Abstract
Inflammatory bowel disease (IBD) is characterized by chronic relapsing inflammatory disorders of the gastrointestinal tract, and includes two major phenotypes: ulcerative colitis and Crohn’s disease. The pathogenesis of IBD is not fully understood as of yet. It is believed that IBD results from complicated interactions between environmental factors, genetic predisposition, and immune disorders. miRNAs are a class of small non-coding RNAs that can regulate gene expression by targeting the 3′-untranslated region of specific mRNAs for degradation or translational inhibition. miRNAs are considered to play crucial regulatory roles in many biologic processes, such as immune cellular differentiation, proliferation, and apoptosis, and maintenance of immune homeostasis. Recently, aberrant expression of miRNAs was revealed to play an important role in autoimmune diseases, including IBD. In this review, we discuss the current understanding of how miRNAs regulate autoimmunity and inflammation by affecting the differentiation, maturation, and function of various immune cells. In particular, we focus on describing specific miRNA expression profiles in tissues and peripheral blood that may be associated with the pathogenesis of IBD. In addition, we summarize the opportunities for utilizing miRNAs as new biomarkers and as potential therapeutic targets in IBD.
Keywords: Autoimmunity, Immune system, Inflammation, Inflammatory bowel disease, miRNA
Core tip: Inflammatory bowel disease (IBD) is characterized by chronic relapsing inflammation in the gastrointestinal tract, but its pathogenesis remains unclear. Further understanding of the molecular mechanisms of IBD is helpful to find new therapeutic strategies. miRNAs play crucial regulatory roles in immune cellular differentiation and maturation, and maintaining immune homeostasis. Aberrant expression of miRNAs is present in IBD. Here, we summarize how miRNAs regulate autoimmunity and inflammation, and describe specific miRNA expression profiles in IBD. We also discuss the opportunities in utilizing miRNAs as new biomarkers and potential therapeutic targets in IBD.
INTRODUCTION
Inflammatory bowel disease (IBD) comprises ulcerative colitis (UC) and Crohn’s disease (CD). IBD is characterized by chronic relapsing inflammation in the gastrointestinal tract, and its incidence and prevalence are increasing[1]. The precise pathogenic mechanism of IBD remains unknown. Accumulated evidence suggests that IBD results from complicated interactions between environmental factors, genetic predisposition, and immune dysregulation. Of these, immune dysregulation is believed to play an important role in the pathogenesis of IBD[2]. Consequently, it is important to uncover the molecular mechanisms that regulate the immune responses in IBD.
miRNAs are a new class of small non-coding RNAs that regulate immune responses in physiologic and pathologic conditions[3]. miRNAs are considered to play significant roles in many biologic processes, including cellular proliferation, differentiation, maturation, and apoptosis[4]. In addition, miRNAs have been implicated in the pathogenesis of common human diseases, such as cardiovascular[5], neurologic[6], and hematologic diseases, cancer[7], and inflammatory and autoimmune diseases[8]. These research findings have led to new insights into IBD pathogenesis.
In this review, we summarize recent findings that miRNAs regulate autoimmunity and inflammation by affecting the differentiation, maturation, and function of various immune cells. We particularly focus on providing evidence of specific miRNA expression profiles in IBD pathogenesis. In addition, we also discuss the possibility for miRNAs as new biomarkers and potential therapeutic targets in IBD.
GENERAL OVERVIEW OF MIRNA
miRNAs are a new class of small (about 22 nucleotides), endogenous, non-coding single-stranded RNA molecules that can negatively regulate target gene expression at the post-transcriptional level[9]. The first miRNA, lin-4, was identified in 1993 in Caenorhabditis elegans[10]. The miRNA sequence database, miRBase, contains 35,828 mature miRNAs in 223 species at time of publication (http://www.mirbase.org/, Release 21, June 2014)[11].
miRNA genes are located either within intronic sequences of protein-coding genes, within intronic or exonic regions of non-coding RNAs, or within intergenic regions[12]. The biogenesis of miRNAs includes two parts: one is transcription in the nucleus, and the other is generation of mature miRNAs in the cytoplasm. First, miRNA is transcribed from the genome by RNA polymerase II or III to generate primary miRNA[13,14]. The primary miRNA is then cleaved by RNase III-type enzyme Drosha to produce a pre-miRNA of approximately 70 nucleotides with a stem-loop structure in the nucleus[15]. Next, the pre-miRNA is exported to the cytoplasm by Exportin 5[16]. Once in the cytoplasm, the pre-miRNA is cleaved by Dicer in cooperation with protein partners, into an approximately 22-nucleotide miRNA duplex[17]. Then, one strand is selected as a functional miRNA, while the passenger strand is degraded. The functional miRNA is loaded into the RNA-induced silencing complex and acts as a guide strand that recognizes the target mRNA by complementary sequences[18]. Full complementarity occurs in plants, resulting in target mRNA degradation. However, incomplete complementary binding occurs in humans, and this leads to mRNA destabilization and translational inhibition[12].
MIRNAS AND THE INNATE IMMUNE SYSTEM
The innate immune system forms the first line of host defense, which is non-specific, and responds to pathogens in a generic way. It is comprised of tissue barriers, immune cells, and immune molecules. The tissue barriers include mechanical (epithelial) barriers, chemical barriers such as antimicrobial peptides, and biologic barriers (commensal flora). The innate immune cells include monocytes/macrophages, dendritic cells (DCs), neutrophils, natural killer (NK) cells, NK T cells, mast cells, eosinophils, and basophils. These cells perform phagocytosis, antigen presentation, and activation of the adaptive immune responses[19]. miRNAs regulate autoimmunity and inflammation by affecting the differentiation, maturation, and function of various immune cells (Figure 1).
Figure 1.
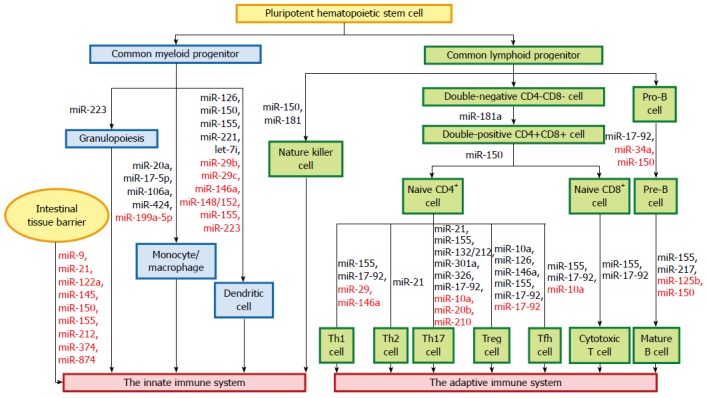
miRNAs and the immune system. miRNAs regulate autoimmunity and inflammation by affecting the differentiation, maturation, and function of various immune cells. The miRNAs in black letters are positive regulators in maintaining the differentiation and function of immune cells, while those in red letters act as negative regulators of these processes.
miRNAs and intestinal tissue barriers
The intestinal mucosa forms a barrier that separates luminal contents from the interstitium. Tissue barriers include tight junctions (TJs), adherens junctions, and desmosomes, which regulate the paracellular permeability of epithelial layers across the apical/basolateral axis[20]. The main protein complexes of TJs are composed of transmembrane proteins such as claudins, occludin and junctional adhesion molecules[21]. Disruption of the intestinal barrier has been shown to be an important pathogenic mechanism contributing to the development of intestinal inflammation[22,23]. McKenna et al[24] reported that miRNAs are important for maintaining the function of intestinal barriers.
miR-21, which is overexpressed in chronic UC, induces the degradation of Ras homolog gene family member (Rho)B mRNA and leads to an increase in intestinal epithelial permeability due to the loss of TJ proteins and ultrastructural changes[25]. miR-150 is significantly elevated in colon tissue in dextran-sulfate-sodium-induced murine experimental colitis and active UC patients. Overexpression of miR-150 results in intestinal epithelial disruption through targeting of c-Myb[26]. Both occludin and claudin-1 have been demonstrated to be involved in miR-874-induced intestinal barrier dysfunction by targeting the 3′-untranslated region of aquaporin 3[27]. miR-9 and miR-374 directly target the 3′-untranslated region of claudin-14 mRNA, leading to claudin-14 mRNA translational repression and decay, in a cooperative manner[28]. miR-145 impairs TJ function by repressing junctional adhesion molecule-1 expression[29]. miR-212 impairs the intestinal epithelial barrier by downregulating zonula occludens-1 protein expression, which is another major component of TJs[30].
Tumor necrosis factor (TNF)-α is an essential mediator of inflammation in the gut. Anti-TNF-α therapy induces remission in patients with severe active CD[31], UC[32], and refractory celiac disease[33]. TNF-α-induced upregulation of miR-122a mediates the degradation of occludin mRNA in enterocytes and influences their permeability[34]. TNF-α-induced miR-155 overexpression inhibits synthesis of zonula occludens-1 by downregulating RhoA expression[35].
miRNAs and monocytes/macrophages
Monocyte/macrophage differentiation is an essential branch of hematopoiesis, which is under the control of a complex network of regulatory factors[36]. During monocytopoiesis, the transcription factor acute myeloid leukemia (AML)1 is upregulated, while miRNAs-17-5p/20a/106a are downregulated. Monocytopoiesis is regulated by a circuitry comprising sequential miRNAs-17-5p/20a/106a, AML1, and monocyte colony-stimulating factor receptor, whereby miRNAs-17-5p/20a/106a act as a master gene complex that negatively regulates AML1 expression[37]. The transcription factor PU.1 upregulates miR-424 expression, and this induces monocyte differentiation via miR-424-dependent translational inhibition of nuclear factor (NF)I-A. This result indicates an important role of miR-424 and its target NFI-A in controlling monocyte/macrophage differentiation[38]. miR-199a-5p targets the activin A receptor type 1B gene, leading to decreased expression of CCAAT/enhancer binding protein α, and eventually, inhibits monocyte/macrophage differentiation[36].
miRNAs and DCs
DCs serve as the most potent antigen-presenting cells, responsible for primary immune responses. Accumulating evidence highlights the importance of specific miRNAs in DC development, antigen-presentation capacity, and cytokine release[39]. miR-146a[40], miR-155[41], and let-7i[42] are involved in the maturation and functional state of DCs, while miR-148/152[43] and miR-223[44] are involved in their antigen-presentation capacity. miR-150 is required for the cross-presentation capacity of Langerhans cells (skin-resident DCs)[45]. In addition, miR-29b, miR-29c[46], miR-126[47], miR-146a[40,48], miR-155, and miR-221[49] have been shown to regulate DC apoptosis and cytokine production.
miRNAs and NK cells
NK cells are cytotoxic lymphocytes that play a vital role in host defense against infection, and they mediate antitumor responses. Recent advances have demonstrated that miRNAs are crucial in NK cell biology[50]. For instance, miR-150 and miR-181 regulate NK cell development[51,52]. Mice lacking miR-150 are defective in generating mature NK cells. On the contrary, transgenic mice with a gain-of-function miR-150 have enhanced NK cell development[51]. miR-181 promotes NK cell development by targeting Nemo-like kinase, which is a inhibitor of Notch signaling[52].
miRNAs and other kind of innate immune cells
Invariant NK T cells are a separate subset of T lymphocytes with innate effector functions. A Dicer-dependent miRNA pathway is important in the regulation of invariant NK T cell differentiation, function, and homeostasis[53]. The normal granulocytic differentiation requires the zinc finger protein growth factor independent-1, which is a transcription inhibitor that regulates the expression of miR-21 and miR-196b during myelopoiesis[54]. In addition, miR-223 plays a crucial role in the regulation of granulocyte differentiation and function, and mediates inflammatory responses[55,56].
miRNAs and activation of the innate immune system
Pattern recognition receptors are critical for the recognition of microorganisms and the induction of immune and inflammatory responses[57]. The families of these proteins include the membrane-bound Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors, and retinoic acid-inducible gene-I-like receptors[58]. Pattern recognition receptors promote downstream signaling cascades. Emerging evidence indicates that miRNAs regulate these processes.
TLRs: miR-146a expression can be induced through exposure to TLR ligands, such as lipopolysaccharide, peptidoglycan, and flagellin, and this induction is controlled by NF-κB. Mice lacking miR-146a are more likely to develop autoimmune diseases, tumorigenesis, and myeloid cell proliferation. miR-146 targets TNF-receptor-associated factor 6 and interleukin (IL)-1-receptor-associated kinase 1, which are key elements of the myeloid differentiation factor 88 pathway, and form a negative feedback mechanism in TLR signaling[59-61]. miR-155 expression is also induced by TLR signaling[61,62]. Unlike miR-146a, miR-155 promotes the immune response. Mice deficient in miR-155 are highly resistant to experimental autoimmune encephalomyelitis[63].
NOD-like receptors: Of this family of proteins, NOD2 functions as an intracellular sensor that contributes to inflammation and immune defense. It has been identified as the strongest single genetic locus in determining susceptibility in CD[64]. A miRs-NOD interaction has been implicated in IBD. These miRNAs include miR-29[65], miR-122[66], miR-146a[67], and miR-192[68]. For example, polymorphisms in NOD2 impair miR-29 expression in DCs, and this results in exaggerated IL-23-induced inflammation[65]. miR-122 targeting of NOD2 has a crucial role in the damage of intestinal epithelial cells induced by lipopolysaccharide[66].
MIRNAS AND THE ADAPTIVE IMMUNE SYSTEM
The adaptive immune system mainly consists of two different lymphocytes (T and B cells), and is highly pathogen specific. The appropriate development and function of these two immune cells are essential when distinguishing foreign from resident antigens. Current studies have indicated that miRNAs play important roles in maintaining the differentiation and function of T and B cells[69].
miRNAs and T-cell regulation
Increasing evidence shows that some specific miRNAs participate in the regulation of crucial immune functions. These immuno-miRs play significant roles in T-cell development, maturation, activation, differentiation, and aging[70]. For example, miR-150[71] and miR-181a[72] are involved in T-cell development, while miR-21[73] and miR-17-92 cluster[74,75] participate in T-cell activation.
miRNAs and T-helper 1/2 cell differentiation
miRNAs have significant effects on T helper (Th) cell differentiation. Naïve T cells can differentiate into Th1, Th2, or Th17 cells after activation[76]. Th1 cells have been associated with the pathogenesis of CD, while Th2 cells have been implicated in UC[19]. miR-155 promotes Th1 differentiation by targeting interferon (IFN)-γ receptor α chain[77]. In contrast, CD4+ T cells deficient in miR-155 display a bias towards Th2 differentiation, which is partly due to increased expression of the Th2-associated transcription factor c-Maf[41,78]. miR-17-92 cluster promotes Th1 differentiation. The function of miR-19b is mediated through phosphatase and tensin homolog, while miR-17 targets transforming growth factor (TGF)-β receptor II and cAMP-responsive element-binding protein 1[79]. miR-29 regulates Th cell differentiation by directly targeting T-box transcription factor T-bet and eomesodermin to suppress IFN-γ production[80]. miR-146a may be a potent inhibitor of Th1 differentiation by targeting protein kinase Cε[81]. miR-21 promotes Th2 differentiation[82].
miRNAs and Th17 cell differentiation
Th17 cells, a new subset of Th cells capable of producing IL-17, play a vital role in the formation of several autoimmune-mediated inflammatory diseases, including IBD[83,84]. Current studies demonstrate that miR-21[85], miR-155[63], miR-301a[86], miR-326[87], miR-17-92 cluster[88], and miR-132/212 cluster[89] act as positive regulators of Th17 differentiation. For example, miR-155 enhances the development of inflammatory T cells (Th1 and Th17 cells), and facilitates Th17 cell formation through cytokines produced by DCs[63]. miR-10a[90], miR-20b[91], and miR-210[92] act as negative regulators of Th17 differentiation. Deletion of miR-210 promotes Th17 differentiation under hypoxic conditions[92].
miRNAs and regulatory T cells
Regulatory T (Treg) cells are another subset of CD4+ T cells that can suppress activity of effector T cells and maintain self-tolerance[76,90]. Treg cells can be classified into two populations, naturally-occurring Treg (nTreg) cells that are generated in the thymus, and inducible Treg (iTreg) cells that arise from naïve CD4+ precursors in the periphery[90]. miRNAs play pivotal roles in the regulation of both Treg cell development and function[93,94]. miR-10a is highly expressed in nTreg cells and can be induced by retinoic acid and TGF-β in iTreg cells[90]. Repression of miR-10a in vitro results in reduced forkhead box (Fox)p3 expression levels, while ablation of miR-10a does not affect the phenotype or number of nTreg cells[93]. miR-155-deficient mice display a marked reduction in the number of Treg cells. Additionally, miR-155 maintains homeostasis of Treg cells by targeting suppressor of cytokine signaling 1 via the IL-2 signaling pathway[95]. miR-146a is important for maintaining suppressive function of Treg cells. Treg cells deficient in miR-146a lead to immunologic intolerance by targeting signal transducer and activator of transcription-1[96]. Silencing of miR-126 can influence the expression of Foxp3 on Treg cells and impair their suppressive function via the PI3K/Akt pathway[97]. miR-17-92 cluster is also involved in Treg cell function. Mice with Treg-specific loss of miR-17-92 cluster develop an exacerbated form of experimental autoimmune encephalomyelitis and fail to achieve clinical remission[74]. However, there are conflicting results. For instance, the study from Jiang et al[79] showed that miR-17-92 cluster prevents Treg cell differentiation and promotes Th1 responses.
miRNAs and follicular helper T cells
Follicular helper T (Tfh) cells are a novel subset of CD4+ T cells that can provide help to B cells, and they are important for germinal center formation[98]. Several studies have demonstrated that miRNAs are crucial for Tfh cell differentiation and function[99,100]. Mice with T-cell-specific loss of miR-17-92 cluster exhibit severely compromised Tfh cell differentiation, germinal center formation, and antibody responses. On the contrary, T-cell-specific miR-17-92 cluster transgenic mice spontaneously accumulate Tfh cells[99]. miR-10a attenuates phenotypic conversion of iTreg cells to Tfh cells by simultaneously targeting Bcl-6, a transcription factor critical for Tfh cell differentiation[101], along with the corepressor Ncor2[90]. Moreover, miR-155 has been reported to promote Tfh cell development[102].
miRNAs and CD8+ T cells
CD8+ T cells or cytotoxic T lymphocytes can devastate various intracellular pathogens and malignancies[103]. Dicer is required for CD8+ T-cell survival and accumulation, but not required for the early steps in CD8+ T-cell activation[104,105]. Dicer and miRNAs such as miR-139 and miR-150 also participate in controlling the cytolytic program, as well as other programs of effector cytotoxic T lymphocyte differentiation[106]. miR-155 is demanded for effector CD8+ T-cell responses to viral and intracellular bacterial infection and cancer. miR-155 has the potential to be a target for immunotherapy for infectious diseases and cancer[103,107,108]. miR-17-92 cluster has dynamic regulation of CD8+ T cells differentiating from naïve to effector and memory states[75,109].
miRNAs and B cells
Ablation of Dicer in early B-cell progenitors leads to a formative block from the pro-B to pre-B transition[110]. miRNAs are also involved in B-cell development and function[110,111], including miR-34a[112], miR-150[113,114], and miR-17-92 cluster[115]. miR-125b inhibits B-cell differentiation in germinal centers[116]. In addition to regulating B-cell differentiation, miR-150[113], miR-155[78], and miR-217[111] regulate B-cell function, including the establishment of B-cell tolerance, as well as antigen-dependent and -independent antibody repertoire diversification.
MIRNAS AND IBD
Abnormal miRNA expressions exist in some diseases, including IBD (Table 1). This offers a new way to improve our comprehension of the mechanism of this disease. Moreover, some specific miRNAs in IBD may serve as potential biomarkers for diagnosis, evaluation indicators of disease activity, or targets for treatment.
Table 1.
Aberrantly expressed miRNAs in inflammatory bowel disease
| Sample type | Expression | miRNAs | Ref. |
| Ulcerative colitis vs healthy controls | |||
| Mucosal tissues | Upregulated | miR-7, miR-16, miR-20b, miR-21, miR-23a, miR-24, miR-29a, miR-29b, miR-31, miR-125b-1*, miR-126, miR-126*, miR-127-3p, miR-135b, miR-146a, miR-150, miR-155, miR-195, miR-206, miR-223, miR-324-3p, miR-424, and let-7f | [25,26,117-122] |
| Downregulated | miR-188-5p, miR-192, miR-200b, miR-215, miR-320a, miR-346, miR-375, and miR-422b; miR-124 (pediatric cases) | [117,119,123,124] | |
| Peripheral blood | Upregulated | miR-16, miR-21, miR-28-5p, miR-103-2*, miR-151-5p, miR-155, miR-188-5p, miR-199a-5p, miR-340*, miR-362-3p, miR-378, miR-422a, miR-500, miR-501-5p, miR-532-3p, miR-769-5p, miR-874, and miRplus-E1271 | [125-127] |
| Downregulated | miR-505* | [126] | |
| Crohn’s disease vs healthy controls | |||
| Mucosal tissues | Upregulated | miR-9, miR-21, miR-22, miR-26a, miR-29b, miR-29c, miR-30b, miR-31, miR-34c-5p, miR-106a, miR-106b, miR-126, miR-126*, miR-127-3p, miR-130a, miR-133b, miR-146a, miR-146b-5p, miR-150, miR-155, miR-181c, miR-196a, miR-196, miR-206, miR-324-3p, miR-375, and miR-424 | [119,121,130,131] |
| Downregulated | miR-7 and miR-141 | [128,129] | |
| Peripheral blood | Upregulated | miR-16, miR-23a, miR-29a, miR-106a, miR-107, miR-126, miR-191, miR-199a-5p, miR-200c, miR-362-3p and miR-532-3p; | [125,133] |
| miR-16, miR-20a, miR-21, miR-30e, miR-93, miR-106a, miR-140, miR-192, miR-195, miR-484, and let-7b (pediatric cases) | |||
miRNAs in UC
miRNAs in mucosal tissues: In 2008, the first profiling study of altered expression of miRNAs in IBD patients was published[117]. Wu and colleagues[117] found a specific miRNA expression pattern: three miRNAs (miR-192, miR-375, and miR-422b) were markedly downregulated, whereas eight miRNAs (miR-16, miR-21, miR-23a, miR-24, miR-29a, miR-126, miR-195, and let-7f) were observably upregulated in active UC tissues. Furthermore, they found that miR-192 participated in the regulation of chemokine production in colonic epithelial cells. Since 2008, some new research in active UC and healthy controls has confirmed the upregulation of miR-21[25,118], miR-29a[119], and miR-126[120], and identified additional upregulated miRNAs, including miR-7, miR-29b, miR-126*, miR-127-3p, miR-135b, miR-223 and miR-324-3p[119], miR-31[119,121], miR-150[26], miR-155[118], miR-146a, miR-206, and miR-424[121], and miR-20b and miR-125b-1*[122]. In contrast, miR-188-5p, miR-215, miR-320a, miR-346[119], and miR-200b[123] were downregulated in colon tissues from active UC patients compared with healthy controls. miR-124 was markedly decreased in pediatric but not in adult UC tissues. Reduced levels of miR-124 in colon tissues appear to increase the expression and activity of signal transducer and activator of transcription-3, and this mediates the pathogenesis of UC in children[124].
miRNAs in peripheral blood: Paraskevi and colleagues[125] found that six miRNAs (miR-16, miR-21, miR-28-5p, miR-151-5p, miR-155, and miR-199a-5p) were remarkably upregulated in blood from UC patients compared with healthy controls. miR-155 had the highest expression level of these six UC-associated miRNAs in peripheral blood. Wu and colleagues[126] found that compared with healthy controls, 12 miRNAs were significantly upregulated, and miRNA-505* was downregulated in blood from active UC patients. Peripheral blood miRNAs may distinguish active UC patients from healthy controls. As compared to active CD patients, ten miRNAs were markedly upregulated, and one miRNA was downregulated in blood from active UC patients[126]. Duttagupta et al[127] completed analyses of miRNA expressions from different hematologic fractions as noninvasive predictors for incidence of UC. They found that seven miRNAs derived from platelets (miR-188-5p, miR-378, miR-422a, miR-500, miR-501-5p, miR-769-5p, and miR-874) were upregulated. This study provides new platelet-derived miRNA biomarkers for clinical application and perception of the potential roles of these miRNAs in the pathogenesis of UC.
miRNAs in CD
miRNAs in mucosal tissues: Most studies of miRNA expression profiles in CD have concentrated on Crohn’s colitis. Fasseu et al[119] found that 23 miRNAs (miR-9, miR-21, miR-22, miR-26a, miR-29b, miR-29c, miR-30b, miR-31, miR-34c-5p, miR-106a, miR-126, miR-126*, miR-127-3p, miR-130a, miR-133b, miR-146a, miR-146b-5p, miR-150, miR-155, miR-181c, miR-196a, miR-324-3p, and miR-375) were remarkably upregulated in colonic tissues from CD patients compared with healthy controls. Five of these miRNAs were specific for patients in an active stage of CD (miR-9, miR-126, miR-130a, miR-181c, and miR-375), whereas the remaining 18 were also upregulated in colonic tissues from inactive CD patients. Huang and colleagues[128] identified that miR-141 was downregulated in inflamed colon tissues from active CD patients. miR-141 inhibited colonic chemokine CXC ligand 12β expression by directly targeting it and blocked colonic immune cell recruitment. Nguyen and colleagues[129] found that only miR-7 was downregulated in eight active colonic CD patients compared to six healthy controls. In addition, miR-206, miR-424[121], miR-106b[130] and miR-196[131] are also upregulated in active colonic CD.
However, is there any tissue-specific miRNA expression profile in the gastrointestinal tract? Wu and colleagues[132] examined miRNA expression patterns in tissues from different intestinal segments in active CD patients. Ten intestine-specific miRNAs (miR-19b, miR-22, miR-23a, miR-26a, miR-31, miR-126, miR-215, miR-320, miR-422b, and let-7d) were identified. Specifically, three of these (miR-22, miR-31, and miR-215) were markedly upregulated in the terminal ileum compared with colon tissue, while miR-19b was downregulated in the terminal ileum. Moreover, miR-23a, miR-26a, miR-126, miR-320, miR-422b, and let-7d showed colon-specific expression. In active colonic CD patients, three miRNAs (miR-23b, miR-106, and miR-191) were upregulated and two (miR-19b and miR-629) were downregulated compared to healthy controls. In active terminal ileal CD patients, four miRNAs (miR-16, miR-21, miR-223, and miR-594) were upregulated in terminal ileal tissues.
miRNAs in peripheral blood: Apart from assessing miRNA expressions in peripheral blood in UC, Paraskevi et al[125] examined miRNA expression patterns in peripheral blood samples from 128 patients with active CD and 162 healthy individuals. Eleven miRNAs (miR-16, miR-23a, miR-29a, miR-106a, miR-107, miR-126, miR-191, miR-199a-5p, miR-200c, miR-362-3p, and miR-532-3p) were markedly upregulated in peripheral blood from CD patients as compared with healthy individuals. There were no significant differences in miRNA expressions in accordance with disease location and phenotype.
Zahm et al[133] examined serum samples from 46 pediatric CD patients and 32 healthy controls by means of a low-density microarray and quantitative reverse transcriptase (qRT) PCR. They found 11 miRNAs (miR-16, miR-20a, miR-21, miR-30e, miR-93, miR-106a, miR-140, miR-192, miR-195, miR-484, and let-7b) that were CD-associated circulating miRNAs. Receiver operating characteristic analyses indicated that these CD-associated miRNAs had promising diagnostic value, with sensitivities of 70%-83% and specificities of 75%-100%. These results demonstrate that circulating miRNAs may be used as novel noninvasive biomarkers in CD.
miRNAs in IBD at different stages
Iborra and colleagues[134] assessed miRNA expression patterns in serum and tissue samples from nine patients with active UC, nine with inactive UC, nine with active CD, and nine with inactive CD, and serum from 33 healthy subjects. They found that two miRNAs (miR-548a-3p and miR-650) were higher, and three (miR-196b, miR-489, and miR-630) were lower in the mucosa of active UC patients compared with inactive UC patients. There were no differences in serum miRNA expression profiles in patients with active UC compared with inactive UC. However, there were differences in serum miRNA expressions between active and inactive CD patients; two serum miRNAs (miR-188-5p and miR-877) were increased, and four serum miRNAs (miR-18a, miR-128, miR-140-5p, and miR-145) were decreased in patients with active CD. Furthermore, four miRNAs (miR-18a*, miR-140-3p, miR-629*, and let-7b) were higher, and three miRNAs (miR-328, miR-422a, and miR-855-5p) were lower in the mucosa of active CD patients compared with inactive CD patients. These results indicate that there are specific miRNA expression patterns associated with different stages of IBD. Further prospective cohort studies in large samples are necessary to validate these findings.
miRNAs as therapy in IBD
miRNA-related therapeutic applications may represent a new and fascinating field in IBD treatment. miRNA-related therapy is based on antisense technology and gene therapy; thus, it involves either miRNA antagonists or miRNA mimics.
miRNA antagonists: miRNA antagonists include anti-miRNA oligonucleotides (AMOs), miRNA sponges, and miRNA masks.
AMOs: AMOs are synthetic anti-miRNA oligonucleotides with reverse complementary sequences to their target miRNAs, which suppress miRNA functions. It is believed that AMOs have a promising future in therapeutic applications. Chemical modifications of AMOs can improve their stability and binding affinity. Common modifications include addition of different 2′-ribose modifications to AMOs (2′-O-methyl and 2′-O-methoxyethyl) and 2′,4′-methylene bridge-locked nucleic acid (LNA). LNA-modified AMOs create high-affinity binding to target mRNAs[135,136]. A study by Janssen et al[137] demonstrated that miravirsen, an LNA-anti-miR-122, is designed to target and inhibit miR-122, and this can reduce viral RNA levels in patients with chronic hepatitis C virus infection. This result proves the possibility of miRNA agents in clinical practice.
miRNA sponges: miRNA sponge technology utilizes plasmid or viral vectors to achieve loss-of-function of miRNAs. The strong promoters can be applied in miRNA sponge vectors for generating high-level expression of the competitive inhibitor transcripts for either transient or long-term inhibition of miRNA function. Considering the merit of sharing a common seed sequence by members of a miRNA family, this technology provides a strong approach for coinstantaneous inhibition of multiple miRNAs of interest with a single inhibitor[138].
miRNA mimicry/replacement therapy: In order to restore miRNA activity, miRNA mimics (synthetic oligonucleotides) and miRNA expression gene vectors are used. MRX34 is a double-stranded miRNA mimic of the naturally occurring miR-34a loaded in liposomal nanoparticles to reestablish its tumor suppressor function. MRX34 was the first miRNA mimic introduced into clinical study for primary as well as metastatic liver cancer in 2013[139,140]. Many miRNAs are downregulated in UC and CD. For example, miR-192, miR-375, miR-422b[117], miR-188-5p, miR-215, miR-320a, miR-346[119], and miR-200b[123] are decreased in UC, and miR-19b and miR-629[132] are decreased in Crohn’s colitis tissues. Theoretically, replenishing these decreased miRNAs by miRNA mimics may provide therapeutic restoration of physiologic functions lost in IBD.
CONCLUSION
In this review, we described the roles of miRNAs as crucial regulators of inflammatory responses and autoimmune disorders, particularly focusing on miRNAs affecting the differentiation, maturation, and function of various immune cells. We also summarized some studies on the current understanding of the connection between miRNAs and IBD. Accumulating evidence suggests that specific miRNA expression profiles exist in IBD, and these miRNAs contribute to the development of inflammation. The definite functions of most miRNAs in IBD have not yet been clarified. Further studies are necessary to validate whether miRNAs could be used to diagnose IBD, distinguish IBD subtypes, and determine the disease activity or location.
Footnotes
Supported by the National Natural Science Foundation of China, No. 81270469.
Conflict-of-interest statement: The authors declare that there are no conflicts of interest related to this work.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 4, 2015
First decision: August 26, 2015
Article in press: November 13, 2015
P- Reviewer: Lakatos PL S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Liu XM
References
- 1.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 4.Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev. 2005;15:563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Wang F, Chen C, Wang D. Circulating microRNAs in cardiovascular diseases: from biomarkers to therapeutic targets. Front Med. 2014;8:404–418. doi: 10.1007/s11684-014-0379-2. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Ji B, Cheng B, Chen J, Bai B. Neuroprotection of microRNA in neurological disorders (Review) Biomed Rep. 2014;2:611–619. doi: 10.3892/br.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrie CH. MicroRNAs in hematological malignancies. Blood Rev. 2013;27:143–154. doi: 10.1016/j.blre.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Iborra M, Bernuzzi F, Invernizzi P, Danese S. MicroRNAs in autoimmunity and inflammatory bowel disease: crucial regulators in immune response. Autoimmun Rev. 2012;11:305–314. doi: 10.1016/j.autrev.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 10.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 11.Van Peer G, Lefever S, Anckaert J, Beckers A, Rihani A, Van Goethem A, Volders PJ, Zeka F, Ongenaert M, Mestdagh P, et al. miRBase Tracker: keeping track of microRNA annotation changes. Database (Oxford) 2014;2014 doi: 10.1093/database/bau080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalla R, Ventham NT, Kennedy NA, Quintana JF, Nimmo ER, Buck AH, Satsangi J. MicroRNAs: new players in IBD. Gut. 2015;64:504–517. doi: 10.1136/gutjnl-2014-307891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek SH, Kim VN. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nat Struct Mol Biol. 2006;13:1097–1101. doi: 10.1038/nsmb1167. [DOI] [PubMed] [Google Scholar]
- 15.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 16.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 17.Koscianska E, Starega-Roslan J, Krzyzosiak WJ. The role of Dicer protein partners in the processing of microRNA precursors. PLoS One. 2011;6:e28548. doi: 10.1371/journal.pone.0028548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gurtan AM, Sharp PA. The role of miRNAs in regulating gene expression networks. J Mol Biol. 2013;425:3582–3600. doi: 10.1016/j.jmb.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6–21. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cichon C, Sabharwal H, Rüter C, Schmidt MA. MicroRNAs regulate tight junction proteins and modulate epithelial/endothelial barrier functions. Tissue Barriers. 2014;2:e944446. doi: 10.4161/21688362.2014.944446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol. 2009;1:a002584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 23.Shen L, Su L, Turner JR. Mechanisms and functional implications of intestinal barrier defects. Dig Dis. 2009;27:443–449. doi: 10.1159/000233282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenna LB, Schug J, Vourekas A, McKenna JB, Bramswig NC, Friedman JR, Kaestner KH. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010;139:1654–1664, 1664.e1. doi: 10.1053/j.gastro.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Ma Y, Shi C, Chen H, Zhang H, Chen N, Zhang P, Wang F, Yang J, Yang J, et al. Overexpression of miR-21 in patients with ulcerative colitis impairs intestinal epithelial barrier function through targeting the Rho GTPase RhoB. Biochem Biophys Res Commun. 2013;434:746–752. doi: 10.1016/j.bbrc.2013.03.122. [DOI] [PubMed] [Google Scholar]
- 26.Bian Z, Li L, Cui J, Zhang H, Liu Y, Zhang CY, Zen K. Role of miR-150-targeting c-Myb in colonic epithelial disruption during dextran sulphate sodium-induced murine experimental colitis and human ulcerative colitis. J Pathol. 2011;225:544–553. doi: 10.1002/path.2907. [DOI] [PubMed] [Google Scholar]
- 27.Zhi X, Tao J, Li Z, Jiang B, Feng J, Yang L, Xu H, Xu Z. MiR-874 promotes intestinal barrier dysfunction through targeting AQP3 following intestinal ischemic injury. FEBS Lett. 2014;588:757–763. doi: 10.1016/j.febslet.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 2012;31:1999–2012. doi: 10.1038/emboj.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adammek M, Greve B, Kässens N, Schneider C, Brüggemann K, Schüring AN, Starzinski-Powitz A, Kiesel L, Götte M. MicroRNA miR-145 inhibits proliferation, invasiveness, and stem cell phenotype of an in vitro endometriosis model by targeting multiple cytoskeletal elements and pluripotency factors. Fertil Steril. 2013;99:1346–1355.e5. doi: 10.1016/j.fertnstert.2012.11.055. [DOI] [PubMed] [Google Scholar]
- 30.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 31.Akobeng AK. Crohn’s disease: current treatment options. Arch Dis Child. 2008;93:787–792. doi: 10.1136/adc.2007.128751. [DOI] [PubMed] [Google Scholar]
- 32.Thukral C, Cheifetz A, Peppercorn MA. Anti-tumour necrosis factor therapy for ulcerative colitis: evidence to date. Drugs. 2006;66:2059–2065. doi: 10.2165/00003495-200666160-00002. [DOI] [PubMed] [Google Scholar]
- 33.Gillett HR, Arnott ID, McIntyre M, Campbell S, Dahele A, Priest M, Jackson R, Ghosh S. Successful infliximab treatment for steroid-refractory celiac disease: a case report. Gastroenterology. 2002;122:800–805. doi: 10.1053/gast.2002.31874. [DOI] [PubMed] [Google Scholar]
- 34.Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323–1333. doi: 10.1053/j.gastro.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian R, Wang RL, Xie H, Jin W, Yu KL. Overexpressed miRNA-155 dysregulates intestinal epithelial apical junctional complex in severe acute pancreatitis. World J Gastroenterol. 2013;19:8282–8291. doi: 10.3748/wjg.v19.i45.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin HS, Gong JN, Su R, Chen MT, Song L, Shen C, Wang F, Ma YN, Zhao HL, Yu J, et al. miR-199a-5p inhibits monocyte/macrophage differentiation by targeting the activin A type 1B receptor gene and finally reducing C/EBPα expression. J Leukoc Biol. 2014;96:1023–1035. doi: 10.1189/jlb.1A0514-240R. [DOI] [PubMed] [Google Scholar]
- 37.Fontana L, Pelosi E, Greco P, Racanicchi S, Testa U, Liuzzi F, Croce CM, Brunetti E, Grignani F, Peschle C. MicroRNAs 17-5p-20a-106a control monocytopoiesis through AML1 targeting and M-CSF receptor upregulation. Nat Cell Biol. 2007;9:775–787. doi: 10.1038/ncb1613. [DOI] [PubMed] [Google Scholar]
- 38.Rosa A, Ballarino M, Sorrentino A, Sthandier O, De Angelis FG, Marchioni M, Masella B, Guarini A, Fatica A, Peschle C, et al. The interplay between the master transcription factor PU.1 and miR-424 regulates human monocyte/macrophage differentiation. Proc Natl Acad Sci USA. 2007;104:19849–19854. doi: 10.1073/pnas.0706963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smyth LA, Boardman DA, Tung SL, Lechler R, Lombardi G. MicroRNAs affect dendritic cell function and phenotype. Immunology. 2015;144:197–205. doi: 10.1111/imm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karrich JJ, Jachimowski LC, Libouban M, Iyer A, Brandwijk K, Taanman-Kueter EW, Nagasawa M, de Jong EC, Uittenbogaart CH, Blom B. MicroRNA-146a regulates survival and maturation of human plasmacytoid dendritic cells. Blood. 2013;122:3001–3009. doi: 10.1182/blood-2012-12-475087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, et al. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Liu F, Jia H, Zhang Q, Yin L, Liu W, Li H, Yu B, Wu J. Inhibition of microRNA let-7i depresses maturation and functional state of dendritic cells in response to lipopolysaccharide stimulation via targeting suppressor of cytokine signaling 1. J Immunol. 2011;187:1674–1683. doi: 10.4049/jimmunol.1001937. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Zhan Z, Xu L, Ma F, Li D, Guo Z, Li N, Cao X. MicroRNA-148/152 impair innate response and antigen presentation of TLR-triggered dendritic cells by targeting CaMKIIα. J Immunol. 2010;185:7244–7251. doi: 10.4049/jimmunol.1001573. [DOI] [PubMed] [Google Scholar]
- 44.Mi QS, Xu YP, Wang H, Qi RQ, Dong Z, Zhou L. Deletion of microRNA miR-223 increases Langerhans cell cross-presentation. Int J Biochem Cell Biol. 2013;45:395–400. doi: 10.1016/j.biocel.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mi QS, Xu YP, Qi RQ, Shi YL, Zhou L. Lack of microRNA miR-150 reduces the capacity of epidermal Langerhans cell cross-presentation. Exp Dermatol. 2012;21:876–877. doi: 10.1111/exd.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong Y, Wu J, Zhao J, Wang H, Liu Y, Chen T, Kan X, Tao Q, Shen X, Yan K, et al. miR-29b and miR-29c are involved in Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid dendritic cells. PLoS One. 2013;8:e69926. doi: 10.1371/journal.pone.0069926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agudo J, Ruzo A, Tung N, Salmon H, Leboeuf M, Hashimoto D, Becker C, Garrett-Sinha LA, Baccarini A, Merad M, et al. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat Immunol. 2014;15:54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park H, Huang X, Lu C, Cairo MS, Zhou X. MicroRNA-146a and microRNA-146b regulate human dendritic cell apoptosis and cytokine production by targeting TRAF6 and IRAK1 proteins. J Biol Chem. 2015;290:2831–2841. doi: 10.1074/jbc.M114.591420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu C, Huang X, Zhang X, Roensch K, Cao Q, Nakayama KI, Blazar BR, Zeng Y, Zhou X. miR-221 and miR-155 regulate human dendritic cell development, apoptosis, and IL-12 production through targeting of p27kip1, KPC1, and SOCS-1. Blood. 2011;117:4293–4303. doi: 10.1182/blood-2010-12-322503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leong JW, Sullivan RP, Fehniger TA. microRNA management of NK-cell developmental and functional programs. Eur J Immunol. 2014;44:2862–2868. doi: 10.1002/eji.201444798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bezman NA, Chakraborty T, Bender T, Lanier LL. miR-150 regulates the development of NK and iNKT cells. J Exp Med. 2011;208:2717–2731. doi: 10.1084/jem.20111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cichocki F, Felices M, McCullar V, Presnell SR, Al-Attar A, Lutz CT, Miller JS. Cutting edge: microRNA-181 promotes human NK cell development by regulating Notch signaling. J Immunol. 2011;187:6171–6175. doi: 10.4049/jimmunol.1100835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fedeli M, Napolitano A, Wong MP, Marcais A, de Lalla C, Colucci F, Merkenschlager M, Dellabona P, Casorati G. Dicer-dependent microRNA pathway controls invariant NKT cell development. J Immunol. 2009;183:2506–2512. doi: 10.4049/jimmunol.0901361. [DOI] [PubMed] [Google Scholar]
- 54.Velu CS, Baktula AM, Grimes HL. Gfi1 regulates miR-21 and miR-196b to control myelopoiesis. Blood. 2009;113:4720–4728. doi: 10.1182/blood-2008-11-190215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fazi F, Rosa A, Fatica A, Gelmetti V, De Marchis ML, Nervi C, Bozzoni I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPalpha regulates human granulopoiesis. Cell. 2005;123:819–831. doi: 10.1016/j.cell.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 56.Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 2008;451:1125–1129. doi: 10.1038/nature06607. [DOI] [PubMed] [Google Scholar]
- 57.Gourbeyre P, Berri M, Lippi Y, Meurens F, Vincent-Naulleau S, Laffitte J, Rogel-Gaillard C, Pinton P, Oswald IP. Pattern recognition receptors in the gut: analysis of their expression along the intestinal tract and the crypt/villus axis. Physiol Rep. 2015;3 doi: 10.14814/phy2.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elia PP, Tolentino YF, Bernardazzi C, de Souza HS. The role of innate immunity receptors in the pathogenesis of inflammatory bowel disease. Mediators Inflamm. 2015;2015:936193. doi: 10.1155/2015/936193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saba R, Sorensen DL, Booth SA. MicroRNA-146a: A Dominant, Negative Regulator of the Innate Immune Response. Front Immunol. 2014;5:578. doi: 10.3389/fimmu.2014.00578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nahid MA, Satoh M, Chan EK. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol Immunol. 2011;8:388–403. doi: 10.1038/cmi.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA. 2007;104:1604–1609. doi: 10.1073/pnas.0610731104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity. 2010;33:607–619. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cuthbert AP, Fisher SA, Mirza MM, King K, Hampe J, Croucher PJ, Mascheretti S, Sanderson J, Forbes A, Mansfield J, et al. The contribution of NOD2 gene mutations to the risk and site of disease in inflammatory bowel disease. Gastroenterology. 2002;122:867–874. doi: 10.1053/gast.2002.32415. [DOI] [PubMed] [Google Scholar]
- 65.Brain O, Owens BM, Pichulik T, Allan P, Khatamzas E, Leslie A, Steevels T, Sharma S, Mayer A, Catuneanu AM, et al. The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity. 2013;39:521–536. doi: 10.1016/j.immuni.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y, Wang C, Liu Y, Tang L, Zheng M, Xu C, Song J, Meng X. miR-122 targets NOD2 to decrease intestinal epithelial cell injury in Crohn’s disease. Biochem Biophys Res Commun. 2013;438:133–139. doi: 10.1016/j.bbrc.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 67.Ghorpade DS, Sinha AY, Holla S, Singh V, Balaji KN. NOD2-nitric oxide-responsive microRNA-146a activates Sonic hedgehog signaling to orchestrate inflammatory responses in murine model of inflammatory bowel disease. J Biol Chem. 2013;288:33037–33048. doi: 10.1074/jbc.M113.492496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chuang AY, Chuang JC, Zhai Z, Wu F, Kwon JH. NOD2 expression is regulated by microRNAs in colonic epithelial HCT116 cells. Inflamm Bowel Dis. 2014;20:126–135. doi: 10.1097/01.MIB.0000436954.70596.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 70.Kroesen BJ, Teteloshvili N, Smigielska-Czepiel K, Brouwer E, Boots AM, van den Berg A, Kluiver J. Immuno-miRs: critical regulators of T-cell development, function and ageing. Immunology. 2015;144:1–10. doi: 10.1111/imm.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghisi M, Corradin A, Basso K, Frasson C, Serafin V, Mukherjee S, Mussolin L, Ruggero K, Bonanno L, Guffanti A, et al. Modulation of microRNA expression in human T-cell development: targeting of NOTCH3 by miR-150. Blood. 2011;117:7053–7062. doi: 10.1182/blood-2010-12-326629. [DOI] [PubMed] [Google Scholar]
- 72.Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 73.Smigielska-Czepiel K, van den Berg A, Jellema P, Slezak-Prochazka I, Maat H, van den Bos H, van der Lei RJ, Kluiver J, Brouwer E, Boots AM, et al. Dual role of miR-21 in CD4+ T-cells: activation-induced miR-21 supports survival of memory T-cells and regulates CCR7 expression in naive T-cells. PLoS One. 2013;8:e76217. doi: 10.1371/journal.pone.0076217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Kouchkovsky D, Esensten JH, Rosenthal WL, Morar MM, Bluestone JA, Jeker LT. microRNA-17-92 regulates IL-10 production by regulatory T cells and control of experimental autoimmune encephalomyelitis. J Immunol. 2013;191:1594–1605. doi: 10.4049/jimmunol.1203567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu T, Wieland A, Araki K, Davis CW, Ye L, Hale JS, Ahmed R. Temporal expression of microRNA cluster miR-17-92 regulates effector and memory CD8+ T-cell differentiation. Proc Natl Acad Sci USA. 2012;109:9965–9970. doi: 10.1073/pnas.1207327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Geginat J, Paroni M, Maglie S, Alfen JS, Kastirr I, Gruarin P, De Simone M, Pagani M, Abrignani S. Plasticity of human CD4 T cell subsets. Front Immunol. 2014;5:630. doi: 10.3389/fimmu.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banerjee A, Schambach F, DeJong CS, Hammond SM, Reiner SL. Micro-RNA-155 inhibits IFN-gamma signaling in CD4+ T cells. Eur J Immunol. 2010;40:225–231. doi: 10.1002/eji.200939381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, et al. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- 79.Jiang S, Li C, Olive V, Lykken E, Feng F, Sevilla J, Wan Y, He L, Li QJ. Molecular dissection of the miR-17-92 cluster’s critical dual roles in promoting Th1 responses and preventing inducible Treg differentiation. Blood. 2011;118:5487–5497. doi: 10.1182/blood-2011-05-355644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CD, Matloubian M, Blelloch R, Ansel KM. MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity. 2011;35:169–181. doi: 10.1016/j.immuni.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Möhnle P, Schütz SV, van der Heide V, Hübner M, Luchting B, Sedlbauer J, Limbeck E, Hinske LC, Briegel J, Kreth S. MicroRNA-146a controls Th1-cell differentiation of human CD4+ T lymphocytes by targeting PRKCΕ. Eur J Immunol. 2015;45:260–272. doi: 10.1002/eji.201444667. [DOI] [PubMed] [Google Scholar]
- 82.Sawant DV, Wu H, Kaplan MH, Dent AL. The Bcl6 target gene microRNA-21 promotes Th2 differentiation by a T cell intrinsic pathway. Mol Immunol. 2013;54:435–442. doi: 10.1016/j.molimm.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gálvez J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014;2014:928461. doi: 10.1155/2014/928461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han L, Yang J, Wang X, Li D, Lv L, Li B. Th17 cells in autoimmune diseases. Front Med. 2015;9:10–19. doi: 10.1007/s11684-015-0388-9. [DOI] [PubMed] [Google Scholar]
- 85.Murugaiyan G, da Cunha AP, Ajay AK, Joller N, Garo LP, Kumaradevan S, Yosef N, Vaidya VS, Weiner HL. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Invest. 2015;125:1069–1080. doi: 10.1172/JCI74347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mycko MP, Cichalewska M, Machlanska A, Cwiklinska H, Mariasiewicz M, Selmaj KW. MicroRNA-301a regulation of a T-helper 17 immune response controls autoimmune demyelination. Proc Natl Acad Sci USA. 2012;109:E1248–E1257. doi: 10.1073/pnas.1114325109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G. MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol. 2009;10:1252–1259. doi: 10.1038/ni.1798. [DOI] [PubMed] [Google Scholar]
- 88.Liu SQ, Jiang S, Li C, Zhang B, Li QJ. miR-17-92 cluster targets phosphatase and tensin homology and Ikaros Family Zinc Finger 4 to promote TH17-mediated inflammation. J Biol Chem. 2014;289:12446–12456. doi: 10.1074/jbc.M114.550723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakahama T, Hanieh H, Nguyen NT, Chinen I, Ripley B, Millrine D, Lee S, Nyati KK, Dubey PK, Chowdhury K, et al. Aryl hydrocarbon receptor-mediated induction of the microRNA-132/212 cluster promotes interleukin-17-producing T-helper cell differentiation. Proc Natl Acad Sci USA. 2013;110:11964–11969. doi: 10.1073/pnas.1311087110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciumè G, Muljo SA, Kuchen S, Casellas R, Wei L, Kanno Y, et al. TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol. 2012;13:587–595. doi: 10.1038/ni.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu E, Wang X, Zheng B, Wang Q, Hao J, Chen S, Zhao Q, Zhao L, Wu Z, Yin Z. miR-20b suppresses Th17 differentiation and the pathogenesis of experimental autoimmune encephalomyelitis by targeting RORγt and STAT3. J Immunol. 2014;192:5599–5609. doi: 10.4049/jimmunol.1303488. [DOI] [PubMed] [Google Scholar]
- 92.Wang H, Flach H, Onizawa M, Wei L, McManus MT, Weiss A. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol. 2014;15:393–401. doi: 10.1038/ni.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jeker LT, Zhou X, Gershberg K, de Kouchkovsky D, Morar MM, Stadthagen G, Lund AH, Bluestone JA. MicroRNA 10a marks regulatory T cells. PLoS One. 2012;7:e36684. doi: 10.1371/journal.pone.0036684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kelada S, Sethupathy P, Okoye IS, Kistasis E, Czieso S, White SD, Chou D, Martens C, Ricklefs SM, Virtaneva K, et al. miR-182 and miR-10a are key regulators of Treg specialisation and stability during Schistosome and Leishmania-associated inflammation. PLoS Pathog. 2013;9:e1003451. doi: 10.1371/journal.ppat.1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, et al. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, Yoshimura A, Baltimore D, Rudensky AY. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142:914–929. doi: 10.1016/j.cell.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin A, Wen Z, Zhou Y, Li Y, Li Y, Luo J, Ren T, Xu L. MicroRNA-126 regulates the induction and function of CD4(+) Foxp3(+) regulatory T cells through PI3K/AKT pathway. J Cell Mol Med. 2013;17:252–264. doi: 10.1111/jcmm.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 99.Kang SG, Liu WH, Lu P, Jin HY, Lim HW, Shepherd J, Fremgen D, Verdin E, Oldstone MB, Qi H, et al. MicroRNAs of the miR-17~92 family are critical regulators of T(FH) differentiation. Nat Immunol. 2013;14:849–857. doi: 10.1038/ni.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baumjohann D, Kageyama R, Clingan JM, Morar MM, Patel S, de Kouchkovsky D, Bannard O, Bluestone JA, Matloubian M, Ansel KM, et al. The microRNA cluster miR-17~92 promotes TFH cell differentiation and represses subset-inappropriate gene expression. Nat Immunol. 2013;14:840–848. doi: 10.1038/ni.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu R, Kagele DA, Huffaker TB, Runtsch MC, Alexander M, Liu J, Bake E, Su W, Williams MA, Rao DS, et al. miR-155 promotes T follicular helper cell accumulation during chronic, low-grade inflammation. Immunity. 2014;41:605–619. doi: 10.1016/j.immuni.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gracias DT, Stelekati E, Hope JL, Boesteanu AC, Doering TA, Norton J, Mueller YM, Fraietta JA, Wherry EJ, Turner M, et al. The microRNA miR-155 controls CD8(+) T cell responses by regulating interferon signaling. Nat Immunol. 2013;14:593–602. doi: 10.1038/ni.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang N, Bevan MJ. Dicer controls CD8+ T-cell activation, migration, and survival. Proc Natl Acad Sci USA. 2010;107:21629–21634. doi: 10.1073/pnas.1016299107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. Aberrant T cell differentiation in the absence of Dicer. J Exp Med. 2005;202:261–269. doi: 10.1084/jem.20050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trifari S, Pipkin ME, Bandukwala HS, Äijö T, Bassein J, Chen R, Martinez GJ, Rao A. MicroRNA-directed program of cytotoxic CD8+ T-cell differentiation. Proc Natl Acad Sci USA. 2013;110:18608–18613. doi: 10.1073/pnas.1317191110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dudda JC, Salaun B, Ji Y, Palmer DC, Monnot GC, Merck E, Boudousquie C, Utzschneider DT, Escobar TM, Perret R, et al. MicroRNA-155 is required for effector CD8+ T cell responses to virus infection and cancer. Immunity. 2013;38:742–753. doi: 10.1016/j.immuni.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lind EF, Elford AR, Ohashi PS. Micro-RNA 155 is required for optimal CD8+ T cell responses to acute viral and intracellular bacterial challenges. J Immunol. 2013;190:1210–1216. doi: 10.4049/jimmunol.1202700. [DOI] [PubMed] [Google Scholar]
- 109.Khan AA, Penny LA, Yuzefpolskiy Y, Sarkar S, Kalia V. MicroRNA-17~92 regulates effector and memory CD8 T-cell fates by modulating proliferation in response to infections. Blood. 2013;121:4473–4483. doi: 10.1182/blood-2012-06-435412. [DOI] [PubMed] [Google Scholar]
- 110.Koralov SB, Muljo SA, Galler GR, Krek A, Chakraborty T, Kanellopoulou C, Jensen K, Cobb BS, Merkenschlager M, Rajewsky N, et al. Dicer ablation affects antibody diversity and cell survival in the B lymphocyte lineage. Cell. 2008;132:860–874. doi: 10.1016/j.cell.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 111.de Yébenes VG, Bartolomé-Izquierdo N, Ramiro AR. Regulation of B-cell development and function by microRNAs. Immunol Rev. 2013;253:25–39. doi: 10.1111/imr.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rao DS, O’Connell RM, Chaudhuri AA, Garcia-Flores Y, Geiger TL, Baltimore D. MicroRNA-34a perturbs B lymphocyte development by repressing the forkhead box transcription factor Foxp1. Immunity. 2010;33:48–59. doi: 10.1016/j.immuni.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xiao C, Calado DP, Galler G, Thai TH, Patterson HC, Wang J, Rajewsky N, Bender TP, Rajewsky K. MiR-150 controls B cell differentiation by targeting the transcription factor c-Myb. Cell. 2007;131:146–159. doi: 10.1016/j.cell.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 114.Zhou B, Wang S, Mayr C, Bartel DP, Lodish HF. miR-150, a microRNA expressed in mature B and T cells, blocks early B cell development when expressed prematurely. Proc Natl Acad Sci USA. 2007;104:7080–7085. doi: 10.1073/pnas.0702409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gururajan M, Haga CL, Das S, Leu CM, Hodson D, Josson S, Turner M, Cooper MD. MicroRNA 125b inhibition of B cell differentiation in germinal centers. Int Immunol. 2010;22:583–592. doi: 10.1093/intimm/dxq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635.e24. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 118.Takagi T, Naito Y, Mizushima K, Hirata I, Yagi N, Tomatsuri N, Ando T, Oyamada Y, Isozaki Y, Hongo H, et al. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J Gastroenterol Hepatol. 2010;25 Suppl 1:S129–S133. doi: 10.1111/j.1440-1746.2009.06216.x. [DOI] [PubMed] [Google Scholar]
- 119.Fasseu M, Tréton X, Guichard C, Pedruzzi E, Cazals-Hatem D, Richard C, Aparicio T, Daniel F, Soulé JC, Moreau R, et al. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One. 2010;5:e13160. doi: 10.1371/journal.pone.0013160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Feng X, Wang H, Ye S, Guan J, Tan W, Cheng S, Wei G, Wu W, Wu F, Zhou Y. Up-regulation of microRNA-126 may contribute to pathogenesis of ulcerative colitis via regulating NF-kappaB inhibitor IκBα. PLoS One. 2012;7:e52782. doi: 10.1371/journal.pone.0052782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin J, Welker NC, Zhao Z, Li Y, Zhang J, Reuss SA, Zhang X, Lee H, Liu Y, Bronner MP. Novel specific microRNA biomarkers in idiopathic inflammatory bowel disease unrelated to disease activity. Mod Pathol. 2014;27:602–608. doi: 10.1038/modpathol.2013.152. [DOI] [PubMed] [Google Scholar]
- 122.Coskun M, Bjerrum JT, Seidelin JB, Troelsen JT, Olsen J, Nielsen OH. miR-20b, miR-98, miR-125b-1*, and let-7e* as new potential diagnostic biomarkers in ulcerative colitis. World J Gastroenterol. 2013;19:4289–4299. doi: 10.3748/wjg.v19.i27.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Y, Xiao Y, Ge W, Zhou K, Wen J, Yan W, Wang Y, Wang B, Qu C, Wu J, et al. miR-200b inhibits TGF-β1-induced epithelial-mesenchymal transition and promotes growth of intestinal epithelial cells. Cell Death Dis. 2013;4:e541. doi: 10.1038/cddis.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Koukos G, Polytarchou C, Kaplan JL, Morley-Fletcher A, Gras-Miralles B, Kokkotou E, Baril-Dore M, Pothoulakis C, Winter HS, Iliopoulos D. MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology. 2013;145:842–852.e2. doi: 10.1053/j.gastro.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Paraskevi A, Theodoropoulos G, Papaconstantinou I, Mantzaris G, Nikiteas N, Gazouli M. Circulating MicroRNA in inflammatory bowel disease. J Crohns Colitis. 2012;6:900–904. doi: 10.1016/j.crohns.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 126.Wu F, Guo NJ, Tian H, Marohn M, Gearhart S, Bayless TM, Brant SR, Kwon JH. Peripheral blood microRNAs distinguish active ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2011;17:241–250. doi: 10.1002/ibd.21450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duttagupta R, DiRienzo S, Jiang R, Bowers J, Gollub J, Kao J, Kearney K, Rudolph D, Dawany NB, Showe MK, et al. Genome-wide maps of circulating miRNA biomarkers for ulcerative colitis. PLoS One. 2012;7:e31241. doi: 10.1371/journal.pone.0031241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang Z, Shi T, Zhou Q, Shi S, Zhao R, Shi H, Dong L, Zhang C, Zeng K, Chen J, et al. miR-141 Regulates colonic leukocytic trafficking by targeting CXCL12β during murine colitis and human Crohn’s disease. Gut. 2014;63:1247–1257. doi: 10.1136/gutjnl-2012-304213. [DOI] [PubMed] [Google Scholar]
- 129.Nguyen HT, Dalmasso G, Yan Y, Laroui H, Dahan S, Mayer L, Sitaraman SV, Merlin D. MicroRNA-7 modulates CD98 expression during intestinal epithelial cell differentiation. J Biol Chem. 2010;285:1479–1489. doi: 10.1074/jbc.M109.057141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu C, Chen J, Xu HG, Zhou X, He Q, Li YL, Jiang G, Shan Y, Xue B, Zhao RX, et al. MIR106B and MIR93 prevent removal of bacteria from epithelial cells by disrupting ATG16L1-mediated autophagy. Gastroenterology. 2014;146:188–199. doi: 10.1053/j.gastro.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brest P, Lapaquette P, Souidi M, Lebrigand K, Cesaro A, Vouret-Craviari V, Mari B, Barbry P, Mosnier JF, Hébuterne X, et al. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in Crohn’s disease. Nat Genet. 2011;43:242–245. doi: 10.1038/ng.762. [DOI] [PubMed] [Google Scholar]
- 132.Wu F, Zhang S, Dassopoulos T, Harris ML, Bayless TM, Meltzer SJ, Brant SR, Kwon JH. Identification of microRNAs associated with ileal and colonic Crohn’s disease. Inflamm Bowel Dis. 2010;16:1729–1738. doi: 10.1002/ibd.21267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zahm AM, Thayu M, Hand NJ, Horner A, Leonard MB, Friedman JR. Circulating microRNA is a biomarker of pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2011;53:26–33. doi: 10.1097/MPG.0b013e31822200cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Iborra M, Bernuzzi F, Correale C, Vetrano S, Fiorino G, Beltrán B, Marabita F, Locati M, Spinelli A, Nos P, et al. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin Exp Immunol. 2013;173:250–258. doi: 10.1111/cei.12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lennox KA, Behlke MA. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther. 2011;18:1111–1120. doi: 10.1038/gt.2011.100. [DOI] [PubMed] [Google Scholar]
- 136.Weiler J, Hunziker J, Hall J. Anti-miRNA oligonucleotides (AMOs): ammunition to target miRNAs implicated in human disease? Gene Ther. 2006;13:496–502. doi: 10.1038/sj.gt.3302654. [DOI] [PubMed] [Google Scholar]
- 137.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 138.Tay FC, Lim JK, Zhu H, Hin LC, Wang S. Using artificial microRNA sponges to achieve microRNA loss-of-function in cancer cells. Adv Drug Deliv Rev. 2015;81:117–127. doi: 10.1016/j.addr.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 139.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469. doi: 10.1016/j.molmed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 140.Chapman CG, Pekow J. The emerging role of miRNAs in inflammatory bowel disease: a review. Therap Adv Gastroenterol. 2015;8:4–22. doi: 10.1177/1756283X14547360. [DOI] [PMC free article] [PubMed] [Google Scholar]


