Abstract
Background
Although ambient air pollution has been linked to reduced lung function in healthy children, longitudinal analyses of pollution effects in asthma are lacking.
Objective
To investigate pollution effects in a longitudinal asthma study and effect modification by controller medications.
Methods
We examined associations of lung function and methacholine responsiveness (PC20) with ozone, carbon monoxide (CO), nitrogen dioxide (NO2) and sulfur dioxide (SO2) levels in 1,003 asthmatic children participating in a 4-year clinical trial. We further investigated whether budesonide and nedocromil modified pollution effects. Daily pollutant concentrations were linked to zip/postal code of residence. Linear mixed models tested associations of within-subject pollutant concentrations with FEV1 and FVC %predicted, FEV1/FVC and PC20, adjusting for seasonality and confounders.
Results
Same-day and 1-week average CO levels were negatively associated with post-bronchodilator %predicted FEV1 (change(95%CI) per IQR: −0.33(−0.49, −0.16), −0.41(−0.62, −0.21), respectively) and FVC (−0.19(−0.25, −0.07), −0.25(−0.43, −0.07)). Longer-term four-month averages of CO were negatively associated with prebronchodilator %predicted FEV1 and FVC (−0.36(−0.62, −0.10), −0.21(−0.42, −0.01)). Four-month averaged CO and ozone levels were negatively associated with FEV1/FVC (p<0.05). Increased four-month average NO2 levels were associated with reduced post-bronchodilator FEV1 and FVC %predicted. Long-term exposures to SO2 were associated with reduced PC20 (%change(95%CI) per IQR:-6(-11,-1.5)). Treatment augmented the negative short-term CO effect on PC20.
Conclusions
Air pollution adversely influences lung function and PC20 in asthmatic children. Treatment with controller medications may not protect but worsens the CO effects on PC20. This clinical trial design evaluates modification of pollution effects by treatment without confounding by indication.
Keywords: asthma, ambient air pollution, airway hyperresponsiveness, inhaled corticosteroids, lung function
Introduction
Over the past thirty years evidence has accumulated demonstrating that ambient air pollution has adverse effects on the respiratory health of asthmatic and non-asthmatic children.1-4 In observational studies of asthmatic children, higher short-term exposures to air pollution have been associated with more symptoms, increased need for reliever medication, hospital admissions, lung function decrements, and airflow obstruction.5-9
Although ambient air pollution has been linked to reduced lung function in healthy children, longitudinal analyses of air pollution effects in asthma are lacking. For instance there are no clinical trials that assessed associations of long-term pollution with lung function, airflow obstruction and airway responsiveness (AHR), and modification of putative pollution effects by controller medications. Pollutants induce adverse effects by affecting oxidant signaling pathways and airway inflammation.10,11 Inhaled corticosteroids (ICS) have been shown to reduce oxidative stress and improve airway function and asthma symptoms.8,12 However, recent observational studies suggest that asthmatic children using inhaled corticosteroids (ICS) may be more vulnerable to the adverse health effects of air pollution compared to those that are not on ICS.13,14 These findings may reflect confounding by indication, since children with more symptomatic asthma may be more likely use an ICS. Only evaluation of pollution effects in the context of a clinical trial can test whether ICS increase or decrease susceptibility to air pollution.
The Childhood Asthma Management Program (CAMP) is such a randomized clinical trial involving eight cities in North America (Albuquerque, New Mexico; Baltimore, Maryland; Boston, Massachusetts; Denver, Colorado; San Diego, California; Seattle, Washington; St. Louis, Missouri; and Toronto-Ontario, Canada). Its main goal was to evaluate the long-term effectiveness and safety of daily inhaled anti-inflammatory medication in children diagnosed with mild-to-moderate asthma.15,16 Using the pre-randomization observational data from this trial, we reported that short-term air pollution exposures increased asthma symptoms and use of relief medication,6 with carbon monoxide and nitrogen dioxide having the strongest associations.
The current paper, investigates in the same CAMP study whether short- and long-term exposures to four of the Environmental Protection Agency's criteria air pollutants (ozone, carbon monoxide (CO), nitrogen dioxide (NO2) and sulfur dioxide (SO2)) are associated with lung function level and AHR in children with asthma. In addition, we investigate whether anti-inflammatory treatment with ICS or nedocromil modifies the effects of pollution on asthma outcomes.
Methods
CAMP study design and methods have been described elsewhere.16 Additionally, detail on all methods used in the present report is provided in an online data supplement. In summary, children enrolled in CAMP were 5–12 years of age and were hyperresponsive to methacholine at study entry. 1,041 Children entered the randomization phase and 311, 312, 418 children received budesonide, nedocromil, and placebo, respectively. All subjects were treated and followed for four years with visits at two and four months after randomization and at four-month intervals thereafter. Each parent or guardian signed a consent form and participants of 7 years of age and older signed an assent form approved by each clinical center's institutional review board.
Outcomes Measures
Spirometry, before and after the bronchodilator administration, was conducted at randomization (RZ) and at follow up visits (n=13) according to the American Thoracic Society Standards. We considered both pre- and post-BD FEV1 and FVC %predicted as outcomes in this current analysis as we investigated short- and long-term effects of air pollution. Additionally, the FEV1/FVC % ratio was used as another measure of airflow obstruction. Using the Wright nebulizer-tidal breathing technique a methacholine challenge was performed annually during the treatment phase. Spirometry was performed 90 seconds after each challenge until FEV1 had fallen by 20% or more (PC20).
Air Pollution Exposure Assessment
Monitoring data on 24-hour averages concentrations of 4 gaseous pollutants (ozone, CO, NO2, and SO2) were obtained for each metropolitan area. The ZIP or postal code centroid coordinates were used to link participants to daily concentrations from the nearest monitor within 50 km that did not have missing data on that day (December 1993 through June 1999).
Statistical Analysis
We fitted a linear mixed model - with random intercepts for each subject - to estimate the associations between lung function (FEV1 and FVC %predicted and FEV1/FVC%) and (log-transformed) PC20 and same day, 1-week and 4-month moving averages of pollution. Number of days from randomization was the time trend of the model. Potential for confounding factors was considered carefully, basing choice of covariates on prior CAMP experience.17,18 To estimate associations across all cities, we constructed a model including city as a covariate, but also compared estimates of this model with study-wide estimates from meta-analyzing city-stratified models. We adjusted for “season” by using sine and cosine functions of time19 and their interactions with city. In addition, we decomposed daily pollution concentrations into between- and within-subject exposures. We report estimates of within-subject exposure effects (on interquartile range scale (IQR)).
To assess potential effect modification of the pollution- outcomes associations by treatment we included a pollutant concentration by treatment interaction into the models while excluding the baseline (RZ) measurements and used ANOVA likelihood ratio to test effect differences across the 3 treatment groups.
We used SAS® software (version 9.2; SAS Institute Inc. 2008, Cary, NC USA) and IBM SPSS statistics (version 20; Armonk, NY USA: IBM Corp 2011) to manage all data. Statistical analysis was performed in IBM SPSS and R programming language (version 2.15.1; 2012-06-22).
Results
All subjects considered in this analysis were randomized into CAMP and followed up during the trial period. A total of 1,003 of the 1,041 total children (96.3%) children were studied. At study entry the mean (SD) age was 9 (2.1) and geometric mean (minmax) for PC20 was 1.1 (0.02-2.5) mg/ml. Table I shows the main characteristics of the participants. 82.5% of the children attended all visits during the 4 years of the trial (median number of completed visits=14 (range: 1-14)). Participants had a median of 14 (range: 1-14) pre-BD and 10 (range: 1-10) post-BD lung function measurements and 4 (range: 0-4) PC20 tests.
Table I.
Demographic characteristics
| N= 1003 | |
|---|---|
| City; n (%) | |
| Albuquerque | 121 (12.1) |
| Baltimore | 126 (12.6) |
| Boston | 123 (12.3) |
| Denver | 141 (14.1) |
| San Diego | 122 (12.2) |
| Seattle | 136 (13.6) |
| Saint Louis | 133 (13.3) |
| Toronto | 101 (10.1) |
|
Sex; n (%) | |
| Males/Females | 602/401 (60/40) |
|
Treatment Group; n (%) | |
| Placebo | 407 (40.6) |
| Budesonide | 298 (29.7) |
| Nedocromil | 298 (29.7) |
|
Ethnicity; n (%) | |
| Caucasians | 677 (67.5) |
| African-Americans | 137 (13.7) |
| Hispanics | 97 (9.7) |
| Other | 92 (9.2) |
|
$Annual Income =>30K USD; n (%) | |
| Yes/No | 728/235 (76/24) |
|
In utero smoking exposure; n (%) | |
| Yes/No | 114/854 (14/86) |
| Pre bronchodilator lung function at randomization; mean (SD) | |
| FEV1 % predicted | 93.8 (14.3) |
| FVC % predicted | 104.0 (13.1) |
| FEV1/FVC % | 79.7 (8.3) |
| Post bronchodilator lung function at randomization; mean (SD) | |
| FEV1 % predicted | 103.0 (12.8) |
| FVC % predicted | 106.5 (12.8) |
| FEV1/FVC % | 85.5 (6.5) |
FEV1 : forced expiratory volume in 1 second; FVC: forced vital capacity; SD: standard deviation
=>30K USD: equal or more than 30,000 United State Dollars at baseline (1993-1995)
Pollution concentrations during December’93-June’99 are summarized by city in Table II. We report the number of observations, percentiles and IQR of daily concentrations of the 4 pollutants. Table E1 shows the IQR of the overall and the within-subject concentration of pollutants.
Table II.
Distribution of 24-hour mean pollution concentrations by city
| Pollutant | City | N | Percentiles | IQR | |||||
|---|---|---|---|---|---|---|---|---|---|
| Valid | Missing | 10 | 25 | 50 | 75 | 90 | |||
| O3 (ppb) | ALB | 1336 | 358 | 13 | 19 | 28 | 36 | 43 | 17 |
| BAL | 1703 | 61 | 7 | 13 | 23 | 33 | 43 | 20 | |
| BOS | 1660 | 62 | 7 | 13 | 21 | 30 | 38 | 17 | |
| DEN | 1669 | 305 | 6 | 13 | 23 | 32 | 41 | 19 | |
| SD | 1664 | 44 | 13 | 20 | 27 | 35 | 41 | 15 | |
| SEA | 1071 | 833 | 7 | 11 | 17 | 22 | 28 | 11 | |
| STL | 1667 | 195 | 7 | 12 | 22 | 32 | 41 | 20 | |
| TOR | 1350 | 64 | 6 | 10 | 17 | 25 | 33 | 14 | |
| TOTAL | 12120 | 1922 | 8 | 14 | 22 | 31 | 39 | 18 | |
| CO (ppm×10) | ALB | 1343 | 351 | 1 | 3 | 7 | 11 | 14 | 8 |
| BAL | 1719 | 45 | 3 | 5 | 7 | 11 | 15 | 6 | |
| BOS | 1660 | 62 | 6 | 8 | 10 | 13 | 16 | 5 | |
| DEN | 1684 | 290 | 4 | 5 | 8 | 12 | 17 | 7 | |
| SD | 1664 | 44 | 4 | 5 | 8 | 11 | 17 | 6 | |
| SEA | 1701 | 203 | 7 | 10 | 14 | 19 | 25 | 9 | |
| STL | 1684 | 178 | 4 | 5 | 7 | 9 | 12 | 4 | |
| TOR | 1350 | 64 | 2 | 6 | 10 | 12 | 15 | 6 | |
| TOTAL | 12805 | 1237 | 4 | 6 | 9 | 12 | 16 | 6 | |
| NO2 (ppb) | ALB | 1307 | 387 | 7 | 11 | 17 | 23 | 30 | 12 |
| BAL | 1719 | 45 | 14 | 18 | 24 | 29 | 36 | 11 | |
| BOS | 1660 | 62 | 14 | 20 | 25 | 32 | 38 | 12 | |
| DEN | 1577 | 397 | 10 | 20 | 29 | 36 | 44 | 17 | |
| SD | 1664 | 44 | 10 | 13 | 19 | 26 | 34 | 13 | |
| SEA | 1255 | 649 | 11 | 15 | 19 | 24 | 30 | 9 | |
| STL | 1707 | 155 | 8 | 13 | 18 | 24 | 28 | 11 | |
| TOR | 1350 | 64 | 13 | 19 | 25 | 32 | 39 | 13 | |
| TOTAL | 12239 | 1803 | 11 | 16 | 22 | 28 | 35 | 13 | |
| SO2 (ppb) | ALB | 25 | 1669 | 0 | 0 | 4 | 16 | 24 | 16 |
| BAL | 1719 | 45 | 2 | 4 | 6 | 9 | 14 | 6 | |
| BOS | 1660 | 62 | 2 | 3 | 5 | 9 | 13 | 5 | |
| DEN | 1571 | 403 | 1 | 2 | 4 | 7 | 10 | 4 | |
| SD | 1454 | 254 | 1 | 2 | 2 | 3 | 5 | 1 | |
| SEA | 1752 | 152 | 2 | 3 | 5 | 7 | 10 | 4 | |
| STL | 1736 | 126 | 1 | 3 | 5 | 9 | 13 | 6 | |
| TOR | 1347 | 67 | 0 | 2 | 4 | 6 | 9 | 4 | |
| TOTAL | 11264 | 2778 | 1 | 2 | 4 | 8 | 12 | 5 | |
ALB: Albuquerque, BAL: Baltimore, BOS: Boston, DEN: Denver, SD: San Diego, SEA: Seattle, STL: Saint Louis, TOR: Toronto, O3: ozone (ppb); CO: carbon monoxide (ppm × 10); NO2: nitrogen dioxide (ppb); SO2: sulfur dioxide (ppb); N: number of observations; IQR: interquartile range, ppb: part per billion; ppm: parts per million
Correlations of 24-hour mean pollution concentrations are shown in Table E2. Overall, ozone was negatively correlated with the other 3 pollutants that were positively correlated with each other. The same pattern of correlation existed in the 8 separate cities (data shown in Table E3 in the online repository). These relationships are expected because ozone is a secondary pollutant of regional origin, whereas the other pollutants are primary and mostly associated with local sources.
Association of pollution with level of lung function
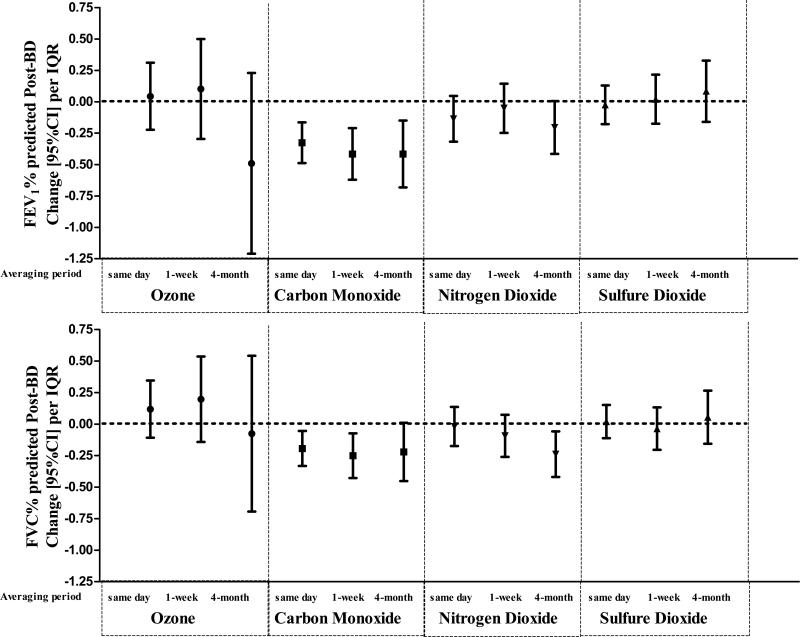
Figure 1 presents the associations of pollution with post-BD FEV1 and FVC %predicted. Same day and 1-week and 4-month moving averages of CO had the most consistent negative associations with %predicted post-BD FEV1 (change (95%CI) per IQR: −0.3(−0.5,−0.2), −0.4(−0.6,−0.2), −0.6(−0.7,−0.1), respectively) and FVC (change (95%CI) per IQR: (−0.2(−0.3, −0.1), −0.3(−0.4, −0.1), −0.2(−0.4,−0.01), respectively). The 4-month average NO2 was also negatively associated with post-BD FEV1 and FVC %predicted (change (95%CI) per IQR: −0.2(−0.4, 0.01) and −0.2 (−0.4,−0.1), respectively). The evidence for negative effects on post-BD lung function was weaker for 4-month average exposure to ozone (all-months) compared to CO or NO2. SO2 was not associated with post-BD FEV1 and FVC %predicted.
Figure 1.
Same day and 1-week and 4-month moving averages of carbon monoxide and 4-month average of nitrogen dioxide had negative associations with post-BD FEV1 (top graphs panel) and FVC (bottom graphs panel). The evidence for adverse effects on post-BD lung function seems weak for 4-month average exposure to ozone (all-months) compared to the traffic pollutants and there were not associations with sulfur dioxide.
The city-wide estimates of the meta-analysis were similar to the estimates given by the model with adjustment for city and city by sine/cosine function of time interactions. Table E4 shows the city-specific and meta-analysis estimates for long-term exposures. Meta-analysis estimates of associations of post-BD FEV1 and FVC %predicted with the 4-month average gas concentrations are comparable to all-cities model estimates in Figure 1.
Table E5 presents the associations of pollution with pre-BD FEV1 (and FVC %predicted. Increases in the average CO levels in the 4-months prior to and including the day of the visit were associated with significant decreases in pre-BD FEV1 (change(95%CI) per IQR: −0.4 (−0.62; −0.10) and FVC (change(95%CI) per IQR: −0.2 (−0.42, −0.01) %predicted. In contrast, compared to their associations with post-BD FEV1, same day and 1-week averages had associations with pre-BD FEV1 (change(95%CI) per IQR: −0.13(−0.29;0.02), −0.2(0.39,0)) and FVC (change(95%CI) per IQR: −0.12 (−0.24, 0.003), −0.15 (−0.30, 0.01)) %predicted that were the same order of magnitude, but somewhat smaller and somewhat weaker in significance. Increases in the NO2 exposures were not associated with reduced pre-BD FEV1 and FVC %predicted. Increase in 4-month SO2 was associated with increases in pre-BD FVC %predicted (change(95%CI) per IQR: 0.23 (0.05,0.42).
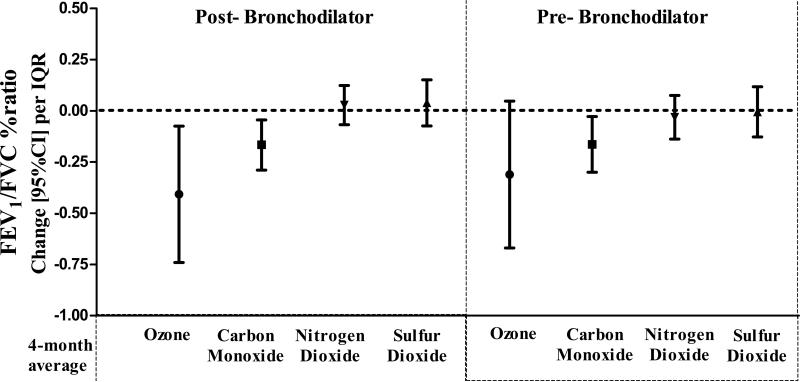
Associations of long-term (4-month average) exposure with FEV1/FVC% are shown in Figure 2. Reduced post-BD FEV1/FVC was associated with increased 4-month averages of ozone and CO, but not with NO2 or SO2 (change (95%CI) per IQR pollution increase:−0.4(−0.8,−0.1), −0.2(−0.3,−0.03), 0.03(−0.1,0.1), 0.03(−0.1,0.1), respectively). Similar associations were found with pre-BD FEV1/FVC% (change(95%CI) per IQR:−0.3(−0.7,0.06), −0.2(−0.3,−0.02), −0.03(−0.1,0.1), −0.01(−0.1,0.1), respectively).
Figure 2.
Reduced post-bronchodilator (BD) FEV1 /FVC (left graph panel) was associated with 4-month averages of ozone and carbon monoxide, but not with nitrogen dioxide or sulfur dioxide. Carbon monoxide also associated with pre-BD ratio (right graph panel).
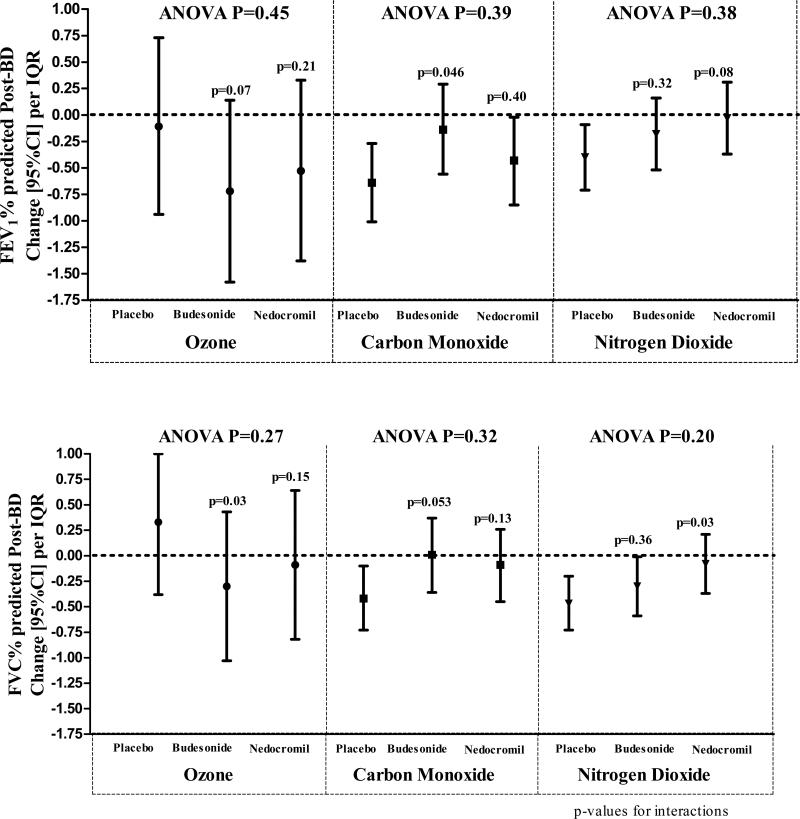
There was weak evidence of modification by treatment of pollution effect on lung function (Figure 3). Although there were differences in the magnitude of long-term pollution effect between placebo and budesonide or placebo and nedocromil (p-values for interactions ranging from 0.03 to 0.50), the overall likelihood ratio tests were not significant (ANOVA p>0.05; tables E6-E9 in the online repository).
Figure 3.
shows long-term (4-month moving average) pollution effect modification by treatment. Although there were differences in the magnitude of long-term pollution effect between placebo and budesonide or placebo and nedocromil (p-values for interactions), the overall likelihood ratio tests (ANOVA) were not significant.
Association of pollution with PC20
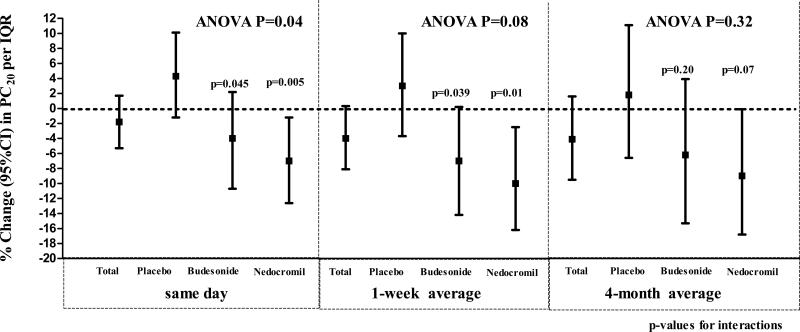
Overall, the only pollutant that was significantly associated with PC20 was the 4-month average SO2 level (%change (95%CI) per IQR: −6(−11,−1.5)). CO had a marginal overall effect on PC20 for all averaging periods (Figure 4). Compared to children on placebo, children on budesonide and nedocromil had a greater drop in PC20 with same day and 1-week average exposures to CO (ANOVA p= 0.04 and 0.08, respectively). This was more prominent for nedocromil. Treatment did not modify associations of SO2with PC20. Tables E10 and E11 in the online repository show the associations for all pollutants with PC20 and the results of interactions with treatment.
Figure 4.
Carbon monoxide had a marginal overall effect on PC20 for all averaging periods. Compared to children on placebo, children on budesonide and nedocromil had a greater drop in PC20 with same day (left graph panel) and 1-week (middle graph panel) average exposures to carbon monoxide (ANOVA p= 0.04 and 0.08, respectively).
Associations of asthma outcomes with warm-month (May – September) ozone were not statistically significant (Table E12 in the online repository). Two-pollutant models showed similar pollutant-asthma outcomes associations as one-pollutant models (Table E13 in the online repository).
In our study, CO was the pollutant with the strongest and most significant associations with lung function and the only pollutant showing associations with both pre- and post-BD lung function with shorter (same day to 1-week)- and longer (4-month)-term exposures (Figure 1, and Table E5). Thus to evaluate whether the longer-term effects were independent of shorter-term effects we put either one-day or one-week averages in the same model with 4-month averages of CO. Since the same-day and 1-week average measurements are included in the original 4-month average estimate, this added to the correlation amongst the measures and introduced co-linearity into our model. To disentangle the shorter- and longer-term averages and their associations with our outcomes, we performed an additional analyses with newly created 4-month averages (ie., calculating the 4-month average leaving out the same day measurements, and calculating the 4-month average leaving out the 1-week average), adjusting for same day and 1-week average CO, respectively. The associations with pre-BD and post-BD lung function from models with single averaging periods, compared to those with shorter (one day or one week) plus longer (4-month) averaging periods are shown in Tables III and IV.
Table III.
Associations of carbon monoxide with FEV1 % predicted: models with single averaging periods are compared to models combining shorter (one day or one week) and longer (4-month) averaging periods
| Pre bronchodilator FEV1 % predicted | ||||||
|---|---|---|---|---|---|---|
| MODEL 1 Change (95%CI) per IQR P-value |
MODEL 2 change (95%CI) per IQR P-value |
MODEL 3 change (95%CI) per IQR P-value |
MODEL 4 change (95%CI) per IQR P-value |
MODEL 5 change (95%CI) per IQR P-value |
||
| Carbon Monoxide | IQR (ppm ×10) | |||||
| Same day | 5 | −0.13 (−0.29, 0.02) P=0.09 |
−0.08 (−0.24, 0.08) P=0.310 |
|||
| 1 week average | 4 | −0.19 (−0.39, 0.001) P=0.05 |
−0.12 (−0.33, 0.09) P=0.269 |
|||
| 4 month average | 3 | −0.36 (−0.62, −0.10) P=0.006 |
||||
| 4 month average excluding same day | 3 | −0.33 (−0.59, −0.06) P=0.016 |
||||
| 4 month average excluding 1 week | 3 | −0.29 (−0.56,−0.02) P=0.037 |
||||
| Post bronchodilator FEV1 %predicted | ||||||
|---|---|---|---|---|---|---|
| MODEL 2 change (95%CI) per IQR P-value |
MODEL 2 change (95%CI) per IQR P-value |
MODEL 2 change (95%CI) per IQR P-value |
MODEL 2 change (95%CI) per IQR P-value |
MODEL 2 change (95%CI) per IQR P-value |
||
| Carbon Monoxide | IQR (ppm ×10) | |||||
| Same day | 5 | −0.33 (−0.49, −0.16) P<0.001 |
−0.28 (−0.45, −0.11) P=0.001 |
|||
| 1 week average | 4 | −0.41 (−0.62, −0.21) P<0.001 |
−0.36 (−0.58, −0.14) P=0.002 |
|||
| 4 month average | 3 | −0.42 (−0.68, −0.15) P=0.002 |
||||
| 4 month average excluding same day | 3 | −0.29 (−0.56, −0.01) P=0.043 |
||||
| 4 month average excluding 1 week | 3 | −0.20 (−0.4, 0.08) P=0.17 |
||||
FEV1: forced expiratory volume in 1 second, IQR: interquartile range, ppm: parts per million, CI: confidence interval
Table IV.
Associations of carbon monoxide with FVC % predicted: models with single averaging periods are compared to models combining shorter (one day or one week) and longer (4-month) averaging periods
| Pre bronchodilator FVC % predicted | ||||||
|---|---|---|---|---|---|---|
| MODEL 1 Change (95%CI) per IQR P-value |
MODEL 2 change (95%CI) per IQR P-value |
MODEL 3 change (95%CI) per IQR P-value |
MODEL 4 change (95%CI) per IQR P-value |
MODEL 5 change (95%CI) per IQR P-value |
||
| Carbon Monoxide | IQR (ppm ×10) | |||||
| Same day | 5 | −0.12 (−0.24, 0.0) P=0.054 |
−0.09 (−0.22,0.03) P=0.142 |
−0.11 (−0.27, 0.06) P=0.15 |
||
| 1 week average | 4 | −0.15 (−0.3, 0.01) P=0.06 |
||||
| 4 month average | 3 | −0.21 (−0.42, −0.01) P=0.042 |
||||
| 4 month average excluding same day | 3 | − 0.17 (−0.39, 0.04) P=0.108 |
||||
| 4 month average excluding 1 week | 3 | −0.15 (−0.37, −0.07) P=0.180 |
||||
| Post bronchodilator FVC % predicted | ||||||
|---|---|---|---|---|---|---|
| MODEL 2 change (95%CI) per IQR P-value |
MODEL 2 change (95%CI) per IQR P-value |
MODEL 2 change (95%CI) per IQR P-value |
MODEL 2 change (95%CI) per IQR P-value |
MODEL 2 change (95%CI) per IQR P-value |
||
| Carbon Monoxide | IQR (ppm ×10) | |||||
| Same day | 5 | −0.19 (−0.33, −0.05) P=0.006 |
−0.17 (−0.32, −0.03) P=0.02 |
|||
| 1 week average | 4 | −0.25 (−0.43, −0.07) P=0.006 |
−0.23 (−0.42, −0.04) P=0.019 |
|||
| 4 month average | 3 | −0.22 (−0.45, 0.01) P=0.061 |
||||
| 4 month average excluding same day | 3 | −0.14 (−0.38, 0.10) P=0.244 |
||||
| 4 month average excluding 1 week | 3 | −0.09 (−0.33, 0.16) P=0.49 |
||||
FVC: forced vital capacity, IQR: interquartile range, ppm: parts per million, CI: confidence interval
Discussion
Short-term adverse effects of pollution on children's pulmonary health have been extensively studied, meta-analyzed, and systematically reviewed. These studies provide strong evidence that short-term exposures to air pollution can increase airflow obstruction in asthmatic and non-asthmatic children,20 and that long-term traffic pollution may increase incident asthma and reduce level of lung function in general populations of children.2,21-24 There are fewer studies considering the effects of long-term exposures to pollution on lung function in asthmatic children,1,2,20 and none that we know of evaluating long-term effects of pollution on lung function and AHR in asthmatic children in the context of a clinical trial.
In this unique asthma intervention trial, increases in the average long-term (4-month) concentrations of ozone, CO and NO2 were all associated with reductions in lung function levels consistent with airflow obstruction, and with some decrease in vital capacity represented by a drop in FVC. There are few air pollution studies including post-BD measurements with which to compare our findings. A recent study of subjects from the Manchester Asthma and Allergy Study (MAAS) birth cohort showed greater long-term pollution (NO2 and PM10) effect on post-BD FEV1 %predicted compared to pre-BD FEV1 %predicted.25 Their findings motivated the hypothesis that the bronchodilator administration might reduce the influence of varying circadian and day-to-day bronchodilator tone on the measurement of FEV1, potentially increasing the power of the study to show pollution influences on lung function. In our study, this may have been the case for shorter-term but not for longer-term cumulative averages of pollution exposure. Focusing on FEV1 as an outcome, with one week and 4-month cumulative averages of CO in the same model, longer-term CO exposure was more consistently associated with lower pre-BD FEV1 %predicted, and short-term CO exposure was more strongly/more consistently associated with lower post-BD lung function measures. Additional unmeasured short-term influences on pre-BD responses that were reversible by bronchodilator administration may have added noise and contributed to the variability in the pre-BD measurement, and this may have resulted in somewhat less robust associations of pollution exposures in the past week with pre-BD FEV1. .
The within-subject variation of gaseous pollutants in our asthma trial was low (i.e., same day CO IQR=0.50 ppm), and while effects were statistically significant (P<0.001), given their small magnitude (for every 0.5 ppm increase in the same day CO concentration, there is a ~0.3 decrease in the average post-BD FEV1 %predicted, which would be equivalent to a patient dropping from 103.0 to 102.7 average FEV1 %predicted post-BD over a 4-year follow up), the lung function responses to pollution may not have short-term clinical relevance. Whether the small pollution-related changes in lung function that we observed have longer-term implications for lung growth and maximum attained lung function in these vulnerable asthmatic children remains to be assessed.
One study in adult asthmatics has reported associations of reduced lung function with short-term exposures to CO, but the mechanisms for this association is not known.26 Endogenous hypoxic-induced CO is a mediator of vasodilation and bronchodilation and high doses of inhaled CO in mice decrease inflammation and AHR.27-33 Exogenous CO at levels encountered by children in our cohort did not have beneficial effects.
Motor vehicles emissions are major sources of CO. These source produces many contaminants - such as fine particles and organic compounds - thus in this case it is likely that CO is a surrogate for other pollutants,2,34,35 and that the observed associations might not be due to CO per se, but due to other pollutants in traffic emission mixtures. Similarly, NO2 may be a marker for complex pollutant mixtures of pollutants emitted by the same sources or related through complex atmospheric reactions. Primary traffic-related pollutants such as elemental/black carbon or freshly emitted primary particles and secondary pollutants, including ozone, are often correlated with NO2.2,34,35 In the present study, air pollutant levels were correlated such that it was difficult to separate out the contributions of the individual pollutants. All effects estimates on asthma outcomes remained significant after controlling for co-pollutants/gases in multi-pollutant models.
Sarnat et al.35 showed relatively strong associations of personal exposure of particles of ambient origin and ambient measurements, but considerably lower associations for gases. In particular, NO2 primarily from traffic emissions, was more strongly associated with personal exposure to traffic particles. This suggests that ambient gases from traffic are associated with personal exposure to particles, and perhaps other compounds from traffic. Since CO is an antioxidant in the lung and not a plausible pollutant to reduce lung function we interpret it as a surrogate for particles and perhaps organic gases from traffic. The two pollutants associated with traffic sources—CO and NO2—were most strongly and consistently associated with reduced level of lung function and more severe hyperresponsiveness in our children with asthma. Those were the same pollutants that influenced asthma exacerbations (i.e., more symptoms and use of relief medication) pre-randomization in this CAMP trial.6 However, SO2 which originates predominately from diesel combustion (diesel fuel content of sulfur was higher during 1990's) and non-traffic fossil fuels (e.g., coal burning power plants and domestic heating), was also associated with enhanced response to metacholine long-term. Most studies investigating the latter association are based on short-term exposures to diesel and they have shown that air pollution may enhance the responsiveness to metacholine as well as to inhaled allergens in sensitized subjects.36-39 One animal study has suggested an increase in AHR with long-term sulfur dioxide exposure, supporting our findings for this pollutant.40 However, our study is unique in the investigation of AHR and its relation with short-term as well as long-term in asthmatic children.
We also found a positive association of long-term exposure to SO2 with pre-BD FVC. Again, experimental and epidemiological studies investigating lung function response to SO2 focus on acute responses and this makes them difficult to be compared with our finding. Most epidemiologic findings show modest negative or null association of SO2 with lung function.41-43 Generally, it is suggested that after acute exposure to SO2 the lung function returns to normal after some minutes to hours and that there is a great deal of inter-individual variation in response to SO2.44-46
Ozone is the most important tropospheric oxidant which is formed through photochemical reactions involving NO2 and hydrocarbons. Cumulative exposure is a function of both the rate and duration of exposure and it has been shown that effects of pollution on children's health have greater impact if the children exercise outdoors.47-50 We also show that longer exposure to ozone is associated with airflow obstruction, indicated by decrease in FEV1/FVC with increase in 4-month average ozone concentration. Ozone and other air pollutants initiate intracellular oxidative stress and are linked to chronic damage and effects on to the human lung with prolonged exposure.51-53 The decrease in post-BD FEV1/FVC may reflect airway wall remodeling process related to repeated exposures to ozone and other pollutants.54 For ozone, associations of reduced lung function with pollution also tended to be stronger for children on budesonide compared to placebo. The latter suggests a less clear relationship between exposure to ozone and airway inflammation. Two recent studies have suggested that children on ICS were more vulnerable to the adverse effects of ozone and other air pollutants.13,14 The authors speculated that the observed associations might be explained by the fact that children on ICS are more likely to have worse asthma and that confounding by indication might exist. The design of our trial prevents confounding by indication because of the double-blinded randomized distribution of treatment to children of similar asthma severity. Although the evidence for the interaction of ICS treatment on ozone mediated effects is weak, it is plausible that the children on the ICS had greater exposure to ozone compared to placebo, either because they were more likely to spend more time outside and exercise more due to better control of their asthma,15,18 or because they had greater minute ventilation because they were able to breathe more deeply when exercising.
In our study, modification of CO effects on AHR by anti-inflammatory treatment suggests that use of controller medication may not protect asthmatic children from pollutant effects. The worsening of AHR with short-term exposure to CO was stronger for children on budesonide and nedocromil compared to placebo, a finding that needs further investigation. Nevertheless, a public health interpretation of this finding is that controller medication use should not be assumed to be sufficient as a preventive measure on days with high pollution levels. Policy for pollution control and advice55 to asthmatic children to avoid outdoor activities on days of high pollution levels remain the most powerful preventive measure.
The present report provides a unique contribution in that it can be considered a meta-analysis of eight large, within-city panel studies. Yet, it does not suffer from many of the challenges associated with meta-analyses in the published literature (e.g., between-study heterogeneity and obvious publication bias). The large and geographically diverse panel of children participating in CAMP trial was followed from December 1993 to June 1999, on average for 4 years. This allowed us to examine the health effects of ambient concentrations of CO, NO2, SO2 and ozone across seasons and geographic regions and results from this study may be applicable to a broad population.
Many studies investigating the long-term effects of pollution have focused on traffic-related exposures and used surrogate measures such as distance to major roads, road density or vehicle density.2,20,21,23,56,57 In this study we measured daily pollutant concentrations to predict long-term (4-month (but also acute (same day) and intermediate (1-week)) effects on asthma severity in children. We acknowledge that exposure is at the zip/postal code rather than the residence level. However, we limited exposure misclassification bias in two ways: 1) by using zip/postal code level concentrations of pollution instead of averaging monitor-specific concentrations by city; and 2) by restricting the period of interested to the period of the trial for which the great majority of the participants attended all visits. In addition, we investigated pollutants that tend to be regional and we also focus on long-term exposure which is less prone to misclassification. Recent evidence supports the value of further investigating, where feasible, whether pollution effects vary by gender.58 Unfortunately, this is outside of the scope of our manuscript and we lack sufficient power for assessment of a three-way interaction (pollution by treatment group by gender).
We conclude that exposure to gaseous pollutants adversely influences level of lung function and AHR in asthmatic children, and treatment use worsens the short-term effects of CO on AHR. The longitudinal evaluation of children treated with daily asthma therapy in a clinical trial enabled us to separate the modification of pollution effects by treatment without confounding by indication.
Supplementary Material
Clinical Implications.
Exposure to gaseous pollutants adversely influences lung function and airway hyperresponsiveness levels in asthmatic children. Anti-inflammatory treatment use may worsen the negative short-term effects of some pollutants on airway hyperresponsiveness.
Capsule Summary.
Gaseous pollutants adversely influence lung function and airway hyperresponsiveness.
The longitudinal evaluation of children on daily asthma therapy in a clinical trial enabled us to separate the modification of pollution effects by treatment without confounding by indication.
Acknowledgements
We dedicate this manuscript to the memory of our friend and colleague Dr. Gail G. Shapiro who passed away unexpectedly during the development of this study. Dr. Shapiro dedicated her life to understanding the causes of childhood asthma and determining the best treatments for asthma. She is deeply missed by her colleagues, patients, and the asthma community.
The Childhood Asthma Management Program trial and CAMP Continuation Study were supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources. The CAMP Continuation Study/Phases 2 and 3 were supported by grants U01HL075232, U01HL075407, U01HL075408, U01HL075409, U01HL075415, U01HL075416, U01HL075417, U01HL075419, U01HL075420, andU01HL075408 from the National Heart, Lung, and Blood Institute. The National Jewish Health site was also supported in part by Colorado CTSA grant UL1RR025780 from NCRR/NIH and UL1TR000154 from NCATS/NIH. C:\lm_camp\misc\campdua.wpd June 1, 2013
Financial support: This study was funded by: the National Institutes of Health (NHLBI P01 HL083069, U01 HL075419, U01 HL65899, R01 HL 086601; NIEHS P01 ES09825, R21 ES020194, P30 ES000002); the U.S. Environmental Protection Agency (RD 83241601, RD 83479801), and the International Initiative for Environment and Public Health Cyprus Program of HSPH. The contents of this publication are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, US EPA does not endorse the purchase of any commercial products or services mentioned in the publication.
Abbreviations
- AHR
airway responsiveness
- BD
bronchodilator
- CAMP
Childhood Asthma Management Program
- CO
Carbon monoxide
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- ICS
Inhaled corticosteroids
- IQR
Interquartile range
- NO2
Nitrogen dioxide
- PC20
Metacholine concentration causing a 20% reduction in FEV1
- RZ
Randomization
- SD
Standard deviation
- SO2
Sulfur dioxide
Footnotes
Authors Contribution: Each living author participated sufficiently in the current work. All authors were involved in the conception, hypotheses delineation, and design of the present article. Management of the data and the analysis was performed by D. Ierodiakonou in consultation with B.A. Coull, A. Zanobetti, D.R. Gold and S.T. Weiss. D. Ierodiakonou wrote the article and all living authors had a substantial involvement in its revision prior to submission.
References
- 1.World Health Organization Effects of air pollution on children' s health and developement. A review of the evidence. [April, 2013];Bonn. 2005 Available at: http://www.euro.who.int/__data/assets/pdf_file/0010/74728/E86575.pdf.
- 2.HEI on the Health Effects of Traffic-Related Air Pollution Traffic-related Air Pollution: A critical Review of the Literature on Emissions, Exposure, and Health Effects. HEI Special Report 17. 2010 [Google Scholar]
- 3.Searing DA, Rabinovitch N. Environmental pollution and lung effects in children. Curr Opin Pediatr. 2011 Jun;23:314–318. doi: 10.1097/MOP.0b013e3283461926. [DOI] [PubMed] [Google Scholar]
- 4.Trasande L, Thurston GD. The role of air pollution in asthma and other pediatric morbidities. J Allergy Clin Immunol. 2005 Apr;115:689–699. doi: 10.1016/j.jaci.2005.01.056. [DOI] [PubMed] [Google Scholar]
- 5.Tzivian L. Outdoor air pollution and asthma in children. J Asthma. 2011 Jun;48:470–481. doi: 10.3109/02770903.2011.570407. [DOI] [PubMed] [Google Scholar]
- 6.Schildcrout JS, Sheppard L, Lumley T, Slaughter JC, Koenig JQ, Shapiro GG. Ambient air pollution and asthma exacerbations in children: an eight-city analysis. Am J Epidemiol. 2006 Sep 15;164:505–517. doi: 10.1093/aje/kwj225. [DOI] [PubMed] [Google Scholar]
- 7.Sunyer J, Spix C, Quenel P, Ponce-de-Leon A, Ponka A, Barumandzadeh T, et al. Urban air pollution and emergency admissions for asthma in four European cities: the APHEA Project. Thorax. 1997 Sep;52:760–765. doi: 10.1136/thx.52.9.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L, Poon R, Chen L, Frescura AM, Montuschi P, Ciabattoni G, et al. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009 Apr;117:668–674. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romieu I, Meneses F, Ruiz S, Sienra JJ, Huerta J, White MC, et al. Effects of air pollution on the respiratory health of asthmatic children living in Mexico City. Am J Respir Crit Care Med. 1996 Aug;154:300–307. doi: 10.1164/ajrccm.154.2.8756798. [DOI] [PubMed] [Google Scholar]
- 10.Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003 Aug;60:612–616. doi: 10.1136/oem.60.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel MM, Chillrud SN, Deepti KC, Ross JM, Kinney PL. Traffic-related air pollutants and exhaled markers of airway inflammation and oxidative stress in New York City adolescents. Environ Res. 2013 Feb;121:71–78. doi: 10.1016/j.envres.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delfino RJ, Zeiger RS, Seltzer JM, Street DH, McLaren CE. Association of asthma symptoms with peak particulate air pollution and effect modification by anti-inflammatory medication use. Environ Health Perspect. 2002 Oct;110:A607–17. doi: 10.1289/ehp.021100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zora JE, Sarnat SE, Raysoni AU, Johnson BA, Li WW, Greenwald R, et al. Associations between urban air pollution and pediatric asthma control in El Paso, Texas. Sci Total Environ. 2013 Mar 15;448:56–65. doi: 10.1016/j.scitotenv.2012.11.067. [DOI] [PubMed] [Google Scholar]
- 14.Lewis TC, Robins TG, Mentz GB, Zhang X, Mukherjee B, Lin X, et al. Air pollution and respiratory symptoms among children with asthma: Vulnerability by corticosteroid use and residence area. Sci Total Environ. 2013 Mar 15;448C:48–55. doi: 10.1016/j.scitotenv.2012.11.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strunk RC. Childhood Asthma Management Program Research Group. Childhood Asthma Management Program: lessons learned. J Allergy Clin Immunol. 2007 Jan;119:36–42. doi: 10.1016/j.jaci.2006.09.038. [DOI] [PubMed] [Google Scholar]
- 16.The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Childhood Asthma Management Program Research Group. Control Clin Trials. 1999 Feb;20:91–120. Anonymous. [PubMed] [Google Scholar]
- 17.Cohen RT, Raby BA, Van Steen K, Fuhlbrigge AL, Celedon JC, Rosner BA, et al. In utero smoke exposure and impaired response to inhaled corticosteroids in children with asthma. J Allergy Clin Immunol. 2010 Sep;126:491–497. doi: 10.1016/j.jaci.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000 Oct 12;343:1054–1063. doi: 10.1056/NEJM200010123431501. Anonymous. [DOI] [PubMed] [Google Scholar]
- 19.Stolwijk AM, Straatman H, Zielhuis GA. Studying seasonality by using sine and cosine functions in regression analysis. J Epidemiol Community Health. 1999 Apr;53:235–238. doi: 10.1136/jech.53.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li S, Williams G, Jalaludin B, Baker P. Panel studies of air pollution on children's lung function and respiratory symptoms: a literature review. J Asthma. 2012 Nov;49:895–910. doi: 10.3109/02770903.2012.724129. [DOI] [PubMed] [Google Scholar]
- 21.Gao Y, Chan EY, Li LP, He QQ, Wong TW. Chronic effects of ambient air pollution on lung function among Chinese children. Arch Dis Child. 2013 Feb;98:128–135. doi: 10.1136/archdischild-2011-301541. [DOI] [PubMed] [Google Scholar]
- 22.Rosenlund M, Forastiere F, Porta D, De Sario M, Badaloni C, Perucci CA. Traffic-related air pollution in relation to respiratory symptoms, allergic sensitisation and lung function in schoolchildren. Thorax. 2009 Jul;64:573–580. doi: 10.1136/thx.2007.094953. [DOI] [PubMed] [Google Scholar]
- 23.Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007 Feb 17;369:571–577. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz J. Lung function and chronic exposure to air pollution: a cross-sectional analysis of NHANES II. Environ Res. 1989 Dec;50:309–321. doi: 10.1016/s0013-9351(89)80012-x. [DOI] [PubMed] [Google Scholar]
- 25.Molter A, Agius RM, de Vocht F, Lindley S, Gerrard W, Lowe L, et al. Long-term exposure to PM10 and NO2 in association with lung volume and airway resistance in the MAAS birth cohort. Environ Health Perspect. 2013 Oct;121:1232–1238. doi: 10.1289/ehp.1205961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canova C, Torresan S, Simonato L, Scapellato ML, Tessari R, Visentin A, et al. Carbon monoxide pollution is associated with decreased lung function in asthmatic adults. Eur Respir J. 2010 Feb;35:266–272. doi: 10.1183/09031936.00043709. [DOI] [PubMed] [Google Scholar]
- 27.Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999 Dec;277:F882–9. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- 28.Cardell LO, Lou YP, Takeyama K, Ueki IF, Lausier J, Nadel JA. Carbon monoxide, a cyclic GMP-related messenger, involved in hypoxic bronchodilation in vivo. Pulm Pharmacol Ther. 1998;11:309–315. doi: 10.1006/pupt.1998.0152. [DOI] [PubMed] [Google Scholar]
- 29.Kinhult J, Uddman R, Cardell LO. The induction of carbon monoxide-mediated airway relaxation by PACAP 38 in isolated guinea pig airways. Lung. 2001;179:1–8. doi: 10.1007/s004080000043. [DOI] [PubMed] [Google Scholar]
- 30.Nakao A, Choi AM, Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med. 2006 Jul-Sep;10:650–671. doi: 10.1111/j.1582-4934.2006.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kourembanas S. Hypoxia and carbon monoxide in the vasculature. Antioxid Redox Signal. 2002 Apr;4:291–299. doi: 10.1089/152308602753666343. [DOI] [PubMed] [Google Scholar]
- 32.Ameredes BT, Otterbein LE, Kohut LK, Gligonic AL, Calhoun WJ, Choi AM. Low-dose carbon monoxide reduces airway hyperresponsiveness in mice. Am J Physiol Lung Cell Mol Physiol. 2003 Dec;285:L1270–6. doi: 10.1152/ajplung.00145.2003. [DOI] [PubMed] [Google Scholar]
- 33.Chapman JT, Otterbein LE, Elias JA, Choi AM. Carbon monoxide attenuates aeroallergen-induced inflammation in mice. Am J Physiol Lung Cell Mol Physiol. 2001 Jul;281:L209–16. doi: 10.1152/ajplung.2001.281.1.L209. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization Health Aspects of Air Pollution with Particulate Matter, Ozone and Nitrogen Dioxide. [May, 2013];Report on a WHO Working Group. 2003 Available at: http://www.euro.who.int/__data/assets/pdf_file/0005/112199/E79097.pdf.
- 35.Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient gas concentrations and personal particulate matter exposures: implications for studying the health effects of particles. Epidemiology. 2005 May;16:385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]
- 36.Jang AS, Choi IS, Lee JH, Park CS, Park CS. Prolonged ozone exposure in an allergic airway disease model: adaptation of airway responsiveness and airway remodeling. Respir Res. 2006 Feb 13;7:24. doi: 10.1186/1465-9921-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svartengren M, Strand V, Bylin G, Jarup L, Pershagen G. Short-term exposure to air pollution in a road tunnel enhances the asthmatic response to allergen. Eur Respir J. 2000 Apr;15:716–724. doi: 10.1034/j.1399-3003.2000.15d15.x. [DOI] [PubMed] [Google Scholar]
- 38.Nordenhall C, Pourazar J, Ledin MC, Levin JO, Sandstrom T, Adelroth E. Diesel exhaust enhances airway responsiveness in asthmatic subjects. Eur Respir J. 2001 May;17:909–915. doi: 10.1183/09031936.01.17509090. [DOI] [PubMed] [Google Scholar]
- 39.Miyabara Y, Takano H, Ichinose T, Lim HB, Sagai M. Diesel exhaust enhances allergic airway inflammation and hyperresponsiveness in mice. Am J Respir Crit Care Med. 1998 Apr;157:1138–1144. doi: 10.1164/ajrccm.157.4.9708066. [DOI] [PubMed] [Google Scholar]
- 40.Song A, Liao Q, Li J, Lin F, Liu E, Jiang X, et al. Chronic exposure to sulfur dioxide enhances airway hyperresponsiveness only in ovalbumin-sensitized rats. Toxicol Lett. 2012 Nov 15;214:320–327. doi: 10.1016/j.toxlet.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Aekplakorn W, Loomis D, Vichit-Vadakan N, Shy C, Wongtim S, Vitayanon P. Acute effect of sulphur dioxide from a power plant on pulmonary function of children, Thailand. Int J Epidemiol. 2003 Oct;32:854–861. doi: 10.1093/ije/dyg237. [DOI] [PubMed] [Google Scholar]
- 42.Peters A, Goldstein IF, Beyer U, Franke K, Heinrich J, Dockery DW, et al. Acute health effects of exposure to high levels of air pollution in Eastern Europe. Am J Epidemiol. 1996 Sep 15;144:570–581. doi: 10.1093/oxfordjournals.aje.a008967. [DOI] [PubMed] [Google Scholar]
- 43.Hoek G, Brunekreef B. Effects of low-level winter air pollution concentrations on respiratory health of Dutch children. Environ Res. 1994 Feb;64:136–150. doi: 10.1006/enrs.1994.1012. [DOI] [PubMed] [Google Scholar]
- 44.Lawther PJ, Macfarlane AJ, Waller RE, Brooks AG. Pulmonary function and sulphur dioxide, some preliminary findings. Environ Res. 1975 Dec;10:355–367. doi: 10.1016/0013-9351(75)90031-6. [DOI] [PubMed] [Google Scholar]
- 45.Winterton DL, Kaufman J, Keener CV, Quigley S, Farin FM, Williams PV, et al. Genetic polymorphisms as biomarkers of sensitivity to inhaled sulfur dioxide in subjects with asthma. Ann Allergy Asthma Immunol. 2001 Feb;86:232–238. doi: 10.1016/S1081-1206(10)62697-X. [DOI] [PubMed] [Google Scholar]
- 46.Nowak D, Jorres R, Berger J, Claussen M, Magnussen H. Airway responsiveness to sulfur dioxide in an adult population sample. Am J Respir Crit Care Med. 1997 Oct;156:1151–1156. doi: 10.1164/ajrccm.156.4.9607025. [DOI] [PubMed] [Google Scholar]
- 47.Millstein J, Gilliland F, Berhane K, Gauderman WJ, McConnell R, Avol E, et al. Effects of ambient air pollutants on asthma medication use and wheezing among fourth-grade school children from 12 Southern California communities enrolled in The Children's Health Study. Arch Environ Health. 2004 Oct;59:505–514. doi: 10.1080/00039890409605166. [DOI] [PubMed] [Google Scholar]
- 48.McConnell R, Berhane K, Gilliland F, London SJ, Islam T, Gauderman WJ, et al. Asthma in exercising children exposed to ozone: a cohort study. Lancet. 2002 Feb 2;359:386–391. doi: 10.1016/S0140-6736(02)07597-9. [DOI] [PubMed] [Google Scholar]
- 49.Spektor DM, Thurston GD, Mao J, He D, Hayes C, Lippmann M. Effects of single- and multiday ozone exposures on respiratory function in active normal children. Environ Res. 1991 Aug;55:107–122. doi: 10.1016/s0013-9351(05)80167-7. [DOI] [PubMed] [Google Scholar]
- 50.Gauderman WJ, Gilliland GF, Vora H, Avol E, Stram D, McConnell R, et al. Association between air pollution and lung function growth in Southern California children: results from a second cohort. Am J Respir Crit Care Med. 2002 Jul 1;166:76–84. doi: 10.1164/rccm.2111021. [DOI] [PubMed] [Google Scholar]
- 51.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Souza MB, Saldiva PH, Pope CA, 3rd, Capelozzi VL. Respiratory changes due to long-term exposure to urban levels of air pollution: a histopathologic study in humans. Chest. 1998 May;113:1312–1318. doi: 10.1378/chest.113.5.1312. [DOI] [PubMed] [Google Scholar]
- 53.Pinkerton KE, Green FH, Saiki C, Vallyathan V, Plopper CG, Gopal V, et al. Distribution of particulate matter and tissue remodeling in the human lung. Environ Health Perspect. 2000 Nov;108:1063–1069. doi: 10.1289/ehp.001081063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chae EJ, Kim TB, Cho YS, Park CS, Seo JB, Kim N, et al. Airway Measurement for Airway Remodeling Defined by Post-Bronchodilator FEV1/FVC in Asthma: Investigation Using Inspiration-Expiration Computed Tomography. Allergy Asthma Immunol Res. 2011 Apr;3:111–117. doi: 10.4168/aair.2011.3.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004 Jun 1;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 56.Brunekreef B, Janssen NA, de Hartog J, Harssema H, Knape M, van Vliet P. Air pollution from truck traffic and lung function in children living near motorways. Epidemiology. 1997 May;8:298–303. doi: 10.1097/00001648-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Perez L, Declercq C, Iniguez C, Aguilera I, Badaloni C, Ballester F, et al. Chronic burden of near-roadway traffic pollution in 10 European cities (APHEKOM network). Eur Respir J. 2013 Mar 21;42(3):594–605. doi: 10.1183/09031936.00031112. [DOI] [PubMed] [Google Scholar]
- 58.Clougherty JE. A Growing Role for Gender Analysis in Air Pollution Epidemiology. Environ Health Perspect. 2009 Oct 16;118:167–176. doi: 10.1289/ehp.0900994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.