, in plants, the molecule involved in cytoplasmic streaming is myosin XI and that it bears similarity to myosin V of mammals and yeast, which is also calcium regulated. Also, myosin XI, at least from tobacco (Nicotiana tabacum), is a processive motor that walks along the actin filament in 35-nm steps (Tominaga et al., 2003). Yokota et al. (1999) isolated a myosin from lily (Lilium longiflorum) pollen tubes that showed motility as well as F-actin-stimulated ATPase activity in basal levels of calcium but was inhibited by calcium concentrations above 1 µm. That study also identified a myosin-associated peptide, shown to be calmodulin, that dissociated in the presence of elevated calcium. In further studies, Tominaga et al. (2012), using an in vitro motility assay in which fluorescently labeled actin filaments were allowed to move over attached myosin, revealed that, in the presence of elevated calcium, the step size became reduced, resulting from the calcium-dependent detachments of the calmodulin light chains. In this example, there are six calmodulin light chains per myosin and they bind to the so-called IQ domain on the neck region, where IQ refers to the first two amino acids (commonly Ile and Gln). When it binds calcium, the ensuing shape change causes calmodulin to detach from the neck region of the myosin. These detachments appear to progressively disable the motor function of myosin, first leading to a shortened power stroke but eventually causing an inhibition of streaming (Tominaga et al., 2012; Tominaga and Ito, 2015). These changes are reversible, so that when the excess calcium is sequestered, calmodulin dissociates from calcium and rebinds the IQ domains on the neck region, restoring the motile activity of the myosin.
While the main focus on myosin XI has been on its role in generating cytoplasmic streaming, it is also important to note that this motor is essential for tip growth in protonemal cells of Physcomitrella patens by a mechanism that is independent of streaming. Normally growing protonemal cells do not exhibit streaming, showing only slow saltatory motion of organelles. However, when the two myosin XI genes are silenced, tip growth is inhibited (Vidali et al., 2010). Localization studies indicate that both myosin and actin aggregate in the cell apex (Vidali et al., 2010) and further that myosin fluctuates in amount, quite possibly controlling the organization of actin (Furt et al., 2013). These are relatively recent results and require more studies to resolve the regulatory mechanisms. Nevertheless, given the presence of the well-established calmodulin-binding sites (the IQ domains) on the neck region of myosin XI, it seems likely that calcium will be involved, possibly in controlling the interaction between myosin and actin and/or in regulating how this complex controls protonemal growth.
To the extent that myosin XI of plants resembles myosin V of yeast and animals, there might be additional ways in which calcium can modulate motor activity. Studies of myosin Va draw attention to the globular tail domain, showing that its position within the three-dimensional scheme of the protein can become a powerful regulator of myosin ATPase activity (Krementsov et al., 2004; Li et al., 2008; Donovan and Bretscher, 2015). At very low calcium concentrations, the activity of myosin Va is blocked, apparently because, at these low levels of calcium, the molecule folds such that the myosin head binds to the tail, causing a loss in ATPase activity (Krementsov et al., 2004; Donovan and Bretscher, 2015). Therefore, somewhat paradoxically, calcium at the physiological level (0.1 µm) is needed to unfold and activate myosin Va ATPase, whereas when the concentration is increased above 1 µm, it inhibits myosin. While it is puzzling how the cell could generate subphysiological concentrations of calcium, we must be careful not to extrapolate too far from in vitro data about what is occurring in vivo. For example, there may be cofactors present in the cell that change the sensitivity to calcium in ways that also modulate the head-to-tail binding activity. The important point, however, is that, similar to myosin Va, myosin XI from both Arabidopsis and tobacco appears to possess the head-to-tail binding that inhibits its ATPase activity (Li and Nebenführ, 2007; Avisar et al., 2012).
While the focus above has been on myosin XI, plants possess a second myosin, designated myosin VIII. Although less well studied than myosin XI, important roles for myosin VIII are suggested from studies showing its association with the expanding cell plate and with the region in the cell cortex to which the cell plate will fuse (Wu and Bezanilla, 2014). A myosin (Radford and White, 1998), likely myosin VIII (Reichelt et al., 1999), also localizes to plasmodesmata, suggesting that it may function in intercellular transport. Given that myosin VIII is also similar to myosin V and that it contains four calmodulin-binding IQ regions (Knight and Kendrick-Jones, 1993), it is reasonable to suggest that it too is regulated by calcium.
Calcium Regulation of Profilin
Profilin is a small (12–15 kD) but abundant protein that binds G-actin or unpolymerized actin and plays an important role in controlling the polymerization of F-actin (Vidali and Hepler, 2001). In particular, the profilin/G-actin complex, when additionally bound with ATP, provides subunits to the barbed or growing end of an actin filament and thus contributes to the elongation of that filament. Given that a significant percentage of actin in plants is present in the depolymerized state (Li et al., 2015), it becomes important to understand how this condition is regulated. It is reasonable to ask, therefore, why doesn’t all the G-actin form into F-actin, given the plentiful supply of ATP? At least one part of the explanation involves calcium. Kovar et al. (2000) show that calcium concentrations of 1 µm or higher, with a saturation at 5 µm, effectively sequester the profilin/G-actin complex and prevent it from undergoing polymerization. The basis for this phenomenon might have more to do with the binding of calcium to actin than to profilin. Actin, at least from animal sources, is known to have a high-affinity site that is occupied by either calcium or magnesium as well as some low-affinity sites (Carlier et al., 1986). When bound with calcium, polymerization is blocked, whereas when bound with magnesium, especially at the low-affinity sites, polymerization is promoted (Carlier et al., 1986). Calcium, under these conditions, might also affect the profilin/actin conformation in a way that renders the complex unfavorable for polymerization (Porta and Borgstahl, 2012). It follows, therefore, in regions of elevated calcium (e.g. the apex of the pollen tube or root hair), that the local ionic conditions will prevent polymerization.
Supporting evidence comes from Snowman et al. (2002), who show that calcium, working through the profilin/actin complex, contributes to the depolymerization of actin during a self-incompatibility reaction in poppy (Papaver rhoeas) pollen. However, the authors are quick to note that the profilin/actin/calcium complex can only account for part of the depolymerization of F-actin; other factors are likely involved (Snowman et al., 2002). Nevertheless, these data help us understand why there is a lack of F-actin in regions of high calcium such as the tip of pollen tubes and root hairs (to be discussed later).
Calcium Regulation of Villin/Gelsolin
Several years ago, Kohno and Shimmen (1987) provided evidence for calcium fragmentation of actin MFs in lily pollen tubes, leading to speculation that a calcium-sensitive actin-binding protein might be involved. Subsequently, two actin-binding proteins were identified, one at 135 kD and the second at 115 kD, which were shown to be homologs of villin (Vidali et al., 1999; Yokota et al., 2003). Although these proteins were initially isolated from pollen, villin has been shown to be present in many plant species and cell types (Klahre et al., 2000; Bao et al., 2012). These observations raised an awareness concerning its ionic regulation, because villin from animal sources (e.g. mammalian intestinal epithelium) typically contains six gelsolin repeats that are well known for their sensitivity to calcium.
Initially, it had been shown that the lily 135-kD villin bound to actin and appeared to participate in the polymerization and bundling of actin MFs. Upon further analysis, it became apparent that the presence of calcium and calmodulin, in the range of 1 µm, reduced this binding and promoted depolymerization (Yokota et al., 2000). More recently, it has become apparent that plant villins, like their animal counterparts, are extremely sensitive to calcium without added calmodulin. Thus, they bind and bundle MFs at 0.1 µm calcium but fragment them at 1 µm calcium (Qu et al., 2014; Huang et al., 2015). At the elevated level of calcium, they may also cap the barbed end, preventing plus end assembly. Structure analysis indicates that, in low calcium (0.1 µm), the six segments of gelsolin form a compact configuration that sterically prevents certain interactions with actin (Burtnick et al., 1997). However, in elevated calcium (1 µm), the N and C termini of the gelsolin complex consisting of the six gelsolin segments, which heretofore were joined, are now released, opening up the gelsolin complex and allowing interactions with actin (Burtnick et al., 1997). In total, there are eight calcium-binding sites on gelsolin, which are divided into two categories (Choe et al., 2002). The first category, called type 1, of which there are two such sites, participate in a shared coordination of calcium between gelsolin and actin. The second category, called type 2, of which there are six (i.e. one on each gelsolin segment), is fully contained within gelsolin. When the calcium concentration increases, the type 2 sites, perhaps especially that on the sixth gelsolin segment (Choe et al., 2002), cause structural rearrangements that promote the binding between gelsolin and actin and further lead to the fragmentation of actin (Burtnick et al., 1997; Choe et al., 2002).
A more systematic analysis of five members of the Arabidopsis villin family shows that villin-5, which is preferentially expressed in pollen, bundles and protects actin filaments from depolymerization in low concentrations of calcium (10 nm), possibly by promoting lateral binding between adjacent filaments (Zhang et al., 2010). However, at 10 µm calcium, it stimulates severing of F-actin and facilitates barbed end capping to prevent new polymerization. Similar results have been obtained with villin-3 (Khurana et al., 2010), villin-2 (Bao et al., 2012), and villin-4 (Zhang et al., 2011). Of the four calcium-sensitive villins in Arabidopsis, villin-2 appears to be the most sensitive, with statistically significant severing at only 0.1 µm calcium (Bao et al., 2012).
However, not all villins display calcium-sensitive severing; parallel studies on villin-1 reveal that, while it stabilizes actin filaments, it does not nucleate, sever, or cap F-actin, being quite insensitive to the calcium concentration (Khurana et al., 2010). Additionally, these authors show that villin-1, while promoting F-actin bundling, fails to protect the bundles against the severing action of villin-3 in the presence of elevated calcium (1–10 µm; Khurana et al., 2010).
In addition to villin, smaller members of that family of proteins, including notably gelsolin, have been identified in plants. Xiang et al. (2007) isolated a particularly small (29-kD) actin-binding protein in lily that contains only two of the gelsolin repeats (G-1 and G-2). But like its larger family members, it proves to be a calcium-sensitive protein that fragments F-actin and caps the plus ends (Xiang et al., 2007). Yet another member of this family of proteins is designated PrABP-80, which was isolated from field poppy pollen (Huang et al., 2004). The protein contains peptides homologous to villin, but the lack of a head piece, together with its inability to bundle F-actin, identifies PrABP-80 as a plant gelsolin. It too exhibits calcium-dependent severing of F-actin as well as plus end capping.
Calcium Regulation of MT-Associated Proteins That Also Bind Actin
At least two proteins, which were originally identified as plant-specific microtubule-associated proteins (MAPs; Hamada, 2014), have been found to bind MFs and to exhibit calcium-sensitive fragmentation of F-actin. The first is MAP18, which in pollen tubes is an F-actin-binding and -severing protein that is activated by an increase in intracellular calcium (Zhu et al., 2013). The second is MICROTUBULE-DESTABILIZING PROTEIN25 (MDP25), which again influences MT behavior (see below) but is also recognized as a powerful modulator of actin MFs (Qin et al., 2014). Like MAP18, MDP25 fragments F-actin in a calcium-sensitive manner. Somewhat curiously, mutants in pollen lacking the wild-type gene grow significantly faster than the wild type; however, they are less successful in causing fertilization (Qin et al., 2014). Also, the mutants, when compared with the wild type, show more prominent arrays of actin in the subapical region of the pollen tube. Structural studies further reveal that MDP25, and possibly MAP18, are plasma membrane-localized proteins that detach and move into the cytosol in the presence of elevated calcium (Li et al., 2011).
Calcium and Proton Regulation of Actin-Depolymerizing Factor and LIM Domain Proteins
ACTIN-DEPOLYMERIZING FACTOR (ADF) is a small (15-kD) actin-binding protein that plays a prime role in the control of actin dynamics and is essential for cell growth and development (Dong et al., 2001; Allwood et al., 2002; Chen et al., 2002; Augustine et al., 2008; Bou Daher et al., 2011). Studies on pollen tubes identified ADF as an abundant actin-binding protein in tobacco (Chen et al., 2002), lily (Allwood et al., 2002), and Arabidopsis (Bou Daher et al., 2011). In tobacco, overexpression analysis indicates that pollen tube growth is inhibited by elevated levels of ADF (Chen et al., 2002). Localization data indicate that, when expressed at only a moderate level, ADF appears to preferentially stain a meshwork of actin close to the tip of the pollen tube. Because ADF is recognized as being regulated by the concentration of protons, Chen et al. (2002) point out that its localization is remarkably close to the position of the alkaline band in the pollen tube. The study in lily, while emphasizing the importance of ADF for actin remodeling, also provides evidence that a related protein, called ACTIN-INTERACTING PROTEIN (AIP), plays a crucial role when working together with ADF. Also in lily, more recent data indicate that both ADF and AIP localize to a subapical region occupied by both the alkaline band and the cortical actin fringe (Lovy-Wheeler et al., 2006). These two proteins also participate in tip growth in protonemata of the moss P. patens (Augustine et al., 2011). ADF/AIP thus stimulates actin severing and the production of new plus ends, which then become focal points for new actin filament formation and growth.
That ADF is regulated by the proton concentration appears well established in both plants and animals (Yonezawa et al., 1985; Carlier et al., 1997; Bernstein et al., 2000; Bernstein and Bamburg, 2004). In plant cells as the pH increases above pH 7, ADF becomes increasingly more effective in severing and promoting actin depolymerization at the minus or pointed end of the actin filament while supporting growth at the plus end (Carlier et al., 1997; Gungabissoon et al., 1998; Allwood et al., 2002; Chen et al., 2002).
In addition to protons, calcium might serve as a regulator of ADF activity, where it participates in the control of phosphorylation, with the Ser residue located at position 6 being the target. In ADFs derived from both tobacco (NtADF1; Chen et al., 2002) and maize (Zea mays; ZmADF3; Smertenko et al., 1998), the phosphorylated state has been found to be inactive, whereas the dephosphorylated state promotes F-actin turnover. From studies of calcium-enhanced phosphorylation of ZmADF3, Smertenko et al. (1998) suggest that this reaction is regulated by a calcium-dependent protein kinase. Consistently, a partially purified fraction from bean (Phaseolus vulgaris) suspension culture cells with phosphorylation activity is enriched in calcium-dependent protein kinases, and this phosphorylation activity is blocked by antibodies to calcium-dependent protein kinase (Allwood et al., 2001). However, such regulation is not ubiquitous; for example, ADF from lily pollen (LlADF1), which retains the conserved Ser at position 6, is not phosphorylated (Allwood et al., 2002). Further complication comes from studies of P. patens, which show that neither a phosphomimetic nor an unphosphorylatable form of ADF supports normal growth; these data suggest that a balance between phosphorylation and dephosphorylation is required to achieve normal growth (Augustine et al., 2008).
A final class of actin-associated proteins to be considered here are the LIM domain proteins, which are suggested to play a role in bundling or stabilizing F-actin (Thomas et al., 2009). Studies on a LIM domain protein from lily pollen tubes (LlLIM1) indicate that low calcium (170 nm) and slightly acidic pH (optimum at 6.25) support the stabilizing and bundling properties of this actin-binding protein (Wang et al., 2008). When conditions deviate from these levels (i.e. the calcium concentration increases and the proton concentration decreases), the stability of F-actin is reduced. Comparing protons and calcium as regulators, Wang et al. (2008) argue that protons are more important.
CALCIUM REGULATION OF MTs
The study by Weisenberg (1972), showing that MTs depolymerized when the calcium concentration exceeds 0.6 µm, sparked an intense interest in the mechanism of action of calcium. In a subsequent breakthrough, Marcum et al. (1978) identified a calcium-dependent regulatory protein, later known as calmodulin. Building support for the idea that calmodulin is the intermediary factor that binds calcium and then stimulates the depolymerization of MTs, they showed that calmodulin plus 10 µm calcium inhibited MT assembly in vitro, whereas without calmodulin the same level of calcium had little effect (Marcum et al., 1978). These authors also showed that low calcium (less than 1 µm) in the presence of calmodulin failed to inhibit MT polymerization. Thus, to depolymerize MTs, both calmodulin and elevated calcium (e.g. 1 µm) are necessary. While these results seem inconsistent with the earlier report showing that only calcium was needed to depolymerize MTs (Weisenberg, 1972), it must be appreciated, especially given the ubiquity of calmodulin, that some of this protein was likely already present in these early in vitro preparations, thereby rendering MTs sensitive to the experimental changes in calcium.
Because tubulin, like actin, is highly conserved, it will come as no surprise that plant MTs are also sensitive to calcium and calmodulin. In relatively early work, Cyr and coworkers showed that MTs in lysed carrot (Daucus carota) cell protoplasts were markedly destabilized by calcium plus calmodulin but not in either calcium or calmodulin alone (Cyr, 1991; Fisher and Cyr, 1993; Durso and Cyr, 1994).
Given the ever-present cortical MTs and their role in controlling cell shape and growth, and given the ability of various growth-modulating stimuli to generate calcium transients, it is reasonable to expect multiple arrays of calcium response elements and signaling pathways that connect these events. I draw attention to one example, namely the ability of roots to respond to mechanical stimuli (i.e. touch) and change their direction of growth. In earlier work, Lee et al. (2005) had shown that mechanical perturbation, through an induced calcium spike (Knight et al., 1991), up-regulated several genes, including notably those for calmodulin and calmodulin-like proteins. More recently, Wang et al. (2011) established a connection between a calmodulin-like protein (CML24) and the organization and orientation of cortical MTs. Plants harboring mutations in CML24 (cml24-2 and cml24-4) showed reduced root length and altered orientation of MTs in epidermal cells. Nevertheless, observations like these are not straightforward to interpret, because calmodulin and its relatives also regulate other activities. For example, CML24 itself binds to the IQ region of myosin VIII (Abu-Abied et al., 2006). These early reports may be only the tip of the iceberg; future work will likely uncover many examples in which calcium, working through appropriate calcium-binding proteins, will affect MTs and MFs in ways that impact plant growth and development.
In addition to calmodulin and related proteins, there are others involved in linking calcium to the control of MT organization and stability; one is MAP18 (Wang et al., 2007). I made reference above to this protein in its role as an actin-binding protein. Although not initially identified as a calcium-binding protein, it is recognized as being the same as PCaP2 and similar to PCaP1, which are calcium-binding proteins shown to be associated with the plasma membrane in Arabidopsis (Kato et al., 2010, 2013; Hamada, 2014). PCaP1, which has been renamed MDP25, is a negative regulator, facilitating the destabilization of cortical MTs in the presence of elevated calcium (0.5 µm; Li et al., 2011; Qin et al., 2012). Because cortical MTs participate in the process of cellulose orientation and deposition, it seems likely that MDP25 plays a role in that process. Specifically, Li et al. (2011) draw attention to in vivo studies showing that blue light causes a rapid reduction in cell elongation in cucumber (Cucumis sativus) hypocotyls while simultaneously inducing an increase in the intracellular calcium (Shinkle and Jones, 1988; Baum et al., 1999). While it can be appreciated that these factors are likely important players, more work is needed to establish the functional relationship between calcium, cortical MT stability/organization, and cell elongation.
Having noted that MAP18 and MDP25 bind to both MTs and MFs, it is important to recognize that the colocalization and coordination of these two cytoskeletal elements may be crucial to several aspects of plant growth and development (Collings, 2008). Indeed, even before these biochemical and physiological studies had emerged, observations from electron micrographs of different plant cells had shown that cortical MTs often possess coaligned MFs (Hardham et al., 1980; Lancelle et al., 1987; Ding et al., 1991a). Although these initial observations were confined to the cortical cytoskeleton, close associations between actin and tubulin have been observed in the preprophase band (Ding et al., 1991b) and the phragmoplast (Kakimoto and Shibaoka, 1988). That the MFs adjacent to MTs are actin has been confirmed, at least in pollen tubes, by immunogold labeling with anti-actin antibody (Lancelle and Hepler, 1991).
In addition to regulating the assembly and structure of MTs in plant cells, calcium contributes to the activity of at least some of the kinesins. The best studied example thus far is KCBP (Reddy and Day, 2011; Ganguly and Dixit, 2013). Reddy and coworkers isolated KCBP from Arabidopsis, showing that it is a minus end-directed motor protein and a member of the class 14 kinesins, a group that is particularly large in plants (Reddy et al., 1996a, 1996b; Narasimhulu et al., 1997; Song et al., 1997). A similar kinesin motor protein emerged from the studies of Oppenheimer et al. (1997) on the zwichel mutant in Arabidopsis, which has impaired trichome development. Reddy and coworkers have further shown that KCBP possesses a calmodulin-binding domain, which together with calcium is involved in the regulation of the motor. Thus, in the absence of calcium, KCBP binds and moves along MTs, whereas in the presence of calcium, the MT-binding affinity as well as ATPase activity decline. More recent work indicates that a calmodulin-binding helix at the C terminus of KCBP is responsible for the negative regulatory activity. When bound with calcium/calmodulin, the helix comes to reside between the motor and the MT, thus blocking motor activity (Vinogradova et al., 2008). Interestingly, KCBP has yet another protein, which has been named KCBP-INTERACTING CALCIUM BINDING PROTEIN (KIC), that contributes to the calcium signal (Reddy et al., 2004). When KIC binds calcium, it too is able to deliver the signal to KCBP and negatively regulate this motor protein. In this instance, KIC is thought to work as an allosteric trap (Vinogradova et al., 2009); when bound to calcium, it sterically prevents the motor from binding to an MT.
KCBP associates with different MT arrays, and under different circumstances. For example, the protein is up-regulated during division and seen to be associated with the preprophase band, the mitotic apparatus, and the phragmoplast (Bowser and Reddy, 1997; Smirnova et al., 1998; Dymek et al., 2006; Buschmann et al., 2015). In addition, KCBP localizes with cortical MTs in cotton (Gossypium hirsutum) fibers (Preuss et al., 2003) and cytoplasmic MTs in elongating spruce (Picea abies) pollen tubes (Lazzaro et al., 2013). Recent studies have identified a KCBP family member in P. patens, where it appears to dimerize and become a processive minus end MT motor (Jonsson et al., 2015). The authors suggest that this activity may compensate for the lack of cytoplasmic dynein in P. patens and higher plants (Jonsson et al., 2015).
I also draw attention to recently published work showing that KCBP interacts with actin MFs as well as MTs (Tian et al., 2015). It has been known for some years that both MTs and MFs, as well as KCBP (Oppenheimer et al., 1997), participate in the control of trichome development. It also has been known that KCBP possesses a MYOSIN TAIL HOMOLOGY DOMAIN4 (MyTH4; Abdel-Ghany et al., 2005), and while it has been thought that this might participate in binding to actin, clear evidence for this activity had not been forthcoming. Tian et al. (2015) now provide evidence that KCBP may be the link between MTs and MFs. First, it is important to note that the N terminus of KCBP includes both MyTH4 and a domain known primarily in the animal literature for its ability to bind to a complex of actin-associated proteins, which includes band 4.1, ezrin, radixin, and moesin, called FERM (Kerber and Cheney, 2011). Using truncated versions of full-length KCBP, Tian et al. (2015) report that the MyTH4 domain binds MTs while the FERM domain binds F-actin. Of course, further work is needed to resolve the functional significance of these interactions. Nevertheless, these results add to our widening appreciation of the likely importance of the interaction between MTs and MFs in the control of various developmental processes (Collings, 2008).
Ideas about the specific function of KCBP have been gleaned from studies involving the microinjection of an affinity-purified antibody that constitutively activates the protein (Narasimhulu et al., 1997; Narasimhulu and Reddy, 1998). This particular antibody, which was raised against a 23-amino acid peptide that contains the calcium/calmodulin-binding domain, interferes with calcium/calmodulin regulation but does not affect MT binding; its presence thus activates KCBP motor activity. Injection of this antibody into dividing stamen hair cells of Tradescantia yields a series of results that depend on the mitotic stage at the time of injection (Vos et al., 2000). When injected during late prophase, the constitutive activation of KCBP induces the breakdown of the nuclear envelope in 2 to 10 min but then arrests cells in metaphase. However, injection of cells in late metaphase did not cause arrest, and moreover, these cells progressed normally into anaphase. Nevertheless, these injected late metaphase cells often failed to form a phragmoplast or complete cytokinesis. At the moment, we do not have a good explanation for these diverse activities, except to say that KCBP, through continuous fine-tuning of its activity, possibly by local calcium gradients, plays a pivotal role in the formation and function of both the mitotic and cytokinetic apparatuses. More recently, Lazzaro et al. (2013) have microinjected the antibody into growing pollen tubes of spruce, observing that MTs become bundled and that the vacuole was repositioned. Cytoplasmic streaming first slows before stopping completely, together with the inhibition of cell elongation. These phenomena might also be influenced by the local ion conditions, where spatially defined gradients of calcium, together with activated calmodulin, are simply overridden by microinjection of the activating antibody.
While KCBP emerges as the best studied calcium-sensitive MT motor protein in plants, there are likely to be others. For example, the calmodulin-binding protein IQD1, recently identified in Arabidopsis, has been found to interact with a KINESIN LIGHT CHAIN-RELATED PROTEIN1 (KLCR1; Bürstenbinder et al., 2013). Further structural studies revealed that GFP-tagged IQD1 localizes to MTs, suggesting that IQD1 recruits KLCR1 and calmodulin to MTs. Further work is warranted, but it seems increasingly clear that calcium and calmodulin will be involved with the activity of plant kinesins other than just KCBP.
Finally, it seems likely that additional examples of calcium/calmodulin regulation will emerge for other plant kinesins. Among the class 14 kinesins, there are several that possess calponin homology domains (for review, see Richardson et al., 2006; Collings, 2008; Reddy and Day, 2011; Schneider and Persson, 2015). These kinesins are interesting for two reasons: first, they are often noted for binding actin and thus could link MTs and MFs (Wills et al., 1994; Schneider and Persson, 2015); and second, the calponin homology domain can be involved in binding to calmodulin (Wills et al., 1994). Should the latter be shown to be present, then there is a strong likelihood that calcium modulation will be found.
INTEGRATED ION/CYTOSKELETAL ACTIVITIES
In the previous sections, I note several different examples in which either actin MFs and/or MTs are controlled in their polymerization or structural organization by changes in calcium, protons, or both. In this section, I attempt to integrate the ion and cytoskeletal activities and to show how these components work together and contribute to our understanding of plant cell growth and development. I will discuss two examples: the first focuses on tip growth, especially in pollen tubes, and the second on cell division.
Tip Growth in Pollen Tubes
One of the best examples showing an interaction between ion fluxes and gradients and the structure and activity of the cytoskeleton occurs in the apex of tip-growing cells, especially the pollen tube (Cole and Fowler, 2006; Hepler et al., 2006; Campanoni and Blatt, 2007; Krichevsky et al., 2007; Cheung and Wu, 2008; Qin and Yang, 2011; Rounds and Bezanilla, 2013; Cai et al., 2015; Hepler and Winship, 2015). It has been known for over 50 years that calcium is essential for pollen tube growth (Brewbaker and Kwack, 1963; Steinhorst and Kudla, 2013). It is also known that pollen tubes grow best in an acidic environment (Holdaway-Clarke et al., 2003). It is widely appreciated that pollen tubes possess a striking gradient of free calcium focused at the extreme apex of the tube (Fig. 1A; Rathore et al., 1991; Miller et al., 1992; Hepler et al., 2006). They also possess a proton gradient, in which the extreme tip exhibits a slightly acidic domain, with an alkaline band being situated a few micrometers back from the tip (Fig. 1B; Feijó et al., 1999). Not only do these standing gradients persist during growth, but in many instances they oscillate in magnitude, with the same period as the growth rate but with differing phase relationships. Thus, the increase in calcium slightly follows the increase in growth rate (Messerli et al., 2000; Cárdenas et al., 2008), as does maximal acidity at the tip (Lovy-Wheeler et al., 2006), whereas the increase in the alkaline band precedes the increase in growth rate (Lovy-Wheeler et al., 2006).
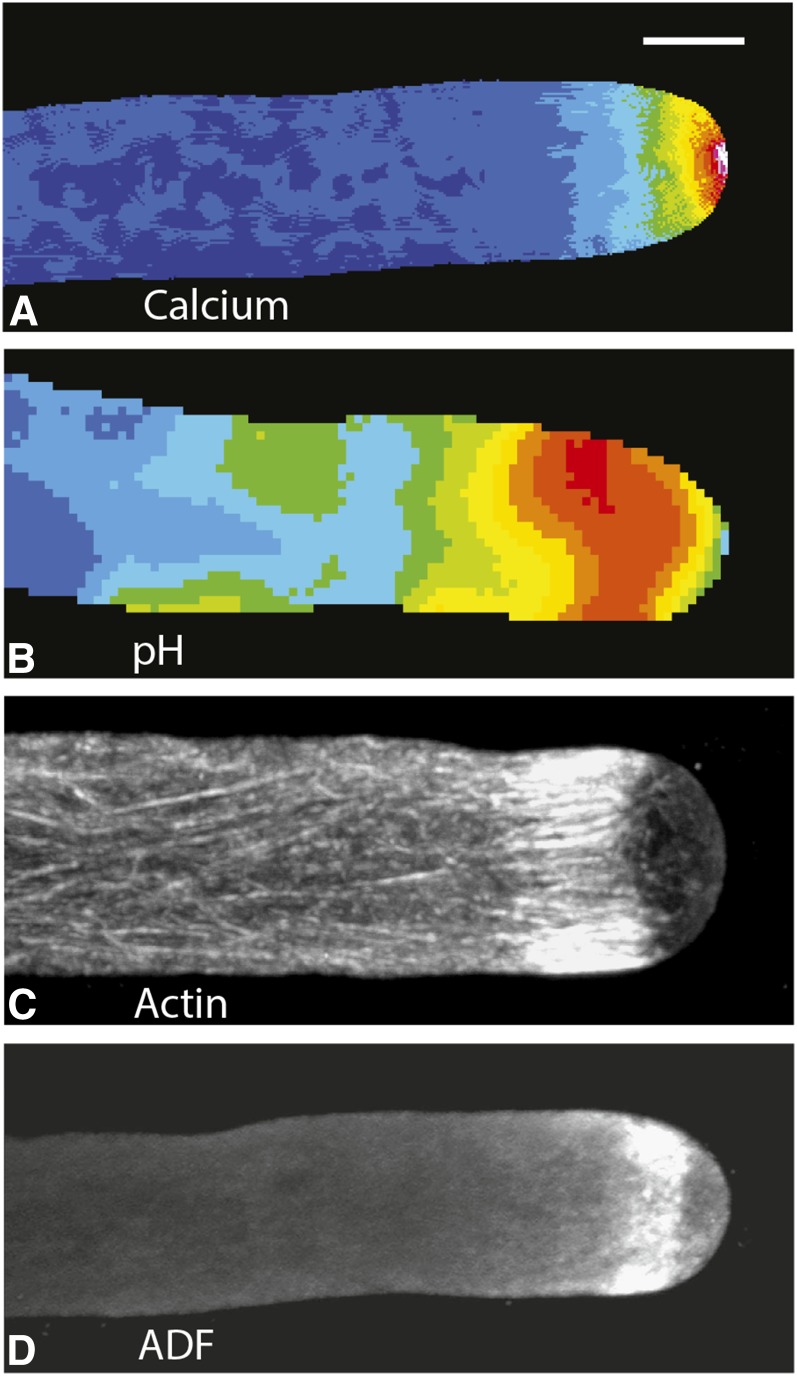
Figure 1.
Lily pollen tubes, which have been treated with different reporters or stains, show the distribution of free calcium (A), pH (B), F-actin (C), and the actin-binding protein ADF (D). A, Microinjection of the calcium-sensitive dye Fura-2-dextran allows one to image the distribution of free calcium in a living lily pollen tube. Ratiometric analysis reveals that the calcium is maximal at the extreme apex, where the concentrations are 1 to 10 µm. Back from the tip, the calcium concentration drops sharply, reaching the basal level of 0.15 µm within 15 to 20 µm. From Rounds et al. (2011). B, In this instance, the living pollen tube has been microinjected with BCECF-dextran, a pH-sensitive dye. The ratiometric image reveals a slightly acidic region at the extreme apex followed by a prominent alkaline band (orange/red; pH 7.5), starting a few micrometers behind the tip and extending rearward for an additional 10 to 20 µm. Thereafter, the pH approaches neutrality. From Rounds et al. (2011). C, In this image, the lily pollen tube has been preserved through rapid freeze fixation, followed by rehydration and staining with an anti-actin antibody. The resulting confocal fluorescence image reveals the striking cortical actin fringe that begins a few micrometers back from the tip and extends rearward for 5 to 10 µm. The MFs in the fringe are organized as a palisade in which the individual elements are aligned parallel to the long axis of the pollen tube. Behind the fringe, the MFs are also longitudinally oriented, but they appear to be much less dense than in the fringe. From Lovy-Wheeler et al. (2005). D, In this example, a lily pollen tube, which has been preserved through rapid freeze fixation and rehydration, has been stained with an antibody to lily ADF1. The stained region starts a few micrometers back from the apex and extends rearward 3 to 5 µm. By comparison with C, it is obvious that ADF colocalizes with the actin fringe, perhaps especially the forward edge of the fringe. Also note that ADF (D) and the actin fringe (C) colocalize with the alkaline band (B). From Lovy-Wheeler et al. (2006). Bar, 10 μm.
The position and localization of the cytoskeleton strongly reflect the corresponding position of the specific ion gradients and the pertinent binding proteins. Thus, actin MFs are generally not observed in the extreme apex of the tube, especially at the polar axis (Fig. 1C; Kroeger et al., 2009). A likely reason is that the calcium concentration at the extreme tip, which can be as high as 10 µm (Messerli et al., 2000), will activate villin/gelsolin and fragment MFs as well as cap newly exposed plus ends, thus preventing further polymerization of actin in this region (Huang et al., 2015). The high calcium will also affect the profilin/G-actin complex and further prevent polymerization (Kovar et al., 2000). Affirmation of that statement derives from the inspection of pollen tubes injected with fluorescent DNase, a probe that binds G-actin; direct imaging reveals a high concentration of G-actin in the apex of the pollen tube (Cárdenas et al., 2008).
The high calcium will also activate calmodulin and inhibit several different proteins or processes, such as MT polymerization and myosin-dependent cytoplasmic streaming. These conclusions find support from the use of TA-calmodulin, a fluorescent derivative that increases its signal in the presence of activated calmodulin. Whereas total calmodulin is evenly spread throughout the pollen tube, those molecules bound with calcium occur predominantly at the tube apex (Rato et al., 2004).
Finally, the high calcium will activate MAP18 and MDP25; as noted earlier, these proteins destabilize MTs and fragment MFs (Li et al., 2011; Zhu et al., 2013; Qin et al., 2014). There are thus several combinations of proteins that, in the presence of calcium, ensure that the extreme apex of the pollen tube will be mostly free of cytoskeletal polymer.
A particularly intriguing cytoskeletal feature of the pollen tube apex is the cortical actin fringe, which is found a few micrometers back from the extreme apex (Fig. 1C; Lovy-Wheeler et al., 2005; Vidali et al., 2009; Dong et al., 2012; Rounds et al., 2014). Although most clearly depicted in lily pollen tubes, the cortical actin fringe has also been observed in tobacco pollen tubes, both in fixed (Lovy-Wheeler et al., 2005) and living (Vidali et al., 2009) preparations. The fringe consists of a palisade of longitudinally oriented MFs positioned close to the plasma membrane (Fig. 1C; Lovy-Wheeler et al., 2006). Because this region, although close to the apex, is nevertheless separated from it, its calcium level is lower than that at the extreme apex. At these lower calcium concentrations, villin might serve as a cross-linking and stabilizing factor to support the formation of MFs. Importantly, this region contains the alkaline band (Fig. 1B; Feijó et al., 1999), which is produced by the closely positioned plasma membrane proton ATPase (Lefebvre et al., 2005; Certal et al., 2008). Notably, this is also where some isoforms of ADF (Fig. 1D) and AIP (Chen et al., 2002; Lovy-Wheeler et al., 2006; Bou Daher et al., 2011) are localized, which because of the alkalinity will enhance the turnover of actin. However, in marked contrast to villin/gelsolin, which caps plus ends, ADF/AIP will promote the growth of new MFs from the newly exposed plus ends. Indeed, the combined colocalization ADF/AIP and the alkaline band seems likely to ensure that the cortical actin fringe remains in a state of constant renewal and growth. Deviations from these conditions, however, will lead to growth inhibition. In a recent study, Wilkins et al. (2015) show that poppy pollen tubes undergoing a self-incompatible induction of programmed cell death exhibit a rapid acidification in the apical cytoplasm, together with an abrupt inhibition of growth. The authors argue that the increased acidity down-regulates ADF and thus prevents actin turnover, which is necessary for normal growth (Wilkins et al., 2015).
Several studies support the importance of the cortical actin fringe for rapid and oscillatory growth of the pollen tube. For example, treatment of the pollen tubes with low concentrations of latrunculin B (2 nm), or injection with low concentrations of profilin or DNase, do not block cytoplasmic streaming but do inhibit growth (Vidali et al., 2001). This low level of latrunculin B destroys the cortical actin fringe but has little effect on the longitudinal cables of actin in the shank of the tube (Vidali et al., 2001, 2009; Rounds et al., 2014). Also, the streaming, which under normal conditions occurs in the reverse fountain pattern, in the presence of 2 nm latrunculin B shifts to a circulatory pattern, with large plastids that heretofore had been prevented from entering the clear zone now freely flowing through the extreme apex of the tube (Vidali et al., 2001). More recently, Dong et al. (2012) have shown a strong correlation between the emergence of rapid growth and the appearance of the fringe. They further note that shorter fringes are associated with more rapid growth. Moreover, when pollen tubes undergo changes in growth direction, the actin fringe is most intense on the faster growing side (Dong et al., 2012). Most recently, Rounds et al. (2014) find, in studies in which growth has been reversibly inhibited, that the fringe reemerges together with the reinitiation of growth. From these results, we see that the apical actin fringe plays a pivotal role in the elongation of the pollen tube, in the maintenance of the apical clear zone, and likely in the generation of the reverse fountain streaming pattern.
Direct inspection of the tube apex using image correlation spectroscopy and fluorescence recovery after photobleaching provides evidence that the vesicles move forward along the cortex, presumably along the MFs of the apical fringe (Bove et al., 2008; Chebli et al., 2013). While these studies and those of Zonia and Munnik (2008) suggest that vesicles are delivered to an annular region at the tube tip and not the polar axis, on balance, the evidence favors the idea that material is actually secreted maximally at or near the polar axis (Lee et al., 2008; McKenna et al., 2009; Rojas et al., 2011; Rounds et al., 2014). These ideas fit with the notion that the apical calcium gradient (Fig. 1A), while effectively destroying the cytoskeleton at the tube tip, stimulates the secretion of vesicles along the polar axis, thereby accounting for the observed elongation of the tube. Therefore, while the apical fringe of actin probably transports vesicles close to the tip, it actually drops them off in an annular zone a micrometer or two back from the apex. For this reason, I suggest that processes of diffusion take over, with the calcium gradient creating a favored spot for vesicle fusion and secretion (Fig. 1A). Thus, as vesicles immediately adjacent to the high point of the calcium gradient fuse and release their contents at the tip plasma membrane, neighboring vesicles will move into the vacated space, creating a diffusive flow from the annular region toward the tube apex.
By marked contrast to the apex, in the shank of the pollen tube only 10 to 20 µm behind the apex in lily, the calcium (Pierson et al., 1996) and proton (Feijó et al., 1999) concentrations are at basal levels (approximately 0.1 µm for both; Fig. 1, A and B); therefore, both MFs and MTs will form structured arrays with less turnover. Although often ignored, it is important to emphasize that electron and fluorescence microscopy studies indicate that well-organized arrays of longitudinally oriented cortical MTs occur in the shank of the pollen tube (Lancelle et al., 1987; Lancelle and Hepler, 1992; Cheung et al., 2008). In addition, we commonly see cortical MTs coaligned with MFs in the pollen tube cortex (Lancelle et al., 1987; Pierson et al., 1989).
The pollen tube thus provides a compelling example in which the ion gradients and the cytoskeleton fit closely together and where the known properties of the actin- and tubulin-binding proteins under specific ion conditions largely account for the observed cytoskeletal organization (Cheung and Wu, 2007; Hepler and Winship, 2015). These observations also underscore the concept of local ionic domains as key regulatory components in large cell systems (Berridge 2006).
Cell Division (Mitosis and Cytokinesis)
For several reasons, it has seemed plausible that calcium contributes to the regulation of both mitosis and cytokinesis. To begin, MTs are a central component of the mitotic and cytokinetic apparatuses. Thus, for chromosomes to move toward the spindle poles during anaphase, the kinetochore MTs must break down, in a process that might contribute to the forces that move the chromosomes (McIntosh et al., 2010). Given the remarkable sensitivity of MTs to calcium at the physiological level (Weisenberg, 1972), it becomes attractive to suggest that small increases in the calcium concentration contribute to their depolymerization. It is additionally pertinent that, even though the nuclear envelope has broken down, the mitotic/meiotic apparatus in different plant and animal cells remains surrounded by the ER (Fig. 2A; Hepler, 1980; Hepler and Wolniak, 1984; Bobinnec et al., 2003; Parry et al., 2005; Whitaker, 2006, 2008), with some elements interpenetrating the mitotic apparatus specifically along the kinetochore MTs (Fig. 2, B and C; Hepler, 1980). During anaphase, this closely positioned ER might elevate the calcium concentration locally around the kinetochore fibers, thereby driving MT depolymerization and contributing to the movement of the chromosomes to the spindle poles (Hepler and Wolniak, 1984; Whitaker, 2006).
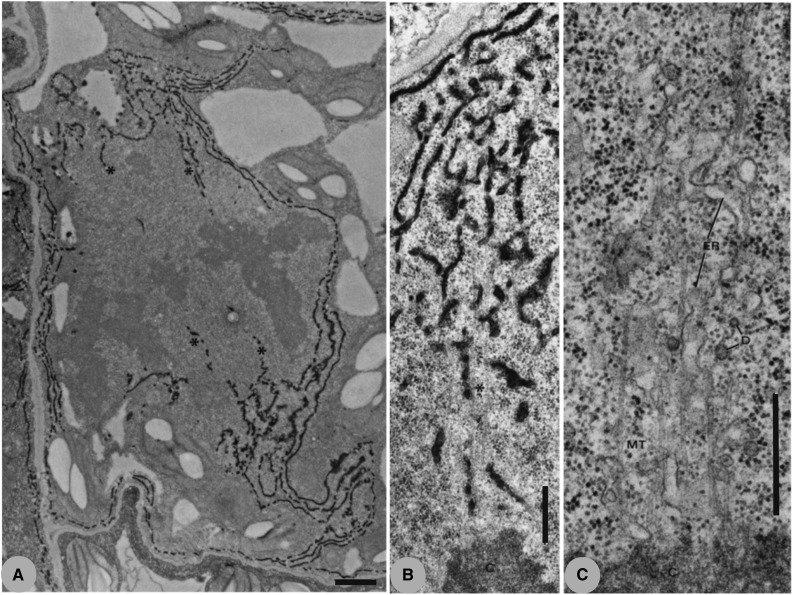
Figure 2.
A, This image shows an electron micrograph of a dividing barley leaf mesophyll cell in metaphase that has been fixed in glutaraldehyde and postfixed in osmium tetroxide plus potassium ferricyanide. The osmium/ferricyanide treatment markedly contrasts the ER and reveals that, while the nuclear envelope has broken down, the mitotic spindle remains surrounded by ER. The image further shows that ER accumulates at the spindle poles and that some elements extend into the spindle interior, usually along kinetochore MTs. Bar = 1 µm. From Hepler (1980). B and C, At higher magnification than in A, these images show that tubular elements of ER extend along the full length of kinetochore MTs. In B, the ER has been contrasted by postfixation with osmium/ferricyanide, whereas in C, only standard glutaraldehyde/osmium fixation has been used. Both images show the close apposition of interpenetrating ER with kinetochore MTs. C also shows some dictyosome vesicles (D). Bars = 0.5 µm. From Hepler (1980).
Supporting evidence comes from studies in which dividing stamen hair cells of Tradescantia have been microinjected with controlled amounts of calcium. By increasing the calcium concentration in an early anaphase cell from a basal level to 1 µm, it is possible to transiently increase chromosome motion 2-fold (Fig. 3A), whereas an increase to approximately 2 µm transiently slows motion (Fig. 3B; Zhang et al., 1990b). Consistently, injection of the elevated level at one end of an anaphase cell slows chromosome motion toward the proximal pole while speeding it toward the distal pole (Fig. 3C; Zhang et al., 1990b). In this experiment, the injection of calcium has likely established a diffusion gradient across the cell, with the concentration being approximately 2 µm at the proximal pole and 1 µm at the distal pole. Taken together, these results support the idea that added calcium facilitates kinetochore MT depolymerization, but this process can only be manipulated over a very limited range. That is, 1 µm enhances depolymerization and accelerates chromosome motion, whereas 2 µm is too drastic and causes excessive decay of the kinetochore fibers so that directed pole-ward motion is slowed or stopped. In support of the above interpretation, we showed subsequently, in cells preinjected with fluorescent brain tubulin to label the MTs, that injection of calcium caused a transient decline in fluorescence (Zhang et al., 1992). Injection of 2 µm calcium causes a distinct decay in the fluorescence of the spindle fibers, whereas injection of 1 µm calcium generates a lesser decline in fluorescence. From these results, it seems reasonable to suggest that chromosome motion is controlled by the local calcium concentration (Zhang et al., 1992).
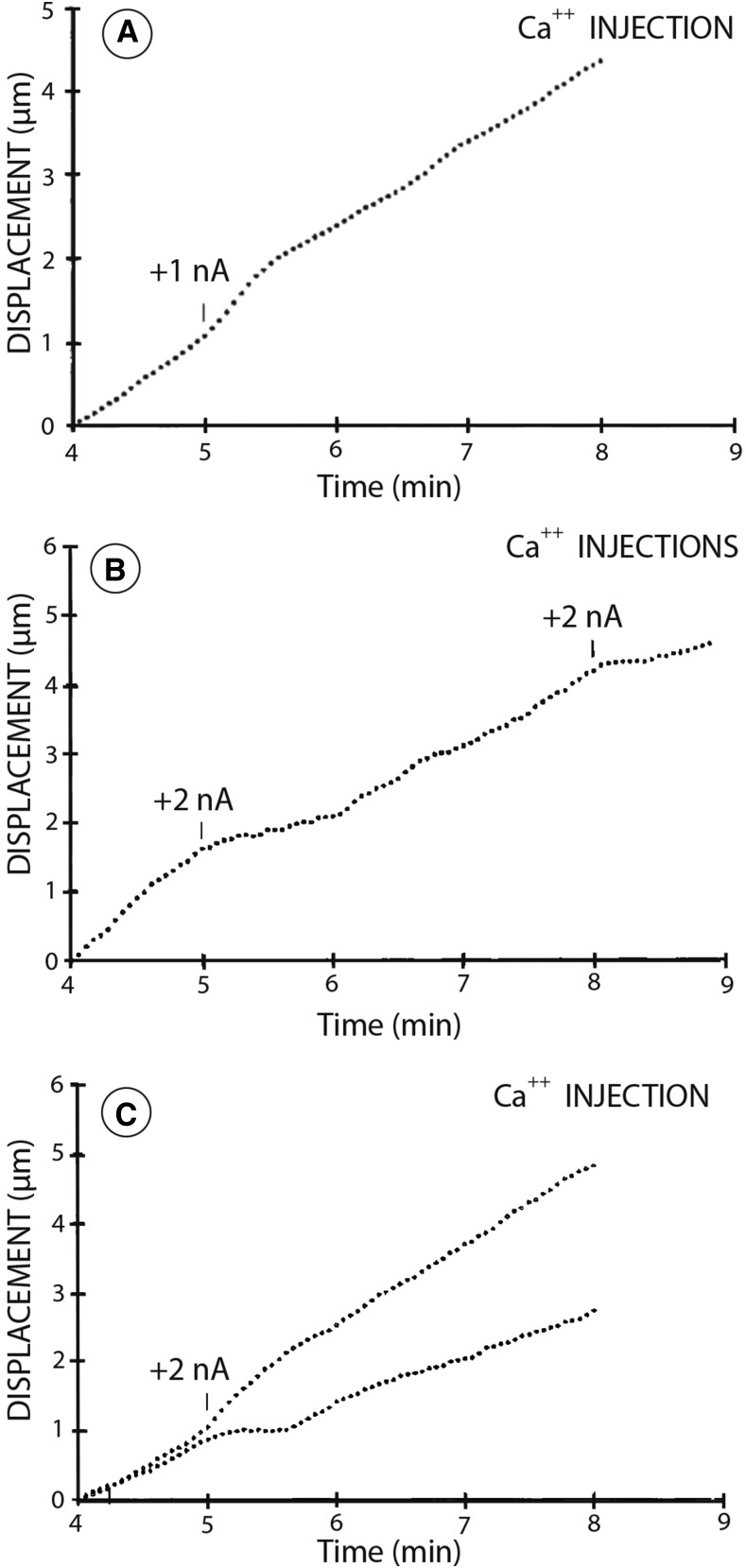
Figure 3.
Iontophoretic injection of controlled amounts of calcium during early anaphase produces a clear effect on chromosome motion. In A and B, the injection needle was inserted into the mid plane of the cell, whereas in C, it was inserted into the spindle pole. The microinjection needle contained 20 mm CaCl2 and 100 mm KCl. The magnitude of the current was then varied in order to produce the desired level of calcium. A, Application of positive current of 1 nA for 10 s, which produces an increase in calcium to approximately 1 µm, causes the chromosomes to increase their rate of motion from 1.1 to 2.1 µm min−1 for approximately 20 s. B, However, application of 2 nA for 10 s causes a brief slowing of chromosome motion. C, Injection of calcium at 2 nA for 10 s to one of the spindle poles causes a brief slowing of chromosomes to the proximal pole while simultaneously accelerating motion to the distal pole. From Zhang et al. (1990b).
There is also reason to suspect that calcium plays a major role in cytokinesis. The phragmoplast, which is a complex of cytoskeletal (MTs and MFs) and membrane (ER and vesicles) elements (Staehelin and Hepler, 1996; McMichael and Bednarek, 2013), gives rise to the new cell plate that eventually separates the recently formed daughter nuclei. Both MTs and MFs are oriented perpendicular to the plane of the plate, with their respective plus ends being proximal to the plate (Kakimoto and Shibaoka, 1988; Ho et al., 2011). For MTs, at least in some examples, the plus ends overlap (Hepler and Jackson, 1968). Through processes not fully understood, during late anaphase the cell plate starts to form in the central part of the cell, like an island emerging in a sea of cytoplasm. It seems likely that the cytoskeletal elements, particularly MTs and their associated kinesin motors (Lee et al., 2001; Lee and Liu, 2013; McMichael and Bednarek, 2013), drive Golgi vesicles to the plane of the plate, where the vesicles fuse to form the new cell wall. During successive stages, the plate grows centrifugally, with new vesicles being added to the expanding edge. At the same time, or more likely in anticipation, the cytoskeletal elements form anew at the edge while decaying in the more central, mature parts of the cell plate.
The placement and fusion of the expanding plate along the side wall involves complex positioning events. The place of fusion is usually forecast by the preprophase band of MTs (Pickett-Heaps and Northcote, 1966). But we also know that MFs associate with the preprophase band (Palevitz, 1987; Cleary et al., 1992) and, indeed, come to mark its edges on the cortical side wall (Sano et al., 2005). As the cell plate approaches the side wall, actin MFs make connections between elements of the expanding phragmoplast and the actin on the edges of the preprophase band site and possibly play a key role in guiding the plate (Valster and Hepler, 1997; Wu and Bezanilla, 2014). The recent work by Wu and Bezanilla (2014) provides important new information showing a coordination of MTs and MFs in the guidance process. Specifically, they provide evidence that myosin VIII binds to the plus ends of MTs. In addition, myosin VIII binds to the preprophase band site on the cell cortex. Yet further pertinent observations show that MFs are polymerized at the phragmoplast mid zone, with some MFs growing normal to the plane of the cell plate while others grow toward the cell periphery. When taken together, Wu and Bezanilla (2014) suggest that the myosin VIII/MT complex links to the preprophase band site via MFs, where the motor activity of myosin VIII, by moving along MFs, effectively reels in the expanding phragmoplast.
The reason for providing the above description is to emphasize the number of potential targets for calcium action and to suggest the likelihood that it plays a role. The assembly and disassembly of both MTs and MFs, the activity of motor proteins (kinesins and myosin VIII), as well as the secretory processes of cell plate fusion are all candidates for regulation by calcium. Supporting evidence involves studies in which BAPTA [1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid] buffers of differing affinities for calcium were microinjected into stamen hair cells undergoing late anaphase and cytokinesis (Jürgens et al., 1994). The results show that all the buffers, but especially dibromo BAPTA (1.5 µm), markedly slow the process of centrifugal cell plate expansion or cause distortion and even complete dissolution of the young cell plate. These results are consistent with local calcium gradients being associated with cell plate formation. Presumably, these local gradients are controlled by the extensive elements of the ER that reside around and among the cytoskeletal elements of the phragmoplast (Hepler, 1982).
Studies on dividing cells in general underscore the concept of domains for the local control of calcium (Whitaker, 2006, 2008). For example, in Drosophila embryos, convincing images show prominent elements of ER surrounding the spindle apparatus (Bobinnec et al., 2003; Parry et al., 2005, 2006); it seems plausible that these membranes locally control the uptake and release of calcium. While some imaging studies show changes in calcium, particularly associated with nuclear envelope breakdown (Parry et al., 2005) and again at the onset of anaphase (Groigno and Whitaker, 1998), overall there are relatively few data, and moreover, the calcium changes observed are small and spatially localized (Parry et al., 2005). This is particularly true in plants, where robust images of calcium transients associated with the stages of cell division are rare, even though these same methods reveal striking ion gradients when applied to pollen tubes. In stamen hair cells, my laboratory reported calcium elevations associated with anaphase in dividing cells injected with the absorbance indicator arsenazo III (Hepler and Callaham, 1987). However, these results could not be confirmed in subsequent studies using the more sensitive indicator Fura-2-dextran (P.K. Hepler, unpublished data). But that does not rule out the possible occurrence of local calcium transients. Given that calmodulin is dispersed uniformly throughout the dividing plant cell (Vos and Hepler, 1998), it would take only small, and highly localized, pulses of calcium to initiate important events such as MT depolymerization. I predict, therefore, that with newer and more sensitive probes, together with those that are targeted to certain domains, such as the ER surfaces along the kinetochore MTs or close to the spindle pole, reproducible calcium transients will at least be detected.
CONCLUSION
Ions, especially calcium, play a major role in the control of the structure and activity of the cytoskeleton and, thus, contribute to many aspects of plant growth and development that are dependent on the cytoskeleton. Prokaryotes evolved mechanisms that markedly lowered the calcium concentration within the cell, with the result that phosphate precipitation and the inhibition of energy metabolism were minimized. In so doing, prokaryotes made possible the subsequent emergence of calcium as a signaling factor. Despite this tight control of calcium in prokaryotic cells and the presence of different signaling pathways, calcium has apparently not been recruited extensively into the regulation of the prokaryotic cytoskeleton. With the emergence of the eukaryotic cell, the calcium paradigm has been taken to a new level, where calcium is now recognized as a universal signaling agent. The factors driving that transformation, which would already have been occurring in prokaryotes, were the evolution of proteins, or specific motifs (EF-hands), that are able to select calcium by several orders of magnitude over closely related magnesium. Also, the much higher speed of reaction inherent with calcium in binding to proteins, compared with magnesium, would have been a major selective advantage favoring the calcium pathways. Given these circumstances, it should come as no surprise that calcium signaling emerged as a common and robust process for many events.
The evolution of the cytoskeleton charts a more checkered course. Although prokaryotic tubulins and actins appeared early, they have undergone significant changes during the progression from prokaryotes to eukaryotes. A major event appears to be the radiation of binding proteins that play pivotal roles in the control of ever so many processes. Perhaps most noticeable are the motor proteins, kinesins, dyneins, and myosins. But also of crucial importance are those proteins controlling nucleation, polymerization, depolymerization, bundling, membrane binding, and association with specific organelles or sites. It is in the activation and deactivation of these proteins and the events they promote where we see calcium and, to a lesser extent, protons performing key functions. If we take villin/gelsolin as a good example, we see that when the calcium concentration is at a basal level (0.1 µm), villin will bundle, stabilize, and even promote the formation of actin MFs. However, when the concentration increases to 1 µm, it fragments MFs and caps their plus ends, preventing MF formation. Thus, a relatively modest increase in the actual number of calcium atoms leads to a profound change in actin structure and activity.
Major factors in the broad scheme of calcium-cytoskeleton interaction are the membrane compartments that sequester calcium and the channels and pumps that control ion movement. Here, it appears that evolutionary processes within eukaryotic cells have produced exquisitely defined systems so that remarkably different events can occur with a single cell, being separated by only a few micrometers. This is the concept of local domains and needs to be part of our thinking as we attempt to unravel the ways in which calcium and protons affect plant growth and development (Berridge, 2006). Despite the fact that we have some impressive probes for examining small structural regions of the cell, we nevertheless run into barriers that prevent us from making the necessary observations. One barrier is the inherent resolution limit of 0.2 µm for the normal light microscope. This barrier, fortunately, is being surmounted (Chen et al., 2014). Together with selective targeting of probes through molecular methods, we can hope for an improved level of spatial detection that could not be dreamed about just a few years ago. It is an exciting time in which the stage is being set for new discoveries. Even with the probes and microscopes we currently have, significant progress has been made in sorting out the ionic and cytoskeletal events that control the growth of the pollen tube. But looking ahead, I would love the opportunity to revisit the dividing cell and see if I could make sense out of the calcium signals as they pertain to the events of mitosis and cytokinesis.
Acknowledgments
Many colleagues have generously helped me during the writing of this article. Through communications, usually by e-mail, I have been made aware of ideas and publications whose inclusion have greatly improved the final product. A few have read early drafts and provided detailed criticisms. I list all these people in alphabetical order and sincerely thank them for the help they provided: Tobias Baskin, University of Massachusetts, Amherst; Magdalena Bezanilla, University of Massachusetts, Amherst; Maurice Bosch, Institute of Biological, Environmental, and Rural Sciences, Aberystwyth, UK; Jon Calame, Eastport, ME; Zac Cande, University of California, Berkeley; Alice Cheung, University of Massachusetts, Amherst; Delfina Dominguez, University of Texas, El Paso; Noni Franklin-Tong, University of Birmingham, Birmingham, UK; Takahiro Hamada, University of Tokyo; Frank Harold, University of Washington, Seattle; Alenka Lovy-Wheeler, Tufts Medical School, Boston; Bo Liu, University of California, Davis; Cornelius Lütz, University of Innsbruck, Innsbruck, Austria, Ursula Lütz-Meindl, University of Salzburg, Salzburg, Austria; Alexander Paredez, University of Washington, Seattle; Anireddy Reddy, Colorado State University, Fort Collins; René Schneider, Max-Planck-Institute, Postdam-Golm, Germany; Louis Tisa, University of New Hampshire, Durham; Luis Vidali, Worcester Polytechnical Institute, Worcester, MA; Patricia Wadsworth, University of Massachusetts, Amherst; Lawrence Winship, Hampshire College, Amherst, MA; Shu-Zon Wu, University of Massachusetts, Amherst; and Dahong Zhang, Oregon State University, Corvallis. I also thank Mike Blatt, Editor-in-Chief of Plant Physiology, for inviting me to write a Founders’ Review. Finally, I thank the National Institutes of Health, the U.S. Department of Agriculture, and especially the National Science Foundation for the many years of prior research support they provided throughout my career.
Glossary
- MT
microtubule
- EF
elongation factor
- MF
microfilament
- ER
endoplasmic reticulum
References
- Abdel-Ghany SE, Day IS, Simmons MP, Kugrens P, Reddy ASN (2005) Origin and evolution of kinesin-like calmodulin-binding protein. Plant Physiol 138: 1711–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Abied M, Golomb L, Belausov E, Huang S, Geiger B, Kam Z, Staiger CJ, Sadot E (2006) Identification of plant cytoskeleton-interacting proteins by screening for actin stress fiber association in mammalian fibroblasts. Plant J 48: 367–379 [DOI] [PubMed] [Google Scholar]
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2008) Molecular Biology of the Cell, Ed 5. Garland Science, New York [Google Scholar]
- Allwood EG, Anthony RG, Smertenko AP, Reichelt S, Drøbak BK, Doonan JH, Weeds AG, Hussey PJ (2002) Regulation of the pollen-specific actin-depolymerizing factor LlADF1. Plant Cell 14: 2915–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allwood EG, Smertenko AP, Hussey PJ (2001) Phosphorylation of plant actin-depolymerising factor by calmodulin-like domain protein kinase. FEBS Lett 499: 97–100 [DOI] [PubMed] [Google Scholar]
- Augustine RC, Pattavina KA, Tüzel E, Vidali L, Bezanilla M (2011) Actin interacting protein1 and actin depolymerizing factor drive rapid actin dynamics in Physcomitrella patens. Plant Cell 23: 3696–3710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine RC, Vidali L, Kleinman KP, Bezanilla M (2008) Actin depolymerizing factor is essential for viability in plants, and its phosphoregulation is important for tip growth. Plant J 54: 863–875 [DOI] [PubMed] [Google Scholar]
- Avisar D, Abu-Abied M, Belausov E, Sadot E (2012) Myosin XIK is a major player in cytoplasm dynamics and is regulated by two amino acids in its tail. J Exp Bot 63: 241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao C, Wang J, Zhang R, Zhang B, Zhang H, Zhou Y, Huang S (2012) Arabidopsis VILLIN2 and VILLIN3 act redundantly in sclerenchyma development via bundling of actin filaments. Plant J 71: 962–975 [DOI] [PubMed] [Google Scholar]
- Baum G, Long JC, Jenkins GI, Trewavas AJ (1999) Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+. Proc Natl Acad Sci USA 96: 13554–13559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Ernstrom GG, Nix P, Davis MW, Jorgensen EM (2008) Protons act as a transmitter for muscle contraction in C. elegans. Cell 132: 149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernander R, Lind AE, Ettema TJ (2011) An archaeal origin for the actin cytoskeleton: implications for eukaryogenesis. Commun Integr Biol 4: 664–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR (2004) A proposed mechanism for cell polarization with no external cues. Cell Motil Cytoskeleton 58: 96–103 [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Painter WB, Chen H, Minamide LS, Abe H, Bamburg JR (2000) Intracellular pH modulation of ADF/cofilin proteins. Cell Motil Cytoskeleton 47: 319–336 [DOI] [PubMed] [Google Scholar]
- Berridge MJ. (2006) Calcium microdomains: organization and function. Cell Calcium 40: 405–412 [DOI] [PubMed] [Google Scholar]
- Bharat TA, Murshudov GN, Sachse C, Löwe J (2015) Structures of actin-like ParM filaments show architecture of plasmid-segregating spindles. Nature 523: 106–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone NW. (2015) The impact of mitochondrial endosymbiosis on the evolution of calcium signaling. Cell Calcium 57: 133–139 [DOI] [PubMed] [Google Scholar]
- Bobinnec Y, Marcaillou C, Morin X, Debec A (2003) Dynamics of the endoplasmic reticulum during early development of Drosophila melanogaster. Cell Motil Cytoskeleton 54: 217–225 [DOI] [PubMed] [Google Scholar]
- Bou Daher F, van Oostende C, Geitmann A (2011) Spatial and temporal expression of actin depolymerizing factors ADF7 and ADF10 during male gametophyte development in Arabidopsis thaliana. Plant Cell Physiol 52: 1177–1192 [DOI] [PubMed] [Google Scholar]
- Bove J, Vaillancourt B, Kroeger J, Hepler PK, Wiseman PW, Geitmann A (2008) Magnitude and direction of vesicle dynamics in growing pollen tubes using spatiotemporal image correlation spectroscopy and fluorescence recovery after photobleaching. Plant Physiol 147: 1646–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowser J, Reddy ASN (1997) Localization of a kinesin-like calmodulin-binding protein in dividing cells of Arabidopsis and tobacco. Plant J 12: 1429–1437 [DOI] [PubMed] [Google Scholar]
- Brewbaker JL, Kwack BH (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot 50: 859–865 [Google Scholar]
- Bürstenbinder K, Savchenko T, Müller J, Adamson AW, Stamm G, Kwong R, Zipp BJ, Dinesh DC, Abel S (2013) Arabidopsis calmodulin-binding protein IQ67-domain 1 localizes to microtubules and interacts with kinesin light chain-related protein-1. J Biol Chem 288: 1871–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick LD, Koepf EK, Grimes J, Jones EY, Stuart DI, McLaughlin PJ, Robinson RC (1997) The crystal structure of plasma gelsolin: implications for actin severing, capping, and nucleation. Cell 90: 661–670 [DOI] [PubMed] [Google Scholar]
- Buschmann H, Dols J, Kopischke S, Peña EJ, Andrade-Navarro MA, Heinlein M, Szymanski DB, Zachgo S, Doonan JH, Lloyd CW (2015) Arabidopsis KCBP interacts with AIR9 but stays in the cortical division zone throughout mitosis via its MyTH4-FERM domain. J Cell Sci 128: 2033–2046 [DOI] [PubMed] [Google Scholar]
- Cai G, Parrotta L, Cresti M (2015) Organelle trafficking, the cytoskeleton, and pollen tube growth. J Integr Plant Biol 57: 63–78 [DOI] [PubMed] [Google Scholar]
- Campanoni P, Blatt MR (2007) Membrane trafficking and polar growth in root hairs and pollen tubes. J Exp Bot 58: 65–74 [DOI] [PubMed] [Google Scholar]
- Carballido-López R. (2006) The bacterial actin-like cytoskeleton. Microbiol Mol Biol Rev 70: 888–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cárdenas L, Lovy-Wheeler A, Kunkel JG, Hepler PK (2008) Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol 146: 1611–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D (1997) Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol 136: 1307–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Pantaloni D, Korn ED (1986) Fluorescence measurements of the binding of cations to high-affinity and low-affinity sites on ATP-G-actin. J Biol Chem 261: 10778–10784 [PubMed] [Google Scholar]
- Case RM, Eisner D, Gurney A, Jones O, Muallem S, Verkhratsky A (2007) Evolution of calcium homeostasis: from birth of the first cell to an omnipresent signalling system. Cell Calcium 42: 345–350 [DOI] [PubMed] [Google Scholar]
- Certal AC, Almeida RB, Carvalho LM, Wong E, Moreno N, Michard E, Carneiro J, Rodriguéz-Léon J, Wu HM, Cheung AY, et al. (2008) Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. Plant Cell 20: 614–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Chakrabarti G (2014) Dimethyl sulphoxide and Ca2+ stimulate assembly of Vibrio cholerae FtsZ. Biochimie 105: 64–75 [DOI] [PubMed] [Google Scholar]
- Chebli Y, Kroeger J, Geitmann A (2013) Transport logistics in pollen tubes. Mol Plant 6: 1037–1052 [DOI] [PubMed] [Google Scholar]
- Chen BC, Legant WR, Wang K, Shao L, Milkie DE, Davidson MW, Janetopoulos C, Wu XS, Hammer JA 3rd, Liu Z, et al. (2014) Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution. Science 346: 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Wong EI, Vidali L, Estavillo A, Hepler PK, Wu HM, Cheung AY (2002) The regulation of actin organization by actin-depolymerizing factor in elongating pollen tubes. Plant Cell 14: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Duan QH, Costa SS, de Graaf BH, Di Stilio VS, Feijó J, Wu HM (2008) The dynamic pollen tube cytoskeleton: live cell studies using actin-binding and microtubule-binding reporter proteins. Mol Plant 1: 686–702 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2007) Structural and functional compartmentalization in pollen tubes. J Exp Bot 58: 75–82 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu HM (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 59: 547–572 [DOI] [PubMed] [Google Scholar]
- Choe H, Burtnick LD, Mejillano M, Yin HL, Robinson RC, Choe S (2002) The calcium activation of gelsolin: insights from the 3A structure of the G4-G6/actin complex. J Mol Biol 324: 691–702 [DOI] [PubMed] [Google Scholar]
- Choi WG, Toyota M, Kim SH, Hilleary R, Gilroy S (2014) Salt stress-induced Ca2+ waves are associated with rapid, long-distance root-to-shoot signaling in plants. Proc Natl Acad Sci USA 111: 6497–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary AL, Gunning BES, Wasteneys GO, Hepler PK (1992) Microtubule and F-actin dynamics at the division site in living Tradescantia stamen hair cells. J Cell Sci 103: 977–988 [Google Scholar]
- Cole RA, Fowler JE (2006) Polarized growth: maintaining focus on the tip. Curr Opin Plant Biol 9: 579–588 [DOI] [PubMed] [Google Scholar]
- Collings DA. (2008) Crossed-wires: interactions and cross-talk between the microtubule and microfilament networks in plants. Plant Cell Monogr 11: 47–79 [Google Scholar]
- Craddock C, Lavagi I, Yang Z (2012) New insights into Rho signaling from plant ROP/Rac GTPases. Trends Cell Biol 22: 492–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyr RJ. (1991) Calcium/calmodulin affects microtubule stability in lysed protoplasts. J Cell Sci 100: 311–317 [Google Scholar]
- Deavours BE, Reddy ASN, Walker RA (1998) Ca2+/calmodulin regulation of the Arabidopsis kinesin-like calmodulin-binding protein. Cell Motil Cytoskeleton 40: 408–416 [DOI] [PubMed] [Google Scholar]
- Decoursey TE. (2003) Voltage-gated proton channels and other proton transfer pathways. Physiol Rev 83: 475–579 [DOI] [PubMed] [Google Scholar]
- Derman AI, Becker EC, Truong BD, Fujioka A, Tucey TM, Erb ML, Patterson PC, Pogliano J (2009) Phylogenetic analysis identifies many uncharacterized actin-like proteins (Alps) in bacteria: regulated polymerization, dynamic instability and treadmilling in Alp7A. Mol Microbiol 73: 534–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MR. (2015) Explaining the “pulse of protoplasm”: the search for molecular mechanisms of protoplasmic streaming. J Integr Plant Biol 57: 14–22 [DOI] [PubMed] [Google Scholar]
- Ding B, Turgeon R, Parthasarathy MV (1991a) Microfilament organization and distribution in freeze substituted tobacco plant tissues. Protoplasma 165: 96–105 [Google Scholar]
- Ding B, Turgeon R, Parthasarathy MV (1991b) Microfilaments in the preprophase band of freeze substituted tobacco root cells. Protoplasma 165: 209–211 [Google Scholar]
- Docampo R, Moreno SNJ (2011) Acidocalcisomes. Cell Calcium 50: 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez DC. (2004) Calcium signalling in bacteria. Mol Microbiol 54: 291–297 [DOI] [PubMed] [Google Scholar]
- Domínguez DC, Guragain M, Patrauchan M (2015) Calcium binding proteins and calcium signaling in prokaryotes. Cell Calcium 57: 151–165 [DOI] [PubMed] [Google Scholar]
- Dong CH, Xia GX, Hong Y, Ramachandran S, Kost B, Chua NH (2001) ADF proteins are involved in the control of flowering and regulate F-actin organization, cell expansion, and organ growth in Arabidopsis. Plant Cell 13: 1333–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Pei W, Haiyun R (2012) Actin fringe is correlated with tip growth velocity of pollen tubes. Mol Plant 5: 1160–1162 [DOI] [PubMed] [Google Scholar]
- Donovan KW, Bretscher A (2015) Head-to-tail regulation is critical for the in vivo function of myosin V. J Cell Biol 209: 359–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle RF, York AL (2002) Bacterial actins? An evolutionary perspective. BioEssays 24: 293–296 [DOI] [PubMed] [Google Scholar]
- Dumetz AC, Chockla AM, Kaler EW, Lenhoff AM (2008) Effects of pH on protein-protein interactions and implications for protein phase behavior. Biochim Biophys Acta 1784: 600–610 [DOI] [PubMed] [Google Scholar]
- Durso NA, Cyr RJ (1994) A calmodulin-sensitive interaction between microtubules and a higher plant homolog of elongation factor-1 α. Plant Cell 6: 893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymek EE, Goduti D, Kramer T, Smith EF (2006) A kinesin-like calmodulin-binding protein in Chlamydomonas: evidence for a role in cell division and flagellar functions. J Cell Sci 119: 3107–3116 [DOI] [PubMed] [Google Scholar]
- Edel KH, Kudla J (2015) Increasing complexity and versatility: how the calcium signaling toolkit was shaped during plant land colonization. Cell Calcium 57: 231–246 [DOI] [PubMed] [Google Scholar]
- Erickson HP. (2007) Evolution of the cytoskeleton. BioEssays 29: 668–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson HP, Anderson DE, Osawa M (2010) FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol Mol Biol Rev 74: 504–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettema TJ, Lindås AC, Bernander R (2011) An actin-based cytoskeleton in archaea. Mol Microbiol 80: 1052–1061 [DOI] [PubMed] [Google Scholar]
- Feijó JA, Sainhas J, Hackett GR, Kunkel JG, Hepler PK (1999) Growing pollen tubes possess a constitutive alkaline band in the clear zone and a growth-dependent acidic tip. J Cell Biol 144: 483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle HH. (2001) pH: signal and messenger in plant cells. Plant Biol 3: 577–591 [Google Scholar]
- Fisher DD, Cyr RJ (1993) Calcium levels affect the ability to immunolocalize calmodulin to cortical microtubules. Plant Physiol 103: 543–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furt F, Liu YC, Bibeau JP, Tüzel E, Vidali L (2013) Apical myosin XI anticipates F-actin during polarized growth of Physcomitrella patens cells. Plant J 73: 417–428 [DOI] [PubMed] [Google Scholar]
- Galkin VE, VanLoock MS, Orlova A, Egelman EH (2002) A new internal mode in F-actin helps explain the remarkable evolutionary conservation of actin’s sequence and structure. Curr Biol 12: 570–575 [DOI] [PubMed] [Google Scholar]
- Ganguly A, Dixit R (2013) Mechanisms for regulation of plant kinesins. Curr Opin Plant Biol 16: 704–709 [DOI] [PubMed] [Google Scholar]
- Garner EC, Campbell CS, Mullins RD (2004) Dynamic instability in a DNA-segregating prokaryotic actin homolog. Science 306: 1021–1025 [DOI] [PubMed] [Google Scholar]
- Groigno L, Whitaker M (1998) An anaphase calcium signal controls chromosome disjunction in early sea urchin embryos. Cell 92: 193–204 [DOI] [PubMed] [Google Scholar]
- Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z (2005) A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol 169: 127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungabissoon RA, Jiang CJ, Drøbak BK, Maciver SK, Hussey PJ (1998) Interaction of maize actin-depolymerising factor with actin and phosphoinositides and its inhibition of plant phospholipase C. Plant J 16: 689–696 [Google Scholar]
- Hamada T. (2014) Microtubule organization and microtubule-associated proteins in plant cells. Int Rev Cell Mol Biol 312: 1–52 [DOI] [PubMed] [Google Scholar]
- Hamel LP, Sheen J, Séguin A (2014) Ancient signals: comparative genomics of green plant CDPKs. Trends Plant Sci 19: 79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardham AR, Green PB, Lang JM (1980) Reorganization of cortical microtubules and cellulose deposition during leaf formation in Graptopetalum paraguayense. Planta 149: 181–195 [DOI] [PubMed] [Google Scholar]
- Harold FM. (2014) In Search of Cell History: The Evolution of Life’s Building Blocks. University of Chicago Press, Chicago [Google Scholar]
- Hashimoto K, Kudla J (2011) Calcium decoding mechanisms in plants. Biochimie 93: 2054–2059 [DOI] [PubMed] [Google Scholar]
- Hepler PK. (1980) Membranes in the mitotic apparatus of barley cells. J Cell Biol 86: 490–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK. (1982) Endoplasmic reticulum in the formation of the cell plate and plasmodesmata. Protoplasma 111: 121–133 [Google Scholar]
- Hepler PK. (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17: 2142–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Callaham DA (1987) Free calcium increases during anaphase in stamen hair cells of Tradescantia. J Cell Biol 105: 2137–2143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Jackson WT (1968) Microtubules and early stages of cell-plate formation in the endosperm of Haemanthus katherinae Baker. J Cell Biol 38: 437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler PK, Lovy-Wheeler A, McKenna ST, Kunkel JG (2006) Ions and pollen tube growth. Plant Cell Monogr 3: 47–69 [Google Scholar]
- Hepler PK, Palevitz BA (1974) Microtubules and microfilaments. Annu Rev Plant Physiol 25: 309–362 [Google Scholar]
- Hepler PK, Wayne RO (1985) Calcium and plant development. Annu Rev Plant Physiol 36: 379–439 [Google Scholar]
- Hepler PK, Winship LJ (2015) The pollen tube clear zone: clues to the mechanism of polarized growth. J Integr Plant Biol 57: 79–92 [DOI] [PubMed] [Google Scholar]
- Hepler PK, Wolniak SM (1984) Membranes in the mitotic apparatus: their structure and function. Int Rev Cytol 90: 169–238 [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Brownlee C (2004) The generation of Ca2+ signals in plants. Annu Rev Plant Biol 55: 401–427 [DOI] [PubMed] [Google Scholar]
- Ho CM, Hotta T, Guo F, Roberson RW, Lee YR, Liu B (2011) Interaction of antiparallel microtubules in the phragmoplast is mediated by the microtubule-associated protein MAP65-3 in Arabidopsis. Plant Cell 23: 2909–2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Keynes RD (1957) Movements of labelled calcium in squid giant axons. J Physiol 138: 253–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Weddle NM, Kim S, Robi A, Parris C, Kunkel JG, Hepler PK (2003) Effect of extracellular calcium, pH and borate on growth oscillations in Lilium formosanum pollen tubes. J Exp Bot 54: 65–72 [DOI] [PubMed] [Google Scholar]
- Huang S, Blanchoin L, Chaudhry F, Franklin-Tong VE, Staiger CJ (2004) A gelsolin-like protein from Papaver rhoeas pollen (PrABP80) stimulates calcium-regulated severing and depolymerization of actin filaments. J Biol Chem 279: 23364–23375 [DOI] [PubMed] [Google Scholar]
- Huang S, Qu X, Zhang R (2015) Plant villins: versatile actin regulatory proteins. J Integr Plant Biol 57: 40–49 [DOI] [PubMed] [Google Scholar]
- Ingerson-Mahar M, Gitai Z (2012) A growing family: the expanding universe of the bacterial cytoskeleton. FEMS Microbiol Rev 36: 256–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson E, Yamada M, Vale RD, Goshima G (2015) Clustering of a kinesin-14 motor enables processive retrograde microtubule-based transport in plants. Nat Plants 1: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens M, Hepler LH, Rivers BA, Hepler PK (1994) Bapta-calcium buffers modulate cell plate formation in stamen hairs of Tradescantia: evidence for calcium gradients. Protoplasma 183: 86–99 [Google Scholar]
- Kakimoto T, Shibaoka H (1988) Cytoskeletal ultrastructure of phragmoplast-nuclei complexes isolated from cultured tobacco cells. Protoplasma (Suppl 2) 95–103 [Google Scholar]
- Kashiyama T, Kimura N, Mimura T, Yamamoto K (2000) Cloning and characterization of a myosin from characean alga, the fastest motor protein in the world. J Biochem 127: 1065–1070 [DOI] [PubMed] [Google Scholar]
- Kato M, Aoyama T, Maeshima M (2013) The Ca2+-binding protein PCaP2 located on the plasma membrane is involved in root hair development as a possible signal transducer. Plant J 74: 690–700 [DOI] [PubMed] [Google Scholar]
- Kato M, Nagasaki-Takeuchi N, Ide Y, Maeshima M (2010) An Arabidopsis hydrophilic Ca2+-binding protein with a PEVK-rich domain, PCaP2, is associated with the plasma membrane and interacts with calmodulin and phosphatidylinositol phosphates. Plant Cell Physiol 51: 366–379 [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Nakayama S, Kretsinger RH (1998) Classification and evolution of EF-hand proteins. Biometals 11: 277–295 [DOI] [PubMed] [Google Scholar]
- Kazmierczak J, Kempe S, Kremer B (2013) Calcium in the early evolution of living systems: a biohistorical approach. Curr Org Chem 17: 1738–1750 [Google Scholar]
- Kerber ML, Cheney RE (2011) Myosin-X: a MyTH-FERM myosin at the tips of filopodia. J Cell Sci 124: 3733–3741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey YM, Wessells NK (1976) Localization of actin filaments in internodal cells of characean algae: a scanning and transmission electron microscope study. J Cell Biol 68: 264–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana P, Henty JL, Huang S, Staiger AM, Blanchoin L, Staiger CJ (2010) Arabidopsis VILLIN1 and VILLIN3 have overlapping and distinct activities in actin bundle formation and turnover. Plant Cell 22: 2727–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuyama M, Tazawa M (1983) Transient increase in intracellular Ca2+ during excitation of tonoplast-free Chara cells. Protoplasma 117: 62–67 [Google Scholar]
- Klahre U, Friederich E, Kost B, Louvard D, Chua NH (2000) Villin-like actin-binding proteins are expressed ubiquitously in Arabidopsis. Plant Physiol 122: 35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AE, Kendrick-Jones J (1993) A myosin-like protein from a higher plant. J Mol Biol 231: 148–154 [DOI] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352: 524–526 [DOI] [PubMed] [Google Scholar]
- Kohno T, Shimmen T (1987) Ca2+-induced fragmentation of actin filaments in pollen tubes. Protoplasma 141: 177–179 [Google Scholar]
- Kovar DR, Drøbak BK, Staiger CJ (2000) Maize profilin isoforms are functionally distinct. Plant Cell 12: 583–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krementsov DN, Krementsova EB, Trybus KM (2004) Myosin V: regulation by calcium, calmodulin, and the tail domain. J Cell Biol 164: 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichevsky A, Kozlovsky SV, Tian GW, Chen MH, Zaltsman A, Citovsky V (2007) How pollen tubes grow. Dev Biol 303: 405–420 [DOI] [PubMed] [Google Scholar]
- Kroeger JH, Daher FB, Grant M, Geitmann A (2009) Microfilament orientation constrains vesicle flow and spatial distribution in growing pollen tubes. Biophys J 97: 1822–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla J, Batistic O, Hashimoto K (2010) Calcium signals: the lead currency of plant information processing. Plant Cell 22: 541–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancelle SA, Cresti M, Hepler PK (1987) Ultrastructure of the cytoskeleton in freeze-substituted pollen tubes of Nicotiana alata. Protoplasma 140: 141–150 [Google Scholar]
- Lancelle SA, Hepler PK (1991) Association of actin with cortical microtubules revealed by immunogold localization in Nicotiana pollen tubes. Protoplasma 165: 167–172 [Google Scholar]
- Lancelle SA, Hepler PK (1992) Ultrastructure of freeze-substituted pollen tubes of Lilium longiflorum. Protoplasma 167: 215–230 [Google Scholar]
- Lazzaro MD, Marom EY, Reddy ASN (2013) Polarized cell growth, organelle motility, and cytoskeletal organization in conifer pollen tube tips are regulated by KCBP, the calmodulin-binding kinesin. Planta 238: 587–597 [DOI] [PubMed] [Google Scholar]
- Lee D, Polisensky DH, Braam J (2005) Genome-wide identification of touch- and darkness-regulated Arabidopsis genes: a focus on calmodulin-like and XTH genes. New Phytol 165: 429–444 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Szumlanski A, Nielsen E, Yang Z (2008) Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J Cell Biol 181: 1155–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YR, Liu B (2013) The rise and fall of the phragmoplast microtubule array. Curr Opin Plant Biol 16: 757–763 [DOI] [PubMed] [Google Scholar]
- Lee YRJ, Giang HM, Liu B (2001) A novel plant kinesin-related protein specifically associates with the phragmoplast organelles. Plant Cell 13: 2427–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre B, Arango M, Oufattole M, Crouzet J, Purnelle B, Boutry M (2005) Identification of a Nicotiana plumbaginifolia plasma membrane H+-ATPase gene expressed in the pollen tube. Plant Mol Biol 58: 775–787 [DOI] [PubMed] [Google Scholar]
- Li J, Blanchoin L, Staiger CJ (2015) Signaling to actin stochastic dynamics. Annu Rev Plant Biol 66: 415–440 [DOI] [PubMed] [Google Scholar]
- Li J, Wang X, Qin T, Zhang Y, Liu X, Sun J, Zhou Y, Zhu L, Zhang Z, Yuan M, et al. (2011) MDP25, a novel calcium regulatory protein, mediates hypocotyl cell elongation by destabilizing cortical microtubules in Arabidopsis. Plant Cell 23: 4411–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JF, Nebenführ A (2007) Organelle targeting of myosin XI is mediated by two globular tail subdomains with separate cargo binding sites. J Biol Chem 282: 20593–20602 [DOI] [PubMed] [Google Scholar]
- Li XD, Jung HS, Wang Q, Ikebe R, Craig R, Ikebe M (2008) The globular tail domain puts on the brake to stop the ATPase cycle of myosin Va. Proc Natl Acad Sci USA 105: 1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hsin J, Zhao L, Cheng Y, Shang W, Huang KC, Wang HW, Ye S (2013) FtsZ protofilaments use a hinge-opening mechanism for constrictive force generation. Science 341: 392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Kunkel JG, Allwood EG, Hussey PJ, Hepler PK (2006) Oscillatory increases in alkalinity anticipate growth and may regulate actin dynamics in pollen tubes of lily. Plant Cell 18: 2182–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovy-Wheeler A, Wilsen KL, Baskin TI, Hepler PK (2005) Enhanced fixation reveals the apical cortical fringe of actin filaments as a consistent feature of the pollen tube. Planta 221: 95–104 [DOI] [PubMed] [Google Scholar]
- Marcum JM, Dedman JR, Brinkley BR, Means AR (1978) Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci USA 75: 3771–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy DW, Kovar DR, Staiger CJ (2001) Actin and actin-binding proteins in higher plants. Protoplasma 215: 89–104 [DOI] [PubMed] [Google Scholar]
- McIntosh JR, Volkov V, Ataullakhanov FI, Grishchuk EL (2010) Tubulin depolymerization may be an ancient biological motor. J Cell Sci 123: 3425–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna ST, Kunkel JG, Bosch M, Rounds CM, Vidali L, Winship LJ, Hepler PK (2009) Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell 21: 3026–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael CM, Bednarek SY (2013) Cytoskeletal and membrane dynamics during higher plant cytokinesis. New Phytol 197: 1039–1057 [DOI] [PubMed] [Google Scholar]
- Meagher RB, McKinney EC, Kandasamy MK (1999) Isovariant dynamics expand and buffer the responses of complex systems: the diverse plant actin gene family. Plant Cell 11: 995–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli MA, Créton R, Jaffe LF, Robinson KR (2000) Periodic increases in elongation rate precede increases in cytosolic Ca2+ during pollen tube growth. Dev Biol 222: 84–98 [DOI] [PubMed] [Google Scholar]
- Miller DD, Callaham DA, Gross DJ, Hepler PK (1992) Free Ca2+ gradient in growing pollen tubes of Lilium. J Cell Sci 101: 7–12 [Google Scholar]
- Narasimhulu SB, Kao YL, Reddy ASN (1997) Interaction of Arabidopsis kinesin-like calmodulin-binding protein with tubulin subunits: modulation by Ca2+-calmodulin. Plant J 12: 1139–1149 [DOI] [PubMed] [Google Scholar]
- Narasimhulu SB, Reddy ASN (1998) Characterization of microtubule binding domains in the Arabidopsis kinesin-like calmodulin binding protein. Plant Cell 10: 957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MR, Chazin WJ (1998) Structures of EF-hand Ca2+-binding proteins: diversity in the organization, packing and response to Ca2+ binding. Biometals 11: 297–318 [DOI] [PubMed] [Google Scholar]
- Nibau C, Wu HM, Cheung AY (2006) RAC/ROP GTPases: ‘hubs’ for signal integration and diversification in plants. Trends Plant Sci 11: 309–315 [DOI] [PubMed] [Google Scholar]
- Oda Y, Fukuda H (2013) Spatial organization of xylem cell walls by ROP GTPases and microtubule-associated proteins. Curr Opin Plant Biol 16: 743–748 [DOI] [PubMed] [Google Scholar]
- Oppenheimer DG, Pollock MA, Vacik J, Szymanski DB, Ericson B, Feldmann K, Marks MD (1997) Essential role of a kinesin-like protein in Arabidopsis trichome morphogenesis. Proc Natl Acad Sci USA 94: 6261–6266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osteryoung KW, Pyke KA (2014) Division and dynamic morphology of plastids. Annu Rev Plant Biol 65: 443–472 [DOI] [PubMed] [Google Scholar]
- Palevitz BA. (1987) Actin in the preprophase band of Allium cepa. J Cell Biol 104: 1515–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palevitz BA, Ash JF, Hepler PK (1974) Actin in the green alga, Nitella. Proc Natl Acad Sci USA 71: 363–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG. (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52: 817–845 [DOI] [PubMed] [Google Scholar]
- Paredez AR, Assaf ZJ, Sept D, Timofejeva L, Dawson SC, Wang CJ, Cande WZ (2011) An actin cytoskeleton with evolutionarily conserved functions in the absence of canonical actin-binding proteins. Proc Natl Acad Sci USA 108: 6151–6156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry H, McDougall A, Whitaker M (2005) Microdomains bounded by endoplasmic reticulum segregate cell cycle calcium transients in syncytial Drosophila embryos. J Cell Biol 171: 47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry H, McDougall A, Whitaker M (2006) Endoplasmic reticulum generates calcium signalling microdomains around the nucleus and spindle in syncytial Drosophila embryos. Biochem Soc Trans 34: 385–388 [DOI] [PubMed] [Google Scholar]
- Pickett-Heaps JD, Northcote DH (1966) Organization of microtubules and endoplasmic reticulum during mitosis and cytokinesis in wheat meristems. J Cell Sci 1: 109–120 [DOI] [PubMed] [Google Scholar]
- Pierson ES, Kengen HMP, Derksen J (1989) Microtubules and actin filaments co-localize in pollen tubes of Nicotiana tabacum L. and Lilium longiflorum Thunb. Protoplasma 150: 75–77 [Google Scholar]
- Pierson ES, Miller DD, Callaham DA, van Aken J, Hackett G, Hepler PK (1996) Tip-localized calcium entry fluctuates during pollen tube growth. Dev Biol 174: 160–173 [DOI] [PubMed] [Google Scholar]
- Porta JC, Borgstahl GE (2012) Structural basis for profilin-mediated actin nucleotide exchange. J Mol Biol 418: 103–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss ML, Delmer DP, Liu B (2003) The cotton kinesin-like calmodulin-binding protein associates with cortical microtubules in cotton fibers. Plant Physiol 132: 154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin T, Li J, Yuan M, Mao T (2012) Characterization of the role of calcium in regulating the microtubule-destabilizing activity of MDP25. Plant Signal Behav 7: 708–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin T, Liu X, Li J, Sun J, Song L, Mao T (2014) Arabidopsis microtubule-destabilizing protein 25 functions in pollen tube growth by severing actin filaments. Plant Cell 26: 325–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Yang Z (2011) Rapid tip growth: insights from pollen tubes. Semin Cell Dev Biol 22: 816–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Jiang Y, Chang M, Liu X, Zhang R, Huang S (2014) Organization and regulation of the actin cytoskeleton in the pollen tube. Front Plant Sci 5: 786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford JE, White RG (1998) Localization of a myosin-like protein to plasmodesmata. Plant J 14: 743–750 [DOI] [PubMed] [Google Scholar]
- Rathore KS, Cork RJ, Robinson KR (1991) A cytoplasmic gradient of Ca2+ is correlated with the growth of lily pollen tubes. Dev Biol 148: 612–619 [DOI] [PubMed] [Google Scholar]
- Rato C, Monteiro D, Hepler PK, Malhó R (2004) Calmodulin activity and cAMP signalling modulate growth and apical secretion in pollen tubes. Plant J 38: 887–897 [DOI] [PubMed] [Google Scholar]
- Reddy ASN, Day IS (2011) Microtubule motor proteins in the eukaryotic green lineage: functions and regulation. Adv Plant Biol 2: 119–141 [Google Scholar]
- Reddy ASN, Narasimhulu SB, Safadi F, Golovkin M (1996a) A plant kinesin heavy chain-like protein is a calmodulin-binding protein. Plant J 10: 9–21 [DOI] [PubMed] [Google Scholar]
- Reddy ASN, Safadi F, Narasimhulu SB, Golovkin M, Hu X (1996b) A novel plant calmodulin-binding protein with a kinesin heavy chain motor domain. J Biol Chem 271: 7052–7060 [DOI] [PubMed] [Google Scholar]
- Reddy VS, Day IS, Thomas T, Reddy ASN (2004) KIC, a novel Ca2+ binding protein with one EF-hand motif, interacts with a microtubule motor protein and regulates trichome morphogenesis. Plant Cell 16: 185–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt S, Knight AE, Hodge TP, Baluska F, Samaj J, Volkmann D, Kendrick-Jones J (1999) Characterization of the unconventional myosin VIII in plant cells and its localization at the post-cytokinetic cell wall. Plant J 19: 555–567 [DOI] [PubMed] [Google Scholar]
- Richardson DN, Simmons MP, Reddy ASN (2006) Comprehensive comparative analysis of kinesins in photosynthetic eukaryotes. BMC Genomics 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas ER, Hotton S, Dumais J (2011) Chemically mediated mechanical expansion of the pollen tube cell wall. Biophys J 101: 1844–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds CM, Bezanilla M (2013) Growth mechanisms in tip-growing plant cells. Annu Rev Plant Biol 64: 243–265 [DOI] [PubMed] [Google Scholar]
- Rounds CM, Hepler PK, Winship LJ (2014) The apical actin fringe contributes to localized cell wall deposition and polarized growth in the lily pollen tube. Plant Physiol 166: 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rounds CM, Winship LJ, Hepler PK (2011) Pollen tube energetics: respiration, fermentation and the race to the ovule. Ann Bot Plants 2011: plr019. doi: 10.1093/aobpla/plr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Higaki T, Oda Y, Hayashi T, Hasezawa S (2005) Appearance of actin microfilament ‘twin peaks’ in mitosis and their function in cell plate formation, as visualized in tobacco BY-2 cells expressing GFP-fimbrin. Plant J 44: 595–605 [DOI] [PubMed] [Google Scholar]
- Schneider R, Persson S (2015) Connecting two arrays: the emerging role of actin-microtubule cross-linking motor proteins. Front Plant Sci 6: 415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmen T, Yokota E (1994) Physiological and biochemical aspects of cytoplasmic streaming. Int Rev Cytol 155: 97–139 [Google Scholar]
- Shinkle JR, Jones RL (1988) Inhibition of stem elongation in Cucumis seedlings by blue light requires calcium. Plant Physiol 86: 960–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smertenko AP, Jiang CJ, Simmons NJ, Weeds AG, Davies DR, Hussey PJ (1998) Ser6 in the maize actin-depolymerizing factor, ZmADF3, is phosphorylated by a calcium-stimulated protein kinase and is essential for the control of functional activity. Plant J 14: 187–193 [DOI] [PubMed] [Google Scholar]
- Smirnova EA, Reddy ASN, Bowser J, Bajer AS (1998) Minus end-directed kinesin-like motor protein, Kcbp, localizes to anaphase spindle poles in Haemanthus endosperm. Cell Motil Cytoskeleton 41: 271–280 [DOI] [PubMed] [Google Scholar]
- Snowman BN, Kovar DR, Shevchenko G, Franklin-Tong VE, Staiger CJ (2002) Signal-mediated depolymerization of actin in pollen during the self-incompatibility response. Plant Cell 14: 2613–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Golovkin M, Reddy ASN, Endow SA (1997) In vitro motility of AtKCBP, a calmodulin-binding kinesin protein of Arabidopsis. Proc Natl Acad Sci USA 94: 322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A, Saw JH, Jørgensen SL, Zaremba-Niedzwiedzka K, Martijn J, Lind AE, van Eijk R, Schleper C, Guy L, Ettema TJ (2015) Complex archaea that bridge the gap between prokaryotes and eukaryotes. Nature 521: 173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staehelin LA, Hepler PK (1996) Cytokinesis in higher plants. Cell 84: 821–824 [DOI] [PubMed] [Google Scholar]
- Steinhorst L, Kudla J (2013) Calcium: a central regulator of pollen germination and tube growth. Biochim Biophys Acta 1833: 1573–1581 [DOI] [PubMed] [Google Scholar]
- Sze H, Li X, Palmgren MG (1999) Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 11: 677–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AR, Brownlee C, Wheeler GL (2012) Proton channels in algae: reasons to be excited. Trends Plant Sci 17: 675–684 [DOI] [PubMed] [Google Scholar]
- Tazawa M, Kishimoto U (1968) Cessation of cytoplasmic streaming in Chara internodes during action potential. Plant Cell Physiol 9: 361–368 [Google Scholar]
- Thomas C, Tholl S, Moes D, Dieterle M, Papuga J, Moreau F, Steinmetz A (2009) Actin bundling in plants. Cell Motil Cytoskeleton 66: 940–957 [DOI] [PubMed] [Google Scholar]
- Tian J, Han L, Feng Z, Wang G, Liu W, Ma Y, Yu Y, Kong Z (2015) Orchestration of microtubules and the actin cytoskeleton in trichome cell shape determination by a plant-unique kinesin. eLife 4: e09351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisa LS, Adler J (1995) Cytoplasmic free-Ca2+ level rises with repellents and falls with attractants in Escherichia coli chemotaxis. Proc Natl Acad Sci USA 92: 10777–10781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisa LS, Sekelsky JJ, Adler J (2000) Effects of organic antagonists of Ca2+, Na+, and K+ on chemotaxis and motility of Escherichia coli. J Bacteriol 182: 4856–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Ito K (2015) The molecular mechanism and physiological role of cytoplasmic streaming. Curr Opin Plant Biol 27: 104–110 [DOI] [PubMed] [Google Scholar]
- Tominaga M, Kojima H, Yokota E, Nakamori R, Anson M, Shimmen T, Oiwa K (2012) Calcium-induced mechanical change in the neck domain alters the activity of plant myosin XI. J Biol Chem 287: 30711–30718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Kojima H, Yokota E, Orii H, Nakamori R, Katayama E, Anson M, Shimmen T, Oiwa K (2003) Higher plant myosin XI moves processively on actin with 35 nm steps at high velocity. EMBO J 22: 1263–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valster AH, Hepler PK (1997) Caffeine inhibition of cytokinesis: effect on the phragmoplast cytoskeleton in living Tradescantia stamen hair cells. Protoplasma 196: 155–166 [Google Scholar]
- van den Ent F, Amos LA, Löwe J (2001) Prokaryotic origin of the actin cytoskeleton. Nature 413: 39–44 [DOI] [PubMed] [Google Scholar]
- Verchot-Lubicz J, Goldstein RE (2010) Cytoplasmic streaming enables the distribution of molecules and vesicles in large plant cells. Protoplasma 240: 99–107 [DOI] [PubMed] [Google Scholar]
- Verret F, Wheeler G, Taylor AR, Farnham G, Brownlee C (2010) Calcium channels in photosynthetic eukaryotes: implications for evolution of calcium-based signalling. New Phytol 187: 23–43 [DOI] [PubMed] [Google Scholar]
- Vidali L, Burkart GM, Augustine RC, Kerdavid E, Tüzel E, Bezanilla M (2010) Myosin XI is essential for tip growth in Physcomitrella patens. Plant Cell 22: 1868–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Hepler PK (2001) Actin and pollen tube growth. Protoplasma 215: 64–76 [DOI] [PubMed] [Google Scholar]
- Vidali L, McKenna ST, Hepler PK (2001) Actin polymerization is essential for pollen tube growth. Mol Biol Cell 12: 2534–2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Rounds CM, Hepler PK, Bezanilla M (2009) Lifeact-mEGFP reveals a dynamic apical F-actin network in tip growing plant cells. PLoS ONE 4: e5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidali L, Yokota E, Cheung AY, Shimmen T, Hepler PK (1999) The 135kDa actin-bundling protein from Lilium longiflorum pollen is the plant homologue of villin. Protoplasma 209: 283–291 [Google Scholar]
- Vinogradova MV, Malanina GG, Reddy ASN, Fletterick RJ (2009) Structure of the complex of a mitotic kinesin with its calcium binding regulator. Proc Natl Acad Sci USA 106: 8175–8179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova MV, Malanina GG, Reddy VS, Reddy ASN, Fletterick RJ (2008) Structural dynamics of the microtubule binding and regulatory elements in the kinesin-like calmodulin binding protein. J Struct Biol 163: 76–83 [DOI] [PubMed] [Google Scholar]
- Vos JW, Hepler PK (1998) Calmodulin is uniformly distributed during cell division in living stamen hair cells of Tradescantia virginiana. Protoplasma 201: 158–171 [Google Scholar]
- Vos JW, Safadi F, Reddy ASN, Hepler PK (2000) The kinesin-like calmodulin binding protein is differentially involved in cell division. Plant Cell 12: 979–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Wan AR, Jauh GY (2008) An actin-binding protein, LlLIM1, mediates calcium and hydrogen regulation of actin dynamics in pollen tubes. Plant Physiol 147: 1619–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu L, Liu B, Wang C, Jin L, Zhao Q, Yuan M (2007) Arabidopsis MICROTUBULE-ASSOCIATED PROTEIN18 functions in directional cell growth by destabilizing cortical microtubules. Plant Cell 19: 877–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang B, Gilroy S, Chehab EW, Braam J (2011) CML24 is involved in root mechanoresponses and cortical microtubule orientation in Arabidopsis. J Plant Growth Regul 30: 467–479 [Google Scholar]
- Watkins NJ, Knight MR, Trewavas AJ, Campbell AK (1995) Free calcium transients in chemotactic and non-chemotactic strains of Escherichia coli determined by using recombinant aequorin. Biochem J 306: 865–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg RC. (1972) Microtubule formation in vitro in solutions containing low calcium concentrations. Science 177: 1104–1105 [DOI] [PubMed] [Google Scholar]
- Whitaker M. (2006) Calcium microdomains and cell cycle control. Cell Calcium 40: 585–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker M. (2008) Calcium signalling in early embryos. Philos Trans R Soc Lond B Biol Sci 363: 1401–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstead B, Gull K (2011) The evolution of the cytoskeleton. J Cell Biol 194: 513–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins KA, Bosch M, Haque T, Teng N, Poulter NS, Franklin-Tong VE (2015) Self-incompatibility-induced programmed cell death in field poppy pollen involves dramatic acidification of the incompatible pollen tube cytosol. Plant Physiol 167: 766–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson RE, Ashley CC (1982) Free Ca2+ and cytoplasmic streaming in the alga Chara. Nature 296: 647–650 [DOI] [PubMed] [Google Scholar]
- Wills FL, McCubbin WD, Gimona M, Strasser P, Kay CM (1994) Two domains of interaction with calcium binding proteins can be mapped using fragments of calponin. Protein Sci 3: 2311–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SZ, Bezanilla M (2014) Myosin VIII associates with microtubule ends and together with actin plays a role in guiding plant cell division. eLife 3: 03498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Huang X, Wang T, Zhang Y, Liu Q, Hussey PJ, Ren H (2007) ACTIN BINDING PROTEIN 29 from Lilium pollen plays an important role in dynamic actin remodeling. Plant Cell 19: 1930–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E, Muto S, Shimmen T (1999) Inhibitory regulation of higher-plant myosin by Ca2+ ions. Plant Physiol 119: 231–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E, Muto S, Shimmen T (2000) Calcium-calmodulin suppresses the filamentous actin-binding activity of a 135-kilodalton actin-bundling protein isolated from lily pollen tubes. Plant Physiol 123: 645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota E, Vidali L, Tominaga M, Tahara H, Orii H, Morizane Y, Hepler PK, Shimmen T (2003) Plant 115-kDa actin-filament bundling protein, P-115-ABP, is a homologue of plant villin and is widely distributed in cells. Plant Cell Physiol 44: 1088–1099 [DOI] [PubMed] [Google Scholar]
- Yonezawa N, Nishida E, Sakai H (1985) pH control of actin polymerization by cofilin. J Biol Chem 260: 14410–14412 [PubMed] [Google Scholar]
- Yutin N, Wolf MY, Wolf YI, Koonin EV (2009) The origins of phagocytosis and eukaryogenesis. Biol Direct 4: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Wadsworth P, Hepler PK (1990a) Microtubule dynamics in living dividing plant cells: confocal imaging of microinjected fluorescent brain tubulin. Proc Natl Acad Sci USA 87: 8820–8824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Callaham DA, Hepler PK (1990b) Regulation of anaphase chromosome motion in Tradescantia stamen hair cells by calcium and related signaling agents. J Cell Biol 111: 171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DH, Wadsworth P, Hepler PK (1992) Modulation of anaphase spindle microtubule structure in stamen hair cells of Tradescantia by calcium and related agents. J Cell Sci 102: 79–89 [Google Scholar]
- Zhang H, Qu X, Bao C, Khurana P, Wang Q, Xie Y, Zheng Y, Chen N, Blanchoin L, Staiger CJ, et al. (2010) Arabidopsis VILLIN5, an actin filament bundling and severing protein, is necessary for normal pollen tube growth. Plant Cell 22: 2749–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiao Y, Du F, Cao L, Dong H, Ren H (2011) Arabidopsis VILLIN4 is involved in root hair growth through regulating actin organization in a Ca2+-dependent manner. New Phytol 190: 667–682 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Yang W, Kirberger M, Lee HW, Ayalasomayajula G, Yang JJ (2006) Prediction of EF-hand calcium-binding proteins and analysis of bacterial EF-hand proteins. Proteins 65: 643–655 [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhang Y, Kang E, Xu Q, Wang M, Rui Y, Liu B, Yuan M, Fu Y (2013) MAP18 regulates the direction of pollen tube growth in Arabidopsis by modulating F-actin organization. Plant Cell 25: 851–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonia L, Munnik T (2008) Vesicle trafficking dynamics and visualization of zones of exocytosis and endocytosis in tobacco pollen tubes. J Exp Bot 59: 861–873 [DOI] [PubMed] [Google Scholar]