Summary
The effectiveness of conventional malaria vector control is being threatened by the spread of insecticide resistance. One promising alternative to chemicals is the use of naturally occurring insect‐killing fungi. Numerous laboratory studies have shown that isolates of fungal pathogens such as Beauveria bassiana can infect and kill adult mosquitoes, including those resistant to chemical insecticides.
Unlike chemical insecticides, fungi may take up to a week or more to kill mosquitoes following exposure. This slow kill speed can still reduce malaria transmission because the malaria parasite itself takes at least eight days to complete its development within the mosquito. However, both fungal virulence and parasite development rate are strongly temperature‐dependent, so it is possible that biopesticide efficacy could vary across different transmission environments.
We examined the virulence of a candidate fungal isolate against two key malaria vectors at temperatures from 10 to 34 °C. Regardless of temperature, the fungus killed more than 90% of exposed mosquitoes within the predicted duration of the malarial extrinsic incubation period, a result that was robust to realistic diurnal temperature variation.
We then incorporated temperature sensitivities of a suite of mosquito, parasite and fungus life‐history traits that are important determinants of malaria transmission into a stage‐structured malaria transmission model. The model predicted that, at achievable daily fungal infection rates, fungal biopesticides have the potential to deliver substantial reductions in the density of malaria‐infectious mosquitoes across all temperatures representative of malaria transmission environments.
Synthesis and applications. Our study combines empirical data and theoretical modelling to prospectively evaluate the potential of fungal biopesticides to control adult malaria vectors. Our results suggest that Beauveria bassiana could be a potent tool for malaria control and support further development of fungal biopesticides to manage infectious disease vectors.
Keywords: Anopheles gambiae, Anopheles stephensi, Beauveria bassiana, biocontrol, extrinsic incubation period, malaria transmission model, Plasmodium falciparum, Plasmodium vivax, temperature
Short abstract
Our study combines empirical data and theoretical modelling to prospectively evaluate the potential of fungal biopesticides to control adult malaria vectors. Our results suggest that Beauveria bassiana could be a potent tool for malaria control and support further development of fungal biopesticides to manage infectious disease vectors.
Introduction
Despite substantial control efforts, malaria remains one of the most important vector‐borne diseases of humans world‐wide (World Health Organization 2013). Currently, malaria control relies heavily on the use of chemical insecticides to suppress adult mosquito vector populations (World Health Organization 2013). Unfortunately, vector control programmes are being undermined by increasing insecticide resistance in malaria‐endemic regions (Ranson et al. 2011; Hemingway 2014; Strode et al. 2014), prompting investigation of alternative technologies.
Recent years have seen a number of studies exploring the potential use of insect‐killing fungal pathogens to target malaria vector populations (Scholte et al. 2003, 2005; Blanford et al. 2005, 2011). Entomopathogenic fungi have a number of features that make them particularly well‐suited for malaria control. Fungal pathogens are effective against mosquito strains that are resistant to available chemical insecticides (Farenhorst et al. 2009, 2010; Blanford et al. 2011). They also infect mosquitoes on contact and so lend themselves to a variety of deployment strategies already in use for conventional insecticides, including treatments for cloth or netting (Scholte et al. 2005; Farenhorst et al. 2011; Mnyone et al. 2012) and indoor residual sprays (Blanford et al. 2011, 2012). Finally, in contrast to chemical insecticides, which generally knock down or kill susceptible mosquitoes within 24 h of exposure (World Health Organization 2006), fungal biopesticides can take more than a week to kill exposed mosquitoes (Blanford et al. 2005; Scholte et al. 2005). Because these individuals continue laying eggs in the days following fungal exposure (Blanford et al. 2011; Mouatcho et al. 2011; Darbro et al. 2012), fungal biopesticides are expected to reduce selection for new resistance phenotypes (Thomas & Read 2007; Read, Lynch & Thomas 2009; Lynch et al. 2012). Thus, fungal pathogens could create opportunities for controlling resistant mosquitoes and/or developing novel insecticide resistance management strategies.
The extent to which such a slow‐acting product such as a fungal biopesticide can provide effective malaria control depends on two factors. First, it depends on the relationship between fungal virulence, defined here as the time required for the fungus to kill the insect, and the parasite's extrinsic incubation period (EIP), which is the amount of time required for malaria to mature within its mosquito vector. Plasmodium parasites require an EIP of at least 8 days before they can be transmitted to new hosts (Detinova 1962; Paaijmans, Read & Thomas 2009; Cator et al. 2013; Mordecai et al. 2013). This provides a window of opportunity for fungal action; as long as the mosquito dies before the malaria parasite matures, there will be no disease transmission. Secondly, biopesticide coverage is important since this determines the probability, and hence timing, of fungal infection. Infecting a mosquito with fungus at or even before the point at which it acquires malaria parasites provides more time for the fungus to work, thus increasing the likelihood that mortality will occur before the end of the EIP (Hancock, Thomas & Godfray 2009; Koella et al. 2009; Read, Lynch & Thomas 2009; Lynch et al. 2012).
Environmental temperature significantly affects fungal virulence (Blanford & Thomas 2001; Thomas & Blanford 2003; Kikankie et al. 2010), which can substantially impact predicted biocontrol efficacy (e.g. Klass, Blanford & Thomas 2007a,b). For example, one study found that while all Anopheles arabiensis mosquitoes died within 13 days of fungal exposure at 25 °C, some individuals survived almost twice as long at 21 °C (Kikankie et al. 2010). Temperature also affects malarial EIP, which ranges from about eight to >50 days as temperatures deviate from the optimum towards the upper and lower thermal extremes (Detinova 1962; Paaijmans, Read & Thomas 2009; Cator et al. 2013; Mordecai et al. 2013). Given that the malaria transmission‐blocking potential of a fungal pathogen is highly dependent on speed of kill relative to the EIP, these thermal sensitivities could significantly impact the effectiveness of this novel control tool. Fungal efficacy could also be directly or indirectly affected by the magnitude of diurnal temperature variation. For example, while Plasmodium chabaudi sporozoite dissemination readily occurs at constant 26 °C, thermal variation of 12 °C (i.e. 26 ± 6 °C) around the same mean temperature prevents dissemination, effectively blocking malaria transmission (Paaijmans et al. 2010). Since diurnal temperature ranges in malaria‐endemic areas frequently exceed 12 °C, these short‐term variations in temperature may further influence mosquito, parasite and pathogen traits (Paaijmans et al. 2010, 2013; Murdock et al. 2012; Murdock, Moller‐Jacobs & Thomas 2013).
To date, fungal virulence in mosquitoes has been evaluated in only a small subset of temperatures (21 and 25 °C, ~27–28 °C (Scholte et al. 2003; Blanford et al. 2005; Kikankie et al. 2010)), and there has been no systematic evaluation of how fungal virulence varies across the full thermal range relevant for malaria transmission (~18–34 °C) (Paaijmans, Read & Thomas 2009; Blanford et al. 2013). There also has been no assessment of how this variation could interact with temperature‐dependent variation in mosquito and malarial traits to affect fungal impact on malaria transmission potential. To address this knowledge gap, we examined how temperature affected survival rates of adult Anopheles stephensi Liston and Anopheles gambiae Giles mosquitoes exposed to the candidate fungal biopesticide Beauveria bassiana (Bals.) Vuill. at temperatures between 10 and 34 °C. In addition, we evaluated the impact of realistic diurnal temperature variation on fungal virulence. We then used these data in combination with published estimates of temperature dependence in EIP, background mosquito mortality rate, biting rate and vector competence to inform an age‐structured model describing mosquito–malaria interactions (Hancock, Thomas & Godfray 2009). We used the model to explore how environmental temperature and daily probability of fungal infection (i.e. biopesticide coverage) might impact the potential of fungal treatments to reduce malaria transmission. We found that fungal biopesticides dramatically reduce the predicted density of malaria‐infectious mosquitoes irrespective of temperature due to differences in the thermal sensitivities between fungal virulence and the EIP of the malaria parasite. The empirical data together with the theoretical predictions provide strong support for the further development of this biocontrol technology.
Materials and methods
Preparation of exposure substrates
We simulated spray treatments on the clay‐mud walls typical of traditional African village dwellings by using an airbrush to apply fungal suspensions to white earthenware clay tiles (World Health Organization 2006; Blanford et al. 2011, 2012). Beauveria bassiana (isolate I93‐825) conidia were suspended in oil (80% Ondina: 20% Isopar M) at concentrations of either 107 or 109 conidia mL−1 following published recommendations (Blanford et al. 2011). We applied the suspensions to tiles at a rate of 80 mL m−2, resulting in application rates (AR) of approximately 8 × 1010 conidia m−2 at the high AR and 8 × 108 conidia m−2 at the low AR. Control tiles were sprayed with blank oil mixture, and all tiles were air‐dried overnight.
Mosquito exposure to fungus
Exposure assays were performed on 3‐ to 5‐day‐old female mosquitoes raised under standard insectary conditions at 26 °C (see Appendix S1 in Supporting Information) and followed standard WHO protocols for testing efficacy of residual insecticide applications (World Health Organization 2006). Groups of approximately 30 mosquitoes were aspirated into a WHO cone in the centre of each tile. After 30 min, the mosquitoes were aspirated into a nylon‐covered cup. A cotton ball saturated with a 10% glucose–0·05% para‐aminobenzoic acid (PABA) solution provided nutrition. Dead mosquitoes were removed daily, dried overnight and placed in sealed tubes with wet cotton balls in a 26 °C incubator. Sporulation was visually assessed approximately 4 days later to confirm cause of death. Unless stated otherwise, there were four replicate cups per treatment. All exposures took place at 26 °C for consistency.
We performed four sets of experiments. First, we evaluated the effect of different constant temperatures on fungal virulence. Following fungal exposure, An. stephensi mosquitoes were moved to incubators maintaining temperatures 10–34 °C. Mosquitoes were evaluated at eleven temperatures in two separate mosquito cohorts (group 1: 20, 22, 24, 26, 28, 30 and 34 °C treatments; group 2: 10, 14, 18, 26 and 32 °C treatments). The 26 °C treatment acted as an internal control.
Secondly, we tested whether the interaction between temperature and fungal virulence was consistent across mosquito species by performing the same assays with An. gambiae mosquitoes at 20, 26 and 32 °C.
Thirdly, we repeated the assays using blood‐fed An. stephensi. Mosquitoes were fed on anaesthetized rats for 30 min just prior to fungal exposure. Fully engorged females were then exposed to tiles as described above and held at temperatures 10–32 °C. We also exposed non‐blood‐fed mosquitoes from the same cohort as an internal control; this group was held at 26 °C. There were three replicate cups per treatment.
Finally, we investigated the impact of realistic diurnal temperature variation on fungal virulence. Following fungal exposure, An. stephensi mosquitoes were placed in incubators either maintaining constant temperatures (20, 26 or 32 °C) or programmed to mimic realistic temperature patterns with a diurnal temperature range (DTR) of 12 °C (e.g. 20 ± 6 °C) (Parton & Logan 1981).
Analysis
All statistical analyses were performed in R (R Development Core Team 2013). We used survreg to fit parametric models (logistic, Gaussian, exponential and Weibull) to each data set and found that the Weibull best described the data. There was evidence that the data did not meet the proportional hazards assumption (overall P < 0·05; cox.zph test of Schoenfeld residuals), so we allowed the Weibull rate and shape parameters to vary with treatment. We fit full Weibull models to each data set and used a backward stepwise procedure (step) to select the model that minimized the Akaike Information Criterion.
The 50% (median) and 90% (LT90) quantiles were predicted from the best‐fit model using predict. For the An. stephensiexperiment, we compared the LT90 predictions to the inverse of the Plasmodium falciparum parasite development rate curve described by Mordecai et al. (2013) as an illustrative metric for overall fungal virulence. In the fluctuating temperature assay, we also used rate summation (Liu, Zhang & Zhu 1995; Paaijmans, Read & Thomas 2009) to estimate the impact of the experimental DTR on malarial extrinsic incubation period (Appendix S1).
Modelling
We utilized the age‐structured model of mosquito–malaria interactions developed by Hancock, Thomas & Godfray (2009) to explore the impact of fungal biopesticides on malaria transmission potential across different temperatures. We combined our empirical mosquito survival distributions together with the curves fit by Mordecai et al. (2013) to parameterize the model at different temperatures. Because we predicted that temperature variation would affect malaria parasite development rate more than fungal virulence, we based our parameterization on performance at constant temperatures. This assumption likely underestimated fungal efficacy but simplified the use of temperature in the model, particularly for other temperature‐dependent traits for which the effects of temperature variation are unclear and rate summation is not necessarily appropriate. We also assumed that human malarial prevalence remained constant, which is a reasonable short‐term assumption given that the mosquito life span is far shorter than the length of the human infectious period (Smith et al. 2005).
The model describes mosquito malaria infection dynamics as a susceptible–exposed–infectious (SEI) process. Adult mosquitoes are recruited to the susceptible class at a constant rate. They then enter the malaria‐exposed class at a rate equal to the product of three constants: the human bite rate, malaria transmission efficiency and proportion of humans with transmissible malaria. Following a fixed delay equal to the length of the malarial EIP, the mosquitoes enter the infectious class. Each class is further divided into mosquitoes that have been infected with fungus and those that have not. Background mortality occurs at a constant rate in all classes, and mosquitoes in the fungus‐infected classes experience additional mortality due to fungal infection (Hancock, Thomas & Godfray 2009). See Table S1 and Appendix S1 for additional information.
Mosquitoes in the model become infected with fungus at a rate determined by the daily probability of fungal infection. In contrast to our laboratory assays, under field conditions, some proportion of mosquitoes would not be expected to encounter the fungus until after they had already been infected with malaria. The timing of fungal exposure and thus the likelihood that malaria infection would precede fungal infection would depend in part on how widely the biopesticide had been applied. By varying the daily probability of fungal infection in the model, we were able to explore how suboptimal coverage (i.e. <100% daily probability of fungal infection) might impact fungal efficacy.
The additional mortality due to fungal infection in the model is specified by the Weibull function:
| (eqn 1) |
where μ F and β are constants and u is the time since fungal infection (Hancock, Thomas & Godfray 2009). Because the model accounts for background mortality in a separate parameter, we used Abbott's correction to subtract control mortality from each survival curve (Abbott 1925). We fit Weibull functions to the corrected curves to parameterize μ F and β for each temperature and fungal application rate (AR) combination (Table S2, Fig. S1). We parameterized the curves based on the non‐blood‐fed An. stephensi assays because they had the most comprehensive temperature coverage. The P. falciparum EIP, bite rate, transmission efficiency and background mortality rate were parameterized based on the curves fit by Mordecai et al. (2013). Fungal biopesticide efficacy was assessed based on the predicted proportional reduction in equilibrium total density of infectious mosquitoes. We also evaluated fungal efficacy against Plasmodium vivax malaria based on the EIP curve described by Cator et al. (2013). Finally, we performed a local sensitivity analysis to evaluate how uncertainty in the parameter estimates affected the model predictions (Hamby 1994).
Results
Mosquito survival following fungal exposure
In all of the experiments, temperature, application rate (AR) and their interaction term were significant predictors of mosquito survival (P < 0·05, Table S3). Median survival times (MSTs) are summarized in Table S4.
Anopheles stephensi in the first experiment had MSTs of 4–14 days at the high AR, with the most rapid mortality occurring at temperatures between 22–28 °C (Fig. 1). Mosquitoes exposed to the low AR generally survived 1–2 days longer, but the relationship between temperature and survival time was qualitatively similar to that of the high AR treatments. Overall mortality was quite low in the control treatments at temperatures <30 °C (<30%) but increased at higher temperatures. Notably, the time required for the fungus to kill 90% of exposed mosquitoes (LT90) was shorter than the P. falciparum extrinsic incubation period (EIP) at both ARs regardless of temperature (Fig. 2).
Figure 1.
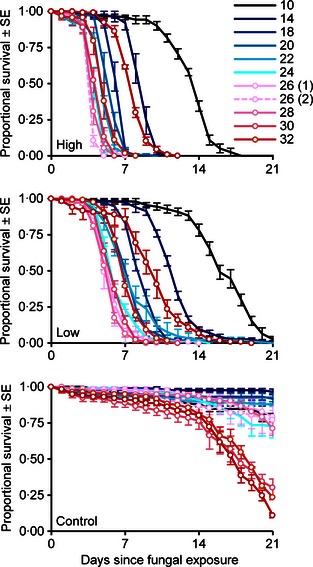
Cumulative proportional survival of Anopheles stephensi maintained at various temperatures (indicated by line colour) following exposure to clay substrates treated with fungus at high or low application rates or with blank oil (‘control’). Lines represent mean daily survival (±SE) from four replicates of approximately 30 mosquitoes in the high and low application rate treatments and 2–4 replicates in the controls (N = 2 for 10 and 26 °C group 2; N = 3 for 14, 18 and 28 °C; N = 4 for all others). The 26 °C treatments were repeated twice.
Figure 2.
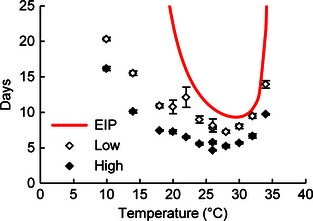
Fungal virulence relative to malarial extrinsic incubation period (EIP). Data points represent the predicted time to 90% mortality (±SE) in mosquitoes exposed to fungus at high or low application rates. The 26 °C treatments were repeated twice with the duplicate points indicating the means for each run. EIP is based on the Plasmodium falciparum curve fit by Mordecai et al. (2013).
The fungal treatment groups in both the An. gambiae and the blood‐fed An. stephensi experiments had similar survival patterns as those described above (Fig. S2). Although diet was a statistically significant predictor of survival at 26 °C (z = −4·80, P < 0·001), predicted MSTs for the two treatments differed by <1 day.
Mosquito survival at fluctuating temperatures was significantly influenced by two‐way interactions among diurnal temperature range (DTR), AR and temperature (P < 0·05, Table S3). However, the absolute impact of thermal variation on MST was relatively small in the fungal treatments, with a DTR of 12 °C increasing MSTs by ≤1 day at each temperature (Fig. 3). In contrast, thermal variation dramatically increased mortality in the control treatment groups at 32 °C (21% vs. 92% survival on day 15). In addition, rate summation predicted that a DTR of 12 °C would slow parasite development at temperatures above 22 °C leading to increases in EIP of up to 5 days. These predicted effects of temperature variation on malaria parasite growth exceeded the effects we observed on fungal virulence such that the addition of temperature variation increased the relative differences between EIP and LT90 (Fig. 4).
Figure 3.
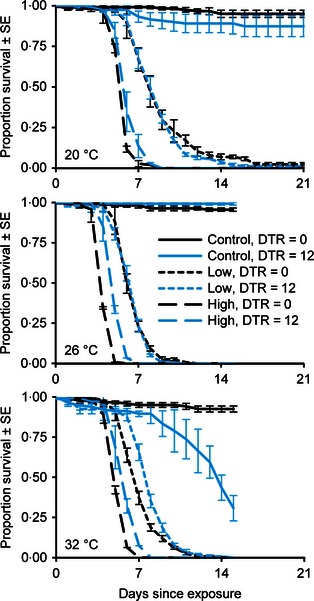
Cumulative proportional survival of adult Anopheles stephensi mosquitoes exposed to different fungal application rates, mean temperatures and diurnal temperature ranges (DTR). Following exposure, mosquitoes were maintained at mean temperatures of 20, 26 or 32 °C (labelled) with either constant (DTR = 0) or fluctuating (DTR = 12) thermal regimes. Data were collected until all fungal treatment mosquitoes were dead (23 days in the 20 °C treatments; graph truncated for consistency). There were N = 4 cups of approximately 30 mosquitoes per cup for all treatments except one (low application rate, 20 °C, DTR = 0), where N = 3.
Figure 4.
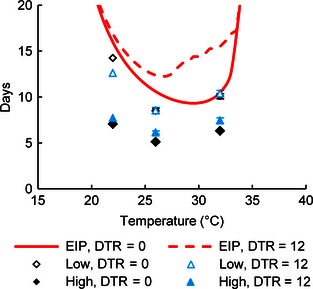
Influence of fluctuating temperatures on fungal virulence and malarial extrinsic incubation period (EIP). Points represent the predicted time to 90% mortality (±SE) for mosquitoes exposed to high or low fungal application rates and subsequently held under constant (DTR = 0) or fluctuating (DTR = 12) temperature conditions. The constant temperature EIP is based on the Plasmodium falciparum curve fit by Mordecai et al. (2013), and the fluctuating temperature EIP (DTR = 12) was predicted using rate summation (see Materials and Methods).
Modelling control potential across different environments
The relative reduction in density of infectious mosquitoes was sensitive to the daily probability of fungal infection, c (Fig. 5). When c = 0·16, reduction in P. falciparum‐infectious mosquito density was >88% at the high AR irrespective of temperature. At lower values of c, control was reduced and the influence of temperature became apparent. Control was greatest under cool conditions (18 °C), declining to a minimum at 32 °C and then rising again as temperature increased. When the model was parameterized for the low fungal AR, the impact of fungus was marginally reduced but the qualitative relationships between temperature and infection probability remained similar (data not shown). Likewise, predicted fungal efficacy against P. vivax malaria was qualitatively similar to the results for P. falciparum; at c = 0·16 and the high AR, the reduction in infectious mosquito density was ≥85% (Fig. S3).
Figure 5.
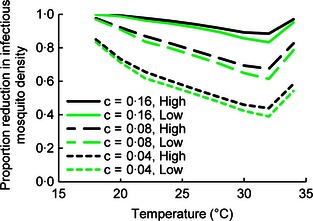
Predicted impact of fungus on Plasmodium falciparum malaria transmission potential at different mean temperatures. Lines represent the predicted proportional reduction in the density of malaria‐infectious mosquitoes relative to no intervention for various daily probabilities of fungal infection (c) at high and low fungal application rates.
Our sensitivity analysis indicated that the proportional reduction in infectious mosquito density was most sensitive to changes in EIP (Fig. S4). However, even at unrealistically low EIP estimates (i.e. 40% reduction in P. falciparum EIP across all temperatures, which, at 30 °C, resulted in an EIP estimate of 5·6 days), the qualitative relationship between temperature and fungal efficacy remained the same, and fungal application was still predicted to reduce infectious mosquito density by >75% at c = 0·16.
Discussion
This study demonstrates that, across the range of mean temperatures associated with malaria transmission, the fungal pathogen B. bassiana has the ability to kill two of the major malaria vectors from Asia and Africa (An. stephensi and An. gambiae, respectively) well within the estimated extrinsic incubation period (EIP) for both P. falciparum and P. vivax malaria. In addition, these results are robust to additional diurnal temperature variation. This apparent robustness is not because the fungus is unaffected by temperature, but rather because fungal virulence is less sensitive to changes in temperature than malaria parasite development rate. Modelling suggests that the fungus could dramatically reduce transmission potential irrespective of environment as long as the daily probability of fungal infection is sufficiently high (≥0·16). As the probability of fungal infection falls, the impact of temperature on control potential becomes more marked, with the exact patterns dependent upon the combined thermal sensitivities of the mosquito, parasite and pathogen. These insights support previous work highlighting the importance of thermal ecology for understanding insect‐pathogen/parasite interactions and predicting the success of microbial biocontrol agents (Thomas & Blanford 2003; Klass, Blanford & Thomas 2007a,b; Lambrechts et al. 2011; Murdock et al. 2012).
We made a number of simplifying assumptions in our analysis, many of which would likely lead us to underestimate fungal efficacy. For example, we assumed that human malaria prevalence remained constant, though long‐term reductions in infectious mosquito density would be expected to decrease the human infection rate, further reducing the probability that mosquitoes would become infected with malaria. In addition, entomopathogenic fungi cause a number of prelethal effects in infected hosts, which could further reduce malaria transmission. Fungal infection inhibits mosquito feeding by decreasing both sensitivity to host odours (George et al. 2011) and feeding propensity (Blanford et al. 2005, 2011; Scholte, Knols & Takken 2006; George et al. 2011), and mosquitoes that do not feed cannot transmit malaria. Fungi have also been shown to increase insect metabolic rates while simultaneously decreasing flight ability (Blanford et al. 2011) and predator evasion behaviour (Arthurs & Thomas 2001), factors which could significantly increase mosquito mortality under field conditions. One study also suggested that, near the end of the EIP, daily mortality rates might be higher in mosquitoes infected with both fungus and malaria than in mosquitoes infected with fungus alone (Blanford et al. 2005; but see Heinig & Thomas 2015). All of these factors could further reduce disease transmission potential. While any increases in mosquito mortality could also increase selection for resistance, modelling studies by Lynch et al. (2012) suggest that fungal pathogens provide evolutionary benefits over fast‐acting chemicals under nearly all scenarios.
Our simplifying treatment of diurnal temperature variation was also conservative. We demonstrated that addition of realistic thermal variation only slightly reduced fungal virulence. While insufficient empirical data exist to fully characterize the effects of temperature variation on malaria parasite growth, previous studies have shown that even a moderate level of diurnal temperature variation can affect both malaria (LaPointe, Goff & Atkinson 2010; Paaijmans et al. 2010) and mosquito traits (Paaijmans et al. 2010, 2013). Rate summation indicated that temperature variation should slow peak parasite growth more than fungal virulence because the temperature dependence of EIP is more strongly nonlinear (i.e. it has a steeper, narrower thermal performance curve) (Ruel & Ayres 1999). Given that our sensitivity analysis demonstrated that the model was most sensitive to variation in EIP, model outputs based on mean temperatures likely underestimate the impact of fungus on malaria transmission potential. This is especially so as rate summation itself tends to underestimate the impact of thermal variation on rate processes when temperature fluctuations exceed critical maximum temperatures (Worner 1992; Klass, Blanford & Thomas 2007b; Paaijmans et al. 2013).
A number of other factors could also influence our predictions. For example, the impact of blood feeding on fungal virulence remains unclear. Mnyone et al. (2011) found that mosquitoes fed on human blood prior to B. bassiana infection survived 2–3 days longer than non‐blood‐fed individuals. However, their blood‐fed controls exhibited similar increases in median survival relative to non‐blood‐fed treatments, so the absolute effect of blood feeding on fungal virulence may have been fairly small. In our study, mosquitoes in the blood feeding experiment survived as long as or longer than those in the non‐blood‐fed experiments, a result consistent with Blanford et al. (2011).
An additional uncertainty is whether the relationships we used to describe the various temperature dependencies are appropriate for all locations. For instance, our results suggested that An. gambiae might be more sensitive (i.e. die more quickly) at high temperatures than An. stephensi. Fungal efficacy might, therefore, vary slightly depending on primary vector species, though research suggests that the B. bassiana isolate used here is also highly virulent against An. arabiensis and An. funestus (Blanford et al. 2011). A recent paper exploring potential effects of local adaptation on vector‐borne disease transmission also noted that complex ‘genotype × genotype × environment’ interactions were possible for systems in which both a parasite and vector evolved in response to local conditions (Sternberg & Thomas 2014). However, few data are available on malaria and mosquito local adaptation, making it difficult to explore the possible significance for transmission or control.
One important question that remains to be addressed is how the daily probability of infection in our model relates to potential delivery of a fungal product in the field. Infection probability depends on several key components, the first of which is the probability of contact per house. Semifield studies have demonstrated probabilities of fungal infection per night (i.e. per house visit) ranging from 23% to 75% depending on delivery strategy (Scholte et al. 2005; Mnyone et al. 2012). The fungal dose acquired by mosquitoes in these semifield studies does not seem to be as high as generally reported from the laboratory, but research currently underway suggests that this problem can be resolved (MBT and collaborators, unpublished data; see also Sternberg, Waite & Thomas 2014). The second component is the proportion of houses receiving the intervention (population coverage). In certain areas, use of bed nets or indoor residual spraying can exceed 80%, although adoption rates can be much lower (World Health Organization 2013). If we assume that 80% of houses are treated with fungus and that mosquitoes are entering houses approximately one in every three days (i.e. once per gonotrophic cycle), we would need approximately a 60% infection probability per house to achieve an effective coverage level of 0·16. Our work suggests this coverage level could significantly reduce the density of malaria‐infectious mosquitoes, even under the least favourable conditions for the fungus. Encouragingly, much lower levels of effective coverage could still have substantial impacts on infectious mosquito density. These arguments are similar to other modelling studies that suggest effective coverage of insecticide‐based interventions is generally much lower than expected from estimates of population coverage and that levels of effective coverage well below 50% can deliver good control (Koella, Saddler & Karacs 2012). Moreover, even partially effective tools can provide a valuable contribution within integrated vector management strategies (Hancock 2009; Thomas et al. 2012).
A number of studies have combined empirical and theoretical approaches to evaluate and improve biocontrol (e.g. Godfray & Waage 1991; Thomas, Wood & Lomer 1995; Fenton et al. 2002; Klass, Blanford & Thomas 2007a; Hancock, Sinkins & Godfray 2011; Lynch et al. 2012; Reilly & Elderd 2014). This study emphasizes environmental temperature as a possible determinant of success. We find that, rather than acting as a simple scaling phenomenon, the influence of temperature depends on net effects across parasite, fungus and mosquito traits that differ in their thermal sensitivities. The importance of temperature is likely to extend beyond fungi to other candidate vector control agents, including Wolbachia (Bian et al. 2013; McGraw & O'Neill 2013; Murdock et al. 2014), microsporidia (Koella, Lorenz & Bargielowski 2009) and even chemicals (Glunt et al. 2014), and to the majority of bacterial, viral and filarial pathogens vectored by mosquitoes. The empirical and theoretical approaches we present here provide a framework for evaluating temperature effects in these other systems.
Data accessibility
Mortality data: Dryad entry doi: 10.5061/dryad.ch206 (Heinig et al. 2015).
Supporting information
Fig. S1. Weibull fits.
Fig. S2. Anopheles gambiae and blood‐fed cumulative proportional survival.
Fig. S3. Plasmodium vivax analyses.
Fig. S4. Sensitivity analyses.
Table S1. Model parameters.
Table S2. Model Weibull estimates.
Table S3. Survival analyses.
Table S4. Median survival times.
Appendix S1. Additional methods.
Acknowledgements
The authors thank Nina Jenkins for fungal culture management; Janet Teeple for insectary support; members of the Read, Thomas and Bjørnstad laboratories for helpful discussion and three reviewers for constructive comments. The research leading to these results received funding from the European Union Seventh Framework Programme FP7/2007‐2013 under grant agreement #306105. This research was also funded in part by NIH‐NIAID (R01AI110793). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences, the National Institute of Allergy and Infectious Diseases, and the National Institutes of Health. The authors declare no conflict of interest.
References
- Abbott, W.S. (1925) A method for computing the effectiveness of an insecticide. Journal of Economic Entomology, 3, 302–303. [PubMed] [Google Scholar]
- Arthurs, S. & Thomas, M.B. (2001) Behavioural changes in Schistocerca gregaria following infection with a fungal pathogen: implications for susceptibility to predation. Ecological Entomology, 26, 227–234. [Google Scholar]
- Bian, G. , Joshi, D. , Dong, Y. , Lu, P. , Zhou, G. , Pan, X. , Xu, Y. , Dimopoulos, G. & Xi, Z. (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science, 340, 748–751. [DOI] [PubMed] [Google Scholar]
- Blanford, S. & Thomas, M.B. (2001) Adult survival, maturation, and reproduction of the desert locust Schistocerca gregaria infected with the fungus Metarhizium anisopliae var acridum . Journal of Invertebrate Pathology, 78, 1–8. [DOI] [PubMed] [Google Scholar]
- Blanford, S. , Chan, B.H.K. , Jenkins, N. , Sim, D. , Turner, R.J. , Read, A.F. & Thomas, M.B. (2005) Fungal pathogen reduces potential for malaria transmission. Science, 308, 1638–1641. [DOI] [PubMed] [Google Scholar]
- Blanford, S. , Shi, W. , Christian, R. , Marden, J.H. , Koekemoer, L.L. , Brooke, B.D. , Coetzee, M. , Read, A.F. & Thomas, M.B. (2011) Lethal and pre‐lethal effects of a fungal biopesticide contribute to substantial and rapid control of malaria vectors. PLoS ONE, 6, e23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford, S. , Jenkins, N.E. , Christian, R. , Chan, B.H. , Nardini, L. , Osae, M. et al (2012) Storage and persistence of a candidate fungal biopesticide for use against adult malaria vectors. Malaria Journal, 11, 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanford, J.I. , Blanford, S. , Crane, R.G. , Mann, M.E. , Paaijmans, K.P. , Schreiber, K.V. & Thomas, M.B. (2013) Implications of temperature variation for malaria parasite development across Africa. Scientific Reports, 3, 1300–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cator, L.J. , Thomas, S. , Paaijmans, K.P. , Ravishankaran, S. , Justin, J.A. , Mathai, M.T. , Read, A.F. , Thomas, M.B. & Eapen, A. (2013) Characterizing microclimate in urban malaria transmission settings: a case study from Chennai, India. Malaria Journal, 12, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbro, J.M. , Johnson, P.H. , Thomas, M.B. , Ritchie, S.A. , Kay, B.H. & Ryan, P.A. (2012) Effects of Beauveria bassiana on survival, blood‐feeding success, and fecundity of Aedes aegypti in laboratory and semi‐field conditions. The American Journal of Tropical Medicine and Hygiene, 86, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detinova, T.S. (1962) Age‐Grouping Methods in Diptera of Medical Importance. World Health Organization, Geneva, CH. [PubMed] [Google Scholar]
- Farenhorst, M. , Mouatcho, J.C. , Kikankie, C.K. , Brooke, B.D. , Hunt, R.H. , Thomas, M.B. , Koekemoer, L.L. , Knols, B.G.J. & Coetzee, M. (2009) Fungal infection counters insecticide resistance in African malaria mosquitoes. Proceedings of the National Academy of Sciences of the United States of America, 106, 17443–17447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farenhorst, M. , Knols, B.G.J. , Thomas, M.B. , Howard, A.F.V. , Takken, W. , Rowland, M. & N'Guessan, R. (2010) Synergy in efficacy of fungal entomopathogens and permethrin against West African insecticide‐resistant Anopheles gambiae mosquitoes. PLoS ONE, 5, e12081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farenhorst, M. , Hilhorst, A. , Thomas, M.B. & Knols, B.G.J. (2011) Development of fungal applications on netting substrates for malaria vector control. Journal of Medical Entomology, 48, 305–313. [DOI] [PubMed] [Google Scholar]
- Fenton, A. , Gwynn, R.L. , Gupta, A. , Norman, R. , Fairbairn, J.P. & Hudson, P.J. (2002) Optimal application strategies for entomopathogenic nematodes: integrating theoretical and empirical approaches. Journal of Applied Ecology, 39, 481–492. [Google Scholar]
- George, J. , Blanford, S. , Domingue, M.J. , Thomas, M.B. , Read, A.F. & Baker, T.C. (2011) Reduction in host‐finding behaviour in fungus‐infected mosquitoes is correlated with reduction in olfactory receptor neuron responsiveness. Malaria Journal, 10, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glunt, K.D. , Paaijmans, K.P. , Read, A.F. & Thomas, M.B. (2014) Environmental temperatures significantly change the impact of insecticides measured using WHOPES protocols. Malaria Journal, 13, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfray, H.C.J. & Waage, J.K. (1991) Predictive modelling in biological control: the mango mealy bug (Rastrococcus invadens) and its parasitoids. Journal of Applied Ecology, 28, 434–453. [Google Scholar]
- Hamby, D.M. (1994) A review of techniques for parameter sensitivity analysis of environmental models. Environmental Monitoring and Assessment, 32, 135–154. [DOI] [PubMed] [Google Scholar]
- Hancock, P.A. (2009) Combining fungal biopesticides and insecticide‐treated bednets to enhance malaria control. PLoS Computational Biology, 5, e1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, P.A. , Sinkins, S.P. & Godfray, H.C.J. (2011) Strategies for introducing Wolbachia to reduce transmission of mosquito‐borne diseases. PLoS Neglected Tropical Diseases, 5, e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock, P.A. , Thomas, M.B. & Godfray, H.C.J. (2009) An age‐structured model to evaluate the potential of novel malaria‐control interventions: a case study of fungal biopesticide sprays. Proceedings of the Royal Society of London Series B‐Biological Sciences, 276, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinig, R.L. & Thomas, M.B. (2015) Interactions between a fungal entomopathogen and malaria parasites within a mosquito vector. Malaria Journal, 14, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinig, R.L. , Paaijmans, K.P. , Hancock, P.A. & Thomas, M.B. (2015) Data from: The potential for fungal biopesticides to reduce malaria transmission under diverse environmental conditions. Dryad Digital Repository, 10.5061/dryad.ch206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway, J. (2014) The role of vector control in stopping the transmission of malaria: threats and opportunities. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 369, 20130431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikankie, C.K. , Brooke, B.D. , Knols, B.G.J. , Koekemoer, L.L. , Farenhorst, M. , Hunt, R.H. , Thomas, M.B. & Coetzee, M. (2010) The infectivity of the entomopathogenic fungus Beauveria bassiana to insecticide‐resistant and susceptible Anopheles arabiensis mosquitoes at two different temperatures. Malaria Journal, 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass, J.I. , Blanford, S. & Thomas, M.B. (2007a) Use of a geographic information system to explore spatial variation in pathogen virulence and the implications for biological control of locusts and grasshoppers. Agricultural and Forest Entomology, 9, 201–208. [Google Scholar]
- Klass, J.I. , Blanford, S. & Thomas, M.B. (2007b) Development of a model for evaluating the effects of environmental temperature and thermal behaviour on biological control of locusts and grasshoppers using pathogens. Agricultural and Forest Entomology, 9, 189–199. [Google Scholar]
- Koella, J.C. , Lorenz, L. & Bargielowski, I. (2009) Microsporidians as evolution‐proof agents of malaria control? Advances in Parasitology (ed. Webster J.P.), pp. 315–327. Academic Press, London, UK. [DOI] [PubMed] [Google Scholar]
- Koella, J.C. , Saddler, A. & Karacs, T.P.S. (2012) Blocking the evolution of insecticide‐resistant malaria vectors with a microsporidian. Evolutionary Applications, 5, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koella, J.C. , Lynch, P.A. , Thomas, M.B. & Read, A.F. (2009) Towards evolution‐proof malaria control with insecticides. Evolutionary Applications, 2, 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambrechts, L. , Paaijmans, K.P. , Fansiri, T. , Carrington, L.B. , Kramer, L.D. , Thomas, M.B. & Scott, T.W. (2011) Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti . Proceedings of the National Academy of Sciences of the United States of America, 108, 7460–7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPointe, D.A. , Goff, M.L. & Atkinson, C.T. (2010) Thermal constraints to the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawai'i. Journal of Parasitology, 96, 318–324. [DOI] [PubMed] [Google Scholar]
- Liu, S.‐S. , Zhang, G.‐M. & Zhu, J. (1995) Influence of temperature variations on rate of development in insects: analysis of case studies from entomological literature. Annals of the Entomological Society of America, 88, 107–119. [Google Scholar]
- Lynch, P.A. , Grimm, U. , Thomas, M.B. & Read, A.F. (2012) Prospective malaria control using entomopathogenic fungi: comparative evaluation of impact on transmission and selection for resistance. Malaria Journal, 11, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw, E.A. & O'Neill, S.L. (2013) Beyond insecticides: new thinking on an ancient problem. Nature Reviews Microbiology, 11, 181–193. [DOI] [PubMed] [Google Scholar]
- Mnyone, L.L. , Kirby, M.J. , Mpingwa, M.W. , Lwetoijera, D.W. , Knols, B.G.J. , Takken, W. , Koenraadt, C.J.M. & Russell, T.L. (2011) Infection of Anopheles gambiae mosquitoes with entomopathogenic fungi: effect of host age and blood‐feeding status. Parasitology Research, 108, 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnyone, L.L. , Lyimo, I.N. , Lwetoijera, D.W. , Mpingwa, M.W. , Nchimbi, N. , Hancock, P.A. et al (2012) Exploiting the behaviour of wild malaria vectors to achieve high infection with fungal biocontrol agents. Malaria Journal, 11, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai, E.A. , Paaijmans, K.P. , Johnson, L.R. , Balzer, C. , Ben‐Horin, T. , de Moor, E. et al (2013) Optimal temperature for malaria transmission is dramatically lower than previously predicted. Ecology Letters, 16, 22–30. [DOI] [PubMed] [Google Scholar]
- Mouatcho, J.C. , Koekemoer, L.L. , Coetzee, M. & Brooke, B.D. (2011) The effect of entomopathogenic fungus infection on female fecundity of the major malaria vector, Anopheles funestus . African Entomology, 19, 725–729. [Google Scholar]
- Murdock, C.C. , Moller‐Jacobs, L.L. & Thomas, M.B. (2013) Complex environmental drivers of immunity and resistance in malaria mosquitoes. Proceedings of the Royal Society of London Series B‐Biological Sciences, 280, 2030–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, C.C. , Paaijmans, K.P. , Cox‐Foster, D. , Read, A.F. & Thomas, M.B. (2012) Rethinking vector immunology: the role of environmental temperature in shaping resistance. Nature Reviews Microbiology, 10, 869–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock, C.C. , Blanford, S. , Hughes, G.L. , Rasgon, J.L. & Thomas, M.B. (2014) Temperature alters Plasmodium blocking by Wolbachia . Scientific Reports, 4, 3932–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans, K.P. , Read, A.F. & Thomas, M.B. (2009) Understanding the link between malaria risk and climate. Proceedings of the National Academy of Sciences of the United States of America, 106, 13844–13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans, K.P. , Blanford, S. , Bell, A.S. , Blanford, J.I. & Read, A.F. (2010) Influence of climate on malaria transmission depends on daily temperature variation. Proceedings of the National Academy of Sciences of the United States of America, 107, 15135–15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans, K.P. , Heinig, R.L. , Seliga, R.A. , Blanford, J.I. , Blanford, S. , Murdock, C.C. & Thomas, M.B. (2013) Temperature variation makes ectotherms more sensitive to climate change. Global Change Biology, 19, 2373–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton, W.J. & Logan, J.A. (1981) A model for diurnal variation in soil and air temperature. Agricultural Meteorology, 23, 205–216. [Google Scholar]
- R Core Team . (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: ISBN 3‐900051‐07‐0, URL http://www.R-project.org/ (Accepted 08/09/2015). [Google Scholar]
- Ranson, H. , N'Guessan, R. , Lines, J. , Moiroux, N. , Nkuni, Z. & Corbel, V. (2011) Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends in Parasitology, 27, 91–98. [DOI] [PubMed] [Google Scholar]
- Read, A.F. , Lynch, P.A. & Thomas, M.B. (2009) How to make evolution‐proof insecticides for malaria control. PLoS Biology, 7, e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly, J.R. & Elderd, B.D. (2014) Effects of biological control on long‐term population dynamics: identifying unexpected outcomes. Journal of Applied Ecology, 51, 90–101. [Google Scholar]
- Ruel, J.J. & Ayres, M.P. (1999) Jensen's inequality predicts effects of environmental variation. Trends in Ecology and Evolution, 14, 361–366. [DOI] [PubMed] [Google Scholar]
- Scholte, E.‐J. , Knols, B.G.J. & Takken, W. (2006) Infection of the malaria mosquito Anopheles gambiae with the entomopathogenic fungus Metarhizium anisopliae reduces blood feeding and fecundity. Journal of Invertebrate Pathology, 91, 43–49. [DOI] [PubMed] [Google Scholar]
- Scholte, E. , Njiru, B.N. , Smallegange, R.C. , Takken, W. & Knols, B.G.J. (2003) Infection of malaria (Anopheles gambiae s.s.) and filariasis (Culex quinquefasciatus) vectors with the entomopathogenic fungus Metarhizium anisopliae . Malaria Journal, 2, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte, E.‐J. , Ng'habi, K. , Kihonda, J. , Takken, W. , Paaijmans, K. , Abdulla, S. , Killeen, G.F. & Knols, B.G.J. (2005) An entomopathogenic fungus for control of adult African malaria mosquitoes. Science, 308, 1641–1642. [DOI] [PubMed] [Google Scholar]
- Smith, D.L. , Dushoff, J. , Snow, R.W. & Hay, S.I. (2005) The entomological inoculation rate and Plasmodium falciparum infection in African children. Nature, 438, 492–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg, E.D. & Thomas, M.B. (2014) Local adaptation to temperature and the implications for vector‐borne diseases. Trends in Parasitology, 30, 115–122. [DOI] [PubMed] [Google Scholar]
- Sternberg, E.D. , Waite, J.L. & Thomas, M.B. (2014) Evaluating the efficacy of biological and conventional insecticides with the new ‘MCD bottle’ bioassay. Malaria Journal, 13, 499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strode, C. , Donegan, S. , Garner, P. , Enayati, A.A. & Hemingway, J. (2014) The impact of pyrethroid resistance on the efficacy of insecticide‐treated bed nets against African anopheline mosquitoes: systematic review and meta‐analysis. PLoS Medicine, 11, e1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, M.B. & Blanford, S. (2003) Thermal biology in insect‐parasite interactions. Trends in Ecology and Evolution, 18, 344–350. [Google Scholar]
- Thomas, M.B. & Read, A.F. (2007) Can fungal biopesticides control malaria? Nature Reviews Microbiology, 5, 377–383. [DOI] [PubMed] [Google Scholar]
- Thomas, M.B. , Wood, S.N. & Lomer, C.J. (1995) Biological control of locusts and grasshoppers using a fungal pathogen: the importance of secondary cycling. Proceedings of the Royal Society of London Series B‐Biological Sciences, 259, 265–270. [Google Scholar]
- Thomas, M.B. , Godfray, H.C.J. , Read, A.F. , van den Berg, H. , Tabashnik, B.E. , van Lenteren, J.C. , Waage, J.K. & Takken, W. (2012) Lessons from agriculture for the sustainable management of malaria vectors. PLoS Medicine, 9, e1001262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (2006) Guidelines for Testing Mosquito Adulticides for Indoor Residual Spraying and Treatment of Mosquito Nets. World Health Organization, Geneva. [Google Scholar]
- World Health Organization . (2013) World Malaria Report 2013. World Health Organization, Geneva. [Google Scholar]
- Worner, S.P. (1992) Performance of phenological models under variable temperature regimes: consequences of the Kaufmann or rate summation effect. Environmental Entomology, 21, 689–699. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Weibull fits.
Fig. S2. Anopheles gambiae and blood‐fed cumulative proportional survival.
Fig. S3. Plasmodium vivax analyses.
Fig. S4. Sensitivity analyses.
Table S1. Model parameters.
Table S2. Model Weibull estimates.
Table S3. Survival analyses.
Table S4. Median survival times.
Appendix S1. Additional methods.
Data Availability Statement
Mortality data: Dryad entry doi: 10.5061/dryad.ch206 (Heinig et al. 2015).


