Abstract
Age-related cataract, an opacity of the eye lens, is the leading cause of visual impairment in the elderly, the etiology of which is related to oxidative stress damage. Oxidation of methionine to methionine sulfoxide is a major oxidative stress product that reaches levels as high as 60% in cataract while being essentially absent from clear lenses. Methionine oxidation results in loss of protein function that can be reversed through the action of methionine sulfoxide reductase A (MsrA), which is implicated in oxidative stress protection and is an essential regulator of longevity in species ranging from Escherichia coli to mice. To establish a role for MsrA in lens protection against oxidative stress, we have examined the levels and spatial expression patterns of MsrA in the human lens and have tested the ability of MsrA to protect lens cells directly against oxidative stress. In the present report, we establish that MsrA is present throughout the human lens, where it is likely to defend lens cells and their components against methionine oxidation. We demonstrate that overexpression of MsrA protects lens cells against oxidative stress damage, whereas silencing of the MsrA gene renders lens cells more sensitive to oxidative stress damage. We also provide evidence that MsrA is important for lens cell function in the absence of exogenous stress. Collectively, these data implicate MsrA as a key player in lens cell viability and resistance to oxidative stress, a major factor in the etiology of age-related cataract.
The eye lens consists of a single layer of epithelial cells that cover concentric layers of elongated fiber cells. The fiber cells nearest the epithelium make up the lens cortex, and the fiber cells in the center of the lens are referred to as the lens nucleus. Lens fiber cells do not turn over and are some of the oldest cells in the body. Damage to lens cells and their components ultimately results in protein aggregation and age-related cataract. Age-related cataract is an opacity of the eye lens that is the major cause of world blindness (1). Among the many factors involved in cataract formation, oxidative stress plays a major role through the oxidation and aggregation of lens proteins (2–5). One major protein modification associated with oxidative stress in the lens is oxidation of methionine residues to methionine sulfoxide. Methionine sulfoxide is barely detectable in young lenses but increases in the lens with age (6). Compellingly, methionine sulfoxide levels increase in cataract (7), and as much as 60% of membrane bound protein methionines are present in an oxidized form (8). Although it has been established that numerous important oxidative stress and other defense systems function in the lens including α-crystallin (9), manganese superoxide dismutase (MnSOD) (10), copper/zinc superoxide dismutase (CuZnSOD) (11), reduced glutathione (12–14), glutathione reductase (15), glutathione S-transferase (16), thiol-transferase (17), catalase (18), and others, most of these systems are protective; that is, they do not reverse oxidative stress damage, and none work directly on oxidized methionines. Oxidation of methionine residues is associated with the loss of numerous protein activities and effects a multitude of biological functions (19–24). Thus, any protective system that could reduce oxidized methionines could play a major role in maintenance of lens transparency.
Unlike most protein modifications, methionine sulfoxide can be converted back to methionine through the action of a class of enzymes known as methionine sulfoxide reductases (Msrs) (25) in a thioredoxin-dependent reaction involving both thioredoxin reductase and NADPH (19, 26). The Msr system is a key repair and defense system that is conserved throughout evolution and influences longevity in species ranging from Escherichia coli to mice. Oxidation of methionine residues results in two forms of methionine sulfoxide, an S and R form. Two separate classes of Msrs, referred to as MsrA and MsrB, have been identified that repair the S and R forms, respectively, of methionine sulfoxide residues (25). Overexpression of MsrA in transgenic flies renders them more resistant to oxidative stress and dramatically increases their lifespan (27). Overexpression of MsrA confers direct protection against peroxide-mediated oxidative stress in yeast and human T-lymphocytes (28). By contrast, E. coli and yeast lacking MsrA are more sensitive to oxidative stress (29, 30), and deletion of the MsrA gene in mice results in increased sensitivity to oxidative stress, a shortened lifespan, and neurological impairment (31).
Increased oxidized methionine content in aging and cataractous lenses suggests a role for methionine sulfoxide in cataract formation. Msr activity has been detected in the lens (32); however, to date, the role of Msrs in lens function or in the development of cataract has not been established. In the present report, we have examined the levels and spatial expression patterns of MsrA in the human lens and have tested the ability of the enzyme to directly protect cultured human lens cells against oxidative stress. The results reveal that high levels of MsrA transcript and protein are found throughout the human lens, that MsrA directly protects lens cells against oxidative stress-induced damage, and that MsrA plays a role in lens cell viability even in the absence of exogenously added stress.
Materials and Methods
Analysis of MsrA Transcript and Protein Levels in Microdissected Components of Whole Human Lenses. The relative levels of MsrA transcript and protein were estimated between microdissected portions of adult human lenses by semiquantitative RT-PCR and Western analysis. Eight clear human lenses were microdissected to remove the lens epithelium from the underlying fiber cells. The fiber cells were further dissected into cortical and nuclear components. RT-PCR was performed by using 100 ng of total RNA as described in ref. 33. The sequences of the primers used in this study are as follows: MsrA forward 5′-AGTACCTGAGCAAGAACCCCA-3′ and MsrA reverse 5′-TCACTCAGACCCCAGAAGACA-3′. MsrA transcript was amplified for 34 PCR cycles with an annealing temperature of 56°C. All reactions were conducted by using reagents contained in a commercial RT-PCR kit (One-Step kit, Invitrogen) according to the manufacturer's protocol. Products were separated by gel electrophoresis, and all products were sequenced to ensure authenticity. Product formation was tested to be linear over the number of PCR cycles indicated. Protein was isolated from microdissected lens epithelia, cortical fibers, and nuclear fibers by sonication, and Western analysis was performed by using MsrA-specific antibody as described in ref. 34.
Spatial Localization of MsrA Protein in an Intact Human Lens. A human lens (<24 h postmortem) from an 18-year-old female was processed, and MsrA protein was visualized by immunohistochemistry as described in ref. 35, using a 1:2,000 dilution of anti-MsrA antibody and streptavidin-conjugated secondary antibody. Complexes were visualized by using the VECTASTAIN Elite Kit (Vector Laboratories) as specified by the manufacturer. Sections were counterstained with hematoxylin, and identical procedures were carried out in the absence of primary antibody as control.
SRA01/04 and 293-FT Cell Culture. Human lens epithelial (HLE) cells (SRA01/04) and 293-FT kidney cells were grown and cultured in DMEM (Invitrogen) supplemented with 15% FBS (Invitrogen), gentamicin (50 units/ml; Invitrogen), penicillin-streptomycin antibiotic mix (50 units/ml; Invitrogen), and Fungizone (5 μl/ml; Invitrogen) at 36.5°C in the presence of 5% CO2.
Creation of MsrA-Overexpressing HLE Cell Lines. MsrA-overexpressing cell lines were created by using the ViraPower Lentiviral Expression System (Invitrogen) as described by the manufacturer. Primers were designed to amplify full-length MsrA transcripts with the exception of the final 9 bp on the 3′ end of the MsrA transcript encoding the last two amino acids and the stop codon. Ablation of the stop codon allowed for fusion of the recombinant protein to a V5-epitope tag used to identify the translated product.
Confirmation of MsrA Overexpression in SRA01/04 HLE Cells. Overexpression of MsrA was confirmed by semiquantitative RT-PCR and Western analysis with an anti-V5 monoclonal antibody (Invitrogen) at a dilution of 1:5,000 as described above.
Short Interfering RNA (siRNA)-Targeted Gene Silencing. Double-stranded siRNAs specific for MsrA were designed and manufactured using Qiagen's (Valencia, CA) 4-for-Silencing service. HLE cells were transfected with siRNA by using the TransMessenger Transfection Reagent kit (Qiagen) according to the manufacturer's protocol. Three different double-stranded siRNA constructs were used in these studies designated as siRNAs 1, 8, and 4 with the following sequences: siRNA 1, CCCCUGUAGCGGCCAAACAUU and UGUUUGGCCGCUACAGGGGUC; siRNA 8, CAAAGUACAAAGGAAUUUAUU and UAAAUUCCUUUGUACUUUGUG; and siRNA 4, CGGGAGGGACAGACUUUCUUU and AGAAAGUCUGUCCCUCCCGGA.
Cell-Viability Assays with 3-(4,5-Carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) Reagent. A CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega) kit containing the tetrazolium compound MTS was used to monitor cell viability according to the manufacturer's protocols. MTS color change was monitored by using an ELX-800 universal plate reader (Bio-Tek, Winooski, VT) set at an absorbance reading of 492 nm.
H2O2 Sensitivity of Control, Overexpressing, and siRNA-Treated HLE Cells. HLE cells were plated in 96-well plates at a density of 20,000 cells per well and treated with multiple concentrations of H2O2 for 24 h in serum-free media. H2O2 treatments were analyzed in sets of four identical treatments, and cell viability was monitored by using MTS assays. For siRNA studies, cells were transfected with 4 μg of siRNA per 500,000 cells or mock-transfected by using all of the transfection reagents in the absence of siRNA. Mean absorbance and standard deviations for each treatment were determined.
Results
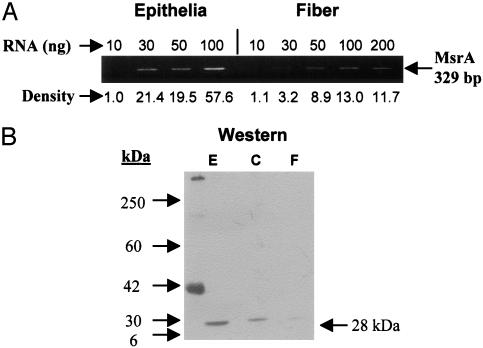
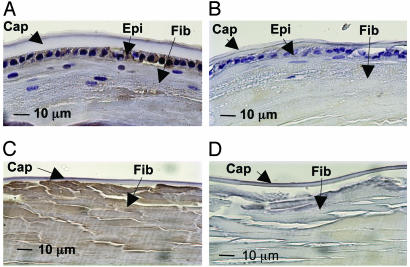
MsrA Transcript and Protein Are Expressed by the Human Lens. Whole human lenses were microdissected and RNA was prepared from isolated lens epithelium and fiber cells, and protein was extracted from lens epithelial, cortical, and nuclear fiber cells. MsrA transcript was detected in the lens epithelium and fiber cells by semiquantitative RT-PCR (Fig. 1A). MsrA transcript is expressed at significantly higher levels in the lens epithelium relative to fiber cells. Consistent with transcript levels, MsrA protein was detected at high levels in the lens epithelium with lower levels in the cortical and nuclear fiber cells by Western analysis (Fig. 1B). Immunohistochemical staining localized MsrA protein to the lens epithelium, cortical, and nuclear fibers (Fig. 2 A and C). No significant staining was observed when using secondary antibody alone (Fig. 2 B and D). These data are in agreement with the levels of MsrA activity detected in subregions of microdissected human lenses (32).
Fig. 1.
Spatial analysis of MsrA transcript and protein levels in microdissected human lenses. (A) Ethidium bromide-stained gels showing the relative levels of MsrA transcript between microdissected lens epithelial cells and fiber cells. (B) Immunoblotting of lens epithelium (E), cortex (C), and fiber (F) extracts with a MsrA-specific antibody using 15 μg of protein.
Fig. 2.
Immunostaining of adult human lens with MsrA-specific antibody. An 18-year-old female human lens was immunostained with anti-MsrA antibody (A and C) or secondary antibody alone (B and D). Peripheral (A and B) and posterior (C and D) portions of the lens are shown. Lens capsule (Cap), epithelium (Epi), and fibers (Fib) are indicated.
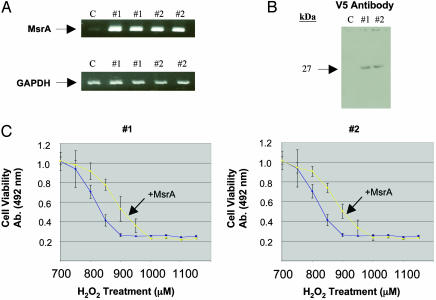
Overexpression of Exogenous MsrA in HLE Cells Confers Resistance to H2O2-Induced Oxidative Damage. To determine the ability of MsrA to protect HLE cells against H2O2-induced oxidative stress, MsrA-overexpressing human lens cell lines were created and exposed to H2O2, and viability was measured by using MTS assays. The overexpressing cells exhibited increased levels of MsrA mRNA (Fig. 3A) and protein (Fig. 3B) relative to uninfected control cells. Overexpression of MsrA protected HLE cells against oxidative stress by as much as 40% over uninfected control cells at concentrations of H2O2 ranging from 800 to 950 μM (Fig. 3C). Oxidation of methionine results in an R and an S form of methionine sulfoxide, of which MsrA is specific for the S form (25). Thus, a 50% increase in cell viability would likely be the maximal amount of protection obtainable through MsrA overexpression.
Fig. 3.
Overexpression of MsrA in HLE cells (SRA01/04). (A) Ethidium bromide-stained gels showing the relative levels of MsrA detected between two separately constructed overexpressing cell lines (#1 and #2) compared with control cells (C). (B) Immunoblotting of 15 μg of protein extracts from control cells (C) and the two MsrA-overexpressing cell lines (#1 and #2) with a mouse monoclonal antibody raised against the 14-aa V5-epitope fused to the C-terminal end of the recombinant MsrA protein. (C) Representative graphs depicting increased resistance to H2O2 stress treatments of the two MsrA-overexpressing cell lines (yellow lines) relative to control cells (blue lines), using MTS cell-viability assays. H2O2 treatments were conducted for 24 h in serum-free media. The absorbance readings and H2O2 concentrations used starting at 700 μM are indicated. Error bars represent standard deviations of eight separate cell-viability assays.
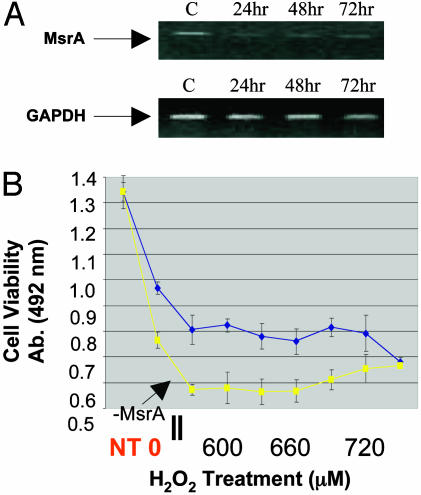
Silencing of Endogenous MsrA Causes Increased Sensitivity of HLE Cells to H2O2-Induced Oxidative Damage. The effects of decreased endogenous MsrA on the sensitivity of HLE cells to H2O2 were examined by siRNA gene silencing. Significant decreases in MsrA transcript levels relative to mock-transfected control cells were detected at 24, 48, and 72 h posttransfection (Fig. 4A). Silencing of endogenous MsrA rendered HLE cells more sensitive to H2O2-induced stress at concentrations ranging from 570 to 720 μM by ≈25% relative to mock-transfected cells (Fig. 4B). The differences in cell viability are not due to the presence of double-stranded RNA molecules because no difference in cell viability was detected when transfections were performed by using a siRNA construct that is ineffective in reducing the transcript levels of MsrA (data not shown).
Fig. 4.
siRNA-mediated MsrA gene suppression in HLE cells (SRA01/04). (A) Ethidium bromide-stained gels showing MsrA-specific gene suppression at 24, 48, and 72 h posttransfection of siRNA relative to mock-transfected control cells (C). (B) Representative graph depicting decreased resistance to H2O2 stress 48 h posttransfection of siRNA (yellow line) relative to mock-transfected control cells (blue line), using MTS cell-viability assays. Untransfected cells are represented by NT in red. siRNA-transfected control cells not exposed to H2O2 are indicated as 0 in red. H2O2 treatments were conducted for 24 h in serum-free media. The absorbance reading and H2O2 concentrations used starting at 540 μM are indicated. Error bars represent standard deviations of eight separate cell-viability assays.
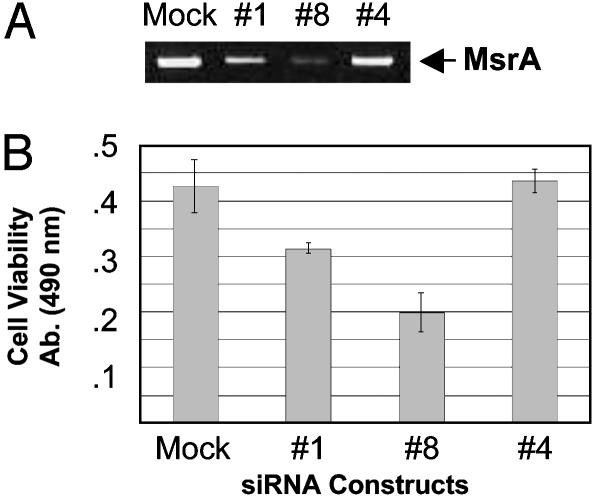
MsrA Is Important for Lens Cell Viability in the Absence of Exogenously Added Oxidative Stress. Decreased MsrA levels resulted in reduced lens cell viability by ≈20% even in the absence of H2O2 (Fig. 4B), suggesting that MsrA could be required for normal cell function. To further explore this possibility, the viability of HLE cells in the absence of exogenously added stress was determined in mock-transfected HLE cells, HLE cells transfected with an siRNA that reduces MsrA transcript levels by ≈50% relative to mock-transfected cells (siRNA #1), an siRNA that reduces MsrA transcript levels by ≈80% relative to mock-transfected cells (siRNA #8), and, as a control, an siRNA that is designed to target the MsrA transcript but that is ineffective in reducing the level of MsrA transcript (siRNA #4). The sequences of the siRNAs are listed in Materials and Methods. The data are shown in Fig. 5. No difference in cell viability was detected between mock-transfected cells and cells transfected with siRNA #4 (Fig. 5B). In contrast, cells transfected with siRNAs #1 and #8 exhibited ≈25% and 54% decreased viability relative to mock-transfected cells (Fig. 5B). The differences in cell viability are proportional to MsrA mRNA levels (compare A with B in Fig. 5), providing further evidence that reduction of MsrA causes reduced viability of HLE cells in the absence of exogenously added stress.
Fig. 5.
Viability of human lens cells treated with MsrA-specific siRNAs in the absence of oxidative stress. (A) Ethidium bromide-stained gel showing MsrA-specific gene suppression at 72 h posttransfection with indicated siRNAs relative to mock-transfected control cells. (B) Representative graph of cell-viability measured by MTS assay 72 h posttransfection with indicated siRNAs and mock-transfected cells examined in the presence of serum. Error bars represent standard deviations of four separate cell-viability assays.
Discussion
Previous studies have demonstrated expression of MsrA in multiple tissues including the kidney, retinal pigmented epithelium, brain, blood, and alveolar macrophages (36). Here, high levels of MsrA transcript and protein were detected in the human lens epithelium and the lens cortical and nuclear fiber cells where there is no protein turnover, suggesting a role for MsrA in defending this region of the lens against oxidation. MsrA has previously been shown to play a role in defense against oxidative stress in multiple nonlens systems (37–39). In this report we also demonstrate that overexpression of MsrA protects lens cells against H2O2-induced oxidative stress, whereas decreased expression of MsrA results in increased sensitivity to oxidative stress and decreased lens cell viability even in the absence of stress.
MsrA transcript exhibits ≈2-fold higher levels in the lens epithelium relative to the terminally differentiated fiber cells (Fig. 1 A). MsrA protein is also present at the highest levels in the lens epithelium relative to cortical fibers, with detectable but lower levels in the nucleus of the lens (Fig. 1B). Immunohistochemical staining for MsrA in a whole human lens paralleled the Western analysis. Intense staining was observed in the lens epithelium with lower levels detected in the cortical fibers (Fig. 2 A and B). The lens epithelium, which is essential for the growth, differentiation, and homeostasis of the entire organ (40, 41), contains the highest levels of enzymes and transport systems in the lens (42–44). It is also the first part of the lens exposed to oxidative stress (43, 44). Multiple studies suggest that the lens epithelium is capable of communicating with the underlying fiber cells (45, 46), and damage to the lens epithelium and its enzyme systems is known to result in cataract formation (6, 47–49). Importantly, oxidative damage to the lens epithelium is believed to be an initiating factor of cataractogenesis (6). The high abundance of MsrA in the lens epithelium and fibers suggests that MsrA plays an important role in defending the lens against oxidative stress. The intense staining for MsrA in the fiber cells of the lens (Fig. 3 C and D) suggests an important function for MsrA in the lens fibers that are particularly prone to oxidative stress damage because they do not turnover and are incapable of replenishing damaged proteins. The high levels of MsrA expression in the fiber cells suggest that the enzyme is possibly involved in the long-term repair and maintenance of crystallins, which constitute ≈40% of the wet weight of the lens and are primarily responsible for lens transparency (50).
Previous studies have revealed the ability of MsrA overexpression to aid cells in defense against oxidative-stress-induced damage including protection of PC12 cells from hypoxia-induced cell death (37) and human T lymphocytes against the cytotoxic effects of H2O2 stress (28). Here we extend these studies to demonstrate that exogenous expression of MsrA in HLE cells confers resistance to H2O2-induced oxidative damage. Overexpression of MsrA to levels approaching a 10-fold increase relative to uninfected control cells (Fig. 4A) resulted in as much as a 40% increase in cell viability over H2O2 concentrations ranging from 800 to 950 μM. This effect appears to be specific for MsrA overexpression because multiple cell lines created from separately prepared viral stocks resulted in similar protection against H2O2-induced stress. In addition, positive control cells created by using a non-MsrA-overexpressing virus exhibited responses to H2O2 exposure that were nearly identical to those of uninfected control cells (data not shown). The concentrations of H2O2 used in this study to induce cell death fall well within the range of concentrations used in many other studies examining H2O2-induced cell death in multiple cell types including HLE cells (51), human retinal pigmented epithelial cells (52), and human neuroblastoma cells (53).
In addition to the protective effects that overexpression of MsrA confers to cells in the presence of oxidative stress, deletion of the MsrA gene in E. coli and yeast renders them more sensitive to oxidative stress conditions (29, 30). Similar effects are observed in mammals. MsrA-deficient mice exhibit a decrease in lifespan of 10% under standard conditions and upwards of 50% under hyperoxic conditions (31). The present work demonstrates that decreased levels of MsrA, through siRNA-mediated gene silencing, in HLE cells results in an ≈25% increase in H2O2 sensitivity over H2O2 concentrations ranging from 570 to 720 μM. Relative to the overexpression studies, the lower levels of H2O2 used in the siRNA studies suggests that the transfection process has a negative effect on cell viability relative to untransfected control cells. This phenomenon is likely to result from an increase in the cell's membrane permeability upon transfection, although the exact mechanism is not known. However, multiple, separately conducted transfections resulted in nearly identical responses of the cells to H2O2, and statistical analysis of each replicate produced the same differences in viability between mock- and siRNA-transfected cells. In addition, cells transfected with a siRNA that is ineffective in decreasing the levels of MsrA exhibited the same responses to H2O2 as the mock-transfected cells. Taken together, these data indicate that the observed differences in cell viability are attributed to decreased levels of MsrA and are not the result of nonspecific effects caused by the presence of double-stranded RNA molecules or the transfection process.
Silencing of the MsrA gene also resulted in a significant decrease in lens cell viability even in the absence of exogenously added stress, providing evidence that MsrA is important for normal lens cell function. Unlike the H2O2 treatments that required the use of serum-free media, these studies were conducted in the presence of serum and therefore indicate that serum starvation is not a factor in the decreased cell viability observed upon transfection of cells when using MsrA siRNAs. This effect was proportional to the level of MsrA silencing elicited by two separate siRNAs and was specific for effective MsrA siRNAs. Because the MTS assay used in this procedure measures the activity of mitochondrial enzymes, this result suggests that MsrA may play a role in mitochondrial function that could include detoxification of superoxide generated during respiration and/or repair of mitochondrial proteins damaged through oxidation. H2O2 is produced at a relatively high rate in cells as a byproduct of normal aerobic metabolism (54) and is known to induce cellular damage through the depletion of ATP, glutathione, and NADPH levels, by the generation of hydroxyl radicals through Fenton reactions (55, 56) and through DNA strand breakage (57). Mitochondria are a main target for reactive oxygen species damage, and H2O2 is known to induce a mitochondrial permeability transition and disrupt the mitochondrial membrane potential, resulting in the release of cytochrome c into the cytosol and thereby triggering cells to undergo apoptosis through the activation of caspase 3 (58). MsrA is reported to consist of a cytosolic and mitochondrial form (59, 60). It is possible that the decrease in cell viability demonstrated in the present report results from multiple phenomenon, and studies are currently underway to determine the exact roles that cytosolic MsrA, mitochondrial MsrA, and MsrBs play in normal cell function.
The present study establishes a potential role for MsrA in defense of lens cells against oxidative stress and in normal lens cell function. These data, in conjunction with increased methionine sulfoxide content in the human lens with age and upon cataract formation, provide evidence that MsrA may be involved in the etiology of cataract formation. Although the targets for MsrA action in the lens have yet to be defined, it has been shown that oxidation of α-crystallin results in a loss of chaperone activity (2, 61) that could play a role in cataract formation (62). Another likely target for MsrA repair are the γ-crystallins, which are rich in methionine residues and are one of the first lens proteins to aggregate in cataract (47). MsrA function depends on the reducing system, and NADPH levels have been shown to decrease rapidly upon cataract formation (63). The targets of MsrA action in the lens have yet to be determined.
Acknowledgments
We thank Dr. Herbert Weissbach for advice and guidance during the course of this work, Drs. Herbert Weissbach and Nat Brot for providing the MsrA antibody used in this study, Nancy Sheets for help in this work, and the West Virginia Eye Bank and the Lions Eye Bank of Oregon for providing the lenses used in this study. This work is in partial fulfillment of the requirements for the Ph.D. degree of J.R.H. at West Virginia University. This work was supported by National Eye Institute–National Institutes of Health Grant EY13022 (to M.K.).
Abbreviations: HLE, human lens epithelial; Msr, methionine sulfoxide reductase; MTS, 3-(4,5-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; siRNA, short interfering RNA.
References
- 1.Kupfer, C. U. B. G. T. (1994) Leading Causes of Visual Impairment Worldwide, Principles and Practice of Ophthalmology Basic Science (Saunders, Philadelphia), pp. 1249-1255.
- 2.Smith, J. B., Jiang, X. & Abraham, E. C. (1997) Free Radical Res. 26, 103-111. [DOI] [PubMed] [Google Scholar]
- 3.McNamara, M. & Augusteyn, R. C. (1984) Exp. Eye Res. 38, 45-56. [DOI] [PubMed] [Google Scholar]
- 4.Bodaness, R. S., Leclair, M. & Zigler, J. S., Jr. (1984) Arch. Biochem. Biophys. 231, 461-469. [DOI] [PubMed] [Google Scholar]
- 5.Zigler, J. S., Jr., Huang, Q. L. & Du, X. Y. (1989) Free Radical Biol. Med. 7, 499-505. [DOI] [PubMed] [Google Scholar]
- 6.Spector, A. (1995) FASEB J. 9, 1173-1182. [PubMed] [Google Scholar]
- 7.Truscott, R. J. & Augusteyn, R. C. (1977) Biochim. Biophys. Acta 492, 43-52. [DOI] [PubMed] [Google Scholar]
- 8.Garner, M. H. & Spector, A. (1980) Proc. Natl. Acad. Sci. USA 77, 1274-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horwitz, J. (1992) Proc. Natl. Acad. Sci. USA 89, 10449-10453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsui, H., Lin, L. R., Ho, Y. S. & Reddy, V. N. (2003) Invest. Ophthalmol. Vis. Sci. 44, 3467-3475. [DOI] [PubMed] [Google Scholar]
- 11.Behndig, A., Karlsson, K., Reaume, A. G., Sentman, M. L. & Marklund, S. L. (2001) Free Radical Biol. Med. 31, 738-744. [DOI] [PubMed] [Google Scholar]
- 12.Packer, L. a. C. E. (1995) Biothiols in Health and Disease (Dekker, New York).
- 13.Harding, J. J., Blakytny, R. & Ganea, E. (1996) Biochem. Soc. Trans. 24, 881-884. [DOI] [PubMed] [Google Scholar]
- 14.Rathbun, W. B., Schmidt, A. J. & Holleschau, A. M. (1993) Invest. Ophthalmol. Vis. Sci. 34, 2049-2054. [PubMed] [Google Scholar]
- 15.Ikebe, H., Susan, S. R., Giblin, F. J., Reddan, J. R. & Reddy, V. N. (1989) Exp. Eye Res. 48, 421-432. [DOI] [PubMed] [Google Scholar]
- 16.Yang, Y., Sharma, R., Cheng, J. Z., Saini, M. K., Ansari, N. H., Andley, U. P., Awasthi, S. & Awasthi, Y. C. (2002) Invest. Ophthalmol. Vis. Sci. 43, 434-445. [PubMed] [Google Scholar]
- 17.Xing, K. Y. & Lou, M. F. (2002) Exp. Eye Res. 74, 113-122. [DOI] [PubMed] [Google Scholar]
- 18.Spector, A., Li, D., Ma, W., Sun, F. & Pavlidis, P. (2002) Invest. Ophthalmol. Vis. Sci. 43, 3251-3264. [PubMed] [Google Scholar]
- 19.Brot, N., Weissbach, L., Werth, J. & Weissbach, H. (1981) Proc. Natl. Acad. Sci. USA 78, 2155-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caldwell, P., Luk, D. C., Weissbach, H. & Brot, N. (1978) Proc. Natl. Acad. Sci. USA 75, 5349-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciorba, M. A., Heinemann, S. H., Weissbach, H., Brot, N. & Hoshi, T. (1997) Proc. Natl. Acad. Sci. USA 94, 9932-9937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson, D. & Travis, J. (1979) J. Biol. Chem. 254, 4022-4026. [PubMed] [Google Scholar]
- 23.Swaim, M. W. & Pizzo, S. V. (1988) J. Leukocyte Biol. 43, 365-379. [DOI] [PubMed] [Google Scholar]
- 24.Vogt, W. (1995) Free Radical Biol. Med. 18, 93-105. [DOI] [PubMed] [Google Scholar]
- 25.Weissbach, H., Etienne, F., Hoshi, T., Heinemann, S. H., Lowther, W. T., Matthews, B., St. John, G., Nathan, C. & Brot, N. (2002) Arch. Biochem. Biophys. 397, 172-178. [DOI] [PubMed] [Google Scholar]
- 26.Moskovitz, J., Weissbach, H. & Brot, N. (1996) Proc. Natl. Acad. Sci. USA 93, 2095-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan, H., Tang, X. D., Chen, M. L., Joiner, M. L., Sun, G., Brot, N., Weissbach, H., Heinemann, S. H., Iverson, L., Wu, C. F., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 2748-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moskovitz, J., Flescher, E., Berlett, B. S., Azare, J., Poston, J. M. & Stadtman, E. R. (1998) Proc. Natl. Acad. Sci. USA 95, 14071-14075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moskovitz, J., Rahman, M. A., Strassman, J., Yancey, S. O., Kushner, S. R., Brot, N. & Weissbach, H. (1995) J. Bacteriol. 177, 502-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moskovitz, J., Berlett, B. S., Poston, J. M. & Stadtman, E. R. (1997) Proc. Natl. Acad. Sci. USA 94, 9585-9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moskovitz, J., Bar-Noy, S., Williams, W. M., Requena, J., Berlett, B. S. & Stadtman, E. R. (2001) Proc. Natl. Acad. Sci. USA 98, 12920-12925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spector, A., Scotto, R., Weissbach, H. & Brot, N. (1982) Biochem. Biophys. Res. Commun. 108, 429-434. [DOI] [PubMed] [Google Scholar]
- 33.Hawse, J. R., Hejtmancik, J. F., Huang, Q., Sheets, N. L., Hosack, D. A., Lempicki, R. A., Horwitz, J. & Kantorow, M. (2003) Mol. Vis. 9, 515-537. [PMC free article] [PubMed] [Google Scholar]
- 34.Kantorow, M., Huang, Q., Yang, X. J., Sage, E. H., Magabo, K. S., Miller, K. M. & Horwitz, J. (2000) Mol. Vis. 6, 24-29. [PMC free article] [PubMed] [Google Scholar]
- 35.Oppermann, B., Zhang, W., Magabo, K. & Kantorow, M. (2001) Invest. Ophthalmol. Vis. Sci. 42, 188-193. [PMC free article] [PubMed] [Google Scholar]
- 36.Moskovitz, J., Jenkins, N. A., Gilbert, D. J., Copeland, N. G., Jursky, F., Weissbach, H. & Brot, N. (1996) Proc. Natl. Acad. Sci. USA 93, 3205-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yermolaieva, O., Xu, R., Schinstock, C., Brot, N., Weissbach, H., Heinemann, S. H. & Hoshi, T. (2004) Proc. Natl. Acad. Sci. USA 101, 1159-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh, V. K. & Moskovitz, J. (2003) Microbiology 149, 2739-2747. [DOI] [PubMed] [Google Scholar]
- 39.St John, G., Brot, N., Ruan, J., Erdjument-Bromage, H., Tempst, P., Weissbach, H. & Nathan, C. (2001) Proc. Natl. Acad. Sci. USA 98, 9901-9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piatigorsky, J. (1981) Differentiation 19, 134-153. [DOI] [PubMed] [Google Scholar]
- 41.Bloemendal, H. (1981) in Molecular and Cellular Biology of the Eye Lens, ed. Bloemendal, H. (Wiley, New York), pp. 1-47.
- 42.Reddy, V. N. (1971) Exp. Eye Res. 11, 310-328. [DOI] [PubMed] [Google Scholar]
- 43.Spector, A. (1982) in Aging of the Lens and Cataract Formation, eds. Sekuler, R., Kline, D. & Dismukes, K. (Liss, New York), pp. 27-43.
- 44.Reddan, J. R. (1982) in Cell Biology of the Eye, ed. McDevitt, D. S. (Academic, New York), pp. 299-375.
- 45.Rae, J. L., Bartling, C., Rae, J. & Mathias, R. T. (1996) J. Membr. Biol. 150, 89-103. [DOI] [PubMed] [Google Scholar]
- 46.Bassnett, S., Kuszak, J. R., Reinisch, L., Brown, H. G. & Beebe, D. C. (1994) J. Cell Sci. 107, 799-811. [DOI] [PubMed] [Google Scholar]
- 47.Brown, N. P. & Bron, N. J. (1996) Lens Disorders: A Clinical Manual of Cataract Diagnosis (Butterworth–Heinemann, Boston), pp. 1-135.
- 48.Harding, J. J. & Crabb, M. J. (1984) in The Lens: Development, Proteins, Metabolism, and Cataract, The Eye, ed. Davson, H. (Academic, Orlando, FL), Vol. 1B, pp. 207-492. [Google Scholar]
- 49.Hightower, K. R. (1995) Curr. Eye Res. 14, 71-78. [DOI] [PubMed] [Google Scholar]
- 50.Delaye, M. & Tardieu, A. (1983) Nature 302, 415-417. [DOI] [PubMed] [Google Scholar]
- 51.Paron, I., D'Elia, A., D'Ambrosio, C., Scaloni, A., D'Aurizio, F., Prescott, A., Damante, G. & Tell, G. (2004) Biochem. J. 378, 929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsui, H., Lin, L. R., Singh, D. P., Shinohara, T. & Reddy, V. N. (2001) Invest. Ophthalmol. Vis. Sci. 42, 2935-2941. [PubMed] [Google Scholar]
- 53.Ruffels, J., Griffin, M. & Dickenson, J. M. (2004) Eur. J. Pharmacol. 483, 163-173. [DOI] [PubMed] [Google Scholar]
- 54.Boveris, A. & Chance, B. (1973) Biochem. J. 134, 707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michiels, C., Raes, M., Toussaint, O. & Remacle, J. (1994) Free Radical Biol. Med. 17, 235-248. [DOI] [PubMed] [Google Scholar]
- 56.Henle, E. S. & Linn, S. (1997) J. Biol. Chem. 272, 19095-19098. [DOI] [PubMed] [Google Scholar]
- 57.Dringen, R. & Hamprecht, B. (1997) Brain Res. 759, 67-75. [DOI] [PubMed] [Google Scholar]
- 58.Tada-Oikawa, S., Oikawa, S., Kawanishi, M., Yamada, M. & Kawanishi, S. (1999) FEBS Lett. 442, 65-69. [DOI] [PubMed] [Google Scholar]
- 59.Hansel, A., Kuschel, L., Hehl, S., Lemke, C., Agricola, H. J., Hoshi, T. & Heinemann, S. H. (2002) FASEB J. 16, 911-913. [DOI] [PubMed] [Google Scholar]
- 60.Vougier, S., Mary, J. & Friguet, B. (2003) Biochem. J. 373, 531-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cherian, M. & Abraham, E. C. (1995) Biochem. Biophys. Res. Commun. 208, 675-679. [DOI] [PubMed] [Google Scholar]
- 62.Brady, J. P., Garland, D., Duglas-Tabor, Y., Robison, W. G., Jr., Groome, A. & Wawrousek, E. F. (1997) Proc. Natl. Acad. Sci. USA 94, 884-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee, S. M., Schade, S. Z. & Doughty, C. C. (1985) Biochim. Biophys. Acta 841, 247-253. [DOI] [PubMed] [Google Scholar]