Abstract
Functional abnormalities in fear circuitry are likely to underlie the pathophysiology of pediatric posttraumatic stress disorder (PTSD), but the few studies to date have yielded conflicting findings. Furthermore, network level functional connectivity and age-related disruptions in fear circuitry have not been thoroughly explored. In a cross-sectional design, 24 healthy and 24 medication-free youth with severe PTSD completed an event-related emotion-processing task during functional MRI. Youth viewed threat and neutral images, half of which were paired with a neutral male face. Group- and age-related differences in brain activation were examined in the medial prefrontal cortex (mPFC), amygdala, and hippocampus. Amygdala functional connectivity was examined using a seed-based approach. PTSD youth showed hyperactivation of the dorsal anterior cingulate cortex (dACC) to threat images. In the dorsomedial PFC (dmPFC), age positively predicted activation in healthy youth but negatively predicted activation in PTSD youth. In the amygdala functional connectivity analysis, PTSD youth showed decreased amygdala–mPFC connectivity to threat images. Furthermore, age positively predicted amygdala–vmPFC connectivity in healthy youth, but negatively predicted connectivity in PTSD youth. Finally, dmPFC activation and amygdala–mPFC connectivity were inversely related to PTSD severity. Pediatric PTSD involves abnormal functional activation and connectivity in fear circuitry. Specifically, dACC hyperactivation is consistent with abnormal promotion of fear responses, whereas reduced amygdala–mPFC connectivity suggests impaired regulation of amygdala responses to threat. Importantly, age-dependent decreases in dmPFC activation and amygdala–vmPFC connectivity may indicate abnormal developmental processes in key emotion pathways in pediatric PTSD.
INTRODUCTION
Pediatric posttraumatic stress disorder (PTSD) is a debilitating illness affecting 5% of youth by the age of 18 years (McLaughlin et al, 2013). Youth with PTSD experience impaired functioning (Carrion et al, 2002), emotional and sleep disturbances (Kovachy et al, 2013), and increased rates of mood and anxiety disorders (Famularo et al, 1996). Abnormalities in threat processing are central to pediatric PTSD; however, the underlying neural substrates and potential developmental abnormalities remain incompletely understood. This information is vital for developing biologically and developmentally informed interventions.
Impaired fear regulation is characteristic of PTSD (Pitman et al, 2012) and neuroimaging studies of adult PTSD suggest abnormalities in the neural circuitry underlying the processing of threat and regulation of fear responses. Common findings in adult PTSD include hyperactivation of the amygdala and dorsal anterior cingulate cortex (dACC) (Pitman et al, 2012), areas important in threat appraisal and the promotion of fear responses. Studies also suggest impaired recruitment of the ventromedial prefrontal cortex (vmPFC) and the hippocampus (Etkin et al, 2011; Patel et al, 2012; Pitman et al, 2012), areas important in the extinction and contextual gating of fear responses. However, studies of functional brain connectivity in adult PTSD have yielded conflicting findings, including abnormal dACC connectivity (Lanius et al, 2004), and both greater (Fonzo et al, 2010; Gilboa et al, 2004; St Jacques et al, 2011) and lower (Stevens et al, 2013) amygdala–mPFC connectivity.
To date, few studies have examined functional brain abnormalities during emotion processing in youth with posttraumatic stress symptoms (PTSS), with varying results. Amygdala hyperactivation has been reported in one study of youth PTSS (Garrett et al, 2012), with negative findings in two others (Crozier et al, 2014; Yang et al, 2004). Surprisingly, none of these studies found group differences in dACC activation. Regarding the mPFC, prior studies have reported decreased rostral ACC (rACC) (Yang et al, 2004) activation but increased vmPFC activation (Garrett et al, 2012) in youth with PTSS. Decreased dorsomedial PFC (dmPFC) activation has also been observed, but only in female youth, suggesting potential sex differences in youth with PTSS (Crozier et al, 2014). Finally, only one reported study has examined functional brain connectivity in youth with PTSS. A study of adolescent sexual assault victims, largely subclinical for PTSD, reported an inverse relationship between PTSS and amygdala–rACC functional connectivity, although no group differences in connectivity were reported (Cisler et al, 2013). These studies provide initial evidence of abnormal prefrontal–amygdala function during emotion processing in pediatric PTSD, yet their differing findings also highlight the need for additional study.
Differences in emotion tasks and sample characteristics may account for some of the variability in functional MRI (fMRI) findings in pediatric PTSD. For example, most of the prior studies have employed emotional and neutral faces. It remains unclear whether similar abnormalities are present during processing of threat imagery and whether threat context alters subsequent face processing in pediatric PTSD. Furthermore, developmental differences in prefrontal–amygdala function may have a role but have not been previously explored in pediatric PTSD. The PFC undergoes structural change into early adulthood (Giedd et al, 1999; Gogtay et al, 2004). These structural changes are paralleled by age-related decreases in amygdala activation and changes in medial prefrontal–amygdala connectivity (Gee et al, 2013b; Vink et al, 2014). Childhood stress may shift these trajectories by increasing amygdala activation, eg (McCrory et al, 2011; Swartz et al, 2015), spurring early development of prefrontal–amygdala connectivity following maternal deprivation (Gee et al, 2013a), or weakening prefrontal–amygdala connectivity following maltreatment experiences (Birn et al, 2014; Herringa et al, 2013). However, it remains unknown whether development of fear circuit function is altered in pediatric PTSD. Finally, prior studies have examined youth with a wide range of PTSS and it remains unclear whether similar abnormalities are present in more severe PTSD. Our prior structural brain analysis in this sample of youth with severe PTSD revealed both overall group differences and potential developmental abnormalities in fear circuit structure, namely decreased vmPFC volume and age-related decline in hippocampus volume (Keding and Herringa, 2015). Thus, we aimed to extend this analysis in the current study to functional brain abnormalities in pediatric PTSD, including age-related effects and functional connectivity analyses.
To address these knowledge gaps, we used fMRI to elucidate abnormalities during emotion processing in a cross-sectional sample of pediatric PTSD compared with non-traumatized healthy youth. We employed an fMRI task using threat-related and neutral images, either of which could be followed by neutral male faces. This allowed the investigation of functional differences in threat processing, while also exploring affective priming effects on subsequent face processing. This task thus expands on previous work by examining both primary and associative mechanisms of altered threat processing in pediatric PTSD. We predicted that pediatric PTSD would be associated with amygdala and dACC hyperactivity, and mPFC hypoactivity to threat-related images. In addition, we predicted that PTSD youth would exhibit decreased functional connectivity between the amygdala and mPFC, but increased amygdala–dACC connectivity, relative to healthy youth. Within these analyses, we examined age-related differences in this circuitry as an indicator of potential developmental abnormalities in pediatric PTSD. We predicted that pediatric PTSD would be characterized by greater amygdala reactivity and weaker amygdala–mPFC connectivity with age, reflecting a developmental shift in fear circuitry toward promotion of fear responses in stress-vulnerable youth.
MATERIALS AND METHODS
Participants
The present sample consisted of 27 healthy non-traumatized youth and 26 youth with PTSD between the ages of 8 and 18 years. Participant recruitment has been previously described (Keding and Herringa, 2015) and is summarized in detail in Supplementary Information. Participant data were excluded after data collection for excessive head motion during the scan, resulting in at least 20% of volumes being censored (n=2 healthy, n=1 PTSD), failure to respond to at least 75% of trials during the scan (n=1 PTSD), or terminating the scan early (n=1 healthy). Thus, final groups for analysis consisted of 24 healthy youth and 24 youth with PTSD (see Table 1 for descriptive statistics). All participants provided written consent or assent with caregiver consent when applicable. The University of Wisconsin Health Sciences Institutional Review Board approved all procedures.
Table 1. Participant Characteristics.
| Healthy | PTSD | |
|---|---|---|
| N | 24 (13F) | 24 (16F) |
| Age | 13.8 (8.1–18.6, ±2.9) | 14.5 (8.1–18.8, ±2.8) |
| Tanner stage | 3.0 (1.0–4.5, ±1.3) | 3.1 (1.0–5.0, ±1.4) |
| IQ | 110.4 (89–133, ±12.0) | 104.4 (87–125, ±11.4) |
| Left handed (n) | 0 | 3 |
| Index trauma (n) | — | Sexual abuse (10) Witnessing violence (3) Traumatic death of loved one (7) Accident (4) |
| Comorbid diagnoses (n) | — | Major depressive disorder (16) Depressive disorder NOS (2) Separation anxiety disorder (4) Social anxiety disorder (6) Generalized anxiety disorder (3) ADHD (4) |
| PTSD duration | — | 44.2 (±38.9) months |
| PTSD Reaction Index | — | 46.7 (±13.6) |
| CAPS-CA past month | — | 66.1 (±19.5) |
| MFQ | 2.7 (0–9, ±2.3) | 24.0 (10–50, ±10.4)a |
| SCARED | 6.9 (1–18, ±4.7) | 31.4 (11–60, ±13.8)a |
| Past psychiatric medication (n) | — | Stimulant (8) Antidepressant (7) Alpha-2 agonist (1) Benzodiazepine (1) |
Abbreviations: CAPS-CA, Clinician-Administered PTSD Scale for Children and Adolescents; MFQ, Mood and Feelings Questionnaire; PTSD, posttraumatic stress disorder; SCARED, Screen for Child Anxiety Related Emotional Disorders.
PTSD and healthy youth did not differ in terms of age, pubertal stage, or IQ. Note that the CAPS-CA was not obtained for the first three PTSD participants.Numbers in parentheses are range and SD unless otherwise indicated.
Differs from healthy group, P<0.05.
Assessments
Each participant and a caregiver reporter underwent a trauma and psychiatric screen by a board-certified child psychiatrist (RJH) with the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS) (Kaufman et al, 1997). A PTSD diagnosis was determined using DSM-IV criteria by combination of the KSADS and Clinician-Administered PTSD Scale for Children and Adolescents (CAPS-CA) (Nader et al, 1998; Weathers et al, 2001). A PTSD diagnosis required at least five DSM-IV symptoms, including at least one from each symptom cluster, following Cohen et al (2011). Using these criteria, most youth (n=20 or 83%) in the PTSD group met the criteria for three symptom clusters (standard DSM-IV PTSD diagnosis). Of the remaining four, two met the criteria for two symptom clusters and two met the criteria for one symptom cluster. The CAPS-CA was not obtained for the first three included participants with PTSD. PTSD severity was additionally examined using the UCLA PTSD Reaction Index (PTSD-RI) (Steinberg et al, 2004). For the PTSD-RI, the greater of youth and caregiver report for each item was used, as this was most strongly correlated with CAPS scores (r=0.81, 0.71, and 0.56 for greater of youth/caregiver, youth only, and caregiver only, respectively). Depressive symptoms over the past 2 weeks were quantified with the Mood and Feelings Questionnaire (MFQ) (Costello and Angold, 1988). Anxiety symptoms over the past 3 months were quantified with the Screen for Child Anxiety Related Emotional Disorders (SCARED) (Birmaher et al, 1997). MFQ and SCARED scores were calculated using the average of youth and caregiver reports. Pubertal stage was assessed by self-report using the Tanner picture-based rating scale (Morris and Udry, 1980). IQ was estimated using the Full-Scale IQ-2 component of the Wechsler Abbreviated Scale of Intelligence-II (Wechsler, 2011).
Experimental Task
All participants completed two mock scans before MRI in which they performed a practice version of the experimental task. The experimental task is an emotion-processing task described previously (Schuyler et al, 2014). Briefly, in an event-related design, youth viewed 16 threat and 16 neutral images from the International Affective Picture Schedule (IAPS) (Lang et al, 2008) for 4 s (see Supplementary Information for stimulus list). Threat images were selected for content relating to trauma types common within the current sample (Table 1); specifically, the majority of the negative images depicted interpersonal violence, injuries, or motor vehicle accidents. Half of all images were followed (3 s post-image offset) by a neutral male face presented for 500 ms, to examine how emotional content affects subsequent face processing (Schuyler et al, 2014). To maximize attention during the task, participants assigned a valence (negative, neutral, or positive) to each IAPS image by pressing a button with their right hand. Task duration was ~8 min. Approximately 1 h after the task, participants provided memory and likability ratings for the eight faces seen during the task, as well as eight novel faces (see Supplementary Information).
Image Acquisition, Preprocessing, and Analysis
Detailed accounts of image acquisition, preprocessing, and analysis are described in Supplementary Information. Images were acquired on a 3.0 T GE Discovery MR750 scanner with an eight-channel radiofrequency head coil (General Electric Medical Systems, Waukesha, WI) at the University of Wisconsin–Madison Department of Psychiatry. Three-dimensional axial high-resolution T1 images were acquired with the following parameters: TE: 3.18 ms, TR: 8.16 ms, TI: 450 ms, slice thickness: 1 mm, 156 slices, flip angle: 12°, FOV: 25.6 cm, and matrix size: 256 by 256 that covered the entire brain. The functional scan was acquired with a gradient echo EPI sequence with the following parameters: TE: 22 ms, TR: 2150 ms, flip angle: 79°, slice thickness: 3 mm, gap: 0.5 mm, 41 sagittal slices, FOV: 224 mm, and matrix size: 64 by 64.
All image preprocessing was performed using AFNI (Cox, 1996) and FSL (Smith et al, 2004). T1 structural images were registered to the MNI 152 2 mm3 template with linear and non-linear warps (FSL's FLIRT/FNIRT). Functional data were slice-time and motion corrected before being aligned to their respective T1-weighted structural images. The first three volumes of EPI time series were removed due to T1-equilibrium effects and the transformation matrix used to register the T1-weighted structural image to the MNI template was then applied to the functional data. All subsequent analyses were performed in MNI space. Volume-to-volume displacement (SSD) was estimated from the six rigid body motion registration parameters. Any functional volume with SSD >1 mm and its preceding volume were censored. All included subjects had 13% or fewer volumes censored. Functional data were smoothed using a 6-mm Gaussian kernel and converted to percent signal change.
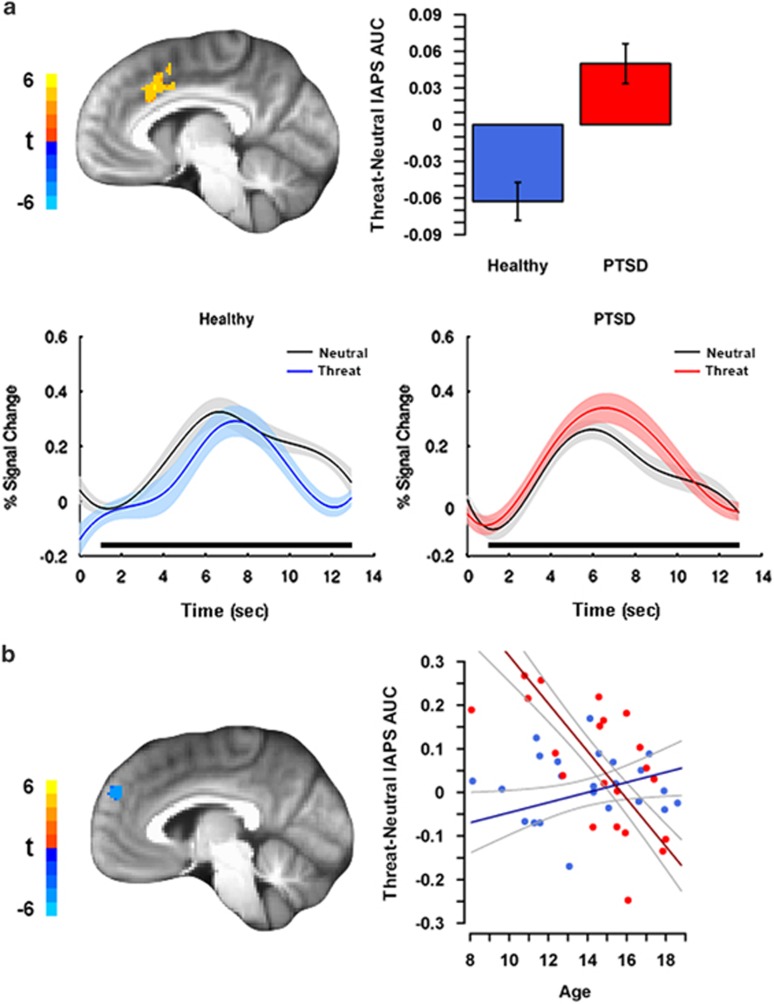
Functional data for individual subjects were analyzed with a general linear model using AFNI's 3dDeconvolve with tent basis functions, to estimate HRF shape for responses to all images. Variables modeled included six motion parameters, threat and neutral IAPS, threat- and neutral-primed faces, and four polynomial drift terms. HRF shape was estimated from 0 to 12.9 s post stimulus (six TRs) using seven tents. Area under the curve (AUC) was the primary dependent measure, calculated by averaging the β-coefficients for tents two through seven. Tent one was excluded from AUC analyses, because signal was expected to be near baseline for all subjects at that time point in the trial (see Figure 1a for example).
Figure 1.
Abnormal medial prefrontal cortex (mPFC) activation to threat in pediatric posttraumatic stress disorder (PTSD) compared with healthy youth. (a) Increased dorsal anterior cingulate cortex (dACC) activation to threat vs neutral images in youth with PTSD. Estimated hemodynamic response curves from dACC by International Affective Picture Schedule (IAPS) valence for each group show specific patterns of activation used to calculate area under the curve (AUC) differences for each group, with black bars showing those time points used for AUC calculation. (b) Age-related differences in left dorsomedial PFC (dmPFC) activation to threat vs neutral images in youth with PTSD compared with healthy youth. A group by age interaction revealed that age positively predicted dmPFC activation in healthy youth, but negatively predicted activation in youth with PTSD. For both panels, color bars correspond to the t-statistic value in each voxel of sagittal views. Plots of extracted average cluster AUC of percent signal change for each effect are shown, with healthy youth in blue and PTSD youth in red. Error bars/bands represent ±1 SE. Scatterplot points are raw data. Note that the voxelwise model included age, sex, and their interactions with group as factors.
Statistical Analysis
Analyses were conducted to examine group differences in activation during the threat-neutral IAPS contrast and for the threat-neutral paired face contrast, to examine contextual priming effects on face processing. Group models were fit using AFNI's 3dttest++. Age, sex, and their two-way interactions with group were included as covariates. A priori masks included the mPFC and, separately, bilateral amygdala/hippocampus (see Supplementary Information). Additional results outside of a priori regions surviving whole brain correction are also reported. Owing to the use of two separate a priori masks, a Bonferroni correction of our α-threshold was performed for the number of masks. Statistical maps were family-wise error (FWE) corrected for multiple comparisons at the cluster level (3dClustSim) using an initial voxel-wise threshold of Puncorrected<0.005. All clusters met a cluster-extent threshold of PFWE<0.025 for a priori regions (mPFC: 99 voxels, amygdala/hippocampus: 28 voxels), and PFWE<0.05 for whole brain (183 voxels).
We performed a psychophysiological interaction (PPI) (Gitelman et al, 2003) to explore group differences in fronto-amygdala connectivity during IAPS images. We separately seeded the left and the right amygdala, identified from the Talairach Daemon atlas (Lancaster et al, 2000). To ensure only those voxels involved in the task were seeded in the PPI analysis, amygdala PPI seeds were functionally defined as those voxels from the anatomical amygdala mask that were active across all subjects in the threat IAPS vs baseline contrast at P<0.1 (Schuyler et al, 2014). As PPI analysis requires an assumed HRF shape, we analyzed individual functional data again with a γ-HRF shape, which yielded similar group differences in activation to the tent function analyses. Individual-level analyses for PPI were conducted with the same model parameters as in the functional analyses, except that HRF shape was assumed and PPI models included regressors for the seed time series and the seed time series by IAPS condition interaction. PPI analyses were restricted to our a priori mPFC search region.
Secondary analyses of cluster activation/connectivity and clinical variables were performed in R (R Core Team, 2014), covarying for age and sex. If clusters survived the more stringent threshold Puncorrected<0.001, these cluster averages were used to increase anatomical specificity in the regression analyses. To examine effects of potentially confounding variables on primary findings, group analyses were repeated including variables such as past medication exposure and general anxiety and depressive symptoms. Within the PTSD group, relationships between cluster averages and clinical measures were conducted, including PTSD severity, illness duration, and general anxiety and depressive symptoms. Finally, the relationship of trauma exposure measures (time elapsed since index trauma and number of trauma types endorsed) with cluster averages was examined to assess for trauma-specific effects.
RESULTS
Participant Characteristics
Participant characteristics are summarized in Table 1. There were no significant differences between groups in age, sex, IQ, or pubertal stage (all P>0.13). Within the PTSD group, the most common index trauma was sexual abuse, followed by traumatic death of a loved one, accident, and witnessing violence. PTSD symptoms averaged 46.7 based on the PTSD-RI, which is indicative of severe PTSD. Of the 24 participants with PTSD, 21 had comorbid psychiatric illness, most commonly depressive disorders (n=18). Findings regarding face memorability and likability ratings can be found in Supplementary Information.
Regional Brain Activation to Threat vs Neutral Images
A summary of activation results is shown in Table 2. PTSD youth showed greater activation in the left dACC than in healthy youth (Figure 1a). In addition, there was a group by age interaction in the left dmPFC. Here, age positively predicted the left dmPFC activation in healthy youth, but negatively predicted the activation in PTSD youth (Figure 1b). No group or group by age effects were observed in the vmPFC or amygdala/hippocampus. Outside of a priori regions, PTSD youth had greater activation in the thalamus and bilateral precentral gyrus than in healthy youth. Finally, no group by sex effects were observed.
Table 2. Summary of Results for Regional Brain Activation and Amygdala Functional Connectivity in PTSD Relative to Healthy Youth.
| Contrast | Effect | Region | BA | Voxels | X | Y | Z | Peak t |
|---|---|---|---|---|---|---|---|---|
| Regional brain activation | ||||||||
| Threat-neutral images | PTSD>healthy | Left dACC | 32, 24, 6 | 565 | −6 | 18 | 38 | 5.01 |
| Left PCG | 6, 44 | 455 | −52 | 4 | 10 | 5.79 | ||
| Thalamus | — | 273 | −6 | −20 | 16 | 3.92 | ||
| Right PCG/STG | 44, 22 | 225 | 52 | 12 | 2 | 4.23 | ||
| Age: PTSD<healthy | Right pMC | 6 | 531 | 14 | 16 | 64 | −5.02 | |
| Right IPL | 40 | 280 | 46 | −42 | 40 | −4.24 | ||
| Left pMC | 6 | 278 | −22 | 0 | 48 | −3.92 | ||
| Left dmPFC | 9 | 189 | −4 | 54 | 30 | −4.2 | ||
| Functional connectivity (PPI) | ||||||||
| Right amygdala seed: threat-neutral images | Age: PTSD>healthy | Right dmPFC | 9 | 82 | 10 | 58 | 34 | 4.18 |
| Left amygdala seed: threat-neutral images | PTSD<healthy | Bilateral rACC/dmPFC | 32, 10, 9 | 93 | −6 | 46 | 12 | −4.22 |
| Left dmPFC | 9, 10 | 90 | −14 | 58 | 8 | −4.22 | ||
| Age: PTSD<healthy | Right vmPFC | 11 | 117 | 8 | 46 | −18 | −4.31 | |
| Left vmPFC | 11 | 81 | −8 | 46 | −16 | −3.79 | ||
Abbreviations: dACC, dorsal anterior cingulate cortex; dmPFC, dorsomedial prefrontal cortex; IPL, inferior parietal lobule; MFG, middle frontal gyrus; PCG, precentral gyrus; pMC, premotor cortex; PPI, psychophysiological interaction; rACC, rostral anterior cingulate cortex; STG, superior temporal gyrus; vmPFC, ventromedial prefrontal cortex.
Peak coordinates (X, Y, Z) are in MNI space, LPI orientation. All contrasts were PTSD minus healthy; thus, a positive t-statistic indicates PTSD>healthy. No group by sex effects were observed in any analysis.
Regional Brain Activation to Contextually Primed Faces
No significant group differences or interactions were detected within the mPFC, amygdala/hippocampus, or whole brain for threat- vs neutral-preceded faces. However, exploratory analysis of threat-preceded faces vs baseline fixation revealed vmPFC hypoactivation in PTSD youth (Supplementary Figure S4).
Amygdala Functional Connectivity During Threat vs Neutral Images
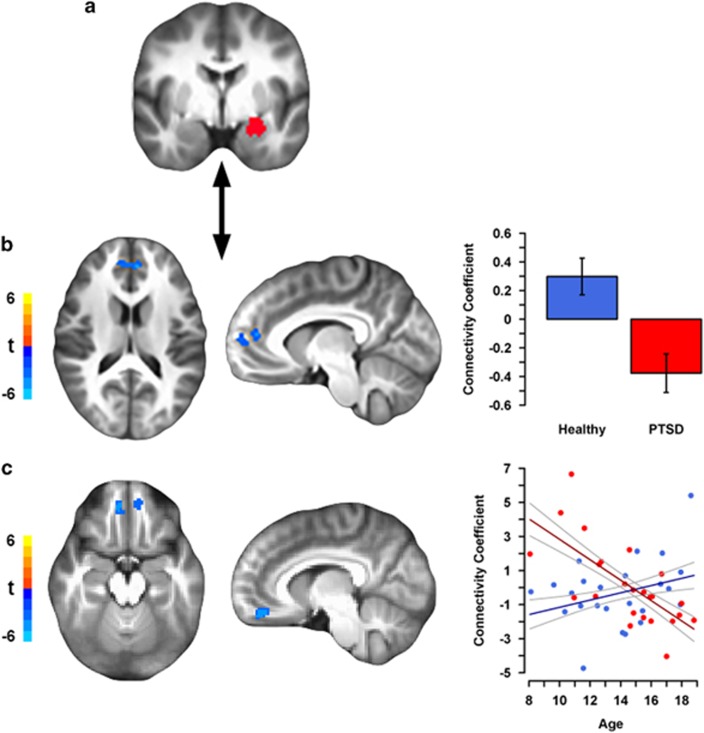
PPI results are summarized in Table 2. Compared with healthy youth, PTSD youth showed reduced connectivity between the left amygdala and bilateral rACC/dmPFC, and an adjacent cluster in the left dmPFC in the threat-neutral IAPS contrast (Figure 2b). No group differences were observed for the amygdala to dACC functional connectivity. In addition, there was a group by age interaction for the left amygdala to bilateral vmPFC connectivity (Figure 2c). Here, the amygdala–vmPFC connectivity increased with age in healthy youth but decreased with age in PTSD youth. For the right amygdala seed, there was a group by age interaction for connectivity to the right dmPFC. The right amygdala–dmPFC connectivity tended to decrease with age in healthy youth, but increased with age in PTSD youth. Finally, no group by sex effects were observed.
Figure 2.
Decreased prefrontal-amygdala connectivity to threat in pediatric posttraumatic stress disorder (PTSD) compared with healthy youth. (a) The left amygdala seed used in the psychophysiological interaction (PPI) analysis. (b) Reduced left amygdala–bilateral rostral anterior cingulate cortex (rACC)/dorsomedial prefrontal cortex (dmPFC) and –left dmPFC (visible in sagittal view) connectivity to threat vs neutral images in youth with PTSD. Data for bar graph come from the rACC/dmPFC cluster, although the left dmPFC plot is comparable. (c) Age-related differences in the left amygdala-bilateral ventromedial PFC (vmPFC) connectivity to threat vs neutral images in youth with PTSD compared with healthy youth. A group by age interaction revealed that age positively predicted amygdala–vmPFC connectivity in healthy youth but negatively predicted connectivity in youth with PTSD. The scatterplot is of connectivity coefficients from the right vmPFC cluster (results from the left vmPFC cluster are comparable). For each figure, axial and sagittal views of regional brain activation are shown on the left. Color bars correspond to the t-statistic value in each voxel. The left hemisphere is shown on the right side of the axial image. Plots of extracted average cluster connectivity coefficients for each effect are shown on the right, with healthy youth in blue and PTSD youth in red. Error bars/bands represent ±1 SE. Scatterplot points are raw data. Note that the voxelwise model included age, sex, and their interactions with group as factors.
Secondary Analyses
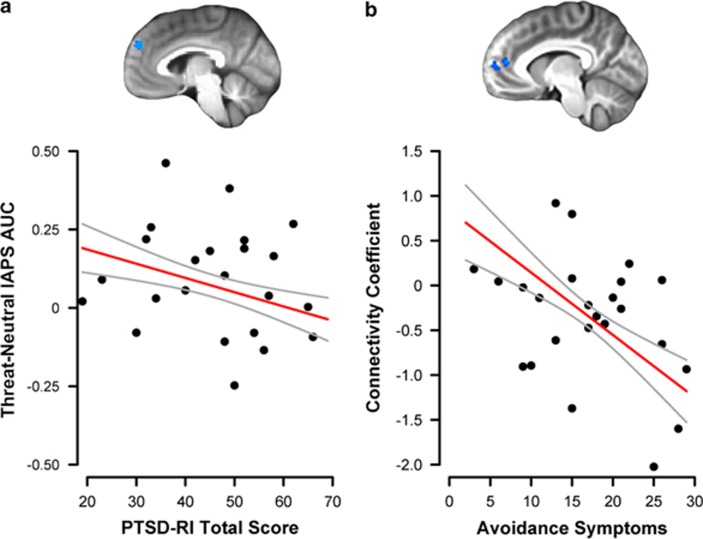
To investigate the relationship between activation or connectivity and PTSD symptom severity among PTSD youth, we regressed average activation/connectivity from each significant cluster on PTSD-RI scores and illness duration. The left dmPFC activation was inversely related to PTSD-RI total scores at a trend level (t19=−1.78, P=0.090; Figure 3) but unrelated to illness duration (P=0.41). Left amygdala–bilateral rACC/dmPFC connectivity was unrelated to PTSD-RI total score (P=0.18) or illness duration (P=0.96), but was inversely related to avoidance symptoms in the model using PTSD symptom clusters (t17=−2.61, P=0.018; Figure 3). No significant relationships were detected between other activation or connectivity clusters and PTSD severity or illness duration (all P>0.14). Finally, primary findings remained largely unchanged when controlled for potentially confounding variables and no significant associations were observed between activation/connectivity and trauma exposure measures, or general anxiety and depressive symptoms (see Supplementary Information).
Figure 3.
Relationship of posttraumatic stress disorder (PTSD) symptom severity to brain activation and connectivity results. (a) Among youth with PTSD, PTSD Reaction Index (PTSD-RI) total score was inversely related (trend level) to the left dorsomedial prefrontal cortex (dmPFC) activation to threat vs neutral images, using the cluster derived from the group contrast. (b) Among youth with PTSD, PTSD-RI avoidance/numbing symptoms were inversely related to the left amygdala–bilateral rostral anterior cingulate cortex (rACC)/dmPFC connectivity to threat vs neutral images, using the cluster derived from the group contrast. Clusters used in each analysis are presented above the scatterplot. Error bands represent ±1 SE. Scatterplot points are raw data.
DISCUSSION
To our knowledge, this is the first reported study to examine the functional neural correlates of emotion processing in pediatric PTSD that incorporates age-related effects and functional connectivity analyses. Our findings suggest that pediatric PTSD is characterized by overt functional abnormalities as well as age-related differences in fear circuitry when viewing threat-related content. Furthermore, nearly all of the group differences remained significant when covarying for potentially confounding variables and were unrelated to trauma exposure measures or general anxiety, or depressive symptoms, suggesting specificity to PTSD. Our results show that youth with PTSD exhibit hyperactivation of the dACC, but not amygdala, to threat images. In addition, PTSD youth showed reduced amygdala–mPFC connectivity, which was inversely related to PTSD severity, suggesting that impaired top-down regulation may contribute to dysregulated responses to threat in pediatric PTSD. In addition to these group differences, we found evidence of age-related functional abnormalities in PTSD youth, which were not accounted for by illness duration or time since trauma. Relative to healthy youth, PTSD youth showed age-related decline in dmPFC activation, which was inversely related to PTSD severity, and age-related decline in amygdala–vmPFC functional connectivity. Together, these novel findings suggest partial overlap with neural findings documented in adult PTSD, but also point to potential developmental abnormalities in fear circuit function in pediatric PTSD.
The present study revealed dACC hyperactivation to threat vs neutral images in youth with PTSD. The dACC is involved in threat appraisal (Kalisch and Gerlicher, 2014) and hyperactivation in adult PTSD is thought to have a role in exaggerated fear learning and expression (Pitman et al, 2012; Shin and Liberzon, 2010). Although dACC hyperactivation has been a relatively consistent finding in adult PTSD (Pitman et al, 2012), it has not previously been reported in pediatric PTSD/PTSS. This may be attributable to differences in emotion task, but could also be related to greater PTSD severity in our sample as compared with prior studies (eg, Cisler et al, 2013; Crozier et al, 2014; Garrett et al, 2012). Interestingly, we did not find evidence of abnormal amygdala–dACC connectivity in pediatric PTSD. Studies of adult PTSD have also failed to detect group differences in amygdala–dACC connectivity during emotion processing, although greater intrinsic amygdala–dACC connectivity has been reported (Brown et al, 2014). On the other hand, the explicit valuation of images in our task may have preferentially elicited connectivity differences between the amygdala and rACC/dmPFC, which is involved in conscious threat appraisal and fear regulation, and is discussed further below.
Surprisingly, we did not find evidence of amygdala hyperactivation in pediatric PTSD, even when separately examining reactivity and recovery periods (see Supplementary Information). Although meta-analyses support amygdala hyperactivation in adult PTSD (Etkin and Wager, 2007; Patel et al, 2012), multiple studies in both adult (Francati et al, 2007) and pediatric (Crozier et al, 2014; Yang et al, 2004) PTSD samples have failed to find such an effect. Although we did not observe group differences in amygdala activation, we did find evidence of reduced amygdala–mPFC functional connectivity in pediatric PTSD. Amygdala–mPFC connectivity was also inversely associated with avoidance symptoms, suggesting that communication between the mPFC and amygdala, and not simply amygdala activation itself, may be an important determinant of dysregulated fear in pediatric PTSD.
As noted, the present findings point to abnormalities in dmPFC function during threat processing in pediatric PTSD, including age-related decline in dmPFC activation and reduced amygdala–dmPFC connectivity. The dmPFC is important in the conscious appraisal of threat and subsequent regulation of emotional responses (Kalisch and Gerlicher, 2014), and is capable of modulating the amygdala based on threat context (Robinson et al, 2012). Furthermore, successful downregulation of negative emotion is associated with amygdala–dmPFC connectivity (Lee et al, 2012), and both anxiety (Kim et al, 2011) and PTSD (Birn et al, 2014) symptoms in adults have been associated with the relative loss of intrinsic amygdala–dmPFC anticorrelation. Our results are consistent with this and suggest that the ability of the dmPFC to modulate amygdala-dependent responses to threat may be compromised in pediatric PTSD, a notion supported by the relationship between dmPFC activation, amygdala–dmPFC connectivity, and PTSD severity.
Interestingly, we found evidence of age-related increases in the right amygdala–dmPFC connectivity in PTSD youth, which differs in part from reduced left amygdala–dmPFC connectivity. There is evidence that negative emotional stimuli are processed preferentially by the left amygdala (Fusar-Poli et al, 2009), suggesting that the left-sided amygdala–dmPFC deficits in PTSD youth may compromise the regulation of emotional responses to threatening stimuli. The significance of age-related increases in the right amygdala–dmPFC remain unclear at this time but could conceivably represent compensatory change in the face of the left-sided deficits.
In addition to reduced amygdala–mPFC connectivity, we also found evidence of age-related decline in amygdala–vmPFC functional connectivity in pediatric PTSD. Interestingly, this effect appears to be driven by greater connectivity in PTSD youth at younger ages, similar to older healthy youth, but lower connectivity in older PTSD youth, similar to younger healthy youth. This may indicate early compensatory development of amygdala–vmPFC connectivity mirroring findings in a maternally deprived sample (Gee et al, 2013a), but also suggest developmental weakening of amygdala–vmPFC connectivity mirroring the effects of childhood maltreatment experiences by late adolescence and early adulthood (Birn et al, 2014; Herringa et al, 2013). Although the exact significance of this apparent developmental shift remains unclear, our prior structural brain study in this sample revealed an inverse relationship between gray matter volume in a similar region of the vmPFC and re-experiencing symptoms (Keding and Herringa, 2015). Fear extinction and putatively safety signaling are mediated by differential signaling between the amygdala, vmPFC, and dACC (Senn et al, 2014). Thus, dACC hyperactivation, combined with age-related decline in amygdala–vmPFC connectivity, could lead to reduced safety signaling and fear overexpression in pediatric PTSD, an imbalance that may become more pronounced over the course of development in afflicted youth.
Our study documents novel findings of functional abnormalities in fear circuitry in pediatric PTSD. However, it is not without limitations. First, although our wide developmental age range of participants permits investigation of age-related effects in pediatric PTSD, our cross-sectional design prohibits causal inference regarding developmental differences or trauma timing on prefrontal development. If our age-related findings are not due to other confounding variables, the age-related findings may suggest that either PTSD chronically affects neural development or that ongoing abnormal neural development contributes to PTSD. Clarification of the effects of pediatric trauma and PTSD on development of fear circuits will be facilitated by hybrid cross-sectional and longitudinal designs in future studies. Second, the present study lacks a trauma-exposed comparison group. As such, it is possible that the abnormalities observed presently in PTSD youth result from trauma exposure rather than PTSD. Future studies including healthy, trauma-exposed youth are merited to explore potentially adaptive brain mechanisms following childhood trauma. Finally, this is a moderate sample size and would merit replication with a larger sample of youth.
In summary, the present findings offer novel insights into the neural underpinnings of emotion dysregulation in pediatric PTSD. Our findings suggest that pediatric PTSD is characterized by abnormal function and connectivity in prefrontal–amygdala circuits underlying threat processing and fear regulation. Although some of these abnormalities bear similarity to adult PTSD findings, age-related differences in fear circuitry also point to abnormal functional brain development in pediatric PTSD. Future studies employing longitudinal measures would be warranted to further explore these possibilities and determine whether timely intervention can restore the normal functioning and development of fear circuits in pediatric PTSD.
FUNDING AND DISCLOSURE
Funding for this study was provided by the National Institute of Mental Health Career Development Award (K08 MH100267, RJH), NARSAD Young Investigator Grant (RJH), American Academy of Child and Adolescent Psychiatry Junior Investigator Award (RJH), University of Wisconsin Institute for Clinical and Translational Research Translational Pilot Grant Award (NIH/NCATS UL1TR000427, RJH), and the University of Wisconsin School of Medicine and Public Health. Additional support for RCW was provided by the National Institutes of Health Training Grant (T32-GM007507) and the National Science Foundation Graduate Research Fellowship (DGE-1256259). Funding sources did not have any direct role in study design, analysis or interpretation of data, or preparation of the manuscript. Both authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
We thank Rachael Meline for her role in participant recruitment and data collection. We also thank Taylor Keding for his role in data collection and valuable comments on earlier drafts of this manuscript. We thank Drs Ned Kalin and Richard Davidson for editorial feedback on the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J et al (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. J Am Acad Child Adolesc Psychiatry 36: 545–553. [DOI] [PubMed] [Google Scholar]
- Birn RM, Patriat R, Phillips ML, Germain A, Herringa RJ (2014). Childhood maltreatment and combat posttraumatic stress differentially predict fear-related fronto-subcortical connectivity. Depress Anxiety 31: 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VM, Labar KS, Haswell CC, Gold AL, Mid-Atlantic MIRECC Workgroup, Beall SK et al (2014). Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 39: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Ray R, Reiss AL (2002). Toward an empirical definition of pediatric PTSD: the phenomenology of PTSD symptoms in youth. J Am Acad Child Adolesc Psychiatry 41: 166–173. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Scott Steele J, Smitherman S, Lenow JK, Kilts CD (2013). Neural processing correlates of assaultive violence exposure and PTSD symptoms during implicit threat processing: a network-level analysis among adolescent girls. Psychiatry Res 214: 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Mannarino AP, Iyengar S (2011). Community treatment of posttraumatic stress disorder for children exposed to intimate partner violence: a randomized controlled trial. Arch Pediatr Adolesc Med 165: 16–21. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Angold A (1988). Scales to assess child and adolescent depression: checklists, screens, and nets. J Am Acad Child Adolesc Psychiatry 27: 726–737. [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Crozier JC, Wang L, Huettel SA, De Bellis MD (2014). Neural correlates of cognitive and affective processing in maltreated youth with posttraumatic stress symptoms: does gender matter? Dev Psychopathol 26: 491–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R (2011). Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15: 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 164: 1476–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famularo R, Fenton T, Kinscherff R, Augustyn M (1996). Psychiatric comorbidity in childhood post traumatic stress disorder. Child Abuse Negl 20: 953–961. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB (2010). Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry 68: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francati V, Vermetten E, Bremner JD (2007). Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety 24: 202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S et al (2009). Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci 34: 418–432. [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A (2012). Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety 29: 449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH et al (2013. a). Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 110: 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M et al (2013. b). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci 33: 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A et al (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2: 861–863. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R et al (2004). Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry 55: 263–272. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ (2003). Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage 19: 200–207. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC et al (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101: 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Burghy CA, Stodola DE, Davidson RJ et al (2013). Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci USA 110: 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Gerlicher AMV (2014). Making a mountain out of a molehill: on the role of the rostral dorsal anterior cingulate and dorsomedial prefrontal cortex in conscious threat appraisal, catastrophizing, and worrying. Neurosci Biobehav Rev 42: 1–8. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36: 980–988. [DOI] [PubMed] [Google Scholar]
- Keding TJ, Herringa RJ (2015). Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology 40: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ (2011). Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex 21: 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovachy B, O'Hara R, Hawkins N, Gershon A, Primeau MM, Madej J et al (2013). Sleep disturbance in pediatric PTSD: current findings and future directions. J Clin Sleep Med 9: 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L et al (2000). Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp 10: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN (2008) International affective picture system (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8. University of Florida,: Gainesville, FL. [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Neufeld RW, Gati JS et al (2004). The nature of traumatic memories: a 4-T FMRI functional connectivity analysis. Am J Psychiatry 161: 36–44. [DOI] [PubMed] [Google Scholar]
- Lee H, Heller AS, van Reekum CM, Nelson B, Davidson RJ (2012). Amygdala-prefrontal coupling underlies individual differences in emotion regulation. Neuroimage 62: 1575–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA et al (2011). Heightened neural reactivity to threat in child victims of family violence. Curr Biol 21: R947–R948. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM et al (2013). Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. J Am Acad Child Adolesc Psychiatry 52: 815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris NM, Udry JR (1980). Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adolesc 9: 271–280. [DOI] [PubMed] [Google Scholar]
- Nader KO, Newman E, Weathers FW, Kaloupek DG, Kriegler JA, Blake DD et al (1998) Clinician-Administered PTSD Scale for Children and Adolescents for DSM-IV. National Center for PTSD: White River Junction, VT. [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA (2012). Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 36: 2130–2142. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW et al (2012). Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13: 769–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available at <http://www.R-project.org>. [Google Scholar]
- Robinson OJ, Charney DR, Overstreet C, Vytal K, Grillon C (2012). The adaptive threat bias in anxiety: amygdala-dorsomedial prefrontal cortex coupling and aversive amplification. Neuroimage 60: 523–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuyler BS, Kral TRA, Jacquart J, Burghy CA, Weng HY, Perlman DM et al (2014). Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Soc Cogn Affect Neurosci 9: 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SBE, Herry C, Grenier F, Ehrlich I, Grundemann J et al (2014). Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81: 428–437. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35: 169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23: S208–S219. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Botzung A, Miles A, Rubin DC (2011). Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J Psychiatr Res 45: 630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Decker KB, Pynoos RS (2004). The University of California at Los Angeles post-traumatic stress disorder reaction index. Curr Psychiatry Rep 6: 96–100. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B et al (2013). Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 47: 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Williamson DE, Hariri AR (2015). Developmental change in amygdala reactivity during adolescence: effects of family history of depression and stressful life events. Am J Psychiatry 172: 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink M, Derks JM, Hoogendam JM, Hillegers M, Kahn RS (2014). Functional differences in emotion processing during adolescence and early adulthood. Neuroimage 91: 70–76. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Keane TM, Davidson JR (2001). Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety 13: 132–156. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2011) Wechsler Abbreviated Scale of Intelligence–Second Edition Manual. Pearson: Bloomington, MN. [Google Scholar]
- Yang P, Wu M-T, Hsu C-C, Ker J-H (2004). Evidence of early neurobiological alternations in adolescents with posttraumatic stress disorder: a functional MRI study. Neurosci Lett 370: 13–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.