Abstract
INSL3 (insulin-like peptide 3) is a relaxin peptide family member expressed by Leydig cells in the vertebrate testis. In mammals, INSL3 mediates testicular descent during embryogenesis but information on its function in adults is limited. In fish, the testes remain in the body cavity, although the insl3 gene is still expressed, suggesting yet undiscovered, evolutionary older functions. Anti-Müllerian hormone (Amh), in addition to inhibiting spermatogonial differentiation and androgen release, inhibits the Fsh (follicle-stimulating hormone)-induced increase in insl3 transcript levels in zebrafish testis. Therefore, the two growth factors might have antagonistic effects. We examine human INSL3 (hINSL3) effects on zebrafish germ cell proliferation/differentiation and androgen release by using a testis tissue culture system. hINSL3 increases the proliferation of type A undifferentiated (Aund) but not of type A differentiating (Adiff) spermatogonia, while reducing the proliferation of Sertoli cells associated with proliferating Aund. Since the area occupied by Aund decreases and that of Adiff increases, we conclude that hINSL3 recruits Aund into differentiation; this is supported by the hINSL3-induced down-regulation of nanos2 transcript levels, a marker of single Aund spermatogonia in zebrafish and other vertebrates. Pulse-chase experiments with a mitosis marker also indicate that hINSL3 promotes spermatogonial differentiation. However, hINSL3 does not modulate basal or Fsh-stimulated androgen release or growth factor transcript levels, including those of amh. Thus, hINSL3 seems to recruit Aund spermatogonia into differentiation, potentially mediating an Fsh effect on spermatogenesis.
Keywords: INSL3, Spermatogonial differentiation, Androgen release, Gene expression, Adult testis, Zebrafish
Introduction
Insulin-like peptide 3 (INSL3) is a relaxin peptide family member expressed in the male reproductive system by Leydig cells during fetal development and adult life. During embryonic development in mammals, INSL3 has a role that is crucial for the success of spermatogenesis in adult life (Kumagai et al. 2002). Knockout mice for INSL3 or its receptor RXFP2 show a cryptorchid phenotype, in which the testes remain inside the body cavity and fail to descend into the scrotum, since the gubernacula do not develop properly (Zimmermann et al. 1999; Nef and Parada 1999; Kumagai et al. 2002). However, although INSL3/RXFP2 are also expressed in the adult testis, information on potential functions in mature testis is incomplete. During the last decade, some studies have suggested a role for INSL3 in spermatogenesis (Kawamura et al. 2004; Pathirana et al. 2012). The effect of INSL3 on germ cell survival has been recorded in rat (Kawamura et al. 2004), whereas a more recent study reported no effects on germ cell apoptosis following RXFP2 ablation in mice (Huang et al. 2012). Nevertheless, testosterone release by cultured mouse Leydig cells increases in response to INSL3 (Pathirana et al. 2012), suggesting an autocrine role of this peptide as previously proposed after the localization of RXFP2 to Leydig cells (Anand-Ivell et al. 2006).
Although more information concerning relaxins has been obtained in the past few years, knowledge of their biological activity mostly comes from studies in mammals and little is known about their role in submammalian vertebrates such as teleost fish, a taxonomic group including nearly 50 % of all vertebrate species (Yegorov et al. 2014). Studies regarding Insl3, other relaxins and their receptors in teleost fish are limited to gene expression data (Good-Ávila et al. 2009; Yegorov et al. 2009; Good et al. 2012). Evidently, no testicular descent occurs in fish and studies directed to finding other, evolutionary older biological activities might also provide new leads for the respective activities of INSL3 in higher vertebrates.
In zebrafish (Danio rerio), for example, insl3 gene expression has been localized to Leydig cells (Good-Ávila et al. 2009). Recent studies with recombinant zebrafish follicle-stimulating hormone (Fsh) have shown that the stimulation of zebrafish testis explants with Fsh increases testicular insl3 mRNA levels, an effect not mediated by the steroidogenic activity of Fsh (García-López et al. 2010) but, instead, by a direct effect on Leydig cells that express both gonadotropins receptors in fish (García-López et al. 2010; Ohta et al. 2007; Chauvigné et al. 2012). On the other hand, recombinant anti-Müllerian hormone (Amh) inhibits the stimulatory effect of Fsh on insl3 mRNA levels in zebrafish (Skaar et al. 2011). This opens up the possibility that, at sites with high levels of Amh, Fsh is less efficient at increasing levels of insl3 mRNA in Leydig cells. Since, in addition to suppressing insl3 mRNA expression, Amh inhibits the differentiation of type A undifferentiated (Aund) spermatogonia and Fsh-stimulated steroidogenesis, we hypothesize that Insl3 stimulates germ cell differentiation and steroidogenesis. This hypothesis is tested as part of our broader goal to understand the effect of Insl3 on testis function.
Materials and methods
Animals
Adult male zebrafish were bred and raised in the aquarium facility of the Department Biology, Utrecht University. The experiments followed the Dutch National regulations for animal use in experimentation. For morphometric/androgen release and mRNA analyses, 8 and 12 animals were used per experiment, respectively.
Human INSL3
Human INSL3 (hINSL3) was synthesized by using the continuous flow Fmoc (N-(9-fluorenyl)methoxycarbonyl)-solid phase methodology together with regioselective disulfide bond formation as previously described (Bathgate et al. 2006) and was obtained as a kind gift from Prof. John D. Wade, University of Melbourne, Victoria, Australia. The peptide was dissolved at a concentration of 100 μg/ml in sterile phosphate-buffered saline (PBS) and aliquots were flash-frozen in liquid N2 and stored at −80 °C. We reasoned that hINSL3 would be biologically active in zebrafish testis because of the following considerations. Two rxfp2 genes (rxfp2a and rxp2b), paralogous to the human RXFP2 gene, are abundantly expressed in zebrafish testis (Good et al. 2012; Yegorov et al. 2014). The specificity of the interaction of hINSL3 with RXFP2 is mainly determined by RXFP2 residues Phe131 and Gln133 interacting with hINSL3 B-chain residue Trp27, RXFP2 residue Trp177 with hINSL3 B-chain residue His12, RXFP2 residue Ile179 with hINSL3 B-chain residue Val19, RXFP2 residues Asp181 and Glu229 with hINSL3 B-chain residue Arg20 and Asp227 with hINSL3 residue Arg16 (Büllesbach and Schwabe 1999, 2004, 2005, 2006; Rosengren et al. 2006; Scott et al. 2007). Alignment of the zebrafish Rxfp2a and Rxfp2b receptor sequences with the human RXFP2 receptor sequence revealed that the zebrafish receptor contains identical residues at the ligand-receptor interaction sites, except for RXFP2 residues Ile179 and Glu229, which are replaced by Val and Ala in the zebrafish Rxfp2a receptor. Human INSL3 should therefore be able to interact with both zebrafish Rxfp2 receptors, as Val179 and Ala229 would not hinder hINSL3 interacting with the Rxfp2a receptor.
Tissue culture
A primary testis tissue culture system was used to study the effects of hINSL3 on germ and somatic cell proliferation, androgen release and testicular mRNA levels, according to protocols previously established (Leal et al. 2009b). The concentration of 100 ng hINSL3/ml was chosen based on a pilot experiment and data published in mammals (Pathirana et al. 2012). To study proliferation and transcript levels, the two testes from each fish were dissected and incubated for 7 days, one under stimulatory conditions (receiving medium containing hINSL3) and the other under basal conditions (receiving only tissue culture medium). The testes were placed on a 0.25-cm2 piece of nitrocellulose membrane (25 μm thickness, 0.22 μm pore size; Millipore, Billerica, Mass., USA), on top of a 700-μl agarose cylinder (1.5 % [w/v] in Ringer’s solution, pH 7.4) that was placed in a 24-well flat-bottom plate (Corning, New York, USA). Medium (1 ml) was added such that the agar cylinder was just not submerged; the medium was refreshed after 4 days. To study androgen release, testes were submerged in 200 μl tissue culture medium in a 96-well plate for ~18 h. All components for the tissue culture studies were freshly prepared according to published protocols (Leal et al. 2009b).
Germ and Sertoli cell proliferation analysis
To evaluate the capacity of hINSL3 to modulate the proliferation activity of various spermatogonial generations and of Leydig and Sertoli cells, 50 μg/ml proliferation marker 5-bromo-2′-deoxyuridine (BrdU; Sigma-Aldrich) was added to the tissue culture medium of the testes during the last 6 h of the 7-day incubation period. During this final period of 6 h, the tissue was submerged in the medium.
In a pulse-chase set-up, zebrafish were exposed in vivo to BrdU dissolved in water (4 mg/ml) for ~12 h per day on 3 consecutive days to allow BrdU incorporation into the DNA of all dividing cells, including slowly dividing, single type Aund spermatogonia, followed by a 4-day chase period, during which the BrdU labeling was cleared from rapidly dividing cell types, including many of the rapidly proliferating spermatogonia (Nóbrega et al. 2010). Subsequently, the testes were dissected and incubated for 4 days ex vivo in the absence or presence of hINSL3 (100 ng/ml), as described above. Importantly, BrdU was not present in the medium during this tissue culture period of 4 days, so that the localization of the proliferation marker at the end of the tissue culture period allowed hINSL3 effects on the dynamics of BrdU to be examined in the germ cells that had taken up the marker previously in vivo.
After the tissue culture period, the testes were fixed at 4 °C overnight in freshly prepared methacarn (60 % [v/v] absolute ethanol, 30 % chloroform and 10 % acetic acid), dehydrated, embedded in Technovit 7100 (Heraeus Kulzer), sectioned at a thickness of 4 μm and used to localize BrdU, as described previously (Leal et al. 2009a). The germ cells/cysts were identified according to previously published morphological criteria (Leal et al. 2009a). In brief (see Fig. 1a), the type Aund spermatogonium is a single germ cell and the largest spermatogonial cell type in zebrafish, with a large nucleus (~9 μm diameter), poorly condensed chromatin and one or two compact nucleoli; it is enveloped by a Sertoli cell, thus forming the initial stage of a spermatogenic cyst. The type Adiff spermatogonia, although morphologically not very different from type Aund, show a smaller (~6 μm diameter) and denser nucleus and occur in groups of 2, 4, or 8 cells (1st, 2nd and 3rd/final generation of type Adiff spermatogonia) in the same stage of development inside a cyst (Fig. 1b), because of the synchronized development among the members of the same germ cell clone based on cytoplasmic bridges remaining from an incomplete cytokinesis during differentiating mitoses. The five generations of type B spermatogonia (16 to 256 cells) show a further reduced nuclear size (~5 μm diameter); the nucleus is slightly elongated/ovoid and clearly contains more heterochromatin than in type A spermatogonia (Fig. 1b).
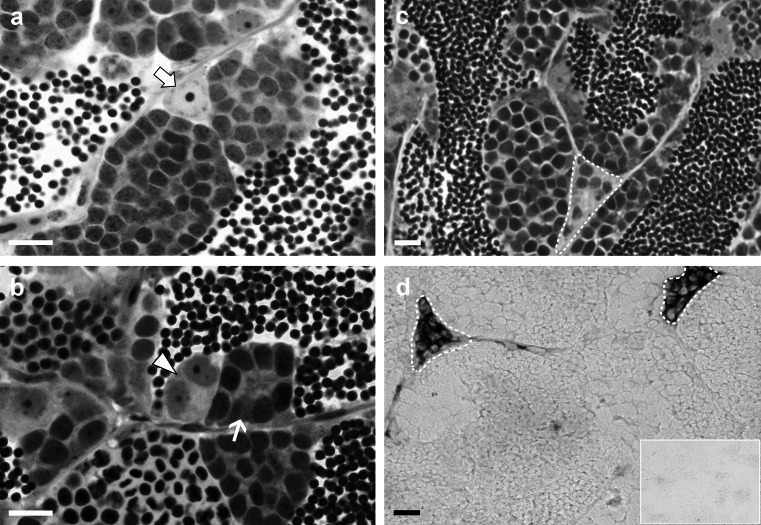
Fig. 1.
Morphological characteristics of zebrafish type A undifferentiated (Aund) and type A differentiating (Adiff) and type B spermatogonia and Leydig cells and in situ hybridization for insl3 mRNA in adult zebrafish testis. a Type Aund spermatogonia (arrow) are single germ cells showing a large and clear nucleus, with poorly condensed chromatin and one or two compact nucleoli. b A cyst containing two type Adiff spermatogonia (arrowhead) that show smaller nuclei stained more intensely by toluidine-blue. Type B spermatogonia (thin arrow) have still smaller, slightly elongated/ovoid nuclei that moreover show a high amount of heterochromatin. c Interstitial space (dashed line) with a group of Leydig cells. d Adult zebrafish testis section showing the detection of insl3 mRNA by in situ hybridization in Leydig cells cluster (dashed lines). Inset in d non-specific staining obtained with the sense probe. a–c Sections were prepared for morphological analysis according to Leal et al. (2009b). Magnification ×1000 (a, b), ×600 (c, d). Bars 10 μm
To quantify proliferation, the mitotic index was determined by examining 100 randomly chosen germ cells/cysts or somatic cells and by discriminating between BrdU-labeled and unlabeled cells. To evaluate the proportion of area occupied by type Aund, Adiff and B spermatogonia, 30 randomly chosen fields were photographed at ×400 magnification by using a conventional microscope equipped with a digital camera. The images were analyzed quantitatively by using ImageJ software (Image Processing and Analysis in Java). With a specific plug-in, a 540-point grid was made to quantify the proportion of the area for the various germ cell types, based on the number of points counted over those germ cell types.
Relative quantification of testicular mRNA levels
The effects of hINSL3 on testicular mRNA levels were investigated after 7 days of testis tissue culture. Total RNA was isolated from the tissue by using an RNAqueous Micro kit (Ambion), according to the manufacturer’s protocol, in order to quantify the mRNA levels of selected testicular genes. The selection included insl3 (García-López et al. 2010), Sertoli cell genes known to modulate spermatogonial proliferation and differentiation behavior, such as amh (Miura et al. 2002; Skaar et al. 2011), gsdf (Sawatari et al. 2007) and igf3 (Morais et al. 2013) and four germ cell markers, namely nanos2 (expressed in single type Aund spermatogonia; Beer and Draper 2013), piwil1 (expressed in all generations of type A spermatogonia; Houwing et al. 2007), sycp3 (expressed in spermatocytes; Chen et al. 2013) and odf3b (expressed in spermatids; Yano et al. 2008). Two micrograms of isolated RNA was taken from each sample to synthesize cDNA as described previously (de Waal et al. 2008). The relative mRNA levels were determined by using real time, quantitative polymerase chain reaction (qPCR) assays, according to published protocols for all genes analyzed (see Table 1), except for nanos2. For detecting nanos2 mRNA (Beer and Draper 2013), primers were designed (Table 1) and validated for specificity and amplification efficiency on serial dilutions of testis cDNA (Bogerd et al. 2001). All qPCRs and calculations were performed as described previously (Bogerd et al. 2001; de Waal et al. 2008; García-López et al. 2010) in 20-μl reaction volumes and quantification cycle (Cq) values were obtained by a Step One Plus Real-Time PCR System (Applied Biosystems) by using default settings. Elongation factor 1α (ef1α) mRNA was used as the endogenous control, since its expression remained stable under both basal and treated conditions.
Table 1.
Primers used for mRNA levels measurement of ef1α (elongation factor 1α), insl3 (insulin-like peptide 3), amh (anti-Müllerian hormone), gsdf, igf3 (two Sertoli cell genes known to modulate spermatogonial proliferation and differentiation), nanos2, piwil1, sycp3, odf3b (four germ cell markers); Fw, forward; Rv, reverse
| Target genes | Primers | Sequence (5′-3′) | Reference |
|---|---|---|---|
| ef1α | AG (Fw) | GCCGTCCCACCGACAAG | Morais et al. 2013 |
| AH (Rv) | CCACACGACCCACAGGTACAG | ||
| insl3 | 2466 (Fw) | TCGCATCGTGTGGGAGTTT | Good et al. 2012 |
| 2467 (Rv) | TGCACAACGAGGTCTCTATCCA | ||
| amh | AD (Fw) | CTCTGACCTTGATGAGCCTCATTT | García-López et al. 2010 |
| AE (Rv) | GGATGTCCCTTAAGAACTTTTGCA | ||
| igf3 | 2680 (Fw) | TGTGCGGAGACAGAGGCTTT | Morais et al. 2013 |
| 2681 (Rv) | CGCCGCACTTTCTTGGATT | ||
| gsdf | 2366 (Fw) | CATCTGCGGGAGTCATTGAAA | García-López et al. 2010 |
| 2367 (Rv) | CAGAGTCCTCCGGCAAGCT | ||
| piwi1l | 2542 (Fw) | GATACCGCTGCTGGAAAAAGG | García-López et al. 2010 |
| 2543 (Rv) | TGGTTCTCCAAGTGTGTCTTGC | ||
| sypc3 | 2730 (Fw) | AGAAGCTGACCCAAGATCATTCC | García-López et al. 2010 |
| 2731 (Rv) | AGCTTCAGTTGCTGGCGAAA | ||
| odf3b | 2791 (Fw) | GATGCCTGGAGACATGACCAA | Leal et al. 2009b |
| 2792 (Rv) | CAAAGGAGAAGCTGGGAGCTTT | ||
| nanos2 | 4817 (Fw) | AAACGGAGAGACTGCGCAGAT | This paper |
| 4818 (Rv) | CGTCCGTCCCTTGCCTTT |
Cellular localization of insl3 mRNA
To localize the cellular expression of insl3 mRNA, in situ hybridization was performed by using digoxigenin (DIG)-labeled cRNA probes previously designed and validated (Good-Ávila et al. 2009). Adult zebrafish testes were fixed in 4 % paraformaldehyde (PFA) in PBS (pH 7.4) at 4 °C overnight and subsequently transferred to 20 % sucrose in PBS until the tissue remained submerged. In situ hybridization was carried out on 10-μm-thick cryosections (Leica cryostat), which were post-fixed with 4 % PFA and then treated with proteinase K (5 μg/mL; Sigma-Aldrich) at room temperature for 5 min. Hybridization, with 500 ng riboprobe per milliliter, was carried out in hybridization buffer (5 × standard sodium citrate, 50 % deionized formamide, 10 % dextran sulfate, 5 × Denhardt’s, 250 μg/ml yeast tRNA, 500 μg calf thymus DNA) at 65 °C overnight. The slides were then washed in 5 × and 0.2 × standard sodium citrate at 55 °C, each for 30 min and blocking was performed with 1 % heat-inactivated goat serum (Vector) in blocking buffer (0.1 M TRIS–HCl, 0.15 M NaCl, pH 7.5) at room temperature for 1 h. Subsequently, the slides were incubated with alkaline-phosphatase-conjugated anti-DIG antibody (Roche) diluted 1:1000 in blocking solution at 4 °C overnight. Color development was conducted by incubating the sections in Nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (Roche) in the dark. After being air-dried, the slides were mounted using Aquamount (Thermo Scientific) and micrographs were taken with an Olympus AX70 microscope.
Androgen release analysis
To determine whether basal or Fsh-stimulated testicular androgen release was modulated by hINSL3, the levels of 11β-hydroxyandrostenedione (OHA), a known precursor of 11-ketotestosterone (11-KT), were measured in testis tissue culture medium using a steroid release bioassay previously adapted for zebrafish testis (García-López et al. 2010). The results were calculated as nanograms of OHA released per milligram of testis tissue when incubated in the absence or presence of 25 ng/ml Fsh and/or 100 ng/ml hINSL3, respectively.
Statistics
The statistical analyses were performed by using the GraphPad Prism 5 software package (GraphPad Software, San Diego, Calif., USA). Differences between control and experimental groups were tested for statistical significance by using the Student’s t-test for paired observation and analysis of variance (post-test Newman-Keuls) for multiple group comparisons. A significance level of P < 0.05 was applied in all analyses. Data are presented as means ± standard error of mean (SEM).
Results
Spermatogonial and somatic cell proliferation
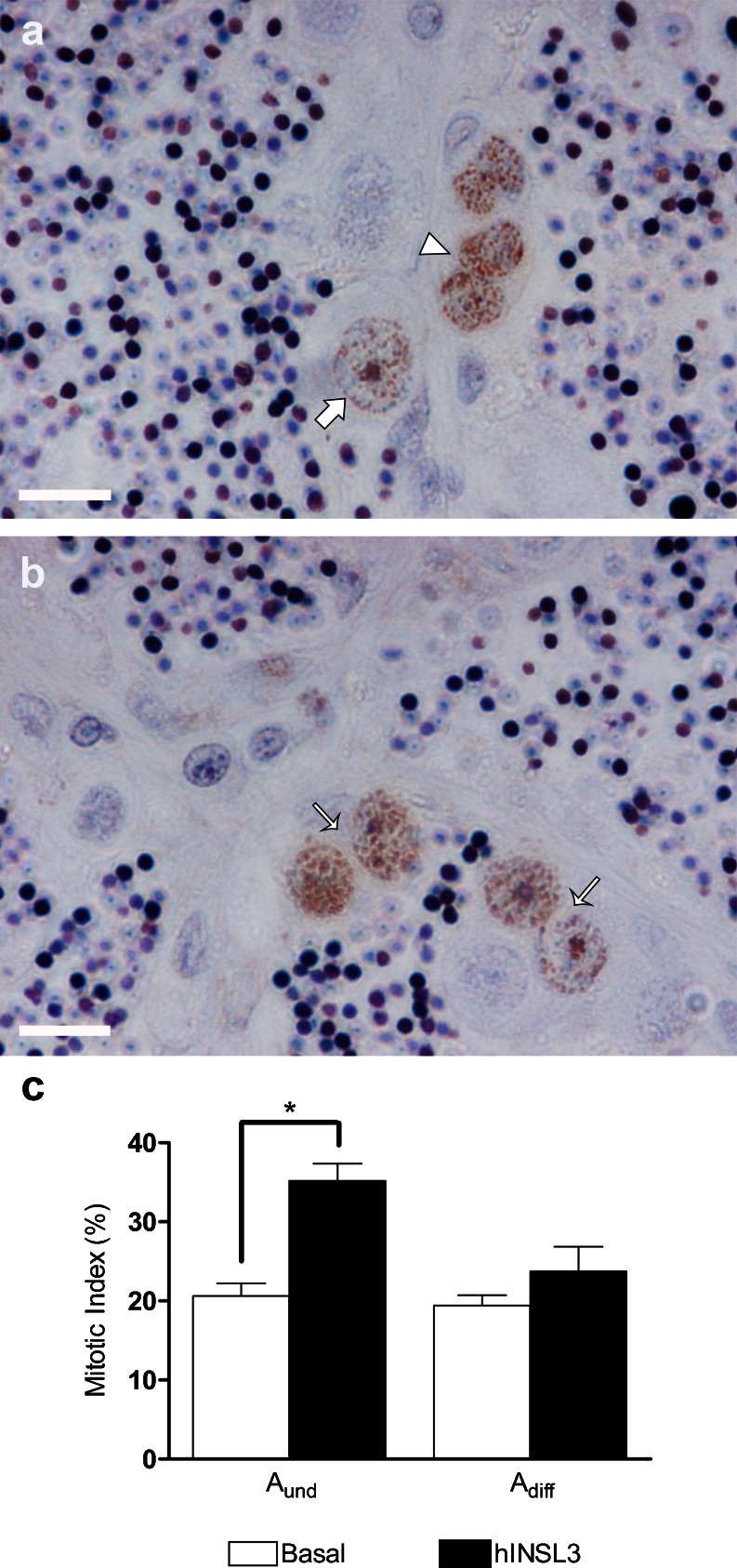
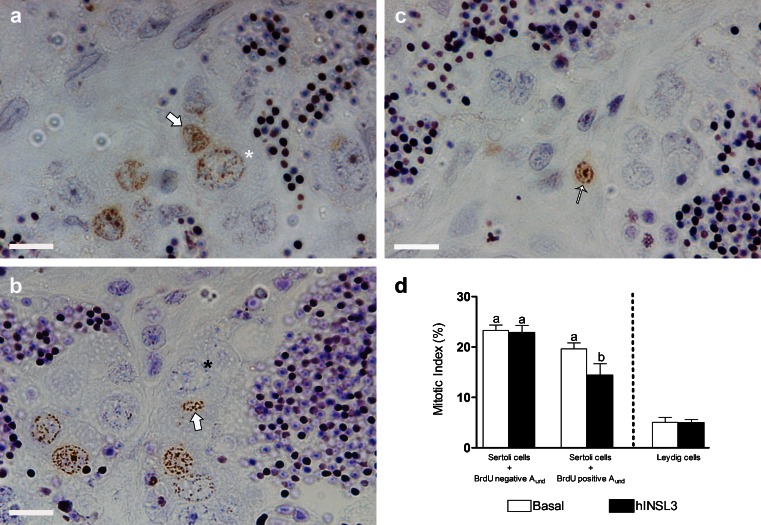
Based on counting BrdU-positive cells, we calculated the mitotic index of type Aund and Adiff spermatogonia (Fig. 2a, b), Leydig cells and Sertoli cells associated with BrdU-positive or BrdU-negative type Aund spermatogonia (Fig. 3a-c). In testis tissue culture, hINSL3 significantly stimulated the proliferation of type Aund spermatogonia, whereas no significant changes were observed for type Adiff spermatogonia (Fig. 2c). Moreover, no effects were observed for Leydig cells or for Sertoli cells associated with BrdU-negative type Aund spermatogonia, whereas a significant decrease in proliferation was found for Sertoli cells associated with BrdU-positive type Aund spermatogonia (Fig. 3d).
Fig. 2.
a, b Testis tissue sections showing BrdU-positive germ cells: type Aund (arrow), type Adiff (thin arrows) and type B (arrowhead) spermatogonia. Magnification ×1000. Bars 10 μm. c Mitotic indices of type Aund and Adiff spermatogonia after incubation for 7 days in the absence (Basal) and presence (hINSL3) of 100 ng hINSL3/ml. *Significant difference (P < 0.05) between treated and control. Results are presented as means ± SEM (n = 8)
Fig. 3.
a, b Testis tissue sections showing BrdU-positive Sertoli cells nuclei (arrows) in association with BrdU-positive (white star) and BrdU-negative (black star) type Aund spermatogonia. c BrdU-positive Leydig cell nucleus (thin arrow). d Mitotic indices of Sertoli cells in association with BrdU-negative or BrdU-positive type Aund spermatogonia and of Leydig cells after incubation for 7 days in the absence (Basal) or presence (hINSL3) of 100 ng hINSL3/ml. Different letters indicate significant differences (P < 0.05) between the absence and presence of hINSL3. Magnification ×1000 (a–c). Bars 10 μm. Results are presented as means ± SEM (n = 8)
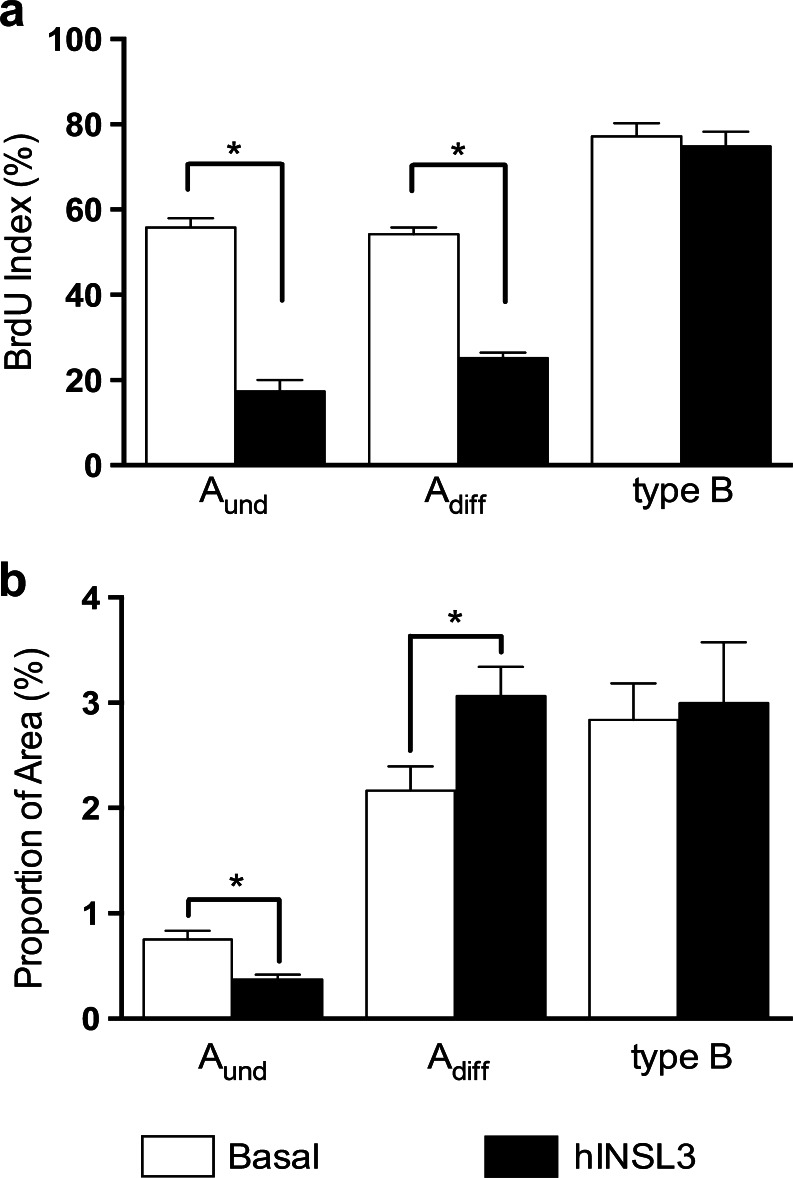
In order to determine whether the increased proliferation activity of type Aund spermatogonia was associated with self-renewal (leading to two single Aund) or differentiation (leading to a pair of Adiff), we labeled these cells with BrdU in vivo via a 3-day pulse and 4-day chase protocol, before the exposure of testis tissue to hINSL3 ex vivo. This allowed the study of the dynamics of spermatogonial proliferation by examining the way that exposure to hINSL3 ex vivo influenced the BrdU indices of type Aund, type Adiff and type B spermatogonia that previously had taken up the BrdU label in vivo. Quantification of the BrdU indices in this type of experiment for the various spermatogonial cell types showed that hINSL3 induced a decrease of approximately three- to two-fold, respectively, for type Aund and Adiff spermatogonia, whereas no change was recorded for type B spermatogonia (Fig. 4a). At the same time, the proportion of the area occupied by type Aund spermatogonia was significantly reduced, whereas an increase was observed in the proportion of type Adiff spermatogonia (Fig. 4b).
Fig. 4.
BrdU indices of type Aund, type Adiff and type B spermatogonia after BrdU exposure in vivo and subsequent tissue culture in the absence (Basal) and presence (hINSL3) of 100 ng hINSL3/ml (a) and volumetric proportion of cysts of type Aund, type Adiff, and type B spermatogonia after tissue culture in the absence (Basal) and presence (hINSL3) of hINSL3 (b). *Significant differences (P < 0.05) between the absence and presence of hINSL3. Results are presented as means ± SEM (n = 8)
Androgen release
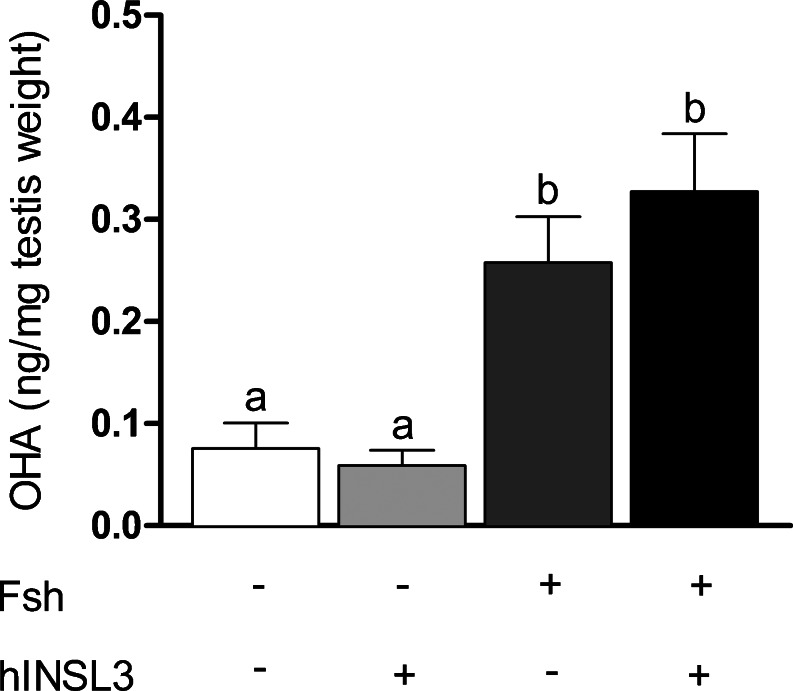
To evaluate whether the effects of hINSL3 on germ cell proliferation and differentiation were associated with a modulation of basal or gonadotropin-stimulated androgen release, we used (recombinant zebrafish) Fsh, which is a potent steroidogenic hormone in fish (reviewed by Schulz et al. 2010). Our results showed that hINSL3 neither modulated basal nor Fsh-stimulated androgen release (Fig. 5).
Fig. 5.
Effects of hINSL3 (100 ng/ml) on basal or follicle-stimulating hormone (Fsh; 25 ng/ml)-stimulated androgen release ex vivo. Results (means ± SEM; n = 8) are presented as nanograms of 11β-hydroxyandrostenedione (OHA) released per milligram (mg) of testis weight
Relative testicular transcript levels and cellular localization of insl3 mRNA
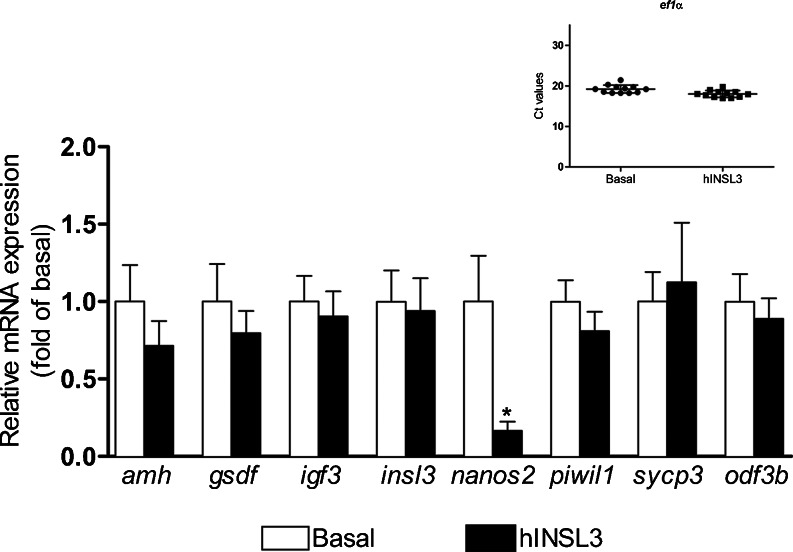
The quantification of the relative transcript levels of selected genes was intended to start an elucidation of the molecular mechanism used by hINSL3 to modulate germ cell proliferation. We analyzed the transcript levels of insl3, three Sertoli cell genes (amh, gsdf and igf3) and a spermatogonial (piwil1), a spermatocyte (sycp3) and a spermatid (odf3b) gene. The qPCR results showed no significant difference between basal and hINSL3-stimulated conditions for these transcripts (Fig. 6). However, transcript levels of nanos2, a marker for single type Aund spermatogonia, was down-regulated six-fold in testis tissue exposed to hINSL3 (Fig. 6).
Fig. 6.
Relative mRNA levels of insl3 and selected Sertoli (amh, gsdf and igf3) and germ cell (nanos2, piwil1, sypc3 and odf3b) genes. Inset Transcript levels of ef1α in the absence (Basal) and presence (hINSL3) of hINSL3; Ct values for the reference gene ef1α. Results are presented as means ± SEM (n = 12)
The cellular localization of insl3 transcripts in zebrafish testis tissue was analyzed by in situ hybridization on cryosections. Transcripts were exclusively observed in the interstitial compartment (Fig. 1d), with a specific hybridization signal exclusively on Leydig cells that often formed clusters in the interstitium (Fig. 1c, d). No signal was observed with the sense insl3 riboprobe (negative control; inset Fig. 1d).
Discussion
Although information is available concerning the evolution and expression of relaxin peptide family members in teleost fish (Good-Ávila et al. 2009; Yegorov et al. 2009; Good et al. 2012), no previous reports exist, to our knowledge, on the biological activity of these peptides in submammalian vertebrates. We reasoned that hINSL3 would be able to interact with both zebrafish Rxfp2 receptors, since the residues identified as important for ligand-receptor interaction with the human receptor for hINSL3 (i.e., RXFP2) are identical in the zebrafish Rxfp2a and Rxfp2b receptors, except for RXFP2 residues Ile179 and Glu229, which are replaced by Val and Ala and most likely do not hinder the interaction of hINSL3 with the zebrafish Rxfp2a receptor. This is supported by our finding that hINSL3 stimulates the differentiation of type Aund spermatogonia (further discussed below). Hence, despite the biological differences in the action of INSL3 in teleosts vs. mammals and their evolutionary distance, paralogous teleost receptors, Rxfp2a and Rxfp2b, are still able to respond to mammalian hINSL3. This suggests that, although the neofunctionalization of certain aspects of INSL3 occurs in mammals (i.e., testicular descent), older conserved functions of INSL3 presumably take place across vertebrates. Whereas spermatogenesis is known not to be dependent on INSL3 function in adult mice (Huang et al. 2012), INSL3 might have as yet to be identified effects on germ cell development in higher vertebrates and the detection of RXFP2 in mammalian germ cells is compatible with the notion that INSL3 modulates germ cell development in a paracrine manner (Huang et al. 2012; Minagawa et al. 2014; Sagata et al. 2015), notwithstanding the known (neo-functionalized) role of INSL3 in mammalian testicular descent.
Using a testis tissue culture approach, we showed that hINSL3 significantly increases the mitotic index of type Aund spermatogonia. Moreover, we propose that these proliferating type Aund spermatogonia are preferentially recruited into differentiation by hINSL3. Type Aund spermatogonia are single germ cells enveloped by one or sometimes two Sertoli cells in zebrafish (Leal et al. 2009a); spermatogonial stem cells (SSC) are part of this germ cell population (Nóbrega et al. 2010). As undifferentiated cells, SSCs have the capacity to produce either more SSCs (self-renewal proliferation) or germ cells committed to the spermatogenic process (differentiating proliferation), the first step being to form a pair of considerably smaller type Adiff spermatogonia. The latter cells represent ~44 % of the cellular volume of Aund, such that a pair of Adiff are slightly smaller than a single Aund (Leal et al. 2009a) and remain connected via a cytoplasmic bridge inside a single cyst, one of the hallmarks of differentiating germ cell divisions. On the other hand, SSC undergoing self-renewal produce two completely separated type Aund daughter cells and the newly generated germ cell needs to recruit its own Sertoli cell to create a new spermatogenic cyst. Consequently, to produce new spermatogenic cysts, Sertoli cell proliferation is also required (França et al. 2015). Here, we found that Sertoli cells contacting BrdU-positive type Aund spermatogonia show reduced proliferation activity when incubated with hINSL3 (Fig. 3d), which we interpret as circumstantial evidence that the Aund spermatogonia are undergoing germ cell differentiation not self-renewal. To obtain more direct evidence for this hypothesis, we used additional molecular and morphological approaches.
Quantifying selected germ cell marker genes show that three out of the four transcripts remain stable, whereas nanos2 mRNA levels are down-regulated in testis tissue exposed to hINSL3. In male mice, SSC maintenance depends on the RNA binding protein NANOS2; its conditional loss in adults results in a loss of SSCs to differentiation, whereas overexpression increases testicular SSC numbers (Sada et al. 2009). Furthermore, miR-34c-mediated down-regulation of the NANOS2 protein enhances murine SSC differentiation (Yu et al. 2014). In teleost fish, Nanos2 appears to play a similar role, as the loss of nanos2 function in tilapia results in germ-cell-deficient testes (Li et al. 2014) and as both protein and transcript are detected in single type Aund spermatogonia (Lacerda et al. 2013; Bellaiche et al. 2014). In the adult zebrafish testis, nanos2 mRNA is expressed exclusively in single, vasa-positive germ cells that are considered to have germ line stem cell-like characteristics in both sexes (Beer and Draper 2013). Hence, a decrease in nanos2 transcript levels in testis tissue incubated with hINSL3 is compatible with a hINSL3-induced differentiation of single type Aund spermatogonia, such as the SSCs in zebrafish.
A second approach that we employed is based on quantitative histology. In agreement with the decreased nanos2 transcript levels, we found a decrease in the area occupied by type Aund spermatogonia and an increased area occupied by Adiff spermatogonia (Fig. 4b). Hence, this increase in type Adiff cells directly suggests that hINSL3 stimulation increases the proliferation of Aund spermatogonia and their differentiation into Adiff spermatogonia but does not change the mitotic activity of Adiff spermatogonia.
We observed that Aund and Adiff spermatogonia pre-labeled with BrdU in vivo lose the BrdU label faster during a subsequent tissue culture period of 4 days when incubated in the presence but not absence of hINSL3 (Fig. 4a). We consider this to be evidence that hINSL3 is involved in the differentiation of type Aund spermatogonia. To explain this, we need to refer to spermatogonial dynamics in zebrafish.
Tracing a BrdU pulse through time, Leal et al. (2009a, 2009b) demonstrated that meiosis and spermiogenesis in zebrafish take 6 days, both in vivo and in tissue culture. The only information available for the duration of the mitotic phase of zebrafish germ cell differentiation indicates that one cell cycle of type Aund spermatogonia takes place within 30 h (Nóbrega et al. 2010). Therefore, we estimate that, during the 96 h of tissue culture for the experiments shown in Fig. 4, three to four spermatogonial divisions might have taken place. Since BrdU is incorporated into the Aund spermatogonia at the beginning of the tissue culture, this means that, in the case of differentiating divisions, the BrdU would have shifted into the Adiff cell pool, leading to a decrease of the BrdU index for type Aund cells. Thus, in conjunction with the reduction of nanos2 transcript levels, the surface area occupied by Aund spermatogonia (Fig. 4b) and the loss of BrdU from the Aund cell pool is the third line of evidence showing that hINSL3 induces the differentiation of single type Aund spermatogonia in zebrafish.
However, the BrdU index of the Adiff spermatogonia is also significantly reduced following exposure to hINSL3 (Fig. 4a). This is unexpected, since their proliferation activity is not changed by hINSL3 (Fig. 2c) but might be explained by the following considerations. The hINSL3-mediated recruitment of type Aund cells into differentiation probably occurs irrespective of their BrdU-labeling status. About 45 % of the Aund spermatogonia do not contain BrdU under control conditions (Fig. 4a: 55 % BrdU-labeled, i.e., 45 % unlabeled) and hINSL3 will have stimulated the production of BrdU-negative Adiff from the initially BrdU-negative Aund spermatogonia. Moreover, initially, BrdU-positive type Aund spermatogonia recruited by hINSL3 into differentiation potentially undergo three or four cell cycles (three cycles from Aund to the third and last generation of Adiff, or four cycles to the first generation of type B spermatogonia), which is associated with an 8- to 16-fold dilution of BrdU that thereby might have become undetectable. We propose that the reduced BrdU index of type Adiff spermatogonia, despite their unchanged mitotic activity, reflects the combined effects of an increased production of BrdU-negative Adiff cells from initially BrdU-negative Aund cells, a loss of BrdU detectability in Adiff cells by dilution attributable to repeated cell cycling and finally a shift of the BrdU label into the B spermatogonia population for the cells that undergo four differentiating cell cycles. Since the proportion of area occupied by Adiff spermatogonia becomes more prominent, although no change is observed for type B spermatogonia (Fig. 4b), we assume that the increased production of type Adiff with undetectable levels of BrdU is the main factor in this regard.
Since type B spermatogonia do not show changes after hINSL3 treatment for the parameters investigated (Fig. 4a, b), other experiments need to be performed to determine whether type B spermatogonia do not respond to hINSL3 or whether potential changes among type B spermatogonia are compensated for by the dynamics of “neighboring” germ cell generations (i.e., type Adiff spermatogonia and primary spermatocytes). However, major changes in the meiotic or post-meiotic germ cell generations do not seem likely considering the relatively stable expression levels of the marker genes sypc3 and odf3b, respectively (Fig. 6).
Previous work has shown that Sertoli cell-derived Amh inhibits spermatogonial differentiation and also reduces Leydig cell insl3 mRNA levels (Skaar et al. 2011). Since our present data suggest that hINSL3 stimulates spermatogonial differentiation, we hypothesize that hINSL3 action might include a down-regulation of amh transcript levels. However, this is not the case. Moreover, transcript levels of gsdf and igf3, coding for growth factors stimulating spermatogonial proliferation in trout (Sawatari et al. 2007) or zebrafish (Morais et al. 2013), are also unaffected by hINSL3, suggesting that a change in the transcript levels of these growth factors is not directly involved in mediating hINSL3 action.
Recent work in mice has shown that INSL3 has an autocrine stimulatory effect on androgen release from Leydig cells (Pathirana et al. 2012) and androgens are known to stimulate spermatogenesis in tissue culture in various fish species (e.g., Miura et al. 1991; Leal et al. 2009b). However, our results show no effect of hINSL3 on basal or Fsh-stimulated androgen release, indicating that hINSL3 effects on zebrafish spermatogonia are not mediated by acutely modulating androgen production.
In summary, in this work, we investigated the potential role of hINSL3 on spermatogenesis in zebrafish, a species in which insl3 gene expression is also found in Leydig cells but in which testicular descent does not occur. Morphometric studies and gene analysis of germ cell markers support the hypothesis that hINSL3 stimulates the differentiating proliferation of type Aund to type Adiff spermatogonia. To our knowledge, this is the first study of the biological activity of a relaxin family member in fish reproduction. Future studies should be directed (1) at investigating whether zebrafish Insl3 shows comparable biological activities and (2) at identifying the testicular cell types expressing receptors for Insl3, as these aspects will lead us towards our larger goal of understanding the effects of Insl3 on testis function in teleosts.
Acknowledgments
The authors thank Henk van de Kant (Utrecht University, Department of Biology) for excellent technical support (BrdU immunostaining).
Footnotes
The authors acknowledge financial support by the European Union (project LIFECYCLE no. FP7/222719), by the Research Council of Norway (project 221648/O30) and by the Brazilian Foundation CAPES (project number BEX: 9802/12-6).
Contributor Information
J. Bogerd, Phone: x31-30-253-3046, Email: j.bogerd@uu.nl, http://www.bio.uu.nl/developmentalbiology
R. W. Schulz, Phone: x31-30-253-3046, Email: r.w.schulz@uu.nl, http://www.bio.uu.nl/developmentalbiology
References
- Anand-Ivell RJ, Relan V, Balvers M, Coiffec-Dorval I, Fritsch M, Bathgate RA, Ivell R. Expression of insulin-like peptide 3 (INSL3) hormone-receptor (LGR8) system in the testis. Biol Reprod. 2006;74:945–953. doi: 10.1095/biolreprod.105.048165. [DOI] [PubMed] [Google Scholar]
- Bathgate RA, Lin F, Hanson NF, Otvos L, Jr, Guidolin A, Giannakis C, Bastiras S, Layfield SL, Ferraro T, Ma S, Zhao C, Gundlach AL, Samuel CS, Tregear GW, Wade JD. Relaxin-3: improved synthesis strategy and demonstration of its high-affinity interaction with the relaxin receptor LGR7 both in vitro and in vivo. Biochemistry. 2006;45:1043–1053. doi: 10.1021/bi052233e. [DOI] [PubMed] [Google Scholar]
- Beer RL, Draper BW. nanos3 maintains germline stem cells and expression of the conserved germline stem cell gene nanos2 in the zebrafish ovary. Dev Biol. 2013;374:308–318. doi: 10.1016/j.ydbio.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Bellaiche J, Lareyre J, Cauty C, Yano A, Allemand I, Le Gac F. Spermatogonial stem cell quest: nanos2, marker of a subpopulation of undifferentiated A spermatogonia in trout testis. Biol Reprod. 2014;90:79. doi: 10.1095/biolreprod.113.116392. [DOI] [PubMed] [Google Scholar]
- Bogerd J, Blomenröhr M, Andersson E, Putten HHAGM van der, Tensen CP, Vischer HF, Granneman JCM, Janssen-Dommerholt C, Goos HJT, Schulz RW (2001) Discrepancy between molecular structure and ligand selectivity of a testicular follicle-stimulating hormone receptor of the African catfish (Clarias gariepinus). Biol Reprod 64:1633–1643 [DOI] [PubMed]
- Büllesbach EE, Schwabe C. Tryptophan B27 in the relaxin-like factor (RLF) is crucial for RLF receptor-binding. Biochemistry. 1999;38:3073–3078. doi: 10.1021/bi982687u. [DOI] [PubMed] [Google Scholar]
- Büllesbach EE, Schwabe C. Synthetic cross-links arrest the C-terminal region of relaxin-like factor in an active conformation. Biochemistry. 2004;43:8021–8028. doi: 10.1021/bi049601j. [DOI] [PubMed] [Google Scholar]
- Büllesbach EE, Schwabe C. LGR8 signal activation by the relaxin-like factor. J Biol Chem. 2005;208:14586–14590. doi: 10.1074/jbc.M414443200. [DOI] [PubMed] [Google Scholar]
- Büllesbach EE, Schwabe C. The mode of interaction of the relaxin-like factor (RLF) with the leucine-rich repeat G protein-activated receptor 8. J Biol Chem. 2006;281:26136–26143. doi: 10.1074/jbc.M601414200. [DOI] [PubMed] [Google Scholar]
- Chauvigné F, Verdura S, Mazón MJ, Duncan N, Zanuy S, Gómez A, Cerda J. Follicle-stimulating hormone and luteinizing hormone mediate the androgenic pathway in Leydig cells of an evolutionary advanced teleost. Biol Reprod. 2012;87:35. doi: 10.1095/biolreprod.112.100784. [DOI] [PubMed] [Google Scholar]
- Chen SX, Bogerd J, Schoonen NE, Martijn J, Waal PP de, Schulz RW (2013) A progestin (17α,20β-dihydroxy-4-pregnen-3-one) stimulates early stages of spermatogenesis in zebrafish. Gen Comp Endocrinol 185:1–9 [DOI] [PubMed]
- França LR, Nóbrega RH, Morais RDVS, Assis LHC, Schulz RW. Sertoli cell structure and function in anamniote vertebrates. In: Griswold MD, editor. Sertoli cell biology. 2. Amsterdam: Elsevier; 2015. pp. 385–407. [Google Scholar]
- García-López A, Jong H de, Nóbrega RH, Waal PP de, Dijk W van, Hemrika W, Taranger GL, Bogerd J, Schulz RW (2010) Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins. Endocrinology 151:2349–2360 [DOI] [PMC free article] [PubMed]
- Good S, Yegorov S, Martijn J, Franck J, Bogerd J. New insights into ligand-receptor pairing and coevolution of relaxin family peptides and their receptors in teleosts. Int J Evol Biol. 2012;2012:310278. doi: 10.1155/2012/310278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good-Ávila SV, Yegorov S, Harron S, Bogerd J, Glen P, Ozon J, Wilson BC. Relaxin gene family in teleosts: phylogeny, syntenic mapping, selective constraint, and expression analysis. BMC Evol Biol. 2009;9:293–311. doi: 10.1186/1471-2148-9-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, Elst H, Filippov DV, Blaser H, Raz E, Moens CB, Plasterk RHA, Hannon GJ, Draper BW, Ketting RF. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell. 2007;129:69–82. doi: 10.1016/j.cell.2007.03.026. [DOI] [PubMed] [Google Scholar]
- Huang Z, Rivas B, Agoulnik AL. Insulin-like peptide 3 signaling is important for testicular descent but dispensable for spermatogenesis and cell survival in adult mice. Biol Reprod. 2012;87:1–8. doi: 10.1095/biolreprod.112.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura K, Kumagai J, Sudo S, Chun SY, Pisarska M, Morita H, Toppari J, Fu P, Wade JD, Bathgate RA, Hsueh AJ. Paracrine regulation of mammalian oocyte maturation and male germ cell survival. Proc Natl Acad Sci U S A. 2004;101:7323–7328. doi: 10.1073/pnas.0307061101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai J, Hsu SY, Matsumi H, Roh JS, Fu P, Wade JD, Bathgate RA, Hsueh AJ. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J Biol Chem. 2002;277:31283–31286. doi: 10.1074/jbc.C200398200. [DOI] [PubMed] [Google Scholar]
- Lacerda SMSN, Costa GMJ, Silva MA, Campos-Junior PHA, Segatelli TM, Peixoto MTD, Resende RR, França LR. Phenotypic characterization and in vitro propagation and transplantation of Nile tilapia (Oreochromis niloticus) spermatogonial stem cells. Gen Comp Endocrinol. 2013;192:95–106. doi: 10.1016/j.ygcen.2013.06.013. [DOI] [PubMed] [Google Scholar]
- Leal MC, Cardoso ER, Nóbrega RH, Batlouni SR, Bogerd J, França LR, Schulz RW. Histological and stereological evaluation of zebrafish (Danio rerio) spermatogenesis with emphasis on spermatogonial generations. Biol Reprod. 2009;81:177–187. doi: 10.1095/biolreprod.109.076299. [DOI] [PubMed] [Google Scholar]
- Leal MC, Waal PP de, García-López A, Chen SX, Bogerd J, Schulz RW (2009b) Zebrafish primary testis tissue culture: an approach to study testis function ex vivo. Gen Comp Endocrinol 162:134–138 [DOI] [PubMed]
- Li M, Yang H, Zhao J, Fang L, Shi H, Li M, Sun Y, Zhang X, Jiang D, Zhou L, Wang D. Efficient and heritable gene targeting in tilapia by CRISPR/Cas9. Genetics. 2014;197:591–599. doi: 10.1534/genetics.114.163667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minagawa I, Sagata D, Pitia AM, Kohriki H, Shibata M, Sasada H, Hasegawa Y, Kohsaka T. Dynamics of insulin-like 3 and its receptor expression in boar testes. J Endocrinol. 2014;220:247–261. doi: 10.1530/JOE-13-0430. [DOI] [PubMed] [Google Scholar]
- Miura T, Yamauchi K, Takahashi H, Nagahama Y. Hormonal induction of all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica) Proc Natl Acad Sci U S A. 1991;88:5774–5778. doi: 10.1073/pnas.88.13.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura T, Miura C, Konda Y, Yamauchi K. Spermatogenesis-preventing substance in Japanese eel. Development. 2002;129:2689–2697. doi: 10.1242/dev.129.11.2689. [DOI] [PubMed] [Google Scholar]
- Morais RDVS, Nóbrega RH, Gómez-González NE, Schmidt R, Bogerd J, França LR, Schulz RW. Thyroid hormone stimulates the proliferation of Sertoli cells and single type A spermatogonia in adult zebrafish (Danio rerio) testis. Endocrinology. 2013;154:4365–4376. doi: 10.1210/en.2013-1308. [DOI] [PubMed] [Google Scholar]
- Nef S, Parada LF. Cryptorchidism in mice mutant for INSL3. Nat Genet. 1999;22:295–299. doi: 10.1038/10364. [DOI] [PubMed] [Google Scholar]
- Nóbrega RH, Greebe CD, Kant H van de, Bogerd J, França LR, Schulz RW (2010) Spermatogonial stem cell niche and spermatogonial stem cell transplantation in zebrafish. PLoS ONE 5:e12808 [DOI] [PMC free article] [PubMed]
- Ohta T, Miyake H, Miura C, Kamei H, Aida K, Miura T. Follicle-stimulating hormone induces spermatogenesis mediated by androgen production in Japanese eel, Anguilla japonica. Biol Reprod. 2007;77:970–977. doi: 10.1095/biolreprod.107.062299. [DOI] [PubMed] [Google Scholar]
- Pathirana IN, Kawate N, Büllesbach EE, Takahashi M, Hatoya S, Inaba T, Tamada H. Insulin-like peptide 3 stimulates testosterone secretion in mouse Leydig cell via cAMP pathway. Regul Pept. 2012;178:102–106. doi: 10.1016/j.regpep.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Rosengren KJ, Zhang S, Lin F, Daly NL, Scott DJ, Hughes RA, Bathgate RAD, Craik DJ, Wade JD. Solution structure and characterization of the LGR8 receptor binding surface of insulin-like peptide 3. J Biol Chem. 2006;281:28287–28295. doi: 10.1074/jbc.M603829200. [DOI] [PubMed] [Google Scholar]
- Sada A, Suzuki A, Suzuki H, Saga Y. The RNA-binding protein NANOS2 is required to maintain murine spermatogonial stem cells. Science. 2009;325:1394–1398. doi: 10.1126/science.1172645. [DOI] [PubMed] [Google Scholar]
- Sagata D, Minagawa I, Kohriki H, Pitia AM, Uera N, Katakura Y, Sukigara H, Terada K, Shibata M, Park EY, Hasegawa Y, Sasada H, Kohsaka T. The insulin-like factor 3 (INSL3)-receptor (RXFP2) network functions as a germ cell survival/anti-apoptotic factor in boar testes. Endocrinology. 2015;156:1523–1539. doi: 10.1210/en.2014-1473. [DOI] [PubMed] [Google Scholar]
- Sawatari E, Shikina S, Takeuchi T, Yoshizaki G. A novel transforming growth factor-β superfamily member expressed in gonadal somatic cells enhances primordial germ cell and spermatogonial proliferation in rainbow trout (Oncorhynchus mykiss) Dev Biol. 2007;301:266–275. doi: 10.1016/j.ydbio.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Schulz RW, França LR, Lareyre J, Le Gac F, Chiarini-Garcia H, Nóbrega RH, Miura T. Spermatogenesis in fish. Gen Comp Endocrinol. 2010;165:390–411. doi: 10.1016/j.ygcen.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Wilkinson TN, Zhang S, Ferraro T, Wade JD, Tregear GW, Bathgate RAD. Defining the LGR8 residues involved in binding insulin-like peptide 3. Mol Endocrinol. 2007;21:1699–1712. doi: 10.1210/me.2007-0097. [DOI] [PubMed] [Google Scholar]
- Skaar KS, Nóbrega RH, Magaraki A, Olsen LC, Schulz RW, Male R. Proteolytically activated, recombinant anti-Müllerian hormone inhibits androgen secretion, proliferation, and differentiation of spermatogonia in adult zebrafish testis organ cultures. Endocrinology. 2011;152:3527–3540. doi: 10.1210/en.2010-1469. [DOI] [PubMed] [Google Scholar]
- Waal PP de, Wang DS, Nijenhuis WA, Schulz RW, Bogerd J (2008) Functional characterization and expression analysis of the androgen receptor in zebrafish (Danio rerio) testis. Reproduction 136:225–234 [DOI] [PubMed]
- Yano A, Suzuki K, Yoshizaki G. Flow-cytometric isolation of testicular germ cells from rainbow trout (Oncorhyncus mykiss) carrying the green fluorescent protein gene driven by trout vasa regulatory regions. Biol Reprod. 2008;78:151–158. doi: 10.1095/biolreprod.107.064667. [DOI] [PubMed] [Google Scholar]
- Yegorov S, Good-Ávila SV, Parry L, Wilson BC. Relaxin family genes in humans and teleosts. Ann N Y Acad Sci. 2009;1160:42–44. doi: 10.1111/j.1749-6632.2009.03842.x. [DOI] [PubMed] [Google Scholar]
- Yegorov S, Bogerd J, Good SV. The Relaxin family peptide receptors and their ligands: new developments and paradigms in the evolution from jawless fish to mammals. Gen Comp Endocrinol. 2014;209:93–105. doi: 10.1016/j.ygcen.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Yu M, Mu H, Niu Z, Chu Z, Zhu H, Hua J. miR-34c enhances mouse spermatogonial stem cells differentiation by targeting Nanos2. J Cell Biochem. 2014;115:232–242. doi: 10.1002/jcb.24655. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Steding G, Emmen JM, Brinkmann AO, Nayerina K, Holstein AF, Engel W, Adham IM. Targeted disruption of INSL3 gene causes bilateral cryptorchidism. Mol Endocrinol. 1999;13:681–691. doi: 10.1210/mend.13.5.0272. [DOI] [PubMed] [Google Scholar]