Abstract
Death receptors of the Tumor Necrosis Factor (TNF) family are found on surface of most cancer cells and their activation typically kills cancer cells through the stimulation of the extrinsic apoptotic pathway. The endogenous ligand for death receptors-4 and -5 (DR4 and DR5) is Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand, TRAIL (Apo2L). Since most untransformed cells are not susceptible to TRAIL-induced apoptosis, death receptor activators have emerged as promising cancer therapeutic agents. One strategy to stimulate death receptors in cancer patients is to use soluble human recombinant TRAIL protein, but this agent has limitations of a short half-life and decoy receptor sequestration. Another strategy that attempted to evade decoy receptor sequestration and to provide improved pharmacokinetic properties was to generate DR4 or DR5 agonist antibodies. The resulting monoclonal agonist antibodies overcame the limitations of short half-life and avoided decoy receptor sequestration, but are limited by activating only one of the two death receptors. Here, we describe a DR4 and DR5 dual agonist produced using Surrobody™ technology that activates both DR4 and DR5 to induce apoptotic death of cancer cells in vitro and in vivo and also avoids decoy receptor sequestration. This fully human anti-DR4/DR5 Surrobody displays superior potency to DR4- and DR5-specific antibodies, even when combined with TRAIL-sensitizing pro-apoptotic agents. Moreover, cancer cells were less likely to acquire resistance to Surrobody than either anti-DR4 or anti-DR5 mono-specific antibodies. Taken together, Surrobody shows promising preclinical pro-apoptotic activity against cancer cells, meriting further exploration of its potential as a novel cancer therapeutic agent.
Keywords: apoptosis, death receptors, antibody therapeutics
INTRODUCTION
Tumor necrosis factor related apoptosis-inducing ligand (TRAIL), also known as Apo2 ligand, belongs to the TNF superfamily of proteins. Many cancer cells have been shown to be sensitive to TRAIL-induced killing, while normal cells are generally resistant, making TRAIL an attractive cancer therapy agent. TRAIL binds to at least five receptors. DR4 (Trail receptor 1) and DR5 (Trail receptor 2) are trans-membrane receptors that have an intracellular death domain (DD) that is essential for recruitment of death-inducing signaling complex (DISC) and subsequent induction of apoptosis via caspase activation. The other three receptors lack the death-inducing activity. DcR1 (decoy receptor 1) and DcR2 (decoy receptor 2) bind TRAIL but lack, or have a truncated death domain, respectively, and are believed to sequester TRAIL and modulate apoptosis triggered by DR4 and DR5. A less studied soluble receptor osteoprotegerin (OpG) has also been shown to bind TRAIL but its role in modulating effects of TRAIL remains poorly understood.
Recombinant human TRAIL activates both DR4 and DR5, but has limited clinical utility because it is rapidly hydrolyzed in blood, resulting in a very short half-life of less than an hour (1–3). In addition, TRAIL can be bound by decoy receptors, which further limits its utility. As an alternative to TRAIL, highly specific agonistic antibodies targeting individual death receptors that avoid decoy receptor binding have been generated and developed as clinical therapeutics (4, 5). These antibodies are generally safe and exhibit substantially improved pharmacokinetic properties compared to TRAIL, with serum half-lives on the order of days, rather than minutes (6–12). However, the specificity of these antibodies has restricted their activity to either DR4 or DR5 and limited their use to cancer cells that express the relevant target receptor. Even with these inherent limitations, some progress has been made with multiple strategies for targeting death receptors in cancer therapy and several agents have been tested in multiple clinical trials, either as single agents or in combination with other chemotherapeutic drugs (13, 14).
To overcome current specificity limitations, we utilized a previously described Surrobody protein scaffold (15) to discover a DR4/5 dual agonist. Surrobodies are derived from a pre-B cell receptor that is composed of a diversified immunoglobulin heavy chain complexed with invariant surrogate light chain that together confer specific high affinity binding to their targets. We screened human Surrobody libraries and discovered a very specific Surrobody with a single heavy chain variable region that binds and activates both DR4 and DR5 with high affinity and potency. The presence of a single heavy chain that is capable of binding both DR4 and DR5 eliminated a common problem of assembly of typical chimeric bispecific antibodies formed from two heavy and two light chains that target two different antigens. Importantly, this dual agonist Surrobody specifically induces apoptosis through both death receptors and, unlike TRAIL, does not bind decoy receptors. In addition, we present preliminary evidence suggesting that Surrobody is less likely to incur resistance compared to monospecific death receptor agonist antibodies. Moreover, we show that this dual agonist Surrobody is capable of synergizing with sensitizing agents to further enhance its clinical potential against apoptosis resistance. Taken together, the overall properties of the death receptor dual agonist Surrobody provides an improvement over monospecific death receptor antibodies, warranting further evaluation as a potential novel agent for cancer therapy.
MATERIALS AND METHODS
Reagents
The death receptor dual agonist Surrobody was provided by Sea Lane Biotechnologies. DR4 antibody was synthesized from patent literature sources (US 7,361,341) and DR5 antibody was synthesized based upon patent literature sources (EP1844077). Surrobody, DR4- and DR5-specific antibodies were expressed in FreeStyle™ 293-F cells (Invitrogen) by transient transfection of heavy chain and light chain plasmids. Cultures were cleared of cells and Surrobody or antibodies purified by a single Protein A chromatography step. The purified Surrobody and antibodies were buffer exchanged into PBS pH 7.4 lacking calcium and magnesium (Gibco), sterile filtered and stored at 2–8 °C. Proteins were analyzed by size exclusion chromatography, reducing and non-reducing SDS-PAGE (Supplementary Figure S1) to assess aggregation, purity and integrity. Anti-DR4-PE, anti-DR5-PE and IgG isotype control antibodies were obtained from eBioscience (cat no. 12-6644-41, 12-9908-41 and 12-4714). Anti-cleaved CASPASE 3 (cat no. 9661), anti-cleaved CASPASE-8 (cat no. 9496), anti-PARP (cat no. 9542) antibodies are from Cell Signaling.
Cell Culture
MDA-MB-231, SKOV3, OVCAR-3, OVCAR-5, MDA-MB-468, Ramos, Jurkat, PC3 and cells were obtained from ATCC and were grown in RPMI medium with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin. HCT116 cells were grown in McCoy media with same additives. For 3D spheroid cell growth, MDA-MB-231 cells were plated at a density of 3,000 cells/well in ultra low attachment 96 well round bottom plates (Corning; cat no. 7007). 267B1 cells were a kind gift from Dr. Dritschilo (16) and were grown in RPMI with 10% fetal bovine serum (FBS), 2 mM glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin. All cell lines were obtained from ATCC and were either cultured less than 6 months or were resuscitated from the expanded ATCC stocks that were less than 6 months old.
Immunoblot Analysis
MDA-MB-231 cells were plated in 12 well plates and the next day they were treated with 1 nM of DR agonist for 3h. In the case of Surrobody, DR4 and DR5 antibodies, protein G was added at 0.5 nM to produce 0.5× molar ratio. 3h post treatment cells were lysed in Laemmli sample buffer and were run on SDS-PAGE, followed by immunoblotting using antibodies directed against cleaved Caspase-3, cleaved Caspase 8, PARP, and actin.
Caspase activity assays
MDA-MB-231 cells were plated at 1500 cells/wells in 40 ul media in 384 well flat bottom plates (Greiner Bio-One). The next day, 10 ul of media containing various concentrations of DR agonists, pre-incubated for 5 min with 0.5× molar ratio of protein G, was added for 3 hrs. For measuring effector caspase activity, 40 ul of the CASPASE-3,7 reagent (Promega; cat. No. G6410) was added directly into wells and the plates were shaken for 30 min at room temperature. Luminescence was measured according to manufacturer’s instructions. For CASPASE-8 activity measurements, 40 ul of CASPASE-Glo 8 reagent was added to wells together with MG132 (as recommended by the manufacturer, at 60 uM final concentration) and the plates were shaken for 1hr at room temperature prior to measuring luminescence.
Cell Viability Assay
For monolayer cultures, all cell lines were plated at 750 cells/384 wells in 50 ul media and the next day the cells were treated with increasing concentrations of DR agonists for 48 hr. To compare relative amounts of cell survival, 30 ul of Cell Titer Glow reagent was added into wells and the plates were shaken for 20 min, followed by detection of luminescence according to manufacturer’s recommendations (Promega Cat. No. G7570). For spheroid cultures, MDA-MB-231 cells were plated at 2000 cells/well in ultralow attachment 96 well plates in 100 ul media. Cells were grown for 8 days, at which time spheroids reached diameters of ~750 nm. Spheroid cultures were treated with DR4, DR5 or death receptor dual agonist at 1000 ng/ml or with TRAIL at 20 ng/ml, which were added to well in 10 uL PBS. Before addition to cells, all the antibodies and Surrobody were incubated for 5 minutes with 0.5× molar ratio of protein G to facilitate clustering. After 2 days, 60 ul of Cell Titer Glow reagent was added and luminescence measurements were recorded. Data were expressed as % survival compared to 100% survival of PBS diluent control.
Generation of DR4, DR5 and TRAIL resistant clones
MDA-MB-231 clones resistant to DR4, DR5, DR4+DR5, and TRAIL were generated by culture for 3 weeks in 10 ml medium containing DR agonists in 100 mm dishes. Briefly, anti-DR4 antibody (500 ng/ml), anti-DR5 antibody (1000 ng/ml), or the combination of anti-DR4 and anti-DR5 (250 ng/ml each), or TRAIL (50 ng/ml) was added to media, which was changed every 3 days for a total of 3 weeks. Before addition to cells, all the monospecific antibodies and Surrobody were incubated for 5 minutes with 0.5× molar ratio of protein G to facilitate clustering. Resistant clones were allowed to grow until they reached sizes visible to the naked eye. The clones were detached using a drop of trysin and recovered cells were transferred into fresh plates and grown under continued selection pressure. At least two clones from each treatment were tested for resistance and the most resistant clone from each treatment was selected for further study.
Tumor xenograft studies
Male, 6 week old nude mice were obtained from Charles River Laboratory. Animals were fed standard mouse chow and tap water, and maintained under 12 h dark/light cycle at 21°C. Male, 6-week-old nude mice were injected bilaterally subcutaneously with 3 × 106 Colo-205 cells in100 uL PBS. When the tumors had reached a volume of ~ 100 mm3, mice were treated intravenously with either PBS or DR4 antibody, DR5 antibody or dual agonist Surrobody at a concentration of 3mg/kg twice a week for a total of four treatments. Tumor dimensions were measured twice weekly using a digital caliper and tumor volume was calculated using the formula (width2 × length)/2. The weight of the mice was also measured twice weekly as a general measurement of health.
Generation of DR4 and DR5 gene knockout cells
pX330 S.Pyogenes Cas9 vector was obtained from Addgene and used to generate vectors containing DR4 or DR5 receptor-targeting guide RNAs according to the previously developed protocols (17). Briefly, pX330 was digested using BbsI, followed by cloning of the annealed oligonucleotides targeting DR4 and DR5 receptor genes. For targeting DR5 receptor 5’-CACCGAGAACGCCCCGGCCGCTTCG-3’ and 5’-AAACCGAAGCGGCCGGGGCGTTCTC-3’ were used, while for targeting DR4 receptor 5’-CACCGCTTCAAGTTTGTCGTCGTCG-3’ and 5’-AAACCGACGACGACAAACTTGAAGC-3’ were used. These vectors were transfected into MDA-MB-231 cells using Lipofectamine 2000 and after five days, the presence of nicking and non-homologous end joining was assayed by PCR amplification of the targeted genomic regions corresponding to either DR4 or DR5 receptors, followed by SURVEYOR assay as previously described (17). Once the presence of nicked clones was confirmed, we selected cells that contained the knockout of DR4 or DR5 by staining with anti-DR4-PE and anti-DR5-PE antibodies, and sorted by FACS into populations that do or do not express the receptors. A pool of sorted DR4 and DR5 knockout cells was used in subsequent cell viability assays.
RESULTS
We assessed a collection of more than 300 unique clones found by panning a human phage displayed Surrobody™ library against either human DR4 or DR5, essentially as described (15, 18) to identify a DR4 and DR5 cross-reactive Surrobody. The resulting cross-reactive clone was reformatted and expressed as a bivalent Surrobody using full-length heavy chains in HEK293 cells as previously described (15). The resulting Surrobody (Supplementary Figure S1 and S2) was transiently expressed at levels yielding routinely nearly 100 mg/L of culture and formed the basis of all subsequent analysis. ELISA-based assays showed the resulting Surrobody bound to both DR4 and DR5, but no binding to DcR1, DcR2, or OPG was detectable, even at concentrations as high as 1000 nM (Supplementary Figure S3). In addition, we developed a “sandwich” ELISA and showed that Surrobody can bind DR4 and DR5 simultaneously (Supplementary Figure S4). This is in sharp contrast to previously reported specific binding of anti-DR4 and anti-DR5 antibodies to DR4 and DR5 receptors, respectively, without any cross-reactivity even at high concentrations (4, 5).
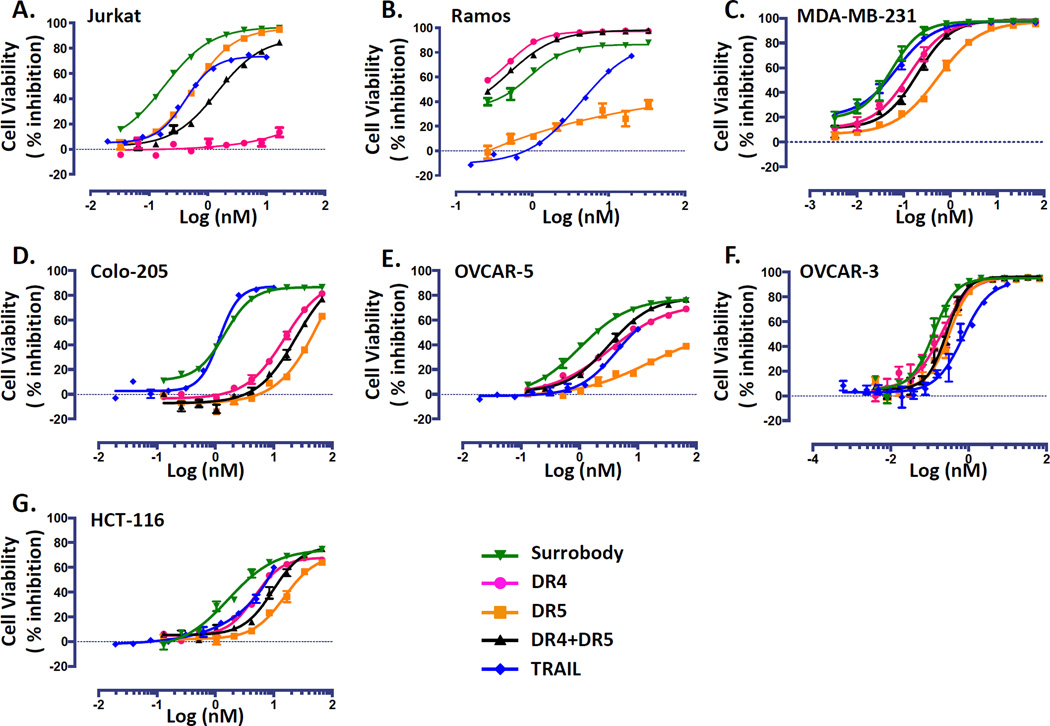
To establish the dual agonist bioactivity of the DR4/DR5-binding Surrobody, we first tested it on Ramos and Jurkat cell lines, which are responsive to only DR4 or DR5 activation, respectively (Figure 1 A, B). As shown, Surrobody was capable of killing both cell lines, similar to TRAIL (Ramos: Surrobody IC50= 0.92 nM vs. TRAIL IC50= 4.27 nM; Jurkat: Surrobody IC50= 0.18 nM vs. TRAIL IC50= 0.83 nM). This is in sharp contrast to previously described DR4 and DR5 monospecific agonist antibodies (patent US 7,361,341 and EP1844077, respectively) that are only capable of killing either Ramos or Jurkat cells, respectively.
Figure 1. Death receptor dual agonist Surrobody is a more potent inducer of tumor cell death than DR4 or DR5 monospecific antibodies.
Various TRAIL-sensitive cancer cell lines were plated at a density of 750 cells/well in 384 wells. The next day cells were cultured with increasing concentrations of monospecific DR4 antibody (pink), monospecific anti-DR5 antibody (orange), the combination of anti-DR4 and anti-DR5 antibodies (black), death receptor dual agonist Surrobody (green), or TRAIL (blue) for 48 hr. Before addition to cells, all the antibodies and Surrobody were incubated for 5 minutes with 0.5× molar ratio of protein G to facilitate clustering. Relative cell viability was estimated from cellular ATP measurements using the Cell Titer Glo reagent (Promega), expressing data as % inhibition of cell survival (mean ± SEM; n= 4). Data are shown for Jurkat (A), Ramos (B), MDA-MB-231 (C), Colo-205 (D), Ovcar-3 (E) and Ovcar-5 (F) and HCT116 (G) cell lines.
Next, we tested the death receptor dual agonist against a broad panel of TRAIL-sensitive human cancer cell lines of multiple tissue origins. The TRAIL-sensitive tumor cell lines we evaluated included the triple negative breast cancer line MDA-MB-231, the colon cancer lines Colo-205 and HCT116, and the ovarian cancer lines Ovcar-3 and Ovcar-5 (Figure 1 C–G). We used flow cytometry for receptor profiling and found that all of these cell lines express both DR4 and DR5 at various levels (Supplementary Table S1 A and B). In general, the tumor cell lines exhibited varied sensitivity to conventional DR4- and DR5-activating antibodies (Figure 1) that did not always correlate with respective DR4 or DR5 expression, consistent with previous reports. Additionally, sensitivity to TRAIL was also varied, with potencies greater than, less than, or comparable to the DR4 or DR5 antibodies. However, in every case, the death receptor dual agonist Surrobody was as potent as or more potent than, the best agonist among TRAIL, anti-DR4 or anti-DR5 monospecific antibodies.
Most interesting was that the death receptor dual agonist Surrobody potency was markedly better than the combined anti-DR4/DR5 antibody mixture. In fact, the mixture of anti-DR4 and anti-DR5 mono-specific antibodies typically resulted in cell killing with potencies intermediate to either anti-DR4 or anti-DR5 alone (Figure 1), rather than producing an additive or synergistic effect.
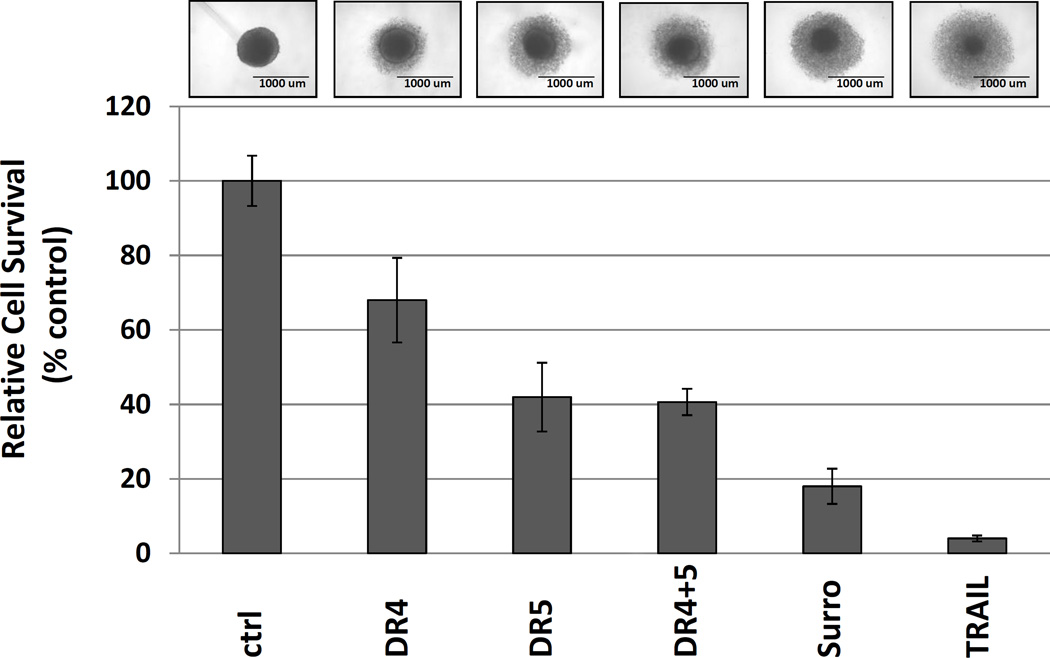
As tumors do not naturally progress as monolayer growths, we tested an in vitro propagated 3-dimensional tumor spheroid culture model as a tumor surrogate to study the effects of death receptor agonists. For this model we selected MDA-MB-231 cells, culturing the cells in ultralow attachment plates, which lack the typical adhesion surface of classical cell culture plastic dishes. Under these growth conditions, the cancer cells form spheres and grow as a single mass. Using this 3D tumor spheroid assay, the dual agonist Surrobody demonstrated significantly more potent suppression of in vitro tumor spheroid growth than either DR4 or DR5 monospecific antibody or even the combination of these antibodies, but slightly less than TRAIL (Figure 2). Again, the combination of monospecific anti-DR4 and anti-DR5 antibodies did not result in additional growth suppression than either antibody alone.
Figure 2. Death receptor dual agonist Surrobody shows superior activity to monospecific anti-DR4 and anti-DR5 antibodies in 3D tumor spheroid culture model.
MDA-MB-231 cells were cultured in ultralow attachment 96 well plates and grown for 8 days to reach a tumor spheroid diameter of ~750 um. The spheroids were treated with indicated DR agonists at 1000 ng/ml (~6nM) or with TRAIL at 20 ng/ml (~1nM) for 2 days and survival assessed by measuring cellular ATP (using Cell Titer Glow reagents). Before addition to the spheroids, all the monospecific antibodies and Surrobody were incubated for 5 minutes with 0.5× molar ratio of protein G to facilitate clustering. Data represent mean ± SD of three spheroids. Representative phase-contrast images of spheroids are shown. Treatment with DR agonists left a core of residual surviving cells surrounded by dead and dying cells.
Based on the previously reported importance of receptor crosslinking to induce receptor clustering (19), we used 0.5× molar ratio of protein G to aid in DR4, DR5 and Surrobody-induced receptor clustering in all of our cell culture assays. To test the importance of receptor clustering, we treated MDA-MB-231 cells with Surrobody that has been cross-linked with increasing molar ratio of protein G and found that it was essential for cell death and that higher receptor clustering increased cell death (Supplementary Figure S5A).
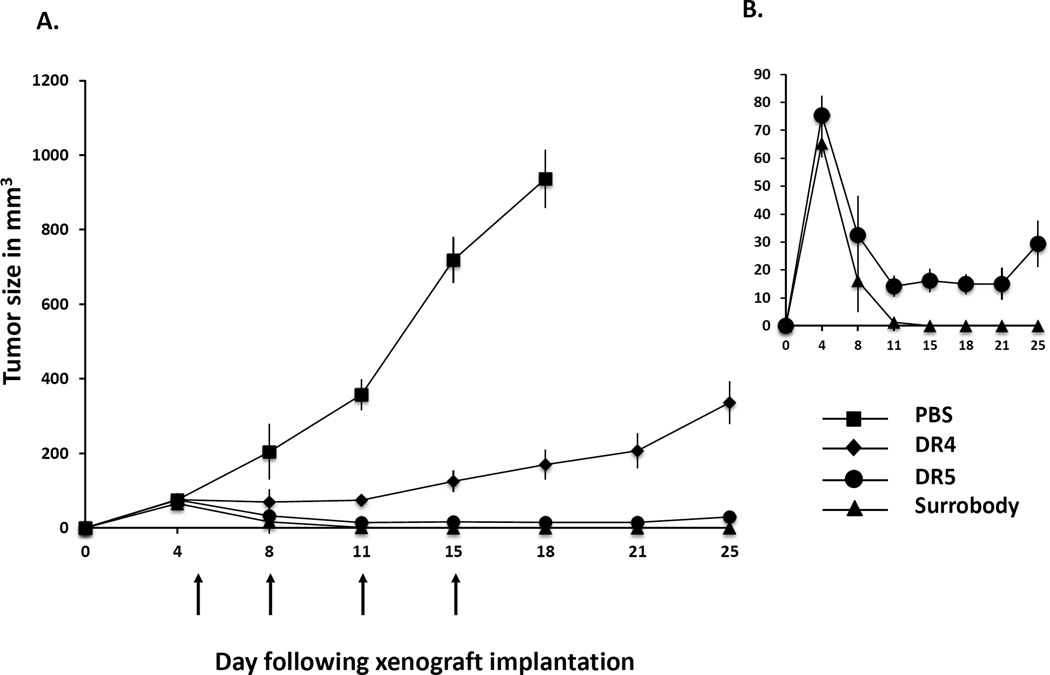
Interestingly, it was previously reported that use of protein G is dispensable in vivo in xenograft studies, since there are other factors such as endogenous Fc receptors that induce death receptor clustering following treatment with anti-DR4 and anti-DR5 antibodies (19–21). To test if Surrobody can induce cell death without protein G clustering in vivo, we compared the anti-tumor activities of DR4 antibody, DR5 antibody and Surrobody in Colo-205 tumor xenograft studies. Briefly, we implanted Colo-205 cells subcutaneously bilaterally into immunocompromised mice and allowed five days for the tumors to grow to approximately 100 mm3 before initiating treatment. Mice received intravenous injection of 3 mg/kg of antibodies twice per week for a total of four treatments. By day 18, the tumors in the PBS vehicle-treated animals reached 1000 mm3 in size that served as an endpoint for termination. In sharp contrast, the tumors of the Surrobody-treated mice began to shrink shortly after the start of treatment and all the tumors completely disappeared by day 15. We found that DR5 antibody and Surrobody displayed similar anti-tumor activity, whereas DR4 antibody reduced the rate of tumor growth, but was unable to eradicate the tumors (Figure 3A). At day 25 following tumor implantation, 10 out of 10 anti-DR4 treated mice still had palpable tumors, with one mouse having a tumor that reached endpoint size of over 1000mm3. Detailed comparison of anti-DR5 antibody and anti-DR4/DR5 Surrobody responses (Figure 3B) showed that 10 out of 10 Surrobody-treated mice achieved complete response with no palpable tumors observed from day 15 until the end of the study. In the case of anti-DR5-treated mice, 3 out of 10 mice showed complete response by day 15, and by the end of the study 4 out of 10 mice showed complete response, while 6 mice still had palpable tumors (Figure 3B).
Figure 3. Death receptor dual agonist inhibits tumor xenograft growth in mice.
A. Three million Colo-205 cells were implanted into flanks of nude mice bilaterally and tumors were allowed to reach approximately 100 mm3 (5 days) before starting intravenous treatment with either PBS vehicle or 3 mg/kg of DR4 antibody, DR5 antibody or dual agonist Surrobody. The mice were treated on day 5, 8, 11 and 15 (indicated by arrows) for a total of four treatments and the tumor size was measured twice weekly. Mean tumor volumes and SEMs are shown for PBS treated (squares), DR4 treated (diamonds), DR5 treated (circles) and Surrobody treated (triangles) mice (10 mice per treatment group). The tumors below the palpitation limit were assigned a volume of 0 mm3. The PBS treated mice were sacrificed at day 18 when the tumors reached the endpoint volume of 1000 mm3, while the mice treated with DR4 antibody, DR5 antibody and Surrobody were sacrificed at 25 days post-implantation. B. The comparison of DR5 antibody- and Surrobody-treated tumor growth curves is shown at a higher volume resolution for easier comparison.
We did not observe any toxicities of Surrobody during the study, inasmuch as the mice looked healthy, did not lose weight, and the histological examination of various tissues at the end of the study did not reveal any toxicities (Supplementary Figure S6). However, given that Surrobody does not bind to mouse death receptor, these observations address non-specific toxicity, but cannot address the toxicity of Surrobody binding to death receptors in normal tissues.
To preliminarily explore the effect of Surrobody on normal cells, we used non-transformed immortalized human prostate epithelial cell line 267B1. We show in Supplementary Figure S5B that higher concentrations of Surrobody were required to induce death of 267B1 cells (IC50 5.83 nM) than any of the tested cancer cell lines (IC50 values ranging from 0.05–1.68 nM). Moreover, Surrobody displayed only a limited ability to induce 267B1 cell death, with a maximum of 40% death at saturating concentrations of antibody. Because the anti-DR4/DR5 Surrobody described here cross-reacts with primate species, preclinical in vivo drug safety studies can be conducted in the future to more definitely assess the impact on normal tissues. The preliminary cell culture data suggest differential sensitivity of transformed vs. non-transformed human cells and thus the possibility of a therapeutic index.
It is highly intriguing that unlike monolayer Colo-205 cells that are more sensitive to anti-DR4 antibody, Colo-205 cells implanted in mice as xenografts displayed higher sensitivity to anti-DR5 antibody. This is similar to our observation that MDA-MB-231 cells display “reversed” sensitivity to anti-DR4 and anti-DR5 antibodies when they were grown in monolayers vs. spheres. It is possible that DR expression levels and signaling pathways change as the cancer cells adapt to their microenvironment from monolayer cultures to more dense and less nutrient accessible 3D-spheres or in-vivo tumors.
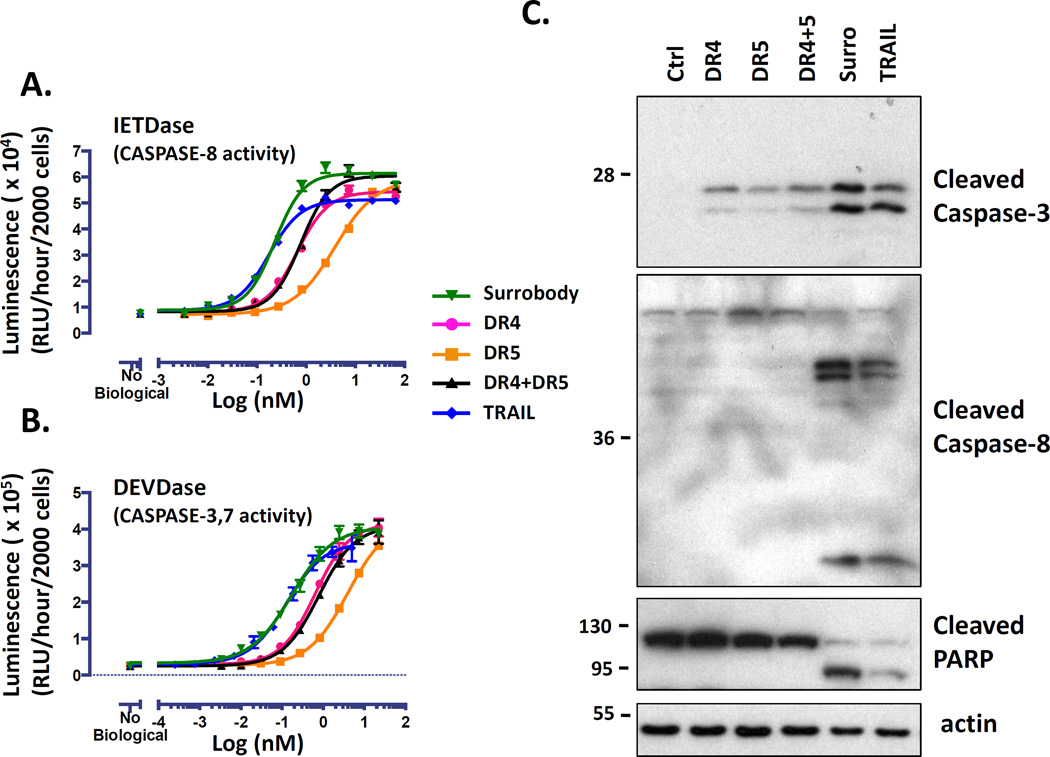
To confirm the apoptotic mechanism of action and provide an opportunity to compare the death receptor dual agonist activities with the single specificity antibodies and TRAIL, we assessed the cellular activation of apoptotic caspases by using luminogenic CASPASE-8 and CASPASE-3 assays. In the first case we used a peptide substrate containing an “IETD”-containing sequence that becomes luminogenic upon proteolytic cleavage by CASPASE-8 (CASPASE 8 Glo). This assay revealed concentration-dependent increases in CASPASE-8 activity in MDA-MB-231 cells following treatment with death receptor dual agonist and the other TRAIL receptor agonists. We found that the death receptor dual agonist Surrobody had comparable potency to TRAIL (IC50 = 0.23 nM vs. 0.17 nM), but greater potency than either of the anti-DR4 or anti-DR5 monospecific antibodies (IC50 = 0.66 nM and 3.9 nM, respectively). To measure the activity of downstream effector caspases, CASPASE-3 and CASPASE-7, we used a second luminogenic peptide substrate that contains “DEVD”-containing sequence (Apo Live Glo). Again the death receptor dual agonist Surrobody induced CASPASE-3/7 activity with similar potency to TRAIL (0.17 nM and 0.13 nM, respectively), and with superior potency to either anti-DR4 or anti-DR5 monospecific antibodies (0.64 nM and 3.98 nM, respectively). Interestingly, the mixture of the DR4 and DR5 monospecific antibodies displayed potencies between those of single antibody treatments (CASPASE-8 IC50 = 0.77 nM; CASPASE-3/7 IC50 = 0.79 nM) (Figure 4A, B).
Figure 4. Death receptor dual agonist Surrobody is a potent inducer of caspase activation.
MDA-MB-231 cells were cultured in 384 wells (2000 cells per well) and treated with increasing concentrations of DR agonists for 6 hr. The activity of CASPASE 8 (A) and CASPASE 3/7 (B) was assessed using CASPASE-8 Glo (contains IETD peptide) and ApoLive-Glo (contains DEVD peptide) reagents, respectively, from Promega, expressing data as relative luminescence units (RLU) generated per hour per 2000 cells (mean ± SEM; n= 4) (C) MDA-MB-231 cells were plated at a density of 70,000 cells/well and the next day they were treated for 3 h with DR agonists at 1 nM. Before addition to cells, all the monospecific antibodies and Surrobody were incubated for 5 minutes with 0.5× molar ratio of protein G to facilitate clustering. Cells were lysed into SDS-sample buffer and lysates were analyzed by SDS-PAGE/immunoblotting using antibodies specific for cleaved CASPASE-8, cleaved CASPASE-3, PARP, or beta-actin. Molecular weight markers are indicated in kilo-Daltons (kDa). Processed CASPASE-8 large (44/42 kDa) and small (18 kDa) subunits are shown. Processed CASPASE-3 large subunits (17/19 kDa) are shown.
We next tested the ability of Surrobody, DR4, DR5 antibodies and TRAIL to induce apoptotic signaling of CASPASE-8, CASPASE-3, and Poly ADP Ribosyl Polymerase (PARP) cleavage. Briefly, MDA-MB-231 cells were treated with DR agonists and the cell lysates were examined by immunoblot analysis for the hallmark proteolytic events associated with apoptotic signaling and caspase activation. We found greater amounts of the apical CASPASE-8 in its cleaved form in cells treated with the Surrobody and TRAIL compared to either the DR4 or DR5 monospecific antibodies alone, or the combination of the DR4 and DR5 monospecific antibodies. This CASPASE-8 processing is readily apparent by the detection of immunoreactive bands migrating at approximately 18 kDa that represent the fully processed form of CASPASE-8. By comparison, the DR4 or DR5 monospecific antibodies, when used alone or combined, mainly produced the partially cleaved forms of CASPASE-8 corresponding to the 44/42 kDa bands (Figure 4C), suggesting less efficient activation of CASPASE-8, which is consistent with the results obtained using CASPASE-8 peptide-based luminescent activity assay (Figure 4A).
Similarly, we found higher levels of cleaved CASPASE-3 in dual agonist Surrobody and TRAIL treated cells by immunoblot analysis using an antibody specific for the 17/19 kDa cleaved form of CASPASE-3 that coincides with the active form of the enzyme. As shown in Figure 4C, treatment with anti-DR4 antibody alone or in combination with anti-DR5 antibody was less effective than Surrobody or TRAIL, which showed similar accumulation of processed CASPASE-3. Notably, the anti-DR5 antibody induced only a minor accumulation of processed CASPASE-3.
Finally we examined Poly ADP Ribosyl Polymerase (PARP) cleavage by the disappearance of the full-length form and appearance of its 89 kDa cleaved fragment. By this measure, Surrobody and TRAIL treatment induced considerably better PARP cleavage than either DR4 or DR5 antibody treatment, whether alone, or in combination (Figure 4C). Immunoblot analysis of beta-actin levels confirmed that the relative changes in the cleaved forms of these proteins were not attributed to protein loading inequalities.
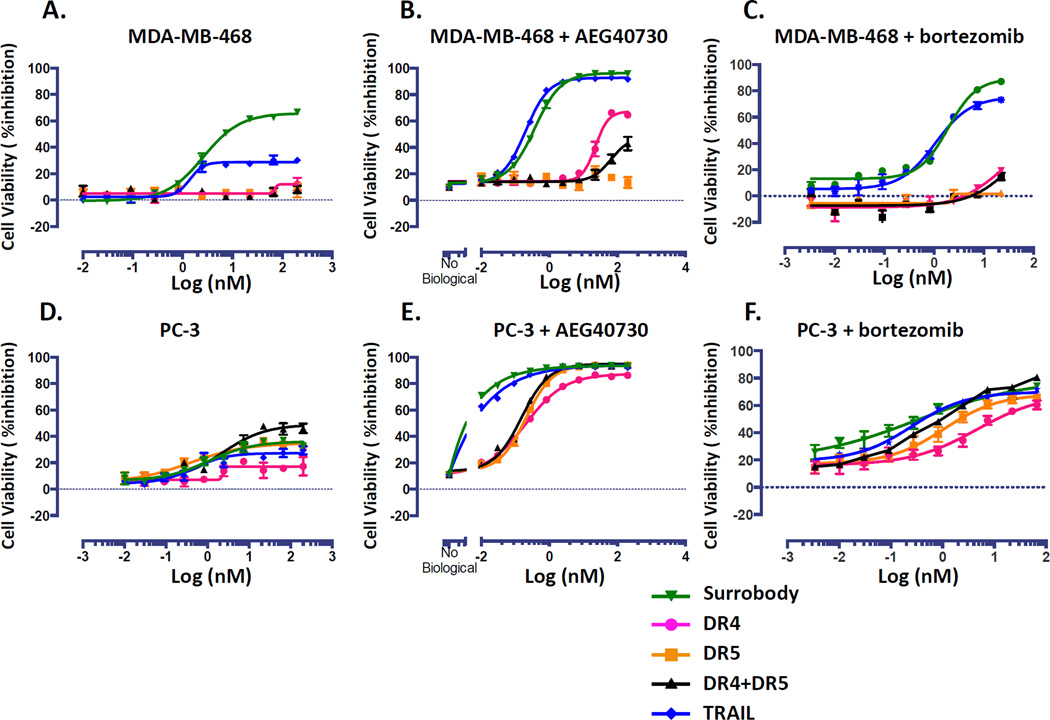
As shown above, the death receptor dual agonist Surrobody is capable of specifically activating apoptotic signal transduction through death receptor engagement. However, it is well known that tumors are heterogeneous in their abilities to fully transduce apoptotic signaling due to insufficient caspase activation or often due to cellular expression of anti-apoptotic proteins. In either case, this results in various levels of resistance. One countermeasure is concomitant treatment or pretreatment with sensitizing agents to enhance or restore apoptotic signaling to reduce or eliminate resistance. To determine whether adding known TRAIL-sensitizing chemical compounds would negate the advantages of the Surrobody compared to mono-specific anti-DR4 or anti-DR5 antibodies, we tested the effects of drug combinations on two TRAIL-resistant tumor cell lines, MDA-MB-468 (ER-negative breast cancer) and PC3 (hormone refractory prostate cancer line). Two different TRAIL-sensitizing agents were assessed – (a) the SMAC mimic, AEG40730 (5), which was previously shown to induce autoubiquitination and proteosomal degradation of inhibitor of apoptosis proteins c-IAP1, c-IAP2 and XIAP that have previously been implicated in TRAIL resistance (5) and (b) the proteasome inhibitor Bortezomib, which has previously been shown to synergize with TRAIL through different mechanisms, including stabilization of tBid (22), and inhibition of the NF-κB pathway (23).
When treated with anti-DR4 or anti-DR5 receptor agonist alone, we observed that PC3 exhibited similar resistance to all agonists while MDA-MB-468 showed a significantly increased sensitivity to the death receptor dual agonist Surrobody when compared to TRAIL, DR4 antibody, DR5 antibody, or a combination of the monospecific antibodies (Figure 5 A, D). Although treatment with AEG40730 alone induced little cell death, pretreatment with AEG40730 sensitized MDA-MB-468 and PC3 cells to DR agonists. In MDA-MB-468, both Surrobody and TRAIL killed nearly all cells, while DR4 antibody-mediated cytotoxicity was only partially effective and DR5 antibody was largely ineffective even in combination with AEG40730 (Figure 5 B). In PC3 cells, all DR agonists were potentiated with AEG40730 inducing nearly complete cell killing. However, the death receptor dual agonist Surrobody and TRAIL exhibited superior potency compared to DR4 and DR5 mono-specific antibodies or the combination of the two mono-specific antibodies (Figure 5E). In addition to SMAC mimetic, we tested the synergy of DR agonists with bortezomib. In this case, TRAIL and Surrobody showed similar potency in inducing death of MDA-MB-468 cells, whereas anti-DR4 and anti-DR5 mono-specific antibodies had only minimal effects (Figure 5C). In the case of PC3 cells, all DR agonists were potentiated only partially with bortezomib (Figure 5F). We conclude, therefore, that even in the presence of TRAIL-sensitizing chemical compounds, the dual DR4/DR5 agonist Surrobody generally shows superior cytotoxic activity compared to mono-specific anti-DR4 or anti-DR5 antibodies.
Figure 5. Smac mimics and bortezomib sensitize TRAIL-resistant tumor cells.
A. TRAIL resistant cells MDA-MB-468 were cultured at a density of 750 cells/well in 384 well plates. The next day cells were cultured with increasing concentrations of monospecific DR4 antibody (pink), monospecific anti-DR5 antibody (orange), the combination of anti-DR4 and anti-DR5 antibodies (black), death receptor dual agonist Surrobody (green), or TRAIL (blue) for 48 hr. Before addition to cells, all the monospecific antibodies and Surrobody were incubated for 5 minutes with 0.5× molar ratio of protein G to facilitate clustering. Relative cell viability was estimated from cellular ATP measurements using the Cell Titer Glo reagent (Promega), expressing data as % inhibition of cell survival (mean ± SEM; n= 4). B and C. The cells were cultured as in A, but they were pre-treated with 10 nM smac mimic AEG40730 or 1 nM bortezomib for 2h before addition of DR agonists. D. TRAIL resistant cells PC3 were cultured, treated and assayed for cell viability as in A. In E and F, PC3 cells were cultured, treated and assayed as in A, but they were pre-treated with 10 nM smac mimic AEG40730 or 1 nM bortezomib for 2h before addition of DR agonists.
Given that resistance is a common problem in cancer therapy, we compared the general ability of the death receptor dual agonist Surrobody and other death receptor agonists to induce resistance in cancer cells following prolonged treatment. To address this question, we plated MDA-MB-231 cells at a very low density and allowed them to grow for 9 days in the presence of increasing concentrations of anti-DR agonists. We observed a number of colonies that survived in cultures treated with the individual DR4 and DR5 monospecific antibodies, a combined mixture of the anti-DR4/anti-DR5 antibodies, and even TRAIL (Supplementary Figure S7). However, MB-MDA-231 cells developed the least resistance to the death receptor dual agonist Surrobody, as reflected by the lowest number of surviving tumor colonies. For example, no viable colonies were detected following treatment with the death receptor dual agonist Surrobody at 1.6 nM, while colonies were readily detectable following treatment with other DR agonists at the same concentration.
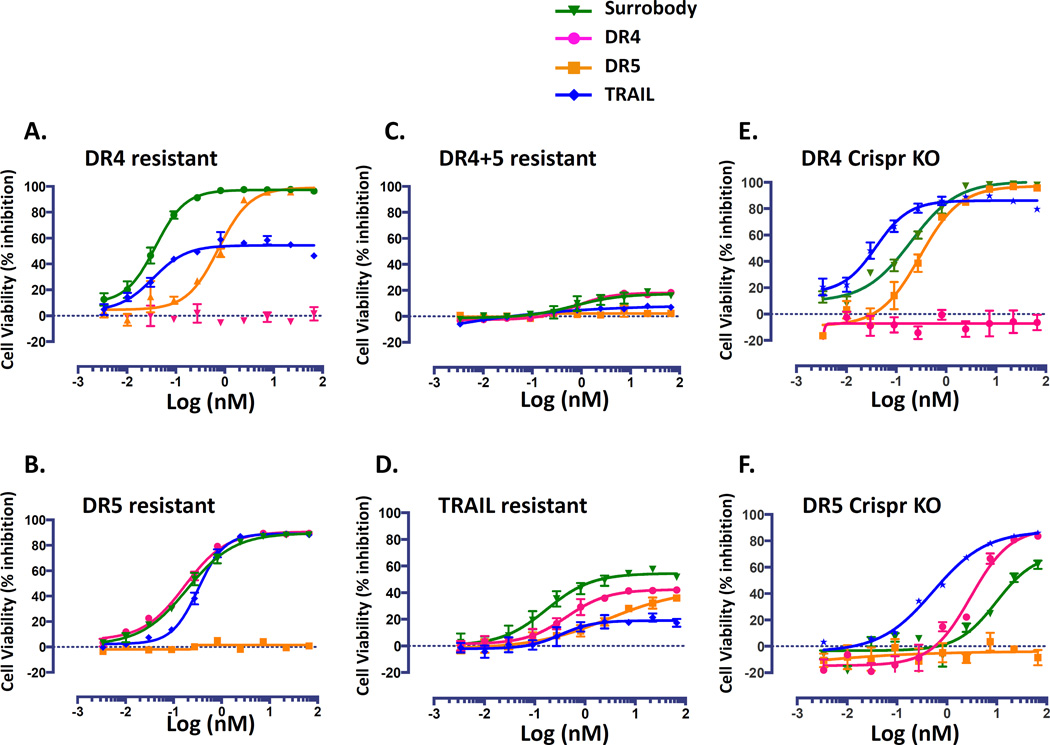
To better understand the characteristics of receptor-induced resistance we profiled the levels of resistance of the survivor clones produced as described above. Briefly, we isolated single MDA-MB-231 clones resulting from treatment with anti-DR4, anti-DR5 or TRAIL for 9 days and then reinforced their resistance by continuing to grow them under the same DR agonist pressure for additional period of 3 weeks. Next, we tested whether these resistant clones were resistant only to the DR agonist used for their selection, or whether they developed resistance to all DR agonists as a general mechanism of apoptotic inhibition (Figure 6). We found that MDA-MB-231 cells that developed resistance to either anti-DR4 or anti-DR5 monospecific antibody pressure did not simultaneously exhibit cross-resistance to the untargeted death receptor. Moreover, we found that the death receptor dual agonist Surrobody retained the ability to kill either of the single receptor-resistant cells.
Figure 6. DR4 and DR5 resistant tumor cell lines remain sensitive to death receptor dual agonists Surrobody.
MDA-MB-231 cell lines resistant to DR4 (A), DR5 (B), DR4+DR5 (C), or TRAIL (D) were created by culturing them for 3 weeks in 500 ng/ml of anti-DR4 antibody, 1000ng/ml of anti-DR5 antibody, 250 ng/ml each of anti-DR4 and anti-DR5, or 50 ng/ml TRAIL. MDA-MB-231 cells that are deficient for either DR4 (E) or DR5 (F) receptors were generated using Crispr gene knockout technology. To assess the sensitivity of these resistant cells to various DR agonists, the cells were plated at a density of 750 cells/well into 384 wells and cultured with increasing concentrations of monospecific anti-DR4 antibody (pink), monospecific anti-DR5 antibody (orange), death receptor dual agonist Surrobody (bright green), or TRAIL (blue) for 48 hr. Before addition to cells, all the monospecific antibodies and Surrobody were incubated for 5 minutes with 0.5× molar ratio of protein G to facilitate clustering. Relative cell viability was estimated from cellular ATP measurements using the Cell Titer Glo reagent (Promega), expressing data as % inhibition of cell survival (mean ± SEM; n= 4).
In the case of DR4-resistant cells, the death receptor dual agonist Surrobody efficacy was superior to TRAIL (which was approximately 50% effective). Also, the Surrobody showed higher potency than the anti-DR5 monospecific antibody against DR4-resistant cells. In the case of the DR5-resistant cell lines, Surrobody, TRAIL, and the anti-DR4 monospecific antibody all displayed potent cytotoxicity. For the resistant cell lines derived from TRAIL pressure, all DR agonists retained some efficacy, but none to the extent achieved by the dual agonist Surrobody. Finally, the resistant cells derived from simultaneous pressure of anti-DR4 and anti-DR5 antibodies remained resistant to all DR agonists tested. Remarkably, under the same resistance selection conditions (3 week incubation with approximately 3.3 nM of the death receptor dual agonist Surrobody), the MDA-MB-231 cells failed to generate any resistant clones, possibly due to differences in potencies between the death receptor dual agonist Surrobody and the monospecific DR4 or DR5 antibodies, or, alternatively, because the mechanism of action of the combination of anti-DR4 and anti-DR5 agonist antibodies differed qualitatively from that of the Surrobody.
We profiled the expression of DR4 and DR5 receptors in resistant cells and found that DR5 resistant cells had a reduced expression of DR5 receptors, while DR4 resistant cells still expressed DR4 receptors (Supplementary Table S1B). This finding suggests that diverse mechanisms can contribute to resistance and may not necessarily include the regulation of death receptor expression. Further investigation would be required to determine if DR4 receptor expressed on the cell surface of DR4 resistant cells cannot signal proper DISC assembly and downstream caspase activation.
To further confirm that Surrobody can induce death in cells that express only one of the death receptors, we used Crispr knockout technology to generate cells deficient in either DR4 or DR5 receptors. We show that Surrobody retained the ability to induce death of cells that were missing either of the DR receptors (Figure 6E, F). In both cases, antibodies directed against the knocked-out DR receptor were completely ineffective, while the Surrobody induced cell death. However, Surrobody was more potent in cells that were DR4-resistant than DR5-resistant, both in the case of treatment-induced resistance (Figure 6A, B) and gene ablation-mediated resistance (Figure 6E, F), suggesting that Surrobody’s ability to induce death of MDA-MB-231 cells may rely more on DR5 than DR4 receptors.
DISCUSSION
Encouraged by the observation that cancer cells exhibit higher sensitivity than normal cells to death receptor activation, several attempts have been made to develop recombinant TRAIL and death receptor agonist antibodies as novel anticancer agents (24). TRAIL therapy has been tested in several clinical trials with limited success, in no small part due to its extremely short serum half-life and probably also due to decoy receptor binding (2). An alternative strategy to overcome these limitations is to develop a long-lived, highly active, dual agonist that avoids decoy receptor sequestration. To this end, we have generated a death receptor dual agonist Surrobody, which binds and activates both DR4 and DR5, avoids decoy receptors, and contains an immunoglobulin Fc for long serum half-life. Importantly, the Surrobody also binds and activates cynomolgus and rhesus DR4 and DR5 in vitro (data not shown), which would be useful in further Surrobody development. Because of its ability to induce apoptosis through either DR4 or DR5, the Surrobody differentiates itself from monospecific antibodies and has demonstrated improved or equipotent activity compared to these agents in our preclinical studies. Moreover, the death receptor dual agonist Surrobody showed greater potency than the combination of monospecific DR4 and DR5 antibodies, suggesting that it has other properties that improve efficacy that remain to be further characterized.
One recently discovered death receptor characteristic important for death-inducing activity is their ability to cluster and form higher order oligomeric molecular complexes that promote more efficient DISC assembly and CASPASE-8 activation (19, 24). Although the composition of these clusters has not been studied in detail, it was previously shown that, in addition to homogeneous DR4 and DR5 complexes, DR4 and DR5 can also form heteromeric complexes in response to TRAIL binding (25). Therefore, one possible explanation for the greater observed potency of the dual agonist Surrobody over the combination of DR4 and DR5 monospecific antibodies could be its ability to more efficiently cluster death receptors, possibly by forming heteromeric complexes containing both DR4 and DR5, leading to more efficient DISC assembly and induction of apoptotic signaling. Consistent with this interpretation, Surrobody treatment of MDA-MB-231 cells, which express functional DR4 and DR5, leads to a greater amount of cleavage of CASPASE-8, CASPASE-3, and PARP compared to either DR4 antibody, DR5 antibody, or their combination.
The dual agonist nature of the Surrobody also provides inherent potential therapeutic benefits not only because various cancers express different levels of DR4 and DR5 receptors and exhibit different sensitivities to their stimulation, but also because it is possible that during disease progression coincidental mutations and epigenetic changes could alter expression of death receptors (26, 27), resulting in dynamic changes in sensitivity to receptor specific agonists. This potential for switching DR4 versus DR5 sensitivity is illustrated, for example, by our comparison of tumor cells grown in monolayer versus 3D-spheroid cultures. Even in cancer cells where both receptors are expressed, the relative ability of DR4 versus DR5 to transmit apoptotic signals can be highly variable (13). For example, the Ramos cells used in this study signal exclusively through DR4, even though DR5 is highly expressed on their cell surface.
In addition, intracellular resistance can disrupt extrinsic apoptotic signaling, with a number of different mechanisms previously implicated in TRAIL resistance, including diminished expression of CASPASE-8 and pro-apoptotic molecules of the BCL-2 family, as well as upregulation of anti-apoptotic molecules such as c-FLIP and IAPs (13). It has previously been shown that SMAC mimics can potentiate TRAIL-induced death of MDA-MB-231 cells (28). Consistent with prior reports (29, 30), we demonstrate that to some extent the extrinsic apoptotic pathway resistance of MDA-MB-468 and PC3 cells can be overcome through IAP inhibition by the SMAC mimic AEG40730 and by proteasome inhibition by bortezomib (Figure 5). Both AEG40730 and bortezomib exhibited better efficacy with Surrobody and TRAIL than with anti-DR4, anti-DR5 or a combination of both antibodies. This observation is also in line with a mechanism of action model where activation of both death receptors (DR4 and DR5), possibly simultaneously, is better able to induce threshold caspase activation leading to apoptotic cell death.
During disease progression, tumors often become more heterogeneous either as a result of accumulating mutations or as a result of microenvironment pressures such as nutrient deprivation and hypoxia. Tumor progression is associated with changing gene expression and it has recently been shown that hypoxia can induce multiple changes in death receptor expression (31, 32). Therefore, a death receptor dual agonist Surrobody may be advantageous compared to monospecific antibodies when tumor progression leads to a change in death receptor expression in any given patient over time. Having dual specificity therefore provides greater opportunity to ensure that at least one of the two active death receptors will be stimulated. Interestingly, we observed that MDA-MB-231 cells grown as a monolayer display differential sensitivity to DR4 and DR5 antibodies compared to when they are grown as 3D-spheroids. Similarly, Colo-205 cells grown as a monolayer showed higher sensitivity to DR4 antibody, but when grown as tumor xenografts in mice, they became more sensitive to DR5 antibody. It is possible that the hypoxic environment of the spheroids and tumors modulates the death receptor expression resulting in different levels of sensitivity to DR4 and DR5 antibodies when compared to monolayer culture. In contrast to monospecific antibodies, the death receptor dual agonist Surrobody retained superior potency in monolayer and spheroid cultures, illustrating the greater flexibility achieved with this agent.
Taken together, our data provide strong support for the continued development of the death receptor dual agonist Surrobody as a novel anti-cancer agent that can activate DR4 and DR5 simultaneously. The dynamic expression of death receptors in response to tumor microenvironment as well as the lack of clear correlation between any single death receptor’s expression and consequential sensitivity to a receptor-specific DR agonist poses a challenge for development of traditional DR4 and DR5 agonists. Similar to TRAIL, the dual specificity of Surrobody is expected to overcome the challenge of patient profiling for death receptor expression. Unlike TRAIL, however, death receptor dual agonist Surrobody avoids the problem of decoy receptor binding and is expected to exhibit a long serum half-life characteristic of antibodies for more sustained duration of activity in vivo. Furthermore, synergistic combinations with other anticancer agents, such as that demonstrated with AEG40730 and bortezomib, may additionally expand the overall clinical utility into patient populations where death receptor activation alone is insufficient to cause tumor cell death. Additionally, detailed preclinical drug safety analysis will be required to assess the promise of dual DR4/DR5 agonist human surrobodies. Altogether, dual agonist DR4/DR5 surrobodies provide a novel approach to the discovery and development of death receptor-targeting anti-cancer therapeutics, which merit additional investigation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Hieu Tran and Sandra Wang for their excellent collective efforts involved in protein production, purification, and characterization of the dual agonist Surrobody and all the necessary related death receptor reagents used in these studies. We would like to thank Dr. Andrea D’Osualdo for guidance in design of vectors and production of DR4 and DR5 crispr-mediated knockout cells. We would also like to thank AEGERA Therapeutics Inc. for generously providing SMAC mimic AEG40730. In addition, we would like to thank Yoav Altman and the Flow Cytometry core of SBMRI for their help in DR expression profiling and sorting of knockout cells.
FINANCIAL SUPPORT: This project was supported by NIH grant CA163743-04 awarded to John C. Reed.
Footnotes
CONFLICT OF INTEREST: We declare no conflict of interest.
REFERENCES
- 1.Herbst RS, Eckhardt SG, Kurzrock R, Ebbinghaus S, O'Dwyer PJ, Gordon MS, et al. Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol. 2010;28:2839–2846. doi: 10.1200/JCO.2009.25.1991. [DOI] [PubMed] [Google Scholar]
- 2.Soria JC, Smit E, Khayat D, Besse B, Yang X, Hsu CP, et al. Phase 1b study of dulanermin (recombinant human Apo2L/TRAIL) in combination with paclitaxel, carboplatin, and bevacizumab in patients with advanced non-squamous non-small-cell lung cancer. J Clin Oncol. 2010;28:1527–1533. doi: 10.1200/JCO.2009.25.4847. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Davis JS, Wu X. Immunoglobulin Fc domain fusion to TRAIL significantly prolongs its plasma half-life and enhances its antitumor activity. Mol Cancer Ther. 2014;13:643–650. doi: 10.1158/1535-7163.MCT-13-0645. [DOI] [PubMed] [Google Scholar]
- 4.Pukac L, Kanakaraj P, Humphreys R, Alderson R, Bloom M, Sung C, et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. British journal of cancer. 2005;92:1430–1441. doi: 10.1038/sj.bjc.6602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams C, Totpal K, Lawrence D, Marsters S, Pitti R, Yee S, et al. Structural and functional analysis of the interaction between the agonistic monoclonal antibody Apomab and the proapoptotic receptor DR5. Cell death and differentiation. 2008;15:751–761. doi: 10.1038/sj.cdd.4402306. [DOI] [PubMed] [Google Scholar]
- 6.Tolcher AW, Mita M, Meropol NJ, von Mehren M, Patnaik A, Padavic K, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 7.Hotte SJ, Hirte HW, Chen EX, Siu LL, Le LH, Corey A, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 8.Camidge DR, Herbst RS, Gordon MS, Eckhardt SG, Kurzrock R, Durbin B, et al. A phase I safety and pharmacokinetic study of the death receptor 5 agonistic antibody PRO95780 in patients with advanced malignancies. Clin Cancer Res. 2010;16:1256–1263. doi: 10.1158/1078-0432.CCR-09-1267. [DOI] [PubMed] [Google Scholar]
- 9.Forero-Torres A, Shah J, Wood T, Posey J, Carlisle R, Copigneaux C, et al. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5) Cancer biotherapy & radiopharmaceuticals. 2010;25:13–19. doi: 10.1089/cbr.2009.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plummer R, Attard G, Pacey S, Li L, Razak A, Perrett R, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 11.Wakelee HA, Patnaik A, Sikic BI, Mita M, Fox NL, Miceli R, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2010;21:376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang H, Reyes AE, 2nd, Eppler S, Kelley S, Damico-Beyer LA. Death receptor 5 agonistic antibody PRO95780: preclinical pharmacokinetics and concentration-effect relationship support clinical dose and regimen selection. Cancer chemotherapy and pharmacology. 2013;72:405–415. doi: 10.1007/s00280-013-2200-3. [DOI] [PubMed] [Google Scholar]
- 13.Dimberg LY, Anderson CK, Camidge R, Behbakht K, Thorburn A, Ford HL. On the TRAIL to successful cancer therapy? Predicting and counteracting resistance against TRAIL-based therapeutics. Oncogene. 2012;32:1341–1350. doi: 10.1038/onc.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Hollander MW, Gietema JA, de Jong S, Walenkamp AM, Reyners AK, Oldenhuis CN, et al. Translating TRAIL-receptor targeting agents to the clinic. Cancer letters. 2013;332:194–201. doi: 10.1016/j.canlet.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Foreman PK, Gore M, Kobel PA, Xu L, Yee H, Hannum C, et al. ErbB3 inhibitory surrobodies inhibit tumor cell proliferation in vitro and in vivo. Mol Cancer Ther. 2012;11:1411–1420. doi: 10.1158/1535-7163.MCT-12-0068. [DOI] [PubMed] [Google Scholar]
- 16.Parda DS, Thraves PJ, Kuettel MR, Lee MS, Arnstein P, Kaighn ME, et al. Neoplastic transformation of a human prostate epithelial cell line by the v-Ki-ras oncogene. The Prostate. 1993;23:91–98. doi: 10.1002/pros.2990230202. [DOI] [PubMed] [Google Scholar]
- 17.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nature protocols. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu L, Yee H, Chan C, Kashyap AK, Horowitz L, Horowitz M, et al. Combinatorial surrobody libraries. Proc Natl Acad Sci U S A. 2008;105:10756–10761. doi: 10.1073/pnas.0805293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graves JD, Kordich JJ, Huang TH, Piasecki J, Bush TL, Sullivan T, et al. Apo2L/TRAIL and the death receptor 5 agonist antibody AMG 655 cooperate to promote receptor clustering and antitumor activity. Cancer cell. 2014;26:177–189. doi: 10.1016/j.ccr.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan-Lefko PJ, Graves JD, Zoog SJ, Pan Y, Wall J, Branstetter DG, et al. Conatumumab, a fully human agonist antibody to death receptor 5, induces apoptosis via caspase activation in multiple tumor types. Cancer biology & therapy. 2010;9:618–631. doi: 10.4161/cbt.9.8.11264. [DOI] [PubMed] [Google Scholar]
- 21.Wilson NS, Yang B, Yang A, Loeser S, Marsters S, Lawrence D, et al. An Fcgamma receptor-dependent mechanism drives antibody-mediated target-receptor signaling in cancer cells. Cancer cell. 2011;19:101–113. doi: 10.1016/j.ccr.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Naumann I, Kappler R, von Schweinitz D, Debatin KM, Fulda S. Bortezomib primes neuroblastoma cells for TRAIL-induced apoptosis by linking the death receptor to the mitochondrial pathway. Clin Cancer Res. 2011;17:3204–3218. doi: 10.1158/1078-0432.CCR-10-2451. [DOI] [PubMed] [Google Scholar]
- 23.Jane EP, Premkumar DR, Pollack IF. Bortezomib sensitizes malignant human glioma cells to TRAIL, mediated by inhibition of the NF-{kappa}B signaling pathway. Mol Cancer Ther. 2011;10:198–208. doi: 10.1158/1535-7163.MCT-10-0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirley S, Morizot A, Micheau O. Regulating TRAIL receptor-induced cell death at the membrane : a deadly discussion. Recent Pat Anticancer Drug Discov. 2011;6:311–323. doi: 10.2174/157489211796957757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kischkel FC, Lawrence DA, Chuntharapai A, Schow P, Kim KJ, Ashkenazi A. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–620. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 26.Elias A, Siegelin MD, Steinmuller A, von Deimling A, Lass U, Korn B, et al. Epigenetic silencing of death receptor 4 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in gliomas. Clin Cancer Res. 2009;15:5457–5465. doi: 10.1158/1078-0432.CCR-09-1125. [DOI] [PubMed] [Google Scholar]
- 27.Horak P, Pils D, Haller G, Pribill I, Roessler M, Tomek S, et al. Contribution of epigenetic silencing of tumor necrosis factor-related apoptosis inducing ligand receptor 1 (DR4) to TRAIL resistance and ovarian cancer. Mol Cancer Res. 2005;3:335–343. doi: 10.1158/1541-7786.MCR-04-0136. [DOI] [PubMed] [Google Scholar]
- 28.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Christian PA, Thorpe JA, Schwarze SR. Velcade sensitizes prostate cancer cells to TRAIL induced apoptosis and suppresses tumor growth in vivo. Cancer biology & therapy. 2009;8:73–80. doi: 10.4161/cbt.8.1.7132. [DOI] [PubMed] [Google Scholar]
- 30.Foster FM, Owens TW, Tanianis-Hughes J, Clarke RB, Brennan K, Bundred NJ, et al. Targeting inhibitor of apoptosis proteins in combination with ErbB antagonists in breast cancer. Breast cancer research : BCR. 2009;11:R41. doi: 10.1186/bcr2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton TR, Henson ES, Azad MB, Brown M, Eisenstat DD, Gibson SB. BNIP3 acts as transcriptional repressor of death receptor-5 expression and prevents TRAIL-induced cell death in gliomas. Cell Death Dis. 2013;4:e587. doi: 10.1038/cddis.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pei GT, Wu CW, Lin WW. Hypoxia-induced decoy receptor 2 gene expression is regulated via a hypoxia-inducible factor 1alpha-mediated mechanism. Biochem Biophys Res Commun. 2009;391:1274–1279. doi: 10.1016/j.bbrc.2009.12.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.