Abstract
BACKGROUND
In a combined animal and human study, we have previously found that a five-day treatment that enhances cortical plasticity also facilitates brain-derived neurotrophic factor (BDNF)-tyrosine receptor kinase B (TrkB) signaling and increases activated TrkB and N-methyl-D-aspartate receptor (NMDAR) association in both the cortex and the peripheral lymphocytes. Patients with Parkinson’s disease (PD) in general show decreased cortical plasticity, as demonstrated by electrophysiological and behavioral studies. Here we test the hypothesis that an exercise program that improves motor function and seems to slow down symptoms’ progression can enhance BDNF-TrkB signaling in lymphocytes.
METHODS
Sixteen patients with PD underwent a four-week Multidisciplinary Intensive Rehabilitation Treatment (MIRT), which included aerobic training, physical and occupational therapy. Blood was collected before, after two- and four-week MIRT. Lymphocytes were isolated to examine BDNF-TrkB signaling induced by incubation with recombinant human BDNF. TrkB signaling complexes, extracellular-signal-regulated kinase-2 and protein-kinase-B were immunoprecipitated; content of immunocomplexes was determined by Western blotting.
RESULTS
After MIRT, all patients showed improvement in motor function. TrkB interaction with NMDAR and BDNF-TrkB signaling increased in peripheral lymphocytes at receptor, intracellular mediators and downstream levels. The decrements in UPDRSII and total scores were significantly correlated with the increases in TrkB signaling at receptor, intracellular mediators and NMDAR interaction levels.
CONCLUSIONS
The significant correlation between reduced UPDRS scores and the changes in lymphocytes’ activity suggest that enhanced BDNF-TrkB signaling in lymphocyte and reduced severity of PD symptoms may be related.
Keywords: Plasticity, LTP, Human, Immune system
Introduction
We have previously found that a five-day course of 5Hz of repetitive transcranial magnetic stimulation (rTMS), a treatment designed to enhance cortical plasticity, upregulates the brain-derived neurotrophic factor (BDNF)-tyrosine kinase B (TrkB) receptor in both the cortex and the peripheral lymphocytes of humans and animals [1]. BDNF-TrkB signaling in lymphocytes is rather stable, as samples collected over time are not altered without a targeted intervention [1]. The increments of BDNF-TrkB signaling in lymphocytes were highly correlated with those in the cortex and with changes in cortical excitability [1], suggesting that BDNF-TrkB signaling in lymphocytes reflects, at least in part, cortical TrkB signaling. Indeed, TrkB, a member of the neurotrophin receptor tyrosine kinase family, is expressed ubiquitously in the nervous system, in different organs and in immune cells. Upon binding of BDNF, TrkB signaling promotes, in general, cell development, maturation, survival [2,3] and, at the cortical level, also facilitates mechanisms related to neuronal plasticity, including long-term potentiation (LTP) and synapses formation [4–6].
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by abnormal motor and non-motor signs. More recently, electrophysiological studies have also revealed decreased long-term potentiation (LTP)-like plasticity in the cortex of patients with PD [7,8]. As a result, PD patients exhibit deficits in short- and long-term retention of newly learned motor skills [9–12] that can be restored by enhancing local LTP-like plasticity with rTMS [12]. A more general improvement in plasticity might be obtained by aerobic exercise, as suggested by recent studies in normal animals [13], in animal model of PD [14], in normal human subjects [15] and in patients with neurodegenerative diseases [16]. In particular, the Multidisciplinary Intensive Rehabilitation Treatment (MIRT), a four-week protocol that includes aerobic exercise as well as functional and goal-directed training [17–21], produces a long-lasting improvement on the motor and non-motor functions of patients with PD [22–25]. Specifically, in a two-year study, the comparison between two groups of patients in the early stages of PD treated with the same amount of rasagiline revealed that UPDRS II and III scores as well as other clinical parameters progressed more slowly in the MIRT group [25]. More importantly, at the end of this study, the percentage of patients requiring an increase in levodopa-equivalent dosage remained lower in the MIRT group (25%) compared to that in other group (80%). Altogether, these results suggest that MIRT might decrease the rate of disease progression although the precise mechanisms remain largely unknown. Nevertheless, it is very plausible that the enhancement of phenomena related to cortical plasticity might play a role in these neuroprotective and neurorestorative effects. If so, based on our finding that changes of BDNF-TrkB signaling are highly correlated in the cortex and in the lymphocytes [1], we could expect that a four-week MIRT in patients with PD will also enhance BDNF-TrkB signaling in lymphocytes.
Materials and Methods
Subjects and clinical assessment
Subjects were sixteen patients with PD with clinical characteristics reported in Table 1. Inclusion criteria were: Mini-Mental State Examination score > 24; ability to walk without physical assistance; ability to perceive visual and auditory cues; right-handedness; no neurological conditions other than PD. Subjects were admitted to the Department of Parkinson’s Disease Rehabilitation at “Moriggia Pelascini” Hospital (Gravedona ed Uniti, Italy), and underwent a four-week MIRT while continuing their pharmacotherapy. We collected blood at three time points: baseline, after two-week MIRT, and after four-week MIRT. Clinical scores were assessed at baseline and after four-week MIRT by the same neurologist and physiotherapist expert in movement disorders and blind to the study design. These included: UPDRS, 6-Minutes Walking Test (6MWT), Berg Balance Scale (BBS), Time Up and Go Test (TUG), Parkinson’s Disease Disability Scale (PDDS), Freezing of Gait Questionnaire (FOGQ). The same neurologist performed all clinical assessments. The study protocol was conducted according to the Declaration of Helsinki, and approved by the local Scientific Committee and Institutional Review Board of “Moriggia Pelascini” Hospital. All subjects gave their informed consent before participation.
TABLE 1.
Characteristics of patients at baseline
| Patient | Age | Gender | Disease Duration (yrs) | Hoehn & Yahr stage | UPDRS III | L-dopa equivalent (mg) |
|---|---|---|---|---|---|---|
| 1 | 77 | M | 16 | 3 | 14 | 1300 |
| 2 | 75 | F | 10 | 3 | 28 | 1100 |
| 3 | 72 | M | 6 | 3 | 21 | 850 |
| 4 | 79 | F | 9 | 3 | 20 | 660 |
| 5 | 61 | M | 5 | 2 | 16 | 600 |
| 6 | 81 | F | 5 | 2 | 18 | 300 |
| 7 | 60 | M | 8 | 3 | 20 | 1100 |
| 8 | 80 | M | 3 | 2.5 | 16 | 800 |
| 9 | 76 | F | 12 | 2.5 | 23 | 1200 |
| 10 | 78 | F | 3 | 2 | 29 | 100 |
| 11 | 73 | M | 5 | 2 | 14 | 200 |
| 12 | 68 | M | 10 | 3 | 20 | 850 |
| 13 | 65 | M | 15 | 3 | 23 | 800 |
| 14 | 65 | M | 12 | 3 | 18 | 640 |
| 15 | 68 | F | 11 | 3 | 16 | 700 |
| 16 | 67 | M | 5 | 3 | 13 | 1240 |
MIRT
MIRT has been fully described in previous publications [17–25]. Briefly, MIRT consisted of four weeks of physical therapy and exercise, with three daily sessions, five days a week. The first was a one-to-one session with a physical therapist, involving muscle stretching, exercises to improve the range of motion of the spinal, pelvic and scapular joints, strengthening of abdominal muscles, postural changes, and balance training on a posturographic platform. The second session included aerobic training on: (1) a treadmill equipped with both visual and auditory cues, (2) a stationary bike, (3) an elliptical machine providing visual feedback, (4) a stabilometric platform. The third was an occupational therapy session to improve autonomy in daily life, use of tools, dressing, transferring from sitting to standing, rolling from supine to sitting, and leg coordination in walking and turning.
Lymphocyte collection, treatment, immunoprecipitation, and Western blotting
Whole blood was collected in EDTA tubes in the morning, between 7 and 8 am, before any activity was performed. Whole blood was layered on an equal volume of Histopaque-1077 at 25°C, and then centrifuged at 400 × g for 30 min (25°C). Lymphocytes (opaque interface) were washed twice with 3 ml of PBS followed by centrifugation at 250 × g for 10 min and resuspension. The pellet was suspended in 400 μl of PBS and 100 μl of glycerol, and stored at −80°. As established in our previous study [1], lymphocytes (100 mg) were thawed gradually and suspended in oxygenated Low-Mg2+ Krebs-Ringer (LMKR) at 4°C. The lymphocyte suspensions were then incubated at 37°C with 50 ng/ml recombinant human BDNF (rhBDNF) containing LMKR or LMKR only for 30 min (total incubation volume was 250 ml). The incubation mixture was aerated with 95% O2/5% CO2 every 10 min for 1 min during the incubation. The ligand stimulation was terminated by adding 1 ml of ice-cold Ca2+-free LMKR containing 0.5 mM EGTA/0.1 mM EDTA, protein phosphatase inhibitors and centrifuge. The resultant lymphocytes were homogenized in 0.25 ml of ice-cold immunoprecipitation buffer. The homogenates were centrifuged at 1000×g for 5 min (4°C), and the supernatant (postmitochondrial fraction) was sonicated for 10 s on ice and solubilized in 0.5% digitonin/0.2% sodium cholate/0.5% NP-40 for 60 min (4°C) with end-to-end rotation. The resultant lysates were cleared by centrifugation at 50,000×g for 5 min and diluted with 0.75 ml of immunoprecipitation buffer and protein concentrations measured by the Bradford method.
TrkB signaling complexes, extracellular-signal-regulated kinase 2 (ERK2) and protein kinase B1 (AKT1) in 100 µg lymphocyte lysate were separately immuoprecipitated by a 2-hr incubation (4°C) with 1 µg immobilized anti-TrkB (for assessment of pY-TrkB; phospholipase C (PLC)-γ1, SRC homology containing protein (Shc) recruitment; and TrkB– N-methyl-D-aspartate (NMDA) receptor interaction), anti-ERK2 (pT202/pY204-ERK2) and anti-AKT (pS473-AKT) followed by addition of 25 μl protein A/G-conjugated agarose beads and incubation at 4°C for 16 hr. The resultant immunocomplexes were pelleted by centrifugation (4°C), washed 3 times with 1 ml of ice-cold PBS, pH7.2 and centrifuged. The resultant immunocomplexes were solubilized by boiling in SDS-PAGE sample preparation buffer. The contents of pY-TrkB, PLC-γ1, Shc, NR1 subunit of NMDAR in the 50% of anti-TrkB, pT202/pY204-ERK2 in anti-ERK2 and pS473-AKT1 in anti-Akt1 immunoprecipitates were determined by Western blotting with specific antibodies as described previously [1]. The blots were stripped and re-probed with anti-TrkB, -ERK2, or -Akt1/2/3 to illustrate even immunoprecipitation efficiency and loading. The signals were detected using a chemiluminescent method and visualized by exposure to x-ray film. The films were scanned and specific bands were quantified by Image J. The data are expressed as ratio of the optical intensity of signaling molecule to the optical intensity of loading control.
Statistical analyses
All data are presented as mean ± standard deviation (SD). For each component of the TrkB signaling pathway we computed the changes between the BDNF-stimulated and unstimulated conditions, as a ratio between the two (i.e., [BDNF-stimulated]/[unstimulated]) at three time points (pre-MIRT, two-week MIRT, and four-week MIRT). Treatment effects were evaluated by repeated measure ANOVA, using time as independent variable with three levels: pre-MIRT, two-week MIRT, and four-week MIRT. We used Mauchly’s test for the verification of sphericity, and reported Greenhouse-Geisser corrected degrees of freedom (df) when sphericity could not be assumed. We used post-hoc tests with Bonferroni correction for multiple comparisons.
Further, we computed changes in BDNF-TrkB signaling as:
We correlated these changes with the differences between clinical scores collected after four-week MIRT and those collected pre-MIRT.
Results
Clinical scores improve after four-week MIRT
The sixteen patients completed the four-week MIRT. As shown in Table 2 and in agreement with our previous results [17–25], all scores reveal significant improvement after MIRT compared to baseline.
TABLE 2.
Clinical scores at baseline and after 4-week MIRT (UPDRS II: Activities of Daily Living; UPDRS III: Motor Examination; UPDRS IV: Complications of Therapy)
| Baseline | Post- MIRT | Difference | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | Mean | SD | Mean | SD | t | p | |
| UPDRS tot | 16 | 43.31 | 11.99 | 29.56 | 9.46 | −13.75 | 4.92 | 11.18 | < 0.01 |
| UPDRS II | 16 | 15.88 | 5.85 | 10.75 | 4.95 | −5.13 | 2.31 | 8.89 | < 0.01 |
| UPDRS III | 16 | 19.31 | 4.71 | 13.06 | 3.28 | −6.25 | 3.28 | 7.63 | < 0.01 |
| UPDRS IV | 16 | 5.13 | 4.76 | 3.44 | 2.76 | −1.69 | 2.55 | 2.65 | 0.009 |
| 6MWT | 16 | 292.38 | 103.46 | 363.63 | 114.64 | 71.25 | 58.38 | −4.73 | < 0.01 |
| BBS | 16 | 44.75 | 7.21 | 52.50 | 4.18 | 7.75 | 4.58 | −6.77 | < 0.01 |
| TUG | 16 | 18.18 | 19.53 | 12.64 | 13.90 | −5.54 | 7.23 | 3.07 | 0.004 |
| PDDS | 16 | 71.13 | 14.53 | 54.25 | 12.12 | −16.88 | 8.34 | 8.09 | < 0.01 |
| FOGQ | 11 | 14.27 | 5.22 | 9.73 | 4.03 | −4.55 | 1.69 | 8.90 | < 0.01 |
TrkB signaling in lymphocytes is enhanced following MIRT
We first evaluated the changes in BDNF-TrkB signaling in lymphocytes following MIRT. The expression of TrkB 145-KDa and 95-KDa, ERK2 or Akt1 remain stable across the three time points, pre-MIRT as well as after two- and four-week MIRT, both under unstimulated and BDNF-stimulated conditions (Fig. 1 A, C). Similarly, the expression of Shc, PLC- γ1, NR1 was not affected by either BDNF stimulation or MIRT (Data not shown).
Figure 1.
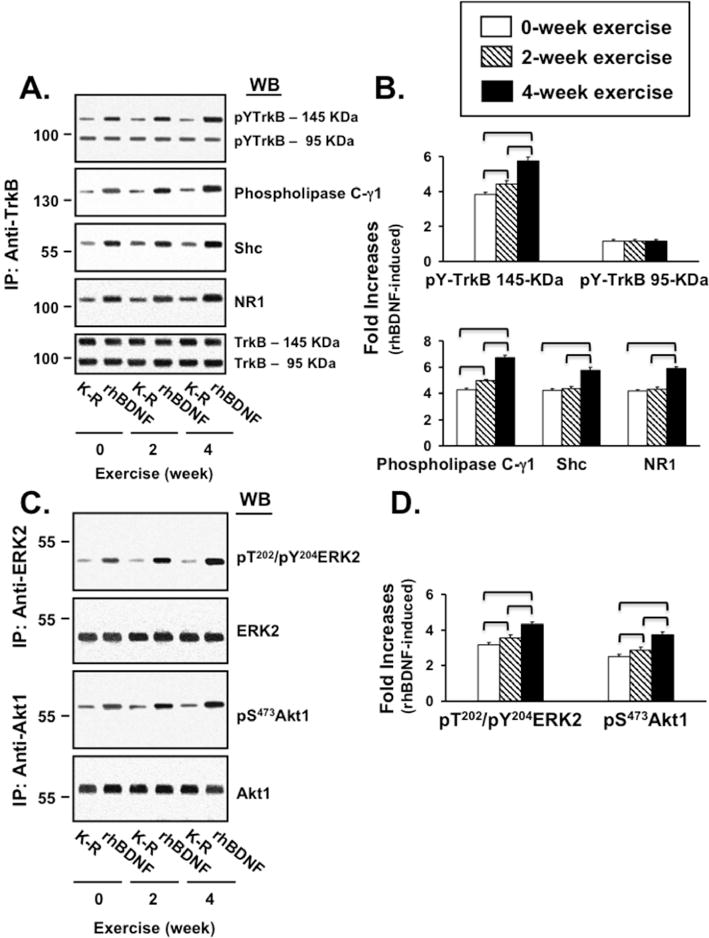
The results of statistical analysis of MIRT effects (repeated measure ANOVA and post-hoc tests) on each component of TrkB signaling are summarized in Table 3. In lymphocytes collected pre-MIRT, BDNF-stimulation increased pY-TrKB 145KDa level by 286 ± 31%, with minimal changes in pY-TrkB 95KDa (19 ± 7%). Following two- and four-week MIRT, the BDNF-induced pY-TrkB 145KDa levels were further increased by 15.6 ± 7.1% and 52.6 ± 10.8%, respectively, without significant increases of pY-TrkB 95KDa levels. Post-hoc tests confirmed that pY-TrKB 145 KDa level pre-MIRT (3.86 ± 0.31) was significantly lower compared to both two- (4.47 ± 0.55) and four-week MIRT (5.89 ± 0.60). The difference between two and four weeks was significant as well (Fig. 1 A, B).
TABLE 3.
Result of ANOVA and post-hoc test: Effect of MIRT on BDNF–TrkB signaling and TrkB–NMDAR recruitment.
| Repeated measure ANOVA | Post-hoc tests, pairwise comparisons, p values* | |||||
|---|---|---|---|---|---|---|
| df○ | F | p | Pre- vs 2-week MIRT | Pre- vs 4-week MIRT | 4- vs 2-week MIRT | |
| pY-TrkB (145KDa) | 2, 30 | 288.45 | <.0001 | <.0001 | <.0001 | <.0001 |
| pY-TrkB (95KDa) | 2, 30 | 0.028 | .972 | >.999 | >.999 | >.999 |
| PLC-γ1 | 2, 30 | 300.60 | <.0001 | <.0001 | <.0001 | <.0001 |
| Shc | 2, 30 | 157.12 | <.0001 | .631 | <.0001 | <.0001 |
| NR1 | 2, 30 | 244.68 | <.0001 | .168 | <.0001 | <.0001 |
| pY-ERK2 | 2, 30 | 224.38 | <.0001 | .007 | <.0001 | <.0001 |
| pS-Akt1 | 1.3, 20.1 | 256.41 | <.0001 | .036 | <.0001 | <.0001 |
Significant results in bold.
Greenhouse-Geisser corrected degrees of freedom when sphericity was not assumed.
p values adjusted for multiple comparison using Bonferroni correction.
Similar results were obtained for PLC- γ1 and Shc (Fig. 1 A, B). Pre-MIRT, BDNF stimulation enhanced pY-TrkB recruitment of PLC- γ1 and Shc by 328 ± 41% and 321 ± 47%, respectively. Importantly, MIRT significantly increased the recruitment of PLC- γ1 and Shc; post-hoc tests confirmed that the levels of recruited PLC- γ1 at two-week MIRT (4.95 ± 0.37) were already significantly higher than pre-MIRT (4.28 ± 0.42). Further significant increase was observed at four-week MIRT (6.75 ± 0.53). On the other hand, Shc recruitment at pre-MIRT (4.22 ± 0.48) and at two-week MIRT (4.33 ± 0.55) were comparable but significantly higher at four-week MIRT (5.78 ± 0.69).
To gain insight into MIRT-induced recruitment of the NMDA receptor, we measured the level of obligatory NMDAR NR1 subunit associated with TrkB (Fig. 1 A, B). Pre-MIRT, BDNF stimulation increased TrkB-NR1 association by 315 ± 22%. TrkB-NMDAR interaction increased with MIRT; post-hoc tests showed that four-week MIRT (5.87 ± 0.47) significantly increased TrkB-NMDAR interaction compared to pre-MIRT (4.15 ± 0.22) and two-week MIRT (4.32 ± 0.44).
BDNF-activated pT/pY-ERK2 and pS473-Akt1 levels also increased with MIRT, as shown in Figure 1 C, D. In lymphocytes collected pre-MIRT, BDNF stimulation increased pT/pY-ERK2 by 224 ± 13% and pS473-Akt1 by 158 ± 19%. MIRT significantly elevated pT/pY-ERK2 and pS473-Akt1. Post-hoc tests showed significant differences between pre-and two-week MIRT for both pT/pY-ERK2 (3.24 ± 0.13 vs 3.46 ± 0.28) and pS473-Akt1 (2.58 ± 0.19 vs 2.76 ± 0.32). Further increases were also observed at four-week MIRT for both pT/pY-ERK2 (4.32 ± 0.25) and pS473-Akt1 (3.74 ± 0.26).
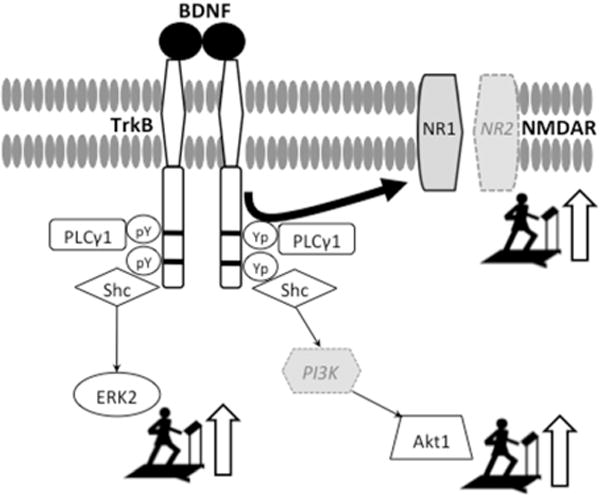
The effects of MIRT on BDNF-TrkB signaling and TrkB-NMDAR interaction are summarized in Figure 2.
Figure 2.
TrkB signaling enhancement in lymphocytes correlates with improvements in UPDRS scores
We found that the decrements in UPDRS total scores were significantly correlated with the increases in TrkB signaling at receptor (TrkB 145KDa), intracellular mediators (PLC-γ1, Shc) and NMDAR interaction levels (NR1). No correlations were found with TrkB 95KDa and downstream effectors (ERK2 and AKT1), which, however, are not exclusive components of BDNF-TrkB signaling, as they participate to other pathways as well, and have not been immunoprecipitated with the TrkB complex. Similar results were found for UPDRS II scores (activity of daily living). Illustrations as well as r and p value of such correlations are reported in the supplemental material (Supplemental Figure 1 A, B). We did not find significant correlations between changes in TrkB signaling components and all the other indices of clinical improvement.
Discussion
The primary and novel result of these studies is that in patients with PD, a four-week MIRT up-regulates BDNF-TrkB signaling in the peripheral lymphocytes at the levels of the receptor, intracellular mediators and downstream effectors. Importantly, these effects were not mediated by increases in the abundance of TrkB and its signaling mediators, since there were no detectable changes in the expression levels of any components in the BDNF-TrkB signaling cascade. This indicates that the four-week MIRT fosters a more efficient BDNF-TrkB signaling in lymphocytes possibly by enhancing BDNF affinity for TrkB [1]. Second, the enhancement of BDNF-TrkB signaling was already present after two weeks of MIRT and further increased at the completion of the treatment. Finally, as in the previous MIRT studies [17–25], motor and non-motor functions improved after the four-week treatment, despite unchanged pharmacological therapy. The new finding is that TrkB signaling enhancement significantly correlated with improvements in the total UPDRS scores and in the activity of daily living, suggesting that the changes in BDNF-TrkB signaling produced a generalized improvement rather than a focal effect on one or a few motor or non-motor signs. This type of general effect seems in accord with the benefit of aerobic exercise on multiple functions [15]. While this study did not include a no-exercise group of patients with PD, it is unlikely that the changes we found are related to the mere passage of time, as suggested by the results of previous studies [1]. In addition, in the present study, the changes through the three time points were progressive and in the same direction of increased TrkB signaling. Based upon these considerations, it is plausible that the changes in lymphocyte BDNF-TrKB signaling are the effects deriving from MIRT and not by the passage of time. Indeed, future studies are warranted to address directly this point.
It is well established that, in neural cells, the activation of the BDNF-TrkB signaling facilitates LTP by triggering phosphorylation and expression of proteins that are markers of synaptic plasticity [6]. However, the roles of TrkB signaling and TrkB-NMDAR interaction in immune cells are far from being completely understood. BDNF-TrkB signaling seems to be critical in the early T-cells development in that it promotes both survival of the precursors and further differentiation of the thymocytes throughout the T-cell differentiation pathway [2,26]. TrkB signaling is also necessary for normal B-cells development in the bone marrow [3]. It is then plausible that BDNF-TrkB signaling in immune cells might be used to promote cells proliferation and differentiation. TrkB-NMDAR interaction, on the other hand, appears to regulate lymphocyte cytokines production (e.g., interleukins, IFN- γ) that, in turn, modulates astrocytes and microglia activation and promotes glutamate clearance after neural damage [27,28]. It has also been shown that NMDAR is expressed in the lymphocytes that infiltrate the brain lesion sites [27]. In our study, significant changes for BDNF-TrkB signaling but not for TrkB-NMDAR interaction were present already after two weeks of treatment, as indicated by the association of NR1 with TrkB. This finding suggests that the enhancement of TrkB signaling precedes the increases in TrkB-NMDAR interaction. Thus, a possible interpretation of the present findings is that the MIRT-induced up-regulation of TrkB signaling and TrkB-NMDAR association in lymphocytes may reflect anti-inflammatory or other processes that in turn promote neurorestoration and recovery of function. Indeed, the results of some studies indicate that inflammation and microglia activation occur in PD [29]. Increased levels of proinflammatory cytokines are present in the serum, spinal fluid and nigrostriatal regions of patients with PD together with activated microglia surrounding dopaminergic neurons and infiltrated peripheral leukocytes [29]. In support of this interpretation, recent studies showed that the motor recovery after 6-hydroxydopamine-induced lesions was delayed in rats with genetically induced lymphocyte deficiency compared to wild-type rats [30]. Nevertheless, further studies are needed to verify whether this is a possible scenario and to define the cascade of effects triggered by the activation of BDNF- TrkB signaling and TrkB-NMDAR association in the lymphocytes. The likelihood of interactions between cortex and lymphocytes in this context has found further support by the recent discovery of a central nervous lymphatic system [31].
Regardless of their precise functions in the lymphocyte, BDNF-TrkB signaling in lymphocytes and in neural cells are highly correlated, as we recently found that they both respond to multiple sessions of rTMS, a treatment that increases LTP-like plasticity [1]. Because of this correlation, we concluded that the enhancement of BDNF-TrkB signaling in the lymphocyte reflects, at least in part, an increase in LTP-related phenomena at the cortical level [1]. Therefore, based upon those conclusions, one might interpret the present data and speculate that MIRT-induced upregulation of the BDNF-TrkB pathway may also occur at cortical level, with an effect on the mechanisms promoting cortical plasticity. Indeed, preliminary results of ongoing electrophysiological studies in our labs show an increase of LTP-like plasticity at the cortical level on a small number of patients that underwent MIRT (Frazzitta, Ghilardi and Quartarone, unpublished data). Nevertheless, caution is needed to interpret these data, as further studies are needed to prove any scenario correct.
In summary, the main result of the present study is that MIRT enhances BDNF-TrkB signaling in lymphocytes of patients with PD, an increase that parallels their improvement in clinical scores. Further studies are needed to define the relationship between these two findings and to determine the mechanisms that link immunity, plasticity and recovery of function in PD.
Supplementary Material
Acknowledgments
This study was supported by grants from the National Institute of Health (NS-054864 to MFG) and the Michael J. Fox Foundation (to HR). The funding sources had no role in data collection, analysis and interpretation.
References
- 1.Wang H-Y, Crupi D, Liu J, et al. Repetitive Transcranial Magnetic Stimulation Enhances BDNF-TrkB Signaling in Both Brain and Lymphocyte. Journal of Neuroscience. 2011;31:11044–54. doi: 10.1523/JNEUROSCI.2125-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maroder M, Bellavia D, Meco D, et al. Expression of trKB Neurotrophin Receptor during T Cell Development. Role of Brain Derived Neurotrophic Factor in Immature Thymocyte Survival. The Journal of Immunology. 1996;157:2864–72. [PubMed] [Google Scholar]
- 3.Schuhmann B, Dietrich A, Sel S, et al. A Role for Brain-Derived Neurotrophic Factor in B Cell Development. Journal of Neuroimmunology. 2005;163:15–23. doi: 10.1016/j.jneuroim.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Lu B. BDNF and Activity-Dependent Synaptic Modulation. Learning & Memory. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramham CR, Messaoudi E. BDNF Function in Adult Synaptic Plasticity: The Synaptic Consolidation Hypothesis. Progress in Neurobiology. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Zhang Z, Su Y, Kang L, Geng D, Wang Y, Luan F, Wang M, Cui H. Magnetic stimulation modulates structural synaptic plasticity and regulates BDNF-TrkB signal pathway in cultured hippocampal neurons. Neurochem Int. 2013 Jan;62(1):84–91. doi: 10.1016/j.neuint.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Koch G. Do Studies on Cortical Plasticity Provide a Rationale for Using Non-Invasive Brain Stimulation as a Treatment for Parkinson’s Disease Patients? Frontiers in Neurology. 2013;4 doi: 10.3389/fneur.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueki Y, Tatsuya M, Mamdouh AK, et al. Altered Plasticity of the Human Motor Cortex in Parkinson’s Disease. Annals of Neurology. 2006;59:60–71. doi: 10.1002/ana.20692. [DOI] [PubMed] [Google Scholar]
- 9.Isaias IU, Moisello C, Marotta G, et al. Dopaminergic Striatal Innervation Predicts Interlimb Transfer of a Visuomotor Skill. Journal of Neuroscience. 2011;31:14458–62. doi: 10.1523/JNEUROSCI.3583-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marinelli L, Crupi D, Di Rocco A, Bove M, Eidelberg D, Abbruzzese G, Ghilardi MF. Learning and Consolidation of Visuo-Motor Adaptation in Parkinson’s Disease. Parkinsonism & Related Disorders. 2009;15(1):6–11. doi: 10.1016/j.parkreldis.2008.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bédard P, Sanes JN. Basal Ganglia-Dependent Processes in Recalling Learned Visual-Motor Adaptations. Experimental Brain Research. 2011;2093:385–93. doi: 10.1007/s00221-011-2561-y. [DOI] [PubMed] [Google Scholar]
- 12.Moisello C, Blanco D, Fontanesi C, et al. TMS Enhances Retention of a Motor Skill in Parkinson’s Disease. Brain Stimulation in press. 2015;8:224–30. doi: 10.1016/j.brs.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaynman S. License to Run: Exercise Impacts Functional Plasticity in the Intact and Injured Central Nervous System by Using Neurotrophins. Neurorehabilitation and Neural Repair. 2005;19:283–95. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- 14.Petzinger GM, Fisher BE, McEwen S, et al. Exercise-Enhanced Neuroplasticity Targeting Motor and Cognitive Circuitry in Parkinson’s Disease. The Lancet Neurology. 2013;12:716–26. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. TRENDS in Cognitive Sciences. 2007;11(8):342–8. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Coelho FG, Vital TM, Stein AM, Arantes FJ, Rueda AV, Camarini R, Teodorov E, Santos-Galduróz RF. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer’s disease. Journal of Alzheimer’s Disease. 2014;39(2):401–8. doi: 10.3233/JAD-131073. [DOI] [PubMed] [Google Scholar]
- 17.Frazzitta G, Maestri R, Bertotti G, Uccellini D, Bazzini G, Abelli P, Aquilani R. Rehabilitation in Parkinson’s Disease: Assessing the Outcome Using Objective Metabolic Measurements. Movement Disorders. 2010;25:609–14. doi: 10.1002/mds.22871. [DOI] [PubMed] [Google Scholar]
- 18.Frazzitta G, Morelli M, Bertotti G, Felicetti G, Pezzoli G, Maestri R. Intensive Rehabilitation Treatment in Parkinsonian Patients with Dyskinesias: A Preliminary Study with 6-Month Followup. Parkinson’s Disease. 2012:1–4. doi: 10.1155/2012/910454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frazzitta G, Bertotti G, Morelli M, et al. Rehabilitation Improves Dyskinesias in Parkinsonian Patients: A Pilot Study Comparing Two Different Rehabilitative Treatments. NeuroRehabilitation. 2012;30:295–301. doi: 10.3233/NRE-2012-0758. [DOI] [PubMed] [Google Scholar]
- 20.Frazzitta G, Bertotti G, Uccellini D, Boveri N, Rovescala R, Pezzoli G, Maestri R. Short- and Long-Term Efficacy of Intensive Rehabilitation Treatment on Balance and Gait in Parkinsonian Patients: A Preliminary Study with a 1-Year Followup. Parkinson’s Disease. 2013:1–5. doi: 10.1155/2013/583278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frazzitta G, Pezzoli G, Bertotti G, Maestri R. Asymmetry and Freezing of Gait in Parkinsonian Patients. Journal of Neurology. 2013;260:71–76. doi: 10.1007/s00415-012-6585-4. [DOI] [PubMed] [Google Scholar]
- 22.Frazzitta G, Maestri R, Ferrazzoli D, Riboldazzi G, Bera R, Fontanesi C, Rossi RP, Pezzoli G, Ghilardi MF. Multidisciplinary intensive rehabilitation treatment improves sleep quality in Parkinson’s disease. Journal of Clinical Movement Disorders. 2015 Apr;2:11. doi: 10.1186/s40734-015-0020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frazzitta G, Bertotti G, Riboldazzi G, et al. Effectiveness of Intensive Inpatient Rehabilitation Treatment on Disease Progression in Parkinsonian Patients: A Randomized Controlled Trial With 1-Year Follow-Up. Neurorehabilitation and Neural Repair. 2012;26:144–50. doi: 10.1177/1545968311416990. [DOI] [PubMed] [Google Scholar]
- 24.Frazzitta G, Balbi P, Maestri R, Bertotti G, Boveri N, Pezzoli G. The Beneficial Role of Intensive Exercise on Parkinson Disease Progression. American Journal of Physical Medicine & Rehabilitation. 2013;92:523–32. doi: 10.1097/PHM.0b013e31828cd254. [DOI] [PubMed] [Google Scholar]
- 25.Frazzitta G, Maestri R, Bertotti G, et al. Intensive Rehabilitation Treatment in Early Parkinson’s Disease: A Randomized Pilot Study With a 2-Year Follow-Up. Neurorehabilitation and Neural Repair. 2015;29:123–31. doi: 10.1177/1545968314542981. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Suarez O, Blanco-Gelaz MA, Lopez ML, et al. Massive Lymphocyte Apoptosis in the Thymus of Functionally Deficient TrkB Mice. Journal of Neuroimmunology. 2002;129:25–34. doi: 10.1016/s0165-5728(02)00166-2. [DOI] [PubMed] [Google Scholar]
- 27.Mashkina AP, Cizkova D, Vanicky I, Boldyrev AA. NMDA receptors are expressed in lymphocytes activated both in vitro and in vivo. Cell Mol Neurobiol. 2010;30(6):901–7. doi: 10.1007/s10571-010-9519-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacheco R, Gallart T, Lluis C, Franco R. Role of glutamate on T-cell mediated immunity. J Neuroimmunol. 2007 Apr;185(1–2):9–19. doi: 10.1016/j.jneuroim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Chao Y, Wong SC, Tan EK. Evidence of inflammatory system involvement in Parkinson’s disease. Biomed Res Int. 2014;2014:308654. doi: 10.1155/2014/308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wheeler CJ, Seksenyan A, Koronyo Y, et al. T-Lymphocyte Deficiency Exacerbates Behavioral Deficits in the 6-OHDA Unilateral Lesion Rat Model for Parkinson’s Disease. Journal of Neurology & Neurophysiology. 2014;5:209. doi: 10.4172/2155-9562.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015 doi: 10.1038/nature14432. published online 01 June 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


