Abstract
Clostridium difficile is the primary cause of nosocomial antibiotic-associated diarrhea in the Western world. The major virulence factors of C. difficile are two exotoxins, toxin A (TcdA) and toxin B (TcdB), which cause extensive colonic inflammation and epithelial damage manifested by episodes of diarrhea. In this study, we explored the basis for an oral antitoxin strategy based on engineered Lactobacillus strains expressing TcdB-neutralizing antibody fragments in the gastrointestinal tract. Variable domain of heavy chain-only (VHH) antibodies were raised in llamas by immunization with the complete TcdB toxin. Four unique VHH fragments neutralizing TcdB in vitro were isolated. When these VHH fragments were expressed in either secreted or cell wall-anchored form in Lactobacillus paracasei BL23, they were able to neutralize the cytotoxic effect of the toxin in an in vitro cell-based assay. Prophylactic treatment with a combination of two strains of engineered L. paracasei BL23 expressing two neutralizing anti-TcdB VHH fragments (VHH-B2 and VHH-G3) delayed killing in a hamster protection model where the animals were challenged with spores of a TcdA− TcdB+ strain of C. difficile (P < 0.05). Half of the hamsters in the treated group survived until the termination of the experiment at day 5 and showed either no damage or limited inflammation of the colonic mucosa despite having been colonized with C. difficile for up to 4 days. The protective effect in the hamster model suggests that the strategy could be explored as a supplement to existing therapies for patients.
INTRODUCTION
Clostridium difficile is an anaerobic, Gram-positive, endospore-forming gastrointestinal pathogen and the leading cause of antibiotic-associated diarrhea (C. difficile-associated disease [CDAD]) in developed nations. The bacterium is transmitted as a spore through the fecal-oral route, and asymptomatic carriage is found in 4 to 20% of the adult population (1). Onset of the disease follows disruption of the endogenous gastrointestinal flora, commonly caused by use of broad-spectrum antibiotics for treatment of a primary condition permitting germination and colonization of C. difficile in the colon (2). Every year, 1 to 3% of all hospitalized North American patients receiving antibiotics as part of their treatment subsequently become infected with C. difficile, making it the most prominent nosocomial infection (3).
Clinical symptoms of CDAD range from mild self-limiting to severe diarrhea, with up to 25% of affected patients experiencing recurrent infections (4). Severe cases of CDAD can lead to pseudomembranous colitis and progress further to toxic megacolon, with a fatal ending in approximately one-third of cases (5).
The toxicity of C. difficile arises primarily from two virulence factors, toxin A (TcdA; 308 kDa) and toxin B (TcdB; 269 kDa), both of which are large, single-subunit exotoxins which share extensive homology (for a review, see reference 6). Both have a modular domain structure with an N-terminal enzymatic domain, a central translocation domain, and a C-terminal receptor binding domain (see Fig. S3 in the supplemental material). The binding domain is thought to be responsible for initial binding to epithelial cells and induces toxin uptake through receptor-mediated endocytosis. Upon lowering of the endosomal pH, the central domain exposes a hydrophobic membrane insertion domain that inserts and translocates the N-terminal catalytic domain from the endosome to the cytosol. The N-terminal enzymatic domain carries a cysteine protease that, through autocatalytic cleavage, releases the domain from the endosome into the cytosol. The released N-terminal glucosyltransferase domain glucosylates the Rho-GTPases in the cytosol, blocking the Rho signaling pathway and leading to cellular shutdown and a loss of cellular barrier function. The causative roles of both TcdA and TcdB have been well established for CDAD, with both toxins inducing epithelial tissue damage and extended colonic inflammation in infected hosts. The precise role of each toxin in CDAD has been debated, but recent experimental evidence with toxin deletion strains points to TcdB being the dominant virulence factor (7, 8).
Recently, with the emergence of new hypervirulent strains, both the severity and mortality of C. difficile outbreaks have risen significantly. The increased virulence was initially identified in the North American isolate BI/NAP1/027 (9) and was manifested in epidemic outbreaks in North American hospitals that subsequently were mirrored on other continents (10, 11). The hypervirulence has been connected with resistance to fluoroquinolones (12) and increased cytotoxicity and highlights the need for new and better treatment strategies for the management of C. difficile infections (CDI).
The primary treatment against CDAD is antibiotics, with metronidazole and vancomycin being the most commonly used ones (13). Although it is effective, the treatment may lead to emergence of resistant strains, and there are concerns that antibiotics inhibit reestablishment of the endogenous bacterial biota, potentially prolonging susceptibility to reinfection at the end of therapy. With the pressing need for improved therapies for CDAD, two alternative treatment strategies currently showing promise are reconstitution of the gastrointestinal flora by fecal transplantation and antibody-based toxin neutralization (14–16).
The use of antibody-based therapies stems from the observation that patients with low antitoxin IgG titers suffer from more severe effects of CDAD and more frequently experience recurrent infections (17, 18). Both intravenous and oral routes of delivery of toxin-neutralizing antibodies have been explored with positive results, but the majority of studies have been conducted in animal models. In humans, intravenous therapy with combined anti-TcdA and -TcdB human monoclonal antibodies (hMAbs) has been shown to significantly reduce the rate of recurrent infections (16). Oral delivery of hyperimmune bovine colostrum (HBC) from cows immunized with C. difficile culture filtrates has also been shown to have potential for both alleviating the effects of CDAD and reducing the frequency of relapse in humans (19, 20). Large-scale therapeutic application, however, has been hampered by the high production costs of hMAbs (intravenous therapies) and HBC (oral therapies) and the high IgG dose requirement (150 to 400 mg/kg of body weight) in order to achieve a therapeutic effect.
Variable domain of heavy chain-only (VHH) antibodies from camelids retain the binding characteristics of the complete antibody, with specificities and affinities comparable to those of conventional IgGs, despite their small size (15 kDa). They are well expressed in bacteria, and their excellent physicochemical stability combined with the possibility to be engineered for improved protease stability makes them ideal choices for passive immunity in the gastrointestinal tract (21, 22).
Lactobacilli are Gram-positive bacteria constituting parts of the normal gastrointestinal flora and are generally recognized as safe (GRAS) for human consumption. They survive gastrointestinal passage and can colonize the intestine, making them suitable vehicles for in situ production and delivery of therapeutic molecules in the small and large intestines (23, 24).
In this study, we explored the development of engineered strains of Lactobacillus for expression of toxin-neutralizing VHH antibody fragments from llamas in the gastrointestinal tract as a means for a rapid and cost-effective form of passive immunization against CDAD.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Escherichia coli DH5α (Invitrogen, Carlsbad, CA) and E. coli K-12 TG-1 (Stratagene, La Jolla, CA) were grown on LB agar plates at 37°C or in LB medium at 37°C with orbital shaking at 220 rpm, unless otherwise stated. Lactobacillus paracasei BL23 was grown in lactobacillus MRS broth (Difco, Sparks, MD) at 37°C without agitation or anaerobically on MRS agar plates (GazPak EZ; BD, Sparks, MD). Antibiotics (Sigma-Aldrich, St. Louis, MO) were added when indicated, at the following concentrations: ampicillin, 100 μg/ml; erythromycin, 300 μg/ml for E. coli and 5 μg/ml for lactobacilli; kanamycin, 100 μg/ml; and chloramphenicol, 50 μg/ml.
Llama immunization and construction of VHH libraries.
C. difficile TcdA and TcdB (List Biologicals, Campbell, CA) were inactivated prior to immunizations by alkylation of the catalytic domain with UDP-2′,3′-dialdehyde (Sigma-Aldrich, St. Louis, MO) (25). Immunizations and library construction were carried out as previously described (26). The prolonged llama immunizations were approved and performed according to the guidelines of Utrecht University Animal Ethical Committee (approval ID 2007.III.01.013/vervolg2). Briefly, two llamas (llamas 19 and 20) received intramuscular injections with 40 μg of C. difficile TcdB in 2 ml phosphate-buffered saline (PBS) mixed with 2 ml Stimune adjuvant (CEDI Diagnostics) on days 0 and 14. Additional boosters with half the amount of toxin were given on days 28 and 35. Blood samples were taken on days 0, 28, and 44 to assess the llama immune response by enzyme-linked immunosorbent assay (ELISA). The neutralizing activities of sera from day 0 (preimmunization) and day 44 were tested in an in vitro neutralization assay. At day 44, 150 ml blood was taken for isolation of RNA from the peripheral blood lymphocytes. Total RNA was isolated (27), and cDNA was synthesized using a SuperScript III first-strand synthesis kit (Invitrogen). The IgG repertoire was amplified and the VHH fraction separated from the conventional IgGs by gel electrophoresis (28). Flanking SfiI and BstEII restriction sites were introduced through a nested PCR, and the VHH fragments were cloned into the SfiI- and BstEII-digested phagemid vector pUR8100. Phagemids were transformed into E. coli K-12 by electroporation, generating library sizes of 107 to 108 CFU.
Selection and screening of VHH fragments binding to toxins.
Phages were recovered from the libraries by infection with the helper phage VCS-M13 and were precipitated with polyethylene glycol as previously described (29), giving phage stocks of approximately 1012 PFU/ml. Panning of phage libraries was carried out separately on immobilized native TcdB (List Biologicals). Wells of a MaxiSorp microtiter plate (Nunc, Rochester, NY) were coated with 100 μl of TcdB (500 ng/ml or 50 ng/ml in PBS) overnight at 4°C. Plates were blocked with 4% skimmed milk (Marvel; Premier Foods, United Kingdom) in PBS (MPBS) for 2 h at room temperature (RT). After washing of the plates, 100 μl (5 × 109 PFU/ml) phage preincubated in 2% MPBS for 30 min was added to each well and incubated at RT with shaking for 2 h. Plates were washed extensively with PBS supplemented with 0.05% Tween 20 (PBS-T), and bound phage were eluted with 100 mM triethylamine (TEA) for 15 min. Phage eluates were neutralized with half the volume of 1 M Tris, pH 7.5, and diluted in PBS before reinfection of E. coli TG-1. Identical conditions were used for a second round of panning on the toxins. After the second round of selection, the phage eluates in E. coli TG-1 were plated on LB agar plates (100 μg/ml ampicillin and 2% glucose), and single colonies were picked for further analysis. The binding specificities of the selected VHH antibodies for TcdB were screened in the first round by using induced periplasmic extracts from the individual E. coli clones. Periplasmic extracts were prepared according to standard protocols (26) and tested for binding to TcdB (2 μg/ml) immobilized on a microtiter plate as previously described (30). The bound VHH antibodies were detected using a rabbit anti-llama IgG antibody (a kind gift from Unilever Research, Vlaardingen, Netherlands) in combination with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody (Dako, Glostrup, Denmark). The best-binding VHH antibodies were identified based on the colorimetric readout (optical density at 405 nm [OD405]), and the clones were sequenced with the M13Rev primer to determine the diversity of the selected VHH antibodies. To further determine the binding specificities and toxin neutralization ability of the isolated VHH clones, 11 unique anti-TcdB VHH antibodies were subcloned into the pAX051 vector for expression and purification by a previously described method (30).
Construction of recombinant Lactobacillus and E. coli.
VHH fragments were excised from the respective E. coli pAX51 expression plasmids by using the restriction enzymes NcoI and NotI (Fermentas, St. Leon-Rot, Germany) and were ligated into the NcoI/NotI-digested Lactobacillus expression vectors pAF100 and pAF900 (31), creating expression plasmids for secretion and cell wall-anchored display (see Table S2 in the supplemental material). Correct insertions of the VHH genes into the plasmids were verified by sequencing of the complete expression cassettes. The expression plasmids were transfected into L. paracasei BL23 by electroporation as previously described (23, 32), generating strains of Lactobacillus expressing the anti-TcdB VHH antibodies, either secreted or cell wall displayed (see Table S2).
The domains of TcdA and TcdB of C. difficile were cloned with a C-terminal vesicular stomatitis virus (VSV) tag and a 6×His tag for expression and purification in E. coli. The catalytic domain of TcdB was amplified by PCR from C. difficile VPI 10463 chromosomal DNA by using primers TxB-frag1-Fw and TxB-frag1-Rv, adding flanking restriction sites for cloning and the coding sequence for a C-terminal VSV tag. The PCR fragment was restriction digested with NcoI and XhoI (Fermentas) and cloned into the NcoI/XhoI-digested plasmid pET28a(+) (Novagen, Madison, WI), generating the plasmid pKA436. Plasmid pKA436 was restriction digested with NcoI and BamHI to excise the TcdB catalytic domain-encoding sequence but maintain the C-terminal VSV and 6×His tag-encoding sequences. The remainder of the TcdB and TcdA domains were likewise PCR amplified with the specified primers (see Table S1 in the supplemental material). The resulting PCR fragments were restriction digested with NcoI and BamHI and cloned into NcoI/BamHI-digested pKA436, giving plasmids pKA432 to pKA439 (see Table S2). The expression plasmids containing the toxin domains were electroporated into E. coli BL21-CodonPlus(DE3)-RIPL (Stratagene, La Jolla, CA), generating strains KKA370 to KKA377 (see Table S2).
Western blotting.
Analysis of relative expression levels and the cellular localization of Lactobacillus-produced VHH antibodies was carried out by Western blotting as previously described (33), with the modifications that concentration of cell culture supernatants was omitted and the supernatants were directly mixed with 2× Laemmli buffer.
ELISA.
Binding of Lactobacillus-produced VHH fragments to antigens was carried out by an ELISA to detect the C-terminal E tag by use of an anti-E-tag antibody, as previously described (33). Complete TcdB (List Biologicals) and E. coli-produced toxin fragments were used as antigens at 2 μg/ml in PBS and were used to coat 96-well microtiter plates (EIR/RIA plates; Costar, Lowell, MA).
For analysis of VHH epitope competition, microtiter plates were coated with 2 μg/ml of the E. coli-produced TcdB binding domain. After blocking, 90-μl VHH antibody-producing Lactobacillus culture supernatants were mixed with 10 μl of nontagged E. coli-produced VHH antibody and added to the wells of a microtiter plate. Binding competitions were carried out in duplicate with 3-fold dilutions of the competing E. coli-produced VHH antibody, covering the range of 12.3 ng/ml to 27 μg/ml. The assays were carried out with the VHH antibody-containing supernatants of each of the four secreting strains (KKA382, KKA440, KKA441, and KKA442) competing with each of the four E. coli-produced VHH antibodies (B2, E2, G3, and D8). Detection of the E tag on VHH antibodies produced by Lactobacillus was carried out as previously described (33).
The antibody response in the llamas immunized with toxins was evaluated by ELISA. Fourfold dilutions of llama sera in PBS (1/500 to 1/32,000) were added to toxin (1 μg/ml)-coated microtiter plates blocked with 1% bovine serum albumin (BSA) in PBS-T. Bound VHH and IgG antibodies were detected with a rabbit anti-llama IgG antibody (1/2,000) in combination with an HRP-conjugated goat anti-rabbit antibody (1/10,000) (Dako).
Flow cytometry.
Analysis of display and binding to TcdB of cell wall-anchored VHH fragments was performed by flow cytometry as described previously (33). Binding to TcdB was carried out with TcdB (List Biologicals) biotinylated with an EZ-Link sulfo-NHS-LC-biotin kit (Thermo Scientific, Rockford, IL) according to the manufacturer's instructions. Lactobacilli displaying cell wall-anchored VHH antibodies were incubated sequentially with biotinylated TcdB (1 μg/ml) and fluorescein isothiocyanate (FITC)-conjugated streptavidin (5 μg/ml) (BioLegend, San Diego, CA) and analyzed using a FACSCalibur machine (Becton Dickinson, Franklin Lakes, NJ).
In vitro neutralization assay.
Neutralization of TcdB by antitoxin VHH antibodies was analyzed on the MA-104 cell line (34), which has previously been characterized for sensitivity to C. difficile TcdA and TcdB (35). TcdA and TcdB (List Biologicals) were titrated on the cell line before use to adjust for batch variation and were used at a level 2- to 4-fold higher than the killing dose unless otherwise stated. MA-104 cells were seeded at 1 × 105 cells per well in a 96-well microtiter plate and incubated for 24 h at 37°C and 5% CO2 in GlutaMAX Dulbecco's modified Eagle medium (DMEM) (Life Technologies, Grand Island, NY) with 10% fetal bovine serum (FBS), reaching 70 to 80% confluence. TcdA and TcdB were mixed with VHH antibodies at various concentrations in serum-free DMEM and incubated on ice for 45 min. Cells were washed with serum-free DMEM, overlaid with 100 μl VHH antibody-toxin mix, and incubated at 37°C for 24 h in 5% CO2. The cytotoxic effect of nonneutralized toxins was scored microscopically by the presence of cells that were showing beginning to complete cell rounding. Complete toxin neutralization was characterized as visually undamaged cells.
Toxin neutralization by llama sera was tested with 4-fold dilutions (1/100 to 1/12,800) of sera incubated with 10 ng/ml TcdB. The serum-toxin mixes were overlaid on washed MA-104 cells, and toxin neutralization was scored as the presence of undamaged cells after 24 h of incubation, as described above.
Adsorption of TcdB by Lactobacillus cell wall-displayed VHH antibody was carried out by incubating 2-fold serial dilutions of Lactobacillus in DMEM (8 × 109 to 1.25 × 108 CFU/ml) with a 5-fold cytotoxic dose of TcdB (50 ng/ml) under mild agitation at 37°C for 1 h. Prior to incubation, Lactobacillus cells were washed three times in DMEM with 25 mM HEPES buffer to bring the pH to 7.2. The DMEM buffer was supplemented with 50 U/ml penicillin, 50 μg/ml streptomycin, and 25 μg/ml gentamicin (all from Life Technologies, Grand Island, NY) to avoid bacterial growth. Lactobacillus and adsorbed TcdB were pelleted by centrifugation at 12,000 rpm for 5 min, and 100 μl of supernatant was transferred to each well of a microtiter plate with washed MA-104 cells. The cytotoxicity of the remaining TcdB in the adsorbed supernatant was recorded as described above.
Purification of VHH and TcdB fragments.
Lactobacillus-produced VHH-G3 secreted from strain KKA382 was purified on a HiTrap anti-E-tag column (GE Healthcare, Buckinghamshire, United Kingdom) by the method previously described for Lactobacillus-produced single-chain variable fragments (scFvs) (33).
E. coli-produced TcdA and TcdB domains were purified by means of the C-terminal 6×His tag. E. coli strains (see Table S2 in the supplemental material) harboring the toxin domain-encoding sequences on a pET28a plasmid were grown in 500 ml YT broth supplemented with 100 mM glucose, 100 μg/ml kanamycin, and 50 μg/ml chloramphenicol at 25°C with orbital shaking at 220 rpm. Cultures were induced with 1 mM isopropyl-β-d-thiogalactopyranoside (Sigma-Aldrich) at an OD600 of 0.3 and grown for a further 4 h. Induced E. coli cultures were pelleted for 10 min at 10,000 × g at 4°C, and the bacterial pellet was lysed with BugBuster HT protein extraction reagent (EMD Millipore, Billerica, MA) according to the manufacturer's instructions. The soluble fractions were adjusted to 30 mM imidazole (Sigma-Aldrich) and 0.5 M NaCl (pH 7.5) and passed through a 0.2-μm filter before being loaded on a 5-ml HisTrap HP column (GE Healthcare). The column was washed with 20 bed volumes of wash buffer (PBS, 30 mM imidazole, 0.5 M NaCl, pH 7.5) and subsequently eluted with 5 bed volumes of elution buffer (PBS, 0.5 M imidazole, 0.5 M NaCl, pH 7.5). The eluate was buffer exchanged with 1× PBS on a HiPrep 26/10 desalting column (GE Healthcare), and toxin domains were concentrated using an Amicon Ultra-16 30K MWCO spin column (Millipore).
Yeast-produced VHH fragments used for the in vivo protection model (VHH-G3, VHH-B2, and VHH-D8) were cloned, expressed, and purified using ion-exchange chromatography (to >95% purity) as a service by BAC BV (GP Naarden, Netherlands).
VHH fragment proteolytic stability.
Water-soluble molecules, including proteases, were extracted from equal amounts of the contents of the small and large intestines of two hamsters. One hundred milligrams of pooled intestinal content was mixed with 1 ml 0.01 M PBS and 300 mg 0.1-mm zirconia beads and homogenized in a FastPrep FP120 homogenizer (Thermo Fisher Scientific, Waltham, MA), using three 20-s pulses at a speed of 4.0 m/s. The solid matter was pelleted by 3 min of centrifugation at a relative centrifugal force (RCF) of 16,000, and the collected supernatant was passed through a 0.2-μm filter. The purified VHH fragments (20 ng/μl in 0.01 M PBS) were mixed with an equal volume of hamster intestinal extract and incubated at 37°C. Samples were taken at time points from 0 to 180 min, mixed with 2× Laemmli loading dye, and denatured by incubation at 100°C for 5 min. Samples were analyzed by Western blotting as previously described, using anti-VHH K212 (BAC BV) mouse immunoglobulin in combination with HRP-conjugated anti-mouse immunoglobulins for detection of the VHH fragments.
C. difficile spore preparation.
Spores of C. difficile 630(Δerm) TcdA− TcdB+ (8) were prepared by the alcohol shock method and stored at −80°C until use (36). A TcdA deletion strain of C. difficile 630(Δerm) was used for the hamster model because it produces the same TcdB toxin as that used for immunization. Spore germination and growth were verified and the optimal dose of infection established in the Syrian golden hamster model of infection.
Prophylactic hamster model.
Six-week-old male Syrian golden hamsters were obtained from Harlan Laboratories, United Kingdom. Hamsters were housed individually under specific-pathogen-free conditions and were given a commercial diet (R-70; Lactahour, Sweden) and water ad libitum. Studies were conducted according to the guidelines of the University of Tartu and approved by the Ethics Committee on Animal Experiments of the Ministry of Agriculture of Estonia.
Hamsters were treated with a single orogastric dose of clindamycin (30 mg/kg) (Sigma-Aldrich) to destabilize the intestinal flora 24 h before challenge with 103 spores of a TcdA− TcdB+ strain of C. difficile 630 (8). Prophylactic treatment with yeast-produced anti-TcdB VHH antibody was started on the same day as clindamycin treatment and was continued for a total of 7 days. One group of hamsters (n = 6) received a mixed dose of 125 μg each of three yeast-produced TcdB-neutralizing VHH fragments (VHH-B2, VHH-G3, and VHH-D8) twice daily by gavage. The two control groups (n = 6 [each]) received either 375 μg of an irrelevant anti-rotavirus VHH antibody (23) twice daily or no VHH antibody.
In an identical prophylactic model, hamsters received Lactobacillus strains expressing cell wall-anchored anti-TcdB VHH antibody twice daily by gavage. The Lactobacillus strains KKA413, KKA416, and KKA101 were grown in MRS medium (Oxoid, United Kingdom) to an OD600 of 1.0, harvested by centrifugation, and washed twice in PBS. Three groups of hamsters (n = 6) received, by gavage, either (i) 5 × 109 CFU of each of two strains of L. paracasei BL23 (KKA413 and KKA416), expressing the cell wall-anchored VHH-B2 and VHH-G3 antibodies, respectively, twice daily; (ii) 1 × 1010 CFU of a nonexpressing strain of L. paracasei BL23 (KKA101) twice daily; or (iii) spores only.
Hamster activity, behavior, and general health, including diarrhea and mortality, were evaluated for the duration of the experiments. The hamster model was terminated on day 5 after the spore challenge to comply with the ethical permit, and surviving hamsters were sacrificed by cervical dislocation. Autopsies of sacrificed hamsters were performed under sterile conditions in a class II microbiological safety cabinet (Jouan, France).
Bacteriological investigations were carried out on fresh samples of heart blood (10 μl) and homogenized tissues of liver, spleen, and small and large intestines by plating on both LAB160 (Lab M Limited, United Kingdom) and MRS (Oxoid, United Kingdom) agar plates. After 72 h of anaerobic (90% N2, 5% CO2, 5% H2) or microaerobic (10% CO2) incubation, the C. difficile and Lactobacillus colonies were identified and enumerated.
Tissue sections for histology were collected from the ileums, ceca, livers, and spleens of the three surviving hamsters. Tissues were fixed in 10% formaldehyde, embedded in paraffin, and stained with hematoxylin and eosin. Signs of inflammation and cellular destruction were examined on coded slides by a pathologist and were scored based on the severity of cellular damage, on a scale of 0 to 5 (no changes, hyperemia, cellular infiltration, necrosis, and pseudomembranes).
Fecal droppings were collected on all days for the duration of the experiment and were analyzed with the Immunocard C. difficile glutamate dehydrogenase (GDH) test (Meridian Bioscience Inc., Cincinnati, OH). For GDH-positive fecal samples, the presence of TcdA or TcdB was analyzed with the immunochromatographic Xpect C. difficile toxin A/B test (Remel, Lenexa, KS). The sensitivity for TcdB in the Xpect C. difficile toxin A/B test is >40.0 ng/ml (0.76 ng/test).
RESULTS
Selection of toxin-neutralizing VHH antibodies.
Two llamas (llamas 19 and 20) were immunized with inactivated TcdB and received three consecutive boosters, at days 14, 28, and 35. The induction of a humoral response was confirmed after the second immunization (day 28) by testing sera for binding to TcdB relative to that of preimmune sera by ELISA (see Fig. S1 in the supplemental material). Postimmunization sera (day 44) were screened for their toxin neutralization titers in a cell-based in vitro assay. A strong toxin-neutralizing response were seen for llama 20, with a serum neutralization titer of 6,400 against TcdB, while llama 19 showed a lower-level response, with a serum neutralization titer of 200 against TcdB (data not shown). Two VHH fragment-specific phage libraries, libraries 19 and 20, were constructed from the pooled peripheral blood lymphocytes from the two llamas, with each library containing between 107 and 108 transformants.
In order to isolate VHH clones with a high binding affinity for toxin B, the phage libraries were subjected to two rounds of panning on native TcdB directly immobilized on microtiter plates, followed by elution with triethylamine buffer. The periplasmic extracts of the induced E. coli clones were screened for binding to TcdB by ELISA. From this analysis, 31 anti-TcdB clones were selected based on toxin binding, their induced periplasmic extracts were tested for in vitro neutralization in a cell-based neutralization assay, and the VHH fragment diversity was determined by sequencing. Eleven unique anti-TcdB VHH fragments showed protection in the initial screen with periplasmic extracts (data not shown).
The 11 selected anti-TcdB VHH fragments were produced in and purified from E. coli and subsequently tested for in vitro neutralization. Sequencing of the anti-TcdB VHH fragments revealed that they fell into 6 separate groups with respect to the amino acid sequence of the CDR3 domain, indicating a high variability of the clones selected from the library. Based on the concentration of VHH antibody giving complete protection against a 4-fold cytotoxic dose of TcdB in a cell-based assay, four VHH fragments (VHH-B2, VHH-E2, VHH-G3, and VHH-D8), belonging to three separate families and providing the best protection, were selected for cloning and expression in L. paracasei BL23 (Table 1).
TABLE 1.
Neutralization of toxin B by VHH fragments in vitro
| VHH clone | Protective concn (μg/ml)a | VHH familyb |
|---|---|---|
| VHH-G1 | >5.12 | V2 |
| VHH-B2 | 1.28 | V1 |
| VHH-D2 | >5.12 | V2 |
| VHH-E2 | 5.12 | V2 |
| VHH-G3 | 0.08–0.32 | V2 |
| VHH-B5 | 0.32 | V2 |
| VHH-D8 | 5.12 | V5 |
| VHH-G9 | >5.12 | V3 |
| VHH-H11 | >5.12 | V4 |
| VHH-A12 | >5.12 | V6 |
| VHH-G12 | >5.12 | V6 |
Concentration of E. coli-produced VHH fragment giving complete neutralization of 4 times the cytotoxic concentration of toxin B (20 ng/ml) in the in vitro neutralization assay.
Based on the sequence divergence of CDR3, the toxin B-neutralizing VHH fragments could be divided into six separate families (V1 to V6).
Construction of anti-TcdB VHH fragment-expressing Lactobacillus strains.
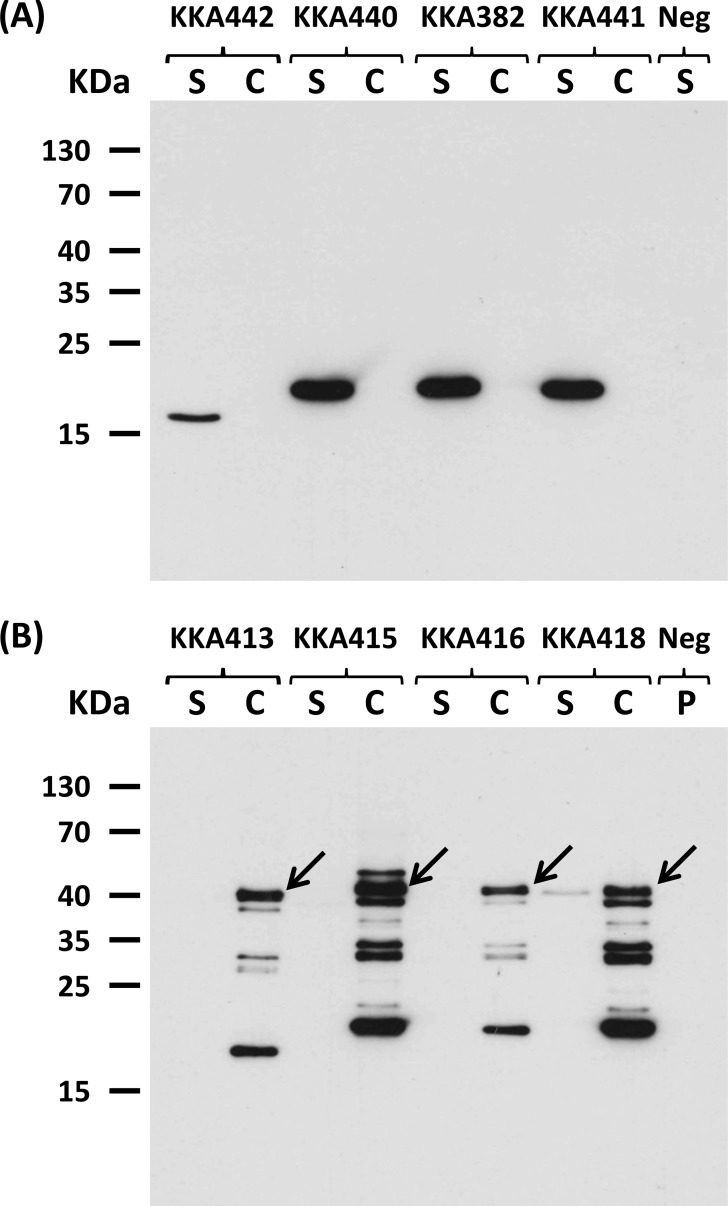
The four anti-TcdB VHH fragments were cloned into two separate expression vectors, either for anchoring and display on the bacterial cell wall or for secretion into the supernatant, essentially as described previously (33) (see Fig. S2 in the supplemental material). Expression and the correct cellular localization of VHH fragments in transformed L. paracasei BL23 were verified by Western blotting to detect the E tag fused to the VHH fragments by use of an anti-E-tag antibody. For the secreted constructs, the VHH fragments were found exclusively in the cell culture supernatant, and no detectable levels were associated with the cell fraction (Fig. 1A). The secreted VHH-B2 antibody was produced at 4 to 5 times lower levels than the other three VHH antibodies. This might have been due to the presence of an arginine in the β-strand within framework 4 of VHH-B2, which has previously been shown to be associated with lower production levels in yeast due to a change in polarity (37). VHH-B2 produced from strain KKA442 also ran as a slightly smaller construct due to a shorter CDR3 domain, consisting of 6 amino acids (aa), in contrast to the 16- or 17-aa CDR3 found for the other three neutralizing VHH fragments. For the anchored constructs, the cell wall anchoring was confirmed by the localization of the VHH fragments within the cell pellet fraction. Only strain KKA418, expressing VHH-D8, showed a faint band in the supernatant fraction, indicating that some shedding from the cell wall or incomplete anchoring occurred (Fig. 1B).
FIG 1.
Expression and cellular localization of Lactobacillus-produced VHH fragments. Detection of TcdB-neutralizing VHH fragments expressed by engineered L. paracasei BL23 strains was performed by immunoblotting of the cell pellet (C) and supernatant (S) fractions. (A) Expression of secreted anti-TcdB VHH fragments by strains KK442 (VHH-B2; 15.20 kDa), KKA440 (VHH-E2; 16.25 kDa), KKA382 (VHH-G3; 16.24 kDa), KKA441 (VHH-D8; 16.74 kDa), and KKA101 (negative control [Neg]). (B) Expression of cell wall-anchored anti-TcdB VHH fragments by strains KK413 (VHH-B2; 39.78 kDa), KKA415 (VHH-E2; 40.78 kDa), KKA416 (VHH-G3; 40.77 kDa), KKA418 (VHH-D8; 41.28 kDa), and KKA101 (negative control). VHH fragments were detected using an anti-E-tag antibody followed by HRP-conjugated anti-mouse immunoglobulins. Arrows indicate the protein bands of expected size. Some degradation of the VHH fragments was seen for the cell wall-anchored constructs due to the crude method for lysing the cells.
Binding of TcdB by Lactobacillus-produced VHH antibodies.
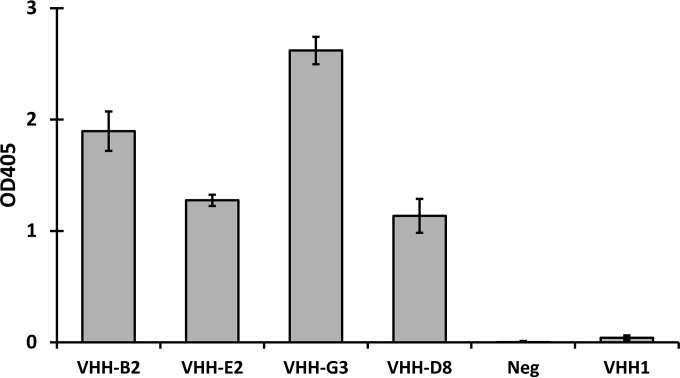
For the secreted constructs, the relative binding levels of the VHH antibodies in the culture supernatants were analyzed by ELISA, using microtiter plates coated with complete TcdB. VHH-G3 from the supernatant of strain KKA383 showed the best binding of the four strains tested. The culture supernatants of strains KKA440 and KKA441, expressing VHH-E2 and VHH-D8, respectively, showed significantly less binding than that of VHH-G3, despite having equal expression levels as analyzed by Western blotting. The VHH-B2 antibody produced from strain KKA442 showed considerable binding despite having an expression level 4- to 5-fold lower than those of the other VHH antibodies as analyzed by Western blotting (Fig. 2).
FIG 2.
Binding of L. paracasei BL23-secreted VHH fragments to TcdB. The relative binding levels of secreted VHH fragments from culture supernatants of engineered strains of L. paracasei BL23 were measured by an ELISA with complete TcdB as the coating antigen.
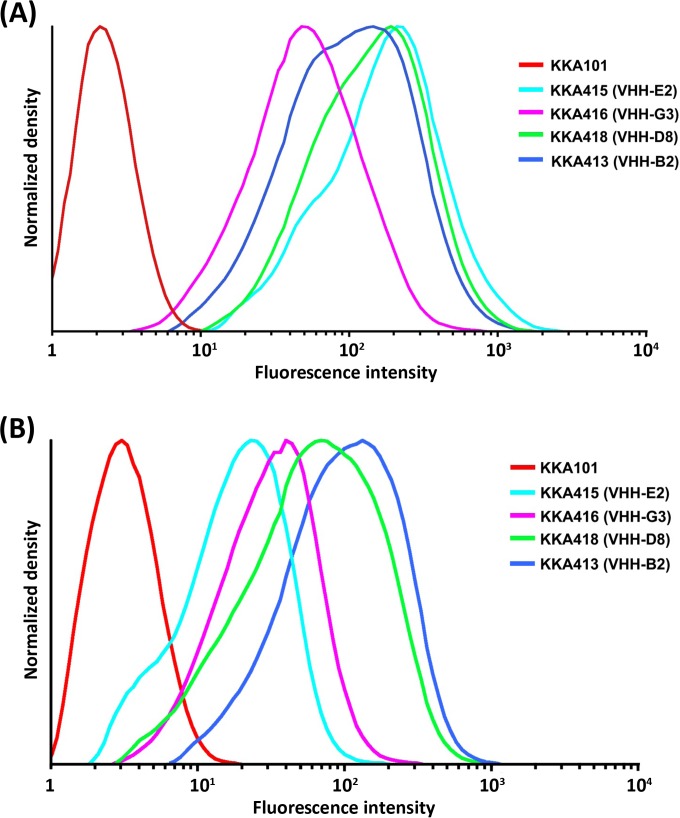
The display and binding of cell wall-anchored VHH antibodies to TcdB were analyzed by flow cytometry using an anti-E-tag antibody recognizing the E tag fused to the VHH fragments (Fig. 3A). The best display was seen for strains KKA415 and KKA418, producing VHH-E2 and VHH-D8, respectively, in accordance with the expression levels observed in Western blots. For comparison, VHH-G3 and VHH-B2, produced by strains KKA413 and KKA416, respectively, had 1- and 2-fold lower levels of display. Toxin binding by cell wall-anchored VHH antibodies was analyzed using biotinylated TcdB (Fig. 3B). All four strains showed significant binding to TcdB, with strains KKA413 and KKA418, expressing VHH-B2 and VHH-D8, respectively, having approximately 2- to 4-fold higher levels of binding to TcdB than those of the other two strains.
FIG 3.
Flow cytometry analysis of the display of cell wall-anchored VHH fragments and their binding to TcdB. (A) Cell wall display of anti-TcdB VHH fragments on the surfaces of L. paracasei BL23 organisms as visualized through the detection of the E tag fused to the VHH fragments by use of a mouse anti-E-tag antibody and an FITC-conjugated rabbit anti-mouse immunoglobulin antibody. (B) Binding of biotinylated TcdB by cell wall-anchored anti-TcdB VHH fragments as detected with phycoerythrin (PE)-conjugated streptavidin.
Mapping of VHH fragment binding to TcdB domains.
Because both TcdA and TcdB belong to the same toxin family and share extended homology, possible cross-reactivity of the anti-TcdB VHH antibodies to TcdA was analyzed by ELISA. All four selected VHH antibodies bound well to TcdB but did not show any cross-reactivity to TcdA (data not shown).
To further narrow down the binding sites, the VHH antibodies produced from Lactobacillus strains were mapped for binding to the three major functional domains constituting the two toxins. Each of the two toxins were cloned and expressed in E. coli as four recombinant fragments, spanning the N-terminal enzymatic domain, the C-terminal receptor binding domain, and (two fragments) the middle transmembrane domain (see Fig. S3A in the supplemental material). The purified toxin fragments from E. coli were used as coating antigens in ELISAs and incubated with the cell culture supernatants of the engineered Lactobacillus strains secreting the VHH fragments. All four VHH fragments produced in Lactobacillus bound exclusively to fragment 4, corresponding to the C-terminal receptor binding domain of TcdB, and showed no cross-reactivity with any of the fragments of the TcdA domains (see Fig. S3B to S3E).
Epitope competition was carried out to analyze if the individual VHH fragments neutralized the toxin activity by binding to distinct sites on the receptor binding domain of the toxin. VHH fragments purified from E. coli were used to compete with Lactobacillus-produced VHH fragments fused to an E tag for binding to TcdB, followed by detection with an anti-E-tag antibody. The binding epitopes of the four VHH fragments corresponded to their respective CDR3 families, with VHH-B2, VHH-G3, and VHH-D8 binding to separate epitopes, while VHH-G3 and VHH-E2 bound to overlapping epitopes (see Fig. S4 in the supplemental material). Each of the four VHH fragments showed epitope self-competition as a validation of the assay. To test a possible synergistic effect of the VHH fragments, combinations of VHH-B2, VHH-G3, and VHH-D8 were tested in the in vitro protection assay as mixtures containing either two or three of the VHH fragments. No discernible additive protective effect was seen for any combination of the VHH fragments compared to the most protective VHH fragment in the mixture used at the same concentration (data not shown).
In vitro neutralization of VHH fragments produced from Lactobacillus.
To test if the TcdB-neutralizing effect was conserved when the VHH fragments were expressed by Lactobacillus, both the secreted and anchored constructs were tested in an in vitro neutralization assay. Because both the supernatants from the Lactobacillus cultures and the direct addition of Lactobacillus to the cell culture assay affected the growth of MA-104 cells, an indirect approach was taken to screen for toxin neutralization.
For the secreted constructs, only strain KKA382, secreting VHH-G3, was analyzed for validation of neutralization. VHH-G3 was purified from the culture supernatant of strain KKA382 through binding to an anti-E-tag column, and the neutralizing capability of affinity-purified VHH-G3 was compared to that of VHH-G3 produced in E. coli. Both VHH-G3 fragments showed identical neutralization of TcdB (with 80 to 320 ng/ml VHH-G3 neutralizing 20 ng/ml TcdB), verifying that the VHH fragment maintained its neutralizing capability when produced by Lactobacillus.
The neutralizing effect of VHH fragments displayed on the Lactobacillus cell surface was analyzed using an adsorption assay. TcdB was incubated with the engineered Lactobacillus strains under conditions of mild agitation, and the supernatant, containing unbound TcdB after removal of Lactobacillus by centrifugation, was assayed for remaining cytotoxicity in the in vitro neutralization assay. An additional four anti-TcdB VHH fragments from the earlier selection, one neutralizing (VHH-B5) and three nonneutralizing (VHH-G1, VHH-D2, and VHH-G9), were expressed on the surfaces of Lactobacillus cells and included in the adsorption assay to analyze if there was a correlation between the in vitro neutralization with soluble VHH antibodies and the adsorption of TcdB when the antibodies were displayed on the cell wall. The adsorption of a 5-fold toxic dose of TcdB was tested on serial dilutions of the engineered Lactobacillus strains. The most efficient adsorption was seen for strain KKA416, displaying VHH-G3, where 2.5 × 108 CFU/ml of engineered bacteria could bind to and remove the cytotoxicity of 50 ng/ml TcdB (Table 2). With an adsorption of at least 80% of the toxin required for neutralization, this corresponds to binding of 360 toxin molecules per Lactobacillus organism. The second best toxin binding was seen with strains KKA413 and KKA417, expressing VHH-B2 and VHH-B5, respectively, with 1 × 109 CFU/ml bacteria providing protection, showing that the three VHH fragments with the highest neutralizing activities also adsorbed the toxin most efficiently when displayed on the cell wall of Lactobacillus. Two VHH fragments, VHH-G1 and VHH-D2, which did not neutralize the toxin as a monomeric soluble form, could adsorb the toxin and confer protection when displayed on the surfaces of lactobacilli. Two of the VHH antibody-displaying strains, KKA415 and KKA419, displaying VHH-E2 and VHH-G9, respectively, did not provide protection at any of the bacterial concentrations tested, indicating that fewer than 11 toxin molecules per Lactobacillus organism were bound by these strains.
TABLE 2.
Adsorption of toxin B by cell wall-displayed VHH fragments
| Strain (VHH fragment) | Protective concn (CFU/ml)a |
|---|---|
| KKA412 (VHH-G1) | 8 × 109 |
| KKA413 (VHH-B2)b | 1 × 109 |
| KKA414 (VHH-D2) | 2 × 109 |
| KKA415 (VHH-E2)b | Not protective |
| KKA416 (VHH-G3)b | 2.5 × 108 |
| KKA417 (VHH-B5)b | 1 × 109 |
| KKA418 (VHH-D8)b | 4 × 109 |
| KKA419 (VHH-G9) | Not protective |
| KKA101 (control) | Not protective |
Protective concentration of engineered L. paracasei BL23 displaying anti-toxin B VHH fragments on the cell wall. The bacteria were tested in 2-fold dilutions from 8 × 109 to 1.25 × 108 CFU/ml for adsorption of 5 times the lethal dose of toxin B (50 ng/ml) in the in vitro neutralization assay.
The VHH fragment was neutralizing as a soluble monomeric form in the in vitro cell-based assay (Table 1).
In vivo protection of anti-TcdB VHH fragments.
The three best in vitro neutralizing VHH fragments (VHH-G3, VHH-D8, and VHH-B2) binding to nonoverlapping epitopes were produced in yeast and tested in the Syrian hamster model of C. difficile disease (38). Hamsters were treated with clindamycin to disrupt the normal gastrointestinal flora for 24 h prior to the challenge with spores of a TcdA− TcdB+ strain of C. difficile 630(Δerm). Post-spore challenge, the hamsters were monitored for 5 days for signs of disease (decreased activity, wet tail, and toxin-positive feces) and, ultimately, death. To mimic a prophylactic treatment, hamsters were treated with a mixture containing 125 μg each of the three neutralizing VHH fragments twice daily for a duration of 6 days, with the first dose given 1 day prior to the spore challenge. No protection was achieved despite the high levels of anti-TcdB VHH antibodies being given continuously during the treatment. The group receiving anti-TcdB VHH antibodies started showing signs of disease at 1 to 2 days post-spore challenge and succumbed to the infection on days 3 and 4, as was also observed for nontreated hamsters receiving spores or hamsters receiving 375 μg of an irrelevant anti-rotavirus VHH antibody (ARP1) twice daily (data not shown). To test if the gastrointestinal environment could degrade the VHH fragments, the VHH-B2 and VHH-G3 fragments were incubated with extracts of hamster intestinal content. Both VHH-B2 and VHH-G3 showed less resistance against proteolysis by gastrointestinal proteases than the ARP1 VHH fragment. VHH-G3 and VHH-B2 were degraded approximately 30-fold and 2-fold faster, respectively, than ARP1 (see Fig. S5 in the supplemental material).
In vivo protection by Lactobacillus-produced VHH fragments.
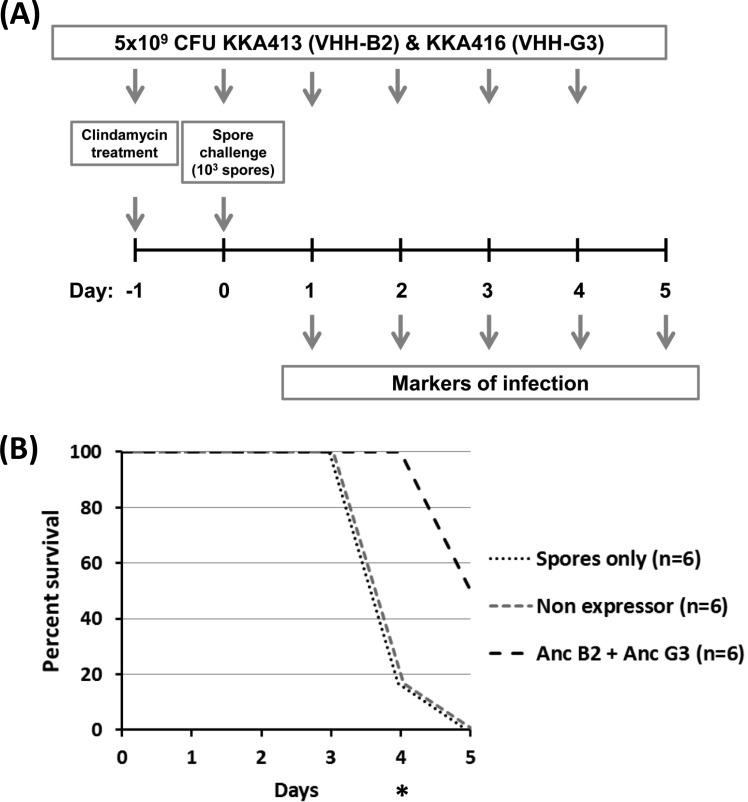
To test if the lack of protection seen with the yeast-purified VHH fragments could be overcome by continuous in situ production of the toxin-neutralizing VHH fragments, the hamster protection model was repeated with engineered Lactobacillus strains expressing toxin-neutralizing VHH fragments. With the yeast-purified VHH fragments failing to provide protection at concentrations exceeding what could likely be achieved by L. paracasei BL23 secreting VHH fragments, we decided to focus on toxin neutralization through cell wall-anchored display by lactobacilli. Two strains of Lactobacillus displaying VHH fragments binding to nonoverlapping epitopes were used in combination. Strains KKA413 and KKA416, displaying VHH-B2 and VHH-G3, respectively, were chosen because they showed the highest in vitro binding and had a higher neutralization activity in the in vitro adsorption assay. Neutralization was tested in a prophylactic hamster protection model receiving a combined dose containing 5 × 109 CFU of each of the two Lactobacillus strains, KKA413 and KKA416, twice daily for the duration of the experiment (Fig. 4A). Spore germination and intestinal colonization by C. difficile were tested by an enzyme immunoassay (EIA) on fecal droppings for the presence of GDH, a cell wall-associated metabolic enzyme produced by C. difficile and used as a marker of vegetative C. difficile (see Table S3 in the supplemental material). TcdB production and the onset of virulence after colonization were detected by an immunochromatographic test for the presence of toxins in fecal droppings. The progression of disease to the onset of diarrhea was monitored through observation of the hamsters for the characteristic wet tail.
FIG 4.
Effect of therapeutic administration of L. paracasei BL23 strains displaying cell wall-anchored VHH fragments neutralizing TcdB in a hamster model of C. difficile infection. (A) Schematic outline of the hamster infection model treated with engineered L. paracasei BL23 strains expressing cell wall-anchored toxin-neutralizing VHH fragments. Clindamycin (30 mg/kg of body weight) was given at day −1 to destabilize the gastrointestinal flora. Hamsters were challenged with 103 spores of C. difficile 630 TcdA− TcdB+ at day 0. A dose of 5 × 109 CFU each of KKA413 (VHH-B2) and KKA416 (VHH-G3) was given twice daily by gavage. The following markers of progression of disease were monitored daily: GDH and TcdB in feces, diarrhea (wet tail), and mortality. (B) Viability of hamsters challenged with spores of C. difficile 630 TcdA− TcdB+. *, P < 0.05. Anc, cell wall anchored.
Hamsters receiving spores only or Lactobacillus harboring an empty expression plasmid started to succumb to the infection on day 4, with 5 of 6 hamsters being dead in both groups (Fig. 4B). For the group receiving the engineered Lactobacillus strains expressing the toxin-neutralizing VHH fragments, all hamsters were alive at day 4 (P < 0.05). At day 5, when the model was terminated, all hamsters in the infected control group and the group receiving the nonexpressing Lactobacillus strain were dead. In the group receiving the Lactobacillus strains expressing the toxin-neutralizing VHH fragments, 3 hamsters died, but the remaining 3 showed no behavioral signs of being infected with C. difficile.
The progression of CDI was rapid for hamsters in the nontreated groups receiving spores only or the nonexpressing lactobacilli. Feces generally tested positive for colonization by C. difficile, toxin production, and diarrhea (wet tail) at day 3, and within 24 h, hamsters succumbed to the infection (see Table S3 in the supplemental material). For the hamsters receiving the engineered Lactobacillus strains, 4 of 6 hamsters showed a delayed progression of infection after colonization and survived for up to 4 days after the detection of GDH in feces. Detection of toxins was similarly delayed, with 2 of the surviving hamsters having toxin-negative feces upon termination of the experiment, despite having tested positive for the presence of vegetative C. difficile by the GDH test for 3 and 4 consecutive days.
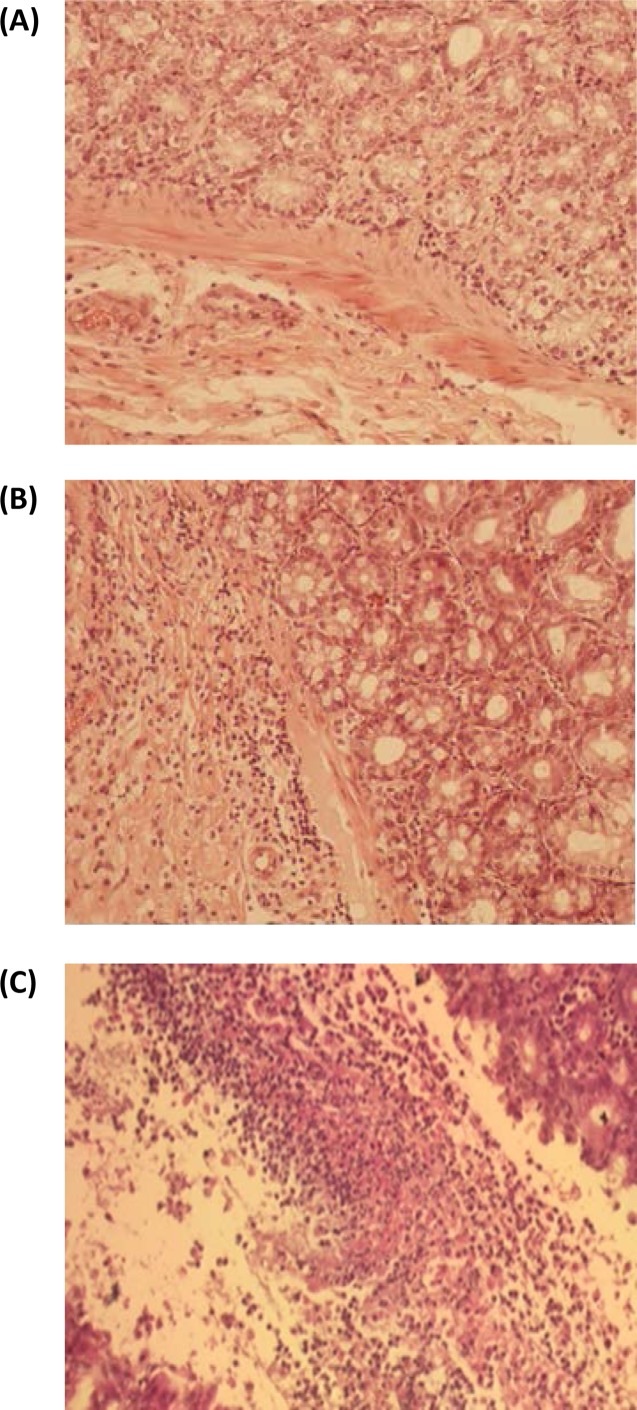
Histological sections from the small and large intestines were analyzed for inflammatory markers and scored for severity on a scale of 0 to 5 (normal, hyperemia, cellular infiltration, necrosis, and pseudomembranes) (39). Unlike in humans, inflammation of the ileal and cecal mucosae has been reported for hamsters with CDI (40, 41). The histology of the small intestines of all three surviving hamsters showed no morphological changes of the mucosa (grade 0). For the large intestine, one of the three surviving hamsters (animal 54-2) showed signs of mild colitis, with lymphocyte and histiocyte infiltration of the colonic mucosa (grade 2) (Fig. 5). The two other surviving hamsters (animals 54-1 and 54-5) showed normal, undamaged mucosae with no morphological changes, despite one of the hamsters (animal 54-5) having had feces positive for TcdB on day 4 after infection. Samples of the blood, spleen, and liver collected at autopsy from all three surviving hamsters were negative for C. difficile. Mild hyperemia was detected by histology for the spleens and livers of all three surviving hamsters.
FIG 5.
Cecum histology of hamsters surviving spore challenge after prophylactic treatment with L. paracasei BL23 strains displaying cell wall-anchored VHH fragments neutralizing TcdB. Hematoxylin- and eosin-stained sections of ceca from different treatment groups were assessed for inflammation and cellular destruction. (A) Normal cecum mucosa of hamster 54-5, with no signs of lesions or inflammation. (B) Mild colitis, with lymphocyte and histiocyte infiltration (grade 2), in the mucosa of the cecum of hamster 54-2. (C) Severe colitis, with necrotic masses with fibrin, macrophages, and neutrophils (pseudomembranes) (grade 5), in the mucosa of the cecum of a nonprotected hamster challenged with C. difficile TcdA− TcdB+ spores.
DISCUSSION
Oral therapy against CDI in humans by use of toxin-neutralizing antibodies was previously explored using hyperimmune bovine colostrum (HBC) and showed therapeutic potential by alleviating the effects of CDAD and reducing the frequency of relapse (19, 20). The aim of the present study was to explore the use of Lactobacillus for expression of toxin-neutralizing antibody fragments to provide in situ neutralization of C. difficile toxins.
In the current study, a broad range of TcdB-neutralizing VHH antibodies was developed. The protective concentrations of anti-TcdB VHH antibodies for neutralizing 20 ng of TcdB in vitro ranged from 80 to 320 ng/ml to 5.12 μg/ml, corresponding to 55- to 220-fold molar excesses of VHH fragments to TcdB for complete neutralization by the best-neutralizing VHH antibody, VHH-G3. This protective range is comparable to or better than that for previously isolated therapeutic anti-TcdB hMAbs tested for neutralization in a similar assay (42), suggesting that the anti-TcdB VHH antibodies isolated in the present study could be suitable for therapeutic use. All four neutralizing anti-TcdB VHH antibodies bound to the cell wall binding domain, indicating that their neutralizing effect most likely arises by blocking toxin binding to the receptor, an interaction that is desirable from a therapeutic perspective because it would prevent uptake of the toxin and the neutralized toxin would remain in the intestine and be eliminated with the feces.
When expressed in Lactobacillus, the anti-TcdB VHH antibodies maintained their neutralizing capabilities in vitro both when secreted into the supernatant and when anchored on the cell wall surface. For adsorption with cell wall-anchored VHH antibodies, a nonneutralizing VHH antibody with a high binding affinity should theoretically be able to immobilize the toxin as efficiently as a neutralizing VHH antibody with an equal binding affinity. Interestingly, it was the two best-neutralizing VHH fragments (VHH-B2 and VHH-G3) that also provided the most efficient binding in the adsorption assay when displayed as cell wall-anchored fragments, whereas the nonneutralizing VHH fragments included in this experiment (VHH-G1, VHH-D2, and VHH-G9) did not appear to bind the VHH antibodies as efficiently. It seems as though VHH-B2 and VHH-G3 not only block epitopes required for toxicity but also have the highest binding affinities among the eight VHH fragments expressed in a cell wall-anchored mode in this study.
The C. difficile hamster model was used to assess the protective effect conferred by toxin-neutralizing VHH antibodies, as it is a well-characterized model which shares some of the recognized features of the human infection. A drawback to this model is the exquisite susceptibility to C. difficile after destabilization of the bacterial flora by use of antibiotics, giving a short course of disease and resulting in a heightened severity and increased mortality compared to those of the disease affecting humans. Therapeutic intervention in the hamster model of CDI has proven very challenging, and the requirement for efficacy of toxin neutralization is very high because it is more of a “prevention of death” model (43). Hamsters may occasionally develop a wet tail, display symptoms of watery diarrhea, lethargy, and irritability, and refuse food, but invariably they will die from the spore challenge unless they are treated. A prophylactic oral treatment model was chosen for the current study because it would be the most likely application for Lactobacillus-mediated toxin neutralization for treatment of CDI.
The failure of the mixture of three yeast-purified TcdB-neutralizing VHH fragments (B2, G3, and D8) to protect animals in the hamster model, despite having shown good in vitro neutralization, was unexpected. The mixture of three neutralizing VHH fragments was given twice daily at doses that would be comparable to the higher range of what could be expected to be secreted from the engineered Lactobacillus strains. In a previous study, a combined dose of 80 mg of chicken IgY polyclonal antibodies against TcdA and TcdB given thrice daily was required to give protection in a prophylactic hamster protection model (44). Although it is not possible to make a direct comparison for toxin neutralization, this dose is approximately 0.6 to 3 times the one used in our study, considering the molecular weight of IgY and that 2 to 10% of total IgY can be expected to be antigen specific (45). VHH-G3 showed very little resistance to proteolytic inactivation compared to a control fragment (ARP1) previously used in an animal model of gastrointestinal infection, which could explain the lack of protection seen with the yeast-purified VHH fragments in the hamster model of CDI. Similarly, the majority (>98%) of bovine-derived anti-C. difficile immunoglobulins were previously found to be degraded in the human gastrointestinal tract when administered orally (46). Engineering of the VHH fragments for improved proteolytic resistance has previously been shown to significantly improve stability and could therefore be a promising approach for further development of the VHH fragments (21, 22). Since the increased neutralization of IgY antibody could be conferred both by a higher proteolytic stability and by its bivalency, we hypothesized that VHH antibody fragments could be more effective if produced continuously and displayed on the surfaces of lactobacilli. A delay in development of infection and partial protection were indeed observed for hamsters orally treated with two engineered strains of L. paracasei BL23, displaying VHH-B2 and VHH-G3. In addition, four of the hamsters receiving engineered Lactobacillus had toxin-negative feces despite being colonized by C. difficile, confirming a possible adsorptive effect of the VHH fragments displayed on the cell wall of Lactobacillus. For the remaining two hamsters, the disease manifested as usual, with the animals succumbing to the infection within 24 h of testing positive for vegetative C. difficile despite receiving Lactobacillus strains expressing toxin-neutralizing antibody fragments. The results suggest a threshold effect where, unless sufficient neutralizing VHH antibodies are present to completely block the toxins, the disease will progress and be fatal.
The complete absence of or very limited mucosal damage in the histological sections from the ceca of the three surviving hamsters, despite the animals having been colonized with C. difficile for up to 4 days, is significant considering the rapid progression of the disease and the extensive damage to the colonic mucosa seen in CDI in hamsters. These results again suggest that binding of toxin to cell wall-displayed VHH fragments has the potential to efficiently neutralize the cytotoxic effects of TcdB.
The observation that the Lactobacillus strains displaying the toxin-neutralizing VHH fragments conferred a protective effect in the hamster protection model, while the yeast-purified VHH fragments failed to have an effect, raises interesting questions. The dose of VHH fragments administered to hamsters was 100-fold lower with engineered lactobacilli than that for purified VHH fragments. Several non-mutually exclusive possibilities may explain why cell wall-anchored expression of the VHH fragments could be advantageous compared to the use of yeast-purified VHH fragments. The continuous production of the VHH fragments on the cell surface of lactobacilli in the gastrointestinal tract could outcompete the ongoing proteolysis of the VHH fragments. Likewise, the anchoring of the VHH fragments on the surfaces of Lactobacillus organisms would markedly increase the footprint of the VHH antibodies bound to toxin and make a larger part of the receptor binding domain inaccessible for binding to the receptor. The bound toxin would also not be free to diffuse in the gastrointestinal tract when immobilized on the cell walls of Lactobacillus organisms. Lastly, the use of a mixture of two Lactobacillus strains expressing VHH antibody fragments, binding two different epitopes, could also contribute to increasing the antibody avidity and promoting agglutination and clearance of the toxins.
Recently, a single intravenous dose of a bispecific antibody composed of two VHH fragments, against both TcdA and TcdB, was shown to reverse fulminant CDI in a mouse model (47). The in vivo neutralizing activity of the bispecific antibody was at least 300-fold more potent than that of the mixture of the individual components, showing the importance of multivalency for toxin neutralization. These results confirm the potential of using VHH antibodies as an affordable antibody-based approach for the treatment of CDI. Although systemic administration of monoclonal antibodies was previously found to be protective against a C. difficile challenge (42, 47), very few studies have reported protection for oral delivery of antibodies, showing the difficulty of this approach (44, 48, 49). Using a similar experimental setup, Kink and Williams (44) observed that hamsters fed daily for 4 consecutive days with a high dose of antitoxin chicken immunoglobulin were protected over a period of 20 days following spore challenge. In the current study, the hamster model had to be terminated at day 5 to comply with the ethical approval, and thus no information on the long-term protection conferred by the engineered Lactobacillus strains could be obtained. However, the surviving hamsters showed limited mucosal damage despite having been colonized by C. difficile for several days, suggesting that these hamsters might have survived for a longer period.
The initial aim of the present study was to produce VHH fragments capable of neutralizing both TcdA and TcdB from C. difficile. Anti-TcdA VHH fragments were therefore also generated through an approach identical to that described here for anti-TcdB VHH fragments (data not shown). These VHH fragments provided only a transient protection against TcdA in vitro that was gradually overcome with time, eventually resulting in complete cell death. This suggests that the binding affinities of the selected VHH fragments were too low to compete with the receptor binding or that not all of the relevant epitopes for preventing toxin processing were blocked. Despite extensive efforts at panning the anti-TcdA VHH libraries for protective clones, no anti-TcdA VHH fragments that could confer complete protection against TcdA, either on their own or used in combinations of multiple VHH fragments, were found. The reason for the lack of neutralizing anti-TcdA VHH antibody despite a neutralizing serum response from the immunized llamas is not evident, as successful isolation of TcdA-neutralizing VHH fragments has been reported previously (47, 50). Slight variations in the antigen used for the immunization could possibly account for the differences, with the two previous studies using the TcdA receptor binding site and a glycosyltransferase-deficient holotoxin of TcdA, respectively, while detoxified toxin A was used in our study.
The study of the therapeutic use of recombinant lactobacilli for treatment of CDI as presented in its current form has some limitations that should be addressed in future studies. The animal model was performed with a small number of hamsters, and the follow-up period was limited to only 5 days for ethical reasons, restricting the information on the long-term effects in the surviving hamsters. Future studies utilizing a more refined hamster model (51) permitting prolongation of the experiment could provide further information on the efficacy of treatment and whether the surviving hamsters were completely protected from the produced TcdB toxin. Likewise, studies with a C. difficile relapse model could be interesting, as this is the application where HBC has proven to be most effective (19, 20). Furthermore, some hamsters tested toxin negative by an enzymatic immunoassay despite being colonized by C. difficile. The use of the more sensitive cytotoxicity assay for this test and typing of the colonizing strains would have confirmed if these hamsters were truly toxin negative and colonized by the strain of C. difficile used for the challenge. In the present study, VHH antibodies were selected against TcdB of C. difficile 630 as proof of principle for the use of Lactobacillus-producing VHH antibodies as a therapy against C. difficile infections. For therapeutic applications, it would be desirable to select VHH antibodies cross-reacting against a broad range of pathogenic strains, including the North American isolate BI/NAP1/027, as well as expressing VHH antibodies neutralizing both toxins A and B. The L. paracasei BL23 strain used in the current study was not selected for long-term colonization and required administration twice daily during the infection, and administration would likely need to be continued for at least a few days after symptoms have disappeared but might even have a positive effect against relapses if administration is prolonged after overcoming the initial infection. For future therapeutic use, a strain colonizing the human gastrointestinal tract should be chosen to improve the delivery of the VHH fragments and make it possible to reduce the frequency of administration. A contained and stable, chromosomally integrated expression system would furthermore be required to enable therapeutic application in humans, but this has already been developed (31).
For therapeutic applications, both intravenous and oral lactobacillus-based deliveries of VHH fragments are promising, and they could even be used in combination. Intravenous delivery of VHH fragments is likely to be the most effective treatment option for fulminant CDI, but this route is more invasive and costly than oral delivery. Oral delivery by engineered lactobacilli, on the other hand, would be more suitable for prophylactic treatment of patients in the risk group for contracting CDI before hospitalization and as a long-term treatment against recurrent CDI. Both the low cost and easy application of engineered Lactobacillus strains would make them ideally suitable for the extensive application required for both prophylactic treatment and prevention of relapse CDAD. In addition, lactobacillus-based oral delivery of VHH fragments could possibly also be used in conjunction with other emerging therapeutic approaches, such as fecal transplantation and a narrow-spectrum antibiotic, such as fidaxomicin (33–35).
In the present study, we have shown that lactobacilli displaying anti-TcdB VHH fragments can inhibit the cytotoxic effect of C. difficile TcdB in the gastrointestinal tract in a prophylactic hamster model. The possibility of in situ neutralization in the hamster model suggests that the strategy could be worth exploring as a supplement to existing therapies for patients. For therapeutic applications, a dual expression strategy where both TcdA and TcdB are targeted and neutralized, possibly through generation of a bispecific antibody, will probably be necessary to provide protection in a clinical setting. Likewise, the selection of VHH antibodies with broad cross-neutralization of toxin types of C. difficile would significantly improve the therapeutic potential of the strategy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the European Union (EU)-funded project LACTOBODY (grant 202162) and by a grant from the Ruth and Richard Juhlin Foundation.
We are grateful to Nigel Minton (School of Molecular Medical Sciences, The University of Nottingham, United Kingdom) for the kind gift of the C. difficile 630 (TcdA− TcdB+) strain.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00870-15.
REFERENCES
- 1.Ozaki E, Kato H, Kita H, Karasawa T, Maegawa T, Koino Y, Matsumoto K, Takada T, Nomoto K, Tanaka R, Nakamura S. 2004. Clostridium difficile colonization in healthy adults: transient colonization and correlation with enterococcal colonization. J Med Microbiol 53:167–172. doi: 10.1099/jmm.0.05376-0. [DOI] [PubMed] [Google Scholar]
- 2.Loo VG, Bourgault AM, Poirier L, Lamothe F, Michaud S, Turgeon N, Toye B, Beaudoin A, Frost EH, Gilca R, Brassard P, Dendukuri N, Beliveau C, Oughton M, Brukner I, Dascal A. 2011. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 365:1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 3.Lucado J, Gould C, Elixhauser A. 2006. Clostridium difficile infections (CDI) in hospital stays, 2009. Healthcare Cost and Utilization Project (HCUP) statistical briefs, statistical brief #124. Agency for Healthcare Research and Quality, Rockville, MD. [PubMed] [Google Scholar]
- 4.Hickson M. 2011. Probiotics in the prevention of antibiotic-associated diarrhoea and Clostridium difficile infection. Ther Adv Gastroenterol 4:185–197. doi: 10.1177/1756283X11399115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyne L. 2010. Clostridium difficile—beyond antibiotics. N Engl J Med 362:264–265. doi: 10.1056/NEJMe0910055. [DOI] [PubMed] [Google Scholar]
- 6.Carter GP, Rood JI, Lyras D. 2012. The role of toxin A and toxin B in the virulence of Clostridium difficile. Trends Microbiol 20:21–29. doi: 10.1016/j.tim.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Lyras D, O'Connor JR, Howarth PM, Sambol SP, Carter GP, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding DN, Rood JI. 2009. Toxin B is essential for virulence of Clostridium difficile. Nature 458:1176–1179. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuehne SA, Cartman ST, Heap JT, Kelly ML, Cockayne A, Minton NP. 2010. The role of toxin A and toxin B in Clostridium difficile infection. Nature 467:711–713. doi: 10.1038/nature09397. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor JR, Johnson S, Gerding DN. 2009. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 136:1913–1924. doi: 10.1053/j.gastro.2009.02.073. [DOI] [PubMed] [Google Scholar]
- 10.Freeman J, Bauer MP, Baines SD, Corver J, Fawley WN, Goorhuis B, Kuijper EJ, Wilcox MH. 2010. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev 23:529–549. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. 2005. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 366:1079–1084. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 12.Gerding DN. 2004. Clindamycin, cephalosporins, fluoroquinolones, and Clostridium difficile-associated diarrhea: this is an antimicrobial resistance problem. Clin Infect Dis 38:646–648. doi: 10.1086/382084. [DOI] [PubMed] [Google Scholar]
- 13.Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH, Society for Healthcare Epidemiology of America, Infectious Diseases Society of America . 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 14.Gough E, Shaikh H, Manges AR. 2011. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis 53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 15.Kassam Z, Lee CH, Yuan Y, Hunt RH. 2013. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol 108:500–508. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 16.Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Gerding DN, Nichol G, Thomas WD Jr, Leney M, Sloan S, Hay CA, Ambrosino DM. 2010. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 17.Kyne L, Warny M, Qamar A, Kelly CP. 2001. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet 357:189–193. doi: 10.1016/S0140-6736(00)03592-3. [DOI] [PubMed] [Google Scholar]
- 18.Kelly CP, Kyne L. 2011. The host immune response to Clostridium difficile. J Med Microbiol 60:1070–1079. doi: 10.1099/jmm.0.030015-0. [DOI] [PubMed] [Google Scholar]
- 19.van Dissel JT, de Groot N, Hensgens CM, Numan S, Kuijper EJ, Veldkamp P, van't Wout J. 2005. Bovine antibody-enriched whey to aid in the prevention of a relapse of Clostridium difficile-associated diarrhoea: preclinical and preliminary clinical data. J Med Microbiol 54:197–205. doi: 10.1099/jmm.0.45773-0. [DOI] [PubMed] [Google Scholar]
- 20.Numan SC, Veldkamp P, Kuijper EJ, van den Berg RJ, van Dissel JT. 2007. Clostridium difficile-associated diarrhoea: bovine anti-Clostridium difficile whey protein to help aid the prevention of relapses. Gut 56:888–889. doi: 10.1136/gut.2006.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmsen MM, van Solt CB, van Zijderveld-van Bemmel AM, Niewold TA, van Zijderveld FG. 2006. Selection and optimization of proteolytically stable llama single-domain antibody fragments for oral immunotherapy. Appl Microbiol Biotechnol 72:544–551. doi: 10.1007/s00253-005-0300-7. [DOI] [PubMed] [Google Scholar]
- 22.Hussack G, Hirama T, Ding W, Mackenzie R, Tanha J. 2011. Engineered single-domain antibodies with high protease resistance and thermal stability. PLoS One 6:e28218. doi: 10.1371/journal.pone.0028218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pant N, Hultberg A, Zhao Y, Svensson L, Pan-Hammarstrom Q, Johansen K, Pouwels PH, Ruggeri FM, Hermans P, Frenken L, Boren T, Marcotte H, Hammarstrom L. 2006. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. J Infect Dis 194:1580–1588. doi: 10.1086/508747. [DOI] [PubMed] [Google Scholar]
- 24.Wells JM, Mercenier A. 2008. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol 6:349–362. doi: 10.1038/nrmicro1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genth H, Selzer J, Busch C, Dumbach J, Hofmann F, Aktories K, Just I. 2000. New method to generate enzymatically deficient Clostridium difficile toxin B as an antigen for immunization. Infect Immun 68:1094–1101. doi: 10.1128/IAI.68.3.1094-1101.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strokappe N, Szynol A, Aasa-Chapman M, Gorlani A, Forsman Quigley A, Hulsik DL, Chen L, Weiss R, de Haard H, Verrips T. 2012. Llama antibody fragments recognizing various epitopes of the CD4bs neutralize a broad range of HIV-1 subtypes A, B and C. PLoS One 7:e33298. doi: 10.1371/journal.pone.0033298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N. 2006. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc 1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 28.Verheesen P, Roussis A, de Haard HJ, Groot AJ, Stam JC, den Dunnen JT, Frants RR, Verkleij AJ, Theo Verrips C, van der Maarel SM. 2006. Reliable and controllable antibody fragment selections from camelid non-immune libraries for target validation. Biochim Biophys Acta 1764:1307–1319. doi: 10.1016/j.bbapap.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Marks JD, Hoogenboom HR, Bonnert TP, McCafferty J, Griffiths AD, Winter G. 1991. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol 222:581–597. [DOI] [PubMed] [Google Scholar]
- 30.Hultberg A, Temperton NJ, Rosseels V, Koenders M, Gonzalez-Pajuelo M, Schepens B, Ibanez LI, Vanlandschoot P, Schillemans J, Saunders M, Weiss RA, Saelens X, Melero JA, Verrips CT, Van Gucht S, de Haard HJ. 2011. Llama-derived single domain antibodies to build multivalent, superpotent and broadened neutralizing anti-viral molecules. PLoS One 6:e17665. doi: 10.1371/journal.pone.0017665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin MC, Pant N, Ladero V, Gunaydin G, Andersen KK, Alvarez B, Martinez N, Alvarez MA, Hammarstrom L, Marcotte H. 2011. Integrative expression system for delivery of antibody fragments by lactobacilli. Appl Environ Microbiol 77:2174–2179. doi: 10.1128/AEM.02690-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marcotte H, Koll-Klais P, Hultberg A, Zhao Y, Gmur R, Mandar R, Mikelsaar M, Hammarstrom L. 2006. Expression of single-chain antibody against RgpA protease of Porphyromonas gingivalis in Lactobacillus. J Appl Microbiol 100:256–263. doi: 10.1111/j.1365-2672.2005.02786.x. [DOI] [PubMed] [Google Scholar]
- 33.Andersen KK, Marcotte H, Alvarez B, Boyaka PN, Hammarstrom L. 2011. In situ gastrointestinal protection against anthrax edema toxin by single-chain antibody fragment producing lactobacilli. BMC Biotechnol 11:126. doi: 10.1186/1472-6750-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitaker AM, Hayward CJ. 1985. The characterization of three monkey kidney cell lines. Dev Biol Stand 60:125–131. [PubMed] [Google Scholar]
- 35.Torres J, Camorlinga-Ponce M, Munoz O. 1992. Sensitivity in culture of epithelial cells from rhesus monkey kidney and human colon carcinoma to toxins A and B from Clostridium difficile. Toxicon 30:419–426. doi: 10.1016/0041-0101(92)90538-G. [DOI] [PubMed] [Google Scholar]
- 36.Baines SD, Freeman J, Wilcox MH. 2005. Effects of piperacillin/tazobactam on Clostridium difficile growth and toxin production in a human gut model. J Antimicrob Chemother 55:974–982. doi: 10.1093/jac/dki120. [DOI] [PubMed] [Google Scholar]
- 37.Gorlani A, Hulsik DL, Adams H, Vriend G, Hermans P, Verrips T. 2012. Antibody engineering reveals the important role of J segments in the production efficiency of llama single-domain antibodies in Saccharomyces cerevisiae. Protein Eng Des Sel 25:39–46. doi: 10.1093/protein/gzr057. [DOI] [PubMed] [Google Scholar]
- 38.Sambol SP, Tang JK, Merrigan MM, Johnson S, Gerding DN. 2001. Infection of hamsters with epidemiologically important strains of Clostridium difficile. J Infect Dis 183:1760–1766. doi: 10.1086/320736. [DOI] [PubMed] [Google Scholar]
- 39.Naaber P, Mikelsaar RH, Salminen S, Mikelsaar M. 1998. Bacterial translocation, intestinal microflora and morphological changes of intestinal mucosa in experimental models of Clostridium difficile infection. J Med Microbiol 47:591–598. doi: 10.1099/00222615-47-7-591. [DOI] [PubMed] [Google Scholar]
- 40.Price AB, Larson HE, Crow J. 1979. Morphology of experimental antibiotic-associated enterocolitis in the hamster: a model for human pseudomembranous colitis and antibiotic-associated diarrhoea. Gut 20:467–475. doi: 10.1136/gut.20.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ni Eidhin DB, O'Brien JB, McCabe MS, Athie-Morales V, Kelleher DP. 2008. Active immunization of hamsters against Clostridium difficile infection using surface-layer protein. FEMS Immunol Med Microbiol 52:207–218. doi: 10.1111/j.1574-695X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- 42.Babcock GJ, Broering TJ, Hernandez HJ, Mandell RB, Donahue K, Boatright N, Stack AM, Lowy I, Graziano R, Molrine D, Ambrosino DM, Thomas WD Jr. 2006. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infect Immun 74:6339–6347. doi: 10.1128/IAI.00982-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Best EL, Freeman J, Wilcox MH. 2012. Models for the study of Clostridium difficile infection. Gut Microbes 3:145–167. doi: 10.4161/gmic.19526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kink JA, Williams JA. 1998. Antibodies to recombinant Clostridium difficile toxins A and B are an effective treatment and prevent relapse of C. difficile-associated disease in a hamster model of infection. Infect Immun 66:2018–2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Nakano T, Sunwoo HH, Paek BH, Chae HS, Sim JS. 1998. Effects of egg and yolk weights on yolk antibody (IgY) production in laying chickens. Poul Sci 77:266–270. doi: 10.1093/ps/77.2.266. [DOI] [PubMed] [Google Scholar]
- 46.Kelly CP, Chetham S, Keates S, Bostwick EF, Roush AM, Castagliuolo I, LaMont JT, Pothoulakis C. 1997. Survival of anti-Clostridium difficile bovine immunoglobulin concentrate in the human gastrointestinal tract. Antimicrob Agents Chemother 41:236–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang Z, Schmidt D, Liu W, Li S, Shi L, Sheng J, Chen K, Yu H, Tremblay JM, Chen X, Piepenbrink KH, Sundberg EJ, Kelly CP, Bai G, Shoemaker CB, Feng H. 2014. A novel multivalent, single-domain antibody targeting TcdA and TcdB prevents fulminant Clostridium difficile infection in mice. J Infect Dis 210:964–972. doi: 10.1093/infdis/jiu196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyerly DM, Bostwick EF, Binion SB, Wilkins TD. 1991. Passive immunization of hamsters against disease caused by Clostridium difficile by use of bovine immunoglobulin G concentrate. Infect Immun 59:2215–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mulvey GL, Dingle TC, Fang L, Strecker J, Armstrong GD. 2011. Therapeutic potential of egg yolk antibodies for treating Clostridium difficile infection. J Med Microbiol 60:1181–1187. doi: 10.1099/jmm.0.029835-0. [DOI] [PubMed] [Google Scholar]
- 50.Hussack G, Arbabi-Ghahroudi M, van Faassen H, Songer JG, Ng KK, MacKenzie R, Tanha J. 2011. Neutralization of Clostridium difficile toxin A with single-domain antibodies targeting the cell receptor binding domain. J Biol Chem 286:8961–8976. doi: 10.1074/jbc.M110.198754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Douce G, Goulding D. 2010. Refinement of the hamster model of Clostridium difficile disease. Methods Mol Biol 646:215–227. doi: 10.1007/978-1-60327-365-7_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.