Abstract
In view of the recent findings of stimulatory effects of GHRH analogs, JI-34, JI-36 and JI-38, on cardiomyocytes, pancreatic islets and wound healing, three series of new analogs of GHRH(1–29) have been synthesized and evaluated biologically in an endeavor to produce more potent compounds. “Agmatine analogs”, MR-356 (N-Me-Tyr1-JI-38), MR-361(N-Me-Tyr1, D-Ala2-JI-38) and MR-367(N-Me-Tyr1, D-Ala2, Asn8-JI-38), in which Dat in JI-38 is replaced by N-Me-Tyr1, showed improved relative potencies on GH release upon subcutaneous administration in vivo and binding in vitro. Modification with N-Me-Tyr1 and Arg29-NHCH3 as in MR-403 (N-Me-Tyr1, D-Ala2, Arg29 -NHCH3 -JI-38), MR-406 (N-Me-Tyr1, Arg29 -NHCH3 -JI-38) and MR-409 (N-Me-Tyr1, D-Ala2, Asn8, Arg29-NHCH3 -JI-38), and MR-410 (N-Me-Tyr1, D-Ala2, Thr8, Arg29-NHCH3 -JI-38) resulted in dramatically increased endocrine activities. These appear to be the most potent GHRH agonistic analogs so far developed. Analogs with Apa30-NH2 such as MR-326 (N-Me-Tyr1, D-Ala2, Arg29, Apa30-NH2 -JI-38), and with Gab30 -NH2, as MR-502 (D-Ala2, 5F-Phe6, Ser28, Arg29, Gab30 -NH2 -JI-38) also exhibited much higher potency than JI-38 upon i.v. administration. The relationship between the GH-releasing potency and the analog structure is discussed. Fourteen GHRH agonists with the highest endocrine potencies were subjected to cardiologic tests. MR-409 and MR-356 exhibited higher potency than JI-38 in activating myocardial repair in rats with induced myocardial infarction. As the previous class of analogs, exemplified by JI-38, had shown promising results in multiple fields including cardiology, diabetes and wound healing, our new, more potent, GHRH agonists should manifest additional efficacy for possible medical applications.
Keywords: hGHRH agonist, hGHRH(1–29), s.c. administration, Cardioprotection
1. Introduction
Human growth hormone-releasing hormone (hGHRH) is a hypothalamic peptide hormone, the first known function of which is stimulation of growth hormone (GH) secretion from the anterior pituitary [16,33]. This 44-amino acid peptide belongs to a family which includes vasoactive intestinal peptide (VIP), pituitary adenylate cyclase-activating peptide (PACAP), secretin, glucagon, glucagon-like peptides-1 and -2 and gastric inhibitory peptide [30]. GHRH regulates production and release of GH through its binding to pituitary GHRH receptors (GHRH-R). This binding induces conformational changes of GHRH-R at its intracellular aspect, which trigger a series of intracellular signaling cascades, leading subsequently to increased transcriptional activities of both GH and GHRH genes [23,28].
For many years, the solitary role of GHRH had been considered to be the regulation of physiological levels of hypothalamic GH and hepatic insulin-like growth factor-I (IGF-I) by activation of the pituitary GH/liver IGF-I axis. In some recent studies, however, under conditions that clearly exclude involvement of GH/IGF-I, GHRH and its analogs have been shown to exert direct effects on a variety of extra-pituitary cells/tissues. Thus, (i) GHRH has been shown to promote the survival of cardio-myocytes in vitro and to protect rat heart against ischemia–reperfusion injury [32]. (ii) GHRH agonist, JI-38, was able to activate myocardial repair after experimental myocardial infarction in rats [19,20]. (iii) Pancreatic islets treated by GHRH agonists, JI-36 or MR-403, and then transplanted into diabetic NOD-SCID mice have been demonstrated to induce normoglycemia more consistently than untreated controls [26,27,36]. (iv) GHRH or its agonist, JI-38, stimulated migration and proliferation of mouse embryonic fibroblasts (MEFs) in vitro and accelerated healing of skin wounds in vivo [10]. (v) A protective role of GHRH agonist, JI-34, in experimental pneumolysin (PLY)-induced lung dysfunction has also been reported [25]. The accumulating results of these studies demonstrate multiple therapeutic roles for GHRH [1] and its agonists in a wide range of medical fields.
Our long-term goal has been to develop GHRH agonists, with appropriate biological and pharmaceutical properties, for use in clinical settings. A major drawback in using native GHRH as a therapeutic agent is its short in vivo half-life. This is a result of its inherent susceptibility to degradation by proteolytic enzymes [4]. Inactivation of native GHRH occurs mainly by dipeptidylaminopeptidase-IV (DDP-IV) which catalyzes cleavage of the first two N-terminal amino acids [14,15]. The deletion of Tyr-Ala dramatically reduces the bioactivity of GHRH, virtually to zero [7,15]. Therefore, based on the sites of hydrolysis of GHRH by these enzymes, and the structural and conformational requirements necessary for substrate effect, we and others have developed various degradation-resistant GHRH agonists [6–8,12,13,21,22,24,37,39,40].
The sequences of the first 29 amino acids of GHRH are highly conserved in different species [2,3,5,11]. This 1–29 amino acid fragment of human GHRH has also been proven to be the shortest sequence to exhibit full activity [9]. Therefore, the structures of most GHRH agonists, including the potent “JI series”, previously reported by us [18], reflect specific modifications of the hGHRH(1–29)NH2 backbone. In view of the discoveries of important effects on cardiomyocytes [20] and pancreatic islets [35] obtained with “JI agonists”, we decided to develop analogs with further augmented potency. In particular, we introduced structural modifications based on incorporation of N-terminal N-Me-Tyr, C-terminal methyl- or ethyl-amide, Apa30- and Gab30-NH2. Here, we report the synthesis of GHRH agonists, designated the “MR series”, with increased endocrine and cardiac activities compared to the previous class of “JI analogs”.
2. Materials and methods
2.1. Synthesis and purification of peptides
Three series of GHRH(1–29) analogs modified at the C-terminal have been synthesized:
-
Five C-terminal Agm GHRH(1–29) analogs (Table 1, Group I) were synthesized on Boc-Agm-SPA-MBHA resin with Boc-chemistry [18,37–40]. Boc amino acid derivatives were used in the synthesis. The side chains of the amino acids were protected by the following groups: Asp, cyclohexyl; Arg, tosyl; Orn, 2-chlorobenzyloxycarbonyl; Ser, Thr and N-Me-Tyr, benzyl; Tyr, 2,6-dichlobenzyloxycarbonyl; Orn, 2-chlorobenzyloxycarbonyl. The side chains of Asn, Gln and Dat were unprotected.
The coupling reactions were achieved with a 3-fold excess of Boc-amino acid and 1-hydroxybenzotriazole. N,N′-diisopropylcarbodiimide is used as a coupling agent. Boc-Gln was coupled with preformed 1-hydroxybenzotriazole ester. In cases where incomplete coupling was found, the coupling procedure was repeated. Acetylation was performed with 30% (v/v) acetic anhydride in dichloromethane for 20 min. Intermediate deblocking was performed with 50% (v/v) trifluoroacetic acid in dichloromethane followed by neutralization with 5% (v/v) diisopropylethylamine in dichloromethane. After completion of the synthesis, the peptide resin was treated by hydrogen fluoride in the presence of 3% cresol at 0 ºC for 2 h. After removal of hydrogen fluoride, the free peptides were precipitated and washed with diethyl ether. The crude C-terminal Agm peptide was then analyzed by HPLC and mass spectrometry (Table 1, Group I).
Nine C-terminal methylamide and two ethylamide hGHRH(1–29)NH2 analogs (Table 1, Group II) were synthesized using the Fmoc peptide synthesis on {3-[(methyl-Fmoc-amino)-methyl]-indol-1-yl} acetyl AM resin. Two C-terminal ethylamide hGHRH(1–29)NH2 analogs were synthesized on {3-[(ethyl-Fmoc-amino)methyl]indol-1-yl}-acetyl AM resin. Before starting the synthesis, the Fmoc group was removed from the resin with 20% piperidine in dimethyl-formamide for 20 min. The side chains of Fmoc-amino acids were protected with acid unstable groups such as β-tert-butyl ester for Asp;tert-butyl (But) for Ser, Thr, N-Me-Tyr and Tyr; Nω-pentamethyldihydrobenzofuran-5-sulfonyl (Pbf) for Arg and D-Arg; Nδ-tert-butoxycarbonyl (Boc) for Orn; Nγ-trityl for Asn and Nδ-trityl for Gln. Dat was unprotected. The coupling of Fmoc amino acid was achieved using HBTU [2-(1H-benzotriazole-1-Yl)-1,1,3,3-tetramethyluronium hex-afluorophosphate] activation performed with 3 equivalents of Fmoc amino acid and HBTU mixed in DMF, followed by addition of 6 equivalents of N,N-diisopropylethylamine (DIPEA) and stirred for 2 min to become a complete solution. The mixture was then immediately added to Fmoc-deblocked resin and incubated for 1–2 h. As an example of synthesis of C-terminal methylamide analog, MR-409: The protected amino acids were coupled to 3.2 g of {3-[(methyl-Fmoc-amino)-methyl]-indol-1-yl} acetyl AM resin in the following order: Fmoc-Arg(Pbf), Fmoc-Asp(OBut), Fmoc-Nle, Fmoc-Ile, Fmoc-Asp(OBut), Fmoc-Gln(Trt), Fmoc-Leu, Fmoc-Leu, Fmoc-Orn(Boc), Fmoc-Arg(Pbf), Fmoc-Ala, Fmoc-Ser(But), Fmoc-Leu, Fmoc-Gln(Trt), Fmoc-Abu, Fmoc-Leu, Fmoc-Val, Fmoc-Orn(Boc), Fmoc-Thr(But), Fmoc-Phe, Fmoc-Ser(But), Fmoc-Asn(Trt), Fmoc-Thr(But), Fmoc-Phe, Fmoc-Ile, Fmoc-Ala, Fmoc-Asp(OBut), Fmoc-D-Ala, Fmoc-N-Me-Tyr(But). After the final coupling, the yield of protected N-Me-Tyr(But)-D-Ala-Asp(OBut)-Ala-Ile-Phe-Thr-Asn(Trt)-Ser(tBu)-Tyr(But)-Arg(Pbf)-Orn(Boc)-Val-Leu-Abu-Gln(Trt)-Leu-Ser(But)-Ala-Arg(Pbf)-Orn(Boc)-Leu-Leu-Gln(Trt)-Asp(OBut)-Ile-Nle-Asp(OBut)-Arg(Pbf)-{3-[(methyl-amino)-methyl]-indol-1-yl}acetyl AM peptide-resin was 11.92 g. The product was then treated with 120 ml of mixed reagent and scavengers containing TFA/phenol/p-cresol/H2O (95:1:1:3 by volume) at room temperature for 3 h. The crude peptide MR-409 was precipitated with tert-butyl methyl ether, washed and dried; it yielded 8.74 g product. The crude MR-409 was then analyzed by HPLC and mass spectrometry (Fig. 1, Group II).
-
Five analogs with C-terminal additional Apa30, Gab30 or Gab31-hGHRH(1–29)NH2 listed in Table 1(Group III) were synthesized by using a Boc method on MBHA resin as described in (I), or using Fmoc synthesis on {3-[(methyl-amino)-methyl]-indol-1-yl}acetyl AM resin as described in (II).
The purification of the crude peptides was performed on a Beckman Gold HPLC System (Beckman Coulter, Inc., Brea, CA) equipped with 127P solvent Module, model 166P UV-VIS Detector, using an XBridge™ reversed phase column (10 mm × 250 mm), packed with C18 silica gel, 300 Å pore size, 5 μm particle size (Waters Co., Milford, MA). The peptides were eluted with a solvent system consisting of solvent A (0.1% aqueous TFA) and solvent B (0.1% TFA in 70% aqueous acetonitrile (MeCN)) in a linear gradient mode of 30–55% of solvent B for 120 min at a flow rate of 5 ml/min. The eluent was monitored at 220 and 280 nm, and the fractions were examined by analytical HPLC using a Hewlett-Packard Model HP-1090 liquid chromatograph and pooled to give maximum purity. Analytical HPLC was carried out on a Supelco Discovery HS C18 reversed-phase column (2.1 mm × 50 mm, C18, 300 Å pore size, 3 μm particle size; Supelco Bellefonte, PA) using gradient elution from 40 to 80% B for 40 min with a solvent system consisting of solvents A and B, defined above, with a flow rate of 0.2 ml/min. The peaks were monitored at 220 and 280 nm. The peptides were judged to be substantially (>95%) pure by analytical HPLC. Molecular masses were determined by Agilent 6210 time-of-flight mass spectrometry in conjugation with 1200 CapLC (Agilent Technologies 6210 Time-of Light LC/MS, Santa Clara, CA). Peptides were eluted on an Agilent Zorbax C18 column (0.5 mm × 150 mm, 300 Å pore size, 5 μm particle size, Agilent, Santa Clara, CA) at a flow rate of 15 μl/min with a linear gradient from 35 to 85% B for 30 min. Solvent A is 0.1% formic acid (FA), Solvent B is 90% aqueous MeCN/0.1% FA. TOF settings are as follow: capillary voltage: 4000 V; drying gas flow: 7 L/min; drying gas temperature: 300 ºC; nebulizer gas: 30 psi; fragmentor voltage: 350 V.
Table 1.
Chemical structures of new hGHRH(1–29) agonists and their calculated molecular weight by mass spectrometry.
| Group I: C-terminal Agm analogs of hGHRH(1–29)NH2
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide | Position of residuea
|
LC–MS
|
||||||||||
| 1 | 2 | 6 | 8 | 12 | 15 | 21 | 27 | 28 | 29 | MW calb | [M+H]c | |
| JI-38 | Dat | Ala | Phe | Gln | Orn | Abu | Orn | Nle | Asp | Agm | 3321.85 | 3322.81 |
| MR-351 | Ac-Me-Tyr | D-Ala | – | – | – | – | – | – | – | – | 3393.90 | 3393.94 |
| MR-356 | N-Me-Tyr | – | – | – | – | – | – | – | – | – | 3351.89 | 3352.08 |
| MR-361 | N-Me-Tyr | D-Ala | – | – | – | – | – | – | – | – | 3351.89 | 3352.81 |
| MR-367 | N-Me-Tyr | D-Ala | – | Asn | – | – | – | – | – | – | 3337.87 | 3338.82 |
| Group II: C-terminal methylamide and ethylamide analogs of hGHRH(1–29)NH2
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide | Position of residued
|
LC–MS
|
|||||||||||
| 1 | 2 | 6 | 8 | 12 | 15 | 21 | 27 | 28 | 29 | MW calb | [M+H]c | ||
| JI-38 | Dat | Ala | Phe | Gln | Orn | Abu | Orn | Nle | Asp | Agm | 3321.85 | 3322.81 | |
| MR-401 | – | D-Ala | – | – | – | – | – | – | – | D-Arg | —NH—CH3 | 3378.87 | 3379.93 |
| MR-403 | N-Me-Tyr | D-Ala | – | – | – | – | – | – | – | Arg | —NH—CH3 | 3407.90 | 3408.97 |
| MR-404 | N-Me-Tyr | D-Ala | Fpa5 | – | – | – | – | – | – | Arg | —NH—CH3 | 3497.85 | 3498.93 |
| MR-405 | N-Me-Tyr | – | – | – | – | – | – | – | Ser | Arg | —NH—CH3 | 3380.91 | 3381.49 |
| MR-406 | N-Me-Tyr | – | – | – | – | – | – | – | – | Arg | —NH—CH3 | 3408.91 | 3409.84 |
| MR-407 | – | – | – | – | – | – | – | – | – | Arg | —NH—CH3 | 3378.88 | 3379.82 |
| MR-408 | – | D-Ala | – | – | – | – | – | – | – | Arg | —NH—CH3 | 3378.88 | 3379.82 |
| MR-409 | N-Me-Tyr | D-Ala | – | Asn | – | – | – | – | – | Arg | —NH—CH3 | 3394.89 | 3395.17 |
| MR-410 | N-Me-Tyr | D-Ala | – | Thr | – | – | – | – | – | Arg | —NH—CH3 | 3381.90 | 3382.91 |
| MR-420 | N-Me-Tyr | D-Ala | – | - | – | – | – | – | – | Arg | —NH—CH2CH3 | 3422.93 | 3423.85 |
| MR-421 | N-Me-Tyr | D-Ala | – | Asn | – | – | – | – | – | Arg | —NH—CH2CH3 | 3408.91 | 3409.84 |
| Group III: C-terminal elongated analogs of hGHRH(1–29)NH2
| ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide | Position of residuee
|
LC–MS
|
||||||||||||
| 1 | 2 | 6 | 8 | 12 | 15 | 21 | 27 | 28 | 29 | 30 | 31 | MW calb | [M+H]c | |
| JI-38 | Dat | Ala | Phe | Gln | Orn | Abu | Orn | Nle | Asp | Agm | 3321.85 | 3322.81 | ||
| MR-326 | N-Me-Tyr | D-Ala | – | – | – | – | – | – | – | Arg | Apa-NH2 | 3493.96 | 3493.98 | |
| MR-327 | – | – | – | – | – | – | – | – | – | Arg | Apa-NH2 | 3463.93 | 3464.96 | |
| MR-502 | – | D-Ala | Fpa5 | Asn | – | – | – | – | Ser | Arg | Gab-NH2 | 3497.86 | 3499.93 | |
| MR-504 | – | D-Abu | – | Asn | – | – | – | – | Ser | Arg | Gab-NH2 | 3420.91 | 3422.99 | |
| MR-702 | – | D-Ala | – | Asn | – | – | – | – | Ser | Arg | Gln | Gab-NH2 | 3538.04 | 3539.03 |
Non-coded amino acids and acyl groups used in the analog peptides are abbreviated as follows: Abu, alpha-aminobutanoyl; Agm, agmatine; N-Me-Tyr, N-methyl-tyrosine.
MW cal indicates calculated molecular weight of GHRH agonists.
[M+H] indicates detected molecular weight by Agilent 6210 time-of-flight LC/MS.
Non-coded amino acids and acyl groups used in the analog peptides are abbreviated as follows: Abu, alpha-aminobutanoyl; Agm, agmatine; Dat, des-amino-tyrosine; Fpa5, pentafluoro-Phe; N-Me-Tyr, N-methyl-tyrosine.
Non-coded amino acids and acyl groups used in the analog peptides are abbreviated as follows: Abu, alpha-aminobutanoyl; Agm, agmatine; Apa, 5-aminopentanoyl; Dat, des-amino-tyrosine; Fpa5, pentafluoro-Phe; Gab, gamma-aminobutanoyl; N-Me-Tyr, N-methyl-tyrosine.
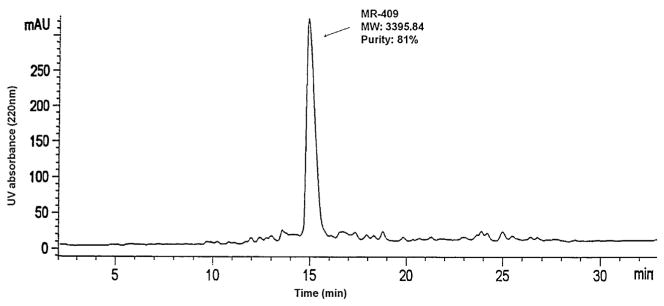
Fig. 1.
HPLC analysis of crude sample MR-409 on Agilent Zorbax 300SB C18 column (2.1 mm × 50 mm, particle size 5 μm), UV detector 220 nm, sample injection 20 μg/25 μl, Eluent A: 0.1% trifluoroacetic acid in water, Eluent B: 0.1% trifluoroacetic acid, 70% acetonitrile in water, linear gradient from 20% B to 60% B in 40 min. MR-409 peak purity: 81%.
2.2. Determination of GH releasing potencies in vivo
Male Sprague-Dawley rats, weighing 200–250 g, were purchased from Charles River Laboratory (Wilmington, MA) for the experiments. All animal procedures were approved by the Veterans Affairs Animal Care and Use Committee and were conducted in accordance with additional institutional guidelines as well. Briefly, groups of 5–6 animals were used in each experiment. The rats were anesthetized with 50 mg/kg of sodium pentobarbital (Nembutal, Lundbeck Inc., Deerfield, IL). Twenty min after inducing anesthesia, GHRH(1–29)NH2, JI-38, or new analogs were injected into the animals intravenously (i.v.) or subcutaneously (s.c.). The peptides were dissolved in 0.01 M acetic acid to get 1 μg/μl stock solution, and then diluted with 5% mannitol. Control rats received 5% mannitol only. Blood samples were collected from the jugular vein at 5, 15 and/or 30 min after the injection and were used for serum preparation. The time points were selected on the basis of our previous studies with GHRH analogs. Concentrations of GH in rat sera were determined by ELISA. The relative potencies of tested peptides were calculated using GHRH(1–29)NH2 and JI-38 for comparison, whose potency was defined as 1. The equivalent dosage of GHRH(1–29)NH2 to reach the same level of serum growth hormone release after injection of the specific tested peptide was determined from a GHRH(1–29)NH2 standard curve using Origin 6.0 software. The relative potency of the tested compounds is the ratio of GHRH(1–29)NH2 equivalence over the actual dosage used for injection.
2.3. Evaluation of receptor binding in vitro
The binding of GHRH(1–29) analogs to membrane receptors of human anterior pituitary cells was determined using 125I-labeled [His1,Nle27]-hGHRH(1-32)NH2 as a radioligand. Normal human pituitary was purchased from the National Hormone and Peptide Program (A. F. Parlow, Los Angeles, County Harbor – UCLA Medical Center, Torrance, CA). Preparation of human pituitary membrane fraction and receptor binding of GHRH(1–29) agonists were performed as previously described [17,38] by using a sensitive in vitro ligand competition assay. In competitive binding analysis, 125I-labeled [His1,Nle27]-hGHRH(1-32)NH2 (0.2 nM) was displaced by hGHRH agonists at 10−6 to 10−12 M. The final binding affinities were expressed as IC50 values and were calculated by using the LIGAND PC computerized curve-fitting program of Munson and Rodbard [31] as modified by McPherson [29]. Relative affinities were compared to hGHRH(1–29)NH2 and calculated as the ratio of IC50 (dose causing 50% inhibition of specific binding to receptors) of the tested peptides to the IC50 of the standard.
2.4. Cardiac assays in rats
2.4.1. Myocardial infarction (MI) model
MI was induced in female 6-month-old Fischer 344 rats by permanent ligation of left ventricular (LV) anterior descending coronary artery as described previously [19,20]. Animals were randomly assigned to receive placebo or one of the following GHRH agonists (MR-356, MR-361, MR-367, MR-403, MR-404, MR-409 and MR-502) starting 2 h post-surgery. All treatment (50 μg/kg) was given subcutaneously twice daily for 4 weeks. The Institutional Animal Care and Use committee of the University of Miami approved all protocols and experimental procedures.
2.4.2. Echocardiographic measurements
Longitudinal evaluation of LV remodeling was obtained by echocardiographic measurements between baseline and 4 weeks following MI. Echocardiographic assessments were performed in anesthetized rats (1–2% isoflurane inhalation) using a Vevo-770 echocardiogram (Visual Sonics Inc., Toronto, Ontario, Canada) equipped with 25 MHz transducer. Cardiac dimensions were recorded from M-mode images using averaged measurements from 3 to 5 consecutive cardiac cycles according to the American Society of Echocardiography [34]. LV end-diastolic and end-systolic volumes and ejection fraction (EF) were calculated from bi-dimensional long-axis parasternal views taken through the infarcted area. All images were analyzed using Vevo 770 3.0.0 software (Visual Sonics).
2.4.3. Morphometric analysis
Slides were prepared with H&E and Masson’s trichrome stain to assess cardiac structure and the presence and extent of myocardial scar, respectively. The percentage of infarcted myocardium was calculated by a previously validated method [20].
2.5. Hormone determination
Serum GH was determined from duplicate samples using the rat Growth Hormone ELISA kit (ALPCO Diagnostics, Mill Valley, CA) according to the manufacturer’s instruction. Absorbance at 405 nm was measured by a Victor 3 Multi-label Counter (Perkin-Elmer, Waltham, MD).
2.6. Statistical analysis
Determination of the significance GH-releasing activity from in vivo experiments was performed by unpaired Student’s t-test or one-way ANOVA, followed by Tukey’s test, using the computer software Sigma Stat (Jandel, San Rafel, CA). Differences were considered significant when p < 0.05. For cardiac assays, all values are shown as mean ±SEM. Significance for these was determined by the unpaired Student’s t test. For a given parameter, p < 0.05 was considered significant. Statistical analysis of cardiac tests was carried out using GraphPad Prism software (San Diego, CA, USA) version 5.0 for Windows.
3. Results
3.1. Design and synthesis of new hGHRH(1–29)NH2 agonists
This series of analogs was designed by modifying JI-38 [18], containing the sequence of [Dat1, Gln8, Orn12,21, Abu15, Nle27, Asp28, Agm29]hGHRH(1–29)NH2, as a model peptide since this analog exhibited high activity in vivo. In each of the new agonistic analogs, Lys12 and Lys21 were replaced by Orn, Gly15 by Abu, and Met27 by Nle. In seven of the new peptides (MR-401, MR-407, MR-408, MR-327, MR-502, MR-504, MR-702), Dat (desamino-Tyr) was conserved at the N-terminus, but most analogs had N-Me-Tyr1. In fourteen analogs, Ala2 was substituted by D-Ala, and in one peptide, Ala2 was replaced by D-Abu. Phe at position 6 was substituted by pentafluoro-Phe (Fpa5) in two compounds. Asn in position 8 and Ser at position 28 were either left unchanged or replaced by Gln and Asp, respectively. We focused on modifications at the C-terminus. As shown in Table 1, the three major groups of the new hGHRH agonists had different C-termini: (I) Agm29; (II) Arg29-methylamide or Arg29-ethylamide, and (III) elongated C-terminus with ω-amino fatty acid amide, such as Apa30-NH2, Gab30-NH2 or Gln30-Gab31-NH2.
The synthesis of C-terminal methylamide peptides on {3-[(methyl-Fmoc-amino)-methyl]-indol-1-yl}acetyl AM resin with Fmoc synthesis was most successful. In the case of synthesis of MR-409, the yield of the crude MR-409 methylamide peptide was very high and manifested good purity (Fig. 1). From 4.4 g of crude MR-409, we obtained 2.1 g peptide with purity over 97%. The total yield calculated from resin was 24%. All synthesized peptides listed in Table 1 were purified by HPLC which led to purity over 95%. Their molecular weights (MW), as determined by mass spectrometry, matched very well with their MW calculated from structures (Table 1 and Fig. 2).
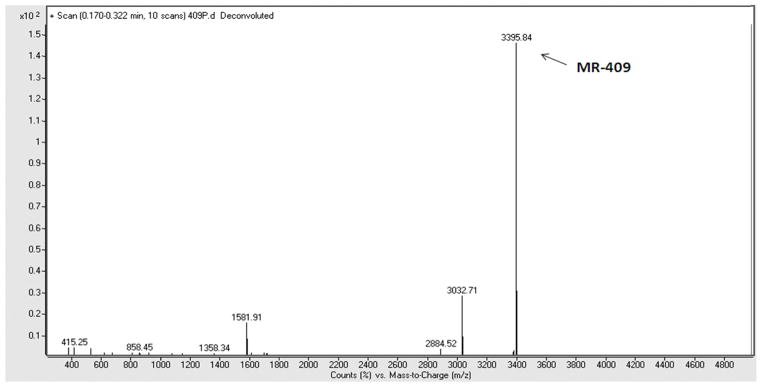
Fig. 2.
Molecular weight of crude sample MR-409 analyzed by Agilent 6210 time-of-flight LC/MS. Expected molecular weight of MR-409 is 3394.89, detected molecular weight of MR-409 is 3395.84.
3.2. Effects of new GHRH analogs on GH release in vivo
The GH-releasing effects of the new GHRH agonists in vivo are summarized in Tables 2 and 3. Most of these new agonists were found to be more potent than JI-38. After i.v. injections, MR-502 in particular, showed a potency 2.1 and 2.9 fold higher than JI-38 at 5 and 15 min, respectively. MR-356, MR-403, MR-504 and MR-702 exhibited slightly improved relative potencies, ranging between 1.1-and 1.6-fold than that of JI-38, in the i.v. test (Table 3). After s.c. administration, the relative potency of MR-403 was 2.7- and 4.0-fold higher than that of JI-38 at 15 and 30 min, respectively. In s.c. tests, MR-326, MR-327, MR-356, MR-361, MR-367, MR-401, MR-404, MR-405, MR-406, MR-407, MR-409, MR-410, MR-420, MR-421 and MR-502 showed higher potencies than JI-38 in the range of 1.0–4.0-fold. Some peptides showed increased potency as compared to JI-38 in both i.v. and s.c. tests. These included MR-356, MR-403 and MR-502.
Table 2.
Effects of subcutaneous administration of hGHRH(1–29)NH2 analogs on GH release in male rats.
| Peptide | Dose (μg/kg) | Serum GH level at selected time after injection (ng/ml)
|
Relative potency
|
|||||
|---|---|---|---|---|---|---|---|---|
| 0 min | 15 min | 30 min | Compared to GHRH(1–29)
|
Compared to JI-38
|
||||
| 15 min | 30 min | 15 min | 30 min | |||||
| GHRH (1–29) | 100 | 34.5 ± 10.2 | 140.9 ± 24.2 | 65.1 ± 16.5 | 1.00 | 1.00 | ||
| 200 | 74.6 ± 14.1 | 240.0 ± 27.2 | 96.2 ± 3.4 | |||||
| JI-38 | 3 | 33.2 ± 2.8 | 230.0 ± 15.1 | 128.7 ± 13.3 | 63.1 | 111.8 | 1.00 | 1.00 |
| MR-326 | 3 | 43.7 ± 6.4 | 309.2 ± 33.5 | 225.9 ± 19.8 | 92.7 | 303.4 | 1.47 | 2.71 |
| MR-327 | 3 | 31.5 ± 3.4 | 258.7 ± 31.1 | 138 ± 27.9 | 73.5 | 126.5 | 1.16 | 1.16 |
| MR-351 | 3 | 30.6 ± 7.2 | 90.8 ± 20.3 | 65.4 ± 10.1 | 18.8 | 33.6 | 0.30 | 0.30 |
| MR-356 | 3 | 25.3 ± 3.9 | 305.4 ± 40.0 | 152.4 ± 21.8 | 91.2 | 150.9 | 1.45 | 1.35 |
| MR-361 | 3 | 46.1 ± 6.3 | 275.9 ± 46.4 | 142.6 ± 18.4 | 80 | 134.1 | 1.27 | 1.20 |
| MR-367 | 3 | 41.1 ± 5.6 | 244.0 ± 25.1 | 141.2 ± 12.3 | 68.1 | 131.7 | 1.08 | 1.18 |
| MR-401 | 3 | 24.6 ± 5.3 | 217.9 ± 37.0 | 204.1 ± 23.7 | 58.8 | 253.4 | 0.93 | 2.27 |
| MR-403 | 3 | 39.4 ± 5.3 | 608.7 ± 35.3* | 281.7 ± 18.6* | 223.8 | 448.9 | 3.55* | 4.02* |
| MR-404 | 3 | 29.5 ± 3.2 | 320.5 ± 32.7 | 151 ± 28.5 | 97.1 | 148.4 | 1.54 | 1.33 |
| MR-405 | 3 | 21.8 ± 5.9 | 227.9 ± 10.5 | 143.3 ± 6.1 | 62.3 | 135.2 | 0.99 | 1.21 |
| MR-406 | 3 | 21.3 ± 4.8 | 492.6 ± 21.8* | 282 ± 17.8* | 170 | 449.7 | 2.69* | 4.02* |
| MR-407 | 3 | 36.7 ± 10.2 | 248.0 ± 40.5 | 135.2 ± 10.8 | 69.6 | 122 | 1.10 | 1.09 |
| MR-408 | 3 | 37.6 ± 15.5 | 213.3 ± 32.0 | 121.2 ± 8.4 | 57.2 | 100.5 | 0.91 | 0.90 |
| MR-409 | 3 | 18.9 ± 3.1 | 427.4 ± 62.4* | 256.7 ± 20.7* | 141.3 | 380.6 | 2.24* | 3.4* |
| MR-410 | 3 | 34.1 ± 1.2 | 334.0 ± 54.0 | 250.4 ± 28.6* | 102.5 | 363.2 | 1.58 | 3.25* |
| MR-420 | 3 | 35.5 ± 7.6 | 256.2 ± 46.0 | 126.1 ± 15.8 | 72.6 | 107.8 | 1.15 | 0.96 |
| MR-421 | 3 | 44.0 ± 8.9 | 252.4 ± 33.3 | 137.3 ± 17.5 | 71.2 | 125.4 | 1.13 | 1.12 |
| MR-502 | 3 | 61.7 ± 8.1 | 266.7 ± 21 | 153.2 ± 33.3 | 76.5 | 152.3 | 1.21 | 1.36 |
| MR-504 | 3 | 25.4 ± 4.1 | 200.0 ± 10.7 | 130.0 ± 19.1 | 52.6 | 113.8 | 0.83 | 1.02 |
| MR-702 | 3 | 43.7 ± 5 | 103.7 ± 23.1 | 85 ± 27.8 | 22.4 | 53.5 | 0.35 | 0.48 |
Data are shown as mean ± SEM.
p < 0.05 vs JI-38 (ANOVA followed by Tukey’s test).
Table 3.
Effects of intravenous administration of GHRH analogs on GH release.
| Injection | Dose (μg/kg) | Serum GH level at selected time after injection (ng/ml)
|
Relative potency
|
|||
|---|---|---|---|---|---|---|
| 0 min | 5 min | 15 min | 5 min | 15 min | ||
| JI-38 | 1 | 48.0 ± 10.4 | 538 ± 58.3 | 198 ± 18.1 | 1 | 1 |
| GHRH(1–29) | 1 | 50.1 ± 6.4 | 441 ± 76.0 | 105 ± 23.7* | 0.82 | 0.53* |
| MR-356 | 1 | 37.8 ± 5.7 | 632 ± 110 | 250 ± 58.1 | 1.17 | 1.26 |
| MR-403 | 1 | 47.7 ± 4.1 | 642 ± 29.7 | 268 ± 21.1 | 1.19 | 1.35 |
| MR-502 | 1 | 59.9 ± 15.9 | 1253 ± 185* | 575 ± 20.9* | 2.05* | 2.90* |
| MR-504 | 1 | 25.7 ± 6.3 | 832 ± 214 | 297 ± 45.6 | 1.55 | 1.50 |
| MR-702 | 1 | 47.7 ± 3.4 | 578 ± 87.7 | 273 ± 40.8 | 1.07 | 1.38 |
Data are shown as mean ± SEM.
p < 0.05 vs JI-38.
3.3. Binding affinities of new hGHRH agonists
The binding affinity of analogs to membrane receptors of human anterior pituitary cells was determined by using 125I-labeled [His1, Nle27]hGHRH(1-32)NH2. The relative affinities of analogs were compared to hGHRH(1–29)NH2 as the standard (IC50 = 4.06 nM; accepted as 1.0) and were found to be much higher. Fourteen analogs showed even higher binding affinities than JI-38 (Table 4).
Table 4.
IC50 values and relative binding affinities of our new GHRH(1–29) agonists to the membrane receptors of human pituitary cells.
| GHRH agonists | IC50 (nM) | Relative affinitya (Binding potency)
|
|
|---|---|---|---|
| Compared to hGHRH(1–29) | Compared to JI-38 | ||
| hGHRH(1–29) | 7.65 | 1.00 | |
| JI-38 | 2.74 | 2.79 | 1.00 |
| MR-326 | 0.98 | 7.81 | 2.80 |
| MR-327 | 2.04 | 3.75 | 1.34 |
| MR-351 | 3.87 | 1.98 | 0.71 |
| MR-356 | 1.02 | 7.50 | 2.69 |
| MR-361 | 1.19 | 6.42 | 2.30 |
| MR-367 | 1.09 | 7.01 | 2.51 |
| MR-401 | 2.60 | 2.94 | 1.05 |
| MR-403 | 0.74 | 10.30 | 3.69 |
| MR-404 | 1.12 | 6.83 | 2.45 |
| MR-405 | 2.51 | 3.04 | 1.09 |
| MR-406 | 0.93 | 8.22 | 2.95 |
| MR-407 | 5.01 | 1.52 | 0.54 |
| MR-408 | 6.32 | 1.21 | 0.53 |
| MR-409 | 1.01 | 7.57 | 2.71 |
| MR-410 | 1.28 | 5.98 | 2.14 |
| MR-420 | 2.70 | 2.83 | 1.01 |
| MR-421 | 3.05 | 2.50 | 0.90 |
| MR-502 | 2.16 | 3.54 | 1.27 |
| MR-504 | 2.85 | 2.68 | 0.96 |
| MR-702 | 3.30 | 2.31 | 0.83 |
Expressed relative to hGHRH(1–29)NH2 = 1 and JI-38 = 1. Values were calculated from duplicate tubes.
3.4. Impact of GHRH agonist on cardiac remodeling
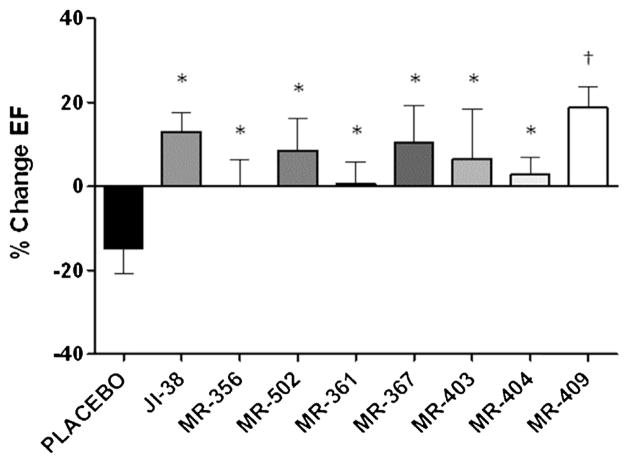
At baseline and after MI, ejection fractions (EF) were similar in all groups. To facilitate comparisons, we calculated the percentage of change in EF at 4 weeks relative to day 2 post-MI in each group. MI control (placebo group) had a negative treatment effect, as EF decreased over time; in contrast, treatment with the new agonists of GHRH preserved or improved EF (Fig. 3). In addition, although echocardiographic measurements at day 2 post-MI revealed that EF tended to be slightly better preserved in JI-38, MR-356 and MR-361 than all other groups, the differences were not statistically significant.
Fig. 3.
Impact of GHRH agonists on cardiac function. Bar graphs showing the percentage of change in ejection fraction (EF%) at 4 weeks relative to day 2 post-MI. All values are shown as mean ± SEM (*p < 0.05, †p < 0.01 vs. Placebo, n = 6–12).
3.5. Impact of GHRH agonists on scar size
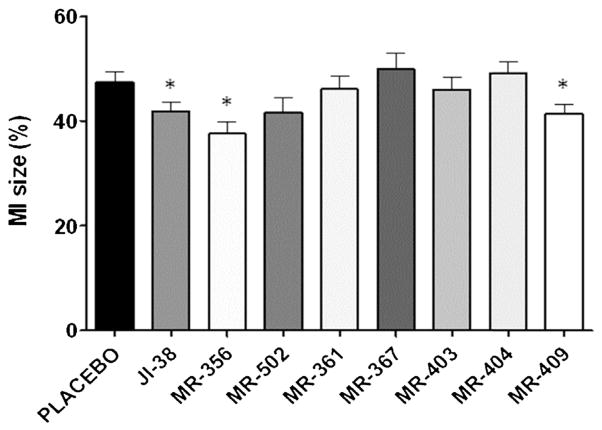
Additionally, the cardioprotective effects of JI-38, MR-356 and MR-409 were confirmed by substantial reductions in the infarct size when compared to the placebo group (Fig. 4); MR-356 and MR-409 results were not significantly different from those of JI-38.
Fig. 4.
Impact of GHRH agonists on myocardial infarct burden (MI%). Bar graphs showing percentage of infarct size. Note that MI% was significantly attenuated in the groups treated with JI-38, MR-356 and MR-409. All values are shown as mean ± SEM (*p < 0.05 vs. Placebo, n = 6–12).
4. Discussion
In our search for hGHRH agonists with greater endocrine and cardiac activities, we have recently synthesized nearly a hundred novel hGHRH analogs and evaluated their endocrine and cardiac properties. Fourteen of the new analogs demonstrated enhanced GH release stimulation in vivo as well as increased hGHRH receptor binding affinities in vitro compared to previous agonist JI-38. The structural design of these new agonists was based on that of our previous potent hGHRH analogs of the “JI” series [18]. Therefore, in our synthesis strategy for the new analogs, several key features of JI-34, JI-36 and JI-38 were maintained. These include substitutions of Orn12, 21, Gln8, Nle27 and Asp28, each of which inhibited isomerization or degradation [18,37–40]; and a replacement of Gly by Abu at position 15, which appears to increase affinity for the GHRH receptor [38]. On the basis of their structures, the selected new hGHRH agonists could be classified into three groups (Table 1). Thus, of the three analogs, which belonged to the Agm29 group, including MR-356 (N-Me-Tyr1-JI-38), MR-361 (N-Me-Tyr1, D-Ala2-JI-38) and MR-367 (N-Me-Tyr1, D-Ala2, Asn8-JI-38), each showed better receptor binding affinity than did JI-38. In particular, MR-356 displayed a high (2.7 fold) increase in GHRH receptor binding affin-ity and 1.4 times greater potency than JI-38, on GH release at 15 min and 30 min after subcutaneous administration. This was slightly better than those of MR-361 and MR-367. Our findings suggest a correlation between the introduction of the N-Me-Tyr1 residue into the peptide structure and the consequent enhancement in receptor binding. Dat1 in JI-38 or acetylated N-Me-Tyr, as in MR-351, showed weaker GH-releasing potency and GH receptor binding affinity than those of N-Me-Tyr1, Agm29 agonists. This implies that the methylation on the alpha amino group of Tyr and the resulting secondary amino group of N-Me-Tyr appear to be favorable for receptor binding and GH-releasing capability. Interestingly, a replacement of Ala2 in MR-356 by D-Ala2 in MR-361 or D-Ala2, Asn8 in MR-367 resulted in a reduced GH-releasing capability and slightly weakened binding affinity. These results suggest that the structure of Ala2, Gln8 in MR-356 is the more favorable addition to Agm29 agonists from the aspect of endocrine potency.
Group II featured a replacement of Agm29 by Arg29-NHCH3 or Arg29-NHCH2CH3 at the C-terminal end of the GHRH agonist and is therefore called the Arg29-NHCH3 or Arg29-NHCH2CH3-GHRH agonist group. The peptide with Arg29-NHCH3, as for example MR-403, is more potent than the peptide with Arg29-NHCH2CH3, as for example MR-420. The most potent compounds of this group are MR-403(N-Me-Tyr1, D-Ala2, Arg29-NHCH3-JI-38), MR-406 (N-Me-Tyr1, Arg29-NHCH3-JI-38) and MR-409 (N-Me-Tyr1, D-Ala2, Asn8, Arg29-NHCH3-JI-38) (Table 1). Among these, MR-403 is the most potent and exhibits the highest GH-releasing potency and binding affinity. Compared with JI-38, the receptor binding affinity of MR-403 is enhanced nearly 3.7-fold and GH-releasing activity in vivo also escalated 3.6–4.0-fold. All three of the group II peptides exhibited significantly increased GH-releasing activities and binding affinities. The replacement of Agm29 with Arg29-NHCH3 yielded a significant increase of GH-release and receptor binding affini-ties. MR-406 appears to be less potent (GH-release) than MR-403 but more than MR-409. Although MR-406 and MR-409 showed slightly lower GH-releasing potency than MR-403 at 15 min after s.c. administration, they all exhibited a significantly higher relative potency than JI-38 at 30 min. The calculated potencies of MR-403, MR-406 and MR-409 at 30 min relative to JI-38 were 4.0, 4.0, and 3.4, respectively, indicating their stability and long half-life in vivo. Since neither N-Me-Tyr at the N-terminal end nor Arg-NHCH3 at the C-terminus are present in JI-38, the use of these as replacements of Dat1 and Agm29 (in JI-38) suggests that both substitutions contribute to the improvement of endocrine activity. However, the C-terminal Arg29-NHCH3, but not the N-terminal N-Me-Tyr, led to increased proteolytic stability, so this structure must be responsible for the higher relative s.c. potencies at 30 min. Aside from their similarity at the N- or C-terminal ends, the major structural differences between the several Arg29-NHCH3 agonists are at positions 2 and 8, where a replacement of Gln8 in MR-403, by Asn8 in MR-409, or a substitution of D-Ala2 in MR-403 with Ala2 in MR-406 slightly decreases the GH-releasing activities and binding affinities. Consequently, our results indicate that D-Ala2, Gln8 in MR-403 is the most favorable form.
The Third Group of agonists has a C-terminal Apa30-NH2 which is represented in MR-326 (N-Me-Tyr1, D-Ala2, Arg29, Apa30-NH2-JI-38), or Gab30-NH2 as in MR-502 (D-Ala2, Fpa56, Asn8, Ser28, Arg29, Gab30-NH2-JI-38). In this group, MR-326 showed the highest potency for stimulating GH release; its relative potency at 15 and 30 min after s.c. injections was 1.5-fold and 2.7-fold greater than that of JI-38, respectively. However with intravenous administration, MR-502 has the highest potency for GH release, even higher than that of MR-403. With repeated s.c. tests, MR-502 showed only 1.2–1.4 times more potent GH release than JI-38. MR-356 and MR-403 showed consistently higher potencies than MR-502 in s.c. tests. The relative potency of GHRH (1–29)-NH2 also appears greater in i.v. tests than in s.c. tests. The great potency of MR-502 in i.v. tests is possibly related to the substitution of Fpa56 for Phe6, and an elongated C-terminal Gab30-NH2.
The substitution with D-Abu2 in MR-504, Fpa56 in MR-404, Ser28 in MR-405 or D-Arg29 in MR-401 did not increase the potencies.
MR-326 and five Arg29-NHCH3 or Arg29-NHCH2CH3 agonists: MR-401, MR-403, MR-406, MR-409, and MR-410 had signifi-cantly higher potencies at 30 min than at 15 min. Most of these agonists except for MR-406 contained D-configuration residues. D-amino acid residues and the specific C-terminal structure improve the resistance to enzymatic degradation and lead to prolonged endocrine potency.
Previously, we reported the beneficial effect of GHRH agonist, JI-38, on cardiac recovery after acute myocardial injury in rats. Consistent with the endocrine assays, cardiac tests with the new GHRH agonists have also demonstrated enhanced activities in the heart, confirming the beneficial role of these agonists in preventing cardiac remodeling following MI. Overall, MR-409 and MR-356 showed a slightly better capability than JI-38 to prevent/reduce ventricular remodeling and improve cardiac function after experimental acute myocardial infarction. Interestingly, MR-367 and MR-404, in spite of having a higher receptor affinity than JI-38 and a comparable one as MR-356 and MR-409, manifested no positive effect on infarct size, suggesting that the various GHRH agonists work in similar but not identical manner on cellular reparative pathways. We have no conclusive explanation for this discrepancy. Since the GHRH signaling pathway in the heart has yet to be fully characterized, it is tempting to propose that MR-367 and MR-404 may act through other intracellular signaling mechanisms, different from those activated by JI-38, MR-356 and MR-409. For instance, it is possible that the signaling induced by MR-367 and MR-404 through the GHRH receptor activates predominantly the PLC/PKC pathway, which results in release of GH from pre-existing vesicles, rather than the adenylate cyclase/PKA pathway, the latter of which was shown to be important in cAMP-dependent protection from barrier dysfunction. Our findings warrant further investigations to elucidate the role of GHRH signal transduction within the heart and its application to cardiac repair. Further tests with these GHRH agonists in the pig MI model are now in progress.
5. Conclusion
In conclusion, we have successfully developed several new GHRH agonists with significantly improved endocrine activities and stability in vivo. Manipulation of the structures of these new agonists reveals that a replacement of Dat1by N-Me-Tyr1 or a C-terminal substitution of Agm by Arg-NHCH3 or Apa30-NH2 can significantly augment the GH-releasing potency and receptor binding affinities. Further studies of these analogs are needed to establish practical medical protocols for clinical regeneration of the heart after myocardial infarction and other medical applications.
Acknowledgments
These studies were supported by the Medical Research Service of the Veterans Affairs Department, Departments of Pathology and Medicine, Division of Hematology/Oncology of the Miller School of Medicine University of Miami, South Florida Veterans Affairs Foundation for Research and Education (all to AVS), NIH RO1 HL-107110 (to JMH) and the L. Austin Weeks Endowment for Urologic Research (NLB) and grants from the Wallace H. Coulter Foundation SB26MT66142E (to AVS) for the development of GHRH agonists for translation research. This work of GH was also supported by TAMOP 4.2.2.A-11/1/KONV-2012-0025 project (GH). This project is co-financed by the European Union and the European Social Fund. Petra Popovics was supported by a stipend program of the Department of Medicine, Dresden and by the Helmholtz Alliance ICEMED (Imaging and Curing Environmental Metabolic Diseases) through the Initiative and Networking Fund of the Helmholtz Association.
Abbreviations
- Abu
α-aminobutanoyl
- Agm
agmatine
- Apa
5-aminopentanoyl
- Dat
des-amino-tyrosine
- EF
ejection fraction
- Fpa5
pentafluoro-Phe
- Gab
gamma-amino-butanoyl
- GH
growth hormone
- GHRH
GH-releasing hormone
- h
human
- IGF
insulin-like growth factor
- i.v
intravenous
- MeCN
acetonitrile
- MI
myocardial infarction
- N-Me-Tyr
N-methyl-tyrosine
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- s.c
subcutaneous
- TOF
time-of-flight
Footnotes
Author’s contributions
R.C., M.Z., and A.V.S. designed the new agonists; N.L.B. and A.V.S. designed the biological experiments; L.S., M.K., M.J., J.H., F.G.R., and P.P. performed in vivo endocrine tests; J.M.H. designed cardiological experiments; R.K.-T. performed cardiological investigations; G.H. performed binding test, T.C. determined hormone levels; W.S. performed HPLC and MS; and R.C., M.Z., M.K., T.C., N.L.B., and A.V.S. wrote the paper.
Conflict of interest
Norman L. Block and Joshua M. Hare are members of the Board of Directors of the biotechnology company, Biscayne Pharmaceuticals. Renzhi Cai, Andrew V. Schally, and Marta Zarandi are listed as coinventors in the patent on GHRH agonists, assigned to the University of Miami and VA. Other authors declare no conflict of interest.
References
- 1.Barron JL, Coy DH, Millar RP. Growth hormone responses to growth hormone-releasing hormone (1–29)-NH2 and a D-Ala2 analog in normal men. Peptides. 1985;6:575–7. doi: 10.1016/0196-9781(85)90124-x. [DOI] [PubMed] [Google Scholar]
- 2.Böhlen P, Esch F, Brazeau P, Ling N, Guillemin R. Isolation and characterization of the porcine hypothalamic growth hormone releasing factor. Biochem Biophys Res Commun. 1983;116:726–34. doi: 10.1016/0006-291x(83)90585-5. [DOI] [PubMed] [Google Scholar]
- 3.Böhlen P, Wehrenberg WB, Esch F, Ling N, Brazeau P, Guillemin R. Rat hypothal-amic growth hormone-releasing factor: isolation, sequence analysis and total synthesis. Biochem Biophys Res Commun. 1984;125:1005–12. doi: 10.1016/0006-291x(84)91383-4. [DOI] [PubMed] [Google Scholar]
- 4.Boulanger L, Lazure C, Lefrançois L, Gaudreau P. Proteolytic degradation of rat growth hormone-releasing factor(1–29) amide in rat pituitary and hypothalamus. Brain Res. 1993;616:39–47. doi: 10.1016/0006-8993(93)90189-t. [DOI] [PubMed] [Google Scholar]
- 5.Brazeau P, Böhlen P, Esch F, Ling N, Wehrenberg WB, Guillemin R. Growth hormone-releasing factor from ovine and caprine hypothalamus: isolation, sequence analysis and total synthesis. Biochem Biophys Res Commun. 1984;125:606–14. doi: 10.1016/0006-291x(84)90582-5. [DOI] [PubMed] [Google Scholar]
- 6.Campbell RM, Bongers J, Felix AM. Rational design, synthesis, and biological evaluation of novel growth hormone releasing factor analogues. Biopolymers. 1995;37:67–88. doi: 10.1002/bip.360370204. [DOI] [PubMed] [Google Scholar]
- 7.Campbell RM, Lee Y, Rivier J, Heimer EP, Felix AM, Mowles TF. GRF analogs and fragments: correlation between receptor binding, activity and structure. Peptides. 1991;12:569–74. doi: 10.1016/0196-9781(91)90103-v. [DOI] [PubMed] [Google Scholar]
- 8.Campbell RM, Stricker P, Miller R, Bongers J, Liu W, Lambros T, et al. Enhanced stability and potency of novel growth hormone-releasing factor (GRF) analogues derived from rodent and human GRF sequences. Peptides. 1994;15:489–95. doi: 10.1016/0196-9781(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 9.Coy DH, Murphy WA, Lance VA, Heiman ML. Differential effects of N-terminal modifications on the biological potencies of growth hormone releasing factor analogues with varying chain lengths. J Med Chem. 1987;30:219–22. doi: 10.1021/jm00384a039. [DOI] [PubMed] [Google Scholar]
- 10.Dioufa N, Schally AV, Chatzistamou I, Moustou E, Block NL, Owens GK, et al. Acceleration of wound healing by growth hormone-releasing hormone and its agonists. Proc Natl Acad Sci USA. 2010;107:18611–5. doi: 10.1073/pnas.1013942107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esch F, Böhlen P, Ling N, Brazeau P, Guillemin R. Isolation and characterization of the bovine hypothalamic growth hormone releasing factor. Biochem Biophys Res Commun. 1983;117:772–9. doi: 10.1016/0006-291x(83)91664-9. [DOI] [PubMed] [Google Scholar]
- 12.Felix AM, Heimer EP, Wang CT, Lambros TJ, Fournier A, Mowles TF, et al. Synthesis, biological activity and conformational analysis of cyclic GRF analogs. Int J Pept Protein Res. 1988;32:441–54. doi: 10.1111/j.1399-3011.1988.tb01375.x. [DOI] [PubMed] [Google Scholar]
- 13.Felix AM, Lu YA, Campbell RM. Pegylated peptides. IV. Enhanced biological activity of site-directed pegylated GRF analogs. Int J Pept Protein Res. 1995;46:253–64. [PubMed] [Google Scholar]
- 14.Frohman LA, Downs TR, Heimer EP, Felix AM. Dipeptidylpeptidase IV and trypsin-like enzymatic degradation of human growth hormone-releasing hormone in plasma. J Clin Invest. 1989;83:1533–40. doi: 10.1172/JCI114049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frohman LA, Downs TR, Williams TC, Heimer EP, Pan YC, Felix AM. Rapid enzymatic degradation of growth hormone-releasing hormone by plasma in vitro and in vivo to a biologically inactive product cleaved at the NH2 terminus. J Clin Invest. 1986;78:906–13. doi: 10.1172/JCI112679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillemin R, Brazeau P, Böhlen P, Esch F, Ling N, Wehrenberg WB. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982;218:585–7. doi: 10.1126/science.6812220. [DOI] [PubMed] [Google Scholar]
- 17.Halmos G, Rekasi Z, Szoke B, Schally AV. Use of radioreceptor assay and cell superfusion system for in vitro screening of analogs of growth hormone-releasing hormone. Receptor. 1993;3:87–97. [PubMed] [Google Scholar]
- 18.Izdebski J, Pinski J, Horvath JE, Halmos G, Groot K, Schally AV. Synthesis and biological evaluation of superactive agonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1995;92:4872–6. doi: 10.1073/pnas.92.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanashiro-Takeuchi RM, Takeuchi LM, Rick FG, Dulce R, Treuer AV, Florea V, et al. Activation of growth hormone releasing hormone (GHRH) receptor stimulates cardiac reverse remodeling after myocardial infarction (MI) Proc Natl Acad Sci USA. 2012;109:559–63. doi: 10.1073/pnas.1119203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanashiro-Takeuchi RM, Tziomalos K, Takeuchi LM, Treuer AV, Lamirault G, Dulce R, et al. Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA. 2010;107:2604–9. doi: 10.1073/pnas.0914138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubiak TM, Friedman AR, Martin RA, Ichhpurani AK, Alaniz GR, Claflin WH, et al. Position 2 and position 2/Ala15-substituted analogs of bovine growth hormone-releasing factor (bGRF) with enhanced metabolic stability and improved in vivo bioactivity. J Med Chem. 1993;36:888–97. doi: 10.1021/jm00059a014. [DOI] [PubMed] [Google Scholar]
- 22.Lance VA, Murphy WA, Sueiras-Diaz J, Coy DH. Super-active analogs of growth hormone-releasing factor (1–29)-amide. Biochem Biophys Res Commun. 1984;119:265–72. doi: 10.1016/0006-291x(84)91647-4. [DOI] [PubMed] [Google Scholar]
- 23.Lin-Su K, Wajnrajch MP. Growth hormone releasing hormone (GHRH) and the GHRH receptor. Rev Endocr Metab Disord. 2002;3:313–23. doi: 10.1023/a:1020949507265. [DOI] [PubMed] [Google Scholar]
- 24.Ling N, Baird A, Wehrenberg WB, Ueno N, Munegumi T, Chiang TC, et al. Synthesis and in vitro bioactivity of human growth hormone-releasing factor analogs substituted at position-1. Biochem Biophys Res Commun. 1984;122:304–10. doi: 10.1016/0006-291x(84)90475-3. [DOI] [PubMed] [Google Scholar]
- 25.Lucas R, Sridhar S, Rick FG, Gorshkov B, Umapathy NS, Yang G, et al. Agonist of growth hormone-releasing hormone reduces pneumolysin-induced pulmonary permeability edema. Proc Natl Acad Sci USA. 2012;109:2084–9. doi: 10.1073/pnas.1121075109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci USA. 2012;109:5022–7. doi: 10.1073/pnas.1201868109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ludwig B, Ziegler CG, Schally AV, Richter C, Steffen A, Jabs N, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci USA. 2010;107:12623–8. doi: 10.1073/pnas.1005098107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayo KE, Miller TL, DeAlmeida V, Zheng J, Godfrey PA. The growth-hormone-releasing hormone receptor: signal transduction, gene expression, and physiological function in growth regulation. Ann N Y Acad Sci. 1996;805:184–203. doi: 10.1111/j.1749-6632.1996.tb17483.x. [DOI] [PubMed] [Google Scholar]
- 29.McPherson GA. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985;14:213–28. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- 30.Montero M, Yon L, Kikuyama S, Dufour S, Vaudry H. Molecular evolution of the growth hormone-releasing hormone/pituitary adenylate cyclase-activating polypeptide gene family. Functional implication in the regulation of growth hormone secretion. J Mol Endocrinol. 2000;25:157–68. doi: 10.1677/jme.0.0250157. [DOI] [PubMed] [Google Scholar]
- 31.Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980;107:220–39. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- 32.Penna C, Settanni F, Tullio F, Trovato L, Pagliaro P, Alloatti G, et al. GH-releasing hormone induces cardioprotection in isolated male rat heart via activation of RISK and SAFE pathways. Endocrinology. 2013;154:1624–35. doi: 10.1210/en.2012-2064. [DOI] [PubMed] [Google Scholar]
- 33.Rivier J, Spiess J, Thorner M, Vale W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature. 1982;300:276–8. doi: 10.1038/300276a0. [DOI] [PubMed] [Google Scholar]
- 34.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 35.Schmid J, Ludwig B, Schally AV, Steffen A, Ziegler CG, Block NL, et al. Modulation of pancreatic islets-stress axis by hypothalamic releasing hormones and 11beta-hydroxysteroid dehydrogenase. Proc Natl Acad Sci USA. 2011;108:13722–7. doi: 10.1073/pnas.1110965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schubert U, Schmid J, Lehmann S, Zhang XY, Morawietz H, Block NL, et al. Transplantation of pancreatic islets to adrenal gland is promoted by agonists of growth-hormone-releasing hormone. Proc Natl Acad Sci USA. 2013;110:2288–93. doi: 10.1073/pnas.1221505110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zarandi M, Csernus V, Bokser L, Bajusz S, Groot K, Schally AV. Synthesis and in vitro and in vivo activity of analogs of growth hormone-releasing hormone (GH-RH) with C-terminal agmatine. Int J Pept Protein Res. 1990;36:499–505. doi: 10.1111/j.1399-3011.1990.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 38.Zarandi M, Horvath JE, Halmos G, Pinski J, Nagy A, Groot K, et al. Synthesis and biological activities of highly potent antagonists of growth hormone-releasing hormone. Proc Natl Acad Sci USA. 1994;91:12298–302. doi: 10.1073/pnas.91.25.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zarandi M, Serfozo P, Zsigo J, Bokser L, Janaky T, Olsen DB, et al. Potent agonists of growth hormone-releasing hormone. Part I Int J Pept Protein Res. 1992;39:211–7. doi: 10.1111/j.1399-3011.1992.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 40.Zarandi M, Serfozo P, Zsigo J, Deutch AH, Janaky T, Olsen DB, et al. Potent agonists of growth hormone-releasing hormone. II. Pept Res. 1992;5:190–3. [PubMed] [Google Scholar]