Abstract
Background
Alcohol-mediated neurodegeneration is associated with white matter (WM) atrophy due to targeting of myelin and oligodendrocytes. However, variability in disease severity suggests co-factors contribute to WM degeneration. We examined the potential co-factor role of the tobacco-specific nitrosamine, nicotine-derived nitrosamine ketone (NNK), since smoking causes WM atrophy and most heavy drinkers consume tobacco products.
Methods
This 8-week study of Long Evans rats had 4 treatment groups: control; NNK-2 mg/kg, 3×/wk in Wks 3–8; ethanol (chronic-26% caloric + binge-2 g/kg, 3×/week in Wks 7–8); and ethanol+NNK. Exposure effects on WM lipid biochemical profiles and in situ distributions were examined using matrix-assisted laser desorption/ionization imaging mass spectrometry (MALDI-IMS) and tandem mass spectrometry.
Results
NNK mainly caused WM fiber degeneration and fiber loss, ethanol caused demyelination, and dual exposures had additive effects. Ethanol and ethanol+NNK decreased WM (including corpus callosum) and/or gray matter (hypothalamus, cortex, medial temporal) levels of several phosphatidylserine (PS), phosphatidylinositol (PI) and sphingolipid (sulfatide; ST) species, while NNK increased or had minimal effect on these lipids. Ethanol+NNK had broader and larger inhibitory effects on phospholipids and sulfatides than ethanol or NNK alone. Principle component analysis clustered control with NNK, and ethanol with ethanol+NNK groups, highlighting the independent ethanol-rather than NNK-driven responses.
Conclusion
Chronic ethanol exposures decreased several phospholipid and sphingolipid species in brain, while concomitant NNK exposures exacerbated these effects. These findings support our hypothesis that tobacco smoking is a pathogenic co-factor in alcohol-mediated WM degeneration.
Key phrases: Imaging mass spectrometry, white matter degeneration, alcohol, tobacco-specific nitrosamine, NNK, MALDI, smoking
Introduction
Chronic heavy alcohol abuse can cause structural and functional brain abnormalities by targeting oligodendrocytes and myelin (Chiappelli et al., 1991; Gnaedinger et al., 1984). This leads to atrophy and degeneration of white matter (WM) (de la Monte, 1988; Harper et al., 1985) and impairments in executive function (de la Monte and Kril, 2014; Jacobus et al., 2013). We hypothesize that alcohol abuse leads to WM degeneration via its inhibitory effects on brain insulin and insulin like growth factor (IGF) signaling pathways which are needed for myelin synthesis and maintenance (Chesik et al., 2008; Cohen et al., 2007; de la Monte et al., 2012). Moreover, the cascade of WM degeneration involves disruption of cellular homeostasis and survival mechanisms due to increased neuro-inflammation, mitochondrial dysfunction, oxidative stress, dysregulated lipid metabolism, and deficits in neurotrophin function (Chesik et al., 2008; Cohen et al., 2007; de la Monte et al., 2012; de la Monte et al., 2009a; Ye et al., 2007). On the other hand, variation in susceptibility to alcohol-mediated neurodegeneration suggests co-factors modulate disease. For example, thiamine deficiency adversely affects cognitive-behavioral functions and brain structure in alcoholics (de la Monte and Kril, 2014). We suspect that chronic exposures to tobacco smoke and related toxins represent additional co-factors. Despite epidemiologic evidence that nearly 80% of heavy drinkers abuse tobacco products, mainly in the form of cigarette smoking (Kalman et al., 2010; Romberger and Grant, 2004), the concept that smoking contributes to alcohol-related neurodegeneration has not been given ample attention. Furthermore, neurocognitive deficits with alterations in WM microstructure and metabolites occur in both heavy drinkers and smokers (Durazzo et al., 2007; Durazzo et al., 2014; Liao et al., 2012; Pfefferbaum et al., 1996). Understanding how tobacco smoke causes WM degeneration, particularly in the context of heavy alcohol abuse could aid in the development of preventive and therapeutic strategies.
Tobacco contains tobacco-specific nitrosamines, including 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone or nicotine-derived nitrosamine ketone (NNK), N-nitrosonornicotine (NNN), nitrosaminoaldehyde (NNAL), N-nitrosoanatabine (NAT), N-nitrosoanabasine (NAB), iso-NNAL, and iso-N-nitrosamino acids (iso-NNAC) which are pro-carcinogenic in chronically high exposure doses (Stepanov and Hecht, 2008). NNK, NNN, and NNAL have the most potent pro-carcinogenic activities, but NNK is one of the most abundantly present nitrosamines in tobacco and tobacco smoke. Moreover, recently we showed that experimental sub-mutagenic exposures to NNK cause steatohepatitis (Zabala et al., 2015). Our interest in studying NNK’s effects on the brain was furthered by the finding that low-level exposures to other nitrosamines cause cognitive impairment and neurodegeneration with deficits in brain insulin/IGF signaling, increased oxidative stress, and reduced levels of myelin-associated gene and protein expression (Andreani et al., 2014; de la Monte et al., 2009d; Tong et al., 2010; Tong et al., 2009), and that nitrosamine exposures exacerbate degenerative effects of alcohol on the brain (Andreani et al., 2014).
Previous studies of ethanol or nitrosamine effects on the brain utilized assays of gene and protein expression, protein phosphorylation, and adducts (Andreani et al., 2014; de la Monte et al., 2009d; Tong et al., 2010; Tong et al., 2009; Zabala et al., 2015). However, additional lipidomics approaches are needed to interrogate the nature of WM atrophy and degeneration since myelin is largely composed of lipids (70–85%) and has a much smaller component of protein (15–30%). Myelin lipids include cholesterol, sphingolipids (sphingomyelin, sulfatide, and cerebroside), phospholipids (phosphatidylserine, phosphatidylinositol, and plasmalogen), and lecithin (Quarles et al., 2006; Schmitt et al., 2014). Mass spectrometry (MS) is a rapidly evolving method of characterizing pathophysiological changes in WM myelin. Furthermore, MS can be coupled with histopathology through the use of matrix-assisted laser desorption/ionization-imaging mass spectrometry (MALDI-IMS). This approach enables in situ localization of specific lipid ions and relative shifts in their abundance and profiles with disease. Herein, we report the use of MALDI-IMS and MS/MS to examine effects of ethanol, NNK, and dual ethanol+NNK exposures on central nervous system (CNS) WM lipids in Long Evans rats. The findings support our hypothesis that smoking, and specifically tobacco nitrosamine exposures can contribute to the pathogenesis of WM degeneration, either alone, or in the setting of heavy alcohol abuse.
Materials and Methods
We conducted an 8-week study of Long Evans rats that included 4 treatment groups: 1) control; 2) NNK (2 mg/kg, i.p. on Mondays, Wednesdays, and Fridays from weeks 3 through 8); 3) ethanol (chronic-26% caloric for 8 weeks + binge- 2g/kg intraperitoneal (i.p.), Tuesdays, Thursdays, and Saturdays in weeks 7 and 8); and 4) ethanol+NNK. Control and NNK-treated rats were pair-fed isocaloric liquid diets containing 0% ethanol throughout the 8 weeks of study. Rats not receiving NNK or ethanol on the designated days were administered saline by i.p. injection (see Supplementary (S) Figure 1). Each of the 4 experimental groups had 8 rats. Basal (7 AM) and binge blood alcohol concentrations were measured (STable 1). NNK exposure was verified by detecting O6-Methylguanine adducts in liver (Zabala et al., 2015). These experiments were approved by the Institutional Animal Care and Use Committee at the Lifespan-Rhode Island Hospital, and the protocols conformed to guidelines established by the National Institutes of Health.
Fresh postmortem brains were sectioned to obtain 3 mm-thick coronal slices including the infundibulum, temporal lobes, and corpus callosum. Cryostat sections (10 µm thick) of frozen tissue blocks were thaw-mounted onto indium tin oxide (ITO)-coated glass slides, vacuum dried, washed with ammonium formate buffer to remove salts and enhance sensitivity for lipid analysis (Angel et al., 2012), and sublimed with 2,5-dehydroxybenzoic acid (DHB) as matrix (Hankin et al., 2007). Imaging was performed using a reflectron geometry MALDI-TOF/TOF mass spectrometer (Ultraflextreme, Bruker Daltonics, Bremen, Germany) (Yalcin and de la Monte, 2015) and visualized with FlexImaging software v4.0. Data collected in the negative ion mode were processed using FlexAnalysis v3.4. Statistical analyses were performed using ClinProTools v3.0. Adjacent sections stained with Luxol fast blue-Hematoxylin and Eosin (LHE) were used to co-register MALDI-TOF results. Phospholipid and sphingolipids were identified from their mass to charge (m/z) product ion ratios in MS/MS spectra and LIPID MAPS tools (http://www.lipidmaps.org/tools/index.html).
Results
Ethanol and NNK cause WM pathology
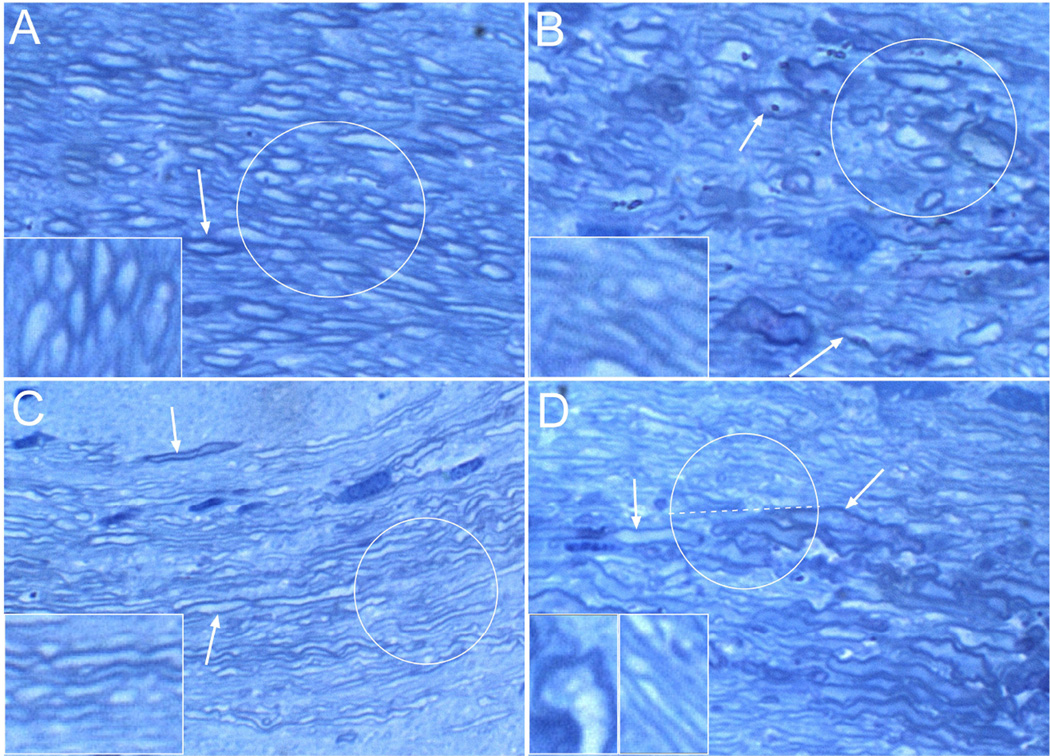
LHE stained histological sections of brain revealed that ethanol or NNK exposures reduced corpus callosum thickness. Toluidine Blue stained 1-micrometer thick sections of glutaraldehyde-fixed Epon embedded tissue revealed abundant well-myelinated fibers in control samples (Figure 1A), and reduced WM fiber densities, marked variation in fiber size, irregular fiber enlargement (dystrophy), and increased abundance of small, thinly myelinated fibers in the NNK group (Figure 1B). Ethanol reduced myelin thickness and decreased the intensity of Toluidine Blue staining (Figure 1C). Ethanol + NNK caused striking fiber loss together with fiber atrophy, dystrophy, and regenerative sprouting which was marked by clusters of small hypo-myelinated fibers (Figure 1D). Therefore, ethanol caused demyelination or hypo-myelination, and also reduced the population of large myelinated axons; NNK caused fiber loss and dystrophy; and ethanol + NNK exposures had additive effects, causing demyelination, axonal degeneration, and fiber loss.
Figure 1. Ethanol and NNK exposures cause structural damage to white matter.
Representative images of corpus callosum white matter from (A) control, (B) NNK-exposed, (C) ethanol-fed, or (D) ethanol+NNK treated Long Evans rats. Brain tissue samples were fixed in glutaraledhyde and embedded in epon. 1-µm thick sections were stained with Toluidine blue. (A) Note the abundant large, well-myelinated fibers (highlighted with the circle and shown at higher magnification in the inset). Arrow points to a thickly myelinated axon. (B) Relative to control, myelinated fibers in NNK-exposed corpora callosa are highly irregular in size and shape due to combined effects of dystrophic enlargement and atrophy. Demyelination marked by reduced intensity of myelin sheath staining (toluidine blue-stained borders; see inset). Upper central arrow shows an enlarge well-myelinated axon, while the lower arrow show an irregularly enlarged partly demyelinated axon. (C) The main effect of chronic ethanol-exposure was demyelination. The majority of axons are thinly myelinated and they appear to be much smaller in caliber than control (circle; also compare inset images). The upper arrow shows some preservation of myelin around one axon, while the lower arrow shows demyelination of a relatively large axon. (D) Ethanol+NNK treatments produced effects corresponding to both NNK and ethanol exposures. Note that in the upper half of the circled fibers and right side of the inset, the fibers mainly show demyelination, whereas in the lower half of the circle, at the arrow tips, and in the left inset, the fibers are mainly dystrophic. Original magnification, 650× (Insets, 1200×).
Ethanol and ethanol+NNK alter WM lipid ion profiles
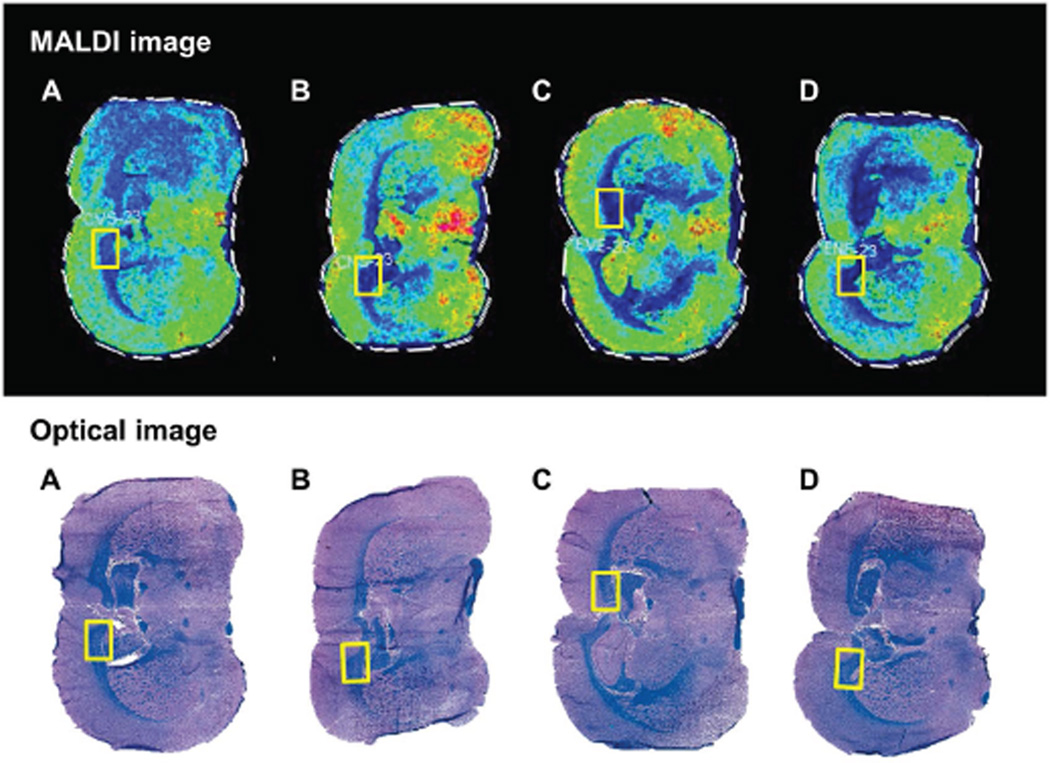
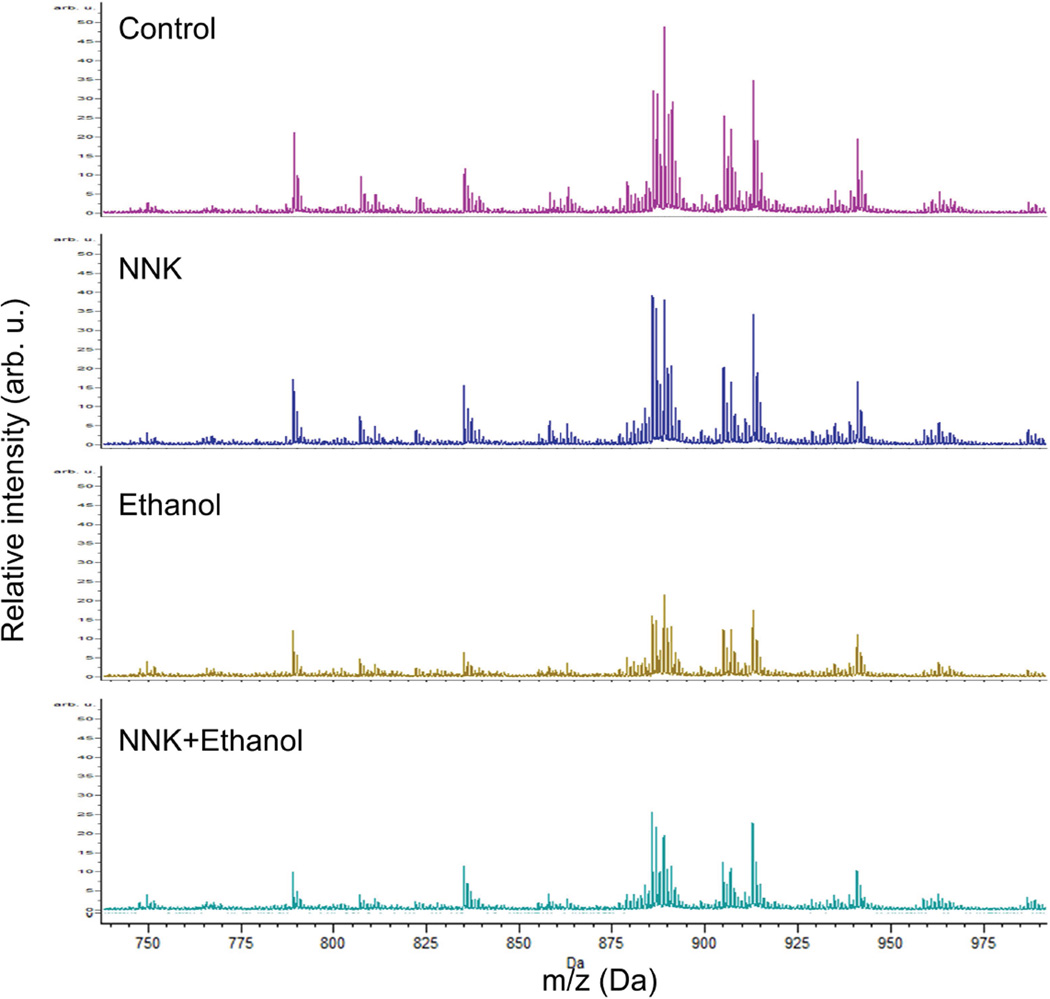
LHE-stained sections were used to identify structural landmarks and delineate standardized ROIs for co-registration with MALDI images. ROIs were restricted to corpus callosum WM (Figure 2) due to its uniform structure and targeting by alcohol (Pfefferbaum et al., 2006). The Peak Statistic report identified 86 distinct m/z lipid ions (m/z=611.79–1046.65) within the ROIs (STable 2). The m/z profiles reflecting relative intensities/abundances of all lipid ion species in brains of the four experimental groups are depicted in Figure 3. The main treatment effects were that 1) ethanol and ethanol+NNK broadly reduced lipid ion intensities relative to control; 2) ethanol+NNK caused greater overall reductions in lipid ion intensities relative to ethanol-alone; and 3) the NNK-only treatment either increased or had minimal effects on lipid ion intensities relative to control. The 13 lipid ions with the highest peak intensities were further characterized with respect to their specific structural identities and expression patterns by MALDI-IMS.
Figure 2. Histology directed MALDI-IMS analysis.
Brains from (A) control, (B) NNK-exposed, (C) ethanol-exposed, and (D) ethanol+NNK-exposed rats were sliced in the coronal plane to obtain a 3 mm slab that flanked the infundibulum of the hypothalamus. Adjacent cryostat sections were (Bottom) formalin-fixed and stained Luxol fast blue/Hematoxylin and Eosin (LHE), or (Top) mounted onto ITO-coated slides, sublimed with DHB and subjected to MALDI-IMS (see methods). In the LHE-stained sections, myelin is blue and gray matter structures are pink. The yellow rectangles represent co-registered regions of interest within the corpus callosum that were used for MALDI-IMS characterization of lipid ions.
Figure 3. Relative lipid ion intensity profiles in corpus callosum.
Lipid ion m/z and intensity were detected by MALDI-TOF. Data acquired by rasterizing across the ROIs (Figure 2) were averaged using ClinProTools software. The spectra reflect relative intensities of lipid ions between m/z 700–1000 Da in corpora callosa of control, and NNK-, ethanol-, or ethanol+NNK-exposed rats. Note ethanol and ethanol+NNK associated suppression of lipid ion intensities relative to control and NNK-treatment.
Structural assignment of WM lipid ions
We performed tandem mass spectrometry (MS/MS) using MALDI-LIFT-TOF/TOF to identify lipid ions directly in tissue. Following laser desorption/ionization and time-of-flight (TOF) m/z detection, the fragmentation patterns of the 13 lipid ions of interest were analyzed (SFigure 2). Those results were analyzed using the LIPID MAPS prediction tool to identify the lipid ions, which were characterized as either phospholipids (phosphatidylserines (PS) and phosphatidylinositols (PI)) or sphingolipids (sulfatides; ST). Lipid ion structural assignments required detection of product ions belonging to both the head group and fatty acid chains (See S-Results and SFigure 3).
Effects of ethanol and NNK exposures on phospholipid and sulfatide ion distributions and intensities in brain
MALDI-IMS revealed that chronic ethanol, NNK, and ethanol+NNK exposures differentially altered phospholipid (PS and PI) and ST levels in the corpus callosum (Table 1) and their distributions in the cerebral cortex, medial temporal structures, and hypothalamus (Figure 4). Following NNK exposure, PI(36:1) was modestly reduced and PS(36:1) was moderately increased, while the other 4 of 5 PI and 2 of 3 PS species were unchanged relative to control. In contrast, ethanol modestly reduced 4 of the 5 PI species and modestly increased PS(36:1), but had no effect on PI(38:5), PS(30:0) or PS(40:6), similar to the findings with respect to NNK. Ethanol+NNK effects were additive with respect to PI in that the reductions in lipid ion intensity were more pronounced than with either ethanol or NNK. On the other hand, for PS, the effects of ethanol+NNK appeared to be interactive and distinct from NNK or ethanol alone. With regard to ST, NNK reduced 3 of the 5 ST species (24:0, 24:0 (OH), 18:0), increased ST(24:1 (OH)), and had no effect on ST(24:1) relative to control. In contrast ethanol reduced all 5 ST species and combined ethanol+NNK further reduced ST levels, indicating additive exposure effects.
Table 1.
MALDI-IMS summary effects of NNK, ethanol, and NNK+ethanol exposures on phospholipid (PI, PS) and sphingolipid (ST) ion intensities (abundances) in rat brains relative to control
| m/z | Lipid assignment | NNK | Ethanol | NNK/Ethanol |
|---|---|---|---|---|
| 705.7 | PS(30:0) | ↔ | ↔ | ↑ |
| 788.8 | PS(36:1) | ↑↑ | ↑ | ↓ |
| 834.8 | PS(40:6) | ↔ | ↔ | ↓ |
| 806.9 | ST(18:0) | ↓ | ↓↓ | ↓↓↓ |
| 888.7 | ST(24:1) | ↔ | ↓ | ↓↓ |
| 890.7 | ST(24:0) | ↓ | ↓ | ↓↓ |
| 904.8 | ST(24:1)(OH) | ↑ | ↓ | ↓↓ |
| 906.9 | ST(24:0)(OH) | ↓ | ↓↓ | ↓↓↓ |
| 857.6 | PI(36:4) | ↔ | ↓ | ↓↓ |
| 862.7 | PI(36:1) | ↓ | ↓ | ↓↓ |
| 883.8 | PI(38:5) | ↔ | ↔ | ↓ |
| 885.5 | PI(38:4) | ↔ | ↓ | ↓↓ |
| 886.8 | PI(38:3) | ↔ | ↓ | ↓↓ |
Tandem mass spectrometry (MS/MS) with MALDI LIFT-TOF/TOF was used to fragment phospholipids and sphingolipids in negative ion mode. The product ion spectra were searched against Lipid Maps database. Differential lipid intensities among groups were determined based on mass spectrum peak intensities and ion distribution map in pseudo-color representing the relative ion intensity.
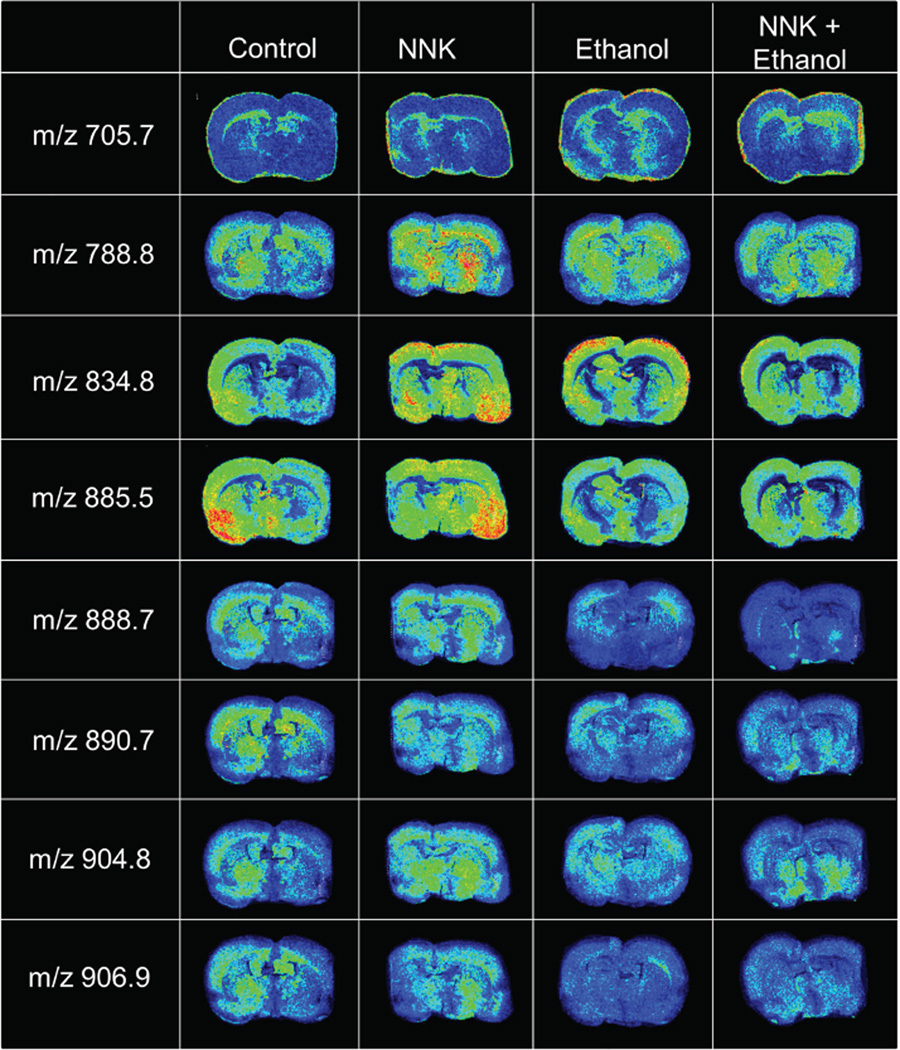
Figure 4. Effects of NNK, ethanol, and ethanol+NNK exposures on WM phospholipid and sulfatide ion profiles.
Representative MALDI-IMS results showing regional distributions and levels of 8 distinct m/z phosphotidylserine (PS), phosphatidylinositol (PI), or sulfatide (ST) species in coronal sections of brain from control, NNK-treated, ethanol-exposed, or ethanol+NNK-exposed rats. Images were obtained in the negative ion mode. See Table 1 for lipid ion identification by MS/MS.
MALDI-IMS
Representative MALDI-IMS images depicting the regional distributions and levels of 8 phospholipid or sulfatide species in brain of control, NNK-treated, ethanol-exposed, or ethanol+NNK-exposed rats are shown in Figure 4. Comparative analysis of visible lipid ion signal distributions and intensities relative to control are summarized below.
PS(30:0) signal intensities were increased by ethanol (corpus callosum and hypothalamus) and ethanol+NNK (corpus callosum), but not NNK.
PS(36:1) was distributed throughout the cortex, corpus callosum, and hypothalamus in all groups. However, the signal intensities in the corpus callosum and hypothalamus were prominently increased by NNK, while modest increases in corpus callosum signals occurred after ethanol exposure.
PS(40:6) levels were moderately abundant in control cortex, hypothalamus and medial temporal structures but virtually undetectable in the corpus callosum. NNK and ethanol increased PS(40:6) in the cortex and hypothalamus. NNK also prominently increased PS(40.6) in medial temporal structures, differentiating its effects from ethanol’s. Paradoxically, ethanol + NNK had no net effect on PS(40:6) relative to control.
PI(38:4) levels in the medial temporal lobe structures, cerebral cortex and hypothalamus were sharply lower in the ethanol and ethanol+NNK relative to control and NNK groups.
ST(24:0) expression in the corpus callosum, cortex, and hypothalamus progressively declined from control, to NNK, followed by ethanol, and then ethanol+NNK treatments. In addition, regional responses varied with different exposures such that the inhibitory effects of NNK were diffuse, whereas ethanol’s effect occurred mainly in the hypothalamus, and ethanol+NNK mainly inhibited ST(24:0) in the cortex.
ST(24:1) had similar expression profiles as ST(24:0) in the control and NNK groups. Ethanol and ethanol+NNK reduced ST(24:1) relative to control and NNK treatments. Ethanol inhibited ST(24:1) expression in the hypothalamus to a greater extent than in the cortex, whereas ethanol+NNK had additive or synergistic effects, virtually depleting ST(24:1) throughout the brain.
ST(24:0)(OH) expression patterns in the control brains were the same as those described for ST(24:0) and ST(24:1). NNK caused modest reductions in overall signal intensity, while ethanol or ethanol+NNK virtually depleted ST(24:0)(OH) signals in nearly all brain regions.
ST(24:1)(OH) was detected in control cortex, corpus callosum, and hypothalamus. NNK globally increased while ethanol modestly reduced their signal intensities. Ethanol+NNK interactive effects were manifested by reduced cortical and corpus callosal, but no effect on hypothalamic signal intensities.
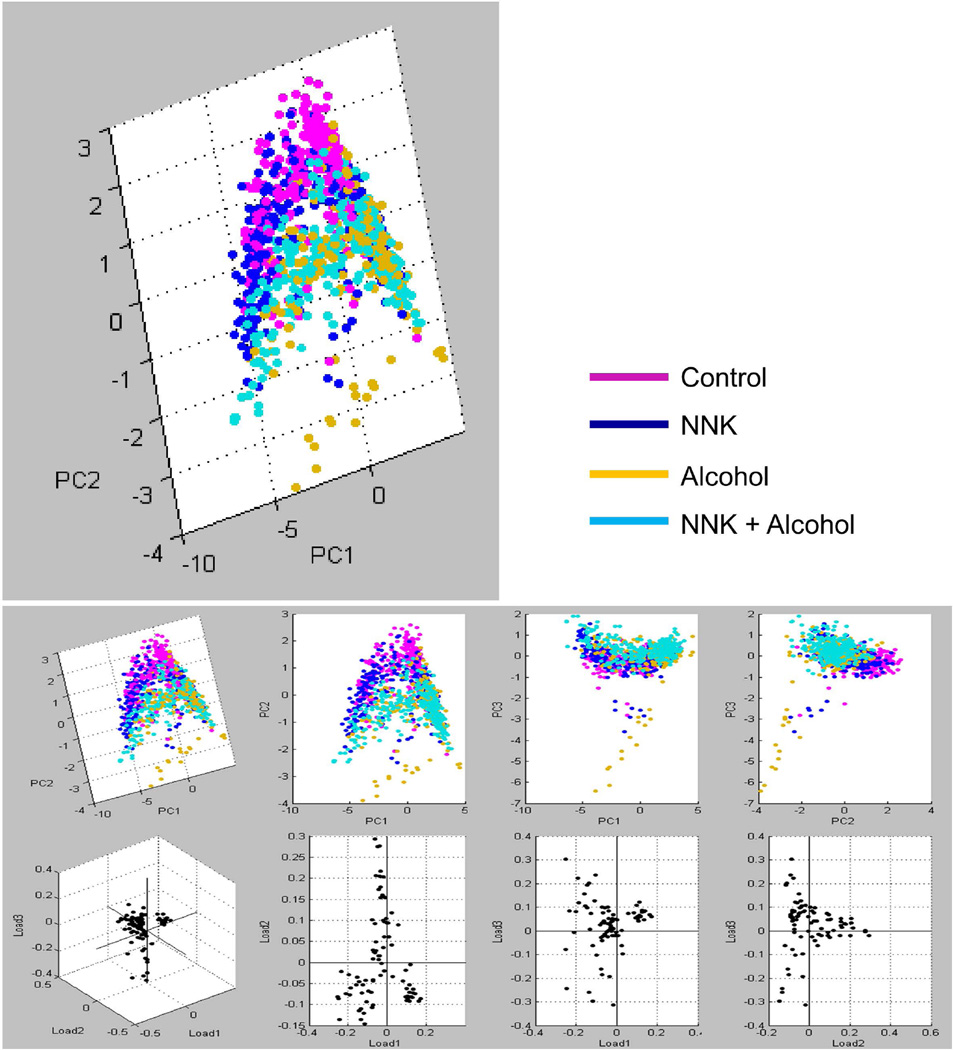
In summary, NNK, ethanol, and ethanol+NNK exposures altered the levels and distributions of phospholipid (PS and PI), and sphingolipid/sulfatide (ST) expression in the brain. NNK enhanced expression of PS(36:1), PS(40:6), PI(38:4), ST(24:1) and ST(24:1)(OH), and modestly reduced the levels of ST(24:0)(OH), and ST(24:0). Ethanol increased lipid ions signals corresponding to PS(30:0) and PS(40.6), but reduced brain levels of PI(38:4), ST(24:0)(OH), ST(24:1), ST(24:0). Combined ethanol+NNK exposures reduced brain levels of ST(24:0)(OH), PI(38:4), ST(24:1), ST(24:0), and ST(24:1)(OH) to degrees that were similar or greater than occurred with ethanol alone. Principle component analysis (PCA) generated two distinct clusters: one showed tight overlap between the control and NNK groups, while the other demonstrated clear overlap between the ethanol and ethanol+NNK treatment groups (Figure 5).
Figure 5.
Principal component analysis (PCA) of IMS data acquired in the negative-ionization mode. ClinProTools was used for PCA of the total MS spectra generated from the ROI in each group. Based on spectral similarities and WM lipid profiles, two distinct groupings were identified: the control with NNK-treated groups formed one cluster (magenta/blue), and the ethanol and ethanol+NNK groups formed the second cluster (yellow/turquoise).
Discussion
WM lipid profiles detected by MALDI-IMS
The corpus callosum was studied because its structure is relatively uniform, and WM, including the corpus callosum, is a target of alcohol-induced neurodegeneration. This study was designed to examine the long-term effects of ethanol, NNK, and ethanol+NNK exposures on lipid ion abundance and profiles in myelin. Myelin, which is most abundant in WM but also present in gray matter, has three main classes of lipids: 1) cholesterol; 2) glycosphingolipids, i.e. cerebrosides (galactosylceramide; galactocerebroside), sulfatides (sulfated galactocerebroside; sulfogalactosylceramide); and gangliosides; and 3) phospholipids, including glycerophospholipids (phosphocholine, phosphoethanolamine, phosphatidylinositol, phosphatidylserine and plasmalogens) and sphingomyelin. Sphingomyelin is composed of ceramide plus a phosphocholine or phosphoethanolamine polar head. Although galactosylceramide is characteristic of myelin (Quarles et al., 2006; Seeley et al., 2008), we elected to examine the effects of ethanol and NNK on the full range of lipids. The rationale was that alterations in less abundant lipid ions could also be important, and ethanol’s or NNK’s effects on lipids may eventually be detectable in plasma membranes of cells outside the CNS.
Ethanol and NNK effects on WM structure in relation to myelin lipids
This study characterizes the effects of chronic ethanol, NNK and ethanol+NNK exposures on WM lipid biochemical alterations in relation to histopathology. Ethanol mainly caused demyelination, while NNK caused axonal degeneration with less prominent demyelination. Combined exposures had additive effects on the loss of myelinated axons, fiber degeneration, and demyelination or hypo-myelination. Correspondingly, MALDI-IMS demonstrated broad reductions in lipid ion peak intensities following ethanol exposure, and further reductions lipid abundance with dual ethanol+NNK exposures. NNK-associated degeneration of myelinated fibers did not produce disproportionate reductions in lipid ion abundance because myelin was lost along with axons, i.e. the losses were balanced. On the other hand, the NNK-associated selected increases in some lipid ion signals could have been due to the accumulation of myelin lipid debris that accompanies axonal loss, i.e. Wallerian degeneration.
The 13 lipid ions selected for analysis by MALDI-LIFT-TOF/TOF and LIPID MAPS were identified as phospholipids (phosphatidylserine and phosphatidylinositol) and sulfatides. Ethanol, NNK, and ethanol+NNK treatments differentially altered the expression of these lipid ions relative to control. Ethanol reduced most of the PI ions and ethanol+NNK had further inhibitory effects. In contrast, NNK’s effects on PI ions were mainly neutral. With regard to PS ions, ethanol and NNK had similar directional effects relative to control, while the dual exposures had different or opposite effects. Therefore, ethanol and NNK altered PI and PS levels in distinct manners, whereas the combined exposures had either additive or interactive effects, possibly due to differential alterations in expression or function of related biosynthetic and degradative enzymes and genes.
Ethanol reduces brain phospholipid levels
Phospholipids are integral components of plasma membranes. Membrane phospholipid content is regulated by phospholipase hydrolysis. Previous studies linked binge alcohol mediated reductions in membrane phospholipids to increased activation of phospholipase A2 neuro-inflammatory pathways, release of pro-oxidative arachidonic acid, and formation of 4-hydroxynonenal-protein adducts in the brain (Adibhatla and Hatcher, 2008; Collins et al., 2014). Therefore, the ethanol and ethanol+NNK associated reductions in WM phospholipid content could have been mediated by increased activation of phospholipases.
Ethanol and NNK Inhibit Sulfatide Expression
Sulfatides are glycosphingolipids synthesized by oligodendrocytes and localized on the extracellular leaflet of myelin plasma membranes (Vos et al., 1994). Sulfatides are synthesized via sulfonation of galactocerobroside, which is formed from ceramide and galactose. Sufatide can be degraded back to ceramide and sulfate via galactosylceramidase and sulfatidase (Eckhardt, 2008; Vos et al., 1994). Sulfatide and galactocerebroside comprise nearly 30% of myelin lipids and are markers of oligodendrocytes. Sulfatides play key roles in protein trafficking, neuronal plasticity, memory, adhesion, myelin maintenance, glial-axonal signaling, insulin secretion, and oligodendrocyte survival (Takahashi and Suzuki, 2012). Reductions in membrane sulfatide content disrupt myelin sheath structure and function and impair neuronal conductivity. For example, mice rendered sulfatide deficient via decreased synthesis exhibit demyelination and loss of axonal function in the CNS (Bosio et al., 1996). Similarly, increased degradation of sulfatide back to ceramide can be problematic due to ceramide accumulation and attendant activation of inflammatory cytokines, increased generation of reactive oxygen species, and apoptosis (Kolesnick and Kronke, 1998). Other studies linked increased ceramide levels in brain to cognitive impairment and neurodegeneration mediated by oxidative stress, neuro-inflammation, insulin resistance, and deficits in oligodendrocyte myelin-associated gene expression (de la Monte et al., 2009a; Tong and de la Monte, 2009). These pathogenic processes are evident in Alzheimer’s and alcoholic brain disease (de la Monte et al., 2012), possibly due to combined effects of sulfatide deficiency (Han et al., 2002) and ceramide accumulation (de la Monte et al., 2012), including in the early stages when WM degeneration emerges (de la Monte, 1989).
The reduced brain sulfatide levels in ethanol and ethanol+NNK treated rats correlate with WM demyelination or hypo-myelination. However, since sulfatides also mediate paranodal glial-axonal signaling, it is possible that the decreased WM sulfatides had a causal role in the NNK and ethanol+NNK treatment associated axonal loss. In light of the to-and-fro enzymatic regulation of sulfatides and ceramides, we hypothesize that the ethanol-associated reductions in brain sulfatide were due to ceramide accumulation since: 1) ethanol exposures alter ceramide levels in the brain (Bae et al., 2014); and 2) chronic ethanol exposure causes brain insulin resistance (Cohen et al., 2007; de la Monte et al., 2008) and insulin resistance states increase ceramide (de la Monte et al., 2012; de la Monte et al., 2009a) and reduce sulfatide (Takahashi and Suzuki, 2012) levels in brain. However, further studies are needed to determine if ethanol and NNK reduce brain sulfatide levels by decreasing ceramide galactosyltransferase and galactocerebroside sulfotransferase, or increasing galactosylceramidase and sulfatidase gene expression or enzyme activity.
Ethanol/NNK Exposures Alter Gray Matter Phospholipid and Sulfatide Expression
Although phospholipids and sphingolipids are largely expressed in WM, the same lipids are present in gray matter structures including cerebral cortex, subcortical nuclei, and brainstem because oligodendrocytes are distributed throughout the brain. The focused MALDI-IMS studies revealed distinct patterns of phospholipid and sphingolipid expression in different brain regions/structures, and differential effects of ethanol, NNK, and ethanol+NNK on their distributions and levels. Although the significance of these regional shifts in brain phospholipid and sphingolipid profiles is not known, the results suggest that neurodegeneration caused by different exposures may be associated with distinct MALDI-IMS lipid ion signatures that could be used as diagnostic aids and indices for monitoring therapeutic responses.
Conclusions
The findings suggest that when combined with histopathological assessments, MALDI-IMS can aid in assigning etiologies of neurodegeneration since the biochemical signatures of demyelination and axonal degeneration appear to be distinct for ethanol, NNK, and combined exposures. The results support the concept that ethanol and tobacco smoke exposures differentially and additively contribute to WM degeneration. Ethanol-mediated reductions in brain phospholipids could be an important mechanism by which membrane integrity is impaired, leading to a range of abnormalities including aberrations in intracellular signaling (Crews et al., 1986; Jackson et al., 2007; Wellner et al., 2013), increased membrane permeability (Manzo-Avalos and Saavedra-Molina, 2010), and ER stress. Broad inhibition of sulfatide expression in brains of ethanol- and NNK-exposed rats marks a fundamental abnormality linked to WM myelin and axonal degeneration, and possibly neural-glial functions needed for plasticity.
Acknowledgement
The authors acknowledge the valuable contributions of Dr. Dale Shannon Cornett of Bruker Daltonics, Inc. Billerica, MA for his on-going support in developing the MALDI-IMS applications in our laboratory. The research was supported by grants AA-11431 and AA-12908 from the National Institutes on Alcohol Abuse and Alcoholism at the National Institutes of Health.
All funding for this research was provided by grants from the National Institutes of Health.
Footnotes
Competing Interest Statement
The authors declare that they have no competing interests.
Author Contribution Statement
EBY: 1) had a major role in establishing the MALDI-IMS methods for lipid analysis in the laboratory; 2) performed all of the MALDI-IMS and MS/MS assays; and 3) prepared the initial draft of the manuscript.
SMD: 1) conceived of the ‘biochemical histopathology’ concept and the eventual utility of MALDI-IMS to enhance diagnostics of metabolic and degenerative diseases; 2) provided critical guidance to EBY; 3) directed and supervised the entire research project; and 4) planned the manuscript and took the lead role in generating all post-initial drafts of the manuscript, including its final version.
KN: Undergraduate honors thesis student who directly assisted EBY in all phases of her efforts.
MT: Responsible for generating the models, harvesting and storing tissue, tissue processing, discussing experimental details and data interpretation.
All authors read and approved of the manuscript prior to its submission.
References
- Adibhatla RM, Hatcher JF. Phospholipase A(2) reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep. 2008;41(8):560–567. doi: 10.5483/bmbrep.2008.41.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreani T, Tong M, de la Monte SM. Hotdogs and Beer: Dietary Nitrosamine Exposure Exacerbates Neurodevelopmental Effects of Ethanol in Fetal Alcohol Spectrum Disorder. JDAR. 2014;3:1–9. 2014. [Google Scholar]
- Angel PM, Spraggins JM, Baldwin HS, Caprioli R. Enhanced sensitivity for high spatial resolution lipid analysis by negative ion mode matrix assisted laser desorption ionization imaging mass spectrometry. Anal Chem. 2012;84(3):1557–1564. doi: 10.1021/ac202383m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae M, Bandaru VV, Patel N, Haughey NJ. Ceramide metabolism analysis in a model of binge drinking reveals both neuroprotective and toxic effects of ethanol. J Neurochem. 2014;131(5):645–654. doi: 10.1111/jnc.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosio A, Binczek E, Stoffel W. Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc Natl Acad Sci U S A. 1996;93(23):13280–13285. doi: 10.1073/pnas.93.23.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesik D, De Keyser J, Wilczak N. Insulin-like growth factor system regulates oligodendroglial cell behavior: therapeutic potential in CNS. J Mol Neurosci. 2008;35(1):81–90. doi: 10.1007/s12031-008-9041-2. [DOI] [PubMed] [Google Scholar]
- Chiappelli F, Taylor AN, Espinosa de los Monteros A, de Vellis J. Fetal alcohol delays the developmental expression of myelin basic protein and transferrin in rat primary oligodendrocyte cultures. Int J Dev Neurosci. 1991;9(1):67–75. doi: 10.1016/0736-5748(91)90074-v. [DOI] [PubMed] [Google Scholar]
- Cohen AC, Tong M, Wands JR, de la Monte SM. Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol Clin Exp Res. 2007;31(9):1558–1573. doi: 10.1111/j.1530-0277.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- Collins MA, Tajuddin N, Moon KH, Kim HY, Nixon K, Neafsey EJ. Alcohol, phospholipase A2-associated neuroinflammation, and omega3 docosahexaenoic acid protection. Mol Neurobiol. 2014;50(1):239–245. doi: 10.1007/s12035-014-8690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Gonzales RA, Palovcik R, Phillips MI, Theiss C, Raizada M. Changes in receptor stimulated phosphoinositide hydrolysis in brain during ethanol administration, aging, and other pathological conditions. Psychopharmacol Bull. 1986;22(3):775–780. [PubMed] [Google Scholar]
- de la Monte S, Derdak Z, Wands JR. Alcohol, insulin resistance and the liver-brain axis. J Gastroenterol Hepatol. 2012;27(Suppl 2):33–41. doi: 10.1111/j.1440-1746.2011.07023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch Neurol. 1988;45(9):990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Quantitation of cerebral atrophy in preclinical and end-stage Alzheimer's disease. Ann Neurol. 1989;25(5):450–459. doi: 10.1002/ana.410250506. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Kril JJ. Human alcohol-related neuropathology. Acta Neuropathol. 2014;127(1):71–90. doi: 10.1007/s00401-013-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Longato L, Tong M, DeNucci S, Wands JR. The liver-brain axis of alcohol-mediated neurodegeneration: role of toxic lipids. Int J Environ Res Public Health. 2009a;6(7):2055–2075. doi: 10.3390/ijerph6072055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Cohen AC, Sheedy D, Harper C, Wands JR. Insulin and insulin-like growth factor resistance in alcoholic neurodegeneration. Alcohol Clin Exp Res. 2008;32(9):1630–1644. doi: 10.1111/j.1530-0277.2008.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Lawton M, Longato L. Nitrosamine exposure exacerbates high fat diet-mediated type 2 diabetes mellitus, non-alcoholic steatohepatitis, and neurodegeneration with cognitive impairment. Mol Neurodegener. 2009d;4:54. doi: 10.1186/1750-1326-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Meyerhoff DJ. The neurobiological and neurocognitive consequences of chronic cigarette smoking in alcohol use disorders. Alcohol Alcohol. 2007;42(3):174–185. doi: 10.1093/alcalc/agm020. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Mattsson N, Weiner MW Alzheimer's Disease Neuroimaging I. Smoking and increased Alzheimer's disease risk: a review of potential mechanisms. Alzheimers Dement. 2014;10(3 Suppl):S122–S1245. doi: 10.1016/j.jalz.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt M. The role and metabolism of sulfatide in the nervous system. Mol Neurobiol. 2008;37(2–3):93–103. doi: 10.1007/s12035-008-8022-3. [DOI] [PubMed] [Google Scholar]
- Gnaedinger JM, Noronha AB, Druse MJ. Myelin gangliosides in developing rats: the influence of maternal ethanol consumption. J Neurochem. 1984;42(5):1281–1285. doi: 10.1111/j.1471-4159.1984.tb02784.x. [DOI] [PubMed] [Google Scholar]
- Han X, D MH, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer's disease: potential role in disease pathogenesis. J Neurochem. 2002;82(4):809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Hankin JA, Barkley RM, Murphy RC. Sublimation as a method of matrix application for mass spectrometric imaging. J Am Soc Mass Spectrom. 2007;18(9):1646–1652. doi: 10.1016/j.jasms.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Kril JJ, Holloway RL. Brain shrinkage in chronic alcoholics: a pathological study. Br Med J (Clin Res Ed) 1985;290(6467):501–504. doi: 10.1136/bmj.290.6467.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SN, Wang HY, Woods AS. In situ structural characterization of glycerophospholipids and sulfatides in brain tissue using MALDI-MS/MS. J Am Soc Mass Spectrom. 2007;18(1):17–26. doi: 10.1016/j.jasms.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res. 2013;214(3):374–381. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalman D, Kim S, DiGirolamo G, Smelson D, Ziedonis D. Addressing tobacco use disorder in smokers in early remission from alcohol dependence: the case for integrating smoking cessation services in substance use disorder treatment programs. Clin Psychol Rev. 2010;30(1):12–24. doi: 10.1016/j.cpr.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Liu T, Chen X, Hao W. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study. Addict Biol. 2012;17(6):977–980. doi: 10.1111/j.1369-1600.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- Manzo-Avalos S, Saavedra-Molina A. Cellular and mitochondrial effects of alcohol consumption. Int J Environ Res Public Health. 2010;7(12):4281–4304. doi: 10.3390/ijerph7124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27(7):994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcohol Clin Exp Res. 1996;20(4):752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Quarles RH, Macklin WB, Morell P. Myelin Formation, Structure and Biochemistry. 6th ed. Philadelphia: Elsevier; 2006. [Google Scholar]
- Romberger DJ, Grant K. Alcohol consumption and smoking status: the role of smoking cessation. Biomed Pharmacother. 2004;58(2):77–83. doi: 10.1016/j.biopha.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Schmitt S, Cantuti Castelvetri L, Simons M. Metabolism and functions of lipids in myelin. Biochim Biophys Acta. 2014 doi: 10.1016/j.bbalip.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Seeley EH, Oppenheimer SR, Mi D, Chaurand P, Caprioli RM. Enhancement of protein sensitivity for MALDI imaging mass spectrometry after chemical treatment of tissue sections. J Am Soc Mass Spectrom. 2008;19(8):1069–1077. doi: 10.1016/j.jasms.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I, Hecht SS. Detection and quantitation of N'-nitrosonornicotine in human toenails by liquid chromatography-electrospray ionization-tandem mass spectrometry. Cancer Epidemiol Biomarkers Prev. 2008;17(4):945–948. doi: 10.1158/1055-9965.EPI-07-2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Suzuki T. Role of sulfatide in normal and pathological cells and tissues. J Lipid Res. 2012;53(8):1437–1450. doi: 10.1194/jlr.R026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, de la Monte SM. Mechanisms of ceramide-mediated neurodegeneration. J Alzheimers Dis. 2009;16(4):705–714. doi: 10.3233/JAD-2009-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Longato L, de la Monte SM. Early limited nitrosamine exposures exacerbate high fat diet-mediated type2 diabetes and neurodegeneration. BMC Endocr Disord. 2010;10(1):4. doi: 10.1186/1472-6823-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong M, Neusner A, Longato L, Lawton M, Wands JR, de la Monte SM. Nitrosamine exposure causes insulin resistance diseases: relevance to type 2 diabetes mellitus, non-alcoholic steatohepatitis, and Alzheimer's disease. J Alzheimers Dis. 2009;17(4):827–844. [PMC free article] [PubMed] [Google Scholar]
- Vos JP, Lopes-Cardozo M, Gadella BM. Metabolic and functional aspects of sulfogalactolipids. Biochim Biophys Acta. 1994;1211(2):125–149. doi: 10.1016/0005-2760(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Wellner N, Diep TA, Janfelt C, Hansen HS. N-acylation of phosphatidylethanolamine and its biological functions in mammals. Biochim Biophys Acta. 2013;1831(3):652–662. doi: 10.1016/j.bbalip.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Yalcin EB, de la Monte SM. Review of Matrix-Assisted Laser Desorption Ionization-Imaging Mass Spectrometry for Lipid Biochemical Histopathology. J Histochem Cytochem. 2015 doi: 10.1369/0022155415596202. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Kollias G, D'Ercole AJ. Insulin-like growth factor-I ameliorates demyelination induced by tumor necrosis factor-alpha in transgenic mice. J Neurosci Res. 2007;85(4):712–722. doi: 10.1002/jnr.21181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabala V, Tong M, Yu R, Ramirez T, Yalcin EB, Balbo S, Silbermann E, Deochand C, Nunez K, Hecht S, de la Monte SM. Potential contributions of the tobacco nicotine-derived nitrosamine ketone (NNK) in the pathogenesis of steatohepatitis in a chronic plus binge rat model of alcoholic liver disease. Alcohol Alcohol. 2015;50(2):118–131. doi: 10.1093/alcalc/agu083. [DOI] [PMC free article] [PubMed] [Google Scholar]