Abstract
Background: Green tea extract (GTE) consumption has been linked to favorable changes in adiposity and bone mineral density (BMD), although it is unknown if these effects are due to green tea catechins or caffeine. The catechol-O-methyltransferase (COMT) genotype may also modify these associations.
Objective: We examined the impact of decaffeinated GTE on body composition (using dual-energy X-ray absorptiometry) and obesity-associated hormones.
Methods: The Minnesota Green Tea Trial was a 12-mo randomized, double-blind, placebo-controlled clinical trial in 937 postmenopausal women (aged 50–70 y) assigned to receive either GTE containing 843 mg (−)-epigallocatechin-3-gallate or placebo. This substudy was conducted in 121 overweight/obese participants [body mass index (BMI) (kg/m2) ≥25.0].
Results: There were no differences in changes in BMI (−0.13 ± 0.11 compared with −0.05 ± 0.11; P = 0.61), total fat mass (−0.30 ± 0.16 compared with −0.12 ± 0.15 kg; P = 0.40), percentage of body fat (−0.15% ± 0.17% compared with −0.15% ± 0.16%; P = 0.99), or BMD (−0.006 ± 0.002 compared with −0.003 ± 0.002 g/cm2; P = 0.49) over 12 mo between women taking GTE (n = 61) and those taking a placebo (n = 60). Interactions were observed between treatment and time for gynoid percentage of fat (%fat) and tissue %fat. Gynoid %fat increased from baseline to month 12 in the placebo group as baseline BMI increased and decreased over time as baseline BMI increased in the GTE group (P-interaction = 0.02). Tissue %fat increased from baseline to month 12 in the placebo group as baseline BMI increased. In the GTE group, tissue %fat decreased during the intervention as baseline BMI increased (P-interaction = 0.04). No changes were seen in circulating leptin, ghrelin, adiponectin, or insulin concentrations. COMT genotype did not modify the effect of GTE on any variable.
Conclusions: Decaffeinated GTE was not associated with overall reductions in adiposity or improvements in BMD in overweight/obese postmenopausal women. However, GTE may be beneficial for reduction in tissue and gynoid %fat in individuals with higher BMI. This clinical trial was registered at www.clinicaltrials.gov as NCT00917735.
Keywords: green tea, obesity, body composition, bone health, postmenopausal women
Introduction
Despite substantial research efforts and public health campaigns, overweight and obesity remain prevalent worldwide (1). Excess adiposity is associated with increased risk of serious health conditions such as cardiovascular disease, type 2 diabetes, and cancer. Conversely, loss of excess fat mass and/or maintenance of a healthy body weight are known to reduce the risk of these diseases (2, 3).
Research on the associations between obesity and metabolic bone diseases such as osteoporosis, a major cause of morbidity and mortality in postmenopausal women, remains inconclusive. Several epidemiologic studies have highlighted the correlation between these conditions (4–8), with many indicating a significant positive relation between body weight and bone mineral density (BMD)7. This correlation is largely attributed to both the increased mechanical load of excess weight and hormonal changes associated with obesity, including increased estrogen and leptin production by adipose tissue, which have been shown to suppress bone resorption and stimulate osteoblastogenesis (9–11). However, evidence has emerged that shows that increased body weight may actually be negatively correlated with BMD after correction for the mechanical loading effect of excess body weight (12) and that obese individuals with a high BMD are still at increased risk of fractures (5). Obesity-associated metabolic derangements including hyperglycemia and insulin resistance have also been linked to reduced bone turnover and osteoblast apoptosis (13).
The etiologies of obesity and metabolic bone disorders involve factors that are both modifiable and nonmodifiable. Dietary intake of natural bioactive foods and beverages, such as green tea, is an adaptable habit that may play a role in reducing the risk of these chronic diseases. Epidemiologic research has suggested that tea intake is associated with both reductions in adiposity and increased BMD (14, 15). Green tea’s antiobesity effects are thought to be due to increases in thermogenesis and fat oxidation through the inhibition of the catechol-O-methyltransferase (COMT) enzyme by green tea catechins (GTCs), resulting in prolonged sympathetic nervous system (SNS) stimulation (16, 17). Proposed mechanisms for improving and/or maintaining bone health include reducing chronic inflammation and oxidative stress, conditions that are also tightly linked with obesity and excess adipose tissue (18).
Genetic variations in COMT enzyme activity have been widely noted. One common polymorphism, a guanine (G) to adenine (A) substitution at position rs4680 has been identified, which produces an amino acid change from valine to methionine. The homozygous low-activity (A/A) genotype is associated with a decrease in enzymatic activity when compared with the high-activity (G/G) genotype (19, 20). Given these effects, the COMT genotype may influence the rate of catechin metabolism and the functional response to dietary catechins, wherein individuals with the low-activity form of the enzyme may have increased thermogenesis, reduced adiposity, and lower body weight due to prolonged SNS effects compared with individuals with the high-activity genotype. Yet, those with the high-activity form of COMT may benefit more from interventions that inhibit the enzyme, because the higher rate of substrate metabolism would be reduced to a greater degree.
Most of green tea’s benefits are attributed to its high concentration of polyphenolic catechins, of which (−)-epigallocatechin-3-gallate (EGCG) is the most abundant and also believed to be the most bioactive (21). On the basis of its potential antiobesity effects, extracts of GTCs have been marketed as herbal supplements for the control of body weight. However, their efficacy has not been consistently proven, and the potential beneficial effects of EGCG on obesity, adipose-related hormone concentrations, and skeletal health remain controversial. In addition, most randomized trials that used green tea included caffeine in the intervention, which is independently known to increase energy expenditure and fat oxidation (22). Therefore, the independent effects of GTCs on these endpoints remain unclear.
The aim of the present study was to determine the effect of caffeine-free green tea extract (GTE) consumption on body composition and BMD in free-living overweight and obese postmenopausal women, a population at risk of both excess weight gain and rapid bone loss. Secondary aims included the measurement of the effects of GTE on obesity-related hormones (insulin, leptin, ghrelin, and adiponectin), correlation of these hormones with body-composition variables, and testing for effect modification by COMT genotype. We hypothesized that GTE supplementation would 1) cause favorable changes in body composition, 2) enhance or maintain measures of BMD, 3), suppress insulin and leptin concentrations, and 4) increase ghrelin and adiponectin concentrations compared with the placebo. We also proposed that GTE supplementation would be particularly beneficial in those with the high-activity form of COMT, due to the inhibitory effects of GTCs on this enzyme. Determining the impact of GTE on body composition, BMD, and obesity-related hormones will advance the understanding of the potential health benefits of GTCs as well as the complex relation between obesity and skeletal health.
Methods
Design.
The Minnesota Green Tea Trial (MGTT) was a randomized, parallel-arm, double-blinded, placebo-controlled study designed to examine the effect of GTE on breast cancer risk factors, including obesity. Details of the study design, eligibility criteria, randomization, blinding, study conduct, and patient flow through the trial have been previously published (23). Briefly, postmenopausal women aged 50 to 70 y and classified as having high breast density (a breast cancer risk factor) were recruited from 2009 to 2013 at clinical centers in the Minneapolis–St. Paul metropolitan area. Exclusionary criteria included the following: any history of breast cancer, proliferative breast disease, or ovarian cancer or any cancer diagnosis within 5 y; previously diagnosed type 1 or type 2 diabetes; consumption of >7 alcoholic drinks/wk [1 alcoholic drink quantified as 12 fluid ounces (fl oz) (355 mL) regular beer, 5 fl oz (148 mL) wine, or 1.5 fl oz (44 mL) liquor]; consumption of > 240 mL green tea/wk; BMI (in kg/m2) <25.0 or >40.0; >4.6 kg weight change during the previous year; current use of menopausal hormone therapy or use within past 6 mo; use of methotrexate or Enbrel (etanercept, Amgen Inc., Thousand Oaks, California); current smoking; history of breast augmentation; positive serology for hepatitis B or C antibodies; or alanine aminotransferase >1.5 times the upper limit of normal (defined as 60 U/L). Eligible participants came to monthly clinic visits for the first 6 mo primarily for hepatic panel monitoring; additional clinic visits occurred at months 9 and 12. MGTT participants were required to come to the University of Minnesota’s Human Nutrition Research Center for a minimum of 5 clinic visits (months 0, 3, 6, 9, and 12) and were given the option of having a hepatic panel assessment at a more convenient Fairview or Park Nicollet clinic during months 1, 2, 4, and 5.
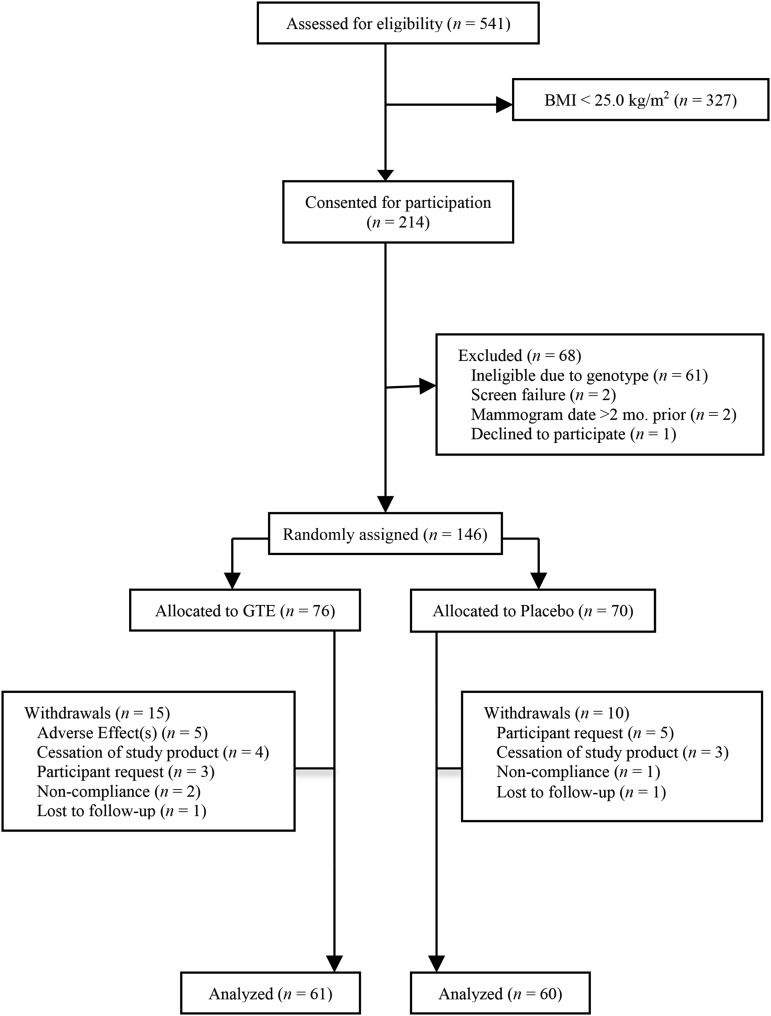
In addition, of 937 women who completed the full study duration, 214 women who were classified as overweight (BMI = 25.0–29.9) or obese (BMI = 30.0–40.0) at the screening clinic visit were consented for body-composition analysis (assessed by DXA) beginning in May 2013. Of these, 146 were randomly assigned to the study (GTE: n = 76; placebo: n = 70). Fifteen women allocated to the GTE group and 10 allocated to the placebo group withdrew from participation; 121 completed the duration of the study (GTE: n = 61; placebo: n = 60) (Figure 1).
FIGURE 1.
CONSORT (Consolidated Standards of Reporting Trials) flow diagram of the recruitment, enrollment, and randomization process. GTE, green tea extract.
Randomization and blinding.
Participants were randomly assigned to receive oral GTE or a placebo in 4 capsules daily for 12 mo. Randomization was performed by the Investigational Drug Services pharmacy at the University of Minnesota Medical Center–Fairview, which used a computer-generated permuted block randomization scheme with blocks of 8 stratified by COMT genotype activity: low (A/A), intermediate (A/G), or high (G/G). Participants and study staff were blinded to treatment allocation throughout the trial. Sample size calculations were performed by using power analysis on the basis of previous reports of GTE’s effect on body fat variables (24–29). The present study has >80% statistical power to detect a 1.5% difference in body fat percentage between the GTE and placebo groups. Institutional review board approval was obtained at each clinical center. All participants provided written informed consent. This trial was registered at clinicaltrials.gov as NCT00917735.
COMT genotyping.
COMT genotype analysis was completed at the University of Minnesota Genomics Center. DNA was extracted from buffy coat samples by the Qiagen DNAeasy Blood and Tissue Kit method (Qiagen). A TaqMan assay was developed for defining the COMT rs4680 polymorphism by using a TaqMan PCR Core Reagent kit (Applied Biosystems). Cell lines with known COMT genotype were used as quality controls with each PCR run.
Study supplement.
Decaffeinated Green Tea Extract Catechin Complex (GTE) and placebo capsules were supplied by Corban Laboratories (Eniva Nutraceutics, Plymouth, Minnesota) in 8 batches. Mean daily catechin content was 1315 ± 116 mg/d (843 ± 44 mg as EGCG), which is approximately equivalent to five 240-mL servings of brewed green tea (30). Placebo capsules were identical in appearance to GTE and contained 816 mg maltodextrin, 808 mg cellulose, and 8 mg magnesium stearate (flow agent). GTE ingredients were analyzed by HPLC (Rutgers University) to demonstrate comparability with the stated catechin contents of the manufacturer. Participants were advised to ingest 2 capsules in the morning hours and 2 in the evening to maintain circulating catechin concentrations throughout each day. Participants in the MGTT were instructed to limit brewed green tea consumption to <240 mL (1 cup)/wk to prevent catechin intake that may confound study results. Participants were not instructed to limit their intake of black tea or other tea types due to their minimal catechin content relative to the amount administered through the GTE intervention.
Compliance assessment.
Compliance to GTE supplementation was assessed by using the following 3 different approaches:
Capsule count. Participants were asked to return all unused capsules in the original bottles every 3 mo. Clinic staff counted the number of returned capsules, calculated the number of unreturned capsules from the number originally dispensed, and calculated compliance as the number of capsules actually consumed divided by the number of capsules the participant should have consumed. By using this method, participants in the MGTT had a 97.2% average compliance rate.
Participant self-monitoring. Each participant filled out a study log indicating the time and date of capsule consumption and returned the study log for monitoring by clinic staff at each visit.
Urinary analysis. Concentrations of epigallocatechin and epicatechin were measured at baseline and at 3, 6, 9, and 12 mo in a random selection of 10% of participants (n = 43 participants randomly assigned to GTE) and were evaluated relative to milligrams of urinary creatinine.
Anthropometric and body-composition measurements.
Body weight was measured to the nearest 0.1 kg at baseline and at months 3, 6, 9, and 12 by using a stand-on digital scale (Scale-Tronix). Standing height was assessed by wall-mounted stadiometer (Seca) to the nearest 0.1 cm at the screening clinic visit and month 12. BMI was calculated by dividing weight in kilograms by height in meters squared (kg/m2).
DXA scans were completed at the University of Minnesota’s Delaware Clinical Research Unit by using a GE Healthcare Lunar iDXA (GE Healthcare) and analyzed with the use of Encore software version 13.6, revision 2. Total body fat was expressed as the percentage of total body mass, and android and gynoid fat were expressed as the percentage of total body fat. Subcutaneous fat was determined by using an algorithm and measurements of total abdominal thickness and the width of the subcutaneous fat layer along the lateral extent of the abdomen along with empirically derived geometric constants to estimate the subcutaneous fat in the android region. Visceral adipose tissue (VAT) was determined in the android region by subtracting subcutaneous fat from total fat. Tissue percentage fat was calculated as follows: fat mass/(total mass – BMC). The android region was defined as the caudal limit placed at the top of the iliac crest and its height set to 20% of the distance from the top of the iliac crest to the base of the skull. The upper limit of the gynoid region was set below the iliac crest a distance 1.5 times the height of the android region. The lower limit was set a distance of 2 times the height of the android region. Central fat distribution was assessed by the android:gynoid fat ratio, calculated as android fat divided by gynoid fat.
Areal BMD was expressed as grams per centimeters squared (g/cm2). T-scores were expressed in SDs by using the peak bone mass from the manufacturer’s reference population. z scores were measured as the deviation from the normal age- and sex-matched means and SDs. Osteoporosis was diagnosed, in accordance with the WHO definition, as a T-score of less than −2.5 as assessed by DXA; osteopenia was diagnosed as BMD of −1.0 to −2.5 (31).
Obesity-associated hormone and glucose homeostasis analysis.
Blood samples for obesity-associated hormone and glucose assessment were collected at the prespecified time points of baseline and month 12, to correspond with the scheduled annual DXA scan, after an overnight fast of >10 h. Whole-blood samples were separated into plasma and serum. Plasma leptin, adiponectin, and ghrelin were measured by using RIA kits manufactured by EMD Millipore (interassay %CV: leptin = 7.1%, adiponectin = 8.9%, ghrelin = 7.8%; intra-assay %CV: leptin = 6.6%, adiponectin = 7.4%, ghrelin = 5.5%). Serum insulin was measured by using a simultaneous 1-step immunoenzymatic chemiluminescent assay (Access Ultrasensitive Insulin assay; Quest Diagnostics; intra-assay %CV: 3–5.0%; interassay %CV = 3.9%). Serum glucose concentrations were measured by using a hexokinase enzymatic reference method (Quest Diagnostics; monthly %CV = 1.4%). HOMA-IR was calculated as fasting insulin (μIU/mL) × fasting glucose (mg/dL)/405.
Dietary assessment.
Participants completed an FFQ at baseline and at month 12 for dietary intake assessment over the previous 12 mo. The Diet History Questionnaire is an FFQ consisting of 176 nutrients, dietary constituents, and food groups and includes portion size and dietary supplement questions. The food list and nutrient database used with the Diet History Questionnaire are based on national dietary data (32). FFQ data were analyzed by using Diet*Calc software developed at the National Cancer Institute.
Physical activity.
Recreational physical activity was assessed at baseline and at month 12 by a validated physical activity questionnaire (33), in which participants were asked about frequency and duration of several types of physical activity. Metabolic equivalent hours (MET-hours), defined as the ratio of work metabolic rate to a standard resting metabolic rate, were computed as the product of average hours per week of each activity multiplied by its MET-hour equivalent. All recorded activities were summed to obtain the total MET-hours of activity performed per week for a given participant.
Statistical analyses.
Differences in baseline demographic and anthropometric characteristics between treatment groups at baseline were assessed by nonpaired Student’s t test for continuous variables and chi-square test for categorical variables. Natural log-transformation was used to normalize the distribution of continuous variables when needed. Two-factor ANOVA was used to compare BMI, adiposity measures, and BMD variables between the GTE and placebo groups and within group at baseline and at month 12. Pairwise comparisons were performed between and within treatment groups. Changes from baseline in BMI, adiposity measures, and BMD variables were calculated by subtracting the baseline from the month 12 values for each participant. Linear regression was used to compare the mean change from baseline in the GTE and placebo groups after adjusting by baseline value, change in BMI, years since menopause, and menopausal hormone therapy use. The same analyses were used to compare adiposity measures and BMD variables between COMT genotype groups.
Obesity-associated hormones, glucose, and HOMA-IR were ln-transformed and 2-factor ANOVA was used to compare geometric means at baseline and at month 12. Pairwise differences were used to compute the P values between and within treatment groups. Similar analyses were performed to compare geometric means between and within COMT genotype at baseline and month 12. Mean changes in obesity-associated hormones, glucose, and HOMA-IR at 12 mo were calculated by subtracting the baseline value from the value at month 12 and analyzed by using linear regression. The explanatory variables included treatment group, baseline value of the variable, change in BMI, COMT genotype, and all possible 2-way interactions. Main effects were kept in the model. Model reduction was considered with the use of backward elimination for 2-way interactions having P values >0.05. Pearson’s correlation was used to evaluate the association between leptin, adiponectin, and insulin and BMI, adiposity measures, and BMD variables at baseline and at month 12.
Data are presented as arithmetic or geometric means and SEMs or 95% CIs for continuous variables or as counts and percentages for categorical variables. All analyses were performed by using the Mixed, GLM, and Freq procedures of SAS, version 9.3 (SAS Institute). Cook’s distance was used to evaluate influential outliers, and residual plots were used to evaluate model assumptions.
Results
Participant characteristics.
Baseline characteristics and demographics were similar for both treatment groups at baseline (Table 1). Physical activity, total energy intake, and intake of caffeine and macro- and micronutrients did not differ at baseline or at month 12. On the basis of the results of the DXA scans, 2.5% of MGTT participants met the criteria for osteopenia (n = 3). No participants met the criteria for osteoporosis (T-score less than −2.5) (31). Baseline characteristics did not differ between participants who completed the study and those who withdrew (data not shown).
TABLE 1.
Baseline characteristics of overweight and obese postmenopausal women randomly assigned to receive GTE or placebo for 12 mo1
| Characteristic | GTE (n = 61) | Placebo (n = 60) | P |
| Age at baseline, y | 60.7 ± 0.60 | 60.0 ± 0.65 | 0.45 |
| Race/ethnicity, n (%) | 0.72 | ||
| Non-Hispanic white | 57 (93.4) | 57 (95.0) | |
| African American | 2 (3.30) | 2 (3.30) | |
| Other | 2 (3.30) | 1 (1.70) | |
| Weight, kg | 74.9 ± 1.12 | 74.1 ± 1.25 | 0.63 |
| Height, cm | 164 ± 0.01 | 164 ± 0.01 | 0.89 |
| BMI, kg/m2 | 27.9 ± 0.34 | 27.6 ± 0.35 | 0.53 |
| Waist circumference, cm | 91.8 ± 7.70 | 91.1 ± 8.50 | 0.63 |
| COMT genotype, n (%) | 0.49 | ||
| Low (A/A) | 20 (32.8) | 21 (35.0) | |
| Intermediate (A/G) | 21 (34.4) | 25 (41.7) | |
| High (G/G) | 20 (32.8) | 14 (23.3) | |
| Time since menopause, y | 9.80 (8.10, 11.8) | 8.50 (7.00, 10.3) | 0.31 |
| MHT use (ever), n (%) | 0.80 | ||
| Yes | 22 (36.1) | 23 (38.3) | |
| No | 39 (63.9) | 37 (61.7) | |
| Length of MHT use, y | 3.50 (1.90, 6.50) | 2.9 (1.60, 5.40) | 0.66 |
| n | 21 | 21 | |
| Use of bone resorption inhibitors, n (%) | 0.66 | ||
| Yes | 3 (4.90) | 2 (3.30) | |
| No | 57 (93.4) | 57 (95.0) | |
| Use of calcium supplements, n (%) | 0.65 | ||
| Yes | 30 (49.2) | 27 (45.0) | |
| No | 31 (50.8) | 33 (55.0) | |
| Physical activity, MET-h/wk | 13.8 (10.7, 17.7) | 16.8 (13.1, 21.7) | 0.27 |
| Alcohol consumption, n (%) | 0.78 | ||
| Yes | 51 (83.6) | 49 (81.7) | |
| No | 10 (16.4) | 11 (18.3) | |
| Alcohol intake (drinkers only),2 drinks/wk | 2.08 (1.54, 2.82) | 2.07 (1.52, 2.82) | 0.98 |
| Energy intake, kcal/d | 1380 (1260, 1520) | 1430 (1300, 1560) | 0.65 |
| Caffeine, mg/d | 141 (89.4, 222) | 161 (102, 254) | 0.68 |
Values are arithmetic means ± SEMs or geometric means (95% CIs) unless otherwise indicated. COMT, catechol-O-methyltransferase; GTE, green tea extract; MET-h, metabolic equivalent hour; MHT, menopausal hormone therapy.
One alcoholic drink was quantified as 12 fluid ounces (355 mL) regular beer, 5 fluid ounces (148 mL) wine, or 1.5 fluid ounces (44 mL) liquor.
Comparison between groups for body-composition and adiposity variables.
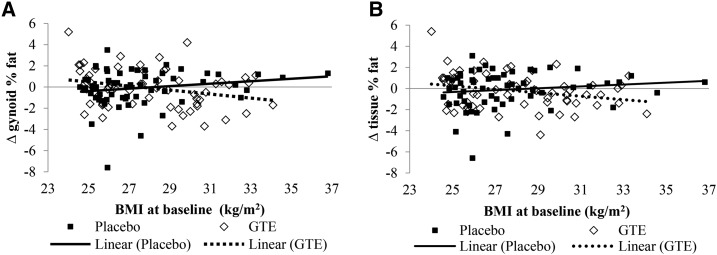
Body-composition and adiposity variables are presented in Table 2. Treatment groups were similar at baseline for most body-composition and adiposity variables, although participants in the GTE group had significantly higher android percentage of fat (%fat) (P = 0.01) and android:gynoid ratio (P = 0.01). No statistically significant differences in change from baseline to month 12 were seen in any body-composition variable between or within treatment groups. Interactions were observed between treatment, time, and baseline BMI for gynoid %fat and tissue %fat (Figure 2). Although the gynoid %fat increased from baseline to month 12 in the placebo group as baseline BMI increased, gynoid %fat decreased over time as baseline BMI increased in the GTE group (Figure 2A; P-interaction = 0.02). Similarly, whereas tissue %fat increased from baseline to month 12 in the placebo group as baseline BMI increased, the opposite occurred in the GTE group: as baseline BMI increased, tissue %fat decreased during the intervention (Figure 2B; P-interaction = 0.04). Android:gynoid ratio did not change from baseline in either study group.
TABLE 2.
Comparison of BMI and adiposity in overweight and obese postmenopausal women randomly assigned to receive GTE or placebo for 12 mo1
| GTE (n = 61) | Placebo (n = 60) | P | |
| BMI, kg/m2 | |||
| Baseline | 28.0 ± 0.34 | 27.6 ± 0.35 | 0.30 |
| Month 12 | 27.9 ± 0.34 | 27.5 ± 0.35 | 0.37 |
| Change from baseline | −0.13 ± 0.11 | −0.05 ± 0.11 | 0.61 |
| Total fat mass, kg | |||
| Baseline | 32.2 ± 0.80 | 30.9 ± 0.80 | 0.25 |
| Month 12 | 31.7 ± 0.80 | 30.9 ± 0.80 | 0.45 |
| Change from baseline | −0.30 ± 0.16 | −0.12 ± 0.15 | 0.40 |
| Total body fat, % | |||
| Baseline | 42.5 ± 0.50 | 41.5 ± 0.50 | 0.21 |
| Month 12 | 42.3 ± 0.50 | 41.4 ± 0.60 | 0.29 |
| Change from baseline | −0.15 ± 0.17 | −0.15 ± 0.16 | 0.99 |
| VAT mass, kg | |||
| Baseline | 1.06 ± 0.07 | 0.88 ± 0.07 | 0.06 |
| Month 12 | 1.04 ± 0.07 | 0.91 ± 0.07 | 0.17 |
| Change from baseline | −0.01 ± 0.02 | 0.03 ± 0.02 | 0.11 |
| Estimated VAT volume, cm3 | |||
| Baseline | 1.12 ± 0.07 | 0.93 ± 0.07 | 0.06 |
| Month 12 | 1.10 ± 0.07 | 0.96 ± 0.07 | 0.17 |
| Change from baseline | −0.01 ± 0.02 | 0.02 ± 0.02 | 0.18 |
| Android fat, % | |||
| Baseline | 48.6 ± 0.80 | 45.5 ± 0.80 | 0.01 |
| Month 12 | 47.4 ± 0.90 | 45.4 ± 0.90 | 0.09 |
| Change from baseline | −0.92 ± 0.43 | −0.27 ± 0.43 | 0.29 |
| Gynoid fat,2 % | |||
| Baseline | 45.6 ± 0.60 | 45.8 ± 0.60 | 0.80 |
| Month 12 | 45.5 ± 0.60 | 45.7 ± 0.60 | 0.81 |
| Change from baseline | −0.06 ± 0.18 | −0.15 ± 0.17 | 0.71 |
| Android:gynoid ratio | |||
| Baseline | 1.06 ± 0.02 | 0.99 ± 0.02 | 0.01 |
| Month 12 | 1.05 ± 0.02 | 0.99 ± 0.02 | 0.03 |
| Change from baseline | −0.009 ± 0.007 | −0.003 ± 0.006 | 0.52 |
| Lean weight, kg | |||
| Baseline | 40.6 ± 0.60 | 40.7 ± 0.60 | 0.99 |
| Month 12 | 40.7 ± 0.50 | 40.7 ± 0.50 | 0.97 |
| Change from baseline | 0.10 ± 0.11 | 0.09 ± 0.11 | 0.91 |
| Lean tissue, % | |||
| Baseline | 54.4 ± 0.50 | 55.3 ± 0.50 | 0.18 |
| Month 12 | 54.6 ± 0.50 | 55.5 ± 0.50 | 0.26 |
| Change from baseline | 0.19 ± 0.17 | 0.18 ± 0.16 | 0.95 |
| Fat tissue,2 % | |||
| Baseline | 32.2 ± 0.80 | 30.9 ± 0.80 | 0.25 |
| Month 12 | 31.7 ± 0.80 | 30.9 ± 0.80 | 0.45 |
| Change from baseline | −0.30 ± 0.16 | −0.12 ± 0.15 | 0.40 |
Values are arithmetic means ± SEMs. BMI change from baseline was adjusted for baseline value, years since menopause, and MHT use. All subsequent change from baseline data were adjusted for baseline value, change in BMI from baseline, years since menopause, and MHT use. GTE, green tea extract; MHT, menopausal hormone therapy; VAT, visceral adipose tissue.
Significant time × treatment interaction.
FIGURE 2.
Scatter plots of interactions between time, treatment group, and baseline BMI for changes in gynoid (A) and tissue (B) %fat in overweight or obese postmenopausal women randomly assigned to GTE or placebo for 12 mo. GTE, green tea extract; %fat, percentage of fat.
No treatment by genotype interactions were observed, so we conducted an exploratory analysis of baseline body-composition variables by COMT genotype, independent of treatment group. A statistically significant difference in lean weight at baseline was observed between participants with the high-activity COMT genotype (n = 34) compared with the intermediate-activity group (n = 46) (P = 0.02). No differences between genotype groups were seen for any other body-composition variable.
Comparison between groups for bone density variables.
No differences were observed between or within treatment groups when comparing change from baseline to month 12 in BMD, T-score, or z score (Table 3). Mean T-scores and z scores were positive in both the GTE and placebo groups at both time points. Results did not differ by COMT genotype.
TABLE 3.
Comparison of bone density measurements in overweight and obese postmenopausal women randomly assigned to receive GTE or placebo for 12 mo1
| GTE (n = 61) | Placebo (n = 60) | P | |
| BMD, g/cm2 | |||
| Baseline | 1.17 ± 0.01 | 1.14 ± 0.01 | 0.07 |
| Month 12 | 1.17 ± 0.01 | 1.14 ± 0.01 | 0.09 |
| Change from baseline | −0.006 ± 0.002 | −0.003 ± 0.002 | 0.49 |
| T-score | |||
| Baseline | 0.95 ± 0.12 | 0.62 ± 0.12 | 0.06 |
| Month 12 | 0.88 ± 0.12 | 0.59 ± 0.12 | 0.09 |
| Change from baseline | −0.07 ± 0.03 | −0.03 ± 0.02 | 0.31 |
| z score | |||
| Baseline | 1.37 ± 0.12 | 1.10 ± 0.12 | 0.11 |
| Month 12 | 1.40 ± 0.12 | 1.12 ± 0.12 | 0.10 |
| Change from baseline | 0.02 ± 0.03 | 0.02 ± 0.03 | 0.99 |
Values are arithmetic means ± SEMs. Changes from baseline data were adjusted for baseline value, BMI change from baseline, years since menopause, and menopausal hormone therapy use. BMD, bone mineral density; GTE, green tea extract.
Comparison of obesity-associated hormone concentrations.
Mean fasting concentrations of obesity-associated hormones and glucose homeostasis markers at baseline and at month 12 are presented in Table 4. Concentrations did not differ between GTE or placebo groups at either time point for any variable, aside from higher circulating adiponectin at baseline in the placebo group compared with the GTE group (P = 0.05), and there were no significant differences in change from baseline between or within treatment groups for any variable. No interaction was observed between GTE and COMT genotype for change from baseline in these variables.
TABLE 4.
Circulating concentrations of obesity-associated hormones and glucose and the HOMA-IR in overweight and obese postmenopausal women randomly assigned to receive GTE or placebo for 12 mo1
| GTE (n = 61) | Placebo (n = 60) | P2 | |
| Insulin, μIU/mL | |||
| Baseline | 6.80 (6.00, 7.80) | 5.9 (5.20, 6.70) | 0.12 |
| Month 12 | 7.50 (6.60, 8.50) | 6.4 (5.70, 7.30) | 0.09 |
| Change from baseline | 0.72 (0.12, 1.32) | 0.50 (−0.11, 1.10) | 0.61 |
| Glucose, mg/dL | |||
| Baseline | 102 (98.3, 105) | 99.0 (95.9, 102) | 0.29 |
| Month 12 | 97.4 (94.3, 101) | 95.7 (92.6, 98.8) | 0.45 |
| Change from baseline3 | −3.10 (−5.60, −0.69) | −4.32 (−6.78, −1.85) | 0.56 |
| HOMA-IR | |||
| Baseline | 1.71 (1.50, 1.96) | 1.44 (1.27, 1.66) | 0.09 |
| Month 12 | 1.81 (1.57, 2.07) | 1.52 (1.32, 1.74) | 0.08 |
| Change from baseline | 0.13 (−0.30, 0.29) | 0.07 (−0.09, 0.24) | 0.61 |
| Adiponectin, μg/mL | |||
| Baseline | 6.60 (5.50, 7.8) | 8.4 (7.10, 10.0) | 0.05 |
| Month 12 | 6.90 (5.80, 8.2) | 8.1 (6.80, 9.70) | 0.21 |
| Change from baseline | 0.54 (−0.32, 1.41) | −0.05 (−0.93, 0.84) | 0.35 |
| Ghrelin, pg/mL | |||
| Baseline | 1060 (935, 1210) | 1120 (985, 1270) | 0.57 |
| Month 12 | 1060 (931.2, 1200) | 1200 (1050, 1360) | 0.19 |
| Change from baseline4 | −31.1 (−119, 56.9) | 84.2 (−5.98, 174) | 0.16 |
| Leptin, μg/L | |||
| Baseline | 31.1 (27.2, 35.6) | 30.9 (27.0, 35.3) | 0.93 |
| Month 12 | 30.4 (26.6, 34.8) | 30.1 (26.3, 34.4) | 0.90 |
| Change from baseline | 0.002 (−2.68, 2.68) | −0.59 (−3.33, 2.15) | 0.76 |
Baseline and month 12 values are geometric means (95% CIs); changes from baseline values are arithmetic means (95% CIs). GTE, green tea extract.
P values for the comparison of the means between GTE and placebo. Within-group comparisons of baseline and month 12 values were not statistically significant.
Associations between obesity-associated hormones and body-composition variables.
Supplemental Table 1 reports Pearson’s correlations between baseline concentrations of leptin, adiponectin, and insulin and BMI, body-composition, and BMD measurements from the pooled group of all participants (n = 121). Fasting plasma leptin concentrations were positively correlated with BMI and body fat measurements and were negatively correlated with percentage of lean tissue (r = −0.270, P < 0.001) and z score (r = −0.314, P < 0.001). The concentration of adiponectin was positively correlated with gynoid %fat (r = 0.264, P = 0.004) and negatively correlated with VAT mass (r = −0.342, P < 0.001). Insulin concentration was positively correlated with BMI and body fat measurements and was negatively correlated with percentage of lean tissue (r = −0.188, P = 0.04). Adiponectin and insulin concentrations were not correlated with BMD. These relations remained similar at month 12 (data not shown).
Discussion
The results of the present study indicate that the intake of 1315 mg GTCs/d (843 mg as EGCG) for 1 y did not affect overall adiposity, fat-free mass, or BMD in postmenopausal overweight or obese women. Similarly, GTE did not alter concentrations of obesity-associated hormones or glucose homeostasis markers compared with the placebo. However, reductions in visceral adiposity in GTE participants nearly reached significance and participants with higher baseline BMI who were randomly assigned to the GTE group showed reduced tissue %fat to a greater degree than those randomly assigned to the placebo group.
Epidemiologic evidence has shown that individuals with the highest degree of tea consumption may have a reduced risk of obesity-related diseases (34, 35), and several randomized trials (typically ≤12 wk in duration) have shown that GTC supplementation may lead to modest decreases in body weight and adiposity. However, results are inconsistent and the clinical relevance of these reductions is a topic of debate (36, 37). Furthermore, most studies examining this relation have included caffeine as part of the intervention, making it impossible to measure the independent effects of GTCs. Although we did not see a change in BMI, total fat mass, or percentage of body fat between treatment groups, trends toward reductions in total VAT mass (P = 0.11) and volume (P = 0.18) in the GTE group suggest that GTCs may inhibit the accumulation of abdominal fat when consumed over the life span. These effects are notable, given that central adiposity is associated with higher risk of metabolic disorders, cardiovascular disease, and some forms of cancer (38). Our results are consistent with evidence from other randomized trials that found an influential effect of GTCs on abdominal and visceral fat (24, 25). In addition, participants with higher baseline BMI who were randomly assigned to receive GTE showed reduced tissue %fat to a greater extent over time compared with those randomly assigned to receive the placebo, indicating that GTE supplementation may be especially beneficial for individuals with a higher degree of adiposity.
As previously stated, the effect of GTCs on body composition has not been consistent across all study populations and trial designs. Genetic variations may at least partially explain these differences in outcomes. The G to A polymorphism at position 4680 of the COMT gene is often cited as playing a potential role in sensitivity to GTCs with respect to energy expenditure and fat oxidation. EGCG has been suggested to inhibit COMT, an enzyme responsible for metabolizing both GTCs and catecholamines such as norepinephrine (39). Reduced activity of COMT may lead to extended effects of these compounds, with resulting increases in SNS activity, fat oxidation, and energy expenditure. However, the results of the present study do not indicate a modifying effect of COMT genotype on body-composition variables. Indeed, there is little in vivo evidence to support a role of COMT in relation to clinically meaningful reductions in body weight and adiposity. Hodgson et al. (40), using targeted catecholamine profiling techniques, determined that GTE did not increase concentrations of norepinephrine, which suggests that GTE supplementation may not alter COMT activity. Furthermore, Lorenz et al. (41) determined that the administration of 750 mg EGCG did not impair the in vivo activity of COMT.
Epidemiologic studies have linked green tea consumption to higher bone density (14) and reduced risk of hip fractures (42). Biological mechanisms are thought to involve the weak estrogenic effect of GTCs (43) and induction of osteoclast apoptosis by EGCG (44), therefore inhibiting bone resorption and leading to increased BMD. We did not observe an effect of GTE on measures of BMD in this study population. The women in the present study had high T-scores and z scores in comparison to the respective reference populations, so it is possible that any favorable effect of GTE on BMD may be best observed in individuals with low bone density. However, many women with normal BMD experience fractures due to poor bone structure, which DXA technology is unable to measure. Shen et al. (45) found that supplementation of 500 mg green tea polyphenols/d (233 mg as EGCG) for 6 mo increased bone-specific alkaline phosphatase (BAP), a glycoprotein found on the surface of osteoblasts that is indicative of bone biosynthesis, in postmenopausal women with osteopenia. Their trial design did not include diagnostic markers of bone health (e.g., T-score), rendering it difficult to determine the clinical relevance of these physiologic changes. Biomarkers including BAP would change long before changes in BMD could be measured by DXA, so additional studies assessing both short- and long-term measures of bone health variables are needed.
In addition to being a fat storage depot, adipose tissue secretes inflammatory markers and adipokines, such as leptin and adiponectin. Research has shown that receptors for adiponectin are present on osteoblasts and may influence proliferation, differentiation, and the mineralization effects of these bone-remodeling cells (46, 47). However, this association is not consistent (48). The impact of leptin on bone metabolism has also been studied, but results remain controversial. We did not observe statistically significant changes in hormone concentrations between treatment groups, in contrast to other studies that showed increases in adiponectin concentrations and improvements in insulin sensitivity with GTE supplementation (49, 50). This could be largely attributed to the lack of change in BMI and body fat mass in our study participants, as well as their general good health status—a pre-existing diagnosis of diabetes (both types 1 and 2) was part of the exclusionary criteria for the MGTT. This was assessed via telephone screening questionnaire (self-report) as well as medication history in the health history questionnaire. Although we did not continuously monitor fasting glucose or insulin concentrations during a participant’s 12-mo intervention period, no participants in the parent study or in this smaller substudy were diagnosed with diabetes mellitus while they were enrolled in the trial. Hormone analysis and correlations to other endpoints were largely exploratory in this data set, and we may not have had sufficient power to detect an effect of GTE on these variables.
It is important to note that the GTE used in this study was decaffeinated (<16 mg caffeine/d), therefore demonstrating the effect of GTCs independent of the well-understood effects of caffeine on fat oxidation and energy expenditure (51). The overall lack of effect of decaffeinated GTE aligns with the results of a previous green tea intervention in 38 obese postmenopausal subjects (52) . This suggests that any beneficial effect of green tea on body weight and adiposity is largely due to its caffeine content or synergism between catechins and caffeine, which is in agreement with a meta-analysis by Phung et al. (37), who observed a statistically significant decrease in body weight, BMI, and waist circumference with interventions incorporating both GTCs and caffeine compared with those with a caffeine-only placebo.
The MGTT is the largest and longest clinical trial investigating the impact of GTE on health outcomes in postmenopausal women, including body composition and bone health. We used the highest daily dosage of GTCs, and assessment of safety of our GTE intervention has been previously published (53). An additional strength of the current study is our use of accurate methods of assessing regional adiposity, which eliminates intra- and interexaminer variation in measurements compared with manual measurements such as waist and hip circumference. Limitations of the current study include the lack of measurements of resting energy expenditure and respiratory quotient, which would have allowed us to form relations between the observed changes in central adiposity, energy, expenditure, and substrate utilization. Last, our genotypic analysis was limited to just 1 polymorphism of 1 enzyme involved in GTC metabolism. It is possible that other enzymes and physiologic pathways may play a role in green tea’s effect on body composition.
In conclusion, the daily consumption of decaffeinated GTE containing 843 mg EGCG for 12 mo was not associated with changes in adiposity, BMD, or obesity-associated hormones. The COMT genotype did not modify these results. However, GTE may be beneficial for reduction in tissue and gynoid %fat in individuals with a higher BMI.
Acknowledgments
We thank Sarah Bedell, Jane Mobeck-Wilson, Kate Ringsak, Amy Brehm, and Ed Smith for their help in carrying out the study coordination, clinical data collection, and laboratory analysis aspects of our study. AMD and MSK contributed to the conception, design, and implementation of the project; AMD and AA contributed to data collection and analytical procedures; AA and LE conducted the statistical analysis; AMD, AA, LE, and MSK interpreted the data; and AMD and MSK wrote the manuscript and had primary responsibility for final content. All authors read and approved the final version of the manuscript.
Footnotes
Abbreviations used: BAP, bone-specific alkaline phosphatase; BMD, bone mineral density; COMT, catechol-O-methyltransferase; EGCG, (–)-epigallocatechin-3-gallate; fl oz, fluid ounce; GTC, green tea catechin; GTE, green tea extract; MET-hour, metabolic equivalent hour; MGTT, Minnesota Green Tea Trial; SNS, sympathetic nervous system; VAT, visceral adipose tissue; %fat, percentage of fat.
References
- 1.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metz JA, Stern JS, Kris-Etherton P, Reusser ME, Morris CD, Hatton DC, Oparil S, Haynes RB, Resnick LM, Pi-Sunyer FX, et al. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: impact on cardiovascular risk reduction. Arch Intern Med 2000;160:2150–8. [DOI] [PubMed] [Google Scholar]
- 3.Hamman RF, Wing RR, Edelstein SL, Lachin JM, Bray GA, Delahanty L, Hoskin M, Kriska AM, Mayer-Davis EJ, Pi-Sunyer X, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006;29:2102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alissa EM, Alnahdi WA, Alama N, Ferns GA. Relationship between nutritional profile, measures of adiposity, and bone mineral density in postmenopausal Saudi women. J Am Coll Nutr 2014;33:206–14. [DOI] [PubMed] [Google Scholar]
- 5.Watts NB; GLOW Investigators. Insights from the Global Longitudinal Study of Osteoporosis in Women (GLOW). Nat Rev Endocrinol 2014;10:412–22. [DOI] [PubMed] [Google Scholar]
- 6.Ong T, Sahota O, Tan W, Marshall L. A United Kingdom perspective on the relationship between body mass index (BMI) and bone health: a cross sectional analysis of data from the Nottingham Fracture Liaison Service. Bone 2014;59:207–10. [DOI] [PubMed] [Google Scholar]
- 7.Zhao LJ, Jiang H, Papasian CJ, Maulik D, Drees B, Hamilton J, Deng HW. Correlation of obesity and osteoporosis: effect of fat mass on the determination of osteoporosis. J Bone Miner Res 2008;23:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu X, Ma X, Lu H, He W, Wang Z, Zhu S. Associations of fat mass and fat distribution with bone mineral density in pre- and postmenopausal Chinese women. Osteoporos Int 2011;22:113–9. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki N, Yano T, Nakazawa N, Yoshikawa H, Taketani Y. A possible role of estrone produced in adipose tissues in modulating postmenopausal bone density. Maturitas 1995;22:9–12. [DOI] [PubMed] [Google Scholar]
- 10.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 1999;140:1630–8. [DOI] [PubMed] [Google Scholar]
- 11.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, Stanczyk FZ, Stephenson HE Jr., Falk RT, Miller R, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst 2003;95:1218–26. [DOI] [PubMed] [Google Scholar]
- 12.Zhao LJ, Liu YJ, Liu PY, Hamilton J, Recker RR, Deng HW. Relationship of obesity with osteoporosis. J Clin Endocrinol Metab 2007;92:1640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wongdee K, Charoenphandhu N. Update on type 2 diabetes-related osteoporosis. World J Diabetes 2015;6:673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muraki S, Yamamoto S, Ishibashi H, Oka H, Yoshimura N, Kawaguchi H, Nakamura K. Diet and lifestyle associated with increased bone mineral density: cross-sectional study of Japanese elderly women at an osteoporosis outpatient clinic. J Orthop Sci 2007;12:317–20. [DOI] [PubMed] [Google Scholar]
- 15.Hodgson AB, Randell RK, Jeukendrup AE. The effect of green tea extract on fat oxidation at rest and during exercise: evidence of efficacy and proposed mechanisms. Adv Nutr 2013;4:129–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem 2011;22:1–7. [DOI] [PubMed] [Google Scholar]
- 17.Westerterp-Plantenga MS. Green tea catechins, caffeine and body-weight regulation. Physiol Behav 2010;100:42–6. [DOI] [PubMed] [Google Scholar]
- 18.Alvehus M, Buren J, Sjostrom M, Goedecke J, Olsson T. The human visceral fat depot has a unique inflammatory profile. Obesity (Silver Spring) 2010;18:879–83. [DOI] [PubMed] [Google Scholar]
- 19.Dawling S, Roodi N, Mernaugh RL, Wang X, Parl FF. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res 2001;61:6716–22. [PubMed] [Google Scholar]
- 20.Syvänen AC, Tilgmann C, Rinne J, Ulmanen I. Genetic polymorphism of catechol-O-methyltransferase (COMT): correlation of genotype with individual variation of S-COMT activity and comparison of the allele frequencies in the normal population and parkinsonian patients in Finland. Pharmacogenetics 1997;7:65–71. [DOI] [PubMed] [Google Scholar]
- 21.Kao YH, Hiipakka RA, Liao S. Modulation of endocrine systems and food intake by green tea epigallocatechin gallate. Endocrinology 2000;141:980–7. [DOI] [PubMed] [Google Scholar]
- 22.Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J. Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disord 2000;24:252–8. [DOI] [PubMed] [Google Scholar]
- 23.Samavat H, Dostal AM, Wang R, Bedell S, Emory TH, Ursin G, Torkelson CJ, Gross MD, Le CT, Yu MC, et al. The Minnesota Green Tea Trial (MGTT), a randomized controlled trial of the efficacy of green tea extract on biomarkers of breast cancer risk: study rationale, design, methods, and participant characteristics. Cancer Causes Control 2015;26:1405–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007;15:1473–83. [DOI] [PubMed] [Google Scholar]
- 25.Wang H, Wen Y, Du Y, Yan X, Guo H, Rycroft JA, Boon N, Kovacs EM, Mela DJ. Effects of catechin enriched green tea on body composition. Obesity (Silver Spring) 2010;18:773–9. [DOI] [PubMed] [Google Scholar]
- 26.Hursel R, Westerterp-Plantenga MS. Green tea catechin plus caffeine supplementation to a high-protein diet has no additional effect on body weight maintenance after weight loss. Am J Clin Nutr 2009;89:822–30. [DOI] [PubMed] [Google Scholar]
- 27.Maki KC, Reeves MS, Farmer M, Yasunaga K, Matsuo N, Katsuragi Y, Komikado M, Tokimitsu I, Wilder D, Jones F, et al. Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. J Nutr 2009;139:264–70. [DOI] [PubMed] [Google Scholar]
- 28.Stendell-Hollis NR, Thomson CA, Thompson PA, Bea JW, Cussler EC, Hakim IA. Green tea improves metabolic biomarkers, not weight or body composition: a pilot study in overweight breast cancer survivors. J Hum Nutr Diet 2010;23:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Auvichayapat P, Prapochanung M, Tunkamnerdthai O, Sripanidkulchai BO, Auvichayapat N, Thinkhamrop B, Kunhasura S, Wongpratoom S, Sinawat S, Hongprapas P. Effectiveness of green tea on weight reduction in obese Thais: a randomized, controlled trial. Physiol Behav 2008;93:486–91. [DOI] [PubMed] [Google Scholar]
- 30.Bhagwat S, Haytowitz DB, Holden JM. USDA Database for the Flavonoid Content of Selected Foods. Beltsville (MD): USDA; 2014 (Report 3). [Google Scholar]
- 31.Kanis JA; WHO Study Group. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. Osteoporos Int 1994;4:368–81. [DOI] [PubMed] [Google Scholar]
- 32. USDA, Human Nutrition Research Center. Continuing Survey of Food Intakes by Individuals 1994–96, 1998. Beltsville (MD): USDA; 2000. [Google Scholar]
- 33.Kriska AM. Modifiable Activity Questionnaire. Med Sci Sports Med 2002;29:S73–8. [Google Scholar]
- 34.Kokubo Y, Iso H, Saito I, Yamagishi K, Yatsuya H, Ishihara J, Inoue M, Tsugane S. The impact of green tea and coffee consumption on the reduced risk of stroke incidence in Japanese population: the Japan public health center-based study cohort. Stroke 2013;44:1369–74. [DOI] [PubMed] [Google Scholar]
- 35.Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr 2013;98(6, Suppl):1676S–81S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurgens TM, Whelan AM, Killian L, Doucette S, Kirk S, Foy E. Green tea for weight loss and weight maintenance in overweight or obese adults. Cochrane Database Syst Rev 2012;12:CD008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phung OJ, Baker WL, Matthews LJ, Lanosa M, Thorne A, Coleman CI. Effect of green tea catechins with or without caffeine on anthropometric measures: a systematic review and meta-analysis. Am J Clin Nutr 2010;91:73–81. [DOI] [PubMed] [Google Scholar]
- 38.Folsom AR, Kushi LH, Anderson KE, Mink PJ, Olson JE, Hong CP, Sellers TA, Lazovich D, Prineas RJ. Associations of general and abdominal obesity with multiple health outcomes in older women: the Iowa Women’s Health Study. Arch Intern Med 2000;160:2117–28. [DOI] [PubMed] [Google Scholar]
- 39.Chen D, Wang CY, Lambert JD, Ai N, Welsh WJ, Yang CS. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: structure-activity relationship and molecular-modeling studies. Biochem Pharmacol 2005;69:1523–31. [DOI] [PubMed] [Google Scholar]
- 40.Hodgson AB, Randell RK, Boon N, Garczarek U, Mela DJ, Jeukendrup AE, Jacobs DM. Metabolic response to green tea extract during rest and moderate-intensity exercise. J Nutr Biochem 2013;24:325–34. [DOI] [PubMed] [Google Scholar]
- 41.Lorenz M, Paul F, Moobed M, Baumann G, Zimmermann BF, Stangl K, Stangl V. The activity of catechol-O-methyltransferase (COMT) is not impaired by high doses of epigallocatechin-3-gallate (EGCG) in vivo. Eur J Pharmacol 2014;740:645–51. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki T, Yoshida H, Hashimoto T, Yoshimura N, Fujiwara S, Fukunaga M, Nakamura T, Yoh K, Inoue T, Hosoi T, et al. Case-control study of risk factors for hip fractures in the Japanese elderly by a Mediterranean Osteoporosis Study (MEDOS) questionnaire. Bone 1997;21:461–7. [DOI] [PubMed] [Google Scholar]
- 43.Miksicek RJ. Commonly occurring plant flavonoids have estrogenic activity. Mol Pharmacol 1993;44:37–43. [PubMed] [Google Scholar]
- 44.Nakagawa H, Wachi M, Woo JT, Kato M, Kasai S, Takahashi F, Lee IS, Nagai K. Fenton reaction is primarily involved in a mechanism of (-)-epigallocatechin-3-gallate to induce osteoclastic cell death. Biochem Biophys Res Commun 2002;292:94–101. [DOI] [PubMed] [Google Scholar]
- 45.Shen CL, Chyu MC, Yeh JK, Zhang Y, Pence BC, Felton CK, Brismee JM, Arjmandi BH, Doctolero S, Wang JS. Effect of green tea and Tai Chi on bone health in postmenopausal osteopenic women: a 6-month randomized placebo-controlled trial. Osteoporos Int 2012;23:1541–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Williams GA, Wang Y, Callon KE, Watson M, Lin JM, Lam JB, Costa JL, Orpe A, Broom N, Naot D, et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology 2009;150:3603–10. [DOI] [PubMed] [Google Scholar]
- 47.Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 2005;331:520–6. [DOI] [PubMed] [Google Scholar]
- 48.Mohiti-Ardekani J, Soleymani-Salehabadi H, Owlia MB, Mohiti A. Relationships between serum adipocyte hormones (adiponectin, leptin, resistin), bone mineral density and bone metabolic markers in osteoporosis patients. J Bone Miner Metab 2014;32:400–4. [DOI] [PubMed] [Google Scholar]
- 49.Liu K, Zhou R, Wang B, Chen K, Shi LY, Zhu JD, Mi MT. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr 2013;98:340–8. [DOI] [PubMed] [Google Scholar]
- 50.Wu AH, Spicer D, Stanczyk FZ, Tseng CC, Yang CS, Pike MC. Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormone levels in healthy postmenopausal women. Cancer Prev Res (Phila) 2012;5:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Astrup A, Toubro S, Cannon S, Hein P, Breum L, Madsen J. Caffeine: a double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers. Am J Clin Nutr 1990;51:759–67. [DOI] [PubMed] [Google Scholar]
- 52.Hill AM, Coates AM, Buckley JD, Ross R, Thielecke F, Howe PR. Can EGCG reduce abdominal fat in obese subjects? J Am Coll Nutr 2007;26:396S–402S. [DOI] [PubMed] [Google Scholar]
- 53.Dostal AM, Samavat H, Bedell S, Torkelson C, Wang R, Swenson K, Le C, Wu AH, Ursin G, Yuan JM, et al. The safety of green tea extract supplementation in postmenopausal women at risk for breast cancer: results of the Minnesota Green Tea Trial. Food Chem Toxicol 2015;83:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]