Abstract
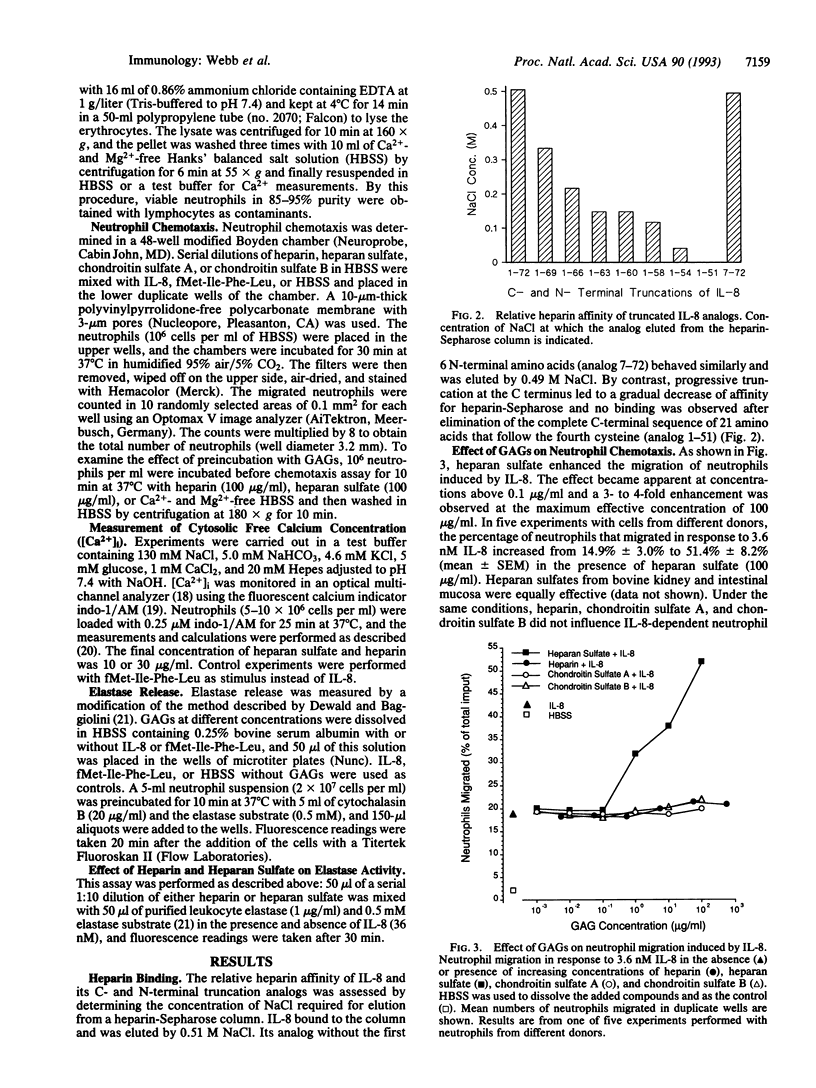
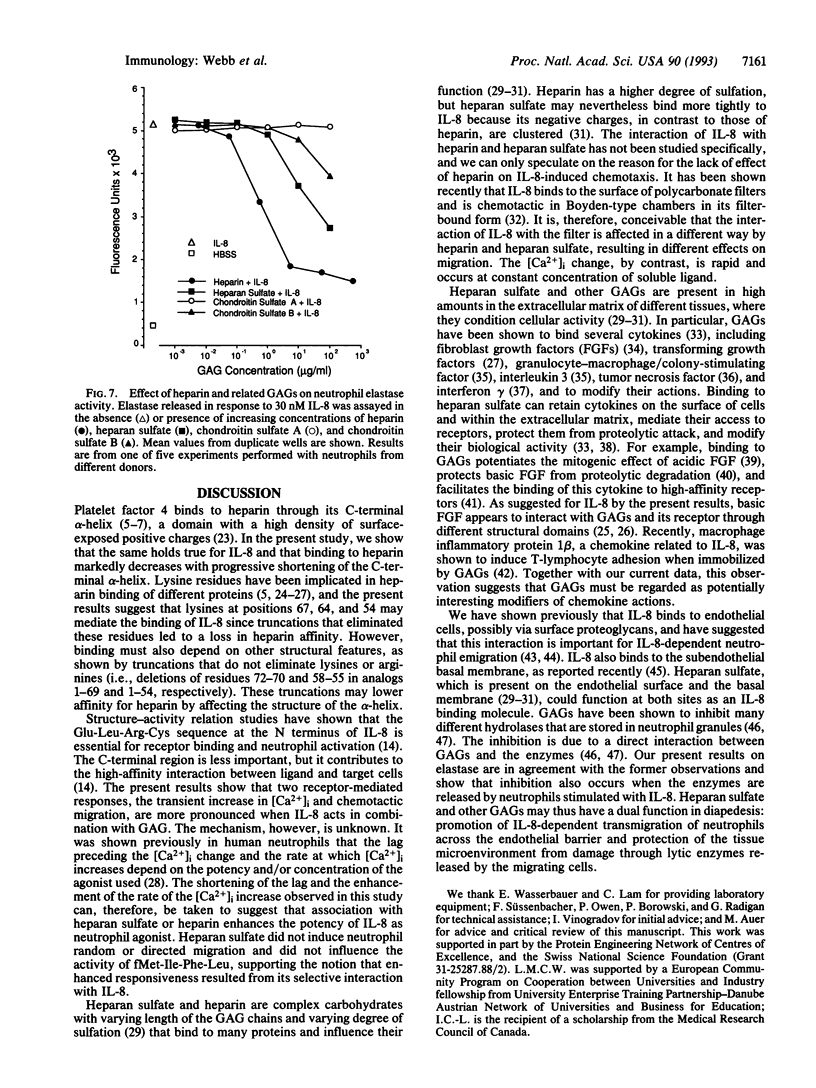
The interaction of interleukin 8 (IL-8) with heparin was studied by using synthetic IL-8 analogs with C- and N-terminal truncations. Elimination of the N-terminal region preceding the first cysteine, which constitutes the IL-8 receptor binding site, did not affect the affinity to heparin-Sepharose. Affinity, however, decreased with progressive truncation at the C terminus, and no binding was observed when the C-terminal alpha-helix was eliminated. The effect of heparin and other glycosaminoglycans on IL-8 activity was also tested. When IL-8 was applied together with heparan sulfate, neutrophil chemotaxis in vitro was enhanced up to 4-fold, and the stimulus-dependent increase in cytosolic free Ca2+ increased markedly in both rate and peak value. Heparin had a similar effect on the Ca2+ response but did not enhance chemotaxis. The glycosaminoglycans by themselves did not elicit neutrophil responses. Their enhancing effect was restricted to stimulation with IL-8 and was not observed when the unrelated chemoattractant fMet-Ile-Phe-Leu was used as the stimulus. Elastase released from stimulated neutrophils was inhibited by heparin, heparan sulfate, and, to a lesser extent, chondroitin sulfate B, confirming previous observations. Taken together, these results suggest that heparan sulfate, which is present on the endothelial cell surface and in the basement membrane, may have a dual function in diapedesis, promotion of IL-8-dependent transmigration of neutrophils, and protection of the tissue microenvironment from damage by lytic enzymes released from the migrating cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avila J. L., Convit J. Physicochemical characteristics of the glycosaminoglycan-lysosomal enzyme interaction in vitro. A model of control of leucocytic lysosomal activity. Biochem J. 1976 Nov 15;160(2):129–136. doi: 10.1042/bj1600129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., Clark-Lewis I. Interleukin-8, a chemotactic and inflammatory cytokine. FEBS Lett. 1992 Jul 27;307(1):97–101. doi: 10.1016/0014-5793(92)80909-z. [DOI] [PubMed] [Google Scholar]
- Baldwin E. T., Weber I. T., St Charles R., Xuan J. C., Appella E., Yamada M., Matsushima K., Edwards B. F., Clore G. M., Gronenborn A. M. Crystal structure of interleukin 8: symbiosis of NMR and crystallography. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):502–506. doi: 10.1073/pnas.88.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A. J., Käser-Glanzmann R., Jakábová M., Lüscher E. F. Characterization of a chondroitin 4 -sulfate proteoglycan carrier for heparin neutralizing activity (platelet factor 4 ) released from human blood platelets. Biochim Biophys Acta. 1972 Dec 29;286(2):312–329. [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Clark-Lewis I., Schumacher C., Baggiolini M., Moser B. Structure-activity relationships of interleukin-8 determined using chemically synthesized analogs. Critical role of NH2-terminal residues and evidence for uncoupling of neutrophil chemotaxis, exocytosis, and receptor binding activities. J Biol Chem. 1991 Dec 5;266(34):23128–23134. [PubMed] [Google Scholar]
- Clore G. M., Appella E., Yamada M., Matsushima K., Gronenborn A. M. Determination of the secondary structure of interleukin-8 by nuclear magnetic resonance spectroscopy. J Biol Chem. 1989 Nov 15;264(32):18907–18911. [PubMed] [Google Scholar]
- Clore G. M., Appella E., Yamada M., Matsushima K., Gronenborn A. M. Three-dimensional structure of interleukin 8 in solution. Biochemistry. 1990 Feb 20;29(7):1689–1696. doi: 10.1021/bi00459a004. [DOI] [PubMed] [Google Scholar]
- Dewald B., Baggiolini M. Evaluation of PAF antagonists using human neutrophils in a microtiter plate assay. Biochem Pharmacol. 1987 Aug 1;36(15):2505–2510. doi: 10.1016/0006-2952(87)90523-5. [DOI] [PubMed] [Google Scholar]
- Eggleton P., Gargan R., Fisher D. Rapid method for the isolation of neutrophils in high yield without the use of dextran or density gradient polymers. J Immunol Methods. 1989 Jul 6;121(1):105–113. doi: 10.1016/0022-1759(89)90425-0. [DOI] [PubMed] [Google Scholar]
- Eriksson A. E., Cousens L. S., Weaver L. H., Matthews B. W. Three-dimensional structure of human basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3441–3445. doi: 10.1073/pnas.88.8.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferran D. S., Sobel M., Harris R. B. Design and synthesis of a helix heparin-binding peptide. Biochemistry. 1992 Jun 2;31(21):5010–5016. doi: 10.1021/bi00136a014. [DOI] [PubMed] [Google Scholar]
- Gallagher J. T., Lyon M., Steward W. P. Structure and function of heparan sulphate proteoglycans. Biochem J. 1986 Jun 1;236(2):313–325. doi: 10.1042/bj2360313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Handin R. I., Cohen H. J. Purification and binding properties of human platelet factor four. J Biol Chem. 1976 Jul 25;251(14):4273–4282. [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Huber A. R., Kunkel S. L., Todd R. F., 3rd, Weiss S. J. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991 Oct 4;254(5028):99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Hébert C. A., Vitangcol R. V., Baker J. B. Scanning mutagenesis of interleukin-8 identifies a cluster of residues required for receptor binding. J Biol Chem. 1991 Oct 5;266(28):18989–18994. [PubMed] [Google Scholar]
- Kernen P., Wymann M. P., von Tscharner V., Deranleau D. A., Tai P. C., Spry C. J., Dahinden C. A., Baggiolini M. Shape changes, exocytosis, and cytosolic free calcium changes in stimulated human eosinophils. J Clin Invest. 1991 Jun;87(6):2012–2017. doi: 10.1172/JCI115230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- Lantz M., Thysell H., Nilsson E., Olsson I. On the binding of tumor necrosis factor (TNF) to heparin and the release in vivo of the TNF-binding protein I by heparin. J Clin Invest. 1991 Dec;88(6):2026–2031. doi: 10.1172/JCI115530. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lortat-Jacob H., Grimaud J. A. Interferon-gamma C-terminal function: new working hypothesis. Heparan sulfate and heparin, new targets for IFN-gamma, protect, relax the cytokine and regulate its activity. Cell Mol Biol. 1991;37(3):253–260. [PubMed] [Google Scholar]
- Loscalzo J., Melnick B., Handin R. I. The interaction of platelet factor four and glycosaminoglycans. Arch Biochem Biophys. 1985 Jul;240(1):446–455. doi: 10.1016/0003-9861(85)90049-9. [DOI] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Du B. Transforming growth factor-beta 1 is a heparin-binding protein: identification of putative heparin-binding regions and isolation of heparins with varying affinity for TGF-beta 1. J Cell Physiol. 1992 Aug;152(2):430–440. doi: 10.1002/jcp.1041520226. [DOI] [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Pratt C. W., Church F. C. Antithrombin: structure and function. Semin Hematol. 1991 Jan;28(1):3–9. [PubMed] [Google Scholar]
- Redini F., Tixier J. M., Petitou M., Choay J., Robert L., Hornebeck W. Inhibition of leucocyte elastase by heparin and its derivatives. Biochem J. 1988 Jun 1;252(2):515–519. doi: 10.1042/bj2520515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R., Gallagher J., Spooncer E., Allen T. D., Bloomfield F., Dexter T. M. Heparan sulphate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature. 1988 Mar 24;332(6162):376–378. doi: 10.1038/332376a0. [DOI] [PubMed] [Google Scholar]
- Rot A. Binding of neutrophil attractant/activation protein-1 (interleukin 8) to resident dermal cells. Cytokine. 1992 Sep;4(5):347–352. doi: 10.1016/1043-4666(92)90077-5. [DOI] [PubMed] [Google Scholar]
- Rot A. Endothelial cell binding of NAP-1/IL-8: role in neutrophil emigration. Immunol Today. 1992 Aug;13(8):291–294. doi: 10.1016/0167-5699(92)90039-A. [DOI] [PubMed] [Google Scholar]
- Rot A., Henderson L. E., Copeland T. D., Leonard E. J. A series of six ligands for the human formyl peptide receptor: tetrapeptides with high chemotactic potency and efficacy. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7967–7971. doi: 10.1073/pnas.84.22.7967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A. Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur J Immunol. 1993 Jan;23(1):303–306. doi: 10.1002/eji.1830230150. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Cerami A. Small cytokine superfamily. Curr Opin Immunol. 1991 Feb;3(1):56–60. doi: 10.1016/0952-7915(91)90077-e. [DOI] [PubMed] [Google Scholar]
- St Charles R., Walz D. A., Edwards B. F. The three-dimensional structure of bovine platelet factor 4 at 3.0-A resolution. J Biol Chem. 1989 Feb 5;264(4):2092–2099. [PubMed] [Google Scholar]
- Sudhalter J., Folkman J., Svahn C. M., Bergendal K., D'Amore P. A. Importance of size, sulfation, and anticoagulant activity in the potentiation of acidic fibroblast growth factor by heparin. J Biol Chem. 1989 Apr 25;264(12):6892–6897. [PubMed] [Google Scholar]
- Talpas C. J., Walz D. A., Lee L. 1H-NMR studies of bovine platelet factor 4: histidine assignments and interactions with heparin. Biochim Biophys Acta. 1991 Jun 24;1078(2):208–218. doi: 10.1016/0167-4838(91)99011-g. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Zhang J. D., Cousens L. S., Barr P. J., Sprang S. R. Three-dimensional structure of human basic fibroblast growth factor, a structural homolog of interleukin 1 beta. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3446–3450. doi: 10.1073/pnas.88.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. B., Katz I. R., Thorbecke G. J., Milot D. C., Holt J. Immunoregulatory activity of peptides related to platelet factor 4. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7571–7574. doi: 10.1073/pnas.86.19.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Tscharner V., Prod'hom B., Baggiolini M., Reuter H. Ion channels in human neutrophils activated by a rise in free cytosolic calcium concentration. 1986 Nov 27-Dec 3Nature. 324(6095):369–372. doi: 10.1038/324369a0. [DOI] [PubMed] [Google Scholar]



