Abstract
The control of renal water excretion occurs in part by regulation of transcription in response to vasopressin in cells of the collecting duct. A systems biology-based approach to understanding transcriptional control in renal collecting duct cells depends on knowledge of what transcription factors and other regulatory proteins are present in the cells' nuclei. The goal of this article is to report comprehensive proteomic profiling of cellular fractions enriched in nuclear proteins from native inner medullary collecting duct (IMCD) cells of the rat. Multidimensional separation procedures and state-of-the art protein mass spectrometry produced 18 GB of spectral data that allowed the high-stringency identification of 5,048 proteins in nuclear pellet (NP) and nuclear extract (NE) fractions of biochemically isolated rat IMCD cells (URL: https://helixweb.nih.gov/ESBL/Database/IMCD_Nucleus/). The analysis identified 369 transcription factor proteins out of the 1,371 transcription factors coded by the rat genome. The analysis added 1,511 proteins to the recognized proteome of rat IMCD cells, now amounting to 8,290 unique proteins. Analysis of samples treated with the vasopressin analog dDAVP (1 nM for 30 min) or its vehicle revealed 99 proteins in the NP fraction and 88 proteins in the NE fraction with significant changes in spectral counts (Fisher exact test, P < 0.005). Among those altered by vasopressin were seven distinct histone proteins, all of which showed decreased abundance in the NP fraction, consistent with a possible effect of vasopressin to induce chromatin remodeling. The results provide a data resource for future studies of vasopressin-mediated transcriptional regulation in the renal collecting duct.
Keywords: aquaporin-2, LC-MS/MS, kidney, transcription, transcription factor, chromatin, mRNA splicing
the inner medullary collecting duct (IMCD) is the final site of transepithelial osmotic water transport along the renal tubule. Water moves into IMCD cells across the apical plasma membrane through the water channel protein, aquaporin-2; it moves out of the cells across the basolateral plasma membrane through two water channels, aquaporin-3 and aquaporin-4 (38). The amount of aquaporin-2 in the apical plasma membrane is generally considered to be the chief determinant of transepithelial water movement (52). Aquaporin-2 is regulated by the peptide hormone vasopressin, which binds to the basolateral plasma membrane and activates a signaling network that is dependent on a rise in intracellular cyclic AMP and generation of aperiodic spike-like increases in intracellular calcium (9, 14, 40, 51, 67). The amount of aquaporin-2 in the apical plasma membrane is controlled by vasopressin in two ways: 1) regulated trafficking to and from the apical plasma membrane (37); and 2) regulation of total aquaporin-2 protein abundance through changes in protein half-life (36, 46), translation (46), and transcription (15, 20). In mouse immortalized cortical collecting duct (mpkCCD) cells, long-term vasopressin exposure (0.1 nM for 5 days) elicited a 23-fold increase in aquaporin-2 protein abundance as measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS), which was associated with a 18-fold increase in aquaporin-2 mRNA abundance as measured using Affymetrix expression microarrays (29). Similarly in rat kidneys, there was a parallel increase in aquaporin-2 protein and mRNA in response to vasopressin administration (15). Thus, the increase in aquaporin-2 protein in collecting duct cells in response to vasopressin can be attributed largely to an increase in aquaporin-2 mRNA abundance. Although a role for regulation of aquaporin-2 mRNA stability cannot yet be ruled out as an explanation for the increase in mRNA abundance in response to vasopressin, accumulated evidence points to transcriptional regulation as the major point of control (34, 62).
Theoretically, regulation of transcription of the aquaporin-2 gene (Aqp2) could involve epigenetic mechanisms or differential binding/activation of transcription factors (or both). While there is thus far little evidence for the former, a number of studies have been published to address transcriptional control via transcription factors (62). In brief, these studies provide presumptive evidence for roles of members of a number of transcription factor subfamilies including AP1, AP2, CREB, ETS, FKHD, GATA, HOX, NFAT, RXR, and SF1 (19, 26, 33, 42, 55, 58, 65, 68). However, the complete transcriptional network that is responsible for the long-term effects of vasopressin in the collecting duct has not been resolved. A key step in this process is the identification of all transcription factors expressed in the collecting duct. Although we have proteomically profiled nuclei from cultured mpkCCD cells (49), comprehensive proteomic profiling has not yet been achieved in nuclear fractions of native collecting duct cells. The depth of previous proteomic profiling of nuclei from native rat IMCD cells, done 6 yr previously, was limited by the sensitivity of the tandem mass spectrometers available (55). Consequently, it was our goal in this study to carry out comprehensive proteomic profiling of nuclear proteins from native rat IMCD cells using three levels of fractionation to obtain comprehensive coverage. The three levels were: 1) isolation of nuclear pellet (NP) and nuclear extract (NE) fractions by a commercially available separation method; 2) use of SDS-PAGE to fractionate the NP and NE samples into 39 fractions each based on molecular weight; and 3) HPLC separation as a component of the LC-MS/MS system. Use of three levels of fractionation spreads out the delivery of tryptic peptides to the mass spectrometer over time, allowing a greater opportunity to detect low-abundance proteins. In total, we identified 5,048 proteins in the combination of the NP and NE fractions, including 369 transcription factors. Based on spectral counting, we identified a small subset of proteins in both fractions that appear to change in abundance in response to short-term treatment with the vasopressin analog, dDAVP.
METHODS
IMCD isolation and protein extraction.
The procedures used are outlined in Table 1. We euthanized 30 male Sprague-Dawley rats according to an approved National Heart, Lung, and Blood Institute (NHLBI) Animal Care and Use Committee protocol (H-0110R3). IMCD suspensions were prepared as previously described with slight modification (11). The kidney inner medullas were dissected, minced, and digested into suspensions by incubation at 37°C for 70–90 min in digestion solution (250 mM sucrose, 10 mM triethanolamine, pH 7.6) containing collagenase B (3 mg/ml; Roche, Indianapolis, IN) and hyaluronidase (3 mg/ml; Worthington, Lakewood, NJ). The resulting inner medullary suspension (whole IM) was subjected to three low-speed centrifugations (at 70 g, 20 s) to separate the IMCD-enriched fraction in the pellet from the non-IMCD fraction in the supernatant. The supernatants containing most of the non-IMCD elements were discarded. The final pellet was resuspended in tubule suspension solution containing (in mM) 118 NaCl, 5 KCl, 4 Na2HPO4, 25 NaHCO3, 2 CaCl2, 1.2 MgSO4, 5.5 glucose, and 5 sodium acetate (300 mOsm). This method has been previously shown to successfully isolate IMCD cells that are viable (12) and vasopressin-responsive (10). A previous study reported that ∼20% of the total protein is derived from non-IMCD elements, chiefly loops of Henle, vasa recta, and interstitial cells (57).
Table 1.
Summary of experimental procedures
| Procedure | Number of Objects |
|---|---|
| Euthanize rats | (10 rats) × 3 days |
| Isolate inner medullas | (20 inner medullas) × 3 days |
| Make IMCD suspensions | (1 suspension) × 3 days |
| Divide suspension into two samples | (2 samples) × 3 days |
| Incubate one sample with dDAVP and one sample with vehicle | (1 dDAVP sample + 1 vehicle sample) × 3 days |
| Fractionate each sample to obtain NE and NP fractions | (1 dDAVP/NE sample + 1 dDAVP/NP sample +1 vehicle/NE sample + 1 vehicle/NP sample) × 3 days |
| Pool samples | 1 dDAVP/NE sample + 1 dDAVP/NP sample +1 vehicle/NE sample + 1 vehicle/NP sample |
| Run SDS-PAGE for each pooled sample | 4 half-gels |
| Slice gels | 39 slices × (1 dDAVP/NE + 1 vehicle/NE) +36 slices × (1 dDAVP/NP + 1 vehicle/NP) |
| In gel trypsinization | 150 samples |
| LC-MS/MS | 150 samples |
See text for details.
The suspended IMCD cells were divided into two volumes and were equilibrated under 95% air/5% carbon dioxide at 37°C for 10 min. To the first aliquot, we added dDAVP (1 nM final concentration for 30 min), and to the second aliquot, we added the same volume of pure suspension fluid, the vehicle (also incubated 30 min). From this point, we followed the fractionation procedures described by Tchapyjnikov et al. (55). In brief, the cells were homogenized (Potter-Elvehjem homogenizer on ice for a total of 2 min in 15 s intervals), centrifuged (1,000 g in 250 mM sucrose buffer containing 10 mM triethanolamine, pH 7.6) with Halt protease/phosphatase inhibitor cocktail (Life Technologies, #78440) to obtain a pellet enriched in nuclei and unbroken cells, and subjected to further fractionation using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Life Technologies, #78835) to obtain fractions labeled NE (nuclear extract), NP (nuclear pellet), and NN (nonnuclear fraction). Total protein concentration was measured (BCA assay, Life Technologies, #23227).
SDS-PAGE and in-gel digestion.
The protein samples (NE and NP with dDAVP and control for each) were concentrated using Amicon Centrifugal Filters, Ultracel 3K (Millipore) to about 400 μg in 110 μl of solution. A 5× concentrate of Laemmli solution (7.5% SDS, 30% glycerol, 50 mM Tris, pH 6.8) with bromophenol blue (1 part 5× concentrate: 4 parts protein sample) was added to the samples. One-dimensional SDS-PAGE was performed using 12% polyacrylamide SDS-Tris-Glycine gels (Bio-Rad). Separate gels were run for the NE and NP fractions, with the control and dDAVP samples being run on different lanes in the same gel. Molecular weight markers (Precision Plus, Bio-Rad) were run in separate lanes. The gels were run for 45 min at 3.00 A and 200 V and were then stained with Imperial protein stain (Thermo Scientific). The gels were destained for 30 min in MS-grade water (JT Baker #JTB-9831-02) and then sliced into 39 equal-sized pieces for the NE fraction or 36 equal-sized pieces for the NP fraction. The slicing used a paper pattern and a razor blade. Each gel piece was diced into 1.5 mm3 blocks with a razor blade. This gave 150 separate samples for LC-MS/MS analysis (39 gel slices × 2 treatments for NE; 36 × 2 treatments for NP). These samples underwent reduction with DTT, alkylation, and in gel trypsinization as described (41) except for the use of 12.5 ng/μl Trypsin Gold (Promega).
After digestion, the peptides were extracted from the gel pieces by four successive washes in 50% ACN/0.5% formic acid. Samples were dried and peptides were then suspended in 0.1% formic acid in MS-grade water. Samples were desalted using C-18 spin columns (Thermo Scientific, #89870) according to the protocol provided with the kit. The resulting samples were dried (Speed-Vac) for storage. Immediately prior to MS analysis, the samples were redissolved in 0.1% formic acid in MS-grade water.
LC-MS/MS.
All samples were analyzed on a nanoflow LC system (Eksigent, Dublin, CA) coupled to a tandem mass spectrometer (Orbitrap Velos Pro Hybrid, Thermo Scientific, San Jose, CA). The sample loading onto a peptide trap cartridge (Agilent Technologies, Palo Alto, CA) occurred at a flow rate of 6 μl/min. The trapped peptides were then fractionated with a reversed-phase PicoFrit column (New Objective, Woburn, MA) using a linear gradient of 5–35% ACN in 0.1% FA. The gradient time was 45 min at a flow rate of 0.25 μl/min. Precursor mass spectra (MS1) were acquired in the Orbitrap at 60,000 resolution and product mass spectra (MS2) were acquired with the ion trap.
To maximize the number of peptide identifications, three algorithms were used to match spectra to peptides, viz. those coded by Mascot (48), SEQUEST (66), and InsPecT (54). The posttranslational modifications allowed were a fixed carbamidomethyl modification on cysteine, variable deamination modifications on asparagine and glutamine, and a variable oxidation modification on methionine. False discovery rate (FDR) at a peptide level was set to 0.01 based on target-decoy analysis (16). To identify ambiguous identifications, we used an in-house program (coded in Java) called ProMatch (55) (http://esbl.nhlbi.nih.gov/Bioinformatic%20Tools.htm). Peptides that only matched to one gene symbol were extracted as a “unique” identification. The peptides that matched to more than one gene symbol were separated as “multiple” identifications. To reconcile the multiple identifications, transcriptomic data from Affymetrix array profiling of rat renal IMCD transcripts was used (57). (Database available at: http://dir.nhlbi.nih.gov/papers/lkem/imcdtr/). If a given gene was not expressed, based on the transcriptomic data (median normalized value < 0.4), its protein product was dropped from consideration. The SEQUEST and Mascot searches were executed within Proteome Discoverer, and peptides with FDR < 0.01 and peptide rank = 1 were retained for further analyses. The InsPecT search was carried on the Biowulf Linux Cluster at the National Institutes of Health (http://biowulf.nih.gov). Raw files (18 GB), search results and all spectra have been uploaded to the ProteomeXchange Consortium (60) via the PRIDE partner repository with the dataset identifier PXD002680. These data are accessible at http://www.ebi.ac.uk/pride/archive/.
Spectral counting.
The number of peptide matches for a given gene symbol were counted and designated as the “spectral count” for that gene symbol. This was the sum of all spectra matching to the gene symbol in all gel slices. The spectral counts in the control samples and the dDAVP samples were compared using the Fisher exact test with the contingency table consisting of the control count, dDAVP count, total count of all peptides in control, and total count of all peptides in dDAVP. For this analysis, peptides that matched to more than one gene symbol were assigned to the gene symbol with the most abundant transcript in the IMCD based on prior data (57).
Bioinformatic analysis.
To assess the apparent molecular weights of each protein identified, we mapped the mass spectrometry output back to the gel slices and then used an in house Java-based software tool, virtualBlot (64), to generate gray-scale images resembling a Western blot for each protein (https://helixweb.nih.gov/ESBL/virtualBlot/). A general classification of protein groups present in the NP and NE fractions was done using the DAVID tool [Database for Annotation, Visualization and Integrated Discovery, version 6.7, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), Bethesda, MD] (27). The software tool ABE (Automated Bioinformatics Extractor, Systems Biology Center, NHLBI, Bethesda, MD) was used to extract Gene Ontology terms without statistical analysis (http://helixweb.nih.gov/ESBL/ABE/). The relationships among proteins that underwent changes in spectral counts in response to dDAVP were identified using STRING Version 10 (http://string-db.org/) (53). This program generates undirected graphs showing functional groups of proteins as clusters or subnetworks. The graphs were visualized using Cytoscape (http://www.cytoscape.org/). A list of transcription factors present in the NE and NP fractions was generated through comparison with a list of all 1,371 transcription factors in the rat genome (URL: http://bioguo.org/AnimalTFDB/).
RESULTS
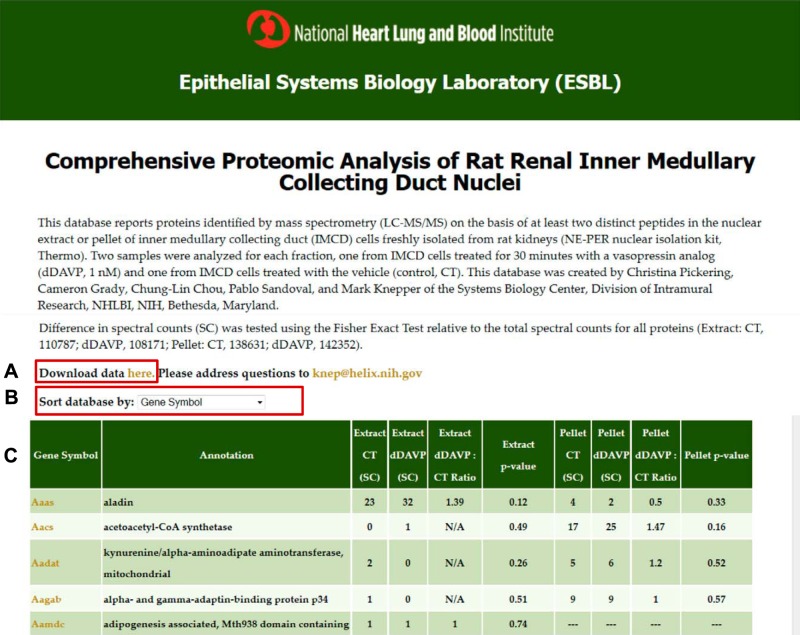
LC-MS/MS analysis of NE and NP fractions from native rat IMCD cells revealed a total of 8,266 proteins with at least one peptide identified and a total of 5,048 proteins when limited to those with two or more unique peptides (peptide FDR was 0.01 using target-decoy approach as described in methods). To provide user-friendly access to the data, we have created a data webpage that lists the proteins identified along with spectral counting data (https://helixweb.nih.gov/ESBL/Database/IMCD_Nucleus/) (Fig. 1). Among the proteins identified from two or more distinct peptides, 2,669 were found in both NE and NP, 1,379 were uniquely found in the NE, and 1,000 were uniquely found in the NP. The webpage also shows the spectral counting data for both vasopressin (dDAVP)-treated and vehicle-treated samples, providing the results of statistical testing using the Fisher exact test to address the likelihood that observed differences in dDAVP versus vehicle counts could have been due to chance alone. We discuss these results further below. Finally, the webpage provides images (“virtual Western blots”) showing the mapping of the MS data to the gel slices from which they were cut (available by clicking on the Gene Symbols).
Fig. 1.
Screenshot of webpage. The user may download the full dataset (A) or sort by different attributes (B). For each protein, the webpage gives several elements of information (C): official gene symbol, annotation (protein name), spectral counts in nuclear extract (NE) fraction for control (CT) and dDAVP conditions, ratio of spectral counts (dDAVP:CT) for NE fraction, P value for NE (Fisher exact test), spectral counts in nuclear pellet (NP) fraction CT and dDAVP conditions, ratio of spectral counts (dDAVP:CT) for NP fraction, P value for NP (Fisher exact test). (For the NE and NP fractions, the P values represent the probability that a difference in spectral counts between control and dDAVP samples could have been obtained by random selection from all spectra found in the respective fraction.) Virtual Western blots may be viewed by clicking on the Gene Symbols for individual proteins. This webpage may be accessed at https://helixweb.nih.gov/ESBL/Database/IMCD_Nucleus/.
Virtual Western blots.
Several examples of virtual western blots are shown in Fig. 2. These blots show data for selected nuclear proteins likely to be relevant to the regulation of Aqp2 transcription. The virtual Western blots show the relative abundances of specific proteins in each of the 39 gel slices from NE and NP fractions (Fig. 2), comparing them to the positions of marker proteins (left) and the calculated molecular weights based on a summation of the residue masses of the component amino acids (right). The calculated molecular weights were extracted from corresponding RefSeq protein records. (Relative abundances are calculated as the sum of the areas under reconstructed chromatograms for all peptides mapping to a given gene symbol for vehicle-treated samples.) Four of six proteins shown map to gel slices consistent with the calculated molecular weight. Three of these proteins (Hdac1, Hist3h3, and Jund) show broad “bands” that spread upward from the calculated molecular weight, presumably because of posttranslational modifications. Two of the proteins have prominent bands at molecular weights below the calculated molecule weight, viz. NF-κB and protein kinase N1 (Fig. 2, last two panels). Both are known to be cleaved physiologically to produce proteins of lower molecular weight. NF-κB (Nfkb1) with calculated molecular weight of 105.5 kDa (p105) is partially proteolyzed by the proteasome to produce p50 (see Uniprot record Q63369.1). Pkn1 (also known as protease-activated kinase 1) with calculated molecular weight of 103.3 kDa is cleaved at three sites along its length (Uniprot record Q63433.2).
Fig. 2.
Examples of virtual Western blots. Each virtual western blot shows protein markers (1st lane), location of protein in gel for NE fraction (2nd lane), location of protein in gel for NP fraction (3rd lane), and calculated molecular weight of protein based on amino acid composition (4th lane).
Gene enrichment analysis.
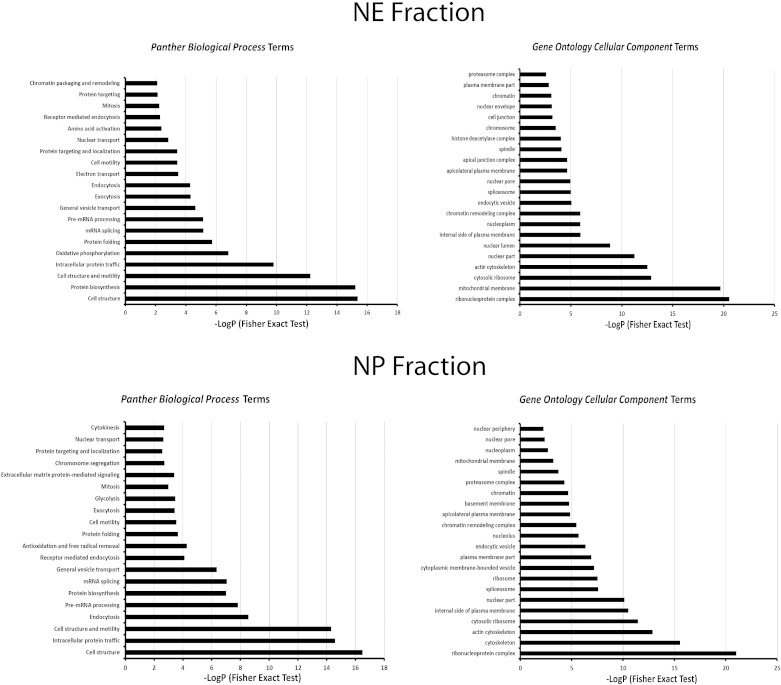
To characterize the proteins found in this study, we extracted Panther biological process terms and Gene Ontology cellular component terms for proteins in the NE and NP fractions (Fig. 3). The background gene list for this analysis was the list of all transcripts detected above threshold in rat IMCD using Affymetrix expression arrays (57). The analysis showed that several protein populations associated with nuclei were enriched in the NE and NP datasets. For example, Panther biological process terms enriched for the NE fraction included “chromatin packaging and remodeling,” “nuclear transport,” “pre-mRNA processing,” “mRNA splicing,” and “protein biosynthesis.” However, it also revealed enrichment of terms consistent with the presence of mitochondrial proteins, endosomal proteins and plasma membrane proteins in the NE fraction. Similarly, Panther biological process terms enriched in the NP fraction included terms consistent with enrichment of nuclear proteins including “cytokinesis,” “nuclear transport,” “chromosome segregation,” “mitosis,” “mRNA splicing,” “protein biosynthesis,” and “pre-mRNA processing”. But again, several of the enriched terms are consistent with nonnuclear structures being present in the analyzed fractions. The presence of nonnuclear structures is predictable from prior data showing the enrichment of marker proteins of the mitochondrion and the basolateral plasma membrane in the pellet from 1,000 g centrifugation of homogenates from mouse mpkCCD cells (63).
Fig. 3.
Enriched Panther biological process terms and Gene Ontology cellular component terms for proteins in the NE fraction (top) and NP fraction (bottom). The background gene list for this analysis was the list of all transcripts detected above threshold in rat inner medullary collecting duct (IMCD) using Affymetrix expression arrays (see text). Analysis was done using the DAVID online tool (Database for Annotation, Visualization and Integrated Discovery version 6.7; NIAID, NIH, Bethesda, MD). The Fisher exact tests compared the fraction of proteins in the NE or NP list associated with a given term to the fraction of proteins coded by the full IMCD transcriptome with that term. The P values report the probability that the fraction in the NE or NP list would be seen by random selection from the transcriptome list.
Expansion of the known proteome of the rat IMCD.
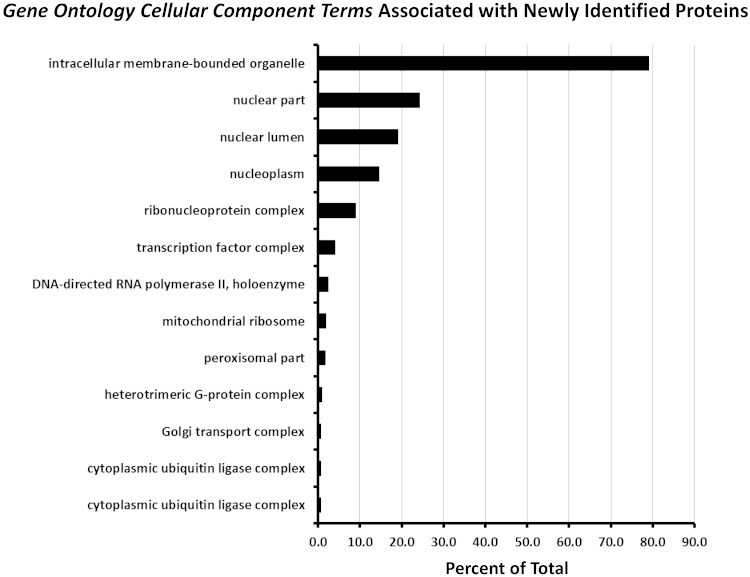
Previous proteomic studies in native rat IMCD cells (1, 2, 6, 21–25, 39, 45, 50, 55, 56, 59, 69, 70) have identified a total of 6,779 proteins (Supplementary Dataset 1).1 The present study found 1,511 proteins (identified with two or more distinct peptides) that were not previously identified by mass spectrometry in rat IMCD cells, resulting in a total of 8,290 proteins identified in all studies up to this one. This number exceeds the number of transcripts identified in microdissected IMCDs by RNA-seq analysis (with RPKM>1), viz. 6,920 transcripts (32). It is close to the number of transcripts detected above background in Affymetrix expression arrays in biochemically purified rat IMCD suspensions, viz. 7,913 (57). Hence, with the newly added data, we have now probably identified nearly all the expressed genes in the rat IMCD. We classified the newly identified proteins in this study based on Gene Ontology cellular component terms (Fig. 4). The largest fraction of newly identified proteins were associated with the terms “intracellular membrane-bounded organelle,” “nuclear part,” “nuclear lumen,” and “nucleoplasm” in accord with the goals of this study. Figure 4 also identifies “transcription factor complex” as an important category of new proteins.
Fig. 4.
Classification of the newly identified proteins in this study based on enriched Gene Ontology cellular component terms. Analysis was done using the online tool DAVID. The graph shows all Gene Ontology cellular component terms with P value < 0.01 (Fisher exact test comparing the fraction of proteins in the newly identified list associated with a given term to the fraction of proteins coded by the full IMCD transcriptome with that term). Horizontal axis shows the percent of the total number of newly identified proteins with a given term.
Transcription factors in rat IMCD.
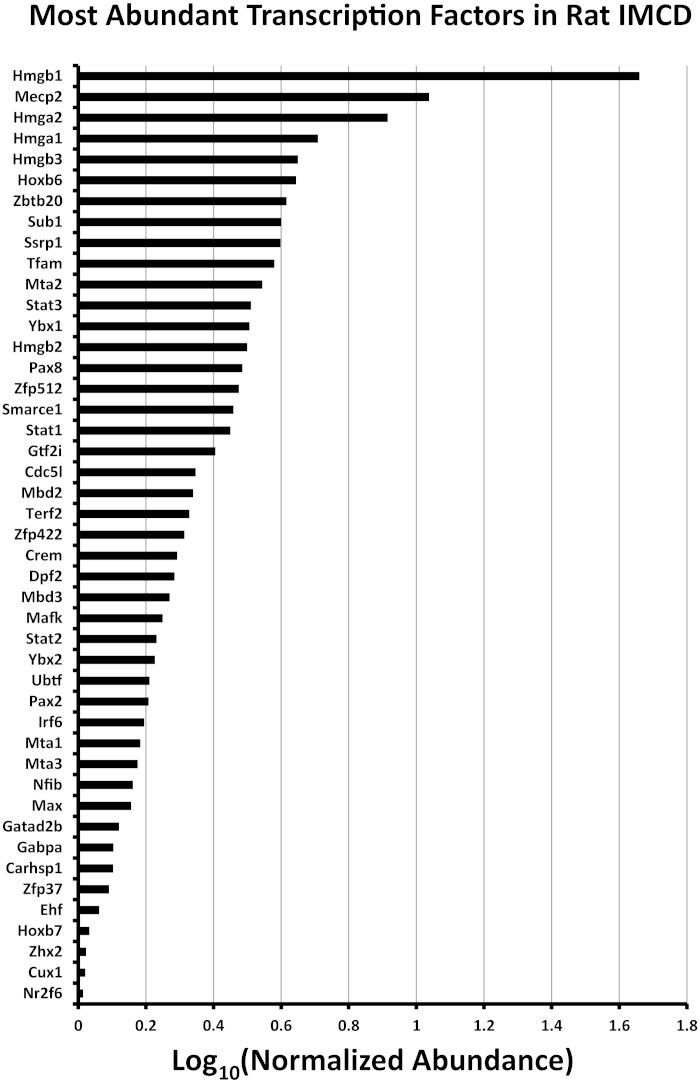
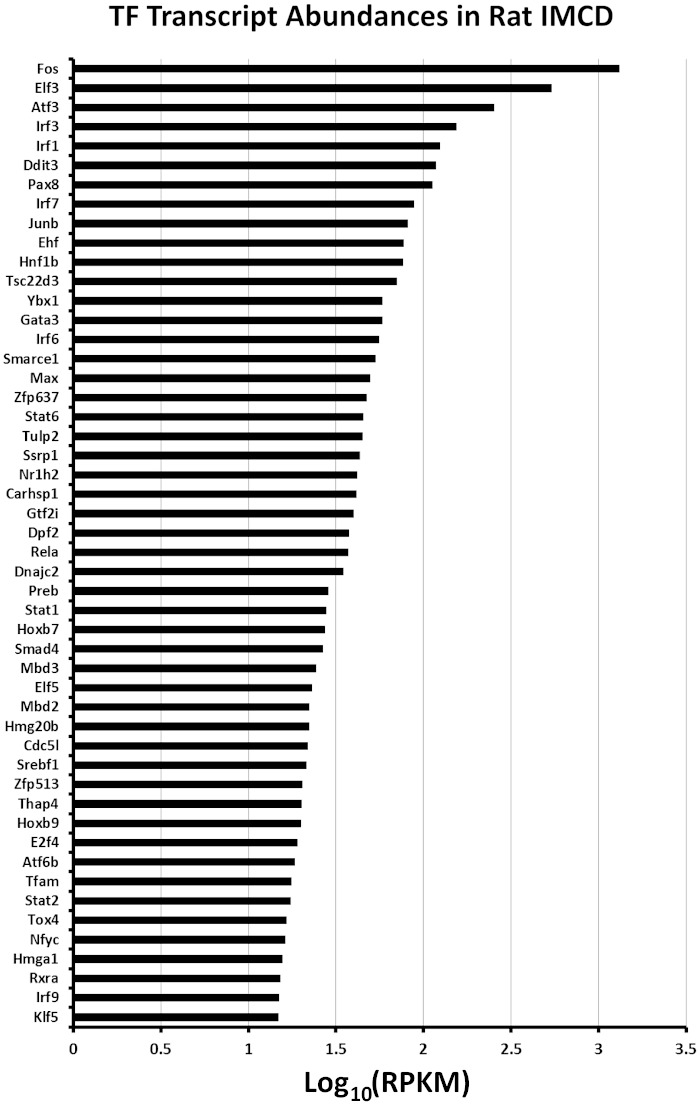
Of the 1,371 transcription factors in the rat genome (URL: bioguo.org/AnimalTFDB/), 369 were found in native IMCD cells in this study. These are listed on an ancillary webpage (https://helixweb.nih.gov/ESBL/Database/IMCD_Nucleus/NE_NP_Webpage_TF.html). By comparison, RNA-seq analysis of microdissected rat IMCDs showed 501 transcription factors expressed at an RPKM of ≥1.0 (32). Figure 5 shows a ranking of the most abundant transcription factors from the point of view of normalized spectral counts, a measure of relative protein abundance in the isolated fractions. Figure 6 shows the top 50 from the perspective of mRNA abundance. A comparison of Figs. 5 and 6 shows that relative protein abundance and relative mRNA abundance were not concordant as has been observed previously in a number of cell types (18, 28, 29, 61). The poor correlation for transcription factors is owing to the broad distribution of protein half-lives among transcription factors in collecting duct cells (46). Indeed, the most abundant transcription factor proteins such as the High Mobility Group proteins near the top of Fig. 5 have very long half-lives, typically > 40 h (46). In contrast, many of the transcription factors with high transcript levels (Fig. 6), such as the AP1 transcription factors, have half-lives that are < 10 h (46).
Fig. 5.
Ranking of the most abundant transcription factors identified in this study from the point of view of relative protein abundance. To generate this graph, spectral counts were normalized by the calculated molecular weights of proteins to provide a measure of protein abundance. Transcription factors are identified by their official gene symbols (vertical axis).
Fig. 6.
Ranking of the most abundant transcription factors (TF) identified in this study from the point of view of transcript abundance. Transcript abundance values in RPKM (reads per kilobase of transcript per million mapped reads) for isolated rat IMCD segments are taken from https://helixweb.nih.gov/ESBL/Database/NephronRNAseq/All_transcripts.html. TFs are identified by their official gene symbols (vertical axis).
Vasopressin-responsive proteins in NP and NE fractions.
Although the main goal of this study was to catalog nuclear proteins expressed in native rat IMCD cells, we chose to analyze NE and NP samples in the presence and absence of 30-min exposure to the V2-selective vasopressin analog dDAVP (1 nM). Protein groups that show apparent changes with vasopressin provide mechanistic clues (i.e., prior data) for future studies of vasopressin action.
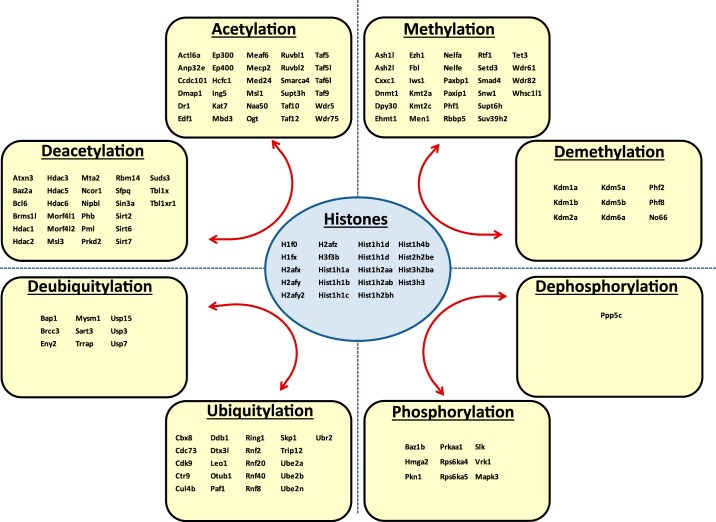
Figure 7 shows the proteins that were significantly altered in abundance in the NP fraction in response to dDAVP (P < 0.005, Fisher exact test) with upregulated proteins shown as green nodes and downregulated proteins as red nodes. These proteins were clustered into groups of related proteins represented as a network generated using the STRING online tool (http://string-db.org/). Out of the 99 vasopressin-regulated NP proteins uploaded to STRING, only 17 lacked connections to any other protein on the list and hence do not appear in Fig. 7. As can be noted, the network generated by STRING is very dense with an edge-to-node ratio of 1.73 (number of edges, 172; number of nodes, 99). By contrast, five random groups of 99 proteins from the full list of 5,048 proteins identified in this study generated STRING networks of lower edge-to-node ratios [0.808, 0.374, 0.616, 0.475, 0.455; 95% confidence interval (0.204, 0.887)], indicating that the dense nature of the vasopressin regulated NP network was unlikely to be due to chance and is therefore likely to represent coordinated physiological changes. Of particular interest was the group labeled “chromatin,” which includes several histones that appear to decrease in abundance in the NP fraction in response to dDAVP. This finding points to the possibility that the transcriptional response to vasopressin in these cells could be in part due to epigenetic changes involving histone modifications that alter nucleosomes to provide access to transcription factors. The multiple histones that decrease in the NP fraction in response to dDAVP contrasts with the lack of transcription factor proteins that exhibit changes in abundance. Figure 8 shows the proteins identified in this study that are involved in chromatin remodeling identified by GO term analysis. This list includes all protein with molecular function or biological process GO terms containing the word “histone” and not the word “binding.” The list includes predominantly proteins involved in posttranslational modification of histones including acetylation (93 proteins), methylation (37 proteins), phosphorylation (10 proteins), and ubiquitylation (30 proteins), as well as 19 histone proteins.
Fig. 7.
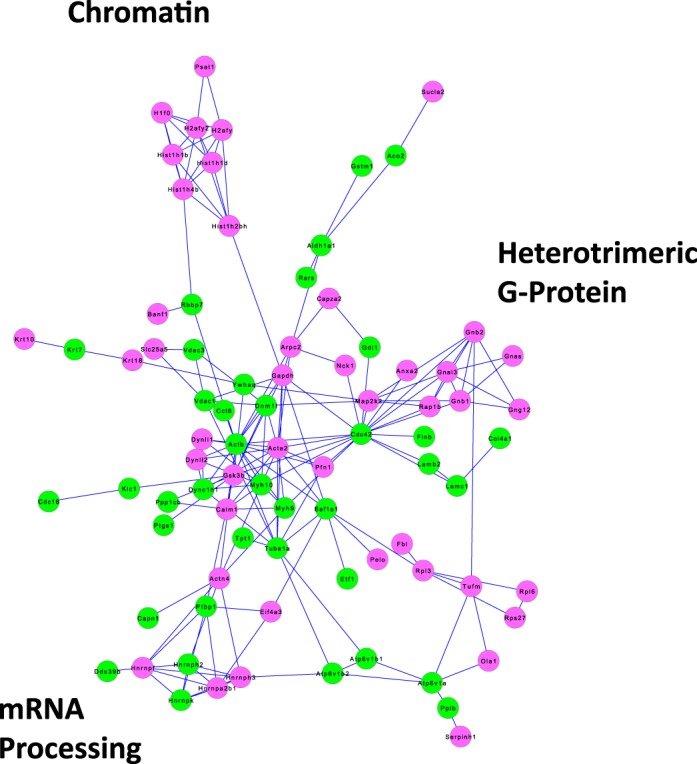
Protein groups regulated by vasopressin in NP fraction of rat IMCD. The network was generated in STRING by inputting gene symbols corresponding to the 99 proteins found to be increased or decreased in the NP fraction in response to a 30 min dDAVP incubation at 1 nM (P < 0.005, Fisher exact test). Those that were increased are designated by green nodes; those that were decreased by red nodes. Proteins are designated by their official gene symbols. Key protein groups are indicated in the margin.
Fig. 8.
Chromatin-modifying proteins identified in nuclear fractions of rat IMCD cells. The list includes all protein identified in this study with molecular function or biological process terms containing the word “histone” and not the word “binding.” In the histone “acetylation” category, only the 30 most abundant are listed; the remainder (in order of abundance) are Mcrs1, Brd4, Cdyl, Tada3, Ing3, Brd1, Kat8, Tex30, Mbip, Ing4, Kat5, Kansl3, Myog, Kansl2, Taf1, Mgea5, Brd8, Brpf3, Kat6a, Epc1, Sap130, Phf20, Brpf1, Brca2.
Figure 9 shows the proteins that were significantly altered in abundance in the NE fraction in response to dDAVP (P < 0.005, Fisher exact test). Out of the 88 vasopressin-regulated NE proteins uploaded to STRING for the NE fraction, only 14 lacked connections to any other protein on the list (These do not appear in Fig. 9). Again, the network generated by the STRING online tool was very dense with an overall edge-to-node ratio of 2.65 (233 edges, 88 nodes), suggesting that vasopressin is coordinately regulating specific groups of protein with shared function. Of particular note in Fig. 9 is the category called “mRNA processing,” which includes a large number of proteins involved with mRNA splicing and transport. If vasopressin produces an overall increase in transcription in native IMCD cells, then it seems possible that it also produces a regulated increase in mRNA splicing and transport, providing a hypothesis for future studies.
Fig. 9.
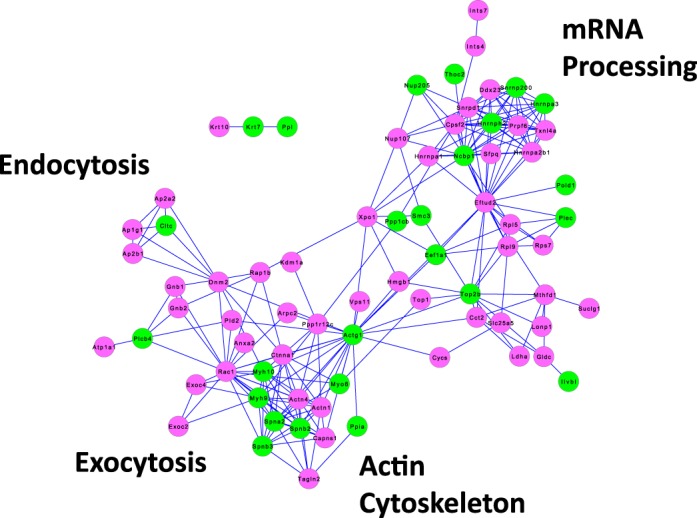
Protein groups regulated by vasopressin in NE fraction of rat IMCD. The network was generated in STRING by inputting gene symbols corresponding to the 88 proteins found to be increased or decreased in the NE fraction in response to a 30 min dDAVP incubation at 1 nM (P < 0.005, Fisher exact test). Those that were increased are designated by green nodes; those that were decreased by red nodes. Proteins are designated by their official gene symbols. Key protein groups are indicated in the margin.
In a prior study (49), we used quantitative LC-MS/MS techniques to identify proteins that undergo vasopressin-induced abundance changes in NE and NP fractions isolated from mouse mpkCCD cells, a cell line that mimics many features of renal collecting duct cells. As in the present study, the observations in mpkCCD cells were made after a 30 min exposure to the vasopressin analog dDAVP or to its vehicle. Table 2 lists the 17 proteins that underwent significant abundance changes in the NE fraction in the present study and were also reported to show significant changes the NE of mpkCCD cells. All but one of these proteins (Rap1b) underwent increases in abundance consistent with translocation into the nucleus in response to dDAVP exposure. Two of these proteins, myosin light chain kinase (Mylk) and protein phosphatase PP1-beta (PPP1cb), are involved in intracellular signaling via changes in protein phosphorylation and potentially are involved in the observed changes in vasopressin-stimulated changes in phosphorylation of nuclear proteins (5). Table 3 shows the six proteins that underwent significant abundance changes in the NP fraction in the present study and were also reported to show significant changes the NP fraction of mpkCCD cells. One of these proteins, Ahnak, was increased both in the NP and NE fraction, consistent with the possibility that it translocases into the nucleus in response to vasopressin. Ahnak is a giant, 5,840-amino acid, highly phosphorylated PDZ domain-containing nuclear protein (also called “Desmoyokin”) that plays roles as a scaffold protein in cell signaling (13). In a previous phosphoproteomics study conducted in suspensions of rat IMCDs, we found coordinated changes in phosphorylation of Ahnak in response to dDAVP with decreases at Ser4506 and Ser4932 and increases at Thr3749, Ser4962, and Ser4973 (21). These phosphorylation sites are just NH2-terminal to the nuclear localization signal domains of Ahnak, raising the possibility that they play a role in differential translocation of Ahnak in response to vasopressin.
Table 2.
Proteins that underwent significant abundance changes in the NE fraction of rat IMCD in the present study and in the NE fraction of mouse mpkCCD cells in a prior study
| This Study |
Schenk et al.* |
||||
|---|---|---|---|---|---|
| Gene Symbol | Annotation | Log2 (dDAVP/Vehicle) | P | Log2 (dDAVP/Vehicle) | P |
| Rap1b | ras-related protein Rap-1b precursor | −0.494 | 0.0015 | −0.636 | 0.0066 |
| Sptan1 | spectrin alpha chain, nonerythrocytic 1 | 0.163 | 0.0003 | 0.529 | 0.0473 |
| Sptbn2 | spectrin beta chain, nonerythrocytic 2 | 0.176 | 0.0013 | 0.746 | 0.0222 |
| Rdx | radixin | 0.176 | 0.0113 | 0.352 | 0.0117 |
| Ahnak | neuroblast differentiation-associated protein AHNAK | 0.202 | 0.0000 | 0.733 | 0.0068 |
| Ppl | periplakin | 0.322 | 0.0000 | 0.956 | 0.0103 |
| Cltc | clathrin heavy chain 1 | 0.367 | 0.0038 | 0.839 | 0.0438 |
| Nup98 | nuclear pore complex protein Nup98-Nup96 proprotein | 0.367 | 0.0333 | 0.199 | 0.0163 |
| Mylk | myosin light chain kinase, smooth muscle | 0.485 | 0.0309 | 0.635 | 0.0092 |
| Mllt4 | afadin | 0.496 | 0.0134 | 0.866 | 0.0365 |
| Hdlbp | vigilin | 0.536 | 0.0479 | 0.272 | 0.0346 |
| Rad21 | double-strand-break repair protein rad21 homolog | 0.546 | 0.0418 | 0.553 | 0.0492 |
| Ppp1cb | serine/threonine-protein phosphatase PP1-beta catalytic subunit | 0.566 | 0.0000 | 0.314 | 0.0247 |
| Arid1b | AT-rich interactive domain-containing protein 1B isoform ×3 | 0.722 | 0.0410 | 0.675 | 0.0289 |
| Rad50 | DNA repair protein RAD50 | 0.816 | 0.0330 | 0.715 | 0.0497 |
| Uaca | uveal autoantigen with coiled-coil domains and ankyrin repeats | 0.895 | 0.0108 | 0.919 | 0.0332 |
| Anapc7 | anaphase-promoting complex subunit 7 | 1.064 | 0.0245 | 0.183 | 0.0417 |
Prior study is Schenk et al. (49). Significance was based on 2-factor analysis.
Table 3.
Proteins that underwent significant abundance changes in the NP fraction of rat IMCD in the present study and in the NP fraction of mouse mpkCCD cells in a prior study
| This Study |
Schenk et al.* |
||||
|---|---|---|---|---|---|
| Gene Symbol | Annotation | Log2 (dDAVP/Vehicle) | P | Log2 (dDAVP/Vehicle) | P |
| Glyr1 | putative oxidoreductase GLYR1 | −0.36 | 0.03203 | −0.15 | 0.02767 |
| Plec | plectin isoform 1 | 0.14 | 0.02756 | 0.69 | 0.00252 |
| Ahnak | neuroblast differentiation-associated protein AHNAK | 0.40 | 0.00002 | 0.70 | 0.00562 |
| Rsu1 | ras suppressor protein 1 | 0.44 | 0.02226 | 0.24 | 0.00228 |
| Arpc2 | actin-related protein 2/3 complex subunit 2 | 0.54 | 0.00059 | 0.61 | 0.04275 |
| Cggbp1 | CGG triplet repeat-binding protein 1 | 3.00 | 0.02147 | 0.38 | 0.02680 |
See Schenk et al. (49). Significance was based on 2-factor analysis.
DISCUSSION
This study is part of a bigger project to use the principles of systems biology to identify the transcriptional network responsible for cell-specific gene expression in collecting duct cells, as well as the basis of vasopressin-mediated regulation of Aqp2 gene expression. The concept underlying systems biology is to study all genes and gene products simultaneously in parallel to identify physiological processes (30). Some of the fundamental tools for data acquisition are protein mass spectrometry (as in this study), oligonucleotide microarrays for transcriptomics as carried out by Uawithya et al. (57) and Khositseth et al. (29), and next-generation sequencing to identify transcriptomes as carried out by Lee et al. (32). The problem can be conceptualized as consisting of two steps: 1) identification of the gene products present in the cell type of interest (here the native IMCD cell); and 2) targeted experiments to sort out the gene products that play specific roles (here, transcriptional regulation of Aqp2 and other genes in the IMCD in response to vasopressin). This paper addresses the former step. A complete or nearly complete accounting of all proteins expressed in the cell type of interest is crucial to the epistemological approach used in systems biology (31). The basic idea is to pose a question and then list all possible (or likely) answers to the question, followed by experiments to prioritize the list. In the immediate context, the questions are “What transcription factor(s) are responsible for cell type-specific gene expression in the IMCD cell?” and “What transcription factor(s) are responsible for vasopressin-mediated regulation of Aqp2 gene transcription?”. Before the present study, the list of possible answers can be said to have contained all 1,371 transcription factors coded by the rat genome. After the current study, the list contains 394 transcription factors including all 369 found in this study plus an additional 42 found in our prior study (55). Although 394 transcription factors may be a somewhat intimidating list, we have eliminated almost 1,000 candidates.
How can this transcription factor list be narrowed down further to address which ones play roles in vasopressin-mediated regulation of gene expression in collecting duct cells? A substantial amount of relevant information already exists, such as DNA sequence-binding preferences for the individual transcription factors (7), prior evidence for regulation of various transcription factors by vasopressin (47), and gene expression lists for collecting duct cell lines, especially the mpkCCD cell line (5, 29, 43, 44, 46, 49, 68). Information from such datasets can, in principle, be integrated using Bayes' rule as done previously for protein kinases in the IMCD (6). Beyond this, new methodologies are now available for the study of physiological control of transcription by selectively deleting transcription factors via genome editing approaches (17) and genome-wide mapping of the binding of DNA sequence-specific transcription factors to regulatory sites on DNA using chromatin immunoprecipitation coupled to deep sequencing (ChIP-seq) (4, 35). Ultimately, it is likely that transcriptional control in the IMCD cell will be found to be mediated by ensembles of transcription factors working in combinatorial fashion (8) rather than one or two working alone. Thus, many of the 394 transcription factors expressed in the IMCD may be involved.
Although the main objective of this work was to proteomically profile IMCD cells, we opted to include replicates with and without vasopressin with a view toward identification of groups of co-regulated proteins. Such groups would be expected to point to specific cellular processes that may be regulated by vasopressin. When the 99 vasopressin-regulated proteins in the NP fraction and the 88 vasopressin-regulated proteins in the NE fraction were input into the STRING algorithm to identify groups of functionally related proteins, we obtained two exceptionally dense networks (Figs. 7 and 9). The resulting graphs pointed to particular protein groups that appear to be regulated by vasopressin. The high density of the NP and NE networks is compatible with coordinated regulation of specific protein groups. Of particular interest in the NP network (Fig. 7) was a group of “chromatin”-related proteins including seven different histones, all of which show decreased abundance in response to vasopressin. This finding raises the possibility that vasopressin, acting through its signaling network, broadly stimulates nucleosome remodeling. It should be feasible to address this possibility through ChIP-seq studies to map specific histone modifications associated with chromatin remodeling in the manner of Barski et al. (3, 4). Similarly, the NE network (Fig. 9) contained a large number of proteins involved in “mRNA processing,” mostly involved in the spliceosome, that might be coordinately regulated by vasopressin.
We previously reported comprehensive proteomic profiling of nuclear fractions from cultured collecting duct cells (mpkCCD line), identifying a number of proteins that appear to translocate into and out of the nucleus (49). The current studies address whether similar responses are seen in native collecting duct cells. Indeed, as shown in Tables 2 and 3, there were a substantial number of proteins that showed similar changes in response to vasopressin in cultured versus native collecting duct cells. One trade-off of using biochemically isolated native collecting duct cells is that the purity of the cells is necessarily < 100%. Thus, some proteins that we report from this analysis may derive from contaminating cell types. For example, aquaporin-1, an extremely abundant water channel in descending limbs of Henle and vasa recta (38) was identified. In general, corroborative studies such as in situ hybridization localization of mRNAs or immunocytochemical localization of proteins would be needed to be sure that any particular protein reported in this study is indeed expressed in the IMCD.
Another caveat is that the nuclear isolation procedures used in this study give nuclear-enriched fractions that nonetheless contain other structures such as basolateral plasma membranes, endoplasmic reticulum, and mitochondria (Figs. 7 and 9) (63). The reader can use annotations of proteins from RefSeq or Uniprot protein records to ascertain the likelihood that any particular protein reported resides in the nuclei of IMCD cells.
Overall, our study has added substantially to the known proteome of rat renal IMCD cells, especially with regard to proteins with functions in the cell nucleus. The number of proteins identified in all proteomic studies of rat IMCD cells now approximates the number of detectable transcripts. The new data provide an information platform for the design and pursuance of additional studies aimed at understanding transcriptional regulation in the renal collecting duct.
FUNDING
The study was carried out in the Division of Intramural Research of the NHLBI (Projects HL-001285 and HL-006129, M. A. Knepper). C. M. Pickering is an undergraduate student from the Department of Chemical Engineering at Auburn University and was a member of the Biomedical Engineering Student Internship Program supported by the National Institute for Biomedical Imaging and Bioengineering (June-August, 2014). C. Grady was a member of the NHLBI Student Summer Internship Program (May-August, 2015). H. J. Jung is the recipient of the Korean Visiting Scientist Training Award, supported by the Korea Health Industry Development Institute of the Korea Ministry of Health and Welfare in cooperation with National Institutes of Health (HI13C1211).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.M.P., C.-L.C., and M.A.K. performed experiments; C.M.P., C.R.G., B.M., M.E., P.C.S., Y.Z., C.-R.Y., H.J.J., C.-L.C., and M.A.K. analyzed data; C.M.P., C.R.G., M.E., P.C.S., Y.Z., C.-R.Y., H.J.J., C.-L.C., and M.A.K. interpreted results of experiments; C.M.P., C.R.G., B.M., Y.Z., and M.A.K. prepared figures; C.M.P., C.-L.C., and M.A.K. drafted manuscript; C.M.P., C.R.G., B.M., M.E., P.C.S., Y.Z., C.-R.Y., H.J.J., C.-L.C., and M.A.K. edited and revised manuscript; C.M.P., C.R.G., B.M., M.E., P.C.S., Y.Z., C.-R.Y., H.J.J., C.-L.C., and M.A.K. approved final version of manuscript; C.-R.Y., C.-L.C., and M.A.K. conception and design of research.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Guanghui Wang of the NHLBI Proteomics Core Facility (Marjan Gucek, Director) for assistance with the mass spectrometry. The authors are grateful to Drs. Jason D. Hoffert, Fahad Saeed, and Trairak Pisitkun for advice. The authors thank Pumipat Tongyoo for advice on the use of the virtualBlot software. This study utilized the high-performance computational capabilities of the Helix Systems at the National Institutes of Health, Bethesda, MD (http://helix.nih.gov).
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Bansal AD, Hoffert JD, Pisitkun T, Hwang S, Chou CL, Boja ES, Wang G, Knepper MA. Phosphoproteomic profiling reveals vasopressin-regulated phosphorylation sites in collecting duct. J Am Soc Nephrol 21: 303–315, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barile M, Pisitkun T, Yu MJ, Chou CL, Verbalis MJ, Shen RF, Knepper MA. Large scale protein identification in intracellular aquaporin-2 vesicles from renal inner medullary collecting duct. Mol Cell Proteom 4: 1095–1106, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Barski A, Zhao K. Genomic location analysis by ChIP-Seq. J Cell Biochem 107: 11–18, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolger SJ, Hurtado PA, Hoffert JD, Saeed F, Pisitkun T, Knepper MA. Quantitative phosphoproteomics in nuclei of vasopressin-sensitive renal collecting duct cells. Am J Physiol Cell Physiol 303: C1006–C1020, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford D, Raghuram V, Wilson JL, Chou CL, Hoffert JD, Knepper MA, Pisitkun T. Use of LC-MS/MS and Bayes' theorem to identify protein kinases that phosphorylate aquaporin-2 at Ser256. Am J Physiol Cell Physiol 307: C123–C139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulyk ML. Analysis of sequence specificities of DNA-binding proteins with protein binding microarrays. Meth Enzymol 410: 279–299, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busser BW, Bulyk ML, Michelson AM. Toward a systems-level understanding of developmental regulatory networks. Curr Opin Genet Dev 18: 521–529, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Champigneulle A, Siga E, Vassent G, Imbert-Teboul M. V2-like vasopressin receptor mobilizes intracellular Ca2+ in medullary collecting tubules. Am J Physiol Renal Fluid Electrolyte Physiol 265: F35–F45, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Chou CL, DiGiovanni SR, Luther A, Lolait SJ, Knepper MA. Oxytocin as an antidiuretic hormone. II. Role of V2 vasopressin receptor. Am J Physiol Renal Fluid Electrolyte Physiol 269: F78–F85, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Chou CL, Rapko SI, Knepper MA. Phosphoinositide signaling in rat inner medullary collecting duct. Am J Physiol Renal Physiol 274: F564–F572, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Chou CL, Yip KP, Michea L, Kador K, Ferraris J, Wade JB, Knepper MA. Regulation of aquaporin-2 trafficking by vasopressin in renal collecting duct: roles of ryanodine-sensitive Ca2+ stores and calmodulin. J Biol Chem 275: 36839–36846, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Davis TA, Loos B, Engelbrecht AM. AHNAK: the giant jack of all trades. Cell Signal 26: 2683–2693, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Ecelbarger CA, Chou CL, Lolait SJ, Knepper MA, DiGiovanni SR. Evidence for dual signaling pathways for V2 vasopressin receptor in rat inner medullary collecting duct. Am J Physiol Renal Fluid Electrolyte Physiol 270: F623–F633, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Ecelbarger CA, Nielsen S, Olson BR, Murase T, Baker EA, Knepper MA, Verbalis JG. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest 99: 1852–1863, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Meth 4: 207–214, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 31: 397–405, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol 19: 1720–1730, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasler U, Jeon US, Kim JA, Mordasini D, Kwon HM, Feraille E, Martin PY. Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J Am Soc Nephrol 17: 1521–1531, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M, Sasaki S, Tsuganezawa H, Monkawa T, Kitajima W, Konishi K, Fushimi K, Marumo F, Saruta T. Expression and distribution of aquaporin of collecting duct are regulated by V2 receptor in rat kidney. J Clin Invest 94: 1778–1783, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffert JD, Pisitkun T, Saeed F, Song JH, Chou CL, Knepper MA. Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics. Mol Cell Proteom 11: M111, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffert JD, Pisitkun T, Saeed F, Wilson JL, Knepper MA. Global analysis of the effects of the V2 receptor antagonist satavaptan on protein phosphorylation in collecting duct. Am J Physiol Renal Physiol 306: F410–F421, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA 103: 7159–7164, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffert JD, van Balkom BW, Chou CL, Knepper MA. Application of difference gel electrophoresis to the identification of inner medullary collecting duct proteins. Am J Physiol Renal Physiol 286: F170–F179, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Hoorn EJ, Hoffert JD, Knepper MA. Combined proteomics and pathways analysis of collecting duct reveals a protein regulatory network activated in vasopressin escape. J Am Soc Nephrol 16: 2852–2863, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hozawa S, Holtzman EJ, Ausiello DA. cAMP motifs regulating transcription in the aquaporin-2 gene. Am J Physiol Cell Physiol 270: C1695–C1702, 1996. [DOI] [PubMed] [Google Scholar]
- 27.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Ideker T, Thorsson V, Ranish JA, Christmas R, Buhler J, Eng JK, Bumgarner R, Goodlett DR, Aebersold R, Hood L. Integrated genomic and proteomic analyses of a systematically perturbed metabolic network. Science 292: 929–934, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Khositseth S, Pisitkun T, Slentz DH, Wang G, Hoffert JD, Knepper MA, Yu MJ. Quantitative protein and mRNA profiling shows selective post-transcriptional control of protein expression by vasopressin in kidney cells. Mol Cell Proteom 10: M110, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knepper MA. Systems biology in physiology: the vasopressin signaling network in kidney. Am J Physiol Cell Physiol 303: C1115–C1124, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knepper MA. Systems biology of diuretic resistance. J Clin Invest 125: 1793–1795, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li SZ, McDill BW, Kovach PA, Ding L, Go WY, Ho SN, Chen F. Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am J Physiol Cell Physiol 292: C1606–C1616, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol 8: 861–867, 1997. [DOI] [PubMed] [Google Scholar]
- 35.Mundade R, Ozer HG, Wei H, Prabhu L, Lu T. Role of ChIP-seq in the discovery of transcription factor binding sites, differential gene regulation mechanism, epigenetic marks and beyond. Cell Cycle 13: 2847–2852, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nedvetsky PI, Tabor V, Tamma G, Beulshausen S, Skroblin P, Kirschner A, Mutig K, Boltzen M, Petrucci O, Vossenkamper A, Wiesner B, Bachmann S, Rosenthal W, Klussmann E. Reciprocal regulation of aquaporin-2 abundance and degradation by protein kinase A and p38-MAP kinase. J Am Soc Nephrol 21: 1645–1656, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Pisitkun T, Bieniek J, Tchapyjnikov D, Wang G, Wu WW, Shen RF, Knepper MA. High-throughput identification of IMCD proteins using LC-MS/MS. Physiol Genomics 25: 263–276, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pisitkun T, Jacob V, Schleicher SM, Chou CL, Yu MJ, Knepper MA. Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. Am J Physiol Renal Physiol 295: F1030–F1043, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA 101: 13368–13373, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rai T, Uchida S, Marumo F, Sasaki S. Cloning of rat and mouse aquaporin-2 gene promoters and identification of a negative cis-regulatory element. Am J Physiol Renal Physiol 273: F264–F273, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Rinschen MM, Yu MJ, Wang G, Boja ES, Hoffert JD, Pisitkun T, Knepper MA. Quantitative phosphoproteomic analysis reveals vasopressin V2-receptor-dependent signaling pathways in renal collecting duct cells. Proc Natl Acad Sci USA 107: 3887, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robert-Nicoud M, Flahaut M, Elalouf JM, Nicod M, Salinas M, Bens M, Doucet A, Wincker P, Artiguenave F, Horisberger JD, Vandewalle A, Rossier BC, Firsov D. Transcriptome of a mouse kidney cortical collecting duct cell line: effects of aldosterone and vasopressin. Proc Natl Acad Sci USA 98: 2712–2716, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sachs AN, Pisitkun T, Hoffert JD, Yu MJ, Knepper MA. LC-MS/MS analysis of differential centrifugation fractions from native inner medullary collecting duct of rat. Am J Physiol Renal Physiol 295: F1799–F1806, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandoval PC, Slentz DH, Pisitkun T, Saeed F, Hoffert JD, Knepper MA. Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol 24: 1793–1805, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanghi A, Zaringhalam M, Corcoran CC, Saeed F, Hoffert JD, Sandoval P, Pisitkun T, Knepper MA. A knowledge base of vasopressin actions in the kidney. Am J Physiol Renal Physiol 307: F747–F755, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savitski MM, Lemeer S, Boesche M, Lang M, Mathieson T, Bantscheff M, Kuster B. Confident phosphorylation site localization using the Mascot Delta Score. Mol Cell Proteom 10: M110, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schenk LK, Bolger SJ, Luginbuhl K, Gonzales PA, Rinschen MM, Yu MJ, Hoffert JD, Pisitkun T, Knepper MA. Quantitative proteomics identifies vasopressin-responsive nuclear proteins in collecting duct cells. J Am Soc Nephrol 23: 1008–1018, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simons BL, Wang G, Shen RF, Knepper MA. In vacuo isotope coded alkylation technique (IVICAT); an N-terminal stable isotopic label for quantitative liquid chromatography/mass spectrometry proteomics. Rapid Commun Mass Spectrom 20: 2463–2477, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Star RA, Nonoguchi H, Balaban R, Knepper MA. Calcium and cyclic adenosine monophosphate as second messengers for vasopressin in the rat inner medullary collecting duct. J Clin Invest 81: 1879–1888, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strange K, Spring KR. Cell membrane water permeability of rabbit cortical collecting duct. J Membr Biol 96: 27–43, 1987. [DOI] [PubMed] [Google Scholar]
- 53.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von MC. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res 43: D447–D452, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanner S, Shu H, Frank A, Wang LC, Zandi E, Mumby M, Pevzner PA, Bafna V. InsPecT: identification of posttranslationally modified peptides from tandem mass spectra. Anal Chem 77: 4626–4639, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Tchapyjnikov D, Li Y, Pisitkun T, Hoffert JD, Yu MJ, Knepper MA. Proteomic profiling of nuclei from native renal inner medullary collecting duct cells using LC-MS/MS. Physiol Genomics 40: 167–183, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trepiccione F, Pisitkun T, Hoffert JD, Poulsen SB, Capasso G, Nielsen S, Knepper MA, Fenton RA, Christensen BM. Early targets of lithium in rat kidney inner medullary collecting duct include p38 and ERK1/2. Kidney Int 86: 757–767, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Uawithya P, Pisitkun T, Ruttenberg BE, Knepper MA. Transcriptional profiling of native inner medullary collecting duct cells from rat kidney. Physiol Genomics 32: 229–253, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uchida S, Matsumura Y, Rai T, Sasaki S, Marumo F. Regulation of aquaporin-2 gene transcription by GATA-3. Biochem Biophys Res Commun 232: 65–68, 1997. [DOI] [PubMed] [Google Scholar]
- 59.van Balkom BW, Hoffert JD, Chou CL, Knepper MA. Proteomic analysis of long-term vasopressin action in the inner medullary collecting duct of the Brattleboro rat. Am J Physiol Renal Physiol 286: F216–F224, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Vizcaino JA, Deutsch EW, Wang R, Csordas A, Reisinger F, Rios D, Dianes JA, Sun Z, Farrah T, Bandeira N, Binz PA, Xenarios I, Eisenacher M, Mayer G, Gatto L, Campos A, Chalkley RJ, Kraus HJ, Albar JP, Martinez-Bartolome S, Apweiler R, Omenn GS, Martens L, Jones AR, Hermjakob H. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat Biotechnol 32: 223–226, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Washburn MP, Koller A, Oshiro G, Ulaszek RR, Plouffe D, Deciu C, Winzeler E, Yates JR 3rd. Protein pathway and complex clustering of correlated mRNA and protein expression analyses in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 100: 3107–3112, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson JL, Miranda CA, Knepper MA. Vasopressin and the regulation of aquaporin-2. Clin Exp Nephrol 17: 751–764, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang CR, Raghuram V, Emamian M, Sandoval PC, Knepper MA. Deep proteomic profiling of vasopressin-sensitive collecting duct cells. II. Bioinformatic analysis of vasopressin signaling. Am J Physiol Cell Physiol 309: C799–C812, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang CR, Tongyoo P, Emamian M, Sandoval PC, Raghuram V, Knepper MA. Deep proteomic profiling of vasopressin-sensitive collecting duct cells. I. Virtual Western blots and molecular weight distributions. Am J Physiol Cell Physiol 309: C785–C798, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasui M, Zelenin SM, Celsi G, Aperia A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol Renal Physiol 272: F443–F450, 1997. [DOI] [PubMed] [Google Scholar]
- 66.Yates JR 3rd, Eng JK, McCormack AL, Schieltz D. Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database. Anal Chem 67: 1426–1436, 1995. [DOI] [PubMed] [Google Scholar]
- 67.Yip KP. Coupling of vasopressin-induced intracellular Ca2+ mobilization and apical exocytosis in perfused rat kidney collecting duct. J Physiol 538: 891–899, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu MJ, Miller RL, Uawithya P, Rinschen MM, Khositseth S, Braucht DW, Chou CL, Pisitkun T, Nelson RD, Knepper MA. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci USA 106: 2441–2446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu MJ, Pisitkun T, Wang G, Shen RF, Knepper MA. LC-MS/MS analysis of apical and basolateral plasma membranes of rat renal collecting duct cells. Mol Cell Proteom 5: 2131–2145, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhao B, Knepper MA, Chou CL, Pisitkun T. Large-scale phosphotyrosine proteomic profiling of rat renal collecting duct epithelium reveals predominance of proteins involved in cell polarity determination. Am J Physiol Cell Physiol 302: C27–C45, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.