Abstract Abstract
Within Dipsadinae, some recent authors have recognized a tribe Nothopsini containing the genera Diaphorolepis, Emmochliophis, Nothopsis, Synophis, and Xenopholis, on the basis of a number of putative morphological synapomorphies. However, molecular results suggest that Nothopsis, Synophis, and Xenopholis do not form a monophyletic group, while the remaining taxa are unsampled in recent molecular phylogenies. Here, DNA-sequence data for some Diaphorolepis and Synophis species are provided for the first time, as well as additional new sequences for Nothopsis and some Synophis species. Including these and other existing data for nothopsine species, previous studies showing that Nothopsini is not a natural group are corroborated. Nothopsini Cope, 1871 is restricted to Nothopsis. Diaphorolepidini Jenner, 1981 is resurrected and re-delimited to include only Diaphorolepis, Emmochliophis, and Synophis. Finally, Xenopholis remains Dipsadinae incertae sedis. Known material of Diaphorolepidini is reviewed to generate revised and expanded descriptions and diagnoses at the tribe, genus, and species level. Numerous cryptic species are likely present in Synophis bicolor and Synophis lasallei. Finally, a new population from the low-elevation cloud forests of SW Ecuador is reported upon, which is genetically and morphologically distinct from all other species, that is here named Synophis zaheri sp. n.
Keywords: Serpentes, Dipsadinae, Nothopsini, Diaphorolepis, Synophis
Introduction
Within Dipsadinae (sensu Pyron et al. 2013), Diaphorolepis, Emmochliophis, Nothopsis, Synophis, and Xenopholis were historically thought to form a monophyletic group on the basis of scutellation, osteological, histological, hemipenial, and respiratory characters (see Sheil and Grant 2001). The group has been referred to as tribe Nothopsini by some authors (Savitzky 1974; Dowling and Duellman 1978). The genera Amastridium, Chersodromus, and Ninia have also been referred to this assemblage (Wallach 1995). Alternatively, Jenner (1981) proposed a tribe Diaphorolepidini containing Diaphorolepis along with Atractus, Chersodromus, Crisantophis, Elapomorphus, Enulius, Gomesophis, Pseudotomodon, Ptychophis, and Sordellina, while Synophis was placed in Philodryadini, and Emmochliophis was not accounted for.
Most subsequent studies have considered Nothopsini to contain only Diaphorolepis, Emmochliophis, Nothopsis, Synophis, and Xenopholis (see Sheil and Grant 2001; Martinez 2011). Some of these taxa, Nothopsis in particular, bear a strong external resemblance to Asian xenodermatids such as Xenodermus (Bogert 1964). In contrast, molecular phylogenetic analyses have strongly supported Nothopsis (Vidal et al. 2010), Synophis (Sheehy 2012), and Xenopholis (Vidal et al. 2010; Pyron et al. 2011; Grazziotin et al. 2012) as dipsadines, as does hemipenial morphology (Zaher 1999). However, these genera do not form a monophyletic group within Dipsadinae in molecular phylogenies, and are widely separated in different dipsadine clades (Vidal et al. 2010; Grazziotin et al. 2012; Sheehy 2012; Pyron et al. 2013).
Thus, the tribe Nothopsini does not appear to represent a natural group, despite the putative morphological synapomorphies uniting the taxa listed above (Savitzky 1974; Ferrarezzi 1994; Wallach 1995; Martinez 2011). Contrastingly, the strength of the molecular results suggests that these likely represent convergence, at least between Nothopsis and Xenopholis. This is not surprising, given the massive ecomorphological diversification exhibited by Dipsadinae following their adaptive radiation in the Neotropics (Cadle 1984a, b, c).
However, Diaphorolepis and Emmochliophis have still not been sampled in any molecular phylogeny, and it is thus unclear where their phylogenetic affinities lie. Morphological evidence suggests that these two genera form a clade with Synophis (see Hillis 1990). Furthermore, there are multiple species of Synophis, with potentially unclear species boundaries (Bogert 1964; Fritts and Smith 1969; Sheil 1998; Sheil and Grant 2001). Here, we report on new material from Diaphorolepis, Synophis, and Nothopsis, present a new molecular phylogeny, and describe a new species of Synophis. We review current knowledge of Diaphorolepis, Emmochliophis, and Synophis, and discuss species limits in these genera. Dipsadine diversity in the Andes is clearly underestimated, and new species are still being discovered in the 21st century (e.g., Salazar-Venezuela et al. 2014; Sheehy et al. 2014; Zaher et al. 2014).
Materials
Molecular phylogeny
Work in Ecuador was carried out under permit number MAE-DNB-CM-2015-0017. We obtained tissue samples of Diaphorolepis wagneri (3 specimens), Synophis bicolor (3), Synophis calamitus (1), Synophis lasallei (1), a new Synophis species (2), and Nothopsis rugosus (1), via fieldwork in Ecuador. The specimens are deposited at the (MZUTI; Tables 1, 2). We also obtained a tissue loan of the holotype of Synophis calamitus from Ecuador (KU 197107; Hillis 1990) from the University of Texas at Austin.
Table 1.
Morphometric data for specimens of Diaphorolepidini species examined or from literature. Codes are: MT; IL; SL; PO; V; SC; D1-3; SVL; TL. Museum codes are given in Sabaj-Perez (2013). Includes data from ReptiliaWebEcuador (Torres-Carvajal et al. 2014).
| Species | Collection | MT | IL | SL | PO | V | SC | D1 | D2 | D3 | SVL | TL | Sex |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diaphorolepis laevis | NMW 14860 | 16 | 10 | 8/9 | 2 | 157 | 84 | 19 | 19 | 17 | 350 | 145 | - |
| Diaphorolepis wagneri | AMNH 49179 | 23 | 10 | 8 | 3 | 194 | 138 | 21 | 19 | 17 | 290 | 153 | M |
| Diaphorolepis wagneri | GML 4-00014 | 25 | 10 | 9 | 2 | 197 | 133 | 21 | 19 | 17 | 355 | 187 | F |
| Diaphorolepis wagneri | KU 75682 | 24 | 10 | 9 | 2 | 196 | 136 | 21 | 19 | 17 | 311 | 142 | F |
| Diaphorolepis wagneri | MECN 2937 | - | - | 9 | 3 | 181 | 133 | 19 | 19 | 17 | 276 | 129 | M |
| Diaphorolepis wagneri | MZUTI 3322 | - | 11 | 8 | 2 | 189 | 141 | 19 | 19 | 17 | 332 | 167 | F |
| Diaphorolepis wagneri | MZUTI 3752 | - | 11 | 8 | 1 | 189 | 134 | 21 | 19 | 17 | 447 | 257 | M |
| Diaphorolepis wagneri | MZUTI 3901 | - | 13 | 9 | 3 | 195 | 131 | 19 | 19 | 17 | 524 | 259 | F |
| Diaphorolepis wagneri | NMW 18915 | - | 13 | 9 | 2 | 191 | 137 | 21 | 19 | 17 | 307 | 146 | M |
| Diaphorolepis wagneri | ZSM 2708/0 | 25 | 12 | 9 | 2 | 193 | 98 | 21 | 19 | 17 | 484 | 200 | F |
| Emmochliophis fugleri | UIMNH 78795 | 16 | 8 | 8 | 2 | 140 | 97 | 19 | 19 | 19 | - | - | M |
| Emmochliophis miops | BMNH 1946.1.12.30 | 13 | 8 | 8 | 1 | 145 | 93 | 19 | 19 | 19 | 251 | 134 | F |
| Eastern Andes | |||||||||||||
| Synophis aff. bicolor | FHGO 9186 | - | 11 | 9 | 2 | 164 | 105 | 19 | 17 | 17 | 379 | 184 | M |
| Synophis aff. bicolor | MZUTI 3529 | - | 11 | 8 | 2 | 163 | 106 | 19 | 19 | 17 | 407 | 202 | M |
| Synophis aff. bicolor | MZUTI 4180 | - | 11 | 9 | 2 | 152 | 100 | 19 | 19 | 18 | 457 | 214 | M |
| Synophis aff. bicolor | UMMZ 91550 | 24/27 | 11 | 9 | 2 | 160 | 103 | - | 19 | 17 | 529 | 235 | F |
| Synophis aff. bicolor | UMMZ 91551 | - | - | 8 | 2 | 161 | 105 | - | 19 | 17 | 535 | 230 | F |
| Synophis aff. bicolor | UMMZ 91552 | - | - | 8 | 2 | 166 | 106 | - | 19 | 17 | 153 | 61 | F |
| Western Andes | |||||||||||||
| Synophis aff. bicolor | BMNH 1940.2.30.31 | - | - | 8 | 2 | 162 | 118 | 21 | 19 | 17 | 408 | 241 | M |
| Synophis aff. bicolor | CAS 23612 | - | - | 8 | 2 | 166 | 100 | - | 19 | 17 | 186 | 80 | F |
| Synophis aff. bicolor | MCZ R-164530 | - | 11 | 9 | 2 | 164 | 116 | - | 19 | 17 | 367 | 208 | - |
| Synophis aff. bicolor | QCAZ 10453 | - | 11 | 8 | 2 | - | - | - | - | - | - | - | - |
| Synophis aff. bicolor | TCWC 66209 | - | 11 | 8 | 2 | 160 | 96 | 21 | 19 | 17 | - | - | - |
| Synophis aff. bicolor | UMMZ 185812 | - | 10 | 8 | 2 | 165 | 105 | - | 19 | 17 | 144 | 66 | - |
| Synophis aff. bicolor | UMMZ 185813 | - | 10 | 8 | 2 | 162 | 122 | - | - | - | 257 | 147 | - |
| Synophis cf. bicolor | MHUA 14133 | >23 | 12 | 8 | 2 | 193 | 127 | - | 19 | - | - | - | M |
| Synophis cf. bicolor | MHUA 14577 | 24 | 11 | 8 | 2 | 190 | 131 | 19 | 19 | 17 | - | - | - |
| Synophis cf. bicolor | MLS2072 | - | 11/10 | 8 | 2 | 184 | 127 | - | 19 | 17 | 407 | 210 | M |
| Synophis bicolor | MECN 6732 | - | 9 | 8 | 2 | 174 | 138 | 19 | 17 | 17 | 361 | 236 | M |
| Synophis bicolor | MECN 6733 | - | 9 | 8 | 2 | 174 | 132 | 19 | 19 | 17 | 406 | 245 | M |
| Synophis bicolor | MECN 8076 | - | 9 | 8 | 2 | 183 | 135 | 19 | 17 | 17 | 376 | 233 | M |
| Synophis bicolor | MZUT 257 | 16 | 9 | 8 | 2 | 180 | 136 | - | 19 | 17 | - | - | - |
| Synophis bicolor | MZUTI 4175 | - | 11 | 8 | 2 | 174 | 143 | 19 | 19 | 17 | 365 | 245 | M |
| Synophis bicolor | UTA R-55956 | - | 9 | 8 | 2 | 176 | 129 | - | 19 | 17 | - | - | - |
| Synophis calamitus | KU 164208 | - | 9 | 8 | 1 | 163 | 125 | 21 | 19 | 17 | 142 | 73 | - |
| Synophis calamitus | KU 197107 | - | 9 | 7 | 1 | 166 | 110 | 21 | 19 | 17 | 149 | 74 | F |
| Synophis calamitus | MZUTI 3694 | - | 11 | 9 | 2 | 166 | 118 | 23 | 19 | 17 | 462 | 265 | M |
| Synophis calamitus | QCAZ 11931 | - | 9 | 8 | 1 | - | - | - | - | - | - | - | - |
| Synophis lasallei | FMNH 81313 | 24 | - | - | 2 | 154 | 112 | - | 21 | - | 292 | 158 | F |
| Synophis lasallei | EPN S.974 | - | - | - | 2 | 156 | 116 | - | 21 | - | 175 | 90 | M |
| Synophis lasallei | EPN S.975 | 24 | - | - | 2 | 155 | 119 | - | 21 | - | 354 | 201 | M |
| Synophis lasallei | FHGO 6489 | - | 11 | 8 | 2 | 147 | 111 | 23 | 21 | 21 | 153 | 86 | M |
| Synophis lasallei | FHGO 8340 | - | 11 | 8 | 2 | 153 | 88 | 21 | 19 | 17 | 415 | 199 | M |
| Synophis lasallei | MCZ R-156873 | - | 11 | 7 | 1 | 147 | 115 | - | - | - | 412 | 206 | - |
| Synophis lasallei | MECN 11250 | - | 10 | 8 | 2 | 153 | 98 | 21 | 19 | 17 | 412 | 196 | F |
| Synophis lasallei | MECN 11262 | - | - | 8 | 2 | 154 | 118 | 21 | 21 | 17 | 306 | 145 | M |
| Synophis lasallei | MECN 2220 | - | 10 | 8 | 2 | 165 | 117 | 19 | 19 | 17 | 294 | 146 | M |
| Synophis lasallei | MLS/CJSP | - | - | - | 2 | 144 | 101 | - | - | - | 300 | 170 | M |
| Synophis lasallei | MZUTI 4181 | - | 11 | 9 | 2 | 156 | 29 | 21 | 21 | 19 | 272 | 42 | M |
| Synophis lasallei | USNM 233061 | - | 11 | 9 | 2 | 156 | 124 | - | 21 | - | 285 | 160 | M |
| Synophis lasallei | USNM 233062 | - | 11 | 8 | 2 | 153 | 126 | - | 22 | 20 | 360 | 200 | - |
| Synophis lasallei | USNM 233063 | - | 11 | 8 | 2 | 151 | 86 | 23 | 21 | 19 | 308 | 197 | M |
| Synophis lasallei | USNM 233064 | - | 11 | 8 | 2 | 151 | - | - | 21 | 19 | 270 | 150 | - |
| Synophis plectovertebralis | UVC 11580 | - | 8 | 8 | 1 | 144 | 91 | 19 | 19 | 17 | 212 | 100 | M |
| Synophis plectovertebralis | UVC 11858 | - | 7 | 7 | 1 | 147 | 79 | 19 | 19 | 17 | 196 | 76.5 | F |
| Synophis zaheri | MZUTI 3353 | - | 8 | 8 | 2 | 166 | 112 | 19 | 19 | 17 | 351 | 184 | M |
| Synophis zaheri | MZUTI 3355 | - | 9 | 8 | 2 | 169 | 111 | 19 | 19 | 17 | 372 | 194 | M |
Table 2.
Vouchered localities for specimens of Diaphorolepidini species examined or from literature. In general, localities are given verbatim as transcribed from the literature, museum records, or field notes. Co-ordinates represent georeferencing attempts from gazetteers under standard guidelines, though some variation from the exact collecting locality will inevitably be present. Similarly, elevations are taken from Google Earth, and may not exactly match the elevations as originally reported. Museum codes are given in Sabaj-Perez (2013). Includes data from ReptiliaWebEcuador (Torres-Carvajal et al. 2014).
| Species | Collection Number | Locality | Latitude | Longitude | Elev. |
|---|---|---|---|---|---|
| Diaphorolepis wagneri | GML 4-00014 | Panama Darien, Cerro Mali, in Serrania del Darien | 8.128557 | -77.253498 | 1268 |
| Diaphorolepis wagneri | MECN 2937 | Canandé, Ecuador | 0.529930 | -79.035410 | 596 |
| Diaphorolepis wagneri | MZUTI 3322 | Milpe, Ecuador | 0.034890 | -78.867130 | 1076 |
| Diaphorolepis wagneri | MZUTI 3901 | Mashpi Lodge, Ecuador | 0.164030 | -78.870730 | 1068 |
| Diaphorolepis wagneri | NMW 18915 | El Palmar, Canar, Ecuador | -2.533300 | -79.333300 | 325 |
| Diaphorolepis wagneri | QCAZ 380 | Ecuador, Cotopaxi, Las Pampas | -0.348360 | -79.076010 | 1238 |
| Diaphorolepis wagneri | QCAZ 381 | Ecuador, Pichincha, Tandapi | -0.415220 | -78.797280 | 1457 |
| Diaphorolepis wagneri | QCAZ 8450 | Ecuador, Cotopaxi, Pucayacu–Sigchos | -0.702730 | -79.056810 | 974 |
| Diaphorolepis wagneri | QCAZ 8782 | Imbabura Lita, Ecuador | 0.815270 | -78.388350 | 865 |
| Diaphorolepis wagneri | UVC 12187 | 18km East of San Jose de Palmar, Colombia | 4.966667 | -76.233333 | 1546 |
| Diaphorolepis wagneri | UVC 5254 | Colombia, Cali, Pichinde, Farallones de Cali | 3.433400 | -76.616680 | 1614 |
| Diaphorolepis wagneri | UVC 5255 | Colombia, Pance, Camino a Corea, Pance, Farallones de Cali | 3.328340 | -76.638650 | 1632 |
| Emmochliophis fugleri | UIMNH 78795 | 4 km. E Río Baba Bridge, 24 km. S Santo Domingo de los Colorados, Pichincha, Ecuador | -0.435562 | -79.246212 | 618 |
| Emmochliophis miops | BMNH 1946.1.12.30 | Parambas (Imbabura), Ecuador | 0.805000 | -78.350833 | 1105 |
| Eastern Andes | |||||
| Synophis aff. bicolor | FHGO 9186 | Río Zopladora, Ecuador | -2.611510 | -78.472174 | 1677 |
| Synophis aff. bicolor | KU 121341 | Ecuador, Pastaza, Mera | -1.457452 | -78.107976 | 1111 |
| Synophis aff. bicolor | MZUTI 3529 | Wild Sumaco, Ecuador | -0.675700 | -77.601290 | 1463 |
| Synophis aff. bicolor | MZUTI 4180 | El Genairo, Ecuador | -4.166181 | -78.94094 | 1212 |
| Synophis aff. bicolor | UMMZ 91550 | Ecuador, Napo-Pastaza, Abitagua | -1.383000 | -78.083000 | 1482 |
| Western Andes | |||||
| Synophis aff. bicolor | BMNH 1940.2.30.31 | Río Solaya, Ecuador | -0.010213 | -78.819510 | 1008 |
| Synophis aff. bicolor | CAS 23612 | Chimborazo, Naranjapata, Ecuador | -2.266667 | -79.083333 | 763 |
| Synophis aff. bicolor | MCZ R-164530 | Ecuador, Pichincha, Tandapi | -0.419803 | -78.801132 | 1714 |
| Synophis aff. bicolor | QCAZ 10453 | Cotopaxi: Naranjito, Bosque Integral Otonga | -0.417820 | -78.988030 | 1655 |
| Synophis aff. bicolor | TCWC 66209 | Ecuador, Cotopaxi, Las Pampas | -0.348360 | -79.076010 | 1238 |
| Synophis aff. bicolor | UMMZ 185812 | Ecuador, Cotopaxi, San Francisco de Las Pampas | -0.440357 | -78.966629 | 1586 |
| Synophis cf. bicolor | MHUA 14577 | Colombia, Dpto. Antioquia, Mpio. Amalfi, V. da La Manguita, Fca. La Esperanza | 6.978611 | -75.044444 | 1394 |
| Synophis cf. bicolor | MLS 2072 | Medellin, Cordillera Central, Colombia | 6.230833 | -75.590556 | 1497 |
| Synophis bicolor | MECN 6732 | Tobar Donoso, Ecuador | 1.189930 | -78.504130 | 229 |
| Synophis bicolor | MECN 6733 | Sendero Awa, Ecuador | 1.164400 | -78.507120 | 257 |
| Synophis bicolor | MZUTI 4175 | Itapoa, Ecuador | 0.46411 | -79.15547 | 267 |
| Synophis bicolor | UTA R-55956 | Ecuador, Esmeraldas, Canton San Lorenzo | 1.03212 | -78.613780 | 318 |
| Synophis calamitus | KU 164208 | 9 km SE Tandayapa, Pichincha Province, Ecuador | -0.047404 | -78.632804 | 2169 |
| Synophis calamitus | KU 197107 | 4 km SE Tandayapa, Pichincha Province, Ecuador | -0.012514 | -78.650697 | 1889 |
| Synophis calamitus | MZUTI 3694 | Tambo Tanda, Ecuador | -0.020108 | -78.651012 | 2048 |
| Synophis lasallei | EPN S.974 | Ecuador, Napo-Pastaza, nr. Río Talin, headwaters of the Río Bobonaza | -1.466670 | -77.883300 | 948 |
| Synophis lasallei | FHGO 6489 | Ceploa, Ecuador | -1.339063 | -77.670660 | 839 |
| Synophis lasallei | FHGO 7770 | Cara del Indio, Ecuador | -3.575695 | -78.451020 | 1207 |
| Synophis lasallei | FHGO 8340 | El Quimi, Ecuador | -3.571852 | -78.516598 | 752 |
| Synophis lasallei | FMNH 81313 | Colombia, Meta, Pico Renjifo, Serrania de la Macarena | 2.476901 | -73.794852 | 520 |
| Synophis lasallei | KU 164221 | 2 km SSW Río Reventador, Ecuador | -0.100000 | -77.600000 | 1479 |
| Synophis lasallei | MCZ R-156873 | Ecuador, Napo Prov., Inecel Station, Cascada San Rafael, Río Quijos | -0.103401 | -77.585487 | 1290 |
| Synophis lasallei | MECN 11250 | Paquisha Alto, Ecuador | -3.909518 | -78.487244 | 1660 |
| Synophis lasallei | MECN 11262 | El Pangui, Ecuador | -3.624502 | -78.586510 | 814 |
| Synophis lasallei | MECN 2220 | Puyo, Ecuador | -1.466780 | -77.983350 | 957 |
| Synophis lasallei | MLS/CJSP | N of Alban, cen. Cundinamarca Dept., cen. Colombia | 4.883333 | -74.450000 | 1983 |
| Synophis lasallei | MZUTI 4181 | Sacha Yaku, Ecuador | -1.407882 | -77.711092 | 974 |
| Synophis lasallei | USNM 233061 | Río Arajuno, headwaters of, tributary of Río Napo, Pastaza, Ecuador | -1.400000 | -77.883300 | 969 |
| Synophis lasallei | USNM 233062 | Río Siquino, tributary of Río Villano, Upper Curaray, Pastaza, Ecuador | -1.455303 | -77.714685 | 576 |
| Synophis lasallei | USNM 233063 | Río Bobonaza, headwaters of, Ecuador | -1.512156 | -77.833454 | 594 |
| Synophis lasallei | WWL 977-978 | Colombia, Meta prov., Villavicencio | 4.150000 | -73.633333 | 539 |
| Synophis plectovertebralis | UVC 11580 | Haciendo San Pedro, 6km S El Queremal, Municipio Dagua, Valle del Cauca, Colombia | 3.483333 | -76.700000 | 1830 |
| Synophis zaheri | MZUTI 3353 | Buenaventura Lodge, Ecuador | -3.647970 | -79.755070 | 874 |
| Synophis zaheri | MZUTI 3355 | Buenaventura Lodge, Ecuador | -3.648820 | -79.756400 | 812 |
We isolated total DNA from liver tissue or tail tips by proteinase K digestion in lysis buffer, followed by protein precipitation with guanidine thiocyanate solution and final DNA precipitation using isopropyl alcohol. We used the following pairs of primers to amplify and sequence four mitochondrial genes (12S, 16S, CYTB, ND4) and one nuclear locus (CMOS): Snake_12S_F (5’-AAACTGGGATTAGATACCCCACTAT-3’), Snake_12S_R (5’-GTRCGCTTACCWTGTTACGACT-3’), Snake_16S_F (5’-CGCCTGTTTAYCAAAAACAT-3’), and Snake_16S_R (5’-CCGGTCTGAACTCAGATCACGT-3’) from Kessing et al. (1989); Snake_Cytb_F (5’-GACCTGTGATMTGAAAACCAYCGTTGT-3’) and Snake_Cytb_R (5’-CTTTGGTTTACAAGAACAATGCTTTA-3’) from Burbrink et al. (2000); Snake_ND4_F (5’-CACCTATGACTACCAAAAGCTCATGTAGAAGC-3’) and Snake_ND4_R (5’-CATTACTTTTACTTGGATTTGCACCA-3’) from Arévalo et al. (1994); and Snake_cmosFs77 (5’-CATGGACTGGGATCAGTTATG-3’) and Snake_cmosRs78 (5’-CCTTGGGTGTGATTTTCTCACCT-3’) from Lawson et al. (2005).
We set up PCR reactions to a total volume of 25 µL containing MgCl2 2–3 mM, dNTPs 200 µM, 0.2 µM of each primer (0.8 µM in the case of ND4) and 1.25 U (16S and Cytb) or 0.625 U (ND4 and c-mos) of Taq DNA polymerase (Invitrogen). Thermocycling parameters consisted of an initial three-minute step at 94 °C; 25 to 30 cycles of 45–60 sec at 94 °C, 45 (16S and c-mos) or 60 (ND4 and Cytb) sec at 53–60 °C, 1 (16S and c-mos) or 2 (ND4 and Cytb) min at 72 °C; and a final extension of 7 min at 72 °C. We used 1.5% agarose gels to visualize the PCR products and QIAquick PCR purification Kit (QIAGEN) to remove unincorporated primers and dNTPs from every PCR reaction before they were sent to Macrogen Inc. for sequencing.
We combined these new data with the publically available sequences for Nothopsis and Xenopholis (Vidal et al. 2010; Grazziotin et al. 2012). We obtained additional sequences of Synophis bicolor from the Museu de Zoologia da Universidade de São Paulo (MHUA 14577 [Museo de Herpetología de la Universidad de Antioquia], from Colombia: 12S, 16S, CYTB, and CMOS) and the University of Texas, Arlington (UTA-R 55956 from Ecuador: CYTB and ND4).
We then included all publically available dipsadine species sampled for these genes. This matrix contains 24% missing data (‘-’), but these have been shown not to have deleterious effects on taxon placement and support in previous analyses (e.g., Pyron et al. 2011). Data were aligned using MAFFT (Katoh and Standley 2013) under the default parameters in Geneious 7.1.9 (Biomatters Ltd.). We determined the optimal partitioning strategy using PartitionFinder (Lanfear et al. 2012). We estimated the phylogeny using MrBayes 3.2.5 (Ronquist et al. 2012), with 4 runs of 4 chains each, run for 20 million generations with the first 25% discarded as burnin. Convergence was assumed as the average standard deviation of split frequencies went to zero and the potential scale reduction factors went to one (Ronquist et al. 2012). The GenBank accession numbers for the new and existing data are given in Appendix I.
Morphological data
Species in Diaphorolepis, Emmochliophis, and Synophis have traditionally been delimited using easily determined external morphological characters (Bogert 1964; Hillis 1990). We relied here on a set of these characters, scored for museum specimens and our new material, to examine and delimit species boundaries (Table 1). For available specimens examined in person, in photographs, or in the literature, we recorded SVL and TL in mm, and counts of supralabials, infralabials, postoculars, ventrals, and subcaudals. We made cursory notes on the hemipenes of some male specimens when they were visible (Zaher 1999; Martinez 2011).
Results
Molecular phylogeny
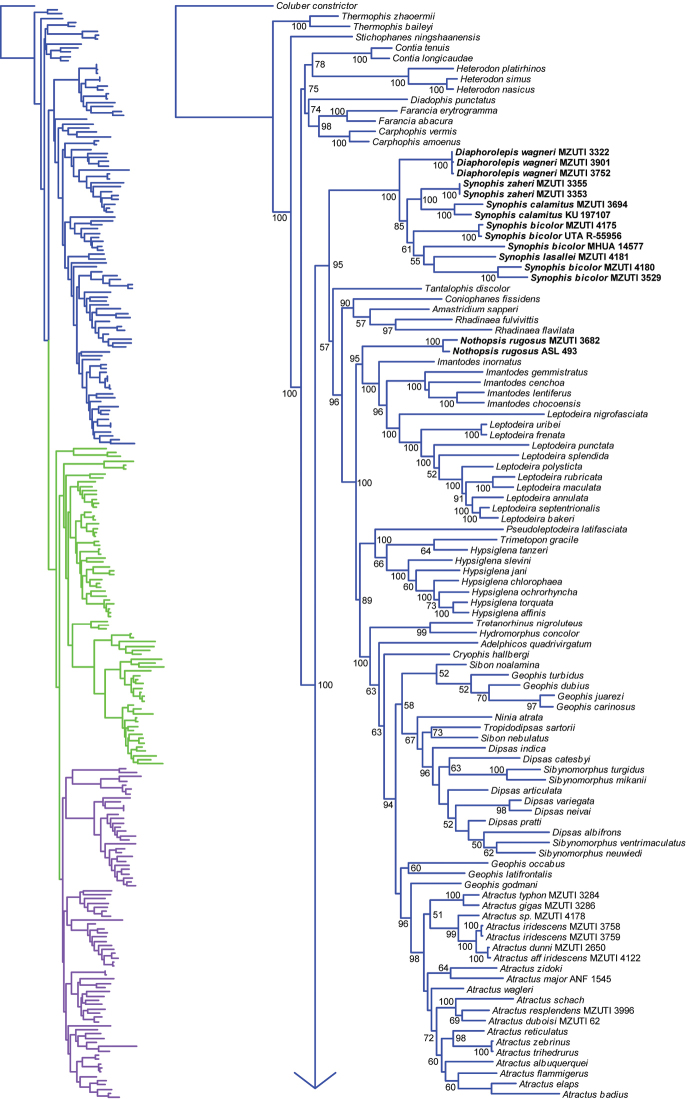
The overall topology and support (Figs 1, 2) is similar to numerous recent studies (Zaher et al. 2009; Vidal et al. 2010; Pyron et al. 2011; Grazziotin et al. 2012). We consider strong support to be posterior probabilities ≥95%, following recent authors (Felsenstein 2004). Overall, there is low support for many backbone nodes, which may reflect inadequate sampling of taxa (only ~250 out of ~900 dipsadine species) or characters (only two independent loci).
Figure 1.
Phylogeny (part) of ~245 dipsadine species plus outgroups, based on partitioned, multi-gene Bayesian inference analysis of 3,462bp of mitochondrial and nuclear DNA. Support values given are posterior probabilities ≥50% from 15 million post-burnin generations.
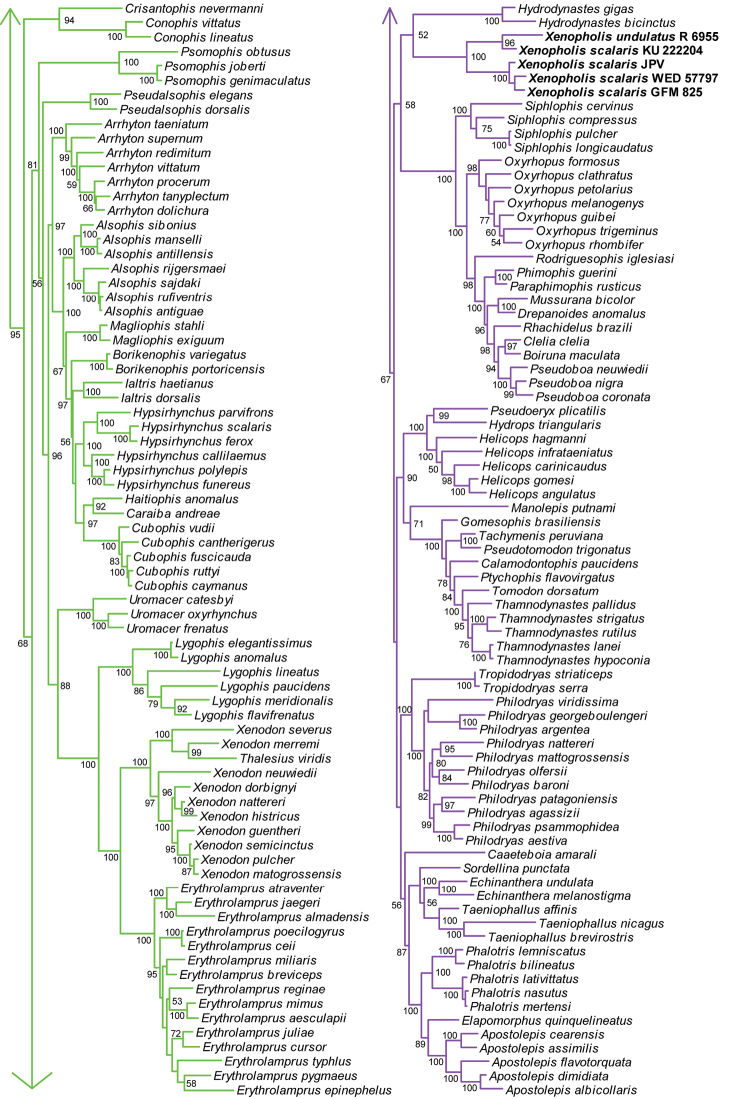
Figure 2.
Phylogeny (part) of ~245 dipsadine species plus outgroups, based on partitioned, multi-gene Bayesian inference analysis of 3,462bp of mitochondrial and nuclear DNA. Support values given are posterior probabilities ≥50% from 15 million post-burnin generations.
Species in Dipsadinae can be broadly grouped into a primarily North American clade (Contia to Carphophis when viewing Fig. 1), a primarily Central American clade (Diaphorolepis to Atractus in Fig. 1), and a primarily South American clade (Crisantophis to Apostolepis in Fig. 2), though many species in the latter two clades range across both Central and South America. Several speciose genera in the primarily Central American clade are non-monophyletic, including Imantodes, Hypsiglena, Geophis, Sibon, Dipsas, Sibynomorphus (Fig. 1), as in previous studies (Grazziotin et al. 2012; Pyron et al. 2013).
In agreement with previous results (Grazziotin et al. 2012; Pyron et al. 2013), we find that Nothopsini is not a natural group (Fig. 1). The genus Nothopsis is strongly supported, and strongly placed with Leptodeira + Imantodes within the Central American clade. Correspondingly, Xenopholis is strongly supported and weakly nested within the South American clade, as the sister lineage to Hydrodynastes. It appears that one Xenopholis scalaris (KU 222204) from a previous study (Pyron et al. 2011) may have been misidentified, and is actually related to Xenopholis undulatus. This specimen is strongly supported as the sister lineage to the sampled Xenopholis undulatus (R-6955), to the exclusion of the three other sampled Xenopholis scalaris, which are strongly supported as a monophyletic group. This specimen is from the Peruvian Amazon and is pictured in Duellman and Mendelson (1995). The specimen pictured resembles the Amazonian Xenopholis scalaris, rather than the more xeric Xenopholis undulatus from the Brazilian shield. Thus, it is possible either that a curatorial or laboratory error occurred at some point, or that there is cryptic genetic diversity in Xenopholis.
A strongly-supported clade comprising Diaphorolepis and Synophis represents the sister to the large, primarily Central American clade that also contains Nothopsis. Monophyly of Synophis with respect to Diaphorolepis is weakly supported. Within a weakly paraphyletic Synophis bicolor, there are three deeply divergent lineages, and the sampled specimen of Synophis lasallei. An apparently new species of Synophis is the strongly-supported sister lineage of Synophis calamitus. The species Synophis plectovertebralis remains unsampled in the molecular phylogeny. Although Emmochliophis is not sampled, we follow previous authors in assuming a close relationship with Diaphorolepis and Synophis, given their strong resemblance (Savitzky 1974; Hillis 1990). Thus, the synapomorphies previously used to diagnose Nothopsini (Savitzky 1974; Wallach 1995) apparently represent convergence in at least three distantly related dipsadine lineages.
Systematics
We seek here to only name clades associated Nothopsini that are strongly supported in our molecular phylogeny. Above the genus level, Nothopsini is not a natural group in any of its recent conformations. We place Nothopsis alone in Nothopsini Cope, 1871. We resurrect and re-delimit the tribe Diaphorolepidini Jenner, 1981 to include only Diaphorolepis, Emmochliophis, and Synophis. The genus Xenopholis is not strongly supported in any supra-generic group and remains incertae sedis in Dipsadinae (see Grazziotin et al. 2012).
Our molecular and morphological data (Tables 1–3; Figs 1, 2) also corroborate previous authors in finding that genus and species boundaries within Diaphorolepidini are unclear and in need of revision (Sheil and Grant 2001). We here provide photographs and range maps of representative material (Figs 3–9). A number of issues are immediately apparent, and can be addressed with our results. We outline these below.
Table 3.
Summary of measured diagnostic characters (external meristic features) for diaphorolepidine species. These data are a summary of Table 1 (omitting some subcaudal scale counts from apparently truncated tails), and can be used to identify ambiguous specimens in the field or collections, and should be updated with new material in the future.
| Species | MT | IL | SL | PO | V | SC | D1 | D2 | D3 |
|---|---|---|---|---|---|---|---|---|---|
| Diaphorolepis laevis | 16 | 10 | 8–9 | 2 | 157 | 84 | 19 | 19 | 17 |
| Diaphorolepis wagneri | 23–25 | 10–13 | 8–9 | 1–3 | 181–197 | 131–141 | 19–21 | 19 | 17 |
| Emmochliophis fugleri | 16 | 8 | 8 | 2 | 140 | 97 | 19 | 19 | 19 |
| Emmochliophis miops | 13 | 8 | 8 | 1 | 145 | 93 | 19 | 19 | 19 |
| Synophis aff. bicolor | 24–27 | 10–11 | 8–9 | 2 | 152–166 | 96–122 | 19–21 | 17–19 | 17–18 |
| Synophis cf. bicolor | 23–24 | 10–12 | 8 | 2 | 184–193 | 127–131 | 19 | 19 | 17 |
| Synophis bicolor | 16 | 9–11 | 8 | 2 | 174–183 | 129–143 | 19 | 17–19 | 17 |
| Synophis calamitus | – | 9–11 | 7–9 | 1–2 | 163–166 | 110–125 | 21–23 | 19 | 17 |
| Synophis lasallei | 24 | 10–11 | 7–9 | 1–2 | 144–165 | 101–126 | 19–23 | 19–22 | 17–21 |
| Synophis plectovertebralis | – | 7–8 | 7–8 | 1 | 144–147 | 79–91 | 19 | 19 | 17 |
| Synophis zaheri | – | 8–9 | 8 | 2 | 166–169 | 111–112 | 19 | 19 | 17 |
Figure 3.
Photographs of some diaphorolepidine species in life: a Synophis zaheri MZUTI 3353 b Synophis zaheri MZUTI 3355 c Synophis calamitus MZUTI 3694 d Synophis aff. bicolor MZUTI 3529 e Synophis lasallei uncat., and f Diaphorolepis wagneri MZUTI 3901.
Figure 9.
Photographs in preservation of some diaphorolepidine species. Upper: Synophis bicolor MZUTI 4175, Middle: Synophis lasallei MZUTI 4181, Lower: Synophis aff. bicolor MZUTI 4180.
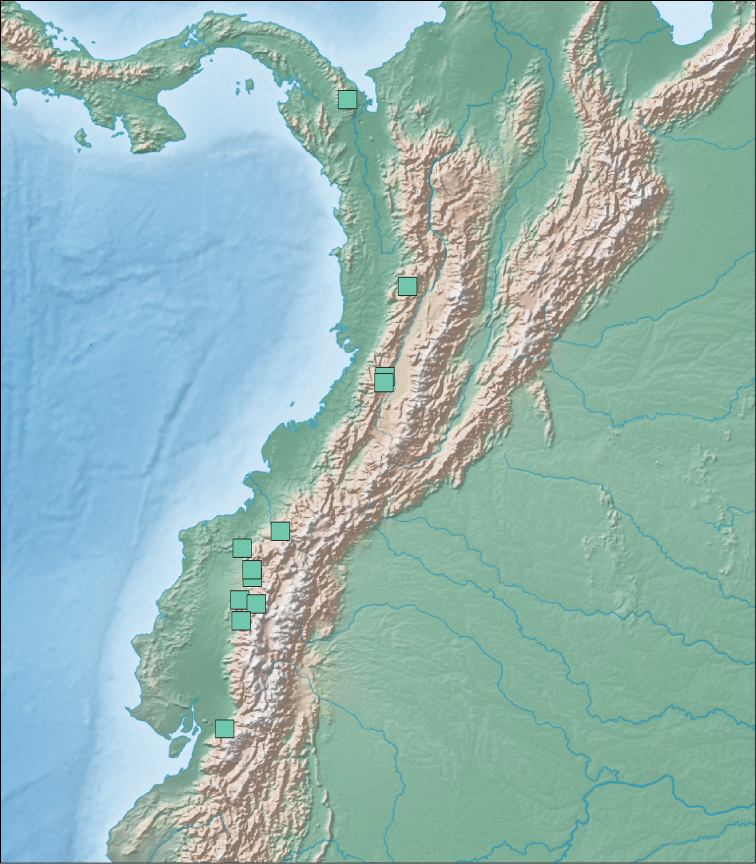
Figure 6.
Map of vouchered localities for Diaphorolepis wagneri (teal squares).
First, the head scalation of Diaphorolepis wagneri has not been accurately characterized by most authors (see Bogert 1964). Additionally, the holotype of Diaphorolepis laevis was incorrectly described with respect to several major characters (Werner 1923). Finally, reviewing museum specimens, including most holotypes, reveals that the current species boundaries and diagnoses are oftentimes inaccurate with respect to the observed range of variation in the relevant characters. In particular, the holotype of Synophis bicolor does not match many populations typically referred to this species (Bogert 1964; Hillis 1990; Sheil and Grant 2001).
In the case of Diaphorolepis wagneri, the postoculars can range from 1–3 (rather than 1–2), as illustrated by Bogert (1964), but not discussed explicitly. Werner (1901) apparently considered the small, lower postocular to be a subocular. Occasionally, the middle postocular will not be in contact with the brille, and resembles a temporal, behind the two remaining postoculars. As noted previously, the nasals are never divided, but only creased (Sheil and Grant 2001), contrary to reports from some previous authors (Bogert 1964; Hillis 1990).
In the case of Diaphorolepis laevis, Werner (1923) diagnosed the species as having fewer ventrals and subcaudals than Diaphorolepis wagneri, and smooth dorsal scales. Examination of the holotype (NMW 14860) reveals that it is indeed keeled, albeit weakly, throughout most of the midbody and posterior dorsal scale rows. This includes a bicarinate vertebral scale row that was previously considered to be diagnostic only of Diaphorolepis wagneri. The specimen appears to have a lighter-colored nuchal collar, though this may be a preservation artifact. The type locality within Colombia is unknown.
In the case of Synophis bicolor, the holotype (MZUT 257) has 180 ventrals, 136 subcaudals, and 9 infralabials, whereas sampled populations from the Andes of Ecuador typically have 152–166 ventrals, 96–122 subcaudals, and 10 or 11 infralabials. The locality of the holotype is unknown. Sampled populations from the Chocó of Ecuador match the holotype more closely, with 174–183 ventrals, 129–143 subcaudals, and 9–11 infralabials. The Chocóan populations typically occur at low to middle elevations (~200–300m), whereas Andean populations occur at higher elevations (~800–1700m). Populations from the northern western Andes of Colombia have 184–193 ventrals, 127–131 subcaudals, and 10–12 infralabials.
These three populations (Chocóan, Colombian Andean, and Ecuadorean Andean; Figs 3D, 4), correspond to three deeply divergent genetic lineages within Synophis bicolor (Fig. 1). A full revision of this species complex is pending further molecular and morphological sampling. We refer to the Chocóan populations as Synophis bicolor, the Ecuadorean Andean populations as Synophis aff. bicolor, and the Colombian Andean populations as Synophis cf. bicolor (using aff. versus cf. somewhat arbitrarily) for the remainder of the paper. The Synophis bicolor group is also weakly paraphyletic with respect to the sampled specimen of Synophis lasallei, which is the sister lineage of the Ecuadorean Andean lineages. The specimen of Synophis lasallei (MZUTI 4181) strongly matches the other Synophis lasallei specimens examined (Table 1), and is thus not a mis-identified Synophis bicolor.
Figure 4.
Photographs of some diaphorolepidine species in life: Synophis bicolor UTA R-55956 (a), and Synophis cf. bicolor MHUA 14577 (b).
Finally, we report here on two specimens of Synophis aff. calamitus from low to middle elevations on the Pacific versant of the Andes in SW Ecuador. These are diagnosable from the species above based on numerous characters, and we here name them:
Synophis zaheri sp. n.
http://zoobank.org/AEE122E3-497B-4DBF-8A2B-79DDD231E42B
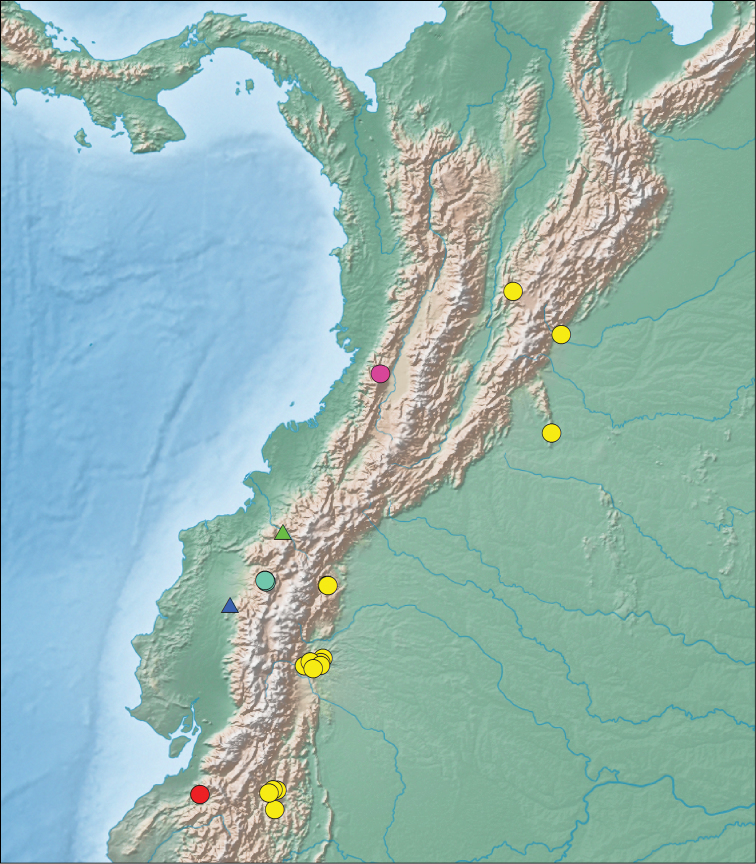
Figure 5.
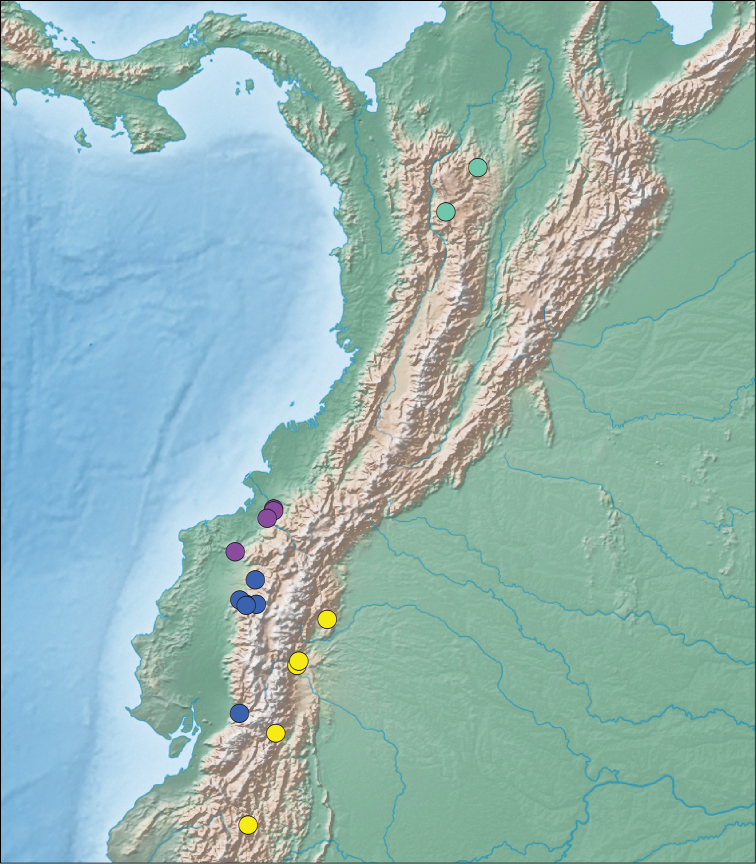
Map of vouchered localities for Synophis lasallei (yellow circles), Synophis plectovertebralis (pink circles), Synophis calamitus (teal circles), Synophis zaheri (red circles), Emmochliophis miops (green triangle) and Emmochliophis fugleri (blue triangle).
Figure 8.
Photographs in preservation of some diaphorolepidine species. Upper: Diaphorolepis wagneri MZUTI 3901, Center: Synophis zaheri MZUTI 3355, Lower: Synophis calamitus MZUTI 3694.
Holotype.
MZUTI 3353 (Fig. 3A), an adult male collected on 30 December 2013 at ~2200h by Alejandro Arteaga, Lucas Bustamante, Rita Hidalgo, Daniel Mideros, and Diana Troya, in the vicinity of Buenaventura Reserve (Fundación Jocotoco), near Piñas, El Oro Province, SW Ecuador, 874m above sea level (-3.65, -79.76; Fig. 5), in a narrow band of cloud forest on the Pacific versant of the Andes.
Paratype.
MZUTI 3355 (Fig. 3B), adult male collected a few minutes after the holotype, a few meters away.
Etymology.
Named after the preeminent Brazilian herpetologist Hussam El-Dine Zaher, for his innumerable contributions to South American herpetology and snake systematics.
Diagnosis.
Synophis zaheri can be differentiated from Diaphorolepis by an unmodified vertebral scale row with a single weak keel (versus a laterally expanded vertebral scale row, bicarinate or smooth); from Emmochliophis by the presence of a loreal (versus absence); from Synophis bicolor by having 166–169 ventrals (versus 174–183) and 111–112 subcaudals (versus 129–143); from Synophis aff. bicolor by having 8 or 9 infralabials (versus 10 or 11) and lighter brown dorsal coloration in life (versus darker black); from Synophis cf. bicolor by having 166–169 ventrals (versus 184–193), 111–112 subcaudals (versus 127–131), and 8 or 9 infralabials (versus 10–12); from Synophis calamitus by having two postoculars (versus one typically) and internasals in contact (versus divided typically); from Synophis lasallei by having 166–169 ventrals (versus 144–165), 19 dorsal scale rows at midbody (versus 21–23 typically), 8 or 9 infralabials (versus 10 or 11), and by having the anteriormost dorsal scale rows smooth (versus keeled); and from Synophis plectrovertebralis by absence of a nuchal collar (versus presence) and two postoculars (versus one).
Description.
Small-sized snakes (351–372mm SVL, 184–194mm TL) with slender bodies and head distinct from neck. Eye large (>1/3 head height), bulbous, and black in life, with pupil not easily distinguishable from iris. Pupil round in preservative (though this may be an effect of fixation). Dorsum coloration grayish-brown with iridescent sheen in life and preservation, no light-colored nuchal collar in adults, and posterior supralabials mostly pigmented (>50%). Ventral coloration primarily bright yellowish-white, extending onto margins of ventral scales and supralabials. Posterior one-third of ventral surface anterior to vent becomes increasingly mottled, and ventral surface of tail color of dorsum. Squamation pattern includes 166–169 ventral scales, 111–112 subcaudals, 19-19-17 dorsal scale rows (scale-row reduction of 2 rows past midbody), anal single, no apical pits, mid-body dorsal scales with weak single keel (first few dorsal scale-rows smooth), vertebral scale row not enlarged, nuchal scales smooth, 8 supralabials, 8 or 9 infralabials, 2 postoculars, loreal present, nasal undivided, fused prefrontals, internasals in contact, and rostral concave. Condition of the vertebrae, which are heavily modified in Emmochliophis and Synophis (Fritts and Smith 1969; Savitzky 1974; Hillis 1990) unknown, pending skeletal preparation or micro-CT scanning. Everted hemipenes are slightly bilobed, semicalyculate, and semicapitate, relatively stout and bulbous, covered in large spines or hooks, similar to that of Diaphorolepis and Synophis aff. bicolor and Synophis lasallei (Bogert 1964; Zaher 1999; Martinez 2011). Both specimens were active by night in primary evergreen foothill forest, with canopy cover between 70 and 100%. The holotype MZUTI 3353 was found on the ground, whereas the paratype MZUTI 3355 was found 50 cm above the ground in a bush. Neither were found close to water, but were active after a rainy day.
In light of this new species and the updated material we have located and examined (Tables 1, 2), we have prepared updated accounts for the tribe and the other species. Hopefully, these will serve as useful descriptive summaries for taxonomic boundaries, species delimitation, and the assignment of new specimens and populations to species-level groups. We focus primarily on the external morphological characters that will be of greatest use for identifying specimens in the field and from preserved collections. In some cases, more detailed information can be found in the original descriptions cited. The tribe name Diaphorolepidini was introduced in the PhD thesis of Jenner (1981), for which availability as a published work is ambiguous. We conservatively continue to credit the name to her, rather than treat it as unavailable and re-describe it ourselves.
Tribe. Diaphorolepidini
Jenner, 1981
Diaphorolepis Jan, 1863 (type genus by original designation)
Emmochliophis Fritts & Smith, 1969
Synophis Peracca, 1896
Etymology.
Apparently from the Greek diaphoros for “differentiated” and lepis for “scales,” likely referring to the enlarged vertebral scale row as compared to the rest of the dorsal scales.
Description.
A group of relatively small-sized (<550mm SVL) dipsadine snakes restricted to the Darien of Panama and northern Andes of South America with fused prefrontals and either an expanded vertebral scale row (Diaphorolepis) or expanded zygapophyses and neural spines in adults (Emmochliophis and Synophis).
Notes.
The tribe name has also been spelled ‘Diaphorolepini’ by Sheehy (2012), but Diaphorolepidini is the correct spelling based on the suffix –lepis, for which the stem is –lepid + –ini. This is a greatly restricted definition of Diaphorolepidini over the original description (Jenner 1981), which included Atractus, Chersodromus, Crisantophis, Elapomorphus, Enulius, Gomesophis, Pseudotomodon, Ptychophis, and Sordellina.
Genus. Diaphorolepis
Jan, 1863
Diaphorolepis laevis Werner, 1923
Diaphorolepis wagneri Jan, 1863 (type species by monotypy)
Etymology.
Apparently from the Greek diaphoros for “differentiated” and lepis for “scales,” likely referring to the enlarged vertebral scale row as compared to the rest of the dorsal scales.
Description.
Relatively small-sized (<550mm SVL) dipsadine snakes restricted to the Darien in Panama and northern Andes of South America, with 16–25 maxillary teeth, 10–13 infralabials, 8 or 9 supralabials, fused prefrontals, internasals in contact, loreal present, 1–3 postoculars, 157–197 ventrals, 84–141 subcaudals, dorsal scales in (19–21)-19-17 rows, and expanded vertebral scale row with weak to strong double keeling.
Notes.
This genus was validly described by Jan (1863), and re-described by Werner (1897). Werner (1901) later incorrectly deemed Jan’s name a nomen nudum, and re-described the genus and type species, designating a neotype. However, this was an error of interpretation, later realized by Werner himself (Werner 1929), and neither the re-description or neotype designation have any nomenclatural validity (see Bogert 1964). The lower subcaudal counts for some specimens likely represent truncated tails.
Diaphorolepis laevis
Werner, 1923
Holotype.
NMW 14860, locality given only as “Colombia.”
Etymology.
Apparently from the Latin laevis for “smooth,” referring to the anterior dorsal scales.
Description.
Relatively small-sized snake (350mm SVL) with 10 infralabials, 8/9 supralabials, 2 postoculars, internasals in contact, fused prefrontals, loreal present, nuchal collar apparently present, 16/18 maxillary teeth, 157 ventrals, 84 subcaudals, 19-19-17 dorsal scale rows, vertebral scale row is enlarged, with single keels on lateral dorsal scale rows and double keels on enlarged vertebral scale row weak to absent anteriorly and weak posteriorly. Uniformly light-colored venter and dark-colored dorsum in preservative. Nothing is known of the hemipenes or vertebrae.
Notes.
Known only from the type specimen. The original description states that the dorsal scales are smooth, but weak keels are evident throughout the posterior portion of the body. A specimen at Harvard, reportedly from Leticia, Amazonas, Colombia, bears the identification Diaphorolepis laevis (MCZ R-143839). Upon examination, this specimen is clearly not Diaphorolepis on the basis of divided prefrontals (versus united in Diaphorolepis), lack of an enlarged bicarinate vertebral scale row (versus presence), and presence of an ocellated dorsal color-pattern (versus uniformly colored dorsum). The overall resemblance is of Dipsas sp.
Diaphorolepis wagneri
Jan, 1863
Holotype.
ZSM 2708/0, locality given only as “Andes of Ecuador.” We revise this by subsequent restriction (sensu Smith 1953) to Milpé, Pichincha province, Ecuador (0.035, -78.87; 1076m), the locality of one of the specimens (MZUTI 3322) examined here.
Description.
Relatively small-sized snakes (276–524mm SVL) with 23–25 maxillary teeth, 10–13 infralabials, 8 or 9 supralabials, 1–3 postoculars with the lower occasionally resembling a subocular and the middle occasionally resembling a temporal, fused prefrontals, internasals in contact, loreal present, incomplete nuchal collar present in juveniles (MZUTI 3322) fading ontogenetically, 181–197 ventrals, 131–141 subcaudals, (19–21)-19-17 dorsal scale rows, strong keels present on dorsal scales, and enlarged, bicarinate vertebral scale row. Uniformly cream-colored venter and dark-brown to black dorsum. Lumbar vertebrae are constricted near the middle, zygapophyses and neural spines are not expanded. The hemipenis has been briefly described (Bogert 1964), but prior to modern classifications of the organ (Zaher 1999), and needs to be examined in more detail. Ranges at low to middle elevations (~300–1600m) along the Pacific versant from the Darien in Panama to central Ecuador.
Etymology.
Most likely after Moritz Wagner, who collected the holotype (see Bauer 2013), and not Johann Andreas Wagner as suggested by previous authors (Beolens et al. 2011).
Notes.
The re-description and neotype designation (NMW 18915) of Werner (1901) have no nomenclatural validity (see Bogert 1964).
Genus. Emmochliophis
Fritts & Smith, 1969
Emmochliophis fugleri Fritts & Smith, 1969 (type species by monotypy)
Emmochliophis miops (Boulenger, 1898)
Etymology.
From the Greek emmochlion for “a socket for a bar” and ophis for “snake,” referring to the unique interlocking vertebrae (Fritts and Smith 1969).
Description.
Relatively small-sized (~250mm SVL) terrestrial snakes restricted to the Pacific Andean slopes of NW Ecuador, with a small number (<17) of maxillary teeth, 8 supralabials, 8 infralabials, fused prefrontals, internasals in contact, loreal absent, fewer than 150 ventrals, fewer than 100 subcaudals, dorsal scales in 19 rows without reduction, trunk vertebrae with lateral expansion of the zygapophyses, and expanded zygapophyses forming a rod-and-groove mechanism in Emmochliophis fugleri, but not in Emmochliophis miops.
Notes.
Both species are known only from the types. The hemipenis of Emmochliophis fugleri has been briefly described (Fritts and Smith 1969), but prior to modern classifications of the organ (Zaher 1999), and needs to be examined in more detail. The organ is unknown in Emmochliophis miops, as the sole known specimen is female (Sheil 1998).
Emmochliophis fugleri
Fritts & Smith, 1969
Holotype.
UIMNH 78795, 4 km. E Río Baba bridge, 24 km. S Santo Domingo de los Colorados, Pichincha, Ecuador, ~600 m.
Etymology.
After Dr. Charles Fugler, who collected the holotype.
Description.
A terrestrial snake from the Pacific Andean slopes of NW Ecuador, diagnosable by 16 maxillary teeth, 8 infralabials, 8 supralabials, 2 postoculars, internasals in contact, loreal absent, nuchal collar absent, 140 ventrals, 97 subcaudals, dorsal scales in 19 rows without reduction, strong keels, and zygapophyses expanded laterally forming rod–and–bar assembly. Type locality is surrounded by banana plantations. Little else is known about the habits or habitat of the species.
Notes.
Known only from the type specimen, a male, collected by C. Fugler in February 1966.
Emmochliophis miops
(Boulenger, 1898)
Synophis miops Boulenger, 1898
Holotype.
BMNH 1946.1.12.30, Paramba, Ecuador (=Parambas, Imbabura fide Lynch and Duellman 1997)
Etymology.
None given by Boulenger (1898); likely from the Greek miops for “myopia,” in reference the species’ small eyes, given as diagnostic by Boulenger.
Description.
Relatively small-sized (~250mm SVL) terrestrial snake from the Pacific Andean slopes of NW Ecuador, diagnosable by 13 maxillary teeth, 8 infralabials, 8 supralabials, 1 postocular, internasals in contact, loreal absent, nuchal collar present, 145 ventrals, 93 subcaudals, dorsal scales in 19 rows without reduction, strong keels, and lateral expansion of the zygapophyses. Type locality is humid subtropical lower montane forest. Little else is known about the habits or habitat of the species. Stomach of type specimen contains remains of a gymnophthalmid lizard (Sheil 1998).
Notes.
Known only from the type specimen, a female, collected by W. F. H. Rosenberg in October 1897. The type specimen was re-described in great detail by Sheil (1998).
Genus. Synophis
Peracca, 1896
Synophis bicolor Peracca, 1896 (type species by monotypy)
Synophis calamitus Hillis, 1990
Synophis lasallei (Nicéforo-Maria, 1950)
Synophis plectovertebralis Sheil & Grant, 2001
Synophis zaheri Pyron, Guayasamin, Peñafiel, Bustamante, & Arteaga, 2015
Etymology.
None given by Peracca (1896); presumably from the Greek syn- for “with” or “together” and ophis for “snake,” though the intended meaning of “with snake” is unclear.
Description.
Relatively small-sized (~300mm SVL) dipsadine snakes of the Andes and Chocó of Colombia and Ecuador, with 16–27 maxillary teeth, 7–11 infralabials, 7–9 supralabials, fused prefrontals, loreal present, 1 or 2 postoculars, 144–184 ventrals, 88–138 subcaudals, dorsal scales in (19–21)-(17–21)-(17–20) rows, neural spine expanded and flattened, laterally expanded zygapophyses, and hemipenes slightly bilobed, semicalyculate, and semicapitate, relatively stout and bulbous, covered in large spines or hooks.
Notes.
On the basis of similar scale counts, but apparently without examining specimens, Amaral (1929) considered the holotype of Synophis bicolor (at the time, the only known specimen from the only known species) to be synonymous with Diaphorolepis wagneri. These snakes are extremely rare, accounting for the paucity of knowledge and unclear species-boundaries. Numerous undescribed species from many new localities are known, and await description (pers. comm., T. Grant, E. Meneses-Pelayo, O. Torres-Carvajal, and J. Arredondo).
Synophis bicolor
Peracca, 1896
Holotype.
MZUT 257, locality given only as “South America.”
Etymology.
None given by Peracca (1896); presumably from the Greek bi-color for “two colors,” referring to the dark dorsum and light venter.
Description.
Small-sized (~200–400mm SVL) dipsadine snakes of the Andes and Chocó of Colombia and Ecuador, diagnosable by 16–27 maxillary teeth, 9–12 infralabials, 8 or 9 supralabials, fused prefrontals, loreal present, 2 postoculars, 152–193 ventrals, 96–143 subcaudals, dorsal scales in (19–21)-(17–19)-(17–18) weakly keeled rows, neural spine expanded and flattened, laterally expanded zygapophyses, and hemipenes slightly bilobed, semicalyculate, and semicapitate, relatively stout and bulbous, covered in large spines or hooks. Populations of this species are found in both lowland Chocóan rainforest and Andean cloud forests. Individuals are often found in leaf litter or in bushes, active at night. One collection from the Pacific Andean slopes of Ecuador (UMMZ 185886–185891) represents clutches of 2, 2, and 8 eggs, with hatchlings 125–132mm SVL. Nothing is known of diet.
Notes.
This is a species complex comprising at least three species-level taxa, which are distinct genetically, geographically, and morphologically (Figs 1, 3D, 4, 7, 9; Tables 1–3).
Figure 7.
Map of vouchered localities for Synophis bicolor populations: Synophis bicolor sensu stricto (purple circles), western Synophis aff. bicolor (blue circles), eastern Synophis aff. bicolor (yellow circles), and Synophis cf. bicolor (teal circles).
First are the Ecuadorean Andean highlands populations (Synophis aff. bicolor), which occur both on both the Pacific and Andean versants (~800–1700m). These are diagnosable by number of ventrals (152–166), subcaudals (96–122), infralabials (10 or 11), and supralabials (8 or 9), in combination. One individual (UMMZ 91550) has 24/27 maxillary teeth. The southernmost individual we examined (MZUTI 4180) has a very low number of ventral scales (152) compared to the remaining populations (160–166). Populations east and west of the Andes may also be a distinct species (O. Torres-Carvajal, pers. comm.), and are presented separately here. Most records from the Pacific versant north of the Río Toachi appear to represent Synophis calamitus (see below); one specimen reported from north of the river (BMNH 1940.2.30.31) may be mis-labeled, mis-identified, or the locality mis-referenced, or the species may be sympatric at some localities north of the river.
Second are the Chocóan populations from NW Ecuador, and presumably SW Colombia (~200–300m). These match the holotype in having 174–183 ventrals, 129–138 subcaudals, 8 supralabials, and typically 9 infralabials, though one specimen from further south (MZUTI 4175) has 11. We revise the type locality of Synophis bicolor by subsequent restriction (sensu Smith 1953) to Tobar Donoso, Carchi Province, Ecuador (1.19, -78.50), locality of several specimens examined here (Tables 1, 2; Figs 1, 4, 7, 9), to cement this association. Thus, this population represents Synophis bicolor sensu stricto in the case of future revision.
Third are the Colombian Andean highland populations (~1400–1500m; see Nicéforo-Maria 1970), which differ from the holotype in having 184–193 ventrals (versus 180), 127–131 subcaudals (versus 136), and 10–12 infralabials (versus 9). This group likely represents a third species, Synophis cf. bicolor. While we refrain from describing these additional Synophis bicolor-group species here based on limited current sampling, the populations described above likely represent at least two (Ecuadorean Andean highland and Colombian Andean Highland) if not three (E and W Ecuadorean and Colombian Andean highland) species.
Synophis calamitus
Hillis, 1990
Holotype.
KU 197107, 4 km SE Tandayapa, Pichincha Province, Ecuador.
Paratype. KU 164208, 9km SE Tandayapa, Pichincha Province, Ecuador.
Etymology.
From the Latin for “calamity,” referring to accidents that befell the original collectors (Hillis 1990).
Description.
A group of relatively small (~450mm SVL) dipsadine snakes of the cloud forests of the Pacific versant of the Andean highlands of Ecuador diagnosable by 9–11 infralabials, 7–9 supralabials, fused prefrontals, internasals separated, loreal present, 1 or 2 postoculars, 163–166 ventrals, 110–125 subcaudals, dorsal scales in (21–23)-19-17 weakly keeled rows, neural spine expanded and flattened, and laterally expanded zygapophyses. Known from middle to high-elevation (~1900–2200m) cloud forests north of the Río Toachi. Nothing is known of diet or reproduction.
Notes.
A detailed description was also provided by Hillis (1990). The hemipenes have likely not been examined. Easily confused with Synophis bicolor; at least one specimen (QCAZ 11931) from near the type locality was originally mis-identified (O. Torres-Carvajal, pers. comm.). We suggest that all populations north of the Río Toachi are likely to represent Synophis calamitus. As mentioned above, one specimen apparently matching Synophis bicolor (BMNH 1940.2.30.31) is known from Río Soloya near Mindo north of Río Toachi, but this may have been mis-labeled, or mis-referenced geographically. The specimen of “Synophis bicolor” examined by Zaher (1999), QCAZ 452, cannot be located (O. Torres-Carvajal, pers. comm.), but originates from Chiriboga, Pichincha Province, Ecuador, north of Río Toachi, and thus may represent an Synophis calamitus. If this is the case, the hemipenes of Synophis calamitus and Synophis lasallei are nearly identical (Zaher 1999; Martinez 2011). Finally, one specimen sequenced here from Tambo Tanda (MZUTI 3694) appears to have aberrantly subdivided head scales, possessing one extra postocular, and 2 extra supralabials and infralabials (Fig. 8), which are misshapen and abnormally small. The badly damaged paratype also appears to have two postoculars on one side (O. Torres-Carvajal, pers. comm.). Thus, we concur with Hillis (1990) that one postocular, 7 or 8 supralabials, and 9 infralabials (along with the divided internasals and smooth anterior dorsal scale-rows) are generally diagnostic of the species, but with rare individual variation.
Synophis lasallei
(Nicéforo-Maria, 1950)
Diaphorolepis lasallei Nicéforo-Maria, 1950
Holotype.
MLS/CJSP uncat., from N of Albán, cen. Cundinamarca Dept., cen. Colombia.
Etymology.
After the Instituto de La Salle, in Bogotá (Nicéforo-Maria 1950).
Description.
Smaller (~300mm SVL) dipsadine snakes of the Amazonian versant of the Andes of Ecuador and Colombia, diagnosable by 24 maxillary teeth, 10 or 11 infralabials, 7–9 supralabials, fused prefrontals, internasals in contact, loreal present, 1 or 2 postoculars, nuchal collar absent, 144–165 ventrals, 101–126 subcaudals, dorsal scales in (19–23)-(19–22)-(17–21) strongly keeled rows even on head and neck, venter dark in some populations, neural spines expanded and flattened, and laterally expanded zygapophyses. Known from low to high elevations (~500–2000m) along the Amazonian versant of the Andes from central Colombia to central Ecuador. Nothing is known of diet or reproduction.
Notes.
The hemipenes are very similar to both Diaphorolepis and Synophis bicolor (Bogert 1964; Zaher 1999; Martinez 2011). Much like Synophis bicolor, this species as currently described has a large geographic and elevational range, with wide variation in phenotype. There is significant variation in the number of dorsal scale rows and reduction thereof. One specimen from Ecuador (MCZ R-156873) has only one postocular and 7 supralabials, but otherwise matches the species. All other specimens have 2 and 8, respectively. Another specimen from Ecuador (MECN 2220) has 165 ventrals and 117 subcaudals with 19-19-17 scale rows, and is thus indistinguishable from Synophis aff. bicolor, with the exception of the strong keels on the nuchal scales and geographic distance from the nearest highland populations of Synophis aff. bicolor. All other specimens of Synophis lasallei have 144–156 ventrals, and most have (21–23)-(21–22)-(19–21) dorsal scale rows. Thus, it seems exceptionally likely that this is a species complex, possibly divided between highland and lowland, or northern and southern populations.
Synophis plectovertebralis
Sheil & Grant, 2001
Holotype.
UVC 11858, from Hacienda San Pedro, about 6 km south El Queremal, Municipio Dagua, Departamento del Valle del Cauca, Colombia.
Paratype.
UVC 11580, from type locality.
Etymology.
From the Latin plecto- for “braided” or “woven” and veretbralis for “vertebrae,” referring to the appearance of the interlocking zygapophyses viewed from above (Sheil and Grant 2001).
Description.
Relatively small (~200mm SVL) dipsadine snakes of the Pacific versant of the Andean Highlands of W Colombia, diagnosable by 24 maxillary teeth, 7 or 8 infralabials, 7 or 8 supralabials, fused prefrontals, internasals in contact, loreal present, 1 postocular, nuchal collar present, 144–147 ventrals, 79–91 subcaudals, dorsal scales in 19-19-17 weakly keeled rows, neural spines expanded and flattened, and laterally expanded zygapophyses forming a partially interlocking complex. The type locality is a middle elevation (~1800m) cloud forest. Both known specimens were collected in moist leaf litter; one was active at night. The stomach of the holotype contained a Ptychoglossus stenolepis (Sauria: Gymnophthalmidae).
Notes.
Known only from the holotype and paratype (apparently juveniles), though other material has apparently been collected in Colombia, near the type locality (T. Grant and E. Meneses-Pelayo, pers. comm.). The hemipenes have not been examined. A more detailed description of the two specimens is provided by Sheil and Grant (2001).
Given our restriction of the name, we also provide the following re-description of the re-delimited Nothopsini. Note that we have not performed a comparative examination of a large series of preserved material, and these data are summarized from the literature (Dunn and Dowling 1957; Savage 2002; Kohler 2008; McCranie 2011) to provide a basis for future revisions.
Tribe. Nothopsini
Cope, 1871
Nothopsis Cope, 1871 (type genus by monotypy)
Nothopsis rugosus Cope, 1871
Nothopsis affinis Boulenger, 1895 (Holotype BMNH 1946.1.15.62, “Salidero, NW Ecuador, 350ft”) [subjective junior synonym of Nothopsis rugosus fide Dunn & Dowling 1957]
Nothopsis torresi Taylor, 1951 (Holotype KU 28710, “’Morehead’ Finca, 5 miles southwest of Turrialba, Costa Rica”) [subjective junior synonym of Nothopsis rugosus fide Dunn & Dowling 1957]
Holotype.
USNM 12427, type locality “Isthmus of Darien [Panama]”
Etymology.
From the Greek nothos for “bastard” and opsis for “appearance,” with Cope (1871) apparently referring to putative mimicry of Bothrops atrox.
Description.
A relatively small-sized (<350mm SVL) dipsadine snake, ranging in Central and South America from Honduras to Colombia and Ecuador, in lowland and middle-elevation rainforests, 250-900m, distinguishable from nearly all other similar or related snakes in the area by the rugose, granular nature of the dorsal scales, in particular lacking differentiation of the cephalic scales with the exception of well-defined internasals and poorly defined frontal and parietals, which are separated by rows of irregular, undifferentiated scales. Color pattern consists of irregular and poorly defined blotches of blackish or light, dark, and yellowish brown. With respect to the characters described here for diaphorolepidine species, Nothopsis rugosus typically exhibits 19–21 maxillary teeth, 9–13 supralabials, 11–16 infralabials, 149–162 ventrals, 81–112 subcaudals, dorsal scales in (24–30)-(26–30)-(22–26) rows, SVL of 151–320mm, and tail length of 61–133mm (see Dunn and Dowling 1957).
Notes.
This taxon has historically been divided up into as many as three species (see Dunn and Dowling 1957), though only a single species is currently recognized. There may be cryptic variation or undiscovered diversity within this group. Note that the family name was originally spelled Nothopidae by Cope (1871), but –ops– is the correct stem from –opsis, and Nothopsidae (and Nothopsini) is thus the correct spelling, as adopted by later authors.
Discussion
Systematics of Diaphorolepidini and Nothopsini
Corroborating previous results, we find that current supra-generic classification in Dipsadinae does not accurately reflect the phylogeny and describe natural groups in many cases (Pyron et al. 2011; Grazziotin et al. 2012). Support for monophyly and placement of many genera is low, and many other genera are apparently non-monophyletic. Efforts to clarify this situation are underway, sampling more taxa and characters (F. Grazziotin, pers. comm.). Only ~250 out of ~900 dipsadine species (Wallach et al. 2014) are sampled here for a few genes, but cryptic and undiscovered diversity is likely much higher in the group, and will require extensive additional sampling of taxa and characters to arrive at a stable phylogenetic and taxonomic resolution. The taxonomy of Dipsadinae has been contentious for quite some time (Cadle 1984a,b,c; Zaher 1999; Zaher et al. 2009; Grazziotin et al. 2012; Sheehy 2012), and will likely require extensive additional sampling of taxa and characters to provide a stable taxonomic resolution.
In particular, we find that Nothopsini is not monophyletic as historically defined, but that Nothopsis is strongly nested within a primarily Central American clade, with Imantodes and Leptodeira. We restrict tribe Nothopsini Cope, 1871 to Nothopsis. We resurrect and re-delimit Diaphorolepidini Jenner, 1981 to include only Diaphorolepis, Emmochliophis, and Synophis. Whereas Emmochliophis remains unsampled in the molecular phylogeny, it appears to be the sister-taxon of Synophis based on morphological data (Hillis 1990). However, our phylogeny suggests that many of the morphological characters previously used to define supra-generic groups in Dipsadinae (see Savitzky 1974; Wallach 1995) are subject to strong and rapid convergence. Thus, future studies may find an alternative placement for this genus. Finally, the genus Xenopholis is weakly nested within a primarily South American clade, and remains Dipsadinae incertae sedis.
Species limits in Diaphorolepidini
Larger sample sizes reveal expanded ranges of diagnostic characters previously used to delimit species in Diaphorolepidini. These will hopefully assist future researchers in describing new taxa, and re-delimiting species boundaries. In particular, both Synophis bicolor and Synophis lasallaei may comprise multiple distinct species. Additional DNA sequencing and meristic and mensural measurements of more specimens should help clarify taxonomic boundaries.
In the case of Synophis bicolor, the Chocóan populations in Ecuador and presumably nearby Colombia match the description of the holotype, and thus likely represent the source of the original specimen, which remains to be re-described in detail. Contrastingly, highland populations in the Andean Highlands of Ecuador and Colombia are morphologically and genetically distinct, and both likely represent undescribed species. In the Ecuadorean Andes, populations of this taxon occur on both the Pacific and Amazonian versants, which may also be distinct from each other. The sampled specimen of Synophis lasallei is weakly nested within the sampled specimens of Synophis bicolor. A wide range of squamation and color pattern is observed in Synophis lasallei, which may represent cryptic species, as well as potential mis-identification of examined specimens. Finally, a cloud-forest population from the Pacific versant in SW Ecuador represents a new species described here as Synophis zaheri, allied to Synophis calamitus. Understanding the geographic distribution and genetic diversity in these taxa will require additional genetic sampling, which is hampered by the rarity of these species.
One of the most distinctive features of diaphorolepidine species is the highly modified condition of the vertebrae, in which the prezygapophyses and postzygapophyses are broadly expanded, forming ridges, and occasionally interlocking (Bogert 1964; Fritts and Smith 1969; Hillis 1990). Given the difficulty of preparing the skeletal material and the extreme rarity of specimens, this was not examined for Synophis zaheri or any additional specimens examined here. However, this may be a crucial character for future systematic revisions in the group, possibly utilizing micro-CT scanning or radiography.
Another possible source of information for delimiting species are the hemipenes. The organs are highly similar in Diaphorolepis and most Synophis species (Bogert 1964; Jenner 1981; Hillis 1990; Zaher 1999). Our observations agree with previous authors that the hemipenes are not strongly differentiated among species, though larger comparative series may reveal characters that serve to better diagnose species-level groups. In particular, the hemipenes are “nearly identical” in Synophis bicolor and Synophis lasallei (Zaher 1999; Martinez 2011), and our examination of Synophis zaheri shows no obvious qualitative differences. It is possible that speciation is primarily ecological or allopatric in this group, and thus there is little physical reproductive isolation.
Conclusions
Higher-level taxonomy in Dipsadinae is still partially unresolved, and many genera and supra-generic groups are either non-monophyletic, or poorly supported and weakly placed. This includes Nothopsini Cope, 1871, which must be restricted to Nothopsis, if it is used at all. We resurrect and re-delimit Diaphorolepidini Jenner, 1981 to include only Diaphorolepis, Emmochliophis, and Synophis. The genus Xenopholis remains Dipsadinae incertae sedis. Revised and expanded diagnoses in Diaphorolepidini support the distinctiveness of all currently recognized taxa. Cryptic species are likely present in Synophis bicolor and Synophis lasallei. A new population from the cloud forest of SW Ecuador is morphologically and genetically distinct, and we here name it Synophis zaheri. We hope that these data will provide a robust platform for future researchers to examine species boundaries in Diaphorolepidini, as additional work clearly remains to be done. This is hampered, however, by the extreme rarity of these species.
Supplementary Material
Acknowledgments
This research was funded in part by the George Washington University (including a grant of time from the Colonial One HPC Initiative), Universidad Tecnológica Indoamérica, and U.S. NSF grants DBI-0905765 and DEB-1441719 to R.A.P. Permits were provided by the Ministerio del Ambiente, Ecuador. We thank T. J. Hibbitts (TCWC), J. Martinez and J. Losos (MCZ), J. A. Campbell, C. J. Franklin, and E. N. Smith (UT Arlington), C. M. Sheehy III (UF), F. Andreone (MZUT), C. Sheil (JCU), T. Grant (USP), A. Savitzky (USU), H. Grillitsch and G. Gassner (NMW), G. Schneider and D. Rabosky (UMMZ), D. Hillis, D. Cannatella, and T. LaDuc (UT Austin), J. Jacobs and K. de Queiroz (NMNH), H. Zaher and F. Grazziotin (MZUSP), J. Daza and J. Arredondo (MHUA), O. Torres-Carvajal (QCAZ), J. Valencia (FHGO), M. Yánez-Muñoz (MECN), J. Culebras (UTI), and D. Troya, D. Mideros, J. Castillo, and R. Hidalgo for access to specimens, data, and pictures.
Appendix I
GenBank accession numbers for Dipsadinae and outgroup species analyzed here.
| Species | 12S | 16S | CYTB | ND4 | CMOS |
|---|---|---|---|---|---|
| Adelphicos quadrivirgatum | - | - | GQ895853 | - | GQ895796 |
| Alsophis antiguae | AF158455 | AF158524 | - | - | - |
| Alsophis antillensis | FJ416691 | FJ416702 | FJ416726 | FJ416800 | - |
| Alsophis manselli | - | AF158528 | FJ416727 | FJ416801 | - |
| Alsophis rijgersmaei | FJ416697 | FJ416708 | FJ416729 | FJ416803 | - |
| Alsophis rufiventris | FJ416698 | FJ416709 | FJ416730 | FJ416804 | - |
| Alsophis sajdaki | - | - | FJ416731 | FJ416805 | - |
| Alsophis sibonius | FJ416692 | FJ416703 | FJ416728 | FJ416802 | - |
| Amastridium sapperi | - | - | GQ334479 | GQ334580 | - |
| Apostolepis albicollaris | JQ598793 | JQ598856 | - | - | JQ598965 |
| Apostolepis assimilis | GQ457781 | GQ457724 | - | - | GQ457843 |
| Apostolepis cearensis | JQ598794 | JQ598857 | - | - | JQ598966 |
| Apostolepis dimidiata | GQ457782 | GQ457725 | JQ598917 | - | GQ457844 |
| Apostolepis flavotorquata | JQ598795 | JQ598858 | GQ895854 | - | GQ895798 |
| Arrhyton dolichura | AF158438 | AF158507 | FJ416721 | FJ416795 | - |
| Arrhyton procerum | AF158452 | AF158521 | FJ416723 | FJ416797 | - |
| Arrhyton redimitum | AF158439 | AF158508 | FJ416720 | FJ416794 | - |
| Arrhyton supernum | AF158436 | AF158505 | FJ416718 | FJ416792 | - |
| Arrhyton taeniatum | AF158453 | AF158522 | FJ416717 | FJ416791 | - |
| Arrhyton tanyplectum | AF158446 | AF158516 | FJ416722 | FJ416796 | - |
| Arrhyton vittatum | AF158437 | AF158506 | FJ416719 | FJ416793 | - |
| Atractus aff. iridescens MZUTI4122 | - | KT944037 | KT944049 | KT944056 | - |
| Atractus albuquerquei | GQ457783 | GQ457726 | JQ598918 | - | GQ457845 |
| Atractus badius | AF158425 | AF158485 | - | - | - |
| Atractus duboisi MZUTI62 | - | KT944041 | - | KT944059 | - |
| Atractus dunni MZUTI2650 | - | KT944038 | KT944050 | KT944057 | - |
| Atractus elaps | - | - | EF078536 | EF078584 | - |
| Atractus flammigerus | AF158402 | AF158471 | - | - | - |
| Atractus gigas MZUTI3286 | - | KT944043 | KT944053 | KT944061 | - |
| Atractus iridescens MZUTI3758 | - | - | KT944052 | - | - |
| Atractus iridescens MZUTI3759 | - | KT944039 | KT944051 | KT944058 | - |
| Atractus major ANF1545 | - | KT944045 | - | - | - |
| Atractus resplendens MZUTI3996 | KT944036 | KT944042 | KT944055 | KT944060 | - |
| Atractus reticulatus | JQ598798 | JQ598886 | - | - | JQ598970 |
| Atractus schach | JQ598799 | AF158486 | - | - | JQ598971 |
| Atractus sp. MZUTI4178 | - | KT944040 | - | - | KT944066 |
| Atractus trihedrurus | GQ457784 | GQ457727 | JQ598919 | - | GQ457846 |
| Atractus typhon MZUTI3284 | - | KT944044 | KT944054 | KT944062 | - |
| Atractus wagleri | - | - | GQ334480 | GQ334581 | - |
| Atractus zebrinus | JQ598800 | JQ598861 | - | - | JQ598972 |
| Atractus zidoki | AF158426 | AF158487 | - | - | - |
| Boiruna maculata | GQ457785 | JQ598862 | GQ895855 | - | GQ895799 |
| Borikenophis portoricensis | FJ416696 | AF158517 | AF471085 | U49308 | AF471126 |
| Borikenophis variegatus | FJ416700 | FJ416711 | FJ416734 | FJ416808 | - |
| Caaeteboia amarali | GQ457807 | GQ457747 | JQ598921 | - | GQ457867 |
| Calamodontophis paucidens | GQ457786 | GQ457728 | - | - | GQ457848 |
| Caraiba andreae | AF158442 | AF158511 | FJ416743 | FJ416817 | - |
| Carphophis amoenus | AY577013 | AY577022 | AF471067 | - | DQ112082 |
| Carphophis vermis | - | - | KP765656 | - | - |
| Clelia clelia | AF158403 | AF158472 | - | - | JQ598973 |
| Coluber constrictor | L01765 | L01770 | EU180432 | AY487040 | AY486937 |
| Coniophanes fissidens | - | - | EF078538 | EF078586 | - |
| Conophis lineatus | GQ457788 | JQ598865 | JQ598924 | - | JQ598975 |
| Conophis vittatus | - | - | GQ895861 | - | GQ895805 |
| Contia longicaudae | - | - | GU112407 | GU112427 | - |
| Contia tenuis | AY577021 | AY577030 | GU112401 | AF402658 | AF471134 |
| Crisantophis nevermanni | GU018152 | GU018169 | - | - | - |
| Cryophis hallbergi | - | - | GQ895863 | EF078544 | GQ895807 |
| Cubophis cantherigerus | AF158405 | AF158475 | AF544669 | FJ416818 | AF544694 |
| Cubophis caymanus | FJ416693 | FJ416704 | FJ416745 | FJ416820 | - |
| Cubophis fuscicauda | FJ416695 | FJ416706 | FJ416747 | FJ416822 | - |
| Cubophis ruttyi | FJ416699 | FJ416710 | FJ416746 | FJ416821 | - |
| Cubophis vudii | AF158443 | AF158512 | FJ416744 | FJ416819 | - |
| Diadophis punctatus | AF544765 | AY577024 | EU193700 | EU193987 | AF471122 |
| Diaphorolepis wagneri MZUTI3322 | - | KR814752 | - | KR814775 | KR814764 |
| Diaphorolepis wagneri MZUTI3752 | - | KR814753 | - | KR814777 | KR814766 |
| Diaphorolepis wagneri MZUTI3901 | - | KR814754 | - | KR814778 | KR814767 |
| Dipsas albifrons | JQ598803 | JQ598866 | JQ598925 | - | - |
| Dipsas articulata | JQ598804 | JQ598867 | - | - | - |
| Dipsas catesbyi | JQ598805 | Z46496 | JQ598926 | EF078585 | JQ598977 |
| Dipsas indica | GQ457789 | GQ457730 | - | - | GQ457850 |
| Dipsas neivai | GQ457790 | GQ457731 | - | - | GQ457851 |
| Dipsas pratti | - | - | GQ334482 | GQ334583 | - |
| Dipsas variegata | AF158406 | AF158476 | - | - | - |
| Drepanoides anomalus | GQ457791 | GQ457732 | GQ895866 | - | GQ895810 |
| Echinanthera melanostigma | JQ598806 | GU018174 | JQ598928 | - | - |
| Echinanthera undulata | JQ598807 | JQ598870 | JQ598929 | - | JQ598978 |
| Elapomorphus quinquelineatus | GQ457794 | GQ457735 | JQ598930 | - | GQ457855 |
| Erythrolamprus aesculapii | GQ457795 | GQ457736 | GQ895871 | - | GQ895814 |
| Erythrolamprus almadensis | JQ598808 | JQ598871 | - | - | JQ598979 |
| Erythrolamprus atraventer | JQ598809 | JQ598872 | - | - | JQ598980 |
| Erythrolamprus breviceps | AF158464 | AF158533 | - | - | - |
| Erythrolamprus ceii | JQ598810 | JQ598873 | - | - | JQ598981 |
| Erythrolamprus cursor | JX905310 | JX905314 | - | - | - |
| Erythrolamprus epinephelus | GU018158 | GU018176 | - | - | - |
| Erythrolamprus jaegeri | GQ457809 | GQ457749 | - | - | GQ457869 |
| Erythrolamprus juliae | AF158445 | AF158514 | - | - | - |
| Erythrolamprus miliaris | JQ598811 | AF158480 | JQ598931 | - | JQ598982 |
| Erythrolamprus mimus | GU018157 | GU018175 | - | - | - |
| Erythrolamprus poecilogyrus | JQ598812 | JQ598875 | - | - | - |
| Erythrolamprus pygmaeus | GU018154 | GU018172 | - | - | - |
| Erythrolamprus reginae | JQ598813 | JQ598876 | - | - | JQ598983 |
| Erythrolamprus typhlus | GQ457811 | GQ457751 | - | - | GQ457871 |
| Farancia abacura | Z46467 | Z46491 | U69832 | DQ902307 | AF471141 |
| Farancia erytrogramma | AY577017 | AY577026 | KP765663 | - | - |
| Geophis carinosus | - | - | GQ895872 | - | GQ895815 |
| Geophis dubius | - | - | KC917319 | - | - |
| Geophis godmani | JQ598814 | JQ598877 | JQ598932 | - | - |
| Geophis juarezi | - | - | KC917315 | - | - |
| Geophis latifrontalis | - | - | KC917322 | - | - |
| Geophis occabus | - | - | KC917323 | - | - |
| Geophis turbidus | - | - | KC917321 | - | - |
| Gomesophis brasiliensis | GQ457796 | GQ457737 | - | - | - |
| Haitiophis anomalus | FJ666091 | FJ666092 | - | - | - |
| Helicops angulatus | GQ457797 | GQ457738 | AF471037 | - | AF471160 |
| Helicops carinicaudus | JQ598815 | - | - | - | JQ598984 |
| Helicops gomesi | GQ457798 | GQ457739 | - | - | GQ457858 |
| Helicops hagmanni | JQ598816 | JQ598878 | - | - | JQ598985 |
| Helicops infrataeniatus | GQ457799 | GQ457740 | JQ598933 | - | GQ457859 |
| Heterodon nasicus | GQ457801 | AY577027 | KP765664 | - | GQ457861 |
| Heterodon platirhinos | AY577019 | AY577028 | GU112412 | AF402659 | JQ598986 |
| Heterodon simus | AY577020 | AY577029 | AF217840 | DQ902310 | AF471142 |
| Hydrodynastes bicinctus | GQ457802 | GQ457742 | JQ598935 | - | GQ457862 |
| Hydrodynastes gigas | GQ457803 | GQ457743 | GQ895873 | - | GQ895816 |
| Hydromorphus concolor | - | - | GQ895874 | - | GQ895817 |
| Hydrops triangularis | GQ457804 | GQ457744 | AF471039 | - | AF471158 |
| Hypsiglena affinis | - | - | GU353241 | EU363055 | - |
| Hypsiglena chlorophaea | EU728577 | EU728577 | EU728577 | EU728577 | - |
| Hypsiglena jani | EU728592 | EU728592 | EU728592 | EU728592 | - |
| Hypsiglena ochrorhyncha | EU728578 | EU728578 | EU728578 | EU728578 | - |
| Hypsiglena slevini | EU728584 | EU728584 | EU728584 | EU728584 | - |
| Hypsiglena tanzeri | - | - | EU728588 | EU363044 | - |
| Hypsiglena torquata | EU728591 | EU728591 | EU728591 | EU728591 | AF471159 |
| Hypsirhynchus callilaemus | AF158440 | AF158509 | FJ416737 | FJ416811 | - |
| Hypsirhynchus ferox | AF158447 | AF158515 | GQ895875 | FJ416816 | GQ895818 |
| Hypsirhynchus funereus | AF158451 | AF158520 | FJ416739 | FJ416813 | - |
| Hypsirhynchus parvifrons | AF158441 | AF158510 | FJ416740 | FJ416814 | - |
| Hypsirhynchus polylepis | AF158450 | AF158519 | FJ416738 | FJ416812 | - |
| Hypsirhynchus scalaris | AF158449 | AF158518 | FJ416741 | FJ416815 | - |
| Ialtris dorsalis | AF158456 | AF158525 | FJ416735 | FJ416809 | - |
| Ialtris haetianus | AF158458 | AF158527 | FJ416736 | FJ416810 | - |
| Imantodes cenchoa | EU728586 | EU728586 | EU728586 | EU728586 | GQ457865 |
| Imantodes chocoensis | - | - | KC176250 | - | - |
| Imantodes gemmistratus | - | - | GQ334487 | EF078557 | - |
| Imantodes inornatus | - | - | GQ334489 | EF078559 | - |
| Imantodes lentiferus | AF158463 | AF158532 | KC176252 | EF078561 | - |
| Leptodeira annulata | GQ457806 | GQ457746 | FJ416713 | FJ416787 | AF544690 |
| Leptodeira bakeri | - | - | GQ334518 | GQ334618 | - |
| Leptodeira frenata | - | - | EF078532 | EF078580 | - |
| Leptodeira maculata | - | - | GQ334524 | GQ334623 | - |
| Leptodeira nigrofasciata | - | - | GQ334526 | EF078581 | - |
| Leptodeira polysticta | EU728590 | EU728590 | EU728590 | EU728590 | - |
| Leptodeira punctata | - | - | EF078530 | EF078577 | - |
| Leptodeira rubricata | - | - | GQ334527 | GQ334631 | - |
| Leptodeira septentrionalis | GU018148 | GU018163 | KC176243 | KC176255 | - |
| Leptodeira splendida | - | - | EF078521 | EF078569 | - |
| Leptodeira uribei | - | - | EF078531 | EF078579 | - |
| Lygophis anomalus | JQ598817 | JQ598879 | - | - | - |
| Lygophis elegantissimus | GQ457808 | GQ457748 | - | - | GQ457868 |
| Lygophis flavifrenatus | JQ598818 | JQ598880 | - | - | - |
| Lygophis lineatus | - | - | - | - | DQ469789 |
| Lygophis meridionalis | GQ457810 | GQ457750 | - | - | GQ457870 |
| Lygophis paucidens | JQ598819 | - | - | - | JQ598987 |
| Magliophis exiguum | FJ416694 | AF158526 | AF471071 | FJ416798 | AF471117 |
| Magliophis stahli | - | - | FJ416725 | FJ416799 | - |
| Manolepis putnami | JQ598820 | JQ598881 | JQ598936 | - | JQ598988 |
| Mussurana bicolor | GQ457787 | GQ457729 | - | - | GQ457849 |
| Ninia atrata | GQ457814 | JQ598882 | JQ598937 | GQ334659 | GQ457874 |
| Nothopsis rugosus ASL493 | GU018159 | GU018177 | - | - | - |
| Nothopsis rugosus MZUTI3682 | - | KR814760 | KR814770 | KR814779 | KR814768 |
| Oxyrhopus clathratus | GQ457815 | GQ457754 | - | - | GQ457875 |
| Oxyrhopus formosus | JQ598821 | AF158482 | - | - | - |
| Oxyrhopus guibei | JQ598822 | JQ627291 | JQ598938 | - | JQ598989 |
| Oxyrhopus melanogenys | JQ598823 | AF158489 | - | - | JQ598990 |
| Oxyrhopus petolarius | GU018144 | GU018170 | GQ334554 | GQ334660 | - |
| Oxyrhopus rhombifer | GQ457816 | GQ457755 | - | - | GQ457876 |
| Oxyrhopus trigeminus | JQ598824 | JQ598884 | JQ598939 | - | - |
| Paraphimophis rusticus | JQ598802 | JQ598864 | JQ598923 | - | JQ598974 |
| Phalotris bilineatus | JQ598827 | JQ598887 | JQ598943 | - | - |
| Phalotris lativittatus | JQ598825 | JQ598885 | - | - | JQ598991 |
| Phalotris lemniscatus | GQ457817 | GQ457756 | JQ598941 | - | GQ457877 |
| Phalotris mertensi | JQ598826 | - | - | - | - |
| Phalotris nasutus | GQ457818 | GQ457757 | GQ895880 | - | GQ895822 |
| Philodryas aestiva | GQ457819 | GQ457758 | - | - | GQ457879 |
| Philodryas agassizii | GQ457823 | GQ457762 | GQ895883 | - | GQ457883 |
| Philodryas argentea | GQ457842 | GQ457780 | JQ598944 | - | GQ457899 |
| Philodryas baroni | JQ598828 | JQ598888 | - | - | - |
| Philodryas georgeboulengeri | - | - | GQ895898 | - | GQ895838 |
| Philodryas mattogrossensis | GQ457820 | GQ457759 | - | - | GQ457880 |
| Philodryas nattereri | JQ598829 | JQ598889 | AF236806 | - | JQ598992 |
| Philodryas olfersii | JQ598830 | AF158484 | JQ598945 | - | JQ598993 |
| Philodryas patagoniensis | GQ457821 | JQ627296 | AF236808 | - | GQ457881 |
| Philodryas psammophidea | GU018149 | GU018168 | - | - | - |
| Philodryas viridissima | AF158419 | AF158474 | AF236807 | - | - |
| Phimophis guerini | GQ457822 | GQ457761 | - | - | GQ457882 |
| Pseudalsophis dorsalis | JQ598832 | JQ598892 | JQ598946 | - | JQ598994 |
| Pseudalsophis elegans | AF158401 | AF158470 | JQ598947 | - | JQ598995 |
| Pseudoboa coronata | GQ457824 | GQ457763 | - | - | GQ457884 |
| Pseudoboa neuwiedii | AF158423 | AF158490 | GQ895884 | - | GQ895825 |
| Pseudoboa nigra | AF544775 | GQ457764 | JQ598948 | - | AF544729 |
| Pseudoeryx plicatilis | GQ457826 | GQ457765 | GQ895885 | - | GQ895826 |
| Pseudoleptodeira latifasciata | EU728579 | EU728579 | EU728579 | EU728579 | - |
| Pseudotomodon trigonatus | GQ457827 | GQ457766 | - | - | GQ457887 |
| Psomophis genimaculatus | GQ457828 | GQ457767 | - | - | GQ457888 |
| Psomophis joberti | GQ457829 | GQ457768 | GQ895887 | - | GQ895828 |
| Psomophis obtusus | JQ598836 | JQ598896 | - | - | - |
| Ptychophis flavovirgatus | GQ457830 | GQ457769 | - | - | GQ457890 |
| Rhachidelus brazili | JQ598837 | JQ598897 | JQ598952 | - | - |
| Rhadinaea flavilata | - | - | AF471078 | - | AF471152 |
| Rhadinaea fulvivittis | - | - | EF078539 | EF078587 | - |
| Rodriguesophis iglesiasi | JQ598831 | JQ598891 | GQ895881 | - | GQ895823 |
| Sibon nebulatus | EU728583 | EU728583 | EU728583 | EU728583 | AF544736 |
| Sibon noalamina | - | KP209376 | - | - | - |
| Sibynomorphus mikanii | GQ457832 | JQ627297 | JQ598954 | - | GQ457892 |
| Sibynomorphus neuwiedi | JQ598838 | JQ598898 | - | - | - |
| Sibynomorphus turgidus | JQ598839 | JQ598899 | - | - | - |
| Sibynomorphus ventrimaculatus | JQ598840 | JQ598900 | - | - | JQ598997 |
| Siphlophis cervinus | JQ598841 | JQ598901 | GQ895888 | - | JQ598998 |
| Siphlophis compressus | GQ457833 | GQ457772 | - | - | GQ457893 |
| Siphlophis longicaudatus | JQ598842 | JQ598902 | - | - | JQ598999 |
| Siphlophis pulcher | GQ457834 | GQ457773 | JQ598955 | - | GQ457894 |
| Sordellina punctata | JQ598843 | JQ598903 | JQ598956 | - | JQ599000 |
| Stichophanes ningshaanensis | KJ719252 | KJ719252 | KJ719252 | KJ719252 | KJ638718 |
| Synophis bicolor MZUTI4180 | - | KT944048 | - | KT944065 | KT944069 |
| Synophis bicolor MHUA14577 | KR814751 | KR814758 | KR814773 | - | KR814769 |
| Synophis bicolor MZUTI3529 | - | KR814759 | KR814771 | KR814780 | KR814762 |
| Synophis bicolor MZUTI4175 | - | KT944046 | - | KT944063 | KT944067 |
| Synophis bicolor UTA R-55956 | - | - | JX398697 | JX398557 | - |
| Synophis calamitus KU197107 | KR814622 | KR814640 | KR814697 | KR814711 | KR814663 |
| Synophis calamitus MZUTI3694 | - | KR814755 | KR814772 | KR814774 | KR814765 |
| Synophis lasallei MZUTI4181 | - | KT944047 | - | KT944064 | KT944068 |
| Synophis zaheri MZUTI3353 | - | KR814756 | - | KR814776 | KR814761 |
| Synophis zaheri MZUTI3355 | - | KR814757 | - | KR814781 | KR814763 |
| Tachymenis peruviana | GQ457835 | GQ457774 | - | - | GQ457895 |
| Taeniophallus affinis | JQ598844 | JQ598905 | JQ598957 | - | GQ457853 |
| Taeniophallus brevirostris | GQ457793 | GQ457734 | JQ598958 | - | GQ457854 |
| Taeniophallus nicagus | JQ598845 | JQ598906 | - | - | JQ599001 |
| Tantalophis discolor | - | - | EF078541 | EF078589 | - |
| Thalesius viridis | AF158468 | AF158538 | - | - | - |
| Thamnodynastes hypoconia | JQ598846 | - | - | - | - |
| Thamnodynastes lanei | GQ457836 | GQ457775 | - | - | - |
| Thamnodynastes pallidus | GU018155 | GU018166 | - | - | - |
| Thamnodynastes rutilus | GQ457837 | GQ457776 | - | - | GQ457896 |
| Thamnodynastes strigatus | JQ598847 | JQ598907 | JQ598959 | - | - |
| Thermophis baileyi | - | - | EU864148 | KF595097 | EU496922 |
| Thermophis zhaoermii | GQ166168 | GQ166168 | GQ166168 | GQ166168 | KF514882 |
| Tomodon dorsatum | GQ457838 | GQ457777 | GQ895892 | - | GQ895833 |
| Tretanorhinus nigroluteus | - | - | GQ895893 | - | GQ895834 |
| Trimetopon gracile | GU018160 | GU018178 | - | - | - |
| Tropidodipsas sartorii | - | - | EF078540 | EF078588 | - |
| Tropidodryas serra | JQ598848 | JQ598908 | JQ598961 | - | - |
| Tropidodryas striaticeps | GQ457839 | GQ457778 | AF236811 | - | - |
| Uromacer catesbyi | AF158454 | AF158523 | FJ416714 | FJ416788 | - |
| Uromacer frenatus | AF158444 | AF158513 | FJ416715 | FJ416789 | - |
| Uromacer oxyrhynchus | FJ416701 | FJ416712 | FJ416716 | FJ416790 | - |
| Xenodon dorbignyi | GQ457812 | GQ457752 | - | - | GQ457872 |
| Xenodon guentheri | JQ598849 | JQ598909 | - | - | - |
| Xenodon histricus | GQ457813 | GQ457753 | JQ598962 | - | GQ457873 |
| Xenodon matogrossensis | JQ598850 | JQ598910 | - | - | - |
| Xenodon merremi | GQ457840 | JQ598911 | JQ598963 | - | GQ457898 |
| Xenodon nattereri | JQ598851 | JQ598912 | - | - | - |
| Xenodon neuwiedii | GQ457841 | GQ457779 | AF236814 | - | - |
| Xenodon pulcher | JQ598852 | JQ598913 | - | - | - |
| Xenodon semicinctus | GU018156 | GU018173 | GQ895877 | - | - |
| Xenodon severus | JQ598853 | Z46474 | JQ598964 | - | - |
| Xenopholis scalaris GFM825 | - | JQ598915 | - | - | - |
| Xenopholis scalaris JPV | GU018145 | GU018164 | - | - | - |
| Xenopholis scalaris KU222204 | - | - | GQ895897 | - | GQ895837 |
| Xenopholis scalaris WED57797 | JQ598854 | - | - | - | JQ599002 |
| Xenopholis undulatus R6955 | JQ598855 | JQ598916 | - | - | JQ599003 |
Citation
Pyron RA, Guayasamin JM, Peñafiel N, Bustamante L, Arteaga A (2015) Systematics of Nothopsini (Serpentes, Dipsadidae), with a new species of Synophis from the Pacific Andean slopes of southwestern Ecuador. ZooKeys 541: 109–147. doi: 10.3897/zookeys.541.6058
References
- Amaral A. (1929) Estudos sobre ofidios neotropicos. XVII. Valor sistemático de varias formas de Ophidios Neotrópicos. Memorias do Instituto Butantan 4: 1–68. [Google Scholar]
- Arévalo E, Davis SK, Sites JW. (1994) Mitochondrial DNA sequence divergence and phylogenetic relationships among eight chromosome races of the Sceloporus grammicus complex (Phrynosomatidae) in central Mexico. Systematic Biology 43: 387–418. doi: 10.1093/sysbio/43.3.387 [Google Scholar]
- Bauer AM. (2013) Book Review: The Eponym Dictionary of Amphibians. Herpetological Review 44: 702–704. [Google Scholar]
- Beolens B, Watkins M, Grayson M. (2011) The Eponym Dictionary of Reptiles. Johns Hopkins University Press, Baltimore, MD. [Google Scholar]
- Bogert CM. (1964) Snakes of the genera Diaphorolepis and Synophis and the colubrid subfamily Xenoderminae. Senckenbergiana Biologica 45: 509–531. [Google Scholar]
- Boulenger GA. (1898) An account of the reptiles and batrachians collected by Mr. W. F. H. Rosenberg in Western Ecuador. Proceedings of the Zoological Society of London 1898: 107–126. doi: 10.1111/j.1096-3642.1898.tb03134.x [Google Scholar]
- Boulenger GA. (1905) Description of new snakes in the collection of the British Museum. Annals and Magazine of Natural History 15: 453–456. doi: 10.1080/03745480509442832 [Google Scholar]
- Burbrink FT, Lawson R, Slowinski JB. (2000) Mitochondrial DNA phylogeography of the polytypic North American rat snake (Elaphe obsoleta): a critique of the subspecies concept. Evolution 54: 2107–2118. doi: 10.1111/j.0014-3820.2000.tb01253.x [DOI] [PubMed] [Google Scholar]
- Cadle JE. (1984a) Molecular systematics of Neotropical xenodontine snakes: I. South-American xenodontines. Herpetologica 40: 8–20. [Google Scholar]
- Cadle JE. (1984b) Molecular systematics of Neotropical xenodontine snakes: II. Central American xenodontines. Herpetologica 40: 21–30. [Google Scholar]
- Cadle JE. (1984c) Molecular systematics of Neotropical xenodontine snakes. III. Overview of xenodontine phylogeny and the history of New World snakes. Copeia 1984: 641–652. doi: 10.2307/1445144 [Google Scholar]
- Cope ED. (1871) Ninth contribution to the herpetology of Tropical America. Proceedings of the Academy of Natural Sciences Philadelphia 23: 200–224. [Google Scholar]
- Dowling HG, Duellman WE. (1978) Systematic herpetology: a synopsis of families and higher categories. HISS Publications, New York. [Google Scholar]
- Duellman WE, Mendelson JR. (1995) Amphibians and reptiles from northern Departamento Loreto, Peru: taxonomy and biogeography. University of Kansas Science Bulletin 55: 329–376. [Google Scholar]
- Dunn ER, Dowling HG. (1957) The neotropical snake genus Nothopsis Cope. Copeia 1957: 255–261. doi: 10.2307/1439148 [Google Scholar]
- Felsenstein J. (2004) Inferring phylogenies. Sinauer Associates, Sunderland, Mass. [Google Scholar]
- Ferrarezzi H. (1994) Uma sinopse dos generos e classificao das serpentes (Squamata): II. familia Colubridae. In: Nascimento LB, Bernardes AT, Cotta GA. (Eds) Herpetologia no Brasil. Pontificia Universidade Catolica de Minas Gerais Belo Horizonte, Minas Gerais, 81–91. [Google Scholar]
- Fritts TH, Smith HN. (1969) A new genus and species of snake from western Ecuador. Transactions of the Kansas Academy of Science 72: 60–66. doi: 10.2307/3627049 [Google Scholar]
- Grazziotin FG, Zaher H, Murphy RW, Scrocchi G, Benavides MA, Zhang YP, Bonatto SL. (2012) Molecular phylogeny of the New World Dipsadidae (Serpentes: Colubroidea): a reappraisal. Cladistics 28: 437–459. doi: 10.1111/j.1096-0031.2012.00393.x [DOI] [PubMed] [Google Scholar]
- Hillis DM. (1990) A new species of xenodontine colubrid snake of the genus Synophis from Ecuador and the phylogeny of the genera Synophis and Emmochliophis. Occasional Papers of the Museum of Natural History University of Kansas 135: 1–9. [Google Scholar]
- International Commission on Zoological Nomenclature: Ride WDL, International Trust for Zoological Nomenclature, Natural History Museum (London England) & International Union of Biological Sciences, General Assembly. (1999) International code of zoological nomenclature = Code international de nomenclature zoologique. International Trust for Zoological Nomenclature, London, xxix, 306 pp. [Google Scholar]
- Jan G. (1863) Elenco Sistemático Degli Ofidi e Disegnati per l’Iconografia Generale. A. Lombardi, Milano. doi: 10.5962/bhl.title.106683 [Google Scholar]
- Jenner JV. (1981) A zoogeographic study and the taxonomy of the xenodontine snakes. PhD Thesis, New York University, New York. [Google Scholar]
- Katoh K, Standley DM. (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing B, Croom H, Martin A, McIntosh C, McMillan WO, Palumbi S. (1989) The simple fool’s guide to PCR. Department of Zoology, University of Hawaii, Honolulu. [Google Scholar]
- Kohler G. (2008) Reptiles of Central America. Herpeton Verlag Elke Kohler, Offenbach, 2nd edition, 400 pp. [Google Scholar]
- Lanfear R, Calcott B, Ho SYW, Guindon S. (2012) PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution 29: 1695–1701. doi: 10.1093/molbev/mss020 [DOI] [PubMed] [Google Scholar]
- Lawson R, Slowinski JB, Crother BI, Burbrink FT. (2005) Phylogeny of the Colubroidea (Serpentes): new evidence from mitochondrial and nuclear genes. Molecular Phylogenetics and Evolution 37: 581–601. doi: 10.1016/j.ympev.2005.07.016 [DOI] [PubMed] [Google Scholar]
- Lynch JD, Duellman WE. (1997) Frogs of the genus Eleutherodactylus in western Ecuador. Systematics, ecology, and biogeography. Special Publication. Natural History Museum, University of Kansas 23: 1–236. [Google Scholar]
- Martinez PMS. (2011) Comparative anatomy of the tribe Nothopsini (Serpentes, Dipsadidae). MSc Thesis Departamento de Zoologia. Universidade de Sao Paulo, Sao Paulo. [Google Scholar]
- McCranie JR. (2011) The snakes of Honduras. SSAR, Salt Lake City, 725 pp. [Google Scholar]
- Nicéforo-Maria H. (1950) Contribucion al conocimiento de los ofidios de Colombia. Revista Academia Colombiana de Ciencias Exactas Fisicas y Naturales Bogotá 7: 518–527. [Google Scholar]
- Nicéforo-María H. (1970) Contribucion al conocimiento de los ofidios de Colombia. Boletín del Instituto de La Salle Bogotá 210: 309–314. [Google Scholar]
- Peracca MG. (1896) Sopra un nuovo genere ed una nuova specie di colubride aglifo dell’America meridionale. Bolletino dei Musei di Zoologia ed Anatomia Comparata della Università di Torino 11: 1–7. [Google Scholar]
- Pyron RA, Burbrink FT, Colli GR, de Oca ANM, Vitt LJ, Kuczynski CA, Wiens JJ. (2011) The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Molecular Phylogenetics and Evolution 58: 329–342. doi: 10.1016/j.ympev.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Pyron RA, Burbrink FT, Wiens JJ. (2013) A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evolutionary Biology 13: . doi: 10.1186/1471-2148-13-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Hohna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) Mrbayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. doi: 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabaj-Perez MH. (2013) Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Version 4.0 (28 June 2013). Electronically accessible at http://www.asih.org/ American Society of Ichthyologists and Herpetologists, Washington, DC. [Google Scholar]
- Salazar-Valenzuela D, Torres-Carvajal O, Passos P. (2014) A new species of Atractus (Serpentes: Dipsadidae) from the Andes of Ecuador. Herpetologica 70: 350–363. doi: 10.1655/HERPETOLOGICA-D-13-00045 [Google Scholar]
- Savage JM. (2002) The Amphibians and Reptiles of Costa Rica: A Herpetofauna Between Two Continents, Between Two Seas. University of Chicago Press, 934 pp. [Google Scholar]
- Savitzky AH. (1974) The relationships of the xenodontine colubrid snakes related to Ninia. MSc Thesis, University of Kansas, Lawrence, KS. [Google Scholar]
- Sheehy CM III. (2012) Phylogenetic relationships and feeding behavior of Neotropical snail-eating snakes (Dipsadinae, Dipsadini). PhD Thesis, University of Texas, Arlington, TX. [Google Scholar]
- Sheehy CM, Yánez-Muñoz MH, Valencia JH, Smith EN. (2014) A new species of Siphlophis (Serpentes: Dipsadidae: Xenodontinae) from the Eastern Andean Slopes of Ecuador. South American Journal of Herpetology 9: 30–45. doi: 10.2994/SAJH-D-12-00031.1 [Google Scholar]
- Sheil CA. (1998) Emmochliophis miops: redescription of Synophis miops (Boulenger, 1898). Journal of Herpetology 32: 604–607. doi: 10.2307/1565222 [Google Scholar]
- Sheil CA, Grant T. (2001) A new species of colubrid snake (Synophis) from western Colombia. Journal of Herpetology 35: 204–209. doi: 10.2307/1566109 [Google Scholar]
- Smith HM. (1953) Revision of type localities. Systematic Zoology 2: 37–41. doi: 10.2307/2411568 [Google Scholar]
- Taylor EH. (1951) A brief review of the snakes of Costa Rica. University of Kansas Science Bulletin 34: 3–188. [Google Scholar]
- Torres-Carvajal O, Salazar-Valenzuela D, Merino-Viteri A. (2014) ReptiliaWebEcuador. Versión 2014.0. Museo de Zoología QCAZ, Pontificia Universidad Católica del Ecuador; http://zoologia.puce.edu.ec/Vertebrados/reptiles/reptilesEcuador [access May 2014] [Google Scholar]
- Vidal N, Dewynter M, Gower DJ. (2010) Dissecting the major American snake radiation: a molecular phylogeny of the Dipsadidae Bonaparte (Serpentes, Caenophidia). Comptus Rendus Biologies 333: 48–55. doi: 10.1016/j.crvi.2009.11.003 [DOI] [PubMed] [Google Scholar]
- Wallach V. (1995) Revalidation of the Genus Tropidodipsas Günther, with notes on the Dipsadini and Nothopsini (Serpentes, Colubridae). Journal of Herpetology 29: 476–481. doi: 10.2307/1565006 [Google Scholar]
- Wallach V, Williams KL, Boundy J. (2014) Snakes of the world: a catalogue of living and extinct species. CRC Press, Boca Raton. doi: 10.1201/b16901 [Google Scholar]
- Werner F. (1897) Ueber einige neue und seltene Reptilien und Frösche der zoologischen Sammlung des bayrischen Staates in München. Sitzungsberichte der Bayerischen Akademie der Wissenschaften zu München, Mathematisch-Physikalische Klasse 27: 203–220. [Google Scholar]
- Werner F. (1901) Uber Reptilien un Batrachier aus Ecuador un Neu-Guinea. Verhandlungen der kaiserlich-königlichen zoologisch-botanischen Gesellschaft in Wien 51: 593–615. [Google Scholar]
- Werner F. (1923) Neue Schlangen des naturhistorischen Museums in Wien. Annalen des Naturhistorischen Museums in Wien 36: 160–166. [Google Scholar]
- Werner F. (1929) Übersicht der Gattungen und Arten der Schlangen der Familie Colubridae. III. Teil (Colubrinae). Mit einem Nachtrag zu den übrigen Familien. Zoologische Jahrbücher Abteilung für Systematik Ökologie und Geographie der Tiere Jena 57: 1–196. [Google Scholar]
- Zaher H. (1999) Hemipenial morphology of the South American xenodontine snakes, with a proposal for a monophyletic Xenodontinae and a reappraisal of colubroid hemipenes. Bulletin of the American Museum of Natural History 240: 1–168. [Google Scholar]
- Zaher H, Grazziotin FG, Cadle JE, Murphy RW, de Moura JC, Bonatto SL. (2009) Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American Xenodontines: a revised classification and descriptions of new taxa. Papeis Avulsos de Zoologia 49: 115–153. doi: 10.1590/S0031-10492009001100001 [Google Scholar]
- Zaher H, Arredondo JC, Valencia JH, Arbaláez E, Rodrigues MT, Marco A. (2014) A new Andean species of Philodryas (Dipsadidae, Xenodontinae) from Ecuador. Zootaxa 3785: 469–480. doi: 10.11646/zootaxa.3785.3.8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.